
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bee Sci. , 11 October 2023
Sec. Bees in Pollination
Volume 1 - 2023 | https://doi.org/10.3389/frbee.2023.1253157
This article is part of the Research Topic Horizons in Bee Science View all 10 articles
As pollinator-dependent crops continue to expand globally, management strategies are needed to meet the current demand for pollination services. Improving the efficiency of pollinators depends on knowledge about crop plant biology as well as pollinator behavior. In this sense, we will review the scope and challenges of implementing a targeted pollination strategy based on the behavioral individual and social plasticity of the honey bee Apis mellifera. Here we summarize current knowledge on the bees´ ability to perceive, learn and generalize floral odors, the bias of their foraging preferences after in-hive experiences and the transfer of food source information within the social context of the colony, all aspects that impact on foraging decisions and can be used to direct pollinators to target crops. We focused on describing how key olfactory cues that mimic crop floral scents are acquired in the hive and propagate among colony mates to guide foraging to specific crops. Knowledge gaps, including volatiles variability between flowers of the same or different crop varieties, alternative managed pollinators, and potential impact on food industry are discussed.
In the last 70 years, the agricultural area devoted to pollinator-dependent crops has increased monotonically (Aizen et al., 2019). Animal pollination, mostly bee pollination, directly affects the yield of 87 of 115 leading single crops (Klein et al., 2007). Given the central place that pollination services have achieved in agriculture, it is necessary to improve the efficiency of pollinators, in particular of those managed by humans, like the honey bee. The challenge of improving pollination services depends on several factors, including knowledge about plant biology and pollination requisites, landscape features, environmental conditions, as well as the pollinator needs (McGregor, 1976; Free, 1993; Delaplane and Mayer, 2000; Abrol, 2012). In particular, improving pollination by managed honey bees requires monitoring the colonies introduced into the crop to assess levels of foraging activity and the resources collected before, during and after the blooming period. This knowledge allows the design of a pollinator management strategy to define the number, placement, and timing of colony introduction to obtain high yields. In addition, healthy and populous colonies are essential to ensure the success of pollination service (McGregor, 1976; Free, 1993; Delaplane and Mayer, 2000; Abrol, 2012).
The crop requirements and the management of its pollinators are covered within the topic known as “managed pollination” or “directed pollination” (Delaplane and Mayer, 2000; Vásquez Romero et al., 2011; Rosa et al., 2018). However, this area does not consider aspects of the individual and social behavior of honey bees, the most commonly managed pollinator worldwide, which directly impacts the pollination services provided. For instance, honey bees exhibit behaviors that advertise and recruit nestmates to the most profitable food sources. In this sense, honey bees have the ability to communicate spatial information about profitable sites through the waggle dance (a figure-of-eight maneuver on the vertical wax combs), and to transfer food-related information, such as scents or tastes, through mouth-to-mouth trophallactic food exchanges among nestmates (von Frisch, 1967; Farina et al., 2005). So far, honey bee plastic behavioral responses to new conditions required by crop pollination management, either by moving hives between environments that offer different floral availability, and/or after the sudden onset of a massive and dominant blooming, are seldom considered for pollination services. Furthermore, honey bees’ orientation and navigation abilities, as well as their capacity to learn floral-related cues, were often neglected. Within the behavioral sciences, these aspects are covered by cognitive ecology (Dukas, 1998), which considers how animals obtain and process information from their environments, and how they relate and use such information to make decisions according to their perception and learning abilities (Healy and Braithwaite, 2000).
Honey bees can visit a wide range of flower types as long as the resources offered are profitable (Visscher and Seeley, 1982; Steffan-Dewenter and Kuhn, 2003). Regardless of their generalist foraging strategy, honey bees exhibit fidelity to a single plant species within the same foraging bout (von Frisch, 1967). Such behavior is known as flower constancy (Free, 1963; von Frisch, 1967) and it implies that experiences with flowers that offer sufficient reward (pollen and/or nectar) encourage bees to keep collecting on the same floral type (Menzel and Erber, 1978; Chittka et al., 1999). It may vary according to the quantity and quality between the food sources (Wells and Wells, 1986). Thus, floral constancy together with the ability to communicate food-related information (location, profitability and chemosensory cues) within the nest (von Frisch, 1967; Farina et al., 2005), make the honey bee an efficient pollinator throughout a broad spectrum of agricultural settings (McGregor, 1976; Free, 1993).
The first attempts to improve food production in agricultural landscapes considering the plastic behavior of the honey bee were reported in the famine time before and during the World War II by different research groups from Germany and the ex-Soviet Union. In that time, different procedures to direct pollinators to target crops were based on the seminal study of von Frisch (1923), showing that recruited forager bees were prone to visit flowers of the same species previously exploited by scouting colony mates. von Frisch observed that the efficiency of the recruitment to a target feeding site depended on the distance to the hive and on the presence of floral odors, which were either emitted by parts of flowers attached to a feeder or diluted in the food. von Frisch noted that the offering of scented food enabled a better recruitment, likely because the chemical properties of the odors were better maintained until the liquid food was shared via trophallaxis with the colony mates. Pioneering practices to promote bee responses to target crops were then based on soaking fragrant flowers in sugar water, which produced a scented solution expected to bias foraging towards the target flowers (Smaragdova, 1933; Gubin, 1936; Gubin, 1938; von Frisch, 1943; von Frisch, 1947). The use of in-hive scented-food stimulation showed increases both in the number of bees that visited the crop (Gubin, 1938; Sorokin, 1938; Komarow, 1939) and in seed yields (Sorokin, 1938; von Frisch, 1947). von Frisch also tested the offering of scented sugar solution outside the hive (i.e., in the crop surroundings; von Frisch, 1943; von Frisch, 1947) and proved that this procedure was effective in increasing both the colony activity level, and the amount of honey produced (von Frisch, 1943), likely as it promotes the display of dances. However, a study of Free (1958) in apple and red clover crops using either the offering of scented sugar solution outside or inside the hive, or the combination of both, showed no evidence of increases in crop yields. Later, Free (1969) tested the extent to which the odor of nectar stored in combs affected foraging preferences in a double-choice test. Although the results were highly variable, Free was able to detect a brief biased response to the odor present in the honeycomb.
Despite their relative success in guiding bees to target crops, procedures that soak fragrant flowers in sugar water have several disadvantages, such as the poor stability of the odor extracted from the flowers and the cost involved in obtaining large quantities of flowers to achieve a stimulus sufficiently intense to modify bee responses. Furthermore, the cutting and crushing of flowers for the syrup preparation may promote the release of unwanted volatiles, related to tissue damage or wilting, being a strong source of variation among results of pioneering studies. For this reason, it is relevant to integrate aspects related to floral odors and honey bee social behavior as part of a targeted pollination strategy. With this in mind, the objective of this review is to summarize some pertinent elements related to individual and social honey bee learning of floral scents that affect foraging responses, which are potentially applicable for guiding bees to target crops to enhance pollination services. Floral volatiles of specific crops, honey bee odor perception, social foraging, and the procedures in the field will also be discussed as necessary components within the targeted pollination framework (Figure 1).

Figure 1 The targeted pollination framework integrates aspects related to floral odors and honey bee social behavior. Floral volatile organic compounds (VOCs) of specific crops are collected and identified to determine a set of potential odor mixtures that mimic the bouquet of the target crop flower. The odorant mimic which honey bees broadly generalize to the natural floral bouquet, but which is also the less discriminating, is selected as the mimic odor to be evaluated in the field. The circulation of sugar syrup scented with the mimic odor inside the colony establishes specific olfactory memories among nestmates. The propagation and persistence of the food-related information at the colony level releases recruiting mechanisms and foraging toward the target crop, consequently improving pollination services (adapted from Farina et al., 2020). Reproduced with permission from Farina and co-workers, Current Biology; published by Cell Press, 2020 (CC-BY 4.0).
Floral bouquets are complex mixtures of volatile organic compounds (VOCs) which are directly involved in plant-pollinator interactions (Knudsen et al., 1993; Raguso, 2008; Pichersky and Dudareva, 2020). Volatile emissions can be altered by several factors, such as cultivar, time of day and pollination status (Rodriguez-Saona et al., 2011; Twidle et al., 2017). Depending on the identity and concentration of the VOCs emitted by the different plant species, diverse specific pollinator groups are attracted (Dobson, 2006). In particular, the olfactory cues that honey bees use to perceive specific flowers has been investigated for different crops, such as oilseed rape (Wadhams et al., 1994), kiwifruit (Twidle et al., 2015), pear (Su et al., 2022) and other Brassicaceae species (Kobayashi et al., 2012), among others. In such studies, honey bee odor detection was assessed by means of electro-antennography (EAG) where the antennal response towards the different floral bouquets is measured. It is well known that, although plants emit large amounts of VOCs, honey bees detect a small subset of these compounds or key odorants (Reinhard et al., 2010; Mas et al., 2020). Moreover, not all odors detected by the peripheral olfactory system are behaviorally meaningful, and most must be learned before they can influence behavior (Riffell et al., 2009b; Menzel, 2012).
Many odors, which initially are neutral to honey bee foragers, may become good predictors of food sources after being learned (von Frisch, 1967; Lindauer, 1970; Gould, 1984; Menzel, 2012). Learning allows individuals to flexibly respond to a changing environment (Menzel, 1999), with varying availability of food sources during the season (Núñez, 1977; Vogel, 1983), being extremely important in species with generalist habits. In this way, honey bees as well other pollinators are able to associate floral cues, such as odors and colors, with the rewards (nectar, pollen) that the source provides (Gould, 1984; Chittka and Thomson, 2001). If bees repeat cue-reward experiences, these associations turn into memories that influence foraging behaviors, by biasing flight orientation (Chaffiol et al., 2005; Nery et al., 2021), landing (Arenas et al., 2007; Arenas et al., 2008), and/or extension of the proboscis (Grüter et al., 2006; Arenas and Farina, 2012). The latter is an innate reflex response that occurs when a bee’s antennae contact the nectar of a flower, leading to an immediate ingestion of the food. In the laboratory, the proboscis extension response (PER) can be evoked by touching the antennae of restrained bees with an enough concentrated sucrose solution (Kuwabara, 1957; Takeda, 1961). Moreover, bees can be trained to associate an odor with a sucrose reward, by means of an olfactory conditioning protocol (Takeda, 1961; Bitterman et al., 1983). Prior to conditioning, bees do not usually respond to the conditioned stimulus (CS), but after successive paired presentations between the odor and a sucrose reward, the previously neutral stimulus now takes control over the proboscis reflex.
Within the PER paradigm, it is possible to train bees to learn that an odor predicts an oncoming reward in the absence of other alternative stimuli, in the so-called absolute conditioning (Giurfa, 2007; Giurfa and Sandoz, 2012). This protocol allows us to test the extent to which the conditioned response can be generalized to different but equivalent stimuli. The phenomenon of generalization is widespread among animal kingdom (Shepard, 1987; Ghirlanda and Enquist, 2003), and it is essential for the foraging behavior of the bees since it enables foragers to respond to different floral odors if they are perceived as similar (Pham-Delegue et al., 1989; Guerrieri et al., 2005). Alternatively, bees trained in a differential conditioning learn not only the characteristics of a reinforced stimulus (rewarded conditioned stimulus, henceforth: CS+), but also those of a nonreinforced one (non-rewarded conditioned stimulus, henceforth: CS-) (Bitterman et al., 1983; Giurfa, 2007; Giurfa and Sandoz, 2012). This protocol allows us to evaluate bees’ abilities to discriminate between both conditioned stimuli. Combined, the two types of conditionings enable investigating how bees learn, generalize and discriminate odorant mixtures.
Previous studies about insect behavior demonstrated that plant-pollinator interactions can be mediated by a few key odorants (Riffell et al., 2009a; Riffell et al., 2009b; Reinhard et al., 2010; Mas et al., 2020). Using the moth Manduca sexta as a study model, it was observed that food source attraction and innate foraging behavior could be elicited by a few of the volatile compounds that conform the natural flower bouquet (Riffell et al., 2009a; Riffell et al., 2009b). The evidence that a few key volatiles are sufficient to account for a complex odor mixture is not limited to innate behaviors but extends to learned responses as well. In honey bees, response to mixtures composed of a few selected key odorants (some of only 3 pure compounds) were sufficient to elicit levels of PER comparable to those evoked by the complete olfactory mixtures of 14 odorants to which the bees were initially conditioned (Reinhard et al., 2010). The key odorant processing of floral scents may be adaptive to maintain stimulus identity in a constantly changing environment while it gives us the possibility for using simple mixtures to mimic the complex floral scent of a species of interest to manipulate odor-mediated responses of pollinators. In fact, it has been recently shown that the conditioning of synthetic mixtures with 3 or 4 constituents could be enough to successfully generalize the natural floral scent of agriculturally important species, such as sunflower, pear, apple, and almond (see Figure 2A as example), which in turn resulted in a bias of the bees’ foraging behavior towards the target crop (Farina et al., 2020; Farina et al., 2022; Farina et al., 2023).
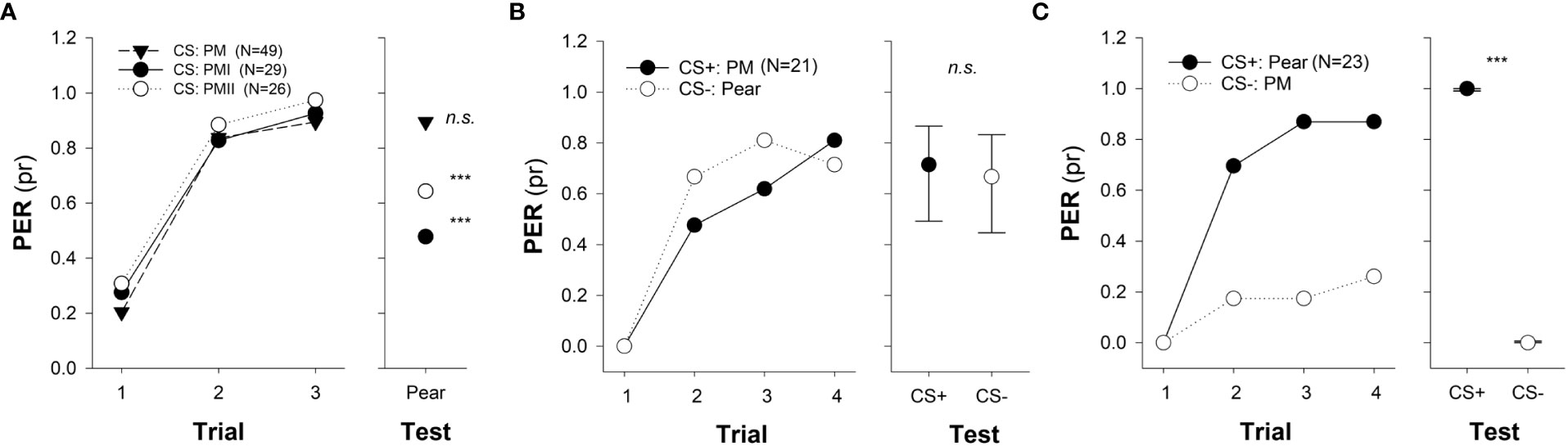
Figure 2 Odor generalization and discrimination of memories from pear mimic odors to natural floral scents. (A) Odor generalization was tested towards the single unrewarded presentation of the pear natural odor (right panel) after one of the pear mimics (PM, PMI or PMII) was used as conditioned stimulus (CS) during an absolute three-trials classical conditioning of the proboscis extension reflex (PER; left panel). Asterisks indicate significant differences between responses obtained at the third conditioning trial and the test (***, p < 0.001). No significant difference (n.s.) indicates that bees could successfully generalize PM to the pear natural scent (test). (B, C) Discrimination was evaluated towards the single presentation of the pear natural odor and the pear mimic (PM) at the test (right panel) after a four-pair-of-trails differential PER conditioning (left panel), for which both odors were used as rewarded (CS+) and non- rewarded stimulus (CS-). (B) Pear natural odor (floral natural scent) was used as CS- and the pear mimic (PM) as CS+. No difference (n.s.) at test indicates that bees could not discriminate between PM and the unrewarded pear natural scent. (C) Pear natural odor (natural floral scent) was used as CS+ and PM as CS-. Asterisks indicate significant differences between tested odors (***, p<0.001). The experimental subjects were all foraging bees and had no previous access to any pear tree. Numbers between brackets indicate sample size. Circles indicate the probability of PER (GLMM predicted data) and bars (in test) show the 95% confidence intervals (adapted from Farina et al., 2022). Reproduced with permission from Farina and co-workers, Scientific Reports; published by Nature Portfolio, 2022 (CC-BY 4.0).
Apart from evaluating the degree of generalization of specific odor mixtures designed to mimic the scent of crop flowers, Farina and coworkers (2020; 2022; 2023) assessed to what extent bees could discriminate them from the respective natural floral scents. Differential PER conditionings using the mimic odor and the natural floral scents both as CS+ and CS- revealed that bees could only discriminate between the stimuli when the natural blend was presented as CS+ and each mimic (either for the sunflower, pear, or apple flower) as CS-. Interestingly, bees failed to distinguish between stimuli if the mimics acted as CS+ and the natural blend as CS-, indicating that discrimination was not symmetric (see Figures 2B, C as example). Such asymmetry between a small subset of key odors that make up a behaviorally effective mixture and the natural floral bouquet denotes the complexity of insect olfactory perception (Sandoz et al., 2001) and suggests that a mimic odor could be more effective in biasing foraging behavior if learned while bees remain naive to crop flower´s olfactory cues than in experienced individuals.
Considering that memories decay in time (Menzel, 1999), some studies focused on the effect of the addition of nonsugar nectar compounds on honey bee olfactory associative learning with the aim to establish more stable long-term memories (Wright et al., 2013; Marchi et al., 2021). On the one hand, the alkaloid caffeine triggers long-term memory in honey bee (Wright et al., 2013), meanwhile the essential amino acid arginine participates in the synthesis of nitric oxide, and therefore promotes protein synthesis during long-term memory formation (Müller, 1996; Müller, 1997). A recent related study showed a positive effect of the combination of caffeine and arginine in the reward, increasing bees’ learning performance and long-term memory formation (Marchi et al., 2021). Thus, the joint administration of nonsugar nectar compounds with synthetic mimic odors could further enhance the persistence of olfactory memories and therefore, the efficacy of a procedure that aims to modify bee´s preferences based on experience.
A honey bee colony can rapidly adjust its foraging behavior and guide its workforce toward the most rewarding flowers in the surrounding environment (Seeley, 1995). Within the hive, social interactions among nestmates facilitate the propagation of food-related information, allowing not only experienced foragers to access information about other available sources, but also new recruits to locate profitable foraging sites via the waggle dance (von Frisch, 1967). There is a consensus that olfactory cues of the discovered resource play an important role in orientation at short distances (von Frisch, 1923; Sherman and Visscher, 2002). On the other hand, further studies support the idea that olfactory cues alone are not sufficient to recruit nestmates (Gould, 1984; Riley et al., 2005) and that orientation of foragers fails if olfactory stimuli from the food source to which they have been trained are relocated beyond 200 meters (Menzel and Greggers, 2013). However, odorants can assist recruits to reach the target if they are learned in the colony and within a recruiting context (Farina et al., 2005; Díaz et al., 2007).
Beside the transmission of spatial information, the dance increases the attention and activity of bees in the vicinity, attracting them to the dancer (von Frisch, 1967; Grüter and Farina, 2009; Balbuena et al., 2012a; Ai and Farina, 2023). Then, more, and highly motivated bees around the dancer can learn the floral odor molecules attached to its body (Moauro et al., 2018). Dancing bees often briefly interrupt the dance and offer food samples to surrounding bees (von Frisch, 1967; Díaz et al., 2007). These oral interactions (i.e. trophalaxis) can be very brief, but just long enough to act as a reward in olfactory learning (Díaz et al., 2007; Farina et al., 2007; Farina and Grüter 2009; Grüter and Farina, 2009). For scented nectars, trophallactic interactions enable the establishment of memories from odors diluted in the food that is being shared, an effective mechanism when scouts forage from distant sources while the odors attached to their body fade during the trip back to the hive. For pollen foragers, cues associated with pollen loads carried on the hind legs of dancers may also be perceived and learned by other foragers (Díaz et al., 2007; Nery et al., 2020) giving selectivity to recruitment (Arenas et al., 2021).
Memorization of olfactory cues within the nest, albeit outside the dancing context, could also assist recruits locate the feeding site (Balbuena et al., 2012b). Olfactory cues could also be learned from scented nectars that are unloaded to the food processor bees (Grüter et al., 2006; Grüter et al., 2009). The food odors learned inside the nest can be retained by colony mates for up to 10–11 days suggesting that olfactory experiences occurring within the colony can propagate to many individuals (Grüter et al., 2009). Moreover, circulation of scented sugar solution biases foraging preferences towards the learned odor, a response that is extended until four days after removing the scented-food stores and the combs where the syrup could have been stored (Arenas et al., 2007; Arenas et al., 2008). It is not trivial to mention that when the odor is not offered in the food but presented as a volatile that aromatizes the nest environment (Arenas et al., 2008), an avoidance rather than an improvement of the landing response towards the exposed odor is observed. These results suggest that the presentation of odors, unpaired with the reward, triggers cognitive processes other than associative learning, which prevents the nectar foragers to visit sources scented with the exposed odor. In summary, although other sensory modalities (e.g. visual) may be much more effective for long-distance flights during searching resources (Chittka and Menzel, 1992; Dyer et al., 2011; Menzel and Greggers, 2013), the use of floral odors via olfactory memories are crucial in the search for food sources when combined with other social interactions occurring in the nest.
Given that olfactory information transfer can also occur when odors are directly provided inside the nest (Arenas et al., 2007; Arenas et al., 2008), the offering of scented food can be used as a standardized procedure to establish specific long-term memories among foragers being part of a targeted pollination strategy. A common practice of beekeepers is feeding colonies with sugar syrup at certain times of the year, for instance during dearth periods of nectar (Geslin et al., 2017a; Sammataro and de Guzman, 2018; FAO et al., 2021). Furthermore, feeding colonies with syrup in the fall can ensure survival through winter and the provision of sugar syrup inside the hive stimulates brood rearing and thereby promotes foraging for pollen (Goodwin, 1997; Sammataro and de Guzman, 2018). Sugar syrup can be offered by means of in-hive feeders of different types, such as division board or top hive feeders. Such supplemental feeding practice is suitable for olfactory conditioning of colonies providing pollination services (Farina et al., 2020; Estravis-Barcala et al., 2021; Farina et al., 2022; Farina et al., 2023). Scented food can be obtained by diluting a small volume of the mimic odor (50 µL) per liter of sucrose solution (50% weight/weight, henceforth: w/w). Scented syrup can be offered using in-hive feeders or even poured over the top of the central frames of the hives (for 1,000-1,500 mL or 500 mL of scented solution, respectively).
Some important aspects to be considered in honey bee management for pollination services are colony size (Goodwin, 1997; Geslin et al., 2017a; Ovinge and Hoover, 2018; Chabert et al., 2021) and the timing of colony introduction into the plots (Free, 1959; Free et al., 1960; Al-Tikrity et al., 1972; Moeller, 1973; Sammataro and de Guzman, 2018). If colonies are introduced into the agricultural setting long before blooming, bees could forage on other attractive non-target flowers and may ignore the crop when it blooms. On the other hand, if colonies are settled when the crop is already in full bloom, they may not be able to learn the cues related to the crop flowers before blooming ends and may not have enough time to learn the landmarks needed to orient themselves. The timing of stimulation is critical as well. Feeding should be done at the beginning of the blooming period to guarantee bees an early access to relevant olfactory information which will assist them in finding the target flowers in a novel environment. It is advisable to perform the stimulation of colonies when the target crop is 10-40% in bloom. A single stimulation event should suffice to guide bees to the target crop. But it should be considered that the number of events may vary with the specific requirements of the crops and the weather conditions.
The targeted pollination strategy has the advantage of being specific to the crop, and usually requires only one application (Farina et al., 2020; Farina et al., 2022; Farina et al., 2023). This method facilitates the propagation of food related information among nestmates, which can be retrieved several days later. These studies demonstrate that specific olfactory memories established within the honey bee colony result in faster foraging that increases crop production, showing the advantages of a targeted pollination approach to enhance pollination services in commercial crops (see Supplementary Table S1).
It should be mentioned that there is a study testing the method of osmoguiding bees with a maceration and cooking of crop flowers, which failed to promote visits to the target pollen (Higuera-Higuera et al., 2023). So far, these results are inconclusive, as some of the assays need more controls to be confirmed.
In addition to the targeted pollination strategy, alternative methods to improve pollination services involve the use of non-crop-specific attractants derived from plant natural extracts or pheromonal compounds, with varying degrees of success (see studies reviewed in Delaplane and Mayer, 2000; Abrol, 2012 and illustrative examples in Supplementary Table S1). Among the first group, the spraying of crop flowers with an essential oil extracted from Lavandula hybrida leaves or with olive pomace extract showed ambiguous results in bee visits but had a positive effect on yield (Meroi Arcerito et al., 2021; Monasterio et al., 2023). Among the latter, the evaluation of commercial attractants based on pheromonal compounds (e.g. Bee Scent, Bee-Here, Pollinus and Polynate) reported mixed results. While some authors documented increases in bee visits (Higo et al., 1995) and seed yields (Przybylska et al., 2021), other studies showed no improvement of either the number of bees on the target crop or fruit set (Schultheis et al., 1994; Ellis and Delaplane, 2009; Williamson et al., 2018). These methods might be limited due to the mode of application. As not only flowers but whole plants were sprayed with the attractant (or attractant dispensers were attached to the branches), bees will not necessarily reach the target flowers and associate nectar and pollen resources with the attractant through learning, reducing the chances of a successful pollination (Knauer and Schiestl, 2015). Also, even when some pheromone-based compounds (Nasonov gland or queen mandibular pheromones) might generate an initial innate response, the repeated exposure to the attractant without a floral reward could result in the losing of the stimulus meaningfulness. This process known as habituation is well documented in bees (Scheiner, 2004).
Another group of attractants is commercialized as food lures (e.g. BeeLure, Beeline and Bee-Q) containing protein, sugars, fats, minerals and/or vitamins. They have been widely tested in several crops with limited success (for example, Rajotte and Fell, 1982; Schultheis et al., 1994; Jayaramappa et al., 2011; Dorjay et al., 2022; Jailyang et al., 2022). These studies do not fall within the scope of this review, as our aim was to focus on cognitive and behavioral aspects of the bee-crop interaction, discarding those attractants which consider bee nutritional matters.
After feeding colonies with scented sugar solution, the effect of the stimulation can be measured both from the bee perspective, on foraging-related activities, and from the crop perspective, on yield (see Supplementary Table S1). To do that, it is relevant to consider the placement of hives within the field, to avoid overlapping treatments (scented and unscented food) and to ensure bees a similar availability of flowers and, therefore, of resources. Although honey bees can forage over vast areas around the nest, up to 10 km or more if food is scarce (Jay, 1986; Beekman and Ratnieks, 2000), they prefer to forage within 1-2 km from their colonies (Seeley, 1995; Aras et al., 1996; Vaissière et al., 2011). Ideally, the groups of treated hives should be at least 2 km apart, which can be difficult to achieve in agricultural settings. Particularly, it has been observed in various crops that the number of foraging bees decreases as distance from the hives increases (Noetzel, 1968; Gary et al., 1976; Johannsmeier et al., 1997; Johannsmeier and Mostert, 2001; Hagler et al., 2011; Cunningham and Le Feuvre, 2013; Chabert et al., 2022), and that honey bees forage at an average distance of 80-1,663 m, and up to more than 6 km, from their colonies in adjacent fields depending on the crop (Gary et al., 1972; Gary et al., 1973; Gary et al., 1975; Gary et al., 1976; Gary et al., 1978; Hagler et al., 2011). Future research should further investigate bee foraging distances in different agricultural scenarios.
Display of waggle dances: To test whether the offering of scented food positively biases bee foraging choice toward the target crop, it is possible to decode waggle dances and reveal the location of their foraging sites (von Frisch, 1967; Visscher and Seeley, 1982). Therefore, waggle dances can be used as indicators to determine the spatial and seasonal ecology of honey bees in both rural and urban landscapes (Couvillon et al., 2014; Balfour and Ratnieks, 2017; Danner et al., 2017; Bänsch et al., 2020a). To that end, two-frame observation hives (each with about 4,000 workers and a mated queen) can be settled in the field. Honey bee dances can be video recorded during the experimental period. The recording times should be equally distributed to morning and afternoon hours to account for pollen and nectar availability of different plant species throughout the day. Based on the angles and the duration of the waggle runs, the observer can then identify dances recruiting toward a given target and perform a daily dance map. Finally, the percentage of dances advertising locations within the target crop at different moments of the experiment can be calculated and the time elapsed since the onset of dances can be measured (Figure 3; Farina et al., 2020).
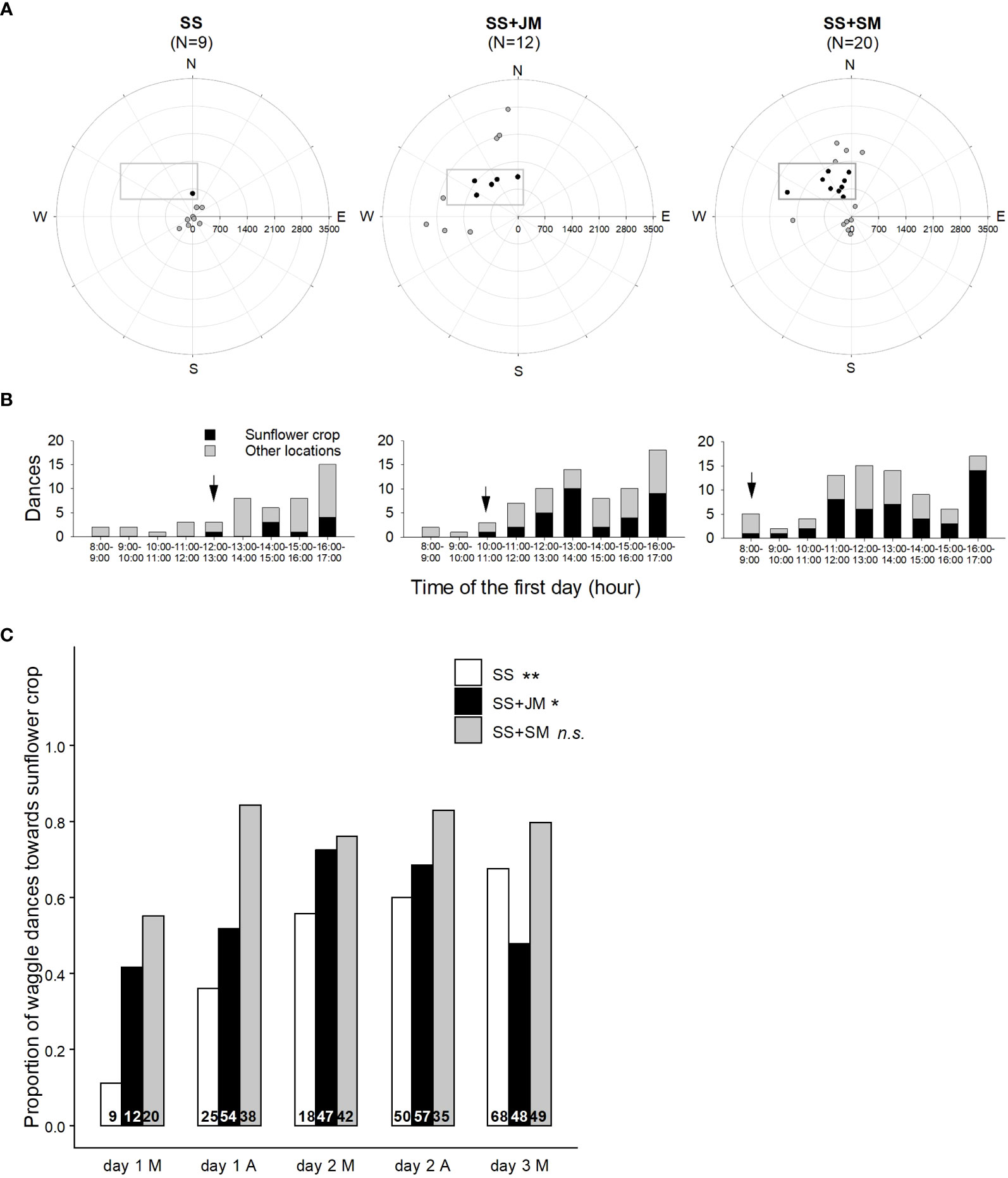
Figure 3 Effect of sunflower mimic odor on the display of waggle dances. (A) Colonies located in two-combs observation hives were fed with unscented sucrose solution (SS), sucrose solution scented with jasmine mimic (SS+JM), or sucrose solution scented with sunflower mimic (SS+SM) in a distant apiary, 2 days before the onset of the stimulation. Food scented with JM, the mimic of a flower that is not available in the surroundings, was offered as control for the unspecific effect of an odor in the solution compared to the specific mimic odor (SM). Radial maps show the foraging locations (circles) decoded by the waggle dances on the first morning of the experiment. Hives (centers) were settled 600 m SE from the sunflower plot (grey rectangles). Dances were categorized according to the location they were indicating, i.e., inside (black circles) or outside of the sunflower plot (gray circles). Numbers between brackets indicate the number of dances observed. The decoded waggle dances revealed the location of their foraging sites which showed that the offering of SM-scented food positively biased bees’ foraging choice toward the sunflower crop. (B) Distribution of waggle dances indicating the sunflower plot (black bars) or other locations (gray bars), displayed on the first day. Black arrow indicates the first dance pointing at the sunflower plot. Dances recruiting toward the target plot occurred earlier in the colony fed SS+SM. During the first morning of the experiment, more than half of the dances in this colony recruited toward the sunflower, and it increased during the afternoon (C) Distribution of waggle dances advertising resources within the sunflower plot in each colony during the mornings (M) and afternoons (A) from 1 to 3 days after moving the colonies. Display of recruiting dances in observation hives were affected by the colony treatment (Fisher’s exact two-sided test). Asterisks indicate significant differences throughout the experimental period for each treatment (**, p < 0.01; *, p < 0.05; n.s., non-significant). Differences in proportions of dancers were noticeable during the first day of the experiment, especially during the afternoon up to 84%, a value that was much higher than those exhibited by the other hives (day 1 A). Differences between colonies fed SS+SM and SS+JM persisted during the rest of the experiment. As SS-treated hive showed an increase in the proportion of dances for the sunflower plot by the end of the experiment, previously observed differences with colonies fed SS+SM were attenuated. Numbers inside bars indicate the number of dances observed. (Adapted from Farina et al., 2020). Reproduced with permission from Farina and co-workers, Current Biology; published by Cell Press, 2020 (CC-BY 4.0).
Hive entrance activity: To evaluate whether the circulation of scented food inside the hives alters foraging activity, the number of incoming bees can be assessed since a large part of these bees is expected to return from foraging sites (Fewell and Winston, 1996; Díaz et al., 2013), while another part is expected to be learning foragers operating orientation flights (Capaldi et al., 2000; Degen et al., 2015). When successful foragers return to the hive and display dances, the activation or reactivation of unemployed foragers is promoted, as well as, in a minor proportion, of those nestmates ready to initiate foraging tasks (Lindauer, 1954; Seeley, 1986; Seeley, 1995; Thom et al., 2007). Incoming foragers at the entrance of the hive can be counted for a short period (1 min) at the same time on consecutive days (Delaplane et al., 2013a; Farina et al., 2020; Estravis-Barcala et al., 2021; Farina et al., 2022; Farina et al., 2023; see Figures 4A, 5A as examples). Ideally, 3 to 5 independent measurements should be done before feeding the colonies to control for environmental conditions, pre-existing colony differences and behavioral inertia (Rodet and Henry, 2014). Then, using a Before-After-Control-Impact (BACI) design is a robust approach to analyze the effect of feeding colonies with scented food on the hive entrance activity by testing the interaction between the periods ‘before’/’after treatment’ and the treatments ‘control’/’scented food’ (Christie et al., 2019; Christie et al., 2020; Wauchope et al., 2021).
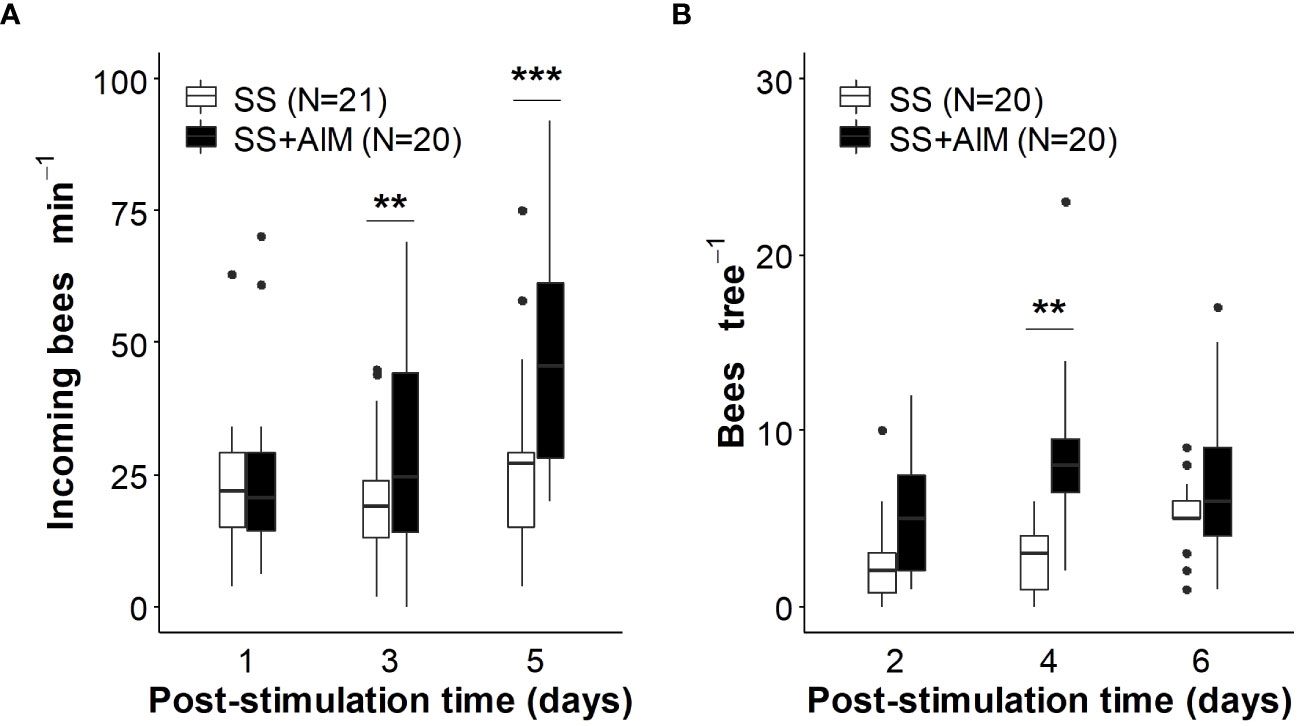
Figure 4 Effect of almond mimic odor on bees´ foraging related activity. Colonies were stimulated with unscented sucrose solution (SS) or almond mimic scented sucrose solution (SS+AlM). (A) The number of incoming bees per minute was monitored up-to 5 days post-stimulation. (B) The density of bees foraging on almond flowers was quantified in trees within 40 m of the treated beehives up-to 6 days post-stimulation. Boxplots (observed data) show the median and interquartile range (IQR), with whiskers showing the maximum value within 1.5 IQR, and individual points mark showing values outside this range. Asterisks indicate significant differences between treatments (**, p < 0.01, ***, p < 0.001, GLMM predicted data). Numbers between brackets indicate sample size (adapted from Farina et al., 2023). Reproduced with permission from Farina and co-workers, Apidologie; published by Springer, 2023 (CC-BY 4.0).
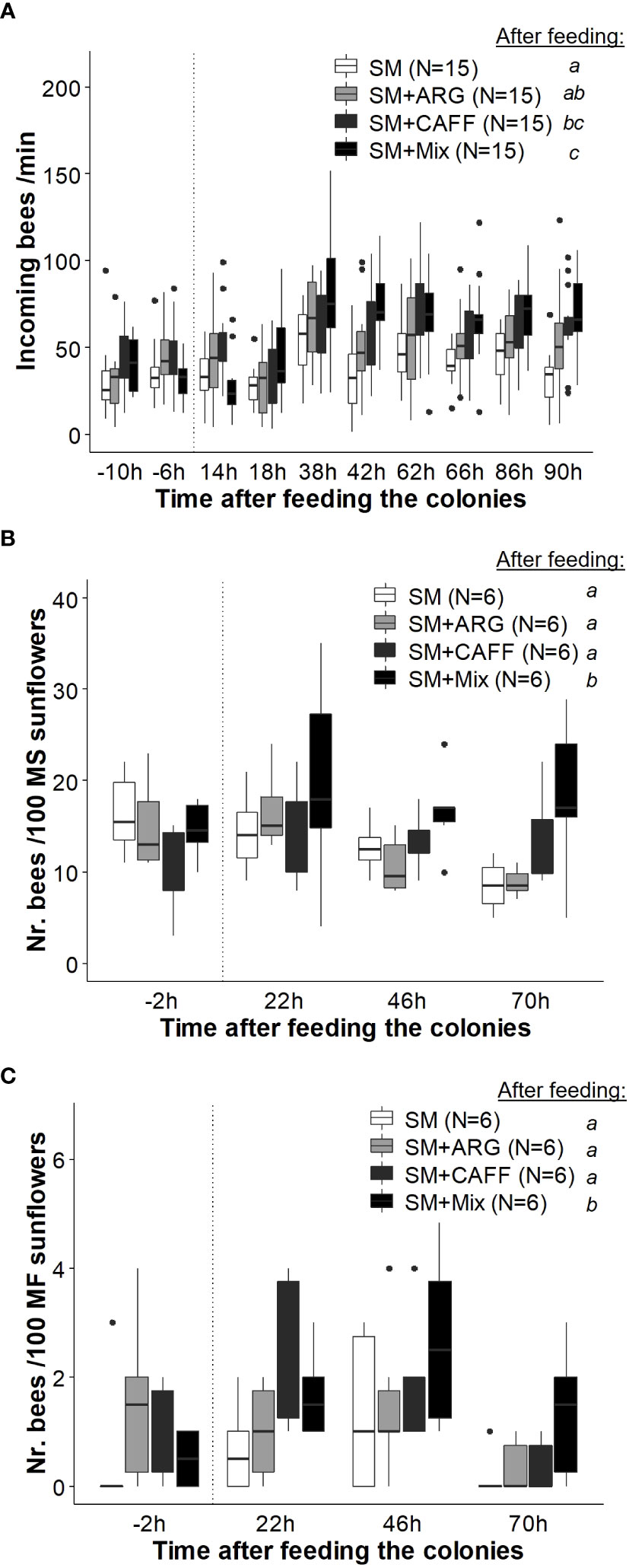
Figure 5 Effect of the sunflower mimic (SM) combined with nectar’s nonsugar compounds on honey bee foraging. Colonies providing pollination services in a field of sunflower hybrid seed production were fed: SM-scented food (as control), and SM-scented food supplemented with either caffeine (SM+CAFF), l-arginine (SM+ARG), or a mixture of both compounds (SM+Mix). (A) Rate of incoming bees before (− 10, − 6 h) and after the offering of the treatments (up to 90 h). (B) Honey bee density on male sterile (MS) sunflower heads in the surroundings of the treated colonies. (C) Honey bees’ density on male fertile (MF) sunflower inflorescences in the surroundings of treated colonies. Boxplots show the median and interquartile range (IQR), with whiskers showing the maximum value within 1.5 IQR, and individual points mark values outside this range. The vertical dotted line indicates the administration of the treatments. Different letters indicate significant differences (p < 0.05) for each treatment after feeding the colonies as assessed with post hoc comparisons. Numbers between brackets indicate sample size (adapted from Estravis-Barcala et al., 2021). Reproduced with permission from Estravis-Barcala and co-workers, Scientific Reports; published by Nature Portfolio, 2021 (CC-BY 4.0).
Pollen collection: The effect of the stimulation with scented food on pollen collection can be assessed by measuring the abundance and weight of corbicular pollen loads. The measurement of these variables is particularly pertinent when honey bees actively exploit this resource in the target crop (Hoover and Ovinge, 2018; Bänsch et al., 2020b), but see below the Case studies section for more discussion. Pollen loads from returning foragers can be collected using conventional pollen traps (frontal-entrance trap), consisting of a wooden structure with a removable metal mesh inside (Delaplane et al., 2013a). The traps should be placed at the hive at the same time on consecutive days, depending on the timing of the crop pollen availability. Ideally, 3 to 5 independent measurements should be done before feeding the colonies as mentioned before. Pollen pellets can be identified as coming either from the target crop or from other competing floral sources based on their color, by comparison with pellets obtained from bees captured foraging on the crop. Finally, the number and weight of the target pollen loads can then be determined (Farina et al., 2020; Farina et al., 2022).
Another way to assess honey bee pollen collection is to quantify the pollen reserves by estimating the amount of stored pollen inside the colony. For this purpose, colonies are thoroughly inspected by sequentially removing frames and recording the area occupied by cells containing pollen on both sides of each frame (Delaplane et al., 2013b; Farina et al., 2023). This measurement must be done before stimulation and at a defined time later, to evince any difference in pollen foraging. The interval of time can be set according to the blooming period of the target crop.
Crop foraging activity: To evaluate whether the offering of scented food affects honey bee foraging intensity on the target crop, densities of foragers visiting the target flowers in the surroundings of the colonies can be assessed. Ideally, foragers can be assessed in all the field, until 2 km away from the colonies (Vaissière et al., 2011). Forager density can be measured by scan sampling on a fixed number of open flowers or inflorescences along a row in herbaceous crops, or in focal trees in orchards (Vaissière et al., 2011; Farina et al., 2020; Estravis-Barcala et al., 2021; Farina et al., 2023; see Figures 5B, C, 4B, respectively, as examples). These measurements must be repeated at the same time (during peak hours of foraging) on consecutive days. As mentioned above for the other variables, ideally, 3 to 5 independent measurements should be done before feeding the colonies as for previous variables. It should be kept in mind that to estimate the floral resources available to honey bees in the field, the recording of flower density or phenology of the crop should be done at the same time as assessing the bee density (Vaissière et al., 2011; Estravis-Barcala et al., 2021).
Although many factors not related to the pollination level during flowering can interfere with the crop production variables, it is possible to evaluate the contribution of honey bee pollination on yield (Vaissière et al., 2011), as long as the crop yield potential is properly controlled with hand pollination treatments (Chabert et al., 2022). Fruit set (the proportion of flowers that develop into mature fruits) is usually correlated to crop yield and it can be strongly affected by pollinator visitation in a wide variety of crop systems (Garibaldi et al., 2016; Reilly et al., 2020). Thus, the effect of the offering of scented food on the target crop yield can be evaluated by means of the fruit set and/or seed set depending on the crop (Estravis-Barcala et al., 2021; Farina et al., 2022). Another way to assess crop yield is by quantifying fruit production (number of fruits and fruit mass) at plant/tree level (Sáez et al., 2020; Estravis-Barcala et al., 2021; Farina et al., 2022). At this small scale, fruit production (number and weight of fruits) should be estimated ideally in specimens in all the field, until 2 km away from the colonies (Vaissière et al., 2011). At larger scales, yield is usually reported by the producers as total fruit weight per unit area (Farina et al., 2020; Farina et al., 2022; see Figure 6 as examples).
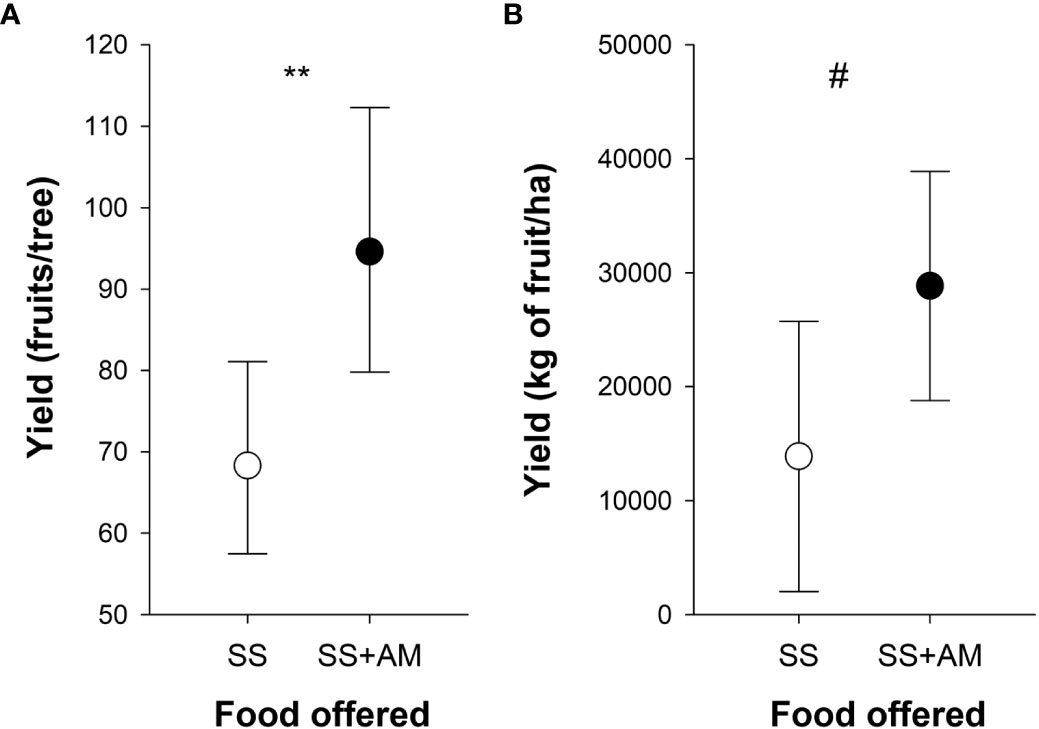
Figure 6 Effect of the apple mimic odor on fruit yield and on plantation yield. (A) Fruit yield was calculated as the counts of fruits (fruit set) per tree in two apple plots, where 30 trees were surveyed. Colonies that provided for each apple plot were fed with apple mimic-scented sucrose solution (SS+AM) or with unscented sucrose solution (SS). (B) Crop yield was obtained either from 11 apple plots provided with 130 colonies in total that had been fed with apple mimic-scented sucrose solution (SS+AM) or from 11 apple plots provided with 139 colonies that had been fed with unscented sucrose solution (SS). Asterisks indicate significant differences between the treatments (**, p < 0.01; #, p = 0.06). Symbols indicate the mean values (GLMM predicted data) and bars show the 95% confidence intervals (adapted from Farina et al., 2022). Reproduced with permission from Farina and co-workers, Scientific Reports; published by Nature Portfolio, 2022 (CC-BY 4.0).
In the last decades, several management methods were developed in an attempt to improve honey bee pollination of crops with ambiguous results (Goodwin, 1997; Delaplane and Mayer, 2000). Recently, the use of mimic odors based on crop floral volatiles has proven to be successful in guiding honey bees toward a target crop, which in turn positively affected foraging activity in systems highly dependent on pollinators, such as sunflower for hybrid seed production, apple, pear and almond, and consequently, increased yields (Farina et al., 2020; Farina et al., 2022; Farina et al., 2023). Although the mentioned species are mass-flowering crops, offering plentiful floral resources for bees, they differ in the type of plantation. While sunflower and almond are usually grown on large-scale monoculture fields and orchards (Farina et al., 2020; Estravis-Barcala et al., 2021; Farina et al., 2023), apple and pear trees are cultivated at a much smaller scale (< 10 ha) and sometime coexist within the same orchard (Díaz et al., 2013; Quinet et al., 2016).
From the bee perspective, olfactory learning of the mimic scents translated into higher levels of foraging activity both at the hive entrance and on the target crop (for sunflower hybrids seed production: Farina et al., 2020; for almond trees: Farina et al., 2023). In the case of apple and pear crops, olfactory memories established within the hive differentially affected bee foraging activity according to the floral resources mainly exploited by bees on these two crops (nectar in apple flowers and pollen in pear flowers; Díaz et al., 2013; Quinet et al., 2016). While the circulation of scented food with the apple mimic promoted a higher number of incoming foragers at the hive (associated with greater activity of nectar foragers), the offering of pear mimic-scented sugar solution did not increase the hive entrance activity, but positively affected pollen collection (Farina et al., 2022). Treatment of colonies with scented foods is expected to increase nectar foraging in the target crop of interest, but not especially to increase pollen foraging. This reasoning is because the odors of the crop flowers are supplied (contingent) with the sugar reward, and not with a pollen reward (Nery et al., 2020; Moreno and Arenas, 2023). However, differences in pollen and nectar foraging patterns in pear and apple tree plantations suggests that the information acquired from sugar syrup can be adjusted and updated based on the availability of resources in the field (Arenas and Kohlmaier, 2019) and thus, be functional to improve pollen collection. Although administration of a scented sucrose solution may activate mainly nectar foragers, a percentage of these bees would have the ability to change their preferred resource by switching to pollen collection (Arenas and Kohlmaier, 2019). This transition is favored especially if the nectar sources visited exhibit a lower productivity than the expected based on foragers´ in-hive experience. Considering that the odors learned predicted a very productive source (i.e., an ad libitum feeder offering a 50% sucrose solution) and that the nectar productivity of pear flowers is relatively low (estimated nectar sugar concentrations: 6.8 ± 0.26% w/w, Díaz et al., 2013; ~10-15% in average depending on the cultivar; Quinet et al., 2016), we speculate that some foragers, initially motivated to collect nectar, may end up collecting pollen. From the early discovery and collection of pollen from pear flowers, which is indeed very productive in terms of pollen reward, the propagation of pollen-related cues (Díaz et al., 2007; Arenas et al., 2021) and information of pollen sources might be guaranteed through the behavioral pathways already described for nectar sources. To this end, higher amounts of pear pollen were collected per foraging bout in the mimic-scented sucrose solution (SS + PM)-treated colony than the control one (Farina et al., 2022). Similarly, almond flowers are also productive in terms of pollen with moderate productivity in nectar values (estimated nectar sugar concentration, 16.7 ± 1.1% w/w; Farina et al., 2023). In this regard, higher areas of pollen reserves were found in colonies fed almond mimic-scented sucrose solution (SS + AlM) than in control colonies (Farina et al., 2023).
From the crop perspective, the offering of scented food increased yield significantly in different sunflower cultivars (i.e., kg of seeds per hectare; Farina et al., 2020), and a higher number of fruits per tree was measured both in pear and apple trees (Farina et al., 2022; see Figure 6A as example). It is worth mentioning that the observed increase in the yield of different apple cultivars at a larger scale (kg of fruits per hectare) resulted in no significant increase (see Figure 6B), suggesting that there may be variation in the extent to which bees generalize the mimic odor to the natural scent of diverse apple varieties. This was not the case for the different lines in sunflower hybrid seed production, where the same formulation was effective in guiding foragers in plots dominated by different hybrid lines (Farina et al., 2020).
Finally, effect of the joint administration of a mimic odor and non-sugar nectar compounds in liquid food was studied in a sunflower field (Estravis-Barcala et al., 2021). Feeding colonies with scented syrup supplemented with both caffeine and arginine resulted in higher foraging activity both at the hive entrance and on the target crop (Figure 5), as well as in increased yields (in terms of seed set and seed mass) compared to the individual effect of the mimic-scented food. Thus, it is suggested that nonsugar compounds, which act as memory enhancers (Marchi et al., 2021), could improve olfactory learning of the mimic odor and its effect on crop pollination.
The growing global demand for pollination services (Aizen et al., 2019) leads to propose new strategies in honey bee management to improve its efficiency in agroecosystems. The implementation of a targeted pollination strategy mediated by honey bee plastic responses integrates aspects related to floral odors and honey bee social behavior, including communication processes. Within this framework, the results so far obtained suggest that conditioning bees to simple synthetic odorant mixtures which mimic specific flowers could enable the establishment of in-hive odor memories that bias bees to the target crop and potentially increase yields (Farina et al., 2020; Farina et al., 2022; Farina et al., 2023). From the growers’ perspective, this method might decrease the honey bee stocking rate by increasing the pollination activity of the honey bee colonies and therefore to save on input costs. While at the same it can help to decrease the detrimental effects of managing too many honey bees at the same location on wild flora and entomofauna (Geslin et al., 2017b; Morales et al., 2017; Russo et al., 2021). Nevertheless, it is worth remarking that there are knowledge gaps to be further investigated. The use of volatile mixtures as odor mimic could be challenging for crops that involve different varieties and this will require a thorough understanding of cultivar-specific floral bouquets (Rodriguez-Saona et al., 2011; Twidle et al., 2017). Additionally, specific crops might present certain characteristics detrimental to pollination by honey bees (i.e., brief blooming period, restrictive floral morphology, lack of nectar as reward). Also, future works are necessary to determine how long the effect of the treatment of feeding colonies with sugar syrup scented with mimic odors lasts. At the same time, it remains to be assessed the extent to which this procedure can be implemented with alternative managed bees for those crops where honey bees are less efficient pollinators. The honey bee is not the most efficient pollinator in many cases (Ne'eman et al., 2010) due to quite limited single visit pollen depositions (Földesi et al., 2021; Page et al., 2021), because they are not especially effective to transfer cross-pollen on cultivars requiring cross-pollination, especially when wild entomofauna is absent (Garibaldi et al., 2013), or they forage on a large area around their nest, resulting in a high probability to be diverted to other competing bloom (Jay, 1986; Quinet et al., 2016; Osterman et al., 2021a). This is of particular interest since many native bees (e.g., bumble bees and solitary bees) are currently reared for agricultural purposes (Osterman et al., 2021b). Lastly, although this procedure has great potential for positive impacts on food industry, research on a proper packaging to maintain the chemical stability of the mixture will also be needed to determine its economic viability. The economic impact of an efficient and sustainable entomophilous pollination procedure for the most high-market valuable crops could improve yields in quantitative and qualitative terms in a global context of increasing demand of pollination services.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This study was supported by grants from the University of Buenos Aires (20020170100078BA), CONICET (PIP 11220200102201CO) and ANPCYT (PICT 2019 2438) of Argentina to WF.
National Scientific and Technical Research Council of Argentina (CONICET) has the intellectual property AR082846B1 and Pat. 20110102441 on the commercial use of the sunflower and apple formulations to improve honey bee pollination efficiency, in which WF and AA are coinventors. CONICET and the University of Buenos Aires (UBA) have filed the patent application PCT/ IB2018/055550 on the commercial use of the pear formulation to improve honey bee pollination efficiency, in which WF is coinventor. CONICET and UBA have filed the patent application PCT/IB2018/055549 on the commercial use of the almond formulation to improve honeybee pollination efficiency, in which WF, FP, and MCEB are coinventors. WF is coinventor and shareholder of ToBEE S.A., the licensee of these technologies.
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frbee.2023.1253157/full#supplementary-material
Abrol D. P. (2012). Pollination biology: biodiversity conservation and agricultural production (New York: Springer).
Ai H., Farina W. M. (2023). In search of behavioral and brain processes involved in honey bee dance communication. Front. Behav. Neurosci. 17, 1140657. doi: 10.3389/fnbeh.2023.1140657
Aizen M. A., Aguiar S., Biesmeijer J. C., Garibaldi L. A., Inouye D. W., Jung C., et al. (2019). Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob. Change Biol. 25, 3516–3527. doi: 10.1111/gcb.14736
Al-Tikrity W. S., Benton A. W., Risius M. L., Clarke J. W. W. (1972). The effect of length of stay of a honeybee colony in a crownvetch field on its foraging behaviour. J. Apic. Res. 11, 51–57. doi: 10.1080/00218839.1972.11099699
Aras P., De Oliveira D., Savoie L. (1996). Effect of a honey bee (Hymenoptera: Apidae) gradient on the pollination and yield of lowbush blueberry. J. Econom. 89 (5), 1080–1083. doi: 10.1093/jee/89.5.1080
Arenas A., Farina W. M. (2012). Learned olfactory cues affect pollen-foraging preferences in honeybees, Apis mellifera. Anim. Behav. 83 (4), 1023–1033. doi: 10.1016/j.anbehav.2012.01.026
Arenas A., Fernández V. M., Farina W. M. (2007). Floral odor learning within the hive affects honeybees’ foraging decisions. Naturwissenschaften 94, 218–222. doi: 10.1007/s00114-006-0176-0
Arenas A., Fernández V. M., Farina W. M. (2008). Floral scents experienced within the colony affect long-term foraging preferences in honeybees. Apidologie 39, 714–722. doi: 10.1051/apido:2008053
Arenas A., Kohlmaier M. G. (2019). Nectar source profitability influences individual foraging preferences for pollen and pollen-foraging activity of honeybee colonies. Behav. Ecol. Sociobiol. 73, 1–10. doi: 10.1007/s00265-019-2644-5
Arenas A., Lajad R., Farina W. (2021). Selective recruitment for pollen and nectar sources in honeybees. J. Exp. Biol. 224 (16), jeb242683. doi: 10.1242/jeb.242683
Balbuena M. S., Arenas A., Farina W. M. (2012a). Floral scents learned inside the honeybee hive have a long-lasting effect on recruitment. Anim Behav. 84, 77–83. doi: 10.1016/j.anbehav.2012.04.008
Balbuena M., Molinas J., Farina W. (2012b). Honey bee recruitment to scented food sources: correlations between in-hive social interactions and foraging decision making. Behav. Ecol. Sociobiol. 66, 445–452. doi: 10.1007/s00265-011-1290-3
Balfour N. J., Ratnieks F. L. (2017). Using the waggle dance to determine the spatial ecology of honey bees during commercial crop pollination. Agric. For. Entomol. 19 (2), 210–216. doi: 10.1111/afe.12204
Bänsch S., Tscharntke T., Ratnieks F. L., Härtel S., Westphal C. (2020a). Foraging of honey bees in agricultural landscapes with changing patterns of flower resources. Agric. Ecosyst. Environ. 291, 106792. doi: 10.1016/j.agee.2019.106792
Bänsch S., Tscharntke T., Wünschiers R., Netter L., Brenig B., Gabriel D., et al. (2020b). Using ITS2 metabarcoding and microscopy to analyse shifts in pollen diets of honey bees and bumble bees along a mass-flowering crop gradient. Mol. Ecol. 29 (24), 5003–5018. doi: 10.1111/mec.15675
Beekman M., Ratnieks F. L. W. (2000). Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 14, 490–496. doi: 10.1046/j.1365-2435.2000.00443.x
Bitterman M. E., Menzel R., Fietz A., Schäfer S. (1983). Classical conditioning of proboscis extension in honeybees (Apis mellifera ). J. Comp. Psychol. 97 (2), 107–119. doi: 10.1037/0735-7036.97.2.107
Capaldi E. A., Smith A. D., Osborne J. L., Fahrbach S. E., Farris S. M., Reynolds D. R., et al. (2000). Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature 403 (6769), 537–540. doi: 10.1038/35000564
Chabert S., Mallinger R. E., Senechal C., Fougeroux A., Geist O., Guillemard V., et al. (2022). Importance of maternal resources in pollen limitation studies with pollinator gradients: A case study with sunflower. Agric. Ecosyst. Environ. 330, 107887. doi: 10.1016/j.agee.2022.107887
Chabert S., Requier F., Chadoeuf J., Guilbaud L., Morison N., Vaissiere B. E. (2021). Rapid measurement of the adult worker population size in honey bees. Ecol. Indic. 122, 107313. doi: 10.1016/j.ecolind.2020.107313
Chaffiol A., Laloi D., Pham-Delègue M. H. (2005). Prior classical olfactory conditioning improves odour-cued flight orientation of honey bees in a wind tunnel. J. Exp. Biol. 208 (19), 3731–3737. doi: 10.1242/jeb.01796
Chittka L., Menzel R. (1992). The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J. Comp. Physiol. A 171 (2), 171–181. doi: 10.1007/bf00188925
Chittka L., Thomson J. D. (2001). Cognitive ecology of pollination: animal behaviour and floral evolution (Cambridge: Cambridge University Press).
Chittka L., Thomson J. D., Waser N. M. (1999). Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86, 361–337. doi: 10.1007/s001140050636
Christie A. P., Abecasis D., Adjeroud M., Alonso J. C., Amano T., Anton A., et al. (2020). Quantifying and addressing the prevalence and bias of study designs in the environmental and social sciences. Nat. Commun. 11 (1), 6377. doi: 10.1038/s41467-020-20142-y
Christie A. P., Amano T., Martin P. A., Shackelford G. E., Simmons B. I., Sutherland W. J. (2019). Simple study designs in ecology produce inaccurate estimates of biodiversity responses. J. Appl. Ecol. 56 (12), 2742–2754. doi: 10.1111/1365-2664.13499
Couvillon M. J., Schürch R., Ratnieks F. L. W. (2014). Waggle dance distances as integrative indicators of seasonal foraging challenges. PloS One 9 (4), e93495. doi: 10.1371/journal.pone.0093495
Cunningham S. A., Le Feuvre D. (2013). Significant yield benefits from honeybee pollination of faba bean (Vicia faba) assessed at field scale. Field Crops Res. 149, 269–275. doi: 10.1016/j.fcr.2013.05.019
Danner N., Keller A., Härtel S., Steffan-Dewenter I. (2017). Honey bee foraging ecology: Season but not landscape diversity shapes the amount and diversity of collected pollen. PloS One 12 (8), e0183716. doi: 10.1371/journal.pone.0183716
Degen J., Kirbach A., Reiter L., Lehmann K., Norton P., Storms M., et al. (2015). Exploratory behaviour of honeybees during orientation flights. Anim. Behav. 102, 45–57. doi: 10.1016/j.anbehav.2014.12.030
Delaplane K. S., Dag A., Danka R. G., Freitas B. M., Garibaldi L. A., Goodwin R. M., et al. (2013a). Standard methods for pollination research with Apis mellifera. J. Apicult Res. 52 (4), 1–28. doi: 10.3896/IBRA.1.52.4.12
Delaplane K. S., van der Steen J., Guzman-Novoa E. (2013b). Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apicult Res. 52 (1), 1–12. doi: 10.3896/IBRA.1.52.1.03
Díaz P. C., Arenas A., Fernández V. M., Martin Susic C., Basilio A. M., Farina W. M. (2013). Honeybee cognitive ecology in a fluctuating agricultural setting of apple and pear trees. Behav. Ecol. 24 (5), 1058–1067. doi: 10.1093/beheco/art026
Díaz P. C., Grüter C., Farina W. M. (2007). Floral scents affect the distribution of hive bees around dancers. Behav. Ecol. Sociobiol. 61, 1589–1597. doi: 10.1007/s00265-007-0391-5
Dobson H. E. (2006). “Relationship between floral fragrance composition and type of pollinator,” in Biology of floral scent. Eds. Dudareva N., Pichersky E. (Boca Raton: CRC Press), 147–198.
Dorjay N., Abrol D. P., Vikram B. (2022). Effect of Bee Attractants on Foraging Activities of Honeybees Apis mellifera, A. dorsata and A. cerana on Cucumis sativus L. and Memordica charantia L. Flowers J. Apic 2, 123–134. doi: 10.17519/apiculture.2022.06.37.2.123
Dukas R. (1998). Cognitive Ecology: The Evolutionary Ecology of Information Processing and Decision Making (Chicago: The University of Chicago Press).
Dyer A. G., Paulk A. C., Reser D. H. (2011). Colour processing in complex environments: insights from the visual system of bees. Proc. R. Soc. B. 278, 952–959. doi: 10.1098/rspb.2010.2412
Ellis A., Delaplane K. S. (2009). An evaluation of Fruit-Boost™ as an aid for honey bee pollination under conditions of competing bloom. J. Apicult. Res. 48 (1), 15–18. doi: 10.3896/IBRA.1.48.1.04
Estravis-Barcala M. C., Palottini F., Farina W. M. (2021). Learning of a mimic odor combined with nectar nonsugar compounds enhances honeybee pollination of a commercial crop. Sci. Rep. 11, 23918. doi: 10.1038/s41598-021-03305-9
FAO, IZSLT, Apimondia, CAAS (2021). Good beekeeping practices for sustainable apiculture (Rome: FAO Animal Production and Health Guidelines No. 25).
Farina W. M., Arenas A., Díaz P. C., Susic Martin C., Corriale M. J. (2022). In-hive learning of specific mimic odours as a tool to enhance honey bee foraging and pollination activities in pear and apple crops. Sci. Rep. 12, 20510. doi: 10.1038/s41598-022-22985-5
Farina W. M., Arenas A., Díaz P. C., Susic Martin C., Estravis-Barcala M. C. (2020). Learning of a mimic odor within honey bee hives improves pollination service efficiency in a commercial crop. Curr. Biol. 30, 1–7. doi: 10.1016/j.cub.2020.08.018
Farina W. M., Grüter C., Diíaz P. C. (2005). Social learning of floral odours inside the honeybee hive. Proc. R. Soc. B. 272 (1575), 1923–1928. doi: 10.1098/rspb.2005.3172
Farina W. M., Grüter C. (2009). “Trophallaxis – a mechanism of information transfer,” in Food Exploitation by Social Insects: Ecological, Behavioral, and Theoretical Approaches. Eds. Hrncir M., Jarau. S. (Boca Raton: CRC Press), 173–187.
Farina W. M., Grüter C., Acosta L., Mc Cabe S. (2007). Honeybees learn floral odors while receiving nectar from foragers within the hive. Naturwissenschaften 94 (1), 55. doi: 10.1007/s00114-006-0157-3
Farina W. M., Palottini F., Estravis-Barcala M. C., Arenas A., Balbuena M. S., González A. (2023). Conditioning honeybees to a mimic odor increases pollination efficiency in an almond self-compatible variety. Apidologie 54 (4), 40. doi: 10.1007/s13592-023-01019-7
Fewell J. H., Winston M. L. (1996). Regulation of nectar collection in relation to honey storage levels by honey bees, Apis mellifera. Behav. Ecol. 7 (3), 286–291. doi: 10.1093/beheco/7.3.286
Földesi R., Howlett B. G., Grass I., Batáry P. (2021). Larger pollinators deposit more pollen on stigmas across multiple plant species - A meta-analysis. J. Appl. Ecol. 58 (4), 699–707. doi: 10.1111/1365-2664.13798
Free J. B. (1958). Attempts to condition bees to visit selected crops. Bee World 39, 221–230. doi: 10.1080/0005772X.1958.11095070
Free J. B. (1959). The effect of moving colonies of honeybees to new sites on their subsequent foraging behaviour. J. Agricult Sci. 53, 1–9. doi: 10.1017/S0021859600030859
Free J. B. (1963). The flower constancy of honeybees. J. Anim Ecol. 32 (1), 119–131. doi: 10.2307/2521
Free J. B. (1969). Influence of the odour of a honeybee colony’s food stores on the behaviour of its foragers. Nature 222 (5195), 778. doi: 10.1038/222778a0
Free J. B., Free N. W., Jay S. C. (1960). The effect on foraging behavior of moving honey bee colonies to crops before or after flowering has begun. J. Econom Entomol. 53, 564–566. doi: 10.1093/jee/53.4.564
Garibaldi L. A., Carvalheiro L. G., Vaissière B. E., Gemmill-Herren B., Hipólito J., Freitas B. M., et al. (2016). Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 351 (6271), 388–391. doi: 10.1126/science.aac7287
Garibaldi L. A., Steffan-Dewenter I., Winfree R., Aizen M. A., Bommarco R., Cunningham S. A., et al. (2013). Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339 (6127), 1608–1611. doi: 10.1126/science.1230200
Gary N. E., Witherell P. C., Lorenzen K. (1978). The distribution and foraging activities of common Italian and “Hy-Queen” honey bees during Alfalfa pollination. Environ. Entomol. 7 (2), 233–240. doi: 10.1093/ee/7.2.233
Gary N. E., Witherell P. C., Marston J. (1972). Foraging range and distribution of honey bees used for carrot and onion pollination. Environ. Entomol. 1 (1), 71–78. doi: 10.1093/ee/1.1.71
Gary N. E., Witherell P. C., Marston J. M. (1973). Distribution of foraging bees used to pollinate alfalfa. Environ. Entomol. 2 (4), 573–578. doi: 10.1093/ee/2.4.573
Gary N. E., Witherell P. C., Marston J. M. (1975). The distribution of foraging honey bees from colonies used for honeydew melon pollination. Environ. Entomol. 4 (2), 277–281. doi: 10.1093/ee/4.2.277
Gary N. E., Witherell P. C., Marston J. M. (1976). The inter-and intra-orchard distribution of honeybees during almond pollination. J. Apic Res. 15 (1), 43–50. doi: 10.1080/00218839.1976.11099832
Geslin B., Aizen M. A., Garcia N., Pereira A. J., Vaissière B. E., Garibaldi L. A. (2017a). The impact of honey bee colony quality on crop yield and farmers’ profit in apples and pears. Agric. Ecosyst. Environ. 248, 153–161. doi: 10.1016/j.agee.2017.07.035
Geslin B., Gauzens B., Baude M., Dajoz I., Fontaine C., Henry M., et al. (2017b). Massively introduced managed species and their consequences for plant-pollinator interactions. Adv. Ecol. Res. 57, 147–199. doi: 10.1016/bs.aecr.2016.10.007
Ghirlanda S., Enquist M. (2003). A century of generalization. Anim Behav. 66 (1), 15–36. doi: 10.1006/anbe.2003.2174
Giurfa M. (2007). Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A 193 (8), 801–824. doi: 10.1007/s00359-007-0235-9
Giurfa M., Sandoz J. C. (2012). Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19 (2), 54–66. doi: 10.1101/lm.024711.111
Goodwin R. M. (1997). Feeding sugar syrup to honey bee colonies to improve pollination: a review. Bee World 78 (2), 56–62. doi: 10.1080/0005772X.1997.11099335
Gould J. L. (1984). “Natural history of honey bee learning,” in The Biology of Learning. Eds. Marler P., Terrace H. S. (Berlin, Heidelberg, New York, Tokyo: Springer-Verlag), 149–180.
Grüter C., Acosta L. E., Farina W. M. (2006). Propagation of olfactory information within the honeybee hive. Behav. Ecol. Sociobiol 60 (5), 707–715. doi: 10.1007/s00265-006-0214-0
Grüter C., Balbuena M. S., Farina W. M. (2009). Retention of long-term memories in different age-groups of honeybee (Apis mellifera) workers. Insect Soc. 56, 385–387. doi: 10.1007/s00040-009-0034-0
Grüter C., Farina W. M. (2009). The honeybee waggle dance: can we follow the steps? Trends Ecol. Evol. 24 (5), 242–247. doi: 10.1016/j.tree.2008.12.007
Gubin A. F. (1936). Bestäubung und Erhöhung der Samenernte bei Rotklee Trifolium pratense L. mit Hilfe der Bienen. Arch. Bienenkunde 17, 209–264.
Gubin A. F. (1938). Pollination and increase in seed yield of red clover Trifolium pratense L. with the help of bees (in Russian). Pschelovodstvo 5, 40–44. H. 7, 15–17.
Guerrieri F., Schubert M., Sandoz J. C., Giurfa M. (2005). Perceptual and neural olfactory similarity in honeybees. PloS Biol. 3 (4), e60. doi: 10.1371/journal.pbio.0030060
Hagler J. R., Mueller S., Teuber L. R., Machtley S. A., Van Deynze A. (2011). Foraging range of honey bees, Apis mellifera, in alfalfa seed production fields. J. Insect Sci. 11 (1), 144. doi: 10.1673/031.011.14401
Healy S., Braithwaite V. (2000). Cognitive ecology: a field of substance? Trends Ecol. Evol. 15 (1), 22–26. doi: 10.1016/S0169-5347(99)01737-1
Higo H. A., Winston M. L., Slessor K. N. (1995). Mechanisms by which honey bee (Hymenoptera: Apidae) queen pheromone sprays enhance pollination. Ann. Entomol. Soc Am. 88 (3), 366–373. doi: 10.1093/aesa/88.3.366
Higuera-Higuera C. A., Esiponsa-Sánchez S. M., Dueñas-Quintero D. M., Palacios-Preciado M., Lozano-Suarez F. E., Solarte-Cabrera V. M., et al. (2023). Valoración de un método de osmoguiado a flores de durazno (Prunus persica) aplicado en abejas Apis mellifera. Rev. UDCA Actualidad Divulgación Científica 26 (1). doi: 10.31910/rudca.v26.n1.2023.2242
Hoover S. E., Ovinge L. P. (2018). Pollen collection, honey production, and pollination services: managing honey bees in an agricultural setting. J. Econ. Entomol. 111 (4), 1509–1516. doi: 10.1093/jee/toy125
Jailyang L., Sharma N. C., Chandel J. S., Rana V. S., Rana K., Chauhan P. (2022). Influence of Bee Scent and other indigenous bee attractants on bee activity and fruiting behaviour of kiwifruit (Actinidia deliciosa A. Chev.). Sci. Hortic. 295, 110869. doi: 10.1016/j.scienta.2021.110869
Jay S. C. (1986). Spatial management of honey bees on crops. Annu. Rev. Entomol. 31 (1), 49–65. doi: 10.1146/annurev.en.31.010186.000405
Jayaramappa K. V., Pattabhiramaiah M., Bhargava H.R. (2011). Influence of bee-attractants on yield parameters of ridge gourd (Luffa acutangula L.)(Cucurbitaceae). World Appl. Sci. J. 15 (4), 457–462.
Johannsmeier M. F., Mostert J. N. (2001). “Crop pollination,” in Beekeeping in South Africa, 3rd ed. Ed. Johannsmeier M. F. (Stellenbosch, South Africa: Agricultural Research Council of South Africa, Plant Protection Research Institute Handbook 14), 235–250.
Johannsmeier M. F., Swart D. J., Morudu T. M. (1997). Honeybees in an avocado orchard: forager distribution, influence on fruit set and colony development. (South African Avocado Growers’ Association Yearbook). 20, 39–41.
Klein A. M., Vaissiere B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 274 (1608), 303–313. doi: 10.1098/rspb.2006.3721
Knauer A. C., Schiestl F. P. (2015). Bees use honest floral signals as indicators of reward when visiting flowers. Ecol. Lett. 18 (2), 135–143. doi: 10.1111/ele.12386
Knudsen J. T., Tollsten L., Bergstrom L. G. (1993). Floral scents—a checklist of volatile compounds isolated by headspace techniques. Phytochemistry 33, 253–280. doi: 10.1016/0031-9422(93)85502-I
Kobayashi K., Arai M., Tanaka A., Matsuyama S., Honda H., Ohsawa R. (2012). Variation in floral scent compounds recognized by honeybees in Brassicaceae crop species. Breed. Sci. 62 (4), 293–302. doi: 10.1270/jsbbs.62.293
Kuwabara M. (1957). Bildung des bedingten ReXexes von Pavlovs Typus bei der Honigbiene, Apis mellifera. J. Fac Hokkaido Univ. Serv VI Zool. 13, 458–464.
Lindauer M. (1954). Temperaturregulierung und Wasserhaushalt im Bienenstaat. Z Vergl. Physiol. 36, 391–432. doi: 10.1111/j.1439-0418.1954.tb00746.x
Lindauer M. S. (1970). Unambiguity of forms and inequality of labels in studies of effect of language on memory for form. Percept. Mot Skills 30 (1), 175–181. doi: 10.2466/pms.1970.30.1.175
Müller U. (1996). Inhibition of nitric oxide synthase impairs a distinct form of longterm memory in the honeybee, Apis mellifera. Neuron 16, 541–549. doi: 10.1016/S0896-6273(00)80073-2
Müller U. (1997). The nitric oxide system in insects. Prog. Neurobiol. 51, 363–381. doi: 10.1016/S0301-0082(96)00067-6
Marchi I. L., Palottini F., Farina W. M. (2021). Combined secondary compounds naturally found in nectars enhance honeybee cognition and survival. J. Exp. Biol. 224, jeb.239616. doi: 10.1242/jeb.239616
Mas F., Horner R. M., Brierley S., Butler R. C., Suckling D. M. (2020). Selection of key floral scent compounds from fruit and vegetable crops by honey bees depends on sensory capacity and experience. J. Insect Physiol. 121, 104002. doi: 10.1016/j.jinsphys.2019.104002
McGregor S. E. (1976). Insect pollination of cultivated crop plants (Vol. 496) (Washington, D.C: Agricultural Research Service, US Department of Agriculture).
Menzel R. (1999). Memory dynamics in the honeybee. J. Comp. Physiol. A 185, 323–340. doi: 10.1007/s003590050392
Menzel R. (2012). The honeybee as a model for understanding the basis of cognition. Nat. Rev. Neurosci. 13, 758–768. doi: 10.1038/nrn3357
Menzel R., Erber J. (1978). Learning and memory in bees. Sci. Am. 239 (1), 102–110. doi: 10.1038/scientificamerican0778-102
Menzel R., Greggers U. (2013). Guidance by odors in honeybee navigation. J. Comp. Physiol. A 199 (10), 867–873. doi: 10.1007/s00359-013-0850-6
Meroi Arcerito F. R., De Feudis L. L., Amarilla L. D., Galetto L., Mitton G., Fernández N., et al. (2021). Fragrance addition improves visitation by honeybees and fruit quality in kiwifruit (Actinidia deliciosa). J. Scie Food Agricult. 101 (12), 5082–5088. doi: 10.1002/jsfa.11153
Moauro M. A., Balbuena M. S., Farina W. M. (2018). Assessment of appetitive behavior in honeybee dance followers. Front. Behav. Neurosci. 12. doi: 10.3389/fnbeh.2018.00074
Moeller F. E. (1973). Timing of placement of colonies of honey bees for pollination of cranberries. J. Econom Entomol. 66, 370–372. doi: 10.1093/jee/66.2.370
Monasterio R., Caselles C., Trentacoste E., Olmo-García L., Carrasco-Pancorbo A., Galmarini C., et al. (2023). Use of olive pomace extract as a pollinator attractant to increase onion (Allium cepa L.) seed crop production. Eur. J. Agron. 149, 126921. doi: 10.1016/j.eja.2023.126921
Morales C. L., Sáez A., Garibaldi L. A., Aizen M. A. (2017). “Disruption of pollination services by invasive pollinator species,” in Impact of Biological Invasions on Ecosystem Services. Eds. Vilà M., Hulme P. E. (Cham: Springer), 203–220.
Moreno E., Arenas A. (2023). Changes in resource perception throughout the foraging visit contribute to task specialization in the honey bee Apis mellifera. Sci. Rep. 13 (1), 8164. doi: 10.1038/s41598-023-35163-y
Ne'eman G., Jürgens A., Newstrom-Lloyd L., Potts S. G., Dafni A. (2010). A framework for comparing pollinator performance: effectiveness and efficiency. Biol. Rev. 85 (3), 435–451. doi: 10.1111/j.1469-185X.2009.00108.x
Nery D., Moreno E., Arenas A. (2020). Pollen reinforces learning in honey bee pollen foragers but not in nectar foragers. J. Exp. Biol. 223 (22), jeb230250. doi: 10.1242/jeb.230250
Nery D., Palottini F., Farina W. M. (2021). Classical olfactory conditioning promotes long-term memory and improves odor-cued flight orientation in the South American native bumblebee Bombus pauloensis. Curr. Zool. 67 (5), 561–563. doi: 10.1093/cz/zoaa073
Noetzel D. M. (1968). Insect Pollination Results on Sunflower (Fargo, USA: Department of Entomology North Dakota State University, Fargo, USA), 108–112.
Núñez J. A. (1977). Nectar flow by melliferous flora and gathering flow by Apis mellifera ligustica. J. Insect Physiol. 23, 265–275. doi: 10.1016/0022-1910(77)90041-5
Osterman J., Aizen M. A., Biesmeijer J. C., Bosch J., Howlett B. G., Inouye D. W., et al. (2021b). Global trends in the number and diversity of managed pollinator species. Agric. Ecosyst. Environ. 322, 107653. doi: 10.1016/j.agee.2021.107653
Osterman J., Theodorou P., Radzevičiūtė R., Schnitker P., Paxton R. J. (2021a). Apple pollination is ensured by wild bees when honey bees are drawn away from orchards by a mass co-flowering crop, oilseed rape. Agric. Ecosyst. Environ. 315, 107383. doi: 10.1016/j.agee.2021.107383
Ovinge L. P., Hoover S. E. (2018). Comparison of honey bee (Hymenoptera: Apidae) colony units of different sizes as pollinators of hybrid seed canola. J. Econ. Entomol. 111 (4), 1535–1541. doi: 10.1093/jee/toy155
Page M. L., Nicholson C. C., Brennan R. M., Britzman A. T., Greer J., Hemberger J., et al. (2021). A meta-analysis of single visit pollination effectiveness comparing honeybees and other floral visitors. Am. J. Bot. 108 (11), 2196–2207. doi: 10.1002/ajb2.1764
Pham-Delegue M. H., Etievant P., Guichard E., Masson C. (1989). Sunflower volatiles involved in honeybee discrimination among genotypes and flowering stages. J. Chem. Ecol. 15, 329–343. doi: 10.1007/BF02027794
Przybylska A., Ćwintal M., Pszczółkowski P., Sawicka B. (2021). Effect of attractants and micronutrient biofortification on the yield and quality of red clover (Trifolium pratense L.) seeds. Agronomy 11 (1), 152. doi: 10.3390/agronomy11010152
Quinet M., Warzée M., Vanderplanck M., Michez D., Lognay G., Jacquemart A. L. (2016). Do floral resources influence pollination rates and subsequent fruit set in pear (Pyrus communis L.) and apple (Malus x domestica Borkh) cultivars? Eur. J. Agro 77, 59–69. doi: 10.1016/j.eja.2016.04.001
Raguso R. A. (2008). Start making scents: the challenge of integrating chemistry into pollination ecology. Entomol. Exp. Appl. 128 (1), 196–207. doi: 10.1111/j.1570-7458.2008.00683.x
Rajotte E. G., Fell R. D. (1982). A commercial bee attractant ineffective in enhancing apple pollination. HortScience 17 (2), 230–231. doi: 10.21273/HORTSCI.17.2.230
Reilly J. R., Artz D. R., Biddinger D., Bobiwash K., Boyle N. K., Brittain C., et al. (2020). Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B. 287 (1931), 20200922. doi: 10.1098/rspb.2020.0922
Reinhard J., Sinclair M., Srinivasan M. V., Claudianos C. (2010). Honeybees learn odour mixtures via a selection of key odorants. PloS One 5 (2), e9110. doi: 10.1371/journal.pone.0009110
Riffell J. A., Lei H., Christensen T. A., Hildebrand J. G. (2009a). Characterization and coding of behaviorally significant odor mixtures. Curr. Biol. 19 (4), 335–340. doi: 10.1016/j.cub.2009.01.041
Riffell J. A., Lei H., Hildebrand J. G. (2009b). Neural correlates of behavior in the moth Manduca sexta in response to complex odors. PNAS 106, (46) 19219–19226. doi: 10.1073/pnas.0910592106
Riley J. R., Greggers U., Smith A. D., Reynolds D. R., Menzel R. (2005). The flight paths of honeybees recruited by the waggle dance. Nature 435, 205–207. doi: 10.1038/nature03526
Rodet G., Henry M. (2014). Analytic partitioning of honeybee (Apis mellifera L.) flight activity at nest entrance: adaptation and behavioural inertia in a changing environment. Ecol. Res. 29 (6), 1043–1051. doi: 10.1007/s11284-014-1191-9
Rodriguez-Saona C., Parra L., Quiroz A., Isaacs R. (2011). Variation in highbush blueberry floral volatile profiles as a function of pollination status, cultivar, time of day and flower part: implications for flower visitation by bees. Ann. Bot. 107 (8), 1377–1390. doi: 10.1093/aob/mcr077
Rosa J. M. D., Arioli C. J., Blochtein B., Agostinetto L., Grutzmacher A. D., Botton M. (2018). Diagnosis of directed pollination services in apple orchards in Brazil. Rev. Bras. Frutic 40 (2), e-234. doi: 10.1590/0100-29452018234
Russo L., de Keyzer C. W., Harmon-Threatt A. N., LeCroy K. A., MacIvor J. S. (2021). The managed-to-invasive species continuum in social and solitary bees and impacts on native bee conservation. Curr. Opin. Insect Sci. 46, 43–49. doi: 10.1016/j.cois.2021.01.001
Sáez A., Aizen M. A., Medici S., Viel M., Villalobos E., Negri P. (2020). Bees increase crop yield in an alleged pollinator-independent almond variety. Sci. Rep. 10 (1), 3177. doi: 10.1038/s41598-020-59995-0
Sammataro D., de Guzman L. (2018). “Honey bee management in the United States,” in Pollination of cultivated plants: A compendium for practitioners, Volume 2. Ed. Roubik D. W. (Rome: Food and Agriculture Organization of the United Nations), 173–181.
Sandoz J. M., Pham-Delegue M. H., Renou R., Wadhams L. J. (2001). Asymmetrical generalisation between pheromonal and oral odours in appetitive olfactory conditioning of the honey bee Apis mellifera L.). J. Comp. Physiol. A 187, 559–568. doi: 10.1007/s003590100228
Scheiner R. (2004). Responsiveness to sucrose and habituation of the proboscis extension response in honey bees. J. Comp. Physiol. A 190, 727–733. doi: 10.1007/s00359-004-0531-6
Schultheis J. R., Ambrose J. T., Bambara S. B., Mangum W. A. (1994). Selective bee attractants did not improve cucumber and watermelon yield. HortScience 29, 155–158. doi: 10.21273/HORTSCI.29.3.155
Seeley T. D. (1986). Social foraging by honeybees: How colonies allocate foragers among patches of flowers. Behav. Ecol. Sociobiol 19, 343–354. doi: 10.1007/BF00295707
Seeley T. D. (1995). The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies (Cambridge: Harvard University Press).
Shepard R. N. (1987). Toward a universal law of generalization for psychological science. Science 237 (4820), 1317–1323. doi: 10.1126/science.3629243
Sherman G., Visscher P. K. (2002). Honeybee colonies achieve fitness through dancing. Nature 419, 920–922. doi: 10.1038/nature01127
Smaragdova N. P. (1933). “Utilization of the responses of the bee for the pollina- tion of plants,” in Red clover pollination and methods of clover seed production. Eds. Gubin A. F., Romashov G. I. (Moscow: Zhizn iZnanie), 219–227.
Sorokin V. (1938). Our experiments on the flight of bees on clover seeds (in Russian). Pschelovodstvo 8, 9.
Steffan-Dewenter I., Kuhn A. (2003). Honeybee foraging in differentially structured landscapes. Proc. R. Soc. Lond. B. 270 (1515), 569–575. doi: 10.1098/rspb.2002.2292
Su W., Ma W., Zhang Q., Hu X., Ding G., Jiang Y., et al. (2022). Honey bee foraging decisions influenced by pear volatiles. Agriculture 12 (8), 1074. doi: 10.3390/agriculture12081074
Takeda K. (1961). Classical conditioned response in the honey bee. J. Insect Physiol. 6 (3), 168–179. doi: 10.1016/0022-1910(61)90060-9
Thom C., Gilley D. C., Hooper J., Esch H. E. (2007). The scent of the waggle dance. PloS Biol. 5, e228. doi: 10.1371/journal.pbio.0050228
Twidle A. M., Mas F., Harper A. R., Horner R. M., Welsh T. J., Suckling D. M. (2015). Kiwifruit flower odor perception and recognition by honey bees, Apis mellifera. J. Agric. Food Chem. 63 (23), 5597–5602. doi: 10.1021/acs.jafc.5b01165
Twidle A. M., Suckling D. M., Seal A. G., Fedrizzi B., Pilkington L. I., Barker D. (2017). Identification of in situ flower volatiles from kiwifruit (Actinidia chinensis var. deliciosa) cultivars and their male pollenisers in a New Zealand orchard. Phytochemistry 141, 61–69. doi: 10.1016/j.phytochem.2017.05.011
Vaissière B., Freitas B. M., Gemmill-Herren B. (2011). Protocol to detect and assess pollination deficits in crops: a handbook for its use (Rome: Food and Agriculture Organization of the United Nations).
Vásquez Romero R. E., Ballesteros Chavarro H. H., Tello Duran J. E., Castañeda Carrillo S. J., Calvo Corredor N. E., Ortega Floréz N. C., et al. (2011). Polinización dirigida con abejas Apis mellifera: Tecnología para el mejoramiento de la producción de cultivos con potencial exportador (Bogotá: Corpoica). 88 pp.
Visscher P. K., Seeley T. D. (1982). Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 63, 1790–1801. doi: 10.2307/1940121
Vogel S. (1983). “Ecophysiology of zoophilic pollination,” in Physiological plant ecology III, Encyclopedia of Plant Physiology. Eds. Lnge O. L., Nobel P. S., Osmond C. B., Ziegier H. (Berlin, Heidelberg: New York: Springer), 559–624.
von Frisch K. (1943). Versuche über die lenkung des bienenfluges durch dufstoffe. (Experiments on the control of the flight of bees through scents). Naturwissenschaften 31, 445–460. doi: 10.1007/BF01468310
von Frisch K. (1947). Duftgelenkte Bienen im Dienste der Landwirtschaft und Imkerei (Wien: Springer). doi: 10.1007/978-3-7091-4485-5
Wadhams L. J., Blight M. M., Kerguelen V., Le Métayer M., Marion-Poll F., Masson C., et al. (1994). Discrimination of oilseed rape volatiles by honey bee: novel combined gas chromatographic-electrophysiological behavioral assay. J. Chem. Ecol. 20, 3221–3231. doi: 10.1007/BF02033722
Wauchope H. S., Amano T., Geldmann J., Johnston A., Simmons B. I., Sutherland W. J., et al. (2021). Evaluating impact using time-series data. Trends Ecol. Evol. 36 (3), 196–205. doi: 10.1016/j.tree.2020.11.001
Wells H., Wells P. H. (1986). Optimal diet minimal uncertainty and individual constancy in the foraging of Honey bees Apis mellifera. J. Anim Ecol. 55, 881–892. doi: 10.2307/4422
Williamson J., Adams C. G., Isaacs R., Gut L. J. (2018). Evaluation of nasonov pheromone dispensers for pollinator attraction in apple, blueberry, and cherry. J. Econ. Entomol. 111 (4), 1658–1663. doi: 10.1093/jee/toy107
Keywords: floral volatiles, learning, mimic odors, honey bee, Apis mellifera, pollinator-dependent crops, foraging behavior
Citation: Farina WM, Arenas A, Estravis-Barcala MC and Palottini F (2023) Targeted crop pollination by training honey bees: advances and perspectives. Front. Bee Sci. 1:1253157. doi: 10.3389/frbee.2023.1253157
Received: 04 July 2023; Accepted: 22 September 2023;
Published: 11 October 2023.
Edited by:
Margie Mayfield, The University of Melbourne, AustraliaReviewed by:
Stan Chabert, University of Florida, United StatesCopyright © 2023 Farina, Arenas, Estravis-Barcala and Palottini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walter M. Farina, d2FsdGVyQGZibWMuZmNlbi51YmEuYXI=
†Present address: M. Cecilia Estravis-Barcala, División Entomología, Museo de La Plata, Universidad Nacional de La Plata, La Plata, Argentina
Florencia Palottini, Instituto de Investigaciones en Biociencias Agrícolas y Ambientales (INBA), CONICET-UBA, Buenos Aires, Argentina
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.