
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anal. Sci. , 13 July 2022
Sec. Forensic Chemistry
Volume 2 - 2022 | https://doi.org/10.3389/frans.2022.934639
This article is part of the Research Topic Lab vs. Lab: Closing the gap between analytical technologies and detection canines View all 3 articles
Cadaver detection dogs (CDDs) are trained to locate human remains and/or objects associated with human remains. This is possible due to their extraordinary olfactory capabilities compared to those of humans. To reinforce this capability, CDDs must be trained and regularly exposed to the target odor in the form of training aids which include—chemical formulations, animal remains, and/or human remains. Currently, the Ontario Provincial Police (OPP) use amputated limbs/feet from consented surgeries performed on diabetic patients as cadaver detection dog training aids. There is limited knowledge about the volatile organic compound (VOC) composition of these training aids and their appropriateness as an alternative to human remains for CDD training purposes, which formed the aim of the current study. VOCs were collected from amputated lower limbs/feet repeatedly using thermal desorption (TD) tubes and analyzed with comprehensive two-dimensional gas chromatography—time-of-flight mass spectrometry (GC×GC-TOFMS). The response of cadaver detection dogs to these training aids was also recorded to understand their alert in the context of the detected VOCs. VOC classes including acids, alcohols, aldehydes, ketones, ester and analogues, ethers, aliphatic, cyclics, sulfur-containing, nitrogen-containing, and halogen-containing VOCs were identified. Of these classes, cyclic VOCs were most abundant followed by nitrogen-containing VOCs while ethers were the least abundant. The most prominent VOCs identified in amputated limbs/feet were decomposition related however, one VOC—sevoflurane, originated from anaesthesia during the surgeries. It was determined that the VOC profile of aged and relatively recent training aids were variable. The aged training aids sampled over time had less variability (compared to more recent training aids). Additionally, the VOC profiles of samples was not found variable owing to the storage conditions—room temperature, refrigerator or freezer. Overall, a 98.4% detection rate was observed for amputated limbs/feet used as CDD training aids and the presence of non-decomposition related VOCs such as sevoflurane did not appear to impact the CDDs’ detection capability. This study highlights that the presence of decomposition VOCs in amputated limbs/feet and their high detection rate by CDDs validates their use as alternative CDD training aids.
Often cases involving missing persons, homicide, or mass disasters result in a search for deceased individuals. There are various search techniques that can be adapted for this type of investigation, including aerial searches, thermal and remote sensing imagery, cadaver detection dogs (CDDs), observing soil and vegetation anomalies, and using geophysics techniques such as probing and ground penetrating radar (Killam, 2004; Dupras et al., 2011; Holland and Connell, 2016). The choice of search method largely depends on the search scenario and terrain, access to resources, and prior knowledge about the event. Of these techniques, law enforcement frequently use CDDs as they are minimally invasive and do not cause disturbance to sensitive areas, unlike, for example, the probing technique (Ruffell et al., 2014). Dogs can access difficult terrain easily and independently whereas using equipment such as a ground-penetrating radar requires expert skills and can be challenging to use in many terrains. Additionally, CDDs are a cheaper alternative to aerial search methods, and are quick, reliable, and efficient in their search. The ability of CDDs to respond to decomposition odor is a learned behaviour. Even with their extraordinary olfactory capability, the CDD needs to be conditioned to express a desirable visual response to the target odor. Thus, constant reinforcement in the form of training using CDD training aids is required (Osterkamp, 2020).
CDD training aids are chemical or natural material sources that are used to train CDDs on decomposition odor (Dargan and Forbes, 2021). Chemical or artificial training aids are commercially available formulations that purportedly resemble the decomposition odor however, studies have shown that they are not ideal for CDD training (Stadler et al., 2012; Tipple et al., 2014). Natural training aids are materials such as tissue obtained from decomposing animal or human remains, or materials such as textile or soil that was in contact with decomposing remains (Dargan and Forbes, 2021). There are no globally acceptable guidelines for training and training aids used for CDDs thus, the choice of training aids is highly dependent upon the organizations’ capability of ethically acquiring appropriate materials. This results in variability in training aid types and their odor which is not always considered representative of human decomposition odor.
Decomposition odor or the “smell of death” is a complex mixture that includes volatile organic compounds (VOCs) that are released as by- or end-products of the process of human decomposition. It is believed that CDDs are able to identify certain decomposition VOCs to locate the presence of human remains in a search environment (Siniscalchi, 2016). However, the exact VOCs that CDDs identify and associate with a decomposition event or a decomposition site is unknown. Since the CDDs are unable to indicate the relevant decomposition related compounds, studies in the recent past have concentrated on chemically profiling the decomposition VOCs using available instrumentation to understand the suite of compounds that comprise the decomposition odor profile. These studies have been conducted on cadavers, human remains, and animal remains (Hoffman et al., 2009; Verheggen et al., 2017; Buis et al., 2019; Deo et al., 2019; Knobel et al., 2019). Studying the VOC profile requires analytical analysis of the decomposition odor. This can be viewed as a two-step process of VOC collection and VOC analysis. For VOC collection, the use of solid phase microextraction (SPME) is preferred in laboratory based studies when samples can be stored in vials, while thermal desorption (TD) tubes are preferred for field collection (Iqbal et al., 2017). For the VOC analysis, conventionally, gas chromatography—mass spectrometry (GC-MS) was used but recent studies have observed a shift to the use of comprehensive two-dimensional gas chromatography—time-of-flight mass spectrometry (GC×GC-TOFMS). This technique allows for better peak capacity and resolution over conventional gas GC-MS (Perrault, 2015; Iqbal et al., 2017; Knobel et al., 2019). In the current study, air samples were collected using TD tubes and analyzed using GC×GC-TOFMS.
Theoretically, the decomposition VOC profile of cadavers constitutes the most accurate odor profile for CDDs, however, realistically, they cannot always be trained on cadavers. Thus, CDD training aids are accepted as an alternative to cadavers for training purposes. Previously, the use of blood and decomposition fluid has been validated for use as CDD training aids however, this has not been done for all training aid types (Rust et al., 2018; Buis et al., 2019). An example of one unvalidated alternative to natural CDD training aids is voluntarily donated limbs by patients undergoing amputation surgeries. This type of ethically and legally acquired training aid is currently used by the Ontario Provincial Police (OPP) Canine Unit. These limbs have not been validated as a cadaver-dog training aid since their VOC profile has not been studied prior to the current research. The objective of this study was to analyze the VOC profile of voluntarily donated amputated limbs from surgeries of diabetic patients. The study also recorded the response of trained CDDs to these training aids. Both the VOC analysis and dog observations were conducted repeatedly on the same training aids, over a span of 18 months to understand any changes that could occur over a period of time.
The experimental data from this study was collected at the OPP Canine Unit, Orillia, Ontario while the chemical analysis was conducted at the Université du Québec à Trois-Rivières (UQTR) Forensic Thanatology Laboratory. This study was conducted on nine training aids (Foot #1–#9) that the OPP uses as CDD training aids. These are amputated lower limbs and/or feet obtained from three voluntary donations following surgeries of diabetic patients conducted in September 2017, December 2017 and January 2019. Samples were handled and dissected by the Anatomy Learning Centre and Teaching Laboratories at Queen’s University, Kingston, Ontario, before being transported to the OPP Canine Unit. The human research ethics approval for working with human remains in the form of CDD training aids was obtained from le comité d'éthique de la recherche avec des etres humain at UQTR with the certificate number CER-19-261-07.12. The animal ethics approval for working with CDDs was obtained from le comité de bons soins aux animaux at UQTR with the certificate number 2020-S.F.2.
The OPP obtained five foot training aids in 2017 (Foot #1–#5) and four in 2019 (Foot #6–#9). The training aids were placed in aerated PVC pipes for exposure of the odor during training but to ensure no contact between the dog/handler and biohazardous material. The PVC pipes were stored in either sealed plastic bags or glass jars in a freezer, refrigerator or at room temperature conditions to be used repeatedly for training purposes and to provide exposure to a range of storage conditions. The VOC samples were collected repeatedly from the same training aids during five different months—December 2019, July 2020, October 2020, March 2021 and May 2021, based on their availability during the visits to the OPP Canine Unit. The repeated sampling of these training aids in different months over a span of 1.5 years generated 29 different types of samples. The CDD trials were held in three different months—July 2020, March 2021 and May 2021 and were based on the availability of CDDs that were operational at the time. The VOC samples for analysis by GC×GC-TOFMS as well as the observational data from the CDDs’ responses were collected on the same day or a day apart for the same training aids. All training aids used in the current study have been summarized in Table 1. This table presents a detailed outline of training aids and their storage condition (color-coded in the table) which were used to study VOC profiles (via TD—GC×GC-TOFMS analysis) and those used for conducting CDD trials (number of CDDs present in that trial indicated by the number in each table cell).
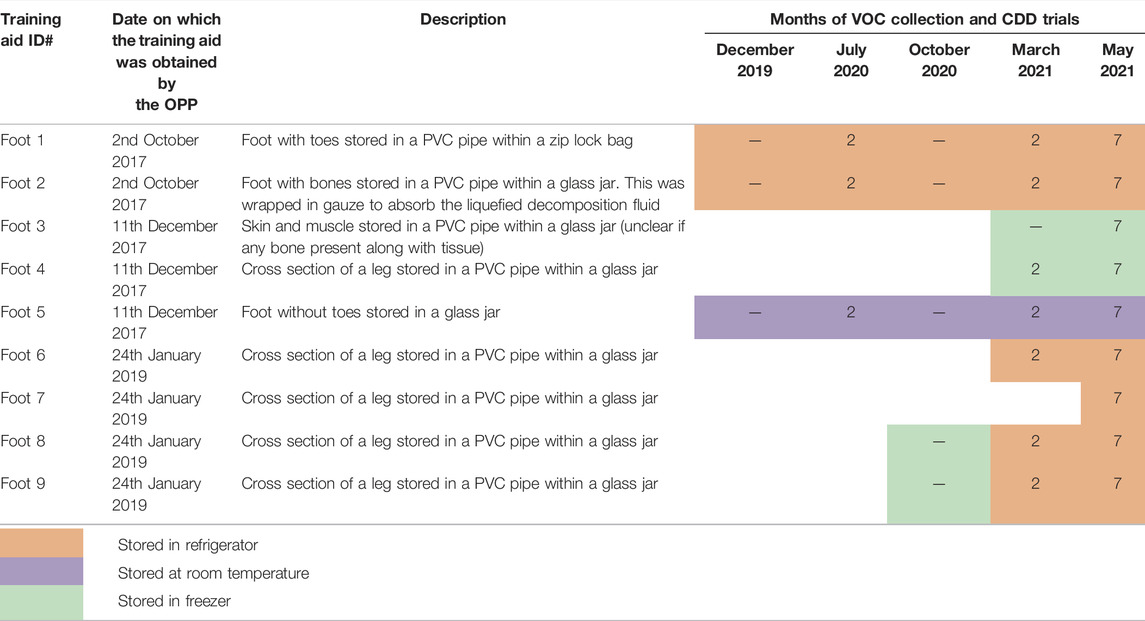
TABLE 1. Summary of the OPP training aids with color-coded storage conditions and the number of CDDs present during each trial (indicated by the number within the table cell) for the current study between December 2019 to May 2021.
The VOC sample collection method was adapted and modified from previously published studies (Deo et al., 2019; Knobel et al., 2019) to suit the requirements of the current samples and instrument type. Prior to VOC collection, the training aids that were stored in the freezer or refrigerator were left at room temperature for a minimum of 24 h to increase the detectable VOCs in the headspace of the storage container. For the VOC collection, a 38 cm × 38 cm × 38 cm aluminum hood was placed over the training aids which were placed on a clean stainless steel table. Following a VOC accumulation period of 10 min in the headspace of the hood, an ACTI-VOC low flow air sampling pump (Markes International Ltd., Llantrisant, United Kingdom) was used to collect air samples onto Tenax TA/Carbograph 5TD sorbent tubes (Markes International Ltd., Llantrisant, United Kingdom). The pump was set to draw air at a constant flow rate of 100 ml/min thus, 500 ml of air sample was collected on every sorbent tube with sampling in duplicates or triplicates (depending on the availability of TD tubes). Control samples were collected using replicas of the storage containers without the training aids to account for background VOCs and contamination or artefacts that could have arisen during the transport and storage of the TD tubes. Once the samples were collected, all the tubes were sealed with brass storage caps, wrapped in aluminum foil and placed inside mason jars for transportation to the laboratory. The sorbent tubes were stored at 4°C until analyzed.
To enable peak area normalization, an internal standard consisting of 0.2 µL of 50 ppm bromobenzene (GC grade, Sigma-Aldrich) in methanol (HPLC Grade, Sigma-Aldrich) was injected using an eVol® XR handheld automated analytical syringe (SGE Analytical Science, Wetherill Park BC, NSW, Australia) onto each sorbent tube prior to analysis. A Markes TD 100-xr multi-tube autosampler (Markes International Ltd., Llantrisant, United Kingdom) was used to thermally desorb the VOCs from the sorbent tubes. Each sorbent tube was heated to 300°C for 4 min to allow thermal desorption of the compounds before being collected onto a general purpose cold trap at -10°C. The trap was desorbed at 300°C for 3 min at a desorption flow rate of 20 ml/min with a 10:1 split. After the sorbent tubes had undergone thermal desorption, they were reconditioned for 60 min at 330°C for subsequent re-use. The thermal desorption unit was connected to a Pegasus® BT 4D GC×GC-TOFMS (LECO, Mississauga, Ontario, Canada) using a transfer line. A 30 m × 0.250 mm inner diameter (ID) × 1.40 μm film thickness Rxi®-624Sil MS column (Restek Corporation, Bellefonte, PA, United States) was used as the first dimension column, and a 2 m × 0.250 mm ID × 0.25 μm film thickness Stabilwax® column (Restek Corporation, Bellefonte, PA, United States) was used as the second dimension column. Helium (high purity, Praxair Canada Inc., Trois-Rivieres, Québec, Canada) was used as the carrier gas at a constant pressure of 17.8 psi. The first dimension oven was set to 35°C and held at this temperature for 5 min before increasing at a rate of 5°C/min to 230°C where it was held for a further 5 min. The offset for the modulator was 15°C and the offset for the second dimension column was 5°C. The modulation period was 5 s with a 1 s hot pulse. The transfer line between the second dimension column and the MS was held at 250°C. An acquisition rate of 200 spectra/s was used to target a mass acquisition range of 29–450 amu. The ion source was held at 250°C, the electron ionization energy was 70 eV.
ChromaTOF® (version 5.51.6.0; LECO) was used for data processing. A 150 signal-to- noise (S/N) ratio was used. The National Institute of Standards and Technology (NIST) Mass Spectral Library was used to establish a list of compounds with a mass spectral match threshold of 70%. Further statistical analysis was conducted by custom R programming scripts (written by the principal statistician in this research) to merge samples and analyze them in accordance with the control samples (Burr, 2022). These scripts are available on GitHub and can be accessed using the link: https://github.com/wesleyburr/GCxGC_Amputated_Limbs/.
Three separate CDD trials (in indoor and outdoor scenarios) were conducted in July 2020, March 2021 and May 2021 which involved exposing CDDs to OPP training aids in their typical training environment. A total of 10 certified CDDs participated in the current study. The number of CDDs present during each of the trials varied greatly and depended upon their availability. A summary of those CDDs present for each trial and further information for each CDD is presented in Table 2.

TABLE 2. Details of CDDs that participated in the trials conducted July 2020, March 2021 and May 2021.
The training aids stored indoors (freezer, refrigerator or room temperature) were used for both indoor and outdoor training. The scenarios involving indoor training were conducted as double-blind trials where the CDD handler and the observers present in the room were unaware of the target odor location. The researcher that placed the training aids in the relevant locations was present outside the room where the trial was conducted to verify the responses called out by the handler without observing the search. The scenarios involving outdoor training were conducted as single blind trials where the CDD handler was unaware of the location of the target odor however, the observers and the researcher knew the location and were in the vicinity to confirm a correct response.
The indoor training was conducted in a variety of rooms where the target odor was hidden by being placed: in a carousel (Figure 1A), inside wooden boxes (Figure 1B), in a wall with holes (Figure 1C), in a mannequin (Figure 1D), in a room with lockers (Figure 1E), in a room with luggage bags (Figure 1F), or inside the furniture within rooms that resembled an apartment. A carousel wheel is a training aid with multiple arms that can hold the target odor to be exposed to the CDDs. A wall with odor holes contains multiple PVC pipes that can contain within them a target odor. For outdoor training, the search areas were approximately an acre either in a woodland area or in a grassland area within the perimeter of the OPP Canine Unit (Figure 2).

FIGURE 1. Indoor search scenarios (A) carousel (B) wooden boxes (C) wall with odor holes (D) mannequin (E) room with lockers (F) room with luggage.
Generally, during each trial, three to four target odors were placed at different locations and the CDDs were worked for no longer than a few minutes followed by breaks to avoid fatigue. The actual duration of search and break frequency given to the CDD was determined by its handler. Additionally, whenever the handler requested it, the CDDs were allowed blank runs in rooms with a similar layout but without a target odor such as in the case of an apartment search scenario. This was an opportunity for the CDDs to become accustomed to the search areas, such as furniture in an apartment, prior to the actual search. Table 3 presents a detailed outline of the training location for each of the training aids across all three trials.
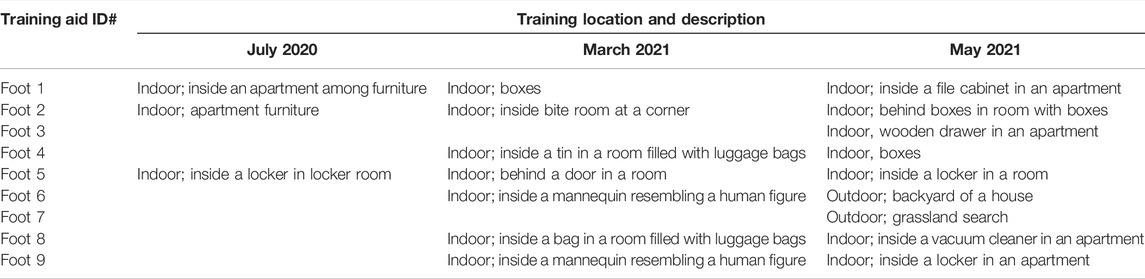
TABLE 3. Details of search location for the training aids during the trials conducted in July 2020, March 2021 and May 2021.
The CDD responses were recorded as:
• True positive (or TP, when the CDD correctly alerted to the target odor),
• Interest (or I, when the CDD showed a behavioural change and narrowed down the target odor but did not alert on the exact location),
• False positive (or FP, when the CDD incorrectly gave a positive alert to a location without a target odor)
• False negative (or FN, when the CDD did not give an alert at a location where there was a target odor or where the handler failed to call the response).
There were no true negative responses recorded in this study as there were no distractor or blank odors (odor samples other than the target odor) used in this trial. Thus, the authors recommend the use of distractor odors to enhance the reliability of the statistical outcome.
Once the CDD responses were recorded, the following statistical measures were used to analyze the data:
•
•
•
Here, when calculating the response rate of the CDDs for each of the training aids, the total number of possible outcomes is equal to the total number of possible responses by the CDDs on the odor. Apart from the statistical data, the false positive events were recorded only as an observation. They could not be used in the statistical estimation of false response rate. This is because, since no distractor odors were used, the false positive events could occur only at random locations and the probability of such event occurring can be infinite. Thus, the false response rate is based only on the false negative responses.
Of the 29 samples analyzed (generated from sampling nine feet training aids over 1.5 years), 1495 different VOCs were identified. All these VOCs were either found to be unique or statistically significant in the training aids compared to controls. The total number of significant compounds detected per sample ranged from a minimum of 23 to a maximum of 430. The detected VOCs belonged to all classes of compounds—acids, alcohols, aldehydes, ketones, ester and analogues, ethers, aliphatic, cyclic, sulfur-containing, nitrogen-containing, and halogen-containing VOCs all of which have been previously identified as VOC classes that comprise decomposition odor (Vass et al., 2004; Statheropoulos et al., 2005; Statheropoulos et al., 2007; Vass et al., 2008; Hoffman et al., 2009; Statheropoulos et al., 2011; Vass, 2012; Rosier et al., 2014; Deo et al., 2019; Dubois et al., 2019; Knobel et al., 2019).
Figure 3 represents the VOC class abundance in all training aids (Foot #1–#9). The overall VOC class abundance trend observed for these training aids indicated that cyclic VOCs (9.4–36.5%; n = 343) were most abundant followed by nitrogen-containing VOCs (5.7–33.3%; n = 197), alcohols (3–21%; n = 161), ester and analogues (0–28.6%; n = 160), aliphatic VOCs (1.9–25%, n = 206), sulfur-containing VOCs (0–22.9%; n = 43), ketones (0–14.2%; n = 145), halogen-containing VOCs (1.3–22.9%, n = 124), acids (0–13%, n = 36), aldehydes (0–6.9%; n = 42), and the least abundant class of ethers (0–4.9%; n = 38). This same trend was observed for both sample groups – those obtained as training aids in 2017 (Foot #1–#5) and those obtained in 2019 (Foot #6–#9). However, not all classes were detected across all of the 29 samples. For example, Foot #1 did not contain acids, alcohols, ester and analogues, ethers, ketones, and sulfur-containing compounds in at least one of the five samples collected in the five different months of analysis. Similarly, in October 2020, ethers were not detected in Foot #2, and aldehydes and ethers were not detected in Foot #5. In March 2021, acids and aldehydes were not detected in Foot #6, and aldehydes were not detected in Foot #7. There was no evident trend in the appearance and disappearance of VOC classes as all the VOC classes were detected in the training aids at some point during the 1.5 years of sampling. Additionally, the storage condition did not appear to influence the VOC class abundance of these training aids.
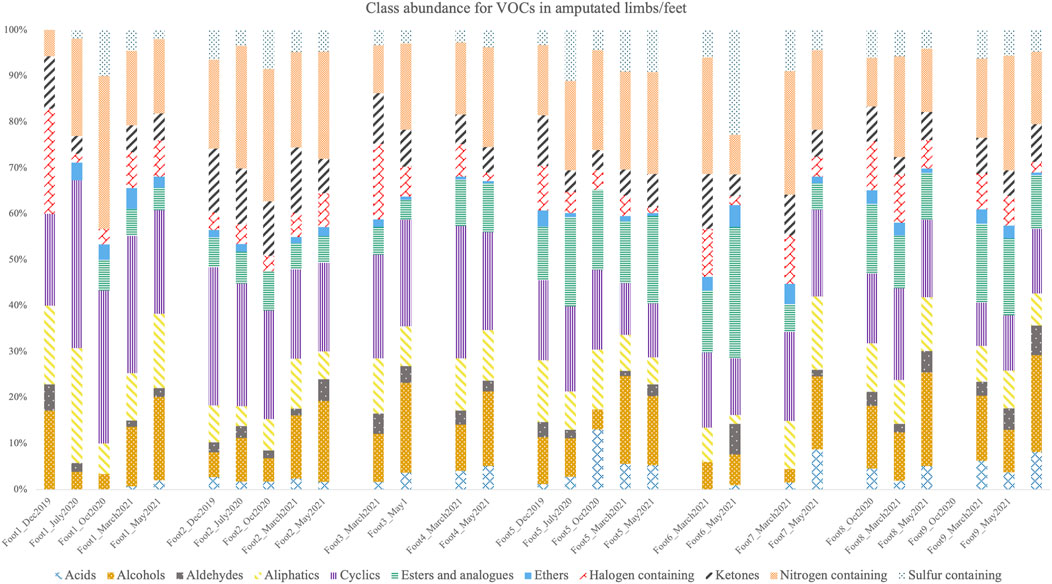
FIGURE 3. VOC class abundance for nine training aids (obtained from amputated limbs/feet) used by OPP as CDD training aids analyzed in five different months between December 2019 to May 2021.
Of the 1495 VOCs detected, Table 4 presents the most prominently occurring VOCs detected across the 29 samples generated from the nine training aids. These VOCs were detected in at least 34% of samples (10 or more samples) ranging up to 93%. A detailed account of the specific sample names which contained these 40 prominent VOCs can be found in the Supplementary Material section as an excel file sheet labelled “40VOCs”. These compounds are relevant because they are VOCs that the CDDs are constantly exposed to during their training sessions. The 40 VOCs listed in Table 4 belonged to the following classes—acids, alcohols, aldehydes, aliphatics, cyclics, ester and analogues, ketones, nitrogen-containing, and sulfur-containing. One additional halogen-containing compound—sevoflurane, was identified as a commonly occurring VOC however, sevoflurane is not known to be a decomposition related compound. A potential source of sevoflurane in these samples is discussed later in Section 4. Ethers were not found among the 40 VOCs, which is consistent with the finding that their abundance is lowest among all VOC classes. Among all of the commonly occurring VOCs, dimethyl trisulfide (DMTS) was detected most frequently in 27 of the 29 samples. The significance of DMTS as a decomposition VOC is discussed in Section 4. Most of the other prominent VOCs detected in amputated limbs/feet CDD training aids have been previously reported in the literature as human decomposition odor related VOCs.
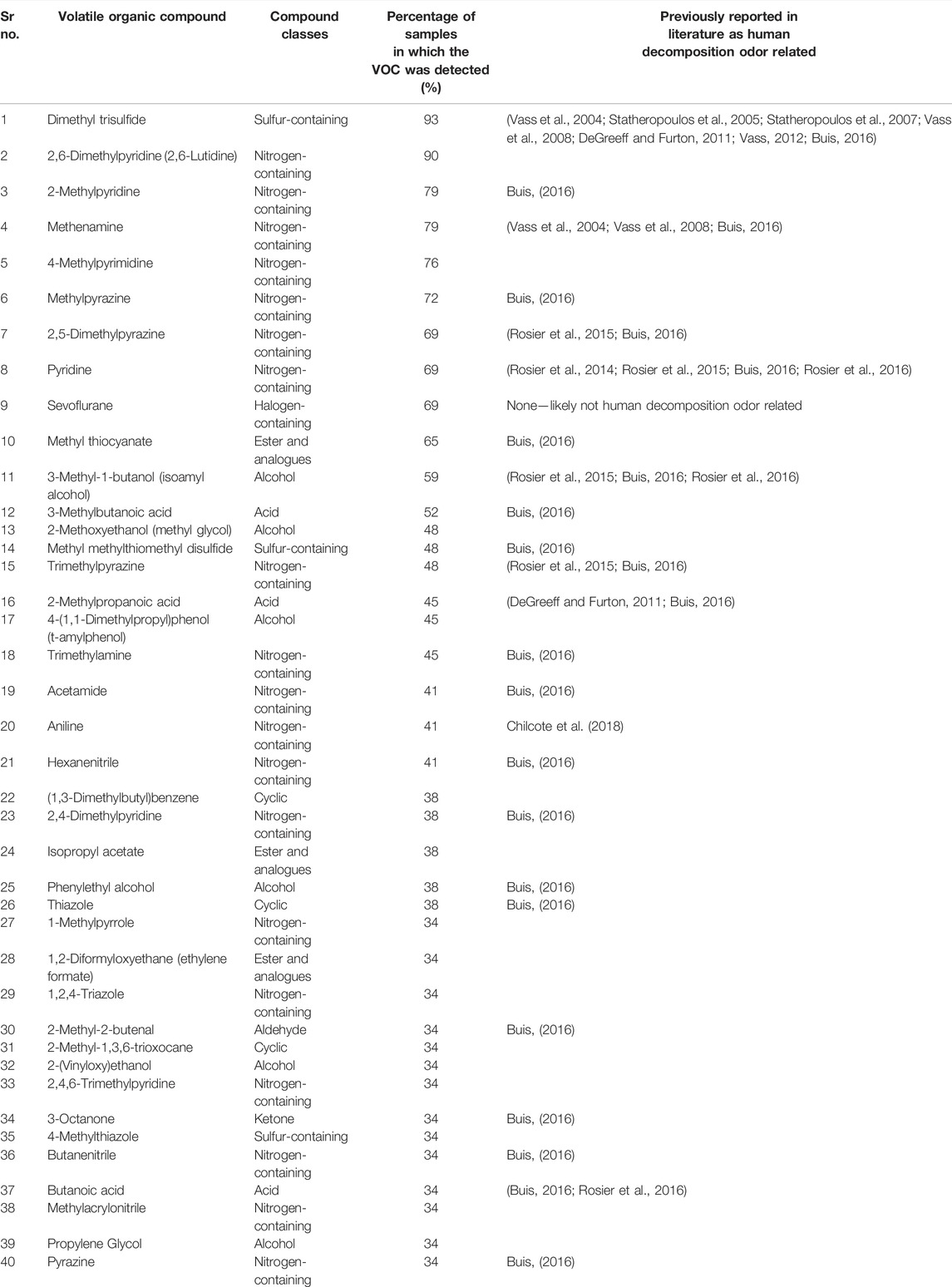
TABLE 4. Prominent VOCs detected in amputated limbs/feet used as training aids by OPP during multiple trials conducted between December 2019 to May 2021.
Principal component analysis (PCA) was performed to cluster and allow for dimension reduction of training aids based on the 40 most prominent VOCs to understand the variability (if any) due to ageing of the training aids. For this, the 29 samples from nine training aids were grouped into two samples sets—training aids obtained in 2017 (Foot #1–#5) samples that had aged for longer than those obtained in 2019 (Foot #6–#9). Figure 4 represents the resulting PCAs of this analysis where, 93% of the variance is explained via (principal component) PC-1 and 3% via PC-2 (cumulative: 96%). The associated loadings plots were used to determine which VOCs were important to the construction of specific principal components (PCs) and samples. Images of loadings can be found in the Supplementary Material. Figure 4 highlights that the training aids obtained in 2017 and 2019 had variability predominantly along PC-1. As evident from the loadings, this variance was owing to the presence of 3-methyl-1-butanol that dominated 2019 samples and most specifically Foot #9 (May 2021). The training aids obtained in 2017 had subtle variability along PC-2 and of these samples, in particular extremes were observed for Foot #4 and #2 (May 2021) owing to the relatively high abundance of trimethylamine, pyrazine and 2,6-lutidine compared to the remaining 2017 samples. Thus, the 2017 and 2019 training aid samples had high inter-sample set variability and the 2019 samples distributed along PC-1 had greater intra-sample set variability compared to those obtained in 2017. The PCA also highlights a cluster of samples, generally belonging to early sampling months of December 2019, July 2020, October 2020. While the samples that became increasingly variable from the cluster belonged to the later sampling months of March 2021 and May 2021.
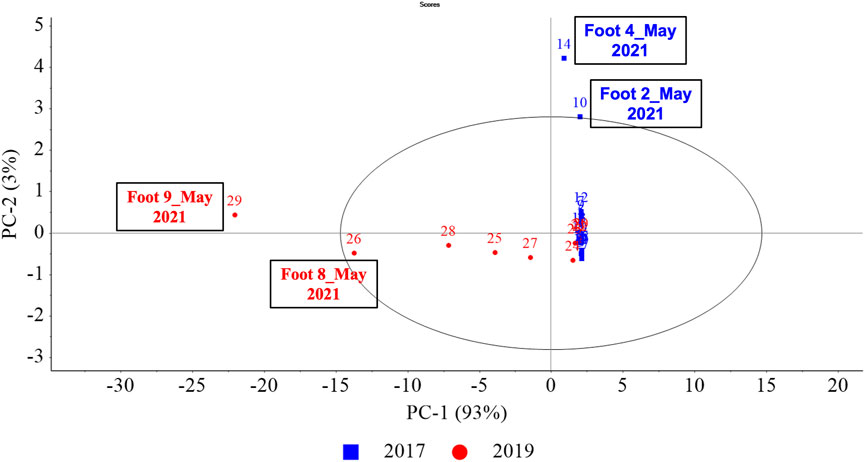
FIGURE 4. (Ageing) PCA scores plot for PC-1, PC-2. PCA scores were calculated using the pre-processed GC×GC-TOFMS normalized peak area of the 40 prominent VOCs in the nine amputated limb/feet samples generating 29 samples (or data points in the above PCA) from analysis conducted in five different months—December 2019, July 2020, October 2020, March 2021 and May 2021. (Here, colour-codes and symbols represent the year OPP acquired these as training aids, blue squares for the year 2017 samples and red circles for the year 2019).
PCA was performed initially on the 2017 training aids (Foot #1–#5) to determine the variation between storage conditions (freezer, refrigerator and room temperature). The 2017 samples were chosen as they included all three storage conditions, while the 2019 samples included mostly refrigerator storage. Foot #8 and #9 were stored in the freezer in October 2020 however, their storage condition was changed to refrigerator prior to the following analysis in March 2021. Thus, analyzing the 2019 samples would have only included two data points for freezer storage, eight for refrigerator storage and none for room temperature. Considering these factors, 2019 samples were not used to analyze the variability due to storage conditions.
Figure 5 represents the PCA of all 2017 samples, attempting to decompose the variability contributed by the storage conditions based on 14 of the 40 most prominent VOCs listed in Table 4. Only 14 VOCs were selected since they were present in over 34% of 2017 samples and thus, they were predominant in 2017 training aids. A detailed account of the specific sample names which contained these 14 prominent VOCs in 2017 training ais can be found in the Supplementary Material section as an excel file sheet labelled “14VOCs”. As evident in Figure 5, PC-1 had the highest explained variability of 76% while PC-2 had an explained variability of 14% (cumulative: 90%). The associated loadings plots were used to determine which VOCs were important to the constructions of specific principal components (PCs) and samples. Images of loadings can be found in the Supplementary Material. Generally, as depicted in Figure 5, the PCA for storage conditions did not reveal clustering based on storage. Relative to all samples, Foot #4 (May 2021) was extreme along with Foot #2 (May 2021), both of which were influenced by the high abundance of 2,6-lutidine; along with Foot #5 (December 2019) which was influenced by the high abundance of methenamine. Thus, all the individual samples had some variability as they were scattered in the PCA scores plot, however the storage conditions did not appear to influence the variability in the sample groups.
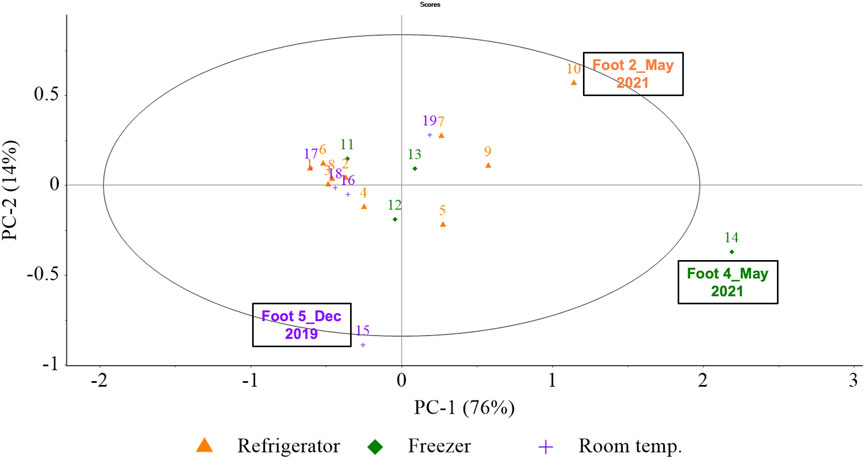
FIGURE 5. (Storage) PCA scores plot for PC-1, PC-2. PCA scores were calculated using the pre-processed GC×GC-TOFMS normalized peak area of the 14 of the 40 prominent VOCs in five amputated limb/feet samples (Foot #1–#5) generating 19 samples (or data points in the above PCA) from analysis conducted in five different months—December 2019, July 2020, October 2020, March 2021 and May 2021. (Here, colour-codes and symbols represent storage condition of the samples, purple crosses for room temperature, orange triangles for refrigerator and green diamonds for freezer).
The response outcomes of each of the CDDs for every training aid that they were exposed to during the CDD trial conducted (during indoor and outdoor search scenarios) in July 2020, March 2021 and May 2021 are presented in Table 5.

TABLE 5. CDD response outcome on amputated limb/feet used as CDD training aids during trials conducted in July 2020, March 2021 and May 2021. (Here, TP = True positive; FN = False negative CDD response, Blank cells = CDD absent from the trial)
As indicated in Table 5, only three of the total number of possible response outcomes were found to be false negative, while the others were true positive. There was no interest response recorded in this study. This table indicates that for most of the training aids, a 100% detection rate was observed with the exception of Foot #5 and #9. The CDD detection rate of Foot #5 (2017, room temperature) was 91% as one (CDD #4) of the two CDDs in the March 2021 trial was not able to detect the presence of the training aid. A 78% detection rate was observed for #Foot 9 (2019, refrigerator) as two (CDD #3 and #5) of the seven CDDs were not able to locate the target odor during this search. Thus, an overall 98.4% detection rate was observed for amputated limbs/feet used as CDD training aids. Additionally, a total of six false positive events in the May 2021 trial occurred at random locations in the training scenarios on Foot#5, #8 and #9. A plausible cause for these false positive results has been discussed in Section 4.
All VOC classes—acids, alcohols, aldehydes, ketones, ester and analogues, ethers, aliphatic, cyclics, sulfur-containing, nitrogen-containing, and halogen-containing compounds, detected in amputated limbs/feet in the current study are known to be present in decomposition odor and have been reported in previous studies. In the current study, cyclic VOCs were the most abundant class followed by nitrogen-containing compounds and ethers were the least abundant class. The cyclic VOC class in this study included both 48% aromatic and 52% other alicyclic compounds of the total VOCs in this class. Several prior studies have classified aromatics as a separate class, and reported aromatics as a dominant VOC class during active and advanced stages of decomposition (Forbes et al., 2014a; Agapiou et al., 2015; Knobel et al., 2019). Nitrogen-containing compounds are produced during the breakdown of amino acids and nucleic acids, while ethers are produced as a result of lipid and sugar degradation (Stefanuto et al., 2017). Even though there is variability in the literature reporting in terms of which classes are more prevalent (Stefanuto et al., 2017), several prior decomposition studies that analyzed VOCs using GC×GC-TOFMS have reported aromatics and nitrogen-containing VOCs as an abundant class, and ethers being less abundant (Focant et al., 2013; Forbes et al., 2014a; Armstrong et al., 2016; Knobel et al., 2019).
Even though sulfur-containing VOCs were not the most abundant class, dimethyl trisulfide (DMTS) was the most prominent VOC among all the VOCs detected. The majority of decomposition odor related studies have determined that sulfur-containing VOCs such as dimethyl sulfide (DMS), dimethyl disulfide (DMDS) and DMTS are the most significant class of VOCs as they are repeatedly reported. Additionally, they are responsible for imparting the characteristic foul decomposition smell, and they are semiochemicals that attract forensically-relevant insects to the decomposing remains (Vass et al., 2004; Forbes and Perrault, 2014; Perrault et al., 2015; Verheggen et al., 2017). Sulfur-containing compounds are produced during bacterial driven breakdown of amino acids that contain sulfur (for e.g., cysteine, methionine) (Dekeirsschieter et al., 2012). Thus, the presence of DMTS in all of the amputated limbs/feet training aids in the current study is significant when comparing their odor profile to cadavers.
Among the other prominent compounds, sevoflurane was detected in 20 of the 29 samples in this study. Sevoflurane is a known anesthetic and is not produced as a result of decomposition of the amputated limbs/feet. Its presence in the samples can be explained by the fact that the amputated limbs/feet were obtained by surgeries conducted on living patients prior to which, anesthesia would have been administered and this compound persisted in the amputated limbs/feet long after they were being used as CDD training aids. The presence of sevoflurane compounds did not interfere with the CDDs’ capability of detecting the target odor which was evident from the overall 98.4% detection rate for CDD training aids in the current study. A major finding of this study was the 40 prominent VOCs that the CDDs are exposed to during their training on amputated limbs. In a future study relating to chemical formulations used as training aids, it can be of interest to incorporate some or all of these 40 chemicals to understand if CDDs respond to the presence of these chemicals individually or in combination.
When constructing PCA, only the 40 most prominent VOCs across the different samples were chosen as the basis for the decomposition into principal components to reduce the impact of singularly present VOCs on the variance reduction. All 1495 VOCs were not incorporated as most of them (77% of VOCs) appeared in only 1–3 samples thus, when conducting PCA, some samples can have a larger influence on specific PCs. It was determined that using a set of VOCs which were largely present across many samples and reducing the influence of a particular sample was suited for this study. The PCA for the 2017 and 2019 training aids highlighted that the sample sets were variable in their VOC profiles. The two sample sets showed both inter- and intra-sample set variability. The inter-sample set variability was distinct as all 2019 samples were spread along PC-1 while, all 2017 samples were spread along PC-2. The inter-sample set variability of 2019 along PC-1 (93% variance) was greater than the intra-sample set variability of 2017 samples along PC-2 (3% variance). This could be because the 2017 samples were much older and had degraded a lot longer which could have resulted in their VOC profiles becoming more generalized over time. Additionally, the major cluster of samples was observed to belong to samples collected early during this study in the months of December 2019, July 2020, October 2020 while the samples further away from the cluster belonged to the later sampling months of March 2021 and May 2021. This could be due to the natural variability in the VOC profile that could have occurred in the samples over time.
The PCA used to understand the variability due to storage conditions did not reveal any variability in the samples owing to their storage conditions. A previous study conducted on blood VOC profiles highlighted that refrigerator and room temperature stored samples were more similar than freezer stored samples as freezing the samples increased the complexity of the VOC profile (Forbes et al., 2014b) however, no such observation was made in the current study. This may be because, even though the samples are stored in their respective storage conditions, they are often removed from storage and used for training purposes. All samples could be stored at ambient temperature for several days before they are placed back into storage. Additionally, during the current study, the training aids were left at room temperature for a minimum of 24 h before VOCs were collected. The fate of the training aids and the process of equilibrating the samples at room temperature prior to sampling could have impacted the VOC profiles and resulted in a natural variability among different samples which could not be attributed to their storage. Thus, the current study found that for the purpose of CDD training, storing the training aids at room temperature, or in a refrigerator or freezer can be acceptable.
Generally, the CDDs had a high detection rate for amputated limbs/feet used as training aids. Only three CDD false negative responses were recorded in this study, one during the search for Foot #5 March 2021 (2017, room temperature) and two during the search for Foot #9 May 2021 (2019, refrigerator) when the CDDs failed to locate the training aids. All VOC classes were identified in both of these samples however, the VOC profile of Foot #9 May 2021 was the most variable among all samples due to the high abundance of 3-Methyl-1-butanol compared to the other samples in this study. Unlike Foot #9 May 2021, there was no significant variability of the VOC profile of the Foot #5 March 2021 sample corresponding with the one false negative observed for this sample in this trial.
The six false positive events observed in this study occurred in the May 2021 trial during either the search for Foot #5 (2017, room temperature) or between the searchers of #8 and #9 (2019, refrigerator). These were only recorded as an observation and could not be used in the statistical estimation of response rate since distractor odors were not used in this trial. When distractor odors are used, the estimation of false response rate can incorporate false negative and false positive responses, and the estimation of detection rate can incorporate true negative and true positive responses for statistical estimations. Thus, the authors recommend the use of distractor odors to enhance the reliability of the statistical outcome. During the May 2021 trial, Foot #8 and #9 were concealed in two separate rooms of the same apartment. Four of the seven CDDs (CDD #3, #5, #7, and #8) gave a false positive response at a third location inside the apartment after successfully locating the first target odor and while still searching for the second. At this instance, the trainer asked the handlers to disregard the alert and continue searching for training purposes. Likewise, during another search for Foot #5 in May 2021 trial, CDD #7 and #10 gave a false positive alert at another location of the apartment after a true positive response. These false positive events occurred at non-target odor locations, and it was suggested by the CDD handlers that the ventilation in the room concentrated the air in that particular corner and the CDDs responded to that. The handlers concluded that this could be due to air becoming saturated and dissipated across the search area as a result of the training aids left in the room for a long period of time (hours). However, since the VOCs were not collected from this location in the apartment, this theory cannot be confirmed. Thus, it is recommended that if a false positive response is recorded at a specific location repeatedly by several CDDs, air samples should be collected from that location for VOC analysis. It is also advised that a specific location and areas in its vicinity should not be re-used to train CDDs on a second training aid to avoid any cross-contamination due to cross ventilation.
This study had several limitations in terms of the fate of the training aids and the number of dogs operational during the trials. In June 2020 and March 2021, a maximum of two CDDs per training aid were operational for the trial. This was resolved during the May 2021 trial when seven CDDs were operational. The sample size (in this case, the number of CDDs available) in any study is important to draw reliable conclusions and make precise estimations of statistical value (Faber and Fonseca, 2014). The other limitation with the fate of the training aid was beyond the control of the principal scientific researchers. Even though each of the samples are stored in their distinct storage conditions long term, they are often removed from storage and used for CDD training, during which time they are exposed to the ambient environment for a limited time. This process can be repeated multiple times for training CDDs and it was difficult to track and incorporate the influence of the fate of each of the training aids on the VOC profiles for the entire duration of this study.
The aim of this study was to analyze the VOC profile and CDD response to amputated lower limbs and/or feet to validate their use as CDD training aids. The study identified all decomposition related classes and multiple decomposition related VOCs in these training aids. One prominent non-decomposition related VOC—sevoflurane (an anesthetic potentially used during amputation surgeries) was also detected. It is of significance to note that if amputated limbs/feet are used as CDD training aids, the CDD could potentially be exposed to some amputation procedure related VOCs however, as determined from the 98.4% detection rate in the current study, their presence likely does not impact CDD detection capability. This study found a variability with ageing of training aids based on their VOC profiles. However, this variability did not seem to impact the CDD responses. Additionally, there was no observed difference in the VOC profiles of samples stored at room temperature and in the refrigerator or freezer. Overall, the presence of decomposition VOCs in amputated limbs/feet and their high detection rate by CDDs validated their use as alternative CDD training aids.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/wesleyburr/GCxGC_Amputated_Limbs/.
The studies involving human participants were reviewed and approved by the Le comité d'éthique de la recherche avec des etres humain at Université du Québec à Trois-Rivières (Certificate number CER-19-261-07.12). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the Le comité de bons soins aux animaux at Université du Québec à Trois-Rivières (Certificate number 2020-S.F.2).
RD: Conceptualization; methodology; writing—original draft; investigation; formal analysis; writing—review and editing. CS: Resources, writing—review and editing. WB: Statistical analysis. BD: Writing—review and editing. SF: conceptualization; methodology; funding acquisition; project administration; supervision; writing-review and editing.
This research was financially supported by the: Canada 150 Research Chair in Forensic Thanatology (C150-2017-12), Natural Science and Engineering Council (NSERC) Discovery Grant Program (RGPIN-2019-06098, 2019), and La Fondation UQTR (2018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors wish to acknowledge Darshil Patel and other members of the Forbes Research Team at Université du Québec à Trois-Rivières for helping with sample and data collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frans.2022.934639/full#supplementary-material
Agapiou, A., Zorba, E., Mikedi, K., McGregor, L., Spiliopoulou, C., and Statheropoulos, M. (2015). Analysis of Volatile Organic Compounds Released from the Decay of Surrogate Human Models Simulating Victims of Collapsed Buildings by Thermal Desorption-Comprehensive Two-Dimensional Gas Chromatography-Time of Flight Mass Spectrometry. Anal. Chim. Acta 883, 99–108. doi:10.1016/j.aca.2015.04.024
Armstrong, P., Nizio, K. D., Perrault, K. A., and Forbes, S. L. (2016). Establishing the Volatile Profile of Pig Carcasses as Analogues for Human Decomposition during the Early Postmortem Period. Heliyon 2 (2), e00070. doi:10.1016/j.heliyon.2016.e00070
Buis, R. C., Rust, L., Nizio, K. D., an Rai, T., Stuart, B. H., and Forbe, S. L. (2019). Investigating the Sensitivity of Cadaver-Detection Dogs to Aged, Diluted Decomposition Fluid. J. Forensic Identif. 69 (3), 367–377.
Buis, R. (2016). The Validation of Human Decomposition Fluid as a Cadaver-Detection Dog Training Aid. Sydney: University of Technology.
Chilcote, B., Rust, L., Nizio, K. D., and Forbes, S. L. (2018). Profiling the Scent of Weathered Training Aids for Blood-Detection Dogs. Sci. Justice 58 (2), 98–108. doi:10.1016/j.scijus.2017.11.006
Dargan, R., and Forbes, S. L. (2021). Cadaver‐detection Dogs: A Review of Their Capabilities and the Volatile Organic Compound Profile of Their Associated Training Aids. WIREs Forensic Sci. 3 (6), e1409. doi:10.1002/wfs2.1409
DeGreeff, L. E., and Furton, K. G. (2011). Collection and Identification of Human Remains Volatiles by Non-contact, Dynamic Airflow Sampling and SPME-GC/MS Using Various Sorbent Materials. Anal. Bioanal. Chem. 401 (4), 1295–1307. doi:10.1007/s00216-011-5167-0
Dekeirsschieter, J., Stefanuto, P.-H., Brasseur, C., Haubruge, E., and Focant, J.-F. (2012). Enhanced Characterization of the Smell of Death by Comprehensive Two-Dimensional Gas Chromatography-Time-Of-Flight Mass Spectrometry (GCxGC-TOFMS). PLOS ONE 7 (6), e39005. doi:10.1371/journal.pone.0039005
Deo, A., Forbes, S. L., Stuart, B. H., and Ueland, M. (2019). Profiling the Seasonal Variability of Decomposition Odour from Human Remains in a Temperate Australian Environment. Aust. J. Forensic Sci. 52, 654–664. doi:10.1080/00450618.2019.1637938
Dubois, L. M., Stefanuto, P.-H., Perrault, K. A., Delporte, G., Delvenne, P., and Focant, J.-F. (2019). Comprehensive Approach for Monitoring Human Tissue Degradation. Chromatographia 82 (5), 857–871. doi:10.1007/s10337-019-03710-3
Dupras, T. L., Schultz, J. J., Wheeler, S. M., and Williams, L. J. (2011). Forensic Recovery of Human Remains: Archaeological Approaches. Boca Raton, Florida: CRC Press.
Faber, J., and Fonseca, L. M. (2014). How Sample Size Influences Research Outcomes. Dent. Press J. Orthod. 19 (4), 27–29. doi:10.1590/2176-9451.19.4.027-029.ebo
Focant, J.-F., Stefanuto, P., Brasseur, C., Dekeirsschieter, J., Haubruge, E., Schotsmans, E., et al. (2013). Forensic Cadaveric Decomposition Profiling by GC×GC-TOFMS Analysis of VOCS. KazNU Chem. Bull. 72 (4), 177–186. doi:10.15328/chemb_2013_4177-186
Forbes, S. L., and Perrault, K. A. (2014). Decomposition Odour Profiling in the Air and Soil Surrounding Vertebrate Carrion. PLOS ONE 9 (4), e95107. doi:10.1371/journal.pone.0095107
Forbes, S. L., Perrault, K. A., Stefanuto, P.-H., Nizio, K. D., and Focant, J.-F. (2014a). Comparison of the Decomposition VOC Profile during Winter and Summer in a Moist, Mid-latitude (Cfb) Climate. PLOS ONE 9 (11), e113681. doi:10.1371/journal.pone.0113681
Forbes, S. L., Rust, L., Trebilcock, K., Perrault, K. A., and McGrath, L. T. (2014b). Effect of Age and Storage Conditions on the Volatile Organic Compound Profile of Blood. Forensic Sci. Med. Pathol. 10 (4), 570–582. doi:10.1007/s12024-014-9610-3
Hoffman, E. M., Curran, A. M., Dulgerian, N., Stockham, R. A., and Eckenrode, B. A. (2009). Characterization of the Volatile Organic Compounds Present in the Headspace of Decomposing Human Remains. Forensic Sci. Int. 186 (1-3), 6–13. doi:10.1016/j.forsciint.2008.12.022
Holland, T. D., and Connell, S. V. (2016). “The Search for and Detection of Human Remains,” in Handbook of Forensic Anthropology and Archaeology (New York, NY: Routledge), 209–222.
Iqbal, M. A., Nizio, K. D., Ueland, M., and Forbes, S. L. (2017). Forensic Decomposition Odour Profiling: A Review of Experimental Designs and Analytical Techniques. TrAC Trends Anal. Chem. 91, 112–124. doi:10.1016/j.trac.2017.04.009
Killam, E. W. (2004). The Detection of Human Remains. Springfeild, Illinois, USA: Charles C Thomas Publisher.
Knobel, Z., Ueland, M., Nizio, K. D., Patel, D., and Forbes, S. L. (2019). A Comparison of Human and Pig Decomposition Rates and Odour Profiles in an Australian Environment. Aust. J. Forensic Sci. 51 (5), 557–572. doi:10.1080/00450618.2018.1439100
Osterkamp, T. (2020). “The Dog’s Nose and Scent,” in Detector Dogs and Scent Movement : How Weather, Terrain, and Vegetation Influence Search Strategies. First edition (Boca Raton, Florida: CRC Press). doi:10.4324/9780429020704-2
Perrault, K. A., Nizio, K. D., and Forbes, S. L. (2015). A Comparison of One-Dimensional and Comprehensive Two-Dimensional Gas Chromatography for Decomposition Odour Profiling Using Inter-year Replicate Field Trials. Chromatographia 78 (15), 1057–1070. doi:10.1007/s10337-015-2916-9
Rosier, E., Cuypers, E., Dekens, M., Verplaetse, R., Develter, W., Van de Voorde, W., et al. (2014). Development and Validation of a New TD-GC/MS Method and its Applicability in the Search for Human and Animal Decomposition Products. Anal. Bioanal. Chem. 406 (15), 3611–3619. doi:10.1007/s00216-014-7741-8
Rosier, E., Loix, S., Develter, W., Van de Voorde, W., Tytgat, J., and Cuypers, E. (2015). The Search for a Volatile Human Specific Marker in the Decomposition Process. PLOS ONE 10 (9), e0137341. doi:10.1371/journal.pone.0137341
Rosier, E., Loix, S., Develter, W., Van de Voorde, W., Tytgat, J., and Cuypers, E. (2016). Time-dependent VOC-Profile of Decomposed Human and Animal Remains in Laboratory Environment. Forensic Sci. Int. 266, 164–169. doi:10.1016/j.forsciint.2016.05.035
Ruffell, A., Pringle, J. K., and Forbes, S. (2014). Search Protocols for Hidden Forensic Objects beneath Floors and within Walls. Forensic Sci. Int. 237, 137–145. doi:10.1016/j.forsciint.2013.12.036
Rust, L., Nizio, K. D., Wand, M. P., and Forbes, S. L. (2018). Investigating the Detection Limits of Scent-Detection Dogs to Residual Blood Odour on Clothing. Forensic Chem. 9, 62–75. doi:10.1016/j.forc.2018.05.002
Siniscalchi, M. (2016). “Olfaction and the Canine Brain,” in Canine Olfaction Science and Law: Advances in Forensic Science, Medicine, Conservation, and Environmental Remediation. Editors T. Jezierski, J. Ensminger, and L. Papet (Boca Raton, Florida: CRC Press), 31–37. doi:10.1201/b20027-5
Stadler, S., Stefanuto, P.-H., Byer, J. D., Brokl, M., Forbes, S., and Focant, J.-F. (2012). Analysis of Synthetic Canine Training Aids by Comprehensive Two-Dimensional Gas Chromatography-Time of Flight Mass Spectrometry. J. Chromatogr. A 1255, 202–206. doi:10.1016/j.chroma.2012.04.001
Statheropoulos, M., Agapiou, A., Zorba, E., Mikedi, K., Karma, S., Pallis, G. C., et al. (2011). Combined Chemical and Optical Methods for Monitoring the Early Decay Stages of Surrogate Human Models. Forensic Sci. Int. 210 (1-3), 154–163. doi:10.1016/j.forsciint.2011.02.023
Statheropoulos, M., Agapiou, A., Spiliopoulou, C., Pallis, G., and Sianos, E. (2007). Environmental Aspects of VOCs Evolved in the Early Stages of Human Decomposition. Sci. Total Environ. 385 (1), 221–227. doi:10.1016/j.scitotenv.2007.07.003
Statheropoulos, M., Spiliopoulou, C., and Agapiou, A. (2005). A Study of Volatile Organic Compounds Evolved from the Decaying Human Body. Forensic Sci. Int. 153 (2), 147–155. doi:10.1016/j.forsciint.2004.08.015
Stefanuto, P.-H., Rosier, E., Tytgat, J., Focant, J.-F., and Cuypers, E. (2017). “Profiling Volatile Organic Compounds of Decomposition,” in Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Editors E. M. Schotsmans, N. Márquez-Grant, and S. L. Forbes (John Wiley and Sons), 39–52. doi:10.1002/9781118953358.ch3
Tipple, C. A., Caldwell, P. T., Kile, B. M., Beussman, D. J., Rushing, B., Mitchell, N. J., et al. (2014). Comprehensive Characterization of Commercially Available Canine Training Aids. Forensic Sci. Int. 242, 242–254. doi:10.1016/j.forsciint.2014.06.033
Vass, A. A. (2012). Odor Mortis. Forensic Sci. Int. 222 (1), 234–241. doi:10.1016/j.forsciint.2012.06.006
Vass, A. A., Smith, R. R., Thompson, C. V., Burnett, M. N., Dulgerian, N., and Eckenrode, B. A. (2008). Odor Analysis of Decomposing Buried Human Remains. J. Forensic Sci. 53 (2), 384–391. doi:10.1111/j.1556-4029.2008.00680.x
Vass, A. A., Smith, R. R., Thompson, C. V., Burnett, M. N., Wolf, D. A., Synstelien, J. A., et al. (2004). Decompositional Odor Analysis Database. J. Forensic Sci. 49 (4), 1–10. doi:10.1520/jfs2003434
Keywords: forensic taphonomy, decomposition odor analysis, comprehensive two-dimensional chromatography (GC×GC-TOFMS), cadaver detection dogs, amputated limbs, training aids
Citation: Dargan R, Samson C, Burr WS, Daoust B and Forbes SL (2022) Validating the Use of Amputated Limbs Used as Cadaver Detection Dog Training Aids. Front. Anal. Sci. 2:934639. doi: 10.3389/frans.2022.934639
Received: 02 May 2022; Accepted: 14 June 2022;
Published: 13 July 2022.
Edited by:
John Vincent Goodpaster, Purdue University Indianapolis, United StatesCopyright © 2022 Dargan, Samson, Burr, Daoust and Forbes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rushali Dargan, cnVzaGFsaWRhcmdhbkBnbWFpbC5jb20=, cnVzaGFsaS5kYXJnYW5AdXF0ci5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.