
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Artif. Intell., 20 August 2021
Sec. Medicine and Public Health
Volume 4 - 2021 | https://doi.org/10.3389/frai.2021.674238
This article is part of the Research TopicFetal-Maternal Monitoring in the Age of Artificial Intelligence and Computer-Aided Decision Support: A Multidisciplinary PerspectiveView all 16 articles
Continuous electronic fetal monitoring and the access to databases of fetal heart rate (FHR) data have sparked the application of machine learning classifiers to identify fetal pathologies. However, most fetal heart rate data are acquired using Doppler ultrasound (DUS). DUS signals use autocorrelation (AC) to estimate the average heartbeat period within a window. In consequence, DUS FHR signals loses high frequency information to an extent that depends on the length of the AC window. We examined the effect of this on the estimation bias and discriminability of frequency domain features: low frequency power (LF: 0.03–0.15 Hz), movement frequency power (MF: 0.15–0.5 Hz), high frequency power (HF: 0.5–1 Hz), the LF/(MF + HF) ratio, and the nonlinear approximate entropy (ApEn) as a function of AC window length and signal to noise ratio. We found that the average discriminability loss across all evaluated AC window lengths and SNRs was 10.99% for LF 14.23% for MF, 13.33% for the HF, 10.39% for the LF/(MF + HF) ratio, and 24.17% for ApEn. This indicates that the frequency domain features are more robust to the AC method and additive noise than the ApEn. This is likely because additive noise increases the irregularity of the signals, which results in an overestimation of ApEn. In conclusion, our study found that the LF features are the most robust to the effects of the AC method and noise. Future studies should investigate the effect of other variables such as signal drop, gestational age, and the length of the analysis window on the estimation of fHRV features and their discriminability.
Continuous electronic fetal monitoring (EFM) is a standard of care during the antepartum and intrapartum periods (American College of Obstetricians and Gynecologists, 2014). EFM involves measuring two signals: fetal heart rate (FHR) and uterine pressure (UP). These two signals make up what is known as cardiotocography (CTG). Non-invasive Doppler ultrasound (DUS) is the preferred FHR acquisition method in clinical settings (Kupka et al., 2020). Uterine pressure is commonly acquired using external sensors that measure the tension in the maternal abdominal wall (Smyth, 1957). There are other acquisition methods: fetal scalp electrocardiography (ECG) for FHR; and intrauterine probes for uterine pressure (Ayres-De-Campos and Nogueira-Reis, 2016). However, these methods are invasive and are typically used only when external monitoring is not possible.
During the antepartum period, FHR monitoring has been shown to provide information about fetal reactivity (Romano et al., 2006) and abnormalities such as intrauterine growth restriction (Signorini et al., 2003; Signorini et al., 2020). During labour, the fetus is exposed to repeated periods of hypoxia during uterine contractions (McNamara and Johnson, 1995). If severe enough, sustained hypoxia can lead to metabolic acidosis and hypoxic-ischemic encephalopathy (HIE). Clinicians assess the risk of acidosis and HIE by visually monitoring the EFM for characteristic FHR patterns such as the baseline, accelerations, and decelerations (American College of Obstetricians and Gynecologists, 2014; Lear et al., 2018). Nevertheless, visual assessment of FHR tracings has low specificity and sensitivity as well as high intra- and inter-observer variability (Farquhar et al., 2020). The application of computerized analysis to quantify FHR signals has been proposed to reduce intra- and inter-observer variability (Keith and Greene, 1994). However, recent studies show that automating the analysis of classical FHR patterns does not yield a significant improvement in the detection of acidosis or HIE (Elliott et al., 2010; Clark et al., 2017; Campanile et al., 2018).
It is thought that the development of new FHR indices reflecting the physiological phenomena of acidosis and HIE could improve the ability to identify fetuses at risk (Hamilton and Warrick, 2013). In this context, fetal heart rate variability (fHRV) shows promise to be an important marker of fetal status (Signorini et al., 2003). Heart rate variability (HRV) quantifies variations in the length of the RR interval in successive heartbeats and has been widely used in adults (Acharya et al., 2006). Most HRV analysis algorithms are based on RR intervals derived from ECG signals (Ramshur 2010). However, it is difficult to use these methods for fetal monitoring since DUS measures of FHR do not provide the RR intervals. For this reason, clinical use of fHRV is generally limited to the visual analysis of FHR variations around its baseline.
The DUS transducer emits an ultrasound wave towards the fetal heart. The movement of the fetal heart changes the frequency of the reflected wave due to the Doppler effect (Hamelmann et al., 2020). As a result, both the amplitude and phase of the reflected wave are modulated and consequently its envelope varies with a frequency related to FHR (Hamelmann et al., 2020). FHR is then estimated from the autocorrelation (AC) of the DUS signal envelope computed over a window several seconds long. The AC, which measures the similarity of the signal to itself across time, will have a maximum at a lag equal to the average RR interval (Kupka et al., 2020). FHR is estimated as the inverse of this average RR interval. Fetal monitors use sliding windows to estimate FHR at a uniform sampling rate.
As a result of the averaging associated with computing the AC method, estimates of fHRV features derived from DUS (
More sophisticated methods have been proposed to improve the estimation of
The availability of large cohorts of perinatal EFM recordings has motivated the development of machine learning (ML) classifiers to improve the early detection of fetal distress and reduce the risk of further injury (Georgieva et al., 2017; Petrozziello et al., 2019). Thus, fHRV features from
This paper analyzes the influence of the AC window length and noise on the estimation and discriminability of some important linear and non-linear fHRV features. These features considered have all been proposed previously for the detection of fetal distress (Signorini et al., 2003). Despite the development of the new sophisticated AC algorithms, we focus on the classical AC method which is the basis of current monitors. The rationale behind this is the desire understand the properties of fHRV computed from EFM data acquired at bedside with current monitors. Thus, our objectives are twofold: 1) To determine how fHRV features computed from
This section describes the methods used for:
1) Simulating RR intervals and associated DUS signals with PSD and ApEn properties similar to those of normal and acidotic fetuses.
2) Estimating PSD and ApEn features.
3) Evaluating differences between fHRV features estimated from
4) Evaluating the discriminability of different simulated fHRV features when applied to normal and acidotic signals.
We used 80
Figure 1 outlines the process for simulating RR intervals, DUS signals, and uniformly sampled FHR. We first generated a sequence of random RR intervals with spectral features for normal or acidosis fetuses similar to those reported by Gonçalves et al., 2013. The 95% confidence intervals (CI) of the power in the low frequency, movement frequency, and high frequency bands reported by Gonçalves et al. are reported in Table 1. Afterwards, we generated the DUS envelope signals corresponding to the simulated RR intervals with added noise. Finally, we applied the AC method with a sliding window to generate uniformly sampled
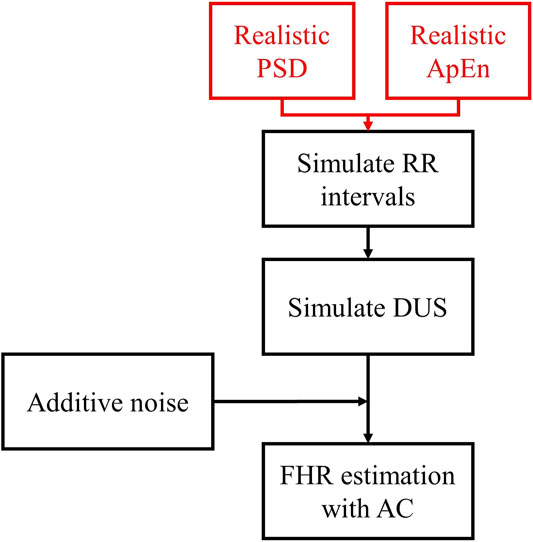
FIGURE 1. Diagram of the simulation of
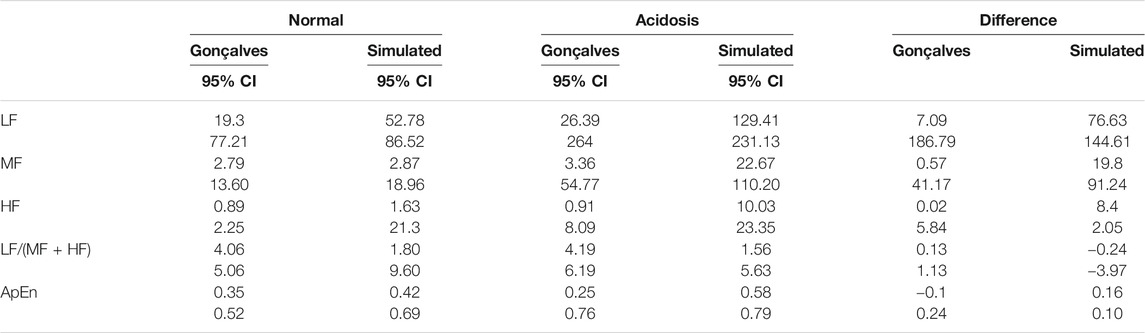
TABLE 1. 95% Confidence intervals (CI) for the fHRV estimates reported by Gonçalves et al., 95% CI of the simulated RR intervals fHRV, and the difference of the limits of the 95% CI between the Normal and Acidosis distributions.
We simulated realizations of RR interval sequences, with controlled fHRV PSD structure and nonlinear complexity, as follows:
We first generated a continuous FHR signal, sampled at 4 Hz, with the desired fHRV spectrum. To do so, we filtered the same white Gaussian noise with three bandpass filters, corresponding to the three bands of interest for fHRV [from (Signorini et al., 2003)]: Low frequency (LF) 0.03–0.15 Hz; Movement frequency (MF) 0.15–0.5 Hz; and High frequency (HF) 0.5–1 Hz band. The three filter outputs were summed in different proportions to generate a signal whose spectrum matched the fHRV spectra reported by Gonçalves et al. (2013).
We then generated a continuous RR interval signal,
1) Sample
2) Repeat for the length of
The resulting
ApEn is a measure of signal complexity, and thus random signals will have higher ApEn compared to periodic signals. To control the ApEn of our simulated RR intervals we modified the MIX process of Ferrario et al. (2006). The original MIX process switches randomly between a periodic signal and a uniformly distributed random signal and so does not permit the control of the realization’s PSD. To do so, we modified the MIX process to switch randomly between
1) A random sequence
2) A semi-periodic signal
The MIX process switching is controlled by a binary random variable
Each RR interval sequence was transformed into a corresponding DUS envelope signal, sampled at 1 kHz, as follows:
1) A template DUS envelope cycle
2) For each RR interval, the selected
3) Consecutive
4) A random additive noise signal,
5) The amplitude of
6) Finally, we generated
Figure 2A shows a segment of a simulated
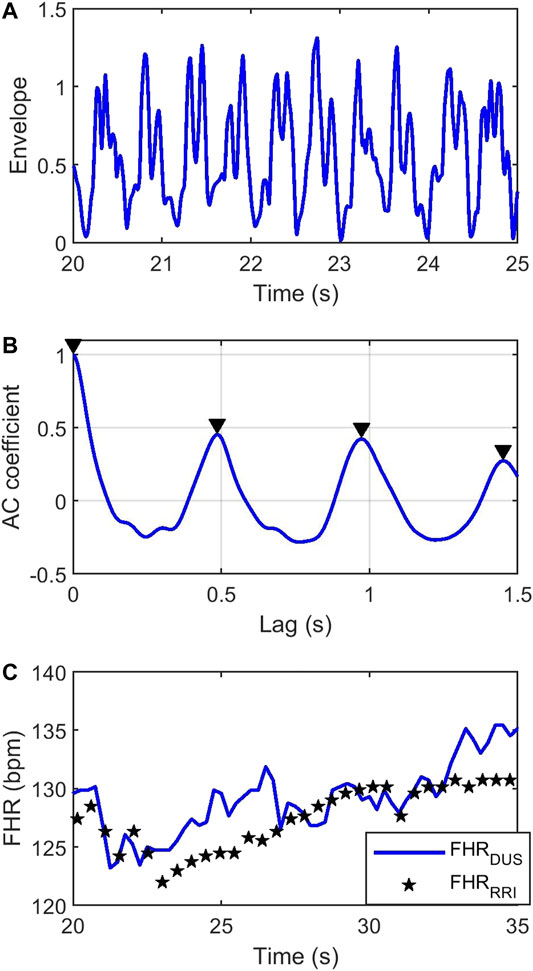
FIGURE 2. (A) Simulated envelope of the
FHR was estimated from the DUS signal by computing its autocorrelation function (AC). The autocorrelation function of a periodic signal is also periodic with the same period. Consequently, the first non-zero maxima in the AC function will reflect the average RR interval. Figure 2B shows the AC coefficient function of the DUS signal in Figure 2A, estimated from a 4s window. The first non-zero-lag peak occurs at ∼0.5 s indicating an FHR = 120 bpm. Sliding the AC window across the signal with steps of 0.25 s will generate an FHR signal sampled at 4 Hz. The blue curve in Figure 2C shows the
Figure 3 shows the procedure used to compare the fHRV estimates from the RR intervals and DUS FHR.
1)
2) These
3) fHRV estimates were obtained from
4) The estimates were compared as a function of AC window length and SNR.
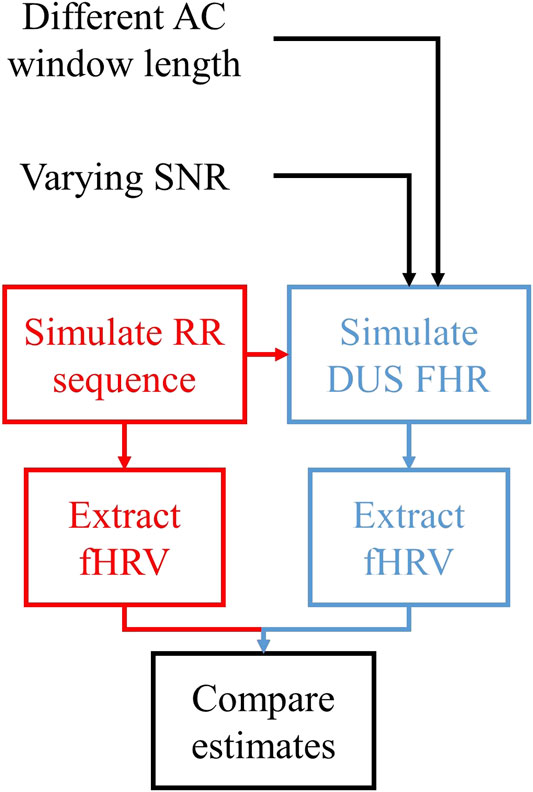
FIGURE 3. Outline of the assessment of the fHRV estimation differences between
We simulated 1,000 Monte Carlo (MC)
The
The PSDs of the non-uniformly sampled
where
ApEn, a measure of the nonlinear complexity of FHR, was estimated as follows:
1) FHR was decimated to 2 Hz, following Gonçalves et al., 2013 who found that sampling the FHR at 2 Hz provided better ApEn estimates than 4 Hz.
2) The function “approximateEntropy” in the Matlab Predictive Maintenance Toolbox was used with an embedding dimension of 2, and radius of 0.2.
Differences between features computed from the RR and DUS signals were quantified in terms of their bias and random differences:
where
Figure 4 describes the procedure used to assess fHRV discriminability. We simulated normal and acidotic
1)
2) The normalized difference between
where
3) The normalized standard deviation of
where
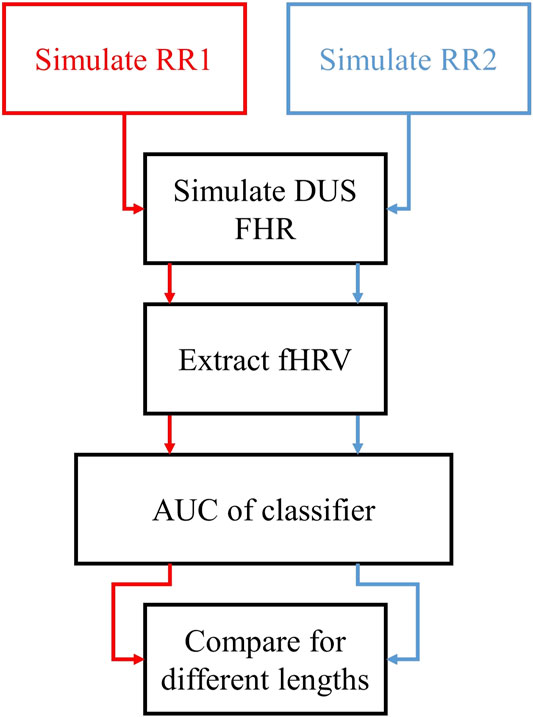
FIGURE 4. Outline of the assessment of the discriminability of fHRV features as functions of the AC window length. We simulated two sets of
We first compared the features of the simulated
We also compared the features of our simulated sequences to those of the 80 subjects in the PhysioNet database. Figure 5 shows boxplots of the normalized
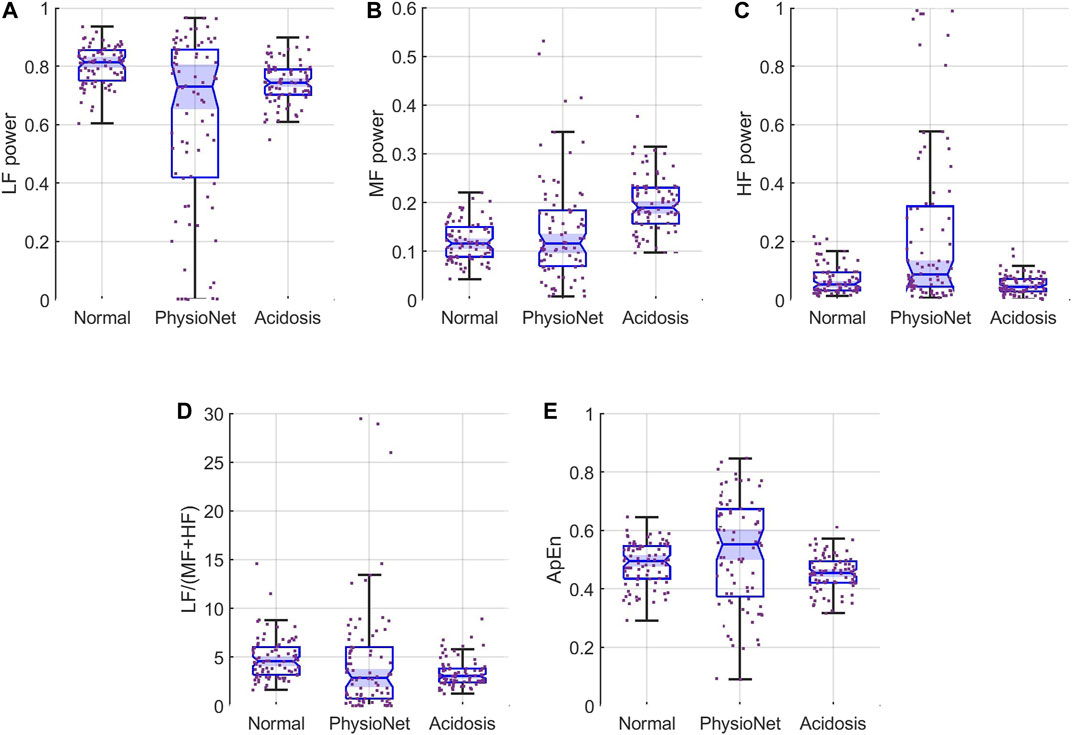
FIGURE 5. Feature distributions for (A) LF, (B) MF, and (C) HF (D) LF/(MF + HF) ratio, and (E) ApEn. Each panel show the samples (scattered points) and boxplots for the features for simulated normal (left), the PhysioNet data (middle), and simulated acidosis
Next we examined the differences between features computed from the simulated

FIGURE 6. Contour plots of the bias difference,
Figures 6C,E show that the MF and HF powers were overestimated for low SNR and short AC windows while for long windows and high SNRs they were underestimated. The bias differences had larger magnitude for HF than for MF. Thus, the HF power was most sensitive to the AC window and additive noise. Figures 6D,F show that the random difference for both MF and HF were larger for low SNR and short AC windows but decreased as the SNR and window length increased.
Figures 6G,H show that LF/(MF + HF) ratio
Figure 6I shows that ApEn was always overestimated with the error decreasing as the SNR and window length increased. The random error of ApEn (Figure 6J) behaved similarly.
We evaluated the discriminability of the features in terms of the AUC of the Neyman-Pearson classifiers for varying AC window length and SNR. As a reference, Table 2 shows the median and 95% CI
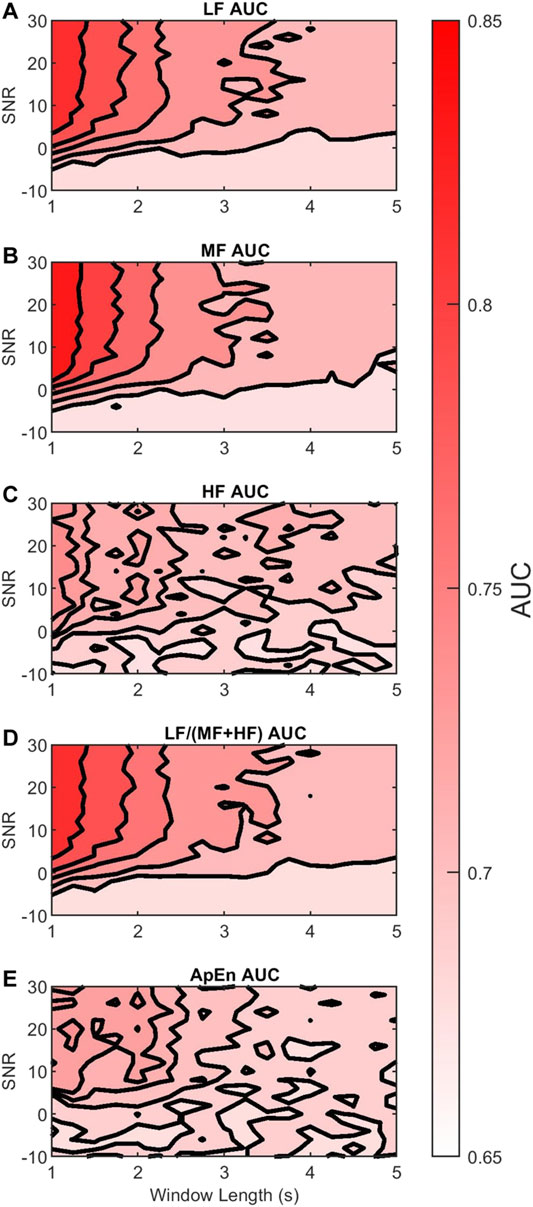
FIGURE 7. Contour plots of the median
Figures 7A,B show that the discriminability of LF and MF was greatest for SNR larger than 0 dB and windows shorter than 2 s. Thus, in this region, their discriminability was not affected much by the AC method. However, outside this region the color contrast is strong, indicating a large drop in discriminability. In contrast, Figure 7C shows that the reduction in HF discriminability was less marked as the window length increased or SNR decreased.
These observations can be contrasted with the results in Table 3. Table 3 shows the 95% CI of the differences between
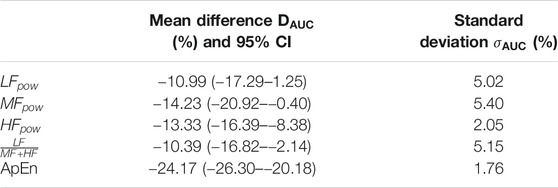
TABLE 3. Mean,
Figure 7D shows that the discriminability of LF/(MF + HF) ratio decreases with longer windows and lower SNR. It follows a similar trend to the LF and MF. The estimated D_AUC showed a decrease of 10.39% of its discriminability, and
Finally, Figure 7E shows that the discriminability of ApEn behaved similarly to HF
This paper has two objectives: 1) to analyze differences in fHRV features estimated from
The results presented in this paper are based on simulations in which we generated artificial RR intervals and the corresponding DUS signals. The significance of our results will depend on the validity of these simulations. We believe they are valid for the following reasons:
First, an important feature of our simulation of RR intervals was that we were able to generate sequences having both power spectral and entropy features similar to those of real data. Table 1 and Figure 5 show that the distribution of fHRV features of our
Secondly, we opted to simulate the envelope of the DUS signals rather than the raw DUS signal itself. Raw DUS signals are subject to multiple artifacts during clinical acquisition: movement of the probe or signal loss introduce noise in the signals (Shakespeare et al., 2001; Jezewski et al., 2017). As a solution, fetal monitors use the envelope of the signal, which serves as a LF filter (Hamelmann et al., 2020). This envelope trades the amount of information contained in the signal, such as the location of specific cardiac events (Shakespeare et al., 2001), for robustness in the estimation of the FHR (Hamelmann et al., 2020). Investigating the effect of extracting the envelope of the signal is out of the scope of this study as we focused specifically on the AC method applied to the DUS envelope. Furthermore, we introduced noise in our signal in two ways: 1) we use 15 DUS envelopes reported in the literature (Hamelmann et al., 2020) as templates, which have intrinsic acquisition noise, and 2) we added bandlimited uniform noise, where the cut-off frequency was set to 7.7 Hz. Thus, even for our simulations with the highest SNR, there is noise inherent to the templates that cannot be removed. This introduces heterogeneity in the signal, each DUS cycle is different from the others.
Finally, it is important to consider the method used to compute
Our experiments aimed to analyze the error of fHRV feature estimation from using the AC method. We found that the length of the autocorrelation window, which determines the extent of signal averaging, had a strong influence on these errors. Longer windows provide more AC averaging, which reduces the effect of additive noise at the cost of beat-to-beat accuracy in the estimation of FHR. In other words, longer averaging windows act as low-pass filters with lower bandwidths. Accordingly, Figure 6A shows underestimation of the LF power for short AC windows and low SNR, but the bias difference increases to be almost zero as AC window length and SNR increase. In contrast, the MF and HF powers were increasingly attenuated as the window length increased. As expected, the AC method attenuates the MF and HF power while increasing the relative magnitude of the LF power. This is in agreement with the findings of Clifford and Tarassenko (2005) which showed that interpolated heart rate signals (without averaging) overestimate LF power with respect to higher frequency bands.
Showing a different behavior, ApEn (Figure 6I) is always overestimated, which might be due to the effect of additive noise. The ApEn is an estimate of a signal irregularity, and it is higher for random than for periodic signals. Thus, adding random noise increases the signal irregularity which directly increases the ApEn. However, Figure 6I shows a decrease when SNR or the window length increase; less noise or more averaging reduces the irregularity in the signal and lowers the ApEn.
In summary, these results show that data from multiple monitors with different parameters may yield different estimates of fHRV. The extent of these differences is documented in our contour plots as a function of window lengths and SNR. Unfortunately, information about the window length used is rarely available for commercial monitors. Unless the manufacturers start to disclose the parameters of their acquisition algorithms, data analysis of such signals must take into account that the variability in the estimated fHRV does not only depend on fetal state but also the CTG monitor.
FHR monitoring during the intrapartum aims to detect fetuses at risk and to use this information to determine whether an emergency cesarean delivery is warranted. Thus, it is important to study how discriminability of certain features is affected by the CTG acquisition methods. Our simulations showed that the discriminability of PSD and ApEn features changed with AC window length and SNR. For all features, the AUC of a Neyman-Pearson classifier decreased as the SNR decreases. This is explained by loss of discriminatory information due to additive noise or large magnitude. Similarly, the AUC decreased as the AC window length increased. This is explained by the loss of discriminatory beat-to-beat information associated with longer AC windows (more averaging).
Two different behaviors can be observed for the five fHRV features analyzed. LF, MF, and LF/(MF + HF) lose less discriminability on average as defined by
The AC method is expected to preserve low frequencies and attenuate high frequencies. Thus, we hypothesized that there might be an LF sub-band that the AC method would not affect. To this end we explored the effects of dividing the LF band into two sub-bands LF1 (0.03–0.072 Hz) and LF2 (0.072–0.15 Hz). Figure 8A shows that by doing so, there was no loss in discriminability for LF1 across different AC window lengths and SNR. In contrast, Figure 8B shows that the discriminability of LF2
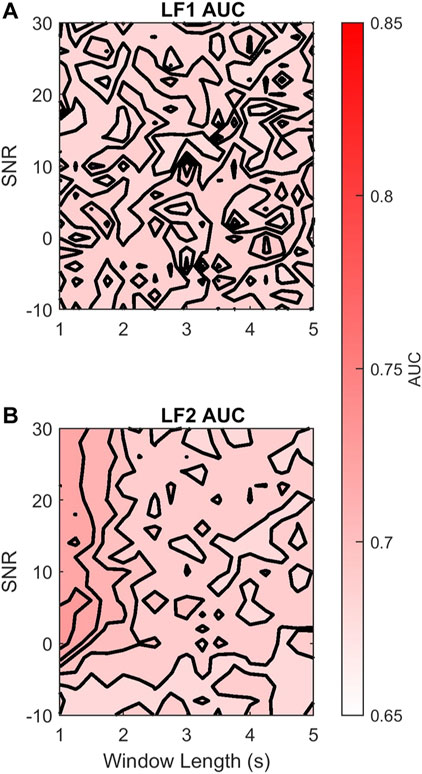
FIGURE 8. Contour plots of the median
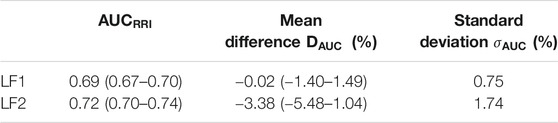
TABLE 4. Median
We believe these results provide important insight into the effects of computing FHR features using the AC method. Nevertheless there are a number of limitations of the work to consider.
First, the reference distributions that we used to define the normal and acidotic classes were estimated from a handful of cases, which might not be enough. Gonçalves et al. (2013) reported features extracted from 21 normal fetuses and six acidotic fetuses. Thus, a larger database would be necessary to better characterize the distributions of both classes. Furthermore, we use 15 DUS templates in our simulations. Although the use of these templates result in variation of the simulated DUS waves, using a larger number of DUS envelopes as templates might produce more realistic DUS simulations.
Secondly, our model only controls the PSD and ApEn of the simulated
Thirdly, our model does not account for signal loss. Implementing signal loss requires to add a different noise model, which behaves as a switch between signal and no signal. We consider that a future study could expand our model to include such a switch using information from real databases. Parameters such as number of drops, or the duration of the artifact can be characterized in their distributions to generate a realistic DUS signal and
Fourthly, our model does not account for the nonstationary behavior of intrapartum FHR signals. The simulated FHR signals were time invariant within a window of 10 min. However, real FHR signals are nonlinear and time-varying. For a single subject, it is expected that the fHRV distribution will vary across labor: increased uterine activity will generate responses in the FHR and fHRV (Warrick and Hamilton, 2012; Lear et al., 2018). Thus, the length of analysis window is an important parameter to consider and optimize: short analysis windows will provide large variability in the estimated features, while long analysis windows will include nonstationary FHR. Another factor that will affect the estimated fHRV is fetal state. It has been shown that fetuses have variable fHRV distributions when they are in quiet and active periods (Signorini et al., 2003). Thus, a long analysis window might contain more than one fetal state, which is also nonstationary behavior. An alternative approach to optimizing the length of the analysis window is to consider time-varying or parameter-varying models to describe FHR and fHRV. Future studies should consider these models in the analysis of intrapartum FHR.
Finally, our model does not consider the effect of gestational age (GA). It has been reported that the distribution of fHRV features vary with GA (Gonçalves et al., 2018). However, no significant difference was reported in PSD or ApEn features for term infants (GA > 36 weeks). Considering that our simulations took as reference fHRV distributions from term infants or intrapartum signals, we believe that our results are valid regardless GA in term infants. Further studies should analyze if the trends of the
Our results demonstrate the susceptibility of fHRV features to the AC method and additive noise in the clinical acquisition of FHR. The dependency of the estimation error on the AC window length, which is part of the proprietary information of the FHR monitor manufacturers, is a limitation in comparing data acquired from different monitors. There is an increasing interest in applying machine learning techniques to FHR tracings on large databases to identify fetuses at risk during antepartum (Signorini et al., 2020) and intrapartum monitoring (Georgieva et al., 2017; Petrozziello et al., 2019). Although the discriminability of fHRV features depends on the AC window length of the FHR monitor and the SNR, it has low variability (<5.4%). Moreover, a feature based on the power below 72 mHz is not affected by the AC method. Thus, understanding the effects of the AC method on fHRV discriminability would potentially lead to a better implementation of ML classifiers of FHR signals when dealing with multiple sources. LF power, MF power, and the LF/(MF + HF) ratio are least affected by the AC method in average but are more influenced by changes in the AC window length and SNR. Classifiers based on these features would benefit from including the fetal monitor model, or acquisition center, as part of the regressor. On the other hand, HF power and ApEn experience the largest loss of discriminability in average, but with lower dependency on AC window length and SNR. Thus, classifiers based on these features would not need to account for differences in the acquisition fetal monitors.
The fetal ECG signals used in this article are openly available as part of the Noninvasive Fetal ECG - The PhysioNet Computing in Cardiology Challenge 2013 (Accession Number: 25401167, URL: https://physionet.org/content/challenge-2013/1.0.0/) and the Abdominal and Direct Fetal ECG Database (Accession Number: 25854665, URL: https://physionet.org/content/adfecgdb/1.0.0/).
JV-C is the first author and the research is part of his PhD. His supervisors PW and RK supported the process during weekly discussions and actively participated in writing the manuscript along with JV-C.
This study was carried thanks to the funding provided by the Bill & Melinda Gates Foundation and the National Institutes of Health.
Author PW was employed by the company PeriGen Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acharya, U. R., Joseph, K. P., Kannathal, N., Lim, C. M., and Suri, J. S. (2006). Heart Rate Variability: a Review. Med. Biol. Eng. Comput. 44, 1031–1051. doi:10.1007/s11517-006-0119 |
Alnuaimi, S. A., Jimaa, S., and Khandoker, A. H. (2017). Fetal Cardiac Doppler Signal Processing Techniques: Challenges and Future Research Directions. Front. Bioeng. Biotechnol. 5, 82. doi:10.3389/fbioe.2017.00082 |
American College of Obstetricians and Gynecologists (2014). “Intrapartum Considerations and Assessment,” in Neonatal Encephalopathy and Neurologic Outcome, Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy (Washington, DC: American Academy of Pediatrics), 87–114.
Ayres-De-Campos, D., and Nogueira-Reis, Z. (2016). Technical Characteristics of Current Cardiotocographic Monitors. Best Pract. Res. Clin. Obstet. Gynaecol. 30, 22–32. doi:10.1016/j.bpobgyn.2015.05.005 |
Campanile, M., D’Alessandro, P., Della Corte, L., Saccone, G., Tagliaferri, S., Arduino, B., et al. (2018). Intrapartum Cardiotocography with and without Computer Analysis: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Maternal-Fetal Neonatal Med. 33, 2284–2290. doi:10.1080/14767058.2018.1542676
Cesarelli, M., Romano, M., Bifulco, P., Fedele, F., and Bracale, M. (2007). An Algorithm for the Recovery of Fetal Heart Rate Series from CTG Data. Comput. Biol. Med. 37, 663–669. doi:10.1016/j.compbiomed.2006.06.003 |
Clark, S. L., Hamilton, E. F., Garite, T. J., Timmins, A., Warrick, P. A., and Smith, S. (2017). The Limits of Electronic Fetal Heart Rate Monitoring in the Prevention of Neonatal Metabolic Acidemia. Am. J. Obstet. Gynecol. 216. doi:10.1016/j.ajog.2016.10.009
Clifford, G. D., and Tarassenko, L. (2005). Quantifying Errors in Spectral Estimates of HRV Due to Beat Replacement and Resampling. IEEE Trans. Biomed. Eng. 52, 630–638. doi:10.1109/tbme.2005.844028 |
Durosier, L. D., Green, G., Batkin, I., Seely, A. J., Ross, M. G., Richardson, B. S., et al. (2014). Sampling Rate of Heart Rate Variability Impacts the Ability to Detect Acidemia in Ovine Fetuses Near-Term. Front. Pediatr. 2. doi:10.3389/fped.2014.00038
Elliott, C., Warrick, P. A., Graham, E., and Hamilton, E. F. (2010). Graded Classification of Fetal Heart Rate Tracings: Association with Neonatal Metabolic Acidosis and Neurologic Morbidity. Am. J. Obstet. Gynecol. 202, 251–258. doi:10.1016/j.ajog.2009.06.026
Farquhar, C. M., Armstrong, S., Masson, V., Thompson, J. M. D., and Sadler, L. (2020). Clinician Identification of Birth Asphyxia Using Intrapartum Cardiotocography Among Neonates with and without Encephalopathy in New Zealand. JAMA Netw. Open 3, e1921363. doi:10.1001/jamanetworkopen.2019.21363 |
Ferrario, M., Signorini, M. G., Magenes, G., and Cerutti, S. (2006). Comparison of Entropy-Based Regularity Estimators: Application to the Fetal Heart Rate Signal for the Identification of Fetal Distress. IEEE Trans. Biomed. Eng. 53, 119–125. doi:10.1109/tbme.2005.859809 |
Georgieva, A., Redman, C. W. G., and Papageorghiou, A. T. (2017). Computerized Data-Driven Interpretation of the Intrapartum Cardiotocogram: a Cohort Study. Acta Obstet. Gynecol. Scand. 96, 883–891. doi:10.1111/aogs.13136 |
Goldberger, A. L., Amaral, L. A. N., Glass, L., Hausdorff, J. M., Ivanov, P. C., Mark, R. G., et al. (2000). PhysioBank, PhysioToolkit, and PhysioNet. Circulation 101, e215-e220. doi:10.1161/01.cir.101.23.e215 |
Gonçalves, H., Amorim-Costa, C., Ayres-De-Campos, D., and Bernardes, J. (2018). Evolution of Linear and Nonlinear Fetal Heart Rate Indices throughout Pregnancy in Appropriate, Small for Gestational Age and Preterm Fetuses: A Cohort Study. Comput. Methods Programs Biomed. 153, 191–199. doi:10.1016/j.cmpb.2017.10.015 |
Gonçalves, H., Costa, A., Ayres-De-Campos, D., Costa-Santos, C., Rocha, A. P., and Bernardes, J. (2013). Comparison of Real Beat-To-Beat Signals with Commercially Available 4 Hz Sampling on the Evaluation of Foetal Heart Rate Variability. Med. Biol. Eng. Comput. 51, 665–676. doi:10.1007/s11517-013-1036-7 |
Hamelmann, P., Vullings, R., Kolen, A. F., Bergmans, J. W. M., Van Laar, J. O. E. H., Tortoli, P., et al. (2020). Doppler Ultrasound Technology for Fetal Heart Rate Monitoring: A Review. IEEE Trans. Ultrason. Ferroelect., Freq. Contr. 67, 226–238. doi:10.1109/tuffc.2019.2943626 |
Hamilton, E. F., and Warrick, P. A. (2013). New Perspectives in Electronic Fetal Surveillance. J. Perinat Med. 41, 83–92. doi:10.1515/jpm-2012-0024 |
Jezewski, J., Matonia, A., Kupka, T., Roj, D., and Czabanski, R. (2012). Determination of Fetal Heart Rate from Abdominal Signals: Evaluation of Beat-To-Beat Accuracy in Relation to the Direct Fetal Electrocardiogram. Biomedizinische Technik/Biomedical Eng. 57, 383–394. doi:10.1515/bmt-2011-0130 |
Jezewski, J., Roj, D., Wrobel, J., and Horoba, K. (2011). A Novel Technique for Fetal Heart Rate Estimation from Doppler Ultrasound Signal. BioMedical Eng. OnLine 10, 92. doi:10.1186/1475-925x-10-92 |
Jezewski, J., Wrobel, J., Matonia, A., Horoba, K., Martinek, R., Kupka, T., et al. (2017). Is Abdominal Fetal Electrocardiography an Alternative to Doppler Ultrasound for FHR Variability Evaluation? Front. Physiol. 8, 305. doi:10.3389/fphys.2017.00305 |
Jonckheere, J., Garabedian, C., Charlier, P., Storme, L., Debarge, V., and Logier, R. (2019). Influence of Averaged Fetal Heart Rate in Heart Rate Variability Analysis. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., 2019, 5979–5982. doi:10.1109/EMBC.2019.8856803 |
Katebi, N., Marzbanrad, F., Stroux, L., Valderrama, C. E., and Clifford, G. D. (2020). Unsupervised Hidden Semi-markov Model for Automatic Beat Onset Detection in 1D Doppler Ultrasound. Physiol. Meas. 41, 085007. doi:10.1088/1361-6579/aba006 |
Keith, R. D. F., and Greene, K. R. (1994). 4 Development, Evaluation and Validation of an Intelligent System for the Management of Labour. Baillière's Clin. Obstet. Gynaecol. 8, 583–605. doi:10.1016/s0950-3552(05)80200-7
Kupka, T., Matonia, A., Jezewski, M., Horoba, K., Wrobel, J., and Jezewski, J. (2020). Coping with Limitations of Fetal Monitoring Instrumentation to Improve Heart Rhythm Variability Assessment. Biocybernetics Biomed. Eng. 40, 388–403. doi:10.1016/j.bbe.2019.12.005
Lear, C. A., Wassink, G., Westgate, J. A., Nijhuis, J. G., Ugwumadu, A., Galinsky, R., et al. (2018). The Peripheral Chemoreflex: Indefatigable Guardian of Fetal Physiological Adaptation to Labour. J. Physiol. 596, 5611–5623. doi:10.1113/jp274937 |
McNamara, H., and Johnson, N. (1995). The Effect of Uterine Contractions on Fetal Oxygen Saturation. BJOG:An Int. J. O&G 102, 644–647. doi:10.1111/j.1471-0528.1995.tb11403.x
Nichols, J. M., Olson, C. C., Michalowicz, J. V., and Bucholtz, F. (2010). A Simple Algorithm for Generating Spectrally Colored, Non-gaussian Signals. Probabilistic Eng. Mech. 25, 315–322. doi:10.1016/j.probengmech.2010.01.005
Peters, C. H. L., Broeke, E. D. M. t., Andriessen, P., Vermeulen, B., Berendsen, R. C. M., Wijn, P. F. F., et al. (2004). Beat-to-beat Detection of Fetal Heart Rate: Doppler Ultrasound Cardiotocography Compared to Direct ECG Cardiotocography in Time and Frequency Domain. Physiol. Meas. 25, 585–593. doi:10.1088/0967-3334/25/2/015 |
Petrozziello, A., Redman, C. W. G., Papageorghiou, A. T., Jordanov, I., and Georgieva, A. (2019). Multimodal Convolutional Neural Networks to Detect Fetal Compromise during Labor and Delivery. IEEE Access 7, 112026–112036. doi:10.1109/access.2019.2933368
Ramshur, J. T. (2010). Design, Evaluation, and Application of Heart Rate Variability Analysis Software (HRVAS). [Master’s thesis]. Memphis, TN: University of Memphis.
Romano, M., Bifulco, P., Cesarelli, M., Sansone, M., and Bracale, M. (2006). Foetal Heart Rate Power Spectrum Response to Uterine Contraction. Med. Bio Eng. Comput. 44, 188–201. doi:10.1007/s11517-006-0022-8 |
Signorini, M. G., Magenes, G., Cerutti, S., and Arduini, D. (2003). Linear and Nonlinear Parameters for the Analysis of Fetal Heart Rate Signal from Cardiotocographic Recordings. IEEE Trans. Biomed. Eng. 50, 365–374. doi:10.1109/tbme.2003.808824 |
Signorini, M. G., Pini, N., Malovini, A., Bellazzi, R., and Magenes, G. (2020). Integrating Machine Learning Techniques and Physiology Based Heart Rate Features for Antepartum Fetal Monitoring. Comput. Methods Programs Biomed. 185, 105015. doi:10.1016/j.cmpb.2019.105015 |
Silva, I., Behar, J., Sameni, R., Zhu, T., Oster, J., Clifford, G. D., et al. (2013). Noninvasive Fetal ECG: The PhysioNet/Computing in Cardiology Challenge 2013. Comput. Cardio. 40, 149–152.
Shakespeare, S. A., Crowe, J., Hayes-Gill, B. R., Bhogal, K., and James, D. K. (2001). The information content of Doppler ultrasound signals from the fetal heart. Med. Biol. Eng. Comput. 39, 619–626. doi:10.1007/BF02345432
Smyth, C. N. (1957). The Guard-Ring Tocodynamometer. Absolute Measurement of Intra-amniotic Pressure by a New Instrument. BJOG:An Int. J. O&G 64, 59–66. doi:10.1111/j.1471-0528.1957.tb02599.x
Valderrama, C. E., Stroux, L., Katebi, N., Paljug, E., Hall-Clifford, R., Rohloff, P., et al. (2019). An Open Source Autocorrelation-Based Method for Fetal Heart Rate Estimation from One-Dimensional Doppler Ultrasound. Physiol. Meas. 40, 025005. doi:10.1088/1361-6579/ab033d |
Keywords: fetal heart rate, cardiotocography, autocorrelation, Doppler ultrasound, classification, fetal heart rate variability
Citation: Vargas-Calixto J, Warrick P and Kearney R (2021) Estimation and Discriminability of Doppler Ultrasound Fetal Heart Rate Variability Measures. Front. Artif. Intell. 4:674238. doi: 10.3389/frai.2021.674238
Received: 01 March 2021; Accepted: 27 July 2021;
Published: 20 August 2021.
Edited by:
Martin Gerbert Frasch, University of Washington, United StatesReviewed by:
Faezeh Marzbanrad, Monash University, AustraliaCopyright © 2021 Vargas-Calixto, Warrick and Kearney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johann Vargas-Calixto, Y2FybG9zLnZhcmdhc2NhbGl4dG9AbWFpbC5tY2dpbGwuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.