
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging , 20 March 2025
Sec. Aging, Metabolism and Redox Biology
Volume 6 - 2025 | https://doi.org/10.3389/fragi.2025.1547883
This article is part of the Research Topic Insights in Aging, Metabolism and Redox Biology: 2024 View all 4 articles
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disease and cause of dementia. AD pathology primarily involves the formation of amyloid β (Aβ) plaques and neurofibrillary tangles containing hyperphosphorylated tau (p-tau). While Aβ targeted treatments have shown clinical promise, other aspects of AD pathology such as microgliosis, astrocytosis, synaptic loss, and hypometabolism may be viable targets for treatment. Among notable novel therapeutic approaches, the Ras homolog (Rho)-associated kinases (ROCKs) are being investigated as targets for AD treatment, based on the observations that ROCK1/2 levels are elevated in AD, and activation or inhibition of ROCKs changes dendritic/synaptic structures, protein aggregate accumulation, inflammation, and gliosis. This review will highlight key findings on the effects of ROCK inhibition in Aβ and ptau pathologies, as well as its effects on neuroinflammation, synaptic density, and potentially metabolism and bioenergetics.
Alzheimer’s disease (AD) is the leading cause of dementia, and in total, AD and other dementias affect 55 million people worldwide. AD diagnosis and staging rely on cognitive, functional, and behavioral tests, cerebrospinal fluid (CSF) and plasma analyses of amyloid β (Aβ) protein and hyperphosphorylated tau (ptau), as well as brain imaging analyses of amyloid and tau. Other biomarkers of AD pathogenesis include CSF or plasma Neurofilament light chain (NfL) and Glial Fibrillary Acidic Protein (GFAP) for the diagnosis of AD, MRI to assess brain atrophy, and positron emission tomography (PET) imaging of Fluorodeoxyglucose (FDG) uptake and synaptic vesicle protein 2A (SV2A) for assessing brain hypometabolism (Jack et al., 2024a; Hansson, 2024; Jack et al., 2024b) and loss of synaptic density (Wang et al., 2024a; Wang et al., 2024b; Mecca et al., 2022a; Mecca et al., 2022b; Mecca et al., 2020). It is well recognized that Aβ plaque accumulation often precedes cognitive symptoms by more than 10 years, with tau hyperphosphorylation, hypometabolism, and neuroinflammation also occurring throughout this clinically latent period. While coexisting pathologies such as alpha-synuclein or TDP43 accumulation may contribute to disease progression, neurofibrillary tangle formation and neurodegeneration generally occur closer to onset of cognitive symptoms. Nonetheless, a subpopulation of people preserve cognition in the presence of multiple pathologies (known as cognitive resilience), with unknown underlying mechanisms (Jack et al., 2024a).
Disease modifying therapies (DMT) for AD have been explored in the past several decades, mainly targeting Aβ, tau, neuroinflammation, enzymes that control neurotransmitter levels, and neurotransmitter receptors to alleviate clinical symptoms. Currently, only antibodies against Aβ have shown clinical promise in early-stage patients, however this treatment excludes those exhibiting amyloid-related imaging abnormality (ARIA) with micro hemorrhage (ARIA-H) and edema (ARIA-E), therefore limiting a broad application of this antibody-based Aβ therapy to patients with AD (Kato et al., 2016). Hence, ongoing studies and clinical trials are exploring tau, neuroinflammation, metabolism and bioenergetics as AD treatment targets.
Targeting Ras homolog (Rho)-associated kinase (ROCK) is among notable novel therapeutic approaches, based on the observation that ROCK activities are elevated in AD (Henderson et al., 2016; Weber and Herskowitz, 2021; Herskowitz et al., 2013), and ROCK activation leads to synaptic and metabolic dysfunction, inflammation, and gliosis (Weber and Herskowitz, 2021; Chong et al., 2017; Martin-Camara et al., 2021; Liu et al., 2007; Li et al., 2012; Jahani et al., 2018; Schinzari et al., 2012; Blazanin et al., 2024; Landry et al., 2020; Weber et al., 2021; Boros et al., 2019; Henderson et al., 2019; Swanger et al., 2015). Furthermore, preclinical studies with the ROCK inhibitor fasudil decreases Aβ and tau in 3xTg and Tau transgenic mice respectively, and lowers phosphorylated tau (ptau) and ameliorates cognitive deficits in APP/PS1 mice (Elliott et al., 2018; Guo et al., 2020a; Wei et al., 2021; Yan et al., 2021; Hamano et al., 2020; Tables 1, 2). Potential mechanisms driving these observations include regulation of AKT and autophagic pathways (Hamano et al., 2020; Preau et al., 2016; Liu H. et al., 2024; Yang et al., 2020; Gao et al., 2016; Gentry et al., 2016; Gurkar et al., 2013). This review will highlight the potential of ROCK inhibitors in the treatment of AD.
ROCKs are serine/threonine kinases and downstream effectors of the Rho-GTPase RhoA. RhoA binding to GTP is facilitated by guanine nucleotide exchange factors (GEFs), and RhoA modulates ROCK activity in response to various signals and stresses. Further regulation by GTPase-activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs) facilitates RhoA inactivation and inhibition (Julian and Olson, 2014; Aguilar et al., 2017). A recent structural study however, also presents the distinct possibility that ROCK2 activity can be constitutive, free of RhoA regulation (Truebestein et al., 2015). ROCK1 and 2, the two isoforms of ROCK, are on different chromosomes in humans. ROCK1 is expressed ubiquitously, and ROCK2 is highly expressed in specific tissues including the brain, heart and skeletal muscle. ROCK isoforms have distinct intracellular locations and effector molecules, variably regulating the cytoskeleton and dendritic morphology (Henderson et al., 2019; Newell-Litwa et al., 2015). ROCK1 and ROCK2 knockout mice exhibit strain-dependent developmental abnormalities and low survival rate, while those survived were largely normal. Mice which survive whole body and tissue specific knockout exhibit protective phenotypes in cardiac hypertrophy, diabetic kidney disease, vascular remodeling and atherosclerosis (Julian and Olson, 2014). C57BL/6J, ROCK1+/−, or ROCK2+/− mice treated by the ROCK inhibitor fasudil, and ROCK2 forebrain excitatory neuron conditional knockout mice under CaMKII-cre, exhibit anxiety-like behavior (Table 1; Weber et al., 2021; Thumkeo et al., 2003; Shimizu et al., 2005; Zhang et al., 2006; Greathouse et al., 2019). These studies suggest that ROCK1 and 2 play an essential role in development, yet demonstrate detrimental effects in certain diseases (Julian and Olson, 2014). Furthermore, ROCK inhibition can be potentially beneficial while long term use may result in unwanted side effects (Weber et al., 2021; Thumkeo et al., 2003; Shimizu et al., 2005; Zhang et al., 2006; Greathouse et al., 2019).
Extensive evidence points to the potential role of ROCK in AD. In postmortem AD brains, it has been demonstrated that ROCK1 and ROCK2 protein levels are elevated compared to controls (Henderson et al., 2016; Herskowitz et al., 2013). Primary neurons exposed to Aβ42 oligomers exhibited elevated pLIMK1, a downstream target of ROCK (Henderson et al., 2016). Furthermore, ROCK1 heterozygous mice exhibited decreased Aβ40 levels in the brain, and knockdown of ROCK1 in primary neurons decreased Aβ40 levels in media after exposure to Aβ42, highlighting a potential pathological mechanism by which ROCK1 exacerbates Aβ accumulation (Henderson et al., 2016). In SH-SY5Y cells however, ROCK1 knockdown increased and ROCK2 knockdown decreased secreted Aβ40, suggesting cell type and culture conditions may impact Aβ processing (Herskowitz et al., 2013). ROCK inhibitor SR3677 treatment in 5xFAD mice decreased Aβ40 and 42 and suppressed BACE1 activity (Herskowitz et al., 2013). Furthermore, ROCK inhibitor fasudil decreased ptau in AD neuro-spheroids (Giunti et al., 2023), and ROCK2 selective inhibitor H-1152 decreased ptau in primary neurons and neuroblastoma cell lines (Hamano et al., 2020). Furthermore, ROCK inhibitor Y-27632 increased dendritic protrusion and density in hippocampal neurons (Swanger et al., 2015; Table 1).
ROCK inhibition activates degradation pathways which remove protein aggregates, as evidenced in the following examples. ROCK inhibitor fasudil elevated AKT1 protein levels in AD neuro-spheroids (Giunti et al., 2023). ROCK2 inhibitor H-1152 elevated LC3II and lowered p62 that are consistent with elevation of autophagy, as well as elevated chymotrypsin-like 20S and trypsin-like 26S proteasomal activities (Hamano et al., 2020). Y27632 also decreased multiple protein aggreates in Neuro2a cells, and the beneficial effects are partially mediated by autophagy and attenuated by proteasome and autophagy inhibitors (Bauer et al., 2009). Both ROCK1 and ROCK2 knockdown exhibited similar outcomes as Y27632 treatment (Bauer et al., 2009). The observation that Y27632 further decreases protein aggregates when combined with ROCK1 and 2 double knockdown is intriguing, suggesting either Y27632 has additional targets than ROCK1/2, or the knockdowns are incomplete (Bauer et al., 2009).
ROCK inhibition mediates protein degradation through various pathways. It has been shown that ROCK and mTOR activities are highly correlated, and ROCK2 knockdown decreased mTOR, an autophagy regulator which downregulates autophagy upon phosphorylation (Gentry et al., 2016). Furthermore, ROCK1 and ROCK2 double knockout in cardiomyocytes promotes starvation-induced autophagy, with increased LC3II and decreased AKT, mTOR, and ULK signaling (Shi et al., 2019). Other autophagy mediators also respond to ROCK inhibition in a stress and cell type specific manner. In H92c cells exposed to high glucose, ROCK inhibitor fasudil increased Beclin1, LC3II/LC3I ratio, and Bcl-2 levels, decreased ROCK1/2 levels. In addition, high glucose-induced apoptosis in these cells is attenuated by fasudil, and fasudil’s anti-apoptotic effect is abolished by autophagy inhibitor 3-MA (Gao et al., 2016). In mice with AAV delivered A53T alpha-synuclein, fasudil treatment elevated LC3II/LC3I ratio, Beclin1 and p-Bcl2/Bcl2 in the brain (Yang et al., 2020).
Effects of ROCK1 or 2 disruption on autophagy are stress and cell type dependent. Disruption of ROCK1 in the heart is cardioprotective against doxorubicin toxicity, associated with decreased accumulation of p62 and LC3II, likely due to decreased autophagy initiation which is mediated by Beclin1 phosphorylation (Shi et al., 2018). In HeLa and 293T cells, ROCK1 interacts with Beclin1 upon starvation (Gurkar et al., 2013). In HeLa and EJ cells, ROCK1 mediates starvation-induced autophagy through phosphorylation of Beclin1, and this is attenuated by ROCK inhibitor Y-27632. Embryonic fibroblasts, EJ cells, and heart tissue from ROCK1 whole body knockout exhibited decreased autophagy following nutrient deprivation (Gurkar et al., 2013). ROCK2 knockout in cardiomyocytes led to compensatory ROCK1 overactivation, associated with inhibition of autophagy, and increased cardiac fibrosis (Shi et al., 2019). This latter work in cardiomyocyte specific ROCK knockout mice further highlights potential developmental and compensatory roles of ROCK, at least in certain tissues, when either ROCKs is disrupted.
Related to autophagy and with potentials to affect mitochondrial function, a recent compound screening study shows that ROCK inhibitors upregulate Parkin-mediated mitophagy (Moskal et al., 2020). The effects of ROCK inhibitors on autophagy and mitophagy flux have largely been studied in vitro. Further investigation is needed to understand whether and how autophagy and mitophagy are responsible at least in part for the effects of ROCK inhibition on Aβ, ptau, synaptic, and neuroinflammatory phenotypes in vivo.
Many in vivo studies have explored the effects of ROCK inhibition on neuroinflammation, cognition, and synaptic phenotypes. In rats, ROCK inhibitor fasudil decreased neuroinflammation induced by i.c.v. injected Aβ1–42, and improved memory, concurrent with attenuation of neuronal loss and NF-κB activation (Song et al., 2013). In 18 month-old rats, fasudil improved learning and memory in water radial-arm and water maze tests (Huentelman et al., 2009). In streptozotocin (STZ) injected rats, fasudil reversed STZ effects on learning and memory, synaptophysin levels, synaptic structural alterations, and levels of p-LIMK2 and p-cofilin (Hou et al., 2012). PI3K inhibitor wortmannin and NOS blocker L-NAME inhibited the effects of Fasudil in attenuating STZ induced changes in memory AChE, TNFα, NFƙB, and eNOS (Kumar and Bansal, 2018).
In 3xTg mice, fasudil treatment decreased Aβ (Elliott et al., 2018). In APP/PS1 mice fasudil decreased Aβ, ptau, and apoptosis; attenuated synaptophysin/Gap43 loss; and improved cognition (Guo et al., 2020a). Tau transgenic mice (rTg4510) treated with fasudil exhibit lower ptau (Hamano et al., 2020). Furthermore, fasudil in APP/PS1 mice increased dendrite arbors (Couch et al., 2010), and in 3xTg mice significantly changed transcriptomes in the rostral cortices with genes downregulated in AD, Parkinson’s disease and Huntington’s disease upregulated by fasudil, including ATP6V1E1 and ATP6V0B which are involved in lysosomal function (Killick et al., 2023). We have shown that fasudil significantly decreased ptau and GFAP in the hippocampus of PS19 mice (Collu et al., 2024; Ouyang et al., 2024). The effects of fasudil on neuroinflammation may be indirectly mediated by ptau or involve regulation of homeostasis of pro-inflammatory and anti-inflammatory astrocytes or microglia (Guo et al., 2020b; Zhang et al., 2013; Ding et al., 2009; Zhao et al., 2023; Chen et al., 2017).
Because of the cell and animal studies on ROCK inhibitors, several promising ROCK inhibiting compounds have been considered for human use and clinical trials (Table 2). Fasudil is the most frequently used ROCK inhibitor in cell and animal models, despite being a weak ROCK inhibitor (IC50 of ∼740 nM for ROCK1 and 2) (Ono-Saito et al., 1999). Fasudil is approved and used for the treatment of cerebral vasospasm in Japan and China (Hamano et al., 2020; Couch et al., 2010; Ono-Saito et al., 1999; Zhao et al., 2015; Guo et al., 2019), and is currently in clinical trials for tauopathies of Progressive Supranuclear Palsy-Richardson Syndrome and Corticobasal Syndrome in the US (NCT04734379). Fasudil is also under compassionate use for amyotrophic lateral sclerosis (ALS) in the US (NCT03792490) and Europe (2017-003676-31). Clinical trials for oral formulation range from 20 to 80 mg 3 times a day or i.v. 30 mg 3 times a day, with a half-life of 5.5 h (PubChem as of 12 December 2024).
Belumosudil (2-[3-[4-(1H-Indazol-5-ylamino)-2-quinazolinyl]phenoxy]-N-(1-methylethyl) acetamide, also known as KD025) is a ROCK2-specific inhibitor with an IC50 = 100 nM (IC50 = 3 µM for ROCK1, PubChem) (Lee et al., 2014; Zanin-Zhorov et al., 2014), and received FDA approval for chronic graft versus host disease (cGvHD) in 2021. ROCK2 knockdown or KD025 treatment decreases STAT3 phosphorylation, production of IL-21 and IL-17 in human CD4+ T cells, and shifts the balance between Th17 and regulatory T cells (Zanin-Zhorov et al., 2014), and is thus considered an immune modulator. Oral administration results in 64% bioavailability and Tmax at 1.26–2.53 h, with mean elimination half-life of 19 h (Belumosudil | C26H24N6O2 | CID 11950170 - PubChem, as of 12 December 2024).
Netarsudil ([4-[(2S)-3-amino-1-(isoquinolin-6-ylamino)-1-oxopropan-2-yl]phenyl] methyl2,4-dimethylbenzoate, also known as AR-13324) has IC50 at 32 nM for ROCK1 and 11 nM for ROCK2 (Dayal et al., 2019), while also inhibits norepinephrine transport. Netarsudil was proven effective in lowering intraocular pressure. The US FDA approved netarsudil for clinical use in 2017 as ophthalmic solution at 0.02% for open-angle glaucoma or ocular hypertension. In vitro incubation of human corneal tissue with netarsudil identified a half-life of 175 min (PubChem as of 12 December 2024), and 16–17 h from aqueous humor in rabbits (Netarsudil Mesylate Monograph for Professionals - Drugs.com as of 12 December 2024).
Ripasudil (4-Fluoro-5-[[(2S)-hexahydro-2-methyl-1H-1,4-diazepin-1-yl] sulfonyl] isoquinoline, also known as K-115)) is another pan-ROCK inhibitor (with the IC50 at 51 and 19 nM68, respectively for ROCK1 and 2) approved as one drop of 0.4% ophthalmic solution twice daily for the treatment of glaucoma and ocular hypertension in 2014 in Japan. It is cleared by the kidney at 7 L/h and biological half-life is 0.455 h (Ripasudil|C15H18FN3O2S|CID 9863672 - PubChem, as of 12 December 2024). Short-term (8 weeks) and long-term (24 months) studies have also observed anti-inflammatory effects in human patients (Wu et al., 2024).
SR3677 [IC50 of 3 nM for ROCK2 and 56 nM for ROCK1 (Feng et al., 2008)] also suppresses β-site APP cleaving enzyme 1 (BACE1) activity, and attenuates Aβ production in an AD mouse brain (Herskowitz et al., 2013). SR3677 EC50 is 57 nM in an assay for Parkin recruitment to damaged mitochondria (Moskal et al., 2020). Considering that ROCK1 and ROCK2 knockdown had opposite effects on endogenous human Aβ levels in SH-SY5Y cells, and that inhibition by SR3677 decreases both endogenous Aβ in SH-SY5Y cells, and Aβ in 5XFAD mouse brains, ROCK2 specific inhibitors such as SR3677 may be of importance in AD treatment. However, this drug has not been approved for human use, and is thus so far not a prime drug repurposing candidate.
NRL-1049 (synonyms: BA-1049) (1-(1-isoquinolin-5-ylsulfonylpiperidin-4-yl)ethanamine; dihydrochloride, IC50 = 0.59 µM and 26 µM for ROCK2 and ROCK1, MedChemExpress website and US20170313680A1, accessed 14 December 2024) exhibits benefits in Ccm1+/−Msh2−/− and Ccm3+/-Trp53−/− cavernous angioma (CA) mouse models which show vascular pathology with ROCK activation (McKerracher et al., 2020). The treatment was initiated from weaning with daily 100 mg/kg/d (Ccm1+/-Msh2−/−) or 10 mg/kg/d (Ccm3+/−Trp53−/−) until 105 and 77 days of age. The underlying mechanisms are not fully understood, and this compound is currently being evaluated in a Phase 1 investigational clinical trial for treating CCM in patients (Morrison et al., 2024).
Additional ROCK inhibitors exist (Ye et al., 2024; Koch et al., 2018; Mani et al., 2022; Feng et al., 2016), and a subset can be found in MedChemExpress (which listed 112 inhibitors and two activators, and comparisons of specificity to ROCK1 or 2 including IC50 when available, as of 15 December 2024) or PubChem.
As discussed above, potential mechanisms of ROCK inhibitors include the potential to upregulate Parkin-mediated mitophagy, which can in turn impact mitochondrial function (Moskal et al., 2020). In a transcription drug effect network analysis, the effects of fasudil on gene expression and levels of LC3II are similar to 2-deoxy-glucose in their regulation of autophagy genes (Iorio et al., 2010). 2-Deoxy-glucose has been shown to target hexokinase, the first enzyme in glycolysis, thus impacting glucose metabolism and the pentose phosphate pathway (Dodson et al., 2013a; Dodson et al., 2022; Benavides et al., 2013; Dodson et al., 2013b). We have shown that in vivo delivery of high fasudil doses in PS19 mice can decrease glycolytic enzyme Pkm1 in broad regions of the brain, and decrease mitochondrial complex IV subunit I in striatum and thalamic regions (Ouyang et al., 2024).
Effects of ROCK inhibition on oxidative stress and metabolism have been shown in multiple tissues. Fasudil administration alongside insulin treatment improved blood flow in patients with obesity-related metabolic syndrome with insulin resistance. In the presence of vitamin C, fasudil no longer exerted additional benefit in blood flow, consistent with a role of oxidative stress in the vascular dysfunction in these patients (Schinzari et al., 2012). ROCK1 is increased in human fatty liver diseases (Schinzari et al., 2012), and overexpression of ROCK1 in the liver promotes adiposity, insulin resistance and lipid accumulation in mice with a high-fat diet (Schinzari et al., 2012). Knockout ROCK1 in the liver attenuated steatosis and hyperglycemia in obese diabetic (ob/ob) mice (Schinzari et al., 2012). Furthermore, the diabetic drug metformin inhibits ROCK1 activity (Schinzari et al., 2012). ROCK inhibition by H1152 or ROCK2 knockdown promotes the generation of insulin-expressing pancreatic beta-like cells from hPSCs (Ghazizadeh et al., 2017). Effects of ROCK inhibition on mitochondria have been shown in cardiomyocytes, where activation of RhoA increases phosphorylation of Drp1 and formation of smaller mitochondria, and ROCK inhibition by Y27632 attenuates RhoA effects on Drp1 phosphorylation and mitochondrial fission (Brand et al., 2018).
Effects of ROCK inhibition on mitochondrial bioenergetics have also been directly measured in corneal endothelial cells (Ho et al., 2022). In these cells, ROCK inhibitor ripasudil upregulated genes for oxidative phosphorylation; concurrent with an increase in basal, maximal, proton leak, ATP production linked, and reserve capacity oxygen consumption rate; while glycolytic rate remained unchanged (Ho et al., 2022). The changes in oxygen consumption rate are associated with increased phosphorylation of AMPK, and are attenuated by AMPK inhibitor dorsomorphin (Ho et al., 2022). Inhibition of mitochondrial function by oligomycin attenuated effects of ripasudil on cell migration (Ho et al., 2022). ROCK inhibitor Y27632 treatment has been shown to enhance hexokinase HK2 localization to the mitochondria in the presence of monensin which facilitates sodium transport across the cell membrane and increases cellular energy demand. This is associated with increased contribution of glycolysis to ATP production (Ho et al., 2025). In senescent fibroblasts, fasudil or Y27632 elevated coupling efficiency based on ATP linked oxygen consumption rate normalized to basal oxygen consumption rate (Park et al., 2018).
Effects of ROCK inhibition on mitochondria dynamics can differ by cell type. In 2D cultured 3T3-L1 cells, ripasudil and Y27632 both attenuated mitochondrial respiration and glycolytic function following suppression by BIM-A87. In SMARCA4-mutant lung cancer cells, ROCK inhibition by KD025 (Belumosudil) suppresses both mitochondrial respiration and adaptive increase in glycolysis induced by inhibition of oxidative phosphorylation (Blazanin et al., 2024). In melanoma cells that had elevated glycolysis, ROCK inhibition by GSK269962A suppressed glycolytic enhancement, oxygen consumption rate, as well as cell proliferation (Murali et al., 2024). ROCK inhibitor Y27632 has also been shown to promote mitochondrial transfer via tunneling nanotubes in retinal pigment epithelium. However, mitochondrial respiration was not enhanced by Y27632 indicating that beneficial effects from ROCK inhibition were mediated by promotion of mitochondrial transfer, not regulation of mitochondrial respiration (Yuan et al., 2024).
In the brain, studies of the effects of ROCK on metabolism have focused on the hypothalamus, where ROCK1 levels are upregulated by leptin, and decreased by a high-fat diet. Overexpression of ROCK1 in the hypothalamus decreases food intake and body weight, and fasudil treatment in prenatal and postnatal rats increases body weight (Huang et al., 2013). In primary neurons, fasudil treatment alleviated the decrease in mitochondrial membrane potential induced by Aβ (Gao et al., 2019). Fasudil and Y27632 have also been shown to attenuate NMDA-induced mitochondrial fragmentation (Martorell-Riera et al., 2015). Knockdown of ROCK1 or Y27632 treatment in PC12 cells attenuated MPP+ induced mitochondrial fission and apoptosis, and Y27632 treatment in vivo attenuated MPTP-induced motor symptoms (Zhang et al., 2019). These studies with MPP+ and MPTP highlight the potential of ROCK inhibition in treating Parkinson’s disease, as MPTP has been shown to induce parkinsonism in humans and mitochondrial dysfunction is suggested to contribute to Parkinson’s disease (Cannon and Greenamyre, 2010; Crabtree and Zhang, 2012).
We have recently reported that high doses of fasudil in PS19 mice resulted in significantly decreased ptau, GFAP, mitochondrial complex I and II protein subunits, and complex I–IV substrate linked oxygen consumption rate in the cortex (Ouyang et al., 2024). Beyond our own study, the effects of ROCK inhibition on mitochondrial electron transport chain function and glycolysis have not been critically investigated in cortical and hippocampal neurons or in the context of AD in vivo. Considering the accumulating evidence demonstrating insufficient mitophagy and mitochondrial bioenergetics in AD, and other neurodegenerative disease, and that ROCK inhibition can affect mitophagy and mitochondrial function in multiple cells, tissues and stress conditions (Moskal et al., 2020; Mani et al., 2022; Chen et al., 2020; Wang et al., 2020; Cai and Jeong, 2020; Redmann et al., 2016; Jeong et al., 2022; John and Reddy, 2021; Weidling and Swerdlow, 2020; Fang et al., 2019; Jia et al., 2024; Carling et al., 2024; Udeochu et al., 2023; Swerdlow, 2023; Liu Y. et al., 2024; Tai et al., 2023; Trushina et al., 2022; Apolloni et al., 2022; Luo et al., 2021; Cunnane et al., 2020), ROCK inhibition may also impact pathogenesis-associated bioenergetic dysfunction in AD and other neurodegenerative diseases.
Metabolic dysregulation is a significant feature of AD (Author Anonymous, 2020; Austad et al., 2021), and decreased glucose metabolism occurs in AD brains (Holscher, 2011; Szablewski, 2017; Goedert and Spillantini, 2006). Among well-established brain imaging procedures for diagnosis of AD and other dementias in standard clinical work-up (Chételat et al., 2020), [18F]FDG (Fluorodeoxyglucose) PET readily demonstrates local cerebral metabolic rate of glucose consumption (Kato et al., 2016). Glycolysis and mitochondrial energy production are intimately linked. Mitochondrial DNA mutations, deficiencies in electron transport chain activities, and deficits in glucose and lipid metabolism in AD support the link between mitochondrial and metabolic dysfunction in AD (Fang et al., 2019; Swerdlow et al., 2017; Swerdlow et al., 2014; Butterfield and Halliwell, 2019; Kunkle et al., 2019; Bloom and Norambuena, 2018; Holper et al., 2019; Swerdlow et al., 2010). The “mitochondrial cascade hypothesis” is based on evidence that mitochondrial dysfunction can exacerbate AD related pathologies (Weidling and Swerdlow, 2020; Holper et al., 2019; Swerdlow and Khan, 2004; Swerdlow, 2020). Despite many studies in vitro and in other disease models, the mechanisms linking ROCK inhibition and metabolic regulation in AD, and the pathways connecting glycolysis, mitochondrial dysfunction, and AD pathology are still largely unclear, primarily due to a lack of tools for detection of these metabolic activities in vivo in distinct cell types and brain regions. The development and application of multiple in vivo imaging tools are essential to further understand and dissect this mechanistic link.
There were demonstrated relationships between FDG PET, brain volumes based on volumetric magnetic resonance imaging (MRI), and tau PET in disease cases and brain regions (Matthews et al., 2024). Hypometabolism has been shown to have high predictive value for cognitive impairment, and/or decline rate (Matthews et al., 2024; Drzezga et al., 2005; Iaccarino et al., 2019; Ou et al., 2019; Koops et al., 2024). Furthermore, distinct patterns of FDG PET have been found for different mixed pathologies (Minoshima et al., 2022). Additionally, a recent study demonstrated more extensive loss of proteins involved in mitochondrial dynamics and bioenergetics in rapidly progressive AD compared to typical AD, while ptau accumulation more closely resembles non-AD control than typical AD, consistent with how ptau accumulation is slower, or that rapid progressive AD and typical AD have different etiologies (Xiyang et al., 2024). Hypometabolic mismatch has also been observed in mixed AD with Lewy body disease (LBD), in that patients with amyloid and alpha-synuclein pathologies exhibit worse hypometabolism relative to tau pathology and brain atrophy (Duong et al., 2024). These new observations further support the importance of developing therapeutics to protect against metabolic and bioenergetic deficits in the brain.
A number of studies have used the concept of targeting bioenergetic and mitochondrial mechanisms as a therapeutic strategy for age-related neurodegenerative diseases (Weidling and Swerdlow, 2020; Fang et al., 2019; Cunnane et al., 2020; Swerdlow et al., 2010; Hill et al., 2019; Rigotto and Basso, 2019). Thus far, a small percentage of AD drug development pipelines target metabolism and bioenergetics (e.g., Two compounds: metformin and tricaprilin, a ketone body precursor compound, in phase III) (Cummings et al., 2024; Cummings et al., 2023). Drugs that target metabolism must be critically evaluated for their potential use in treating AD, either alone or in combination with other therapeutic strategies. Recent clinical trials, evoke and evoke+, are randomized, double-blind, placebo-controlled phase 3 trials investigating the efficacy, safety, and tolerability of once-daily oral semaglutide in early-stage symptomatic AD (Cummings et al., 2025), and this glucagon-like peptide-1 receptor agonist is already approved for the treatment of type 2 diabetes and obesity (Fessel, 2024). While the outcomes of the trial will be available in the near future, it is anticipated to achieve 30% improvement of AD, as benefit to cognitive function in neurodegenerative disorders is expected following treatment (Fessel, 2024; Fan et al., 2020). Interestingly, there have been studies linking GLP-1 and the ROCK signaling pathway in the peripheral tissues (Fan et al., 2020; Kong et al., 2014). Both GLP-1 and Y27632 decrease glucotoxicity-induced increase of stress fibers, and GLP-1 inhibited glucotoxicity-induced activation of RhoA/ROCK (Kong et al., 2014). Furthermore, GLP-1 agonist liraglutide has been shown to ameliorate myocardial hypertrophy in hypertensive rats, potentially through ROCK2 decrease (Fan et al., 2020), as in H9C2 cells, GLP-1 decreased ROCK2, and Y27632 further decreased AngII effects on hypertrophic gene ANP expression when combined with GLP-1 (Fan et al., 2020).
Current FDA approved AD treatments are antibodies which decrease cerebral Aβ, including aducanumab, lecanemab and donanemab, and both lecanemab and donanemab have shown clinical benefits in clinical trials (Gandy, 2023). These advances provide hope for effective AD treatment, while also further revealing challenges and opportunities. Antibody treatment has serious side effects including the occurrence of amyloid related imaging abnormality (ARIA) with micro hemorrhage and edema (brain swelling or bleeding), and might not be as effective for patients with greater tau pathology (Gueorguieva et al., 2023a; Sims et al., 2023; Reardon, 2023; Manly and Deters, 2023; Gueorguieva et al., 2023b).
Most clinical trials have not used the FDA-approved FDG PET as an endpoint measurement. [18F]FDG is a radioactive glucose analog widely used in clinical and research settings worldwide for PET imaging to assess metabolic activity and detect abnormalities in tissues, particularly for cancer, cardiac, and neurological conditions. Mechanistically, fluorodeoxyglucose crosses the blood-brain barrier and is phosphorylated by hexokinase to fluorodeoxy-6-phosphate, acting as a competitive substrate with glucose at the first step of glycolysis. Because of the deoxy substitution, it cannot be further metabolized through the glycolysis pathway, and thus remains in the tissue to be detected through imaging.
PET imaging with suitable radioligands can quantify the distribution of proteins of interest through the measurement of radioactivity distribution in living subjects. Such radioligands may reveal, non-invasively, differences in quantity and distribution between healthy and diseased states. To determine in vivo effects of ROCK inhibition, we have developed the first-in-class ROCK ligand [11C]ROCK201 for imaging studies to determine the target occupancy (Figure 1; Zheng et al., 2022). We have evaluated the imaging characteristics of this brain permeable ROCK2 radiotracer in rodent and nonhuman primate brains, and demonstrated fast brain kinetics and specific binding signals. This development will facilitate the study of ROCK, the effects of ROCK inhibition on Aβ, ptau, and metabolism, how these pathologies act together in AD progression, and whether ROCK inhibition attenuates AD.
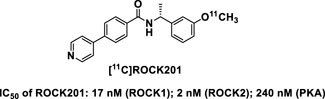
Figure 1. Structure of the first-in-class ROCK ligand [11C]ROCK201 for imaging studies. IC50 for ROCK1, ROCK2 and PKA are indicated, demonstrating higher affinity to ROCKs.
Metabolic and bioenergetic deficits are significant features of AD, thus intervention strategies for AD ideally not only address Aβ and ptau pathology but also protect against synaptic loss, neuroinflammation, metabolic and bioenergetic deficiencies. Studies addressing this objective are still in urgent need. ROCK inhibitors have shown effects on autophagy, mitophagy, synapse and neuroinflammation, in addition to well-established results concerning pathogenic Aβ and ptau. Furthermore, some ROCK inhibitors have been approved for human use in treating other diseases, and thus have the potential to serve as effective drugs for AD treatment. Overall, ROCK inhibitors have a broad range of effects, including as immune modulators (graft vs. host disease), in attenuating Aβ production, in recruiting Parkin to damaged mitochondria, and in the treatment of intra-ocular pressure. ROCK inhibitors target multiple downstream signaling pathways, including but not limited to: cytoskeleton regulation (e.g., myosin light chain phosphatase and cofilin); NFkB and STAT3 pathways; the expression of angiotensin-converting enzyme, eNOS, IL21 and 17; autophagy pathway proteins (mTOR, Beclin1, Bcl2, LC3II, p62, ATP6V1E1 and ATP6V0B); HK2 and Parkin targeting to the mitochondria; Drp1 phosphorylation, and levels of the glycolytic enzyme PKM1 (Figure 2).
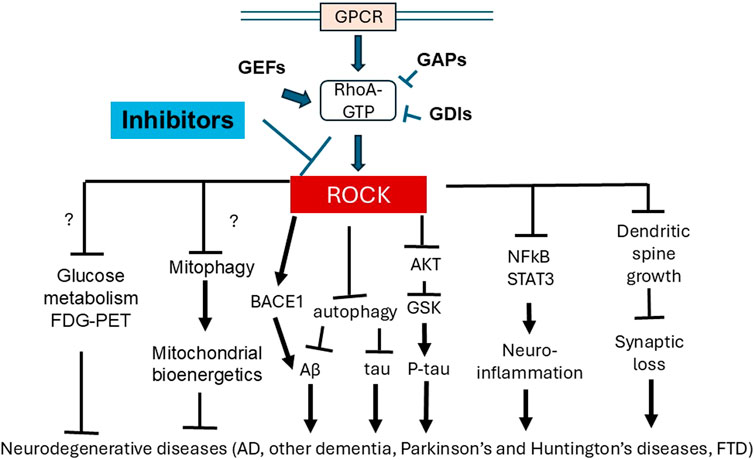
Figure 2. Inhibition of ROCK have multiple downstream functions which can provide benefit for neurodegenerative diseases. ROCK are downstream effectors of the Rho-GTPase RhoA, which binds to GTP, and regulated by guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs). Because of a converging role of ROCK in regulating multiple downstream functions, ROCK inhibitors have been developed, which have demonstrated extensive pre-clinical ability to ameliorate AD pathology, including Aβ and ptau (potentially through BACE, autophagy and AKT regulation), neuroinflammation (potentially through NFkB and STAT3 regulation) and synaptic loss (potentially through cytoskeleton regulation and dendritic spine growth). ROCK inhibition in non-neuronal tissue and disease models has also shown effects on metabolism including glycolysis and mitochondrial bioenergetics, but effects of ROCK inhibition on neuronal metabolism remains to be explored. FDG-PET can aid future studies to elucidate these potential effects of ROCK inhibition on metabolism.
Major barriers in developing ROCK inhibitor-based AD therapies are as follows:
1. Research on AD therapies remain predominately focused on Aβ and ptau, which are AD specific biomarkers, while neuroinflammation, synaptic loss, metabolism and mitochondrial function are implicated in many diseases and conditions beyond AD. These pathologies not particularly specific to AD prevent commitment of effort and resources to study their contribution to disease pathogenesis and their potential as drug targets. AD has broad pathological phenotypes and it remains a possibility that cognitive resilience is dependent on phenotypes and regulation beyond Aβ and ptau. Thus, investigating AD pathogenesis processes and regulation needs to be broader than simply on Aβ and ptau.
2. Determining metabolic and mitochondrial bioenergetic changes in a cell type specific manner, in vivo, with single cell resolution is still technically challenging. Super resolution non-invasive imaging of mitochondrial membrane potential, ATP/ADP ratio, or complex activities is still lacking. Detecting changes in these parameters in vivo may enhance our conceptual understanding of cognitive neuroscience and neurodegenerative disease by facilitating the measurement of real-time activities while cognitive tests are being performed, and longitudinally during disease progression.
3. ROCK1 and 2 knockout mice exhibit developmental abnormalities and excitatory neuron specific ROCK2 knockout mice exhibit anxiety behavior. ROCK2 knockout has been shown to lead to overactivation of ROCK1 and inhibition of autophagy. Some evidence also suggests that current ROCK inhibitors may have off-target effect as represented by the observation that Y-27632 further decreases protein aggregates when ROCK1 and 2 are both knocked down. Similarities and differences among ROCK inhibitors concerning brain penetration, off-target effects, on-target side effects, systemic effects to peripheral tissues, dose, frequency, and duration of treatment, and effectiveness in subtypes and substages of AD, need to be further evaluated in both AD animal models and human AD iPSC/neuro-spheroids to define the beneficial window of treatment. As has been said, “the dose makes the poison.”
4. ROCK1/2 target multiple substrates and enzymes, and inhibiting ROCK may have broad side effects. ROCK is involved in various signaling pathways that control cell migration, proliferation, and apoptosis. Systemic inhibition of ROCK may have unexpected consequences that impair bodily functions, such as vascular regulation and immune responses. The multi-target properties of ROCK inhibition make it challenging to control the dose and duration of treatment to modulate specific pathways. Yet, multi-target drugs may be what we need to treat a multi-faceted disease such as AD. Indeed, there are ample examples of multi-target drugs including but not limited to GLP-1 agonists for treating diabetes.
With the advance of artificial intelligence and the ability to process big data, we expect to see great strides in this field. As some of the ROCK inhibitors are in human clinical trials or approved to be used for illnesses other than AD, analyses of data available in human populations will also provide crucial information for accelerating the refinement of therapies for AD treatment. We hope that some of the studies will soon benefit AD patients.
CZ: Writing–original draft, Writing–review and editing. WX: Writing–original draft, Writing–review and editing. JZ: Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by UAB Nathan Shock Center P30 AG050886 (JZ), I01 BX004730 and I01 BX003527 Merit Awards from the Biomedical Laboratory Research and Development of the Veterans Affairs Office of Research and Development, Cure Alzheimer Fund, and RF1AG063913 from the NIH (WX), R01AG072895 (JZ), R01ES034846 (JZ), R21AG081687 (JZ), as well as funding from the Canadian Institutes of Health Research (CIHR507113) and the Alzheimer’s Association (CZ).
Authors thank Jane Paterson for editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aguilar, B. J., Zhu, Y., and Lu, Q. (2017). Rho GTPases as therapeutic targets in Alzheimer’s disease. Alzheimers Res. Ther. 9 (1), 97. doi:10.1186/s13195-017-0320-4
Apolloni, S., Milani, M., and D’Ambrosi, N. (2022). Neuroinflammation in friedreich’s ataxia. Int. J. Mol. Sci. 23 (11), 6297. doi:10.3390/ijms23116297
Austad, S. N. B. S., Buford, T. W., Carter, C. S., Smith, Jr D. L., Darley-Usmar, V., and Zhang, J. (2021). Targeting whole body metabolism and mitochondrial bioenergetics in the drug development for Alzheimer’s disease. Acta Pharm. Sin. B 12 (19), 511–531. doi:10.1016/j.apsb.2021.06.014
Author Anonymous, (2020). 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. doi:10.1002/alz.12068
Bauer, P. O., Wong, H. K., Oyama, F., Goswami, A., Okuno, M., Kino, Y., et al. (2009). Inhibition of Rho kinases enhances the degradation of mutant huntingtin. J. Biol. Chem. 284 (19), 13153–13164. doi:10.1074/jbc.M809229200
Benavides, G. A., Liang, Q., Dodson, M., Darley-Usmar, V., and Zhang, J. (2013). Inhibition of autophagy and glycolysis by nitric oxide during hypoxia-reoxygenation impairs cellular bioenergetics and promotes cell death in primary neurons. Free Radic. Biol. Med. 65, 1215–1228. doi:10.1016/j.freeradbiomed.2013.09.006
Blazanin, N., Liang, X., Mahmud, I., Kim, E., Martinez, S., Tan, L., et al. (2024). Therapeutic modulation of ROCK overcomes metabolic adaptation of cancer cells to OXPHOS inhibition and drives synergistic anti-tumor activity. bioRxiv, doi:10.1101/2024.09.16.613317
Bloom, G. S., and Norambuena, A. (2018). Alzheimer’s disease as a metabolic disorder. OCL 25, D403. doi:10.1051/ocl/2018044
Boros, B. D., Greathouse, K. M., Gearing, M., and Herskowitz, J. H. (2019). Dendritic spine remodeling accompanies Alzheimer’s disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol. Aging 73, 92–103. doi:10.1016/j.neurobiolaging.2018.09.003
Brand, C. S., Tan, V. P., Brown, J. H., and Miyamoto, S. (2018). RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes. Cell Signal 50, 48–57. doi:10.1016/j.cellsig.2018.06.012
Butterfield, D. A., and Halliwell, B. (2019). Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 20 (3), 148–160. doi:10.1038/s41583-019-0132-6
Cai, Q., and Jeong, Y. Y. (2020). Mitophagy in Alzheimer’s disease and other age-related neurodegenerative diseases. Cells 9 (1), 150. doi:10.3390/cells9010150
Cannon, J. R., and Greenamyre, J. T. (2010). Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog. Brain Res. 184, 17–33. doi:10.1016/S0079-6123(10)84002-6
Carling, G. K., Fan, L., Foxe, N. R., Norman, K., Wong, M. Y., Zhu, D., et al. (2024). Alzheimer’s disease-linked risk alleles elevate microglial cGAS-associated senescence and neurodegeneration in a tauopathy model. Neuron 112 (23), 3877–3896.e8. doi:10.1016/j.neuron.2024.09.006
Chen, G., Kroemer, G., and Kepp, O. (2020). Mitophagy: an emerging role in aging and age-associated diseases. Front. Cell Dev. Biol. 8, 200. doi:10.3389/fcell.2020.00200
Chen, J., Sun, Z., Jin, M., Tu, Y., Wang, S., Yang, X., et al. (2017). Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non-specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF-κB pathway. J. Neuroimmunol. 305, 108–114. doi:10.1016/j.jneuroim.2017.02.010
Chételat, G., Arbizu, J., Barthel, H., Garibotto, V., Law, I., Morbelli, S., et al. (2020). Amyloid-PET and (18)F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 19 (11), 951–962. doi:10.1016/s1474-4422(20)30314-8
Chong, C. M., Ai, N., and Lee, S. M. (2017). ROCK in CNS: different roles of isoforms and therapeutic target for neurodegenerative disorders. Curr. Drug Targets 18 (4), 455–462. doi:10.2174/1389450117666160401123825
Collu, R., Yin, Z., Giunti, E., Daley, S., Chen, M., Morin, P., et al. (2024). Effect of the ROCK inhibitor fasudil on the brain proteomic profile in the tau transgenic mouse model of Alzheimer’s disease. Front. Aging Neurosci. 16, 1323563. doi:10.3389/fnagi.2024.1323563
Couch, B. A., DeMarco, G. J., Gourley, S. L., and Koleske, A. J. (2010). Increased dendrite branching in AbetaPP/PS1 mice and elongation of dendrite arbors by fasudil administration. J. Alzheimers Dis. 20 (4), 1003–1008. doi:10.3233/jad-2010-091114
Crabtree, D. M., and Zhang, J. (2012). Genetically engineered mouse models of Parkinson’s disease. Brain Res. Bull. 88 (1), 13–32. doi:10.1016/j.brainresbull.2011.07.019
Cummings, J., Zhou, Y., Lee, G., Zhong, K., Fonseca, J., and Cheng, F. (2024). Alzheimer’s disease drug development pipeline: 2024. Alzheimers Dement. 10, e12465. doi:10.1002/trc2.12465
Cummings, J., Zhou, Y., Lee, G., Zhong, K., Fonseca, J., and Cheng, F. (2023). Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement. (N Y) 9 (2), e12385. doi:10.1002/trc2.12385
Cummings, J. L., Atri, A., Feldman, H. H., Hansson, O., Sano, M., Knop, F. K., et al. (2025). Evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimers Res. Ther. 17 (1), 14. doi:10.1186/s13195-024-01666-7
Cunnane, S. C., Trushina, E., Morland, C., Prigione, A., Casadesus, G., Andrews, Z. B., et al. (2020). Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 19 (9), 609–633. doi:10.1038/s41573-020-0072-x
Dayal, N., Mikek, C. G., Hernandez, D., Naclerio, G. A., Yin Chu, E. F., Carter-Cooper, B. A., et al. (2019). Potently inhibiting cancer cell migration with novel 3H-pyrazolo[4,3-f]quinoline boronic acid ROCK inhibitors. Eur. J. Med. Chem. 180, 449–456. doi:10.1016/j.ejmech.2019.06.089
Ding, J., Yu, J. Z., Li, Q. Y., Wang, X., Lu, C. Z., and Xiao, B. G. (2009). Rho kinase inhibitor fasudil induces neuroprotection and neurogenesis partially through astrocyte-derived G-CSF. Brain Behav. Immun. 23 (8), 1083–1088. doi:10.1016/j.bbi.2009.05.002
Dodson, M., Benavides, G., Darley-Usmar, V., and Zhang, J. (2022). Differential effects of 2-deoxyglucose and glucose deprivation on 4-hydroxynonenal dependent mitochondrial dysfunction in primary neurons. Front. Aging 3, 812810. doi:10.3389/fragi.2022.812810
Dodson, M., Darley-Usmar, V., and Zhang, J. (2013a). Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med. 63, 207–221. doi:10.1016/j.freeradbiomed.2013.05.014
Dodson, M., Liang, Q., Johnson, M. S., Redmann, M., Fineberg, N., Darley-Usmar, V. M., et al. (2013b). Inhibition of glycolysis attenuates 4-hydroxynonenal-dependent autophagy and exacerbates apoptosis in differentiated SH-SY5Y neuroblastoma cells. Autophagy 9 (12), 1996–2008. doi:10.4161/auto.26094
Drzezga, A., Grimmer, T., Riemenschneider, M., Lautenschlager, N., Siebner, H., Alexopoulus, P., et al. (2005). Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J. Nucl. Med. 46 (10), 1625–1632.
Duong, M. T., Das, S. R., Khandelwal, P., Lyu, X., Xie, L., McGrew, E., et al. (2024). Hypometabolic mismatch with atrophy and tau pathology in mixed Alzheimer and Lewy body disease. Brain, awae352. doi:10.1093/brain/awae352
Elliott, C., Rojo, A. I., Ribe, E., Broadstock, M., Xia, W., Morin, P., et al. (2018). A role for APP in Wnt signalling links synapse loss with β-amyloid production. Transl. Psychiatry 8 (1), 179. doi:10.1038/s41398-018-0231-6
Fan, S., Xiong, Q., Zhang, X., Zhang, L., and Shi, Y. (2020). Glucagon-like peptide 1 reverses myocardial hypertrophy through cAMP/PKA/RhoA/ROCK2 signaling. Acta Biochim. Biophys. Sin. 52 (6), 612–619. doi:10.1093/abbs/gmaa038
Fang, E. F., Hou, Y., Palikaras, K., Adriaanse, B. A., Kerr, J. S., Yang, B., et al. (2019). Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 22 (3), 401–412. doi:10.1038/s41593-018-0332-9
Feng, Y., LoGrasso, P. V., Defert, O., and Li, R. (2016). Rho kinase (ROCK) inhibitors and their therapeutic potential. J. Med. Chem. 59 (6), 2269–2300. doi:10.1021/acs.jmedchem.5b00683
Feng, Y., Yin, Y., Weiser, A., Griffin, E., Cameron, M. D., Lin, L., et al. (2008). Discovery of substituted 4-(pyrazol-4-yl)-phenylbenzodioxane-2-carboxamides as potent and highly selective Rho kinase (ROCK-II) inhibitors. J. Med. Chem. 51 (21), 6642–6645. doi:10.1021/jm800986w
Fessel, J. (2024). All GLP-1 agonists should, theoretically, cure Alzheimer’s dementia but dulaglutide might Be more effective than the others. J. Clin. Med. 13 (13), 3729. doi:10.3390/jcm13133729
Gandy, S. (2023). News and views: anti-amyloid antibodies and novel emerging approaches to Alzheimer’s disease in 2023. Mol. Neurodegener. 18 (1), 66. doi:10.1186/s13024-023-00656-x
Gao, H., Hou, F., Dong, R., Wang, Z., Zhao, C., Tang, W., et al. (2016). Rho-Kinase inhibitor fasudil suppresses high glucose-induced H9c2 cell apoptosis through activation of autophagy. Cardiovasc Ther. 34 (5), 352–359. doi:10.1111/1755-5922.12206
Gao, Y., Yan, Y., Fang, Q., Zhang, N., Kumar, G., Zhang, J., et al. (2019). The Rho kinase inhibitor fasudil attenuates Aβ1-42-induced apoptosis via the ASK1/JNK signal pathway in primary cultures of hippocampal neurons. Metab. Brain Dis. 34 (6), 1787–1801. doi:10.1007/s11011-019-00487-0
Gentry, E. G., Henderson, B. W., Arrant, A. E., Gearing, M., Feng, Y., Riddle, N. C., et al. (2016). Rho kinase inhibition as a therapeutic for progressive supranuclear palsy and corticobasal degeneration. J. Neurosci. 36 (4), 1316–1323. doi:10.1523/JNEUROSCI.2336-15.2016
Ghazizadeh, Z., Kao, D. I., Amin, S., Cook, B., Rao, S., Zhou, T., et al. (2017). ROCKII inhibition promotes the maturation of human pancreatic beta-like cells. Nat. Commun. 8 (1), 298. doi:10.1038/s41467-017-00129-y
Giunti, E., Collu, R., Daley, S., Querfurth, H., Morin, P., Killick, R., et al. (2023). Reduction of phosphorylated tau in Alzheimer’s disease induced pluripotent stem cell-derived neuro-spheroids by rho-associated coiled-coil kinase inhibitor fasudil. J. Alzheimers Dis. 96 (4), 1695–1709. doi:10.3233/jad-230551
Goedert, M., and Spillantini, M. G. (2006). A century of Alzheimer’s disease. Science 314 (5800), 777–781. doi:10.1126/science.1132814
Greathouse, K. M., Henderson, B. W., Gentry, E. G., and Herskowitz, J. H. (2019). Fasudil or genetic depletion of ROCK1 or ROCK2 induces anxiety-like behaviors. Behav. Brain Res. 373, 112083. doi:10.1016/j.bbr.2019.112083
Gueorguieva, I., Willis, B. A., Chua, L., Chow, K., Ernest, C. S., Wang, J., et al. (2023b). Donanemab exposure and efficacy relationship using modeling in Alzheimer’s disease. Alzheimers Dement. (N Y) 9 (2), e12404. doi:10.1002/trc2.12404
Gueorguieva, I., Willis, B. A., Chua, L., Chow, K., Ernest, C. S., Shcherbinin, S., et al. (2023a). Donanemab population pharmacokinetics, amyloid plaque reduction, and safety in participants with Alzheimer’s disease. Clin. Pharmacol. Ther. 113 (6), 1258–1267. doi:10.1002/cpt.2875
Guo, H., Zhao, Z., Zhang, R., Chen, P., Zhang, X., Cheng, F., et al. (2019). Monocytes in the peripheral clearance of amyloid-β and Alzheimer’s disease. J. Alzheimers Dis. 68 (4), 1391–1400. doi:10.3233/jad-181177
Guo, M. F., Zhang, H. Y., Li, Y. H., Gu, Q. F., Wei, W. Y., Wang, Y. Y., et al. (2020b). Fasudil inhibits the activation of microglia and astrocytes of transgenic Alzheimer’s disease mice via the downregulation of TLR4/Myd88/NF-κB pathway. J. Neuroimmunol. 346, 577284. doi:10.1016/j.jneuroim.2020.577284
Guo, M. F., Zhang, H. Y., Zhang, P. J., Liu, X. Q., Song, L. J., Wei, W. Y., et al. (2020a). Fasudil reduces β-amyloid levels and neuronal apoptosis in APP/PS1 transgenic mice via inhibition of the Nogo-A/NgR/RhoA signaling axis. J. Integr. Neurosci. 19 (4), 651–662. doi:10.31083/j.jin.2020.04.243
Gurkar, A. U., Chu, K., Raj, L., Bouley, R., Lee, S. H., Kim, Y. B., et al. (2013). Identification of ROCK1 kinase as a critical regulator of Beclin1-mediated autophagy during metabolic stress. Nat. Commun. 4, 2189. doi:10.1038/ncomms3189
Hamano, T., Shirafuji, N., Yen, S. H., Yoshida, H., Kanaan, N. M., Hayashi, K., et al. (2020). Rho-kinase ROCK inhibitors reduce oligomeric tau protein. Neurobiol. Aging 89, 41–54. doi:10.1016/j.neurobiolaging.2019.12.009
Hansson, O. (2024). A clinical perspective on the revised criteria for diagnosis and staging of Alzheimer’s disease. Nat. Aging 4 (8), 1029–1031. doi:10.1038/s43587-024-00675-3
Henderson, B. W., Gentry, E. G., Rush, T., Troncoso, J. C., Thambisetty, M., Montine, T. J., et al. (2016). Rho-associated protein kinase 1 (ROCK1) is increased in Alzheimer’s disease and ROCK1 depletion reduces amyloid-β levels in brain. J. Neurochem. 138 (4), 525–531. doi:10.1111/jnc.13688
Henderson, B. W., Greathouse, K. M., Ramdas, R., Walker, C. K., Rao, T. C., Bach, S. V., et al. (2019). Pharmacologic inhibition of LIMK1 provides dendritic spine resilience against β-amyloid. Sci. Signal 12 (587), eaaw9318. doi:10.1126/scisignal.aaw9318
Herskowitz, J. H., Feng, Y., Mattheyses, A. L., Hales, C. M., Higginbotham, L. A., Duong, D. M., et al. (2013). Pharmacologic inhibition of ROCK2 suppresses amyloid-β production in an Alzheimer’s disease mouse model. J. Neurosci. 33 (49), 19086–19098. doi:10.1523/JNEUROSCI.2508-13.2013
Hill, B. G., Shiva, S., Ballinger, S., Zhang, J., and Darley-Usmar, V. M. (2019). Bioenergetics and translational metabolism: implications for genetics, physiology and precision medicine. Biol. Chem. 401 (1), 3–29. doi:10.1515/hsz-2019-0268
Ho, W. T., Chang, J. S., Chen, T. C., Wang, J. K., Chang, S. W., Yang, M. H., et al. (2022). Inhibition of Rho-associated protein kinase activity enhances oxidative phosphorylation to support corneal endothelial cell migration. FASEB J. 36 (7), e22397. doi:10.1096/fj.202101442RR
Ho, W. T., Chang, J. S., Lei, C. J., Chen, T. C., Wang, J. K., Chang, S. W., et al. (2025). ROCK inhibitor enhances resilience against metabolic stress through increasing bioenergetic capacity in corneal endothelial cells. Invest Ophthalmol. Vis. Sci. 66 (1), 51. doi:10.1167/iovs.66.1.51
Holper, L., Ben-Shachar, D., and Mann, J. J. (2019). Multivariate meta-analyses of mitochondrial complex I and IV in major depressive disorder, bipolar disorder, schizophrenia, Alzheimer disease, and Parkinson disease. Neuropsychopharmacology 44 (5), 837–849. doi:10.1038/s41386-018-0090-0
Holscher, C. (2011). Diabetes as a risk factor for Alzheimer’s disease: insulin signalling impairment in the brain as an alternative model of Alzheimer’s disease. Biochem. Soc. Trans. 39 (4), 891–897. doi:10.1042/BST0390891
Hou, Y., Zhou, L., Yang, Q. D., Du, X. P., Li, M., Yuan, M., et al. (2012). Changes in hippocampal synapses and learning-memory abilities in a streptozotocin-treated rat model and intervention by using fasudil hydrochloride. Neuroscience 200, 120–129. doi:10.1016/j.neuroscience.2011.10.030
Huang, H., Lee, D. H., Zabolotny, J. M., and Kim, Y. B. (2013). Metabolic actions of Rho-kinase in periphery and brain. Trends Endocrinol. Metab. 24 (10), 506–514. doi:10.1016/j.tem.2013.06.003
Huentelman, M. J., Stephan, D. A., Talboom, J., Corneveaux, J. J., Reiman, D. M., Gerber, J. D., et al. (2009). Peripheral delivery of a ROCK inhibitor improves learning and working memory. Behav. Neurosci. 123 (1), 218–223. doi:10.1037/a0014260
Iaccarino, L., Sala, A., and Perani, D.Alzheimer’s Disease Neuroimaging Initiative (2019). Predicting long-term clinical stability in amyloid-positive subjects by FDG-PET. Ann. Clin. Transl. Neurol. 6 (6), 1113–1120. doi:10.1002/acn3.782
Ida, Y., Sato, T., Umetsu, A., Watanabe, M., Furuhashi, M., Hikage, F., et al. (2022). Addition of ROCK inhibitors alleviates prostaglandin-induced inhibition of adipogenesis in 3T3L-1 spheroids. Bioeng. (Basel) 9 (11), 702. doi:10.3390/bioengineering9110702
Iorio, F., Bosotti, R., Scacheri, E., Belcastro, V., Mithbaokar, P., Ferriero, R., et al. (2010). Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc. Natl. Acad. Sci. U. S. A. 107 (33), 14621–14626. doi:10.1073/pnas.1000138107
Jack, C. R., Andrews, J. S., Beach, T. G., Buracchio, T., Dunn, B., Graf, A., et al. (2024a). Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 20 (8), 5143–5169. doi:10.1002/alz.13859
Jack, C. R., Andrews, S. J., Beach, T. G., Buracchio, T., Dunn, B., Graf, A., et al. (2024b). Revised criteria for the diagnosis and staging of Alzheimer’s disease. Nat. Med. 30 (8), 2121–2124. doi:10.1038/s41591-024-02988-7
Jahani, V., Kavousi, A., Mehri, S., and Karimi, G. (2018). Rho kinase, a potential target in the treatment of metabolic syndrome. Biomed. Pharmacother. 106, 1024–1030. doi:10.1016/j.biopha.2018.07.060
Jeong, Y. Y., Han, S., Jia, N., Zhang, M., Sheshadri, P., Tammineni, P., et al. (2022). Broad activation of the Parkin pathway induces synaptic mitochondrial deficits in early tauopathy. Brain 145 (1), 305–323. doi:10.1093/brain/awab243
Jia, N., Ganesan, D., Guan, H., Jeong, Y. Y., Han, S., Nissenbaum, M., et al. (2024). Mitochondrial bioenergetics stimulates autophagy for pathological tau clearance in tauopathy neurons. bioRxiv. doi:10.1101/2024.02.12.579959
John, A., and Reddy, P. H. (2021). Synaptic basis of Alzheimer’s disease: focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res. Rev. 65, 101208. doi:10.1016/j.arr.2020.101208
Julian, L., and Olson, M. F. (2014). Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases 5, e29846. doi:10.4161/sgtp.29846
Kaneko, Y., Ohta, M., Inoue, T., Mizuno, K., Isobe, T., Tanabe, S., et al. (2016/01/19 2016). Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci. Rep. 6 (1), 19640. doi:10.1038/srep19640
Kato, T., Inui, Y., Nakamura, A., and Ito, K. (2016). Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res. Rev. 30, 73–84. doi:10.1016/j.arr.2016.02.003
Killick, R., Elliott, C., Ribe, E., Broadstock, M., Ballard, C., Aarsland, D., et al. (2023). Neurodegenerative disease associated pathways in the brains of triple transgenic Alzheimer’s model mice are reversed following two weeks of peripheral administration of fasudil. Int. J. Mol. Sci. 24 (13), 11219. doi:10.3390/ijms241311219
Koch, J. C., Tatenhorst, L., Roser, A. E., Saal, K. A., Tonges, L., and Lingor, P. (2018). ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol. Ther. 189, 1–21. doi:10.1016/j.pharmthera.2018.03.008
Kong, X., Yan, D., Sun, J., Wu, X., Mulder, H., Hua, X., et al. (2014). Glucagon-like peptide 1 stimulates insulin secretion via inhibiting RhoA/ROCK signaling and disassembling glucotoxicity-induced stress fibers. Endocrinology 155 (12), 4676–4685. doi:10.1210/en.2014-1314
Koops, E. A., Dutta, J., Hanseeuw, B. J., Becker, J. A., Van Egroo, M., Prokopiou, P. C., et al. (2024). Elevated locus coeruleus metabolism provides resilience against cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 21, e14385. doi:10.1002/alz.14385
Kumar, M., and Bansal, N. (2018). Fasudil hydrochloride ameliorates memory deficits in rat model of streptozotocin-induced Alzheimer’s disease: involvement of PI3-kinase, eNOS and NFκB. Behav. Brain Res. 351, 4–16. doi:10.1016/j.bbr.2018.05.024
Kunkle, B. W., Grenier-Boley, B., Sims, R., Bis, J. C., Damotte, V., Naj, A. C., et al. (2019). Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51 (3), 414–430. doi:10.1038/s41588-019-0358-2
Landry, T., Shookster, D., and Huang, H. (2020). Tissue-specific approaches reveal diverse metabolic functions of rho-kinase 1. Front. Endocrinol. 11, 622581. doi:10.3389/fendo.2020.622581
Lee, J. H., Zheng, Y., von Bornstadt, D., Wei, Y., Balcioglu, A., Daneshmand, A., et al. (2014). Selective ROCK2 inhibition in focal cerebral Ischemia. Ann. Clin. Transl. Neurol. 1 (1), 2–14. doi:10.1002/acn3.19
Li, C. B., Li, X. X., Chen, Y. G., Gao, H. Q., Bao, C. M., Liu, X. Q., et al. (2012). Myocardial remodeling in rats with metabolic syndrome: role of Rho-kinase mediated insulin resistance. Acta Biochim. Pol. 59 (2), 249–254. doi:10.18388/abp.2012_2146
Liu, H., Zhang, P., Yu, J., Wang, J., Yu, J., and Guo, M. (2024a). Fasudil improves cognitive function in APP/PS1 transgenic mice by promoting mitophagy and inhibiting NLRP3 inflammasome activation. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 40 (8), 696–703.
Liu, P. Y., Chen, J. H., Lin, L. J., and Liao, J. K. (2007). Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J. Am. Coll. Cardiol. 49 (15), 1619–1624. doi:10.1016/j.jacc.2006.12.043
Liu, Y., Kwok, W., Yoon, H., Ryu, J. C., Stevens, P., Hawkinson, T. R., et al. (2024b). Imbalance in glucose metabolism regulates the transition of microglia from homeostasis to disease-associated microglia stage 1. J. Neurosci. 44 (20), e1563232024. doi:10.1523/JNEUROSCI.1563-23.2024
Luo, H., Krigman, J., Zhang, R., Yang, M., and Sun, N. (2021). Pharmacological inhibition of USP30 activates tissue-specific mitophagy. Acta Physiol. 232, e13666. doi:10.1111/apha.13666
Mani, S., Jindal, D., Chopra, H., Jha, S. K., Singh, S. K., Ashraf, G. M., et al. (2022). ROCK2 inhibition: a futuristic approach for the management of Alzheimer’s disease. Neurosci. Biobehav Rev. 142, 104871. doi:10.1016/j.neubiorev.2022.104871
Manly, J. J., and Deters, K. D. (2023). Donanemab for Alzheimer disease-who benefits and who is harmed? JAMA 330 (6), 510–511. doi:10.1001/jama.2023.11704
Martin-Camara, O., Cores, A., Lopez-Alvarado, P., and Menendez, J. C. (2021). Emerging targets in drug discovery against neurodegenerative diseases: control of synapsis disfunction by the RhoA/ROCK pathway. Eur. J. Med. Chem. 225, 113742. doi:10.1016/j.ejmech.2021.113742
Martorell-Riera, A., Segarra-Mondejar, M., Reina, M., Martinez-Estrada, O. M., and Soriano, F. X. (2015). Mitochondrial fragmentation in excitotoxicity requires ROCK activation. Cell Cycle 14 (9), 1365–1369. doi:10.1080/15384101.2015.1022698
Matthews, D. C., Kinney, J. W., Ritter, A., Andrews, R. D., Toledano Strom, E. N., Lukic, A. S., et al. (2024). Relationships between plasma biomarkers, tau PET, FDG PET, and volumetric MRI in mild to moderate Alzheimer’s disease patients. Alzheimers Dement. (N Y) 10 (3), e12490. doi:10.1002/trc2.12490
McKerracher, L., Shenkar, R., Abbinanti, M., Cao, Y., Peiper, A., Liao, J. K., et al. (2020). A brain-targeted orally available ROCK2 inhibitor benefits mild and aggressive cavernous angioma disease. Transl. Stroke Res. 11 (3), 365–376. doi:10.1007/s12975-019-00725-8
Mecca, A. P., Chen, M. K., O’Dell, R. S., Naganawa, M., Toyonaga, T., Godek, T. A., et al. (2022a). Association of entorhinal cortical tau deposition and hippocampal synaptic density in older individuals with normal cognition and early Alzheimer’s disease. Neurobiol. Aging 111, 44–53. doi:10.1016/j.neurobiolaging.2021.11.004
Mecca, A. P., Chen, M. K., O’Dell, R. S., Naganawa, M., Toyonaga, T., Godek, T. A., et al. (2020). In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 16 (7), 974–982. doi:10.1002/alz.12097
Mecca, A. P., O’Dell, R. S., Sharp, E. S., Banks, E. R., Bartlett, H. H., Zhao, W., et al. (2022b). Synaptic density and cognitive performance in Alzheimer’s disease: a PET imaging study with [(11) C]UCB-J. Alzheimers Dement. 18 (12), 2527–2536. doi:10.1002/alz.12582
Minoshima, S., Cross, D., Thientunyakit, T., Foster, N. L., and Drzezga, A. (2022). (18)F-FDG PET imaging in neurodegenerative dementing disorders: insights into subtype classification, emerging disease categories, and mixed dementia with copathologies. J. Nucl. Med. 63 (Suppl. 1), 2S–12S. doi:10.2967/jnumed.121.263194
Morrison, L., Gutierrez, J., Ayata, C., Lopez-Toledano, M., Carrazana, E., Awad, I., et al. (2024). Current and future treatment options for cerebral cavernous malformations. Stroke Vasc. Interventional Neurology 4 (3), e001140. doi:10.1161/SVIN.123.001140
Moskal, N., Riccio, V., Bashkurov, M., Taddese, R., Datti, A., Lewis, P. N., et al. (2020). ROCK inhibitors upregulate the neuroprotective Parkin-mediated mitophagy pathway. Nat. Commun. 11 (1), 88. doi:10.1038/s41467-019-13781-3
Murali, V. S., Rajendran, D., Isogai, T., DeBerardinis, R. J., and Danuser, G. (2024). RhoA activation promotes glucose uptake to elevate proliferation in MAPK inhibitor resistant melanoma cells. bioRxiv. doi:10.1101/2024.01.09.574940
Newell-Litwa, K. A., Badoual, M., Asmussen, H., Patel, H., Whitmore, L., and Horwitz, A. R. (2015). ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J. Cell Biol. 210 (2), 225–242. doi:10.1083/jcb.201504046
Ono-Saito, N., Niki, I., and Hidaka, H. (1999). H-series protein kinase inhibitors and potential clinical applications. Pharmacol. Ther. 82 (2-3), 123–131. doi:10.1016/s0163-7258(98)00070-9
Ou, Y. N., Xu, W., Li, J. Q., Guo, Y., Cui, M., Chen, K. L., et al. (2019). FDG-PET as an independent biomarker for Alzheimer’s biological diagnosis: a longitudinal study. Alzheimers Res. Ther. 11 (1), 57. doi:10.1186/s13195-019-0512-1
Ouyang, X., Collu, R., Benavides, G. A., Tian, R., Darley-Usmar, V., Xia, W., et al. (2024). ROCK inhibitor fasudil attenuates neuroinflammation and associated metabolic dysregulation in the tau transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 21, 183–200. doi:10.2174/0115672050317608240531130204
Park, J. T., Kang, H. T., Park, C. H., Lee, Y. S., Cho, K. A., and Park, S. C. (2018). A crucial role of ROCK for alleviation of senescence-associated phenotype. Exp. Gerontol. 106, 8–15. doi:10.1016/j.exger.2018.02.012
Preau, S., Delguste, F., Yu, Y., Remy-Jouet, I., Richard, V., Saulnier, F., et al. (2016). Endotoxemia engages the RhoA kinase pathway to impair cardiac function by altering cytoskeleton, mitochondrial fission, and autophagy. Antioxid. Redox Signal 24 (10), 529–542. doi:10.1089/ars.2015.6421
Reardon, S. (2023). Alzheimer’s drug donanemab helps most when taken at earliest disease stage, study finds. Nature 619 (7971), 682–683. doi:10.1038/d41586-023-02321-1
Redmann, M., Darley-Usmar, V., and Zhang, J. (2016). The role of autophagy, mitophagy and lysosomal functions in modulating bioenergetics and survival in the context of redox and proteotoxic damage: implications for neurodegenerative diseases. Aging Dis. 7 (2), 150–162. doi:10.14336/ad.2015.0820
Rigotto, G., and Basso, E. (2019). Mitochondrial dysfunctions: a thread sewing together Alzheimer’s disease, diabetes, and obesity. Oxid. Med. Cell Longev. 2019, 7210892. doi:10.1155/2019/7210892
Schinzari, F., Tesauro, M., Rovella, V., Di Daniele, N., Gentileschi, P., Mores, N., et al. (2012). Rho-kinase inhibition improves vasodilator responsiveness during hyperinsulinemia in the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 303 (6), E806–E811. doi:10.1152/ajpendo.00206.2012
Shi, J., Surma, M., and Wei, L. (2018). Disruption of ROCK1 gene restores autophagic flux and mitigates doxorubicin-induced cardiotoxicity. Oncotarget 9 (16), 12995–13008. doi:10.18632/oncotarget.24457
Shi, J., Surma, M., Yang, Y., and Wei, L. (2019). Disruption of both ROCK1 and ROCK2 genes in cardiomyocytes promotes autophagy and reduces cardiac fibrosis during aging. FASEB J. 33 (6), 7348–7362. doi:10.1096/fj.201802510R
Shimizu, Y., Thumkeo, D., Keel, J., Ishizaki, T., Oshima, H., Oshima, M., et al. (2005). ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J. Cell Biol. 168 (6), 941–953. doi:10.1083/jcb.200411179
Sims, J. R., Zimmer, J. A., Evans, C. D., Lu, M., Ardayfio, P., Sparks, J., et al. (2023). Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330 (6), 512–527. doi:10.1001/jama.2023.13239
Song, Y., Chen, X., Wang, L. Y., Gao, W., and Zhu, M. J. (2013). Rho kinase inhibitor fasudil protects against β-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci. Ther. 19 (8), 603–610. doi:10.1111/cns.12116
Swanger, S. A., Mattheyses, A. L., Gentry, E. G., and Herskowitz, J. H. (2015). ROCK1 and ROCK2 inhibition alters dendritic spine morphology in hippocampal neurons. Cell Logist. 5 (4), e1133266. doi:10.1080/21592799.2015.1133266
Swerdlow, R. H. (2020). The mitochondrial hypothesis: dysfunction, bioenergetic defects, and the metabolic link to Alzheimer’s disease. Int. Rev. Neurobiol. 154, 207–233. doi:10.1016/bs.irn.2020.01.008
Swerdlow, R. H. (2023). The Alzheimer’s disease mitochondrial cascade hypothesis: a current overview. J. Alzheimers Dis. 92 (3), 751–768. doi:10.3233/JAD-221286
Swerdlow, R. H., Burns, J. M., and Khan, S. M. (2010). The Alzheimer’s disease mitochondrial cascade hypothesis. J. Alzheimers Dis. 20 (Suppl. 2), S265–S279. doi:10.3233/JAD-2010-100339
Swerdlow, R. H., Burns, J. M., and Khan, S. M. (2014). The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim. Biophys. Acta 1842 (8), 1219–1231. doi:10.1016/j.bbadis.2013.09.010
Swerdlow, R. H., and Khan, S. M. (2004). A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med. Hypotheses 63 (1), 8–20. doi:10.1016/j.mehy.2003.12.045
Swerdlow, R. H., Koppel, S., Weidling, I., Hayley, C., Ji, Y., and Wilkins, H. M. (2017). Mitochondria, cybrids, aging, and Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 146, 259–302. doi:10.1016/bs.pmbts.2016.12.017
Szablewski, L. (2017). Glucose transporters in brain: in Health and in Alzheimer’s disease. J. Alzheimers Dis. 55 (4), 1307–1320. doi:10.3233/JAD-160841
Tai, Y. H., Engels, D., Locatelli, G., Emmanouilidis, I., Fecher, C., Theodorou, D., et al. (2023). Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nat. Metab. 5 (8), 1364–1381. doi:10.1038/s42255-023-00838-3
Thumkeo, D., Keel, J., Ishizaki, T., Hirose, M., Nonomura, K., Oshima, H., et al. (2003). Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol. Cell Biol. 23 (14), 5043–5055. doi:10.1128/MCB.23.14.5043-5055.2003
Truebestein, L., Elsner, D. J., Fuchs, E., and Leonard, T. A. (2015). A molecular ruler regulates cytoskeletal remodelling by the Rho kinases. Nat. Commun. 6, 10029. doi:10.1038/ncomms10029
Trushina, E., Trushin, S., and Hasan, M. F. (2022). Mitochondrial complex I as a therapeutic target for Alzheimer’s disease. Acta Pharm. Sin. B 12 (2), 483–495. doi:10.1016/j.apsb.2021.11.003
Udeochu, J. C., Amin, S., Huang, Y., Fan, L., Torres, E. R. S., Carling, G. K., et al. (2023). Tau activation of microglial cGAS-IFN reduces MEF2C-mediated cognitive resilience. Nat. Neurosci. 26, 737–750. doi:10.1038/s41593-023-01315-6
Wang, J., Huang, Q., Chen, X., You, Z., He, K., Guo, Q., et al. (2024b). Tau pathology is associated with synaptic density and longitudinal synaptic loss in Alzheimer’s disease. Mol. Psychiatry 29 (9), 2799–2809. doi:10.1038/s41380-024-02501-z
Wang, J., Huang, Q., He, K., Li, J., Guo, T., Yang, Y., et al. (2024a). Presynaptic density determined by SV2A PET is closely associated with postsynaptic metabotropic glutamate receptor 5 availability and independent of amyloid pathology in early cognitive impairment. Alzheimers Dement. 20 (6), 3876–3888. doi:10.1002/alz.13817
Wang, W., Zhao, F., Ma, X., Perry, G., and Zhu, X. (2020). Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol. Neurodegener. 15 (1), 30. doi:10.1186/s13024-020-00376-6
Weber, A. J., Adamson, A. B., Greathouse, K. M., Andrade, J. P., Freeman, C. D., Seo, J. V., et al. (2021). Conditional deletion of ROCK2 induces anxiety-like behaviors and alters dendritic spine density and morphology on CA1 pyramidal neurons. Mol. Brain 14 (1), 169. doi:10.1186/s13041-021-00878-4
Weber, A. J., and Herskowitz, J. H. (2021). Perspectives on ROCK2 as a therapeutic target for Alzheimer’s disease. Front. Cell Neurosci. 15, 636017. doi:10.3389/fncel.2021.636017
Wei, W., Wang, Y., Zhang, J., Gu, Q., Liu, X., Song, L., et al. (2021). Fasudil ameliorates cognitive deficits, oxidative stress and neuronal apoptosis via inhibiting ROCK/MAPK and activating Nrf2 signalling pathways in APP/PS1 mice. Folia Neuropathol. 59 (1), 32–49. doi:10.5114/fn.2021.105130
Weidling, I. W., and Swerdlow, R. H. (2020). Mitochondria in Alzheimer’s disease and their potential role in Alzheimer’s proteostasis. Exp. Neurol. 330, 113321. doi:10.1016/j.expneurol.2020.113321
Wu, J., Wei, J., Chen, H., Dang, Y., and Lei, F. (2024). Rho kinase (ROCK) inhibitors for the treatment of glaucoma. Curr. Drug Targets 25 (2), 94–107. doi:10.2174/0113894501286195231220094646
Xiyang, Y., Gao, J., Ding, M., Ren, X., Appleby, B. S., Leverenz, J. B., et al. (2024). Exacerbated mitochondrial dynamic abnormalities without evident tau pathology in rapidly progressive Alzheimer’s disease. J. Alzheimers Dis. 102, 1074–1083. doi:10.1177/13872877241295403
Yan, Y., Gao, Y., Fang, Q., Zhang, N., Kumar, G., Yan, H., et al. (2021). Inhibition of rho kinase by fasudil ameliorates cognition impairment in APP/PS1 transgenic mice via modulation of gut microbiota and metabolites. Front. Aging Neurosci. 13, 755164. doi:10.3389/fnagi.2021.755164
Yang, Y. J., Bu, L. L., Shen, C., Ge, J. J., He, S. J., Yu, H. L., et al. (2020). Fasudil promotes α-synuclein clearance in an AAV-mediated α-synuclein rat model of Parkinson’s disease by autophagy activation. J. Park. Dis. 10 (3), 969–979. doi:10.3233/JPD-191909
Ye, Q., Li, X., Gao, W., Gao, J., Zheng, L., Zhang, M., et al. (2024). Role of Rho-associated kinases and their inhibitor fasudil in neurodegenerative diseases. Front. Neurosci. 18, 1481983. doi:10.3389/fnins.2024.1481983
Yuan, J., Chen, F., Jiang, D., Xu, Z., Zhang, H., and Jin, Z. B. (2024). ROCK inhibitor enhances mitochondrial transfer via tunneling nanotubes in retinal pigment epithelium. Theranostics 14 (15), 5762–5777. doi:10.7150/thno.96508
Zanin-Zhorov, A., Weiss, J. M., Nyuydzefe, M. S., Chen, W., Scher, J. U., Mo, R., et al. (2014). Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A. 111 (47), 16814–16819. doi:10.1073/pnas.1414189111
Zhang, H., Li, Y., Yu, J., Guo, M., Meng, J., Liu, C., et al. (2013). Rho kinase inhibitor fasudil regulates microglia polarization and function. Neuroimmunomodulation 20 (6), 313–322. doi:10.1159/000351221
Zhang, Q., Hu, C., Huang, J., Liu, W., Lai, W., Leng, F., et al. (2019). ROCK1 induces dopaminergic nerve cell apoptosis via the activation of Drp1-mediated aberrant mitochondrial fission in Parkinson’s disease. Exp. Mol. Med. 51 (10), 1–13. doi:10.1038/s12276-019-0318-z
Zhang, Y. M., Bo, J., Taffet, G. E., Chang, J., Shi, J., Reddy, A. K., et al. (2006). Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 20 (7), 916–925. doi:10.1096/fj.05-5129com
Zhao, H., Li, X., Zheng, Y., Zhu, X., Qi, X., Huang, X., et al. (2023). Fasudil may alleviate alcohol-induced astrocyte damage by modifying lipid metabolism, as determined by metabonomics analysis. PeerJ 11, e15494. doi:10.7717/peerj.15494
Zhao, Y., Tseng, I.-C., Heyser, C. J., Rockenstein, E., Mante, M., Adame, A., et al. (2015). Appoptosin-mediated caspase cleavage of tau contributes to progressive supranuclear palsy pathogenesis. Neuron 87 (5), 963–975. doi:10.1016/j.neuron.2015.08.020
Keywords: Alzheimer’s disease, fasudil, amyloid, phosphorylated tau, glucose metabolism, PET imaging, mitochondria, synapse
Citation: Zheng C, Xia W and Zhang J (2025) Rock inhibitors in Alzheimer’s disease. Front. Aging 6:1547883. doi: 10.3389/fragi.2025.1547883
Received: 18 December 2024; Accepted: 28 February 2025;
Published: 20 March 2025.
Edited by:
Xuejun Wang, University of South Dakota, United StatesReviewed by:
Jui-Heng Tseng, Arizona State University, United StatesCopyright © 2025 Zheng, Xia and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Zheng, Y2hhby56aGVuZ0BjYW1oLmNh, Y2guemhlbmdAdXRvcm9udG8uY2E=; Weiming Xia, d2VpbWluZy54aWFAdmEuZ292 Jianhua Zhang, Smlhbmh1YXpoYW5nQHVhYm1jLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.