- 1College of Orthopedics and Traumatology, Henan University of Chinese Medicine, Zhengzhou, China
- 2Department of Rheumatology, Henan Province Hospital of Traditional Chinese Medicine, Zhengzhou, Henan Province, China
Elderly-onset rheumatoid arthritis (EORA) is a distinct subtype of rheumatoid arthritis characterized by heightened treatment challenges due to immune aging and the complexity of comorbidities. This review systematically summarizes the definition, clinical features, epidemiological trends, therapeutic challenges, and the potential applications of biologic agents in EORA. It primarily focuses on the efficacy, safety, and individualized treatment strategies associated with various biologic agents. Studies indicate that biologics, such as TNF-α inhibitors, IL-6 inhibitors, and JAK inhibitors, can significantly reduce inflammation and improve joint function in EORA patients. However, their long-term use is closely linked to increased risks of infections, thrombosis, and malignancies, underscoring the importance of personalized treatment approaches and dynamic monitoring. Moreover, the advent of novel biologic agents, including IL-17 and IL-23 inhibitors, as well as second-generation JAK inhibitors, offers additional therapeutic options for refractory patients and demonstrates substantial potential in optimizing both efficacy and safety. With the rapid progress of precision medicine and artificial intelligence (AI) technologies, gene profiling, biomarker analysis, and AI-assisted decision-making are gradually steering EORA treatment towards more personalized and precise strategies. However, the high cost of treatment and the limited accessibility of these technologies remain significant barriers in clinical practice. Future research should focus on validating the long-term safety of novel therapies and refining individualized treatment strategies to enhance patient outcomes and quality of life.
1 Introduction
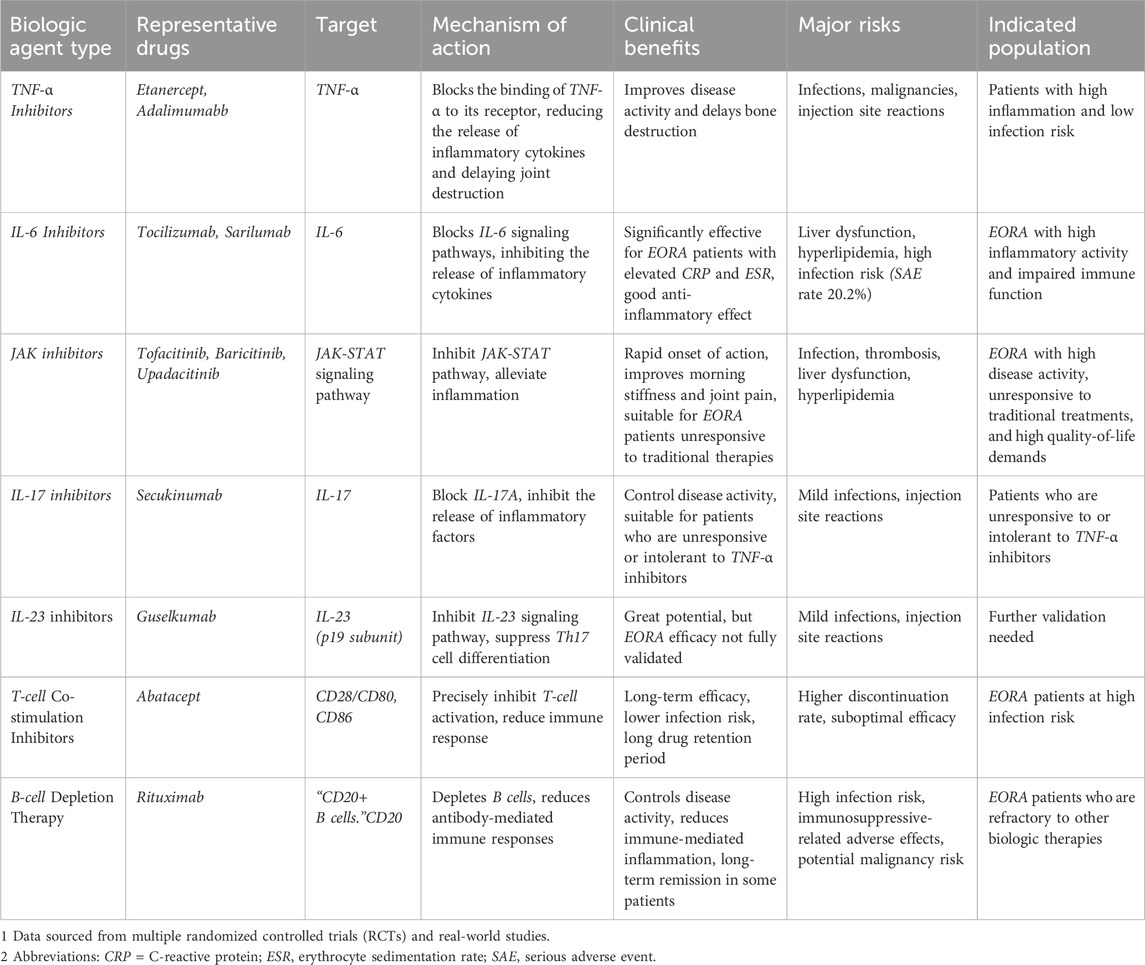
With the accelerated aging of the global population, there has been increasing attention on elderly-onset rheumatoid arthritis (EORA). As a distinct subtype of rheumatoid arthritis (RA), EORA differs significantly from young-onset rheumatoid arthritis (YORA) in terms of pathophysiological mechanisms, clinical manifestations, and therapeutic needs. The physiological characteristics and chronic comorbidities of EORA patients make disease management more complex and increase treatment risks (Pavlov-Dolijanovic et al., 2023). Although biologic agents have brought new hope to the treatment of RA, research specifically targeting EORA patients remains limited, particularly in terms of the efficacy, safety, and individualized treatment strategies of different biologic agents (Baghdadi et al., 2015). Therefore, this review aims to summarize the current application of biologic agents in the treatment of EORA, focusing on their efficacy, safety, and individualized treatment strategies, while providing recommendations for optimizing clinical practice. Specifically, Table 1 summarizes the efficacy and safety data of various biologic agents in the treatment of EORA, offering crucial insights for clinical decision-making. As illustrated in Figure 1, the mechanisms of biologic agents in the treatment of elderly-onset rheumatoid arthritis are depicted, offering a comprehensive understanding of their therapeutic effects and underlying pathways.
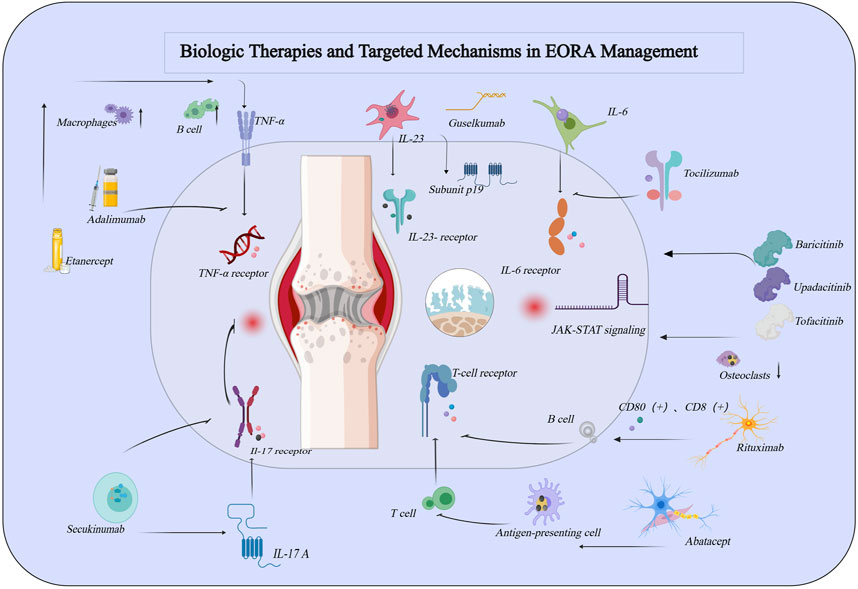
Figure 1. Schematic Representation of the Role of Common Biologic Agents in the Pathogenesis of Rheumatoid Arthritis. This figure was created using MedPeer (medpeer.cn).
2 Definition, epidemiology, and clinical features of EORA
EORA refers to rheumatoid arthritis RA that is first diagnosed in individuals aged 60 years or older. Compared to YORA, EORA exhibits significant differences in both pathological mechanisms and clinical manifestations, primarily due to the effects of immunosenescence and inflammaging. Immunosenescence refers to the gradual decline of immune function with age, leading to a reduced ability to fight infections and an increased susceptibility to autoimmune reactions, while inflammaging describes a state of chronic low-grade inflammation that results from the overexpression of pro-inflammatory factors, such as TNF-α and IL-6, in the elderly population. In EORA patients, inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), are often elevated, and the disease tends to predominantly affect larger joints (e.g., the shoulders and knees), which exacerbates functional disability and significantly reduces quality of life (Targońska-Stępniak et al., 2021; Yoshii et al., 2020). The prevalence of EORA is estimated at approximately 1.5%–2% in the elderly population, with an increasing trend as individuals age. It is particularly common among females, especially postmenopausal women, in whom the decline in estrogen levels contributes to immune dysregulation, thereby increasing the risk of developing the disease (Tan et al., 2016; Turesson et al., 2015). Moreover, EORA patients frequently present with multiple chronic comorbidities, including cardiovascular diseases (e.g., hypertension and coronary artery disease) and metabolic disorders (e.g., diabetes and hyperlipidemia). These comorbidities complicate treatment regimens and require careful consideration of drug metabolism and safety (Jin et al., 2017; Vicente et al., 2021). In conclusion, the distinct pathological mechanisms and epidemiological characteristics of EORA highlight the need for personalized treatment strategies, which could provide new directions for future clinical research and therapeutic practice (Sato et al., 2021; Liu et al., 2024).
3 Application of biologic agents in EORA
In recent years, biologic agents have offered a diverse range of treatment options for patients with EORA. These medications target specific immune pathways, effectively inhibiting inflammatory responses, reducing disease activity, and delaying joint and bone damage (Dalal et al., 2019). However, due to the unique physiological characteristics and chronic comorbidities of EORA patients, the use of biologics presents an increased risk of adverse effects and poses additional management challenges. Currently, the biologic agents commonly used in clinical practice can be classified into seven major categories: TNF-α inhibitors, IL-6 inhibitors, JAK inhibitors, B-cell depletion therapies, T-cell co-stimulation blockers, IL-17 inhibitors, and IL-23 inhibitors. In the following sections, we will explore the mechanisms of action, efficacy, safety profiles, and reasons for discontinuation for each class of these biologics, providing a comprehensive guide to optimizing clinical treatment.
3.1 TNF-α inhibitors
3.1.1 Adalimumab
Adalimumab is a fully human monoclonal antibody that specifically targets and blocks the binding of TNF-α to its receptors. This mechanism of action inhibits the release of pro-inflammatory cytokines, reduces systemic inflammation, and helps to slow the progression of cartilage and bone damage (Filippini et al., 2010). Due to its targeted action, adalimumab is considered a key therapeutic option for patients with EORA. Clinical studies demonstrate that in patients aged ≥65 years, adalimumab achieves a 38.8% improvement in DAS28 scores, comparable to the 37.5% improvement observed in patients aged 18–65 years (p > 0.05). However, improvements in functional scores (HAQ) tend to be somewhat lower in elderly patients (see Table 1), which may be attributed to factors such as delayed treatment initiation, age-related immune system decline, and the greater burden of comorbidities (Veerasubramanian et al., 2024). While adalimumab is highly effective in halting bone damage and improving quality of life, its safety profile in elderly patients—especially those with compromised immune function—remains a significant concern. The most commonly reported adverse effects of adalimumab include an increased risk of infections, such as reactivation of tuberculosis and fungal infections, as well as malignancies, including skin cancer and lymphoma. These risks are particularly pronounced in older individuals who may already be immunosuppressed (Cometi et al., 2020). Studies indicate that the relative risk of severe infections in elderly patients treated with TNF-α inhibitors is 1.59 (95% CI: 1.45–1.76), with heightened risk in patients with underlying cardiovascular disease or a history of malignancy (Pavlov-Dolijanovic et al., 2023). Additionally, secondary treatment failure frequently occurs, with initial efficacy often diminishing over time, leading to eventual discontinuation of therapy. The discontinuation rate of adalimumab in elderly patients (21.8%) is significantly higher than in younger patients (16.9%, p < 0.05). This difference is primarily attributed to side effects, poor treatment tolerance, and the high costs associated with biologic therapies (Gossec et al., 2024). From a clinical management perspective, adalimumab is most suitable for patients with high disease activity but relatively low risks of infection and malignancy. For patients at an elevated risk of infection, alternative biologic agents such as abatacept or tocilizumab should be prioritized (Jiang et al., 2024; Choi et al., 2023). Given the potential for serious side effects, long-term monitoring of elderly patients on adalimumab therapy should focus on assessing disease activity (DAS28, HAQ), inflammatory markers (CRP, ESR), and any adverse events. Treatment regimens should be adjusted accordingly to balance efficacy and safety, with careful consideration given to dosing intervals and potential modifications based on individual patient response.
3.1.2 Etanercept
Etanercept is a soluble tumor necrosis factor receptor fusion protein that binds competitively to TNF-α, thereby inhibiting its interaction with cell surface receptors. This mechanism effectively suppresses inflammation and delays joint and bone damage (Filippini et al., 2010). Etanercept has shown sustained efficacy in preventing bone destruction and improving inflammatory markers. Its relatively lower risk of infections provides a therapeutic advantage in elderly patients. However, some studies have reported suboptimal functional improvement (e.g., in HAQ scores) among elderly patients, which may be attributed to the burden of comorbidities and delayed treatment initiation. Regarding safety, the primary adverse effects associated with etanercept include infections (such as pneumonia and reactivation of tuberculosis) and injection site reactions. Etanercept is associated with a lower risk of infections than adalimumab (OR = 0.79, 95% CI: 0.46–1.34), and is generally better tolerated (D'Arcy et al., 2021). Nevertheless, etanercept may still be linked to an increased risk of skin cancer and lymphoma, requiring regular monitoring of the skin and lymphatic system in patients. The discontinuation rate of etanercept is primarily influenced by infection risks and injection site reactions, with the high cost of treatment also contributing to reduced patient adherence. Despite these challenges, etanercept typically demonstrates a higher drug retention rate in EORA patients compared to adalimumab, particularly in those with high disease activity but a lower risk of infections (Jiang et al., 2024). Overall, both adalimumab and etanercept are effective TNF-α inhibitors that improve DAS28 scores and slow bone damage in EORA patients. However, they differ significantly in terms of safety profiles and patient suitability. Adalimumab carries a higher risk of infections and malignancies (see Table 1), making it more appropriate for patients with a low risk of infection. In contrast, etanercept, with its lower infection risk and better tolerability, is generally better suited for elderly patients with fewer comorbidities. Although injection site reactions are more common with etanercept, its overall tolerability is superior to that of adalimumab. Long-term follow-up should focus on dynamically monitoring disease activity (e.g., DAS28 and HAQ scores), inflammatory markers, and adverse events. Treatment regimens should be adjusted based on individual patient responses to minimize the cumulative impact of potential side effects.
3.2 IL-6 inhibitors
Tocilizumab is a monoclonal antibody that targets interleukin-6 (IL-6), inhibiting the IL-6 signaling pathway. This action reduces the release of pro-inflammatory cytokines, alleviating both joint inflammation and systemic inflammatory responses. Due to its targeted mechanism of action, tocilizumab is an important therapeutic option for EORA patients, particularly those with significantly elevated CRP and ESR levels who have not responded adequately to conventional DMARDs. A prospective study in Japan demonstrated that in patients aged ≥65 years, the improvement in the Clinical Disease Activity Index (CDAI) with tocilizumab was significantly higher than in younger patients (p = 0.03), confirming its superior efficacy in EORA (see Table 1) (Specker et al., 2022). However, despite its notable efficacy, the safety risks associated with tocilizumab should not be overlooked. In elderly patients, the incidence of serious adverse events (SAEs) is as high as 20.2%, which is significantly higher than in younger patients (11.5%, p < 0.0001). Common adverse events include bacterial pneumonia, fungal infections, liver dysfunction, and hyperlipidemia (Kondo et al., 2020). These risks are particularly pronounced in elderly patients with impaired immune function, underscoring the importance of individualized treatment strategies in EORA. Accordingly, comprehensive infection screening should be performed prior to treatment, and liver function, blood lipid levels, and other relevant parameters should be closely monitored during therapy to ensure the early detection and management of potential adverse effects. Liver dysfunction and infections are among the primary reasons for discontinuation of tocilizumab, highlighting the drug’s inherent risks and the need for flexible, personalized treatment adjustments. To optimize efficacy while minimizing risks, the dosing frequency and dosage of tocilizumab should be tailored to the patient’s immune status, comorbidities, and infection risks. For patients at higher risk of infections, a more conservative treatment approach should be considered, such as reducing the dosing frequency or lowering the dosage, while intensifying monitoring of treatment response and adverse events. Multidisciplinary management can help mitigate the adverse effects of treatment, extend drug retention, and improve long-term outcomes. Therefore, treatment decisions for EORA patients should not only be based on the drug’s efficacy but also take into account individual risks and treatment tolerance. This ensures that therapy remains both appropriate and safe. Furthermore, Sarilumab, another IL-6 inhibitor, has shown promise in reducing inflammation and improving joint function. However, clinical trial data specific to EORA patients is currently lacking, and further research is needed to assess its efficacy and safety in elderly populations.
3.3 JAK inhibitors
3.3.1 Tofacitinib
Tofacitinib is an oral Janus kinase (JAK) inhibitor that modulates the JAK-STAT signaling pathway, effectively reducing both joint and systemic inflammation in patients with EORA. This mechanism makes it a valuable therapeutic option for patients who have not responded adequately to conventional DMARDs or TNF-α inhibitors. Clinical studies have shown that tofacitinib provides comparable improvements in DAS28 scores in elderly patients as it does in younger individuals, demonstrating its effectiveness in disease control. However, concerns about its safety, especially in the elderly, have emerged. Compared to younger patients, elderly individuals (≥65 years) receiving tofacitinib have a significantly higher risk of serious adverse events (SAEs) — 17.56 per 100 patient-years versus 8.44 per 100 patient-years in younger cohorts. Additionally, elderly patients are at twice the risk of herpes zoster infections (6.40 vs 3.76 per 100 patient-years), and face elevated risks of venous thromboembolism and malignancy, particularly at the 10 mg dose. Notably, the risk of venous thromboembolism in this group is substantially increased, with a hazard ratio (HR) of 5.02 (95% CI: 1.44–17.47) (Shih et al., 2024). Given these risks, careful monitoring is essential when using tofacitinib, especially in elderly patients who typically have a reduced immune response and multiple comorbidities. The oral formulation of tofacitinib offers convenience, particularly for older patients who may struggle with adherence to injectable therapies. However, when compared to other JAK inhibitors, like upadacitinib, tofacitinib shows somewhat weaker effects in improving quality of life and reducing morning stiffness (Duran et al., 2022). Especially in patients with higher disease activity, clinical practice requires balancing effective treatment with monitoring for potential safety concerns. Adjustments to treatment, including dose modifications or closer monitoring for infections and thromboembolic events, may be necessary to ensure that the benefits of tofacitinib outweigh the risks (Wang et al., 2024). In summary, while tofacitinib represents a promising therapeutic option for EORA patients, its potential safety issues necessitate vigilant monitoring and individualized treatment strategies to mitigate risks while optimizing outcomes.
3.3.2 Baricitinib
Baricitinib is a selective inhibitor of Janus kinases 1 (JAK1) and 2 (JAK2) that modulates the JAK-STAT signaling pathway, effectively reducing both joint and systemic inflammation in patients with EORA. It has demonstrated significant efficacy in patients with high disease activity, particularly in those who have not responded adequately to conventional DMARDs. Long-term studies show that both 2 mg and 4 mg doses of baricitinib consistently improve DAS28 scores and ACR20 response rates, with a notable advantage in controlling inflammation (Taylor et al., 2022). However, despite its strong therapeutic benefits, safety concerns with baricitinib should be carefully considered. In elderly patients, the incidence of serious infections is 5.5 cases per 100 patient-years, compared to 2.1 cases per 100 patient-years in younger patients. Pneumonia (0.6 cases per 100 patient-years) and herpes zoster (0.3 cases per 100 patient-years) are the most common infections observed. Additionally, there is an increased risk of venous thromboembolism (VTE), with deep vein thrombosis occurring at a rate of 0.49 cases per 100 patient-years and pulmonary embolism at 0.26 cases per 100 patient-years. The incidence of major adverse cardiovascular events (MACE) is 0.5 cases per 100 patient-years, highlighting the need for close monitoring of infection and thrombotic risks when using baricitinib (Keystone et al., 2015). Compared to tofacitinib, baricitinib carries a more pronounced risk of lipid abnormalities, especially in elderly patients who may require more frequent monitoring of lipid profiles. Despite these safety concerns, the rapid onset of action and stable long-term efficacy of baricitinib make it an important option for treating patients with high disease activity in EORA. In clinical practice, it is crucial to monitor not only infection risks but also lipid levels and cardiovascular health throughout treatment to ensure an optimal balance between efficacy and safety. Baricitinib is particularly well-suited for patients with higher quality-of-life demands, as it is especially effective in alleviating morning stiffness and joint pain (Genovese et al., 2016). This makes it complementary to other JAK inhibitors, such as upadacitinib, which may target different aspects of the disease. Ultimately, treatment with baricitinib requires individualized dosing (2 mg or 4 mg), tailored to each patient’s specific risk profile, along with regular monitoring of relevant blood parameters and infection indicators. By doing so, clinicians can maximize the therapeutic benefits of baricitinib while minimizing potential safety risks (Virtanen et al., 2024).
3.3.3 Upadacitinib
Upadacitinib is a novel, selective JAK1 inhibitor that, due to its high selectivity, significantly reduces the risk of off-target adverse effects. In multiple clinical trials, upadacitinib has shown a rapid onset of action and potent anti-inflammatory effects, particularly in improving pain, morning stiffness, and overall quality of life, outperforming adalimumab. The SELECT-MONOTHERAPY trial results showed that upadacitinib monotherapy significantly increased the ACR20 response rate at 14 weeks (68% vs 41%, p < 0.001), with a corresponding improvement in DAS28-CRP scores (Kiełbowski et al., 2024). Furthermore, the SELECT-EARLY trial revealed that upadacitinib outperformed methotrexate (MTX) monotherapy in both efficacy and radiographic improvements, highlighting its potential as an early treatment option for EORA (Strand et al., 2021). Compared to baricitinib, upadacitinib shows superior efficacy in relieving morning stiffness and pain, along with significant improvements in both ACR20 and DAS28 scores. These factors make it a competitive option for patients with high disease activity in EORA. However, safety concerns with upadacitinib should not be overlooked. Its primary adverse events include infections (including upper respiratory infections and herpes zoster) and liver dysfunction (see Table 1). Research showns that the incidence of serious adverse events is 4.7%, with venous thromboembolism occurring in 0.6% of patients and liver function abnormalities in 0.8% (Fleischmann et al., 2019). While upadacitinib may present a slightly lower infection risk than tofacitinib and baricitinib, pneumonia and lipid abnormalities still require close monitoring, particularly in elderly patients. To minimize adverse reactions, reducing the dose from 30 mg to 15 mg and regularly monitoring liver function, blood lipids, and infection risks are recommended. This approach can help optimize long-term therapeutic efficacy while minimizing safety concerns (Smolen et al., 2019). Consequently, upadacitinib is particularly suited for EORA patients with higher quality-of-life demands, especially those seeking better control over morning stiffness and joint pain. However, clinical use should be individualized, with close monitoring to maintain a balance between efficacy and safety based on each patient’s specific risk profile.
3.4 IL-17 inhibitors
3.4.1 Secukinumab
Secukinumab is a fully humanized monoclonal antibody that targets interleukin-17A (IL-17A). It works by blocking the binding of IL-17A to its receptor, thereby inhibiting the release of pro-inflammatory cytokines and reducing joint and systemic inflammation in patients with EORA. In treating EORA, secukinumab not only effectively controls disease activity but also offers an important alternative for patients who do not respond to or cannot tolerate traditional TNF-α inhibitors (Sato et al., 2006). Clinical studies have consistently demonstrated secukinumab’s significant efficacy in treating EORA. For example, a Phase III trial showed that after 12 weeks of treatment, secukinumab significantly increased the ACR20 response rate to over 60%, outperforming the placebo group. Further meta-analyses revealed that in patients with inadequate response to TNF-α (TNF-IR), secukinumab achieved ACR50 and ACR70 response rates that were 1.94 and 2.11 times higher than those of the placebo group, respectively (p < 0.01). Importantly, this efficacy was sustained over the long term, leading to substantial improvements in disease remission and functional recovery (Wu et al., 2019; Genovese et al., 2010). These findings highlight secukinumab’s potential not only in improving short-term symptoms but also in playing a critical role in long-term disease management strategies for EORA patients. In terms of safety, secukinumab has shown good tolerability, particularly concerning infection risk. The most common adverse events are mild infections (such as upper respiratory tract infections) and injection site reactions (see Table 1), while the incidence of severe infections remains relatively low—around 2.5%–3.0%, which is lower than the infection rates typically seen with TNF-α inhibitors. This relatively lower infection risk makes secukinumab an attractive option for elderly EORA patients (Kunwar et al., 2016; Chabaud et al., 1999). However, injection site reactions remain a frequent reason for discontinuation, highlighting the importance of targeted management and patient education to improve adherence and overall treatment experience. Secukinumab’s value in the treatment of EORA lies in its favorable balance between efficacy and safety. It is particularly well-suited for patients with high disease activity and a low infection risk. In clinical practice, selecting the appropriate patients and addressing injection site reactions can further enhance both the treatment’s effectiveness and patient satisfaction, making secukinumab a strong treatment option for EORA patients.
3.5 IL-23 inhibitors
3.5.1 Guselkumab
Guselkumab is a fully humanized monoclonal antibody that targets interleukin-23 (IL-23) by binding to its p19 subunit thereby inhibiting its signaling. This action prevents the differentiation of Th17 cells and the associated pro-inflammatory responses. IL-23 activates Th17 cells through the JAK-STAT pathway, which contributes to the inflammatory processes and bone loss observed in rheumatoid arthritis (RA). Studies have shown that IL-23 levels are significantly elevated in the serum and synovial fluid of RA patients, with the levels correlating closely with disease activity and bone erosion, supporting the potential of IL-23 as a therapeutic target for RA. In terms of efficacy, guselkumab has demonstrated strong anti-inflammatory effects in other immune-mediated diseases, such as psoriasis and psoriatic arthritis. However, its clinical efficacy for the treatment of EORA remains inconclusive. A Phase II clinical trial showed guselkumab failed to significantly improve DAS28 scores or meet primary efficacy endpoints, suggesting that its effectiveness in RA treatment requires further investigation. This suggests that, while guselkumab has shown promise in managing other immune-mediated diseases, its potential application in EORA still requires more robust clinical data to support its use (Yuan et al., 2019; Pastor-Fernández et al., 2020). From a safety perspective, guselkumab has generally shown good tolerability. The most common adverse events include mild infections (e.g., upper respiratory tract infections) and injection site reactions, while the incidence of severe infections is low, comparable to other biologic agents (see Table 1). However, since IL-23 plays a crucial role in maintaining immune tolerance, long-term inhibition of IL-23 may increase the risk of autoimmune diseases or infections. Therefore, regular monitoring of patients—especially those in the EORA population, who have a higher risk of infection—is critical during treatment. Although current clinical evidence does not fully support the widespread use of guselkumab in the treatment of EORA, its mechanism of inhibiting the IL-23/IL-17 axis offers a promising new therapeutic approach. As more clinical research and data accumulate, guselkumab may become an important therapeutic option for patients with EORA.
3.6 T-cell Co-stimulation inhibitors
3.6.1 Abatacept
Abatacept is a T-cell co-stimulation inhibitor that works by selectively blocking T-cell activation, thereby reducing the transmission of inflammatory signals. It is particularly well-suited for EORA, who often have compromised immune systems and a higher risk of infections. As such, abatacept has become a key treatment option in the management of EORA (Nawrot et al., 2018). Clinical studies have shown that there is no significant difference in treatment outcomes between RA patients aged ≥65 years and those aged <65 years, with abatacept demonstrating comparable efficacy in both groups (Serhal et al., 2020). This suggests that abatacept offers stable and reliable efficacy in elderly patients, especially in those with impaired immune function. Additionally, abatacept has a notably higher drug retention rate compared to other biologics, with a median retention period of 254.1 weeks—significantly longer than that of TNF-α inhibitors (184 weeks) and JAK inhibitors (139.1 weeks). Its 2-year retention rate is 66.3%, further emphasizing its sustained effectiveness in long-term treatment (Rasch et al., 2003). These findings suggest that abatacept not only effectively controls disease activity in the short term but also provides long-lasting therapeutic benefits, which is particularly important for elderly patients who require ongoing treatment. Despite its strong efficacy and safety profile, inadequate response remains the primary reason for treatment discontinuation in elderly patients, with approximately 66.1% of patients stopping treatment due to insufficient effectiveness (Manfredi et al., 2024). Other factors such as smoking, joint erosion, and diabetes have also been identified as major risk factors for discontinuation (Sugihara, 2022). These considerations highlight the need for personalized treatment plans that take into account individual patient characteristics, in order to better manage disease progression and reduce the likelihood of premature discontinuation. Additionally, the economic burden of treatment can significantly affect adherence, particularly when the costs are high. Therefore, it is essential to factor in the patient’s immune status, comorbidities, and financial situation when developing a treatment strategy. Regular monitoring of disease activity and health status is also crucial to ensure timely adjustments to the treatment plan, maximizing efficacy while minimizing adverse effects (Ebina et al., 2019). With its low infection risk and long drug retention period, abatacept provides a safe and effective treatment option for elderly EORA patients, particularly those with compromised immune systems and a higher risk of infections. This positions it as one of the most clinically valuable therapeutic options for this patient population.
3.7 B-cell depletion therapy
3.7.1 Rituximab
Rituximab is a monoclonal antibody that targets CD20 on B cells, leading to their depletion and a subsequent reduction in antibody-mediated immune responses. This mechanism makes rituximab a particularly effective treatment option for EORA patients, especially those who have failed to respond to other biologic therapies. By inhibiting B cell-driven immune responses, rituximab not only controls disease activity but also helps modulate the overall immune system, offering a valuable alternative for EORA patients who are refractory to other treatments. While rituximab may not be as effective as certain other biologics, such as tocilizumab, in improving specific biomarkers like PON1 activity, it has demonstrated significant benefits in controlling disease activity and alleviating immune-mediated inflammation. Clinical studies have shown that rituximab can effectively improve Disease Activity Score (DAS28-CRP) and Clinical Disease Activity Index (CDAI) scores, with some patients achieving long-term remission. These improvements are reflected in reduced joint inflammation, lower disease activity, and improved quality of life for patients (Razmjou et al., 2024). However, rituximab use carries significant safety risks, particularly regarding infections. It is crucial to closely monitor for early signs of bacterial and viral infections during treatment, with timely prophylactic measures being essential. Additionally, rituximab’s immunosuppressive effects may result in infusion-related reactions and other long-term adverse events related to immune suppression, including an increased risk of malignancies (see Table 1). Severe infections or poor drug tolerance are the primary reasons for discontinuation, underscoring the need for individualized patient management, particularly during the initiation and maintenance phases of treatment. In EORA patients, who often have naturally declining immune function, special attention should be given to monitoring immune status and infection risks to ensure the safety of the treatment. Despite the safety concerns, rituximab’s ability to control disease activity and reduce immune-mediated inflammation makes it an effective treatment option for many EORA patients, especially those who have not responded to other biologics (Pappas et al., 2014). To optimize its therapeutic benefits and minimize risks, clinical management should emphasize close monitoring, timely interventions, and careful patient selection. This approach will help ensure the most effective and safe use of rituximab in the treatment of EORA.
4 Clinical translation challenges of novel biologic therapies and the integration of precision medicine
Although novel biologic agents have demonstrated promising efficacy in clinical trials, their clinical translation faces several significant challenges. One of the most critical hurdles is integration of precision medicine into routine clinical practice, particularly in addressing patient heterogeneity, genetic backgrounds, and complex comorbidities. As the demand for personalized treatments grows, future therapeutic strategies will increasingly depend on selecting biologic agents based on a patient’s genetic profile, immune status, and disease characteristics, with the goal of achieving better and more predictable treatment responses. Precision medicine not only broadens the options for treating EORA but also lays the foundation for developing individualized treatment approaches. By combining genomics, artificial intelligence (AI), and clinical data, future treatments will be more personalized and accurate, allowing for better prediction of patient responses to therapies and potential adverse events. For example, AI models can incorporate a patient’s genetic information to predict the risks of complications such as thrombosis or infections, enabling timely adjustments to the treatment plan. This approach has the potential to significantly enhance treatment efficacy and improve patients’ quality of life (Mertz et al., 2023; Topol, 2019). However, despite the theoretical promise of novel biologic agents and precision medicine, high costs and technological barriers remain significant obstacles to their widespread application. The high cost of developing and deploying these biologic therapies, especially in low- and middle-income countries, limits access to these treatments. As a result, future research must focus on reducing the economic burden of these therapies, while promoting technological innovations to enhance accessibility, allowing more patients to benefit from these advances. In conclusion, technological innovation, supportive policies, and the ongoing development of precision medicine have the potential to improve the long-term prognosis of EORA patients and enhance their quality of life through the clinical use of novel biologic agents and personalized treatment strategies. Additionally, future treatments will focus not only on controlling disease but also on delivering personalized health management tailored to each patient’s needs.
5 Conclusion
As the global population ages, EORA has become a major focus in rheumatoid arthritis (RA) research. Recent advances in biologic therapies and precision medicine have significantly improved treatment options for EORA patients, especially those who fail to respond to traditional therapies. Novel biologic agents, such as IL-17 inhibitors, IL-23 inhibitors, second-generation JAK inhibitors, and B-cell depletion therapies, have shown strong efficacy in modulating immune responses. These treatments not only reduce disease activity and systemic inflammation but also mitigate long-term risks of infections, thrombosis, and malignancies.The integration of precision medicine has further advanced the treatment of EORA. By leveraging genetic profiling and artificial intelligence (AI)-assisted decision-making, clinicians can now tailor treatment plans to the unique needs of individual patients, enhancing both efficacy and safety. The identification of genetic markers like HLA-DRB1*04 and AI-based drug response predictions provides a solid foundation for personalized therapies. Despite the promising potential of novel biologic therapies and precision medicine, their widespread adoption faces significant challenges, particularly in low- and middle-income countries. Future research should focus on long-term safety, further validation of efficacy, and strategies to reduce treatment costs, ensuring broader patient access. With ongoing technological innovations and policy support, individualized treatment strategies have the potential to greatly improve long-term prognosis and quality of life for EORA patients. In summary, the combination of emerging biologic therapies and precision medicine provides an optimistic outlook for treating EORA. In the future, research should focus on overcoming the challenges of large-scale implementation, optimizing treatment protocols, and further improving patient outcomes and quality of life.
Author contributions
YJL: Conceptualization, Investigation, Writing–original draft, Writing–review and editing. YJL: Writing–original draft, Resources. YT: Writing–original draft, Visualization. HG: Resources, Writing–review and editing. QM: Methodology, Resources, Writing–review and editing. JC: Writing–review and editing, Validation. JM: Conceptualization, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by: 1. The National Natural Science Foundation of China (No. 81804050). 2. The Joint Fund of the Henan Provincial Science and Technology Research and Development Program (Project for the Cultivation of Advantageous Disciplines) (No. 232301420079). 3. The Henan Traditional Chinese Medicine Discipline Top Talent Program (豫卫中医〔2021〕15号). 4. The 2024 Henan Province Natural Science Foundation (General Program) (No. 242300420110), for the study of the mechanism of Guizhi Shaoyao Zhimu Decoction in regulating the pathogenesis of rheumatoid arthritis by intervening in the gut microbiota and/or intestinal barrier function based on the “Gut-Joint” axis. 5. The Rheumatology Youth Cultivation Program (No. 202327-009) of the Chinese Association of Traditional Chinese Medicine.
Acknowledgments
The authors would like to acknowledge the support of Henan University of Chinese Medicine and Henan Provincial Hospital of Traditional Chinese Medicine throughout the research process. Special thanks to Professor MA JUNFU for their insightful feedback on the manuscript and to GU HUIMIN for their assistance with data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baghdadi, L. R., Woodman, R. J., Shanahan, E. M., and Mangoni, A. A. (2015). The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PloS one 10 (2), e0117952. doi:10.1371/journal.pone.0117952
Chabaud, M., Durand, J. M., Buchs, N., Fossiez, F., Page, G., Frappart, L., et al. (1999). Human interleukin-17: AT cell–derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis and Rheumatism Official J. Am. Coll. Rheumatology 42 (5), 963–970. doi:10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E
Choi, S. R., Shin, A., Ha, Y. J., Lee, Y. J., Lee, E. B., and Kang, E. H. (2023). Comparative risk of infections between JAK inhibitors versus TNF inhibitors among patients with rheumatoid arthritis: a cohort study. Arthritis Res. and Ther. 25 (1), 129. doi:10.1186/s13075-023-03111-w
Cometi, L., Bruni, C., Passavanti, S., Tofani, L., Bartoli, F., Fiori, G., et al. (2020). Risk of malignancy and biologic therapy in rheumatic inflammatory diseases: a single-center experience. Rheumatology Immunol. Res. 1 (1), 39–45. doi:10.2478/rir-2020-0001
Dalal, D. S., Duran, J., Brar, T., Alqadi, R., Halladay, C., Lakhani, A., et al. (2019). Efficacy and safety of biological agents in the older rheumatoid arthritis patients compared to Young: a systematic review and meta-analysis. Seminars arthritis rheumatism 48 (5), 799–807. doi:10.1016/j.semarthrit.2018.07.009
D'Arcy, M. E., Beachler, D. C., Pfeiffer, R. M., Curtis, J. R., Mariette, X., Seror, R., et al. (2021). Tumor necrosis factor inhibitors and the risk of cancer among older Americans with rheumatoid arthritis. Cancer Epidemiol. Biomarkers and Prev. 30 (11), 2059–2067. doi:10.1158/1055-9965.epi-21-0125
Duran, E., Unaldi, E., Bilgin, E., Bolek, E. C., Yardimci, G. K., Farisogullari, B., et al. (2022). AB0416 Cardiovascular event, venous thromboembolizm, and infection risk with tofacitinib in rheumatoid arthritis patients aged≥ 60 years. Ann. Rheumatic Dis. 81 (Suppl. 1), 1336.2–1336. doi:10.1136/annrheumdis-2022-eular.4309
Ebina, K., Hashimoto, M., Yamamoto, W., Hirano, T., Hara, R., Katayama, M., et al. (2019). Drug tolerability and reasons for discontinuation of seven biologics in elderly patients with rheumatoid arthritis-The ANSWER cohort study. PLoS One 14 (5), e0216624. doi:10.1371/journal.pone.0216624
Filippini, M., Bazzani, C., Favalli, E. G., Marchesoni, A., Atzeni, F., Sarzi-Puttini, P., et al. (2010). Efficacy and safety of anti-tumour necrosis factor in elderly patients with rheumatoid arthritis: an observational study. Clin. Rev. allergy and Immunol. 38, 90–96. doi:10.1007/s12016-009-8142-1
Fleischmann, R., Pangan, A. L., Song, I. H., Mysler, E., Bessette, L., Peterfy, C., et al. (2019). Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis and rheumatology 71 (11), 1788–1800. doi:10.1002/art.41032
Genovese, M. C., Kremer, J., Zamani, O., Ludivico, C., Krogulec, M., Xie, L., et al. (2016). Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374 (13), 1243–1252. doi:10.1056/NEJMoa1507247
Genovese, M. C., Van den Bosch, F., Roberson, S. A., Bojin, S., Biagini, I. M., Ryan, P., et al. (2010). LY2439821, a humanized anti–interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis and Rheumatism 62 (4), 929–939. doi:10.1002/art.27334
Gossec, L., Kerschbaumer, A., Ferreira, R. J. O., Aletaha, D., Baraliakos, X., Bertheussen, H., et al. (2024). EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. rheumatic Dis. 83 (6), 706–719. doi:10.1136/ard-2024-225531
Jiang, Z., Zou, Y., Li, G., Zhao, S., and Zhang, W. (2024). Comparisons of infection events associated with tumor necrosis factor inhibitors in patients with inflammatory arthritis: a systematic review and network meta-analysis. Front. Pharmacol. 15, 1376262. doi:10.3389/fphar.2024.1376262
Jin, S., Li, M., Fang, Y., Li, Q., Liu, Ju., Duan, X., et al. (2017). Chinese Registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Res. and Ther. 19 (1), 251. doi:10.1186/s13075-017-1457-z
Keystone, E. C., Taylor, P. C., Drescher, E., Schlichting, D. E., Beattie, S. D., Berclaz, P. Y., et al. (2015). Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann. rheumatic Dis. 74 (2), 333–340. doi:10.1136/annrheumdis-2014-206478
Kiełbowski, K., Plewa, P., Bratborska, A. W., Bakinowska, E., and Pawlik, A. (2024). JAK inhibitors in rheumatoid arthritis: immunomodulatory properties and clinical efficacy. Int. J. Mol. Sci. 25 (15), 8327. doi:10.3390/ijms25158327
Kondo, N., Fujisawa, J., and Endo, N. (2020). Subcutaneous tocilizumab is effective for treatment of elderly-onset rheumatoid arthritis. Tohoku J. Exp. Med. 251 (1), 9–18. doi:10.1620/tjem.251.9
Kunwar, S., Dahal, K., and Sharma, S. (2016). Anti-IL-17 therapy in treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatol. Int. 36, 1065–1075. doi:10.1007/s00296-016-3480-9
Liu, T., Meng, W., Wang, W., Sun, G., Chen, Xi, Lu, Y., et al. (2024). A cross-sectional study of predictive factors of health literacy among rheumatoid arthritis patients in China. Front. Psychol. 15, 1390442. doi:10.3389/fpsyg.2024.1390442
Manfredi, A., Fornaro, M., Bazzani, C., Perniola, S., Cauli, A., Rai, A., et al. (2024). Retention rate of biologic and targeted synthetic anti-rheumatic drugs in elderly rheumatoid arthritis patients: data from GISEA registry. Front. Med. 11, 1349533. doi:10.3389/fmed.2024.1349533
Mertz, P., Wollenschlaeger, C., Chasset, F., Dima, A., and Arnaud, L. (2023). Rheumatoid vasculitis in 2023: changes and challenges since the biologics era. Autoimmun. Rev. 22, 103391. doi:10.1016/j.autrev.2023.103391
Nawrot, J., Boonen, A., Peeters, R., Starmans, M., and van Onna, M. (2018). Rheumatologists’ views and experiences in managing rheumatoid arthritis in elderly patients: a qualitative study. J. Rheumatology 45 (5), 590–594. doi:10.3899/jrheum.170773
Pappas, D. A., Kremer, J. M., Reed, G., Greenberg, J. D., and Curtis, J. R. (2014). Design characteristics of the CORRONA CERTAIN study: a comparative effectiveness study of biologic agents for rheumatoid arthritis patients. BMC Musculoskelet. Disord. 15, 113. doi:10.1186/1471-2474-15-113
Pastor-Fernández, G., Mariblanca, I. R., and Navarro, M. N. (2020). Decoding IL-23 signaling cascade for new therapeutic opportunities. Cells 9 (9), 2044. doi:10.3390/cells9092044
Pavlov-Dolijanovic, S., Bogojevic, M., Nozica-Radulovic, T., Radunovic, G., and Mujovic, N. (2023). Elderly-onset rheumatoid arthritis: characteristics and treatment options. Medicina 59 (10), 1878. doi:10.3390/medicina59101878
Rasch, E. K., Hirsch, R., Paulose-Ram, R., and Hochberg, M. C. (2003). Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis and Rheumatism Official J. Am. Coll. Rheumatology 48 (4), 917–926. doi:10.1002/art.10897
Razmjou, A. A., Kremer, J. M., Pappas, D. A., Curtis, J. R., Wang, J., Shahbazian, A., et al. (2024). Disease response in rheumatoid arthritis across four biologic therapies associates with improvement in paraoxonase-1 activity and oxylipins. RMD open 10 (4), e004829. doi:10.1136/rmdopen-2024-004829
Sato, K., Suematsu, A., Okamoto, K., Yamaguchi, A., Morishita, Y., Kadono, Y., et al. (2006). Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203 (12), 2673–2682. doi:10.1084/jem.20061775
Sato, S., Matsumoto, H., Temmoku, J., Fujita, Y., Matsuoka, N., Yashiro-Furuya, M., et al. (2021). Sustained long-term retention rates of abatacept in combination with conventional synthetic disease-modifying antirheumatic drugs in elderly patients with rheumatoid arthritis. Med. Kaunas. Lith. 57 (9), 914. doi:10.3390/medicina57090914
Serhal, L., Lwin, M. N., Holroyd, C., and Edwards, C. J. (2020). Rheumatoid arthritis in the elderly: characteristics and treatment considerations. Autoimmun. Rev. 19 (6), 102528. doi:10.1016/j.autrev.2020.102528
Shih, P. C., Hung, P. C., Leong, P. Y., Hsu, J. N., Yang, C. C., Wei, J. C. C., et al. (2024). Incidence and risk factors of discontinuation of tofacitinib and biologic disease-modifying anti-rheumatic drugs among patients with rheumatoid arthritis: a population-based cohort study. Clin. Rheumatol. 43, 3625–3637. doi:10.1007/s10067-024-07161-6
Smolen, J. S., Pangan, A. L., Emery, P., Rigby, W., Tanaka, Y., Vargas, J. I., et al. (2019). Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 393 (10188), 2303–2311. doi:10.1016/S0140-6736(19)30419-2
Specker, C., Aringer, M., Burmester, G. R., Killy, B., Hofmann, M. W., Kellner, H., et al. (2022). The safety and effectiveness of tocilizumab in elderly patients with rheumatoid arthritis and in patients with comorbidities associated with age. Clin. Exp. Rheumatol. 40 (9), 1657–1665. doi:10.55563/clinexprheumatol/f7ff6q
Strand, V., Tundia, N., Wells, A., Buch, M. H., Radominski, S. C., Camp, H. S., et al. (2021). Upadacitinib monotherapy improves patient-reported outcomes in rheumatoid arthritis: results from SELECT-EARLY and SELECT-MONOTHERAPY. Rheumatology 60 (7), 3209–3221. doi:10.1093/rheumatology/keaa770
Sugihara, T. (2022). Treatment strategies for elderly-onset rheumatoid arthritis in the new era. Mod. Rheumatol. 32 (3), 493–499. doi:10.1093/mr/roab087
Tan, T. C., Gao, X., Thong, B. Y.-H., Leong, K. P., Lian, T. Y., Law, W. G., et al. (2016). Comparison of elderly- and young-onset rheumatoid arthritis in an Asian cohort. Int. J. rheumatic Dis. 20 (6), 737–745. doi:10.1111/1756-185X.12861
Targońska-Stępniak, B., Grzechnik, K., Kolarz, K., Gągoł, D., and Majdan, M. (2021). Systemic inflammatory parameters in patients with elderly-onset rheumatoid arthritis (EORA) and young-onset rheumatoid arthritis (YORA)-An observational study. J. Clin. Med. 10 (6), 1204. doi:10.3390/jcm10061204
Taylor, P. C., Takeuchi, T., Burmester, G. R., Durez, P., Smolen, J. S., Deberdt, W., et al. (2022). Safety of baricitinib for the treatment ofrheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann. rheumatic Dis. 81 (3), 335–343. doi:10.1136/annrheumdis-2021-221276
Topol, E. J. (2019). High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25 (1), 44–56. doi:10.1038/s41591-018-0300-7
Turesson, C., Turesson, C., Bergström, U., Bergström, U., and Pikwer, M. (2015). High serum cholesterol predicts rheumatoid arthritis in women, but not in men: a prospective study. Arthritis Res. and Ther. 17, 284. doi:10.1186/s13075-015-0804-1
Veerasubramanian, P. K., Wynn, T. A., Quan, J., and Karlsson, F. J. (2024). Targeting TNF/TNFR superfamilies in immune-mediated inflammatory diseases. J. Exp. Med. 221 (11), e20240806. doi:10.1084/jem.20240806
Vicente, G. N. S., Pereira, I. A., de, C., da, M., Licia, M. H., Carnieletto, A. P., et al. (2021). Cardiovascular risk comorbidities in rheumatoid arthritis patients and the use of anti-rheumatic drugs: a cross-sectional real-life study. Adv. rheumatology 61 (1), 38. doi:10.1186/s42358-021-00186-4
Virtanen, A., Spinelli, F. R., Telliez, J. B., O'Shea, J. J., Silvennoinen, O., and Gadina, M. (2024). JAK inhibitor selectivity: new opportunities, better drugs? Nat. Rev. Rheumatol. 20, 649–665. doi:10.1038/s41584-024-01153-1
Wang, X., Yang, J., Yu, L. Y., Zhang, J., Zhang, X., and Shen, H. L. (2024). Effect of disease duration on the use of tofacitinib: a real-world study in elderly patients with rheumatoid arthritis. Clin. Rheumatol. 43 (9), 2807–2815. doi:10.1007/s10067-024-07084-2
Wu, D., Hou, S. Y., Zhao, S., Hou, L. X., Jiao, T., Xu, N. N., et al. (2019). Meta-analysis of IL-17 inhibitors in two populations of rheumatoid arthritis patients: biologic-naive or tumor necrosis factor inhibitor inadequate responders. Clin. Rheumatol. 38, 2747–2756. doi:10.1007/s10067-019-04608-z
Yoshii, I., Chijiwa, T., and Sawada, N. (2020). Efficacy and safety of targeted strategy for treating rheumatoid arthritis patients aged 75 Years or older. Tohoku J. Exp. Med. 250 (1), 13–23. doi:10.1620/tjem.250.13
Keywords: elderly-onset rheumatoid arthritis, biologic agents, infection, individualized treatment, review
Citation: Li Y, Liu Y, Tian Y, Gu H, Meng Q, Cui J and Ma J (2025) The research progress of biologics in elderly-onset rheumatoid arthritis (EORA). Front. Aging 5:1511812. doi: 10.3389/fragi.2024.1511812
Received: 15 October 2024; Accepted: 04 December 2024;
Published: 23 January 2025.
Edited by:
Xin Zhang, School of Medicine, Duke University, United StatesReviewed by:
Yuzhou Gan, Peking University People’s Hospital, ChinaJianan Zhao, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2025 Li, Liu, Tian, Gu, Meng, Cui and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junfu Ma, emhvbmdndW9kZWZ1d2FAMTYzLmNvbQ==
 Yujie Li
Yujie Li Yifan Liu1
Yifan Liu1 Junfu Ma
Junfu Ma