- 1Department of Microbiology Immunology and Parasitology, UNIFESP Federal University of São Paulo, São Paulo, Brazil
- 2Department of Immunology, University of Miami, Miami, FL, United States
Ageing has been associated with comorbidities, systemic low-grade of inflammation, and immunosenescence. Hypertension is the most common morbidity and anti-hypertensives are used for more than 50%. Angiotensin-converting enzyme 1 inhibitors (ACEi) and angiotensin II receptor blockers (ARB) control blood pressure but also seem to play a role in comorbidities such as Alzheimer’s disease, sarcopenia and cancer. The impact of anti-hypertensives in comorbidities is due to the expression of renin-angiotensin system (RAS) in several tissues and body fluids. Angiotensin-converting enzyme 1 (ACE1) has been linked to oxidative stress, metabolism, and inflammation. The levels and activity of ACE1 are under genetic control and polymorphisms have been correlated with susceptibility to Alzheimer’s disease. In addition, some results found that ACEi and ARB users present delayed cognitive decline and reduced risk of dementia. Regarding to sarcopenia, RAS has been linked to the catabolic and anabolic pathways for muscle mass maintenance. In some studies, older adults using ACEi were highly benefited by exercise training. In cancer, RAS and its products have been shown to play a role since their inhibition in animal models modulates tumor microenvironment and improves the delivery of chemotherapy drugs. Clinically, the incidence of colorectal cancer is reduced in patients using ACEi and ARB. During the pandemic COVID-19 it was found that ACE2 receptor plays a role in the entry of SARS-CoV-2 into the host cell. ACE1 genotypes have been linked to an increased risk for COVID-19 and severe disease. In some studies COVID-19 patients taking ARB or ACEi presented better outcome.
Introduction
Ageing is a complex process which has been associated with comorbidities, systemic low-grade of inflammation, and changes in the frequency/function of immune cells (immunosenescence) (Bueno et al., 2014; Alves et al., 2018; Alves and Bueno, 2019; Pawelec et al., 2021). A comorbidity with high impact in older individuals is hypertension which can affect 27% of individuals younger than 60 years and 74% of older adults with more than 80 years (Lloyd-Jones et al., 2005). Hypertension in the older adults has been linked to an increased risk of ischemic and hemorrhagic strokes, vascular dementia, Alzheimer’s disease, coronary artery disease, atrial fibrillation, chronic kidney disease and retinal diseases (Perry et al., 2000; Vaccarino et al., 2000; Bulpitt et al., 2003; Rosendorff et al., 2007). Non-pharmacological approaches includes healthy diet, physical activity, non-smoking, avoidance of high intake of alcohol, among others. However, pharmacological interventions can be required, and the benefits on cardiovascular outcomes can be reached by thiazide diuretics, angiotensin-converting enzyme inhibitor (ACEi), angiotensin II receptor blockers (ARB), and calcium channel blocker (CCB). [reviewed in (Oliveros et al., 2020)].
In this mini-review it is not our goal to discuss hypertension and its treatment, since there are excellent articles on the field (Pont and Alhawassi, 2017; Oliveros et al., 2020; Li et al., 2022) and the topic itself would require a new whole article. Instead, our aim is to discuss angiotensin-converting enzyme 1 (ACE1) expression and the possible link with age-related diseases such as Alzheimer’s disease, sarcopenia, cancer, and COVID-19. In addition, we will describe findings on how ACE1 inhibition/blockade can interfere with the outcome of older patients from these conditions. However, it has to be pointed that the benefits observed by the use of anti-hypertensives in some age-related diseases can be linked, at least in part, to the control of high blood pressure.
The angiotensin-converting enzyme 1 (ACE1) is a dipeptidyl carboxylase which can be inhibited leading thus to the reduction of blood pressure via the renin-angiotensin system (RAS). In a summary definition, RAS is composed by a vasoconstrictor, pro-inflammatory ACE1/AngII/AT1R axis, and a vasodilating anti-inflammatory ACE2/Ang1-7/MasR axis. In addition to the blood pressure control, ACE1 and its peptide substrates affect cardiovascular and renal function, hematopoiesis, reproduction, and the immunity (Bernstein et al., 2012; Bernardi et al., 2016). ACE1 expression has been observed not only in tissues since its soluble form was found in urine, serum, seminal fluid, amniotic fluid, and cerebrospinal fluid (Hooper, 1991). Because of its wide expression in several tissues and body fluids, ACE1 has been associated to some diseases development/progression.
Recently it has been shown, mainly in experimental models, that ACE1 can interfere with several processes in the organism through mechanisms such as oxidative stress, metabolism, and inflammation (de Cavanagh et al., 1999; de Cavanagh et al., 2000; Engeli et al., 2000; de Cavanagh et al., 2001; Godsel et al., 2003; Kuno et al., 2003; Muller et al., 2003). It has also been suggested that ACE1 influences age-related diseases (i.e., Alzheimer’s disease—AD, sarcopenia, cancer) (Hu et al., 1999; Kehoe et al., 1999; Carl-McGrath et al., 2004; Yoshihara et al., 2009; Zhang et al., 2019; MacLachlan et al., 2022). ACE1 levels are under genetic control and many studies have focused on insertions and deletions polymorphisms in intron 16 of the ACE gene as a marker for a functional polymorphism. Many single nucleotide polymorphisms have been detected in the ACE1 gene in recent years and the search for the locations of functional polymorphisms currently represents a topic of extensive investigation. For example, it has been shown that ACE1 polymorphisms are correlated with susceptibility to AD (Hu et al., 1999; Kehoe et al., 1999) but the associated mechanisms are still poorly understood. In addition, it was found recently that in normal ageing, ACE1 expression is increased in brain homogenates and this expression is unchanged in the early stages of AD (MacLachlan et al., 2022) Regarding sarcopenia, Yoshihara et al. (2009) found a weak correlation between ACE1 polymorphism and physical function in aged individuals. In cancer (gastric or colorectal), the same patient presented higher expression of ACE1 in tumor microenvironment than in healthy tissues (Carl-McGrath et al., 2004; Zhang et al., 2019).
In the immune system, our group found that ACE1 (CD143) is expressed not only in all subsets of CD4+ and CD8+ T cells (naive, central memory, effector memory, and effector memory re-expressing RA), but also in B cells (naive, unswitched memory, switched memory, double negative) and in myeloid cells from PBMC of ageing individuals (Bueno et al., 2022). Within the immune system, ACE1 has been shown to have both beneficial and detrimental effects, due to its role in signalling pathways (Gonzalez-Villalobos et al., 2013). In older adults with significant hypertension it was found that ACE1 inhibitors (ACEi) interfere with cytokines secretion by stimulated T cells in culture (Shasha et al., 1991). In PBMC from controls, it was also observed that ACEi in culture suppress the production of cytokines by monocytes and T cells, confirming the role of ACE in inflammatory signalling pathways (Constantinescu et al., 1998). These findings suggest a role played by ACE1 in the age-related chronic diseases via inflammation, but the associated mechanisms are not completely understood yet. It is important to know that the effects of the treatment of individuals with age-associated conditions and/or diseases with ACEi is strictly dose-, host-, and disease-dependent.
SARS-CoV-2 has been the cause of more than 6 million of deaths worldwide (WHO, 2023) and as the first step for the entry into the host cell the virus binds the human angiotensin-converting enzyme (ACE)2 receptor. Moreover, polymorphisms of ACE1/ACE2 and inhibition/blockade of ACE1 have been associated with patient outcome from COVID-19. Therefore, considering the major impact of infections on aged adults, mainly in those with chronic diseases this mini-review will focus on ACE1 and ACEi and their possible correlation with important age-related conditions such as Alzheimer’s disease, sarcopenia, cancer and SARS-CoV-2 infection. Figure 1.
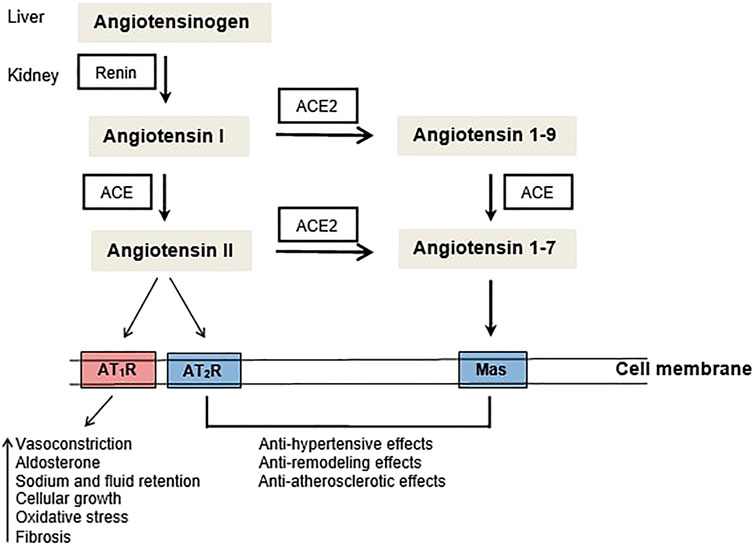
FIGURE 1. The renin-angiotensin system (RAS): ACE - angiotensin converting enzyme, AT1R - Angiotensin II type 1 receptor, AT2R - Angiotensin II type 2 receptor.
ACE1 and cognition/dementia/Alzheimer’s disease
The extension of life expectancy has been related to an increase in cases of dementia and loss of cognitive functions. The most common type of dementia in older adults is AD which has been characterized by reduced synaptic strength, synaptic loss, and neurodegeneration. The hallmark of this disease is the accumulation of senile plaques and neurofibrillary tangles that are respectively associated with changes in the cleavage in amyloid precursor protein (APP)/production of the beta-amyloid (Aβ) and tau protein hyperphosphorylation (Sengoku, 2020). However, it has been proposed that AD clinical symptoms cannot be explained only by the neuropathology of abnormal protein accumulation. As the AD pathogenesis is not completely understood, the current treatments lead to a modest effect in cognition, and therefore it is desirable to develop therapies that can modify the disease development and progress (Cummings et al., 2019). A potential target is the RAS which has been suggested to play a role in AD. ACE inhibitors (ACEi) or blockers of Angiotensin II receptors (ARB) have been shown to delay the cognitive decline and reduce the risk of dementia [reviewed in (Loera-Valencia et al., 2021)].
Yasar et al. (2018) found that cognitively normal older adults (mean age 67.8 years) with elevated ACE1 serum levels at the study entry, presented a worse 1 year evolution in processing speed and work memory suggesting a role played by RAS in neurodegenerative diseases. This hypothesis is reinforced by findings of circulating AngII in regions without blood brain barrier (BBB) and the expression of RAS components in brain. Moreover, ACE1 (acts in the generation of AngII) is expressed in the frontal and temporal lobe and its expression is altered in patients with AD (Arregui et al., 1982; Savaskan et al., 2001; Miners et al., 2008). In opposition, MacLachlan et al. found that in normal ageing, human brain homogenates presented increase of ACE1 and AngII protein levels and decrease of ACE1 activity whereas in early stages of AD it was observed increased ACE1 activity and unchanged ACE1 and AngII protein levels (MacLachlan et al., 2022). In post-mortem brain of older adults (78.5 years) it was found that ACE1 activity correlated inversely with ACE2 activity in AD. Brain homogenates displayed significantly reduced activity of ACE2 which was related to the disease stage. ACE2 activity correlated inversely with total insoluble amyloid β (Aβ) levels, β-secretase activity and protein tau (ptau) load. Authors suggested that the imbalance between classical ACE1 axis and the regulatory ACE2 axis of RAS plays a role in AD pathogenesis (Kehoe et al., 2016). In older adults with memory complaints or AD and no anti-hypertensive treatment, it was found that ACE1 levels and activity in cerebral spinal fluid (CSF) and serum were reduced in patients with AD. Lower ACE1 in CSF correlated with lower CSF Aβ levels, indicating more brain pathology. Reduced ACE1 in CSF was also linked to lower CSF tau and ptau levels (Jochemsen et al., 2014).
Regarding patients using anti-hypertensives, Yasar et al. (2008) evaluated women (70–80 years) for 9 years and observed that the use of ACEi was associated with 59%–85% lower risk of impairment in speed of processing, executive functioning, and verbal memory. In another study, the use of ACEi during 3 years of follow-up showed that anti-hypertensives with BBB crossing status were associated with 65% less decline in instrumental activities of daily living per year compared to other anti-hypertensive drugs. Anti-hypertensives with no BBB crossing status were associated with increased risk of dementia by 20% per year of medicament exposure (Sink et al., 2009). In cognitively normal hypertension-treated older adults (65–84 years and follow-up of 3.5 years) the use of BBB crossing ACEi was associated with a reduced risk of mild cognitive impairment (Solfrizzi et al., 2013). In a meta-analysis (14 cohorts/6 countries) with a total of 12,849 (50–90 years) individuals it was shown that anti-hypertensive BBB crossing drugs were associated with a better memory recall over a follow-up of 3 years (Ho et al., 2021). Fazal et al. (2017) evaluated hypertensive patients using at the time of Alzheimer’s diagnostic centrally acting ACEi (C-ACEi, n = 1,207), non-centrally acting ACEi (NC-ACEi, n = 143) and 3,910 using neither. Improved cognition was observed in patients using C-ACEi over the first 9 months after diagnosis in comparison with NC-ACEi. Long-term differences were not found in cognition and survival between the groups.
In conclusion, anti-hypertensive medications reduce the risk of cognitive impairment but it remains unclear whether the benefits are reached via blood pressure control alone or via action on the RAS components. However, Soto et al. (2013) found in patients (mean age 77.8 years) with AD and using ACEi that the slow rate of cognitive decline over 4 years of follow-up was independent of hypertension at baseline or developed subsequently. In addition, human cortical neuron cell line cultured with Losartan displayed decline in the activation of tau kinases, production of p-tau and reactive oxygen species (De Dios et al., 2022) suggesting reduction of neurotoxicity and neuron death which are causes of dementia.
ACE1 and sarcopenia/exercise
Essential daily activities can be impaired in older adults because of physical limitations. During the aging process muscle mass can be reduced and the remaining muscle may present reduced quality. Sarcomeric proteins, collagen, fibers and myocites relies on the balance between catabolic and anabolic pathways for muscle mass maintenance. In addition, the replacement of muscle fibers by fibroblasts, extracellular matrix proteins, and conective tissue caracterizes the histological quality and quantity of the muscle. The renin-angiotensin system (RAS) modulates all these processes via protein turnover, cellular apoptosis, and collagen metabolism (Song et al., 2005; Burks et al., 2011; Cabello-Verrugio et al., 2011; Cabello-Verrugio et al., 2015).
Decreased physical function in the ageing population has been related with disability, loss of independence, higher risk of cardiovascular morbidity and mortality (Newman et al., 2006; Shaw et al., 2006; Studenski et al., 2011). In addition, chronic diseases (i.e., hypertension) could exacerbate the process of sarcopenia in aged individuals (Brown et al., 2000; Nelson et al., 2004). In hypertensive middle-aged patients and also in healthy ageing males it was shown an inverse correlation between blood pressure and ACE1 activity in vastus lateralis muscle suggesting that ACE1 levels in muscle are influenced by hemodynamic factors (Reneland et al., 1999). However, in spite of ACE1 activity identification in vastus lateralis muscle, no correlation was found between these measurements and age (Reneland and Lithell, 1994). The evaluation of ACE1-mRNA transcripts in vastus lateralis muscle by Schaufelberger et al. (1998) found no difference in ACE1-mRNA transcripts between hypertensive patients and control individuals nor differences in ACE1 gene expression was observed due to ACEi treatment. More recently it has been suggested that ACE1 inhibitors (ACEi) could improve physical function not only via regulation of blood pressure but also through direct effects on body composition and secreted factors (Carter et al., 2005). In older adults the muscle wasting linked to AngII is due to the decrease of the growth factor 1 (IGF-1) and since the use of ACEi was capable to elevate the levels of IGF-1, it has been suggested a beneficial role played by ACEi (Song et al., 2005; Giovannini et al., 2010). Buford et al. (2012) evaluated older adults (70–89 years old) with mild to moderate functional deficits and found that ACEi users were highly benefited by 12 months exercise training than non-users. In opposition, Sumukadas et al. (2014) observed that in functionally impaired older individuals (n = 170, mean age-75 years), ACEi did not enhance the effect of 20 weeks exercise training. In agreement, Kostka et al. (2021) found that even though older patients (79–82 years old) not taking ACEi presented a negative correlation between muscle power/muscle contraction and ACE activity, patients taking ACEi showed no consistent association with handgrip strength, muscle power, muscle contraction velocity, and functional performance. Witham et al. (2014) followed 639 individuals (mean age 65 years) during 4.4 years and observed no difference in grip strength change per year in ACEi users. However, in patients with knee osteoarthritis, a study of 8 years follow-up of 4,295 individuals (mean age 61.2 years) showed that ACEi use (12.8% of participants) was correlated with a reduced risk of frailty (Veronese et al., 2019). Preliminary findings have also indicated beneficial effects of other RAS inhibitors such as ARBs and renin inhibitors, mostly because of their inhibitory effects on local inflammation and oxidative stress.
Sarcopenia diagnostic is complex and should consider not only muscle loss and impaired function, but also how the age-related decline in cognition affects for example grip strength and gait speed. The results from ACEi effects on muscle mass and function are controversial and are probably linked to differences in the enrolled populations, parameters used to assess muscle mass and strength, period of intervention and follow-up, anti-hypertensive used and protocols of physical activity. Further studies are required to support the evidence of ACEi and ARB beneficial effect in preventing sarcopenia.
ACE1 and cancer
Cancer is highly incident in ageing individuals, and considering that age is a risk factor for hypertension and requires the use of anti-hypertensive drugs, these pharmacological treatments have been studied to determine whether they interfere with cancer development and progression (Ribatti et al., 2007).
Renin-angiotensin system (RAS) and its products have been linked to tumor growth since AT2, the active fraction derived from angiotensin activity is associated to cellular growth, angiogenesis induction, and activation of AT1R receptor. Intracellular signalling pathways of AT1R are linked to fibroblasts growth factor, epidermal growth factor, tumor growth factor (TGF-) beta, platelets-derived growth factor, nitric oxide synthase, protein kinase C, angiopoiethin 2, and metalloprotease [reviewed in (George et al., 2010)]. Experimental studies have linked the RAS to cancer development. In addition, ACEi or ARBs inhibit experimental tumor growth [reviewed in (George et al., 2010)]. Although the antitumoral mechanism associated with ACEi, and ARBs has not been elucidated yet, it has been proposed a role for Ang II-dependent signalling in inhibiting angiogenesis, inflammation, and cellular proliferation (Escobar et al., 2004; Hillers-Ziemer et al., 2022). Inhibition of the RAS pathway, indeed, actively modulates the tumor microenvironment, modifies the tumor stroma, reduces the stiffness of the matrix and ultimately improves the delivery of chemotherapeutic drugs, suggesting that ACEi and ARBs could provide a complementary treatment in cancer patients. A change in the immune milieu, and in the modulation of macrophages, CD8+ T cells and T regulatory cells has also been reported (Escobar et al., 2004; Hillers-Ziemer et al., 2022).
In human lung adenocarcinoma, it was observed that genes encoding for ACE and for the angiotensin II receptor were repressed whereas genes encoding for angiotensinogen were overexpressed (Goldstein et al., 2017). In opposition, AGTR1 (encode AT1 angiotensin II receptor) mRNA was increased in estrogen receptor-positive breast cancer and hormone-independent prostate cancer (Rhodes et al., 2009).
Data with aggregated results of randomized controlled trials (RCT) aim to evaluate the possible increased risk of developing cancer in patients using ACEi or ARBs. In five RCT it was observed a discrete increase in the risk of cancer with the use of ARBs. Patients with cancer such as breast, prostate and lung presented a higher occurrence of new lung cancer if receiving ARB (Sipahi et al., 2010). However, in a meta-analysis of 70 RCT (324,168 participants) it was not found any difference in the risk of cancer with ARB or ACEi versus placebo (Bangalore et al., 2011). The study of 15 multicenter double-blind RCT with 138,769 participants and a follow-up of 12 months showed no site-specific cancer incidence (lung, breast, prostate) in patients using ARB versus controls (Collaboration, 2011). In a systemic review (meta-analysis–12 publications) Datzman et al. (2019) evaluated ARB and carcinogenesis as primary outcome and concluded that there is no correlation between ARB therapy and the increase in the risk of lung cancer. However, a large cohort in Caucasian patients showed an augmented risk of lung cancer (14% after 5 years) associated with ACEi therapy and increase related to years of using the medicaments (Hicks et al., 2018). Studies in patients with advanced non-small cell lung cancer (NSCLC) showed no detrimental effect of RAS-blockers in the patient outcome (Aydiner et al., 2015; Menter et al., 2017).
ACE1 and colorectal cancer
Colorectal cancer (CRC) is highly incident in older individuals and a significant percentage of patients suffers from cardiovascular diseases. Data from 2005 to 2008 with 12,648 metastatic CRC patients showed that 52% were 65 years old or more and 48.3% were hypertensive. In this study antibiotics and anti-hypertensives were the most used medicaments (61.7% and 49.7% respectively) (Fu et al., 2011). The use of anti-hypertensive drugs (ACEi and ARBs) have been extensively evaluated in CRC because there is more convincing evidence of cancer risk reduction in patients taking these medicaments. A meta-analysis (6 studies, 113,048 patients) performed by Dai et al. (2015) found that the incidence of CRC was significantly reduced in patients using ACEi/ARB than in non-users. Moreover, this study showed that patients using ACEi/ARB presented a better outcome in CRC. In patients with negative CRC colonoscopy, the use of ACEi or ARB (for at least 180 days) was evaluated and correlated with tumor development between 3 and 36 months after the negative diagnosis. In 3 years of follow-up, patients using ACEi/ARB (n = 30,856, 61–78 years) showed significantly lower incidence of CRC than non-users (Asgharzadeh et al., 2018). A retrospective study with 13,982 patients (65 years or more) diagnosed with CRC found that the use of ACEi, beta blockers and thiazide diuretics was associated with reduced mortality. It was also found correlation between adherence to therapy (anti-hypertensive) and decreased specific mortality to CRC. The authors suggested that these drugs could be used in addition to the anti-tumor therapy for the treatment of CRC in stages I-III (Cheung et al., 2020; Balkrishnan et al., 2021). Morris et al. (2016) evaluated patients with rectal cancer treated with surgery and adjuvant radiology and found that users of ACEi/ARB presented a significant positive correlation between anti-hypertensive drugs and complete pathological response after therapy. Authors concluded that ACEi/ARB can modulate the tumor response to neoadjuvant therapy em patients with in situ rectal cancer in advanced stage. Engineer et al. (2013) found that patients (60–65 years) with stage III CRC, taking ACEi/ARB drugs or beta blockers and treated with chemotherapy or radiotherapy presented decrease in the tumor progression, less hospitalization, and reduced mortality in comparison with non-users. In contrast, Zeman et al. (2022) (n = 112, mean 62 years) observed that patients taking ACEi or ARB, and with metastasis in regional lymph node, history of adenocarcinoma, neoadjuvant therapy, and rectal resection presented reduced survival when compared with non-users. The small number of patients and the more severe disease could be the reason for the difference in Zeman’s results.
In summary, the association between the use of anti-hypertensive drugs and the risk and prognosis of cancer remains inconclusive. In colorectal cancer, the benefit of medicaments such as ACEi and ARB are more evident and some authors suggest that these medicaments could be used in association with chemotherapy, radiotherapy, and check-point blockade with the aim to improve the efficacy of the anti-tumor therapy.
ACE1 and COVID-19
Considering that COVID-19 and other infectious diseases have a major impact in aged adults, mainly in those with chronic diseases (i.e., hypertension and users of ACEi or ARBs) the association between SARS-CoV-2 infection/outcome and renin-angiotensin system (RAS) has been proposed.
On cell surface, SARS-CoV-2 binds the human angiotensin-converting enzyme (ACE)2 receptor and the transmembrane protease (TMPRSS)2 contributes for the fusion between the virus membrane and the cell membrane with subsequent entry of the virus into the host cell. ACE2 and TMPRSS2 are expressed extensively in organs such as lung, heart, kidney, gastrointestinal tract, among others and have been linked to the SARS-CoV-2 widespread infection (Kwenandar et al., 2020; Yuki et al., 2020; Zou et al., 2020; Cao et al., 2021; Hariyanto et al., 2021). Using systematic review with meta-analysis, several studies have shown some level of correlation between polymorphisms of RAS-related genes and increased risk of developing severe COVID-19 (Dieter et al., 2022; Dobrijevic et al., 2022; Gupta et al., 2022; Saengsiwaritt et al., 2022). Yamamoto et al. (2021) in a Medline database search found a higher link between ACE1 DD genotype and severe COVID-19 whereas small studies show that the ACE1 II genotype is a risk factor. Confirming the association between ACE and COVID-19, it was found that patients (n = 1,686, mean age 65.6 years) infected with SARS-CoV-2 and taking ARB or ACEi at hospital admission required less use of ventilation and vasopressors compared with non-users. However, this association was observed only for males and authors suggested that sex-based differences in RAS dysregulation may explain these results since males presented higher plasma ACE1 and AngII than females at baseline and early period of admission (Rocheleau et al., 2022). In another study it was evaluated whether the use of ACEi/ARB (outpatient and in-hospital) had any association with COVID-19 mortality. It was found that these medicaments were independently associated with a reduced risk of in-hospital mortality. Moreover, African American patients whose display higher prevalence of ACE D allele and consequent more severe COVID-19, showed a significant reduction in in-hospital mortality (Li et al., 2021).
In summary, there are few results until now, but they suggest that the individual genetic background and the treatment with ACEi or ARB are associated with a better clinical outcome in COVID-19 disease. More studies are needed, but using the findings obtained until now it will be possible to stratify the individual risk for disease severity and maybe to include ACEi or ARB therapy for those patients with higher risk of worst outcome.
Concluding remarks
ACE1 expression has a wide tissue and body fluids distribution and interferes with several biological processes. During the ageing process, ACE1 expression/activity is changed in several tissues which has been linked to age-related diseases (Alzheimer’s, sarcopenia, cancer). In addition, ACE expression in lymphoid and myeloid cells of older individuals has been recently shown suggesting that ACE can also influence immunity. Old individuals are more likely hypertensive and use angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) and these medicaments can somehow interfere with the outcome from age-related diseases. Some results on cognition impairment and dementia have shown a reduced risk on these conditions in individuals using ACEi or ARBs. In contrast, for muscle wasting, muscle power, sarcopenia and frailty there are contradictory results regarding the benefit of using ACEi or ARBs. In carcinogenesis, the dual colorectal cancer CRC - ACEi or ARBs has been widely studied since the benefits of these drugs for the patient outcome are reported in several clinical trials. In SARS-CoV2 infection the virus entry into the host cells relies on ACE2 expression and some preliminary results show the better outcome of patients taking ACEi or ARB. The precise mechanisms that link the use ACEi or ARBs and age-related diseases are unknown yet but it is possible to stratify each patient risk for disease development/progression and in addition to evaluate ACE polymorphism. Data from each patient could be used to decide whether or not to include ACEi or ARB to other therapies (precision medicine).
Author contributions
VB and DF contributed equaly for this mini-review. They searched on literature, discussed the main points of the theme, and wrote the review.
Funding
CAPES PrInt UNIFESP no 88881.310735/2018-01.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alves, A. S., and Bueno, V. (2019). Immunosenescence: participation of T lymphocytes and myeloid-derived suppressor cells in aging-related immune response changes. Einstein (Sao Paulo) 17 (2), eRB4733. doi:10.31744/einstein_journal/2019RB4733
Alves, A. S., Ishimura, M. E., Duarte, Y. A. O., and Bueno, V. (2018). Parameters of the immune system and vitamin D levels in old individuals. Front. Immunol. 9, 1122. doi:10.3389/fimmu.2018.01122
Arregui, A., Perry, E. K., Rossor, M., and Tomlinson, B. E. (1982). Angiotensin converting enzyme in Alzheimer's disease increased activity in caudate nucleus and cortical areas. J. Neurochem. 38 (5), 1490–1492. doi:10.1111/j.1471-4159.1982.tb07930.x
Asgharzadeh, F., Hassanian, S. M., Ferns, G. A., Khazaei, M., and Hasanzadeh, M. (2018). The therapeutic potential of angiotensin-converting enzyme and angiotensin receptor inhibitors in the treatment of colorectal cancer: Rational strategies and recent progress. Curr. Pharm. Des. 24 (39), 4652–4658. doi:10.2174/1381612825666190111145140
Aydiner, A., Ciftci, R., and Sen, F. (2015). Renin-Angiotensin system blockers may prolong survival of metastatic non-small cell lung cancer patients receiving erlotinib. Med. Baltim. 94, e887. doi:10.1097/MD.0000000000000887
Balkrishnan, R., Desai, R. P., Narayan, A., Camacho, F. T., Flausino, L. E., and Chammas, R. (2021). Associations between initiating antihypertensive regimens on stage I-III colorectal cancer outcomes: A medicare seer cohort analysis. Cancer Med. 10 (15), 5347–5357. doi:10.1002/cam4.4088
Bangalore, S., Kumar, S., Kjeldsen, S. E., Makani, H., Grossman, E., Wetterslev, J., et al. (2011). Antihypertensive drugs and risk of cancer: Network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 12, 65–82. doi:10.1016/s1470-2045(10)70260-6
Bernardi, S., Michelli, A., Zuolo, G., Candido, R., and Fabris, B. (2016). Update on RAAS modulation for the treatment of diabetic cardiovascular disease. J. Diabetes Res. 2016, 8917578. doi:10.1155/2016/8917578
Bernstein, K. E., Ong, F. S., Blackwell, W. L., Shah, K. H., Giani, J. F., Gonzalez-Villalobos, R. A., et al. (2012). A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 65 (1), 1–46. doi:10.1124/pr.112.006809
Brown, M., Sinacore, D. R., Ehsani, A. A., Binder, E. F., Holloszy, J. O., and Kohrt, W. M. (2000). Low-intensity exercise as a modifier of physical frailty in older adults. Arch. Phys. Med. Rehabil. 81 (7), 960–965. doi:10.1053/apmr.2000.4425
Bueno, V., Destro, P., Teixeira, D., and Frasca, D. (2022). Angiotensin converting enzyme (ACE) expression in leukocytes of older adults. medRxiv. doi:10.1101/2022.07.27.22278062
Bueno, V., Sant'Anna, O. A., and Lord, J. M. (2014). Ageing and myeloid-derived suppressor cells: Possible involvement in immunosenescence and age-related disease. Age (Dordr) 36 (6), 9729. doi:10.1007/s11357-014-9729-x
Buford, T. W., Manini, T. M., Hsu, F. C., Cesari, M., Anton, S. D., Nayfield, S., et al. (2012). Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J. Am. Geriatr. Soc. 60 (7), 1244–1252. doi:10.1111/j.1532-5415.2012.04045.x
Bulpitt, C. J., Beckett, N. S., Cooke, J., Dumitrascu, D. L., Gil-Extremera, B., Nachev, C., et al. (2003). Results of the pilot study for the hypertension in the very elderly trial. J. Hypertens. 21 (12), 2409–2417. doi:10.1097/00004872-200312000-00030
Burks, T. N., Andres-Mateos, E., Marx, R., Mejias, R., Van Erp, C., Simmers, J. L., et al. (2011). Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Transl. Med. 3, 82ra37. doi:10.1126/scitranslmed.3002227
Cabello-Verrugio, C., Acuña, M. J., Morales, M. G., Becerra, A., Simon, F., and Brandan, E. (2011). Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem. Biophys. Res. Commun. 410, 665–670. doi:10.1016/j.bbrc.2011.06.051
Cabello-Verrugio, C., Morales, M. G., Rivera, J. C., Cabrera, D., and Simon, F. (2015). Renin-angiotensin system: An old player with novel functions in skeletal muscle. Med. Res. Rev. 35, 437–463. doi:10.1002/med.21343
Cao, W., Feng, Q., and Wang, X. (2021). Computational analysis of TMPRSS2 expression in normal and SARS-CoV-2-infected human tissues. Chem. Biol. Interact. 346, 109583. doi:10.1016/j.cbi.2021.109583
Carl-McGrath, S., Lendeckel, U., Ebert, M., Wolter, A. B., Roessner, A., and Rocken, C. (2004). The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int. J. Oncol. 25, 1223–1232. doi:10.3892/ijo.25.5.1223
Carter, C. S., Onder, G., Kritchevsky, S. B., and Pahor, M. (2005). Angiotensin-converting enzyme inhibition intervention in elderly persons: Effects on body composition and physical performance. J. Gerontol. 60, 1437–1446. doi:10.1093/gerona/60.11.1437
Cheung, K. S., Chan, E. W., Seto, W. K., Wong, I. C. K., and Leung, W. K. (2020). ACE (Angiotensin-Converting enzyme) inhibitors/angiotensin receptor blockers are associated with lower colorectal cancer risk: A territory-wide study with propensity score analysis. Hypertension 76 (3), 968–975. doi:10.1161/HYPERTENSIONAHA.120.15317
Collaboration, A. R. B. T. (2011). Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J. Hypertens. 29, 623–635. doi:10.1097/HJH.0b013e328344a7de
Constantinescu, C. S., Goodman, D. B., and Ventura, E. S. (1998). Captopril and lisinopril suppress production of interleukin-12 by human peripheral blood mononuclear cells. Immunol. Lett. 62 (1), 25–31. doi:10.1016/s0165-2478(98)00025-x
Cummings, J. L., Tong, G., and Ballard, C. (2019). Treatment combinations for Alzheimer's disease: Current and future pharmacotherapy options. J. Alzheimers Dis. 67 (3), 779–794. doi:10.3233/JAD-180766
Dai, Y. N., Wang, J. H., Zhu, J. Z., Lin, J. Q., Yu, C. H., and Li, Y. M. (2015). Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers therapy and colorectal cancer: A systematic review and meta-analysis. Cancer Causes Control 26 (9), 1245–1255. doi:10.1007/s10552-015-0617-1
Datzmann, T., Fuchs, S., Andree, D., Hohenstein, B., Schmitt, J., and Schindler, C. (2019). Systematic review and meta-analysis of randomised controlled clinical trial evidence refutes relationship between pharmacotherapy with angiotensin-receptor blockers and an increased risk of cancer. Eur. J. Intern Med. 64, 1–9. doi:10.1016/j.ejim.2019.04.019
de Cavanagh, E. M., Ferder, L., Carrasquedo, F., Scrivo, D., WassermAnn, A., Fraga, C. G., et al. (1999). Higher levels of antioxidant defenses in enalapril-treated versus non-enalapril-treated hemodialysis patients. Am. J. Kidney Dis. 34, 445–455. doi:10.1016/s0272-6386(99)70071-5
de Cavanagh, E. M., Inserra, F., Ferder, L., and Fraga, C. G. (2000). Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R572–R577. doi:10.1152/ajpregu.2000.278.3.R572
de Cavanagh, E. M., Inserra, F., Toblli, J., Stella, I., Fraga, C. G., and Ferder, L. (2001). Enalapril attenuates oxidative stress in diabetic rats. Hypertension 38, 1130–1136. doi:10.1161/hy1101.092845
De Dios, L., Collazo, C., and Inostroza-Nieves, Y. (2022). Renin-angiotensin-system increases phosphorylated tau and Reactive Oxygen Species in human cortical neuron cell line. Biochem. Biophys. Rep. 32, 101355. doi:10.1016/j.bbrep.2022.101355
Dieter, C., Brondani, L. A., Leitão, C. B., Gerchman, F., Lemos, N. E., and Crispim, D. (2022). Genetic polymorphisms associated with susceptibility to COVID-19 disease and severity: A systematic review and meta-analysis. PLoS One 17 (7), e0270627. doi:10.1371/journal.pone.0270627
Dobrijevic, Z., Robajac, D., Gligorijevic, N., Šunderic, M., Penezic, A., Miljuš, G., et al. (2022). The association of ACE1, ACE2, TMPRSS2, IFITM3 and vdr polymorphisms with COVID-19 severity: A systematic review and meta-analysis. EXCLI J. 21, 818–839. doi:10.17179/excli2022-4976
Engeli, S., Negrel, R., and Sharma, A. M. (2000). Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension 35, 1270–1277. doi:10.1161/01.hyp.35.6.1270
Engineer, D. R., Burney, B. O., Hayes, T. G., and Garcia, J. M. (2013). Exposure to ACEI/ARB and β-blockers is associated with improved survival and decreased tumor progression and hospitalizations in patients with advanced colon cancer. Transl. Oncol. 6, 539–545. doi:10.1593/tlo.13346
Escobar, E., Rodríguez-Reyna, T. S., Arrieta, O., and Sotelo, J. (2004). Angiotensin II, cell proliferation and angiogenesis regulator: Biologic and therapeutic implications in cancer. Curr. Vasc. Pharmacol. 2 (4), 385–399. doi:10.2174/1570161043385556
Fazal, K., Perera, G., Khondoker, M., Howard, R., and Stewart, R. (2017). Associations of centrally acting ACE inhibitors with cognitive decline and survival in Alzheimer`s disease. B J. Psych. Open 3 (3), 158–164. doi:10.1192/bjpo.bp.116.004184
Fu, A. Z., Zhao, Z., Gao, S., Barber, B., and Liu, G. G. (2011). Comorbid conditions in patients with metastatic colorectal cancer. World J. Oncol. 2 (5), 225–231. doi:10.4021/wjon370e
George, A. J., Thomas, W. G., and Hannan, R. D. (2010). The renin-angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer 10 (11), 745–759. doi:10.1038/nrc2945
Giovannini, S., Cesari, M., Marzetti, E., Leeuwenburgh, C., Maggio, M., and Pahor, M. (2010). Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J. Nutr. Health Aging 14 (6), 457–460. doi:10.1007/s12603-010-0036-7
Godsel, L. M., Leon, J. S., Wang, K., Fornek, J. L., Molteni, A., and Engman, D. M. (2003). Captopril prevents experimental autoimmune myocarditis. J. Immunol. 171, 346–352. doi:10.4049/jimmunol.171.1.346
Goldstein, B., Trivedi, M., and Speth, R. C. (2017). Alterations in gene expression of components of the renin-angiotensin system and its related enzymes in lung cancer. Lung Cancer Int. 2017, 6914976. doi:10.1155/2017/6914976
Gonzalez-Villalobos, R. A., Shen, X. Z., Bernstein, E. A., Janjulia, T., Taylor, B., Giani, J. F., et al. (2013). Rediscovering ACE: Novel insights into the many roles of the angiotensin-converting enzyme. J. Mol. Med. Berl. 91 (10), 1143–1154. doi:10.1007/s00109-013-1051-z
Gupta, K., Kaur, G., Pathak, T., and Banerjee, I. (2022). Systematic review and meta-analysis of human genetic variants contributing to COVID-19 susceptibility and severity. Gene 844, 146790. doi:10.1016/j.gene.2022.146790
Hariyanto, T. I., Rizki, N. A., and Kurniawan, A. (2021). Anosmia/hyposmia is a good predictor of coronavirus disease 2019 (COVID-19) infection: A meta-analysis. Int. Arch. Otorhinolaryngol. 25 (1), e170–e174. doi:10.1055/s-0040-1719120
Hicks, B. M., Filion, K. B., Yin, H., Sakr, L., Udell, J. A., and Azoulay, L. (2018). Angiotensin converting enzyme inhibitors and risk of lung cancer: Population based cohort study. BMJ 363, k4209. doi:10.1136/bmj.k4209
Hillers-Ziemer, L. E., Kuziel, G., Williams, A. E., Moore, B. N., and Arendt, L. M. (2022). Breast cancer microenvironment and obesity: Challenges for therapy. Cancer Metastasis Rev. 41 (3), 627–647. doi:10.1007/s10555-022-10031-9
Ho, J. K., Moriarty, F., Manly, J. J., Larson, E. B., Evans, D. A., Rajan, K. B., et al. (2021). Blood-brain barrier crossing renin-angiotensin drugs and cognition in the elderly: A meta-analysis. Hypertension 78 (3), 629–643. doi:10.1161/HYPERTENSIONAHA.121.17049
Hooper, N. M. (1991). Angiotensin converting enzyme: Implications from molecular biology for its physiological functions. Int. J. Biochem. 23 (7-8), 641–647. doi:10.1016/0020-711x(91)90032-i
Hu, J., Miyatake, F., Aizu, Y., Nakagawa, H., Nakamura, S., Tamaoka, A., et al. (1999). Angiotensin-converting enzyme genotype is associated with Alzheimer disease in the Japanese population. Neurosci. Lett. 277, 65–67. doi:10.1016/S0304-3940(99)00827-7
Jochemsen, H. M., Teunissen, C. E., Ashby, E. L., van der Flier, W. M., Jones, R. E., Geerlings, M. I., et al. (2014). The association of angiotensin-converting enzyme with biomarkers for Alzheimer's disease. Alzheimers Res. Ther. 6 (3), 27. doi:10.1186/alzrt257
Kehoe, P. G., Russ, C., McIlroy, S., Williams, H., Holmans, P., Holmes, C., et al. (1999). Variation in DCP1, encoding ACE, is associated with susceptibility to Alzheimer disease. Nat. Genet. 21, 71–72. doi:10.1038/5009
Kehoe, P. G., Wong, S., Al Mulhim, N., Palmer, L. E., and Miners, J. S. (2016). Angiotensin-converting enzyme 2 is reduced in Alzheimer's disease in association with increasing amyloid-β and tau pathology. Alzheimers Res. Ther. 8 (1), 50. doi:10.1186/s13195-016-0217-7
Kostka, J., Sikora, J., Guligowska, A., and Kostka, T. (2021). Quadriceps muscle power and optimal shortening velocity are inversely related to angiotensin converting enzyme activity in older men. F1000Res 10, 184. doi:10.12688/f1000research.51208.2
Kuno, A., Yamada, T., Masuda, K., Ogawa, K., Sogawa, M., Nakamura, S., et al. (2003). Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology 124, 1010–1019. doi:10.1053/gast.2003.50147
Kwenandar, F., Japar, K. V., Damay, V., Hariyanto, T. I., Tanaka, M., Lugito, N. P. H., et al. (2020). Coronavirus disease 2019 and cardiovascular system: A narrative review. Int. J. Cardiol. Heart Vasc. 29, 100557. doi:10.1016/j.ijcha.2020.100557
Li, M., Zhou, M., Yang, Y., Liu, Y., Yin, C., Geng, W., et al. (2022). Multi-trajectories of systolic and diastolic hypertension and coronary heart disease in middle-aged and older adults. Front. Public Health 10, 1017727. doi:10.3389/fpubh.2022.1017727
Li, S., Sarangarajan, R., Jun, T., Kao, Y. H., Wang, Z., Hao, K., et al. (2021). In-hospital use of ACE inhibitors/angiotensin receptor blockers associates with COVID-19 outcomes in African American patients. J. Clin. Invest. 131 (19), e151418. doi:10.1172/JCI151418
Lloyd-Jones, D. M., Evans, J. C., and Levy, D. (2005). Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA 294 (4), 466–472. doi:10.1001/jama.294.4.466
Loera-Valencia, R., Eroli, F., Garcia-Ptacek, S., and Maioli, S. (2021). Brain renin-angiotensin system as novel and potential therapeutic target for Alzheimer's disease. Int. J. Mol. Sci. 22 (18), 10139. doi:10.3390/ijms221810139
MacLachlan, R., Kehoe, P. G., and Miners, J. S. (2022). Dysregulation of ACE-1 in normal aging and the early stages of Alzheimer's disease. J. Gerontol. A Biol. Sci. Med. Sci. 77, 1775–1783. doi:10.1093/gerona/glac083
Menter, A. R., Carroll, N. M., Sakoda, L. C., Delate, T., Hornbrook, M. C., Jain, R. K., et al. (2017). Effect of angiotensin system inhibitors on survival in patients receiving chemotherapy for advanced nonsmall-cell lung cancer. Clin. Lung Cancer 18, 189–197. e183. doi:10.1016/j.cllc.2016.07.008
Miners, J. S., Ashby, E., Van Helmond, Z., Chalmers, K. A., Palmer, L. E., Love, S., et al. (2008). Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer's disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 34 (2), 181–193. doi:10.1111/j.1365-2990.2007.00885.x
Morris, Z. S., Saha, S., Magnuson, W. J., Morris, B. A., Borkenhagen, J. F., Ching, A., et al. (2016). Increased tumor response to neoadjuvant therapy among rectal cancer patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Cancer 122 (16), 2487–2495. doi:10.1002/cncr.30079
Muller, D. N., Fiebeler, A., Park, J. K., Dechend, R., and Luft, F. C. (2003). Angiotensin II and endothelin induce inflammation and thereby promote hypertension-induced end-organ damage. Clin. Nephrol. 60 (1), S2–S12.
Nelson, M. E., Layne, J. E., Bernstein, M. J., Nuernberger, A., Castaneda, C., Kaliton, D., et al. (2004). The effects of multidimensional home-based exercise on functional performance in elderly people. J. Gerontol. A Biol. Sci. Med. Sci. 59 (2), 154–160. doi:10.1093/gerona/59.2.m154
Newman, A. B., Simonsick, E. M., Naydeck, B. L., Boudreau, R. M., Kritchevsky, S. B., Nevitt, M. C., et al. (2006). Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 295, 2018–2026. doi:10.1001/jama.295.17.2018
Oliveros, E., Patel, H., Kyung, S., Fugar, S., Goldberg, A., Madan, N., et al. (2020). Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 43 (2), 99–107. doi:10.1002/clc.23303
Pawelec, G., Picard, E., Bueno, V., Verschoor, C. P., and Ostrand-Rosenberg, S. (2021). MDSCs, ageing and inflammageing. Cell. Immunol. 362, 104297. doi:10.1016/j.cellimm.2021.104297
Perry, H. M., Davis, B. R., Price, T. R., Applegate, W. B., Fields, W. S., Guralnik, J. M., et al. (2000). Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: The systolic hypertension in the elderly program (SHEP). JAMA 284 (4), 465–471. doi:10.1001/jama.284.4.465
Pont, L., and Alhawassi, T. (2017). Challenges in the management of hypertension in older populations. Adv. Exp. Med. Biol. 956, 167–180. doi:10.1007/5584_2016_149
Reneland, R., Haenni, A., Andersson, P. E., Andrén, B., and Lithell, H. (1999). Skeletal muscle angiotensin-converting enzyme and its relationship to blood pressure in primary hypertension and healthy elderly men. Blood Press 8 (1), 16–22. doi:10.1080/080370599438347
Reneland, R., and Lithell, H. (1994). Angiotensin-converting enzyme in human skeletal muscle. A simple in vitro assay of activity in needle biopsy specimens. Scand. J. Clin. Lab. Invest. 54, 105–111. doi:10.3109/00365519409086516
Rhodes, D. R., Ateeq, B., Cao, Q., Tomlins, S. A., Mehra, R., Laxman, B., et al. (2009). AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc. Natl. Acad. Sci. U. S. A. 106 (25), 10284–10289. doi:10.1073/pnas.0900351106
Ribatti, D., Conconi, M. T., and Nussdorfer, G. G. (2007). Nonclassic endogenous novel [corrected] regulators of angiogenesis. Pharmacol. Rev. 59 (2), 185–205. doi:10.1124/pr.59.2.3
Rocheleau, G. L. Y., Lee, T., Mohammed, Y., Goodlett, D., Burns, K., Cheng, M. P., et al. (2022). Renin-angiotensin system pathway therapeutics associated with improved outcomes in males hospitalized with COVID-19. Crit. Care Med. 50 (9), 1306–1317. doi:10.1097/CCM.0000000000005589
Rosendorff, C., Beeri, M. S., and Silverman, J. M. (2007). Cardiovascular risk factors for Alzheimer's disease. Am. J. Geriatr. Cardiol. 16 (3), 143–149. doi:10.1111/j.1076-7460.2007.06696.x
Saengsiwaritt, W., Jittikoon, J., Chaikledkaew, U., and Udomsinprasert, W. (2022). Genetic polymorphisms of ACE1, ACE2, and TMPRSS2 associated with COVID-19 severity: A systematic review with meta-analysis. Rev. Med. Virol. 32 (4), e2323. doi:10.1002/rmv.2323
Savaskan, E., Hock, C., Olivieri, G., Bruttel, S., Rosenberg, C., Hulette, C., et al. (2001). Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer's dementia. Neurobiol. Aging 22 (4), 541–546. doi:10.1016/s0197-4580(00)00259-1
Schaufelberger, M., Drexler, H., Schieffer, E., and Swedberg, K. (1998). Angiotensin-converting enzyme gene expression in skeletal muscle in patients with chronic heart failure. J. Card. Fail 4 (3), 185–191. doi:10.1016/s1071-9164(98)80005-5
Sengoku, R. (2020). Aging and Alzheimer's disease pathology. Neuropathology 40 (1), 22–29. doi:10.1111/neup.12626
Shasha, S. M., Nusam, D., Labin, L., Kristal, B., Steinberger, O., Barzilai, M., et al. (1991). Effect of converting enzyme inhibitor captopril on T cell functions in essential hypertension. Nephron 59 (4), 586–590. doi:10.1159/000186648
Shaw, L. J., Olson, M. B., Kip, K., Kelsey, S. F., Johnson, B. D., Mark, D. B., et al. (2006). The value of estimated functional capacity in estimating outcome: Results from the NHBLI-sponsored women's ischemia syndrome evaluation (WISE) study. J. Am. Coll. Cardiol. 47, S36–S43. doi:10.1016/j.jacc.2005.03.080
Sink, K. M., Leng, X., Williamson, J., Kritchevsky, S. B., Yaffe, K., Kuller, L., et al. (2009). Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the cardiovascular health study. Arch. Intern Med. 169 (13), 1195–1202. doi:10.1001/archinternmed.2009.175
Sipahi, I., Debanne, S. M., Rowland, D. Y., Simon, D. I., and Fang, J. C. (2010). Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 11, 627–636. doi:10.1016/s1470-2045(10)70106-6
Solfrizzi, V., Scafato, E., Frisardi, V., Seripa, D., Logroscino, G., Kehoe, P. G., et al. (2013). Angiotensin-converting enzyme inhibitors and incidence of mild cognitive impairment. The Italian Longitudinal Study on Aging. Aging. Age (Dordr). 35 (2), 441–453. doi:10.1007/s11357-011-9360-z
Song, Y. H., Li, T., Du, J., Mitch, W. E., Rosenthal, N., and Delafontaine, P. (2005). Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J. Clin. Investig. 115, 451–458. doi:10.1172/JCI22324
Soto, M. E., van Kan, G. A., Nourhashemi, F., Gillette-Guyonnet, S., Cesari, M., Cantet, C., et al. (2013). Angiotensin-converting enzyme inhibitors and Alzheimer's disease progression in older adults: Results from the réseau sur la Maladie d'Alzheimer français cohort. J. Am. Geriatr. Soc. 61 (9), 1482–1488. doi:10.1111/jgs.12415
Studenski, S., Perera, S., Patel, K., Rosano, C., Faulkner, K., Inzitari, M., et al. (2011). Gait speed and survival in older adults. JAMA 305, 50–58. doi:10.1001/jama.2010.1923
Sumukadas, D., Band, M., Miller, S., Cvoro, V., Witham, M., Struthers, A., et al. (2014). Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 69 (6), 736–743. doi:10.1093/gerona/glt142
Vaccarino, V., Holford, T. R., and Krumholz, H. M. (2000). Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J. Am. Coll. Cardiol. 36 (1), 130–138. doi:10.1016/s0735-1097(00)00687-2
Veronese, N., Stubbs, B., Smith, L., Maggi, S., Jackson, S. E., Soysal, P., et al. (2019). Angiotensin-converting enzyme inhibitor use and incident frailty: A longitudinal cohort study. Drugs Aging 36 (4), 387–393. doi:10.1007/s40266-019-00642-3
WHO (2023). WHO coronavirus (COVID-19). Avaliable At: https://covid19.who.int (Accessed January 4, 2023).
Witham, M. D., Syddall, H. E., Dennison, E., Cooper, C., McMurdo, M. E., and Sayer, A. A. (2014). ACE inhibitors, statins and thiazides: No association with change in grip strength among community dwelling older men and women from the hertfordshire cohort study. Age Ageing 43 (5), 661–666. doi:10.1093/ageing/afu008
Yamamoto, N., Nishida, N., Yamamoto, R., Gojobori, T., Shimotohno, K., Mizokami, M., et al. (2021). Angiotensin-converting enzyme (ACE) 1 gene polymorphism and phenotypic expression of COVID-19 symptoms. Genes. (Basel) 12 (10), 1572. doi:10.3390/genes12101572
Yasar, S., Varma, V. R., Harris, G. C., and Carlson, M. C. (2018). Associations of angiotensin converting enzyme-1 and angiotensin II blood levels and cognitive function. J. Alzheimers Dis. 63 (2), 655–664. doi:10.3233/JAD-170944
Yasar, S., Zhou, J., Varadhan, R., and Carlson, M. C. (2008). The use of angiotensin-converting enzyme inhibitors and diuretics is associated with a reduced incidence of impairment on cognition in elderly women. Clin. Pharmacol. Ther. 84 (1), 119–126. doi:10.1038/sj.clpt.6100483
Yoshihara, A., Tobina, T., Yamaga, T., Ayabe, M., Yoshitake, Y., Kimura, Y., et al. (2009). Physical function is weakly associated with angiotensin-converting enzyme gene I/D polymorphism in elderly Japanese subjects. Gerontology 55 (4), 387–392. doi:10.1159/000222429
Yuki, K., Fujiogi, M., and Koutsogiannaki, S. (2020). COVID-19 pathophysiology: A review. Clin. Immunol. 215, 108427. doi:10.1016/j.clim.2020.108427
Zeman, M., Skałba, W., Szymański, P., Hadasik, G., Żaworonkow, D., Walczak, D. A., et al. (2022). Risk factors for long-term survival in patients with ypN+ M0 rectal cancer after radical anterior resection. BMC Gastroenterol. 22 (1), 141. doi:10.1186/s12876-022-02226-9
Zhang, K., Mao, T., He, Z., Wu, X., Peng, Y., Chen, Y., et al. (2019). Angiotensin I-converting enzyme gene plays a crucial role in the pathology of carcinomas in colorectal cancer. Artif. Cells Nanomed Biotechnol. 47 (1), 2500–2506. doi:10.1080/21691401.2019.1626402
Keywords: ageing, angiotensin-converting enzyme (ACE), Alzheimer’s disease, sarcopenia, cancer, COVID-19
Citation: Bueno V and Frasca D (2023) Mini-review: Angiotensin- converting enzyme 1 (ACE1) and the impact for diseases such as Alzheimer’s disease, sarcopenia, cancer, and COVID-19. Front. Aging 4:1117502. doi: 10.3389/fragi.2023.1117502
Received: 06 December 2022; Accepted: 11 January 2023;
Published: 23 January 2023.
Edited by:
Leena P. Bharath, Merrimack College, United StatesReviewed by:
Carlos F. Sánchez-Ferrer, Autonomous University of Madrid, SpainCopyright © 2023 Bueno and Frasca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valquiria Bueno, dmJ1ZW5vQHVuaWZlc3AuYnI=
 Valquiria Bueno
Valquiria Bueno Daniela Frasca
Daniela Frasca