
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Antibiot., 21 May 2024
Sec. Pharmacology
Volume 3 - 2024 | https://doi.org/10.3389/frabi.2024.1388039
This article is part of the Research TopicAntibiotic Treatment of Lyme DiseaseView all articles
 Amy D. Thompson1*
Amy D. Thompson1* Desiree N. Neville2
Desiree N. Neville2 Laura L. Chapman3
Laura L. Chapman3 Fran Balamuth4
Fran Balamuth4 Meagan M. Ladell5
Meagan M. Ladell5 Anupam B. Kharbanda6
Anupam B. Kharbanda6 Rachael Aresco7
Rachael Aresco7 Lise E. Nigrovic7 on behalf of Pedi Lyme Net
Lise E. Nigrovic7 on behalf of Pedi Lyme NetBackground: The 2018 Infectious Disease Committee of the American Academy of Pediatrics stated that up to 3 weeks or less of doxycycline is safe in children of all ages. Our goal was to examine trends in doxycycline treatment for children with Lyme disease.
Methods: We assembled a prospective cohort of children aged 1 to 21 years with Lyme disease who presented to one of eight participating Pedi Lyme Net centers between 2015 and 2023. We defined a Lyme disease case with an erythema migrans (EM) lesion or positive two-tier Lyme disease serology categorized by stage: early-localized (single EM lesion), early-disseminated (multiple EM lesions, cranial neuropathy, meningitis, and carditis), and late (arthritis). We compared doxycycline treatment by age and disease stage and used logistic regression to examine treatment trends.
Results: Of the 1,154 children with Lyme disease, 94 (8.1%) had early-localized, 449 (38.9%) had early-disseminated, and 611 (53.0%) had late disease. Doxycycline treatment was more common for older children (83.3% ≥ 8 years vs. 47.1% < 8 years; p < 0.001) and with early-disseminated disease (77.2% early-disseminated vs. 52.1% early-localized or 62.1% late; p < 0.001). For children under 8 years, doxycycline use increased over the study period (6.9% 2015 to 67.9% 2023; odds ratio by year, 1.45; 95% confidence interval, 1.34–1.58).
Conclusion: Young children with Lyme disease are frequently treated with doxycycline. Prospective studies are needed to confirm the safety and efficacy of doxycycline in children younger than 8 years, especially for those receiving courses longer than 3 weeks.
For many years, tetracycline use in children under 8 years of age was limited because of concerns about tooth staining and enamel hypoplasia (Stultz and Eiland, 2019). A recent review of the available evidence suggests that the risks from the newer tetracycline antibiotics (e.g., doxycycline) are very low (Ravindra et al., 2023). In 2018, the Infectious Disease Committee of the American Academy of Pediatrics stated that short courses (up to 3 weeks) of oral doxycycline are safe in patients of all ages (Kimberlin et al., 2018). Then, in 2020, the Infectious Disease Society of America (IDSA), American College of Rheumatology (ACR), and American Academy of Neurology (AAN) suggested that doxycycline be considered a first-line treatment option for Lyme disease-associated meningitis and cranial neuropathy (Lantos et al., 2021). The impact of these guidelines on antibiotic prescribing for Lyme disease, especially for the youngest children, has not been evaluated.
To this end, we assembled a multi-center cohort of children with Lyme disease presenting to one of the eight participating Pedi Lyme Net centers. We identified children diagnosed with confirmed Lyme disease and examined trends in doxycycline treatment over time by patient age and disease stage.
We performed a prospective cohort study at eight pediatric emergency departments (EDs), located in Lyme disease endemic areas, participating in the Pedi Lyme Net clinical research network (Nigrovic et al., 2020). The study staff approached children undergoing evaluation for potential Lyme disease to obtain informed consent for study participation. The study protocol was approved by the institutional review board at each participating center with permission for data sharing.
The parent study enrolled children aged 1 to 21 years of age undergoing evaluation for Lyme disease between June 1, 2015 and December 31, 2023. We limited this sub-study to those children with confirmed Lyme disease and available documentation of antibiotic treatment.
We obtained patient demographics and clinical history from providers and patients at the time of enrollment. Approximately 1 month after enrollment, we reviewed the medical record to abstract results of diagnostic testing as well as the antibiotics prescribed with duration (in days). When antibiotic treatment prescribed and/or duration was not available from the medical record, the study staff performed phone follow-up to clarify.
We defined a case of Lyme disease by either provider-diagnosed erythema migrans (EM) lesion or a positive two-tier serology within 30 days of enrollment in a child with symptoms compatible with acute Lyme disease (Mead et al., 2019). Although specific Lyme disease assays varied by study site (Maulden et al., 2020), we utilized the results available to the medical providers making antibiotic treatment decisions. We categorized all Lyme disease cases as one of the following stages after review of the prospectively recorded clinical history and physical examination: early-localized (single EM lesion), early-disseminated (multiple EM lesions, cranial neuropathy, meningitis, carditis), and late (arthritis) (Lantos et al., 2021).
Our primary outcome was any treatment with doxycycline. We included all antibiotics prescribed within 30 days of enrollment, documented by medical record review or phone follow-up.
Continuous data were reported using medians and interquartile ranges and categorical data using proportions with associated 95% confidence intervals (CI). First, we compared children treated with any doxycycline to children not receiving doxycycline. Second, we compared the proportion of children treated with doxycycline overall by age (<8 years of age vs. ≥ 8years of age) and by disease stage (early-localized vs. early-disseminated vs. late stage) using chi-square test. Using binary logistic regression, we examined trends in doxycycline use overall and by age as well as disease stage over the study period.
All analyses were performed using SPSS version 29.0.0 (IBM Corp; Armonk, NY, USA).
Of the 1,154 children with Lyme disease, 1,127 (97.7%) patients had their antibiotic treatment documented. Of these, the median patient age was 8 years [interquartile range (IQR), 6–12 years], and 727 (63.0%) were male patients. The majority of children presented during the peak Lyme disease season between June and October (771, 66.8%). Overall, 81 (7.0%) had an EM lesion alone, 967 (83.8%) had positive two-tier Lyme disease serology alone, and 106 (9.2%) had both. Overall, 94 (8.1%) had early-localized, 449 (38.9%) had early-disseminated, and 611 (53.0%) had late Lyme disease.
Of the 1,127 with antibiotic treatment documented, 756 (67.1%) received doxycycline. Overall, 683 (90.3%) received doxycycline at the index encounter, and 73 (9.7%) were switched to doxycycline after another initial antibiotic. The median duration of doxycycline treatment was 21 days (IQR, 14–28 days). Of the 353 children who received more than 3 weeks of doxycycline, 124 (35.1%) were under 8 years of age.
Next, we compared children treated with doxycycline to those who did not receive doxycycline (Table 1). Children treated with doxycycline were older and more likely to have early-disseminated disease.
Overall, the usage of doxycycline increased over the 9-year study period (Figure 1). Between 2015 and 2023, doxycycline use increased from 40.0% to 79.3% overall [odds ratio (OR) by year 1.26, 95% confidence interval 1.20–1.33], from 6.9% to 67.9% for children under 8 years (OR by year 1.45, 95% CI 1.34–1.58), and from 42.1% to 84.7% for children with early-disseminated disease (OR 1.30, 95% CI 1.18–1.42).
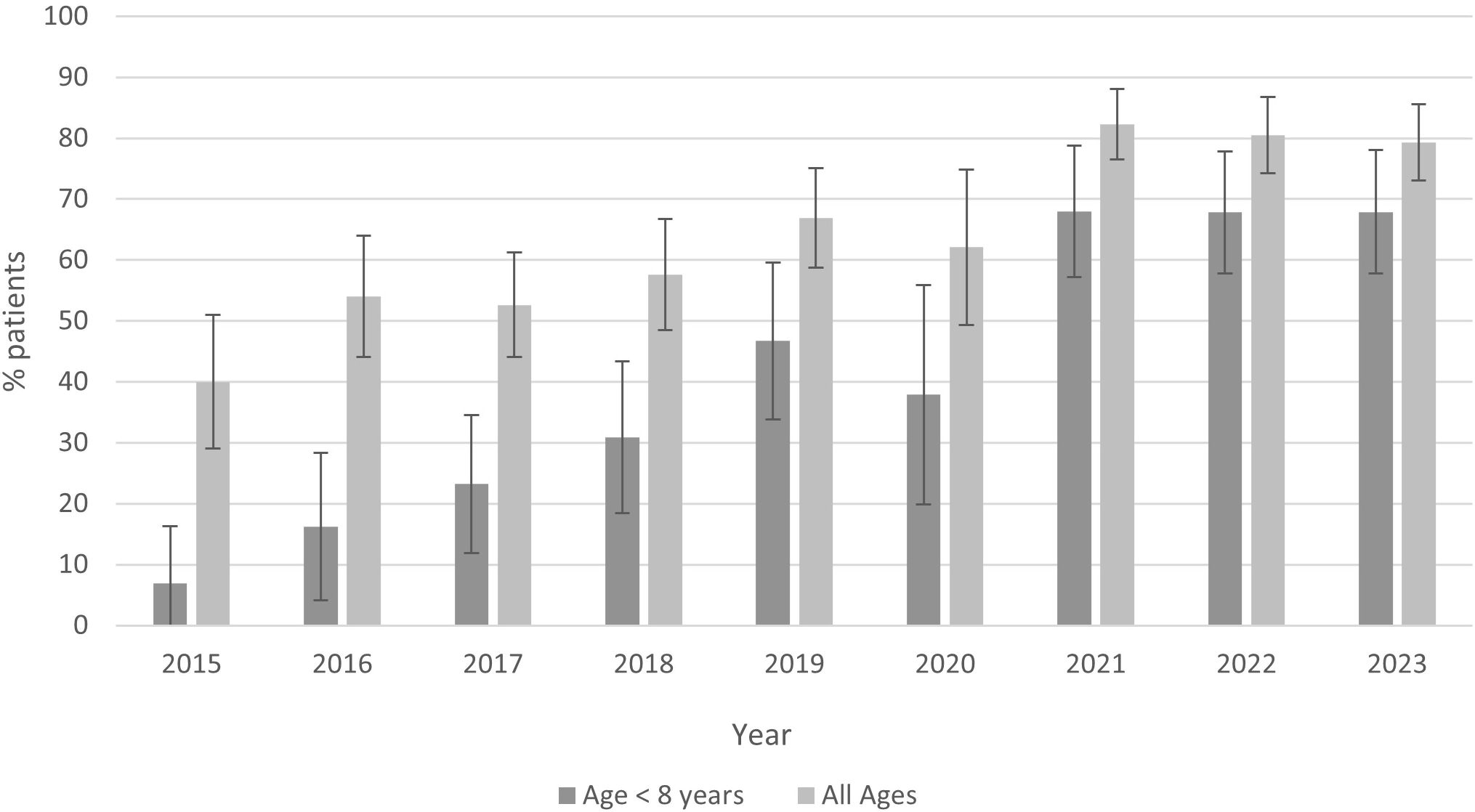
Figure 1 Proportion of children with Lyme disease treated with doxycycline overall and for children <8 years of age (2015–2023).
Consistent with the new safety recommendations (Kimberlin et al., 2018) and Lyme disease treatment guidelines (Lantos et al., 2021), two-thirds of children with confirmed Lyme disease were treated with doxycycline in our multi-center prospective cohort study. Between 2015 and 2023, doxycycline utilization increased substantially, especially for children younger than 8 years of age and those with early-disseminated disease.
Tetracyclines, including doxycycline, are used to treat a wide range of infections and have a low risk of associated serious adverse events (Smith and Leyden, 2005; Agwuh and MacGowan, 2006). However, tetracycline has a high binding affinity for calcium, which can cause enamel hypoplasia and teeth staining (Sánchez et al., 2004). The risk is highest when the exposure occurs before the enamel of the permanent tooth has calcified (under 8 years), especially with higher doses and longer duration of therapy (Conchie et al., 1970; Grossman et al., 1971). Given the risk for dental staining, tetracyclines have not been recommended for children under 8 years of age. Doxycycline, a newer tetracycline with a lower affinity for calcium binding (Forti and Benincori, 1969), does not appear to have the same dental effects at the currently recommended dosages (Cross et al., 2016; Stultz and Eiland, 2019; Ravindra et al., 2023).
While intravenous ceftriaxone has long been the recommended treatment for Lyme neuroborreliosis (Wormser et al., 2006), parenteral antibiotic therapy has been associated with a high rate of complications from both the antibiotic and the commonly required peripherally inserted central catheter line (Thompson et al., 2012). With its high oral availability and known activity against Borrelia burgdorferi, doxycycline provides an attractive treatment alternative (Saivin and Houin, 1988; Wormser and Halperin, 2008). Several European clinical trials in adults (Borg et al., 2005; Ljøstad et al., 2008; Kortela et al., 2021; Arnason and Skogman, 2022) and a retrospective study in children (Lopez et al., 2019) reported good outcomes for patients with Lyme meningitis treated with doxycycline. In 2020, the Infectious Disease Society of America, American Academy of Neurology, and American College of Rheumatology recommended either oral doxycycline or parenteral ceftriaxone as a first-line treatment for Lyme meningitis (Lantos et al., 2021). An ongoing prospective study is comparing the efficacy of doxycycline to ceftriaxone for the treatment of pediatric Lyme meningitis on short- and long-term clinical outcomes (Nigrovic et al., 2023a).
Consistent with previous studies, we observed an increase in doxycycline treatment for children with Lyme disease that began before the publication of the recent clinical guidelines. For children with Lyme meningitis diagnosed at one of the 24 pediatric centers located in endemic areas that contributed data to Pediatric Health Information System (PHIS), ceftriaxone utilization declined from 48% in 2015 to 9% in 2020 (Roelf et al., 2021). In a single-center retrospective study of 32 children under 8 years of age with Lyme disease treated with doxycycline, none had treatment failure documented in the medical record (Brown et al., 2023). Although two caregivers reported dental staining on a follow-up telephone survey, this was not based on an in-person dental examination. The current guidelines suggest limiting doxycycline to 3 weeks or less. However, a substantial minority of children under 8 years of age with Lyme disease were treated with a longer course. Although no adverse events were reported, further studies are needed to evaluate the safety of doxycycline courses longer than 3 weeks for young children.
The black-legged tick that transmits Lyme disease can also carry other bacteria, viruses, or parasites that can cause human disease. Doxycycline effectively treats two of the most common bacterial co-infecting pathogens, Anaplasma and Erlichia (Krause et al., 2021). Therefore, clinicians may select doxycycline to cover Borrelia as well as other bacterial coinfections. Using a multiplex high-definition polymerase chain reaction assay (HDPCR), we previously found that only a minority of children with Lyme disease had a tick-borne coinfection, suggesting that the primary reason doxycycline was prescribed was for Lyme disease treatment (Nigrovic et al., 2023b). The optimal approach to the diagnosis and treatment of children with potential tick-borne co-infections requires further study.
Our study has several limitations. First, enrollment was limited to the times when the study staff were available. However, the study patients had similar clinical characteristics to eligible children who were not enrolled. Second, as we did not collect the reason that a specific Lyme disease treatment was prescribed, we cannot take into account antibiotic allergies or concerns for a tick-borne co-infection. Lastly, we only performed a short-term clinical follow-up by review of medical record and phone follow-up and may have failed to identify all treatment complications, including dental staining. Further studies are needed to better understand the potential adverse effects of longer courses of doxycycline for Lyme disease treatment, especially for the youngest children.
In this eight-center multicenter study, children with Lyme disease were commonly prescribed doxycycline with increasing utilization over the study period (2015–2023), especially for children under 8 years of age and those with early-disseminated Lyme disease. Prospective studies are needed to confirm the safety and efficacy of doxycycline in children, especially for children younger than 8 years receiving it for longer than 3 weeks.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board at each participating center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardian or by the patient if ≥ 18 years.
AT: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. DN: Writing – review & editing, Project administration, Investigation, Data curation. LC: Writing – review & editing, Project administration, Investigation, Data curation. FB: Writing – review & editing, Project administration, Investigation, Data curation. ML: Writing – review & editing, Project administration, Investigation, Data curation. AK: Writing – review & editing, Project administration, Investigation, Data curation. RA: Writing – review & editing, Project administration, Data curation. LN: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the Global Lyme Alliance (LEN). The funder had no role in the research conduct or results interpretation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agwuh K. N., MacGowan A. (2006). Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 58, 256–265. doi: 10.1093/jac/dkl224
Arnason S., Skogman B. H. (2022). Effectiveness of antibiotic treatment in children with Lyme neuroborreliosis-a retrospective study. BMC Pediatr. 22, 332. doi: 10.1186/s12887-022-03335-w
Borg R., Dotevall L., Hagberg L., Maraspin V., Lotric-Furlan S., Cimperman J., et al. (2005). Intravenous ceftriaxone compared with oral doxycycline for the treatment of Lyme neuroborreliosis. Scand. J. Infect. Dis. 37, 449–454. doi: 10.1080/00365540510027228
Brown K., Corin S., Handel A. S. (2023). Doxycycline for the treatment of lyme disease in young children. Pediatr. Infect. Dis. J. 42, e470–e472. doi: 10.1097/INF.0000000000004128
Conchie J. M., Munroe J. D., Anderson D. O. (1970). The incidence of staining of permanent teeth by the tetracyclines. Can. Med. Assoc. J. 103, 351–356.
Cross R., Ling C., Day N. P., McGready R., Paris D. H. (2016). Revisiting doxycycline in pregnancy and early childhood–time to rebuild its reputation? Expert Opin. Drug Saf. 15, 367–382. doi: 10.1517/14740338.2016.1133584
Forti G., Benincori C. (1969). Doxycycline and the teeth. Lancet 293, 782. doi: 10.1016/S0140-6736(69)91787-5
Grossman E. R., Walchek A., Freedman H., Flanagan C. (1971). Tetracyclines and permanent teeth: the relation between dose and tooth color. Pediatrics 47, 567–570. doi: 10.1542/peds.47.3.567
Kimberlin D. W., Jackson M. A., Long S. S., Lyme disease (2018). “Committee on infectious diseases; american academy of pediatrics,” in Red Book. Eds. Kimberlin D. W., Brady M. T., Jackson M. A., Long S. S. (American Academy of Pediatrics, Elk Grove Village, IL), 516–525.
Kortela E., Kanerva M. J., Puustinen J., Hurme S., Airas L., Lauhio A., et al. (2021). Oral doxycycline compared to intravenous ceftriaxone in the treatment of Lyme neuroborreliosis: a multicenter, equivalence, randomized, open-label trial. Clin. Infect. Dis. 72, 1323–1331. doi: 10.1093/cid/ciaa217
Krause P. J., Auwaerter P. G., Bannuru R. R., Branda J. A., Falck-Ytter Y. T., Lantos P. M., et al. (2021). Clinical practice guidelines by the Infectious Diseases Society of America (IDSA): 2020 guideline on diagnosis and management of babesiosis. Clin. Infect. Dis. 72, e49–e64. doi: 10.1093/cid/ciab050
Lantos P. M., Rumbaugh J., Bockenstedt L. K., Falck-Ytter Y. T., Aguero-Rosenfeld M. E., Auwaerter P. G., et al. (2021). Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Clin. Infect. Dis. 72, e1–e48. doi: 10.1093/cid/ciaa1215
Ljøstad U., Skogvoll E., Eikeland R., Midgard R., Skarpaas T., Berg Å., et al. (2008). Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: a multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurol. 7, 690–695. doi: 10.1016/S1474-4422(08)70119-4
Lopez S. M., Campfield B. T., Nowalk A. J. (2019). Oral management for pediatric Lyme meningitis. J. Pediatr. Infect. Dis. Soc 8, 272–275. doi: 10.1093/jpids/piy072
Maulden A. B., Garro A. C., Balamuth F., Levas M. N., Bennett J. E., Neville D. N., et al. (2020). Two-Tier Lyme disease serology test results can vary according to the specific First-Tier test used. J. Pediatr. Infect. Dis. Soc 9, 128–133. doi: 10.1093/jpids/piy133
Mead P., Petersen J., Hinckley A. (2019). Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 68, 703. doi: 10.15585/mmwr.mm6832a4
Nigrovic L. E., Chun T. H., Vargas S. E., Caffrey A. R., Halperin J. J., Race J. A., et al. (2023a). Protocol: Comparative effectiveness and complications of intravenous ceftriaxone compared with oral doxycycline in Lyme meningitis in children: a multicentre prospective cohort study. BMJ Open 13, e071141. doi: 10.1136/bmjopen-2022-071141
Nigrovic L. E., Neville D. N., Balamuth F., Levas M. N., Bennett J. E., Kharbanda A. B., et al. (2020). Pediatric lyme disease biobank, United States 2015–2020. Emerg. Infect. Dis. 26, 3099–3101. doi: 10.3201/eid2612.200920
Nigrovic L. E., Neville D. N., Chapman L., Balamuth F., Levas M. N., Thompson A. D., et al. (2023b). Multiplex high-definition polymerase chain reaction assay for the diagnosis of tick-borne infections in children. Open Forum Infect. Dis. 10, ofad121. doi: 10.1093/ofid/ofad121
Ravindra D., Huang G., Hallett K., Burgner D. P., Gwee A., Silva M. J. (2023). Antibiotic exposure and dental health: A systematic review. Pediatrics 52, 2023061350. doi: 10.1542/peds.2023-061350
Roelf K. M., Garro A., Monuteaux M. C., Nigrovic L. E. (2021). Changes in antibiotic treatment for children with lyme meningitis 2015–2020. Hosp Pediatr. 11, e243–e248. doi: 10.1542/hpeds.2021-005909
Saivin S., Houin G. (1988). Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 15, 355–366. doi: 10.2165/00003088-198815060-00001
Sánchez A. R., Rogers R. S. III, Sheridan P. J. (2004). Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int. J. Dermatol. 43, 709–715. doi: 10.1111/j.1365-4632.2004.02108.x
Smith K., Leyden J. J. (2005). Safety of doxycycline and minocycline: a systematic review. Clin. Ther. 27, 1329–1342. doi: 10.1016/j.clinthera.2005.09.005
Stultz J. S., Eiland L. S. (2019). Doxycycline and tooth discoloration in children: changing of recommendations based on evidence of safety. Ann. Pharmacother. 53, 1162–1166. doi: 10.1177/1060028019863796
Thompson A. D., Cohn K. A., Shah S. S., Lyons T., Welsh E. J., Hines E. M., et al. (2012). Treatment complications in children with Lyme meningitis. Pediatr. Infect. Dis. J. 31, 1032–1035. doi: 10.1097/INF.0b013e31825eb3c7
Wormser G. P., Dattwyler R. J., Shapiro E. D., Halperin J. J., Steere A. C., Klempner M. S., et al. (2006). The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43, 1089–1134. doi: 10.1086/508667
Keywords: Lyme disease, children, doxycycline, dental, Borrelia burgdorferi
Citation: Thompson AD, Neville DN, Chapman LL, Balamuth F, Ladell MM, Kharbanda AB, Aresco R and Nigrovic LE (2024) Increased usage of doxycycline for young children with Lyme disease. Front. Antibiot. 3:1388039. doi: 10.3389/frabi.2024.1388039
Received: 19 February 2024; Accepted: 10 April 2024;
Published: 21 May 2024.
Edited by:
Peter J. Gwynne, Tufts University, United StatesReviewed by:
Jon Blevins, University of Arkansas for Medical Sciences, United StatesCopyright © 2024 Thompson, Neville, Chapman, Balamuth, Ladell, Kharbanda, Aresco and Nigrovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy D. Thompson, QW15LlRob21wc29uQG5lbW91cnMub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.