- 1Department of Infectious Diseases, Communicable Diseases Centre, Hamad Medical Corporation, Doha, Qatar
- 2Department of Medical Education, Weill Cornell Medicine – Qatar, Education City, Qatar Foundation, Doha, Qatar
- 3Laboratory Services, Philadelphia Department of Public Health, Philadelphia, PA, United States
- 4The Life Science Centre, School of Science and Technology, Örebro University, Örebro, Sweden
- 5Department of Microbiology and Immunology, Weill Cornell Medicine – Qatar, Education City, Qatar Foundation, Doha, Qatar
Introduction: Among Gram-negative bacteria (GNB), Enterobacterales (Enterobacterales), such as Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae), are the most clinically relevant pathogens in healthcare settings. Infections secondary to these pathogens are widely common but multidrug resistance (MDR) in Enterobacterales has become a significant challenge with increased morbidity, mortality, and cost of management. The escalating global prevalence of MDR in Enterobacterales has led to limited treatment options, raising an urgent need for novel antimicrobial therapy(s) and detailed studies exploring underlying resistance mechanisms. In Enterobacterales, the prime antimicrobial resistance mechanism against β-lactam antibiotics is mainly the production of β-lactamases, particularly extended-spectrum β-lactamases (ESBLs). Although the Gulf region is witnessing major challenges from infections secondary to MDR GNB, the extent of the problem has not been fully evaluated. Therefore, this review aims to address the prevalence and genetic characterization of ESBL-producing Enterobacterales in the Gulf Cooperation Council (GCC) countries.
Methods: PubMed® (National Library of Medicine, Bethesda, MD, USA) search was conducted, which looked for academic articles discussing the epidemiology of MDR Enterobacterales in the GCC countries, published in the last 5 years.
Results and conclusions: In GCC countries there is a high prevalence rate of MDR Enterobacterales, particularly ESBLs. Prevalence rates of ESBL-producing Enterobacterales among the Enterobacterales in general clinical samples in the GCC region is 21.6%–29.3%, with a slightly higher prevalence rate in intensive care unit patients (17.3–31.3%) and in patients with urinary tract infections (25.2%–31.7%). ESBL carriers have also been noted in the general community. ESBL-producing Enterobacterales from the GCC region show high levels of resistance to ampicillin, aztreonam, third-/fourth-generation cephalosporins, fluoroquinolones, and trimethoprim-sulfamethoxazole. Intermediate resistance rates are observed against nitrofurantoin, piperacillin/tazobactam, and gentamicin, with increasing resistance observed against tigecycline. The isolates demonstrate low-level resistance to carbapenems, fosfomycin, colistin, and amikacin. Enterobacterales isolates that are concomitant ESBL producers and are carbapenem resistant have been increasingly reported and demonstrate alarmingly increased antibiotic resistance patterns compared with ESBL Enterobacterales. The most prevalent genes for ESBL resistance in the Enterobacterales isolates in the GCC region are: blaCTX-M (subtype group 1) followed by/co-dominated by blaTEM and blaSHV, whereas the most common carbapenem-resistant genes are blaOXA-48 and blaNDM-1.
1 Introduction
Over recent decades, antimicrobial resistance (AMR) has risen as a global concern because of substantial increases in morbidity and mortality, and because of the associated costs of management (Bassetti et al., 2017; Naylor et al., 2018). At its forefront are Gram-negative bacteria, (GNB) such as Enterobacterales (Enterobacterales), Pseudomonas aeruginosa, and Acinetobacter baumannii, which have become increasingly resistant to most conventional and broad-spectrum antimicrobial agents, including carbapenems (Nordmann and Poirel, 2019). A subset of the GNB family, the order of Enterobacterales consists of Gram-negative bacilli that predominantly colonize the human gastro-intestinal tract. In healthcare settings, the clinically important pathogens encompass Escherichia coli, Klebsiella species, Enterobacter species, Proteus species, Citrobacter species, Salmonella species, Shigella species, and Serratia marcescens. Following the discovery of antibiotics in the last century, these bacteria have accumulated various AMR mechanisms, enabling them to become multidrug resistant, resulting in a noticeable increase in morbidity and mortality (Exner et al., 2017). Owing to their capacity to disseminate as healthcare-associated infections (HCAIs) together with the rapid acquisition of diverse mechanisms of resistance, the World Health Organization has listed cephalosporin- and carbapenem-resistant Enterobacterales as multidrug-resistant priority pathogens in need of immediate advances in research and development and newer therapeutic options (WHO, 2017).
1.1 Mechanisms of resistance in Enterobacterales
According to an agreed international consensus, multidrug-resistant GNB refer to pathogens that are non-susceptible to at least one agent from three different antimicrobial classes, where extensively drug-resistant (XDR) bacteria are susceptible to only two or less antimicrobial classes, and pandrug-resistant (PDR) bacteria are not susceptible to all routinely tested antimicrobials (Exner et al., 2017). This is widely evident in Enterobacterales, which express resistance to several classes, including penicillins, cephalosporins, aminoglycosides, quinolones, and sulfonamides, as well as broad-spectrum agents, such as tigecycline, carbapenems, and polymyxins. This resistance can be gained either intrinsically via chromosomal inheritance/through de novo mutations or, more commonly, extrinsically via plasmid-mediated mobile horizontal gene transfer (Ruppe et al., 2015) (Partridge, 2015). Resistance genes can be transferred between chromosomes and plasmids via mobile genetic elements, such as insertion sequences, integron systems, or transposons (Partridge, 2015).
One of the key resistance mechanisms in Enterobacterales is the production of β-lactamases, particularly extended-spectrum β-lactamases (ESBLs), which hydrolyze β-lactam-based antibiotics such as penicillins, cephalosporins, and monobactams (Bush, 2018). As per the pivotal Ambler classification system, β-lactamases are categorized into classes A to D based on their amino acid sequences (Ruppe et al., 2015). Although classes A, C, and D are serine-based proteases, class B are zinc-based metallo-β-lactamases (MBLs) (Walther-Rasmussen and Høiby, 2007). Class A β-lactamases include penicillinases (TEM, SHV), ESBLs (TEM, SHV, and CTX-M), and carbapenemases (KPC, GES, IMI, SME, SFC, and NMC-A) [7, 9]. Historically, genomic studies on class A β-lactamases were the first to be described, which include penicillinases encoded by blaTEM, blaSHV, and blaCTX-M. Penicillinases are named as such because of their hydrolyzing effect on benzylpenicillin and ampicillin (Walther-Rasmussen and Høiby, 2007). After the introduction of third-generation cephalosporins, certain penicillinases acquired ESBL phenotype via amino acid substitutions in their active sites (Partridge, 2015). Hence, ESBL-producing GNB are resistant to most β-lactams except for a subset of drugs, including carbapenems, which became the sine qua non for the management of ESBL infections, particularly for invasive disease (Ruppe et al., 2015). Despite ESBLs conferring resistance to penicillins, third-generation cephalosporins, and monobactams, they remain vulnerable to β-lactamase inhibitors such as clavulanic acid, tazobactam, and sulbactam, which are commonly used in β-lactam–β-lactamase inhibitor therapies (BLBLIs), albeit with raised concerns regarding using these agents for serious invasive diseases (Samarasinghe, 2019; Burillo and Bouza, 2022).
Exploring β-lactamases and other resistance mechanisms expressed by antimicrobial- and multidrug-resistant pathogens highlights the genetic and environmental components of resistance. Accumulated resistance can be either acquired from mobile elements from the environment or from continuous antibiotic exposure, which results in advantageous de novo mutations that potentiate the survival and evolution of resistant pathogens (Huttner et al., 2013; Goodman et al., 2016). Factors that drive AMR are increased use of antimicrobials in agriculture and in the animal industry, including the intermediate environment; host factors, such as comorbidities/immune status; healthcare-associated aspects, such as poor adherence to infection control/prevention practices; and inappropriate and excessive antibiotics exposure (Aslam et al., 2021). Furthermore, AMR is further aggravated by the propagation of resistant pathogens facilitated by international travel and population diversity. This context is best highlighted in the Gulf Cooperation Council (GCC) region, which hosts a diverse expatriate population and is at a crossroads for frequent international travel. As an example, in Qatar, previous studies demonstrate significant rates of community ESBL urinary tract infections (UTIs) where immigrant food handlers were carriers for the resistant pathogens (Eltai et al., 2018a; Eltai et al., 2018c; Andres et al., 2020).
1.2 Antimicrobial resistance in the Gulf Cooperation Council region
The problem of antibiotic resistance in Gram-negative pathogens is rising globally and in the GCC region. In a recent study of isolates from Saudi Arabia, 90.1% of the tested Gram-negative isolates were multidrug resistant, with 2.7% being XDR, which is comparable to similarly high MDR rates of 74.5% and 97.5% and XDR rates of 1.5% and 7.7% in Egypt and Sudan, respectively, with the ESBL gene blaCTX-M as the predominant gene (100%) (Azab et al., 2021). Among Enterobacterales, another study from Saudi Arabia identified 81%, 18.2%, and 2.8% of Enterobacterales as MDR, XDR, and PDR, respectively (Bandy and Tantry, 2021). Similar to global trends, increased multidrug resistance (MDR) rates in the GCC region have also led to higher morbidity, mortality, and economic costs. For example, studies show that Klebsiella pneumoniae and MDR Enterobacterales isolates are linked to 42% and 84% of intensive care unit (ICU) mortalities, respectively, in Saudi Arabia, and any increase in MDR rates will further exacerbate the problem (Al Bshabshe et al., 2020; Alkofide et al., 2020). In the United Arab Emirates, it was noted that hospitalized patients with ESBL UTIs have longer hospital stay durations and are treated with a larger number of antibiotics than their counterparts with non-ESBL infections (Ranjan Dash et al., 2018). MDR infections are also at the forefront of medical concerns in the wake of the global COVID-19 pandemic. COVID-19, especially in severe cases, is frequently associated with excessive antimicrobial prescribing and increased observed AMR (Beovic et al., 2020; Abu-Rub et al., 2021; Baiou et al., 2021; Langford et al., 2022), evidently leading a viscous cycle of increased rates of multidrug-resistant bacteria, along with the development of potentially new XDR and PDR strains. In a recent study in Qatar on critically ill COVID-19 patients, an incidence rate of 4.5 per 1,000 ICU days was identified for MDR Gram-negative bacterial infections. MDR K. pneumoniae (23.5%) and E. coli (12%) were among the major bacteria identified in this cohort, with an ESBL rate of 82.6% and 91.7%, respectively (Baiou et al., 2021).
Although there have been multiple studies that have evaluated the problem of AMR in the region from different perspectives, this comprehensive review of data published in the last 5 years aims to produce an updated report on the prevalence and genomic epidemiology of ESBL-producing Enterobacterales in the GCC region.
2 Methods, data collection, and analysis
Available publications pertaining to the prevalence of MDR Enterobacterales in the Middle East were searched in PubMed® (National Library of Medicine, Bethesda, MD, USA) (Moher et al., 2010). The following Boolean search terms were used for the search: “Specified country” AND (“Enterobacteriaceae” OR “E. coli” OR “Escherichia coli” OR “Klebsiella pneumoniae” OR “K. pneumoniae”) AND (“resistant” OR “resistance “OR “multidrug resistant”). A similar search with the term ESBL was also carried out. Relevant papers that were published within the last 5 years were included with no age restrictions. The following six countries were included in this paper: Bahrain, Kuwait, Qatar, Oman, Saudi Arabia, and United Arab Emirates. When multiple publications were present, the most recent data/publications that covered the objectives were included. For purposeful inclusion in this study, ESBL Enterobacterales are defined as Enterobacterales that are resistant to β-lactam antibiotics, such as penicillins, oxyimino-cephalosporins (e.g., ceftriaxone, ceftazidime, cefepime, and cefotaxime), and monobactams, as confirmed and reported by routine internal microbiological susceptibility testing (Burillo and Bouza, 2022). Mixed source indicates all clinical specimens, such as lower respiratory tract infections, blood, urine, wound infections, and others.
3 Data and discussion
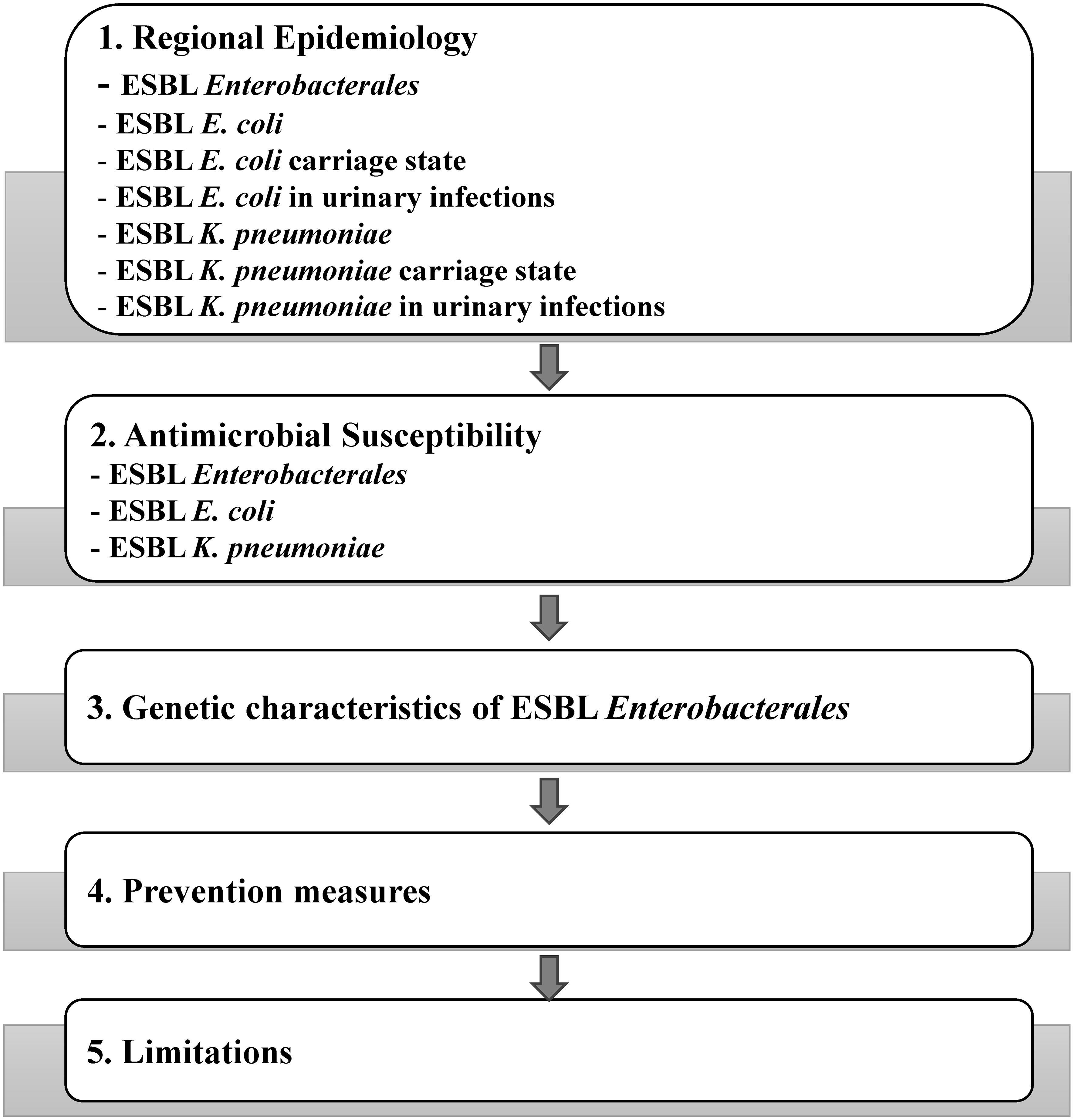
The schematic of the data being discussed in this review is shown in Figure 1.
3.1 Regional epidemiology
3.1.1 ESBL Enterobacterales
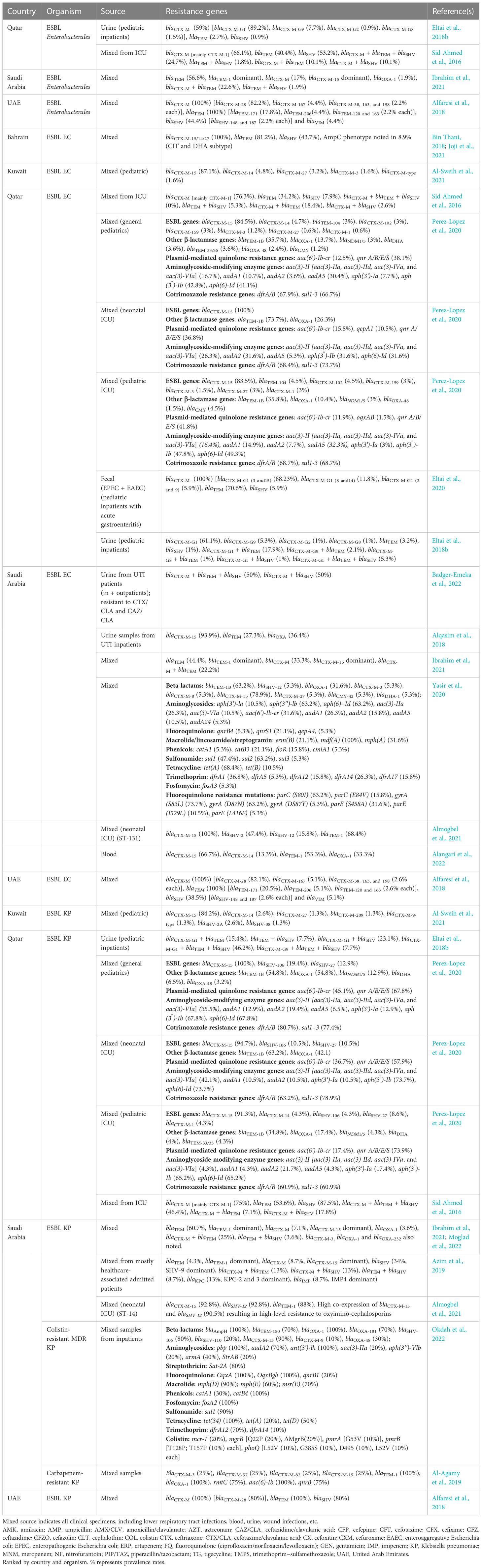
Overall, the prevalence rate of ESBL-Enterobacterales among Enterobacterales in general clinical mixed samples was 21.6%–29.3%, with a higher prevalence rate of 17.3%–31.3% in ICU patients and 25.2%–31.7% in patients with UTIs in the GCC region (Table 1). The country-specific prevalence rates of ESBL Enterobacterales and associated antimicrobial susceptibility data, where applicable, are detailed in Table 1.
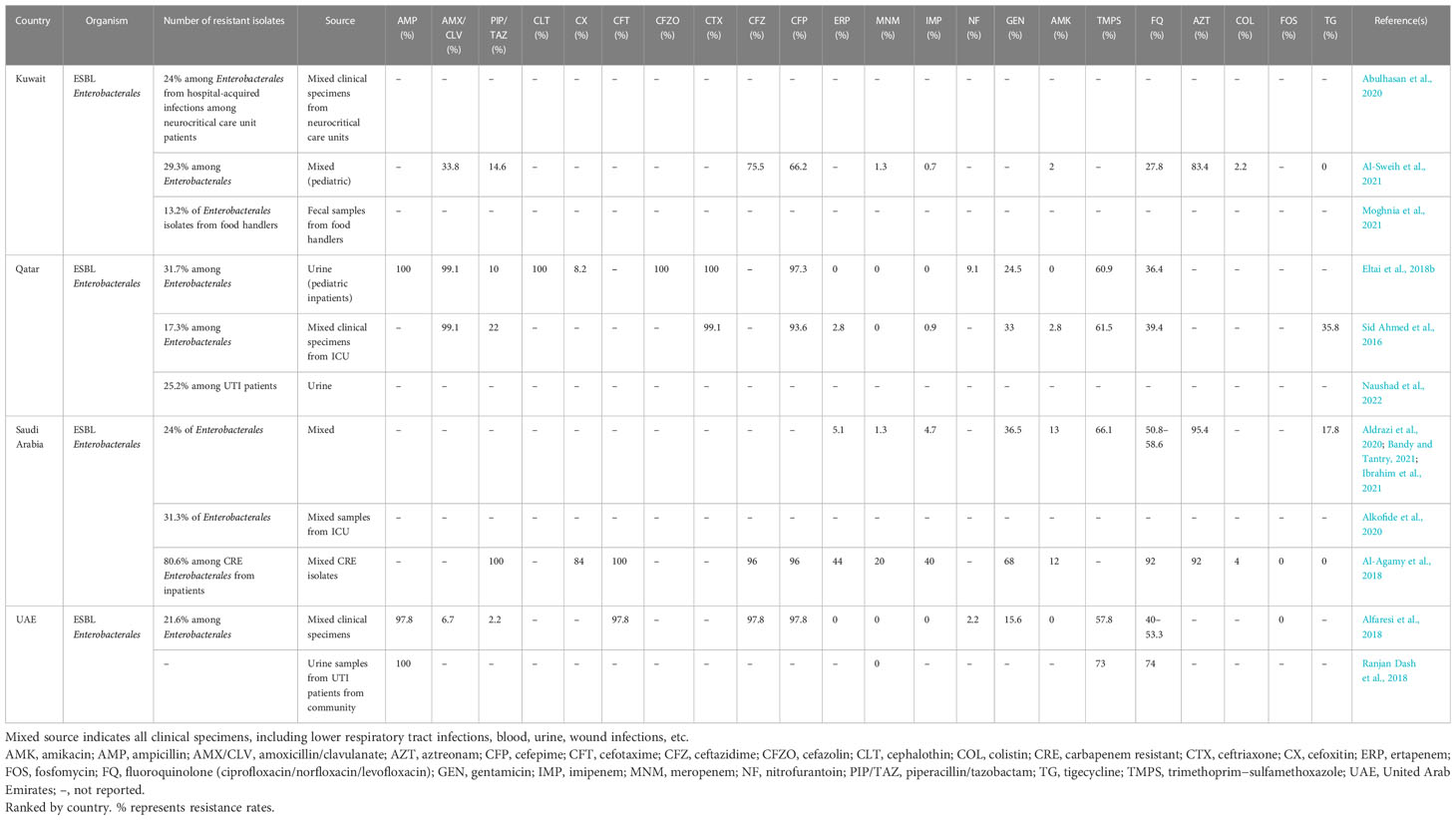
Table 1 ESBL Enterobacterales prevalence rate in the GCC region and antimicrobial resistance profile.
Furthermore, as an indicator of the prevalence of ESBL Enterobacterales in the general community, 13.2% of food handlers, who are mainly immigrants, were identified as ESBL Enterobacterales carriers in a study conducted in Kuwait in 2021 (Moghnia et al., 2021). When compared with the GCC region, a higher ESBL prevalence has been noted in countries like Thailand (38.4%), Vietnam (55.1%), Cambodia (40.6%), and the Philippines (36.8%) (Suwantarat and Carroll, 2016; Caron et al., 2018; Siriphap et al., 2022).
Globally, among Gram-negative bacterial infections, E. coli (33%) is the most isolated organism followed by K. pneumoniae (19.2%), hence we decided to focus on these two pathogens exclusively in this review (Karlowsky et al., 2022). As a regional representation, in a longitudinal study over a 6-year period from a large hospital in Saudi Arabia encompassing almost 33,000 GNB isolates, ESBLs were predominated by E. coli and K. pneumoniae at 20% and 26%, respectively (Al-Tawfiq et al., 2020).
3.1.2 E. coli
In the GCC countries, E. coli more commonly demonstrate the non-carbapenem-resistant ESBL phenotype when compared with K. pneumoniae, which is similar to some other regions, such as the USA, Canada, and Asia, but in contrast to parts of Europe, where ESBL non-carbapenem-resistant K. pneumoniae is more dominant (Karlowsky et al., 2022). Although exceptions to this can also be seen, such as in a previous study from Saudi Arabia (Al-Tawfiq et al., 2020). Overall, ESBL E. coli is the dominant organism among ESBL Enterobacterales in the GCC region, with a prevalence rate ranging from 34% to 86.6% (Supplementary Table 1). Specifically, in case of general mixed samples in the GCC region (excluding ICU patients and urine samples), the prevalence rate of ESBL E. coli among total E. coli infections has been reported to be 20%–71.3%. Supplementary Table 1 details country-specific prevalence rates of ESBL E. coli and associated antimicrobial susceptibility data, where applicable, which have been ranked according to country and sample type. Kuwait tops the list with the largest number of ESBL non-carbapenem-resistant E. coli cases in general clinical samples at 71.3% (Supplementary Table 1) (Karlowsky et al., 2022). Among carbapenem-resistant E. coli, a study from Saudi Arabia showed a 60% prevalence rate of ESBL E. coli among E. coli infections (Al-Agamy et al., 2018). Higher prevalence rates were noted in ICU patients, especially among infants, at 79.2% (Almogbel et al., 2021).
Previous reports from Saudi Arabia show that the rates of MDR isolates in mixed clinical samples, especially ESBL E. coli and K. pneumoniae, were seen to be increasing in the country from 2010 to 2015 (Farah et al., 2019). Specifically, ESBL E. coli rates increased from 32% in 2010 to 41% in 2015 in mixed samples (Farah et al., 2019). However, this increasing trend seems to have reversed, with another study showing a decrease in ESBL E. coli prevalence rate from 56% in 2016 to 50% in 2017 (Aldawsari et al., 2020). Recent studies from Saudi Arabia also show a lower prevalence of ESBL E. coli, ranging from 31.1% to 36.6% (Ibrahim et al., 2019; Aldrazi et al., 2020; Bandy and Tantry, 2021; Ibrahim et al., 2021).
In comparison to general mixed samples, because of expected pathogen, host, and environmental factors, the prevalence rates of ESBL E. coli are higher in most studies analyzing specific samples from critical patients, as seen for respiratory samples [100% among respiratory E. coli infections (ICU patients)], endotracheal samples (75% among MDR E. coli), and blood samples (52.2% among total bloodstream infection-causing E. coli) (Bandy and Almaeen, 2020; Kabrah et al., 2021; Sannathimmappa et al., 2021) (Supplementary Table 1).
3.1.3 ESBL E. coli carriage state
Among healthy carriers, studies carried out in Qatar and Kuwait identified an ESBL E. coli prevalence rate of 9% among commensal E. coli and of 18.8% among E. coli isolates from food handlers, respectively (Eltai et al., 2018c; Moghnia et al., 2021) (Supplementary Table 1). This poses a significant risk factor, as any failures in hand/food hygiene among these food handlers could lead to rapid dissemination of these ESBL/carbapenem-resistant (Supplementary Table 1) strains in the community, and, subsequently, increase transfer of resistance genes among prevalent bacteria, with the potential for secondary infections (Moghnia et al., 2021). These data also reinforce the need for strict and active monitoring of food hygiene protocols and consistent testing of relevant staff to prevent related hospital and community spread. The potential spread of these MDR bacteria in hospital environments can be exemplified by the latter study from the GCC region where ESBL E. coli isolates from healthy food handlers in Kuwait represented 71.2% of food handlers working in healthcare settings and 28.8% of community food handlers (p = 0.001) (Moghnia et al., 2021).
3.1.4 ESBL E. coli in urinary infections
For urinary infections, in the GCC region, an overall prevalence rate of 4%–50% was seen for ESBL E. coli among urinary infection-causing E. coli (Supplementary Table 1). A higher ESBL E. coli prevalence among uropathogenic E. coli was seen in hospitalized patients (23.5%–50%) (Kabrah et al., 2021; Mohammed et al., 2022) when compared with studies looking at both inpatients and outpatients (4–33.5%) (Balkhi et al., 2018; Alzahrani et al., 2020; Bazaid et al., 2021; Abalkhail et al., 2022; Badger-Emeka et al., 2022) or specifically outpatients (11.1% in female outpatients and 23% in patients with UTIs in the community) (Ranjan Dash et al., 2018; Alasmary, 2021) (Supplementary Table 1). A study from Saudi Arabia noted a lower ESBL E. coli prevalence rate among uropathogens in children (3.23%) and adults (4.82%) than in elderly patients (8.33%) samples from emergency department (Alanazi et al., 2018) (Supplementary Table 1). A study from Bahrain, encompassing over 3,000 non-repetitive urinary samples from a large tertiary care hospital, showed the one of the highest ESBL E. coli prevalence in the GCC region in relation to urinary infections, with rates of ESBL E. coli infections increasing from 37.3% in 2018 to 39% in 2019 (Saeed et al., 2021). Another alarming factor is that the majority of ESBL E. coli and carbapenem-resistant E. coli cases in Bahrain are hospital acquired (51% and 5.9%, respectively) rather than community acquired (37.6% and 0.5%, respectively) (Saeed et al., 2021); however, in the United Arab Emirates, it was observed that > 75% of the ESBL E. coli and K. pneumoniae cases were from the community (Dash et al., 2008). Similar to mixed clinical samples, one study from Saudi Arabia noted a marked decline in ESBL uropathogenic E. coli cases, decreasing from 100% in 2017 to 4% in 2019, among a cohort of both inpatients and outpatients, including emergency and intensive care patients (Badger-Emeka et al., 2022). The authors attribute this decrease to the adoption of antimicrobial stewardship programmes in some hospitals, in addition to the implementation of stringent regulation for prescription antibiotic dispensing in community pharmacies by the Saudi Arabia Ministry of Health (Badger-Emeka et al., 2022). Another study in Saudi Arabia also noted a similar decrease in ESBL E. coli and K. pneumoniae among uropathogenic E. coli and K. pneumoniae, from 23.7% and 40%, respectively, in 2015 to 17.6% and 16.7%, respectively, in 2019 in a cohort composed largely of outpatients (Bazaid et al., 2021). Kuwait showed a variable trend, with a study reporting increasing prevalence of ESBL E. coli from approximately 18% in 2017 to 37% in 2019, followed by a decrease to 22% in 2021 (Al Benwan and Jamal, 2022).
3.1.5 ESBL K. pneumoniae
ESBL K. pneumoniae formed the second most dominant organism among ESBL Enterobacterales in the region, with a prevalence rate of 11.1%–52.8% (Supplementary Table 2). The ESBL K. pneumoniae prevalence rate among K. pneumoniae isolates in the GCC region ranged from 13%–52.4% for general mixed clinical samples to 7%–87.5% for samples from ICU patients (Supplementary Table 2), with the prevalence increasing to 90.5% among carbapenem-resistant K. pneumoniae isolates isolated from inpatients. Supplementary Table 2 details country-specific prevalence rates of ESBL K. pneumoniae/spp. and associated antimicrobial susceptibility data, where applicable, which have been ranked according to country and sample type. Kuwait reported the highest prevalence of ESBL non-carbapenem-resistant K. pneumoniae (52.4%) among K. pneumoniae isolates in marked contrast to lower values reported by the United Arab Emirates and Qatar (20.8% and 28.2%, respectively) in case of general mixed samples (Karlowsky et al., 2022) (Supplementary Table 2). The high prevalence rate of ESBL (non-carbapenem-resistant) K. pneumoniae (52.4%) in Kuwait, with an equally alarming high rate of ESBL (non-carbapenem-resistant) E. coli (71.3%), is similar to the prevalence rates reported by countries like India, Thailand, Vietnam, and Mexico (Karlowsky et al., 2022). One study from Saudi Arabia noted that the prevalence rate of ESBL K. pneumoniae showed a modest increase from 22% in 2016 to 26% in 2017 in the country (Aldawsari et al., 2020), whereas another study, looking at isolates from 2014 to 2018, noted that 57.5%–77.8% of the K. pneumoniae isolates in the study were resistant to third- and fourth-generation cephalosporins (Al-Zalabani et al., 2020).
The prevalence of ESBL K. pneumoniae/spp. among Klebsiella isolates across diverse clinical sample types is similar to the range shown by general mixed samples, with prevalence rates of 10%–100% in blood samples, 5.5% among respiratory K. pneumoniae infections (ICU patients), 17.1% among MDR K. pneumoniae in endotracheal samples, 30% among emphysematous pyelonephritis-causing K. pneumoniae, and 25% and 34.3% among total K. pneumoniae/spp. in the case of surgical site and device-associated healthcare infections, respectively (Supplementary Table 2) (Balkhy et al., 2020; Bandy and Almaeen, 2020; El-Saed et al., 2020; Kabrah et al., 2021; Sannathimmappa et al., 2021; Bazaid et al., 2022; Robles-Torres et al., 2022).
3.1.6 K. pneumoniaecarriage state
Among healthy carriers, a study from Kuwait identified a prevalence rate of ESBL K. pneumoniae of 4% among K. pneumoniae from food handlers (Moghnia et al., 2021), indicative of a lower carrier rate of ESBL K. pneumoniae in the general population compared with carrier rates of ESBL E. coli of 18.8% among E. coli isolates from food handlers (Moghnia et al., 2021) (Supplementary Tables 1, 2).
3.1.7 K. pneumoniae in urinary infections
In case of ESBL K. pneumoniae urinary infections, multiple published studies are available from Saudi Arabia and one study each from the United Arab Emirates, Oman, Kuwait, Qatar, and Bahrain (Supplementary Table 2). The prevalence of ESBL K. pneumoniae among K. pneumoniae causing urinary infections ranged from 10% to 20% in general/mixed patients (adults) (Supplementary Table 2), whereas a higher prevalence rate was noted in the pediatric population, ranging from 29.2% to 39.5% (Bazaid et al., 2022; Mohammed et al., 2022). Similar to reports of ESBL E. coli, Kuwait showed a mixed trend, with one study reporting an increasing prevalence of ESBL K. pneumoniae, from approximately 33% in 2017 to 50% in 2019, followed by a decrease, to 42%, in 2021 (Al Benwan and Jamal, 2022).
3.2 Antimicrobial susceptibility
Beta-lactamase Gram-negative producers in the region have been shown to have significantly higher resistance rates to cephalosporins, amoxicillin/clavulanate, piperacillin/tazobactam, nitrofurantoin, aztreonam, ciprofloxacin, and trimethoprim/sulfamethoxazole than non-β-lactamase producers (Ibrahim et al., 2019).
3.2.1 ESBL Enterobacterales
ESBL Enterobacterales collectively in the GCC region, in general, showed a > 95% resistance to ampicillin, with high resistance rates to aztreonam (83.4%–95.4%), trimethoprim/sulfamethoxazole (57.8%–73%), and fluoroquinolone (27.8%–74%), as listed in Table 1. Intermediate levels of resistance were seen against gentamicin (15.6%–36.5%), piperacillin/tazobactam (2.2%–22%), and nitrofurantoin (2.2%–9.1%). One study each from Kuwait, Qatar, and Saudi Arabia evaluated the resistance rates of the ESBL Enterobacterales isolates against tigecycline, and 0%, 35.8%, and 17.8% of the tested isolates were identified as resistant, respectively (Table 1). Low resistance rates were seen against carbapenems (0%–5.1%), amikacin (0%–13%), and colistin (2.2%), with absolute sensitivity to fosfomycin (Table 1). ESBL and carbapenem-resistant Enterobacterales isolates showed comparatively higher resistance rates against piperacillin/tazobactam (100%), cefoxitin (84%), fluoroquinolone (92%), gentamicin (68%), and colistin (4%) compared with ESBL Enterobacterales (Table 1). These isolates still showed absolute susceptibility to fosfomycin and tigecycline. One study from Qatar identified that ESBL Enterobacterales from ICU patients in the region demonstrated high susceptibility (99.1%) against two new antibiotic combinations of ceftazidime/avibactam and ceftolozane/tazobactam, which could be potentially alternative therapeutic options for treatment of such infections (Ahmed et al., 2022).
3.2.2 ESBL E. coli
ESBL E. coli isolates in the region (excluding studies primarily dealing with urinary isolates and concomitant ESBL and carbapenem-resistant isolates) showed a similar profile as depicted by the ESBL Enterobacterales, with high–medium resistance rates to ampicillin (94.7%–100%), amoxicillin/clavulanate (5.1%–97.3%), aztreonam (79%–100%), trimethoprim/sulfamethoxazole (52.1%–78.9%), and fluoroquinolone (0%–77%), with lower resistance rates against piperacillin/tazobactam (0%–15.4%), carbapenems (0%–20%), nitrofurantoin (0%–31.6%), gentamicin (0%–32%), amikacin (0%–15.8%), colistin (0%–1%), and tigecycline (0%–4.1%), and absolute sensitivity to fosfomycin, as listed in Supplementary Table 1. Isolates from Saudi Arabia were particularly noteworthy, with higher resistance rates toward nitrofurantoin (1%–31.6%), amikacin (0%–15.8%), trimethoprim/sulfamethoxazole (56.2%–78.9%), fluoroquinolone (0%–77%), and tigecycline (0–4.1%) compared with data from Kuwait, Bahrain, Oman, and the United Arab Emirates, where the values for these countries ranged from 0% to 9.9% for nitrofurantoin, 0% to 1.9% for amikacin, 52.1% to 66.8% for trimethoprim/sulfamethoxazole, 39.6% to 59% for fluoroquinolone, and with 0% for tigecycline (Supplementary Table 1). The isolates from the United Arab Emirates also showed a atypical low resistance rate to amoxicillin clavulanate at 5.1%, compared with higher resistance rates ranging from 9% to 97.3% in Bahrain, Qatar, Kuwait, and Saudi Arabia (Supplementary Table 1). ESBL and carbapenem-resistant E. coli isolates showed higher resistance rates to piperacillin/tazobactam (100%), fluoroquinolone (100%), and gentamicin (50%) (Supplementary Table 1) than ESBL E. coli isolates.
ESBL E. coli isolates from urinary infections generally showed, overall, a high resistance to most antibiotics, including ampicillin (47.8%–100%), aztreonam (99.2%–100%), amoxicillin/clavulanate (33.3%–100%), trimethoprim/sulfamethoxazole (33.3%–82%), fluoroquinolone (0%–100%), nitrofurantoin (0%–52.2%), gentamicin (13.7%–63.6%), and tigecycline (0%–77.1%) (Supplementary Table 1), except for piperacillin/tazobactam, amikacin, and fosfomycin, that had low resistance rates of 3%–27.1%, 0%–7.7%, and 1.2%–3.7%, respectively (Supplementary Table 1). Studies from Bahrain, Oman, Saudi Arabia, and the United Arab Emirates investigating ESBL E. coli isolates from urinary infections also reported almost absolute sensitivity to carbapenems in the region (Supplementary Table 1). Concerningly, a study from Saudi Arabia reported a colistin resistance rate of 58.4% among ESBL E. coli in a cohort of patients with UTIs (Abalkhail et al., 2022). In Oman, a decrease in resistance was seen in E. coli isolates to cephalosporins, aminoglycosides, amoxicillin-clavulanic acid, cotrimoxazole, piperacillin/tazobactam, nitrofurantoin, and carbapenems from 2013 to 2018, which the authors attribute to increased prescription of fluoroquinolones, thus effectively putting a halt to resistance development toward other drugs (Al Mamari et al., 2022).
3.2.3 ESBL K. pneumoniae
ESBL K. pneumoniae in the GCC region (excluding studies primarily dealing with urinary isolates) also showed overall high resistance rates to most antibiotics, including ampicillin (50%–100%), aztreonam (88.2%–100%), amoxicillin/clavulanate (20%–89.7%), trimethoprim/sulfamethoxazole (0%–93.3%), nitrofurantoin (0%–81.6%), fluoroquinolone (0%–56.4%), and gentamicin (28.6%–90%) (Supplementary Table 2), and intermediate resistance to amikacin (0%–50%) and piperacillin/tazobactam (0%–35.3%) (Supplementary Table 2). Lower rates of resistances were seen against carbapenems (0%–14.63%) and colistin (2.7%) (Supplementary Table 2). Few studies from the region also reported absolute sensitivity to fosfomycin (Al-Agamy et al., 2018; Alfaresi et al., 2018; Karlowsky et al., 2022) (Supplementary Table 2). Although multiple studies from the region reported absolute sensitivity to tigecycline (Al-Agamy et al., 2018; Al Rahmany et al., 2019; Al-Sweih et al., 2021; Bazaid et al., 2022), a few studies from Saudi Arabia reported higher resistance rates, ranging from 8% to 23.4%, for tigecycline (Aldrazi et al., 2020; Jalal et al., 2023). ESBL and carbapenem-resistant K. pneumonia isolates showed comparatively higher resistance rates to piperacillin/tazobactam (100%), fluoroquinolone (89.5%), and colistin (5.3%) than ESBL K. pneumonia (Supplementary Table 2). In a 10-year study (2011–2021), K. pneumonia isolates in Saudi Arabia were noted as becoming increasingly resistant to ceftazidime, cefotaxime, and cefepime, with resistance rates of 29.9%, 26.2%, and 53.9%, respectively, in 2011 and resistance rates of 84.9%, 85.1%, and 85.8%, respectively, in 2021 (Jalal et al., 2023).
ESBL K. pneumoniae isolates from urinary infections once again showed high rates of resistance to ampicillin (58.3%–100%), amoxicillin/clavulanate (69%–100%), nitrofurantoin (14%–57%), trimethoprim/sulfamethoxazole (14%–85%), fluoroquinolone (0%–48%), gentamicin (0%–83.3%), amikacin (0%–57%), tigecycline (0%–41.7%), and cefoxitin (8.3%–100%) (Supplementary Table 2), and showed lower rates of resistance to only piperacillin/tazobactam (7%–17%) and carbapenems (0%–7%) (Supplementary Table 2), with absolute sensitivity to colistin, as reported in one study from Saudi Arabia (Alzahrani et al., 2020). In Oman, a decrease in resistance was seen in K. pneumoniae isolates to cephalosporins and ciprofloxacin, whereas an increasing resistance was noted toward amikacin, amoxicillin-clavulanic acid, cotrimoxazole, nitrofurantoin, and carbapenems from 2013 to 2018. The authors attribute this to a decline in the number of prescriptions of cephalosporins, which subsequently reduced further resistance development against them (Al Mamari et al., 2022).
Despite the reported alternative options for ESBL infections, such as nitrofurantoin, quinolones, and cotrimoxazole, regional genetic variations must also be taken into consideration. For example, high rates of glucose-6-phosphate dehydrogenase (G6PD) deficiency is prevalent in the region, particularly in the Bahraini population (rates of G6PD deficiency in newborn males and females were 18% and 10%, respectively) (Al-Arayyed et al., 2007; Saeed et al., 2021). These observations preclude the liberal use of drugs such as ciprofloxacin, nitrofurantoin, and trimethoprim-sulfamethoxazole for fear of inducing hemolytic crises in G6PD-deficient recipients (Saeed et al., 2021).
3.3 Genetic characteristics
Among the studies from different countries in the GCC region, it was clear that the blaCTX-M gene is the dominant ESBL gene among ESBL Enterobacterales (39.6%–100%), E. coli (55.5%–100%), and K. pneumoniae (32.1%–100%) in this region across different sample types and clinical settings (Table 2). Specifically, the enzymes belonging to the CTX-M group 1, which include CTX-M1, CTX-M-15, and CTX-M-28 enzymes (Dehshiri et al., 2018), are predominant. The blaCTX-M-15 gene was reported as being the most prevalent ESBL gene in isolates in the gulf region, and this is still the case in many studies from the region (Alqasim et al., 2018; Bin Thani, 2018; Ibrahim et al., 2021). However, this is being replaced by blaCTX-M-28 in some countries in the GCC region (e.g., the United Arab Emirates), and is similar to trends being reported in countries like South Korea (Yoo et al., 2010; Alfaresi et al., 2011; Alfaresi et al., 2018). Both genes are extremely similar to each other, with a difference of only one amino acid, and they both belong to the same CTX-M group 1 (Alfaresi et al., 2018). The genes identified in ESBL Enterobacterales isolates from the GCC region and specifically in ESBL E. coli and K. pneumoniae are detailed in Table 2. Colistin resistance genes (mcr-1 and mcr-9) have also been reported in Enterobacterales isolates from the region (Perez-Lopez et al., 2020; Tsui et al., 2020a; Tsui et al., 2020b).
Previously, a meta-analysis of studies from the GCC region on E. coli strains collected between 2013 and 2019 from the region identified that the main resistance genes in the region included CTX−M (53.8%), followed by TEM (40.6%), NDM−1 (28.4%), OXA (24.3%), VIM (8.5%), and SHV (7.8%) (Bindayna et al., 2022). As evidenced in Table 2, this is largely still the case for ESBL E. coli and K. pneumoniae, where CTX−M dominates (55.5%–100% and 32.1%–100%, respectively), followed by TEM (27.3%–100% and 26%–100%, respectively), and SHV (5.3%–100% and 3.6%–100%, respectively) (Table 2).
3.4 Prevention measures
One of the main aims of introducing antimicrobial stewardship programmes (ASPs) in healthcare is to combat the growing rates of AMR. Over the last decade, most GCC countries introduced ASPs in healthcare settings, with various implementation challenges (McGowan and Gerding, 1996; Hashad et al., 2020). In some countries in the region, the implementation of ASPs is showing success, as evidenced by the incidence of MDR Enterobacteriaceae, which have declined in response to strict implementation of ASPs in Bahrain. The adoption of strict policies in 2014 in Bahrain led to a decrease in the incidence of MDR Enterobacteriaceae from a high of 45.4 cases per 10,000 patient admissions to 23.2 cases per 10,000 patient admissions (Saeed et al., 2019). In addition, to combat the increasing AMR threat, many countries in the world, including GCC countries, have adopted the Global Action Plan on AMR (GAPAMR), the implementation of which is monitored by the Tracking AMR Country Self-Assessment Survey (TrACSS 2022 country reports, 2022). Saudi Arabia and Oman have demonstrated nationwide implementation of most indicators of national progress and capacity on tackling AMR, at levels exceeding the global average for many of these indicators. Regionally, Qatar has achieved nationwide implementation of three of the five indicators in the human health sector. Kuwait, the United Arab Emirates, and Bahrain have demonstrated success in implementing two of the five indicators. Although substantial progress has been made in tackling AMR in the GCC region in the last few years, this still remains an area of concern and needs continuous attention and efforts.
3.5 Limitations
One of the fundamental limitations of this review is that the prevalence of MDR, microbiological characteristics, the distribution of isolates, and ESBL activity are very difficult to compare between studies because of the paucity of reports from certain countries and because of the heterogeneity in studies, with specific differences in sample sources (i.e., community, hospital outpatient/inpatients, and critical care), sample types (i.e., urine, respiratory, wound and bloodstream infections), collection periods, regional and local guidelines for classifying intermediate resistance as resistant/susceptible, and type of analytical methods used (Bandy and Tantry, 2021). In addition, there exists inherent limitations in individual studies, including small sample sizes and studied populations. These differences make comparisons between studies susceptible to conclusive biases. Nevertheless, the review compiled the majority of its data from recent and available published literature in the studied subject from all regional countries, which will hopefully pave the way for future research.
4 Summary and conclusions
The GCC countries have a substantial expatriate population, ranging from approx 40-90%. The region is an important destination for travel, tourism, and global trade, and frequently hosts international or religious events, such as Umrah and Hajj. These factors, along with an embedded culture of inappropriate, excessive antimicrobial prescribing, and poor antibiotic regulations, spearhead the propagation of AMR (Sonnevend et al., 2015; Saeed et al., 2019).
The prevalence rates of ESBL-Enterobacterales in the GCC region predominated by E. coli and K. pneumoniae are similar to many global regions with intermediate regional prevalence; however, higher prevalence rates were observed in patients with UTIs and in samples from critical care. The prevalence of ESBL in the community, including carrier state, is alarming, and, therefore, there is a need for critical surveillance, exploration of underlying precipitating factors, and development of public health preventive mechanisms to halt its spread.
Owing to the increased morbidity, mortality, and economic costs associated with MDR infections, predictors for the acquisition of MDR infections would be invaluable tools to assist its management (Baiou et al., 2021; El-Sokkary et al., 2021). Another area of focus should be the development of novel therapeutics against these organisms, an area that has been significantly neglected in the past decade. A distinctive fact is that Enterobacterales isolates that are ESBL producing and carbapenem resistant are being increasingly reported in the region, which warrants concomitant control and prevention measures, and the introduction of novel therapeutic agents.
Promisingly, however, there is a growing research focus in this field in almost all GCC countries, which should strengthen the monitoring process in these countries. Furthermore, strict antimicrobial stewardship is also being implemented in almost all GCC countries, which will hopefully control and monitor the appropriate prescribing of antimicrobials on a long-term basis.
Author contributions
HH, HA-H, LA, MA-O, MAS, HH, HM, BS, AS, and SS analyzed the data and wrote/reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This publication was made possible by NPRP grant (NPRP12S-0219-190109) from the Qatar National Research Fund (a member of Qatar Foundation).
Acknowledgments
The authors are also grateful for the support provided by the Department of Medical education at Weill Cornell Medicine—Qatar.
Conflict of interest
Author HH was employed by the company Hamad Medical Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frabi.2023.1177954/full#supplementary-material
References
Abalkhail A., Alyami A. S., Alrashedi S. F., Almushayqih K. M., Alslamah T., Alsalamah Y. A., et al. (2022). The prevalence of multidrug-resistant escherichia coli producing ESBL among Male and female patients with urinary tract infections in Riyadh region, Saudi Arabia. Healthcare (Basel) 10. doi: 10.3390/healthcare10091778
Abdallah F. B., Lagha R., Al-Sarhan B., Mabrouk I., Alhomrani M., Gaber A., et al. (2020). Molecular characterization of multidrug resistant e. coli associated to urinary tract infection in taif, Saudi Arabia. Pak J. Pharm. Sci. 33, 2759–2766.
Abulhasan Y. B., Abdullah A. A., Shetty S. A., Ramadan M. A., Yousef W., Mokaddas E. M. (2020). Health care-associated infections in a neurocritical care unit of a developing country. Neurocrit. Care 32, 836–846. doi: 10.1007/s12028-019-00856-8
Abu-Rub L. I., Abdelrahman H. A., Johar A. A., Alhussain H. A., Hadi H. A., Eltai N. O. (2021). Antibiotics prescribing in intensive care settings during the COVID-19 era: a systematic review. Antibiotics (Basel) 10. doi: 10.3390/antibiotics10080935
Ahmed M. A. S., Ibrahim E. B., Hamid J. M., Daghfal J., Alyazidi M. A., Abdelwahab A. H., et al. (2022). Evaluation of in vitro activity of Ceftazidime/Avibactam and Ceftolozane/Tazobactam against ESBL-producing enterobacterales isolated from intensive care units from Qatar. Oman Med. J. 37, e422. doi: 10.5001/omj.2022.89
Al-Agamy M. H., Aljallal A., Radwan H. H., Shibl A. M. (2018). Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive escherichia coli and klebsiella pneumoniae in Riyadh hospitals. J. Infect. Public Health 11, 64–68. doi: 10.1016/j.jiph.2017.03.010
Al-Agamy M. H., El-Mahdy T. S., Radwan H. H., Poirel L. (2019). Cooccurrence of NDM-1, ESBL, RmtC, AAC(6')-ib, and QnrB in clonally related klebsiella pneumoniae isolates together with coexistence of CMY-4 and AAC(6')-ib in enterobacter cloacae isolates from Saudi Arabia. BioMed. Res. Int. 2019, 6736897. doi: 10.1155/2019/6736897
Alanazi M. Q., Alqahtani F. Y., Aleanizy F. S. (2018). An evaluation of e. coli in urinary tract infection in emergency department at KAMC in Riyadh, Saudi Arabia: retrospective study. Ann. Clin. Microbiol. Antimicrob. 17, 3.
Alangari A., Jaffal A. A., Alyousef A. M., Alyousef A. A. (2022). Comparative metabolic characterization of extraintestinal pathogenic escherichia coli blood isolates from Saudi Arabia. J. Trop. Med. 2022, 1745835. doi: 10.1155/2022/1745835
Al-Arayyed S., Sultan B., Shome D. K., Bapat J. P. (2007). Neonatal screening for genetic blood diseases. Bahrain Med. Bull. 29, 88–90.
Alasmary M. Y. (2021). Antimicrobial resistance patterns and ESBL of uropathogens isolated from adult females in najran region of Saudi Arabia. Clin. Pract. 11, 650–658. doi: 10.3390/clinpract11030080
Al Benwan K., Jamal W. (2022). Etiology and antibiotic susceptibility patterns of urinary tract infections in children in a general hospital in Kuwait: a 5-year retrospective study. Med. Princ. Pract. 31, 562–569. doi: 10.1159/000527640
Al Bshabshe A., Al-Hakami A., Alshehri B., Al-Shahrani K. A., Alshehri A. A., Al Shahrani M. B., et al. (2020). Rising klebsiella pneumoniae infections and its expanding drug resistance in the intensive care unit of a tertiary healthcare hospital, Saudi Arabia. Cureus 12, e10060. doi: 10.7759/cureus.10060
Aldawsari A., Tawfik K., Al-Zaagi I. C. (2020). Antimicrobial-resistant bacteria and prescription of antibiotics at a tertiary care hospital in Riyadh, Saudi Arabia. Cureus 12, e12098. doi: 10.7759/cureus.12098
Aldrazi F. A., Rabaan A. A., Alsuliman S. A., Aldrazi H. A., Alabdalslam M. J., Alsadiq S. A., et al. (2020). ESBL expression and antibiotic resistance patterns in a hospital in Saudi Arabia: do healthcare staff have the whole picture? J. Infect. Public Health 13, 759–766. doi: 10.1016/j.jiph.2019.12.001
Alfaresi M. S., Elkoush A. A., Alshehhi H. M., Abdulsalam A. I. (2011). Molecular characterization and epidemiology of extended-spectrum beta-lactamase-producing escherichia coli and klebsiella pneumoniae isolates in the united Arab Emirates. Med. Princ. Pract. 20, 177–180. doi: 10.1159/000319912
Alfaresi M., Kim Sing G., Senok A. (2018). First report of bla(CTX-M-28) in enterobacteriaceae isolates in the united Arab Emirates. J. Pathog. 2018, 1304793–1304793. doi: 10.1155/2018/1304793
Alfouzan W., Dhar R., Abdo N. M., Alali W. Q., Rabaan A. A. (2021). Epidemiology and microbiological profile of common healthcare associated infections among patients in the intensive care unit of a general hospital in Kuwait: a retrospective observational study. J. Epidemiol. Glob. Health 11, 302–309. doi: 10.2991/jegh.k.210524.001
Al-Garni S. M., Ghonaim M. M., Ahmed M. M. M., Al-Ghamdi A. S., Ganai F. A. (2018). Risk factors and molecular features of extended-spectrum beta-lactamase producing bacteria at southwest of Saudi Arabia. Saudi Med. J. 39, 1186–1194. doi: 10.15537/smj.2018.12.23273
Alkofide H., Alhammad A. M., Alruwaili A., Aldemerdash A., Almangour T. A., Alsuwayegh A., et al. (2020). Multidrug-resistant and extensively drug-resistant enterobacteriaceae: prevalence, treatments, and outcomes - a retrospective cohort study. Infect. Drug Resist. 13, 4653–4662. doi: 10.2147/IDR.S283488
Al Mamari Y., Sami H., Siddiqui K., Tahir H. B., Al Jabri Z., Al Muharrmi Z., et al. (2022). Trends of antimicrobial resistance in patients with complicated urinary tract infection: suggested empirical therapy and lessons learned from a retrospective observational study in Oman. Urol. Ann. 14 (4), 345–352. doi: 10.4103/ua.ua_67_22
Almogbel M., Altheban A., Alenezi M., Al-Motair K., Menezes G. A., Elabbasy M., et al. (2021). CTX-M-15 positive escherichia coli and klebsiella pneumoniae outbreak in the neonatal intensive care unit of a maternity hospital in ha'il, Saudi Arabia. Infect. Drug Resist. 14, 2843–2849. doi: 10.2147/IDR.S317079
Alqasim A., Abu Jaffal A., Alyousef A. A. (2018). Prevalence of multidrug resistance and extended-spectrum β-lactamase carriage of clinical uropathogenic escherichia coli isolates in Riyadh, Saudi Arabia. Int. J. Microbiol. 2018, 3026851. doi: 10.1155/2018/3026851
Al Rahmany D., Albeloushi A., Alreesi I., Alzaabi A., Alreesi M., Pontiggia L., et al. (2019). Exploring bacterial resistance in northern Oman, a foundation for implementing evidence-based antimicrobial stewardship program. Int. J. Infect. Dis. 83, 77–82. doi: 10.1016/j.ijid.2019.04.004
Al-Sweih N., Jamal W., Mokaddas E., Habashy N., Kurdi A., Mohamed N. (2021). Evaluation of the in vitro activity of ceftaroline, ceftazidime/avibactam and comparator antimicrobial agents against clinical isolates from paediatric patients in Kuwait: ATLAS data 2012-19. JAC Antimicrob. Resist. 3, dlab159. doi: 10.1093/jacamr/dlab159
Al-Tawfiq J. A., Rabaan A. A., Saunar J. V., Bazzi A. M. (2020). Antimicrobial resistance of gram-negative bacteria: a six-year longitudinal study in a hospital in Saudi Arabia. J. Infect. Public Health 13, 737–745. doi: 10.1016/j.jiph.2020.01.004
Alzahrani M. A., Ali M. S., Anwar S. (2020). Bacteria causing urinary tract infections and its antibiotic susceptibility pattern at tertiary hospital in Al-baha region, Saudi Arabia: a retrospective study. J. Pharm. Bioallied Sci. 12, 449–456. doi: 10.4103/JPBS.JPBS_294_19
Alzahrani M. A., Sadoma H. H. M., Mathew S., Alghamdi S., Malik J. A., Anwar S. (2021). Retrospective analysis of antimicrobial susceptibility of uropathogens isolated from pediatric patients in tertiary hospital at Al-baha region, Saudi Arabia. Healthcare (Basel) 9. doi: 10.3390/healthcare9111564
Al-Zalabani A., Althobyane O. A., Alshehri A. H., Alrehaili A. O., Namankani M. O., Aljafri O. H. (2020). Prevalence of klebsiella pneumoniae antibiotic resistance in Medina, Saudi arabi -2018. Cureus 12, e9714. doi: 10.7759/cureus.9714
Andres P.-L., Sathyavathi S., Hassan A.-M., Kin Ming T., Mohammad Rubayet H., Mohammed S., et al. (2020). Molecular characterization of extended-spectrum β-Lactamase-Producing escherichia coli and klebsiella pneumoniae among the pediatric population in Qatar. Front. Microbiol. 11, 581711. doi: 10.3389/fmicb.2020.581711
Aslam B., Khurshid M., Arshad M. I., Muzammil S., Rasool M., Yasmeen N., et al. (2021). Antibiotic resistance: one health one world outlook. Front. Cell Infect. Microbiol. 11, 771510. doi: 10.3389/fcimb.2021.771510
Azab K. S. M., Abdel-Rahman M. A., El-Sheikh H. H., Azab E., Gobouri A. A., Farag M. M. S. (2021). Distribution of extended-spectrum beta-lactamase (ESBL)-encoding genes among multidrug-resistant gram-negative pathogens collected from three different countries. Antibiotics (Basel) 10. doi: 10.3390/antibiotics10030247
Azim N. S. A., Nofal M. Y., Alharbi M. A., Al-Zaban M. I., Somily A. M. (2019). Molecular-diversity, prevalence and antibiotic susceptibility of pathogenic klebsiella pneumoniae under Saudi condition. Pak J. Biol. Sci. 22, 174–179. doi: 10.3923/pjbs.2019.174.179
Badger-Emeka L. I., Al-Sultan A. A., Bohol M. F. F., Al-Anazi M. R., Al-Qahtani A. A. (2021). Genetic analysis, population structure, and characterisation of multidrug-resistant klebsiella pneumoniae from the Al-hofuf region of Saudi Arabia. Pathogens 10. doi: 10.3390/pathogens10091097
Badger-Emeka L. I., Kausar N., Estrella E., Angeles G. B. (2022). A three-year look at the phylogenetic profile, antimicrobial resistance, and associated virulence genes of uropathogenic escherichia coli. Pathogens 11. doi: 10.3390/pathogens11060631
Baiou A., Elbuzidi A. A., Bakdach D., Zaqout A., Alarbi K. M., Bintaher A. A., et al. (2021). Clinical characteristics and risk factors for the isolation of multi-drug-resistant gram-negative bacteria from critically ill patients with COVID-19. J. Hosp. Infect. 110, 165–171. doi: 10.1016/j.jhin.2021.01.027
Balkhi B., Mansy W., Alghadeer S., Alnuaim A., Alshehri A., Somily A. (2018). Antimicrobial susceptibility of microorganisms causing urinary tract infections in Saudi Arabia. J. Infect. Dev. Ctries 12, 220–227. doi: 10.3855/jidc.9517
Balkhy H. H., El-Saed A., Alshamrani M. M., Alsaedi A., Nasser W. A., Gammal A. E., et al. (2020). High burden of resistant gram negative pathogens causing device-associated healthcare infections in a tertiary care setting in Saudi arabi -2016. J. Glob Antimicrob. Resist. 23, 26–32. doi: 10.1016/j.jgar.2020.07.013
Bandy A., Almaeen A. H. (2020). Pathogenic spectrum of blood stream infections and resistance pattern in gram-negative bacteria from aljouf region of Saudi Arabia. PloS One 15, e0233704. doi: 10.1371/journal.pone.0233704
Bandy A., Tantry B. (2021). ESBL activity, MDR, and carbapenem resistance among predominant enterobacterales isolated in 2019. Antibiotics (Basel) 10. doi: 10.3390/antibiotics10060744
Bassetti M., Poulakou G., Ruppe E., Bouza E., Van Hal S. J., Brink A. (2017). Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 43, 1464–1475. doi: 10.1007/s00134-017-4878-x
Bazaid A. S., Aldarhami A., Gattan H., Barnawi H., Qanash H., Alsaif G., et al. (2022). Antibiogram of urinary tract infections and sepsis among infants in neonatal intensive care unit. Children (Basel) 9. doi: 10.3390/children9050629
Bazaid A. S., Saeed A., Alrashidi A., Alrashidi A., Alshaghdali K., Alreshidi T., et al. (2021). Antimicrobial surveillance for bacterial uropathogens in ha'il, Saudi Arabia: a five-year multicenter retrospective study. Infect. Drug Resist. 14, 1455–1465. doi: 10.2147/IDR.S299846
Beovic B., Dousak M., Ferreira-Coimbra J., Nadrah K., Rubulotta F., Belliato M., et al. (2020). Antibiotic use in patients with COVID-19: a 'snapshot' infectious diseases international research initiative (ID-IRI) survey. J. Antimicrob. Chemother. 75, 3386–3390. doi: 10.1093/jac/dkaa326
Bindayna K. M., Joji R. M., Ezzat H., Jahrami H. A. (2022). Antibiotic-resistance genes in e. coli strains in GCC countries: a meta-analysis. Saudi J. Med. Med. Sci. 10, 1–11. doi: 10.4103/sjmms.sjmms_638_21
Bin Thani A. S. (2018). Identification of novel DNA sequence associated with pathogenicity island III536 locus in uropathogenic escherichia coli isolate and distribution of virulence determinants in beta-lactam resistant isolates. Microb. Pathog. 123, 393–397. doi: 10.1016/j.micpath.2018.07.035
Burillo A., Bouza E. (2022). Controversies over the management of infections caused by amp-c- and ESBL-producing enterobacterales : what questions remain for future studies? Curr. Opin. Infect. Dis. 35, 575–582. doi: 10.1097/QCO.0000000000000863
Bush K. (2018). Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 62 (10), e01076-18. doi: 10.1128/AAC.01076-18
Caron Y., Chheang R., Puthea N., Soda M., Boyer S., Tarantola A., et al. (2018). Beta-lactam resistance among enterobacteriaceae in Cambodia: the four-year itch. Int. J. Infect. Dis. 66, 74–79. doi: 10.1016/j.ijid.2017.10.025
Dash N., Mansour A. L. Z., Nora A.-K., Shehhi F., Jalila A.-N., Senok A., et al. (2008). Distribution and resistance trends of community associated urinary tract pathogens in sharjah, UAE. Microbiol. Insights 2008, 41–45. doi: 10.4137/MBI.S780
Dehshiri M., Khoramrooz S. S., Zoladl M., Khosravani S. A., Parhizgari N., Motazedian M. H., et al. (2018). The frequency of klebsiella pneumonia encoding genes for CTX-m, TEM-1 and SHV-1 extended-spectrum beta lactamases enzymes isolated from urinary tract infection. Ann. Clin. Microbiol. Antimicrob. 17, 4. doi: 10.1186/s12941-018-0256-y
El-Saed A., Balkhy H. H., Alshamrani M. M., Aljohani S., Alsaedi A., Al Nasser W., et al. (2020). High contribution and impact of resistant gram negative pathogens causing surgical site infections at a multi-hospital healthcare system in Saudi arabi -2016. BMC Infect. Dis. 20, 275. doi: 10.1186/s12879-020-4939-6
El-Sokkary R., Uysal S., Erdem H., Kullar R., Pekok A. U., Amer F., et al. (2021). Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: an international ID-IRI survey. Eur. J. Clin. Microbiol. Infect. Dis. 40, 2323–2334. doi: 10.1007/s10096-021-04288-1
Eltai N. O., Al Thani A. A., Al-Ansari K., Deshmukh A. S., Wehedy E., Al-Hadidi S. H., et al. (2018a). Molecular characterization of extended spectrum [beta] -lactamases enterobacteriaceae causing lower urinary tract infection among pediatric population. Antimicrobial Resistance Infection Control 7. doi: 10.1186/s13756-018-0381-6
Eltai N. O., Al Thani A. A., Al-Ansari K., Deshmukh A. S., Wehedy E., Al-Hadidi S. H., et al. (2018b). Molecular characterization of extended spectrum β -lactamases enterobacteriaceae causing lower urinary tract infection among pediatric population. Antimicrobial Resistance Infection Control 7, 90. doi: 10.1186/s13756-018-0381-6
Eltai N. O., Al Thani A. A., Al Hadidi S. H., Al Ansari K., Yassine H. M. (2020). Antibiotic resistance and virulence patterns of pathogenic escherichia coli strains associated with acute gastroenteritis among children in Qatar. BMC Microbiol. 20, 54. doi: 10.1186/s12866-020-01732-8
Eltai N. O., Yassine H. M., Al Thani A. A., Abu Madi M. A., Ismail A., Ibrahim E., et al. (2018c). Prevalence of antibiotic resistant escherichia coli isolates from fecal samples of food handlers in Qatar. Antimicrob. Resist. Infect. Control 7, 78. doi: 10.1186/s13756-018-0369-2
Exner M., Bhattacharya S., Christiansen B., Gebel J., Goroncy-Bermes P., Hartemann P., et al. (2017). Antibiotic resistance: what is so special about multidrug-resistant gram-negative bacteria? GMS Hyg Infect. Control 12, Doc05. doi: 10.3205/dgkh000290
Farah S. M., Alshehri M. A., Alfawaz T. S., Alasmeri F. A., Alageel A. A., Alshahrani D. A. (2019). Trends in antimicrobial susceptibility patterns in king fahad medical city, Riyadh, Saudi Arabia. Saudi Med. J. 40, 252–259. doi: 10.15537/smj.2019.3.23947
Goodman K. E., Simner P. J., Tamma P. D., Milstone A. M. (2016). Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant enterobacteriaceae (CRE). Expert Rev. Anti Infect. Ther. 14, 95–108. doi: 10.1586/14787210.2016.1106940
Hashad N., Perumal D., Stewart D., Tonna A. P. (2020). Mapping hospital antimicrobial stewardship programmes in the gulf cooperation council states against international standards: a systematic review. J. Hosp Infect. 106, 404–418. doi: 10.1016/j.jhin.2020.09.004
Huttner A., Harbarth S., Carlet J., Cosgrove S., Goossens H., Holmes A., et al. (2013). Antimicrobial resistance: a global view from the 2013 world healthcare-associated infections forum. Antimicrob. Resist. Infect. Control 2, 31–31. doi: 10.1186/2047-2994-2-31
Ibrahim M. E., Abbas M., Al-Shahrai A. M., Elamin B. K. (2019). Phenotypic characterization and antibiotic resistance patterns of extended-spectrum beta-lactamase- and AmpC beta-Lactamase-Producing gram-negative bacteria in a referral hospital, Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. 2019, 6054694. doi: 10.1155/2019/6054694
Ibrahim M. E., Algak T. B., Abbas M., Elamin B. K. (2021). Emergence of bla TEM, bla CTX-m, bla SHV and bla OXA genes in multidrug-resistant enterobacteriaceae and acinetobacter baumannii in Saudi Arabia. Exp. Ther. Med. 22, 1450. doi: 10.3892/etm.2021.10885
Jalal N. A., Al-Ghamdi A. M., Momenah A. M., Ashgar S. S., Bantun F., Bahwerth F. S., et al. (2023). Prevalence and antibiogram pattern of klebsiella pneumoniae in a tertiary care hospital in makkah, Saudi Arabia: an 11-year experience. Antibiotics (Basel) 12. doi: 10.3390/antibiotics12010164
Joji R. M., Al-Mahameed A. E., Jishi T. A., Fatani D. I., Saeed N. K., Jaradat A., et al. (2021). Molecular detection of plasmid-derived AmpC beta-lactamase among clinical strains of enterobacteriaceae in Bahrain. Ann. Thorac. Med. 16, 287–293. doi: 10.4103/atm.ATM_523_20
Kabrah A. (2022). Extended-spectrum beta-lactamase and carbapenem-resistant gram-negative pathogens in makkah, Saudi Arabia. Ethiop J. Health Sci. 32 (6), 1221–1230. doi: 10.4314/ejhs.v32i6.20
Kabrah A. M., Kabrah S. M., Bahwerth F. S., Alredaini N. F. (2021). Antibiotic resistance profile of common bacteria isolated from blood stream, lower respiratory tract and urinary infections in intensive care unit in Saudi Arabia: a retrospective study. Ethiop J. Health Sci. 31, 1231–1240. doi: 10.4314/ejhs.v31i6.19
Karlowsky J. A., Lob S. H., Deryke C. A., Siddiqui F., Young K., Motyl M. R., et al. (2022). Prevalence of ESBL non-CRE escherichia coli and klebsiella pneumoniae among clinical isolates collected by the SMART global surveillance programme from 2015 to 2019. Int. J. Antimicrob. Agents 59, 106535. doi: 10.1016/j.ijantimicag.2022.106535
Lagha R., Ben Abdallah F., Aah A. L., Amor N., Hassan M. M., Mabrouk I., et al. (2021). Molecular characterization of multidrug resistant klebsiella pneumoniae clinical isolates recovered from king abdulaziz specialist hospital at taif city, Saudi Arabia. J. Infect. Public Health 14, 143–151. doi: 10.1016/j.jiph.2020.12.001
Langford B. J., Soucy J. R., Leung V., So M., Kwan A. T. H., Portnoff J. S., et al. (2023). Antibiotic resistance associated with the COVID-19 pandemic: a systematic review and meta-analysis. Clin. Microbiol. Infect. 29(3), 302–309. doi: 10.1016/j.cmi.2022.12.006
Mcgowan J. E. Jr., Gerding D. N. (1996). Does antibiotic restriction prevent resistance? New Horiz. 4, 370–376.
Moghnia O. H., Rotimi V. O., Al-Sweih N. A. (2021). Monitoring antibiotic resistance profiles of faecal isolates of enterobacteriaceae and the prevalence of carbapenem-resistant isolates among food handlers in Kuwait. J. Glob. Antimicrob. Resist. 25, 370–376. doi: 10.1016/j.jgar.2021.04.009
Moglad E., Alanazi N., Altayb H. N. (2022). Genomic study of chromosomally and plasmid-mediated multidrug resistance and virulence determinants in klebsiella pneumoniae isolates obtained from a tertiary hospital in Al-kharj, KSA. Antibiotics (Basel) 11. doi: 10.3390/antibiotics11111564
Mohammed D., Isa H. M., Ali M. F. (2022). Microbial etiology and antibiotic resistance patterns of urinary tract pathogens in hospitalized infants in Bahrain: a tertiary care center experience. Cureus 14, e29236. doi: 10.7759/cureus.29236
Moher D., Liberati A., Tetzlaff J., Altman D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. doi: 10.1016/j.ijsu.2010.02.007
Mutair A. A., Alhumaid S., Alawi Z. A., Zaidi A. R. Z., Alzahrani A. J., Al-Tawfiq J. A., et al. (2021). Five-year resistance trends in pathogens causing healthcare-associated infections at a multi-hospital healthcare system in Saudi arabi -2019. J. Glob Antimicrob. Resist. 25, 142–150. doi: 10.1016/j.jgar.2021.03.009
Naushad V. A., Purayil N. K., Wilson G. J., Chandra P., Joseph P., Khalil Z., et al. (2022). Epidemiology of urinary tract infection in adults caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteriaceae - a case-control study from Qatar. IJID Reg. 3, 278–286. doi: 10.1016/j.ijregi.2022.05.001
Naylor N. R., Atun R., Zhu N., Kulasabanathan K., Silva S., Chatterjee A., et al. (2018). Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob. Resist. Infect. Control 7, 58–58. doi: 10.1186/s13756-018-0336-y
Nordmann P., Poirel L. (2019). Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin. Infect. Dis. 69, S521–S528. doi: 10.1093/cid/ciz824
Okdah L., Aldosary M. S., Almazyed A., Alkhurayb H. M., Almossallam M., Al Obaisi Y. S., et al. (2022). Genomic characterization of colistin-resistant isolates from the king fahad medical city, kingdom of Saudi Arabia. Antibiotics (Basel) 11. doi: 10.3390/antibiotics11111597
Partridge S. R. (2015). Resistance mechanisms in enterobacteriaceae. Pathology 47, 276–284. doi: 10.1097/PAT.0000000000000237
Perez-Lopez A., Sundararaju S., Al-Mana H., Tsui K. M., Hasan M. R., Suleiman M., et al. (2020). Molecular characterization of extended-spectrum beta-Lactamase-Producing escherichia coli and klebsiella pneumoniae among the pediatric population in Qatar. Front. Microbiol. 11, 581711. doi: 10.3389/fmicb.2020.581711
Ranjan Dash N., Albataineh M. T., Alhourani N., Khoudeir A. M., Ghanim M., Wasim M., et al. (2018). Community-acquired urinary tract infections due to extended-spectrum β -lactamase-producing organisms in united Arab Emirates. Travel Med. Infect. Dis. 22, 46–50. doi: 10.1016/j.tmaid.2018.01.007
Robles-Torres J. I., Castellani D., Trujillo-Santamaria H., Teoh J. Y., Tanidir Y., Campos-Salcedo J. G., et al. (2022). Prognosis of extended-Spectrum-Beta-Lactamase-Producing agents in emphysematous pyelonephritis-results from a Large, multicenter series. Pathogens 11. doi: 10.3390/pathogens11121397
Ruppe E., Woerther P. L., Barbier F. (2015). Mechanisms of antimicrobial resistance in gram-negative bacilli. Ann. Intensive Care 5, 61. doi: 10.1186/s13613-015-0061-0
Saeed N. K., Al Khawaja S., Al-Biltagi M. (2021). Antimicrobial susceptibilities of urinary extended-spectrum beta-lactamase escherichia coli to fosfomycin. Oman Med. J. 36, e314. doi: 10.5001/omj.2021.95
Saeed N. K., Alkhawaja S., Azam N., Alaradi K., Al-Biltagi M. (2019). Epidemiology of carbapenem-resistant enterobacteriaceae in a tertiary care center in the kingdom of Bahrain. J. Lab. Physicians 11, 111–117. doi: 10.4103/JLP.JLP_101_18
Saleem M., Syed Khaja A. S., Hossain A., Alenazi F., Said K. B., Moursi S. A., et al. (2023). Pathogen burden among ICU patients in a tertiary care hospital in hail Saudi Arabia with particular reference to beta-lactamases profile. Infect. Drug Resist. 16, 769–778. doi: 10.2147/IDR.S394777
Samarasinghe S. (2019). The distribution of ESBL-producing enterobacteriaceae: Leicestershire UK compared to worldwide. Am. J. Biomed. Sci. Res. 3 (1), AJBSR.MS.ID.000636. doi: 10.34297/AJBSR.2019.03.000636
Sannathimmappa M. B., Nambiar V., Aravindakshan R., Al-Kasaby N. M. (2021). Profile and antibiotic-resistance pattern of bacteria isolated from endotracheal secretions of mechanically ventilated patients at a tertiary care hospital. J. Educ. Health Promot 10 (1), 195. doi: 10.4103/jehp.jehp_1517_20
Shaaban O. A., Mahmoud N. A., Zeidan A. A., Kumar N., Finan A. C. (2021). Prevalence and resistance patterns of pediatric urinary tract infections in Bahrain. Cureus 13, e20859. doi: 10.7759/cureus.20859
Sid Ahmed M. A., Bansal D., Acharya A., Elmi A. A., Hamid J. M., Sid Ahmed A. M., et al. (2016). Antimicrobial susceptibility and molecular epidemiology of extended-spectrum beta-lactamase-producing enterobacteriaceae from intensive care units at hamad medical corporation, Qatar. Antimicrob. Resist. Infect. Control 5, 4. doi: 10.1186/s13756-016-0103-x
Siriphap A., Kitti T., Khuekankaew A., Boonlao C., Thephinlap C., Thepmalee C., et al. (2022). High prevalence of extended-spectrum beta-lactamase-producing escherichia coli and klebsiella pneumoniae isolates: a 5-year retrospective study at a tertiary hospital in northern Thailand. Front. Cell Infect. Microbiol. 12, 955774. doi: 10.3389/fcimb.2022.955774
Sonnevend A., Ghazawi A. A., Hashmey R., Jamal W., Rotimi V. O., Shibl A. M., et al. (2015). Characterization of carbapenem-resistant enterobacteriaceae with high rate of autochthonous transmission in the Arabian peninsula. PloS One 10, e0131372. doi: 10.1371/journal.pone.0131372
Suwantarat N., Carroll K. C. (2016). Epidemiology and molecular characterization of multidrug-resistant gram-negative bacteria in southeast Asia. Antimicrob. Resist. Infect. Control 5, 15. doi: 10.1186/s13756-016-0115-6
TrACSS 2022 country reports. (2022). Global database for tracking antimicrobial resistance (AMR) country self- assessment survey (TrACSS). (World Health Organization). Available at: https://amrcountryprogress.org/#/country-profile-view.
Tsui C. K. M., Sundararaju S., Al Mana H., Hasan M. R., Tang P., Perez-Lopez A. (2020a). Draft genome sequence of an extended-spectrum beta-Lactamase-Producing klebsiella oxytoca strain bearing mcr-9 from Qatar. Microbiol. Resour Announc 9. doi: 10.1128/MRA.00429-20
Tsui C. K. M., Sundararaju S., Mana H. A., Hasan M. R., Tang P., Perez-Lopez A. (2020b). Plasmid-mediated colistin resistance encoded by mcr-1 gene in escherichia coli co-carrying bla(CTX-M-15) and bla(NDM-1) genes in pediatric patients in Qatar. J. Glob Antimicrob. Resist. 22, 662–663. doi: 10.1016/j.jgar.2020.06.029
Walther-Rasmussen J., Høiby N. (2007). Class a carbapenemases. J. Antimicrobial Chemotherapy 60, 470–482. doi: 10.1093/jac/dkm226
WHO (2017) WHO publishes list of bacteria for which new antibiotics are urgently needed. Available at: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
Yasir M., Farman M., Shah M. W., Jiman-Fatani A. A., Othman N. A., Almasaudi S. B., et al. (2020). Genomic and antimicrobial resistance genes diversity in multidrug-resistant CTX-m-positive isolates of escherichia coli at a health care facility in jeddah. J. Infect. Public Health 13, 94–100. doi: 10.1016/j.jiph.2019.06.011
Yoo J. S., Byeon J., Yang J., Yoo J. I., Chung G. T., Lee Y. S. (2010). High prevalence of extended-spectrum beta-lactamases and plasmid-mediated AmpC beta-lactamases in enterobacteriaceae isolated from long-term care facilities in Korea. Diagn. Microbiol. Infect. Dis. 67, 261–265. doi: 10.1016/j.diagmicrobio.2010.02.012
Keywords: Enterobacterales, antibiotic resistance, multidrug resistance, Gulf Cooperation Council, E. coli, K. pneumoniae
Citation: Hadi HA, Al-Hail H, Aboidris LE, Al-Orphaly M, Ahmed MAS, Samuel BG, Mohamed HA, Sultan AA and Skariah S (2023) Prevalence and genetic characterization of clinically relevant extended-spectrum β-lactamase-producing Enterobacterales in the Gulf Cooperation Council countries. Front. Antibiot. 2:1177954. doi: 10.3389/frabi.2023.1177954
Received: 02 March 2023; Accepted: 17 May 2023;
Published: 26 June 2023.
Edited by:
Hsin-Yao Wang, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Telma Susana Lopes De Sousa, University of Trás-os-Montes and Alto Douro, PortugalWan-Ying Lin, Syu Kang Sport Clinic, Taiwan
Jia-Ruei Yu, Chang Gung Memorial Hospital, Taiwan
Copyright © 2023 Hadi, Al-Hail, Aboidris, Al-Orphaly, Ahmed, Samuel, Mohamed, Sultan and Skariah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali A. Sultan, YWxzMjAyNkBxYXRhci1tZWQuY29ybmVsbC5lZHU=; Sini Skariah, c2lzMjAxM0BxYXRhci1tZWQuY29ybmVsbC5lZHU=
†These authors have contributed equally to this work and share first authorship
 Hamad A. Hadi
Hamad A. Hadi Hissa Al-Hail2†
Hissa Al-Hail2† Leena Elsheikh Aboidris
Leena Elsheikh Aboidris Hana Adam Mohamed
Hana Adam Mohamed Ali A. Sultan
Ali A. Sultan Sini Skariah
Sini Skariah