
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Antibiot., 09 February 2023
Sec. Antibiotic Development
Volume 2 - 2023 | https://doi.org/10.3389/frabi.2023.1135485
The rise in antimicrobial resistance and the decline in new antibiotics has created a great need for novel approaches to treat drug resistant bacterial infections. Increasing the burden of antimicrobial resistance, bacterial virulence factors allow for survival within the host, where they can evade host killing and antimicrobial therapy within their intracellular niches. Repurposing host directed therapeutics has great potential for adjuvants to allow for more effective bacterial killing by the host and antimicrobials. To this end, phosphoinositide 3-kinase inhibitors are FDA approved for cancer therapy, but also have potential to eliminate intracellular survival of pathogens. This review describes the PI3K pathway and its potential as an adjuvant target to treat bacterial infections more effectively.
We are approaching a future where the antibiotics that we rely on today will no longer be effective (Reardon, 2014). Consequently, there is an urgent need to find new therapeutics for multi drug resistant bacteria (De Oliveira et al., 2020). However, finding novel antibiotics to replace our existing arsenal has proven to be difficult (Silver, 2011). To answer this unmet need for novel antibacterials, there have been investigations into the potential of adjuvant therapeutics that inhibit bacterial drug resistance or improve immune system clearance of bacteria (Abdul-Ghani et al., 2006; Wright, 2016; Liu et al., 2019; Chang et al., 2021). These therapeutics do not directly kill the bacteria but allow for better clearance of the infection by the host and/or common antibiotics (Wright, 2016,) (Zumla et al., 2016; Chiang et al., 2018; Kaufmann et al., 2018), which could improve the therapeutic outcome in patients with severe or chronic bacterial infections (Kilinc et al., 2021; Wallis et al., 2022). Although not yet approved by the FDA, there are a variety of host directed therapeutics being investigated to help treat bacterial infections (Kaufmann et al., 2018; Barker et al., 2019; Liu et al., 2021; Wallis et al., 2022).
A host kinase that has promise as a potential adjuvant therapeutic target is phosphoinositide 3-kinase (PI3K) (Kiran et al., 2016; Paik et al., 2019; Adefemi et al., 2020; Kilinc et al., 2021). PI3K is dysfunctional in a wide range of cancers and inhibition of PI3K has proven effective to mitigate the carcinogenic upregulation of PI3K that leads to uncontrolled cellular growth (Yang et al., 2019). The advantage of repurposing PI3K inhibitors for infectious disease treatment is they are already FDA approved for cancer therapy and there is abundance research into PI3K inhibitors (Garber, 2014; Zhang et al., 2020; Mishra et al., 2021; Vanhaesebroeck et al., 2021; Richardson et al., 2022). There are several classes and isotypes of PI3Ks used ubiquitously throughout the body. However, class 1 and 3 PI3Ks are those involved specifically in macrophage killing of bacteria (Gillooly et al., 2001). In addition, class 1 PI3Ks are important for neutrophil migration, and it has been shown that aberrant migration in aged neutrophils is corrected in the presence of PI3K inhibitors (Sapey et al., 2014).
Inhibition of PI3K has potential as an adjuvant because bacterial pathogens manipulate the PI3K pathway to invade host cells and survive intracellularly (Krachler et al., 2011; Ogawa et al., 2011; Van Avondt et al., 2015; Ledvina et al., 2018). Depending on the stage of infection, bacterial manipulation of the PI3K pathway results in different outcomes that range from facilitating bacterial uptake into the host cells to inhibiting phagosome maturation and lysosomal fusion (Gillooly et al., 2001; Vergne et al., 2003; Vergne et al., 2004; Vergne et al., 2005; Cano et al., 2015). Obligate and non-obligate intracellular pathogens, Chlamydia trachomatis and Mycobacteria tuberculosis respectively, are examples of bacterial species that can survive and replicate intracellularly through manipulation of the PI3K pathway (Bai et al., 2014; Brooks et al., 2017; Sah and Lutter, 2020). PI3K manipulation by these species results in infections that are not only protected from host immune killing but are recalcitrant to antibiotic treatment (Hartkoorn et al., 2007; Bastidas and Valdivia, 2016; Ellis et al., 2019; Yu et al., 2020). In addition, facultative intracellular bacteria Klebsiella pneumoniae and Salmonella typhimurium can manipulate the PI3K pathway to avoid phagosome maturation and survive within macrophages for several days (Oelschlaeger and Tall, 1997; Cano et al., 2015; Bengoechea and Sa Pessoa, 2019). Utilizing PI3K inhibitors as adjuvants in combination with antibiotics for these infections would eliminate intracellular bacteria (Oghumu and Satoskar, 2013; Kimmey and Stallings, 2016) to allow more effective host bacterial clearance and antibiotic treatment (Cano et al., 2015). This review provides a brief overview of 1) PI3K function; 2) the various PI3K inhibitors; 3) how bacteria can manipulate PI3K to their advantage and 4) how PI3K inhibitors have potential as adjuvants to eliminate pathogens from their protective niches.
Phosphoinositol 3-kinases (PI3Ks) are lipid kinases that reside in the plasma membrane of mammalian cells and consist of three subunits: two regulatory subunits p85 and p55; and a catalytic subunit p110 (Yang et al., 2019) (Figure 1). PI3Ks become activated after a transmembrane protein (ie. receptor tyrosine kinases (RTK)) signals the p85 regulatory subunit to bind and activate the p110 catalytic subunit (Yang et al., 2019). The catalytic subunit then phosphorylates phosphatidylinositol-4,5-bisphosphate (PtdIns-4,5-P2 or PIP2) to phosphatidylinositol-3,4,5-triphosphate (PtdIns-3,4,5-P3 or PIP3) (Fruman et al., 2017). Following phosphorylation, PIP3 is used as a secondary messenger to recruit and activate cytosolic proteins (ie. AKT and PDK-1) for a variety of purposes (Fruman et al., 2017) that vary between different cell types and PI3K isotypes (Leahy et al., 2012).
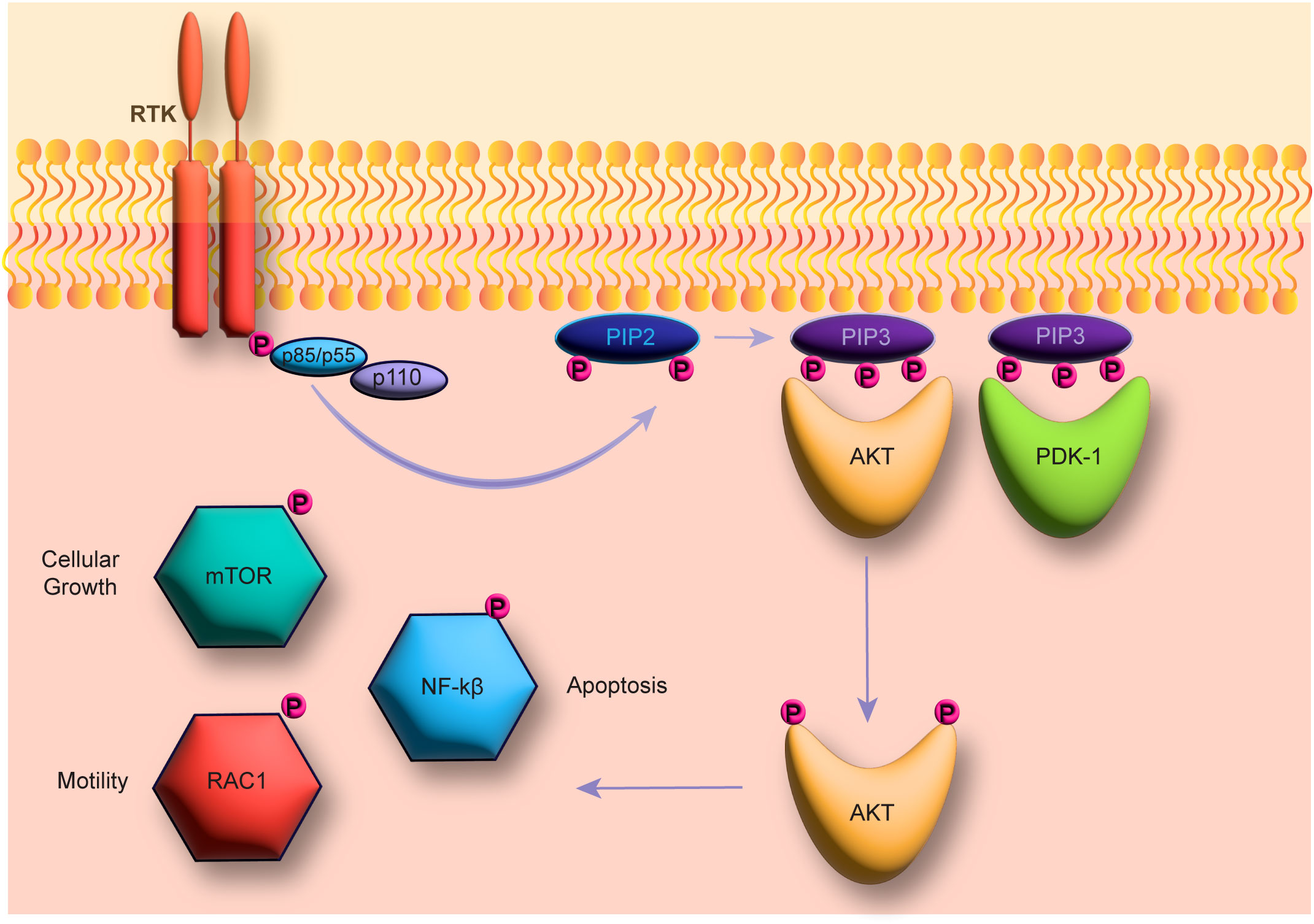
Figure 1 The PI3K pathway. The figure shows the PI3K pathway beginning with receptor tyrosine kinase (RTK) induction and phosphorylation of the catalytic PI3K subunit p110. The catalytic subunit then catalyzes the phosphorylation from PIP2 to PIP3, which in turn can then activate AKT and PDK-1 to activate downstream pathways (ie. mTOR, NF-kβ, and RAC-1).
There are 3 classes of PI3Ks and of these, class 1 is the most extensively studied due to having a major role in cancer development (Yang et al., 2019) (Table 1). This class is therefore the target of most therapeutic inhibitors in clinical development. Although not as well characterized as class 1, class 2 PI3Ks also have been shown to have role in cancer development (Gulluni et al., 2017; Gulluni et al., 2019; He et al., 2021). Specifically, this class has been shown to be important for migration of prostate cancer cells (Mavrommati et al., 2016). Class 3 PI3Ks are involved in membrane trafficking, endosome-lysosome maturation, and autophagosome formation (Jean and Kiger, 2014). Class 3 has been shown to play an important role in autophagy in the liver and heart (Jaber et al., 2012). However, this class has not yet been shown to have any role in disease or cancer (Jean and Kiger, 2014). Although PI3Ks are ubiquitous throughout the body, with select isotypes found in various compartment that have unique roles (Leahy et al., 2012).
There are 4 class 1 PI3Ks isotypes that are named after their catalytic subunit proteins (Table 1). Class 1A consists of isotypes p110α, p110β, and p110δ, while class 1B consists of one isotype, p110γ (Yang et al., 2019). Isotypes p110α and p110β are constitutively expressed throughout the body and have many cellular functions (Leahy et al., 2012; Yang et al., 2019). Conversely, PI3K isotypes p110δ and p110γ are only found in immune cells and they can activate or repress downstream pathways (Fruman et al., 2017). Interestingly, p110γ are expressed in lymphocytes and are responsible for chemotaxis, making this specific PI3K isotype an excellent target for adjuvant immunotherapy to limit intracellular survival of pathogens (Leahy et al., 2012).
Class 2 PI3Ks have 3 isotypes: PI3K-C2α, PI3K-C2β, and PI3K-C2γ (Islam et al., 2020) (Table 1). Of these isotypes, PI3K-C2α, PI3K-C2β are found throughout the body, while PI3K-C2γ has been shown to be isolated to liver, pancreas, and reproductive organs (Islam et al., 2020). Isotype Class 2 PI3Ks are responsible for glucose uptake by the liver and have a role in blood pressure regulation (Koch et al., 2021). Studies with PI3K-C2β knock out mice revealed that because of its role in glucose uptake, this enzyme has potential as a target for diabetes treatment (Koch et al., 2021).
There are 4 class 1 PI3Ks isotypes that are named after their catalytic subunit proteins (Table 1). Class 1A consists of isotypes p110α, p110β, and p110δ, while class 1B consists of one isotype, p110γ (Yang et al., 2019). Isotypes p110α and p110β are constitutively expressed throughout the body and have many cellular functions (Leahy et al., 2012; Yang et al., 2019). Conversely, PI3K isotypes p110δ and p110γ are only found in immune cells and they can activate or repress downstream pathways (Fruman et al., 2017). Interestingly, p110γ are expressed in lymphocytes and are responsible for chemotaxis, making this specific PI3K isotype an excellent target for adjuvant immunotherapy to limit intracellular survival of pathogens (Leahy et al., 2012).
Class 2 PI3Ks have 3 isotypes: PI3K-C2α, PI3K-C2β, and PI3K-C2γ (Islam et al., 2020) (Table 1). Of these isotypes, PI3K-C2α, PI3K-C2β are found throughout the body, while PI3K-C2γ has been shown to be isolated to liver, pancreas, and reproductive organs (Islam et al., 2020). Isotype Class 2 PI3Ks are responsible for glucose uptake by the liver and have a role in blood pressure regulation (Koch et al., 2021). Studies with PI3K-C2β knock out mice revealed that because of its role in glucose uptake, this enzyme has potential as a target for diabetes treatment (Koch et al., 2021).
Class 3 has just a single isotype that is named after the catalytic subunit Vps34, with a corresponding regulatory subunit named Vsp15 (Jean and Kiger, 2014) (Table 1). Although insulin does not affect the activity of class 3 PI3Ks, it also has promise as a target for diabetes treatment because of its role in the feedback loop of glucose homeostasis (Nemazanyy et al., 2015). Interestingly, this therapy would target the regulatory subunit, not the usual therapeutic catalytic subunit because knocking down the regulatory subunit Vsp15, not the catalytic subunit Vsp34 has been shown to increase insulin sensitivity (Nemazanyy et al., 2015).
Considering the ubiquitous nature of PI3Ks throughout the body, when designing therapeutics for inhibition of PI3K classes, the specific isotype and its functions must be thoroughly investigated. For infectious disease adjuvants, focusing on classes and isotypes with functions in lymphocytes would result in a more specific therapeutic effect with less unwanted side effects. Therefore, designing specific inhibitors for class 1 isotypes p110δ and p110γ would be ideal for this purpose. Furthermore, it would be advantageous to repurpose PI3K inhibitors as adjuvants for bacterial infections with the extensive amount of research and development into inhibitors of class 1 PI3Ks as cancer therapeutics (Leahy et al., 2012).
PI3Ks are ubiquitous throughout the entire body and have been shown to be important for many physiological processes (Fruman et al., 2017). Research in the late 1980’s revealed that PtdIns-3,4,5-P3, the product of PI3K activation is central for malignant cancerous growth (Whitman et al., 1988; Auger et al., 1989). The PI3K/AKT/mTOR pathway is dysregulated in almost all cancer types leading to uncontrolled growth (Hoxhaj and Manning, 2020). Specifically, activation of the PI3K pathway in cancer cells is responsible for proliferation, invasion, metastasis, and angiogenesis (Rascio et al., 2021). Since the PI3K pathway is a driver of uncontrolled growth and spread of a variety of cancers, there are currently academic and clinical efforts in place to develop PI3K, AKT, and mTOR inhibitors as cancer therapeutics (Yang et al., 2019; Castel et al., 2021).
One important role for PI3K in the liver is regulation of glucose uptake and glycogen storage (Jean and Kiger, 2014; Koch et al., 2021). This makes PI3Ks possible targets for to treat the symptoms of diabetes (Maffei et al., 2018). Similarly, patients with a mutation in PI3K resulting in SHORT syndrome have difficulties with glucose homeostasis mimicking type 1 diabetes (Fruman et al., 2017). However, unlike diabetes, this defect does not affect the production of insulin but the ability of insulin to activate PI3K (Fruman et al., 2017). Its role in glucose regulation makes inhibiting PI3K for cancer therapy problematic because inhibition causes a release of glucose that in turn initiates the release of insulin. The consequence of this insulin release is re-activation of PI3K in tumor cells and ineffective chemotherapy (Fruman et al., 2017). However, only certain classes and isotypes have a role in glucose metabolism and their roles are unique (Maffei et al., 2018). For example, class 2 PI3Ks are activated by insulin (Koch et al., 2021), class 3 PI3Ks are involved in glucose feedback loop (Nemazanyy et al., 2015), and class 1A PI3Ks are the isotypes responsible for glucose regulation in the liver (Maffei et al., 2018). Furthermore, class 1A isotypes p110α and p110β are the major regulators of glucose in the liver, while class 1B p110γ regulates glucose in immune cells (Maffei et al., 2018).
Lastly, relevant to this review, PI3K has a major role in the innate and adaptive immune system. For professional phagocytes such as macrophages and neutrophils, PI3K plays a major role in both phagocytosis and killing of intracellular bacteria within phagosomes (Gillooly et al., 2001). Class 1A isotype p110δ and class 1B isotype p110γ and are the isotypes used specifically in immune cells (Okkenhaug, 2013). In addition to each isotype having a unique role, PI3Ks can activate or repress downstream genes depending on the cell type and associated receptor (Fruman et al., 2017). For example, class 1 Pi3Ks are involved in the phagosome cup formation, while class 3 PI3K is involved in the maturation (Koyasu, 2003). Specifically, class 1B isotype p110γ allows for neutrophil migration by producing PtdIns-3,4,5-P3 at the leading edge of the cell (Jean and Kiger, 2014). In addition, class 1A isotype p110δ, class 1B isotype p110γ, and class 3 PI3K Vps34 are responsible for bacterial clearance by immune cells (Thi and Reiner, 2012).
The extensive list of roles for PI3Ks make them attractive targets for cancer therapy, immune therapy, and diabetes treatment. When looking for therapeutic alternatives to complement our classical antibiotic therapy for drug resistant bacterial infections, targeting the PI3K specific isotypes is an intriguing possibility. Combination therapy with antibiotics and isotype selective PI3K inhibitors has the potential of increasing therapeutic outcome of drug resistant bacterial infections without interfering with PI3Ks in other cell types leading to unwanted side effects.
PI3K inhibitors are an exciting target for cancer therapy because upregulation of the PI3K/AKT/mTOR pathway is present in almost all cancers (Yang et al., 2019). PI3K upregulation is responsible for uncontrolled growth, increased chemotaxis, and invasiveness of cancer cells (Yang et al., 2019). Since the discovery of its important role in cancer development, PI3K inhibitors have been increasingly developed and optimized (Macara et al., 1984; Sugimoto et al., 1984; Whitman et al., 1985; Whitman et al., 1988).
In 1957 wortmannin, the first PI3K inhibitor was discovered after isolation from the fungal species Penicillium wortmannin (Cleary and Shapiro, 2010) (Figure 2A). Similarly, Eli Lilly developed LY294002 as a reversible broad inhibitor of PI3k (Cleary and Shapiro, 2010) (Figure 2B). The development of LY294002 was based on optimizing the naturally occurring flavonoid quercetin (Figure 2C), that can inhibit a broad range of host kinases (Abdul-Ghani et al., 2006; Imai et al., 2012). These inhibitors have been used extensively in laboratories studying the cellular functions of PI3K but due to their poor solubility and physiochemical characteristics these inhibitors have not been used therapeutically (Cleary and Shapiro, 2010). After the discovery of PI3K inhibitors, efforts to improve their pharmacological characteristics resulted in several new inhibitors being developed (Cleary and Shapiro, 2010). Many studies have worked to improve the physiochemical characteristics of wortmannin and LY294002 through analog design resulting in more stable forms of wortmannin and more soluble analogs of LY294002 (Cleary and Shapiro, 2010).
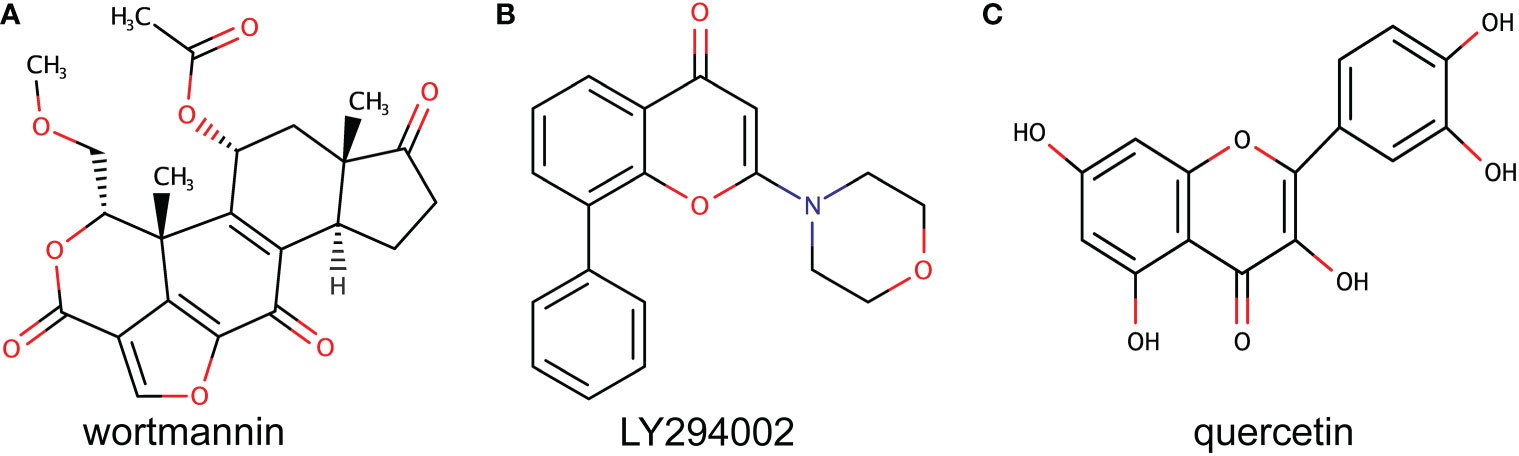
Figure 2 Original PI3K inhibitors. The figure shows the chemical structure of the first two discovered PI3K inhibitors. (A) shows wortmannin that was discovered from Penicillium wortmannin. (B) shows the chemical structure of the synthetic LY294002 designed based on the natural flavonoid quercetin shown in (C).
In addition to the broad inhibitors of PI3K, therapeutics have been developed with the ability to bind both PI3K and the very similar catalytic subunit of mTOR (Cleary and Shapiro, 2010). Although they have not yet acquired FDA approval, there are dual PI3K and mTOR inhibitors in phase 3 of clinical development increasing the effectiveness of PI3K/AKT/mTOR pathway inhibition (Cleary and Shapiro, 2010; Yang et al., 2019). These have shown promise over therapeutics that solely inhibit mTOR, which has a negative feedback loop that activates PI3K and AKT when inhibited. However, it is uncertain at this point whether dual inhibition of both PI3K and mTOR is superior to the inhibition of PI3K alone (Cleary and Shapiro, 2010). In addition, multiple trials for dual inhibitors have been terminated due to low tolerability and adverse side effects (Yang et al., 2019). If the tolerability can be improved, perhaps dual PI3K/mTOR inhibitors could be beneficial for cancer therapy because studies have shown PI3K to be involved in chemotherapy resistance (Cleary and Shapiro, 2010; Garber, 2014).
In recent years, isotype selective inhibitors have been more frequently pursued for development because they have been shown to have fewer side effects than broad-spectrum inhibitors (Cleary and Shapiro, 2010). For example, the PI3K isotype in the liver that is important for sending signals from the insulin receptor is p110α. Broad-spectrum PI3K inhibition can cause hyperglycemia by releasing extra glucose that in turn causes a large release of insulin (Fruman et al., 2017). Therefore, avoiding inhibition of PI3K isotype p110α can eliminate the hyperglycemic side effects that accompany broad PI3K inhibition (Fruman et al., 2017). In addition, it would be optimal to target specific isotypes when eliminating intracellular bacterial survival because p110γ and p110δ are the main PI3K isotypes for lymphocyte signaling (Cleary and Shapiro, 2010). This reveals great promise for avoiding unwanted side effects when using specific inhibitors to eliminate intracellular survival of bacterial pathogens.
Currently, there are isotype selective inhibitors that have been approved by the FDA (Table 2) and others are in stage 3 clinical trials (Garber, 2014). Specifically, in 2014 Gilead had the first PI3K isotype selective inhibitor Zydelig™ (Idelalisib) approved by the FDA for treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and follicular lymphoma (Garber, 2014). Zydelig™ is the only approved inhibitor specifically targeting the p110δ isotype of PI3K (Garber, 2014; Yang et al., 2019). To target PI3K p110α, Novartis acquired approval for Alpelisib™ (BYL719), for treatment of breast cancer (Lee et al., 2022). In addition to these very specific inhibitors, Copiktra® (Duvelisib) and Aliqopa™ (Copanlisib) that have been approved shown to inhibit both p110γ and p110δ isotypes (Garber, 2014; Flinn et al., 2019; Yang et al., 2019). With these current approvals and more in various stages of clinical trials, there are many options to repurpose for adjuvant therapeutics.
Development of p110γ selective inhibitors has been slow because long term cancer treatment with these therapeutics can dampen the immune response to bacterial infections (Yang et al., 2019). However, acute inhibition of PI3K does not have the same effects on the immune system as long-term inhibition (Adefemi et al., 2020). Although T cell activation is inhibited, short term acute PI3K inhibition enhances the myeloid immune response to infections resulting in better infection control (Adefemi et al., 2020). Interestingly, it has been shown that chronic inflammation in elderly patients causes aberrant migration of neutrophils and PI3K inhibitors can help improve chemotaxis accuracy (Sapey et al., 2014). Aberrant neutrophil migration has also been observed in patients with severe sepsis (Patel et al., 2018). This exciting potential therapeutic application for PI3K inhibitors needs to be explored more thoroughly because the devastating mortality rates associated with sepsis (Vincent et al., 2002; Fleischmann-Struzek et al., 2020). Overall, selective inhibitors are optimal to repurpose as adjuvants for bacterial infection treatment because the PI3K isotypes used by the immune system are not ubiquitous throughout the body and the treatment length for bacterial infections is shorter than cancer therapy.
Bacterial pathogens can evade clearance by the host immune system by manipulating the cellular processes that facilitate clearance of invading organisms (Petit and Lebreton, 2022). There are a variety of mechanisms used by different bacterial species to survive, replicate, and hide within the host cells (Martinez et al., 2018). These virulence factors allow the pathogen to colonize and proliferate within a host organism and evade killing by antimicrobials (Liu et al., 2020; Petit and Lebreton, 2022). PI3Ks are targeted by many bacterial pathogens and therefore would be an advantageous target for host immune therapy and more effective treatment of chronic bacterial infections.
Intracellular bacteria can evade host immune clearance, reside, and replicate within macrophages and epithelial cells (Xue et al., 2010).. This ability to replicate and survive within the host is paramount to their success as a pathogen (Allwood et al., 2011; Mitchell et al., 2016). These pathogens are very difficult to treat because they can hide within the mammalian cells to evade antibiotic treatments (Kamaruzzaman et al., 2017; Tucker et al., 2021).
Francisella tularensis is potential bioterrorism agent that causes fatal pneumoniae and no vaccine is available (Maurin, 2015). F. tularensis has an intracellular life cycle where it escapes from the phagosome and replicates within the macrophage cytosol (Celli and Zahrt, 2013; Ledvina et al., 2018) (Figure 3A). F. tularensis uses OpiA, its own bacterial PI3K to promote bacterial escape into the cytosol and this kinase has been shown to be resistant to the fungal PI3K inhibitor wortmannin (Ledvina et al., 2018). Furthermore, it has been suggested that additional intracellular bacteria, may harbor their own bacterial PI3K to facilitate phagosomal escape because OpiA family proteins (OFP) have been found in the genomes of intracellular replicating Legionella, Vibrio, and Rickettsia genera (Ledvina et al., 2018). Another potential bioterrorism agent, Bacillus anthracis replicates in the cytosol of host cells and manipulates the host PI3Ks to allow for intracellular replication (Xue et al., 2010). B. anthracis spores invade lung epithelial cells to replicate then escape to spread the infection (Xue et al., 2010) (Figure 3B). Xue et al. showed that treatment of A549 cells with the PI3K inhibitors LY294002 or wortmannin resulted in a drastic reduction in B. anthracis spore internalization, revealing the importance of PI3K activation by this species (Xue et al., 2010).
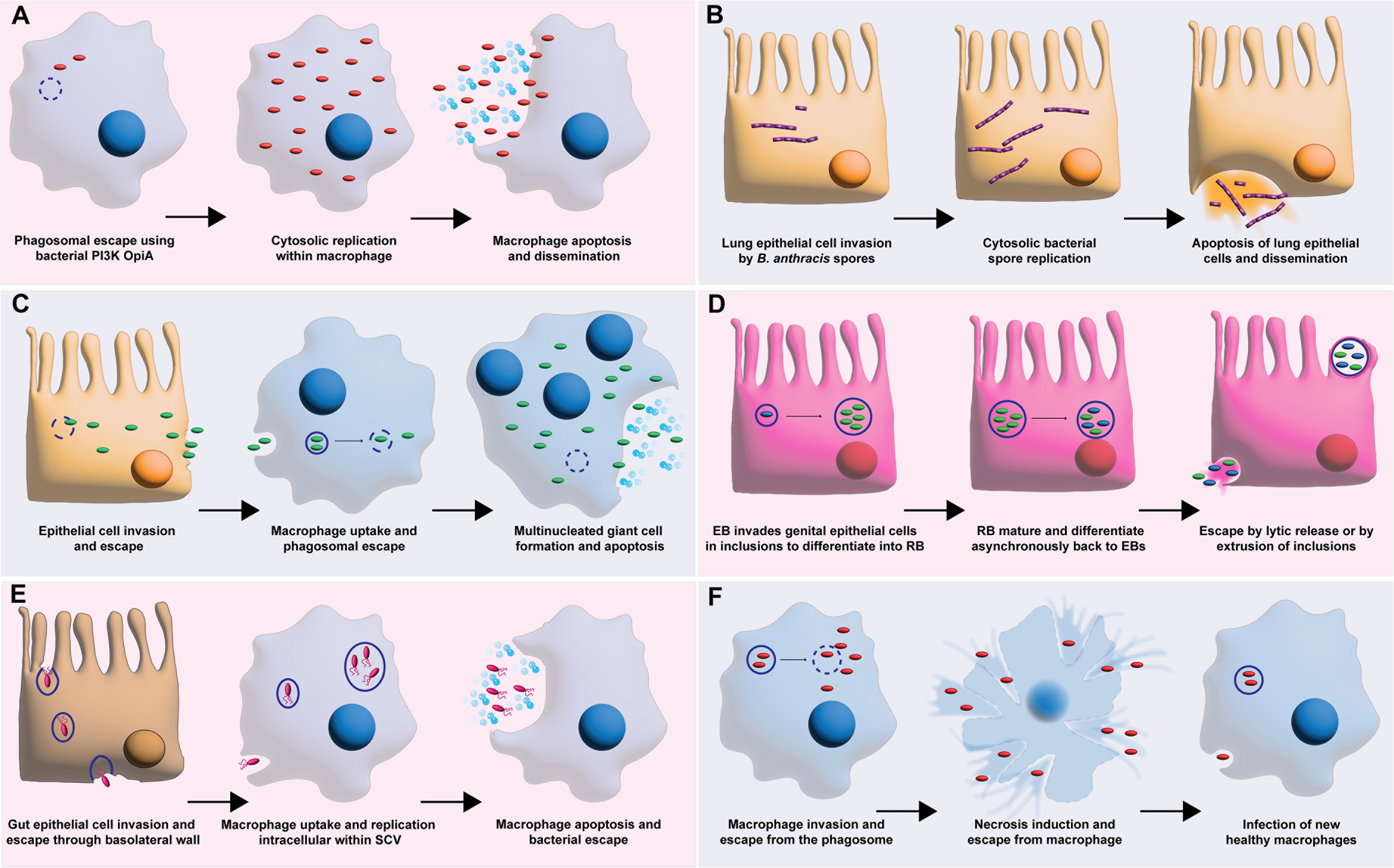
Figure 3 Bacterial intracellular survival strategies manipulating PI3K. (A) shows F. tularensis escape from the phagosome using its own bacterial PI3K OpiA. This allows for subsequent cytosolic replication, macrophage apoptosis, and bacterial dissemination. (B) shows B. anthracis spore invasion of lung epithelial cell attained by manipulation of PI3K. The resulting invasion leads to spore replication and epithelial cell apoptosis. (C) shows B. pseudomallei epithelial cell invasion with subsequent macrophage uptake facilitated by hijacking of PI3K pathway. Intracellular replication then leads to multinucleated giant macrophage cells, apoptosis, and bacterial escape. (D) shows C. trachomatis elementary body (EB) invasion of genital epithelial cells facilitated by manipulation of PI3K. This results in intracellular replication of reticulate bodies (RB) and infection spread. (E) shows S. typhimurium gut epithelial cell invasion facilitated by PI3K manipulation to invade macrophages to survive and replicate within the salmonella containing vacuole (SCV) before macrophage apoptosis leads to bacterial escape. (F) shows M. tuberculosis evasion of phagosome maturation leading to replication and escape from the phagosome, necrosis induction in the macrophage, and release to infect new macrophages.
Burkholderia pseudomallei is a U. S. Tier 1 select agent for which there is no vaccine and high mortality rates are associated with the infections (Adefemi et al., 2020). This species is a facultative intracellular pathogen that can invade and replicate in both professional phagocytes and non-phagocytic cells (Figure 3C) (Adefemi et al., 2020). Specifically, B. pseudomallei can invade both epithelial cells and macrophages by using PI3K to hijack the host cell actin, ultimately creating multinucleated giant cells leading to apoptosis and escape (Hii et al., 2008; Pflughoeft et al., 2019). Interestingly, Ganesan et al. found that treatment with chloroquine in combination with doxycycline resulted in greater murine survival from B. pseudomallei infections by inhibiting an enzyme downstream to PI3K, glycogen synthase kinase-3β (Ganesan et al., 2020). This study shows the therapeutic benefit of repurposing host targeting drugs as adjuvants with a common antibiotic for treatment of bacterial infections (Ganesan et al., 2020).
The obligate intracellular Chlamydia species ability to manipulate host cell kinases is necessary for its success and survival as a pathogen (Sah and Lutter, 2020). More specifically, inhibition of PI3Ks by Chlamydia species allows for cell invasion, suppression of the host apoptosis and the acquisition of nutrients necessary for survival (Sah and Lutter, 2020) Once inside the host cell Chlamydia trachomatis differentiates from elementary bodies to reticulate bodies within inclusions that will eventually be either extruded from the cell or released by bacterial lysis after the cells mature and differentiate back into elementary bodies (Figure 3D) (Adefemi et al., 2020). In C. trachomatis, an effector protein TepP can recruit and activate PI3K on membranes to initiate bacterial invasion (Carpenter et al., 2017). Interestingly, the infectious elementary bodies of C. trachomatis that spread the infection have the highest amount of TepP proteins (Carpenter et al., 2017).
Salmonella species are facultative intracellular pathogens that have the ability to use multiple mechanisms to invade and replicate within host cells (Boumart et al., 2014) Salmonella typhimurium manipulates PI3K to pass through the gut epithelial lining, escape through the basolateral wall, and invade macrophages to replicate within the Salmonella containing vacuole (SCV) (Figure 3E) (Hurley et al., 2014). These species invade non-phagocytic cells by two mechanisms: the Zipper mechanism, shared by Listeria monocytogenes and the Trigger mechanism, shared by Shigella flexneri (Boumart et al., 2014). S. typhimurium manipulation of PI3K allows it to highjack macrophages, using them to disseminate the infection (Garcia-Gil et al., 2018). The S. typhimurium effector protein SopB activates PI3K pathway in B cells to facilitate survival (Roppenser et al., 2013; Garcia-Gil et al., 2018), by not allowing them to form the NLRCR4 inflammasome and fight the infection (Garcia-Gil et al., 2018).
Mycobacterium tuberculosis not only survives within macrophages but replicates within the phagosome (Liu et al., 2016). M. tuberculosis uses a recombinant leucine-responsive regulatory protein (rLpr) to increase activation of PI3K via the toll-like receptor 2 (TLR-2) (Liu et al., 2016). In addition, the marker for phagolysosome fusion Rab7 is targeted by M. tuberculosis, not allowing the phagosome to mature to the late stage (Nguyen and Yates, 2021). Following replication within the phagosome, the phagosomal membrane is permeabilized allowing the bacteria to escape to the cytosol, triggering necrosis of the macrophage and allowing escape of M. tuberculosis to infect other macrophages (Figure 3F) (Behar et al., 2010).
Some pathogens are not all traditionally considered when investigating bacterial survival within host cells (Sendi and Proctor, 2009; Silva, 2012). However, these pathogens can manipulate the immune system to their advantage to allow survival and spread of the infection (Silva, 2012; Silva and Pestana, 2013). Therefore, when considering adjuvant PI3K therapy these pathogens should also be considered potential targets.
Staphylococcus aureus is a dangerous pathogen with community acquired infections easily spread due to their ability to invade healthy individuals (Boyle-Vavra and Daum, 2007; DeLeo et al., 2009; Bukharie, 2010; Yoong and Pier, 2010; Turner et al., 2019). Skin infections caused by S. aureus can quickly invade and disseminate leading to systemic infection with sepsis (Edwards et al., 2010). Although traditionally thought of as an extracellular pathogen, S. aureus has been shown to invade non-phagocytic mammalian cells (Nakagawa et al., 2017). For example, keratinocyte invasion by S. aureus induces lysis continued invasion of the dermis layer. When engulfed by macrophages and neutrophils partial evasion of phagolysosome killing leads to escape and dissemination of infection (Oviedo-Boyso et al., 2011; Hommes and Surewaard, 2022) (Figure 4A). Interestingly, S. aureus internalization decreases in bovine epithelial cells when treated with PI3K inhibitors (Oviedo-Boyso et al., 2011). Escherichia coli K1 has been shown to invade endothelial cells to cross the blood brain barrier and cause bacterial meningitis (Reddy et al., 2000). E. coli has been shown to manipulate the human brain microvascular endothelial cells and force micropinocytosis (Loh et al., 2017) (Figure 4B). Attachment to the epithelial cell lining of the blood brain barrier by E. coli activates several signaling pathways including the PI3K pathway (Loh et al., 2017).
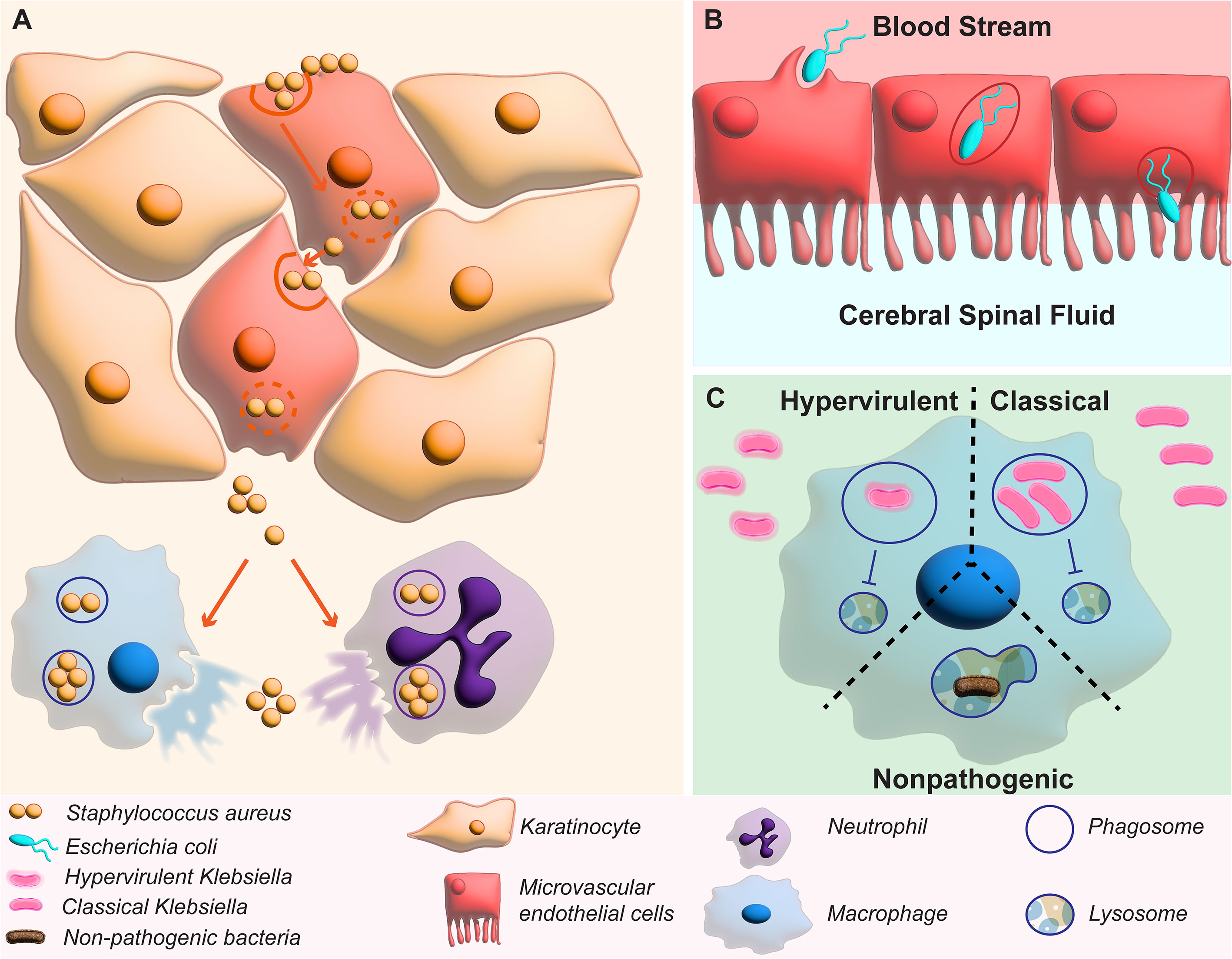
Figure 4 Manipulation of PI3K by non-traditional intracellular bacteria. (A) shows how manipulation of PI3K by S. aureus leads to invasion of keratinocytes of the dermis to spread the infection and can replicate within phagocytes in macrophages and neutrophils. (B) shows E. coli invasion of microvascular endothelial cells to pass the blood brain barrier. (C) shows how activation of PI3K by hypervirulent and classical K. pneumoniae inhibit phagolysosome fusion that usually kills non-pathogenic bacteria.
A close relative to E. coli, K. pneumoniae is historically know an extracellular pathogen, unable to survive for extended periods in intracellular compartments like its close relative E. coli (Belon and Blanc-Potard, 2016). However, studies have now shown that K. pneumoniae is able to survive for up to 48 hours within the vacuole once engulfed by macrophages (Oelschlaeger and Tall, 1997). This vacuole, named the Klebsiella containing vacuole (KCV) does not fuse with lysosomes and therefore deviates from the canonical endocytic pathway used by macrophages to clear engulfed pathogens (Bengoechea and Sa Pessoa, 2019) (Figure 4C). This lack of maturation of the phagosome also results in less activation of the adaptive immune system because less antigens are presented on the surface of macrophages (Cano et al., 2015). Although it is unclear the factor that allows K. pneumoniae to persist within the phagosome, the capsule does not appear to play a major role because both hypervirulent and classical K. pneumoniae can manipulate the PI3K pathway to stop phagosome maturation by inhibiting fusion with the lysosome compartment (Figure 4C) (Cano et al., 2015). These classical intracellular pathogens have been shown to display less intracellular survival in the presence of AKT inhibitors targeting the enzyme immediately downstream to PI3K in the PI3K/AKT/Rab14 axis (Bengoechea and Sa Pessoa, 2019). In addition, it has recently been revealed that K. pneumoniae manipulates PI3K through the mammalian protein SARM1 (sterile α and HEAT armadillo motif-containing protein) revealing a potential target upstream for future therapeutic development (Feriotti et al., 2022). With the many bacterial species that manipulate PI3K to invade the host and survive intracellularly, repurposing PI3K inhibitors to release them from their protective niches would be beneficial to allow the host immune system and antibiotics to be more effective at treating these infections.
With the variety of pathogens that manipulate the PI3K pathway to evade host immune killing (Krachler et al., 2011; Cano et al., 2015; Liu et al., 2016; Lacoma et al., 2017; Garcia-Gil et al., 2018), investigating the potential as adjuvant therapeutics to eliminate intracellular bacterial survival is important for the fight against drug resistant bacterial species. PI3K inhibitors provided in a short, acute dose can promote bacterial pathogen clearance (Adefemi et al., 2020). Using PI3K inhibitors can release the pathogens from their niches used to hide from the immune system (Wong et al., 2019) and disseminate infection (Chen et al., 2002). In combination with antibiotics, PI3K inhibitors have the potential to behave as adjuvants allowing more effective antibiotic therapy at lower doses.
Studies have shown that acute PI3K treatment can improve the early-stage progression of infections (Adefemi et al., 2020). Many genera of bacteria manipulate PI3K to avoid phagolysosome fusion and PI3K inhibitors allow for efficient fusion of the lysosome and bacterial clearance (Figure 5A). This has been used to show that PI3K/AKT inhibition can eliminate intracellular S. typhimurium and M. tuberculosis (Bengoechea and Sa Pessoa, 2019). Interestingly, inhibition of PI3K in M. tuberculosis by isotype specific inhibitors has the potential to decrease the characteristic late-stage infection IL-17A induced pathology by interfering with Th17 differentiation (Leisching, 2019). Shapira et al. performed high-content screening of kinase inhibitors and found inhibition of PI3K controls autophagy and apoptosis decreasing intracellular survival (Shapira et al., 2020). This effect has also been seen with the facultative intracellular K. pneumoniae that manipulates the PI3K pathway to avoid phagolysosome fusion of late-stage endosomes (Cano et al., 2015). Treatment with a PI3K inhibitor revealed a decrease in K. pneumoniae intracellular survival within macrophages (Cano et al., 2015; Bengoechea and Sa Pessoa, 2019). Inhibiting bacterial pathogen survival in host immune cells has great potential for treatment of chronic infections.
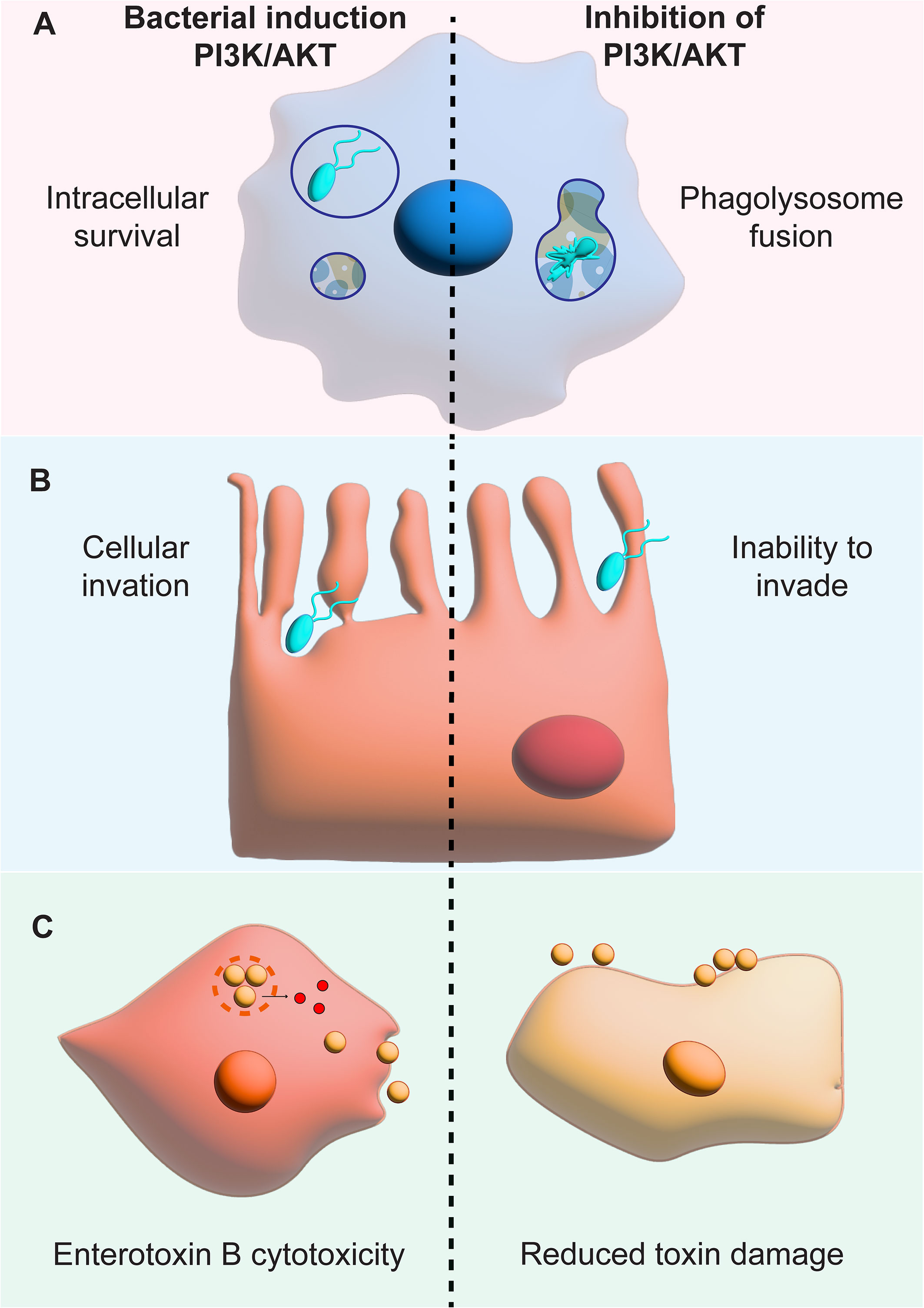
Figure 5 PI3K inhibition decreases bacterial invasion and survival within host cells. The figure shows the effects of PI3K manipulation next to the effects of PI3K inhibition. (A) shows that bacterial PI3K manipulation used by S. typhimurium, M. tuberculosis and K. pneumoniae to inhibit phagolysosome fusion can be stopped by PI3K inhibitors leading to fusion and bacterial death. (B) shows how the invasion of epithelial cells by E. coli is stopped by PI3K inhibitors. (C) reveals the benefits of PI3K inhibition on the keratinocyte survival in the presence of the Enterotoxin B toxin.
PI3K inhibition also has the potential to decrease bacterial invasion and survival within non-phagocytic cells like epithelial cells. Testing PI3K inhibitors when infecting intestinal cells with S. typhimurium, Huang et al. revealed that this species uses PI3K activation to decrease inflammation. This decreased inflammation allows S. typhimurium to survive and PI3K inhibition led to decreased bacterial survival within intestinal epithelial cells (Huang et al., 2005). Like S. typhimurium, PI3K manipulation is also important for epithelial cell invasion by Helicobacter pylori and Listeria monocytogenes revealing the potential of PI3K inhibitors for a variety of intracellular pathogens (Figure 5B) (Booth et al., 2003). Furthermore, the penetration of the blood brain barrier by E. coli leading to meningitis can be stopped by using PI3K inhibitors (Loh et al., 2017). These studies reveal the potential of PI3K therapeutics for non-phagocytic cells revealing a broader application for adjuvants for a variety of infections and bacterial species.
PI3K inhibitors have also shown promise in treating pathogens that do not have a true intracellular stage (Whitman et al., 1988; Cano et al., 2015; Yang et al., 2019). With these infections, inflammation and toxic damage can be mitigated by using PI3K inhibitors. For example, the natural PI3K inhibitor deguelin reduces Staphylococcal Enterotoxin B induction of T-cell proliferation toxicity (Whitfeild SJ et al., 2017) (Figure 5C). However, more work is needed to understand all the potential effects of PI3K treatment for bacterial infections. For example, the treatment of an E. coli induced endotoxemic mice with PI3K inhibitors lead to a decrease in mouse survival because of increased LPS-induced inflammation (Schabbauer et al., 2004). This data reveal that PI3K inhibitors can also be repurposed to protect from the effects of bacterial toxins during infection but much research is needed to pursue these applications.
This review provides an overview of the role of PI3K pathway in intracellular bacterial survival and how repurposing PI3K inhibitors can potentially help eliminate these difficult to treat bacterial infections. PI3K inhibition in combination with antibiotics and the host immune system can lead to more effective treatment of many bacterial infections. However, much research is needed to explore the potential of PI3K inhibitors as adjuvant therapeutics for intracellular bacterial pathogens.
The review design, writing, figures, and editing was performed by RF.
K99AI163295 to RF.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdul-Ghani R., Serra V., Gyorffy B., Jurchott K., Solf A., Dietel M., et al. (2006). The PI3K inhibitor LY294002 blocks drug export from resistant colon carcinoma cells overexpressing MRP1. Oncogene. 25 (12), 1743–1752. doi: 10.1038/sj.onc.1209201
Adefemi F., Fruman D. A., Marshall A. J. (2020). A case for phosphoinositide 3-Kinase-Targeted therapy for infectious disease. J. Immunol. 205 (12), 3237–3245. doi: 10.4049/jimmunol.2000599
Allwood E. M., Devenish R. J., Prescott M., Adler B., Boyce J. D. (2011). Strategies for intracellular survival of burkholderia pseudomallei. Front. Microbiol. 2. doi: 10.3389/fmicb.2011.00170
Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. (1989). PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 57 (1), 167–175. doi: 10.1016/0092-8674(89)90182-7
Aung K. T., Yoshioka K., Aki S., Ishimaru K., Takuwa N., Takuwa Y. (2019). The class II phosphoinositide 3-kinases PI3K-C2alpha and PI3K-C2beta differentially regulate clathrin-dependent pinocytosis in human vascular endothelial cells. J. Physiol. Sci. 69 (2), 263–280. doi: 10.1007/s12576-018-0644-2
Bai W., Liu H., Ji Q., Zhou Y., Liang L., Zheng R., et al. (2014). TLR3 regulates mycobacterial RNA-induced IL-10 production through the PI3K/AKT signaling pathway. Cell Signal. 26 (5), 942–950. doi: 10.1016/j.cellsig.2014.01.015
Barker W. T., Nemeth A. M., Brackett S. M., Basak A. K., Chandler C. E., Jania L. A., et al. (2019). Repurposing eukaryotic kinase inhibitors as colistin adjuvants in gram-negative bacteria. ACS Infect. Dis. 5 (10), 1764–1771. doi: 10.1021/acsinfecdis.9b00212
Bastidas R. J., Valdivia R. H. (2016). Emancipating chlamydia: Advances in the genetic manipulation of a recalcitrant intracellular pathogen. Microbiol. Mol. Biol. Rev. 80 (2), 411–427. doi: 10.1128/MMBR.00071-15
Behar S. M., Divangahi M., Remold H. G. (2010). Evasion of innate immunity by mycobacterium tuberculosis: is death an exit strategy? Nat. Rev. Microbiol. 8 (9), 668–674. doi: 10.1038/nrmicro2387
Belon C., Blanc-Potard A. B. (2016). Intramacrophage survival for extracellular bacterial pathogens: MgtC as a key adaptive factor. Front. Cell Infect. Microbiol. 6. doi: 10.3389/fcimb.2016.00052
Bengoechea J. A., Sa Pessoa J. (2019). Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol. Rev. 43 (2), 123–144. doi: 10.1093/femsre/fuy043
Bogani G., Chiappa V., Bini M., Ronzulli D., Indini A., Conca E., et al. (2022). BYL719 (alpelisib) for the treatment of PIK3CA-mutated, recurrent/advanced cervical cancer. Tumori. doi: 10.1177/03008916211073621
Booth J. W., Telio D., Liao E. H., McCaw S. E., Matsuo T., Grinstein S., et al. (2003). Phosphatidylinositol 3-kinases in carcinoembryonic antigen-related cellular adhesion molecule-mediated internalization of neisseria gonorrhoeae. J. Biol. Chem. 278 (16), 14037–14045. doi: 10.1074/jbc.M211879200
Boumart Z., Velge P., Wiedemann A. (2014). Multiple invasion mechanisms and different intracellular behaviors: a new vision of salmonella-host cell interaction. FEMS Microbiol. letters. 361 (1), 1–7. doi: 10.1111/1574-6968.12614
Boyle-Vavra S., Daum R. S. (2007). Community-acquired methicillin-resistant staphylococcus aureus: the role of panton-valentine leukocidin. Lab. Invest. 87 (1), 3–9. doi: 10.1038/labinvest.3700501
Brooks A. B., Humphreys D., Singh V., Davidson A. C., Arden S. D., Buss F., et al. (2017). “MYO6 is targeted by salmonella virulence effectors to trigger PI3-kinase signaling and pathogen invasion into host cells,” in Proceedings of the national academy of sciences of the united states of America, vol. 114. , 3915–3920. doi: 10.1073/pnas.1616418114
Bukharie H. A. (2010). A review of community-acquired methicillin-resistant staphylococcus aureus for primary care physicians. J. Family Community Med. 17 (3), 117–120. doi: 10.4103/1319-1683.74320
Cano V., March C., Insua J. L., Aguilo N., Llobet E., Moranta D., et al. (2015). Klebsiella pneumoniae survives within macrophages by avoiding delivery to lysosomes. Cell Microbiol. 17 (11), 1537–1560. doi: 10.1111/cmi.12466
Carpenter V., Chen Y. S., Dolat L., Valdivia R. H. (2017). The effector TepP mediates recruitment and activation of phosphoinositide 3-kinase on early chlamydia trachomatis vacuoles. mSphere. 2 (4), e00207-17. doi: 10.1128/mSphere.00207-17
Castel P., Toska E., Engelman J. A., Scaltriti M. (2021). The present and future of PI3K inhibitors for cancer therapy. Nat. Cancer. 2 (6), 587–597. doi: 10.1038/s43018-021-00218-4
Celli J., Zahrt T. C. (2013). Mechanisms of francisella tularensis intracellular pathogenesis. Cold Spring Harb. Perspect. Med. 3 (4), a010314. doi: 10.1101/cshperspect.a010314
Chang M., Mahasenan K. V., Hermoso J. A., Mobashery S. (2021). Unconventional antibacterials and adjuvants. Acc Chem. Res. 54 (4), 917–929. doi: 10.1021/acs.accounts.0c00776
Chen Y. H., Chen S. H., Jong A., Zhou Z. Y., Li W., Suzuki K., et al. (2002). Enhanced escherichia coli invasion of human brain microvascular endothelial cells is associated with alternations in cytoskeleton induced by nicotine. Cell Microbiol. 4 (8), 503–514. doi: 10.1046/j.1462-5822.2002.00209.x
Chiang C. Y., Uzoma I., Moore R. T., Gilbert M., Duplantier A. J., Panchal R. G. (2018). Mitigating the impact of antibacterial drug resistance through host-directed therapies: Current progress, outlook, and challenges. mBio. 9 (1), e01932-17. doi: 10.1128/mBio.01932-17
Cleary J. M., Shapiro G. I. (2010). Development of phosphoinositide-3 kinase pathway inhibitors for advanced cancer. Curr. Oncol. Rep. 12 (2), 87–94. doi: 10.1007/s11912-010-0091-6
DeLeo F. R., Diep B. A., Otto M. (2009). Host defense and pathogenesis in staphylococcus aureus infections. Infect. Dis. Clin. North Am. 23 (1), 17–34. doi: 10.1016/j.idc.2008.10.003
De Oliveira D. M. P., Forde B. M., Kidd T. J., Harris P. N. A., Schembri M. A., Beatson S. A., et al. (2020). Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 33 (3). doi: 10.1128/CMR.00181-19
Edwards A. M., Potts J. R., Josefsson E., Massey R. C. (2010). Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PloS Pathog. 6 (6), e1000964. doi: 10.1371/journal.ppat.1000964
Ellis M. J., Tsai C. N., Johnson J. W., French S., Elhenawy W., Porwollik S., et al. (2019). A macrophage-based screen identifies antibacterial compounds selective for intracellular salmonella typhimurium. Nat. Commun. 10 (1), 197. doi: 10.1038/s41467-018-08190-x
Feriotti C., Sa-Pessoa J., Calderon-Gonzalez R., Gu L., Morris B., Sugisawa R., et al. (2022). Klebsiella pneumoniae hijacks the toll-IL-1R protein SARM1 in a type I IFN-dependent manner to antagonize host immunity. Cell Rep. 40 (6), 111167. doi: 10.1016/j.celrep.2022.111167
Fleischmann-Struzek C., Mellhammar L., Rose N., Cassini A., Rudd K. E., Schlattmann P., et al. (2020). Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 46 (8), 1552–1562. doi: 10.1007/s00134-020-06151-x
Flinn I. W., Cherry M. A., Maris M. B., Matous J. V., Berdeja J. G., Patel M. (2019). Combination trial of duvelisib (IPI-145) with rituximab or bendamustine/rituximab in patients with non-Hodgkin lymphoma or chronic lymphocytic leukemia. Am. J. Hematol. 94 (12), 1325–1334. doi: 10.1002/ajh.25634
Fruman D. A., Chiu H., Hopkins B. D., Bagrodia S., Cantley L. C., Abraham R. T. (2017). The PI3K pathway in human disease. Cell. 170 (4), 605–635. doi: 10.1016/j.cell.2017.07.029
Ganesan N., Embi N., Hasidah M. S. (2020). Potential of repurposing chloroquine as an adjunct therapy for melioidosis based on a murine model of burkholderia pseudomallei infection. Trop. Biomed. 37 (2), 303–317.
Garber K. (2014). Kinase inhibitors overachieve in CLL. Nat. Rev. Drug Discovery 13 (3), 162–164. doi: 10.1038/nrd4259
Garcia-Gil A., Galan-Enriquez C. S., Perez-Lopez A., Nava P., Alpuche-Aranda C., Ortiz-Navarrete V. (2018). SopB activates the akt-YAP pathway to promote salmonella survival within b cells. Virulence. 9 (1), 1390–1402. doi: 10.1080/21505594.2018.1509664
Gillooly D. J., Simonsen A., Stenmark H. (2001). Phosphoinositides and phagocytosis. J. Cell Biol. 155 (1), 15–17. doi: 10.1083/jcb.200109001
Gulluni F., De Santis M. C., Margaria J. P., Martini M., Hirsch E. (2019). Class II PI3K functions in cell biology and disease. Trends Cell Biol. 29 (4), 339–359. doi: 10.1016/j.tcb.2019.01.001
Gulluni F., Martini M., De Santis M. C., Campa C. C., Ghigo A., Margaria J. P., et al. (2017). Mitotic spindle assembly and genomic stability in breast cancer require PI3K-C2alpha scaffolding function. Cancer Cell. 32 (4), 444–59 e7. doi: 10.1016/j.ccell.2017.09.002
Hartkoorn R. C., Chandler B., Owen A., Ward S. A., Bertel Squire S., Back D. J., et al. (2007). Differential drug susceptibility of intracellular and extracellular tuberculosis, and the impact of p-glycoprotein. Tuberculosis (Edinb). 87 (3), 248–255. doi: 10.1016/j.tube.2006.12.001
Hawkins P. T., Stephens L. R. (2015). PI3K signalling in inflammation. Biochim. Biophys. Acta 1851 (6), 882–897. doi: 10.1016/j.bbalip.2014.12.006
He Y., Sun M. M., Zhang G. G., Yang J., Chen K. S., Xu W. W., et al. (2021). Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 6 (1), 425. doi: 10.1038/s41392-021-00828-5
Hii C. S., Sun G. W., Goh J. W., Lu J., Stevens M. P., Gan Y. H. (2008). Interleukin-8 induction by burkholderia pseudomallei can occur without toll-like receptor signaling but requires a functional type III secretion system. J. Infect. Dis. 197 (11), 1537–1547. doi: 10.1086/587905
Hommes J. W., Surewaard B. G. J. (2022). Intracellular habitation of staphylococcus aureus: Molecular mechanisms and prospects for antimicrobial therapy. Biomedicines. 10 (8), 1804. doi: 10.3390/biomedicines10081804
Hoxhaj G., Manning B. D. (2020). The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer. 20 (2), 74–88. doi: 10.1038/s41568-019-0216-7
Huang F. C., Li Q., Cherayil B. J. (2005). A phosphatidyl-inositol-3-kinase-dependent anti-inflammatory pathway activated by salmonella in epithelial cells. FEMS Microbiol. letters. 243 (1), 265–270. doi: 10.1016/j.femsle.2004.12.013
Hurley D., McCusker M. P., Fanning S., Martins M. (2014). Salmonella-host interactions - modulation of the host innate immune system. Front. Immunol. 5. doi: 10.3389/fimmu.2014.00481
Imai Y., Yamagishi H., Ono Y., Ueda Y. (2012). Versatile inhibitory effects of the flavonoid-derived PI3K/Akt inhibitor, LY294002, on ATP-binding cassette transporters that characterize stem cells. Clin. Transl. Med. 1 (1), 24. doi: 10.1186/2001-1326-1-24
Islam S., Yoshioka K., Aki S., Ishimaru K., Yamada H., Takuwa N., et al. (2020). Class II phosphatidylinositol 3-kinase alpha and beta isoforms are required for vascular smooth muscle rho activation, contraction and blood pressure regulation in mice. J. Physiol. Sci. 70 (1), 18. doi: 10.1186/s12576-020-00745-2
Jaber N., Dou Z., Chen J. S., Catanzaro J., Jiang Y. P., Ballou L. M., et al. (2012). Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc. Natl. Acad. Sci. United States America. 109 (6), 2003–2008. doi: 10.1073/pnas.1112848109
Jean S., Kiger A. A. (2014). Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 127 (Pt 5), 923–928. doi: 10.1242/jcs.093773
Juhasz G., Hill J. H., Yan Y., Sass M., Baehrecke E. H., Backer J. M., et al. (2008). The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in drosophila. J. Cell Biol. 181 (4), 655–666. doi: 10.1083/jcb.200712051
Kamaruzzaman N. F., Kendall S., Good L. (2017). Targeting the hard to reach: challenges and novel strategies in the treatment of intracellular bacterial infections. Br. J. Pharmacol. 174 (14), 2225–2236. doi: 10.1111/bph.13664
Kaufmann S. H. E., Dorhoi A., Hotchkiss R. S., Bartenschlager R. (2018). Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discovery 17 (1), 35–56. doi: 10.1038/nrd.2017.162
Kilinc G., Saris A., Ottenhoff T. H. M., Haks M. C. (2021). Host-directed therapy to combat mycobacterial infections. Immunol. Rev. 301 (1), 62–83. doi: 10.1111/imr.12951
Kimmey J. M., Stallings C. L. (2016). Bacterial pathogens versus autophagy: Implications for therapeutic interventions. Trends Mol. Med. 22 (12), 1060–1076. doi: 10.1016/j.molmed.2016.10.008
Kiran D., Podell B. K., Chambers M., Basaraba R. J. (2016). Host-directed therapy targeting the mycobacterium tuberculosis granuloma: a review. Semin. Immunopathol. 38 (2), 167–183. doi: 10.1007/s00281-015-0537-x
Koch P. A., Dornan G. L., Hessenberger M., Haucke V. (2021). The molecular mechanisms mediating class II PI 3-kinase function in cell physiology. FEBS J. 288 (24), 7025–7042. doi: 10.1111/febs.15692
Koyasu S. (2003). The role of PI3K in immune cells. Nat. Immunol. 4 (4), 313–319. doi: 10.1038/ni0403-313
Krachler A. M., Woolery A. R., Orth K. (2011). Manipulation of kinase signaling by bacterial pathogens. J. Cell Biol. 195 (7), 1083–1092. doi: 10.1083/jcb.201107132
Krag C., Malmberg E. K., Salcini A. E. (2010). PI3KC2alpha, a class II PI3K, is required for dynamin-independent internalization pathways. J. Cell Sci. 123 (Pt 24), 4240–4250. doi: 10.1242/jcs.071712
Lacoma A., Cano V., Moranta D., Regueiro V., Dominguez-Villanueva D., Laabei M., et al. (2017). Investigating intracellular persistence of staphylococcus aureus within a murine alveolar macrophage cell line. Virulence. 8 (8), 1761–1775. doi: 10.1080/21505594.2017.1361089
Leahy J. W., Buhr C. A., Johnson H. W., Kim B. G., Baik T., Cannoy J., et al. (2012). Discovery of a novel series of potent and orally bioavailable phosphoinositide 3-kinase gamma inhibitors. J. medicinal Chem. 55 (11), 5467–5482. doi: 10.1021/jm300403a
Ledvina H. E., Kelly K. A., Eshraghi A., Plemel R. L., Peterson S. B., Lee B., et al. (2018). A phosphatidylinositol 3-kinase effector alters phagosomal maturation to promote intracellular growth of francisella. Cell Host Microbe 24 (2), 285–95 e8. doi: 10.1016/j.chom.2018.07.003
Lee S., Kim M. S., Jeong J. W., Chae J. W., Koo T. S., Maeng H. J., et al. (2022). Bioanalysis of alpelisib using liquid chromatography–tandem mass spectrometry and application to pharmacokinetic study. J. Anal. Sci. Technol 13 (2022). doi: 10.1186/s40543-022-00340-7
Leisching G. R. (2019). PI3-kinase deltagamma catalytic isoforms regulate the Th-17 response in tuberculosis. Front. Immunol. 10. doi: 10.3389/fimmu.2019.02583
Liu Y., Jia Y., Yang K., Wang Z. (2020). Heterogeneous strategies to eliminate intracellular bacterial pathogens. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00563
Liu Y., Li J. Y., Chen S. T., Huang H. R., Cai H. (2016). The rLrp of mycobacterium tuberculosis inhibits proinflammatory cytokine production and downregulates APC function in mouse macrophages via a TLR2-mediated PI3K/Akt pathway activation-dependent mechanism. Cell Mol. Immunol. 13 (6), 729–746. doi: 10.1038/cmi.2015.58
Liu Y., Li R., Xiao X., Wang Z. (2019). Antibiotic adjuvants: an alternative approach to overcome multi-drug resistant gram-negative bacteria. Crit. Rev. Microbiol. 45 (3), 301–314. doi: 10.1080/1040841X.2019.1599813
Liu Y., Tong Z., Shi J., Li R., Upton M., Wang Z. (2021). Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics. 11 (10), 4910–4928. doi: 10.7150/thno.56205
Loh L. N., McCarthy E. M. C., Narang P., Khan N. A., Ward T. H. (2017). Escherichia coli K1 utilizes host macropinocytic pathways for invasion of brain microvascular endothelial cells. Traffic. 18 (11), 733–746. doi: 10.1111/tra.12508
Macara I. G., Marinetti G. V., Balduzzi P. C. (1984). “Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity: possible role in tumorigenesis,” in Proceedings of the national academy of sciences of the united states of America, vol. 81. , 2728–2732. doi: 10.1073/pnas.81.9.2728
Maffei A., Lembo G., Carnevale D. (2018). PI3Kinases in diabetes mellitus and its related complications. Int. J. Mol. Sci. 19 (12), 4098. doi: 10.3390/ijms19124098
Magagnoli M., Carlo-Stella C., Santoro A. (2020). Copanlisib for the treatment of adults with relapsed follicular lymphoma. Expert Rev. Clin. Pharmacol. 13 (8), 813–823. doi: 10.1080/17512433.2020.1787829
Martinez E., Siadous F. A., Bonazzi M. (2018). Tiny architects: biogenesis of intracellular replicative niches by bacterial pathogens. FEMS Microbiol. Rev. 42 (4), 425–447. doi: 10.1093/femsre/fuy013
Maurin M. (2015). Francisella tularensis as a potential agent of bioterrorism? Expert Rev. anti-infective Ther. 13 (2), 141–144. doi: 10.1586/14787210.2015.986463
Mavrommati I., Cisse O., Falasca M., Maffucci T. (2016). Novel roles for class II phosphoinositide 3-kinase C2beta in signalling pathways involved in prostate cancer cell invasion. Sci. Rep. 6, 23277. doi: 10.1038/srep23277
Mazza S., Maffucci T. (2011). Class II phosphoinositide 3-kinase C2alpha: what we learned so far. Int. J. Biochem. Mol. Biol. 2 (2), 168–182.
Mishra R., Patel H., Alanazi S., Kilroy M. K., Garrett J. T. (2021). PI3K inhibitors in cancer: Clinical implications and adverse effects. Int. J. Mol. Sci. 22 (7). doi: 10.3390/ijms22073464
Mitchell G., Chen C., Portnoy D. A. (2016). Strategies used by bacteria to grow in macrophages. Microbiol. Spectr. 4 (3), 10. doi: 10.1128/microbiolspec.MCHD-0012-2015
Nakagawa S., Matsumoto M., Katayama Y., Oguma R., Wakabayashi S., Nygaard T., et al. (2017). Staphylococcus aureus virulent PSMalpha peptides induce keratinocyte alarmin release to orchestrate IL-17-Dependent skin inflammation. Cell Host Microbe 22 (5), 667–77 e5. doi: 10.1016/j.chom.2017.10.008
Nemazanyy I., Montagnac G., Russell R. C., Morzyglod L., Burnol A. F., Guan K. L., et al. (2015). Class III PI3K regulates organismal glucose homeostasis by providing negative feedback on hepatic insulin signalling. Nat. Commun. 6, 8283. doi: 10.1038/ncomms9283
Nguyen J. A., Yates R. M. (2021). Better together: Current insights into phagosome-lysosome fusion. Front. Immunol. 12. doi: 10.3389/fimmu.2021.636078
Nurnberg B., Beer-Hammer S. (2019). Function, regulation and biological roles of PI3Kgamma variants. Biomolecules. 9 (9), 427. doi: 10.3390/biom9090427
Oelschlaeger T. A., Tall B. D. (1997). Invasion of cultured human epithelial cells by klebsiella pneumoniae isolated from the urinary tract. Infect. Immun. 65 (7), 2950–2958. doi: 10.1128/iai.65.7.2950-2958.1997
Ogawa M., Mimuro H., Yoshikawa Y., Ashida H., Sasakawa C. (2011). Manipulation of autophagy by bacteria for their own benefit. Microbiol. Immunol. 55 (7), 459–471. doi: 10.1111/j.1348-0421.2011.00343.x
Oghumu S., Satoskar A. R. (2013). PI3K-gamma inhibitors in the therapeutic intervention of diseases caused by obligate intracellular pathogens. Commun. Integr. Biol. 6 (2), e23360. doi: 10.4161/cib.23360
Ohashi Y., Tremel S., Masson G. R., McGinney L., Boulanger J., Rostislavleva K., Johnson C. M., Niewczas I., Clark J., Williams R. L. (2020). Membrane characteristics tune activities of endosomal and autophagic human VPS34 complexes. Elife. 9:e058281. doi: 10.7554/eLife.58281
Okkenhaug K. (2013). Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 31, 675–704. doi: 10.1146/annurev-immunol-032712-095946
Oviedo-Boyso J., Cortes-Vieyra R., Huante-Mendoza A., Yu H. B., Valdez-Alarcon J. J., Bravo-Patino A., et al. (2011). The phosphoinositide-3-kinase-Akt signaling pathway is important for staphylococcus aureus internalization by endothelial cells. Infect. Immun. 79 (11), 4569–4577. doi: 10.1128/IAI.05303-11
Paik S., Kim J. K., Chung C., Jo E. K. (2019). Autophagy: A new strategy for host-directed therapy of tuberculosis. Virulence. 10 (1), 448–459. doi: 10.1080/21505594.2018.1536598
Patel J. M., Sapey E., Parekh D., Scott A., Dosanjh D., Gao F., et al. (2018). Sepsis induces a dysregulated neutrophil phenotype that is associated with increased mortality. Mediators Inflamm. 2018, 4065362. doi: 10.1155/2018/4065362
Petit T. J. P., Lebreton A. (2022). Adaptations of intracellular bacteria to vacuolar or cytosolic niches. Trends Microbiol. 30 (8), 736–748. doi: 10.1016/j.tim.2022.01.015
Pflughoeft K., Hau D., Thorkildson P., AuCoin D. (2019). Burkholderia pseudomallei. Eds. Sunit K., Singh J. H. K. (Springer Nature Switzerland AG: Defense Against Biological Attacks: Springer Cham), 185–212.
Rascio F., Spadaccino F., Rocchetti M. T., Castellano G., Stallone G., Netti G. S., Ranieri E.. (2021). The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers (Basel). 13 (16), 3949. doi: 10.3390/cancers13163949
Reddy M. A., Prasadarao N. V., Wass C. A., Kim K. S. (2000). Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 275 (47), 36769–36774. doi: 10.1074/jbc.M007382200
Richardson N. C., Kasamon Y., Pazdur R., Gormley N. (2022). The saga of PI3K inhibitors in haematological malignancies: survival is the ultimate safety endpoint. Lancet Oncol. 23 (5), 563–566. doi: 10.1016/S1470-2045(22)00200-5
Roppenser B., Kwon H., Canadien V., Xu R., Devreotes P. N., Grinstein S., et al. (2013). Multiple host kinases contribute to akt activation during salmonella infection. PloS One 8 (8), e71015. doi: 10.1371/journal.pone.0071015
Sah P., Lutter E. I. (2020). Hijacking and use of host kinases by chlamydiae. Pathogens. 9 (12), 3949. doi: 10.3390/pathogens9121034
Sapey E., Greenwood H., Walton G., Mann E., Love A., Aaronson N., et al. (2014). Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 123 (2), 239–248. doi: 10.1182/blood-2013-08-519520
Schabbauer G., Tencati M., Pedersen B., Pawlinski R., Mackman N. (2004). PI3K-akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler. Thromb. Vasc. Biol. 24 (10), 1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce
Sendi P., Proctor R. A. (2009). Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol. 17 (2), 54–58. doi: 10.1016/j.tim.2008.11.004
Shapira T., Rankine-Wilson L., Chao J. D., Pichler V., Rens C., Pfeifer T., et al. (2020). High-content screening of eukaryotic kinase inhibitors identify CHK2 inhibitor activity against mycobacterium tuberculosis. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.553962
Silva M. T. (2012). Classical labeling of bacterial pathogens according to their lifestyle in the host: inconsistencies and alternatives. Front. Microbiol. 3. doi: 10.3389/fmicb.2012.00071
Silva M. T., Pestana N. T. (2013). The in vivo extracellular life of facultative intracellular bacterial parasites: role in pathogenesis. Immunobiology. 218 (3), 325–337. doi: 10.1016/j.imbio.2012.05.011
Silver L. L. (2011). Challenges of antibacterial discovery. Clin. Microbiol. Rev. 24 (1), 71–109. doi: 10.1128/cmr.00030-10
Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. (1984). “Evidence that the rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol,” in Proceedings of the national academy of sciences of the united states of America, vol. 81. , 2117–2121. doi: 10.1073/pnas.81.7.2117
Thi E. P., Reiner N. E. (2012). Phosphatidylinositol 3-kinases and their roles in phagosome maturation. J. Leukoc. Biol. 92 (3), 553–566. doi: 10.1189/jlb.0212053
Tucker A. N., Carlson T. J., Sarkar A. (2021). Challenges in drug discovery for intracellular bacteria. Pathogens. 10 (9), 1172. doi: 10.3390/pathogens10091172
Turner N. A., Sharma-Kuinkel B. K., Maskarinec S. A., Eichenberger E. M., Shah P. P., Carugati M., et al. (2019). Methicillin-resistant staphylococcus aureus: an overview of basic and clinical research. Nat. Rev. Microbiol. 17 (4), 203–218. doi: 10.1038/s41579-018-0147-4
Utermark T., Rao T., Cheng H., Wang Q., Lee S. H., Wang Z. C., et al. (2012). The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 26 (14), 1573–1586. doi: 10.1101/gad.191973.112
Valet C., Chicanne G., Severac C., Chaussade C., Whitehead M. A., Cabou C., et al. (2015). Essential role of class II PI3K-C2alpha in platelet membrane morphology. Blood. 126 (9), 1128–1137. doi: 10.1182/blood-2015-03-636670
Van Avondt K., van Sorge N. M., Meyaard L. (2015). Bacterial immune evasion through manipulation of host inhibitory immune signaling. PloS Pathog. 11 (3), e1004644. doi: 10.1371/journal.ppat.1004644
Vanhaesebroeck B., Perry M. W. D., Brown J. R., Andre F., Okkenhaug K. (2021). PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discovery 20 (10), 741–769. doi: 10.1038/s41573-021-00209-1
Vergne I., Chua J., Deretic V. (2003). Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198 (4), 653–659. doi: 10.1084/jem.20030527
Vergne I., Chua J., Lee H. H., Lucas M., Belisle J., Deretic V. (2005). “Mechanism of phagolysosome biogenesis block by viable mycobacterium tuberculosis,” in Proceedings of the national academy of sciences of the united states of America. (New York: Rockefeller University Press) 102, 4033–4038. doi: 10.1073/pnas.0409716102
Vergne I., Fratti R. A., Hill P. J., Chua J., Belisle J., Deretic V. (2004). Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell. 15 (2), 751–760. doi: 10.1091/mbc.e03-05-0307
Vincent J. L., Abraham E., Annane D., Bernard G., Rivers E., Van den Berghe G. (2002). Reducing mortality in sepsis: new directions. Crit. Care 6 Suppl 3 (Suppl 3), S1–18. doi: 10.1186/cc1860
Wallis R. S., O'Garra A., Sher A., Wack A. (2022). Host-directed immunotherapy of viral and bacterial infections: past, present and future. Nat. Rev. Immunol 23(2), 121–133. doi: 10.1038/s41577-022-00734-z
Wang H., Lo W. T., Vujicic Zagar A., Gulluni F., Lehmann M., Scapozza L., et al. (2018). Autoregulation of class II alpha PI3K activity by its lipid-binding PX-C2 domain module. Mol. Cell. 71 (2), 343–51 e4. doi: 10.1016/j.molcel.2018.06.042
Whitfeild SJ R. J., Griffiths G., Williamson E. D., Cater A. J. (2017). “The akt pathway inhibitor degeulin prevents staphylococcal enterotoxin b induced splenocyte proliferation and inflammation,” in Advances in bioscience and biotechnology (Scientific Research). doi: 10.4236/abb.2017.81001
Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. (1988). Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 332 (6165), 644–646. doi: 10.1038/332644a0
Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. (1985). Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 315 (6016), 239–242. doi: 10.1038/315239a0
Wong W. F., Chambers J. P., Gupta R., Arulanandam B. P. (2019). Chlamydia and its many ways of escaping the host immune system. J. Pathog. 2019, 8604958. doi: 10.1155/2019/8604958
Wright G. D. (2016). Antibiotic adjuvants: Rescuing antibiotics from resistance. Trends Microbiol. 24 (11), 862–871. doi: 10.1016/j.tim.2016.06.009
Xue Q., Jenkins S. A., Gu C., Smeds E., Liu Q., Vasan R., et al. (2010). Bacillus anthracis spore entry into epithelial cells is an actin-dependent process requiring c-src and PI3K. PloS One 5 (7), e11665. doi: 10.1371/journal.pone.0011665
Yang Q., Modi P., Newcomb T., Queva C., Gandhi V. (2015). Idelalisib: First-in-Class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma. Clin. Cancer Res. 21 (7), 1537–1542. doi: 10.1158/1078-0432.CCR-14-2034
Yang J., Nie J., Ma X., Wei Y., Peng Y., Wei X. (2019). Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol. Cancer. 18 (1), 26. doi: 10.1186/s12943-019-0954-x
Yang Z., Zou X., Feng P., Zhan H., Xiong D., Lang J. (2019). Inhibition of the PI3K/AKT signaling pathway or overexpression of Beclin1 blocks reinfection of streptococcus pneumoniae after infection of influenza a virus in severe community-acquired pneumonia. Inflammation. 42 (5), 1741–1753. doi: 10.1007/s10753-019-01035-9
Yoong P., Pier G. B. (2010). Antibody-mediated enhancement of community-acquired methicillin-resistant staphylococcus aureus infection. Proc. Natl. Acad. Sci. United States America. 107 (5), 2241–2246. doi: 10.1073/pnas.0910344107
Yu K., Song L., Kang H. P., Kwon H. K., Back J., Lee F. Y. (2020). Recalcitrant methicillin-resistant staphylococcus aureus infection of bone cells: Intracellular penetration and control strategies. Bone Joint Res. 9 (2), 49–59. doi: 10.1302/2046-3758.92.BJR-2019-0131.R1
Zhang M., Jang H., Nussinov R. (2020). PI3K inhibitors: review and new strategies. Chem. Sci. 11 (23), 5855–5865. doi: 10.1039/d0sc01676d
Keywords: kinase inhibitor, drug resistant bacteria, adjuvant antibiotics, intracellular bacteria multiplication, bacterial invasion and survival
Citation: Fleeman R (2023) Repurposing inhibitors of phosphoinositide 3-kinase as adjuvant therapeutics for bacterial infections. Front. Antibiot. 2:1135485. doi: 10.3389/frabi.2023.1135485
Received: 31 December 2022; Accepted: 30 January 2023;
Published: 09 February 2023.
Edited by:
Waleed Younis, South Valley University, EgyptReviewed by:
Abhishek Mishra, Houston Methodist Research Institute, United StatesCopyright © 2023 Fleeman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renee Fleeman, UmVuZWUuRmxlZW1hbkB1Y2YuZWR1
†ORCID: Renee Fleeman, orcid.org/0000-0001-7103-461X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.