
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 17 April 2025
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1569682
 Celestin Munyaneza*
Celestin Munyaneza* Ferdinand Bizimana
Ferdinand Bizimana Felicitas Mukumbo
Felicitas Mukumbo Sandrine Gatesi
Sandrine Gatesi Ephrem Sibomana
Ephrem Sibomana Severin Munyampuhwe
Severin Munyampuhwe Marie Fausta Dutuze
Marie Fausta DutuzeBackground: Although zoonotic diseases pose significant health and economic threats globally, rural communities in developing countries are more vulnerable due to the increased proximity between animals and humans and the lack of knowledge about these diseases. This study assessed the knowledge, attitudes, practices (KAP), and risk factors regarding zoonotic diseases among smallholder livestock farmers in Bugesera district of Rwanda.
Methods and materials: A convenient sample of 155 livestock smallholder farmers was selected from eight of the fifteen sectors of the district. Data were collected through interviews using a semi-structured questionnaire. Descriptive analyses including frequencies and means were used to summarize the data. Pearson’s chi-square test was used to examine associations between knowledge and socio-demographic variables and between knowledge and practices.
Results: Findings showed that 50.3% of respondents knew diseases could be transmitted from animals to humans and just 13.5% recognized reverse zoonotic transmission - humans to animals. When specifically asked if they knew about brucellosis, tuberculosis, and Rift Valley fever; 88, 79, and 41% of respondents, respectively, reported being familiar with these diseases though many were unaware of their zoonotic nature. Risky attitudes and practices were prevalent, including the lack of isolation for sick animals (70.97%) and failure to quarantine newly introduced animals (83.87%). While 81.94% vaccinated their animals, only 16.54% could specify at least one vaccinated disease, and none knew the date of their animals’ next vaccination date. Other poor practices were reported, with 64.52% not separating animal and human utensils, and only 25.81% of cattle owners reported using artificial insemination. Additionally, 34.46% consumed raw non-boiled milk, and 24.5% did not use mosquito nets. Regarding roaming animals in the neighborhood, 79% of rats, 55% bats, 68% dogs, 67% cats, and 5.2% monkeys.
Conclusion: The study revealed low awareness and high-risk practices regarding zoonotic diseases among smallholder livestock farmers in Bugesera district, posing a significant One Health concern. Therefore, educational programs to improve KAP and strengthen zoonotic disease prevention efforts in this district.
Livestock plays a crucial role in rural livelihoods and the economies of smallholder farmers in developing countries, including Rwanda (1, 2). In Rwanda, livestock accounts for 4% of the national Gross Domestic Product (GDP) and around 50% of private households own at least one animal (3). Most of these farm households are considered smallholder farmers and significantly contribute to the overall country’s livestock production. This population of smallholder farmers has limited economic resources and often lacks proper training in livestock management and formal education. They typically raise animals across generations, primarily for subsistence and cultural reasons, rather than for significant commercial purposes.
In Rwanda, owning livestock is driven by several reasons including economic and nutritional benefits to the households as well as manure production. In addition, cattle in particular holds cultural value and social significance (4). For instance, households’ prosperity is traditionally linked with the number of cows owned, cows are given as dowry in traditional wedding ceremonies, and cows and sometimes small ruminants are exchanged between friends to enhance social cohesion. While modernization is diminishing these practices in urban areas, they are still deeply rooted in rural areas. This strong connection between Rwandans and livestock increases the risk of zoonotic disease transmission, particularly in rural settings (5–7).
Several zoonotic diseases are reported in Rwanda including Rift Valley Fever (RVF), brucellosis, bovine tuberculosis, leptospirosis, rabies, anthrax, monkeypox, and Marburg. Rift Valley Fever is endemic with epizootic surges. A study conducted in the Eastern province showed RVF seroprevalence rates varying from 7.9 to 36.9% in cattle across all six districts (8). Severe RVF outbreaks occurred in 2018 and 2022 in both animal and human populations, with the Eastern and Southern provinces most affected (9–11). Brucellosis prevalence in cattle has ranged from 1.7 to 18.9% over the past decade (12). In goats, the prevalence of 10.7%. was reported (13). Bovine tuberculosis prevalence, as assessed at Nyabugogo abattoir was found to be 0.5% based on culture and postmortem results (14). A study on estimates of foodborne pathogens including Campylobacter spp., nontyphoidal Salmonella enterica, Cryptosporidium spp., Brucella spp., and Mycobacterium bovis due to consumption of raw milk and other dairy products, respectively, showed estimates of 71; 121; 12; 0.3; and 13 cases per 100.000 population (15). A study conducted on asymptomatic adult humans in Gisagara and Huye districts showed a high seroprevalence of leptospirosis of 40.1% (16). For rabies, although no research has been conducted to determine the prevalence, in 2016, 413 dog bites were documented leading to one human death (17). For anthrax, prevalence data is also limited, its presence was noted in both lowland and highland agro-ecological zones (18). Recently in 2024, Rwanda experienced monkeypox for the first time, primarily affecting rural areas with frequent human-animal interactions. Still in 2024, from September to December, Rwanda also faced the first outbreak of Marburg with 66 confirmed cases and 15 deaths. Furthermore, Rwanda shares borders with countries where other severe viral zoonotic hemorrhagic fevers including Ebola virus disease, Crimean-Congo hemorrhagic fever (CCHF), and Lassa fever have been reported such as Uganda, Democratic Republic of the Congo (DRC), and Tanzania (19–22).
Zoonotic diseases are associated with significant losses in both human and animal populations. The losses include sicknesses and/or deaths, reduced animal productivity, financial burdens of prevention, treatment, and control measures, and economic losses from trade bans or restrictions on the trade of animals and/or animal products (5, 23–28). These consequences are more exacerbated in local smallholder farmers who usually rely on their livestock as a main source of income and/or proteins from animal sources.
Many zoonotic diseases are considered important occupational health hazards among livestock keepers (5), as various management practices such as those implying direct contact with animals, handling aborted materials with bare hands, and consumption of unpasteurized-infected milk increase their exposure to these diseases (29).
Thus, for effective prevention of zoonotic diseases, it is essential to enhance knowledge and promote appropriate attitudes and practices, particularly among populations that have daily interactions with livestock. This approach would help mitigate zoonotic diseases as occupational hazards and reduce their prevalence in both human and animal populations (30). In this perspective, several studies have shown that a large number of livestock farmers are not aware of zoonotic diseases, and that some of their attitudes and practices expose them to the risk of zoonotic transmission (31, 32).
Although Rwanda has selected 6 priority zoonotic diseases in 2017 and strengthened their surveillance programs, these diseases remain a public health concern. These include (i) viral hemorrhagic fevers (Ebola, Marburg, Yellow fever, and CCHF), (ii) High Pathogenic Avian Influenza (HPAI), (iii) RVF, (iv) brucellosis, (v) sleeping sickness, and rabies (33, 34). Studies conducted in different districts of the country focused mainly on seroprevalence of these diseases (7–9, 12, 35–40). Very few studies were conducted on knowledge, attitudes, and practices (KAP) about these zoonotic diseases (41–43). The existing studies on KAP related to zoonotic diseases have focused on individual diseases, and thus did not provide a comprehensive status on KAP levels within the populations of study regarding the zoonotic diseases present in their environment.
The present study aimed to assess the level of KAP toward zoonotic diseases among smallholder livestock farmers in Bugesera district of Rwanda.
This study was conducted in Bugesera District, which is located in the Southwest part of the Eastern province of Rwanda. The district spans an area of 1,337 km2 and consists of 15 administrative sectors. In 2023, the population of Bugesera is estimated to be 551,103 inhabitants located in 137,777 households (44). The district belongs to the climatic zone of tropical savannahs with the annual rainfall variable ranging between 850 and 1,200 mm. Agriculture in Bugesera district is predominantly rain-fed, making it highly vulnerable to weather fluctuations. The district covers a total land area of 120,400 hectares, with approximately 72,300 hectares, or 60.05% of the total area, dedicated to agricultural use (45). Of 137,777 households, 59 and 42% are involved in crop and livestock farming, respectively. The households raising cows, goats, sheep, pigs, rabbits, chickens and other poultry are, respectively, 13.8, 24.0, 1.2, 8.3, 5.6, 14.5 and 1.1%. The number of cows, goat, sheep, pigs, rabbits, chicken, and other poultry are, respectively, 34,412; 100,332; 4,533; 24,459; 17,307; 313,761 and 5,439 (44).
The study was conducted in 8 out of 15 administrative sectors of Bugesera District (Figure 1). These sectors are Gashora, Kamabuye, Mayange, Musenyi, Ngeruka, Nyamata, Rweru, and Shyara. The study population comprised of smallholder farmers who raise any type of domestic animal species namely cattle, goat, sheep, poultry, pig, and rabbit in the selected sectors. The sectors were selected based on the highest numbers of cattle population with the total cattle population. Cattle were considered as the reference species due to their significant role transmission of endemic zoonotic diseases such as Rift Valley fever (RVF), brucellosis, and tuberculosis, and high economic losses associated with them in cases of outbreaks (8, 9, 12, 14). In addition, its cultural value and its long-life cycle imply that cattle farmers usually keep the ‘farmer’ status for a long period as opposed to other species namely small ruminants, poultry, and pig farmers who are less consistent and abandon this business easily if productivity and production conditions are not optimal. Within the selected sectors, a convenient sample size of 155 smallholder farmers was determined based on financial and technical resources. A multi-stage sampling method was applied to a total of 49,011 households across the selected sectors—Gashora (5,131), Kamabuye (4,622), Mayange (6,617), Musenyi (7,123), Ngeruka (6,961), Nyamata (8,778), Rweru (6,399), and Shyara (3,380). The sample of 155 households was proportionally distributed among these sectors, with allocations of 15, 14, 19, 22, 21, 28, 24, and 12 households, respectively. Within each sector, households were selected randomly. The inclusion criteria consisted of belonging to a household that owns livestock in the same compound as humans and being an adult that was 18 years of age or older.
A cross-sectional study was conducted in November 2023. The data were collected using a semi-structured questionnaire that was written in English and then translated in Kinyarwanda, with interviews conducted in Kinyarwanda. The questionnaire comprised diverse questions on farmer’s socio-demographic information, KAP, and risk factors associated with zoonotic diseases and it was pre-tested to ensure its validity.
The data obtained from the questionnaires were recorded using Microsoft Excel 2021. Statistical analysis was done using R 4.4.1 statistical software. Socio-demographic data were summarized using gtsummary R package. These were presented as frequencies and percentages. Pearson’s chi-square was used to assess the difference in levels of knowledge in different categories of socio-demographic characteristics. Additionally, the association between practices and knowledge was evaluated. Throughout the statistical analyses, statistical significance was considered significant if p-value ≤0.05.
The necessary ethical permission for conducting this study was obtained from the Rwanda National Research and Ethic Committee (RNEC) with identification number of IRB 00001973 of IORG0001100 – No 893/RNEC/2022. Participants gave their consent by signing a consent form before the interviews.
A little more than half (53%) of the respondents were females while the remaining were males. 5.2, 71, and 23.8% were, respectively, aged between 18 and 29, 30 and 60, and above 60 years. Regarding educational level, 29.9% did not have formal education, 55.6% had primary level, 13% secondary level, and 1.3% university level. The respondents had various experiences with livestock, namely, less than 5 years, 5 to 9 years, 10 to 19 years, and 20 and over years with the respective proportions of 21, 13, 33, and 33%. Most of the farmer households (83%) owned cattle, while 64, 43, and 32% kept goats, chickens, and pigs, respectively. A small proportion (7.7%) kept sheep and very few farmers kept rabbits and other poultry, including turkeys and ducks (5.8 and 3.9%, respectively). Few households kept dogs (7.10%) and cats (5.1%) (Table 1).
Farmers mentioned various reasons for keeping livestock including the production of animal products such as milk, eggs, and meat for home consumption, selling animal products, selling live animals, saving money, production of manure, and others. Producing manure was mentioned as the main reason for keeping cattle (53.2%) among cattle keepers, followed by milk for home consumption (30.6%). The main reason for rearing goats and sheep was saving money with 42.7% of goat keepers and 63.6% of sheep keepers, respectively. Producing manure was the second reason for rearing goats and sheep with 32.2 and 27.2% of owner households, respectively. The two main reasons for rearing pigs were saving money and manure with 43.9 and 31.7% of households, respectively. The main reason for rearing chicken was for eggs for home consumption with 70.6% while 3.4% aimed at manure production. Rabbit keepers aimed at meat and manure production with the proportion of 42.8 and 28.5%, respectively. Cats and dogs were solely kept for protection.
As shown in Table 2, a little more than half of the respondents (50.3%) were aware that some diseases can be transmitted from animals to humans while only 13.5% of the respondents knew that diseases can be transmitted from humans to animals. 52.2% have heard of either diseases transmitted from animals to humans and/or humans to animals. Except for three respondents (1.9%), all those who acknowledged the possibility of human-to-animal disease transmission were also aware of animal-to-human transmission. Given their epidemiological importance in Rwanda, brucellosis, tuberculosis, and Rift Valley fever were specifically targeted by specific questions about them. To these questions, respondents showed levels of knowledge that were, respectively, of 88, 79, and 41%. Other diseases mentioned by respondents were gastro-intestinal diseases (3.8%), anthrax (2.6%), and rabies (1.3%). Due to the lack of knowledge, gastro-intestinal diseases were mentioned using broad terms without specifying diseases. The main sources of information included veterinarians (24%), radio (12%), other farmers (37%), local government officials (1.3%), schools (1.3%), and hospitals (1.3%).
Table 3 indicates that factors such as sector, gender, age group, experience in livestock rearing, membership in a farmer cooperative, and visits from livestock professionals did not significantly influence knowledge levels about zoonotic diseases.
The majority of farmers (71 and 84.5%, respectively) do not isolate sick animals from healthy ones or quarantine newly introduced animals. While 82% reported vaccinating their animals, only 13.5% could name at least one vaccinated disease, and none knew the date of the next vaccination. Additionally, only 25.6% of cattle owners use artificial insemination. While nearly all farmers (99.3%) use farm manure, only 3.2% wear PPE, such as gloves, when handling it. Approximately 65.1% of respondents assist animals during parturition, but only 17.1% use protective measures, posing a potential risk of infection (Table 4). When asked about the disposal of abortive materials, 75% reported burying them, while 20% either discarded them in the bushes or fed them to dogs, and 5% disposed of them in toilets.
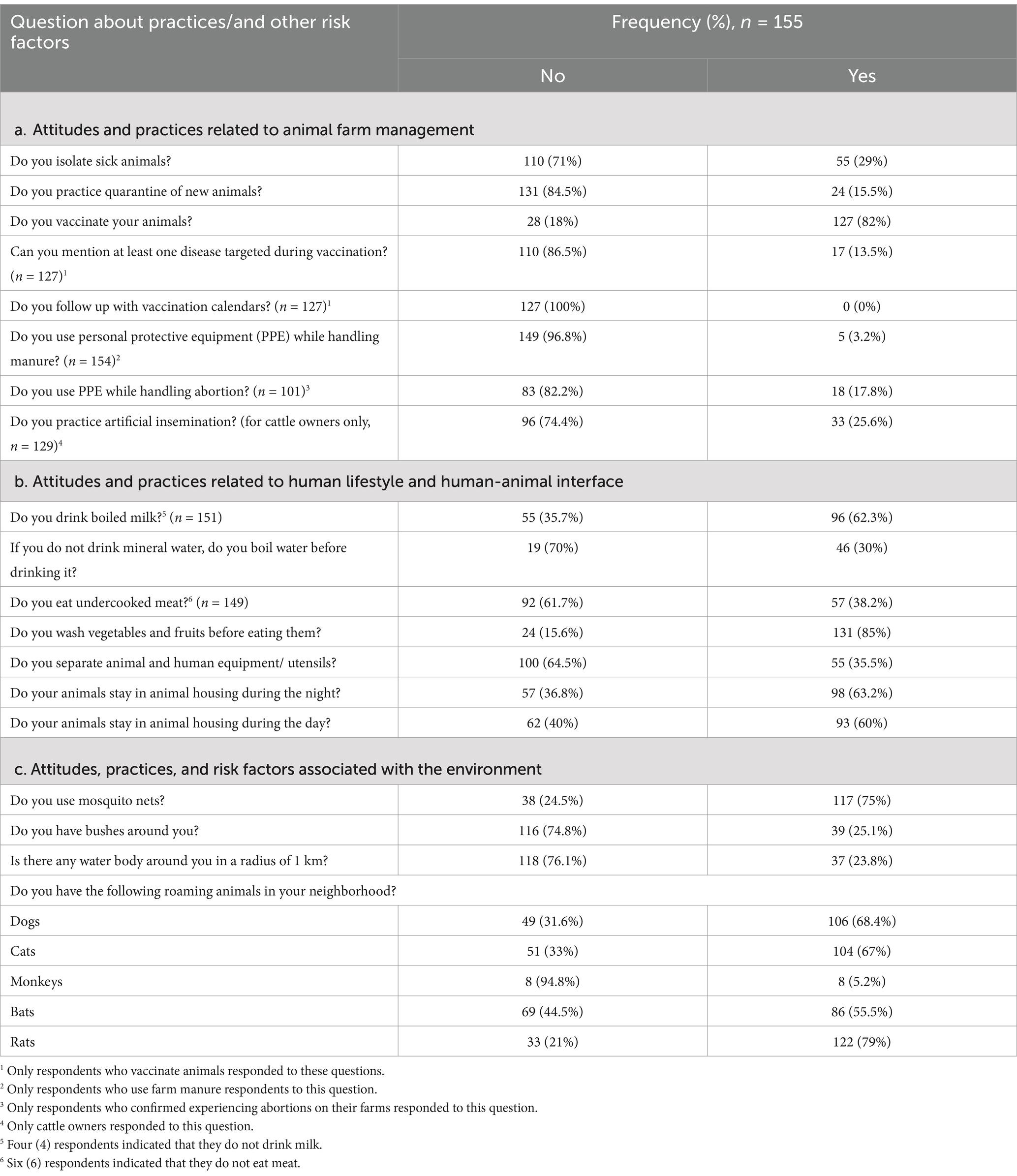
Table 4. Frequencies of respondents according to the practices associated with zoonotic disease transmission.
As shown in Table 4, approximately 62.3% of respondents boil milk before drinking it. Although none of the respondents reported drinking mineral water, only 30% boil tap or lake water before consuming it. Additionally, 38.2% of the respondents who eat meat (149 individuals) reported consuming undercooked meat occasionally. Approximately 85% of respondents wash raw vegetables and/or fruits before consumption. However, more than half of the farmers (64.5%) do not separate animal and human utensils, including cleaning and kitchen utensils.
About 40% of cattle farmers did not have animal houses for daytime use, and 36.8% lacked houses for nighttime use. More animals were housed at night than during the day. Chickens were the most commonly housed animals, with 71.43% housed during the day and 73.33% at night, followed by cattle (70.59% during the day and 73.33% at night), and small ruminants (59.26% during the day and 76.92% at night). Alternative housing used at night included kitchens (20% of cattle, 100% of rabbits, and 76.92% of small ruminants), storage rooms for human use (11.5% of small ruminants), and human houses (75% of pigs and 11.54% of small ruminants). All dogs and cats were roaming freely around the houses during the day and night, occasionally sleeping in the kitchen.
Regarding environmental risk factors, 75% of respondents reported using mosquito nets, while 24.8% live near bushes and 23.8% live less than 1 km from a water body. Additionally, respondents indicated the presence of roaming animals in their neighborhoods, including dogs (68.4%), cats (67%), monkeys (5.2%), bats (55.5%), and rats (79%) (Table 4).
Table 5 shows significant differences in knowledge about zoonotic diseases in relation to both vaccinating animals and boiling milk. A higher proportion of smallholder farmers who vaccinate their animals (45%) are aware of zoonotic diseases, compared to those who are not (5.8%). Similarly, a larger proportion of respondents who boil milk before drinking it (40%) have heard of zoonotic diseases, compared to those who have not (6.4%). No relationship was found between other practices and the level of knowledge about zoonotic diseases.
The demographic composition of the respondents, particularly the predominance of older and less-educated individuals, reflects the characteristics of smallholder livestock farming in Bugesera district. This should be taken into consideration while designing effective interventions to improve KAP regarding zoonotic diseases. The relatively small livestock numbers align with the typical smallholder farming systems, where farmers primarily raise animals for subsistence, cultural, and economic purposes. The findings also corroborate the Fifth Population and Housing Census of Rwanda conducted in 2012, which reported that most livestock farmers in Rwanda operate on a small scale and that sheep are relatively uncommon in the Eastern Province (44). As expected, cattle were found to be the most commonly owned animal species (83%), reflecting their significant cultural and economic importance to farmers. Beyond the historical preference for cattle compared to other livestock species in Rwanda, the number of cattle per household has increased due to the Girinka (One Cow per Poor Family) program, a social and agricultural initiative launched by the Rwandan government in 2006 (46). This program provides cows to vulnerable families to enhance nutrition, income, and agricultural productivity, with 450,000 cows distributed across the country as of 2021.
While Girinka has made important contributions to food security by improving nutrition, increasing agricultural productivity, reducing poverty, enhancing social cohesion - since beneficiaries are expected to pass on the first female calf to another vulnerable family- and promoting environmental sustainability (46, 47), it should also be accompanied by awareness campaigns on zoonotic diseases, given that cattle are main drivers of the most endemic zoonotic in Rwanda such as RVF, brucellosis, and tuberculosis (9, 11, 36, 48, 49).
A considerable proportion of the households kept livestock for their products, including milk, meat and eggs for sale and home consumption while other farmers raised livestock for producing manure. This interaction between human and domestic animals can influence attitudes and practices that expose livestock farmers to risks of contracting zoonotic diseases. Several studies have revealed that animal products such as milk, meat, eggs and manure can be a direct or indirect sources of zoonosis, if not handled or consumed correctly (50, 51). Although dogs and cats were raised for protection, they are known reservoirs of zoonotic diseases such as rabies, leptospirosis, salmonellosis, and leishmaniosis (52–56). This highlights the need for increased awareness about the possible zoonotic diseases that are related to different animal species owned by smallholder farmers, and appropriate biosecurity measures to mitigate the risk of zoonotic transmission.
Approximately half (50.3%) of the respondents demonstrated awareness of zoonotic disease transmission from animals to humans, but knowledge of reverse transmission (human-to-animal) was notably lower (13.5%). It was also found that except 3 respondents (1.9%), all respondents who know about human–to–animal transmission, are also aware of animal–to–human transmission. This may reveal that humans are generally concerned about human health and less about animal health, especially in rural areas where animal welfare is still not well known. 88,79, and 41% of respondents indicated having heard of brucellosis, tuberculosis, and RVF, however many of these are not aware of the zoonotic potential of these diseases. The increased awareness of these diseases may be attributed to their prevalence and economic impact in the region, as previous studies have documented significant seroprevalence rates for brucellosis (1.7–18.9%) (36, 57) and RVF (7.9–36.9%) (8, 9, 11) in Rwandan livestock populations. A prevalence of bovine tuberculosis in Nyabugogo abattoir was also found to be 0.5% (14). The more frequently diseases are reported in a geographic area, the greater the awareness among local inhabitants. When outbreaks occur - particularly those causing significant losses - they tend to receive high attention through various channels, including veterinarians, extension workers, radio broadcasts, and peer-to-peer farm communications. In addition, these three diseases are among the 6 selected priority zoonotic diseases at national level in 2017, alongside viral hemorrhagic fevers (Ebola, Marburg, Yellow fever, and CCHV), HPAI, and rabies (33, 34). This has increased the number of control programs specifically targeting them, which make them more familiar to the public ad especially to farmers.
Additional zoonotic diseases mentioned by respondents were anthrax (2.6%), rabies (1.35) and gastro-intestinal zoonotic diseases (3.8%). The low level of knowledge about anthrax and rabies is associated with low prevalence rates and limited research on them in Rwanda. Gastrointestinal zoonotic diseases were mentioned by respondents using general terms without specifying particular diseases. Under this group could be classified diseases caused by bacteria and parasites such as Campylobacter spp., Salmonella spp., Escherichia coli, Cryptosporidium spp., Toxoplasma gondii, and others. Categorizing them using broad terms is a sign of lack of knowledge, which is a significant challenge since the diseases are usually associated with poor hygienic conditions which mostly characterize smallholder farms. In addition, poor practices associated with transmission of those diseases were reported such as failure to wash raw vegetables and fruits before consumption (15.6%), and failure to boil tap and/or lake water before drinking (70%).
The main sources of information were found to be other farmers (37%), veterinarians (24%), and radio (12%). This shows that educational programs such training of trainers (ToT) in which community leaders are educated to educate others can be effective. It is also positive to find that veterinarians constitute a reliable source of information, thus they can also plan more educational programs about zoonotic disease prevention. Although not specifically covered in this study, social media usage can be considered an important teaching channel due to its growing influence, along with the widespread coverage of mobile phones and the internet across the country. As of early 2025, mobile phone usage in Rwanda has continued to expand, reaching 92% of the population, while internet penetration accounts for 34.2% of the population (58).
Although no significant difference was observed, gender disparities in knowledge were observed, with men demonstrating slightly higher awareness of zoonotic disease transmission. This may be attributed to differences in access to information and involvement in livestock management activities. Similar trends have been found in a study conducted to determine the level of KAP regarding RVF in the eastern province which showed that 78.7% of male have heard of RVF as opposed to 59.7% in female population (41). Older individuals and those with more years of livestock experience were also more knowledgeable, likely due to accumulated exposure to farming risks and traditional knowledge. However, participation in farmer cooperatives and visits from livestock officers did not significantly influence knowledge levels, suggesting a need for more targeted and effective education programs.
Despite the potential health risks associated with livestock management, many farmer in the study did not implement critical biosecurity measures. The majority (71%) did not isolate sick animals, and 84.5% did not quarantine newly introduced animals, increasing the likelihood of disease spread within farms. Comparisons with other studies indicate that poor quarantine practices are common in pastoral communities in Africa, as reported in Kenya (59) and South Africa (60).
Vaccination coverage among farmers was relatively high at 82%, but very few (13.5%) could specify the diseases their animals were vaccinated against, and none were aware of the next vaccination date for their animals. This knowledge gap undermines the effectiveness of vaccination programs, as adherence to proper vaccination schedules is crucial for effective disease prevention. The discrepancy between high vaccination rates and the lack of commitment to following vaccination schedules may be attributed to the fact that for most major diseases, vaccination campaigns are conducted and subsidized by the government. As a result, farmers may not feel personally responsible for keeping track of or following up on their animals’ vaccination schedules. Strategies should be implemented to increase farmers’ accountability regarding animal vaccination. Additionally, artificial insemination use among cattle farmers was low (25.81%), a concerning trend given that natural breeding increases the risk of transmission of reproductive zoonoses such as brucellosis (50).
Risky food consumption behaviors were also observed, with 35.7% of respondents consuming unboiled milk and 61.7% eating undercooked meat. Given that the study population primarily consists of low-income smallholder farmers, consuming pasteurized milk may not be feasible; however, boiling milk before consumption is a practical and effective measure to prevent zoonotic transmission. Similarly, while 98.05% of respondents consumed vegetables, only 86.75% washed them before eating, increasing the risk of exposure to foodborne zoonotic bacteria and parasites such as Campylobacter spp., Salmonella spp., Escherichia coli, Cryptosporidium spp., Toxoplasma gondii, and others (61).
Environmental factors play a crucial role in the transmission of zoonotic diseases. Approximately 75.48% of respondents in the study reported using mosquito nets, but a significant proportion lived near bushes (24.84%) or water bodies (23.86%). These environmental conditions increase the risk of exposure to vector-borne zoonotic diseases. While some of these diseases have already been reported in the area, others have not yet been detected, but the district is at risk due to its geographic features, such as low altitude, high temperatures, and the presence of numerous water bodies, all of which are favorable to the mosquito life cycle. Several zoonotic mosquito-borne diseases, such as Rift Valley Fever (RVF), which is endemic and causes severe outbreaks, as well as other bunyaviral infections like Bunyamwera and Batai, have been reported in Rwanda (9, 11), and other bunyaviral zoonotic infections such Bunyamwera, Batai, are reported in Rwanda (9). A study that aimed at determining occurrence of other arboviruses including chikungunya virus (CHIKV), o’nyong-nyong virus (ONNV), dengue virus (DENV), West Nile virus (WNV), Zika virus (ZIKV), Rift Valley fever virus (RVFV) and CCHFV showed that in a sample of 2,294 febrile human patients that were put in 230 pools, ONNV infection was detected in 12 pools (5.2%) while ZIKV was detected in three pools (1.3%). Other arboviruses were not detected in this study (62). Given that Rwanda is sharing borders with Uganda, Tanzania, and DRC where those diseases are reported, the risk of transmission is high.
A significant number of roaming animals, including rats (79%), bats (55.5%), stray dogs (68.4%), cats (67%), and monkeys (5.2%), were reported by respondents, posing a risk for zoonotic disease transmission. Rats serve as reservoirs for leptospirosis, salmonellosis, hantaviruses, toxoplasmosis, and plague. Cats can transmit toxoplasmosis, rabies, salmonellosis, campylobacteriosis, and leptospirosis. Stray dogs are potential carriers of rabies, leptospirosis, leishmaniasis, campylobacteriosis, and scabies. Monkeys contribute to the spread of rabies, Marburg virus disease, Ebola virus disease, monkeypox, yellow fever, tuberculosis, leptospirosis, and gastrointestinal parasites such as Giardia, Cryptosporidium, and Entamoeba spp. Bats are natural reservoirs of rabies, EVD, MVD, histoplasmosis, leptospirosis, severe acute respiratory syndrome (SARS), SARS-CoV-2 (COVID-19), and other coronaviruses (63–66). The presence of these animals significantly increases the risk of zoonotic disease outbreaks, especially since some of these diseases already reported in Rwanda.
Limitations of this study include the use of a convenience sample, which was determined based on available financial and technical resources, potentially affecting the generalizability of the findings. Additionally, some survey questions were structured as simple ‘Yes/No’ responses, whereas a Likert scale could have provided a more nuanced understanding of respondents’ behaviors. For example, questions such as “Do you wash vegetables and fruits?,” “Do you boil milk before drinking?,” and “Do you boil water before drinking?” assumed consistent practices, whereas a frequency-based scale (e.g., always, often, sometimes, rarely, never) would have better captured variations in behavior. This limitation may have led to an overestimation or underestimation of certain practices, impacting the accuracy of the study’s conclusions.
Overall, this study highlights significant gaps in KAP related to zoonotic diseases among smallholder livestock farmers in Bugesera district. The findings emphasize the urgent need for tailored educational programs focusing on disease awareness, biosecurity practices, and safe food handling. Strengthening vaccination programs, promoting AI adoption, and implementing stricter animal movement controls are also crucial measures to reduce zoonotic risks. Future research should include serological surveys of key zoonotic diseases among both livestock and human populations to better understand transmission dynamics and inform policy decisions.
Zoonotic diseases can have negative economic and social impact on livestock farmers and other stakeholders. This study assessed the knowledge, attitudes and practices among livestock keepers in Bugesera district. The results highlighted the crucial contributions of livestock toward the farmers’ livelihoods through the provision of food for family consumption, manure for fertilizing cropland, and generating income for daily or emergency expenses. However, some critical gaps in knowledge, attitudes and practices required to minimize the risk of zoonotic disease transmission were also revealed. This poses a risk to health, well-being and socio-economic stability in smallholder livestock farming communities.
The results from the study show that the knowledge about zoonotic diseases is still low. Some farmers do not know that a disease can be transmitted from animals to humans and vice versa. Others do not know the most endemic zoonotic diseases in Rwanda including brucellosis, RVF and toxoplasmosis. Farmers still have some attitudes and practices that expose them to risk of contracting zoonotic diseases. Those include drinking raw milk and undercooked meat, assisting animal parturition without protection, sharing utensils with animals, and staying in the same house with animals. Some preventions of zoonotic diseases are not respected. These include biosecurity measures such as vaccination and quarantine, using mosquito nets. Other factors can put livestock keepers to risk of zoonotic diseases including having roaming animals such as dogs, cats, bats and monkeys, having bushes around the house. Hence, there is a need for intervention to teach the farmers about zoonotic diseases and how they can be prevented. This study is crucial for guiding intervention. More studies are recommended, these include screening the most common diseases such as brucellosis, tuberculosis and RVF among the livestock keepers and their animals.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Rwanda National Research and Ethic Committee (RNEC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FB: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition, Software. FM: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SG: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. ES: Conceptualization, Investigation, Supervision, Writing – review & editing. SM: Conceptualization, Investigation, Supervision, Writing – review & editing. MD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was fully funded by the Rwanda Institute for Conservation Agriculture (RICA) following the call for research and extension projects proposals No 01/2022.
We would like to express our gratitude to the livestock keepers for sharing their valuable information for this study, to the livestock officers who guided the data collection process, and to the enumerators for their dedicated efforts in gathering the data. We also extend our sincere thanks to RICA for the essential support, including funding, which made this study possible.
The authors declare that the research was conducted without any commercial or financial relationships that could be perceived as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1569682/full#supplementary-material
1. Herrero, M, Grace, D, Njuki, J, Johnson, N, Enahoro, D, Silvestri, S, et al. The roles of livestock in developing countries. Animal. (2013) 7:3–18. doi: 10.1017/S1751731112001954
2. Irabona, J. Role of livestock farming development in Gatsibo region Socioeconomy – A case study. IJIRYE. (2023) 7:83–90. doi: 10.18576/ijye/070203
3. National Institute of Statistics Rwanda. GDP National Accounts (third quarter 2024). (2025). Available online at: https://www.statistics.gov.rw/publication/gdp-national-accounts-third-quarter-2024 (Accessed January 8, 2025).
4. Kim, SK, Tiessen, K, Beeche, A, Mukankurunziza, J, and Kamatari, A. Soil fertility and manure management—lessons from the knowledge, attitudes, and practices of Girinka farmers in the district of Ngoma, Rwanda. Agroecol Sustain Food Syst. (2013) 37:631–58. doi: 10.1080/21683565.2012.762636
6. Osoro, EM, Munyua, P, Omulo, S, Ogola, E, Ade, F, Mbatha, P, et al. Strong association between human and animal Brucella Seropositivity in a linked study in Kenya, 2012–2013. Am J of Trop Med Hygiene. (2015) 93:224–31. doi: 10.4269/ajtmh.15-0113
7. Rujeni, N, and Mbanzamihigo, L. Prevalence of brucellosis among women presenting with abortion/stillbirth in Huye, Rwanda. J Trop Med. (2014) 2014:1–3. doi: 10.1155/2014/740479
8. Umuhoza, T, Berkvens, D, Gafarasi, I, Rukelibuga, J, Mushonga, B, and Biryomumaisho, S. Seroprevalence of Rift Valley fever in cattle along the Akagera-Nyabarongo rivers, Rwanda. J S Afr Vet Assoc. (2017) 88:1–5. doi: 10.4102/jsava.v88i0.1379
9. Dutuze, MF, Ingabire, A, Gafarasi, I, Uwituze, S, Nzayirambaho, M, and Christofferson, RC. Identification of Bunyamwera and possible other Orthobunyavirus infections and disease in cattle during a Rift Valley fever outbreak in Rwanda in 2018. Am J Trop Med Hyg. (2020) 103:183–9. doi: 10.4269/ajtmh.19-0596
10. Griffith, EF, Schurer, JM, Mawindo, B, Kwibuka, R, Turibyarive, T, and Amuguni, JH. The use of drones to deliver Rift Valley fever vaccines in Rwanda: perceptions and recommendations. Vaccine. (2023) 11:605. doi: 10.3390/vaccines11030605
11. Nsengimana, I, Juma, J, Roesel, K, Gasana, MN, Ndayisenga, F, Muvunyi, CM, et al. Genomic epidemiology of Rift Valley fever virus involved in the 2018 and 2022 outbreaks in livestock in Rwanda. Viruses. (2024) 16:1148. doi: 10.3390/v16071148
12. Djangwani, J, Ooko Abong, G, Gicuku Njue, L, and DWM, K. Brucellosis: prevalence with reference to east African community countries – A rapid review. Veterinary Med Sci. (2021) 7:851–67. doi: 10.1002/vms3.425
13. Habimana, JP, Ntivuguruzwa, JB, Uwimana, AL, Ugirabe, A, Gasana, E, and Van Heerden, H. Seroprevalence and risk factors associated with brucellosis in goats in Nyagatare district. Rwanda. (2023). Available at: https://www.biorxiv.org/content/10.1101/2023.05.01.538860v1 (Accessed April 09, 2025).
14. Habarugira, G, Rukelibuga, J, Nanyingi, MO, and Mushonga, B. Bovine tuberculosis in Rwanda: prevalence and economic impact evaluation by meat inspection at Société des abattoirs de Nyabugogo-Nyabugogo abattoir, Kigali. J S Afr Vet Assoc. (2014) 85:5. doi: 10.4102/jsava.v85i1.1062
15. Sapp, AC, Nane, GF, Amaya, MP, Niyonzima, E, Hategekimana, JP, VanSickle, JJ, et al. Estimates of disease burden caused by foodborne pathogens in contaminated dairy products in Rwanda. BMC Public Health. (2023) 23:657. doi: 10.1186/s12889-023-15204-x
16. Ntabanganyimana, E, Giraneza, R, Dusabejambo, V, Bizimana, A, Hamond, C, Iyamuremye, A, et al. Sero-prevalence of anti-Leptospira antibodies and associated risk factors in rural Rwanda: A cross-sectional study. Coburn J, editor. PLoS Negl Trop Dis. (2021) 15:e0009708. doi: 10.1371/journal.pntd.0009708
18. Feed The Future. Rwanda: Livestock disease management and food safety brief. (2016). Available at: https://livestocklab.ifas.ufl.edu/media/livestocklabifasufledu/pdf-/pdfs-by-country-pre2019/Rwanda_Brief_LivestockDisFS_final.pdf (Accessed April 09, 2025).
19. Rugarabamu, S, Mwanyika, GO, Rumisha, SF, Sindato, C, Lim, HY, Misinzo, G, et al. Seroprevalence and associated risk factors of selected zoonotic viral hemorrhagic fevers in Tanzania. Int J Infect Dis. (2021) 109:174–81. doi: 10.1016/j.ijid.2021.07.006
20. Balinandi, S, Mulei, S, Whitmer, S, Nyakarahuka, L, Cossaboom, CM, Shedroff, E, et al. Crimean-Congo hemorrhagic fever cases diagnosed during an outbreak of Sudan virus disease in Uganda, 2022–23. PLoS Negl Trop Dis. (2024) 18:e0012595. doi: 10.1371/journal.pntd.0012595
21. Balinandi, S, Von Brömssen, C, Tumusiime, A, Kyondo, J, Kwon, H, Monteil, VM, et al. Serological and molecular study of Crimean-Congo hemorrhagic fever virus in cattle from selected districts in Uganda. J Virol Methods. (2021) 290:114075. doi: 10.1016/j.jviromet.2021.114075
22. Nyakarahuka, L, Whitmer, S, Klena, J, Balinandi, S, Talundzic, E, Tumusiime, A, et al. Detection of sporadic outbreaks of Rift Valley fever in Uganda through the National Viral Hemorrhagic Fever Surveillance System, 2017–2020. Am J Trop Med Hygiene. (2023) 108:995–1002. doi: 10.4269/ajtmh.22-0410
23. Halliday, JEB, Allan, KJ, Ekwem, D, Cleaveland, S, Kazwala, RR, and Crump, JA. Endemic zoonoses in the tropics: a public health problem hiding in plain sight. Vet Rec. (2015) 176:220–5. doi: 10.1136/vr.h798
24. Mwinyi, MO, Kayunze, KA, Sitali, DC, Simuunza, MC, and Muma, JB. Socio-economic impact of brucellosis on livestock farmers in southern and Western provinces, Zambia (2016). J. Tech. Res. Appl. 4:204–9.
25. Bukachi, SA, Wandibba, S, and Nyamongo, IK. The socio-economic burden of human African trypanosomiasis and the coping strategies of households in the South Western Kenya foci. PLoS Negl Trop Dis. (2017) 11:e0006002. doi: 10.1371/journal.pntd.0006002
26. Lokamar, PN, Kutwah, MA, Atieli, H, Gumo, S, and Ouma, C. Socio-economic impacts of brucellosis on livestock production and reproduction performance in Koibatek and Marigat regions, Baringo County, Kenya. BMC Vet Res. (2020) 16:61. doi: 10.1186/s12917-020-02283-w
27. Mekonnen, SA, Gezehagn, A, Berju, A, Haile, B, Dejene, H, Nigatu, S, et al. Health and economic burden of foodborne zoonotic diseases in Amhara region, Ethiopia. PLoS One. (2021) 16:e0262032. doi: 10.1371/journal.pone.0262032
28. Kiiza, D, Denagamage, T, Serra, R, Maunsell, F, Kiker, G, Benavides, B, et al. A systematic review of economic assessments for brucellosis control interventions in livestock populations. Prev Vet Med. (2023) 213:105878. doi: 10.1016/j.prevetmed.2023.105878
29. Yongabi Anchang, K, Avery, L, and Pertiwiningrum, A. A commentary on occupational infectious diseases due to agricultural practices in sub-Saharan Africa. Biomass Bioenergy. (2014) 70:99–111. doi: 10.1016/j.biombioe.2014.02.037
30. Zinsstag, J, Schelling, E, Roth, F, Bonfoh, B, de Savigny, D, and Tanner, M. Human benefits of animal interventions for zoonosis control. Emerg Infect Dis. (2007) 13:527–31. doi: 10.3201/eid1304.060381
31. Ntirandekura, J, Matemba, L, Ngowi, H, Kimera, S, and Karimuribo, E. Knowledge, perceptions and practices regarding brucellosis in pastoral communities of Kagera region in Tanzania. J Adv Vet Anim Res. (2018) 5:343. doi: 10.5455/javar.2018.e285
32. Tebug, S. Factors associated with milk producer\s awareness and practices in relation to zoonoses in northern Malawi. Vet World. (2013) 6:249. doi: 10.5455/vetworld.2013.249-253
33. Rwanda Biomedical Center. Rwanda one health zoonotic diseases prioritization. (2017). Available online at: https://www.rbc.gov.rw/fileadmin/user_upload/RWANDA_OH_ZOONOTIC_DISEASES_PRIORITIZATION_REPORT.pdf (Accessed April 9, 2025).
34. World Health Organization. Joint external evaluation of IHR core capacities of the Republic of Rwanda. WHO/WHE/CPI/REP/201822. (2018). Available at: https://iris.who.int/handle/10665/274353 (Accessed April 09, 2025).
35. Gafirita, J, Kiiza, G, Murekatete, A, Ndahayo, LL, Tuyisenge, J, Mashengesho, V, et al. Seroprevalence of brucellosis among patients attending a district hospital in Rwanda. Am J Trop Med Hygiene. (2017) 97:831–5. doi: 10.4269/ajtmh.16-0632
36. Manishimwe, R. Comparison between rose Bengal plat test and competitive enzyme linked immunosorbent assay to detect bovine brucellosis in Kigali City, Rwanda. JDVAR. (2015) 2:94–7. doi: 10.15406/jdvar.2015.02.00038
37. Ndazigaruye, G, Mushonga, B, Kandiwa, E, Samkange, A, and Segwagwe, BE. Prevalence and risk factors for brucellosis seropositivity in cattle in Nyagatare district, Eastern Province, Rwanda. J S Afr Vet Assoc. (2018) 89:1–8. doi: 10.4102/jsava.v89i0.1625
38. Migambi, P, Gasana, M, Uwizeye, CB, Kamanzi, E, Ndahindwa, V, Kalisvaart, N, et al. Prevalence of tuberculosis in Rwanda: results of the first nationwide survey in 2012 yielded important lessons for TB control. PLoS One. (2020) 15:e0231372. doi: 10.1371/journal.pone.0231372
39. Kiiza, D, Biryomumaisho, S, Robertson, ID, and Hernandez, JA. Seroprevalence of and risk factors associated with exposure to Brucella Spp. in dairy cattle in three different Agroecological zones in Rwanda. Am J Trop Med Hygiene. (2021) 104:1241–6. doi: 10.4269/ajtmh.20-1426
40. Ntivuguruzwa, JB, Kolo, FB, Gashururu, RS, Umurerwa, L, Byaruhanga, C, and van Heerden, H. Seroprevalence and associated risk factors of bovine brucellosis at the wildlife-livestock-human Interface in Rwanda. Microorganisms. (2020) 8:1553. doi: 10.3390/microorganisms8101553
41. Smith, LJ, Schurer, JM, Ntakiyisumba, E, Shyaka, A, and Amuguni, JH. Rift Valley fever knowledge, mitigation strategies and communication preferences among male and female livestock farmers in Eastern Province, Rwanda. PLoS Negl Trop Dis. (2021) 15:e0009705. doi: 10.1371/journal.pntd.0009705
42. Ntampaka, P, Nyaga, PN, Niragire, F, Gathumbi, JK, and Tukei, M. Knowledge, attitudes and practices regarding rabies and its control among dog owners in Kigali city, Rwanda. PLoS One. (2019) 14:e0210044. doi: 10.1371/journal.pone.0210044
43. Sebera, E, Kiiza, F, Iradukunda, J, Mukarwego, C, Twagirumukiza, E, and Bubanje, V. Knowledge, attitude, and practices towards prevention of tuberculosis among HIV-positive patients at Kibagabaga District hospital, Rwanda, 2021. RPHB. (2024) 5:36–44. doi: 10.4314/rphb.v5i1.4
44. National Institute of Statistics of Rwanda (NISR). Fifth Rwanda population and housing census, 2022 (2023). 176 p. Available at: https://www.statistics.gov.rw/publication/main_indicators_2022 (Accessed April 09, 2025).
45. National Institute of Statistics of Rwanda (NISR). Seasonal agricultural survey annual report, December 2021. (2021). Available at: https://www.statistics.gov.rw/publication/1754 (Accessed April 09, 2025).
46. Rwanda Governance Board. Assessing Girinka Programme (2006–2016). (2018). Available at: https://www.rgb.rw/fileadmin/user_upload/RGB/Publications/HOME_GROWN_SOLUTIONS/GIRINKA_REPORT_2018.pdf (Accessed April 09, 2025).
47. Vincent, Kayigema. An assessment of the Girinka (one cow per poor family) program and poverty alleviation in Rwanda: a case study of Bugesera district. (2013). Available online at: https://researchspace.ukzn.ac.za/items/6b46f819-77df-4d50-9747-8a8c15a12ce1 (Accessed April 09, 2025).
48. Habimana, JP, Ntivuguruzwa, JB, Uwimana, AL, Ugirabe, A, Gasana, E, and Van Heerden, H. Seroprevalence and risk factors associated with brucellosis in goats in Nyagatare district, Rwanda. (2023). Available at: https://www.biorxiv.org/content/10.1101/2023.05.01.538860v1 (Accessed April 09, 2025).
49. Ntivuguruzwa, JB, Michel, A, Byaruhanga, C, Gashururu, R, Kolo, FB, and vanHeerden, H. Awareness and occupational exposure to brucellosis and other zoonotic diseases among abattoir Workers in Rwanda. (2021). Available online at: https://www.researchsquare.com/article/rs-1012737/v1 (Accessed April 19, 2024).
50. Khurana, SK, Sehrawat, A, Tiwari, R, Prasad, M, Gulati, B, Shabbir, MZ, et al. Bovine brucellosis – a comprehensive review. Vet Q. (2021) 41:61–88. doi: 10.1080/01652176.2020.1868616
51. Kimble, JB, Noronha, L, Trujillo, JD, Mitzel, D, Richt, JA, and Wilson, WC. Rift Valley fever. Vet Clin N Am Food Anim Pract. (2024) 40:293–304. doi: 10.1016/j.cvfa.2024.01.004
52. Chomel, B. Emerging and re-emerging zoonoses of dogs and cats. Animals. (2014) 4:434–45. doi: 10.3390/ani4030434
54. Bradley, EA, and Lockaby, G. Leptospirosis and the environment: A review and future directions. Pathogens. (2023) 12:1167. doi: 10.3390/pathogens12091167
55. Mann, S, Frasca, K, Scherrer, S, Henao-Martínez, AF, Newman, S, Ramanan, P, et al. A review of Leishmaniasis: current knowledge and future directions. Curr Trop Med Rep. (2021) 8:121–32. doi: 10.1007/s40475-021-00232-7
56. Galán-Relaño, Á, Valero Díaz, A, Huerta Lorenzo, B, Gómez-Gascón, L, Mena Rodríguez Ma, Á, Carrasco Jiménez, E, et al. Salmonella and salmonellosis: an update on public health implications and control strategies. Animals. (2023) 13:1–22. doi: 10.3390/ani13233666
57. Ntivuguruzwa, JB, Kolo, FB, Gashururu, R, Uwibambe, E, Musanayire, V, Ingabire, A, et al. Molecular characterization of Brucella spp. from seropositive herds of cattle farmed at the wildlife–livestock–human interface in Rwanda. Front Vet Sci. (2022) 9:1017851. doi: 10.3389/fvets.2022.1017851
58. Rwanda Information Society Authority (RISA). Digital 2025: Rwanda. (2025). Available online at: https://datareportal.com/reports/digital-2025-rwanda (Accessed April 9, 2025).
59. Obonyo, MO, Farr, M, Hikufe Hikufe, E, Rubanzana, W, Owiny, MO, and Roka, ZG. Investigation of anthrax in an endemic region in Kenya: a mixed methods approach. Pan Afr Med J. (2018). 30:12. doi: 10.11604/pamj.supp.2018.30.1.15279
60. Ngoshe, YB, Etter, E, Gomez-Vazquez, JP, and Thompson, PN. Knowledge, attitudes, and practices of communal livestock farmers regarding animal health and zoonoses in far northern KwaZulu-Natal, South Africa. IJERPH. (2022) 20:511. doi: 10.3390/ijerph20010511
61. Mpatswenumugabo, JP, Mukasafari, MA, Ndahetuye, JB, Wredle, E, and Båge, R. A systematic literature review of milk consumption and associated bacterial zoonoses in East Africa. J Appl Microbiol. (2023) 134:lxad080. doi: 10.1093/jambio/lxad080
62. Rusanganwa, V, Lwande, OW, Bainda, B, Chiyo, PI, Seruyange, E, Bucht, G, et al. Arbovirus surveillance in febrile patients attending selected health facilities in Rwanda. Infection Ecol Epidemiol. (2024) 14:2289872. doi: 10.1080/20008686.2023.2289872
63. Himsworth, CG, Parsons, KL, Jardine, C, and Patrick, DM. Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis. (2013) 13:349–59. doi: 10.1089/vbz.2012.1195
64. Pal, M, and Tolawak, D. A comprehensive review on major zoonotic parasites from dogs and cats. Int J Med Parasitol Epidemiol Sci. (2023) 4:3–11. doi: 10.34172/ijmpes.2023.02
65. Gerhold, RW, and Jessup, DA. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health. (2013) 60:189–95. doi: 10.1111/j.1863-2378.2012.01522.x
Keywords: smallholder livestock farmers, zoonoses, one health, knowledge, attitudes, practices, risk factors
Citation: Munyaneza C, Bizimana F, Mukumbo F, Gatesi S, Sibomana E, Munyampuhwe S and Dutuze MF (2025) Knowledge, attitudes, practices (KAP), and risk factors toward zoonotic diseases among smallholder livestock farmers in Bugesera district of Rwanda. Front. Public Health. 13:1569682. doi: 10.3389/fpubh.2025.1569682
Received: 01 February 2025; Accepted: 31 March 2025;
Published: 17 April 2025.
Edited by:
Adwoa Asante-Poku, University of Ghana, GhanaReviewed by:
Molalegne Bitew, Health Biotechnology at Bio and Emerging Technology Institute (BETin) of Ethiopia, EthiopiaCopyright © 2025 Munyaneza, Bizimana, Mukumbo, Gatesi, Sibomana, Munyampuhwe and Dutuze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Celestin Munyaneza, bXVuZXphY2VsQHlhaG9vLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.