
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Public Health , 29 January 2025
Sec. Public Mental Health
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1541866
This article is part of the Research Topic The Intersection of Psychology, Healthy Behaviors, and its Outcomes View all 79 articles
Introduction: Harmonica playing mimics pursed-lip breathing and strengthens respiratory muscles. Combined with music therapy, it may improve both pulmonary and mental health in chronic obstructive pulmonary disease (COPD) patients, though its effects are not well understood. This protocol outlines a randomized controlled trial (RCT) to evaluate the effectiveness of integrating harmonica playing into pulmonary rehabilitation (PR) programs.
Methods and analysis: This single-center, two-arm RCT will be conducted at a tertiary hospital in Guangzhou, China. A total of 248 adult patients (with a clinical diagnosis of COPD but without severe comorbidities, significant cognitive impairments, and prior experience with the intervention components) will be randomized in a 1:1 ratio to either a harmonica-integrated PR group (intervention) or a standard PR group (control) for 6 months of home-based, tele-supervised training. The intervention will incorporate harmonica sessions in addition to standard PR exercises (breathing and physical exercises). Both groups will undergo in-hospital training sessions, supplemented by daily home practice under remote supervision by PR staff. The primary outcome is lung function (measured by FEV1%), while secondary outcomes include respiratory muscle strength, exercise capacity, fatigue, dyspnea, symptom burden, mental health, self-efficacy, quality of life, social support, adherence, and patient satisfaction. Statistical analyses will employ mixed-effects models with an intention-to-treat approach.
Conclusion: This trial will evaluate the efficacy of a harmonica-integrated, home-based PR program with tele-supervision for COPD patients on lung function, respiratory muscle strength, exercise capacity, and overall health. If effective, it could offer a novel, affordable, and accessible home-based PR approach for COPD management.
Trial registration number: ClinicalTrials.gov: NCT05995847.
Chronic obstructive pulmonary disease (COPD), the third leading cause of death globally, is characterized by dyspnea, cough, and sputum production, often accompanied by peripheral muscle dysfunction, cardiovascular disease, anxiety, depression, and chronic fatigue (1, 2). Pulmonary rehabilitation (PR), a cornerstone of non-pharmacological COPD treatment, typically integrates self-management education, respiratory training, psychosocial support, and structured exercise, effectively improving lung function, alleviating symptoms, and enhancing quality of life (3).
Respiratory training is essential in PR, strengthening respiratory muscles and alleviating dyspnea (4). However, certain traditional exercises (e.g., Positive Expiratory Pressure Therapy, Threshold Inspiratory/Expiratory Muscle Training) can be monotonous and difficult to sustain over time, particularly due to their repetitive, non-interactive nature (5, 6). Harmonica playing provides a novel alternative by combining pursed-lip and diaphragmatic breathing with controlled airflow, simulating respiratory training through an engaging, interactive musical activity that reduces monotony and enhances motivation (7, 8). Beyond physiological benefits, music induces positive emotions, reduces stress, and fosters a sense of accomplishment (9). Furthermore, harmonicas are cost-effective (10), with a 24-hole tremolo model from a reputable Chinese brand priced around USD 4.30. This is much cheaper than respiratory training devices like the XEEK Kuner (USD 430) and Breath Home (USD 428). Portable and requiring no extra equipment or space (11), the harmonica further minimizes costs, making it a financially advantageous adjunct to PR.
However, the benefits of harmonica playing in improving health outcomes for COPD patients remain incompletely explored. Three preliminary studies have suggested potential improvements in respiratory muscle strength, exercise capacity, breathing control, and quality of life (12–14). Nevertheless, these studies suffer from significant methodological limitations, including lack of randomization (12), small sample sizes (from 9 to 13) (12–14), and inconsistent practice regimens (10–40 min daily) without standardized guidelines on home practice duration or frequency (12–14). The studies had inadequate follow-up periods (13) and no systematic post-intervention monitoring in others (12, 14), thereby limiting the assessment of long-term impacts. Moreover, they mainly assessed physiological outcomes, like spirometry, inspiratory and expiratory muscle strength, and breathlessness, ignoring functional measures like the six-minute walk distance (6MWD) (15) and inadequately addressing psychological and social factors such as adherence and wellbeing (12–14). These gaps underscores the need for a robust randomized controlled trial with comprehensive outcome measures to evaluate harmonica-integrated PR for COPD.
This study utilizes a rigorous randomized controlled trial (RCT) design with a large sample size, extended intervention period, standardized training protocol, and comprehensive outcomes, addressing the limitations of previous research. It aims to provide robust evidence on the physiological, functional, and psychological benefits of a harmonica-integrated, tele-supervised, home-based PR program for COPD patients. The primary outcome is lung function, with secondary outcomes including respiratory muscle strength, exercise capacity, fatigue, dyspnea, mental health, self-efficacy, quality of life, adherence, and satisfaction—addressing both physiological and psychosocial factors. Findings may inform global COPD management guidelines, positioning harmonica-integrated PR as a cost-effective, accessible, and engaging alternative, particularly in areas with limited access to traditional rehabilitation services.
The primary objective of this study is to assess the effectiveness of a harmonica-integrated, tele-supervised, home-based PR program on lung function in patients with COPD, compared to a standard PR program without harmonica integration. Secondary objectives include evaluating the intervention's effects on respiratory muscle strength, exercise capacity, fatigue, dyspnea, symptom burden, mental health, self-efficacy, quality of life, social support, adherence, and satisfaction.
A single-center, two-arm study will be conducted at a tertiary hospital in Guangzhou, China to compare the effects of a harmonica-integrated, tele-supervised home-based PR program with those of a standard PR program on lung function and overall health in COPD patients. A total of 248 adult participants, clinically diagnosed with COPD but free of severe comorbidities, significant cognitive impairments, and prior exposure to the intervention components will be enrolled. The intervention will span 6 months, with clinical assessments performed at baseline (T0, the day the intervention begins), and 1 month (T1), 3 months (T2), and 6 months (T3) post-baseline. Figure 1 illustrates the participant flow through the study. This study registered at ClinicalTrials.gov (NCT05995847).
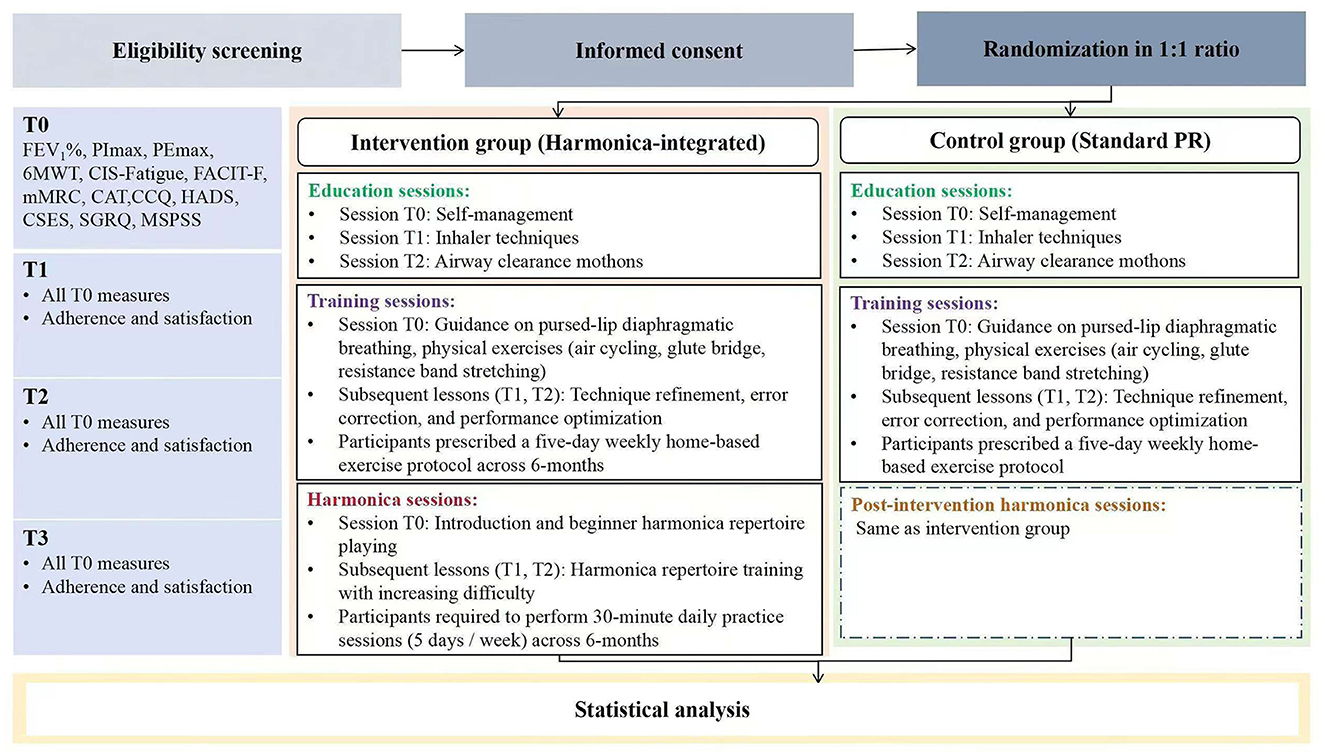
Figure 1. Flowchart of the randomized controlled trial protocol. FEV1%, Forced expiratory volume in one second of predicted; PImax, maximum inspiratory pressure; PEmax, maximum expiratory pressure; 6MWT, Six-Minute Walking Test; CIS-Fatigue, Checklist Individual Strength-Fatigue; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; mMRC, modified Medical Research Council; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; CCQ, Clinical Chronice Obstructive Pulmonary Disease Questionnaire; HADS, Hospital Anxiety and Depression Scale; CSES, Chronic Obstructive Pulmonary Disease Self-Efficacy Scal; SGRQ, St. George's Respiratory Questionnaire; MSPSS, Multidimensional Scale of Perceived Social Support; T0, baseline; T1, 1 month post-baseline; T2, 3 months post-baseline; T3, 6 months post-baseline.
Eligible patients must have a clinical diagnosis of COPD and a post-bronchodilator forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio of < 70%. Exclusion criteria include recent COPD exacerbations (within 6 weeks), resting oxygen saturation (SpO2) below 88% on room air or inadequate oxygenation despite supplementation; as well as severe dyspnea with Modified Medical Research Council (mMRC) grade 4, recent myocardial infarction, unstable angina, severe arrhythmias (e.g., ventricular arrhythmias or rapid atrial fibrillation), or pulmonary hypertension with right heart failure. Additional exclusions include comorbidities affecting physical activity (e.g., neurological disorders, cancer, significant musculoskeletal issues), cognitive impairments, and inability to communicate in Chinese. Furthermore, those with prior participation in PR within 6 months, or regular experience playing harmonica or similar wind instruments, are excluded to ensure baseline equivalence, minimize learning and behavioral biases, and avoid psychological effects, thereby enhancing the validity and reliability of the study outcomes.
Based on an anticipated minimum clinically important difference (MCID) of a 4% increase in percent predicted FEV1 (FEV1%) and a standard deviation of 10% (16), each group will require 99 participants to achieve 80% power at a 0.05 two-tailed significance level. Considering a 20% dropout rate, the study plans to enroll 248 patients (124 per group) (17).
Participants will be recruited using a comprehensive, multifaceted approach to ensure a broad and inclusive reach. Recruitment efforts will include advertisements in the hospital's outpatient clinics, referrals from healthcare providers, and telephone outreach to former outpatient and inpatient COPD patients. In addition, we will employ online recruitment strategies, such as WeChat and community outreach. The research team will meticulously screen interested individuals based on stringent inclusion and exclusion criteria to determine their eligibility. Enrollment will occur on a rolling basis, allowing eligible candidates to join the study as they meet the criteria and space is available. All eligible candidates will receive detailed information about the study through an information letter and an informative flier. Those who agree to participate will provide written informed consent as part of the enrollment process.
Participants will be randomly assigned in a 1:1 ratio to either the intervention or control group using a computer-generated randomization sequence developed by an independent statistician unaffiliated with the recruitment process. To ensure allocation concealment, the sequence will be sealed in opaque, sequentially numbered envelopes. Upon enrollment, the recruitment coordinator, who does not have prior access to the allocation sequence, will open the corresponding sealed envelope to assign each participant to their respective group. The recruitment coordinator will then inform participants of their group allocation and provide the necessary materials, including disease education manuals, rehabilitation training diaries, elastic bands, and portable pulse oximeters for monitoring oxygen saturation during exercises. Participants allocated to the harmonica-integrated group will additionally receive a harmonica. Additionally, the recruitment coordinator will communicate the time and location of each session to the participants.
This study employs a partially blinded design to minimize potential biases. Care providers responsible for delivering the interventions—including rehabilitation instructors, harmonica instructors, home-training supervisors, and home-training recorders—are blinded to group allocations and the overall study protocol. They are informed only of the specific treatments they are to administer without knowledge of the study protocol and participants' group assignments. Outcome assessors conducting evaluations are blinded to the group allocations to ensure objective assessment of study outcomes. The data analyst performing the final data analysis is also blinded to group assignments to maintain objectivity in data interpretation. Although the recruitment coordinators are aware of group assignments for administrative purposes, they do not engage in any intervention, assessment, or follow-up tasks to minimize potential biases. Due to the nature of the intervention, patients cannot be blinded. However, potential biases will be minimized by standardizing intervention protocols and using objective outcomes as primary measures.
The intervention group will undergo a six-month program including education sessions, training sessions, and harmonica sessions. Training and harmonica sessions will be conducted in person at the hospital, followed by daily home-based exercises with continuous tele-supervision and feedback delivered via WeChat. Details are listed in Table 1.
The intervention group will participate in three sequential 45-min educational sessions designed to enhance self-management. The initial session (T0) covers disease-specific knowledge and rehabilitation strategies. The second session (T1) focuses on optimal inhaler use, and the final session (T2) introduces advanced airway clearance techniques, building on prior learning. The curriculum progresses incrementally, tailored to participants' learning abilities to gradually improve disease self-management. Additionally, at baseline, each patient will receive disease education manuals for home study.
The intervention group will participate in three instructor-led training sessions focused on respiratory optimization. The initial session (T0) will provide personalized instruction in breathing techniques (pursed-lip and diaphragmatic breathing) and physical exercises (air cycling exercises, hip bridge exercises, seated stretching, standing stretching, and lower limb stretching), as illustrated in Figure 2. Subsequent sessions (T1 and T2) will concentrate on refining techniques, correcting errors, and optimizing performance. Additionally, participants will adhere to a home-based exercise regimen prescribed for 5 days each week.

Figure 2. Training exercises included in the program. (A) Air cycling exercises; (B) hip bridge exercises; (C) seated stretching; (D) standing stretching; (E) lower limb stretching.
Participants in the intervention group will receive three progressive harmonica lessons (T0, T2, T3), conducted by professional music instructors, designed to optimize skill development. The initial lesson (T0) will cover harmonica fundamentals, including instrument anatomy, maintenance and cleaning, harmonica-based breathing techniques, and basic performance skills, establishing a foundation for subsequent lessons. At baseline, simple songs will be selected to build patients' confidence. Participants will be provided with structured written materials outlining practice methods and musical exercises. Subsequent lessons (T2 and T3) will introduce classical songs of increasing difficulty, building on foundational skills and progressively enhancing technical proficiency and enjoyment. Throughout the six-month intervention, participants will engage in a daily 30-min continuous (no-break) practice of a specified set of songs.
Upon randomization, a WeChat group will be established for each intervention cohort, including participants themselves, their family members (if they choose), a home-training supervisor and recorder, a rehabilitation instructor, and a harmonica instructor. For participants with limited device familiarity or access, one-on-one personalized WeChat training will be conducted at baseline, including video tutorials, content uploads, and interaction with PR staff. Family members will be trained if needed to assist with remote monitoring.
Participants will upload daily videos of their training and harmonica playing, report training duration, and complete rehabilitation training diaries documenting exercise and harmonica practice times. The supervisor and recorder will independently review the videos to verify adherence, and discrepancies will be resolved through double-checking the video recordings. Additionally, the supervisor and recorder will send daily reminders, track attendance, and note any absences. Rehabilitation and harmonica instructors will conduct real-time video assessments of participants' techniques, providing immediate feedback, personalized guidance, and addressing participant queries.
The control group will participate in a six-month program, which is similar to the intervention group in structure but excludes the harmonica sessions. This group will undergo the same educational and rehabilitation training sessions as the intervention group. The specific activities will be detailed in Table 1.
After randomization, control group participants will be added to a dedicated WeChat group comprising participants, their family members (optional), a home-training recorder and supervisor, and a rehabilitation instructor. The same recording methods and daily prompts will be applied as in the intervention group. The home-training supervisor will review uploaded videos, monitor adherence, send daily reminders, and track training completion. The rehabilitation instructor will conduct real-time video assessments to ensure proper adherence to the training protocols.
Primary and secondary outcomes will be assessed at four key intervals: baseline (T0), 1 month (T1), 3 months (T2), and 6 months (T3) post-baseline, as shown in Table 2. Secondary outcomes related to “adherence and satisfaction” will be measured only at T1, T2, and T3.
The primary outcome of this study is the change in lung function, measured as FEV1% 48 h after discontinuing inhaled bronchodilators. Measurements will be obtained using the portable X1 spirometer (serial no. X1231100300024) from Saikemed (Xiamen) Medical Equipment Co., Ltd. Predicted FEV1 values will be calculated based on the “Compilation of Normal Lung Function Values Across China” (Mu Kuijin & Liu Shiwuan, eds.): for males, FEV1 = −1.087 – 0.029 × A + 0.033 × H; for females, FEV1 = −0.753 – 0.022 × A + 0.026 × H, where A is age (years) and H is height (cm) (18). A clinically significant change is defined as a ≥4% improvement in FEV1%, a recognized MCID in COPD research (16). COPD severity will be classified according to the ATS/ERS criteria (2005/2019) based on post-bronchodilator FEV1%: mild (≥70%), moderate (60–69%), moderate-to-severe (50–59%), severe (35–49%), and very severe (< 35%) (19).
Secondary outcomes will encompass changes in respiratory muscle strength, exercise capacity, fatigue, breathlessness, clinical symptoms, clinical control, mental health, self-efficacy, quality of life, social support, adherence and satisfaction. Each outcome will be measured using validated tools accordingly to ensure reliable and actionable insights.
Inspiratory and expiratory muscle strength will be measured as PImax (maximum inspiratory pressure) and PEmax (maximum expiratory pressure) using the X1 spirometer (serial no. X1231100300024).
The 6-min walk test (6MWT) is a validated measure of exercise capacity in COPD patients, demonstrates reliable performance in the Chinese population and correlates with disease severity and mortality (20). Participants will complete a 6-min walk along a standardized flat corridor, with total distance walked serving as the primary outcome metric. Heart rate and perceived exertion (Borg scale) will be assessed pre- and post-test to evaluate exercise intensity and physiological recovery. Predicted 6MWD (meters) will be calculated using Enright and Sherrill's formulas based on body mass index (BMI) and age: for males, 6MWD = 1,140 – 5.61 × BMI – 6.94 × age; for females, 6MWD = 1,017 - 6.24 × BMI – 5.83 × age. These values will provide a reference for interpreting the actual distance walked (21).
Fatigue will be measured using the Chinese version of the Checklist Individual Strength-Fatigue (CIS-Fatigue; Cronbach's alpha of 0.88) and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F; Cronbach's alpha of 0.90) scales. Both scales are validated in Chinese populations and are widely used in assessing fatigue in chronic illness (22, 23). The CIS-Fatigue, a 20-item subscale of the Checklist Individual Strength, employs a 7-point Likert scale with total scores ranging from 8 to 56, where scores < 26, 27–35, and >36 represent normal, mild, and severe fatigue, respectively. The FACIT-F, a 13-item self-reported scale rated on a 5-point Likert scale (“not at all” to “very much”), measures fatigue-related tiredness, weakness, and activity limitations over the preceding seven days. Higher scores on both scales indicate greater fatigue (24).
The mMRC Dyspnea Scale, with a Cronbach's alpha of 0.82 in China, will assess breathlessness severity on a 0–4 scale. A score of 0 indicates no breathlessness, while 1 represents breathlessness when hurrying or walking uphill. Scores of 2 and 3 correspond to slower walking or stopping for breath after 100 meters or a few minutes on level ground, respectively. A score of 4 indicates breathlessness when dressing or an inability to leave the house. This scale is commonly used in Chinese COPD research and clinical practice, showing strong correlations with pulmonary function and quality of life (25).
Clinical symptoms will be evaluated using the Chinese version of the COPD Assessment Test (CAT; Cronbach's alpha of 0.805), a 5-point scale with 8 items assessing cough, sputum, dyspnea, chest tightness, confidence, activity, sleep, and energy. Scores range from 0 to 40: 0–10 (mild), 11–20 (moderate), 21–30 (severe), and 31–40 (very severe) impact. The CAT, validated in the Chinese COPD population, is sensitive to clinical changes and strongly correlates with health-related quality of life (26).
Clinical control will be assessed using the Chinese version of the Clinical COPD Questionnaire (CCQ; Cronbach's alpha of 0.90) (27), a 10-item tool evaluating symptoms, function, and mental state. Items are scored on a 7-point scale (0 = no impairment, 6 = most severe impairment), with higher scores indicating poorer disease control. It is validated in Chinese COPD patients and correlates with clinical outcomes and quality of life (28).
Anxiety and depression will be assessed using the Chinese version of the Hospital Anxiety and Depression Scale (HADS; Cronbach's alpha of 0.78–0.93) (29), a 4-point scale (0–3) tool with 14 items, divided into two subscales: anxiety (HADS-Anxiety 7 items) and depression (HADS-Depression, 7 items). Each subscale is scored from 0 to 21, with categories: 0–7 (normal), 8–10 (mild), 11–14 (moderate), and 15–21 (severe). The HADS is widely validated in Chinese COPD clinical settings as a reliable measure of psychological distress (30).
Self-efficacy will be measured using the Chinese version of the Chronic Obstructive Pulmonary Disease Self-Efficacy Scale (CSES; Cronbach's alpha > 0.80), which is validated for use in Chinese populations (31). This 20-item, 10-point scale (0–10) assesses symptom management, physical activity, and psychological coping, with total scores ranging from 0 to 200. Higher scores indicate greater self-efficacy in managing COPD (32).
The Chinese version of the St. George's Respiratory Questionnaire (SGRQ; Cronbach's alpha of 0.97) will be used to assess subjective quality of life (33). It has been validated for use in COPD patients in China and is an essential tool for capturing the multidimensional impact of COPD on patients' lives, including respiratory symptoms (e.g., cough, sputum, dyspnea), activity limitations (e.g., walking, sports, chores), and disease impacts (e.g., walking, sports, chores). Scores, ranging from 0 (no impairment) to 100 (worst health), are calculated as a weighted average of the individual item scores, with higher scores indicating a greater disease impact (34).
The Chinese version of the Multidimensional Scale of Perceived Social Support (MSPSS; Cronbach's alpha of 0.91) (35) will assess perceived social support from family, friends, and significant others using a 12-item, 7-point Likert scale (1–7). Subscale scores range from 4 to 28, and total scores from 12 to 84, with higher scores indicating greater perceived support. It has shown reliability in the Chinese population and is an effective tool for assessing social support in COPD patients (36).
Adherence and satisfaction will be assessed at T1, T2, and T3. Adherence will be quantified by recording attendance and exercise completion. Satisfaction will be assessed using a study-specific questionnaire designed to comprehensively capture participants' perceptions.
Data will be collected using a dual-entry and verification process, incorporating range checks, logic tests, and source document validation to ensure data integrity (37). All analyses will be conducted with R software version 4.4.0. The primary analysis will follow the intention-to-treat principle (ITT), including all randomized participants in their assigned groups (38). Baseline characteristics will be summarized using descriptive statistics, with continuous variables compared using independent t-tests and categorical variables using chi-square tests between the harmonica-integrated PR group and the standard PR care group. Primary and secondary outcomes will be analyzed using mixed-effects models to account for the repeated measures design (39). Sensitivity analyses will include per-protocol analyses focusing on participants who fully adhered to the intervention. Effect sizes and 95% confidence intervals will be reported alongside p-values. All statistical tests will be two-tailed, with significance set at p < 0.05. Subgroup analyses will be conducted based on pre-specified baseline characteristics as necessary. Cohen's D will be used as the effect size metric, with common thresholds (small: 0.2, medium: 0.5, large: 0.8) (40).
By leveraging the respiratory benefits of harmonica playing—which simulates respiratory training and incorporates the advantages of music therapy—along with the convenience of home-based training and the instant support of tele-supervision, this study aims to evaluate the effectiveness of a harmonica-integrated, tele-supervised home-based PR program. This innovative approach may offer an accessible, affordable, and enjoyable non-pharmacological treatment option for patients with COPD.
With a robust sample size of 248 participants, a six-month intervention, standardized protocols, and comprehensive outcome measures, the study enhances statistical power, generalizability, and long-term assessment (41, 42). Key design features include a partially blinded methodology, where care providers, outcome assessors, and data analysts are unaware of group assignments, minimizing bias and ensuring objective evaluation (43). The home-based approach improves accessibility for remote or mobility-limited patients, reduces travel and costs, and may lower infection risks compared to clinic settings (44, 45). Tele-supervision involves roles such as home-training recorder, supervisor, and instructors, providing real-time guidance and technique correction. Communication via WeChat fosters a supportive community, while video recordings ensure objective adherence data (46). Harmonica training progresses from basic music theory and breathing techniques to advanced playing skills, promoting mastery and enjoyment. Concurrently, comprehensive PR sessions include breathing exercises (e.g., pursed-lip, diaphragmatic) and physical activities (e.g., air cycling, hip bridge, resistance band stretching) to enhance respiratory and overall muscle function (41, 47). Regular technical assessments and introductory guidance support effective home-based training.
This intervention offers scalable advantages over traditional rehabilitation methods by utilizing widely accessible technologies such as smartphones and WeChat, ensuring broad feasibility and seamless integration with existing healthcare systems (48). The harmonica's low cost and portability facilitate implementation in resource-limited settings without the need for specialized equipment. Tele-supervision and remote monitoring enhance efficiency, particularly in areas with limited rehabilitation services (49). To address unfamiliarity with equipment and limited access, one-on-one personalized WeChat training will provided at baseline, including video tutorials, content uploads and interacting with PR staff, with family members trained if needed to assist in remote monitoring.
Several limitations and challenges need attention and efforts to mitigate. The lack of participant blinding may introduce bias, especially for subjective outcomes. Emphasizing objective measures (e.g., lung function tests), blinded data analysts, strict quality control, and adherence to standardized protocols can partially mitigate this. Nonetheless, self-reported outcomes (e.g., satisfaction, quality of life, mental health) remain susceptible to expectation bias, requiring cautious interpretation of related outcomes. Self-selection bias, due to voluntary participation and the novelty of harmonica use, may also affect findings. Recording baseline characteristics and recruiting a diverse participant pool may mitigate this. While video evidence can reduce adherence bias, misreporting may still occur, enhanced supervision and daily confirmations could address this. The six-month duration may increase dropout rates, particularly among severe COPD patients. Regular follow-ups and motivational support may help, but additional recruitment may be necessary if dropout rates jeopardize statistical power. Furthermore, the single-center approach may limit generalizability, necessitating multi-center trials for broader applicability in the future. Failure to stratify participants by Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage may lead to underrepresentation of certain stages, particularly GOLD 4, and potentially skew subgroup analyses. As treatment responses vary by stage, this lack of stratification could confound efficacy assessment. Given COPD's patient heterogeneity, not stratifying may result in inaccurate evaluations of intervention effectiveness among patients at different stages, affecting overall treatment judgment. Future study designs or RCTs should account for this. The absence of long-term follow-up will restrict the assessment of enduring effects, extended follow-up should be included in future trials. While time equivalence between the intervention and control groups is challenging, the primary activities (breathing and exercise training) ensure general time equivalence. Future analyses should consider additional time demands on the intervention group. While volume, frequency, and density are well-defined in our intervention, objective measures of intensity (e.g., breath pressure, mouth/tongue effort) remain unquantified. Inter-participant variability in technique may therefore introduce differences in actual training load, potentially impacting the findings and warranting cautious interpretation. Future research may explore device-based measures (e.g., breath pressure sensors, digital harmonicas) to better capture and standardize the intensity component of this intervention. Moreover the optimal duration and frequency for clinical outcomes are not yet established and should be explored in future trials.
This study aims to enhance COPD rehabilitation by integrating harmonica playing into a home-based, tele-supervised program. By addressing existing research gaps, it seeks to provide robust empirical evidence for a novel, cost-effective, and accessible intervention strategy for COPD management. The findings may promote music-based therapies in chronic disease management and encourage multidisciplinary approaches that combine respiratory therapy with psychological and social support, thereby expanding and improving COPD rehabilitation strategies and patient outcomes.
QZ: Writing – review & editing. XL: Writing – original draft. WC: Writing – review & editing. DF: Writing – review & editing. JuL: Writing – review & editing. JiL: Writing – review & editing, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangzhou Science and Technology Bureau, the Guangdong Zhong Nanshan Medical Foundation, and the First Affiliated Hospital of Guangzhou Medical University Joint Funding Project for Basic and Applied Basic Research (Fund Number: 202201020462).
We gratefully acknowledge and thank the Guangzhou Science and Technology Bureau, the Guangdong Zhong Nanshan Medical Foundation, and the First Affiliated Hospital of Guangzhou Medical University Joint Funding Project for financial support of this study. We acknowledge Haibo Xu for his invaluable assistance with the Institutional Review Board application.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. (2022) 399:2227–42. doi: 10.1016/S0140-6736(22)00470-6
2. Petousi N, Pavord ID, Couillard S. The Lancet COPD Commission: broader questions remain. Lancet. (2023) 401:1569–70. doi: 10.1016/S0140-6736(23)00556-1
3. Schneeberger T, Abdullayev G, Koczulla AR. Pneumologische rehabilitation. Rehabilitation. (2023) 62:232–47. doi: 10.1055/a-2043-6767
4. Rochester CL, Spruit MA, Holland AE. Pulmonary Rehabilitation in 2021. JAMA. (2021) 326:969–70. doi: 10.1001/jama.2021.6560
5. Shi L, Liu F, Liu Y, Wang R, Zhang J, Zhao Z, Zhao J. Biofeedback respiratory rehabilitation training system based on virtual reality technology. Sensors. (2023) 23:9025. doi: 10.3390/s23229025
6. Daynes E, Houchen-Wolloff L, Barradell AC, Greening NJ, Singh SJ. The training to improve dyspnoea study- patient experiences of using a high frequency airway oscillating device. Int J Chron Obstruct Pulmon Dis. (2024) 19:1345–55. doi: 10.2147/COPD.S443186
7. Lewis A, Conway J, Middleton J, Startup CK, Wyatt J. Playing the harmonica with chronic obstructive pulmonary disease. A qualitative study. Chron Respir Dis. (2022) 19:14799731221083315. doi: 10.1177/14799731221083315
8. de Witte M, Pinho ADS, Stams GJ, Moonen X, Bos AER, van Hooren S. Music therapy for stress reduction: a systematic review and meta-analysis. Health Psychol Rev. (2022) 16:134–59. doi: 10.1080/17437199.2020.1846580
9. Kaasgaard M, Rasmussen DB, Andreasson KH, Hilberg O, Løkke A, Vuust P, et al. Use of Singing for Lung Health as an alternative training modality within pulmonary rehabilitation for COPD: a randomised controlled trial. Eur Respir J. (2022) 59:2101142. doi: 10.1183/13993003.01142-2021
10. Albright RH, Fleischer AE, A. Primer on cost-effectiveness analysis. Clin Podiatr Med Surg. (2024) 41:313–21. doi: 10.1016/j.cpm.2023.07.006
12. Alexander JL, Wagner CL. Is harmonica playing an effective adjunct therapy to pulmonary rehabilitation? Rehabil Nurs. (2012) 37:207–12. doi: 10.1002/rnj.33
13. Hart MK, Stewardson E, Jamil AK, Tecson KM, Millard MW. Usefulness of harmonica playing to improve outcomes in patients with chronic obstructive pulmonary disease. Proc (Bayl Univ Med Cent). (2020) 33:178–82. doi: 10.1080/08998280.2019.1704135
14. Okamoto J, Furukawa Y, Kobinata N, Yoshikawa H, Araki F, Yagyu A, et al. Combined effect of pulmonary rehabilitation and music therapy in patients with chronic obstructive pulmonary disease. J Phys Ther Sci. (2021) 33:779–83. doi: 10.1589/jpts.33.779
15. Li J, Li X, Deng M, Liang X, Wei H, Wu X. Features and predictive value of 6-min walk test outcomes in interstitial lung disease: an observation study using wearable monitors. BMJ Open. (2022) 12:e055077. doi: 10.1136/bmjopen-2021-055077
16. Donohue JF. Minimal clinically important differences in COPD lung function. COPD. (2005) 2:111–24. doi: 10.1081/copd-200053377
17. Alghamdi SM, Janaudis-Ferreira T, Alhasani R, Ahmed S. Acceptance, adherence and dropout rates of individuals with COPD approached in telehealth interventions: a protocol for systematic review and meta-analysis. BMJ Open. (2019) 9:e026794. doi: 10.1136/bmjopen-2018-026794
18. Pulmonary Function Group of the Chinese Medical Association for Respiratory Diseases. Guidelines for the examination of lung function (second part) – pulmometer examination. Chin J Tuberculosis Respir Dis. (2014) 37:481–6. doi: 10.3760/cma.j.issn.1001-0939.2014.07.001
19. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. (2005) 26:948–68. doi: 10.1183/09031936.05.00035205
20. Zhu Y, Zhang Z, Du Z, Zhai F. Mind-body exercise for patients with stable COPD on lung function and exercise capacity: a systematic review and meta-analysis of RCTs. Sci Rep. (2024) 14:18300. doi: 10.1038/s41598-024-69394-4
21. Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. (1998) 158:1384–7. doi: 10.1164/ajrccm.158.5.9710086
22. Yang CC, Chen HT, Luo KH, Watanabe K, Chuang HY, Wu CW, et al. The validation of Chinese version of workplace PERMA-profiler and the association between workplace well-being and fatigue. BMC Public Health. (2024) 24:720. doi: 10.1186/s12889-024-18194-6
23. Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. (1994) 38:383–92. doi: 10.1016/0022-3999(94)90099-x
24. Cai T, Chen J, Ni F, Zhu R, Wu F, Huang Q, et al. Psychometric properties of the Chinese version of the functional assessment of chronic illness therapy-fatigue (FACIT-F) among patients with breast cancer. Health Qual Life Outcomes. (2023) 21:91. doi: 10.1186/s12955-023-02164-4
25. Siu DCH, So CT, Lau CWL, Hui EHM, Fung A, Chan TM, et al. The Manchester respiratory activities of daily living questionnaire: reliability and validity of the Chinese version with pictorial enhancement. Int J Chron Obstruct Pulmon Dis. (2021) 16:91–100. doi: 10.2147/COPD.S283769
26. Liu M, Li Y, Yin D, Wang Y, Fu T, Zhu Z, et al. COPD assessment test as a screening tool for anxiety and depression in stable COPD patients: a feasibility study. COPD. (2023) 20:144–52. doi: 10.1080/15412555.2023.2174843
27. Lin WC, Huang TY, Liu CY, Yeh ML Yu CH, Hwang SL. Validation of the clinical COPD questionnaire in Taiwan. COPD. (2016) 13:360–6. doi: 10.3109/15412555.2015.1094456
28. van der Molen T, Willemse BW, Schokker S. ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the clinical COPD questionnaire health. Qual Life Outcomes. (2003) 1:13. doi: 10.1186/1477-7525-1-13
29. Yang Z, Huang X, Liu X, Hou J, Wu W, Song A, et al. Psychometric properties and factor structure of the Chinese version of the hospital anxiety and depression scale in people living with HIV. Front Psychiatry. (2019) 10:346. doi: 10.3389/fpsyt.2019.00346
30. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. (2003) 1:29. doi: 10.1186/1477-7525-1-29
31. Wang X, Liu Y, Liu Y, Zhang J, Liu L, Matarese M, et al. Exploring patients with COPD self-care behaviours and self-efficacy and their interconnections: a network analysis. J Clin Nurs. (2024) 23:17378. doi: 10.1111/jocn.17378
32. Wigal JK, Creer TL, Kotses H. The COPD self-efficacy scale. Chest. (1991) 99:1193–6. doi: 10.1378/chest.99.5.1193
33. Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George respiratory questionnaire. Chest. (2007) 132:456–63. doi: 10.1378/chest.06-0702
34. Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Wang C, et al. Validation and clinical interpretation of the St George's Respiratory Questionnaire among COPD patients, China. Int J Tuberc Lung Dis. (2009) 13:181−9.
35. Yang X, Xue M, Pauen S, He H. Psychometric properties of the Chinese version of multidimensional scale of perceived social support. Psychol Res Behav Manag. (2024) 17:2233–41. doi: 10.2147/PRBM.S463245
36. Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. (1990) 55:610–7. doi: 10.1080/00223891.1990.9674095
37. Koo TK Li MY, A. Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. (2016) 15:155–63. doi: 10.1016/j.jcm.2016.02.012
38. Tripepi G, Chesnaye NC, Dekker FW, Zoccali C, Jager KJ. Intention to treat and per protocol analysis in clinical trials. Nephrology (Carlton). (2020) 25:513–7. doi: 10.1111/nep.13709
39. Yu Z, Guindani M, Grieco SF, Chen L, Holmes TC, Xu X, et al. Beyond t test and ANOVA: applications of mixed-effects models for more rigorous statistical analysis in neuroscience research. Neuron. (2022) 110:21–35. doi: 10.1016/j.neuron.2021.10.030
40. Rogliani P, Laitano R, Ora J, Beasley R, Calzetta L. Strength of association between comorbidities and asthma: a meta-analysis. Eur Respir Rev. (2023) 32:220202. doi: 10.1183/16000617.0202-2022
41. Frei A, Radtke T, Dalla Lana K, Brun P, Sigrist T, Spielmanns M, et al. Effectiveness of a long-term home-based exercise training program in patients with COPD after pulmonary rehabilitation: a multicenter randomized controlled trial. Chest. (2022) 162:1277–86. doi: 10.1016/j.chest.2022.07.026
42. Hariton E, Locascio JJ. Randomised controlled trials-the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG. (2018) 125:1716. doi: 10.1111/1471-0528.15199
43. Juul S, Gluud C, Simonsen S, Frandsen FW, Kirsch I, Jakobsen JC. Blinding in randomised clinical trials of psychological interventions: a retrospective study of published trial reports. BMJ Evid Based Med. (2021) 26:109. doi: 10.1136/bmjebm-2020-111407
44. Eze ND, Mateus C, Cravo Oliveira Hashiguchi T. Telemedicine in the OECD: An umbrella review of clinical and cost-effectiveness, patient experience and implementation. PLoS One. (2020) 15:e0237585. doi: 10.1371/journal.pone.0237585
45. Vallier JM, Simon C, Bronstein A, Dumont M, Jobic A, Paleiron N, et al. Randomized controlled trial of home-based vs. hospital-based pulmonary rehabilitation in post COVID-19 patients. Eur J Phys Rehabil Med. (2023) 59:103–10. doi: 10.23736/S1973-9087.22.07702-4
46. Arensman RM, Geelen RH, Koppenaal T, Veenhof C, Pisters MF. Measuring exercise adherence in patients with low back pain: development, validity, and reliability of the EXercise Adherence Scale (EXAS). Physiother Theory Pract. (2022) 38:928–37. doi: 10.1080/09593985.2020.1818337
47. Silva CMDSE, Gomes Neto M, Saquetto MB, Conceição CSD, Souza-Machado A. Effects of upper limb resistance exercise on aerobic capacity, muscle strength, and quality of life in COPD patients: a randomized controlled trial. Clin Rehabil. (2018) 32:1636–44. doi: 10.1177/0269215518787338
48. Chang J, Mai Y, Zhang D, Yang X, Li A, Yan W, et al. Media use behavior mediates the association between family health and intention to use mobile health devices among older adults: cross-sectional study. J Med Internet Res. (2024) 26:e50012. doi: 10.2196/50012
Keywords: chronic obstructive pulmonary disease, harmonica playing, music therapy, pulmonary rehabilitation, tele-supervision, home-based training
Citation: Zeng Q, Lin X, Chen W, Fong DYT, Li J and Li J (2025) Effectiveness of a harmonica-integrated, tele-supervised home-based pulmonary rehabilitation program on lung function and comprehensive health outcomes in patients with chronic obstructive pulmonary disease: a randomized controlled trial protocol. Front. Public Health 13:1541866. doi: 10.3389/fpubh.2025.1541866
Received: 08 December 2024; Accepted: 13 January 2025;
Published: 29 January 2025.
Edited by:
Yibo Wu, Peking University, ChinaReviewed by:
Sampath Kumar Amaravadi, University of Chichester, United KingdomCopyright © 2025 Zeng, Lin, Chen, Fong, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaying Li, amxpNDY1QGpoLmVkdQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.