
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 21 February 2025
Sec. Children and Health
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1513526
Objective: To assess health inequities associated with retinoblastoma across various Socio-Demographic Index (SDI) regions and evaluate whether these inequities have decreased from 1990 to 2021, with the aim of enhancing awareness and guiding government policies.
Design: Population-based demographic analysis.
Participants: Children diagnosed with retinoblastoma from 204 countries and territories.
Methods: The estimates and their 95% uncertainty interval (UI) for disability-adjusted life-years (DALYs) of retinoblastoma were extracted from Global Burden of Disease study (GBD) 2021. The age-standardized DALYs and the average annual percentage change (AAPC) were evaluated.
Main measures: The Slope Index of Inequality (SII) and concentration index were computed to quantify the absolute and relative cross-national health inequality.
Results: All SDI regions and the majority of countries experienced a significant decline in age-standardized DALYs from 1990 to 2021. The decrease was more rapid in middle to high SDI regions than in low to low-middle SDI regions. Globally, the 2–4 years age group had the highest DALYs rate, consistent with trends in low to middle SDI regions. In contrast, the highest DALYs rate in high and high-middle SDI regions was found in the 12–23 months age group. The SII was −40.81 (95% CI −36.04 to −45.58) DALYs per 100,000 population in 1990 and − 30.32 (95% CI −27.18 to −33.47) DALYs per 100,000 population in 2021. The concentration index increased from −0.37 (95% CI −0.46 to −0.28) in 1990 to −0.45 (95% CI −0.53 to −0.36) in 2021, although this increase did not reach statistical significance (p = 0.256).
Conclusion: Despite advancements in retinoblastoma management, the overall burden of the disease-related DALY remains disproportionately concentrated in poorer populations. The health inequalities are persisting and widening. This underscores the limitations of current efforts. Until progress benefits everyone, the vision of equitable healthcare remains imperfect.
Retinoblastoma is the most common primary eye malignancy in children, affecting over 8,000 children globally each year (1). This childhood cancer is highly curable when detected early, making it a critical indicator of healthcare efficacy across different regions (2, 3). Despite its treatability, significant global disparities exist in the detection and treatment outcomes of retinoblastoma, primarily influenced by socioeconomic factors (4–9). Advances in screening, detection, and management in high-income countries have led to dramatic improvements in disease outcomes, with nearly all children in these regions surviving (10, 11). However, such advancements have not been mirrored in many lower-income settings, where patients often present with advanced disease, and some even have distant metastasis at the time of initial diagnosis (12–14).
Efforts to address inequities in retinoblastoma treatment have been ongoing for decades (15–18). Various countries and international organizations have implemented initiatives aimed at improving early detection and access to treatment. These initiatives emphasize international cooperation, standardizing treatment protocols, and leveraging technology to improve outcomes globally (7, 15–23). Evaluating their impact on reducing inequities is crucial for providing policymakers with essential decision-making information. However, few studies have examined trends in these inequities, particularly using globe salvage or eye preservation as comparative indicators between regions (8). These measures do not comprehensively represent the treatment outcomes of retinoblastoma, as the primary goal is to ensure disease-free survival for the child, with globe preservation being secondary (8, 24). Thus, a unified, global analysis of retinoblastoma burden and health disparities is urgently needed to enhance awareness and guide government policies.
The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2021 has, for the first time, documented the burden of retinoblastoma (25). This study uses disability-adjusted life years (DALYs) as a key metric to provide a comprehensive estimation of disease burden. DALYs combine years of life lost (YLL) due to retinoblastoma and years lived with disability (YLD) resulting from retinoblastoma, offering a more scientifically robust measure than globe salvage rates.
As recommended by the World Health Organization (WHO), the slope index of inequality (SII) and the concentration index are the two most common measures used to summarize health inequality across subgroups (26). Both measures’ strengths lie in their population-weighted calculation, yielding a single number that accurately reflects inequality across all subgroups, accounting for the size of each subgroup (26).
In this study, we utilized data from the GBD 2021 database to (1) assess health inequities associated with retinoblastoma across various Socio-Demographic Index (SDI) regions and (2) analyze trends in these health disparities using the SII and concentration index from 1990 to 2021. This analysis seeks to provide valuable insights for policymakers to develop targeted strategies to mitigate health disparities and improve outcomes for children with retinoblastoma worldwide.
This analysis is based on data from the 2021 Global Burden of Disease (GBD) study, coordinated by the Institute for Health Metrics and Evaluation (IHME). The 2021 GBD study provides the largest and most recent comparative estimates of global disease burden, including incidence, prevalence, and disability-adjusted life-years (DALYs) for 371 diseases and injuries, at both country and subnational levels from 1990 to 2021. The methodology employed in the GBD 2021 has been extensively documented in prior studies (25, 27).
We extracted estimates and 95% uncertainty intervals (UI) for DALYs to measure the burden of retinoblastoma [GBD cause code: B.10.1; International Classification of Diseases (ICD)-10 code: C69.2-C69.22]. The Socio-Demographic Index (SDI) was used as an indicator of a nation’s or region’s socioeconomic status, which correlates significantly with overall health indicators. The SDI considers factors such as the fertility rate of women under 25, average educational attainment, and lag-distributed income per capita in each country or territory.
The GBD 2021 provided non-zero burden estimates for retinoblastoma in children under 9 years old, while the default age-standardized DALY rates were estimated across the entire age range from 0 to 99+ years. To facilitate comparisons of disease burden across different years and regions, we estimated age-standardized DALY rates and corresponding 95% confidence intervals (CIs) for children aged 0–9 years, using crude rates for two age subgroups (0–4 years and 5–9 years) and the world standard population. We examined the relationship between the burden of retinoblastoma-related DALY in 2021 and the SDI at the national level using Spearman’s rank correlation. We also investigated the distribution of DALY rates across age groups (<1 year, 12–23 months, 2–4 years, and 5–9 years) in different SDI regions to determine which age groups experience the highest burden of retinoblastoma. We employed Joinpoint regression to estimate average annual percentage change (AAPC) as a measure of temporal trends (28). AAPC quantify the average annual increase or decrease of a specific variable over a defined period. For our study period from 1990 to 2021, AAPC were calculated from the weighted average of slope coefficients obtained from the Joinpoint regression model.
Total DALYs and age-standardized DALY rates were extracted for the analysis of inequality. The Slope Index of Inequality (SII) and the concentration index were used to measure absolute and relative income-related inequality across countries, respectively. The SII was determined by performing a regression analysis on national DALY rates against a relative position scale associated with the SDI. This scale was defined by the midpoint of the cumulative population distribution ranked by SDI. Heteroscedasticity was adjusted using iteratively reweighted least squares. The concentration index was obtained by numerically integrating the area under the Lorenz concentration curve, which was constructed using the cumulative proportion of DALYs and the cumulative relative distribution of the population ranked by SDI. The concentration index was calculated as twice the area between the 45° diagonal line (line of equality) and the Lorenz curve.
AAPCs were calculated using the Joinpoint Regression Program (version 5.0.2). The SII and concentration index were computed and visualized using Stata MP16.0 and R software V.4.3.3. All other analyses and visualizations were executed using R software V.4.3.3.
Globally, the DALYs for retinoblastoma decreased from 276,066 in 1990 to 243,204 in 2021. The age-standardized DALYs for retinoblastoma dropped from 23 per 100,000 population in 1990 to 18.64 per 100,000 population in 2021, with an average annual decrease of −0.67%. In both 1990 and 2021, the highest age-standardized DALYs were in the low SDI region, with 56.69 per 100,000 population in 1990 and 39.38 per 100,000 population in 2021. Conversely, the high SDI region recorded the lowest age-standardized DALYs for both years, at 3.19 per 100,000 population in 1990 and 1.32 per 100,000 population in 2021 (Table 1). All SDI regions experienced a significant decline in age-standardized DALYs from 1990 to 2021 (all AAPC p-values <0.001). The decrease was more rapid in high-middle (AAPC = −3.2%) and high SDI (AAPC = −2.9%) regions compared to middle (AAPC = −2.09%), low (AAPC = −1.14%), and middle-low SDI (AAPC = −0.99%) regions (Table 1).
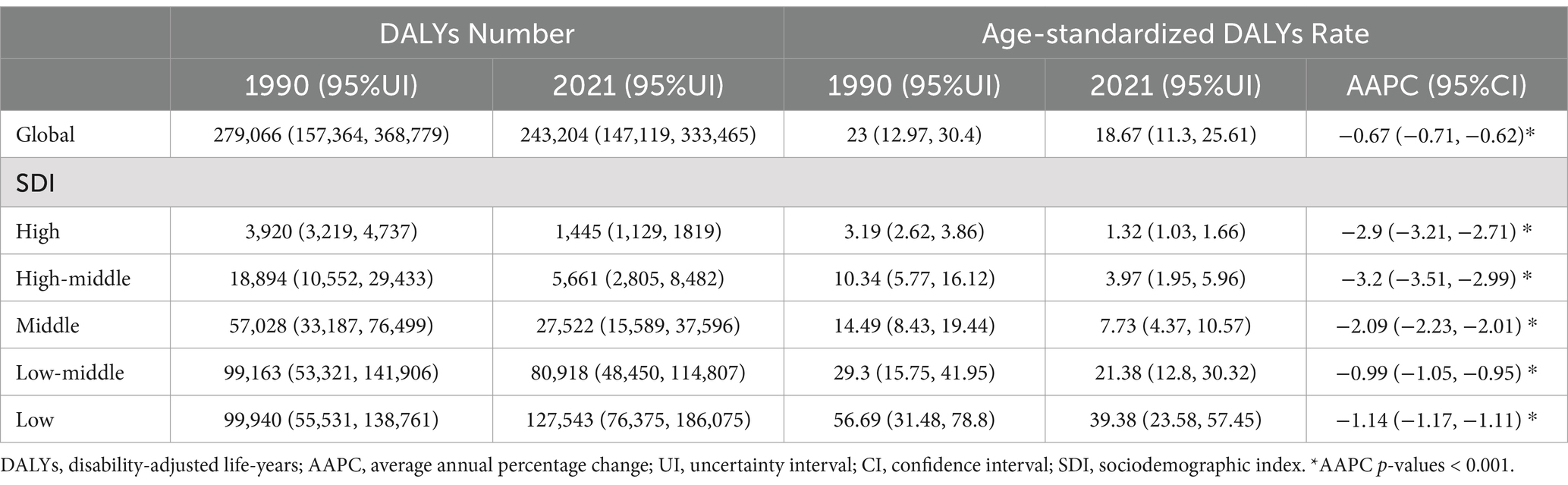
Table 1. Global and regional burden of retinoblastoma and the AAPC of age-standardized DALYs rate, 1990–2021.
At the national level, among the top 10 countries with the highest age-standardized DALYs for retinoblastoma in 2021, Tokelau had the highest value at 177.98. The remaining nine countries were in Africa: Malawi (177.15), Kenya (159.78), Eritrea (97.80), Mozambique (94.27), Comoros (84.03), Tanzania (77.22), Djibouti (69.25), Madagascar (67.75), and Somalia (63.85). In contrast, wealthy island or peninsular nations, such as Seychelles (<0.001), Saint Kitts and Nevis (0.002), Qatar (0.003), and Bermuda (0.01), exhibited the lowest age-standardized DALYs (Figure 1A).
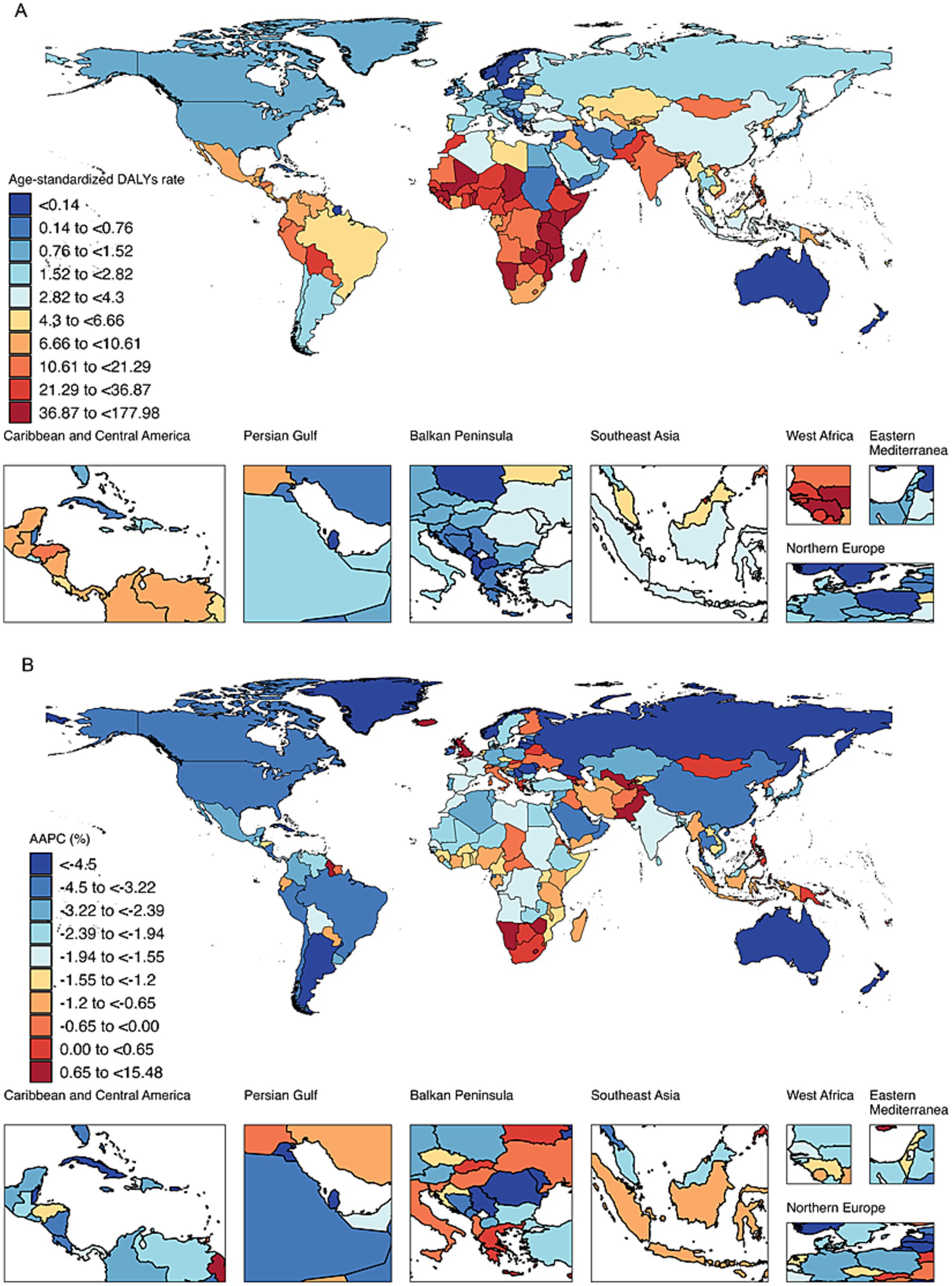
Figure 1. (A) Spatial distribution of age-standardized DALYs for retinoblastoma in 2021. DALYs, disability-adjusted life years. (B) Spatial distribution of average annual percentage change of age-standardized DALYs for retinoblastoma from 1990 to 2021. DALYs, disability-adjusted life years. AAPC, average annual percentage change.
From 1990 to 2021, the majority of countries (165 out of 204) exhibited a decreasing trend in age-standardized DALYs for retinoblastoma. Among these, Cuba showed the most significant decline with an AAPC of −11.50%, followed by Kuwait (AAPC = −10.59%) and Lithuania (AAPC = −10.14%). Among the countries exhibiting an increasing trend, Tokelau experienced the most rapid rise with an AAPC of 15.48%, followed by Armenia (AAPC = 9.68%) and Georgia (AAPC = 6.74%; Figure 1B).
At national level in 2021, the association between the SDI and DALYs revealed a significant negative correlation (Spearman’s ρ = −0.71, p < 0.001). Globally, the 2–4 years age group had the highest DALY rate, a trend consistent in low, low-middle, and middle SDI regions. In contrast, the highest DALY rate in high and high-middle SDI regions was found in the 12–23 months age group (Figure 2).

Figure 2. Age-specific DALYs Rate across different SDI regions in 2021. DALYs, disability-adjusted life-years; SDI, Sociodemographic Index.
Significant absolute and relative SDI-related inequalities in the burden of retinoblastoma were observed, with a disproportionately higher burden shouldered by countries with lower SDI (Figure 3). The SII was −40.81 (95% CI −36.04 to −45.58) DALYs per 100,000 population in 1990 and − 30.32 (95% CI −27.18 to −33.47) DALYs per 100,000 population in 2021. This decline indicates that the absolute inequality in the age-standardized burden of retinoblastoma between high and low SDI countries narrowed during this period (Figure 3). Relative inequality analysis showed that the absolute values for the concentration index remained above 0.37 from 1990 to 2021, indicating a reasonably high level of relative inequality. The values exhibited an increasing trend between 1990 (−0.37, 95% CI −0.46 to −0.28) and 2021 (−0.45, 95% CI −0.53 to −0.36), although this increase did not reach statistical significance (diff = −0.07, 95% CI −0.2 to 0.05, p = 0.256; Figure 4).

Figure 3. Absolute SDI-related health inequality, presented using regression lines, for age-standardized DALYs rate of retinoblastoma across 204 counties and territories, 1990 vs. 2021. And temporal trend of Slope Index of Inequality from 1990 to 2021. DALYs, disability-adjusted life-years; SDI, Sociodemographic Index. SII, Slope Index of Inequality.
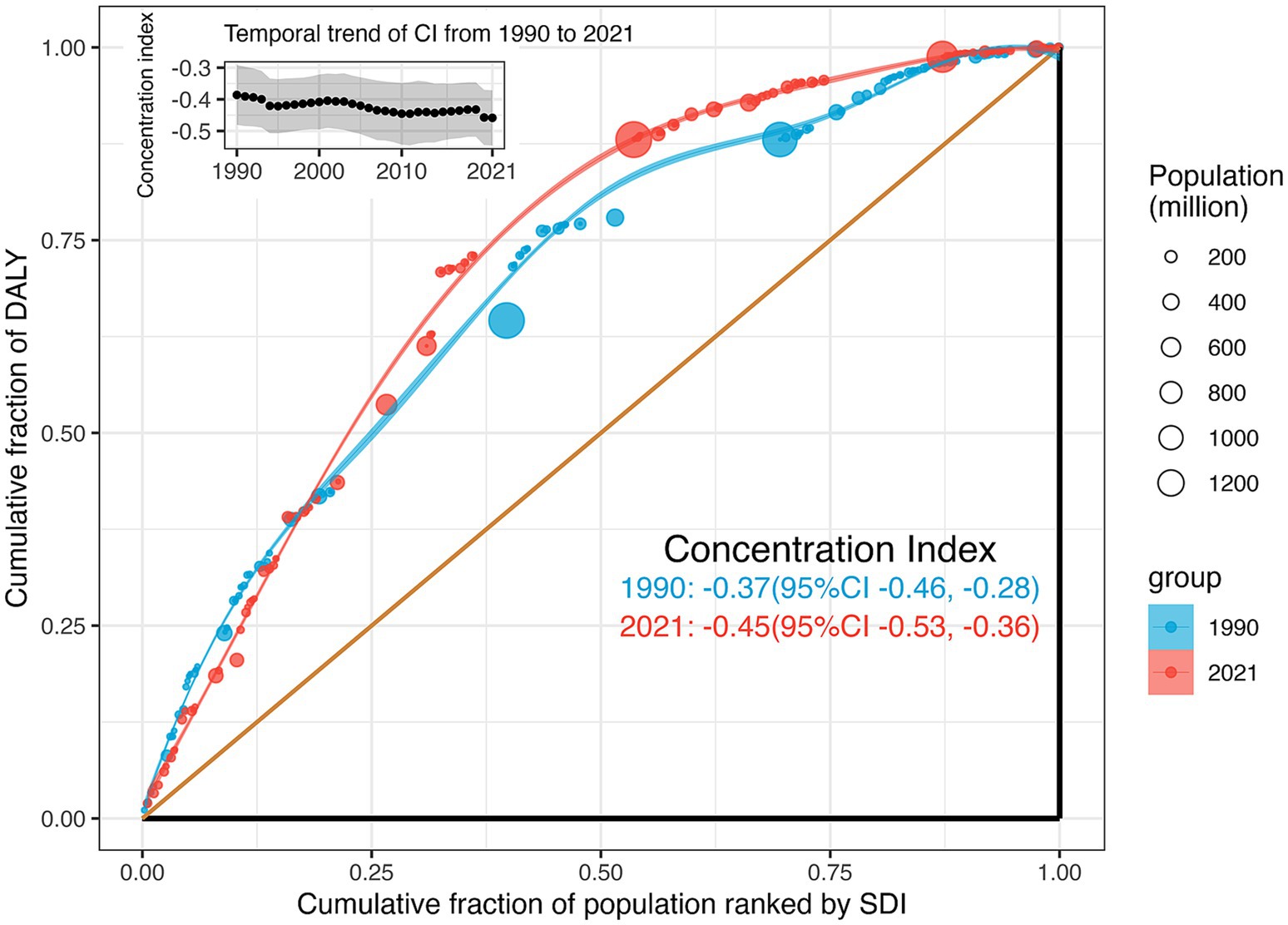
Figure 4. Relative SDI-related health inequality, presented using concentration curves, for age-standardized DALYs rate of retinoblastoma across 204 counties and territories, 1990 vs. 2021. And temporal trend of the concentration index from 1990 to 2021. DALYs, disability-adjusted life-years; SDI, Sociodemographic Index.
This secondary analysis of data from the GBD 2021 offers a comparative overview of the global burden of retinoblastoma, socio-economic-related inequalities, and temporal trends from 1990 to 2021. Our study found that, despite a reduction in the global age-standardized burden of retinoblastoma, health inequalities related to this disease remain significant and persistent.
Our study confirmed a global decline in the burden of retinoblastoma-related DALY from 1990 to 2021. This decline is likely due to the establishment of specialized referral centers, advancements in genetic understanding, and the introduction of chemotherapy in recent decades (29). Despite this overall global decrease, the age-standardized DALYs and the extent of this decline varied significantly at the national level. Regions with middle to high SDI experienced more pronounced decreases compared to low and middle-low SDI regions, reflecting health inequities across different socioeconomic contexts.
We found that DALY rates were highest in the 12–23 months age group in high and middle-high SDI regions, whereas in regions with lower SDI, DALY rates peaked in the 2–4-year age group. These findings align with previous research indicating that the age at onset of retinoblastoma is younger in high-income regions compared to low-income regions (4, 7). The Global Retinoblastoma Study Group previously reported that the median age at diagnosis of retinoblastoma worldwide is 24 months: 31 months in low-income countries and 14 months in high-income countries (4).
Previous studies by the Global Retinoblastoma Study Group (4), the American Joint Committee on Cancer Ophthalmic Oncology Task Force (7), and meta-analyses by Wong et al. (8) have focused on the relationships between global retinoblastoma presentation and income level. These studies demonstrated that higher income levels are associated with increased overall survival, better globe salvage rates, younger age at diagnosis, and lower proportions of locally advanced disease and distant metastasis. However, these studies typically employed simple measures of inequality, comparing health outcomes between selected regions. However, “complex measures” recommended by the WHO offer a more comprehensive view of inequality across all regions or countries (26). These measures can provide a deeper understanding of disparities in health outcomes related to retinoblastoma.
In this study, we used two WHO-recommended measures: the SII and the concentration index. These measures rely on the cross-national health gradient and the SDI ordering. By weighting population size, they provide a comprehensive description of inequality across all subgroups and track trends over time. This approach is crucial for monitoring health inequalities and evaluating the impact of social policies. Effective social policies that reduce poverty, enhance educational opportunities, or create jobs can decrease the size of disadvantaged subgroups (26). Therefore, assessing the impact of such policies on health inequality is essential for policymakers.
By calculating the SII and the concentration index, we found that the burden of retinoblastoma was negatively associated with socioeconomic level. From 1990 to 2021, this inequality saw a slight reduction in absolute terms (SII) but remained consistently high in relative terms (concentration index). To our knowledge, these findings have not been previously reported.
The disparity in trends between absolute and relative eye health inequality can be attributed to differences in their computational methods (30). Absolute inequality, quantified by the SII, represents the absolute difference in predicted values of a health indicator between the highest and lowest SDI levels, considering the entire SDI distribution through a regression model. As SII is influenced by measurement scales, a reduction in SII alongside decreases in the age-standardized DALY rate is anticipated. Conversely, relative inequality, measured by the concentration index, assesses proportional health differences among socioeconomic groups. Negative values indicate a concentration among the disadvantaged, and positive values among the advantaged. Despite a theoretical range of ±1, practical values rarely exceed 0.5, with 0.2 to 0.3 indicating high relative inequality (26). Since 1990, the concentration index for retinoblastoma has consistently exceeded −0.37, reaching its peak at −0.45 in 2021, indicating a persistently high level of relative inequality and slight worsening over time. Thus, our findings underscore the ongoing severity of health inequality in retinoblastoma.
According to the WHO, there are significant disparities in childhood cancer outcomes both between and within countries, with socioeconomic status being a major driver of these inequities (31). Identified by the WHO as a “tracer cancer, “retinoblastoma serves as a marker for global healthcare transformation and progress (3, 23). Despite ongoing efforts to improve retinoblastoma outcomes globally, our study reveals the persistent and severe nature of health inequalities in this disease, necessitating further reflection and action. This requires collective investment, particularly in addressing the current gaps in research funding. Our findings highlight the urgent need for targeted interventions to improve affordable early detection strategies and enhance treatment accessibility in underserved populations. Standardizing treatment protocols and leveraging technological advancements are crucial to narrowing health inequalities and improving survival rates for children with retinoblastoma worldwide. These efforts must be supported by global health policies that prioritize equitable resource allocation, particularly in low-income regions, where increased investment in healthcare infrastructure is essential for ensuring timely access to diagnostic and treatment services. Additionally, fostering international collaborations to share research, expertise, and best practices in retinoblastoma treatment, while advocating for affordable diagnostic technologies, is key to advancing global health outcomes. Policy recommendations should focus on establishing screening programs and ensuring access to services, particularly in low- and middle-SDI regions, to move closer to achieving equitable access to treatment and care for all. Until progress benefits everyone, the vision of equitable healthcare remains imperfect. International collaborations.
This secondary analysis has several strengths. It uses the latest data and standard methods for a comprehensive global assessment of health equity. The insights provided by this study are difficult to obtain from isolated regional studies. However, the study has limitations. First, the GBD data source and methodology have certain weaknesses. Limited data quality may introduce biases into the analysis. Additionally, the study is constrained by the ecological fallacy since all conclusions are based on national-level data rather than individual-level data. Second, this study focuses on analyzing the outcomes of past global efforts to improve health inequities related to retinoblastoma. It does not evaluate the impact of specific policies on these inequities, which is crucial for future policy development. Finally, although the WHO recommends using the concentration index, it is more susceptible to being influenced by changes in the SDI of populous countries. Future analyses should consider these factors to ensure a balanced assessment.
In conclusion, despite advancements in retinoblastoma management, the overall burden of the disease-related DALY remains disproportionately concentrated in poorer populations. Furthermore, this health inequality has not significantly improved over the past few decades, underscoring the limitations of current efforts. This persistent disparity highlights the urgent need for targeted interventions focused on underserved communities to achieve the goal of eliminating deaths from this rare yet curable cancer and enhancing the quality of life for affected individuals.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
XLi: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. YC: Data curation, Validation, Writing – original draft. XZ: Data curation, Writing – review & editing. XLiu: Formal analysis, Validation, Writing – review & editing. YZ: Formal analysis, Validation, Writing – review & editing. XW: Validation, Writing – review & editing. JL: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Guides Local Science and Technology Development Fund Projects (YDZJSX2024B013) and the Doctoral Project Fund of Shanxi Eye Hospital affiliated to Shanxi Medical University (B202403). The sponsor or funding organization had no role in the design or conduct of this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
SDI, Demographic Index; UI, Uncertainty Interval; DALYs, Disability-Adjusted Life Years; GBD, Global Burden of Disease study; AAPC, Average Annual Percentage Change; SII, Slope Index of Inequality; YLL, Years of Life Lost; YLD, Years Lived with Disability; WHO, World Health Organization; IHME, Health Metrics and Evaluation; ICD, International Classification of Diseases; CIs, Confidence Intervals.
1. Dimaras, H, Corson, TW, Cobrinik, D, White, A, Zhao, J, Munier, FL, et al. Retinoblastoma. Nat Rev Dis Primers. (2015) 1:15021. doi: 10.1038/nrdp.2015.21
2. Delhiwala, KS, Vadakkal, IP, Mulay, K, Khetan, V, and Wick, MR. Retinoblastoma: An update. Semin Diagn Pathol. (2016) 33:133–40. doi: 10.1053/j.semdp.2015.10.007
3. Lam, CG. Retinoblastoma as a lens for correctable disparities worldwide. Lancet Glob Health. (2022) 10:e1074–5. doi: 10.1016/s2214-109x(22)00290-x
4. Fabian, ID, Abdallah, E, and Abdullahi, SU. Global retinoblastoma presentation and analysis by National Income Level. JAMA Oncol. (2020) 6:685–95. doi: 10.1001/jamaoncol.2019.6716
5. Chantada, GL, Doz, F, Orjuela, M, Qaddoumi, I, Sitorus, RS, Kepak, T, et al. World disparities in risk definition and management of retinoblastoma: a report from the international retinoblastoma staging working group. Pediatr Blood Cancer. (2008) 50:692–4. doi: 10.1002/pbc.21427
6. Truong, B, Green, AL, Friedrich, P, Ribeiro, KB, and Rodriguez-Galindo, C. Ethnic, racial, and socioeconomic disparities in retinoblastoma. JAMA Pediatr. (2015) 169:1096–104. doi: 10.1001/jamapediatrics.2015.2360
7. Tomar, AS, Finger, PT, Gallie, B, Kivelä, TT, Mallipatna, A, Zhang, C, et al. Global retinoblastoma treatment outcomes: association with National Income Level. Ophthalmology. (2021) 128:740–53. doi: 10.1016/j.ophtha.2020.09.032
8. Wong, ES, Choy, RW, Zhang, Y, Chu, WK, Chen, LJ, Pang, CP, et al. Global retinoblastoma survival and globe preservation: a systematic review and meta-analysis of associations with socioeconomic and health-care factors. Lancet Glob Health. (2022) 10:e380–9. doi: 10.1016/s2214-109x(21)00555-6
9. Rajeshuni, N, Whittemore, AS, Ludwig, CA, Mruthyunjaya, P, and Moshfeghi, DM. Racial, ethnic, and socioeconomic disparities in retinoblastoma enucleation: a population-based study, SEER 18 2000-2014. Am J Ophthalmol. (2019) 207:215–23. doi: 10.1016/j.ajo.2019.04.015
10. Munier, FL, Beck-Popovic, M, Chantada, GL, Cobrinik, D, Kivelä, TT, Lohmann, D, et al. Conservative management of retinoblastoma: challenging orthodoxy without compromising the state of metastatic grace. “alive, with good vision and no comorbidity”. Prog Retin Eye Res. (2019) 73:100764. doi: 10.1016/j.preteyeres.2019.05.005
11. MacCarthy, A, Birch, JM, Draper, GJ, Hungerford, JL, Kingston, JE, Kroll, ME, et al. Retinoblastoma: treatment and survival in Great Britain 1963 to 2002. Br J Ophthalmol. (2009) 93:38–9. doi: 10.1136/bjo.2008.139626
12. Kivelä, T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. (2009) 93:1129–31. doi: 10.1136/bjo.2008.150292
13. Nyamori, JM, Kimani, K, Njuguna, MW, and Dimaras, H. The incidence and distribution of retinoblastoma in Kenya. Br J Ophthalmol. (2012) 96:141–3. doi: 10.1136/bjophthalmol-2011-300739
14. Leal-Leal, C, Flores-Rojo, M, Medina-Sansón, A, Cerecedo-Díaz, F, Sánchez-Félix, S, González-Ramella, O, et al. A multicentre report from the Mexican retinoblastoma group. Br J Ophthalmol. (2004) 88:1074–7. doi: 10.1136/bjo.2003.035642
15. Chaugule, SS, Honavar, SG, and Finger, PT. Surgical ophthalmic oncology: A collaborative open access reference. Cham: Springer International Publishing (2019).
16. Torres-Netto, EA, Gabel-Obermaier, C, Gabel, P, Gloor, B, Wiedemann, P, Taylor, H, et al. Twenty years of International Council of Ophthalmology fellowships: description of the programme and the impact on more than 1100 awardees. Br J Ophthalmol. (2021) 105:1318–24. doi: 10.1136/bjophthalmol-2020-316484
17. Chantada, GL, Dunkel, IJ, and Schaiquevich, PS Twenty-year collaboration between North American and South American retinoblastoma programs[J]. JCO Glob Oncol. 2:437.
18. Li, N, Wang, S, and Zhao, G Meeting the Unmet Needs in Rare Cancer: Advancing Treatment Options for Retinoblastoma and Beyond[J]. JAMA. 332:1618–1620.
19. Tomar, AS, Finger, PT, Gallie, B, Mallipatna, A, Kivelä, TT, Zhang, C, et al. A multicenter, international collaborative study for American joint committee on Cancer staging of retinoblastoma: part II: treatment success and globe salvage. Ophthalmology. (2020) 127:1733–46. doi: 10.1016/j.ophtha.2020.05.051
20. Tomar, AS, Finger, PT, Gallie, B, Mallipatna, A, Kivelä, TT, Zhang, C, et al. A multicenter, international collaborative study for American joint committee on Cancer staging of retinoblastoma: part I: metastasis-associated mortality. Ophthalmology. (2020) 127:1719–32. doi: 10.1016/j.ophtha.2020.05.050
21. Khedekar, A, Devarajan, B, Ramasamy, K, Muthukkaruppan, V, and Kim, U. Smartphone-based application improves the detection of retinoblastoma. Eye (Lond). (2019) 33:896–901. doi: 10.1038/s41433-018-0333-7
22. Munson, MC, Plewman, DL, Baumer, KM, Henning, R, Zahler, CT, Kietzman, AT, et al. Autonomous early detection of eye disease in childhood photographs. Sci Adv. (2019) 5:eaax6363. doi: 10.1126/sciadv.aax6363
23. World Health Organization. Cure all framework: WHO global initiative for childhood cancer: Increasing access, advancing quality, saving lives. (2021). Available at: https://www.who.int/publications/i/item/9789240025271 (accessed Feb 8, 2025).
24. Abramson, DH, Fabius, AW, and Issa, R. Advanced unilateral retinoblastoma: the impact of ophthalmic artery chemosurgery on enucleation rate and patient survival at MSKCC. PLoS One. (2015) 10:e0145436. doi: 10.1371/journal.pone.0145436
25. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the global burden of disease study 2021. Lancet (London, England). (2024) 403:2100–32. doi: 10.1016/s0140-6736(24)00367-2
26. World Health Organization. Handbook of health inequality monitoring with a special focus on low- and middle-income countries. WHO: Geneva. (2013).
27. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
28. Kim, HJ, Fay, MP, Feuer, EJ, and Midthune, DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. (2000) 19:335–51.
29. Fabian, ID, Onadim, Z, Karaa, E, Duncan, C, Chowdhury, T, Scheimberg, I, et al. The management of retinoblastoma. Oncogene. (2018) 37:1551–60. doi: 10.1038/s41388-017-0050-x
30. Asada, Y. A framework for measuring health inequity. J Epidemiol Community Health. (2005) 59:700–5. doi: 10.1136/jech.2004.031054
31. World Health Organization. Childhood cancer inequalities in the WHO European Region 2022 report. WHO: Geneva. (2022). Available at: https://www.who.int/europe/publications/m/item/childhood-cancer-inequalities-in-the-who-european-region-2022-report (Accessed February 8, 2025).
Keywords: retinoblastoma, health inequality, global burden of disease study, demographic index, slope index, concentration index
Citation: Li X, Chang Y, Zhao X, Liu X, Zhang Y, Wang X and Li J (2025) Global health inequities in retinoblastoma: a 1990–2021 analysis across socio-demographic index regions. Front. Public Health. 13:1513526. doi: 10.3389/fpubh.2025.1513526
Received: 18 October 2024; Accepted: 03 February 2025;
Published: 21 February 2025.
Edited by:
M. Ashwin Reddy, Barts Health NHS Trust, United KingdomReviewed by:
Thiago Gonçalves dos Santos Martins, Federal University of São Paulo, BrazilCopyright © 2025 Li, Chang, Zhao, Liu, Zhang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junhong Li, anVuaG9uZ2xpMjAyMkAxNjMuY29t; Xi Li, ZHJsaXhpMjAyMEBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.