- 1Yusra Institute of Pharmaceutical Sciences, Yusra Medical and Dental College, Islamabad, Pakistan
- 2School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons (RCSI) University of Medicine and Health Sciences, Dublin, Ireland
- 3Communication and Media Studies, Fatima Jinnah Women University, Rawalpindi, Pakistan
- 4Department of Pharmacy, Quaid-i-Azam University, Islamabad, Pakistan
- 5Sabin Vaccine Institute, Washington, DC, United States
- 6Health Services Academy, Islamabad, Pakistan
- 7Global Health Department, Health Services Academy, Islamabad, Pakistan
- 8Armed Forces Institute of Pathology, Rawalpindi, Pakistan
Background and aims: The general population have depicted concern about the safety and efficacy of the vaccine and its long-term effects on human health. Pakistan being on the verge of the pandemic is in more demand for vaccination and immunization. Therefore, this study aimed to evaluate the COVID-19 vaccines side effects among the general population.
Methods: A cross-sectional face-to-face study was carried out among individuals who received either the first dosage or both doses of vaccination in twin cities (Islamabad and Rawalpindi) of Pakistan. Data was collected through a self-administered questionnaire. The questionnaire included three sections (socio-demographic, medical history, vaccine, and immunization) with 20 questions. The collected data was analyzed in SPSS (version 25) using descriptive statistics, the chi-square test, and the odd ratio.
Results: A total of 2,618 participants were included and of them, females (55.3%; n = 1,449) were more than males. The majority of the participants reported the use of precautionary medicines including vitamin C (1,319; 50.4%) followed by paracetamol (n = 1,249; 47.7%) and mineral supplements (n = 616; 23.5%) for COVID-19. In this study, 3.8% (n = 99) were unvaccinated and the first and second doses of the vaccine was received by 2,519 and 2,239 of the participants, respectively. Different types of side effects were highlighted in the current study. The most frequently reported side effects after the first dose of COVID-19 were fever (n = 997), pain at the injection site (n = 994), muscle pain (n = 620), and fatigue (n = 482). Additionally, pain at the injection site (n = 852), fever (n = 815), and muscle pain (n = 601) were commonly reported after the 2nd dose of COVID-19. The lowest reported side effects were swollen lymph nodes and anaphylactic shock. In the current study, people who were previously immunized with the flu and pneumonia vaccine had a lower risk of developing side effects (p < 0.05).
Conclusion: This study highlights important information about side effects reported due to the COVID-19 vaccinations. Moreover, the use of precautionary medications was also highlighted. These findings could have a valuable impact on designing future comparative studies and developing policies/guidelines for pandemic preparedness.
Introduction
The new coronavirus COVID-19 is the most recent conflict in the series of pandemics, which originated in the Wuhan region of China in December 2019 (1, 2). It was not only spreading fear of a new virus but rather the chances of the resurgence of other known viruses, likewise Poliovirus increased dramatically, especially in low middle-income countries like Pakistan where the prevalence surged (3, 4). Most nations, including Pakistan, implemented strict precautionary measures to counter and control the spread of the epidemic, such as mandatory mask usage along quarantine approach work from home strategy, and smart lockdown (5–7). Being conducive to the halt of spread, these strategies are unable to ensure long-term protection and immunity against COVID-19 infection (8). Therefore, therapeutic, and preventative treatments seem to be paramount in managing COVID-19 infections (9).
Vaccines are considered to be a crucial step in controlling any disease. The development of COVID-19 vaccines was crucial and believed to be a vital weapon to fight the pandemic (10, 11). However, the World Health Organization (WHO) has listed vaccine hesitancy as one of the top 10 global health issues since 2019; it is fueled by false information about the efficiency and safety of vaccines (12, 13). A new challenge of misinformation and disinformation spread on social media platforms, which were later called as infodemic (10, 14). Side effects directly impact vaccine hesitancy and data from February 2022 shows that only 10.6% of the people from the global south compared to 61.9% of the World’s population received COVID-19 vaccine shots (15).
The Pakistani government has approved the following COVID-19 vaccines: the mRNA-based BNT162b2 (commercial name: Comirnaty, Pfizer—BioNTech); the inactivated virus-based BBIBP-CorV (commercial name: Covilo, BBIBP-CorV); CoronaVac (commercial name: CoronaVac, Sinovac); and the adenoviral vector-based ChAdOx1-S (commercial name: Vaxzevria, Oxford—AstraZeneca), Gam-COVID-Vac (commercial name: Sputnik V, Gamaleya), mRNA-1273 (commercial name: Spikevax, Moderna—NIAID) and AD5-nCOV (commercial name: Convidecia, CanSino). The COVID-19 vaccination recipients reported injection site or local reactions more commonly than systemic reactions and significant adverse effects were uncommon, according to global safety studies on vaccine reactogenicity (16). Females and the younger population group are more prone to report more adverse effects than the other groups (17, 18). A Plethora of people around the globe expressed their concerns and reluctance to get vaccinated despite published safety data on COVID-19 vaccines, and studies have revealed a correlation between vaccination intention and favorable vaccination attitudes (19).
The most common reason for vaccine hesitation among various population groups is an aversion to the potential negative effects of immunizations (20). Similarly, a recent systematic analysis found that increasing the public’s understanding of vaccines’ effectiveness, and honesty regarding their side effects, are vital strategies to improve vaccine uptake (21). The current study is related to the assessment of the COVID-19 vaccine’s side effects and acceptance amongst the Pakistani population. This study aims to collect data through the quantitative methodology to assess the type and frequency of side effects experienced by individuals after being administered 1st and 2nd dose of COVID-19 vaccines, respectively. This study also intends to configure which vaccines were preferred by individuals in twin cities and which side effects were most prevalent. This knowledge would also give people more confidence to acquire COVID-19 vaccinations, hence increasing vaccine acceptance. Therefore, this study aimed to investigate the COVID-19 vaccine’s side effects and acceptance among the general population in the Twin Cities (Rawalpindi and Islamabad) of Pakistan.
Materials and methods
Study design and setting
A 6-month cross-sectional face-to-face questionnaire-based study was carried out among the general population in Twin cities (Rawalpindi and Islamabad) of Pakistan. Islamabad-Rawalpindi is the fourth-largest metropolitan area in Pakistan.
Inclusion and exclusion criteria
This study included the general population with age ≤ 20 years, residing in selected study settings, and vaccination status. The individuals who did not belong to the Islamabad/ Rawalpindi areas and were non-consented to participate were excluded from this study.
Sample size
According to the World Population Review, the population of Islamabad and Rawalpindi was 1,163,580 and 2,280,730 in 2021, respectively (22, 23). The total population was 3,525,490. This information was entered into the Raosoft sample size calculator and a minimum required sample size was 385 with an assumed 50% response distribution, 5% error margin, and 95% confidence level (24). We distributed a questionnaire with 2,650 participants to provide a broad perspective, avoid missing data, and account for the response rate.
Data collection method and study tool (questionnaire)
A self-administered questionnaire was prepared through a literature search (16–18, 21). Initially, the questionnaires were prepared in English and translated into the Pakistan national language (Urdu) then back to the English language to ensure consistency. Three academic expert researchers then examined the questionnaire to assess its validity, appropriateness, consistency, and adequacy. The questionnaire was tested with 20 participants at the Yusra Institute of Pharmaceutical Sciences (YIPS), Islamabad, and Rawalpindi Medical University, Rawalpindi, Pakistan to verify the conception of the questionnaire’s language and suitability for reliably measuring the variables under observation. Participants in the pilot study were excluded from the final sample. Minor modifications were made following the suggestions. Finally, the questionnaire was distributed among eligible participants visiting COVID-19 vaccination camps in various hospital settings in Islamabad and Rawalpindi. The data for this study was gathered for 8 months starting from September 1, 2020, to April 30, 2021.
The final questionnaire comprised 3 core sections with 20 questions (Supplementary Data Sheet 1). The first section was about the socio-demographic characteristics (sex, age, marital status, education, employment, and residence). The second section included medical history. The third section contained vaccines and immunization-related questions (previous immunization status, COVID-19 test, results, vaccination status against COVID-19, type of vaccine, dose status of vaccine, and side effects after the first and second dose of COVID-19).
Statistical analysis
Descriptive statistics were utilized to assess the participant’s data. Data was analyzed using Microsoft Excel (Microsoft Corp,) and IBM-SPSS version 25 (Chicago, Illinois, U.S.A.). All of the quantitative variables in the study were presented in the form of frequencies and percentages. The chi-square test and odd ratio were also used for analysis purposes. Statistical significance is defined as a p-value of less than 0.05.
Results
Sociodemographic information
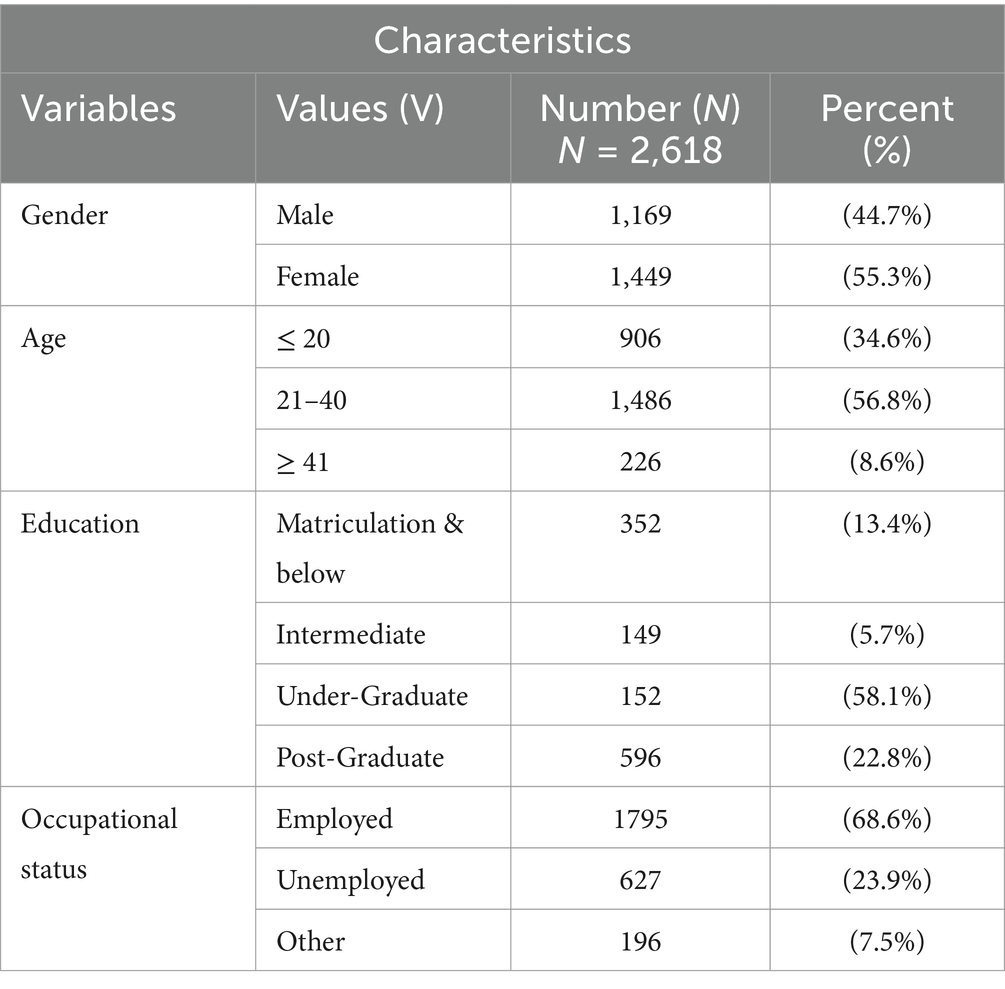
This study involved a total of 2,618 participants, and among them, 44% were male (n = 1,169) and 55.3% were female (n = 1,449). Of them, 34.6% of the participants were ≤ 20 years of age, 56.8% of participants were grouped in the age group of 21–40 years, and 8.6% of participants were grouped in the ≥41 year age group. Most of the participants had bachelor-level degrees (58.1%), followed by post-graduates (22.8%), intermediate (5.7%), and matriculation (13.4%). About, 68.6% of the study participants were employed while 23.9% of the participants were unemployed (Table 1).
Types of precautionary medicines
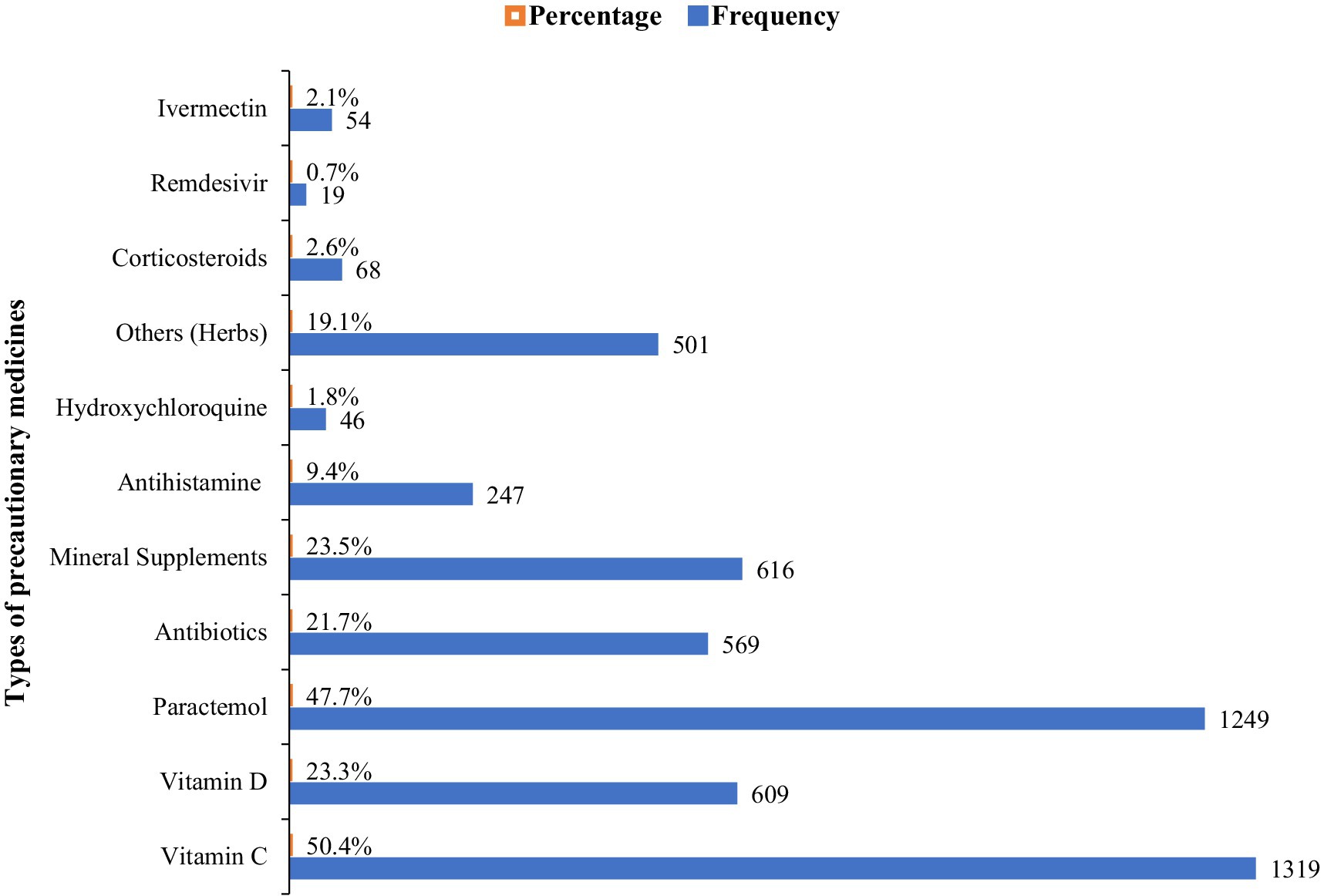
The majority of the participants reported the use of precautionary medicines for COVID-19 including vitamin C, vitamin D, analgesic antipyretic drugs (paracetamol), antibiotics (macrolides, cephalosporins, and fluoroquinolones groups), mineral supplements (zinc, magnesium, calcium, iron, selenium), herbs, anti-allergic (antihistamine), hydroxychloroquine, corticosteroids, redeliver and ivermectin. Among them, vitamins showed the highest frequency (50.4%) followed by paracetamol (47.7%), mineral supplements (23.5%), and vitamin D (23.3%). While remdesivir and hydroxychloroquine showed the lowest frequency (Figure 1).
Types of vaccines
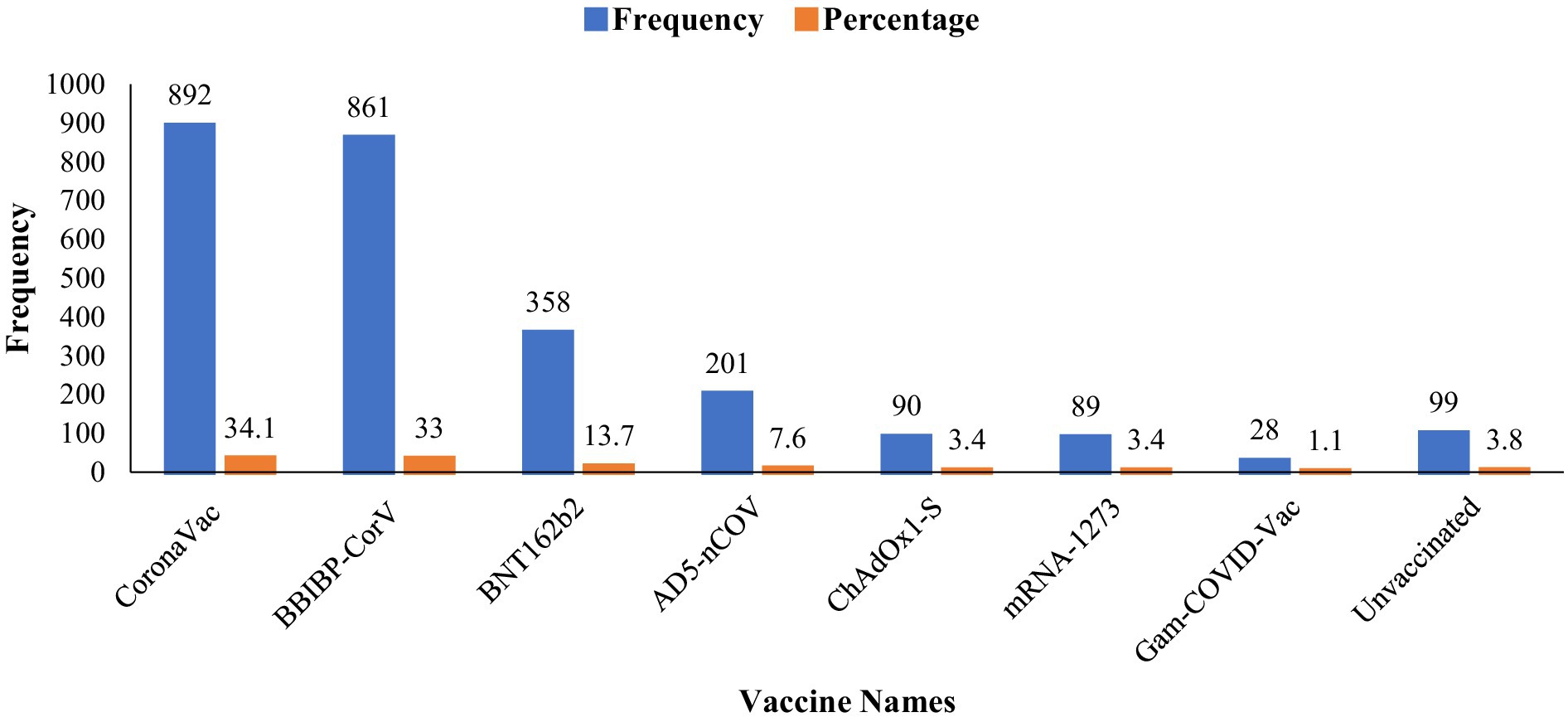
Different types of vaccines were analyzed in this study, including BBIBP-CorV, CoronaVac, ChAdOx1-S, BNT162b2, AD5-nCOV, mRNA-1273, and Gam-COVID-Vac. Among them, CoronaVac showed the highest frequency (n = 892; 34.1%), followed by BBIBP-CorV (n = 861; 33%). Meanwhile, mRNA-1273 and ChAdOx1-S had a low usage rate of only 3.4%. Lastly, Gam-COVID-Vac had the lowest frequency (n = 28; 1.1%). Moreover, ninety-nine (n = 99; 3.8%) participants in this study were unvaccinated (Figure 2).
Trend of side effects after the first and second doses of vaccines
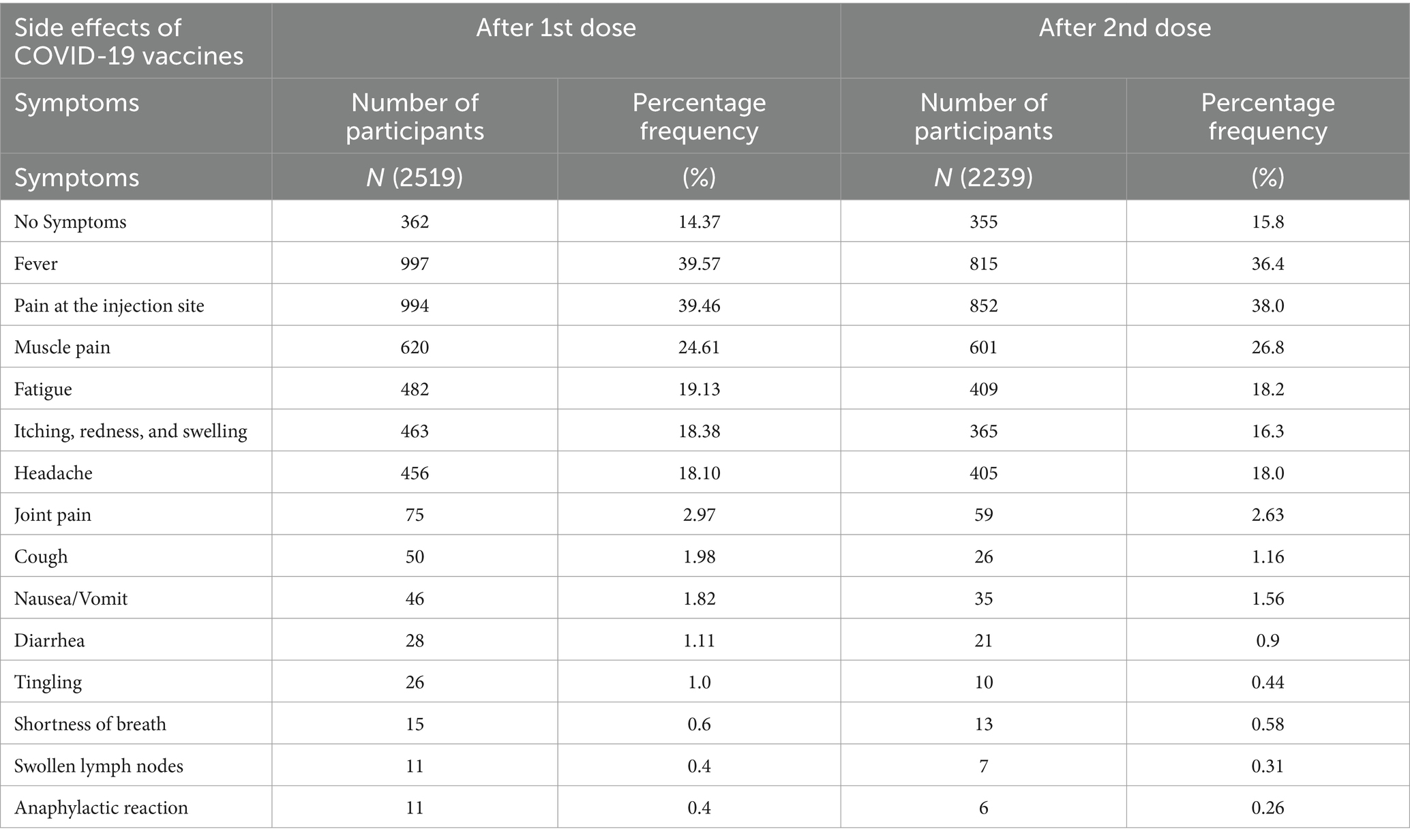
This study also determined several types of side effects of vaccines after the first and second vaccine doses. Fever, Pain at the injection site, muscle pain, fatigue, itching, redness and swelling, headache, joint pain, cough, nausea/vomiting, diarrhea, tingling, shortness of breath, swollen lymph nodes, and anaphylactic reaction were the common symptoms that reported by the participants. Among them, after the first dose of the vaccine, fever showed the highest frequency (n = 997; 39.57%) while after the second dose of the vaccine, pain at the injection site showed the highest frequency (n = 852; 38%). Swollen lymph nodes and anaphylactic shock showed the lowest frequency (n = 11; 0.4%) after the first dosage of vaccine, while after the second dose of vaccine anaphylactic shock showed the lowest frequency (n = 6; 0.26%) (Table 2).
Relationship between COVID-19 vaccine-related side effects and previously immunized participants with flu and pneumonia vaccine
The present study has also analyzed and compared the previously immunized participants with the flu vaccine and pneumonia vaccine in relationship to their side effects after the first and second doses of COVID-19 vaccines. The has revealed that the people who were previously immunized with the flu vaccine had a lower risk of developing tingling (p-value = 0.00), fever (p-value = 0.01), pain at the injection site (p-value = 0.01) and anaphylactic shock (p-value = 0.02) after the first dose of the vaccine because the p-value is lower than 1% as shown in Table 3. The participants immunized with the flu vaccine also reported a lower chance of developing headache (p-value = 0.02), joint pain (p-value = 0.05), diarrhea (p-value = 0.00), swelling, itching, and redness at the injection site (p-value = 0.04) after the second dose of the vaccine.
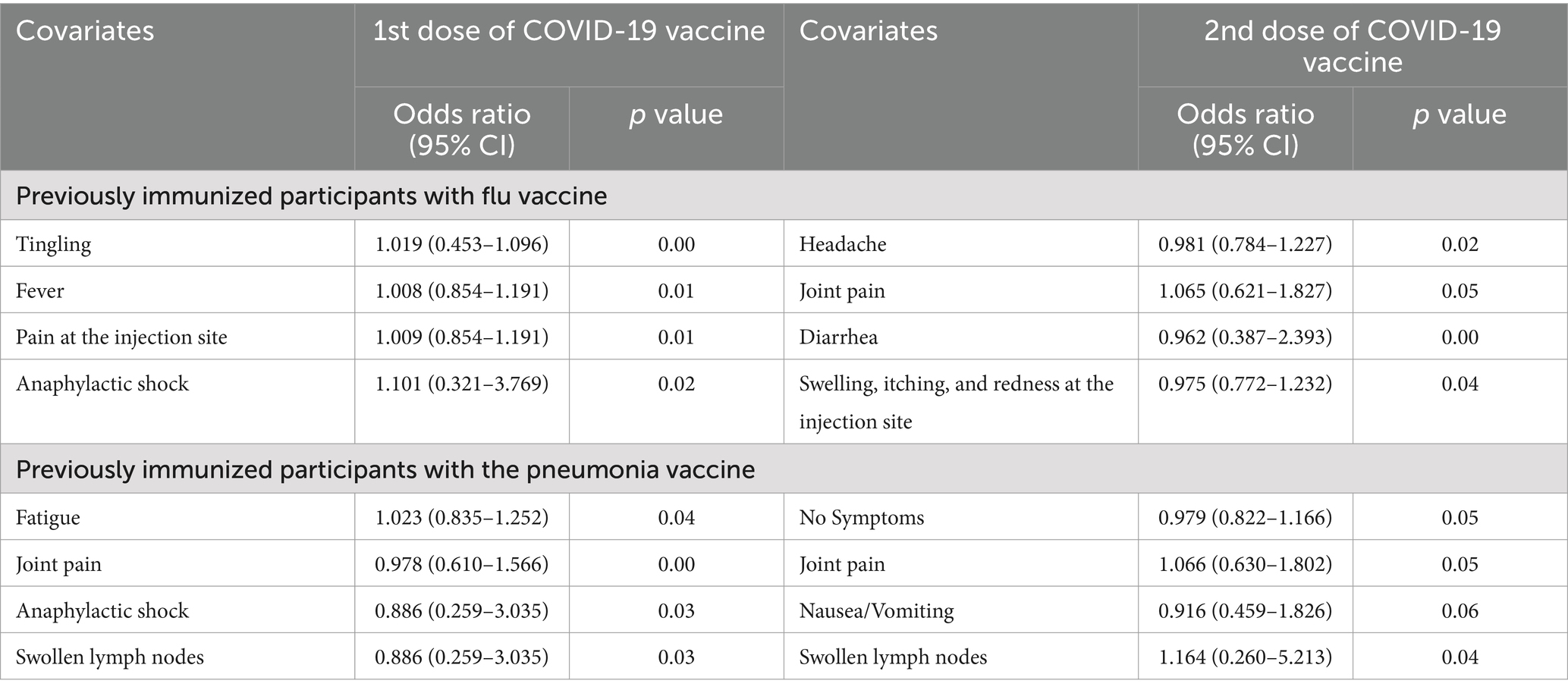
Table 3. Relationship between COVID-19 vaccine-related side effects and previously immunized participants with flu and pneumonia vaccine.
Similarly, the participants who were previously immunized with the pneumonia vaccine had a lower risk of developing fatigue (p-value = 0.04), joint pain (p-value = 0.00), anaphylactic shock (p-value = 0.03) and swollen lymph nodes (p-value = 0.03) after the first dose of the as shown in Table 3, and also such people had a lower chance of developing joint pain (p-value = 0.05), nausea and vomiting (p-value = 0.06), swollen lymph nodes (p-value = 0.04) after second dose of vaccine.
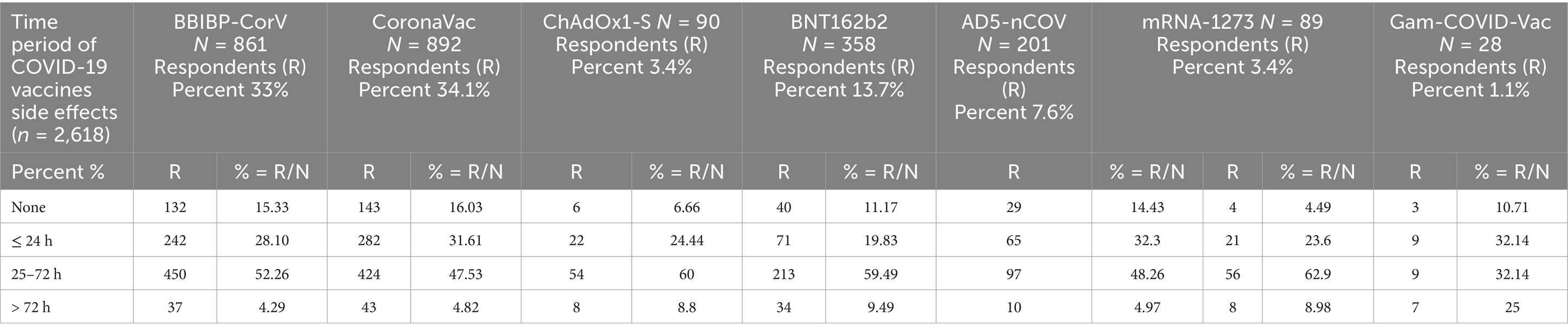
Period of COVID-19 vaccine side effects
This study also investigated the span of side effects linked with several COVID-19 vaccinations, which focus on the period in which they affect. These findings were made about a variety of COVID-19 vaccines. It has been showed that both BBIBP-CorV and CoronaVac vaccines have higher percentages for vaccine-related side effects that were prolonged for 25 to 72 h with percentage values of 52.26 and 47.53%, respectively, while this value was lowest (32.14%) for Gam-COVID-Vac as shown in Table 4.
Discussion
The present study evaluated the side effects and acceptance of COVID-19 vaccines among the Pakistani population in the Rawalpindi/Islamabad regions. Studies have shown that the side effects which are associated with vaccines are a major problem resulting in vaccine hesitancy (25). Therefore, to achieve the maximum acceptance of any vaccine, its adverse side effects should be tackled effectively (26).
In this study, most of the participants took precautionary medicines to avoid vaccination including immunity-boosting vitamins and minerals supplements, paracetamol, antibiotics, antiviral and antiparasitic drugs, steroids, anti-allergic, and other herbal remedies. This depicts that most of the participants were aware of the disease-preventive role of vitamin C. Several studies have reported that vitamin C is critical in boosting the adaptive immune response of the body (27). Several studies have reported that vitamin C plays a major role in preventing COVID-19 infection, and its progression, and significantly reduced COVID-related mortality rates (28). About, 57% of the participants took only one precautionary medicine, while only 3 participants out of 2,618 took all 11 COVID-preventive medications. Studies have reported that taking multiple medications at the same time can lead to adverse side effects due to the chemical interaction of the drugs (29, 30). Therefore, it is important to be cautious while taking such multiple medication regimens. Moreover, it is also reported in prior studies that social media including online health literacy information was used during COVID-19. Such practice leads to COVID-19 misinformation and malpractices (31, 32). Therefore, interdisciplinary, multilevel approaches are needed to involve government and public health organizations and social media companies to control and prevent misinformation and promote better public health (31).
It was reported in this study that most of the sample population were vaccinated individuals. Both BBIBP-CorV and CoronaVac were the most frequently selected vaccines, while Gam-COVID-Vac was the least selected. It was also revealed that only 4% of the study participants were unvaccinated individuals. This data shows that vaccine acceptance was higher in the participants. Several studies have reported that vaccine acceptance is associated with awareness and knowledge of individuals about the disease and its vaccination (33). Furthermore, the acceptance of any particular vaccination is also directly associated with the strong acceptance of the vaccine (34). It is recommended that easy vaccination techniques that are comfortable for the public and enhanced interactions between patients or caregivers and healthcare staff are useful strategies for increasing vaccine acceptability (35).
This study also analyzed different side effects that were reported after the first and second doses of vaccines. Fever, pain at the injection site, muscle pain, fatigue, itching, redness and swelling, headache, joint pain, cough, nausea/vomiting, diarrhea, tingling, shortness of breath, swollen lymph nodes and anaphylactic reaction among them; pain at injection site showed the highest percentage (37.16%) and cough showed the lowest percentage (1.16%) after first dose of BBIBP-CorV. Fever showed the highest percentage (34.35%), and cough showed the lowest percentage (1.01%) after the second dose of the BBIBP-CorV. Pain at the injection site showed the highest percentage (41.14%) and cough showed the lowest percentage (1.79%) after the first dose of the CoronaVac vaccine. Fever showed the highest percentage (30.17%), and cough showed the lowest percentage (1.21%) after the second dose of the CoronaVac vaccine. Fever showed the highest percentage (47.7%) and cough showed the lowest percentage (4.44%) after the first dose of the ChAdOx1-S vaccine. Fever showed the highest percentage (48.23%) and cough showed the lowest percentage (1.17%) after the second dose of the ChAdOx1-S vaccine. Pain at the injection site showed the highest percentage (45.53%) and cough and joint pain showed the lowest percentage (2.23%) after the first dose of the BNT162b2 vaccine. Fever showed the highest percentage (43.15%) and cough showed the lowest percentage (0.29%) after the second dose of the BNT162b2 vaccine. Fever showed the highest percentage (65.16%) and cough showed the lowest percentage (3.37%) after the first dose of the mRNA-1273 vaccine. Fever showed the highest percentage (65.47%), and cough showed the lowest percentage (1.19%) after the second dose of the mRNA-1273 vaccine. Fever showed the highest percentage (53.57%), no symptoms (3.57%), cough (10.71%), and joint pain (0%) after the first dose of the Gam-COVID-Vac. Fever showed the highest percentage (42.30%), and cough showed the lowest percentage (3.84%) after the second dose of Gam-COVID-Vac. Data from several studies have reported that the COVID-19 vaccines, like other vaccines, can have some side effects including myalgia, malaise, fever, and headache and these symptoms generally resolve within a few days without causing any severe damage to the body (36, 37).
The majority of adverse reactions lasting for 25 to 72 h were for BBIBP-CorV (52.26%) and CoronaVac (47.53%) vaccinations, respectively, while symptoms persisting for more than 72 h were 4.29 and 4.82%. Similar trends were observed with the ChAdOx1-S and BNT162b2 vaccines, with adverse effects lasting 25 to 72 h being the most common (60 and 59.49%, respectively), and those lasting more than 72 h being the least common (8.8 and 9.49%). These data highlight the temporal heterogeneity of side effects among COVID-19 vaccinations, allowing for a deeper comprehension of the immunogenic features of these vaccines.
Limitation and strength
Like every study, this study also had some limitations. Causal inferences cannot be made due to the cross-sectional nature of the study. The study only included participants from the Islamabad-Rawalpindi of Pakistan, limiting the findings’ generalizability. However, Islamabad-Rawalpindi is the fourth-largest resident area in Pakistan. Thus, it is expected that the outcomes in the other areas would not differ considerably. The current study also depends on self-reported data, which is susceptible to bias. However, despite this limitation, we believe that our findings are sound and provide baseline data that will assist healthcare policymakers and researchers in the future. This is the first study in Pakistan with a significant sample size on the side effects of COVID-19 vaccinations in the general population. This study also highlighted the need for multicenter studies in another region of Pakistan to further explore the COVID-19 side effects scenario.
Conclusion
This study provided data about the side effects experienced by individuals in twin cities of Pakistan after being administered with the first and second doses of COVID-19 vaccines. It is concluded that the most prevalent side effects experienced were pyrexia or fever followed by headache and chills. CoronaVac was the most common choice for vaccine followed by BBIBP-CorV and ChAdOx1-S. The most prevalent precautionary medicines that were taken by individuals to minimize or avoid COVID-19 infection were vitamins and paracetamol. The longest time for a side effect to be present was 24–48 h. Therefore, it is concluded that the vaccine’s side effects are mild to moderate, and it is safe to administer and take multiple doses of COVID-19 vaccine to prevent the disease from spreading and to develop immunity from the virus. However, there is a need to perform further studies on larger populations that can cross-verify the consistency of these results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the National Institute of Health, Islamabad, Pakistan, and Rawalpindi Medical University, Rawalpindi, Pakistan (approved on 19 August 2020, with the reference number 163/IREF/RMU-2020) (Supplementary Data Sheet 2).
Author contributions
SZ: Investigation, Project administration, Software, Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HQ: Conceptualization, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. IQ: Conceptualization, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. ZK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. TI: Validation, Visualization, Writing – original draft, Writing – review & editing. NA: Data curation, Methodology, Writing – original draft, Writing – review & editing. KH: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. TS: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. SA: Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Validation, Visualization, Writing – original draft, Writing – review & editing. SJ: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. AI: Resources, Visualization, Writing – original draft, Writing – review & editing. HA: Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1420291/full#supplementary-material
References
1. Zhu, H, Wei, L, and Niu, P. The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy. (2020) 5:1–3. doi: 10.1186/s41256-020-00135-6
2. Singhal, T. A review of coronavirus Disease-2019 (COVID-19). Indian J Pediatr. (2020) 87:281–6. doi: 10.1007/s12098-020-03263-6
3. Shrestha, AB, Nawaz, MH, Shrestha, S, and Pokharel, P. Poliomyelitis amidst COVID-19 pandemic in Pakistan: A perspective. Ann Med Surg. (2023) 85:333–4. doi: 10.1097/MS9.0000000000000168
4. Burkholder, B, Wadood, Z, Kassem, AM, Ehrhardt, D, and Zomahoun, D. The immediate impact of the COVID-19 pandemic on polio immunization and surveillance activities. Vaccine. (2023) 41:A2–A11. doi: 10.1016/j.vaccine.2021.10.028
5. Güner, R, and Hasanoğlu, I. COVID-19: prevention and control measures in community. Turk J Med Sci. (2020) 50:571–7. doi: 10.3906/sag-2004-146
6. Al-Shammari, AA, Ali, H, Alahmad, B, Al-Refaei, FH, Al-Sabah, S, Jamal, MH, et al. The impact of strict public health measures on COVID-19 transmission in developing countries: the case of Kuwait. Front Public Health. (2021) 9:757419. doi: 10.3389/fpubh.2021.757419
7. Ur Rehman, K, Andleeb, S, Alfarraj, S, Ali Alharbi, S, and Mahmood, A. Assessment of risk management and control measures against coronavirus disease. Saudi J Biol Sci. (2021) 28:3013–20. doi: 10.1016/j.sjbs.2021.02.042
8. Khalid, A, and Ali, S. COVID-19 and its challenges for the healthcare system in Pakistan. Asian Bioeth Rev. (2020) 12:551–64. doi: 10.1007/s41649-020-00139-x
9. Lam, S, Lombardi, A, and Ouanounou, A. COVID-19: a review of the proposed pharmacological treatments. Eur J Pharmacol. (2020) 886:173451. doi: 10.1016/j.ejphar.2020.173451
10. Skafle, I, Nordahl-Hansen, A, Quintana, DS, Wynn, R, and Gabarron, E. Misinformation about COVID-19 vaccines on social media: rapid review. J Med Internet Res. (2022) 24:e37367. doi: 10.2196/37367
11. Megha, KB, Nayar, SA, and Mohanan, PV. Vaccine and vaccination as a part of human life: in view of COVID-19. Biotechnol J. (2022) 17:e2100188. doi: 10.1002/biot.202100188
12. Kanozia, R, and Arya, R. Fake news”, religion, and COVID-19 vaccine hesitancy in India, Pakistan, and Bangladesh. Media Asia. (2021) 48:313–21. doi: 10.1080/01296612.2021.1921963
13. Dubé, È, Ward, JK, Verger, P, and MacDonald, NE. Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health. Annu Rev Public Health. (2021) 42:175–91. doi: 10.1146/annurev-publhealth-090419-102240
14. Briand, SC, Cinelli, M, Nguyen, T, Lewis, R, Prybylski, D, Valensise, CM, et al. Infodemics: a new challenge for public health. Cell. (2021) 184:6010–4. doi: 10.1016/j.cell.2021.10.031
15. Our World in Data. (2022). Coronavirus (COVID-19) vaccinations. Available online at: https://ourworldindata.org/covid-vaccinations.
16. Anand, P, and Stahel, VP. The safety of COVID-19 mRNA vaccines: a review. Patient Saf Surg. (2021) 15:1–9. doi: 10.1186/s13037-021-00291-9
17. Al-Qazaz, HK, and Al-Obaidy, LM. COVID-19 vaccination, do women suffer from more side effects than men? A retrospective cross-sectional study. Pharm Pract. (2022) 20:01–10. doi: 10.18549/PharmPract.2022.2.2678
18. Alemayehu, A, Demissie, A, Yusuf, M, Abdullahi, Y, and Abdulwehab, R. COVID-19 vaccine side effect: age and gender disparity in adverse effects following the first dose of AstraZeneca COVID-19 vaccine among the vaccinated population in eastern Ethiopia: a community-based study. SAGE Open Med. (2022) 10:20503121221108616. doi: 10.1177/20503121221108616
19. Cordina, M, and Lauri, MA. Attitudes towards COVID-19 vaccination, vaccine hesitancy and intention to take the vaccine. Pharm Pract. (2021) 19:2317. doi: 10.18549/PharmPract.2021.1.2317
20. Cascini, F, Pantovic, A, Al-Ajlouni, Y, Failla, G, and Ricciardi, W. Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: a systematic review. EClinicalMedicine. (2021) 40:101113. doi: 10.1016/j.eclinm.2021.101113
21. Riad, A, and Pokorná, A. Prevalence of COVID-19 vaccine side effects among healthcare Workers in the Czech Republic. J Clin Med. (2021) 10:71428. doi: 10.3390/jcm10071428
22. World Population Review. (2021). Islamabad Population Data. Available online at: https://worldpopulationreview.com/cities/pakistan/islamabad (Accessed April 17, 2022).
23. World Population Review. (2021). Rawalpindi Population Data. Available online at: https://worldpopulationreview.com/cities/pakistan/rawalpindi (Accessed April 17, 2022).
24. Raosoft. (2025). Sample size calculator. Available online at: http://www.raosoft.com/samplesize.html (Accessed January 3, 2025).
25. Galagali, PM, and Kinikar, AA. Vaccine hesitancy: obstacles and challenges. Curr Pediatr Rep. (2022) 10:241–8. doi: 10.1007/s40124-022-00278-9
26. Abbas, S, Abbas, B, Amir, S, and Wajahat, M. Evaluation of adverse effects with COVID-19 vaccination in Pakistan. Pak J Med Sci. (2021) 37:1959–64. doi: 10.12669/pjms.37.7.4522
27. Patterson, T, Isales, CM, and Fulzele, S. Low level of vitamin C and dysregulation of vitamin C transporter might be involved in the severity of COVID-19 infection. Aging Dis. (2021) 12:14–26. doi: 10.14336/AD.2020.0918
28. Shahbaz, U, Fatima, N, Basharat, S, Bibi, A, Yu, X, Hussain, MI, et al. Role of vitamin C in preventing of COVID-19 infection, progression, and severity. AIMS Microbiol. (2022) 8:108–24. doi: 10.3934/microbiol.2022010
29. Bishara, D, Kalafatis, C, and Taylor, D. Emerging and experimental treatments for COVID-19 and drug interactions with psychotropic agents. Ther Adv Psychopharmacol. (2020) 10:2045125320935306. doi: 10.1177/2045125320935306
30. Varghese, GM, John, R, Manesh, A, Karthik, R, and Abraham, OC. Clinical management of COVID-19. Indian J Med Res. (2020) 151:401–10. doi: 10.4103/ijmr.IJMR_957_20
31. Joseph, AM, Fernandez, V, Kritzman, S, Eaddy, I, Cook, OM, Lambros, S, et al. COVID-19 misinformation on social media: a scoping review. Cureus. (2022) 14:e24601. doi: 10.7759/cureus.24601
32. Bin Naeem, S, and Kamel Boulos, MN. COVID-19 misinformation online and health literacy: a brief overview. Int J Environ Res Public Health. (2021) 18:8091. doi: 10.3390/ijerph18158091
33. Joshi, A, Kaur, M, Kaur, R, Grover, A, Nash, D, and El-Mohandes, A. Predictors of COVID-19 vaccine acceptance, intention, and hesitancy: a scoping review. Front Public Health. (2021) 9:698111. doi: 10.3389/fpubh.2021.698111
34. Roy, DN, Biswas, M, Islam, E, and Azam, MS. Potential factors influencing COVID-19 vaccine acceptance and hesitancy: a systematic review. PLoS One. (2022) 17:e0265496. doi: 10.1371/journal.pone.0265496
35. Habersaat, KB, and Jackson, C. Understanding vaccine acceptance and demand-and ways to increase them. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2020) 63:32–9. doi: 10.1007/s00103-019-03063-0
36. Sprent, J, and King, C. COVID-19 vaccine side effects: the positives about feeling bad. Sci Immunol. (2021) 6:p. eabj9256. doi: 10.1126/sciimmunol.abj9256
Keywords: vaccine, precautionary medication, immunization, infodemic, side effects, COVID-19, Pakistan
Citation: Zaidi S, Qayyum HA, Qayyum IA, Khan Z, Islam T, Ahmed N, Hopkins KL, Sommers T, Akhtar S, Khan SA, Javed S, Ikram A and Akhtar H (2025) COVID-19 vaccines side effects among the general population during the pandemic: a cross-sectional study. Front. Public Health. 13:1420291. doi: 10.3389/fpubh.2025.1420291
Edited by:
Ritthideach Yorsaeng, Chulalongkorn University, ThailandReviewed by:
Larry Ellingsworth, Novavax, Inc., United StatesRamzi Mukred Saeed, University of Marburg, Germany
Zamira Shabani, Luigj Gurakuqi University of Shkodër, Albania
Copyright © 2025 Zaidi, Qayyum, Qayyum, Khan, Islam, Ahmed, Hopkins, Sommers, Akhtar, Khan, Javed, Ikram and Akhtar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hashaam Akhtar, SGFzaGFhbWFraHRhckBnbWFpbC5jb20=
†ORCID: Hashaam Akhtar, https://orcid.org/0000-0003-1913-831X
 Samana Zaidi1
Samana Zaidi1 Zakir Khan
Zakir Khan Naveed Ahmed
Naveed Ahmed Shahzad Ali Khan
Shahzad Ali Khan Sumbal Javed
Sumbal Javed Aamer Ikram
Aamer Ikram Hashaam Akhtar
Hashaam Akhtar