
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Public Health , 15 January 2025
Sec. Radiation and Health
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1517147
This article is part of the Research Topic Advances in Radiation Research and Applications: Biology, Environment and Medicine View all 7 articles
A correction has been applied to this article in:
Corrigendum: Physical parameters and biological factors affect the abscopal effect of combining radiotherapy with immunotherapy: an update on preclinical works
 Wangcai Ren1†
Wangcai Ren1† Jialing Wen2†
Jialing Wen2† Gang Guo1,2
Gang Guo1,2 Wenchao Gu3
Wenchao Gu3 Shenke Zhang4,5
Shenke Zhang4,5 Chang Liu6,7
Chang Liu6,7 Kensuke Osada8
Kensuke Osada8 Takashi Shimokawa6
Takashi Shimokawa6 Qiaojuan Wang1,2
Qiaojuan Wang1,2 Yue Wang1,2
Yue Wang1,2 Xuanzhang Tu1
Xuanzhang Tu1 Chen Li9*
Chen Li9* Li Sui2*
Li Sui2* Liqiu Ma1,5,8*
Liqiu Ma1,5,8*In the process of radiotherapy for cancer patients, there is an extremely low probability phenomenon that the distal tumor/metastasis away from the irradiation field undergoes regression after localized radiation therapy, which is called the abscopal effect. Enhancing the incidence of this phenomenon possesses profound significance for the investigation of metastatic cancer treatment. Currently, the underlying mechanisms of the abscopal effect remain unclear. Radiation-induced immunogenic cell death is considered one of the potential mechanisms for the abscopal effect. From this perspective, we explored how physical parameters and biological factors influence this process. Differences between patients with respect to physical factors and intrinsic biological factors that activate the immune response (acquired factors) may affect the induction of the abscopal effect.
Cancer is one of the most significant diseases affecting human health. According to estimates by the International Agency for Research on Cancer (IARC), one in five individuals worldwide are projected to develop cancer in their lifetime, thus making cancer prevention one of the important public health challenges of the 21st century (1). Radiotherapy, which is recognized as one of the main types of oncological treatment, primarily exerts its tumoricidal effects by disrupting the DNA double-strand of cancer cells, thereby achieving effective control over local tumors (2). The efficacy of radiotherapy for distant metastatic cancers is limited; however, strategies that induce the abscopal effect may increase this efficacy (3, 4).
The abscopal effect, which was first proposed by Mole (5), refers to the phenomenon where in several patients treated with radiotherapy, the volume of distant tumors that are not located within the irradiation field is significantly reduced or tumor regression occurs following local tumor irradiation. Numerous scholars have explored this phenomenon (6–8). A preclinical study in animals indicated that this effect was tumor-specific and not observed in immunodeficient nude mice, thus revealing the involvement of T cells in the mechanism underlying this effect (7). A case report on cutaneous melanoma in 2012 further increased interest in this phenomenon. In this report, after treatment with ipilimumab, the patient’s metastatic chest lesions were subjected to palliative radiotherapy totaling 2,850 cGy; at two months post-radiotherapy, a reduction in the metastatic lesions located outside of the irradiation field was observed. Additionally, changes in the proportions of immune cells and the levels of cell surface antigens in the blood were detected, thereby indicating an enhanced antitumor immune response (8). Thus, the activation of the antitumor immune response may be the key to the induction of the abscopal effect via radiotherapy.
Immunotherapy is known to work synergistically with other therapies to improve treatment efficacy, and RT combined with immunotherapy has been shown to enhance anti-tumor activity (9–11). Active immunotherapy, which enhances anti-tumor immunity by activating the patient’s immune system, has been shown to increase the incidence of the abscopal effect when combined with radiotherapy (12, 13). However, due to the unclear mechanism of the abscopal effect, the key factors that increase the probability of inducing the abscopal effect have yet to be identified.
From this mini review, we discuss the physical and biological factors affecting the abscopal effect and propose potential mechanisms for its induction. Our aim is to provide a scientific basis for optimizing radiotherapy-immunotherapy combination therapy protocols aimed at curing metastatic cancers.
Numerous preclinical studies have revealed the significant impact of various radiation factors, including radiation dose and fractionation, on the efficiency of the abscopal effect. Formenti et al. (14) underscored the importance for researchers to focus on the investigation of the optimal radiation dose and fractionation. A preclinical study performed by the team of the National Institutes for Quantum Science and Technology reported that there is an optimal dose range for the abscopal effect generated with carbon-ion radiotherapy combined with an anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody (15). Yamamoto et al. (16) utilized a mouse model of osteosarcoma to investigate the antitumor effects of photon beam (180 kV, 15 mA) irradiation at different doses and fractions (8 Gy × 3, or 16 Gy × 1) combined with an anti-CTLA-4 antibody on both local and distant tumors. These findings indicated that the combination of high-dose photon beam irradiation with an anti-CTLA-4 antibody significantly enhances the antitumor effect on distant non-irradiated tumors and prolongs the overall survival of mice. Ghaffari-Nazari et al. (17) used a mouse model with a bilateral CT26 colon cancer tumor to assess the impact of different photon beam (6 MV, 3.5 Gy/min) radiation schemes (16 Gy × 1, 10 Gy × 2, and 3 Gy × 10) at biologically equivalent doses on distant non-irradiated tumors. Their study revealed that single high-dose radiotherapy combined with an anti-programmed cell death-ligand 1 (PD-L1) antibody may be the most effective strategy to induce the abscopal effect. However, some preclinical studies have noted that although low-dose photon irradiation is insufficient to directly kill tumors, it can activate and alter the immune environment and modulate the tumor stroma, thereby increasing the efficacy of immunotherapy (18–20). To delve deeper into the influence of dose fractionation on the probability of inducing the abscopal effect, Dewan et al. (21) compared the therapeutic outcomes of different photon beam (6 MV, 600 cGy/min) radiation protocols (20 Gy × 1, 8 Gy × 3, or 6 Gy × 5) in a bilateral tumor-bearing mouse model developed by using murine mammary carcinoma (TSA) cells. Research has revealed that radiotherapy results in comparable control over the primary tumor when combined with an anti-CTLA-4 antibody. Notably, only the fractionated treatments (8 Gy × 3, 6 Gy × 5) successfully induced the abscopal effect. This may be due to the fact that fractionated doses promote the proliferation of effector T cells and reduce regulatory T cell levels, thereby enhancing systemic anti-tumor immunity (22).
In addition to the radiation dose and fractionation, the radiation dose rate is also an important radiation physical parameter. A preclinical study performed by Tinganelli et al. reported that carbon-ion irradiation in ultra-high dose rate (FLASH, >40 Gy/s) reduces lung metastasis in an osteosarcoma mouse model (23). Whether the irradiation dose rate is one of the key factors affecting the induction of the abscopal effect requires further verification by subsequent experiments in the future. Moreover, Liu et al. investigated the abscopal effect induced by different qualities of radiation in a Lewis lung adenocarcinoma tumor-bearing mouse model and reported that, compared with photon radiation, carbon-ion radiation significantly enhanced the abscopal effect (24). These studies suggest that the abscopal effect can be more effectively induced by selecting appropriate radiation physical parameters (such as radiation dose, fractionation, dose rates, and radiation quality, among other factors) for treatment.
The induction of the abscopal effect is not only influenced by physical parameters but also significantly affected by biological factors, which can be divided into intrinsic and acquired factors. Intrinsic factors include the genetic background of the host and tumor and the level of tumor immunogenicity, among other factors. These factors form the basis for individual variability in responses to treatment. Acquired factors, on the other hand, involve changes that are induced by therapeutic interventions such as radiotherapy and immunotherapy. For example, radiotherapy can increase the immunogenicity of cancer cells, whereas immunotherapy can activate a systemic antitumor immune response.
As one of the most advanced immunotherapeutic approaches, immune checkpoint inhibitors target checkpoint pathways such as the CTLA-4 and PD-1/PD-L1 pathways, thus promoting the body’s production of an effective antitumor immune response and preventing the immune evasion of tumors. Studies have reported synergistic enhancement of the abscopal effect when immune checkpoint inhibitors are combined with localized radiotherapy (8, 25, 26). The systemic antitumor effect of radiation typically relies on the activation of tumor-specific CD8+ T cells initiated by antigen-presenting cells (APCs). Immunotherapeutic strategies that enhance APC activation are also commonly employed to augment the abscopal effect (27).
The host’s genetic background significantly influences the efficacy of radiotherapy combined with immunotherapy against cancer metastasis. A preclinical study based on carbon-ion radiotherapy combined with dendritic cells (DCs, which are a type of APC) demonstrated that the host’s genetic background has a substantial effect on the effectiveness of combined therapy. Specifically, the therapy effectively suppressed lung metastasis in Th1-dominant mice but was less effective in Th2-dominant mice (28).
The impact of tumor’s genetic background on the abscopal effect has also been investigated. Strigari et al. (29) reported that the abscopal effect induced by photon radiotherapy may depend on the status of the tumor suppressor p53 within the tumor. In an experiment involving the transplantation of wild-type (wt)-p53 or p53-null HCT116 human colon cancer cells into athymic female nude mice, after 20 Gy electron irradiation of the local tumor, non-irradiated wt-p53 distant tumors exhibited significant inhibition of tumor growth, whereas p53-null tumors did not show a noticeable difference.
Furthermore, the immunogenicity of the tumor may also influence the induction of the abscopal effect. Lai et al. (30) categorized tumors into high or low immunogenicity groups based on the number of infiltrating immune cells and the expression of MHC-I molecules. They evaluated different tumor models with varying degrees of immunogenicity (including high and low immunogenicity) via sham irradiation, a single dose of 15 Gy, and three fractions of 5 Gy. The results revealed a positive correlation between tumor immunogenicity and the abscopal effect of radiation therapy. In highly immunogenic tumors (colorectal carcinoma MC38 cells and OVA-expressing EL4 thymic lymphoma E.G7-OVA cells), a single dose of 15 Gy radiation effectively induced the abscopal effect. In poorly immunogenic tumors (Lewis lung carcinoma LL/2 cells and melanoma B16-F10 cells), radiation therapy failed to induce the abscopal effect.
In conclusion, the efficiency of combination therapy in inducing the abscopal effect may be significantly influenced by biological factors such as the genetic background of the host or tumor. Therefore, it is imperative to fully consider the role of these biological factors before initiating clinical studies on the combination of radiotherapy and immunotherapy.
The influence of physical and biological factors on the occurrence of the abscopal effect remains an enigmatic subject. Scholars are persistently employing various mouse models to investigate the mechanisms by which radiation therapy induces the abscopal effect, with the goal of elucidating the mechanism of this effect. It is widely accepted that the immune response induced by radiation, which is known as immunogenic cell death (ICD), may be one of the underlying mechanisms for the abscopal effect (31). Radiotherapy can indirectly kill cancer cells by inducing tumor-targeted immune responses. When tumor cells are irradiated and enter the death phase, they transition from a non-immunogenic to an immunogenic state, thereby triggering an antitumor immune response in the body. This process is referred to as ICD (32).
During the occurrence of ICD, cancer cells express or release damage-associated molecular patterns (DAMPs), such as calreticulin (CRT), high mobility group protein 1 (HMGB1) and adenosine 5′-triphosphate (ATP), on the cell membrane or release them extracellularly. CRT, which is located on the endoplasmic reticulum, moves to the cell membrane surface as a result of radiation stress and binds to the CD91 receptor on APCs, thereby releasing an “eat me” signal. ATP, which is distributed in the cytoplasmic matrix, is released from inside of the cell to the outside of the cell after radiation, after which it binds to the P2RX7 receptor and functions as a “find me” signal, thereby recruiting dendritic cells, macrophages, and other phagocytic cells to gather at the tumor site. HMGB1 is a non-histone chromatin-binding protein located in the cell nucleus that interacts with DNA and regulates gene transcription. When the cell enters the death phase, HMGB1 is released from the nucleus into the outside, and extracellular HMGB1 activates the corresponding signaling pathways by binding to different pattern recognition receptors (such as TLR2, TLR4, and RAGE), thus promoting the maturation of DCs. Mature DCs “teach” cytotoxic T cells after phagocytosing antigens, which allows them to surveil and eliminate tumors. Additionally, natural killer (NK) cells that are recruited during the ICD process can kill cancer cells by releasing granzymes and perforins (32–34). The in vivo antitumor immune activation response that is elicited by radiotherapy plays a vital role throughout the body, thus benefiting patients with metastatic cancer (35, 36). Furthermore, this process is an organic process in which physical factors mediate the response to acquired factors in the body. The increase in the immunogenicity of cancer cells mediated by radiotherapy further promotes the activation of the systemic antitumor immune response, which may induce an abscopal effect, as shown in Figure 1.
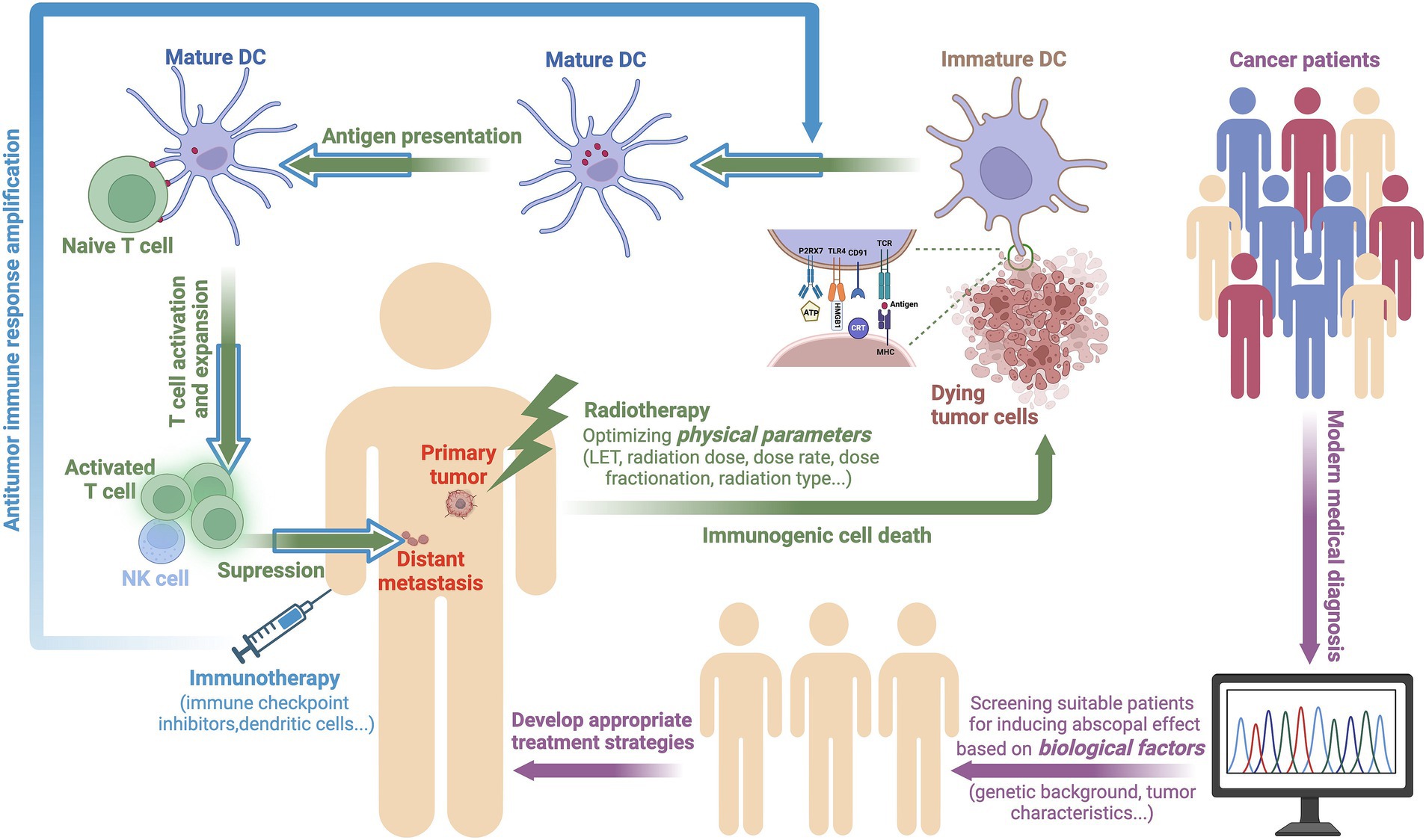
Figure 1. A potential mechanism by which physical and biological factors affect the occurrence of the abscopal effect. For patients possessing abscopal effects that are likely to be induced according to genetic backgrounds and tumor characteristics, optimized radiotherapy may be able to induce ICD in cancer cells, thereby triggering an antitumor immune response. During ICD, cancer cells release DAMPs, such as CRT, HMGB1, and ATP under radiation-induced stress. These DAMPs promote the maturation of DCs, thereby activating T cells to surveil and eliminate tumors. Immunotherapy further amplifies this antitumor immune response, thus extending its therapeutic impact (created with BioRender.com).
A substantial body of preclinical research has indicated that different physical parameters of radiation can have varying impacts on the activation of the biological body’s acquired immune response (Table 1); specifically, different physical parameters can affect the expression levels of DAMPs from tumor cells. Onishi et al. (37) irradiated two types of human cervical cancer cells (HeLa and SiHa) and human esophageal squamous cancer cells (KYSE70) with carbon ion (C-ion) beams at the same biologically equivalent dose but with different LET values (13 keV/μm or 70 keV/μm), and the results revealed that the expression level of HMGB1 in the culture medium at 72 h after irradiation increased with increasing LET. Moreover, the expression levels of DAMPs in various cancer cells exposed to photons (X-ray and γ-ray) and proton radiation were observed to change in a dose-dependent manner, whereas there appears to be an optimal dose range in which C-ion radiation induces the maximum expression of DAMPs (38–40).
In addition to dose and LET, radiation type also influences the release of DAMPs. Huang et al. (38) conducted a comparative analysis of CRT following X-ray, proton beam, and C-ion beam irradiation of human tumor cells. These results demonstrated that all forms of radiation led to increased CRT on the cell membrane. Notably, the CRT induced by C-ion beam was most pronounced at the same physical dose (41). Similarly, C-ion beam exposure can induce greater expression of CRT than can photons or protons at the same equivalent biological dose (within a certain range). However, the situation appears to be different for HMGB1. Multiple studies have shown that, compared with X-rays, C-ions are equally effective at inducing HMGB1 release at iso-survival doses (41, 42).
In addition, the biological factors of the body itself can also affect the activation of the acquired immune response under the same radiation physical parameters; specifically, at the same dose and type of radiation, the expression levels of immune-related molecules on the surfaces of tumor cells with different genetic backgrounds still differ. Huang et al. (38) compared CRT exposure on the cell membrane after irradiation of human tumor cells with photons, protons, and carbon ions and reported that the increase in CRT on the cell membrane is tumor dependent. Radiosensitive tumor cells, such as nasopharyngeal carcinoma (CNE-2) and tongue squamous cell carcinoma (Tca-8113) cells, have greater CRT expression on the plasma membrane after radiotherapy than do radioresistant tumor cells, such as glioblastoma (U251) and lung adenocarcinoma (A549) cells; that is, the expression level of immunogenicity-related molecules is affected by the characteristics of the tumor. Such differences may be due to the differences in the immunogenicity of tumor cells under the action of radiation.
In summary, the differences in the efficacy of physical factors and biological factors themselves in activating the acquired immune response may lead to differences in the induction of the abscopal effect in the body. In other words, the key to inducing the abscopal effect may depend on the body’s ability to produce an acquired immune response.
To date, research has demonstrated that tumor irradiation can activate an antitumor immune response within the body. However, there is no evidence to suggest that radiotherapy alone can stably induce the abscopal effect (43, 44). The probability of inducing this effect can be increased by combining radiotherapy with immunotherapy, thereby prolonging the survival of patients (45). The primary task that remains in elucidating this mechanism is the determination of the optimal radiation physics parameters, including the type of radiation, dosage, and number of fractions. In addition, the sequence in which radiation and immune drugs are administered can also affect the immune landscape. For example, pretreatment with immune checkpoint inhibitors may help to prime the immune system before radiation exposure, thus potentially leading to a more robust systemic response (46, 47). Conversely, the initial administration of radiotherapy may help to create a favorable environment for subsequent immunotherapy by enhancing antigen presentation and T-cell activation (48). By incorporating contemporary medical diagnostic techniques, the biological characteristics of patients (such as genetic backgrounds and tumor features) are assessed to identify the indications for combined therapy and a suitable patient cohort. Consequently, an appropriate strategy for the integration of radiotherapy and immunotherapy can be formulated to maximize the antitumor effects of both treatment modalities (Figure 1).
Particle therapy is a novel type of efficient radiotherapy that is based on cutting-edge radiation technology. Although particle therapy is gaining traction in cancer treatment, reports on its combination with immunotherapy to induce abscopal effects remain relatively scarce. It is necessary to further strengthen the evaluation of the abscopal effect induced by particle therapy combined with immunotherapy. The exploration of the optimal treatment strategy for the combination of particle radiotherapy and immunotherapy will effectively elucidate the mechanisms underlying the abscopal effect, thus facilitating its transformation from a rare phenomenon to a stable and effective tool for the treatment of metastatic disease.
In conclusion, the combination of radiotherapy with immunotherapy demonstrates great promise for the treatment of cancer. The identification of optimal radiation parameters and the selection of suitable patients for combined therapy are critical steps toward maximizing therapeutic benefits. The potential of the use of particle therapy in combination with immunotherapy to induce the abscopal effect is a novel research field that requires further investigation. Furthermore, a more in-depth understanding of the mechanisms of the abscopal effect will provide a theoretical basis for its clinical application, thus offering a novel and potent approach to combating metastatic cancer.
WR: Software, Visualization, Writing – original draft. JW: Software, Writing – review & editing. GG: Supervision, Writing – review & editing. WG: Formal analysis, Writing – review & editing. SZ: Formal analysis, Writing – review & editing. ChaL: Formal analysis, Writing – review & editing. QW: Formal analysis, Writing – review & editing. YW: Formal analysis, Writing – review & editing. XT: Formal analysis, Writing – review & editing. CheL: Supervision, Writing – review & editing. LS: Methodology, Supervision, Writing – review & editing. LM: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing, Funding acquisition, Resources, Writing – original draft. KO: Writing – review & editing, Investigation, Resources. TS: Writing – review & editing, Investigation, Resources.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by JSPS KAKENHI 26861029 (L. Ma) and 17K16423 (L. Ma), CNNC Basic Research Foundation CNNC-JCYJ-202224 (L. Ma), CIAE President’s Strategic Grant YZ212404000801 (L. Ma), and Grant-in-Aid for Young Scientifics from Gunma University 100215001E0205040400 (L. Ma).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Santivasi, WL, and Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal. (2014) 21:251–9. doi: 10.1089/ars.2013.5668
3. Ganesh, K, and Massagué, J. Targeting metastatic cancer. Nat Med. (2021) 27:34–44. doi: 10.1038/s41591-020-01195-4
4. Link, B, Crigna, AT, Hölzel, M, Giordano, FA, and Golubnitschaja, O. Abscopal effects in metastatic Cancer: is a predictive approach possible to improve individual outcomes? J Clin Med. (2021) 10:5124. doi: 10.3390/jcm10215124
5. Mole, RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. (1953) 26:234–41. doi: 10.1259/0007-1285-26-305-234
6. Antoniades, J, Brady, LW, and Lightfoot, DA. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int J Radiat Oncol Biol Phys. (1977) 2:141–7. doi: 10.1016/0360-3016(77)90020-7
7. Demaria, S, Ng, B, Devitt, ML, Babb, JS, Kawashima, N, Liebes, L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. (2004) 58:862–70. doi: 10.1016/j.ijrobp.2003.09.012
8. Postow, MA, Callahan, MK, Barker, CA, Yamada, Y, Yuan, J, Kitano, S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. (2012) 366:925–31. doi: 10.1056/NEJMoa1112824
9. Mahmood, J, Shukla, HD, Soman, S, Samanta, S, Singh, P, Kamlapurkar, S, et al. Immunotherapy, radiotherapy, and hyperthermia: a combined therapeutic approach in pancreatic Cancer treatment. Cancers. (2018) 10:469. doi: 10.3390/cancers10120469
10. Hiniker, SM, Maecker, HT, and Knox, SJ. Predictors of clinical response to immunotherapy with or without radiotherapy. J Radiat Oncol. (2015) 4:339–45. doi: 10.1007/s13566-015-0219-2
11. van der Burg, SH, Arens, R, Ossendorp, F, van Hall, T, and Melief, CJM. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. (2016) 16:219–33. doi: 10.1038/nrc.2016.16
12. Dagoglu, N, Karaman, S, Caglar, HB, and Oral, EN. Abscopal effect of radiotherapy in the immunotherapy era: systematic review of reported cases. Cureus. (2019) 11:e4103. doi: 10.7759/cureus.4103
13. Ngwa, W, Irabor, OC, Schoenfeld, JD, Hesser, J, Demaria, S, and Formenti, SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. (2018) 18:313–22. doi: 10.1038/nrc.2018.6
14. Formenti, SC. Optimizing dose per fraction: a new chapter in the story of the Abscopal effect? Int J Radiat Oncol. (2017) 99:677–9. doi: 10.1016/j.ijrobp.2017.07.028
15. Ma, L, Li, Y, Sakamoto, Y, Xie, L, Suzuki, S, Yoshida, Y, et al. Optimal radiation dose to induce an abscopal effect by combining carbon-ion radiotherapy and anti-CTLA4 antibody. Neoplasia. (2025) 60:101099. doi: 10.1016/j.neo.2024.101099
16. Yamamoto, J, Takahashi, Y, Minami, K, Tamari, K, Katsuki, S, Takenaka, W, et al. High dose local photon irradiation is crucial in anti-CTLA-4 antibody therapy to enhance the Abscopal response in a murine pancreatic carcinoma model. Cancers. (2022) 14:2087. doi: 10.3390/cancers14092087
17. Ghaffari-Nazari, H, Alimohammadi, M, Alimohammadi, R, Rostami, E, Bakhshandeh, M, Webster, TJ, et al. Radiation dose and schedule influence the abscopal effect in a bilateral murine CT26 tumor model. Int Immunopharmacol. (2022) 108:108737. doi: 10.1016/j.intimp.2022.108737
18. Klug, F, Prakash, H, Huber, PE, Seibel, T, Bender, N, Halama, N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. (2013) 24:589–602. doi: 10.1016/j.ccr.2013.09.014
19. De Palma, M, Coukos, G, and Hanahan, D. A new twist on radiation oncology: low-dose irradiation elicits immunostimulatory macrophages that unlock barriers to tumor immunotherapy. Cancer Cell. (2013) 24:559–61. doi: 10.1016/j.ccr.2013.10.019
20. Arnold, KM, Flynn, NJ, Raben, A, Romak, L, Yu, Y, Dicker, AP, et al. The impact of radiation on the tumor microenvironment: effect of dose and fractionation schedules. Cancer Growth Metastasis. (2018) 11:1179064418761639. doi: 10.1177/1179064418761639
21. Dewan, MZ, Galloway, AE, Kawashima, N, Dewyngaert, JK, Babb, JS, Formenti, SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res Off J Am Assoc Cancer Res. (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
22. Buchwald, ZS, Wynne, J, Nasti, TH, Zhu, S, Mourad, WF, Yan, W, et al. Radiation, immune checkpoint blockade and the Abscopal effect: a critical review on timing, Dose and Fractionation. Front Oncol. (2018) 8:612. doi: 10.3389/fonc.2018.00612
23. Tinganelli, W, Weber, U, Puspitasari, A, Simoniello, P, Abdollahi, A, Oppermann, J, et al. FLASH with carbon ions: tumor control, normal tissue sparing, and distal metastasis in a mouse osteosarcoma model. Radiother Oncol. (2022) 175:185–90. doi: 10.1016/j.radonc.2022.05.003
24. Liu, R, Geng, Y, Luo, H, Zhang, Q, Yang, Z, Sun, S, et al. Carbon ion irradiation combined with PD-1 inhibitor trigger abscopal effect in Lewis lung cancer via a threshold dose. J Cancer. (2023) 15:2245. doi: 10.21203/rs.3.rs-2871494/v1
25. Golden, EB, Demaria, S, Schiff, PB, Chachoua, A, and Formenti, SC. An Abscopal response to radiation and Ipilimumab in a patient with metastatic non-small cell lung Cancer. Cancer Immunol Res. (2013) 1:365–72. doi: 10.1158/2326-6066.CIR-13-0115
26. Twyman-Saint Victor, C, Rech, AJ, Maity, A, Rengan, R, Pauken, KE, Stelekati, E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. (2015) 520:373–7. doi: 10.1038/nature14292
27. Younes, AI, Barsoumian, HB, Sezen, D, Verma, V, Patel, R, Wasley, M, et al. Addition of TLR9 agonist immunotherapy to radiation improves systemic antitumor activity. Transl Oncol. (2021) 14:100983. doi: 10.1016/j.tranon.2020.100983
28. Ma, L, Sakamoto, Y, Ando, K, Fujita, H, Takahashi, A, Takeshima, T, et al. Th balance-related host genetic background affects the therapeutic effects of combining carbon-ion radiation therapy with dendritic cell immunotherapy. Int J Radiat Oncol Biol Phys. (2022) 112:780–9. doi: 10.1016/j.ijrobp.2021.10.141
29. Strigari, L, Mancuso, M, Ubertini, V, Soriani, A, Giardullo, P, Benassi, M, et al. Abscopal effect of radiation therapy: interplay between radiation dose and p53 status. Int J Radiat Biol. (2014) 90:248–55. doi: 10.3109/09553002.2014.874608
30. Lai, J-Z, Zhu, Y-Y, Liu, Y, Zhou, L-L, Hu, L, Chen, L, et al. Abscopal effects of local radiotherapy are dependent on tumor immunogenicity. Front Oncol. (2021) 11:690188. doi: 10.3389/fonc.2021.690188
31. Tang, J, Malachowska, B, Wu, X, and Guha, C. Repurposing radiation therapy for Immuno-oncology. Clin Oncol. (2021) 33:683–93. doi: 10.1016/j.clon.2021.08.015
32. Garg, AD, Dudek-Peric, AM, Romano, E, and Agostinis, P. Immunogenic cell death. Int J Dev Biol. (2015) 59:131–40. doi: 10.1387/ijdb.150061pa
33. Kroemer, G, Galluzzi, L, Kepp, O, and Zitvogel, L. Immunogenic cell death in Cancer therapy. Annu Rev Immunol. (2013) 31:51–72. doi: 10.1146/annurev-immunol-032712-100008
34. Ma, L. From photon beam to accelerated particle beam: Antimetastasis effect of combining radiotherapy with immunotherapy. Front Public Health. (2022) 10:847119. doi: 10.3389/fpubh.2022.847119
35. McLaughlin, M, Patin, EC, Pedersen, M, Wilkins, A, Dillon, MT, Melcher, AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. (2020) 20:203–17. doi: 10.1038/s41568-020-0246-1
36. Keisari, Y, and Kelson, I. The potentiation of anti-tumor immunity by tumor abolition with alpha particles, protons, or carbon ion radiation and its enforcement by combination with Immunoadjuvants or inhibitors of immune suppressor cells and checkpoint molecules. Cells. (2021) 10:228. doi: 10.3390/cells10020228
37. Onishi, M, Okonogi, N, Oike, T, Yoshimoto, Y, Sato, H, Suzuki, Y, et al. High linear energy transfer carbon-ion irradiation increases the release of the immune mediator high mobility group box 1 from human cancer cells. J Radiat Res (Tokyo). (2018) 59:541–6. doi: 10.1093/jrr/rry049
38. Huang, Y, Dong, Y, Zhao, J, Zhang, L, Kong, L, and Lu, JJ. Comparison of the effects of photon, proton and carbon-ion radiation on the ecto-calreticulin exposure in various tumor cell lines. Ann Transl Med. (2019) 7:542. doi: 10.21037/atm.2019.09.128
39. Gameiro, SR, Jammeh, ML, Wattenberg, MM, Tsang, KY, Ferrone, S, and Hodge, JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. (2014) 5:403–16. doi: 10.18632/oncotarget.1719
40. Golden, EB, Frances, D, Pellicciotta, I, Demaria, S, Helen Barcellos-Hoff, M, and Formenti, SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Onco Targets Ther. (2014) 3:e28518. doi: 10.4161/onci.28518
41. Ando, K, Fujita, H, Hosoi, A, Ma, L, Wakatsuki, M, Seino, K, et al. Intravenous dendritic cell administration enhances suppression of lung metastasis induced by carbon-ion irradiation. J Radiat Res (Tokyo). (2017) 58:446–55. doi: 10.1093/jrr/rrx0005
42. Yoshimoto, Y, Oike, T, Okonogi, N, Suzuki, Y, Ando, K, Sato, H, et al. Carbon-ion beams induce production of an immune mediator protein, high mobility group box 1, at levels comparable with X-ray irradiation. J Radiat Res (Tokyo). (2015) 56:509–14. doi: 10.1093/jrr/rrv007
43. Janopaul-Naylor, JR, Shen, Y, Qian, DC, and Buchwald, ZS. The Abscopal effect: a review of pre-clinical and clinical advances. Int J Mol Sci. (2021) 22:11061. doi: 10.3390/ijms222011061
44. Nabrinsky, E, Macklis, J, and Bitran, J. A review of the Abscopal effect in the era of immunotherapy. Cureus. (2022) 14:e29620. doi: 10.7759/cureus.29620
45. Bernstein, MB, Krishnan, S, Hodge, JW, and Chang, JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. (2016) 13:516–24. doi: 10.1038/nrclinonc.2016.30
46. Grimaldi, AM, Simeone, E, Giannarelli, D, Palla, M, Muto, P, Falivene, S, et al. The abscopal effect: efficacy of radiotherapy in patients on progression after treatment with ipilimumab 3 mg/kg. J Immunother Cancer. (2013) 1:O23. doi: 10.1186/2051-1426-1-S1-O23
47. Nelson, BE, Adashek, JJ, Lin, SH, and Subbiah, V. On target methods to induce abscopal phenomenon for off-target effects: from happenstance to happenings. Cancer Med. (2023) 12:6451–65. doi: 10.1002/cam4.5454
Keywords: abscopal effect, physical parameters, biological factors, radiotherapy, immunogenic cell death
Citation: Ren W, Wen J, Guo G, Gu W, Zhang S, Liu C, Osada K, Shimokawa T, Wang Q, Wang Y, Tu X, Li C, Sui L and Ma L (2025) Physical parameters and biological factors affect the abscopal effect of combining radiotherapy with immunotherapy: an update on preclinical works. Front. Public Health. 12:1517147. doi: 10.3389/fpubh.2024.1517147
Received: 25 October 2024; Accepted: 20 December 2024;
Published: 15 January 2025.
Edited by:
Yi Xie, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Anggraeini Puspitasari Kokko, HollandPTC, NetherlandsCopyright © 2025 Ren, Wen, Guo, Gu, Zhang, Liu, Osada, Shimokawa, Wang, Wang, Tu, Li, Sui and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Li, bGljaGVuQG5pcnAuY2hpbmFjZGMuY24=; Li Sui, bGlzdWlAY2lhZS5hYy5jbg==; Liqiu Ma, bWEubGlxaXVAcXN0LmdvLmpw
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.