- 1Unit of Cell and Gene Therapy, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Epidemiology and Public Health Unit, Institute of Biomedical Technologies - National Research Council, Segrate (MI), Italy
- 3Dino Ferrari Center, Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy
- 4Quality Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Department of Biomedical Sciences for Health, University of Milan, Milan, Italy
Introduction: Human induced pluripotent stem cells (hiPSCs), derived from reprogrammed adult somatic cells, hold significant promise for disease modelling, personalized medicine, drug discovery, and regenerative therapies. Public awareness and understanding of hiPSCs are crucial for advancing research in this field. However, limited data exists on the general population’s knowledge and attitudes toward their use.
Methods: This study aimed to assess the awareness and perceptions of hiPSCs among Italian adults through a web-based survey conducted via the EUSurvey platform, using a snowball sampling approach. The survey included demographic information and mandatory questions on knowledge, awareness, and concerns regarding hiPSC technology, with responses collected on a 3-point scale. Statistical analysis was performed using chi-squared tests, with significance set at p ≤ 0.05.
Results: Out of 1874 respondents, the majority were aged 18–35 years (40.5%), female (63.4%), and university-educated (67.2%). Among those familiar with hiPSCs (54.1%, n = 1,201), 95.3% expressed willingness to donate blood samples for hiPSC generation to treat individuals with incurable diseases. Concerns about current research and therapeutic applications were low (less than 20%), but nearly half of the respondents were hesitant or opposed to the use of hiPSCs in animal experiments and their commercialization by pharmaceutical companies. Increased skepticism was observed in older, less educated, religious individuals, and those who were not blood donors. Overall, the Italian public shows strong support for hiPSC-based therapies, though reservations exist around specific ethical and economic issues.
Discussion: These findings underscore the importance of addressing public concerns through targeted educational campaigns, not only in Italy but globally, to foster a more informed and supportive environment for advancing stem cell research and its clinical applications worldwide. Similar studies have been conducted in Japan, the United States, and Sweden, but there remains a need for all countries to engage with their citizens to better understand how stem cell research is perceived locally. Such engagement is crucial for guiding international strategies in personalized medicine and regenerative therapies, ensuring that emerging technologies are met with both ethical integrity and public trust.
1 Introduction
Human induced pluripotent stem cells (hiPSCs) are generated from adult somatic cells (e.g., skin fibroblasts or blood cells) by genetic reprogramming to an embryonic state. When the genes encoding specific transcription factors are introduced into adult cells, they acquire the key features of the stem status, i.e., the ability to differentiate into almost every cell type and to self-renew. hiPSCs were first reported in Japan in 2007 when Professor Yamanaka reprogrammed mouse fibroblasts (1), followed by human fibroblasts (2). Because of their pluripotent stem state, hiPSCs offer an unlimited supply of a broad variety of patient-specific cells for research and therapeutic applications.
The discovery of hiPSCs overcame the ethical concerns associated with human embryonic stem cells (hESCs), which can be derived from the inner cell mass of a 5-to 7-day-old blastocyst and whose production involves the destruction of human embryos (3, 4). hESCs are considered the gold standard of pluripotent stem cells, but their research is associated with many public and political controversies, as people have different scientific, religious, and moral beliefs; additionally, the utilization of these early-stage embryos has generated much debate and controversy among pro-life movement supporters and stem-cell research opponents (5).
In the field of stem cell research, at the beginning of the 21st century, human multipotent stem cells isolated from fetal and adult sources, such as amniotic fluid (6, 7) and cord blood (8–11), were proposed for research and clinical applications as they do not raise any particular ethical concerns. However, it is still controversial whether these cells can be expanded indefinitely and, most importantly, whether they are pluripotent, i.e., have the potential to differentiate into any of the three germ layers (12, 13).
hiPSCs combine the best properties of hESCs and adult stem cells, avoiding most ethical debates, since no embryos or oocytes are used to generate hiPSCs. Notably, they provide a virtually unlimited source of stem cells as they can be propagated indefinitely and have the intrinsic potential to differentiate into any cell type. For these reasons, hiPSCs hold great potential for disease modeling, personalized therapies, drug discovery, and regenerative medicine.
Nowadays, hiPSCs can be derived from a variety of cellular sources, including umbilical cord blood (14). Significant advancements in reprogramming techniques, such as Sendai virus-mediated reprogramming and non-viral methods, have enhanced the efficiency and safety of hiPSC generation (15). These methods facilitate the conversion of somatic cells into a pluripotent state, thereby expanding the potential for diverse applications in regenerative medicine and personalized therapies. The use of patient-derived hiPSCs can provide large quantities of disease-relevant cells, including even inaccessible ones such as neurons and cardiomyocytes, for disease modeling to study the etiology of human diseases and to develop therapeutic strategies (16). Furthermore, since hiPSCs can be derived from patients themselves, the rapid development of genome editing technologies may allow the correction of disease-causing gene mutations in patient-derived hiPSCs or the introduction of specific mutations into cells from healthy donors (17). Regarding the use of hiPSCs for drug discovery, it is worth mentioning that the identification of a successful therapeutic drug requires a large number of tests and that the failure rate of drug discovery and development is even greater than 90%, resulting in a long, high-risk, and costly process (18). Therefore, the scalability of hiPSC culture and their ability to duplicate indefinitely without undergoing replicative senesce allow disease phenotypes to be consistently recapitulated at a culture scale in order to meet the demands of drug screening (19, 20).
In the field of regenerative medicine, hiPSCs are a promising prospect for cell therapy in a wide range of diseases for which there are currently no cures or effective therapies. The basic paradigm in the use of hiPSCs for cell therapy purposes is to first differentiate them into the desired cell type of interest and then to transplant the resulting specialized tissue-specific cells into patients. The differentiation step is crucial because if undifferentiated hiPSCs are directly injected, they would form tumors (called teratomas) due to their highly proliferative nature and broad differentiation potential (21, 22).
In principle, hiPSCs can be derived directly from individual patients who require therapy; this procedure has the advantage of ensuring an identical immune status and minimizing the risk of transplant rejection. However, producing autologous clinical-grade cells for individual patients would pose major logistical and scientific difficulties, and wide-scale application is likely to be financially prohibitive. An alternative to personalized hiPSC therapy would be to create a bank of clinical-grade hiPSC lines that can be expanded and differentiated for use in a large number of patients (23). Ideally, such a bank would be constituted by hiPSCs obtained from healthy, O-blood-group volunteer donors, selected to maximize the chance of human leukocyte antigens matching and thereby to minimize the risk of allograft rejection. Assuming that a single pluripotent stem cell line can provide cells or tissues for a large number of potential recipients, some studies have been undertaken to calculate the number of hiPSC donors required to cover enough of the population in different countries (1, 23, 24); thus, cells from single donors could potentially last forever and be used by everyone for different purposes.
When a donor signs the informed consent form, it is very important to rigorously explain to him/her all relevant aspects of their own cell donation, including the rights, risks, benefits, research aims, and commercial implications, etc. Moreover, since an individual, from whom hiPSCs have been derived, may want to know about the fate of his/her cells, even the collection of hiPSCs still raises ethical concerns regarding privacy, confidentiality, use, religion, patenting, etc.
In Italy, the ethical debate on stem cells, particularly embryonic ones, is strongly active. Historically, Italy has been a vocal opponent of embryonic stem cell research, considering it morally unacceptable because it involves the destruction of human embryos. Italian law also reflects a conservative stance on embryonic stem cell research: Law 40/2004 restricts the use of human embryos in research and prohibits the cloning and the creation of embryos for research purposes (25). Nevertheless, Italian researchers actively and strongly support stem cell research for basic, translational, and clinical purposes, contributing to global advances in stem cell science.
Despite the fact that stem cell research is permitted and supported in Italy, it raises confusion among the general public, perhaps because it is not easy to understand the major differences between embryonic stem cells and induced pluripotent stem cells. This is an important point because awareness and knowledge regarding hiPSCs can significantly influence the formation of attitudes among the general population and health professionals, which can later be reflected in the course of further research in this field. Thus, surveys to understand the interests of the general public can be a useful vehicle for effectively communicating issues related to hiPSCs. The involvement of citizens and nonspecialist members of the public in gathering opinions on activities related to scientific research occurs mainly through digital technologies, known as “citizen science” (26), and has increased significantly in recent decades (27–32). Although some studies of public opinion on stem cell research have been carried out in the USA, Japan, and Europe (32–35), to our knowledge, no online survey has focused on public attitudes toward hiPSC study participants and recipients as well as possible concerns in the general Italian population.
Based on these premises, the aim of this work was to investigate the knowledge, awareness, and hope in hiPSCs among the Italian adult population using an online survey. In order to obtain a strong and recognized outcome, we decided to use the European online survey management system built for the creation and publication of globally accessible forms to survey the general public. This platform has demonstrated a high consistency and a strong reliability (36).
2 Methods
2.1 Sample recruitment and study population
The hiPSC web-based survey was implemented using the European Commission’s official open-source management tool EUSurvey, an online platform supported by the European Commission’s ISA program, which promotes interoperability solutions for European public administrations. The survey was available online from January 17, 2021 to June 21, 2021, and it was accessible to everyone. The survey was shared using a snowball sampling strategy (37), mainly through social media platforms (e.g., WhatsApp), mailing lists, and word of mouth, which were chosen as the best communication tools to reach the entire population. The survey was specifically designed to maximize the number of participants not pertaining to the Italian scientific community. To this end, the text of the survey was written, as much as possible, in layman’s language, resulting in a simple structure and quick reading to facilitate understanding and participation. Inclusion criteria were as follows: aged >18 years; had access to a mobile phone, computer, or tablet with internet connection; had the ability to write and speak Italian; and provided online consent to participate in the study.
2.2 Variables collected and data transformation
The hiPSC web-based survey was designed as a collaborative project of a working group including biologists, epidemiologists, clinicians, and public health professionals after a comprehensive literature review of existing research (32–34), with the aim of improving knowledge of Italian attitudes toward the use of hiPSCs. Participants were asked to complete the web survey with mandatory questions after reading an introductory page and accepting the option to give consent to participate.
The introductory section defined hiPSCs, focusing on their ability to self-renew and differentiate into several cell types. This section was followed by three comprehension and knowledge questions: (1) This is the first time that I have heard about hiPSCs; (2) hiPSCs obtained from a blood sample can differentiate into all of the mature cell types that constitute the adult human body; (3) hiPSCs obtained from a blood sample can generate undifferentiated daughter cells indefinitely. The possible responses for these three questions were “True,” “False,” and “I do not know.”
The survey then continued with seven questions to assess personal views and possible concerns about hiPSC technology: (4) I would donate a blood sample for the generation of hiPSCs to treat only my relatives, close friends, or myself; (5) I would donate a blood sample for the generation of hiPSCs to treat anyone with an untreatable disease who needed therapy to replace damaged cells or tissues; (6) I would accept that hiPSCs obtained from my blood sample would be used in experiments on animals; (7) I am concerned about the current research and therapeutic applications of these new stem cells; (8) I am concerned about the management of my personal data in relation to the storage and use of the new stem cells derived from my blood cells; (9) I would accept that the new stem cells from my blood might be used for the development of therapies or other applications that are currently unpredictable but regulated; (10) I would donate a blood sample for the generation of hiPSCs, even if they might be acquired in the future by a pharmaceutical company for the development of treatments for incurable diseases. The possible responses to these seven statements were “I agree,” “I disagree,” and “I do not know.”
Finally, the survey included questions to register participants’ personal information, which was then transformed, where appropriate, into the following: gender (men, women, and other); age (18–35, 36–55, and > 55 years); educational level (no university degree vs. university degree); marital status (married, civil union, single, separated/divorced, widow/widower, and I prefer not to answer); employment status (worker with a permanent position, self-employed, worker with a temporary position, unemployed, housewife, retired, and student); work sector (healthcare vs. other); region of origin (northern Italy, central Italy, southern Italy plus islands, and foreign countries); blood donor (current, former, and not a blood donor); willingness to donate organs after death (for organ donation and against organ donation); relatives or friends suffering from rare diseases with no cure (no, experience with relatives and friends, and personal experience); religion (religious vs. nonreligious); frequency of accessing news information (daily, at least once a week, at least once a month, and never); and sources of news information (television, printed newspaper, radio, online newspaper, social networks, and other). The content of the survey is included in Annex 1.
2.3 Statistical analysis
The characteristics of the sample were presented using frequencies and percentages. The Chi-squared or Fisher’s exact tests were used to compare the characteristics of participants according to age groups and to possible survey answers. Participants who responded negatively to both comprehension and knowledge (questions 2 and 3) were excluded from further analysis, resulting in a final sample of 1,201 subjects (64.1%). The responses “I do not know,” “disagree,” and “agree” (reference category) were the dependent variables. Tests were not adjusted for multiple comparisons because of the exploratory nature of the study. Analyses were performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Armonk, NY). All p-values are two-tailed, and a p-value ≤0.05 was considered statistically significant.
2.4 Ethics
This project was reviewed by the internal Ethics Committee (Comitato Etico Milano Area 2) and received a favorable opinion (protocol no. 540 on February 26, 2021). The survey study contained only anonymized data from the participants; therefore, the research team had no contact with the participants after their participation in the study. The study was designed, conducted, and reported in accordance with the Declaration of Helsinki, as revised in 2013. Data were handled and stored in accordance with the European Union General Data Protection Regulation (EU GDPR) 2016/679.
3 Results
Compared to the Italian population as a whole, the hiPSC study enrolled younger people, especially for those in the following age groups: 18–25 years old (14.3% vs. 9.4%), 26–35 years old (26.2% vs. 12.7%), and > 65 years old (7.5% vs. 26.5%). Meanwhile, the distributions for those aged 36–45, 46–55, and 56–65 years old who completed the study were quite similar to those of the general population. Data on the age distribution of the Italian population were taken from the ISTAT website (38) (Supplementary Figure 1).
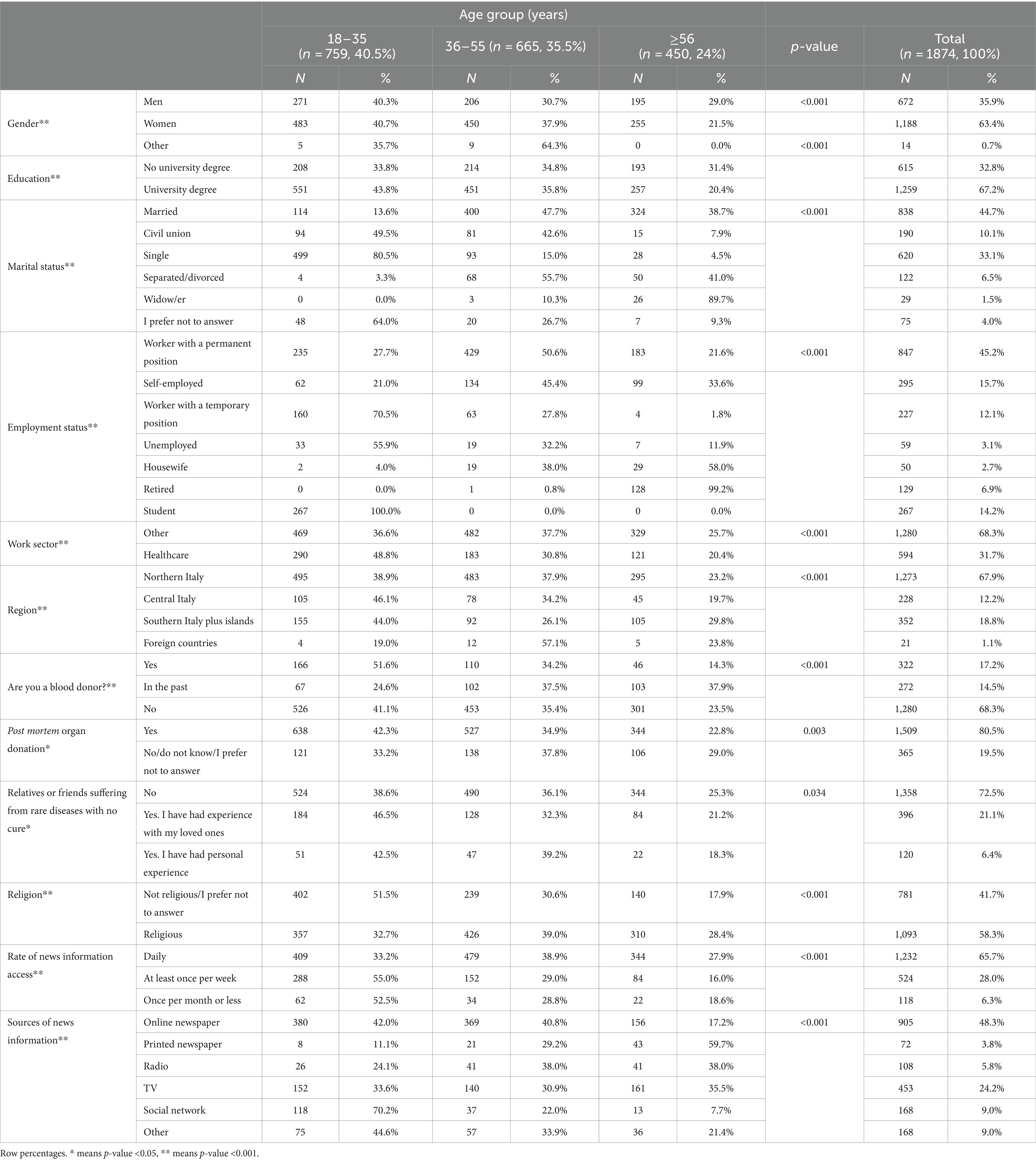
Table 1 summarizes the personal characteristics of the 1874 participants who completed the survey by age group. The majority of the sample was aged 36 years or older (59.5%), 63.4% were women (n = 1,188), 67.2% had a university degree or postgraduate qualification, and 44.7% were married. Respondents were mostly employed with a stable job (45.2%) and did not work in the health sector (68.3%); 67.9% of participants were from northern Italy. The majority of the sample was not a blood donor (68.3%), and 80.5% were willing to donate their organs after death. Approximately 66% of respondents reported daily access to news information sources, with online newspapers (48.3%) being the main source, followed by television (24.2%). Statistically significant differences were found among age groups.
Table 2 shows that compared to those who were unaware of hiPSCs prior to the survey, those who were aware of hiPSCs (question 1) were more likely to be younger, have a university degree, be single, have a temporary job, be a student, work in the health sector, be nonreligious, have a high rate of news information access, and be informed mainly through printed and online newspapers.
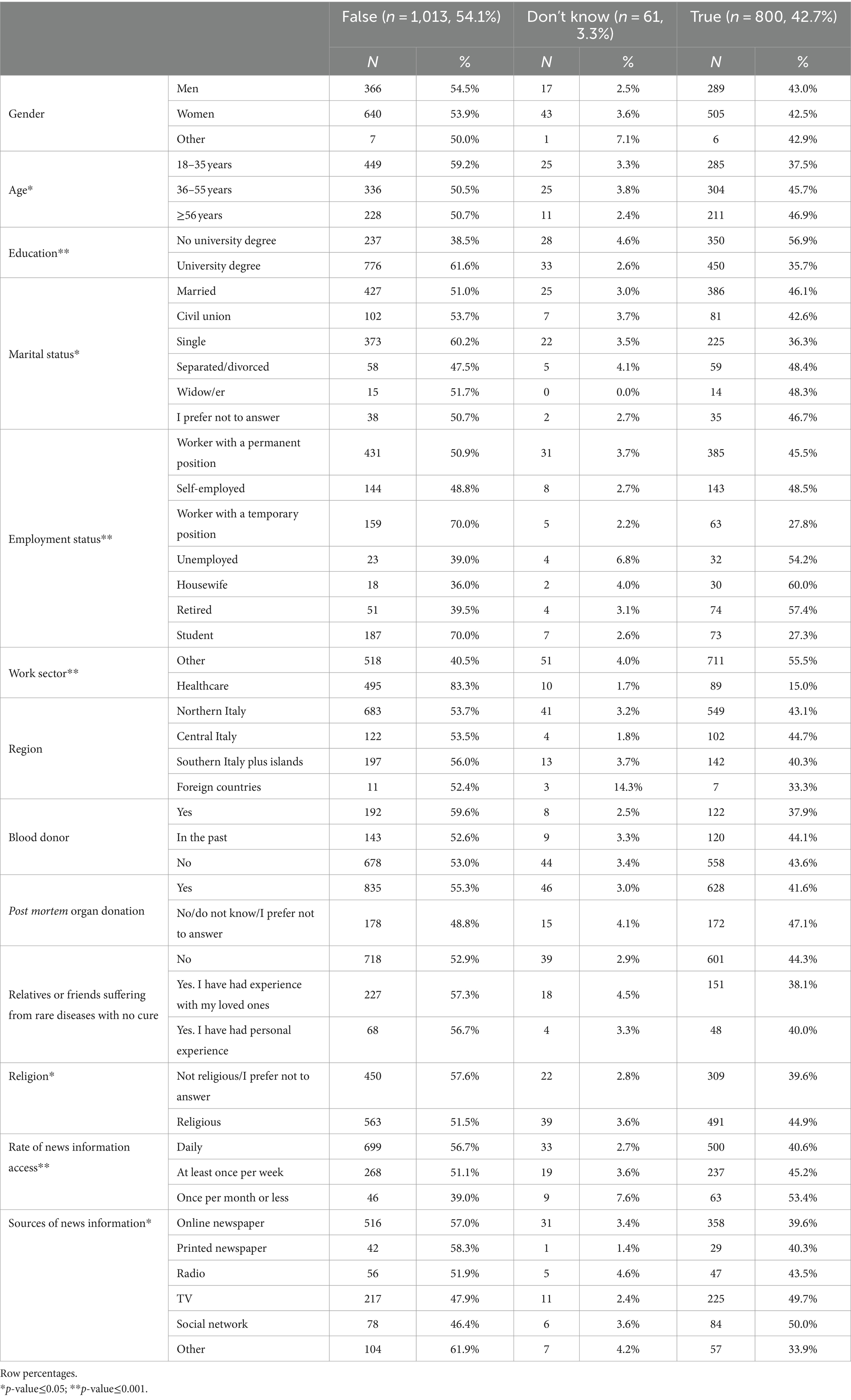
Table 2. Characteristics of the study participants by response to question 1 “This is the first time that I have heard about hiPSCs” (n = 1874).
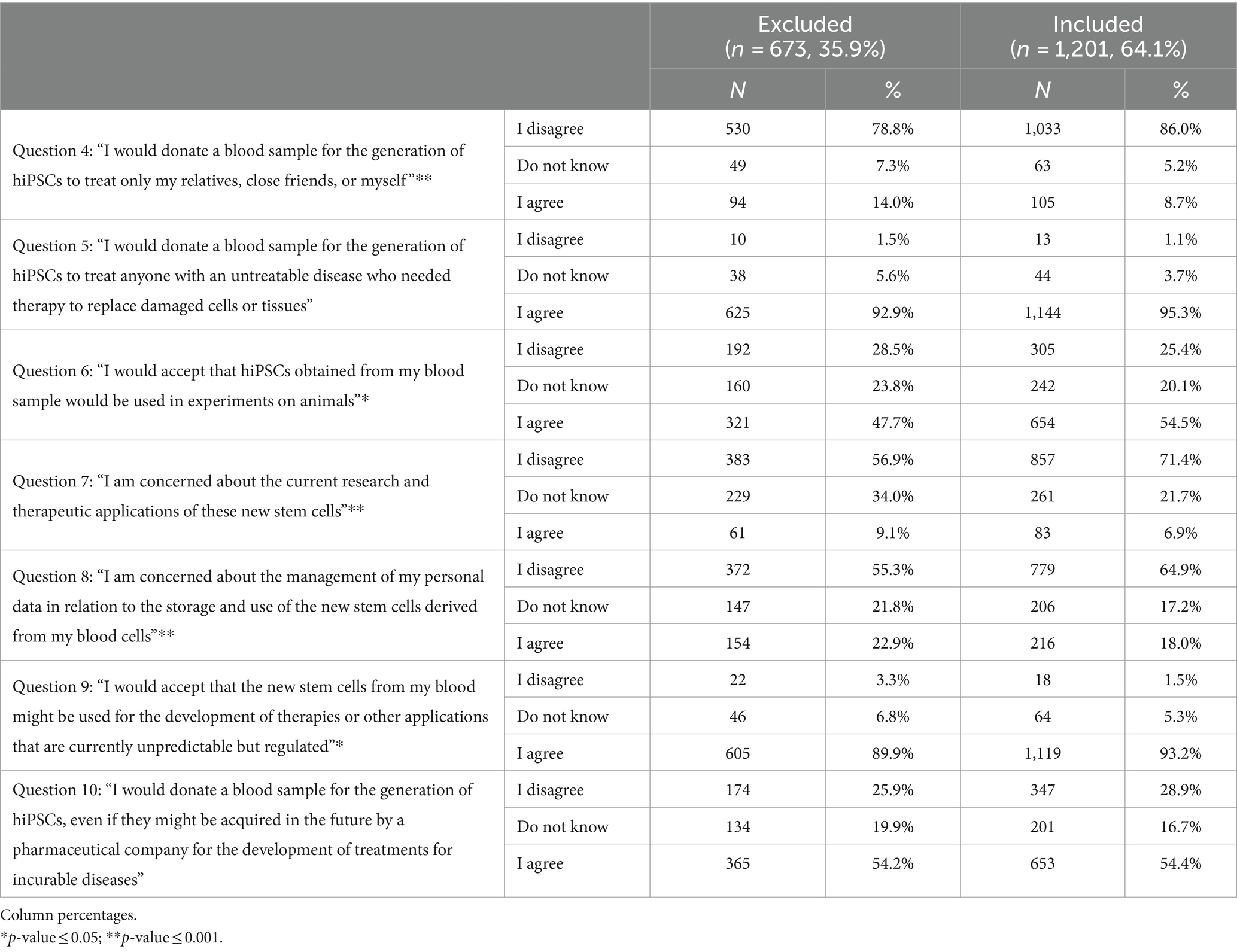
When we compared the characteristics of the included and excluded participants, we found that those included in the analysis were less likely to not report their gender as well as more likely to have a university degree, to be employed in the healthcare sector, to be nonreligious, and to have a higher rate of news information access (Supplementary Table 1). In addition, the included participants were less likely than the excluded participants to be willing (“I agree”) to donate cells for the treatment of family and friends only (question 4), to be concerned about current research and therapeutic applications of new stem cells (question 7), and to be concerned about the management of their personal data (question 8); meanwhile, they were more likely to agree to their use in animal experiments (question 6) and in the development of therapies or other applications (question 9) (Table 3).
For the subsequent analyses, only the included participants were included as the sample. Figures 1A,B; Supplementary Table 2 shows that the majority of the sample (86.0%) disagreed with statement 4, “I would donate a blood sample for the generation of hiPSCs to treat only my relatives, close friends, or myself.” Younger people (18–35 years) were more likely to disagree than the oldest groups, as were graduates of university compared to those without a university degree, while married people and widowers as well as housewives were more likely to agree. Those in favor of post-mortem organ donation were less likely to support donating their organs only to friends and relatives, as were those with personal experience regarding rare diseases for which there is no treatment. Religious people and those who receive news information once per month or less were more likely to donate only to relatives and friends. Table 4 shows that 95.3% of people agreed that everyone should be treated with these new cells (question 5), with widows being less likely to agree than married/cohabiting, single, and separated/divorced individuals, and post-mortem organ donors being more likely to agree than those who do not wish to donate their organs.
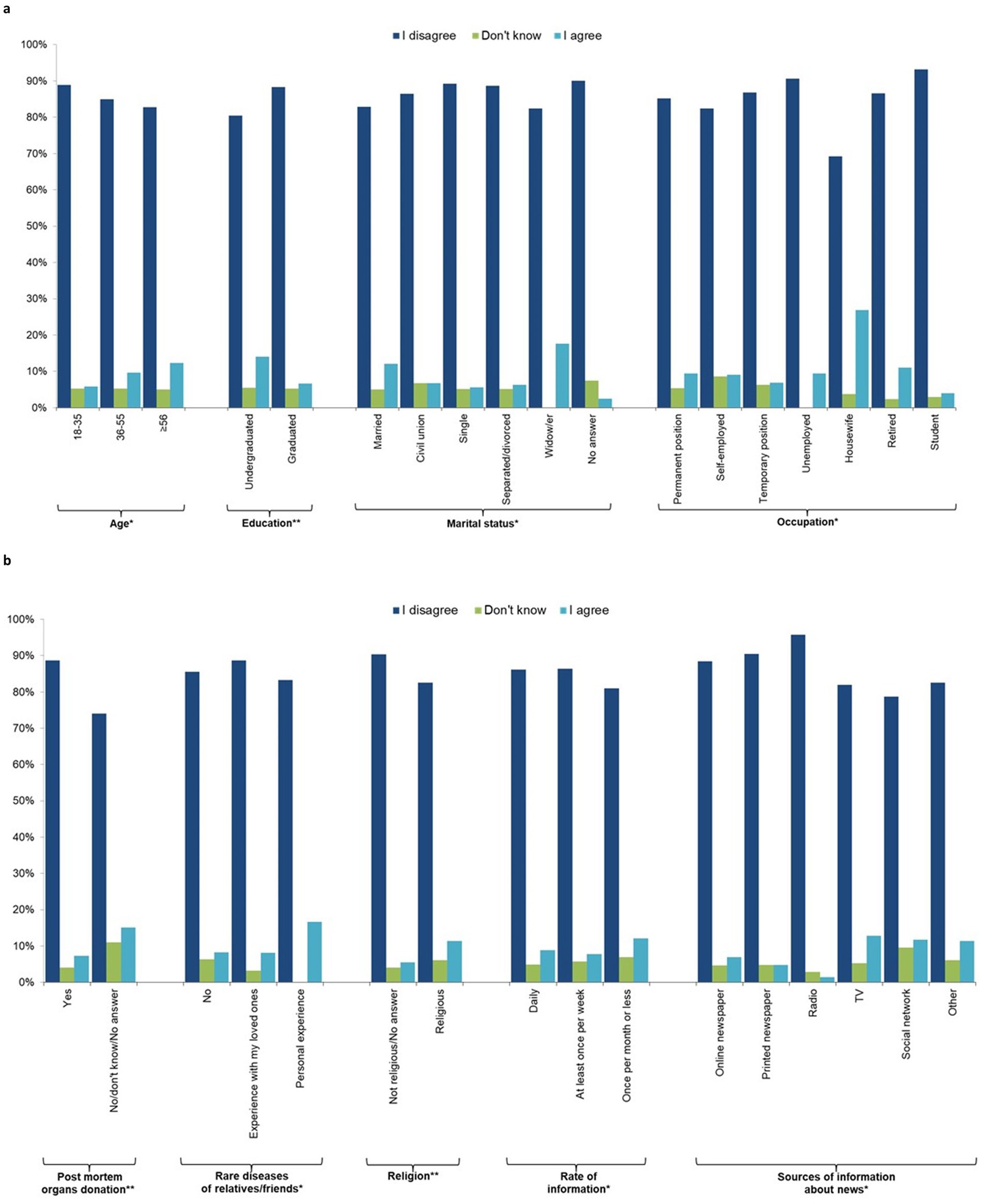
Figure 1. Individual characteristics of the study participants by response to question 4 “I would donate a blood sample for the generation of hiPSCs to treat only my relatives, close friends or me” (n = 1,201, 64.1%). (a) socio-demographic characteristics, (b) behavioural characteristics.
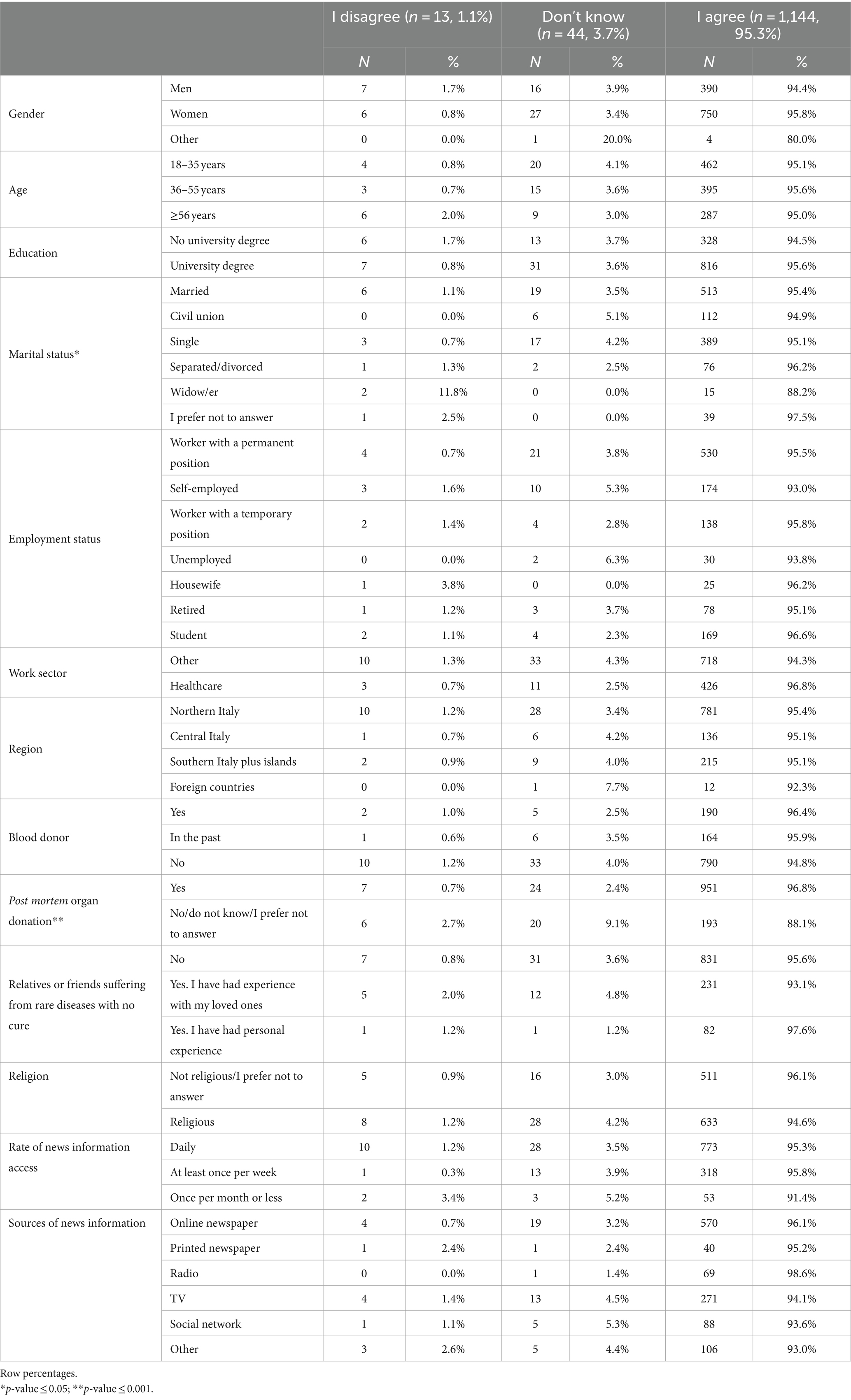
Table 4. Characteristics of the study participants by response to question 5 “I would donate a blood sample for the generation of hiPSCs to treat anyone with an untreatable disease who needed therapy to replace damaged cells or tissues” (n = 1,201, 64.1%).
In response to question 6 on the use of these cells in animal experiments, just over half (54.5%) of the sample agreed, with the other half split between those who disagreed (25.4%) and those who answered “I do not know” (20.1%). Men, younger people, university graduates, people with a temporary job, students, people working in the health sector, and post-mortem organ donors were more likely to agree, as were single people and those who preferred not to answer (p-value≤0.05) (Figure 2; Supplementary Table 3).
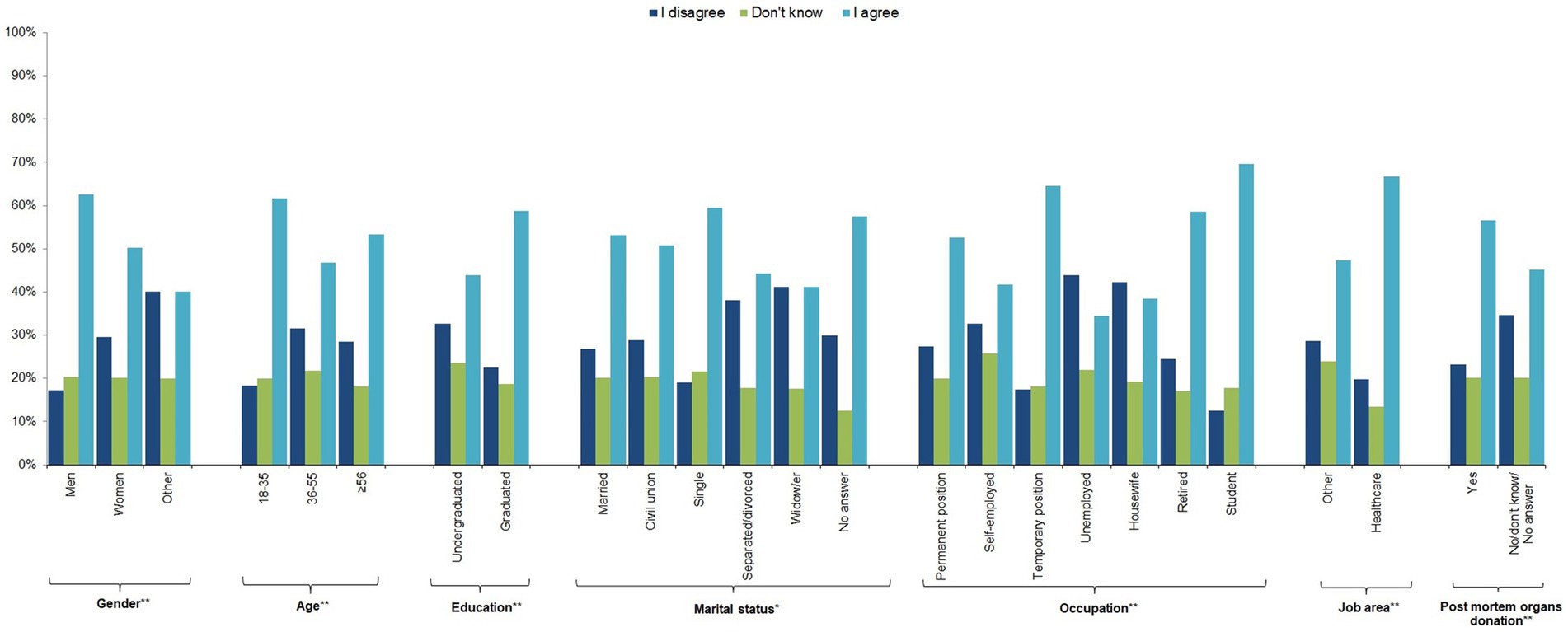
Figure 2. Individual characteristics of the study participants by response to question 6 “I would accept that hiPSCs obtained from my blood sample would be used in experiments on animals” (n = 1,201, 64.1%).
Those most concerned about current research and therapeutic applications of new stem cells (question 7, Figures 3A,B; Supplementary Table 4) were the minority of the sample (6.9%), and 21.7% answered “I do not know.” The most concerned people were those who did not indicate their gender, older people (≥56 years), separated/divorced or widowed people, housewives, people not working in the health sector, those not willing to donate their organs after death, and religious people.
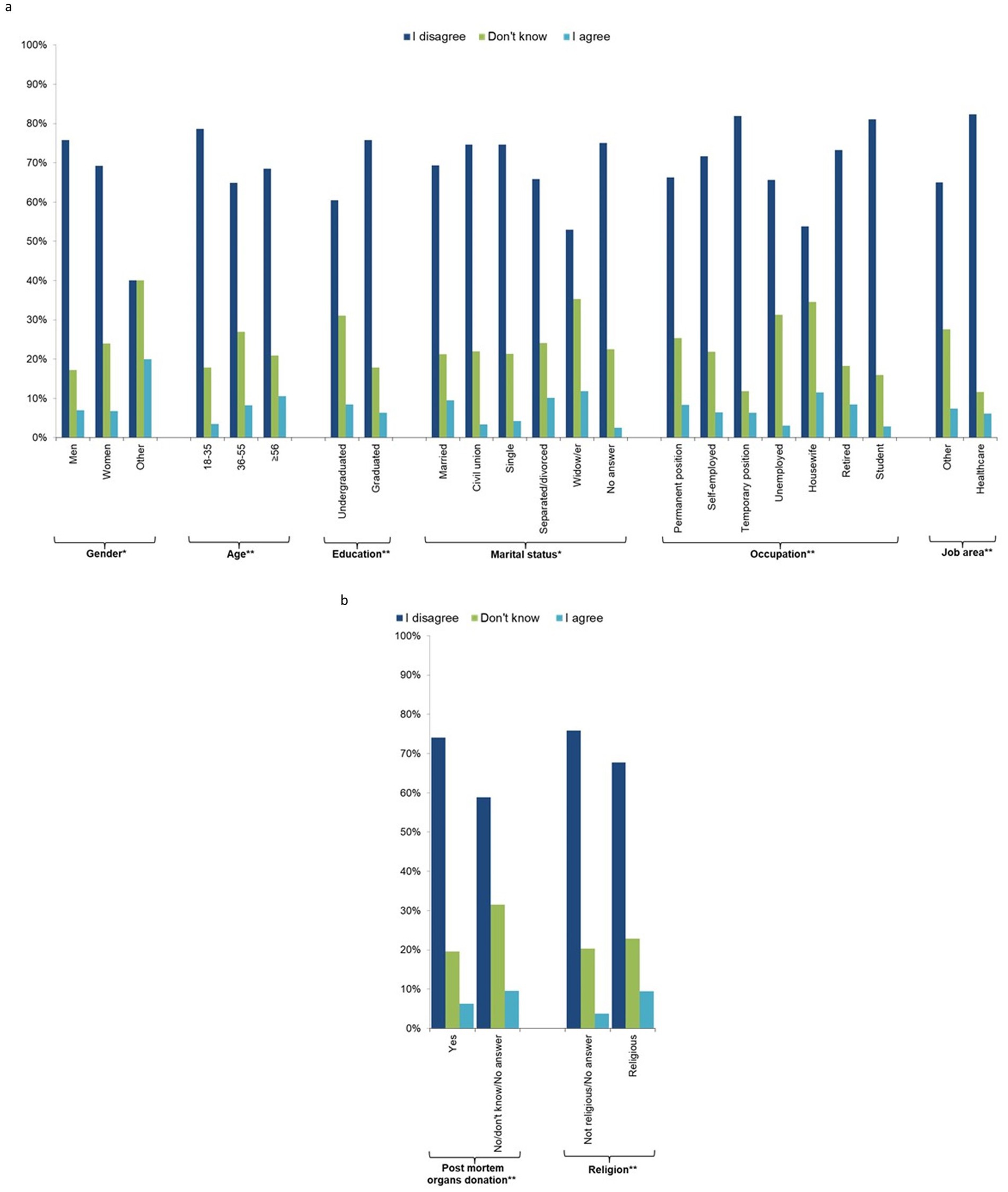
Figure 3. Individual characteristics of the study participants by response to question 7 “I am concerned about the current research and therapy applications of these new stem cells” (n = 1,201, 64.1%). (a) socio-demographic characteristics, (b) behavioural characteristics.
The majority (65%) of the sample was not concerned about the management of their personal data (question 8), 17.2% did not know, and 18.0% were concerned. There was a decreasing trend with age, with the youngest (18–35 years) being less concerned than those aged ≥56 years. In addition, university graduates, those who preferred not to provide their marital status, widow/ers, housewives, retired people, those not working in the health sector, religious people, those who would not donate or were uncertain about post-mortem organ donation, and those who get their news from the radio were more concerned about the management of their personal data (Figure 4; Supplementary Table 5).
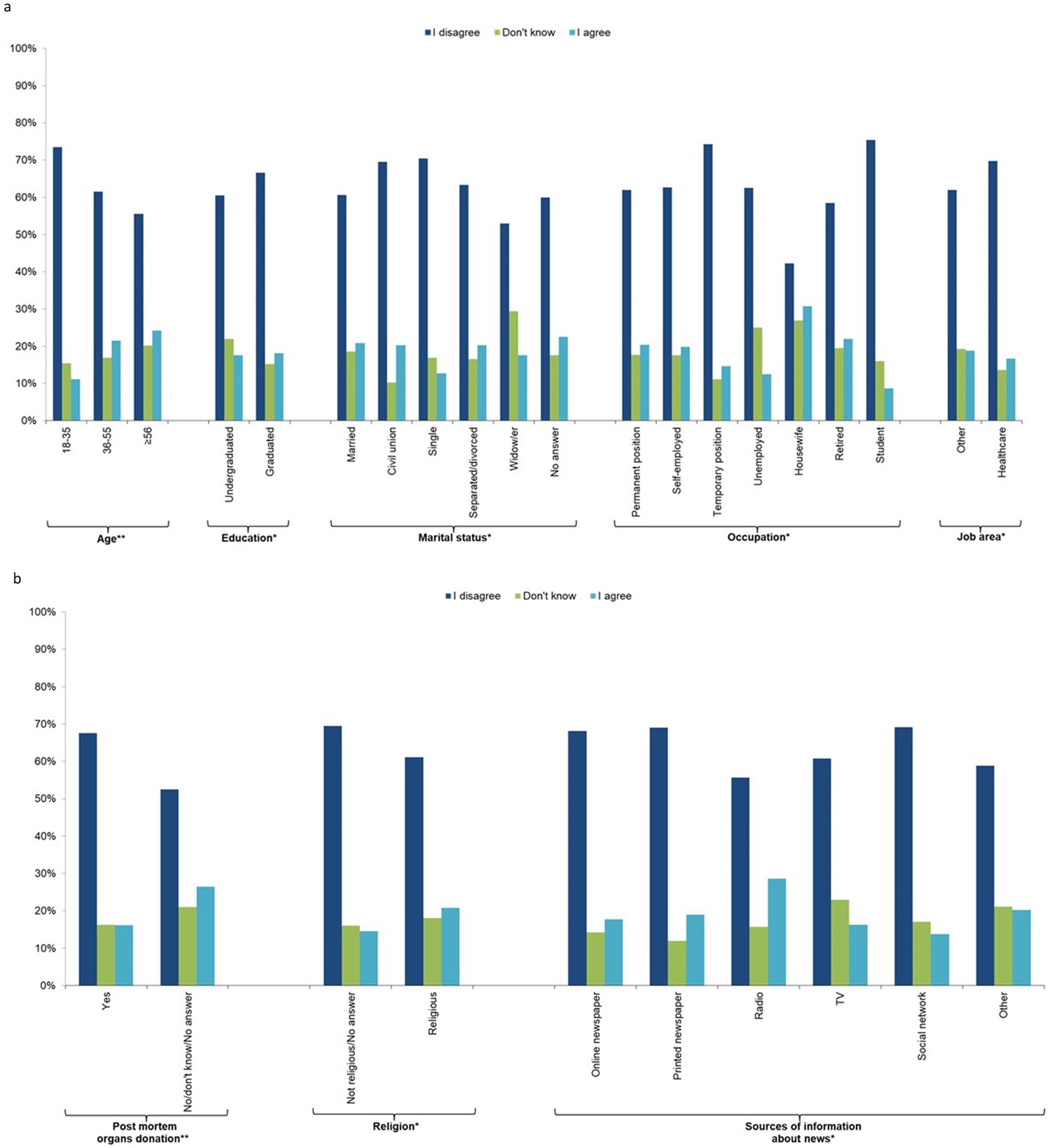
Figure 4. Individual characteristics of the study participants by response to question 8 “I am concerned about the management of my personal data in relation to storage and use of the new stem cells derived from my blood cells” (n = 1,201, 64.1%). (a) socio-demographic characteristics, (b) behavioural characteristics.
Participants who responded positively to question 9, “I would accept that the new stem cells from my blood might be used for the development of therapies or other applications that are currently unpredictable but regulated” (Table 5), were almost the whole sample (93.2%). People who lived in the regions of southern Italy, on the islands, or abroad and who were not post-mortem organ donors were more likely to disagree or respond “I do not know.”
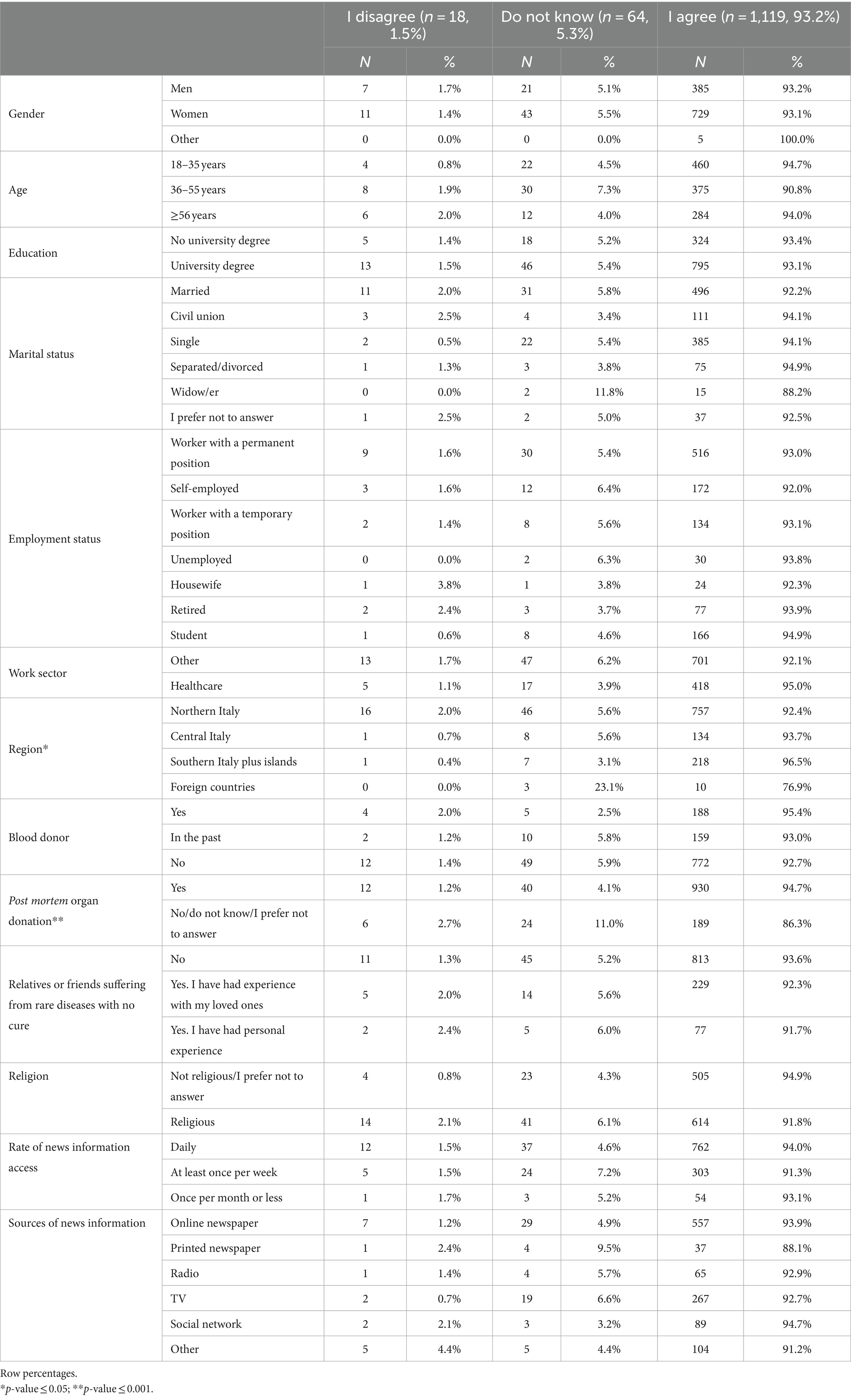
Table 5. Characteristics of the study participants by response to question 9 “I would accept that the new stem cells from my blood might be used for the development of therapies or other applications that are currently unpredictable but regulated” (n = 1,201. 64.1%).
Table 6 shows that just over half of the sample (54.4%) agreed to donate a blood sample for the generation of hiPSCs, even if they might be purchased in the future by a pharmaceutical company for the development of treatments for incurable diseases (question 10). People who did not specify their gender, those aged ≥56 years, those who were not willing to donate their organs after death, and those who got their information from printed newspapers or other sources were more likely to disagree or respond “I do not know.”
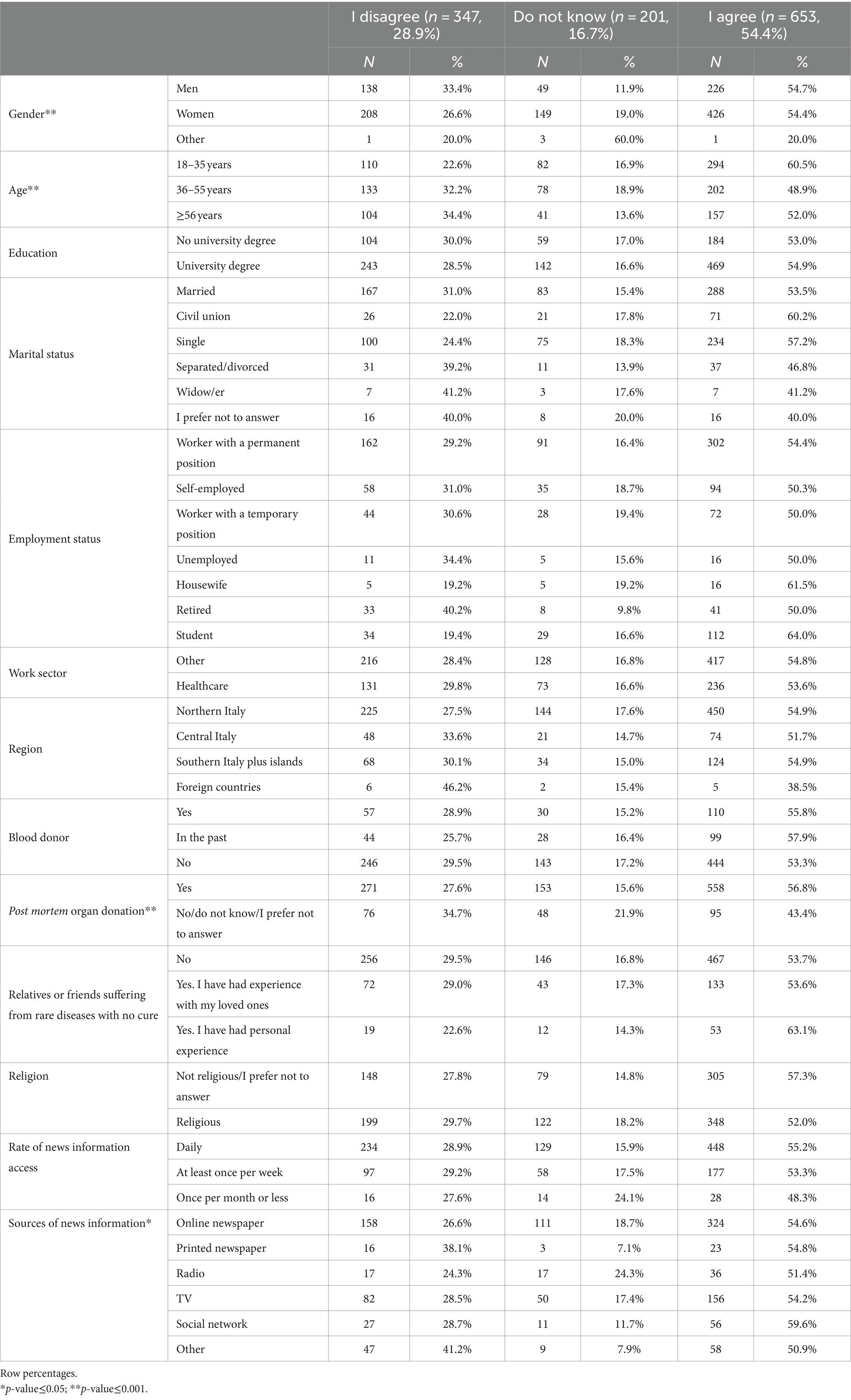
Table 6. Characteristics of the study participants by response to question 10 “I would donate a blood sample for the generation of hiPSCs, even if they might be acquired in the future by a pharmaceutical company for the development of treatments for incurable diseases” (n = 1,201. 64.1%).
4 Discussion
4.1 Summary of main findings
hiPSCs have great promise for cell therapy in the field of regenerative medicine, offering a cure or effective treatment for a variety of diseases for which there are currently no viable treatments. Combining the best aspects of hESCs and adult stem cells, hiPSCs circumvent most ethical controversies because they are generated without the use of embryos or oocytes. Importantly, hiPSCs offer an almost limitless supply of stem cells because of their ability to differentiate into any type of cell and their potential to be propagated indefinitely (17).
The present study provides a snapshot of the characteristics of an Italian sample aimed at exploring knowledge, awareness, and hope in hiPSCs. To the best of our knowledge, this is the first online survey to investigate public attitudes and concerns regarding hiPSC study participants and recipients in the general Italian population.
Looking at the responses to the survey, in the first question on knowledge of hiPSCs, 54.1% of Italian respondents had heard of hiPSCs. This is interesting, but it is worth noting that 67.2% of our respondents were university graduates and 31.7% worked in the health sector; perhaps this can explain the high percentage of people who were aware of hiPSCs.
A similar question was posed by Shineha et al. to respondents from six different countries. The authors surveyed knowledge of hiPSCs in France, Germany, the UK, the USA, South Korea, and Japan among 600 respondents, 100 from each country. In Germany and the UK, only 12 and 15% of respondents knew about hiPSCs, respectively. In France, the USA, and South Korea, 30% of respondents were aware of hiPSCs, while 97% of Japanese respondents were aware of hiPSCs. This result is remarkable, showing that the population of Japan has been educated about this type of cell and its use in research and therapy since its discovery in 2007 by Professor Yamanaka in their country (29).
As expected, those who had heard about hiPSCs were more likely to be young, to have a university degree, to have a temporary job, to be a student, to work in the health sector, and to have a high rate of news information access. The demographic information regarding education and work sectors provided predictable results, as people with a university degree and those working in the health sector were likely to have studied these cell types during their lives. It should be noted that people with a high rate of news information access also were more aware of these cells, even though they may never have heard of it at university or school.
A survey conducted by Ishihara et al. examined the awareness and interest in hiPSCs and regenerative medicine among 2,396 Japanese high school and university students. The results showed that over 80% of the students recognized hiPSCs and regenerative medicine. However, many of them expressed concerns about the side effects, safety, and costs associated with regenerative treatments as well as supported the need for enhanced education to improve understanding of hiPSCs (39). These results are consistent with our findings, although, again, the level of knowledge in Japan may be influenced by Professor Yamanaka’s Nobel Prize achievement.
The majority of the sample (86.0%) disagreed with the statement “I would donate a blood sample for the generation of hiPSCs to treat only my relatives, close friends, or myself.” However, 95.3% of people agreed that everyone, not just relatives or close friends, should be treated with these new cells, and they would donate them to anyone. This is the key finding of our work: people, regardless of their level of knowledge about stem cells, would donate cells to treat anyone who needed them due to incurable diseases.
When asked about the use of hiPSCs in animal experiments, just over half of respondents agreed, while the rest were evenly divided between those who disagreed and those who were unsure. It should be noted that the latter group still has a chance to understand the importance of animal testing in the development of new therapies via education. For example, even if innovative three-dimensional models are becoming increasingly used in drug screening (40, 41), animal testing remains a crucial part of the research process, providing valuable insights that cannot yet be fully replicated by alternative methods. In fact, regulatory requirements often mandate animal testing to ensure the safety and efficacy of new drugs and treatments before human trials.
Nevertheless, question 6 reflects the issues surrounding the use of animals in scientific research, with ethical, scientific, and social concerns, as well as active involvement of the general public and animal rights activist associations. Recently, Bearth and colleagues conducted a survey aimed at investigating public views on animal testing and potential alternatives in Switzerland, and they showed that animal testing was accepted mainly for medicines and food (42).
There have always been conflicting opinions on the issue of animal testing, with major problems raised by animal rights associations; in addition, public debate has often suffered from misleading information that is disseminated by individuals or groups who oppose animal testing (43).
In Italy, for example, a neuroscientist at the University of Turin and Tilburg University faced controversy with animal rights activists over his research using primates to study visual perception and cortical blindness. Despite these challenges, he defended the scientific need for animal models to understand neurological diseases and to develop therapies. This case highlights the wider debate between the scientific community and animal rights movements over animal experimentation (44).
Moving forward to question 7, 65% of the sample population was not concerned about the management of their personal data. In general, there was a decreasing trend with age, with the youngest being less concerned than those aged ≥56 years. This is an interesting point, given the current importance of managing personal data in the age of social media.
In the last question, we found that just over half of the sample population would agree to donate a blood sample for the generation of hiPSCs, even if they might be purchased in the future by a pharmaceutical company for the development of treatments for incurable diseases. It is important to note that while 1,144 respondents agreed with the statement “I would donate a blood sample for the generation of hiPSCs to treat anyone with an untreatable disease who needed therapy to replace damaged cells or tissues,” when the role and profit of pharmaceutical companies come into play, this number drops to 653. This finding indicates that 42.9% of those who said they would be willing to donate to anyone would reconsider their position if a pharmaceutical corporation got involved.
Thus, we identified specific subgroups of people who were more concerned and skeptical about hiPSCs, such as older, less educated, non-blood donors and religious individuals. In fact, we found that post-mortem organ donors were more likely to agree that stem cells should be donated to everyone, used to treat incurable diseases, and used in animal experiments than nondonors, who were more concerned about the use and handling of their personal data and future applications. Therefore, we hypothesized that those who choose to donate their organs are by nature altruistic toward other people and very knowledgeable about the progress of science. Similarly, we observed that young people often agreed more with the use of these cells and, in general, had fewer concerns regarding this technology than the older age groups. Again, we speculated that young people may be more receptive to the use of new technologies, even in the field of healthcare.
4.2 Limitations and strengths of the study
This study had some limitations that must be addressed. For example, there was intrinsic selection bias in this study due to the voluntary participation in the hiPSC web-based survey. Although the high level of participation allowed us to obtain a fairly balanced sample, some of the characteristics of the sample were not sufficiently representative of the Italian adult population as would be expected in a web survey using snowball sampling. In particular, as this method does not use random selection, it is prone to error and sample bias because not all members of a group have an equal chance of being selected and participants are likely to involve people who are similar to themselves (37). Indeed, we found an over-representation of women, young respondents with high levels of education, and participants from the healthcare sector. Furthermore, the majority of respondents (67.9%) were from northern Italy, which may not represent the views of the whole country, especially the south, as there are significant regional differences for historical, socio-cultural and administrative reasons that may have influenced public attitudes. In particular, based on previous research (45–47), we can speculate that this geographical bias may have led to an overestimation of the percentage of general attitudes toward willingness to donate blood samples for hiPSC generation in the present sample compared to the general population.
Therefore, as the sample was self-selected, the results should be generalized with caution.
Future research should take these limitations into account by opting for different recruitment approaches (e.g., using simple random sampling to select a random set of participants from a large population in which everyone has an equal chance of being selected), or by following a set of best practices to increase the reliability of snowball sampling research.
Moreover, the cross-sectional and observational design of the study provides only a snapshot of the community at the time of the survey and it is not possible to draw causal inferences.
Another weakness of this study is that the data were self-reported, which may have introduced measurement error and recall bias (e.g., misunderstanding of the survey questions, etc.). However, it is reasonable to assume that non-differential misclassification may have occurred, where the likelihood of misclassifying exposure is independent of the possible survey responses (outcomes) and vice versa, increasing the similarity between the exposed and unexposed groups. In addition, the use of an anonymous online survey (no personal contact and no identifying information), significantly reduced the likelihood of social desirability bias, which is the tendency of respondents to answer in a way that is more socially acceptable to others.
Nevertheless, the hiPSC web survey has several strengths. First, to the best of our knowledge, this is the first online survey to focus on public attitudes toward hiPSCs and possible concerns in the general Italian population. Second, it reached a large sample of adults covering all regions of Italy, which provides a robust dataset for statistical analysis. Third, the survey was designed to take into account some information on sociodemographic characteristics and behaviors based on previous literature reports adding depth to the data interpretation. Fourth, the survey was implemented using EUSurvey, an open-source management tool developed by the European Commission that guarantees complete anonymity of participants and their personal data as well as a high level of data security.
Fifthly, the study draws comparisons with similar research carried out in other countries, such as Japan, the USA and Sweden. This adds an international perspective and increases the relevance of the finding.
Finally, the use of web-based surveys as an alternative method for obtaining public opinion on various public health issues can overcome the higher costs and interviewer bias typical of traditional methods (e.g., telephone surveys), as they are a cheap and scalable means of efficiently and rapidly involving a large number of people in a study, regardless of geographical distance (48).
5 Conclusion
In conclusion, this work demonstrates that people are generally willing to donate cells for the development of new therapies to cure incurable diseases, but some are concerned about data management, animal testing, and potential profits for pharmaceutical companies; these concerns are particularly evident in some specific subgroups of individuals. Furthermore, this work highlights the importance of surveys based on citizen engagement to accurately identify groups of individuals who are the least receptive to certain topics. Such insights can enable the precise targeting and dissemination of campaigns designed to elevate awareness of sensitive research issues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study did not require ethical approval as it involved the administration of a survey without the use of animal or human specimens. All data were collected anonymously, and no sensitive personal information was recorded.
Author contributions
NE: Data curation, Writing – original draft, Formal analysis. FP: Data curation, Writing – original draft, Formal analysis, Methodology. VP: Formal analysis, Writing – original draft, Supervision. SCo: Formal analysis, Writing – original draft, Data curation. MB: Data curation, Writing – original draft, Conceptualization, Validation. CM: Conceptualization, Data curation, Writing – original draft. SCa: Writing – original draft, Validation. LL: Validation, Writing – original draft, Conceptualization, Data curation, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Fondazione Regionale per la Ricerca Biomedica (Regione Lombardia), Project nr. CP2_10/2018. This research was also funded by Ricerca Corrente by the Italian Ministry of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1491257/full#supplementary-material
References
1. Okita, K, Ichisaka, T, and Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature. (2007) 448:313–7. doi: 10.1038/nature05934
2. Takahashi, K, Tanabe, K, Ohnuki, M, Narita, M, Ichisaka, T, Tomoda, K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72. doi: 10.1016/j.cell.2007.11.019
3. Park, SJ, Kim, YY, Han, JY, Kim, SW, Kim, H, and Ku, S-Y. Advancements in human embryonic stem cell research: clinical applications and ethical issues. Tissue Eng Regen Med. (2024) 21:379–94. doi: 10.1007/s13770-024-00627-3
4. Robertson, JA. Human embryonic stem cell research: ethical and legal issues. Nat Rev Genet. (2001) 2:74–8. doi: 10.1038/35047594
5. Lo, B, and Parham, L. Ethical issues in stem cell research. Endocr Rev. (2009) 30:204–13. doi: 10.1210/er.2008-0031
6. Bossolasco, P, Montemurro, T, Cova, L, Zangrossi, S, Calzarossa, C, Buiatiotis, S, et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. (2006) 16:329–36. doi: 10.1038/sj.cr.7310043
7. Loukogeorgakis, SP, and De Coppi, P. Concise review: amniotic fluid stem cells: the known, the unknown, and potential regenerative medicine applications. Stem Cells Dayt Ohio. (2017) 35:1663–73. doi: 10.1002/stem.2553
8. Barilani, M, Lavazza, C, Viganò, M, Montemurro, T, Boldrin, V, Parazzi, V, et al. Dissection of the cord blood stromal component reveals predictive parameters for culture outcome. Stem Cells Dev. (2015) 24:104–14. doi: 10.1089/scd.2014.0160
9. Montemurro, T, Viganò, M, Ragni, E, Barilani, M, Parazzi, V, Boldrin, V, et al. Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur J Cell Biol. (2016) 95:228–38. doi: 10.1016/j.ejcb.2016.04.003
10. Morello, W, Budelli, S, Bernstein, DA, Montemurro, T, Montelatici, E, Lavazza, C, et al. First clinical application of cord blood mesenchymal stromal cells in children with multi-drug resistant nephrotic syndrome. Stem Cell Res Ther. (2022) 13:420. doi: 10.1186/s13287-022-03112-7
11. Montemurro, T, Lavazza, C, Montelatici, E, Budelli, S, La Rosa, S, Barilani, M, et al. Off-the-shelf cord-blood mesenchymal stromal cells: production, quality control, and clinical use. Cells. (2024) 13:1066. doi: 10.3390/cells13121066
12. Quesenberry, PJ, Dooner, G, Colvin, G, and Abedi, M. Stem cell biology and the plasticity polemic. Exp Hematol. (2005) 33:389–94. doi: 10.1016/j.exphem.2004.11.005
13. Quesenberry, PJ, Wen, S, Goldberg, LR, and Dooner, MS. The universal stem cell. Leukemia. (2022) 36:2784–92. doi: 10.1038/s41375-022-01715-w
14. Barilani, M, Cherubini, A, Peli, V, Polveraccio, F, Bollati, V, Guffanti, F, et al. A circular RNA map for human induced pluripotent stem cells of foetal origin. EBioMedicine. (2020) 57:102848. doi: 10.1016/j.ebiom.2020.102848
15. Al Abbar, A, Ngai, SC, Nograles, N, Alhaji, SY, and Abdullah, S. Induced pluripotent stem cells: reprogramming platforms and applications in cell replacement therapy. BioRes Open Access. (2020) 9:121–36. doi: 10.1089/biores.2019.0046
16. Cao, L, Tan, L, Jiang, T, Zhu, X-C, and Yu, J-T. Induced pluripotent stem cells for disease modeling and drug discovery in neurodegenerative diseases. Mol Neurobiol. (2015) 52:244–55. doi: 10.1007/s12035-014-8867-6
17. Shi, Y, Inoue, H, Wu, JC, and Yamanaka, S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. (2017) 16:115–30. doi: 10.1038/nrd.2016.245
18. Sun, D, Gao, W, Hu, H, and Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm Sin B. (2022) 12:3049–62. doi: 10.1016/j.apsb.2022.02.002
19. Rivera-Ordaz, A, Peli, V, Manzini, P, Barilani, M, and Lazzari, L. Critical analysis of cGMP large-scale expansion process in bioreactors of human induced pluripotent stem cells in the framework of quality by design. BioDrugs. (2021) 35:693–714. doi: 10.1007/s40259-021-00503-9
20. Elitt, MS, Barbar, L, and Tesar, PJ. Drug screening for human genetic diseases using iPSC models. Hum Mol Genet. (2018) 27:R89–98. doi: 10.1093/hmg/ddy186
21. Abad, M, Mosteiro, L, Pantoja, C, Cañamero, M, Rayon, T, Ors, I, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. (2013) 502:340–5. doi: 10.1038/nature12586
22. Knoepfler, PS. Deconstructing stem cell Tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells Dayt Ohio. (2009) 27:1050–6. doi: 10.1002/stem.37
23. Taylor, CJ, Peacock, S, Chaudhry, AN, Bradley, JA, and Bolton, EM. Generating an iPSC Bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. (2012) 11:147–52. doi: 10.1016/j.stem.2012.07.014
24. Pappas, DJ, Gourraud, P-A, Gall, CL, Laurent, J, Trounson, A, DeWitt, N, et al. Proceedings: human leukocyte antigen Haplo-homozygous induced pluripotent stem cell Haplobank modeled after the California population: evaluating matching in a multiethnic and admixed population. Stem Cells Transl Med. (2015) 4:413–8. doi: 10.5966/sctm.2015-0052
25. Beltrame, L. The Italian way to stem cell research: rethinking the role of Catholic religion in shaping Italian stem cell research regulations. Dev World Bioeth. (2017) 17:157–66. doi: 10.1111/dewb.12104
26. Bonney, R, Cooper, C, and Ballard, H. The theory and practice of citizen science: launching a new journal. Citiz Sci Theory Pract. (2016) 1:1. doi: 10.5334/cstp.65
27. Wardropper, CB, Dayer, AA, Goebel, MS, and Martin, VY. Conducting conservation social science surveys online. Conserv Biol. (2021) 35:1650–8. doi: 10.1111/cobi.13747
28. Yui, H, Kamisato, A, Muto, K, Yashiro, Y, Watanabe, S, Kiya, Y, et al. Attitudes towards human fetal tissue research: survey of researchers and the public in Japan. Regen Ther. (2023) 24:78–84. doi: 10.1016/j.reth.2023.05.007
29. Shineha, R, Inoue, Y, and Yashiro, Y. A comparative analysis of attitudes toward stem cell research and regenerative medicine between six countries - a pilot study. Regen Ther. (2022) 20:187–93. doi: 10.1016/j.reth.2022.04.007
30. Crane, AT, Shen, FX, Brown, JL, Cormack, W, Ruiz-Estevez, M, Voth, JP, et al. The American public is ready to accept human-animal chimera research. Stem Cell Rep. (2020) 15:804–10. doi: 10.1016/j.stemcr.2020.08.018
31. Middleton, A, Milne, R, Thorogood, A, Kleiderman, E, Niemiec, E, Prainsack, B, et al. Attitudes of publics who are unwilling to donate DNA data for research. Eur J Med Genet. (2019) 62:316–23. doi: 10.1016/j.ejmg.2018.11.014
32. Grauman, Å, Hansson, M, Nyholm, D, Jiltsova, E, Widner, H, van Vliet, T, et al. Attitudes and values among the Swedish general public to using human embryonic stem cells for medical treatment. BMC Med Ethics. (2022) 23:138. doi: 10.1186/s12910-022-00878-6
33. Nisbet, MC. Public opinion about stem cell research and human cloning. Public Opin Q. (2004) 68:131–54. doi: 10.1093/poq/nfh009
34. Shineha, R, Kawakami, M, Kawakami, K, Nagata, M, Tada, T, and Kato, K. Familiarity and prudence of the Japanese public with research into induced pluripotent stem cells, and their desire for its proper regulation. Stem Cell Rev Rep. (2010) 6:1–7. doi: 10.1007/s12015-009-9111-z
35. Einsiedel, E, Premji, S, Geransar, R, Orton, NC, Thavaratnam, T, and Bennett, LK. Diversity in public views toward stem cell sources and policies. Stem Cell Rev Rep. (2009) 5:102–7. doi: 10.1007/s12015-009-9063-3
36. EUSurvey. Available at: https://ec.europa.eu/eusurvey/home/welcome
37. Baltar, F, and Brunet, I. Social research 2.0: virtual snowball sampling method using Facebook. Internet Res. (2012) 22:57–74. doi: 10.1108/10662241211199960
38. Popolazione residente al 1° gennaio: Per fascia di età. Available at: http://dati.istat.it/Index.aspx?QueryId=42869 (Accessed October 1, 2024).
39. Ishihara, K, Ichinomiya, A, Inami, M, Hashimoto, T, Yuzawa, R, Ishizu, M, et al. Recognition of, interest in, and understanding of induced pluripotent stem cells and regenerative medicine in Japanese students. Regen Ther. (2016) 5:96–106. doi: 10.1016/j.reth.2016.09.003
40. Ravi, M, Paramesh, V, Kaviya, S, Anuradha, E, and Solomon, FDP. 3D cell culture systems: advantages and applications. J Cell Physiol. (2015) 230:16–26. doi: 10.1002/jcp.24683
41. Weinhart, M, Hocke, A, Hippenstiel, S, Kurreck, J, and Hedtrich, S. 3D organ models-revolution in pharmacological research? Pharmacol Res. (2019) 139:446–51. doi: 10.1016/j.phrs.2018.11.002
42. Bearth, A, Wiesner, L, and Siegrist, M. Public views of animal testing and alternatives in chemical risk assessment. Food Chem Toxicol. (2024) 188:114644. doi: 10.1016/j.fct.2024.114644
43. Petetta, F, and Ciccocioppo, R. Public perception of laboratory animal testing: historical, philosophical, and ethical view. Addict Biol. (2021) 26:e12991. doi: 10.1111/adb.12991
44. Italian blindness researchers planning monkey studies threatened after health ministry releases names. Available at: https://www.science.org/content/article/italian-blindness-researchers-planning-monkey-studies-threatened-after-health-ministry
45. Costa, G, Marinacci, C, Caiazzo, A, and Spadea, T. Individual and contextual determinants of inequalities in health: the Italian case. Int J Health Serv Plan Adm Eval (2003) 33:635–667; discussion 743–749. doi: 10.2190/AM8R-K0DY-F7PM-3RNP
46. Franzini, L, and Giannoni, M. Determinants of health disparities between Italian regions. BMC Public Health. (2010) 10:296. doi: 10.1186/1471-2458-10-296
47. Romano, V, Milne, R, and Mascalzoni, D. Italian public’s views on sharing genetic information and medical information: findings from the “your DNA, your say” study. Wellcome Open Res. (2021) 6:180. doi: 10.12688/wellcomeopenres.16909.1
Keywords: human induced pluripotent stem cells, internet-based survey, public attitude, citizen participation, stem cell research
Citation: Elia N, Prinelli F, Peli V, Conti S, Barilani M, Mei C, Castaldi S and Lazzari L (2024) Public attitudes toward the use of human induced pluripotent stem cells: insights from an Italian adult population. Front. Public Health. 12:1491257. doi: 10.3389/fpubh.2024.1491257
Edited by:
Shyam Kishor Sah, University of California, Berkeley, United StatesReviewed by:
Kshitiz Raj Shrestha, Independent researcher, Kathmandu, NepalRachel Steeg, Fraunhofer UK Research, United Kingdom
Nelson Monteiro, E&M Biolab, United States
Copyright © 2024 Elia, Prinelli, Peli, Conti, Barilani, Mei, Castaldi and Lazzari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenza Lazzari, bG9yZW56YS5sYXp6YXJpQHBvbGljbGluaWNvLm1pLml0
†These authors have contributed equally to this work
 Noemi Elia
Noemi Elia Federica Prinelli
Federica Prinelli Valeria Peli
Valeria Peli Silvia Conti
Silvia Conti Mario Barilani
Mario Barilani Cecilia Mei
Cecilia Mei Silvana Castaldi4,5
Silvana Castaldi4,5 Lorenza Lazzari
Lorenza Lazzari