- 1Department of Nursing and Midwifery, Dessie Health Science College, Dessie, Ethiopia
- 2Department of Environmental Health, College of Medicine and Health Sciences, Debre Markos University, Debre Markos, Ethiopia
- 3Department of Environmental Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 4National Centre for Epidemiology and Population Health, The Australian National University, Canberra, ACT, Australia
Introduction: Visceral Leishmaniasis, also known as kala-azar, is a potentially fatal, neglected tropical disease caused by the protozoan parasite Leishmania and transmitted through infected sandflies. It is one of the major global public health problems and contributors to economic crisis among people. Though different studies investigated human visceral leishmaniasis in Eastern Africa, the findings were inconsistent and inconclusive enough, and there is no representative data on this devastating public health concern. Therefore, this systematic review and meta-analysis aimed to determine the pooled prevalence and risk factors associated with human visceral leishmaniasis in Eastern Africa.
Methods: The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) guidelines were followed for this study. Databases such as PubMed/MEDLINE, CINAHL, LIVIVO, African Journals Online, African Index Medicus (AIM), HINARI, Science Direct, Web of Science, Cochrane Library, Google Scholar, Semantic Scholar, and Google were used to retrieve all the relevant articles. The search was carried out from 23 May 2024 to 17 July 2024. Data were analyzed using STATA 17 software to determine the pooled prevalence of human visceral leishmaniasis with a 95% confidence interval using a random-effects model.
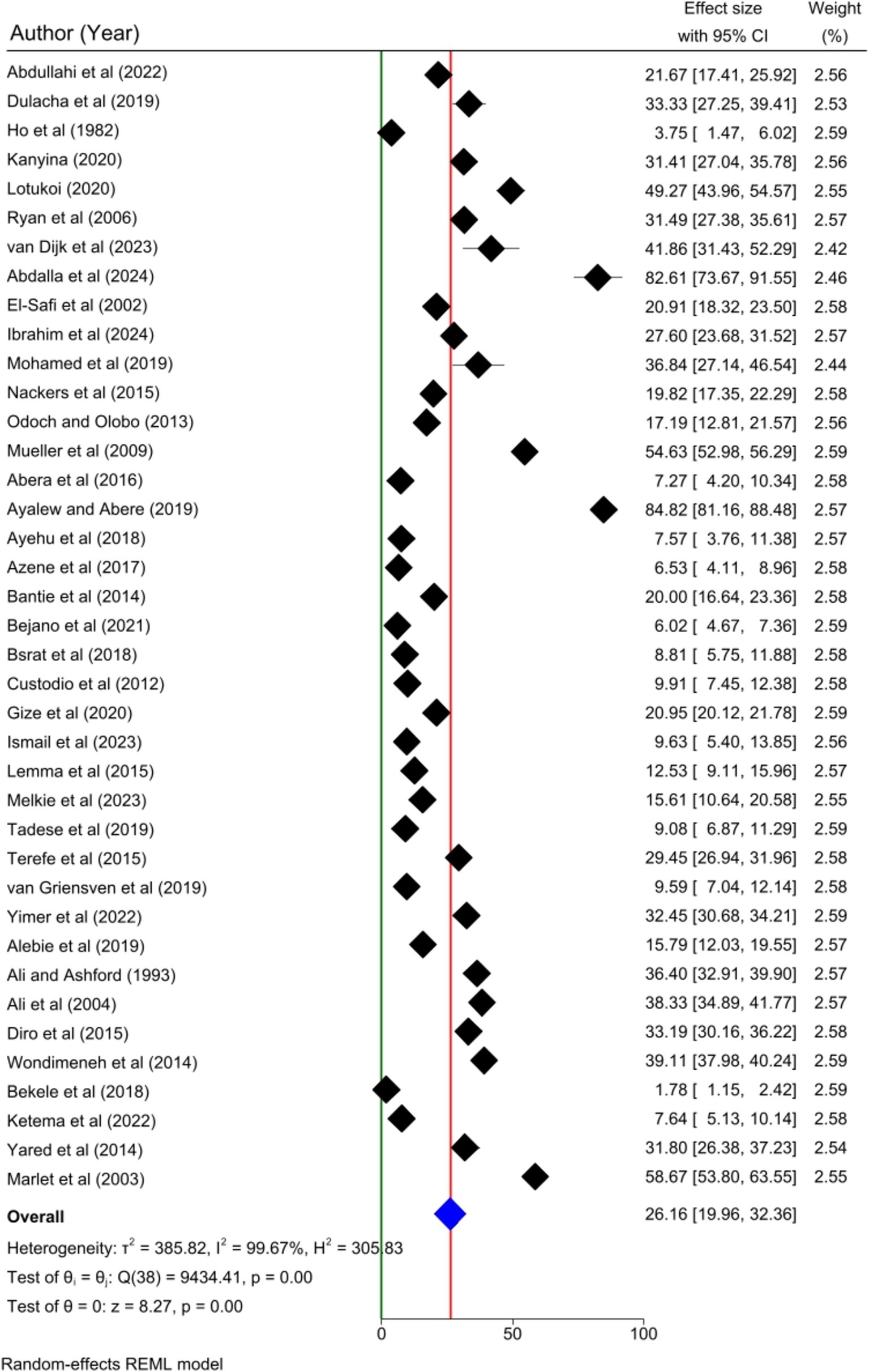
Result: In this meta-analysis, thirty-nine articles with 40,367 study participants were included. The overall pooled prevalence of human visceral leishmaniasis in Eastern Africa was 26.16% [95%; CI: 19.96, 32.36%; I2 = 99.67%; p = 0.00]. Gender, age, family size, presence of termite hill/mound, presence of cattle/domestic animals, outdoor sleeping, presence of VL infected family member/s, and presence of water source/pathway near home were the risk factors significantly associated with human visceral leishmaniasis.
Conclusion: The recorded pooled prevalence of human visceral leishmaniasis in Eastern Africa underscores the urgent need for comprehensive intervention strategies. This includes rigorous health education for residents, covering the disease’s cause, transmission, vector breeding sites, and prevention mechanisms.
Introduction
Visceral leishmaniasis (VL), known as kala-azar, is a potentially fatal, neglected tropical disease caused by the protozoan parasite Leishmania. Transmitted through the bite of infected sandflies, VL primarily affects impoverished communities (1–4). VL is characterized by severe symptoms including fever, weight loss, fatigue, weakness, loss of appetite, enlarged liver and spleen, anemia, and swollen lymph nodes (2, 5). It is the most serious form of leishmaniasis, posing a life-threatening risk if left untreated (6). The parasite, Leishmania donovani, thrives in humans and sandflies, making human populations in Asia and Eastern Africa particularly vulnerable (1, 4, 7, 8). This neglected disease disproportionately impacts the poorest communities, highlighting the urgent need for improved prevention, diagnosis, and treatment strategies to combat its devastating effects (5).
VL is endemic in 80 countries with an estimated annual global incidence of 50,000–90,000 worldwide (9, 10). According to the 2022 World Health Organization (WHO) report, about 85% of global VL cases were reported from seven countries: Brazil, Ethiopia, India, Kenya, Somalia, South Sudan and Sudan (10). The 2017 World Health Organization (WHO) report revealed significant burden of VL in Eastern Africa, with South Sudan reporting the highest number of cases (3474) (11) followed by Sudan with 2902 (12), Ethiopia with 2,141 (13), and Somalia 1,166 cases (14). The disease has also been reported in Kenya, Uganda, and Eritrea (15–17). This trend continued in 2022, with Eastern Africa accounting for a staggering 73% of global VL cases. Alarmingly, half of these cases occurred in children under the age of 15, underscoring the devastating impact of this neglected disease on young lives (18).
A comprehensive global analysis of blood donor data revealed a pooled prevalence of visceral leishmaniasis at 7% (19). Different systematic reviews and meta-analysis conducted on human VL in Iran revealed that the pooled prevalence was 2–3% (2, 20). Moreover, systematic reviews and meta-analysis of studies conducted in Ethiopia, where VL is endemic, revealed a pooled prevalence of the disease ranging from 9.44 to 21%, 9.44% (21) to 21% (22), underscoring the high burden of VL within the country. VL imposes a significant economic burden on affected families in different countries. The costs associated with healthcare, including informal payments for accessing providers, diagnostics and medication, and transport costs for multiple visits can be substantial. These high direct expenses often force individuals to adopt coping strategies like selling or renting assets and taking out loans, which further exacerbating their financial hardship (23–25). Despite the goal of universal health coverage, remote areas with endemic visceral leishmaniasis (VL) face significant obstacles. Their weak and under-resourced health systems struggle to integrate complex VL diagnostic and treatment services into their limited basic healthcare packages. This challenge is further compounded by factors like economically driven migration or massive population displacements during conflicts, exacerbating the burden of the disease (26). Despite being a neglected tropical disease, visceral leishmaniasis (VL) has been prioritized for elimination by 2030 under the Sustainable Development Goals (SDGs), specifically Target 3.3 (27).
Preventing visceral leishmaniasis in Eastern Africa is a complex challenge due to the numerous interconnected risk factors associated with the disease. Factors such as age, sex, environmental conditions like the presence of certain trees or termite mounds, animal ownership such as cattle and dogs, living conditions, awareness levels, occupation, and education all contribute to the disease’s spread. To effectively address the impact and severity of VL, a multi-faceted approach is crucial, considering the interplay of these various risk factors (28–42). Behavioral factors, such as sleeping outdoors, neglecting bed nets, and inadequate insecticide spraying, along with housing conditions like wall type, have been identified as significant risk factors for VL in various studies (29, 31, 32, 34, 36–38, 43–45).
Although many primary studies have been carried out on human visceral leishmaniasis among the countries of Eastern Africa, the findings have not been consistent with the prevalence ranging from 1.8 to 84.8%. Moreover, the findings were not conclusive, and these could hamper the assessments of ongoing intervention efforts and activities (46, 47). Moreover, no study provides evidence of the pooled prevalence of human visceral leishmaniasis in Eastern Africa. Therefore, this systematic review and meta-analysis aimed to estimate the pooled prevalence of human visceral leishmaniasis and identify its risk factors in Eastern Africa. This systematic review and meta-analysis underscore the critical need for prioritizing primary prevention of human visceral leishmaniasis in Eastern Africa. This evidence-based information will be invaluable for policymakers, healthcare planners, and other stakeholders in developing and implementing comprehensive strategies to prevent, control, and mitigate the impact of VL. These insights can contribute to a future where the burden of this neglected tropical disease is significantly reduced in Eastern Africa.
Materials and methods
Study registration
The protocol of this systematic review and meta-analysis was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database under registration number CRD42023427719.
Search strategy
The search was carried out from 23 May 2024 to 17 July 2024 by three independent authors (AKG, CD, and LB). The identified articles were imported into Endnote version 8 software, where duplicate entries were removed. All the relevant articles were selected based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (48).
In this systematic review and meta-analysis, published and unpublished studies were searched from different electronic databases such as PubMed/MEDLINE, Science Direct, Cochrane Library, LIVIVO, CINAHL, African Journals Online, Web of Science, African Index Medicus (AIM), HINARI, Semantic Scholar, Google, and Google Scholar. In addition, gray literature was also identified from digital libraries and repositories of different universities. The search was carried out using the following keywords: “prevalence,” “epidemiology,” “Visceral Leishmaniasis,” “Kala-Azar,” “Kala Azar “, “Black Fever “, “Black Disease,” Dumdum Fever *, “Black Sickness,” “Leishmania infantum,” “Leishmania donovani,” Human Leishmaniosis,” “Human Leishmaniasis,” “Leishmaniasis,” “Leishmaniases,” “associated factor*,” “risk factor*,” determinant*, predictor*, cause*, Burundi, Comoros, Djibouti, Ethiopia, Eritrea, Kenya, Rwanda, Seychelles, Somalia, South Sudan, Sudan, Tanzania, and Uganda. Using the appropriate Boolean operators: “AND” or “OR,” all keywords were combined.
Inclusion criteria
This systematic review and meta-analysis included all published and unpublished studies conducted from January 1982 to July 2024 in countries of Eastern Africa that investigated human visceral leishmaniasis. The studies were conducted both in community and institutional settings and employed cross-sectional, cohort, and case–control designs. Only studies reported in English were included.
Population
All studies conducted on all groups of human population were included.
Exposure
People infected with human VL.
Comparisons
People that were not infected with human VL.
Exclusion criteria
This systematic review and meta-analysis excluded studies that lacked full text, were unidentified, were abstracts, editorials, irretrievable, letters, or did not report human visceral leishmaniasis as the primary outcome.
Measurement of the outcome
This study aimed to determine the pooled prevalence of human visceral leishmaniasis in countries of Eastern Africa. This was calculated by dividing the number of study participants with VL by the total sample size and multiplying by 100. Additionally, the systematic review and meta-analysis investigated factors associated with VL.
Operational definition
Visceral Leishmaniasis: Also known as kala-azar, is a potentially fatal, neglected tropical disease caused by the protozoan parasite Leishmania, transmitted through infected sandflies and characterized by severe symptoms including fever, weight loss, fatigue, weakness, loss of appetite, enlarged liver and spleen, anemia, and swollen lymph nodes (1–5).
Data extraction procedure
A standard data extraction template consisting of several details such as author name, year of publication, country, study setting, study design, method of VL detection, sample size, and prevalence was prepared. Duplicate articles were removed after the relevant articles for inclusion were carefully screened. Three independent authors (AKG, BD, and LB) were involved to undertake the required data extraction activities.
Quality assessment
Using the Joana Brigg Institute (JBI) checklist of critical appraisal for cross-sectional studies, the quality of each article was critically evaluated (49) (Supplementary material). With scores measured on a scale of 100%, the quality of each article was independently assessed by three authors (AKG, CD, and GB). For further analysis, articles having a quality score of above 50% were included (50, 51). A mean score was calculated from the evaluation results of all the reviewers for an agreement in case of any differences when undertaking the quality assessment.
Study selection
Overall, 1,107 studies were identified from an electronic database and reference searching. An Endnote 8 reference manager was used. Two hundred and ninety-seven duplicated articles were removed. The total number of articles excluded based on their titles and abstracts due to the failure to meet the inclusion criteria was 751. Moreover, ten articles were removed as they did not report the outcome of interest and six articles were excluded as they failed to meet quality assessment methods. In this meta-analysis, 39 full-text articles were included to estimate the pooled prevalence of human VL in Eastern Africa by following the PRISMA 2020 guideline (Figure 1).
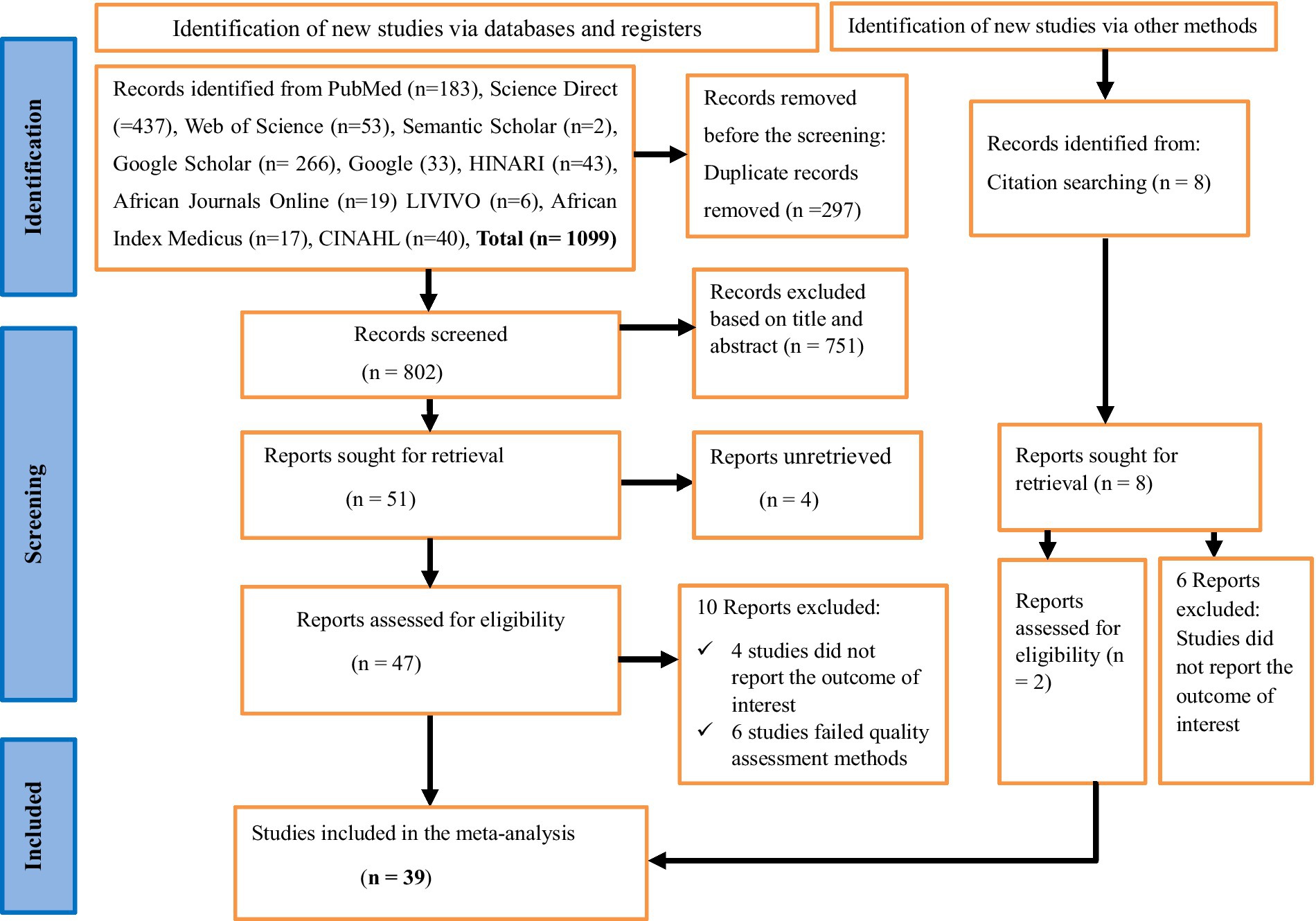
Figure 1. A PRISMA flow chart showing study selection for systematic review and meta-analysis of the prevalence of human visceral leishmaniasis and its risk factors in Eastern Africa, 2024.
Statistical analysis procedures
After the data were extracted, analysis was made using STATA (Corporation, College Station, Texas, United States) version 17 software after the extracted data were successfully imported in to it. Heterogeneity within the included studies was assessed using the Higgs I2 test, with values of 75, 50, and 25% showing high, moderate, and low levels of heterogeneity, respectively (52). With a 95% confidence interval, a restricted maximum-likelihood (53) method of random-effects model was used to determine the pooled prevalence of human visceral leishmaniasis in Eastern Africa. The odds ratio was computed to show the strength of the association between human VL (the outcome variable) and its risk factors.
The pooled prevalence of human visceral leishmaniasis was presented using a forest plot. To determine the influence of an individual study on the pooled prevalence estimate of VL, sensitivity analysis was performed. Sub-group analysis was also done to identify the possible sources of heterogeneity based on the year of publication (before 2016 and 2016 and after), country category (Ethiopia, Kenya, Sudan, and other countries), study setting (healthcare facility-based and community-based), study design (cross-sectional, case–control and cohort), and sample size category (lower than 1,000, and 1,000 and above). Additionally, a funnel plot and Egger’s test were used to determine the presence of potential publication bias (54).
Results
Characteristics of the included studies
In this meta-analysis, twenty-six cross-sectional (28–31, 33, 34, 36–38, 40–45, 47, 55–64), eight cohort (46, 65–71), and five case–control (32, 35, 39, 72) studies with a total of 40,367 study subjects were included. The highest prevalence of human VL was 84.8% (46), and the lowest prevalence was 1.8% (47) among the included studies. Regarding the country in which the studies were conducted, twenty-four were in Ethiopia (3, 29–34, 37, 41–47, 56–58, 64–66, 69–71), seven studies were in Kenya (28, 35, 38, 60, 61, 63, 72), five studies were in Sudan (36, 39, 55, 59, 62), two studies were in Uganda (40, 68), and the other study was conducted in Somalia (67). Based on study setting, twenty-two of the included studies (28–31, 33–41, 43, 47, 56–60, 63, 72) were community-based and the remaining fifteen studies (32, 42, 44–46, 55, 61, 62, 65–71) were healthcare facility-based. Regarding the diagnostic method/s of VL, twenty-five studies used serological examination (3, 29–32, 34, 35, 37, 39, 40, 44, 45, 47, 55, 56, 60, 61, 63–67, 70–72), five studies used immunological test (33, 41, 57–59), six studies (28, 36, 38, 43, 46, 69) unspecified the diagnostic method/s used, and the remaining three studies used molecular diagnosis (62), a combination of serological and molecular diagnosis (42), and a combination of serological and parasitological examination (68). Regarding the year of publication, twenty-three of the included studies (28–31, 33–36, 38, 41, 42, 44–47, 56, 61, 62, 64, 66, 70–72) were published in 2016 and after, and the remaining 16 studies (3, 32, 37, 39, 40, 43, 55, 57–60, 63, 65, 67–69) were published before 2016. Based on the sample size, thirty-two studies had a sample size of lower than1000 (28–32, 34–46, 55–63, 65, 67, 72), and the remaining seven studies had a sample size of 1,000 and above (33, 47, 66, 68–71) (Table 1).
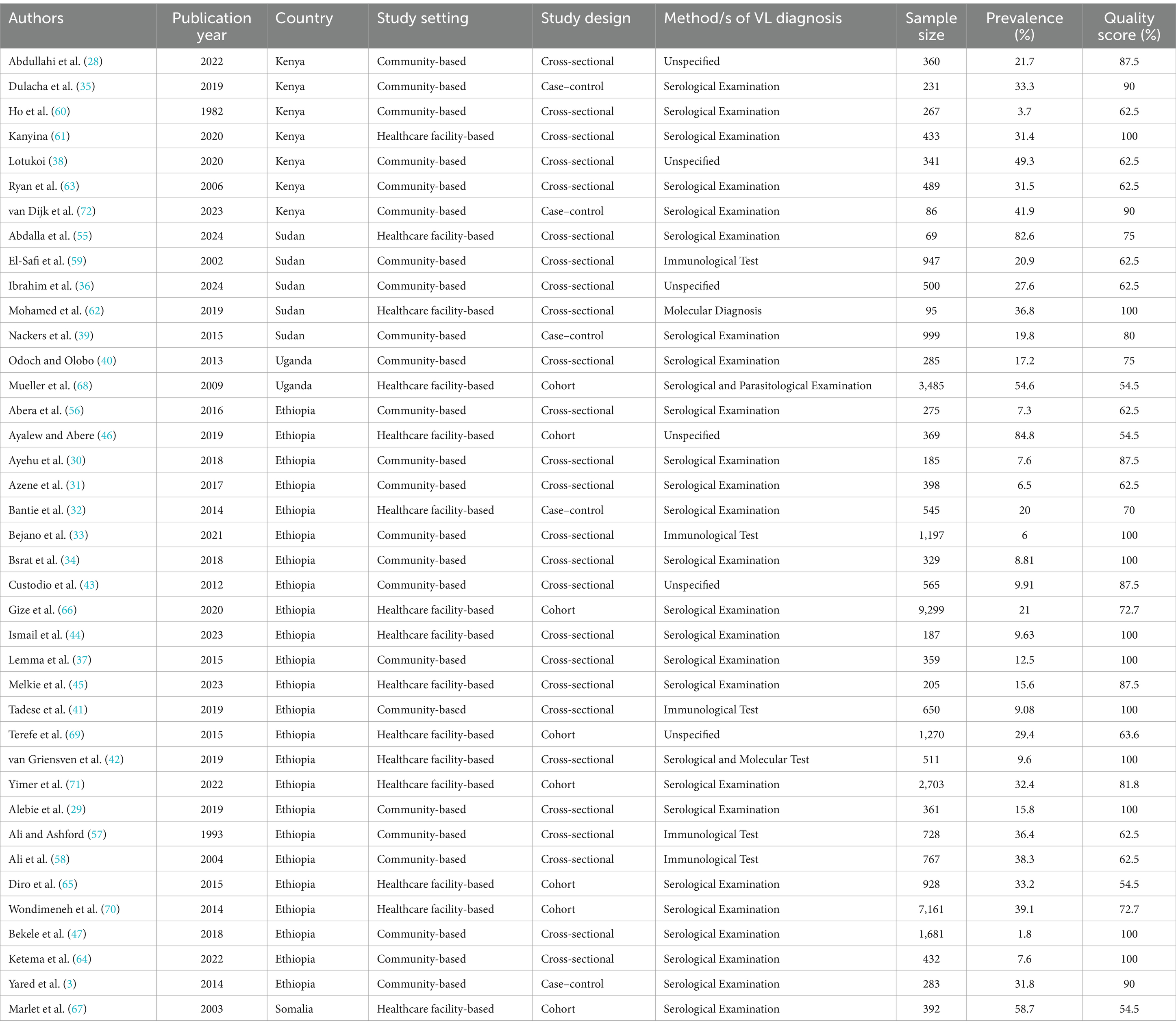
Table 1. Characteristics of the included studies to determine the pooled prevalence of human VL in Eastern Africa, 2024.
Meta-analysis
Pooled prevalence of human visceral leishmaniasis
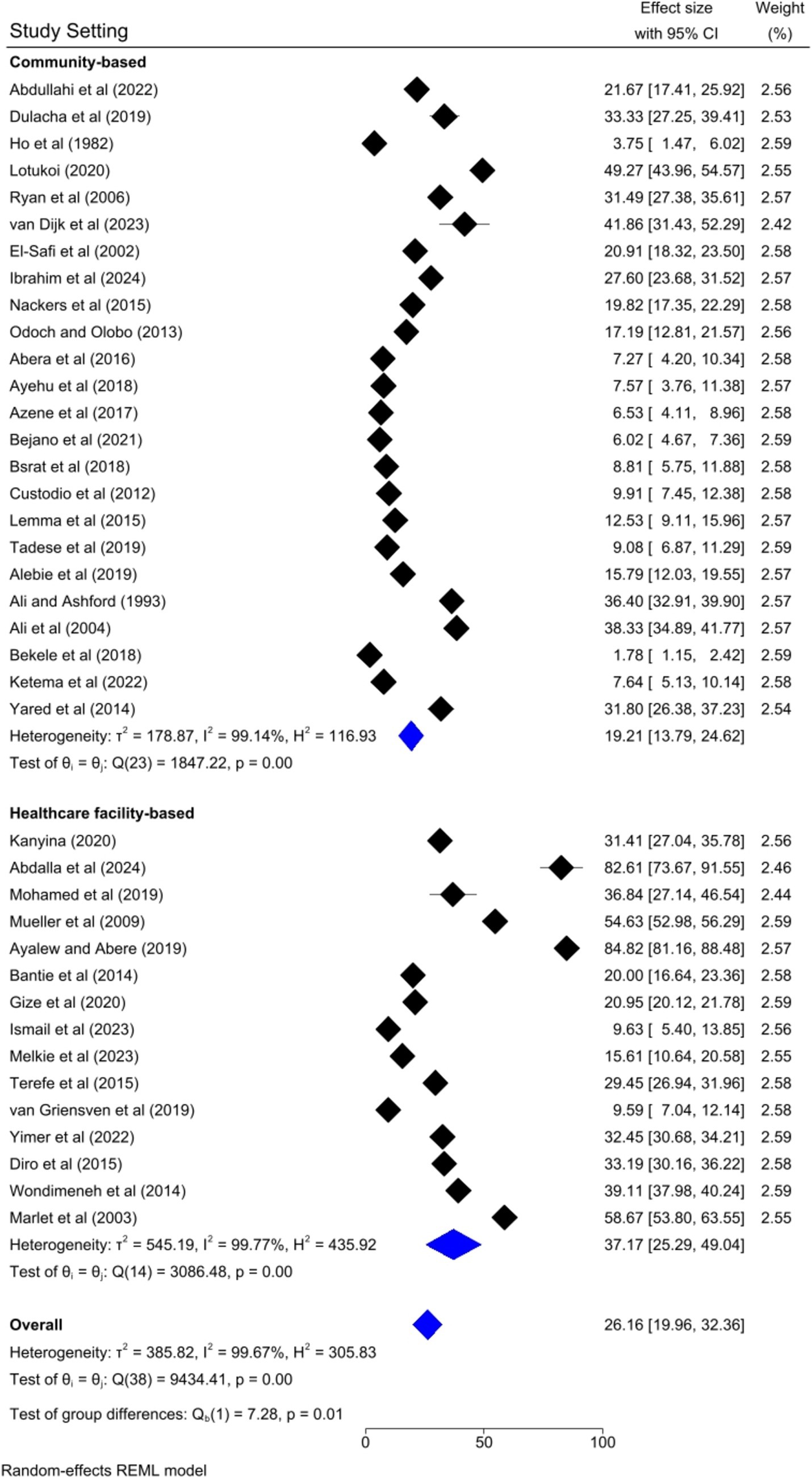
Thirty-nine articles were included to determine the pooled prevalence of human visceral leishmaniasis in this meta-analysis. The pooled prevalence of human visceral leishmaniasis in Eastern Africa was 26.16% [95%; CI: 19.96, 32.36%; I2 = 99.67%; p = 0.00]. A random effects model was employed to estimate the pooled prevalence of human VL (Figure 2).
Test for publication bias
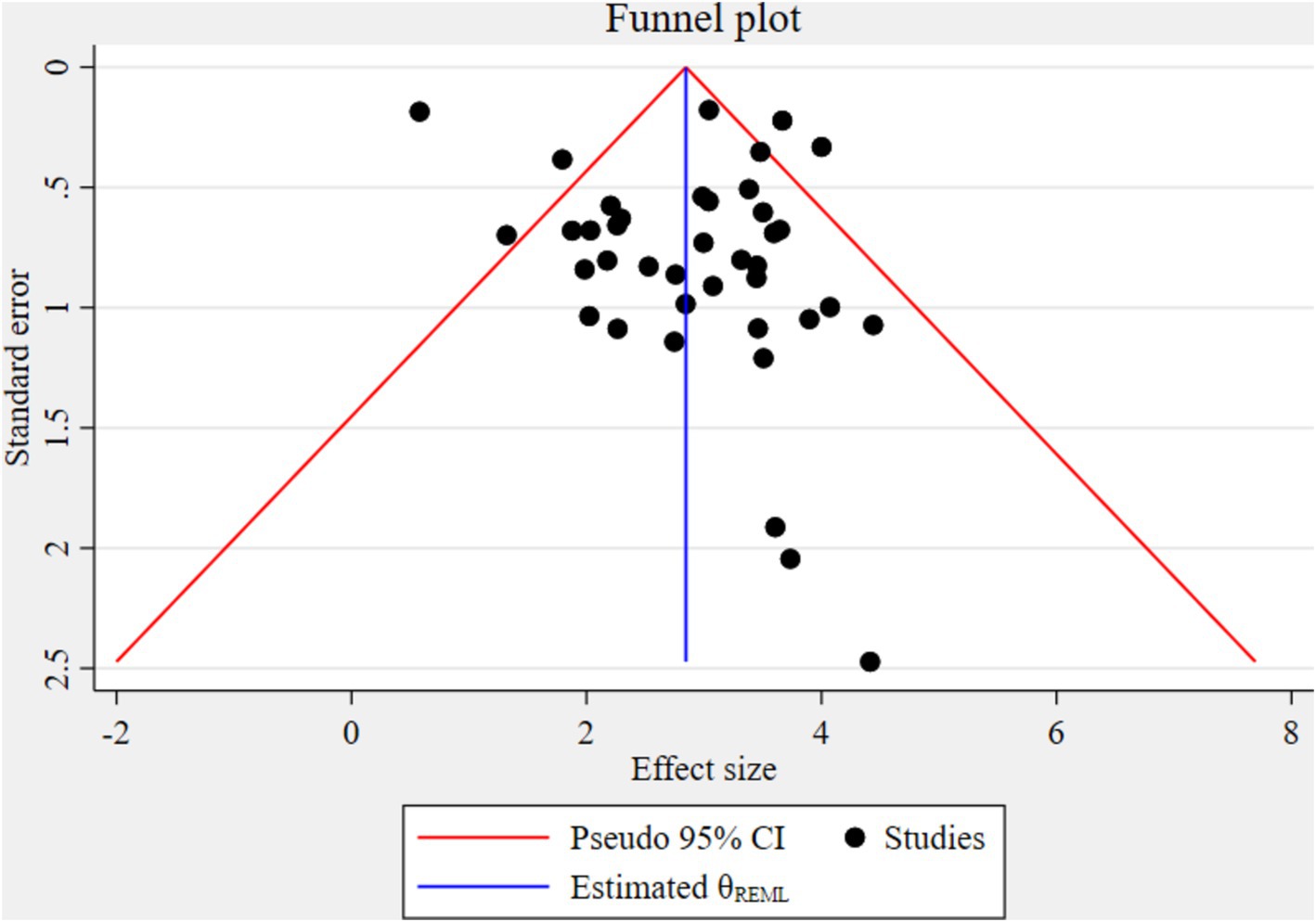
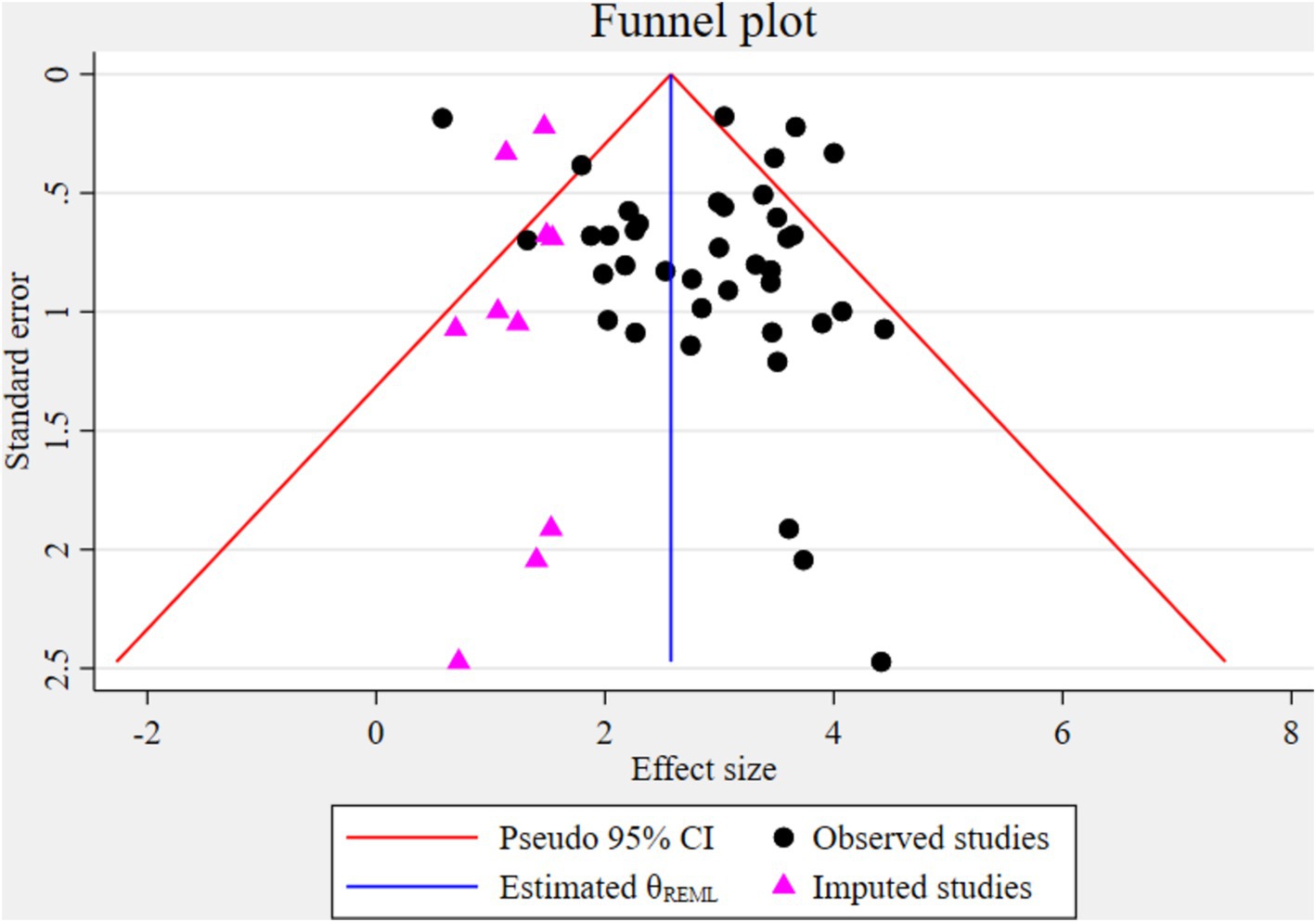
The funnel plot indicated significant publication bias (Figure 3). Statistically, Eggers’s test result also depicted statistically significant publication bias (small studies effect) (p = 0.028). A trim and fill analysis was conducted to identify the source of publication bias, resulting in a notable variation in the adjusted point estimate of the pooled odds ratio (OR = 2.58; 95% CI: 2.26–2.89), compared to the initial or observed point estimate (OR = 2.85; 95% CI: 2.52–3.18) (Figure 4).
Sensitivity analysis
The impact of individual studies on the pooled estimate of human VL was successfully evaluated by performing a sensitivity analysis. The finding revealed that none of the included studies affected the pooled estimate (Figure 5).
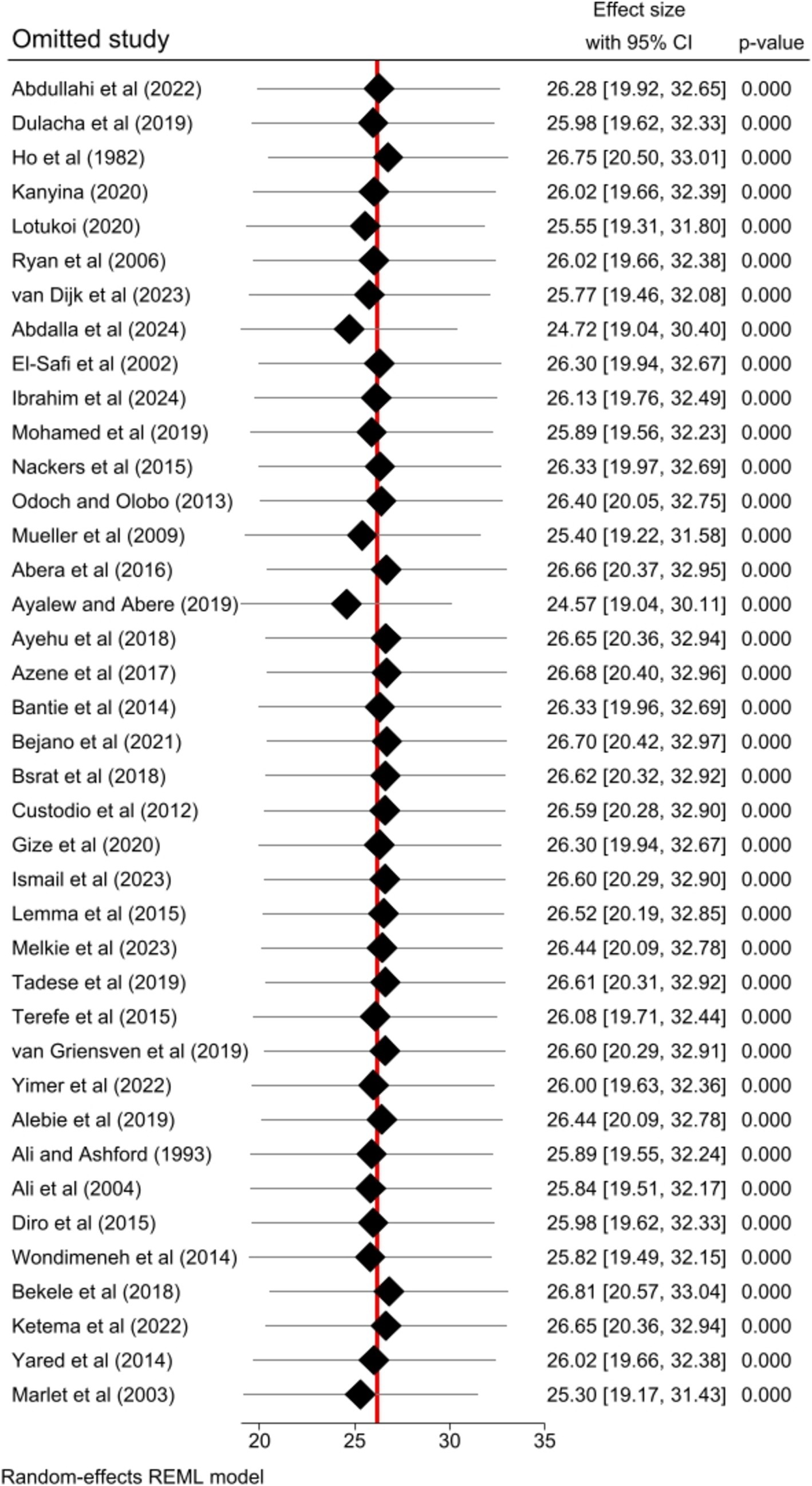
Figure 5. Sensitivity analysis result of the included studies for the pooled prevalence of human VL in Eastern Africa, 2024.
Subgroup analysis
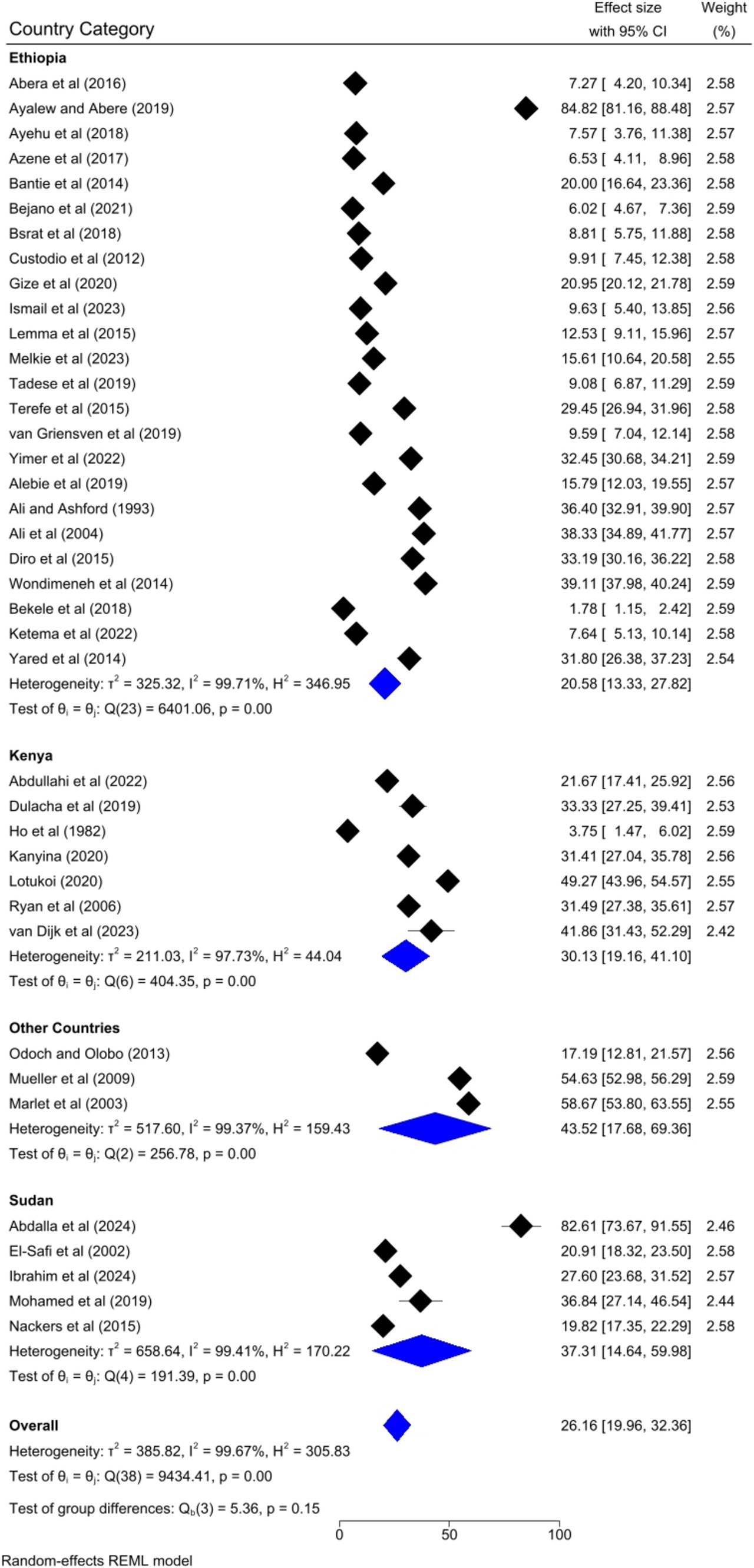
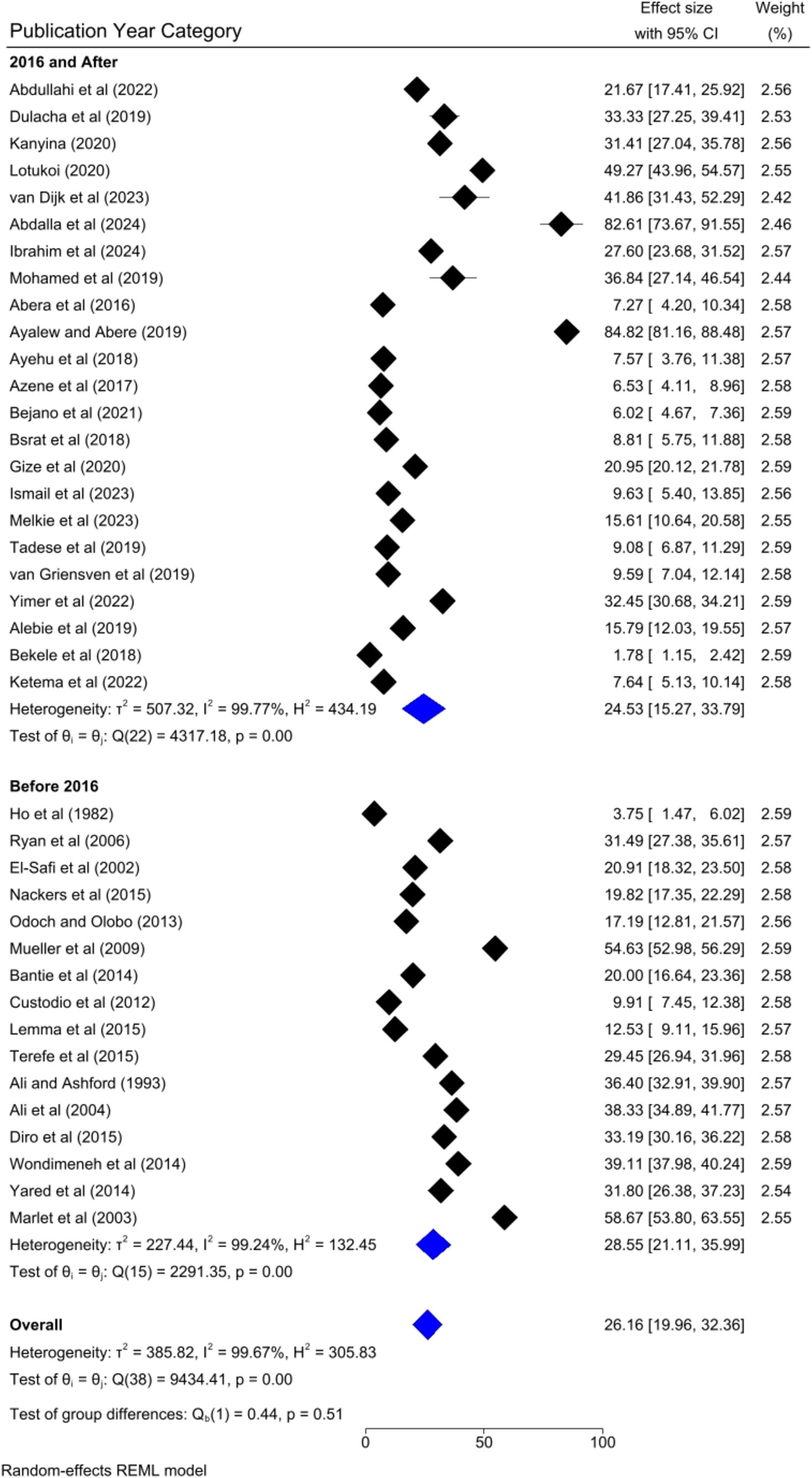
Based on the country category in which the studies were conducted, the highest human VL pooled prevalence was registered in studies conducted in other countries (i.e., Uganda and Somalia) [43.52, 95% CI: 17.68–69.36%] as compared to studies conducted in Sudan [37.31, 95% CI: 14.64–59.98%], Kenya [30.13, 95% CI: 9.161–41.10%], and Ethiopia [20.58, 95% CI: 13.33–27.82%] (Figure 6). Based on the study setting in which the studies were conducted, the highest pooled prevalence of human VL was recorded among studies conducted at the healthcare facility [37.17, 95% CI: 25.29–49.04%], compared to studies conducted in the community [19.21, 95% CI: 13.79–24.62%] (Figure 7). Regarding the articles’ publication year category, the highest pooled human VL was observed among studies published before 2016 [28.55, 95% CI: 21.11–35.99%] as compared to those studies published in 2016 and after [24.53, 95% CI: 15.27–33.79%] (Figure 8).
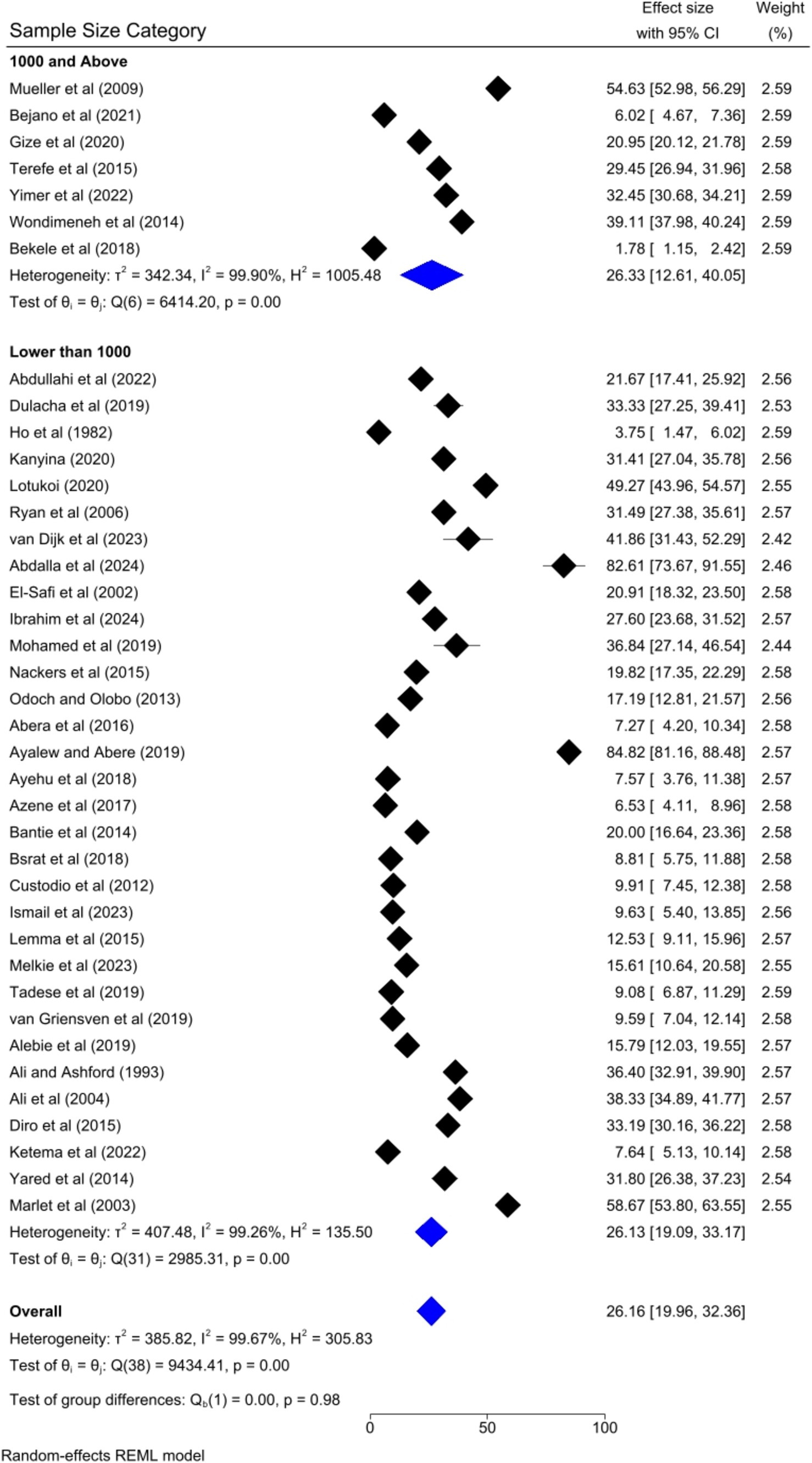
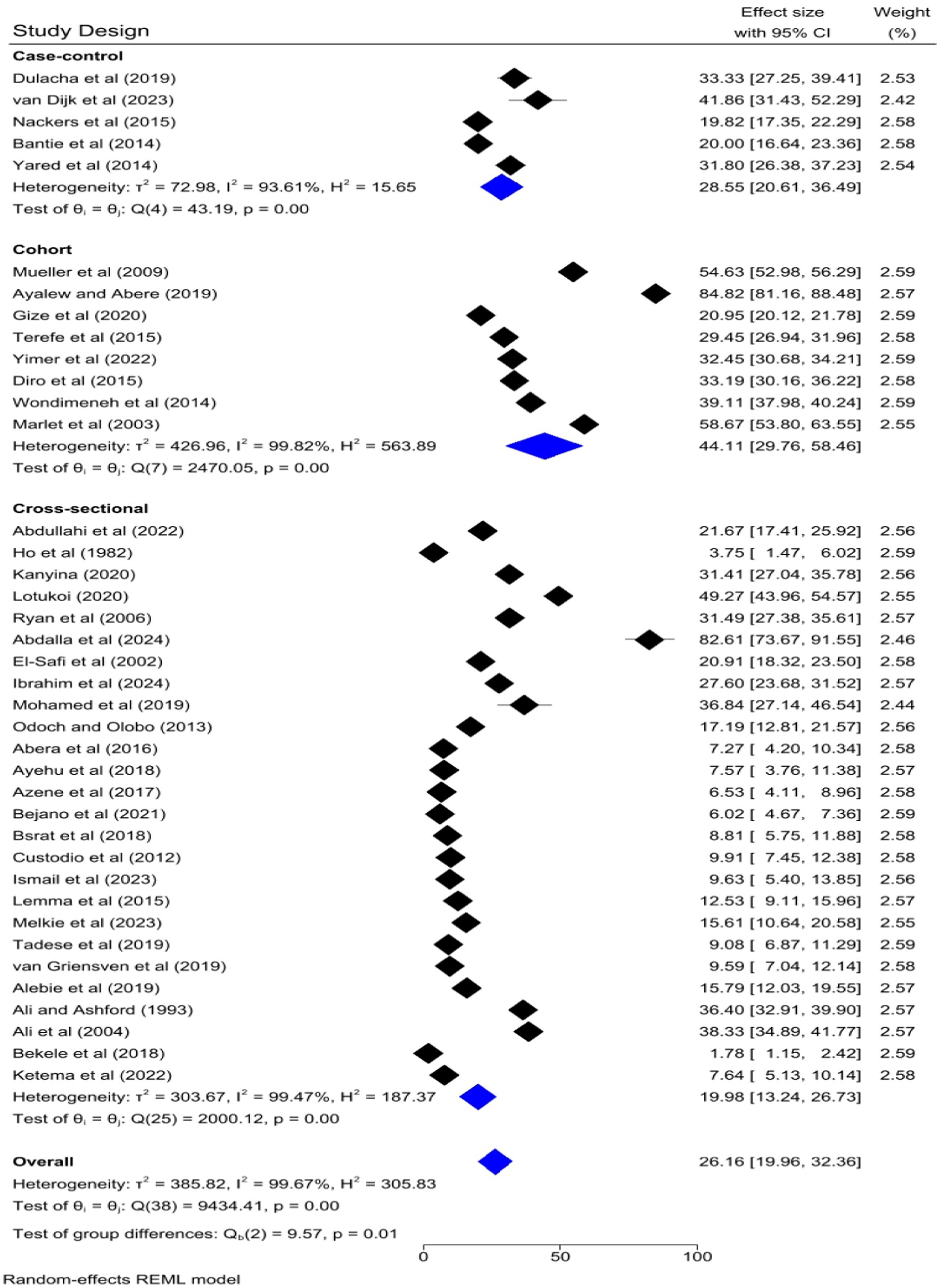
The pooled prevalence of VL was slightly higher among studies that had a sample size of 1,000 and above [26.33, 95% CI: 12.61–40.05%] as compared to studies that had a sample size of lower than 1,000 [26.13, 95% CI: 19.09–33.17%] (Figure 9). Regarding the study design, the highest pooled prevalence of VL was registered among cohort studies [44.11, 95% CI: 29.76–58.46] followed by case–control [28.55, 95% CI: 20.61–36.49%] and cross-sectional studies [19.98, 95% CI: 13.24–26.73%] (Figure 10).
Meta-regression
To identify the source of heterogeneity by considering, country category, study setting, study design, publication year category, and sample size category as factors, a univariate meta-regression analysis was performed. However, statistical significance was demonstrated by two of these variables (Table 2).

Table 2. A univariate meta-regression analysis to pinpoint the factors associated with the heterogeneity of human VL pooled prevalence in Eastern Africa, 2024.
Risk factors associated with human visceral leishmaniasis in Eastern Africa
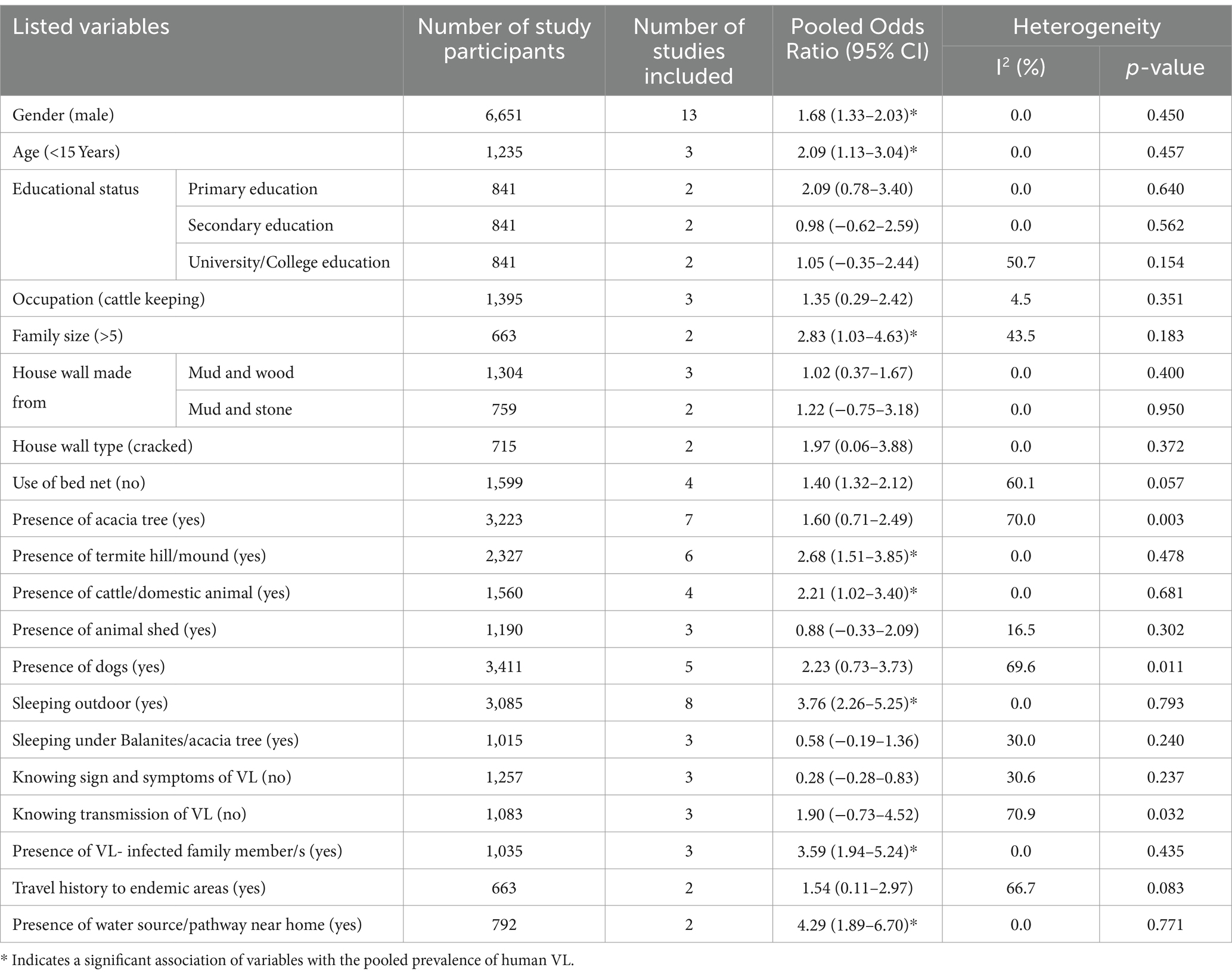
Twenty-three factors were repeatedly presented in the included articles of this meta-analysis. The factors were gender, age (< 15 years), educational status (primary education, secondary education, university/college education), family size >5, occupation (cattle keeping), house wall made from (mud and wood, mud and stone), house wall type (cracked), use of bed net, presence of acacia tree, presence of termite hill/mound, presence of cattle/domestic animal, presence of animal shed, presence of dogs, outdoor sleeping, sleeping under Balanites/acacia tree, knowing sign and symptoms of VL, knowing transmission of VL, presence of VL- infected family member/s, travel history to endemic areas, and presence of water source/pathway near home (Table 3).
The association between gender and human VL among the thirteen studies (29, 32–35, 38–41, 43, 45, 64, 72) has been assessed. The result showed a significant association in nine of the included studies. According to this meta-analysis, the odds of VL infection were 68% higher among males compared to females [POR = 1.68; 95% CI: 1.33–2.03]. The association between age below 15 years and VL among the three studies (29, 32, 34) has been determined. The result showed a significant association in two of the studies. According to the results of the random effect meta-analysis, the odds of VL infection were 2.09 times higher among people aged below 15 years compared to people aged 15 years and above [POR = 2.09; 95% CI: 1.13–3.04]. Two studies (35, 64) were considered to determine the association between family size >5 and VL. One of them was significantly associated with the outcome of interest. The odds of VL infection were 2.83 times higher among people who had a family of more than five as compared to their counterparts [POR = 2.83; 95% CI: 1.03–4.63].
Based on the findings of six studies (28, 29, 31, 32, 35, 64) the association between VL and the presence of termite hill/mound was assessed. In five of these studies, a positive association was found. According to the results of this meta-analysis, the odds of VL infection were 2.68 times higher among people who had termite hill/mound near their home compared to their counterparts [POR = 2.68; 95% CI: 1.51–3.85] (Table 3). The link between the presence of cattle/domestic animals and the outcome variable VL was assessed with four articles (30–32, 64). Their link was significant in three of the included studies. This finding revealed that the odds of VL infection were 2.21 times higher among people who had cattle/domestic animals compared to their counterparts [POR = 2.21; 95% CI: 1.02–3.40].
Eight articles (29, 31, 32, 34, 37, 38, 43, 44) were included to identify the association between sleeping outdoors and the pooled prevalence of VL. Seven of the included studies had a significant association with VL. Based on the results of the meta-analysis, it was revealed that the odds for the occurrence of VL infection among people who practiced outdoor sleeping were 3.76 times higher when compared to people who did not practice outdoor sleeping [POR = 3.76; 95% CI: 2.26–5.25]. The association between the presence of VL- infected family member/s and the prevalence of VL was assessed by the included three studies (31, 45, 64). Two of the included studies showed a positive association with the prevalence of VL. The result of this meta-analysis found that the odds of VL infection were 3.6 times higher among people who had VL- infected family member/s compared to those who did not have VL- infected family member/s [POR = 3.59; 95% CI: 1.94–5.24].
The association between the presence of water source/pathway near home and VL infection was determined by two studies (28, 64). The association was positive in both of the studies. The random-effect meta-analysis of this study revealed that the odds of VL infection were 4.29 times higher among people whose home is near to water source/pathway compared to their counterparts [POR = 4.29; 95% CI: 1.89–6.70] (Table 3).
Discussion
Visceral leishmaniasis is a devastating parasitic disease that has continued to be a global burden to have a substantial impact on health that primarily affects the poorest and most marginalized communities in the world (25, 73). The burden of VL is particularly high in South Asia, East Africa, and the Mediterranean basin. The economic and social consequences of VL are severe, as the disease disproportionately affects individuals who are already vulnerable (10, 25). VL is a major public health problem, particularly in Eastern Africa, where it is considered a neglected tropical disease. The disease is endemic in countries of Eastern Africa like Ethiopia, Kenya, Sudan, and South Sudan, with high transmission rates in rural communities (25, 74, 75). The impact of VL in East Africa is compounded by factors like poverty, poor sanitation, conflict, and displacement (26).
The pooled prevalence of human visceral leishmaniasis in Eastern Africa was 26.16% [95%; CI: 19.96, 32.36%]. However, the report is higher than the pooled prevalence of VL obtained from a global systematic review and meta-analysis done among blood donors (7%) (19) and peoples with human immunodeficiency virus (HIV) (6%) (76). Moreover, the figure is higher than the findings of systematic reviews and meta-analysis pooled prevalence report of human VL in Iran conducted by Rahmanian et al. (3%) (20) and Rostamian et al. (2%) (2). The finding is also higher than the reported pooled prevalence of visceral leishmaniasis in Ethiopia by Haftom et al. (9.44%) (21) and Ayalew Assefa (16%) (22). The high prevalence and the pattern might reflect the lack of and inefficient access to affordable and active drugs, incorrect prescribing, and poor compliance undermine case management and perpetuate anthroponotic infection, and inadequate and unsustainable vector control (77). Other evidence suggests that this trend is continued due to the continuing widespread conflict in Eastern Africa, which destroyed housing and health care infrastructure. This in turn resulted in forced migrations to endemic areas that promote the emergence of VL (78). Moreover, the lack of advancement in diagnostics in field and facility settings, proficient vaccination of the disease, and unaffordability of the new advanced technologies contribute to the pattern to continue (79, 80).
Regarding subgroup analysis, the pooled prevalence of visceral leishmaniasis across the study setting is significantly heterogeneous after a univariate meta-regression. This might be because community-based studies often include a broader and more diverse population, including asymptomatic individuals or those with mild symptoms who might not seek medical care. In contrast, healthcare facility studies typically involve patients already symptomatic and seeking treatment, leading to higher observed prevalence rates. Moreover, community-based studies might use random sampling, while healthcare facility studies often use convenience sampling, which can introduce bias.
The univariate meta-regression analysis also found that the sample size category was a significant factor for the heterogeneity of the pooled prevalence of visceral leishmaniasis in Eastern Africa. This might be due to the statistical power in which the studies with smaller sample sizes often have less statistical power which leads to greater variability in prevalence estimates. This variability can contribute to heterogeneity when these studies are pooled with larger studies. Moreover, the sampling errors might be the reason for the significant heterogeneity of the pooled prevalence as smaller studies are more susceptible to sampling error, where the sample may not accurately represent the broader population. This can result in prevalence estimates that differ significantly from those of larger, more representative studies.
The odds of VL infection were higher among males compared to females. This finding is supported by a systematic review and meta-analysis report in Ethiopia in which males were 67% at higher risk of VL infection as compared to females (21), and a study conducted in India and Nepal which revealed that males were at greater risk of VL infection by a factor of 2.4 as compared to females (81). This might be because, socio-culturally, men are often more exposed to environments where sandflies, the vectors of the disease, are prevalent. This is because men are more likely to engage in outdoor activities such as farming, herding, or sleeping outside, which increases their risk of being bitten by infected sandflies which results in VL infection (82, 83).
The odds of VL infection were higher among people aged below 15 years compared to people aged 15 years and above. This finding is supported by the fact that Eastern Africa accounted for 73% of global VL cases, half of which occurred in children aged under 15 years in 2022 according to the WHO (84). This might be due to people aged less than 15 years being more likely to engage in activities that increase their exposure to sandfly bites, such as playing outdoors, which further elevates their risk. However, contrary to this finding, a cross-sectional study conducted in Southeastern Nepal revealed that the risk of VL infection among people aged ≥15 years was 5.5 times greater as compared to people aged <15 years (4). This inconsistency might be due to the difference in outdoor movement practiced by people under fifteen years and their counterparts. The odds of VL infection were 2.83 times higher among people who had a family of more than five as compared to their counterparts. This finding is supported by a study conducted in Nepal which elucidated that the risk of VL infection among families ≥6 in number was greater by a factor of 4.4 compared to their counterparts (4). Similarly, another study conducted in Nepal evidenced that large size households (>5 persons) were 3.6 times at greater risk of VL infection (85).
The odds of VL infection were higher among people who had termite hills/mounds near their homes compared to their counterparts. This finding is supported by a systematic review that explored the burrows of mammals, caves, crevices, termite hills, and walls with cracks that may serve as breeding sites for sand flies which later increase the transmission and infection of VL (86). The finding is also supported by a study conducted in Ethiopia in which the presence of termite hills was a risk factor for visceral leishmaniasis (87). Moreover, the presence of termite hills is one of the major landscape factors associated with increasing the risk of leishmaniasis infection. The presence of termite hill increases the risk of infection by a factor of 2.4 (88). This might be due to the roles as breeding and resting sites for sandflies, the primary vectors of the disease. This is evidenced by a study conducted in Colombia (89) in which termite hills provide an ideal microhabitat with stable temperature and humidity, which are conducive to the lifecycle of sandflies. These insects thrive in such environments, increasing the likelihood of human-sandfly interactions, especially in rural areas where people live or work near termite mounds. Consequently, individuals in these areas are at a higher risk of being bitten by infected sandflies, leading to a greater incidence of visceral leishmania.
The odds of VL infection were higher among people who had cattle/domestic animals compared to their counterparts. This aligns with findings from a systematic review of South Asian VL (90) and a study in India (91), which indicated that livestock ownership increases the risk of VL infection. In this meta-analysis, it was revealed that the odds for the occurrence of VL infection among people who practiced outdoor sleeping were 3.76 times higher when compared to people who did not practice outdoor sleeping. This finding is supported by a systematic review on VL (86) and a study conducted in India (92) which evidenced that sleeping outside was associated with increased risk of VL. This might be due to the increased exposure to sandflies which are most active during the night and sleeping outdoors increases the likelihood of being bitten by these insects, which can carry the Leishmania parasite. Moreover, lack of protective measures such as insecticide-treated bed nets or screens, and environmental factors of the outdoor environments (like vegetation, humidity, and temperature) especially in endemic areas, often provide ideal breeding grounds for and attract sandflies to outdoor sleeping areas which increase the chance of sandfly biting and risk of VL infection in the end.
The odds of VL infection higher among people who had VL- infected family member/s compared to those who did not have VL- infected family member/s. This finding is supported by a systematic review and meta-analysis of factors associated with visceral leishmaniasis in the Americas (93), and a systematic review of environmental and socioeconomic risk factors associated with visceral leishmaniasis (86). Another study conducted in Nepal supported this finding and evidenced that the proximity to previous VL cases was a strong risk factor for VL by 3.79-fold (85). Moreover, this is also congruent with another study conducted in India (92) in which living in the same household with a VL case was associated with a markedly elevated risk of VL. The reason for this might be the shared environmental and behavioral factors within households, such as living conditions and exposure to sandflies (the vector for VL), contribute to the increased risk of the disease.
The odds of VL infection were higher among people whose homes are near water source/pathway compared to their counterparts. This finding is supported by a study in Nepal, which demonstrated that living near water sources, such as ponds, significantly increased the risk of VL infection by a factor of 3.7 (4). Similarly, a study in India also found that proximity to water bodies increased the risk of VL infection (91).
Limitations of the study
Even though the systematic review and meta-analysis was conducted based on the latest PRISMA guideline, it faced limitations due to the substantial heterogeneity among included studies. The disparity in sample sizes, with some studies involving large participants, while others involved small participants, introduced potential bias and limited the generalizability of findings. Furthermore, the contrasting study settings, with some studies conducted in healthcare facilities and others in community settings, created substantial variability in exposure to risk factors and VL prevalence. This heterogeneity made it challenging to draw robust conclusions about the pooled prevalence of VL and its associated risk factors. Despite these limitations, this review provides valuable insights into the complexities of VL transmission and highlights the need for further research to address the most effective intervention mechanism/s.
Conclusion
In this study, the recorded pooled prevalence of human visceral leishmaniasis in Eastern Africa was higher (26.16%). Gender, age, family size, presence of termite hill/mound, presence of cattle/domestic animals, outdoor sleeping, presence of VL infected family member/s, and presence of water source/pathway near home were the risk factors significantly associated with human visceral leishmaniasis. While further research is needed to determine the most effective and timely intervention methods for visceral leishmaniasis, this study highlights the urgent need for comprehensive intervention strategies in Eastern Africa. This includes rigorous health education for residents, covering the disease’s cause, transmission, vector, and breeding sites, with strong collaboration between the WHO, government officials, non-governmental organizations (NGOs), and healthcare providers. Additionally, promoting health-seeking behavior for prompt treatment and implementing prevention strategies like avoiding outdoor sleep between dusk and dawn, wearing protective clothing, and using insect repellents are crucial steps toward controlling VL in the region.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Supervision, Validation, Writing – review & editing. LB: Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing. BD: Investigation, Software, Supervision, Validation, Visualization, Writing – review & editing. CD: Funding acquisition, Methodology, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1488741/full#supplementary-material
Abbreviations
OR, Odds Ratio; POR, Pooled Odds Ratio; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; VL, Visceral Leishmaniasis; WHO, World Health Organization.
References
1. Scarpini, S, Dondi, A, Totaro, C, Biagi, C, Melchionda, F, Zama, D, et al. Visceral Leishmaniasis: epidemiology, diagnosis, and treatment regimens in different geographical areas with a focus on pediatrics. Microorganisms. (2022) 10:1887. doi: 10.3390/microorganisms10101887
2. Rostamian, M, Bashiri, H, Yousefinejad, V, Bozorgomid, A, Sohrabi, N, Raeghi, S, et al. Prevalence of human visceral leishmaniasis in Iran: a systematic review and meta-analysis. Comp Immunol Microbiol Infect Dis. (2021) 75:101604. doi: 10.1016/j.cimid.2020.101604
3. Yared, S, Deribe, K, Gebreselassie, A, Lemma, W, Akililu, E, Kirstein, OD, et al. Risk factors of visceral leishmaniasis: a case control study in North-Western Ethiopia. Parasit Vectors. (2014) 7:1–11. doi: 10.1186/s13071-014-0470-1
4. Schenkel, K, Rijal, S, Koirala, S, Koirala, S, Vanlerberghe, V, Van der Stuyft, P, et al. Visceral leishmaniasis in southeastern Nepal: a cross-sectional survey on Leishmania donovani infection and its risk factors. Trop Med Int Health. (2006) 11:1792–9. doi: 10.1111/j.1365-3156.2006.01735.x
5. Chappuis, F, Sundar, S, Hailu, A, Ghalib, H, Rijal, S, Peeling, RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. (2007) 5:873–82. doi: 10.1038/nrmicro1748
6. Lyons, S, Veeken, H, and Long, J. Visceral leishmaniasis and HIV in Tigray, Ethiopia. Trop Med Int Health. (2003) 8:733–9. doi: 10.1046/j.1365-3156.2003.01088.x
7. World Health Organization. WHO bi-regional consultation on the status of Leishmaniasis control and surveillance in East Africa. Nairobi, Kenya: World Health Organization (2018).
8. Abongomera, C, van Henten, S, Vogt, F, Buyze, J, Verdonck, K, and van Griensven, J. Prognostic factors for mortality among patients with visceral leishmaniasis in East Africa: systematic review and meta-analysis. PLoS Negl Trop Dis. (2020) 14:e0008319. doi: 10.1371/journal.pntd.0008319
9. Ruiz-Postigo, JA, Jain, S, Madjou, S, Agua, JFV, Maia-Elkhoury, AN, Valadas, S, et al. Global leishmaniasis surveillance, 2022: assessing trends over the past 10 years/surveillance mondiale de la leishmaniose, 2022: evaluation des tendances des 10 dernieres annees. Wkly Epidemiol Rec. (2023) 98:471–88.
10. WHO. Leishmaniasis (2023). Available at: https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis (accessed July 22, 2024).
11. WHO. Leishmaniasis country profile—2015: South Sudan. (2017). Available at: https://leishinfowho-cc55.es/wp-content/uploads/2022/07/pdfs/country-profiles/South-Sudan/LEISHMANIASIS_CP_SSD_2015.pdf (accessed July 25, 2024).
12. WHO. Leishmaniasis country profile—2015: Sudan. (2017) Available at: https://leishinfowho-cc55.es/wp-content/uploads/2022/07/pdfs/country-profiles/Sudan/LEISHMANIASIS_CP_SDN_2015.pdf (accessed July 25, 2024).
13. WHO. Leishmaniasis country profile—2015: Ethiopia. (2017) Available at: https://leishinfowho-cc55.es/wp-content/uploads/2022/07/pdfs/country-profiles/Ethiopia/LEISHMANIASIS_CP_ETH_2015.pdf (accessed July 25, 2024).
14. WHO. Leishmaniasis country profile—2015: Somalia. (2017) Available at: https://leishinfowho-cc55.es/wp-content/uploads/2022/07/pdfs/country-profiles/Somalia/LEISHMANIASIS_CP_SOM_2015.pdf (accessed July 25, 2024).
15. WHO. Leishmaniasis country profile—2015: Uganda. (2018) Available at: https://leishinfowho-cc55.es/wp-content/uploads/2022/07/pdfs/country-profiles/Uganda/LEISHMANIASIS_CP_UGA_2015.pdf (accessed July 25, 2024).
16. WHO. Leishmaniasis country profile—2015: Kenya. (2017) Available at: https://leishinfowho-cc55.es/wp-content/uploads/2022/07/pdfs/country-profiles/Kenya/LEISHMANIASIS_CP_KEN_2017.pdf (accessed July 25, 2024).
17. Alamin, AA. Visceral Leishmaniasis in a non-endemic region of Eritrea. Cureus. (2020) 12:e11318. doi: 10.7759/cureus.11318
18. WHO. New framework launched to eliminate visceral leishmaniasis in eastern Africa. (2024) Available at: https://www.who.int/news/item/12-06-2024-new-framework-launched-to-eliminate-visceral-leishmaniasis-in-eastern-africa (accessed October 22, 2024).
19. Asfaram, S, Fakhar, M, Soosaraei, M, Hosseini Teshnizi, S, Mardani, A, Banimostafavi, ES, et al. Global status of visceral leishmanial infection among blood donors: a systematic review and meta-analysis. Transfus Apher Sci. (2017) 56:748–54. doi: 10.1016/j.transci.2017.09.007
20. Rahmanian, V, Rahmanian, K, Sotoodeh, JA, and Bokaie, S. Systematic review and meta-analysis of human visceral leishmaniasis in Iran. Acta Fac Med Naiss. (2019) 36:279–93. doi: 10.5937/afmnai1904279R
21. Haftom, M, Petrucka, P, Gemechu, K, Nesro, J, Amare, E, Hailu, T, et al. Prevalence and risk factors of human leishmaniasis in Ethiopia: a systematic review and meta-analysis. Infect Dis Ther. (2021) 10:47–60. doi: 10.1007/s40121-020-00361-y
22. Assefa, A. Leishmaniasis in Ethiopia: a systematic review and meta-analysis of prevalence in animals and humans. Heliyon. (2018) 4:e00723. doi: 10.1016/j.heliyon.2018.e00723
23. Anoopa Sharma, D, Bern, C, Varghese, B, Chowdhury, R, Haque, R, Ali, M, et al. The economic impact of visceral leishmaniasis on households in Bangladesh. Trop Med Int Health. (2006) 11:757–64. doi: 10.1111/j.1365-3156.2006.01604.x
24. Sarnoff, R, Desai, J, Desjeux, P, Mittal, A, Topno, R, Siddiqui, NA, et al. The economic impact of visceral leishmaniasis on rural households in one endemic district of Bihar, India. Trop Med Int Health. (2010) 15:42–9. doi: 10.1111/j.1365-3156.2010.02516.x
25. Okwor, I, and Uzonna, J. Social and economic burden of human Leishmaniasis. Am J Trop Med Hyg. (2016) 94:489–93. doi: 10.4269/ajtmh.15-0408
26. Alvar, J, den Boer, M, and Dagne, DA. Towards the elimination of visceral leishmaniasis as a public health problem in East Africa: reflections on an enhanced control strategy and a call for action. Lancet Glob Health. (2021) 9:e1763–9. doi: 10.1016/S2214-109X(21)00392-2
27. Wamai, RG, Kahn, J, McGloin, J, and Ziaggi, G. Visceral leishmaniasis: a global overview. J Glob Health Sci. (2020) 2:2–3. doi: 10.35500/jghs.2020.2.e3
28. Abdullahi, B, Mutiso, J, Maloba, F, Macharia, J, Riongoita, M, and Gicheru, M. Climate change and environmental influence on prevalence of visceral leishmaniasis in west Pokot county, Kenya. J Trop Med. (2022) 2022:1–6. doi: 10.1155/2022/1441576
29. Alebie, G, Worku, A, Yohannes, S, Urga, B, Hailu, A, and Tadesse, D. Epidemiology of visceral leishmaniasis in Shebelle zone of Somali region, eastern Ethiopia. Parasit Vectors. (2019) 12:209. doi: 10.1186/s13071-019-3452-5
30. Ayehu, A, Aschale, Y, Lemma, W, Alebel, A, Worku, L, Jejaw, A, et al. Seroprevalence of asymptomatic Leishmania donovani among laborers and associated risk factors in agricultural camps of west Armachiho District, Northwest Ethiopia: a cross-sectional study. J Parasitol Res. (2018) 2018:5751743. doi: 10.1155/2018/5751743
31. Azene, W, Menkir, S, Kebede, A, and Gashaw, F. Visceral leishmaniasis and associated risk factors in Libo Kemkem, Bura Egzi Abhier ab Kebele, Northwestern Ethiopia. EC Microbiol. (2017) 7:162–72.
32. Bantie, K, Tessema, F, Massa, D, and Tafere, Y. Factors associated with visceral leishmaniasis infection in North Gondar zone, Amhara region, north West Ethiopia, case control study. Sci J Public Health. (2014) 2:560–8. doi: 10.11648/j.sjph.20140206.20
33. Bejano, S, Shumie, G, Kumar, A, Asemahagn, E, Damte, D, Woldie, S, et al. Prevalence of asymptomatic visceral leishmaniasis in human and dog, Benishangul Gumuz regional state, Western Ethiopia. Parasit Vectors. (2021) 14:1–8. doi: 10.1186/s13071-020-04542-z
34. Bsrat, A, Berhe, M, Gadissa, E, Taddele, H, Tekle, Y, Hagos, Y, et al. Serological investigation of visceral Leishmania infection in human and its associated risk factors in Welkait District, Western Tigray, Ethiopia. Parasite Epidemiol Control. (2018) 3:13–20. doi: 10.1016/j.parepi.2017.10.004
35. Dulacha, D, Mwatha, S, Lomurukai, P, Owiny, MO, Matini, W, Irura, Z, et al. Epidemiological characteristics and factors associated with visceral leishmaniasis in Marsabit County, northern Kenya. J Interv Epidemiol Public Health. (2019) 2:3. doi: 10.37432/JIEPH.2019.2.1.14
36. Ibrahim, B, Elmadani, M, Hemeda, S, Gadallh, A, Abdallh, M, and Elawad Ahmed, AE. Prevalence of visceral Leishmaniasis among wildlife rangers in Dinder National Park, Sudan. medRxiv. (2024):2024-06. doi: 10.1101/2024.06.24.24309386
37. Lemma, W, Tekie, H, Yared, S, Balkew, M, Gebre-Michael, T, Warburg, A, et al. Sero-prevalence of Leishmania donovani infection in labour migrants and entomological risk factors in extra-domestic habitats of Kafta-Humera lowlands-kala-azar endemic areas in the Northwest Ethiopia. BMC Infect Dis. (2015) 15:1–8. doi: 10.1186/s12879-015-0830-2
38. Lotukoi, JA. Exposure factors associated with visceral leishmaniasis (kala-azar) in Loima Sub-County of Turkana County. Kenya: JKUAT-COHES (2020).
39. Nackers, F, Mueller, YK, Salih, N, Elhag, MS, Elbadawi, ME, Hammam, O, et al. Determinants of visceral leishmaniasis: a case-control study in Gedaref state, Sudan. PLoS Negl Trop Dis. (2015) 9:e0004187. doi: 10.1371/journal.pntd.0004187
40. Odoch, WD, and Olobo, JO. Prevalence of kala-azar infection in Pokot county, Amudat district, northeastern Uganda. Open. J Epidemiol. (2013) 2013:203–8. doi: 10.4236/ojepi.2013.34029
41. Tadese, D, Hailu, A, Bekele, F, and Belay, S. An epidemiological study of visceral leishmaniasis in north East Ethiopia using serological and leishmanin skin tests. PLoS One. (2019) 14:e0225083. doi: 10.1371/journal.pone.0225083
42. van Griensven, J, van Henten, S, Mengesha, B, Kassa, M, Adem, E, Endris Seid, M, et al. Longitudinal evaluation of asymptomatic Leishmania infection in HIV-infected individuals in north-West Ethiopia: a pilot study. PLoS Negl Trop Dis. (2019) 13:e0007765. doi: 10.1371/journal.pntd.0007765
43. Custodio, E, Gadisa, E, Sordo, L, Cruz, I, Moreno, J, Nieto, J, et al. Factors associated with Leishmania asymptomatic infection: Results from a cross-sectional survey in highland northern Ethiopia. PLoS Negl Trop Dis. (2012) 6:e1813. doi: 10.1371/journal.pntd.0001813
44. Ismail, A, Yared, S, Dugassa, S, Abera, A, Animut, A, Erko, B, et al. Sero-prevalence of visceral leishmaniasis and its associated factors among asymptomatic individuals visiting Denan health center, southeastern Ethiopia. Trop Dis Travel Med Vaccines. (2023) 9:8. doi: 10.1186/s40794-023-00196-8
45. Melkie, I, Yimer, M, Alemu, G, and Tegegne, B. Asymptomatic Leishmania donovani infection and associated factors among blood donors attending at Metema district blood Bank, Northwest Ethiopia: a cross-sectional study. Arch Public Health. (2023) 81:62. doi: 10.1186/s13690-023-01082-7
46. Ayalew, S, and Abere, M. Determinant factors and spatial distribution of visceral Leishmaniasis in North Gondar in selected hospitals. J Dis. (2019) 6:81–9. doi: 10.18488/journal.99.2019.62.81.89
47. Bekele, F, Belay, T, Zeynudin, A, and Hailu, A. Visceral leishmaniasis in selected communities of Hamar and Banna-Tsamai districts in lower Omo Valley, south West Ethiopia: sero-epidemological and leishmanin skin test surveys. PLoS One. (2018) 13:e0197430. doi: 10.1371/journal.pone.0197430
48. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. Statement: an updated guideline for reporting systematic reviews. BMJ. Australia: The Joanna Briggs Institute. (2020) 2021:372. doi: 10.1136/bmj.n71
49. Peters, MD, Godfrey, CM, McInerney, P, Soares, CB, Khalil, H, and Parker, D. The Joanna Briggs institute reviewers' manual 2015: methodology for JBI scoping reviews. (2015).
50. Moola, S, Munn, Z, Tufanaru, C, Aromataris, E, Sears, K, Sfetcu, R, et al. Systematic reviews of etiology and risk. Joanna Briggs Institute reviewer’s manual. 5 : The Joanna Briggs Institute Adelaide, Australia; (2017). p. 217–69.
51. Munn, Z, Moola, S, Lisy, K, Riitano, D, and Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
52. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
53. Thompson, SG, and Sharp, SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. (1999) 18:2693–708. doi: 10.1002/(SICI)1097-0258(19991030)18:20<2693::AID-SIM235>3.0.CO;2-V
54. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
55. Abdalla, AOA, Mohammed, AA, Khaier, MM, and Zainaldeen, AA. Prevalence of Leishmania donovani infection in humans and dogs in Gadarif state, Sudan: a diagnostic comparison. Sudan J Med Sci. (2024) 19:119–31. doi: 10.18502/sjms.v19i1.15788
56. Abera, A, Tasew, G, Tsegaw, T, Kejella, A, Mulugeta, A, Worku, D, et al. Visceral leishmaniasis in Benishangul-Gumuz regional state, Western Ethiopia: reemerging or emerging? Am J Trop Med Hyg. (2016) 95:104–8. doi: 10.4269/ajtmh.15-0738
57. Ali, A, and Ashford, RW. Visceral leishmaniasis in Ethiopia. I. Cross-sectional leishmanin skin test in an endemic locality. Ann Trop Med Parasitol. (1993) 87:157–61. doi: 10.1080/00034983.1993.11812749
58. Ali, A, Gebre-Michael, T, Mengistu, G, and Balcha, F. A survey on leishmaniasis and the leishmanin skin test profile in lower Awash Valley, Northeast Ethiopia. Ethiop J Health Dev. (2004) 18:159–63.
59. El-Safi, SH, Bucheton, B, Kheir, MM, Musa, HAA, Moawia, E-O, Hammad, A, et al. Epidemiology of visceral leishmaniasis in Atbara River area, eastern Sudan: the outbreak of Barbar El Fugara village (1996–1997). Microbes Infect. (2002) 4:1439–47. doi: 10.1016/S1286-4579(02)00026-6
60. Ho, M, Siongok, TK, Lyerly, WH, and Smith, DH. Prevalence and disease spectrum in a new focus of visceral leishmaniasis in Kenya. Trans R Soc Trop Med Hyg. (1982) 76:741–6. doi: 10.1016/0035-9203(82)90095-5
61. Kanyina, EW. Characterization of visceral leishmaniasis outbreak, Marsabit County, Kenya, 2014. BMC Public Health. (2020) 20:446. doi: 10.1186/s12889-020-08532-9
62. Mohamed, NS, Osman, HA, Muneer, MS, Samy, AM, Ahmed, A, Mohammed, AO, et al. Identifying asymptomatic Leishmania infections in non-endemic villages in Gedaref state, Sudan. BMC Res Notes. (2019) 12:1–7. doi: 10.1186/s13104-019-4608-2
63. Ryan, JR, Mbui, J, Rashid, JR, Wasunna, MK, Kirigi, G, Magiri, C, et al. Spatial clustering and epidemiological aspects of visceral leishmaniasis in two endemic villages, Baringo District, Kenya. Am J Trop Med Hyg. (2006) 74:308–17. doi: 10.4269/ajtmh.2006.74.308
64. Ketema, H, Weldegebreal, F, Gemechu, A, and Gobena, T. Seroprevalence of visceral leishmaniasis and its associated factors among asymptomatic pastoral community of dire district, Borena zone, Oromia region, Ethiopia. Front Public Health. (2022) 10:917536. doi: 10.3389/fpubh.2022.917536
65. Diro, E, Lynen, L, Assefa, M, Takele, Y, Mengesha, B, Adem, E, et al. Impact of the use of a rapid diagnostic test for visceral leishmaniasis on clinical practice in Ethiopia: a retrospective study. PLoS Negl Trop Dis. (2015) 9:e0003738. doi: 10.1371/journal.pntd.0003738
66. Gize, A, Workineh, A, and Hailu, T. A trend prevalence of visceral Leishmaniasis in west Armachiho District, Amhara region, Northwest Ethiopia. Trop Dis Travel Med Vaccines. (2020) 6:23. doi: 10.1186/s40794-020-00125-z
67. Marlet, M, Wuillaume, F, Jacquet, D, Quispe, K, Dujardin, J, and Boelaert, M. A neglected disease of humans: a new focus of visceral leishmaniasis in Bakool, Somalia. Trans R Soc Trop Med Hyg. (2003) 97:667–71. doi: 10.1016/S0035-9203(03)80099-8
68. Mueller, Y, Mbulamberi, DB, Odermatt, P, Hoffmann, A, Loutan, L, and Chappuis, F. Risk factors for in-hospital mortality of visceral leishmaniasis patients in eastern Uganda. Trop Med Int Health. (2009) 14:910–7. doi: 10.1111/j.1365-3156.2009.02305.x
69. Terefe, Y, Afera, B, Bsrat, A, and Syoum, Z. Distribution of human leishmaniasis (VL) and its associated risk factors, in Metemma, Ethiopia. Epidemiol Res Int. (2015) 2015:630812:1–5. doi: 10.1155/2015/630812
70. Wondimeneh, Y, Takele, Y, Atnafu, A, Ferede, G, and Muluye, D. Trend analysis of visceral leishmaniasis at Addis Zemen health center, Northwest Ethiopia. Biomed Res Int. (2014) 2014:545393:1–5. doi: 10.1155/2014/545393
71. Yimer, M, Nibret, E, and Yismaw, G. Updates on prevalence and trend status of visceral leishmaniasis at two health facilities in Amhara regional state, Northwest Ethiopia: a retrospective study. Biochem Res Int. (2022) 2022:1–7. doi: 10.1155/2022/3603892
72. van Dijk, N, Carter, J, Kiptanui, D, Mens, PF, and Schallig, HD. A case-control study on risk factors for visceral Leishmaniasis in west Pokot County. Kenya: Tropical Medicine & International Health. (2023).
73. Bamorovat, M, Sharifi, I, Khosravi, A, Aflatoonian, MR, Agha Kuchak Afshari, S, Salarkia, E, et al. Global dilemma and needs assessment toward achieving sustainable development goals in controlling Leishmaniasis. J Epidemiol Global Health. (2024) 14:22–34. doi: 10.1007/s44197-024-00190-z
74. WHO. Global leishmaniasis update, 2006–2015: a turning point in leishmaniasis surveillance Geneva (2017) Available at: https://www.who.int/publications/i/item/who-wer9238 (accessed July 24, 2024).
75. Reithinger, R, Brooker, S, and Kolaczinski, JH. Visceral leishmaniasis in eastern Africa – current status. Trans R Soc Trop Med Hyg. (2007) 101:1169–70. doi: 10.1016/j.trstmh.2007.06.001
76. Kantzanou, M, Karalexi, MA, Theodoridou, K, Kostares, E, Kostare, G, Loka, T, et al. Prevalence of visceral leishmaniasis among people with HIV: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. (2023) 42:1–12. doi: 10.1007/s10096-022-04530-4
77. Murray, HW, Berman, JD, Davies, CR, and Saravia, NG. Advances in leishmaniasis. Lancet. (2005) 366:1561–77. doi: 10.1016/S0140-6736(05)67629-5
78. Hotez, PJ, and Kamath, A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. (2009) 3:e412. doi: 10.1371/journal.pntd.0000412
79. van Griensven, J, and Diro, E. Visceral leishmaniasis: recent advances in diagnostics and treatment regimens. Infect Dis Clin. (2019) 33:79–99. doi: 10.1016/j.idc.2018.10.005
80. Ejazi, SA, and Ali, N. Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects. Expert Rev Anti-Infect Ther. (2013) 11:79–98. doi: 10.1586/eri.12.148
81. Picado, A, Ostyn, B, Singh, SP, Uranw, S, Hasker, E, Rijal, S, et al. Risk factors for visceral leishmaniasis and asymptomatic Leishmania donovani infection in India and Nepal. PLoS One. (2014) 9:e87641. doi: 10.1371/journal.pone.0087641
82. Hotez, PJ, Molyneux, DH, Fenwick, A, Kumaresan, J, Sachs, SE, Sachs, JD, et al. Control of neglected tropical diseases. N Engl J Med. (2007) 357:1018–27. doi: 10.1056/NEJMra064142
83. van Henten, S, Adriaensen, W, Fikre, H, Akuffo, H, Diro, E, Hailu, A, et al. Cutaneous leishmaniasis due to Leishmania aethiopica. EClinicalMedicine. Addis Ababa, Ethiopia: The World Health Organization. (2018) 6:69–81. doi: 10.1016/j.eclinm.2018.12.009
84. World Health Organization. New framework launched to eliminate visceral leishmaniasis in eastern Africa. (2024).
85. Uranw, S, Hasker, E, Roy, L, Meheus, F, Das, ML, Bhattarai, NR, et al. An outbreak investigation of visceral leishmaniasis among residents of Dharan town, eastern Nepal, evidence for urban transmission of Leishmania donovani. BMC Infect Dis. (2013) 13:1–9. doi: 10.1186/1471-2334-13-21
86. Valero, NNH, and Uriarte, M. Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: a systematic review. Parasitol Res. (2020) 119:365–84. doi: 10.1007/s00436-019-06575-5
87. Gadisa, E, Tsegaw, T, Abera, A, Elnaiem, D-e, den Boer, M, Aseffa, A, et al. Eco-epidemiology of visceral leishmaniasis in Ethiopia. Parasit Vectors. (2015) 8:381. doi: 10.1186/s13071-015-0987-y
88. Wijerathna, T, Gunathilaka, N, Gunawardena, K, and Rodrigo, W. Socioeconomic, demographic and landscape factors associated with cutaneous leishmaniasis in Kurunegala District, Sri Lanka. Parasit Vectors. (2020) 13:244. doi: 10.1186/s13071-020-04122-1
89. Vivero, RJ, Torres-Gutierrez, C, Bejarano, EE, Peña, HC, Estrada, LG, Florez, F, et al. Study on natural breeding sites of sand flies (Diptera: Phlebotominae) in areas of Leishmania transmission in Colombia. Parasit Vectors. (2015) 8:116. doi: 10.1186/s13071-015-0711-y
90. Bern, C, Courtenay, O, and Alvar, J. Of cattle, sand flies and men: a systematic review of risk factor analyses for south Asian visceral leishmaniasis and implications for elimination. PLoS Negl Trop Dis. (2010) 4:e599. doi: 10.1371/journal.pntd.0000599
91. Saha, S, Ramachandran, R, Hutin, YJ, and Gupte, MD. Visceral leishmaniasis is preventable in a highly endemic village in West Bengal, India. Trans R Soc Trop Med Hyg. (2009) 103:737–42. doi: 10.1016/j.trstmh.2008.10.006
92. Barnett, PG, Singh, S, Bern, C, Hightower, AW, and Sundar, S. Virgin soil: the spread of visceral leishmaniasis into Uttar Pradesh, India. Am J Trop Med Hyg. (2005) 73:720–5. doi: 10.4269/ajtmh.2005.73.720
Keywords: Eastern Africa, human, outdoor sleeping, termite hill, visceral leishmaniasis
Citation: Geto AK, Berihun G, Berhanu L, Desye B and Daba C (2024) Prevalence of human visceral leishmaniasis and its risk factors in Eastern Africa: a systematic review and meta-analysis. Front. Public Health. 12:1488741. doi: 10.3389/fpubh.2024.1488741
Edited by:
Ram Raghavan, University of Missouri, United StatesReviewed by:
Medhavi Sudarshan, Patliputra University, IndiaSamiur Rahim, University of Dhaka, Bangladesh
Agbajelola Victor, University of Missouri, United States
Copyright © 2024 Geto, Berihun, Berhanu, Desye and Daba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abebe Kassa Geto, YWJlYmVrYXNzYTIxMjlAZ21haWwuY29t
 Abebe Kassa Geto
Abebe Kassa Geto Gete Berihun
Gete Berihun Leykun Berhanu
Leykun Berhanu Belay Desye
Belay Desye Chala Daba
Chala Daba