- 1School of Public Health, Guangdong Pharmaceutical University, Guangzhou, China
- 2Department of Foodborne Diseases and Food Safety Risk Surveillance, Guangzhou Center for Disease Control and Prevention, Guangzhou, China
- 3Department of Public Health, Xiaolou Town Health Center, Guangzhou, China
- 4School of Public Health, Jinan University, Guangzhou, China
- 5Department of Physical and Chemical Inspection, Guangzhou Center for Disease Control and Prevention, Guangzhou, China
Background: Aflatoxin B1 (AFB1), a potent carcinogen produced by Aspergillus species, is a prevalent contaminant in oil crops, with prolonged exposure associated with liver damage. Home-made peanut oil (HMPO) produced by small workshops in Guangzhou is heavily contaminated with AFB1. Despite the enactment of the Small Food Workshops Management Regulations (SFWMR), no quantitative assessment has been conducted regarding its impact on food contamination and public health. The study aims to assess the impact of SFWMR on AFB1 contamination in HMPO and liver function in the population.
Method: AFB1 contamination in HMPO were quantified using high-performance liquid chromatography and liver function data were obtained from the health center located in a high-HMPO-consumption area in Guangzhou. Interrupted time series and mediation analyses were employed to assess the relationship between the implementation of SFWMR, AFB1 concentrations in HMPO, and liver function among residents.
Result: The AFB1 concentrations in HMPO were 1.29 (0.12, 6.58) μg/kg. The average daily intake of AFB1 through HMPO for Guangzhou residents from 2010 to 2022 ranged from 0.25 to 1.68 ng/kg bw/d, and the Margin of Exposure ranged from 238 to 1,600. The implementation of SFWMR was associated with a significant reduction in AFB1 concentrations in HMPO, showing an immediate decrease of 2.865 μg/kg (P = 0.006) and a sustained annual reduction of 2.593 μg/kg (P = 0.034). Among residents in the high-HMPO-consumption area, the implementation of SFWMR was significantly associated with a reduction in the prevalence of liver function abnormality (PR = 0.650, 95% CI: 0.469–0.902). Subgroup analysis revealed that this reduction was significantly associated with the implementation of SFWMR in the female (PR = 0.484, 95% CI: 0.310–0.755) and in individuals aged ≥ 60 years (PR = 0.586, 95% CI: 0.395–0.868). Mediation analysis demonstrated that AFB1 concentrations in HMPO fully mediated the relationship between the implementation of SFWMR and the liver function abnormality (PR = 0.981, 95% CI: 0.969–0.993).
Conclusion: In Guangzhou, the public health issue arising from AFB1 intake through HMPO warrants attention. The implementation of SFWMR had a positive impact on the improvement of AFB1 contamination in HMPO and the liver function. Continued efforts are necessary to strengthen the enforcement of the regulations. The exposure risks to AFB1 among high-HMPO-consumption groups also demand greater focus.
1 Introduction
Aflatoxins (AFs) are a group of secondary metabolites produced by Aspergillus species (primarily Aspergillus flavus and Aspergillus parasiticus) (1), with aflatoxin B1 (AFB1) being particularly notable due to its widespread presence and toxicity (2). Approximately 4.5 billion people worldwide are exposed to aflatoxin-contaminated food, particularly in low and middle-income countries in subtropical regions (3, 4). The 2004 outbreak of acute aflatoxicosis in Kenya was among the most significant epidemics of human aflatoxin poisoning recorded in mycotoxin history (5). Tanzania had experienced hundreds of cases of aflatoxicosis in the districts of Kiteto, Chemba, and Kondoa for the three consecutive years since 2016 (6). AFB1 is known for its potent carcinogenic properties and is classified as a Group I carcinogen by the International Agency for Research on Cancer (7). The physicochemical stability of AFB1 renders it resistant to degradation at conventional cooking temperatures (8), making dietary intake the primary route of human exposure (9). Furthermore, due to its lipophilic nature, foods rich in fat content, such as peanuts and corn, are more susceptible to fungal contamination and subsequent AFB1 production (10).
In China, peanuts are the primary oil crop, with ~52% of the total yield allocated for oil extraction, and peanut oil accounts for over 25% of the annual vegetable oil production (11). The consumption of peanut oil in China exhibits distinct regional preferences, primarily in the East China and South China (12). Guangdong Province leads South China with a per capita daily peanut oil consumption of 19.43 grams (13). Local customs and dietary habits have contributed to the widespread presence of small food workshops producing and selling peanut oil across various regions of Guangdong Province (14, 15). Home-made peanut oil (HMPO) is an edible vegetable oil produced operating in rudimentary facilities by private workshops. Unlike industrially processed peanut oil, HMPO retains more of the natural flavor of peanuts and is therefore a preferred choice among consumers. However, the absence of refining, detoxification processes, and product inspection increases the risk of AFB1 contamination in HMPO (16).
The effects of AFB1 on liver function are primarily characterized by direct hepatocyte damage and impaired energy metabolism (17). AFB1 impacts liver function through multiple mechanisms. First, it disrupts mitochondrial bioenergetics and membrane potential (18), promotes mitochondrial cholesterol transport (19, 20), and induces mitochondrial autophagy (21), cumulatively leading to mitochondrial dysfunction. Mitochondrial dysfunction subsequently triggers excessive reactive oxygen species production and compromises antioxidant defenses, intensifying oxidative stress in hepatocytes (22). Additionally, experimental evidence suggests that AFB1 contributes to hepatic inflammation via dysregulated intestinal flora (23), release of damage-associated molecular patterns and cytokines, and immune cell activation (24). These mechanisms directly damage hepatocytes and indirectly impair liver function by disrupting overall hepatic metabolic processes.
Long-term consumption of AFB1 contaminated HMPO is associated with liver damage (25). In regions with high HMPO consumption, such as Guangzhou, routine liver function monitoring is essential. Guangzhou, situated in the south-central part of Guangdong Province, experiences a subtropical monsoon climate, with an average summer temperature of 28°C and an annual precipitation of 64 inches (26). Such warm and humid conditions favor the growth and toxin production of Aspergillus flavus in peanut raw materials. A previous study indicated severe AFB1 contamination in HMPO in Guangzhou (27). A subsequent food safety risk assessment identified HMPO as the primary dietary source of AFB1 exposure for residents in peripheral areas (14).
Small food workshops face challenges, including inadequate management and production inconsistencies, largely due to their limited scale, inconspicuous locations, and rudimentary production process. To enhance food safety management and protect public health, the Guangdong Provincial Government introduced the Small Food Workshops Management Regulations (SFWMR) (28). The regulations build on the Food Safety Law by providing clear guidelines for registering and supervising small food workshops. While existing study has primarily focused on the qualitative interpretation of the regulations (29–31), there has been no quantitative analyses of their implementation effects, particularly with respect to food contamination and consumer health. Our study addresses this gap by offering scientific evidence to evaluate the effectiveness of SFWMR in enhancing food safety and safeguarding public health.
In these contexts, our study aims to evaluate the impact of SFWMR implementation on AFB1 concentrations in HMPO and liver function among residents of the high-consumption area, and to explore the mediation effect of AFB1 concentrations in HMPO in the relation between SFWMR implementation and liver function.
2 Materials and methods
2.1 Sampling of home-made peanut oil
Information on HMPO samples for this study was obtained from the Food Safety Risk Monitoring System of the Guangzhou Center for Disease Control and Prevention. HMPO consumption in Guangzhou is concentrated in peripheral districts. Sampling focused on the months of June to August, a period characterized by high temperatures and humidity. From 2010 to 2022, streets in peripheral districts were designated as sampling units, and street types were stratified based on their distance from the center of each district. Two central streets and one remote street were randomly selected as sampling sites in each peripheral area, resulting in 15 streets selected as sampling sites for HMPO. The distribution of sampling sites during the study period is presented in Figure 1. The sampling process involved two individuals, acting as consumers, procuring HMPO from small workshops to ensure the representativeness of the samples. A total of 590 HMPO samples were included in this study. After collection, the samples were sealed, refrigerated at 4°C, and analyzed within 48 h.
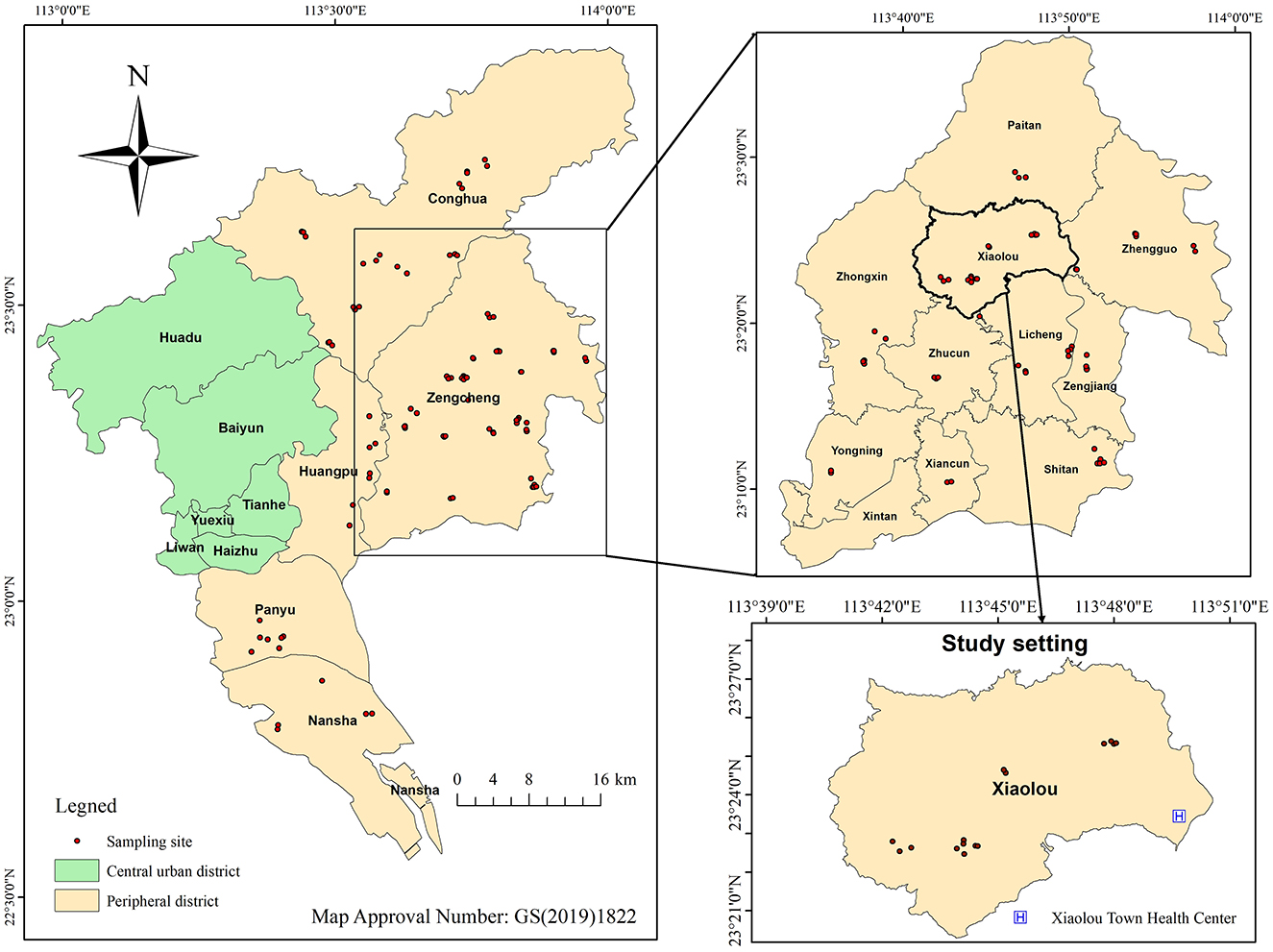
Figure 1. Distribution of sampling sites for home-made peanut oil from 2010 to 2022 and study setting.
2.2 Detection of AFB1 in HMPO
The detection of AFB1 was conducted using high-performance liquid chromatography (HPLC) in accordance with the methodologies specified in Determination of Aflatoxins B1, B2, G1, and G2 in Foods (GB/T 5009.23-2006) (32) and Determination of Aflatoxins B and G Groups in Foods (GB 5009.22-2016) (33). The methodology outlined in GB 5009.22-2016 has been described in our previous study (14). The experimental procedures following GB/T 5009.23-2006 are as follows.
2.2.1 Chemicals and instruments
Aflatoxin B1 standard (Purity > 98.0%, HPLC grade) was purchased from Sigma-Aldrich, USA; Acetonitrile, trifluoroacetic acid and hexane, all HPLC grade, were purchased from Merck, Germany; Ultrapure water; C18 reversed-phase column (Jiangsu Hming Technology Co., Ltd., China); A liquid chromatography system (SHIMADZU, Japan) equipped with RF-20A fluorescence detector; Milli-Q ultrapure water machine (Millipore, USA); Vortex mixer (IKA, Germany); Centrifuge (SIGMA, Germany); Nitrogen blower (Beijing Tongtailian Technology Development Co., Ltd., China); Electronic balance (METTLER-TOLEDO, USA).
2.2.2 Sample extraction and purification
A 20.0 g aliquot of the HMPO sample was mixed with 80.0 mL of an acetonitrile-water (84:16) solution for 30 min, and then filtered through qualitative filter paper. An 8.0 mL portion of the filtrate was transferred to a multifunctional purification column for further processing. Subsequently, 2.0 mL of the purified solution was evaporated to dryness under nitrogen in a 60°C water bath. To the residue, 200.0 μL of hexane and 100.0 μL of trifluoroacetic acid were added, vortexed for 30 s, and derivatized at 40°C for 15 min. After derivatization, the sample was dried at room temperature, dissolved in 200.0 μL of a water-acetonitrile (85:15) solution, mixed and centrifuged. The supernatant was then collected for further analysis.
2.2.3 Liquid chromatography condition
The mobile phase consisted of water and acetonitrile. Gradient elution was programmed as follows: starting with 15% acetonitrile at 0 min, increasing to 17% at 6 min, further increasing to 25% at 8 min, and returning to 15% at 14 min. A C18 reversed-phase column (125 mm × 2.1 mm, 5.0 μm particle size) was employed, with a flow rate of 0.5 mL/min and a column temperature of 30°C. The injection volume was 25.0 μL. Detection was performed at an excitation wavelength of 360 nm and an emission wavelength of 440 nm.
2.2.4 Method validation
Weighed AFB1 standard and diluted with acetonitrile in a 10 mL volumetric flask to prepare a series of AFB1 standard working solutions at concentrations of 0.50, 2.50, 5.00, 25.00, 50.00, and 100.00 μg/L. The standard curve was expressed as y = 0.213x + 0.085, with an R-squared value of 0.9992 (Supplementary Figure S1).
The sensitivity of the method was assessed by determining the limit of detection (LOD) and limit of quantification (LOQ). The LOD and LOQ were 0.10 μg/kg and 0.30 μg/kg, determined at 3 times and 10 times the signal-to-noise ratio (S/N), respectively.
The spiked recovery experiment was conducted using blank samples. Six portions of each blank sample were spiked with low (5.00 μg/kg), intermediate (25.00 μg/kg) and high (50.00 μg/kg) levels of the AFB1 standard. The average spiked recovery percentages ranged from 90.48 to 97.27%, with relative standard deviations (RSDs) of 1.92–3.24%, meeting the requirements for trace analysis (Supplementary Table S1).
2.2.5 Data processing
AFB1 detection values exceeding the acceptable limit of 20.0 μg/kg were regarded as exceeding the standard (34), and values below LOD were categorized as non-detect (ND). If the proportion of ND values was < 60%, these values were replaced with half of the LOD. Conversely, if the proportion of ND values was ≥60%, the values were substituted with the LOD (35).
2.3 Dietary exposure analysis
The deterministic exposure assessment model was employed to calculate the estimated dietary intake (EDI) of AFB1 through HMPO. C represents the AFB1 concentrations detected in HMPO samples; IR represents the daily intake of edible oil in the population, estimated from the consumption data in the Guangzhou Statistical Yearbook (36); BW represents the average body weight. According to the Report on Nutrition and Chronic Diseases of the Chinese Population, the average body weight was 66.2 kg for males and 57.3 kg for females (37). After applying the sex ratio of the Guangzhou population (38), the average body weight was determined to be 62.0 kg. The equation is as follows:
2.4 Margin of exposure
Risk characterization for genotoxic and carcinogenic compounds, such as AFB1, is based on the margin of exposure (MOE), calculated by dividing 10% of the lower confidence limit of the benchmark dose (BMDL10) of AFB1 by the estimated dietary intake, as shown in Equation 2. The BMDL10 for AFB1 is 400 ng/kg bw/d (39). A smaller MOE indicates a greater risk of exposure to genotoxic and carcinogenic compounds. The European Food Safety Authority considers BMDL10 to be derived from animal studies. Due to inherent uncertainties, the MOE ≥ 10,000 indicates a low public health concern and a low priority for risk management (40).
2.5 Setting and collection of population data
The study setting was Xiaolou Town, Zengcheng District, Guangzhou. Xiaolou is a traditional agricultural town with a population of ~30,000 residents (41). Figure 1 and Supplementary Table S2 demonstrate the presence of numerous HMPO workshops in the area, establishing it as a representative high-HMPO-consumption region in Guangzhou. The Xiaolou Town Health Center is the sole health institution designated to provide the National Basic Public Health Service Program in the town (42) and offers annual health check-ups to residents. The study included participants from 20 local administrative villages, ensuring broad representativeness. Health data for residents from 2010 to 2022 were retrieved from the medical examination system of Xiaolou Town Health Center, and study variables included gender, age and liver enzyme levels. Individuals with a history of hepatitis B were excluded from the study.
AFB1 exposure is strongly associated with abnormal liver function, as indicated by elevated blood levels of liver enzymes, particularly aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (43–45). These enzymes are abundant in liver cells and are released into the bloodstream upon liver cell damage, serving as bioindicators of abnormal liver function (46, 47). Liver function abnormality was defined according to the Reference Intervals for Common Clinical Biochemistry Tests (WS/T 404) as elevated AST or ALT levels, with AST > 40 U/L or ALT > 50 U/L for males and AST > 35 U/L or ALT > 40 U/L for females (48).
2.6 Statistical analysis
Interrupted Time Series Analysis (ITSA) is a statistical method used to evaluate the effectiveness of interventions through segmented regression models. Small Food Workshops Management Regulations have been in effect since October 1, 2015 (28). Considering the lag in the implementation effects and the annual nature of the data analyzed, the year 2016 was designated as the intervention breakpoint.
The continuous outcome variable was analyzed using a segmented linear regression model. The regression equation is as follows:
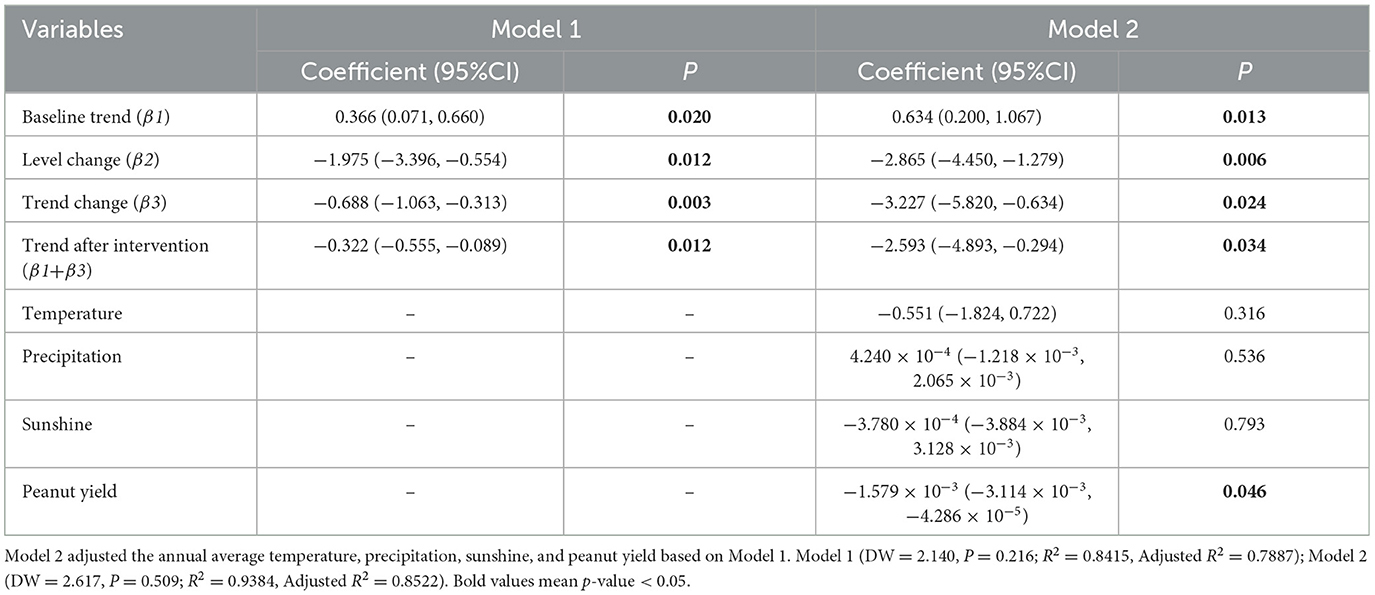
In Equation 3, Yt represents the outcome variable, which in this study denotes the AFB1 contamination in HMPO. Tt is a count variable for the year. Xt is an indicator variable for the intervention, with a value of 0 before the implementation of SFWMR and 1 after. (Tt−T) represents the difference between the year count variable and the intervention breakpoint. εt is the random error term. The values assigned to these variables are detailed in Supplementary Table S3. β0 is the intercept. β1 indicates the trend before the intervention. β2 represents the level change after the intervention. β3 represents the difference in the slope between pre- and post-intervention periods. (β1+β3) reflects the trend after the intervention. We fitted both an unadjusted model and a model adjusted for potential confounders, including average annual temperature, rainfall, sunlight, and peanut yield.
Considering that the prevalence of liver function abnormality in this study was >10%, the binary outcome variable was analyzed using the log-binomial regression model (49). The regression equation is as follows:
In Equation 4, Yt represents whether the resident has liver function abnormality. The prevalence ratio (PR) was employed to quantify the intervention effect in the segmented log-binomial regression model. The PR was calculated by comparing the post-intervention fitted values with the counterfactual outcomes. Specifically, the prevalence ratio PRt, at each time point during the intervention period was calculated, and the geometric mean of PRt was used to obtain the overall PR for the intervention period (50). Furthermore, stratified analyses were conducted by gender and age ≥ 60 years, with age stratification following the definition of older adults as stated by the World Health Organization (51). The Durbin-Watson method was used to detect first-order autocorrelation in the ITSA model. In the presence of autocorrelation, the Newey-West method was applied to adjust the standard errors of the parameter estimates.
Mediation analysis was conducted to investigate the role of AFB1 contamination in HMPO as a mediator of the relationship between the implementation of SFWMR and liver function abnormality in residents. The formula is as follows:
Where Y represents whether the resident has liver function abnormality. X indicates whether the resident was exposed to the implementation of SFWMR. M represents the annual AFB1 contamination in HMPO. C refers to potential confounding factors, including gender and age. The direct effect (DE) and indirect effect (IE) were described using the prevalence ratio, with the calculation of PR detailed in Equation 9. Mediation effects were evaluated using 1,000 bootstrap samples.
Microsoft Excel 2021 was employed for data organization and cleaning. Categorical variables were summarized as frequency and percentage. Continuous variables with a normal distribution were summarized using mean and standard deviation, while those with a skewed distribution were reported as median and interquartile range. All statistical analyses were conducted in R (version 4.1.3). P < 0.05 (two-tailed) was considered statistically significant.
3 Results
3.1 Contamination of AFB1 in HMPO
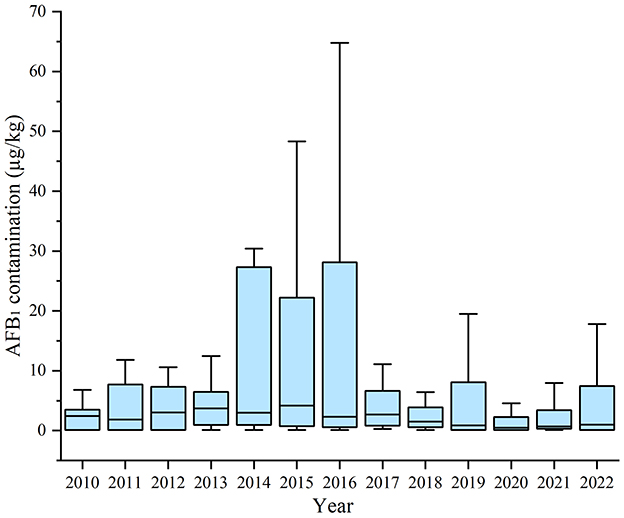
The AFB1 contamination in HMPO were 1.29 (0.12, 6.58) μg/kg. Additionally, 67 samples exceeded the AFB1 standard, resulting in an exceedance rate of 11.36%. The variation in AFB1 contamination in HMPO between years was statistically significant (P = 0.019). After 2016, the AFB1 contamination in HMPO decreased from 2.72 (0.49, 7.47) μg/kg to 0.98 (0.12, 6.30) μg/kg (P = 0.011) (Figure 2; Supplementary Table S4).
3.2 EDI and MOE
As shown in Figure 3, the average daily intake of AFB1 through HMPO among Guangzhou residents from 2010 to 2022 ranged from 0.25 to 1.68 ng/kg bw/d. The MOE ranged from 238 to 1,600, which was lower than 10,000.
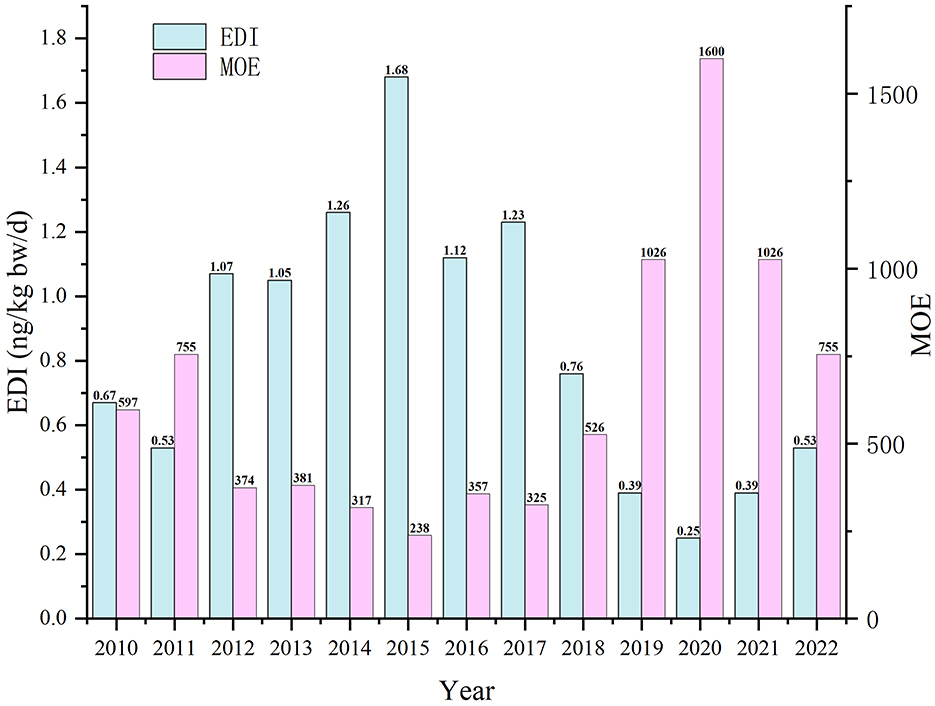
Figure 3. Estimated dietary intake of AFB1 through HMPO and margin of exposure in Guangzhou from 2010 to 2022.
3.3 Liver function information of population
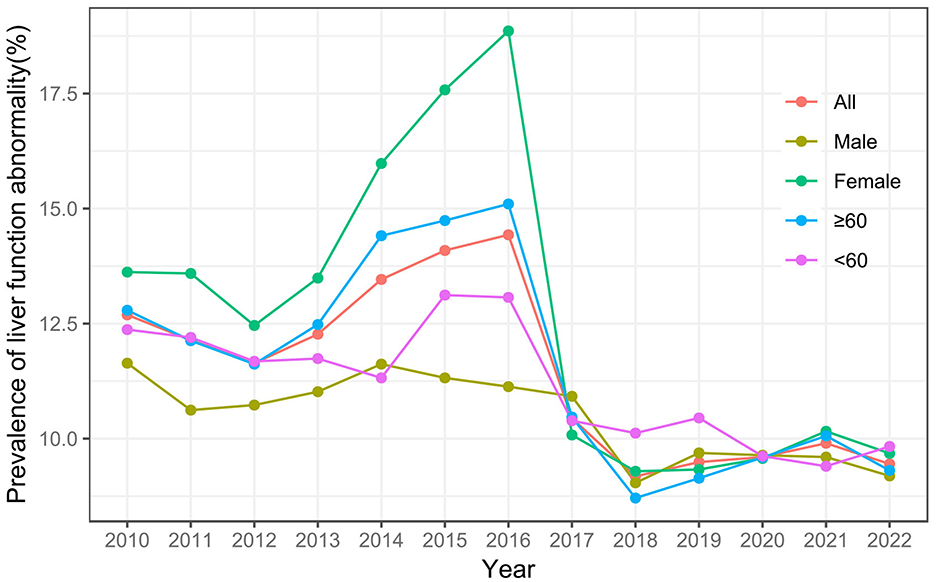
The study included 21,828 residents, comprising 11,558 females (52.95%) and 10,270 males (47.05%). A total of 15,501 (71.01%) participants were aged ≥ 60 years and 6,327 (28.99%) were aged < 60 years. The mean age of the population was 64.34 ± 13.43 years. Of these residents, 2,289 (10.49%) were identified with liver function abnormality (Supplementary Table S5). Prior to 2016, both females, and individuals aged ≥ 60 years exhibited a higher prevalence of liver function abnormality, with an increasing trend. After 2016, the prevalence of liver function abnormality decreased and stabilized across all groups (Figure 4).
3.4 ITSA of AFB1 Levels in HMPO
According to the adjusted model, prior to the implementation of SFWMR, AFB1 contamination in HMPO exhibited an increasing annual trend of 0.634 μg/kg (P = 0.013). In the first year following the implementation of SFWMR, AFB1 levels in HMPO decreased by 2.865 μg/kg (P = 0.006). After the implementation, there was a continued annual decrease in the AFB1 levels of 2.593 μg/kg (P = 0.034). Compared to the counterfactual scenario, the trend decreased by 3.227 μg/kg per year (P = 0.024) (Table 1; Figure 5).
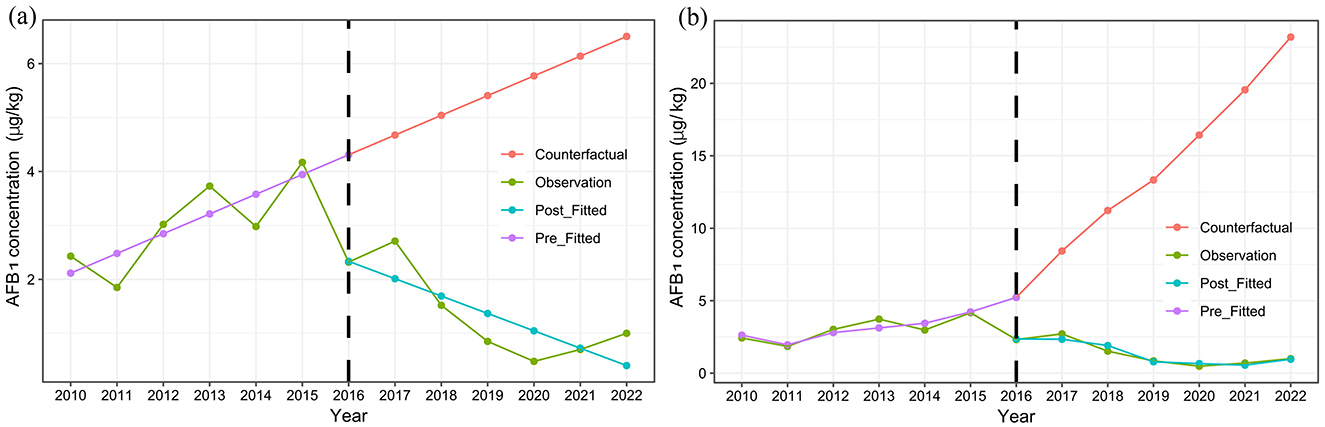
Figure 5. Trend of changes in AFB1 concentration in HMPO before and after the implementation of SFWMR. (A) is Model 1 and (B) is Model 2.
3.5 ITSA of liver function in population
In the entire population, the implementation of SFWMR was associated with a reduction in the prevalence of liver function abnormality compared to the counterfactual outcome (PR = 0.650, 95% CI: 0.469–0.902). When stratified by gender and age, significant associations were observed in both the female group (PR = 0.484, 95% CI: 0.310–0.755) and the age ≥ 60 years group (PR = 0.586, 95% CI: 0.395–0.868). However, no significant associations were found in the male group or among participants aged < 60 years (Figures 6, 7).
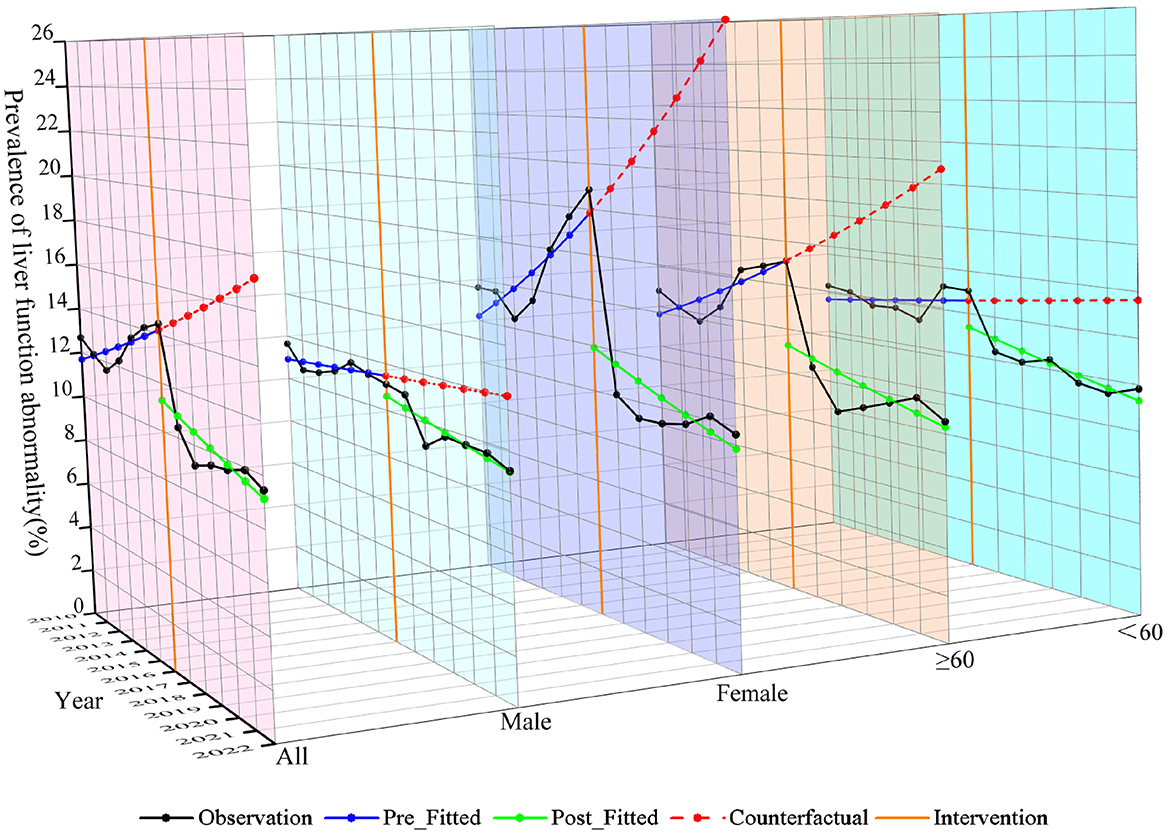
Figure 6. Trend of changes in prevalence of liver function abnormality among population before and after the implementation of SFWMR.
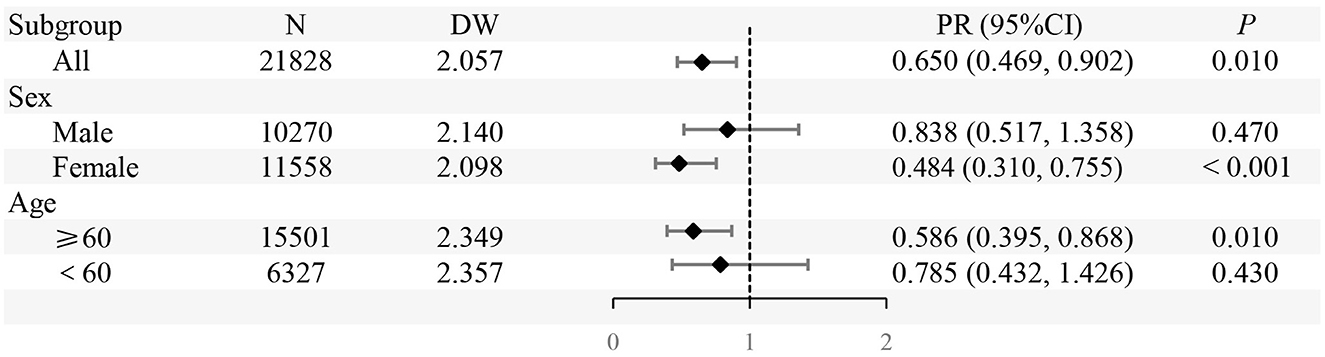
Figure 7. The prevalence ratio (PR) of prevalence of liver function abnormality in different subgroups before and after the implementation of SFWMR compared to the counterfactual outcome.
3.6 Mediation analysis
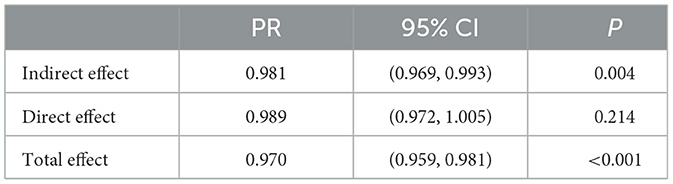
As shown in Table 2, the implementation of SFWMR had a total effect on liver function abnormality (PR = 0.970, 95% CI: 0.959–0.981). The analysis revealed an indirect effect of AFB1 contamination in HMPO on the relationship between the implementation of SFWMR and the liver function abnormality (PR = 0.981, 95% CI: 0.969–0.993). AFB1 contamination in HMPO fully mediated the relationship between the implementation of SFWMR and liver function abnormality.
4 Discussion
4.1 AFB1 contamination of HMPO
Aflatoxin contaminants in food are widely recognized as a significant health concern (52). In this study, AFB1 was detected in HMPO samples from Guangzhou from 2010 to 2022 by HPLC, with an exceedance rate of 11.36%. This rate is comparable to the national average of 11.11% (53) and lower than rates reported in other areas of Guangdong Province, including Huizhou (19.72%) (54), Heyuan (11.82%) (55), and the western region of Guangdong (18.7%) (15). This variability in regulatory enforcement could partly explain the disparities in exceedance rates between Guangzhou and other cities in Guangdong Province. While the SFWMR specifies the requirements for obtaining a food workshop registration certificate, the specific application processes, inspection criteria, and acceptance scopes are tailored by each prefecture-level city according to their local circumstances (56).
However, the exceedance rate in Guangzhou is higher than the rate reported in Tianjin (6.67%) (57). This discrepancy may be attributed to regional differences in climate and soil characteristics. Guangzhou's warm and humid subtropical climate provides optimal conditions for the growth of Aspergillus molds that produce toxins (58). Moreover, the predominantly clay-based soil in Guangzhou contrasts with the sandy soil prevalent in Tianjin (59). The high water retention capacity of clay facilitates the growth of Aspergillus species (60), thereby increasing the risk of peanut contamination before harvesting. Contaminated peanuts introduce AFB1 into the product during the pressing process (61).
4.2 AFB1 dietary exposure and MOE
Dietary intake is the main route of AFB1 exposure. Our study observed that the average daily intake of AFB1 through HMPO ranged from 0.25 to 1.68 ng/kg bw/d for Guangzhou residents. The MOEs were all below 10,000, consistent with the findings of HE, indicating that the health risks associated with HMPO warrant attention (25). A deterministic exposure assessment was performed based on the general population in our study. Notably, age was found to be an important factor influencing the EDI of AFB1, with children experiencing the highest exposure risk (14, 62). Given their higher exposure levels and the potential for synergistic hepatotoxic effects from chronic AFB1 intake, coupled with their relatively immature immune systems, children are particularly vulnerable (63).
4.3 Impact of SFWMR on AFB1 contamination in HMPO
Based on the ITSA, we evaluated the time-variant impact of SFWMR on AFB1 contamination in HMPO. In the counterfactual scenario, AFB1 contamination in HMPO would have increased yearly. Aflatoxin contamination in food is predominantly due to improper handling of raw materials (64). A study in Kenya showed that the source of peanuts and the presence of defective nuts were the primary contributors to increased aflatoxin contamination in the cottage industry (65). Small workshops often purchase large quantities of peanut raw materials at one time. Their storage facilities lack temperature control and dehumidification systems, rendering peanuts highly susceptible to mold contamination. Additionally, these workshops often fail to effectively remove moldy peanuts and do not perform oil refining treatments (55). A research demonstrated that the refining process can significantly reduce AFB1 content in peanut oil (66). Thus, incorporating the refining process into the standard production of HMPO is recommended.
After the implementation of SFWMR, there was a significant reduction in AFB1 contamination in HMPO, and this downward trend persisted in subsequent years. This decrease can be attributed to the impact of SFWMR. For instance, article 14 of the regulations explicitly prohibits small food workshops from producing edible oils that do not meet food safety standards and defines penalties for non-compliance. Secondly, the regulations standardize the registration process of small food workshops by requiring on-site inspection of storage facilities, production environments, layout of functional areas, refining equipment, and product labels. Workshops must also provide qualified AFB1 inspection reports for HMPO issued by the third-party testing institution during the inspection. Furthermore, the regulations establish the production license publicity system, which promotes the healthy development of small food workshops by leveraging market forces through consumer choice (28). Our study emphasized the positive impact of these comprehensive intervention measures in standardizing HMPO production in small food workshops. In Africa, small-scale operators and unorganized markets present key challenges to effective mycotoxin regulation (67). The practices implemented in Guangzhou may serve as a reference for these regions.
4.4 Impact of SFWMR on liver function in the population
Aflatoxin contamination in HMPO in Guangdong urgently requires urgent attention, highlighting the need for enhanced public health management of consumers (25). The study evaluated the potential health impact of SFWMR in a region characterized by high HMPO consumption, focusing on liver function. Our findings indicated that the implementation of SFWMR was associated with a decrease in the prevalence of liver function abnormality. Moreover, mediation analysis revealed that the protective effect of SFWMR implementation on liver function operates through the reduction of AFB1 contamination in HMPO. From the disease prevention perspective, the implementation of SFWMR reduced AFB1 exposure and played the primary preventive role in improving liver function in the population. Evidence from animal experiments and epidemiologic studies demonstrates an association between exposure to AFB1 and abnormal liver function. In rats, chronic exposure to AFB1 resulted in hepatocellular damage and significantly elevated serum levels of liver enzymes and oxidative stress markers (46). Population-based studies in Saudi Arabia and Ghana similarly reported a significant association between AFB1 exposure and liver function enzyme levels (45, 68). In addition, AFB1 exposure acts synergistically with hepatitis B virus infection to cause liver dysfunction (25). Mechanistically, the hepatotoxicity of AFB1 arises from its active metabolite, AFB1-8,9-epoxide, which induces mitochondrial dysfunction, oxidative stress, and inflammatory responses, ultimately leading to apoptosis and necrosis of hepatocytes (17).
Additionally, subgroup analysis revealed that the implementation of SFWMR resulted in significant improvements in liver function for females and individuals aged ≥ 60 years. This improvement may be attributed to consumer habits. HMPO, which has a lower oil yield, is infrequently used by commercial food service establishments, but is preferred by females and older adults for home cooking. Males and younger individuals, particularly those working outdoors, tend to consume less HMPO. This preference aligns with Mills' findings, which indicate that females and older individuals are more likely to consume homemade meals (69). Consequently, after the implementation of SFWMR, AFB1 intake among females and older adults significantly decreased, resulting in a more pronounced protective effect on liver function. AFB1 exposure is not only related to food contamination but also to consumption. This highlights the need for greater attention to high-HMPO-consumption groups.
4.5 Significance of SFWMR
Small food workshops face supervision challenges due to their low entry thresholds, broad distribution, and mobility. Despite the enactment of the National Food Safety Standard for Maximum Levels of Mycotoxins in Food in China, supervising small food workshops remains problematic (15). These small workshops are crucial for supporting local agriculture and sideline products, addressing employment issues, and increasing residents' incomes. They also contribute significantly to preserving regional characteristics and dietary culture. The implementation of SFWMR is not a restriction on entrepreneurial freedom. On the contrary, it provides a framework for standardizing productions and encourages small food workshops to upgrade and renovate their facilities to obtain food production licenses. The implementation of SFWMR address legislative and regulatory gaps for small food workshops, enhancing food safety through legal thinking and methods (70).
4.6 Limitation
The study analyzed the implementation effects of SFWMR from the perspective of food contamination and the health impacts on consumers. However, our study had several limitations. Firstly, although ALT and AST are crucial for liver function assessment and hepatocellular damage monitoring, their elevation may also indicate non-hepatic tissue damage, potentially leading to false positives (71). To enhance the specificity of liver disease diagnosis, other biomarkers and imaging tests can be used in tandem. Gamma-glutamyltransferase (GGT) and Alkaline phosphatase (ALP) exhibit distinct expression patterns compared to ALT and AST in liver disease (44). GGT, concentrated in the liver and biliary system, often rises in cholestatic diseases and liver injury. ALP, present in the hepatobiliary system, bones, placenta, and intestines, may indicate bone or liver disease (47). Additionally, imaging tests such as ultrasound, Computed Tomography scans, and Magnetic Resonance Imaging are important tools for assessing liver function. They provide visual information about the structure and morphology of the liver and help identify the presence and progression of liver diseases. The combined use of these biomarkers and imaging tests can help differentiate liver from non-liver diseases and improve diagnostic accuracy (72). Secondly, the liver function data were obtained from the health examination system, which may introduce selection bias. Finally, although the single-group ITSA method used in this study provides reliable inferences, the ecological study design limits the determination of causality. Future follow-up studies will address these limitations by incorporating additional biochemical and imaging tests, increasing the sample size and establishing a control group to more comprehensively evaluate the effectiveness of SFWMR implementation.
5 Conclusion
In Guangzhou, the public health concern arising from AFB1 intake through HMPO requires considerable attention. The enactment of SFWMR significantly contributed to reducing AFB1 contamination in HMPO and improving liver function. These outcomes highlight the efficacy of regulatory interventions in addressing food safety hazards and promoting public health. Continued efforts are necessary to strengthen the enforcement of the regulations. The exposure risks to AFB1 among high-HMPO-consumption groups also demand greater focus.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Guangzhou Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
JL: Visualization, Writing – original draft, Methodology, Data curation, Formal analysis. YL: Writing – review & editing, Conceptualization. YWa: Investigation, Supervision, Writing – review & editing, Data curation, Formal analysis. JZ: Investigation, Writing – review & editing, Supervision. YWu: Writing – review & editing, Data curation, Investigation. YZ: Writing – review & editing, Investigation, Supervision. LaL: Writing – review & editing, Investigation, Supervision. YO: Writing – review & editing, Data curation. LH: Writing – review & editing, Data curation. SW: Data curation, Writing – review & editing. XG: Writing – review & editing, Resources. LiL: Resources, Writing – review & editing. RP: Writing – review & editing. ZB: Writing – review & editing. WZ: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by the Guangzhou Science and Technology Key Program (202103000039) and the Guangzhou Health Science and Technology General Guidance Project (20251A011061).
Acknowledgments
The authors wish to express their gratitude to Dr. Florence for her thorough review and correction of the language and grammar in this article. Her contributions significantly enhanced the clarity and coherence of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1484414/full#supplementary-material
References
1. Okechukwu VO, Adelusi OA, Kappo AP, Njobeh PB, Mamo MA. Aflatoxins: Occurrence, biosynthesis, mechanism of action and effects, conventional/emerging detection techniques. Food Chem. (2024) 436:137775. doi: 10.1016/j.foodchem.2023.137775
2. Joseph Owuor L, Solomon O, Dora AOO. Aflatoxin b1: chemistry, environmental and diet sources and potential exposure in human in kenya. In:Xi-Dai L, , editor. Aflatoxin b1 Occurrence, Detection and Toxicological Effects. Rijeka: IntechOpen (2019). p. Ch. 1.
3. Hamid AS, Tesfamariam IG, Zhang Y, Zhang ZG. Aflatoxin b1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention. Oncol Lett. (2013) 5:1087–92. doi: 10.3892/ol.2013.1169
4. Kortei NK, Annan T, Dzikunoo J, Agbetiameh D. Exposure assessment and risk characterization of aflatoxins intake through consumption of maize (zea mays) in different age populations in the volta region of Ghana. Int J Food Contamin. (2022) 9:13. doi: 10.1186/s40550-022-00099-0
5. Omara T, Kiprop AK, Wangila P, Wacoo AP, Kagoya S, Nteziyaremye P, et al. The scourge of aflatoxins in Kenya: a 60-year review (1960 to 2020). J Food Qual. (2021) 2021:8899839. doi: 10.1155/2021/8899839
6. Kinyenje E, Kishimba R, Mohamed M, Mwafulango A, Eliakimu E, Kwesigabo G. Aflatoxicosis outbreak and its associated factors in Kiteto, Chemba and Kondoa districts, Tanzania. PLoS Glob Public Health. (2023) 3:e0002191. doi: 10.1371/journal.pgph.0002191
7. Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, et al. A review of human carcinogens–part F: chemical agents and related occupations. Lancet Oncol. (2009) 10:1143–4. doi: 10.1016/S1470-2045(09)70358-4
8. Liu Y, Galani Yamdeu JH, Gong YY, Orfila C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr Rev Food Sci Food Saf. (2020) 19:1521–60. doi: 10.1111/1541-4337.12562
9. Pickova D, Ostry V, Malir F. A recent overview of producers and important dietary sources of aflatoxins. Toxins. (2021) 13:186. doi: 10.3390/toxins13030186
10. Kumar A, Pathak H, Bhadauria S, Sudan J. Aflatoxin contamination in food crops: causes, detection, and management: a review. Food Prod Proc Nutr. (2021) 3:1–9. doi: 10.1186/s43014-021-00064-y
11. Boshou L. A review on progress and prospects of peanut industry in China. China J Oil Crop Sci. (2020) 42:161–6. doi: 10.19802/j.issn.1007-9084.2020115
12. Liwei Z, Liaowei W. Development status, existing problems and policy recommendations of peanut industry in China. China Oils Fats. (2020) 45:116–22. doi: 10.12166/j.zgyz.1003-7969/2020.11.024
13. Qin M, Liang J, Yang D, Yang X, Cao P, Wang X, et al. Spatial analysis of dietary exposure of aflatoxins in peanuts and peanut oil in different areas of China. Food Res Int. (2021) 140:109899. doi: 10.1016/j.foodres.2020.109899
14. Zhang W, Liu Y, Liang B, Zhang Y, Zhong X, Luo X, et al. Probabilistic risk assessment of dietary exposure to aflatoxin B(1) in Guangzhou, China. Sci Rep. (2020) 10:7973. doi: 10.1038/s41598-020-64295-8
15. Qi N, Yu H, Yang C, Gong X, Liu Y, Zhu Y. Aflatoxin B(1) in peanut oil from Western Guangdong, China, during 2016-2017. Food Addit Contam Part B Surveill. (2019) 12:45–51. doi: 10.1080/19393210.2018.1544173
16. Bordin K, Sawada MM, Rodrigues CEdC, da Fonseca CR, Oliveira CAF. Incidence of aflatoxins in oil seeds and possible transfer to oil: a review. Food Eng Rev. (2014) 6:20–8. doi: 10.1007/s12393-014-9076-9
17. Moloi TP, Ziqubu K, Mazibuko-Mbeje SE, Mabaso NH, Ndlovu Z. Aflatoxin B1-induced hepatotoxicity through mitochondrial dysfunction, oxidative stress, and inflammation as central pathological mechanisms: a review of experimental evidence. Toxicology. (2024) 509:153983. doi: 10.1016/j.tox.2024.153983
18. Xu F, Li Y, Cao Z, Zhang J, Huang W. AFB1-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition. Ecotoxicol Environ Saf. (2021) 216:112213. doi: 10.1016/j.ecoenv.2021.112213
19. Lin J-X, Xu C-Y, Wu X-M, Che L, Li T-Y, Mo S-M, et al. Rab7a-mTORC1 signaling-mediated cholesterol trafficking from the lysosome to mitochondria ameliorates hepatic lipotoxicity induced by aflatoxin B1 exposure. Chemosphere. (2023) 320:138071. doi: 10.1016/j.chemosphere.2023.138071
20. Che L, Huang J, Lin J-X, Xu C-Y, Wu X-M, Du Z-B, et al. Aflatoxin B1 exposure triggers hepatic lipotoxicity via p53 and perilipin 2 interaction-mediated mitochondria-lipid droplet contacts: an in vitro and in vivo assessment. J Hazard Mater. (2023) 445:130584. doi: 10.1016/j.jhazmat.2022.130584
21. Ren X-L, Han P, Meng Y. Aflatoxin B1-induced COX-2 expression promotes mitophagy and contributes to lipid accumulation in hepatocytes in vitro and in vivo. Int J Toxicol. (2020) 39:594–604. doi: 10.1177/1091581820939081
22. Jobe MC, Mthiyane DM, Dludla PV, Mazibuko-Mbeje SE, Onwudiwe DC, Mwanza M. Pathological role of oxidative stress in aflatoxin-induced toxicity in different experimental models and protective effect of phytochemicals: a review. Molecules. (2023) 28:5369. doi: 10.3390/molecules28145369
23. Ye L, Chen H, Tsim KWK, Shen X, Li X, Li X, et al. Aflatoxin B1 induces inflammatory liver injury via gut microbiota in mice. J Agric Food Chem. (2023) 71:10787–97. doi: 10.1021/acs.jafc.3c02617
24. Zhang L-Y, Zhan D-L, Chen Y-Y, Wang W-H, He C-Y, Lin Y, et al. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: an in vitro, ex vivo, and in vivo study. Arch Toxicol. (2019) 93:3305–20. doi: 10.1007/s00204-019-02572-w
25. He Z, Chen Z, Mo Y, Lu X, Luo Y, Lin S, et al. Assessment of the adverse health effects of aflatoxin exposure from unpackaged peanut oil in Guangdong, China. Toxins. (2023) 15:646. doi: 10.3390/toxins15110646
26. Gongfu Z, Pingchia K. Guangzhou: Encyclopedia Britannica (2024). Available at: https://www.britannica.com/place/Guangzhou (accessed July 7, 2024).
27. Yanyan W, Kuncai C, Yufei L, Yan L. Contamination level and exposure assessment of aflatoxin b1 in loose-packed peanut oil extracted by a native method in individual workshops in Guangzhou. South China J Prev Med. (2023) 49:132–6. doi: 10.12183/j.scjpm.2023.0132
28. The Standing Committee of the Guangdong Provincial People's Congress. Regulations on the Management of Small Food Workshops and Food Stalls in Guangdong Province (2015). Available at: https://flk.npc.gov.cn/detail2.html?NDAyOGFiY2M2MTI3Nzc5MzAxNjEyN2RmYWYyMDFlOGY (accessed July 7, 2024).
29. Yaping L, Jiaoni S. Local legislation, departmental interest and food safety: a case study of provincial legislation on small and medium sized food enterprises. Acad Res. (2021) 43–53+177. doi: 10.3969/j.issn.1000-7326.2021.02.008
30. Wenying W, Jiangjun J, Weihao Y. A study on the supervision system of food production and processing small workshops and vendors - based on relevant policy and regulations analysis in 22 provinces and cities. Food Ind. (2016) 78–83. Available at: https://d.wanfangdata.com.cn/periodical/Ch9QZXJpb2RpY2FsQ0hJTmV3UzIwMjQxMTA1MTcxMzA0EgxzcGoyMDE2MDIwMjIaCGJidm8zcGg3
31. Ming Y, Xiaohan Z. A study of local legislation on stall keepers and pedlars:An empirical analysis based on 20 local legislative documents. J Pingdingshan Univ. (2018) 33:59–63. doi: 10.3969/j.issn.1673-1670.2018.04.013
32. GB/T 5009.23-2006. Determination of Aflatoxins B1, B2, G1, and G2 in Foods. Beijing: Ministry of Health of the People's Republic of China (2006).
33. GB 5009.22-2016. Determination of Aflatoxins B and G Groups in Foods. Beijing: National Health Commission of the People's Republic of China (2016).
34. GB 2761-2017. National Food Safety Standards – Maximum Levels of Fungal Toxins in Food. Beijing: National Medical Products Administration (2017).
35. Euro G-F, editor. Report on a workshop in the frame of gems-food euro, eur/hfa target 22. In: Proceedings of the Second Workshop on Reliable Evaluation of Low-Level Contamination of Food. Kulmbach (1995).
36. Guangzhou Statistical Bureau. Guangzhou Statistical Yearbook (2023). Available at: https://tjj.gz.gov.cn/datav/admin/home/www_nj/ (accessed July 7, 2024).
37. Yuejiao L. Report on nutrition and chronic disease status of Chinese residents. Food Nutr China. (2020) 26:2. doi: 10.19870/j.cnki.11-3716/ts.2020.12.001
38. The People's Government of Guangzhou Municipality. Bulletin of the Seventh National Population Census of Guangzhou Municipality [1] (no.3) - Sex Composition of the Population (2021). Available at: http://www.gz.gov.cn/zwgk/sjfb/tjgb/content/post_7286236.html (accessed July 7, 2024).
39. Schrenk D, Bignami M, Bodin L, Chipman JK, Del Mazo J, Grasl-Kraupp B, et al. Risk assessment of aflatoxins in food. EFSA J. (2020) 18:e06040. doi: 10.2903/j.efsa.2020.6040
40. Authority EFS. International frameworks dealing with human risk assessment of combined exposure to multiple chemicals. EFSA J. (2013) 11:3313. doi: 10.2903/j.efsa.2013.3313
41. People's Government of Xiaolou Town. Overview of Xiaolou Town (2024). Available at: https://www.zc.gov.cn/jg/jdbscjzzf/xlz/qygk/ (accessed July 7, 2024).
42. Zengcheng District Healthcare Bureau. Zengcheng District National Basic Public Health Service Organization Information Publicity (2023). Available at: https://www.zc.gov.cn/zx/tzgg/qwsjkj/content/post_9289048.html (accessed July 7, 2024).
43. Liao S, Shi D, Clemons-Chevis CL, Guo S, Su R, Qiang P, et al. Protective role of selenium on aflatoxin B1-induced hepatic dysfunction and apoptosis of liver in ducklings. Biol Trace Elem Res. (2014) 162:296–301. doi: 10.1007/s12011-014-0131-4
44. Hall P, Cash J. What is the real function of the liver ‘function' tests? Ulster Med J. (2012) 81:30–6.
45. Farag R, AlAyobi D, Kwon H, EL-Ansary A. Relationship between aflatoxin B1 exposure and etiology of liver disease in Saudi ARABIAN patients. J Pure Appl Microbiol. (2018) 12: 1147–3. doi: 10.22207/JPAM.12.3.13
46. Hatipoglu D, Keskin E. The effect of curcumin on some cytokines, antioxidants and liver function tests in rats induced by aflatoxin B1. Heliyon. (2022) 8:e09890. doi: 10.1016/j.heliyon.2022.e09890
47. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. (2005) 172:367–79. doi: 10.1503/cmaj.1040752
48. National Health Commission of the People's Republic of China. Reference Interval for Commonly Used Biochemical Test Items in Clinical Practice (2012). Available at: http://www.nhc.gov.cn/wjw/s9492/201301/9b2e494990ce4825a48b4b476f6f928b.shtml (accessed July 7, 2024).
49. Chen W, Shi J, Qian L, Azen SP. Comparison of robustness to outliers between robust poisson models and log-binomial models when estimating relative risks for common binary outcomes: a simulation study. BMC Med Res Methodol. (2014) 14:82. doi: 10.1186/1471-2288-14-82
50. Travis-Lumer Y, Goldberg Y, Levine SZ. Effect size quantification for interrupted time series analysis: implementation in R and analysis for COVID-19 research. Emerg Themes Epidemiol. (2022) 19:9. doi: 10.1186/s12982-022-00118-7
51. Amuthavalli Thiyagarajan J, Mikton C, Harwood RH, Gichu M, Gaigbe-Togbe V, Jhamba T, et al. The un decade of healthy ageing: Strengthening measurement for monitoring health and wellbeing of older people. Age Ageing. (2022) 51:afac147. doi: 10.1093/ageing/afac147
52. Alameri MM, Kong AS, Aljaafari MN, Ali HA, Eid K, Sallagi MA, et al. Aflatoxin contamination: an overview on health issues, detection and management strategies. Toxins. (2023) 15:246. doi: 10.3390/toxins15040246
53. Bolei Y, Xiujuan Z, Gang W, Chenxi Z. Investigation of aflatoxin B1 and cyclopiazonic acid in bulk peanut oil in China. China Oils Fats. (2020) 45:34–7+53. doi: 10.12166/j.zgyz.1003-7969/2020.09.007
54. Guoying Y, Sichao L, Lingling L. Analysis on aflatoxin B1 contamination in vegetable oils. J Prev Med Inf. (2017) 33:593–6. Available at: https://d.wanfangdata.com.cn/periodical/Ch9QZXJpb2RpY2FsQ0hJTmV3UzIwMjQxMTA1MTcxMzA0EhF5Znl4cWJ6ejIwMTcwNjAyMRoIbXV5ZWdtbmE%3D
55. Lijuan W, Bicong C, Yuanbiao H, Zifan C. Analysis and suggestions on sampling inspection data of edible vegetable oil in Heyuan in 2021. China Food Saf Mag. (2022) 30–3. doi: 10.16043/j.cnki.cfs.2022.31.048
56. Guangdong Provincial Drug Administration. Explanations on the Relevant Contents of the Measures for the Registration and Administration of Small Food Workshops in Guangdong Province (2016). Available at: https://mpa.gd.gov.cn/gkmlpt/content/2/2109/post_2109077.html#1879 (accessd July 7, 2024).
57. Yi Z, Xiaohui L, Yiping X, Kang A, Mingyue Z. Contamination status of some fungal toxins in commercially available vegetable oil in Tianjin in 2017. Occup Health. (2018) 34:3353–6. doi: 10.13329/j.cnki.zyyjk.2018.0939
58. Liu C, Van der Fels-Klerx HJ. Quantitative modeling of climate change impacts on mycotoxins in cereals: a review. Toxins. (2021) 13:276. doi: 10.3390/toxins13040276
59. Soilinfo. Data Sourced From National Soil Information Service Platform of China (2024). Available at: http://www.soilinfo.cn (accessed July 7, 2024).
60. Nji QN, Babalola OO, Mwanza M. Soil Aspergillus species, pathogenicity and control perspectives. J Fungi. (2023) 9:766. doi: 10.3390/jof9070766
61. Vasseghian Y, Moradi M, Dragoi E-N, Khaneghah AM. A review on mycotoxins detection techniques in edible oils. Int J Environ Anal Chem. (2022) 102:2125–39. doi: 10.1080/03067319.2020.1750607
62. Fang L, Zhao B, Zhang R, Wu P, Zhao D, Chen J, et al. Occurrence and exposure assessment of aflatoxins in Zhejiang Province, China. Environ Toxicol Pharmacol. (2022) 92:103847. doi: 10.1016/j.etap.2022.103847
63. Khan R, Ghazali FM, Mahyudin NA, Samsudin NIP. Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: a review. J Fungi. (2021) 7:606. doi: 10.3390/jof7080606
64. Shabeer S, Asad S, Jamal A, Ali A. Aflatoxin contamination, its impact and management strategies: an updated review. Toxins. (2022) 14:307. doi: 10.3390/toxins14050307
65. Ndung'u J, Makokha A, Onyango C, Mutegi C, Wagacha J, Christie M, et al. Prevalence and potential for aflatoxin contamination in groundnuts and peanut butter from farmers and traders in Nairobi and Nyanza Provinces of Kenya. J Appl Biosci. (2013) 65:89579. doi: 10.4314/jab.v65i0.89579
66. Lu T, Guo Y, Zeng Z, Wu K, Li X, Xiong Y. Identification and detoxification of AFB1 transformation product in the peanut oil refining process. Food Control. (2023) 149:109726. doi: 10.1016/j.foodcont.2023.109726
67. Chilaka CA, Obidiegwu JE, Chilaka AC, Atanda OO, Mally A. Mycotoxin regulatory status in Africa: a decade of weak institutional efforts. Toxins. (2022) 14:442. doi: 10.3390/toxins14070442
68. Jolly PE, Jiang Y, Ellis WO, Awuah RT, Appawu J, Nnedu O, et al. Association between aflatoxin exposure and health characteristics, liver function, hepatitis and malaria infections in ghanaians. J Nutr Environ Med. (2007) 16:242–57. doi: 10.1080/13590840701703918
69. Mills S, Adams J, Wrieden W, White M, Brown H. Sociodemographic characteristics and frequency of consuming home-cooked meals and meals from out-of-home sources: cross-sectional analysis of a population-based cohort study. Public Health Nutr. (2018) 21:2255–66. doi: 10.1017/S1368980018000812
70. Haoran X. Extending the legislativetentacles to the front line of food safety - interpretation of the Guangdong Province food production and processing small workshop and food stall management regulations. Voice People. (2015) 25:27–8. Available at: https://d.wanfangdata.com.cn/periodical/Ch9QZXJpb2RpY2FsQ0hJTmV3UzIwMjQxMTA1MTcxMzA0EhdRS0MyMDE1MjAxNTExMDQwMDA1NTg1NxoIeTl0YWlmNDU%3D
71. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgraduate Med J. (2016) 92:223–34. doi: 10.1136/postgradmedj-2015-133715
Keywords: small food workshop, home-made peanut oil, liver function, aflatoxin B1, interrupted time series analysis
Citation: Lei J, Li Y, Wang Y, Zhou J, Wu Y, Zhang Y, Liu L, Ou Y, Huang L, Wu S, Guo X, Liu L, Peng R, Bai Z and Zhang W (2024) The impact of small food workshops management regulations on aflatoxin B1 in home-made peanut oil and the liver function of high-consumption area residents: an interrupted time series study in Guangzhou, China. Front. Public Health 12:1484414. doi: 10.3389/fpubh.2024.1484414
Received: 21 August 2024; Accepted: 09 December 2024;
Published: 20 December 2024.
Edited by:
Ahmed Noah Badr, National Research Centre, EgyptReviewed by:
Amal Hathout, National Research Centre, EgyptZeinab Khaled Hamza, National Research Centre, Egypt
Md Atiqul Haque, Hajee Mohammad Danesh Science & Technology University, Bangladesh
Salah Salem, National Research Centre, Egypt
Copyright © 2024 Lei, Li, Wang, Zhou, Wu, Zhang, Liu, Ou, Huang, Wu, Guo, Liu, Peng, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Zhang, Z3pjZGN6aGFuZ3d3QGZveG1haWwuY29t
 Jiangbo Lei
Jiangbo Lei Yan Li2
Yan Li2 Yanyan Wang
Yanyan Wang