- 1Department of Geriatrics, The Third People’s Hospital of Yunnan Province, Kunming, Yunnan, China
- 2Department of Endocrinology, The First People’s Hospital of Yunnan Province, Kunming, Yunnan, China
Background: Sarcopenia, sarcopenic obesity, and hypertension are all widespread public health problems in middle-aged and older populations, and their association is controversial. The purpose of this study is to analyze the relationship between obesity, sarcopenia, and sarcopenic obesity with hypertension in a middle-aged and older community population in China through a large-scale longitudinal design.
Methods: In this cohort study with 7 years of follow-up, the study population was drawn from participants in the China Health and Retirement Longitudinal Study (CHARLS) in 2011 and followed up in 2013, 2015, and 2018. The diagnostic criteria for sarcopenia were based on the consensus recommendations issued by the Asian Working Group for Sarcopenia (AWGS) in 2019. The diagnosis of obesity is based on body mass index and waist circumference. Sarcopenic obesity is defined as the coexistence of sarcopenia and obesity. Cox proportional risk regression models were used to analyze the association of obesity, sarcopenia, and sarcopenic obesity with hypertension.
Results: A total of 7,301 participants with a mean age of 58 ± 8.8 were enrolled in the study, and 51.9% females. A total of 1,957 participants had a new onset of hypertension after 7 years of follow-up. In a multifactorial analysis, obesity and sarcopenic obesity were associated with hypertension; hazard ratios (HRs) and 95% confidence intervals (CIs) were 1.67 (1.43 ~ 1.96), p < 0.001, and 1.61 (1.09 ~ 2.37), p = 0.017. Sarcopenia and hypertension were not significantly associated; the HR and 95% CI were 1.17 (0.9 ~ 1.52), p = 0.23.
Conclusion: There is no significant correlation between sarcopenia and hypertension, but obesity and sarcopenic obesity increase the risk of hypertension. Targeted management of middle-aged and older people with sarcopenic obesity is needed in public health efforts.
1 Introduction
Hypertension is a common chronic disease in the older population, and according to the Global Burden of Hypertension Disease Report (1), it is projected that the global burden of disease from hypertension may exceed 1.6 billion people by 2025. Hypertension increases the risk of diseases such as cardiovascular disease, stroke, kidney disease, and retinopathy, reduces quality of life, and significantly increases the risk of death (2–4). Hence, delving deeper into the risk factors for hypertension becomes crucial for early detection and intervention.
Sarcopenia is a chronic condition associated with aging that is characterized by a reduction in skeletal muscle mass with a concomitant decline in muscle strength or somatic function and is associated with adverse events such as falls and fractures, increasing disability and mortality rates, and seriously affecting the quality of life of older people (5). A large longitudinal cohort study from China Health and Retirement Longitudinal Study (CHARLS) found that higher grip strength was an independent protective factor for hypertension in a middle-aged and older population (6). Sarcopenic obesity is the presence of sarcopenia and obesity in the same individual, with the two acting synergistically to affect health and lead to serious adverse outcomes (7–10). It has been established that obesity significantly increases the risk of hypertension through metabolic disorders, insulin resistance, and atherosclerosis (11). In addition, in obese patients, fat stored in the viscera, in particular, releases pro-inflammatory cytokines that cause the body to be in a state of chronic inflammation, and the aging process is associated with chronic low-grade inflammation called inflammatory aging (12). Inflammatory aging plays a prominent role in age-related chronic diseases such as atherosclerosis, insulin resistance, and sarcopenia, while this low-grade inflammation is one of the key factors contributing to the reduction of skeletal muscle mass (13). Numerous animal studies have provided compelling evidence for a causal role of inflammation in the pathogenesis of hypertension (14). Inflammation promotes intrarenal angiotensin II production by infiltrating inflammatory cells and renal tubular epithelial cells, which in turn further causes hypertension (15). There is some mechanistic overlap between sarcopenia, obesity, and sarcopenic obesity and hypertension.
A large number of studies have confirmed that obesity is a risk factor for hypertension (16–18), but there are discrepancies in the association between sarcopenia and sarcopenic obesity with hypertension. Myasthenia gravis obesity was similarly found to be a risk factor for hypertension in a US cohort study (19) that included 1,019 participants. However, a study by Dos Santos et al. (20) found that sarcopenia and sarcopenic obesity were not associated with arterial blood pressure. In an Iranian cross-sectional study (21), it was shown that obesity was associated with hypertension, whereas sarcopenia and sarcopenic obesity were not significantly associated with hypertension. Different studies may have inconsistent results due to differences in race, geography, lifestyle, and dietary habits. China accounts for more than 1/6 of the world’s population, and it is unclear whether sarcopenia and sarcopenic obesity are risk factors for hypertension in Chinese community populations. For this reason, we conducted a large 7-year CHARLS-based cohort study aimed at investigating the longitudinal association between sarcopenia and sarcopenic obesity and new-onset hypertension in a middle-aged and older Chinese community population, to provide a new evidence for the management of hypertension risk in such populations.
2 Methods
2.1 Study population
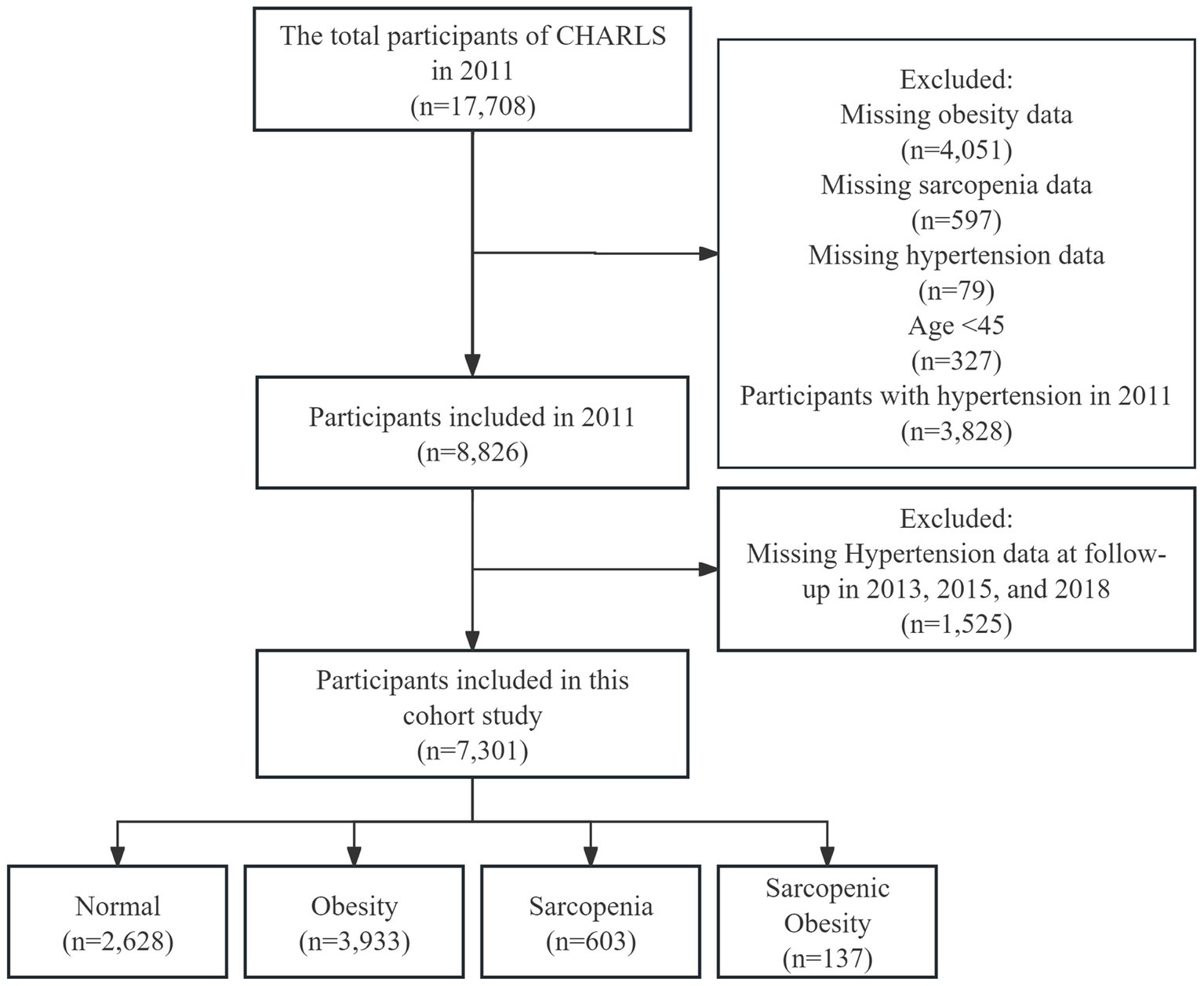
Participants in this cohort study were drawn from the China Health and Retirement Longitudinal Study (CHARLS) 2011, 2013, 2015 and 2018 survey, which collects a high-quality microdata set representative of middle-aged and older Chinese adults aged 45 years and older at the household and individual level to analyze the aging of the Chinese population (22). We developed a few exclusion criteria: (1) missing data on obesity (4,051), sarcopenia (597), and hypertension (79) at baseline; (2) age < 45 years (327); (3) with hypertension at baseline (3,828); and (4) missing data on hypertension at follow-up in 2013, 2015, and 2018 (1,525). Finally, 7,301 participants were included in this cohort study (Figure 1).
The CHARLS study was approved by the Peking University Biomedical Ethics Committee (approval number: IRB00001052-11015), and each participant signed an informed consent form (22). The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations for cohort studies (23).
2.2 Assessment of obesity, sarcopenia, and sarcopenic obesity
Obesity status was assessed using body mass index (BMI) and waist circumference; specifically, obesity was defined when BMI was ≥28 kg/m2 (24) or when waist circumference was ≥85 cm for men and ≥ 80 cm for women (25). The participants’ height and weight were measured in the standing position using Seca™ 213 height meters (China Seca Hangzhou Co., Ltd.) and Omron™ HN-286 weight scales (Kerui Technology Yangzhou Co., Ltd.), measuring waist circumference Using a Soft Ruler.
The assessment of sarcopenia status was based on the consensus recommendations issued by the Asian Working Group for Sarcopenia (AWGS) in 2019 (26), which are more appropriate for the Chinese population. Specifically, sarcopenia was defined as a decrease in skeletal muscle mass combined with a decrease in physical performance or a decrease in muscle strength. As there were no data on DXA or BIA in CHARLS, we employed the muscle mass equations that have been demonstrated to exhibit satisfactory concordance with DXA in the Chinese population to calculate the appendicular skeletal muscle mass (ASM) of the participants (27):
The skeletal muscle mass index (SMI) was calculated as follows: SMI = ASM / height2. The cut-off value for low SMI was defined as the lowest 20% of SMI by sex in the study population: 7 kg/m2 for male and 5.28 kg/m2 for female. Muscle strength was assessed using grip strength by using the Yuejian™ WL-1000 dynamometer (Nantong Yuejian Physical Testing Equipment Co., Ltd.), and participants took turns measuring grip strength twice for each hand. As recommended by the AWGS 2019, low muscle strength was defined as a maximum grip strength value of <28 kg in males or < 18 kg in females in four measurements. Physical function was assessed by 5 chair stand tests and 5 m gait speed, with low physical function defined as 5 chair stand tests ≥12 s or gait speed <1.0 m/s. The diagnostic criteria for sarcopenic obesity are based on an the most recent expert consensus published in 2022 by the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO): sarcopenic obesity is defined as the coexistence of obesity and sarcopenia (28, 29).
2.3 Assessment of hypertension
Hypertension was assessed based on the question in the questionnaire, “Have you been diagnosed with hypertension by a doctor?” and the time of the onset of hypertension at the follow-up was derived from the question, “When was the condition first diagnosed or known by yourself?” Hypertension was considered to be present when answering yes to the above question or when the time variable was not missing. For missing time to diagnosis of hypertension (n = 73), incident time was defined as the mean time to incident hypertension in other participants during the follow-up period.
2.4 Covariates
The covariates included in our cohort study were demographic characteristics, lifestyle, chronic diseases, and other blood test indicators. Demographic characteristics included gender, age, education, marital status, and place of residence. Lifestyle included smoking and drinking. Chronic disease data came from the questionnaire, “Have you been diagnosed with a chronic disease by a doctor?” The results of the questionnaire include dyslipidemia, diabetes mellitus, and kidney disease. Other blood test data included estimated glomerular filtration rate (eGFR), total triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c).
2.5 Statistical analysis
All participants were divided into four groups: normal, obesity, sarcopenia, and sarcopenic obesity. Continuous variables were expressed as the mean ± standard deviation (SD) or median and interquartile range (IQR), and categorical variables were expressed as counts and percentages. The t-tests, chi-square tests, ANOVA, or non-parametric tests were used to analyze the baseline characteristics of participants. Univariate and multivariate Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for hypertension in different groups. Covariates included in the multifactor Cox proportional hazards regression model include: gender, age, education, marital status, residence, smoking, drinking, dyslipidemia, diabetes mellitus, kidney disease, eGFR, TG, HDL-c, and LDL-c. Cumulative risk curves for the development of hypertension were plotted using Kaplan–Meier curves, and differences between groups were analyzed using log-rank tests. Finally, considering that age, smoking, and renal function status are all strongly associated with hypertension and that gender may also be a potential influence (30), we performed subgroup analyses of participants with different gender, age, smoking, and eGFR status to explore interactions.
All the analyses were performed with the statistical software Stata/MP version 17.0 (StataCorp LP, College Station, TX, USA) and Free Statistics software versions 1.9.2. The level of statistical significance was set at p < 0.05 (two-sided).
3 Results
3.1 Baseline characteristics of study participants
This cohort study ultimately included 7,301 participants, with a mean age of 58 years (SD 8.8) and 51.9% females. Table 1 shows the baseline characteristics of all participants. At baseline, 3,933 participants were diagnosed with obesity, 603 with sarcopenia, and 137 with sarcopenic obesity. Overall, obese participants had the highest prevalence of diabetes and dyslipidemia and had higher TG and lower HDL-c, while sarcopenia participants had the highest HDL-c levels, all p < 0.001. 84.7% of the sarcopenic obesity group were female, and the mean age was 70.5 ± 9.1, which was significantly higher than the mean age of the other groups, p < 0.001. In addition, participants in the sarcopenic obesity group were less likely to be married, less likely to smoke, had the least amount of diabetes and kidney disease, and had the lowest LDL-c levels, all p < 0.05.
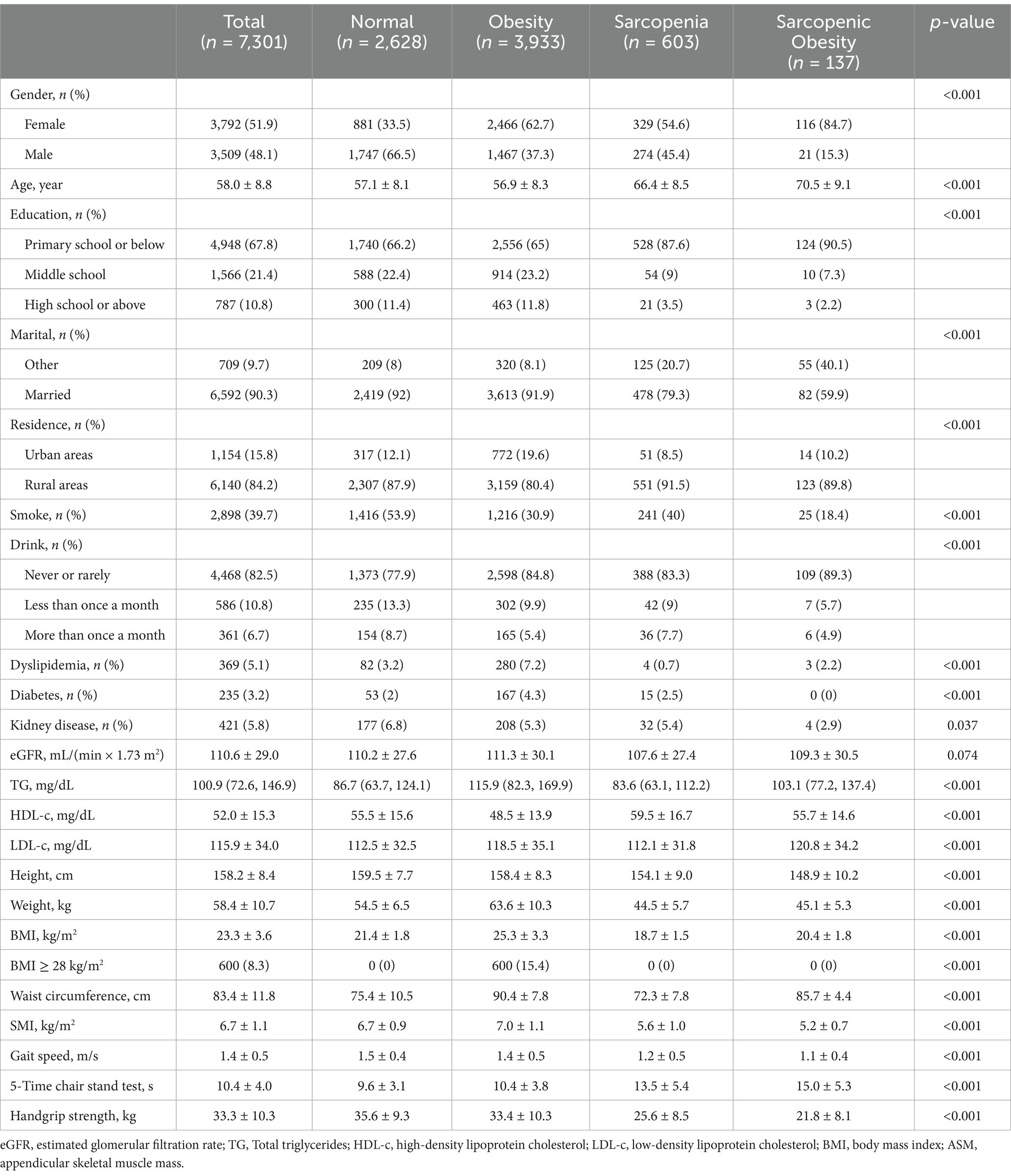
Table 1. Baseline characteristics of participants in this study according to obesity, sarcopenia, and sarcopenic obesity.
3.2 Association between obesity, sarcopenia, and sarcopenic obesity with hypertension
After following up for 7 years, there were 1,957 cases of hypertension, corresponding to 26.8% of all participants. Table 2 show the prevalence, HR, and 95%CI of hypertension in obesity, sarcopenia, and sarcopenic obesity participants, using normal participants as references. The prevalence of hypertension in normal, obesity, sarcopenia, and sarcopenic obesity participants were 19.9, 31.5, 25.4, and 29.9%. In univariate analyses, the HRs and 95% CIs for hypertension in participants with obesity, sarcopenia, and sarcopenic obesity were 1.7 (1.54 ~ 1.88), 1.33 (1.11 ~ 1.59), and 1.63 (1.19 ~ 2.24), respectively, all p < 0.01. In multivariate analysis after adjustment for all covariates, obesity and sarcopenic obesity were associated with hypertension; HRs and 95% CIs were 1.67 (1.43 ~ 1.96) and 1.61 (1.09 ~ 2.37), both p < 0.05. Sarcopenia and hypertension were not significantly associated, HR and 95% CI was 1.17 (0.9 ~ 1.52), p = 0.23. Kaplan–Meier curves showed that the cumulative risk of hypertension increased over time; Participants with obesity and sarcopenic obesity possessed a higher cumulative risk of hypertension (log-rank test, p < 0.0001) (Figure 2).
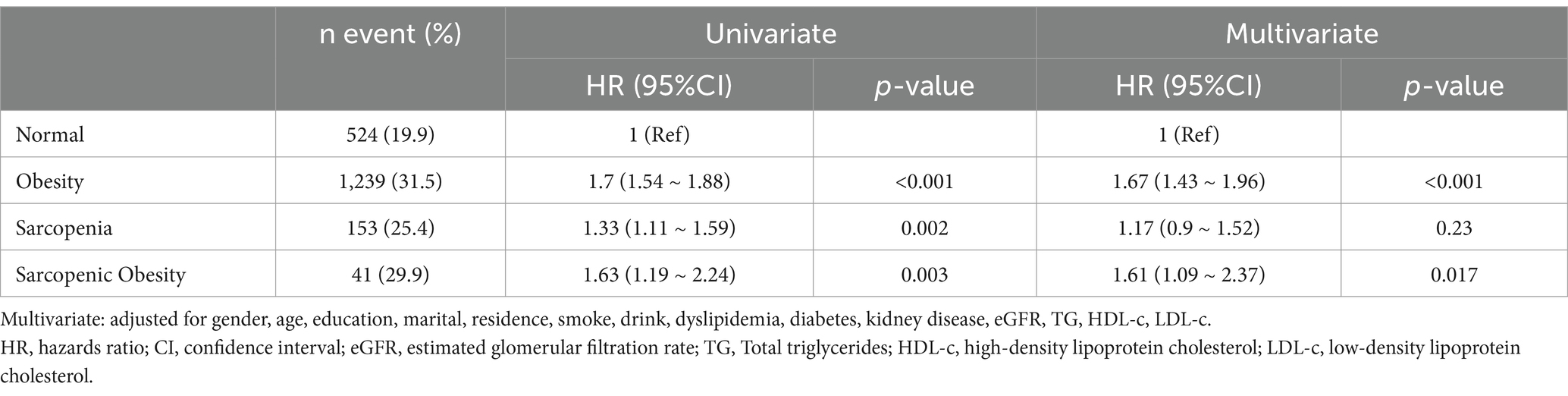
Table 2. Univariate and multivariate analysis the relationship between obesity, sarcopenia and sarcopenic obesity with hypertension.

Figure 2. The cumulative risk of hypertension increases over time (log-rank test, p < 0.0001). Light blue indicates normal population, yellow indicates obesity, dark blue indicates sarcopenia, and red indicates sarcopenic obesity.
Figure 3 shows the results of the multifactorial subgroup analysis. The association between obesity, sarcopenia, and sarcopenic obesity with hypertension was similar among participants of different genders, smoking, and eGFR status. However, we found that the effects of sarcopenia and sarcopenic obesity on hypertension appeared to differ between those aged less than 60 years and those aged more than 60 years.
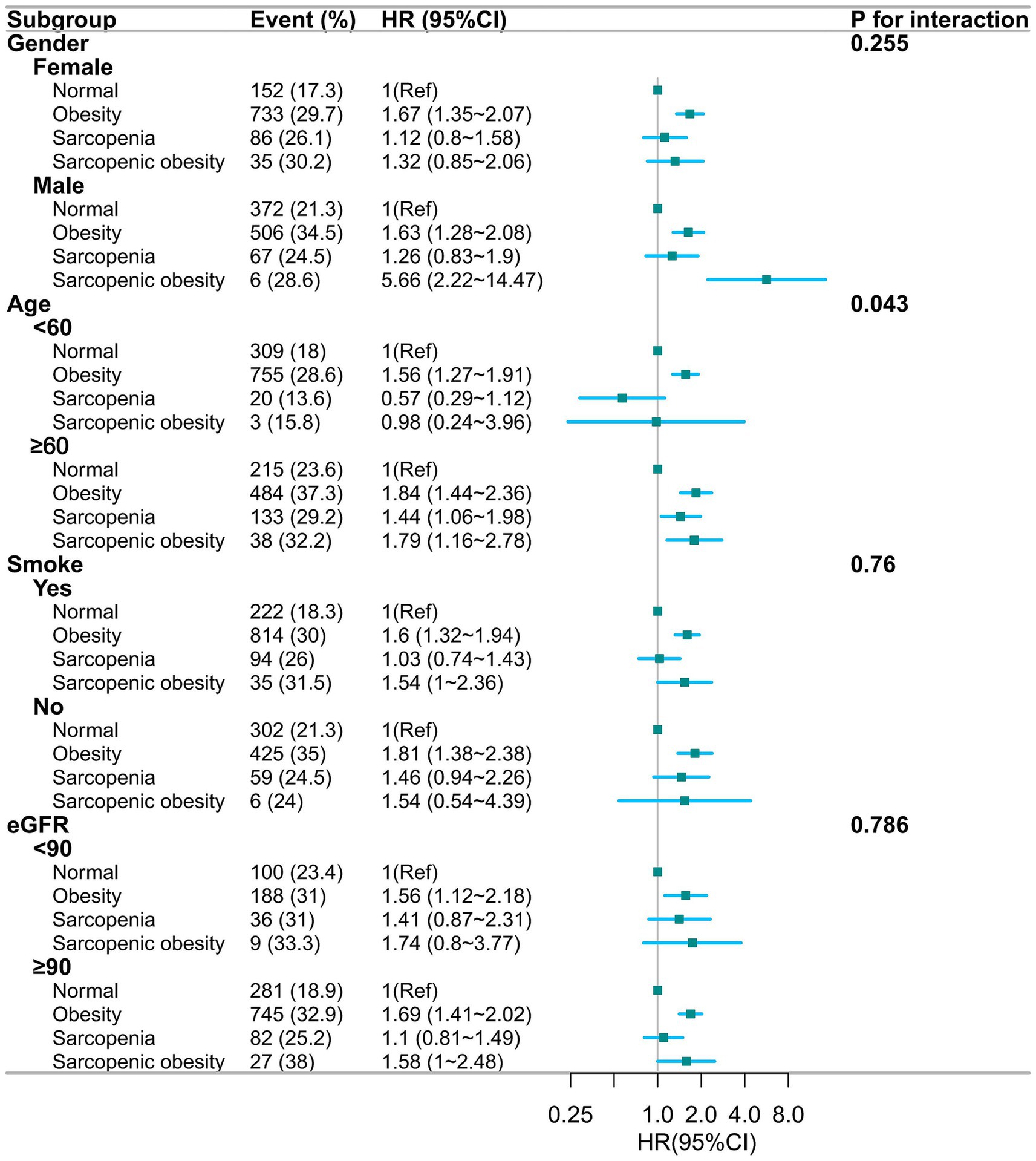
Figure 3. Forest plot of multifactorial subgroup analyses based on gender, age, smoking, and eGFR grouping, adjusted for gender, age, education, marital, residence, smoke, drink, dyslipidemia, diabetes, kidney disease, eGFR, TG, HDL-c, LDL-c. HR, hazards ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; TG, Total triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol.
4 Discussion
In our cohort study, the diagnostic criteria for sarcopenia were based on the recommendations of AWGS 2019, and the diagnostic criteria for sarcopenic obesity were based on the expert consensus published jointly by ESPEN and EASO in 2022. We used the representative CHARLS database to analyze the associations between obesity, sarcopenia, and sarcopenic obesity with the risk of new-onset hypertension in a middle-aged and older Chinese community-based population. After adjusting for all covariates, obesity and sarcopenic obesity were significantly associated with hypertension, and there was no significant association between sarcopenia and hypertension.
In our study, sarcopenic obesity increased the risk of hypertension, and sarcopenia was not significantly associated with hypertension. A cross-sectional study by Dutra et al. (31) of women in a Brazilian community found that sarcopenic obesity was a risk factor for hypertension, and that the prevalence of sarcopenic obesity was higher than in other studies, but the number of subjects enrolled was relatively small, which makes it difficult to discuss the prevalence. A large cross-sectional study in northern China (32) included 14,926 adults aged 35 ~ 74 years and found a high prevalence of sarcopenic obesity (65.1%), which is associated with hypertension, diabetes mellitus, and lipid metabolic abnormalities. However, a cross-sectional survey by Pasdar et al. in Iranian Kurds (21) showed that sarcopenia and sarcopenic obesity were not associated with hypertension. Another cross-sectional study, also from northern China, including 1,082 participants (33), found that men of advanced age, physical inactivity, diabetes mellitus, and absence of hypertension may have a higher prevalence of sarcopenia, and this study had a smaller sample size and was limited to the local area of Bengbu. Another cross-sectional study in Korea (34) found that subjects with obese sarcopenia appeared to have a greater risk of hypertension than subjects with obesity or sarcopenia alone. So the association between sarcopenia, sarcopenic obesity, and hypertension may vary in different countries because sarcopenia may be influenced by economic level, medical level, and genetic factors.
Definitions of obesity and measurement techniques (BMI and waist circumference), while consistent with standard practice, have some limitations. For example, BMI does not distinguish between fat and muscle mass, which may affect its accuracy in identifying sarcopenic obesity. In our study population, the majority of both obesity and sarcopenic obesity were abdominal obesity, with only 15.4% of obesity participants having a BMI ≥28 kg/m2 and all sarcopenic obesity participants having a BMI <28 kg/m2. Similar to our findings, an epidemiological study on sarcopenic obesity based on the Western Cohort Population conducted in China (35) found that defining obesity by waist circumference found a prevalence of 7.22% for sarcopenic obesity, whereas the prevalence of sarcopenic obesity in obesity defined by BMI was only 0.63%. To our knowledge, in a retrospective cohort study in China that included 74,955 participants (36), abdominal obesity was found to be about two times more prevalent than hypertension in those with normal abdominal circumference, which is a risk factor for hypertension. Similarly, in a cohort study of risk factors for hypertension in the United States (37), which included 3,475 participants, centripetal obesity significantly increased the risk of developing hypertension.
Our study also found a significant gender difference in the prevalence of sarcopenic obesity, with a prevalence of 84.7% (116/137) in females. This gender difference may be related to age-related changes in sex hormone levels; the mean age of the sarcopenic obesity group was 70.5 ± 9.1 years, and most of the women were in menopause. In addition, women (62.7%) were more likely than men (37.3%) to be purely obese participants in our baseline profile, which may also account for the gender difference. It has been found that a decrease in estrogen levels associated with menopause is associated with a decrease in muscle mass and an increase in total body fat (38). During menopause, changes in hormone levels may lead to the release of pro-inflammatory cytokines, and this induced systemic mild inflammation can promote the loss of muscle mass. In men, testosterone can promote muscle regeneration by activating satellite cells, enhancing muscle protein synthesis, and increasing androgen receptor expression (39). Decreasing testosterone levels with increasing age may have a side effect on muscle mass and fat distribution in older adults (40).
The pathogenesis of obesity and hypertension is complex. Obesity and sarcopenic obesity have been found to be associated with the development of hypertension through the following common underlying mechanisms (e.g., chronic inflammation, insulin resistance). Both obesity and sarcopenic obesity trigger the release of inflammatory factors (41), which promotes muscle protein catabolism and accelerates the progression of sarcopenia. These inflammatory factors can also affect the sympathetic nervous system and the renin-angiotensin-aldosterone system, leading to elevated blood pressure. Low serum lipocalin levels in obese patients contribute to hypertension by increasing insulin resistance, and there is growing evidence that insulin resistance is associated with sarcopenia (42). Our study found that sarcopenia alone did not increase the risk of hypertension, but sarcopenic obesity increased the risk of hypertension, which is consistent with the findings of Dutra et al. (31). Our study implies a possible threshold effect when sarcopenia increases the risk of hypertension only when combined with obesity, while the risk of hypertension is not increased in sarcopenic populations without combined obesity. The specific thresholds and mechanisms of BMI or waist circumference that lead to an increased risk of hypertension in the sarcopenia population remain to be further investigated.
In our subgroup analyses, the effects of sarcopenia and sarcopenic obesity on hypertension differed significantly between those aged >60 and < 60 years. This may be related to the age characteristics of our study population. In our study, <60 years of age accounted for fewer participants with sarcopenia (147/603) and sarcopenic obesity (19/137). Sarcopenia and hypertension share a variety of potential pathogenic mechanisms (e.g., insulin resistance, decreased physical activity, chronic inflammation, inadequate protein intake). Chronic inflammation, particularly the production of catabolic cytokines (41), is a major mechanism leading to sarcopenia and age-related chronic diseases, including hypertension (34). The common pathway of reduced physical activity with age, leading to skeletal muscle mitochondrial autophagy hyperfunction and the accumulation of oxidative toxicants, which leads to muscle damage, may explain why hypertension is more prevalent in participants with sarcopenia or sarcopenic obesity over the age of 60 years, and the prevalence increases with age. However, the interaction of age with this needs further study.
To the best of our knowledge, this is the first representative cohort study with a large sample size based on a community-based population of middle-aged and older people in China. We found that sarcopenia is not associated with the risk of hypertension, but sarcopenic obesity increases the risk of hypertension. The findings of this study highlight the significant impact of sarcopenic obesity on the risk of hypertension among middle-aged and older people. This suggests that targeted interventions for middle-aged and older individuals with sarcopenic obesity are needed in public health efforts to reduce the incidence of hypertension, thereby lowering the risks of complications such as cardiovascular diseases, stroke, and kidney diseases. However, there are some limitations to our study. Firstly, although the formula for ASM has been validated in a Chinese population and has shown good agreement with DXA in various studies, muscle mass was estimated using the formula, not DXA or bioimpedance analysis. This is because there is no DXA or BIA data in CHARLS. This formula has been reported to be in good agreement with DXA and has been used in many studies on sarcopenia, e.g., in Chen et al. (43), Luo et al. (44), Gao et al. (45), Zhou et al. (46) to assess muscle mass. Secondly, the diagnosis of hypertension was based on a questionnaire, not on the results of multiple measurements of blood pressure on different dates, certain hypertension susceptibility factors such as genetic susceptibility, sodium intake, physical activity, environmental and psychological factors were not considered. Thirdly, BMI does not distinguish between fat and muscle mass, which may affect its accuracy in identifying sarcopenic obesity.
5 Conclusion
There is no significant correlation between sarcopenia and hypertension, but obesity and sarcopenic obesity increase the risk of hypertension. Targeted management of middle-aged and older people with sarcopenic obesity is needed in public health efforts.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://charls.pku.edu.cn/.
Ethics statement
This study was based on publicly available datasets (CHARLS). Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or the patients’/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. The CHARLS study was approved by the Peking University Biomedical Ethics Committee (approval number: IRB00001052-11015), and each participant signed an informed consent form.
Author contributions
RD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. GJ: Data curation, Investigation, Writing – original draft. ZD: Data curation, Funding acquisition, Investigation, Writing – original draft. HY: Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WH: Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific Research Fund of Yunnan Provincial Department of Education (grant number: 2024J0866), Third People’s Hospital of Yunnan Province (grant number: 2024SSYKT01).
Acknowledgments
This study was based on the China Health and Retirement Longitudinal Study (CHARLS). Authors would like to thank the CHARLS research team, field staff, and all the CHARLS participants for their time and efforts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Egan, BM, Kjeldsen, SE, Grassi, G, Esler, M, and Mancia, G. The global burden of hypertension exceeds 1.4 billion people: should a systolic blood pressure target below 130 become the universal standard? J Hypertens. (2019) 37:1148–53. doi: 10.1097/hjh.0000000000002021
2. Parati, G, Lackland, DT, Campbell, NRC, Owolabi, MO, Bavuma, C, Mamoun Beheiry, H, et al. How to improve awareness, treatment, and control of hypertension in Africa, and how to reduce its consequences: a call to action from the world hypertension league. Hypertension. (2022) 79:1949–61. doi: 10.1161/hypertensionaha.121.18884
3. Chen, X, Liu, L, Liu, M, Huang, X, Meng, Y, She, H, et al. Hypertensive retinopathy and the risk of stroke among hypertensive adults in China. Invest Ophthalmol Vis Sci. (2021) 62:28. doi: 10.1167/iovs.62.9.28
4. Murray, CJL, Aravkin, AY, Zheng, P, Abbafati, C, Abbas, KM, Abbasi-Kangevari, M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
5. Jones, C, Chen, KM, Weeks, B, Qi, M, and Moyle, W. Healthy beat acupunch exercise program: validation and feasibility study for older adults with reduced physical capacity or probable sarcopenia. Explore. (2021) 17:498–504. doi: 10.1016/j.explore.2020.05.010
6. Luo, JH, Zhang, TM, Yang, LL, Cai, YY, and Yang, Y. Association between relative muscle strength and hypertension in middle-aged and older Chinese adults. BMC Public Health. (2023) 23:2087. doi: 10.1186/s12889-023-17007-6
7. Atkins, JL, and Wannamathee, SG. Sarcopenic obesity in ageing: cardiovascular outcomes and mortality. Br J Nutr. (2020) 124:1102–13. doi: 10.1017/s0007114520002172
8. Liu, C, Wong, PY, Chung, YL, Chow, SK, Cheung, WH, Law, SW, et al. Deciphering the "obesity paradox" in the elderly: a systematic review and Meta-analysis of Sarcopenic obesity. Obes Rev. (2023) 24:e13534. doi: 10.1111/obr.13534
9. Wannamethee, SG, and Atkins, JL. Sarcopenic obesity and Cardiometabolic health and mortality in older adults: a growing health concern in an ageing population. Curr Diab Rep. (2023) 23:307–14. doi: 10.1007/s11892-023-01522-2
10. Yoshimura, Y, Wakabayashi, H, Nagano, F, Matsumoto, A, Shimazu, S, Shiraishi, A, et al. The applicability of the Espen and Easo-defined diagnostic criteria for Sarcopenic obesity in Japanese patients after stroke: prevalence and association with outcomes. Nutrients. (2022) 14:4205. doi: 10.3390/nu14194205
11. Kim, YJ, Moon, S, Yu, JM, and Chung, HS. Implication of diet and exercise on the management of age-related sarcopenic obesity in Asians. Geriatr Gerontol Int. (2022) 22:695–704. doi: 10.1111/ggi.14442
12. Franceschi, C, Garagnani, P, Parini, P, Giuliani, C, and Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
13. Franceschi, C, Garagnani, P, Vitale, G, Capri, M, and Salvioli, S. Inflammaging and 'Garb-Aging'. Trends Endocrinol Metab. (2017) 28:199–212. doi: 10.1016/j.tem.2016.09.005
14. Vaziri, ND, and Rodríguez-Iturbe, B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. (2006) 2:582–93. doi: 10.1038/ncpneph0283
15. Okamura, A, Rakugi, H, Ohishi, M, Yanagitani, Y, Takiuchi, S, Moriguchi, K, et al. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens. (1999) 17:537–45. doi: 10.1097/00004872-199917040-00012
16. Hall, JE, do Carmo, JM, da Silva, AA, Wang, Z, and Hall, ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. (2019) 15:367–85. doi: 10.1038/s41581-019-0145-4
17. Mouton, AJ, Li, X, Hall, ME, and Hall, JE. Obesity, hypertension, and cardiac dysfunction: novel roles of Immunometabolism in macrophage activation and inflammation. Circ Res. (2020) 126:789–806. doi: 10.1161/circresaha.119.312321
18. Hall, JE, do Carmo, JM, da Silva, AA, Wang, Z, and Hall, ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. (2015) 116:991–1006. doi: 10.1161/circresaha.116.305697
19. Ma, J, Hwang, SJ, McMahon, GM, Curhan, GC, McLean, RR, Murabito, JM, et al. Mid-adulthood cardiometabolic risk factor profiles of Sarcopenic obesity. Obesity. (2016) 24:526–34. doi: 10.1002/oby.21356
20. dos Santos, EP, Gadelha, AB, Safons, MP, Nóbrega, OT, Oliveira, RJ, and Lima, RM. Sarcopenia and Sarcopenic obesity classifications and Cardiometabolic risks in older women. Arch Gerontol Geriatr. (2014) 59:56–61. doi: 10.1016/j.archger.2014.03.012
21. Pasdar, Y, Darbandi, M, Rezaeian, S, Najafi, F, Hamzeh, B, and Bagheri, A. Association of Obesity, sarcopenia, and Sarcopenic obesity with hypertension in adults: A Cross-sectional study from Ravansar, Iran during 2014-2017. Front Public Health. (2021) 9:705055. doi: 10.3389/fpubh.2021.705055
22. Zhao, Y, Hu, Y, Smith, JP, Strauss, J, and Yang, G. Cohort profile: the China health and retirement longitudinal study (Charls). Int J Epidemiol. (2014) 43:61–8. doi: 10.1093/ije/dys203
23. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. The strengthening the reporting of observational studies in epidemiology (Strobe) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
24. Chen, C, and Lu, FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. (2004) 17:1–36.
25. Wang, Z, Ma, J, and Si, D. Optimal cut-off values and population means of waist circumference in different populations. Nutr Res Rev. (2010) 23:191–9. doi: 10.1017/s0954422410000120
26. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
27. Wen, X, Wang, M, Jiang, CM, and Zhang, YM. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr. (2011) 20:551–6.
28. Donini, LM, Busetto, L, Bischoff, SC, Cederholm, T, Ballesteros-Pomar, MD, Batsis, JA, et al. Definition and diagnostic criteria for Sarcopenic obesity: Espen and Easo consensus statement. Obes Facts. (2022) 15:321–35. doi: 10.1159/000521241
29. Donini, LM, Busetto, L, Bischoff, SC, Cederholm, T, Ballesteros-Pomar, MD, Batsis, JA, et al. Definition and diagnostic criteria for Sarcopenic obesity: Espen and Easo consensus statement. Clin Nutr. (2022) 41:990–1000. doi: 10.1016/j.clnu.2021.11.014
30. Messerli, FH, Williams, B, and Ritz, E. Essential Hypertension. Lancet. (2007) 370:591–603. doi: 10.1016/s0140-6736(07)61299-9
31. Dutra, MT, Martins, KG, Vieira Dos Reis, DB, de Oliveira, SA, and Mota, MR. Association between adiposity indices and blood pressure is stronger in Sarcopenic obese women. Curr Hypertens Rev. (2019) 15:161–6. doi: 10.2174/1573402114666181031145341
32. Yin, T, Zhang, JX, Wang, FX, Zhao, JH, Zhao, Y, Liu, L, et al. The association between Sarcopenic obesity and hypertension, diabetes, and abnormal lipid metabolism in Chinese adults. Diabetes Metab Syndr Obes. (2021) 14:1963–73. doi: 10.2147/dmso.S308387
33. Yao, J, Wang, Y, Yang, L, Ren, M, Li, L, and Wang, H. Prevalence of possible sarcopenia in community-dwelling older Chinese adults: a cross-sectional study. BMJ Open. (2022) 12:e067425. doi: 10.1136/bmjopen-2022-067425
34. Han, K, Park, YM, Kwon, HS, Ko, SH, Lee, SH, Yim, HW, et al. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and nutrition examination surveys (Knhanes) 2008-2010. PLoS One. (2014) 9:e86902. doi: 10.1371/journal.pone.0086902
35. Hu, F, Zhang, G, Xu, Z, Zuo, Z, Huang, N, Ge, M, et al. The diagnostic agreement of Sarcopenic obesity with different definitions in Chinese community-dwelling middle-aged and older adults. Front Public Health. (2024) 12:1356878. doi: 10.3389/fpubh.2024.1356878
36. Li, H, Shi, Z, Chen, X, Wang, J, Ding, J, Geng, S, et al. Relationship between obesity indicators and hypertension-diabetes comorbidity in an elderly population: A retrospective cohort study. BMC Geriatr. (2023) 23:789. doi: 10.1186/s12877-023-04510-z
37. Ong, KL, Tso, AW, Lam, KS, and Cheung, BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. (2008) 51:1142–8. doi: 10.1161/hypertensionaha.107.105205
38. Messier, V, Rabasa-Lhoret, R, Barbat-Artigas, S, Elisha, B, Karelis, AD, and Aubertin-Leheudre, M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas. (2011) 68:331–6. doi: 10.1016/j.maturitas.2011.01.014
39. LeBlanc, ES, Wang, PY, Lee, CG, Barrett-Connor, E, Cauley, JA, Hoffman, AR, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. (2011) 96:3855–63. doi: 10.1210/jc.2011-0312
40. Yeap, BB. Are declining testosterone levels a major risk factor for ill-health in aging men? Int J Impot Res. (2009) 21:24–36. doi: 10.1038/ijir.2008.60
41. Kalinkovich, A, and Livshits, G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
42. Knudson, JD, Payne, GA, Borbouse, L, and Tune, JD. Leptin and mechanisms of endothelial dysfunction and cardiovascular disease. Curr Hypertens Rep. (2008) 10:434–9. doi: 10.1007/s11906-008-0082-2
43. Chen, Y, Liao, J, Zeng, Y, Ma, H, Jiang, C, Yu, S, et al. The combined effect of diabetes mellitus and sarcopenia on depression and cognitive function: insights from the Charls cohort, 2011-2020. Eur Geriatr Med. (2024) 15:1881–90. doi: 10.1007/s41999-024-01039-1
44. Luo, YX, Zhou, XH, Heng, T, Yang, LL, Zhu, YH, Hu, P, et al. Bidirectional transitions of sarcopenia states in older adults: the longitudinal evidence from Charls. J Cachexia Sarcopenia Muscle. (2024) 15:1915–29. doi: 10.1002/jcsm.13541
45. Gao, K, Cao, LF, Ma, WZ, Gao, YJ, Luo, MS, Zhu, J, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. (2022) 44:101264. doi: 10.1016/j.eclinm.2021.101264
Keywords: obesity, sarcopenia, sarcopenic obesity, hypertension, CHARLS
Citation: Du R, Yuan J, Huang Y, Jiang G, Duan Z, Yang H and Huang W (2025) Sarcopenia is not associated with hypertension, but sarcopenic obesity increases risk of hypertension: a 7-year cohort study. Front. Public Health. 12:1479169. doi: 10.3389/fpubh.2024.1479169
Edited by:
Humberto Muzi-Filho, Federal University of Rio de Janeiro, BrazilReviewed by:
Leucio Vieira, Federal University of Pernambuco, BrazilRegina Souza Aires, Federal University of Pernambuco, Brazil
Copyright © 2025 Du, Yuan, Huang, Jiang, Duan, Yang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Yang, eWFuZ2hvbmcwNTI5QDEyNi5jb20=; Wei Huang, SHVhbmd3ZWk3OTdAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Runfen Du
Runfen Du Junchao Yuan2†
Junchao Yuan2† Hong Yang
Hong Yang