- 1Ethiopian Field Epidemiology Training Program, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
- 2Department of Epidemiology, Mekelle University, Mekelle, Ethiopia
- 3Afar Public Health Institute, Samara, Ethiopia
- 4Department of Epidemiology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Introduction: Ethiopia is a global hotspot for child malnutrition, with an estimated 1.2 million children under five affected by severe acute malnutrition (SAM) in 2022. In response, the country has integrated SAM into its broader disease surveillance system. In January 2022, the Dubti District Health Office in the Afar Region detected an unusual surge in SAM cases through its surveillance system. This study aimed to assess the extent of the outbreak and identify the associated risk factors.
Methods: We conducted an unmatched case–control study involving 258 mother–child dyads from five affected kebeles in the Dubti District of the Afar Region Ethiopia. The descriptive study included all 442 SAM cases from the line list, while 86 cases and 168 controls were selected using a simple random sampling method for the analytic study. The data were entered into EpiData software (version 3.1) and analyzed using SPSS software (version 25.0). Binary logistic regression (LR) analysis was performed to identify risk factors for SAM. Statistically, the results were summarized using an adjusted odds ratio (AOR), 95% confidence intervals (CIs), and a p-value of <0.05.
Results: The median age of the cases was 22 months, with an interquartile range of 12–34 months. A total of 39 deaths were reported, with a case fatality rate (CFR) of 8.82%. The identified SAM risk factors included households with more than five members (AOR = 3.341, 95% CI: 1.475–7.563), more than five under-five children (AOR = 4.442, 95% CI: 2.000–9.866), lack of vaccination (AOR = 3.641, 95% CI: 1.618–8.198), pneumonia (AOR = 5.61, 95% CI: 2.488–12.651), diarrhea (AOR = 4.68, 95% CI: 2.169–10.097), lack of access to sanitation and hygiene (AOR = 3.18, 95% CI: 1.462–6.934), and household food insecurity (AOR = 9.46, 95% CI: 2.095–42.712).
Conclusion: The study revealed a significant outbreak of SAM, with a CFR of 8.82%. The outbreak was associated with factors such as large family sizes, having multiple under-five children, a lack of vaccination, pneumonia, and diarrhea. These findings emphasize the urgent need to safeguard essential child health services, water supply, sanitation and hygiene, and household food security.
Introduction
Child undernutrition includes various nutritional disorders, such as underweight, wasting, stunting, and micronutrient deficiencies (1). It is a critical issue for child survival and significantly impacts both cognitive and physical development (2, 3). Undernutrition can manifest in acute, chronic, or mixed forms. Severe acute malnutrition (SAM), characterized by severe wasting and recent weight loss, is a severe form of protein-energy deficiency (1, 4).
The causes of malnutrition can be categorized into three types of causes, namely immediate, underlying, and basic (5). Basic causes of malnutrition are rooted in the political, social, and economic environment. Underlying causes include insufficient food access, inadequate maternal and child care, and poor water, sanitation, and hygiene (WASH) conditions. Immediate causes include inadequate dietary intake and acute illnesses (5–7).
Malnutrition contributes to 50% of all child deaths. Malnourished children face an increased risk of hospitalization and contracting infectious diseases, such as diarrhea, acute respiratory infections, measles, and malaria (8).
Globally, in 2022, it was reported that 45 million under-five children were wasted, with only 7.3 million receiving treatment for SAM, and 149 million were stunted (9, 10). According to the United Nations International Children’s Emergency Fund (UNICEF), nearly 40 million under-five children are at risk of SAM, with approximately one child developing SAM every minute in 15 crisis-hit countries, including Afghanistan, Haiti, Yemen, Burkina Faso, Chad, the Democratic Republic of the Congo, Kenya, Madagascar, Mali, Niger, Nigeria, Somalia, South Sudan, Sudan, and Ethiopia (11).
The World Health Organization (WHO) reported that 11 million under-five children were acutely malnourished during 2022, specifically in seven countries of the Greater Horn of Africa, including Djibouti, Somalia, Sudan, South Sudan, Ethiopia, Kenya, and Uganda. The WHO also reported that the SAM rates were 5–24% in Somalia, 2.7% in Sudan, 1.3–6.1% in South Sudan, 2–12.3% in Kenya, 0.9–4.5% in Uganda, and 11.1–14.7% in Djibouti (12).
In Ethiopia, a UNICEF report indicates that approximately 4.7 million under-five children are malnourished, including 1.2 million children with SAM. In addition, 5.5 million children are stunted, with 1.8 million experiencing severe stunting. Undernutrition accounts for 45% of all child deaths in the country (13).
Ethiopia has pledged to end child malnutrition by 2030 through initiatives such as integrating nutritional surveillance into the national Integrated Diseases Surveillance and Response system, incorporating targets into the National Health Sector Transformation Plan, and adopting the United Nations’ Sustainable Development Goal 2 (14, 15). As a result, significant progress has been made in reducing wasting, stunting, and underweight (16). The prevalence rates of stunting, severe wasting, and underweight in the country are 37, 7, and 21%, respectively (17). However, recent conflicts, droughts, and environmental changes have exacerbated nutritional problems, particularly in three conflict-affected regions of Ethiopia: Tigray, Amhara, and Afar (18).
SAM is a weekly reportable condition under public health emergency management (PHEM). In January 2022, the Dubti District Health Office in the Afar Region noted an unusual surge in SAM cases through its routine surveillance system, following the reporting of 17 SAM-related deaths. A multidisciplinary team, comprising field epidemiologists, health officers, PHEM officers, and health information technicians, was subsequently deployed. Data were analyzed, an action threshold level was established, and an outbreak was confirmed, leading to a prompt response.
Materials and methods
Study setting
An unmatched case–control study was conducted in five SAM-affected kebeles (such as Korile, Dembel, Gumtameli, Sekoyta, and Galimeda), which are small administrative units in Dubti District of Afar, Northeastern Ethiopia, from 1 May 2022 to 30 May 2022. This area is located 600 km from Addis Ababa, the capital city of Ethiopia. The district has 13 kebeles and 10,992 households. Based on 2007 census data, the total population of the district for 2022–2023 was estimated to be 49,173. The district is one of the hotspot areas for under-five malnutrition in the region, characterized by recurrent droughts and pastoral communities that rely on livestock production. The prevalence rates of wasting, stunting, and underweight were estimated to be 16.2, 43.1, and 24.8%, respectively (19).
Study population
All under-five children living in the five malnutrition-affected kebeles in Dubti District comprised the study population. Cases were defined as children aged 6–59 months with a weight-for-height score (WFH) of less than −3 standard deviations (SDs), a mid-upper arm circumference (MUAC) of less than 110 mm, or bilateral pitting edema (20). Controls were defined as children of the same age with WFH score greater than −2 SDs and/or MUAC greater than 125 mm (21). Children with congenital anomalies, including Down syndrome, and physical deformities that interfered with the standard anthropometric procedure, as well as those whose mothers or caregivers failed to provide informed consent, were excluded from the study.
Sample size determination
We used all SAM cases identified in the line list for descriptive analysis. For the analytic study, the sample size was calculated using Epi-Info software version 7.1.1.0 based on the following assumptions: power (80%), 95% confidence interval (CI), a case-to-control ratio of 1:2, and findings from a previous study that identified prelacteal feedings as risk factors for SAM (19). Therefore, by considering the percentage of controls exposed (78.6%), the percentage of cases exposed (93.3%), an odds ratio of 3.81, and a 10% non-response rate, the final calculated sample size was 258, comprising 86 cases and 172 controls.
Mathematically, non-response rate.
where N = sample size, P1 = percentage of cases exposed (93.3%), P2 = percentage of controls exposed (78.6%), the odds ratio (OR) = 3.81, r = ratio of cases to controls (1:2), Zβ = 80%, Zα/2 = 1.96, p− = (P1 + r × P2)/(r + 1), and q− = 1–p−.
Sampling procedure
All five affected kebeles—Korile, Dembel, Gumtameli, Sekoyta, and Galimeda—were purposefully selected for the investigation. To describe the SAM outbreak by person, place, and time, we utilized the entire line list, which included all SAM reports submitted to the Dubti District Health Office during the outbreak period. However, when investigating the factors associated with the SAM outbreak, all SAM cases in the line list were identified and assigned unique identification numbers. SAM-affected children in these kebeles were then selected using a simple random sampling technique from the line list. These children were then traced back to their communities for data collection. Controls—children who did not meet the standard case definition of SAM—were also recruited using a simple random sampling technique from neighbors living in the same residential areas. For every SAM-affected child, two neighbor controls were recruited.
Confirmation of the outbreak
A SAM outbreak occurs when the number of SAM cases exceed the threshold during a normal season in a specific area. The WHO recommends various threshold calculation techniques for weekly reportable diseases, such as the 75th percentile, mean + 2 SDs, cumulative sum, and a constant case count (22). Considering that Dubti District is an area endemic for child undernutrition, we used the mean + 2 SD method, which adds 2 SDs to the average number of reported SAM cases over the past 5 years. Using District Health Information Software, the current data (2021/2022) were compared to the average weekly SAM reports from 2017 to 2021 to determine whether the action threshold was surpassed.
Data collection procedure and measurement
We used a structured questionnaire adapted from the literature (19, 21, 23–27) and conducted a house-to-house survey to collect data from mothers/caregivers through face-to-face interviews, immunization cards, and anthropometric measurements. The child’s age was estimated using an immunization card, a birth certificate, or information recalled by the mothers or caregivers. Dietary diversity was assessed through 24-h food recall of seven WHO-recommended food items.
Dietary diversity was assessed using the dietary diversity score (DDS) based on 24-h food recall, in accordance with the WHO’s minimum dietary diversity recommendations. A child was considered to have a diversified diet if they consumed four or more food items from the following seven WHO-recommended food groups: (1) grains, roots, and tubers; (2) legumes and nuts; (3) dairy products, such as milk, yogurt, and cheese; (4) flesh foods, including meat, fish, poultry, and liver/organ meats; (5) eggs; (6) vitamin A-rich fruits and vegetables; and (7) other fruits and vegetables. A DDS of ≥4 was considered indicative of a diversified diet (26).
Household food security was measured using the Household Food Insecurity Access Scale (HFIAS). The HFIAS consists of two types of related questions: nine occurrence questions that ask about experiences of food insecurity in the past 4 weeks (30 days) and 9 severity questions that inquire about the frequency of these experiences. Furthermore, the HFIAS categorizes household food insecurity into four categories: category one, food security if [(Q1a = 0 or Q1a = 1) and Q2 = 0 and Q3 = 0 and Q4 = 0 and Q5 = 0 and Q6 = 0 and Q7 = 0 and Q8 = 0 and Q9 = 0]; category two, mildly food insecure access if [(Q1a = 2 or Q1a = 3 or Q2a = 1 or Q2a = 2 or Q2a = 3 or Q3a = 1 or Q4a = 1) and Q5 = 0 and Q6 = 0 and Q7 = 0 and Q8 = 0 and Q9 = 0]; category three, moderately food insecure access if [(Q3a = 2 or Q3a = 3 or Q4a = 2 or Q4a = 3 or Q5a = 1 or Q5a = 2 or Q6a = 1 or Q6a = 2) and Q7 = 0 and Q8 = 0 and Q9 = 0]; and category four, severely food insecure access if [Q5a = 3 or Q6a = 3 or Q7a = 1 or Q7a = 2 or Q7a = 3 or Q8a = 1 or Q8a = 2 or Q8a = 3 or Q9a = 1 or Q9a = 2 or Q9a = 3], as described in detail in the HFIAS (24). Households in category 1, with an HFIAS score of 0–1, were considered food secure, whereas those in categories two, three, or four were classified as food insecure (27).
The child’s vaccination status was assessed using an immunization card and information recalled by the mothers. A child who received all of the vaccines recommended for their age was considered fully immunized (28). A child who presented with a cough, fast breathing, and/or danger signs, based on the integrated management of newborn and child illness classification, was diagnosed with pneumonia (29). Furthermore, diarrhea was defined as passing three or more loose or liquid stools per day (30). Five nurses with a Bachelor of Science degree who had experience in under-five nutritional surveys and two supervisors with a master’s degree in Public Health participated in the data collection.
Anthropometric measurement
The control children underwent standardized anthropometric measurements. Briefly, weight was recorded using a calibrated portable scale to the nearest 0.1 kg, with participants wearing light clothing. Height was measured with a calibrated portable stadiometer to the nearest 0.1 cm. The participants stood without shoes, in a Frankfurt position, with their heels, buttocks, shoulders, and heads touching a vertical support. For the children aged 6–23 months, recumbent length was measured. The mid-upper arm circumference (MUAC) was determined by measuring the circumference of the upper arm at its midpoint, with the arm bent at a right angle (20).
Data quality control
We used the English version of the questionnaire, which was translated into the local language, Afarigna, and then back into English. The questionnaire was pretested with 5% of the sample to ensure clarity, completeness, and consistency. Anthropometric indices were measured and interpreted according to the WHO 2006 growth standards (20). The data collectors received 3 days of training on the data collection tool and procedure, as well as on protecting data confidentiality.
Data analysis
The data were entered into EpiData software version 3.1 and analyzed using SPSS software version 25.0. A chi-squared test was conducted to assess differences in the baseline sociodemographic and economic characteristics of the cases and controls. However, when the conditions for the chi-squared test were violated—specifically, when the expected values in at least 80% of the cells were less than five or when any cell had an expected value less than one—Fisher’s exact test was conducted. A binary logistic regression (LR) analysis model was applied to identify risk factors for SAM. Variables with corresponding p-values <0.25 in the bivariable binary LR analysis were further analyzed. Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were calculated, and a p-value of <0.05 was considered statistically significant. The overall model fit was assessed using the Hosmer–Lemeshow goodness-of-fit test, with a p-value of >0.05. In addition, the data were assessed for collinearity with a variance inflation factor of less than 5.
Ethical consideration
Ethical clearance was obtained from the Afar Public Health Institute, ethical approval number APH015/2022. Informed consent was obtained from all individual participants included in the study. The data confidentiality was assured via the de-identification of personal identifier information and the storage of the file in a secure folder. Children who met the case definition of SAM during control selection were linked to nearby health facilities for nutritional intervention.
Results
Descriptive epidemiology
Description of the SAM cases by person
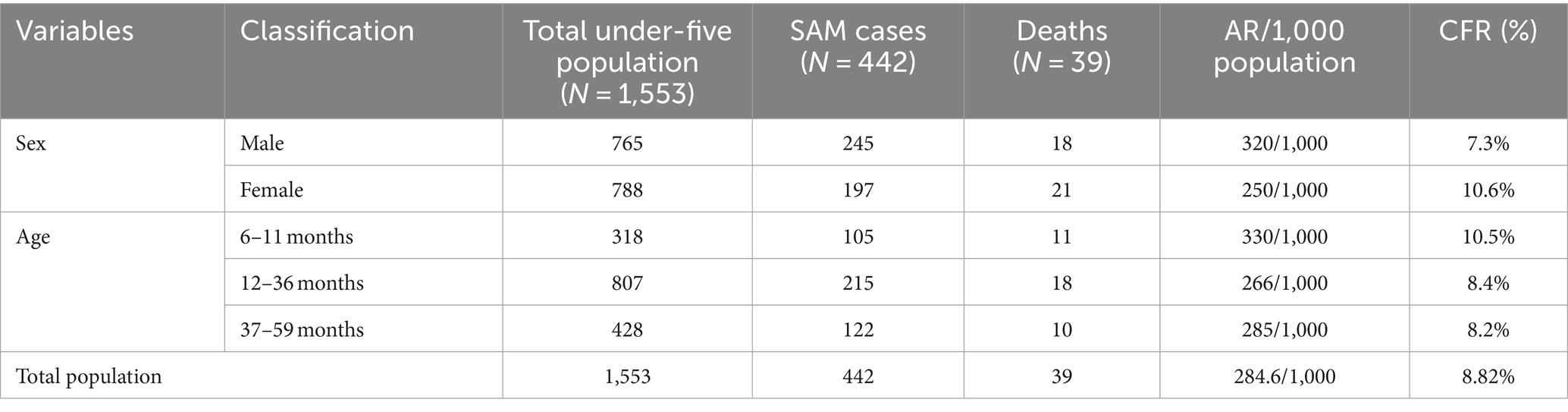
A total of 442 SAM cases were reported in this outbreak. Of these, 245 (55.4%) were male patients. The median age of the cases was 22 months, with an interquartile range of 12–34 months. A total of 191 (43.2%) children presented with diarrhea, 138 (31.2%) with fever, and 185 (41.9%) with pneumonia.
The incidence of SAM was 284.6 per 1,000 population. The male patients had the highest attack rate (AR; 320/1,000 population), followed by those aged 6–11 months (330/1,000 population). Furthermore, 39 SAM-related deaths were recorded, resulting in a case fatality rate (CFR) of 8.82%. The highest CFR was observed among the female patients (10.6%), followed by those aged 6–11 months (10.5%; Table 1).
Description of the SAM cases by place
A total of 260 (58.9%) cases were reported from Galimeda, followed by Korile with 59 cases (13.3%) and Debel with 45 cases (10.2%). Similarly, the highest AR was observed in Galimeda (350/1,000 population), followed by Korile (309/1,000 population) and Debel (300/1,000 population; Table 2).
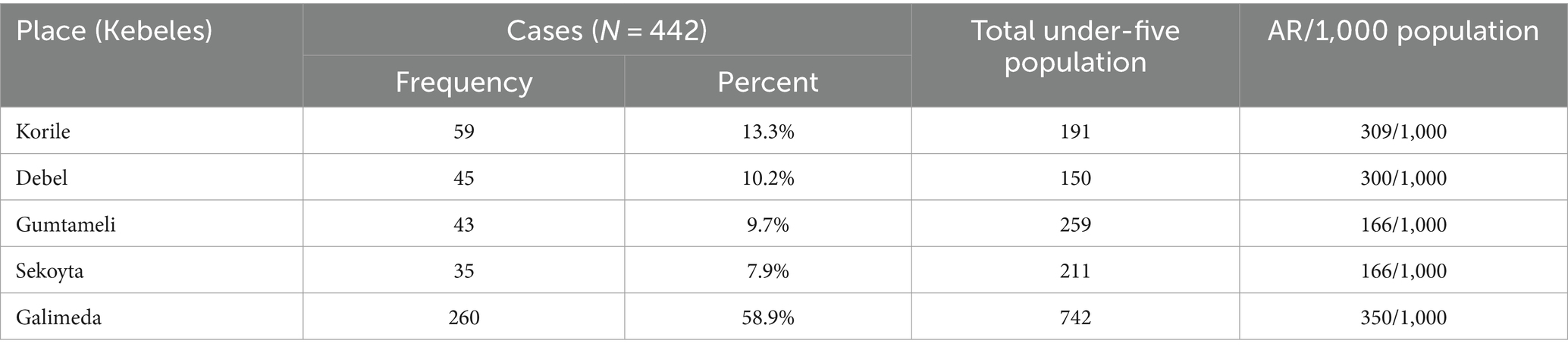
Table 2. SAM rates by affected Kebeles in Dubti District, Awsiresu Zone, Afar, Northeastern Ethiopia, 2022.
Description of the SAM outbreak by time
Using the mean + 2 SDs method, it was found that the threshold level was surpassed from epidemiological week (Epi-week) 45 in 2021 to Epi-week 17 in 2022, confirming the SAM outbreak (Figure 1).
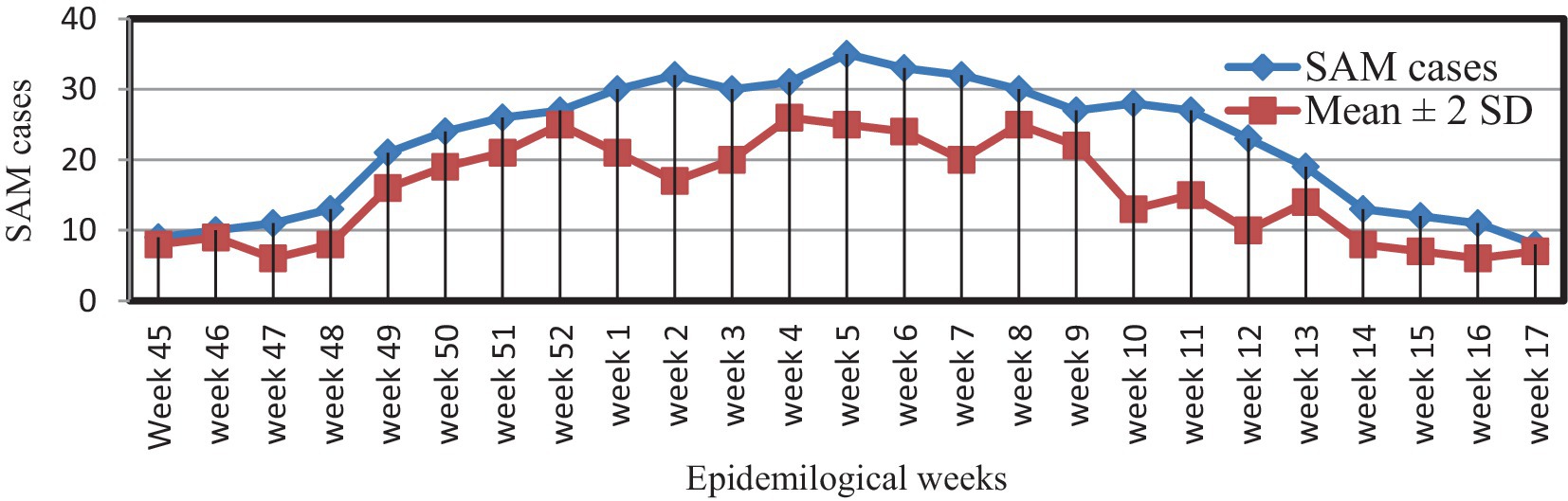
Figure 1. Weekly SAM reports and action thresholds in Dubti District, Awsiresu Zone, Afar Region, Northeastern Ethiopia, 2022.
The SAM outbreak began in Epi-week 45 in 2021 and continued through Epi-week 5 in 2022, when it dropped below the action threshold level in Epi-week 17 in 2022. The epidemic curve suggested a continuous common-source type of outbreak (Figure 2).
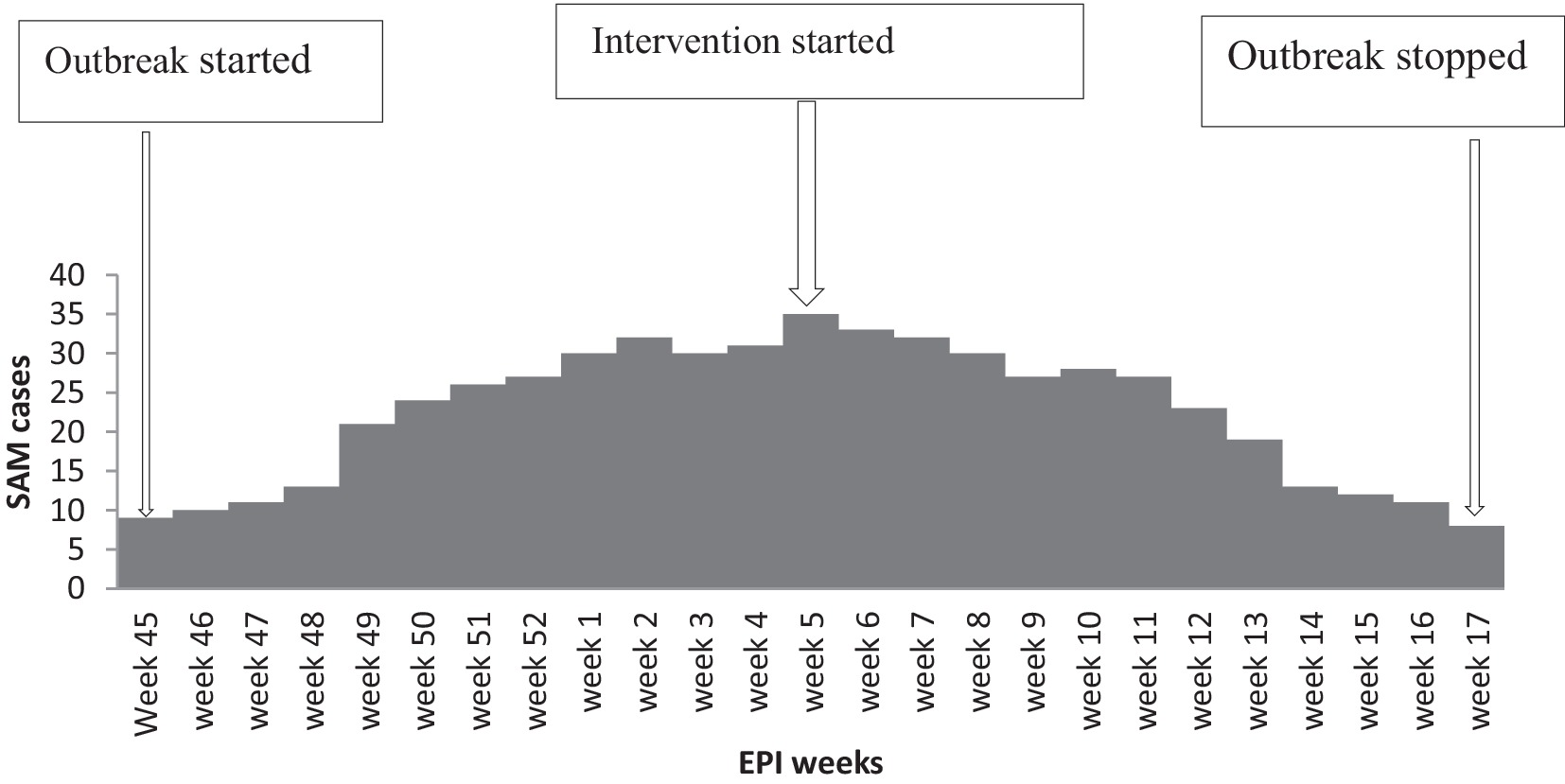
Figure 2. Epidemic curve depicts the onset of SAM in Dubti District, Awsiresu Zone, Afar Region, Northeastern Ethiopia, 2022.
Analytic study
Sociodemographic characteristics
The study had 258 mother–child pairs (86 cases and 172 controls), with 84 cases and 168 controls willing to participate. The response rate was 97.7%. The median ages of the cases and controls were 24 and 26 months, respectively. There was a statistically significant difference in educational level, occupation, family size, and the number of children under 5 years of age between cases and controls at a p-value of <0.005 (Table 3).
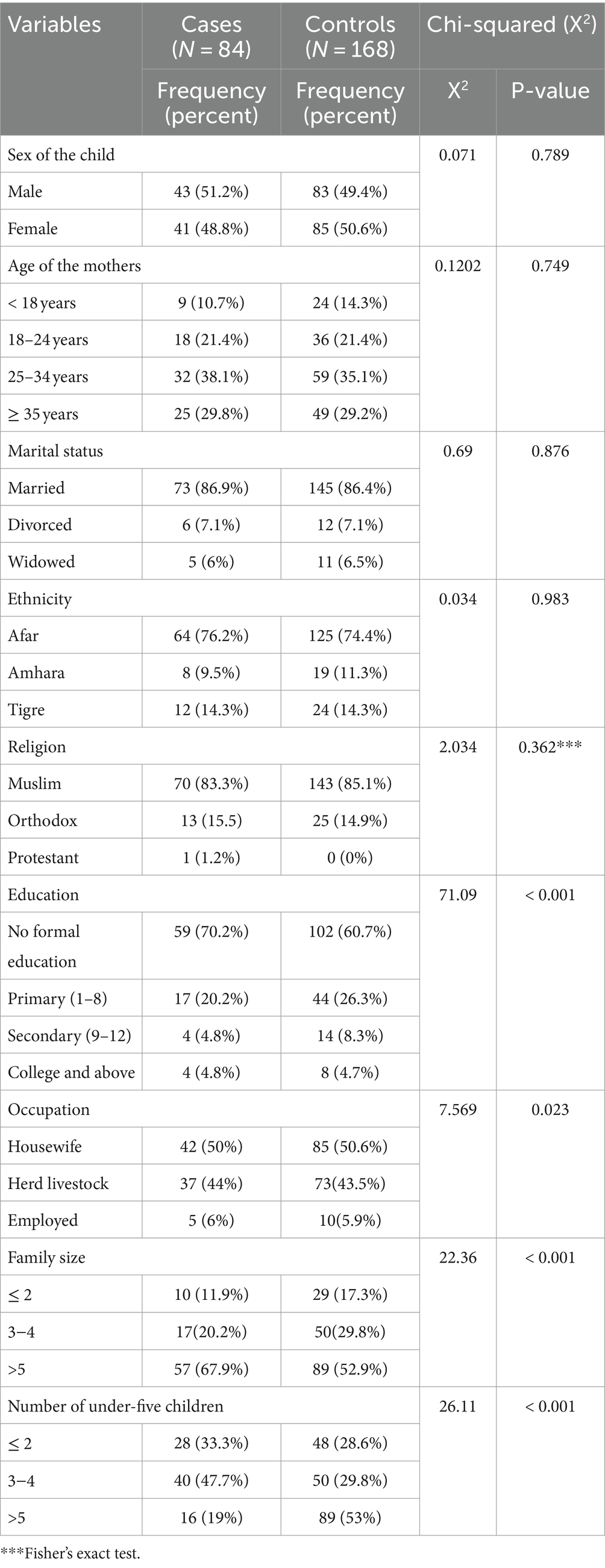
Table 3. Sociodemographic characteristics of the mothers/caregivers in Dubti District, Awsiresu Zone, Afar Region, Northeast Ethiopia, 2022.
Household food security status
According to the HFIAS, four (4.8%) of the households among the cases and 27 (16.1%)of the households among the controls were food secure, whereas 80 (95.2%) households among the cases and 141 (83.9%) households among the controls were food insecure. Among the food-insecure households in the case group, 37 (44%) were classified as mildly food insecure, 25 (29.8%) as moderately food insecure, and 18 (21.4%) as severely food insecure. Furthermore, 70 (41.7%), 51 (30.3%), and 20 (11.9%) of the food-insecure households in the control group were classified as mildly insecure, moderately insecure, and severely food insecure, respectively (Table 4).

Table 4. Household food security status in Dubti District, Awsiresu Zone, Afar Region, Northeast Ethiopia, 2022.
Child dietary diversity practices
According to the WHO’s minimum dietary diversity recommendation, 32 (38%) of the cases and 92 (54.8%) of the controls met these standards. Grains, roots, and tubers were the most commonly consumed foods, with 546 (4.3%) of the cases and 140 (76.2%) controls consuming them. This was followed by legumes and nuts, with 46 (54.8%) of the cases and 135 (65.5%) of the controls consuming them. Among the severely malnourished children, 20 (23.8%), 26 (31%), and 23 (27.4%) consumed eggs, flesh foods, and vitamin A-rich fruits and vegetables, respectively (Table 5).
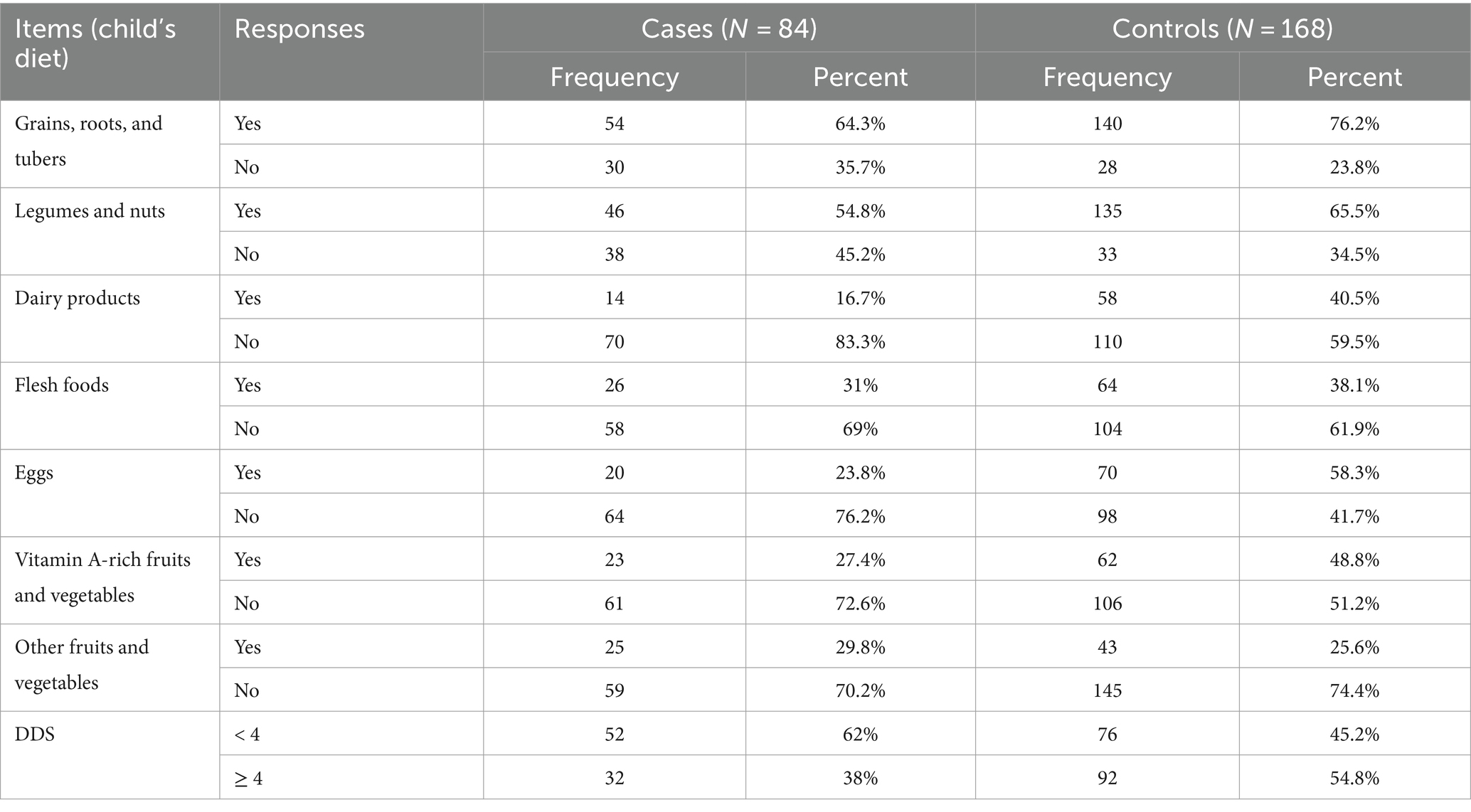
Table 5. Dietary diversity practices among the children in the Dubti district, Awsiresu Zone, Afar Region, Northeast Ethiopia, 2022.
Child feeding practices
Breastfeeding was initiated within 1 h of delivery for 67 (79.8%) of the cases and 138 (82.1%) of the controls. During the first 6 months of life, 55 (65.8%) of the cases and 108 (64.3%) of the controls were fed only breast milk. In addition, 33 (35.7%) of the cases and 111 (66%) of the controls received more than four feeds per day (Table 6).
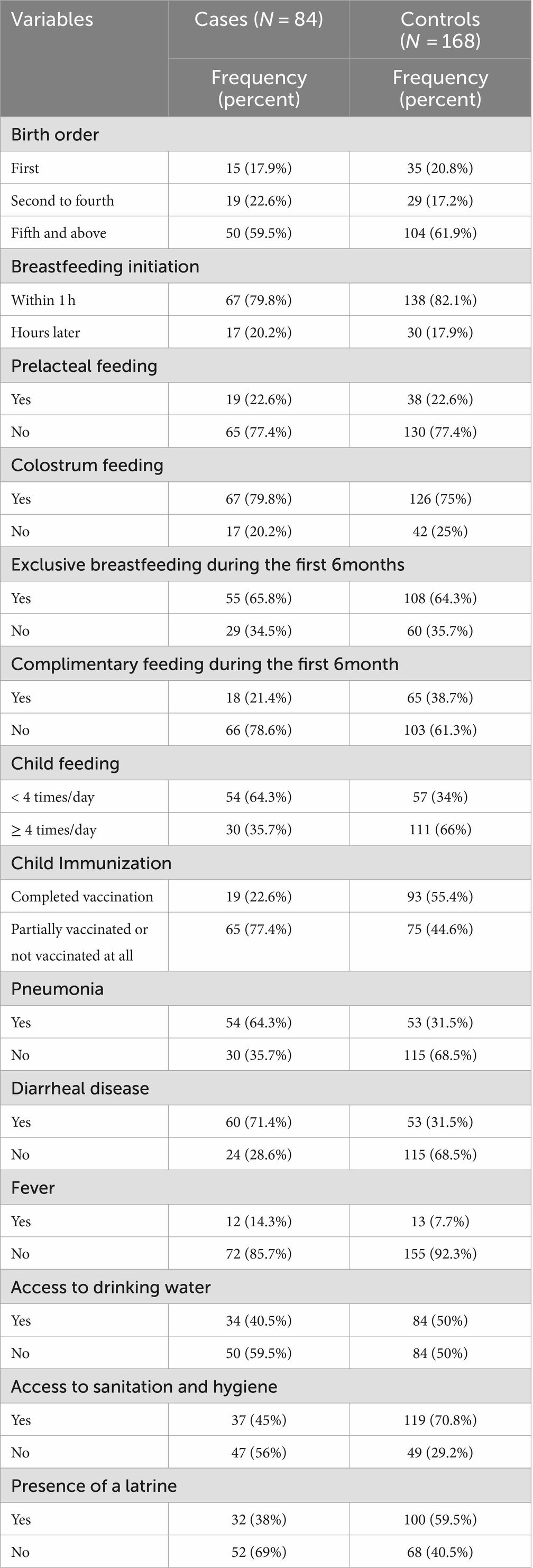
Table 6. Household access to WASH, child feeding practices, and health-related characteristics in Dubti District, Awsiresu Zone, Afar Region, Northeast Ethiopia, 2022.
Child immunization and medical illness
A total of 19 (22.6%) of the cases and 93(55.4%) of the controls were fully immunized for their age. Furthermore, 60 (71.4%) of the cases and 53 (31.5%) of the controls had diarrhea, while 54 (64.3%) of the cases and 53 (31.5%) of the controls had acquired pneumonia (Table 6).
Household access to WASH
A total of 34 (40.5%) of the households in the case group and 84 (50%) of the households in the control group reported having access to safe drinking water. Similarly, 37 (45%) of the households in the case group and 119 (70.8%) of the households in the control group reported access to sanitation and hygiene (Table 6).
Factors associated with the SAM outbreak
After controlling for potential confounding factors, the children in families with more than five members had 3.34 times greater odds of experiencing SAM compared to the children in smaller families (AOR = 3.34, 95% CI: 1.475−7.563). Similarly, households with more than five under-five children had 4.4 times greater odds of SAM than their counterparts (AOR = 4.44, 95% CI: 2.000−9.866). Compared to the fully vaccinated children, unvaccinated children were 3.6 times more likely to experience SAM (AOR = 3.64, 95% CI: 1.618 ~ 8.198). The children with a history of pneumonia had a 5.6-fold greater risk of experiencing SAM (AOR = 5.61, 95% CI: 2.488−12.651), while those with diarrheal disease had a 4.7-fold greater chance of experiencing SAM (AOR = 4.68, 95% CI: 2.169−10.097; Table 7).

Table 7. Factors associated with the SAM outbreak in Dubti District, Awsiresu Zone, Afar Region, Northeast Ethiopia, 2022.
Discussion
We aimed to describe the extent of the SAM outbreak and identify risk factors associated with the current outbreak in the Dubti District of the Afar Region. A total of 442 cases and 39 deaths were reported. The AR was the highest among male patients (320/1,000 population), infants aged 6–11 months (330/1,000 population), and residents of Galimeda (350/1,000 population). The outbreak spanned 23 weeks, and the epidemic curve suggested a continuous common-source type of outbreak. A large family size, a high number of under-five children, a lack of vaccination, a lack of access to sanitation and hygiene, acute illnesses such as pneumonia, diarrheal disease, and household food insecurity were the factors associated with this outbreak.
The reported case fatality rate (CFR) for SAM in the current outbreak was 8.82%. The findings are consistent with CFRs reported in studies conducted in Addis Ababa, Ethiopia (10%) (31); at Felege Hiwot Hospital, Bahr Dar, Ethiopia (11.3%) (32); in Nigeria (8.5%) (33); and at St. Mary’s Hospital, Uganda (12.6%) (34). However, the CRF in this study was higher than the observed CFR in studies conducted at Hiwot Fana Specialized Hospital, Ethiopia (2.1%) (35), and in rural Jharkhand and Odisha, eastern India (1.2%) (36). It also exceeds the WHO’s and Ethiopia’s target for SAM management, which reports a CFR of less than 5% (37, 38). Variations in the demographic and underlying clinical characteristics of children, treatment protocols, resource availability, medical supplies, and quality of care may have contributed to the observed differences in CFR rates across various settings. Furthermore, the findings suggest a need to improve the quality of care as there may be gaps in healthcare providers’ adherence to treatment protocols, training, and resource availability.
The children in families with more than five members had 3.34 times greater odds of experiencing SAM compared to the children in smaller families. Similarly, the odds of SAM were 29.4% higher in households with more than five under-five children. These findings are consistent with studies conducted in the Libo Kemekem district, Amhara region (39), Benna Tsemay district, southern Ethiopia (40), and Bangladesh (41). This may be attributed to increased economic strain and the sharing of limited food among family members in households with larger family sizes and more children, which can lead to poor nutritional status.
The odds of SAM were 5.6 times greater among the children who had pneumonia compared to their counterparts. Pneumonia was a common comorbidity among severely malnourished children in a study conducted in Bangladesh (42). This may be attributed to malnutrition weakening the body’s immune system, reducing physical activity, and increasing susceptibility to pneumonia. In addition, insensible dehydration due to rapid breathing or fever, combined with decreased appetite from pneumonia, may have contributed to SAM.
Lack of access to sanitation and hygiene was associated with a 3.2-fold greater odds of SAM. Similarly, children with diarrheal disease had a 3.6-fold greater chance of experiencing SAM. These findings are consistent with studies conducted in the districts of Dermot, Kalafo, and Enebsie Sarmidr in Ethiopia (43–45), as well as Vadodara, India (46). This may be explained by appetite loss, poor digestion, malabsorption, and electrolyte loss due to diarrheal disease, which can result in acute weight loss and malnutrition.
In the present study, the immunization status of the children was associated with the development of SAM. The non-immunized children had a 4.7-fold greater risk of experiencing SAM. This finding aligns with studies conducted in the Benishangul-Gumz (47) and Somali regional states of Ethiopia (48) and Zambia (49). This may be explained by the fact that non-immunized children are more likely to contract pneumonia and diarrheal disease due to missed vaccinations.
Children from food-insecure households were 9.5 times more likely to develop SAM compared to their counterparts. Several studies support the positive association between household food insecurity and SAM. Specifically, household food insecurity was associated with a fourfold increased risk of SAM in studies conducted in Leqa Dulacha District, Oromia region, Ethiopia, and in two districts (Terai and Jhapa) in Nepal (21, 23). In addition, the likelihood of a child developing SAM was 1.8 times greater among food-insecure households in a study conducted in Mao City, Chad (25). This may be attributed to food-insecure households experiencing food shortages, which lead to insufficient dietary intake for children in terms of both quantity and quality, thereby increasing their risk of severe acute malnutrition.
Limitations of the study
The study has some limitations. First, potential recall bias might have affected the reporting of past events. However, we mitigated this by using reference calendars, such as holidays, to assist the mothers/caregivers in their recall. Second, owing to the reciprocal causation relationships between SAM and pneumonia as well as between SAM and diarrhea, a child may have acquired diarrheal disease and pneumonia after developing SAM. However, we addressed this by asking the mothers/caregivers about the temporal sequence of these conditions. Third, recumbent length measurements for young children aged 6–23 months may have been influenced by their inability to lie completely straight, thus potentially affecting the reliability of the results. To mitigate this bias, we strictly adhered to standardized anthropometric procedures and involved two individuals in the measurement process to ensure the maximum validity of the measurements and the reliability of the results. Finally, this study did not examine specific missed vaccines associated with SAM or the potential relationship between family income/wealth index and SAM.
Areas for further research
Future research could delve deeper into the relationship between the family income/wealth index and SAM. In addition, investigating the specific child vaccines that were missed and their potential association with SAM would provide valuable insights for targeted interventions. Finally, future research should aim to conduct longitudinal studies to establish more definitive causal relationships between SAM and pneumonia, as well as SAM and diarrhea. By exploring these areas, researchers can contribute to a more comprehensive understanding of the factors contributing to SAM and develop more effective prevention and treatment strategies.
Conclusion
The AR was the highest among male patients, infants aged 6–11 months, and residents of Galimeda. The epidemic curve suggested a continuous common-source type of outbreak. In this study, the CFR was higher than the WHO’s and Ethiopia’s targets for SAM management. Risk factors for the current outbreak included households with more than five members, more than five under-five children, lack of vaccination, diarrheal disease, pneumonia, limited access to sanitation and hygiene, and household food insecurity. The findings demonstrate the need for multisectoral and multidisciplinary collaboration to improve essential child health services, access to WASH, and household food security through economic empowerment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by institutional review board of Afar Public Health Institute. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. DW: Methodology, Supervision, Writing – original draft, Writing – review & editing. FW: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. KM: Methodology, Supervision, Writing – original draft, Writing – review & editing. AE: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the Afar Public Health Institute and the Dubti District Health Office for providing support letters to conduct this study. We also acknowledge the contributions of the investigation team, as well as the data collectors and supervisors who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
All the claims expressed in this article and its contents are solely the responsibility of the authors and do not necessarily represent the official views of affiliated institutions.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1475104/full#supplementary-material
Abbreviations
AOR, adjusted odds ratio; AR, attack rate; CFR, case fatality rate; CI, confidence interval; Epi-week, epidemiological week; HFIAS, Household Food Insecurity Access Scale; LR, logistic regression; MDT, multidisciplinary team; MUAC, mid-upper arm circumference; PHEM, Public Health Emergency Management; SAM, severe acute malnutrition; SD, standard deviation; WASH, water supply, sanitation, and hygiene; WFH, weight for height; WHO, World Health Organization.
References
1. Zhang, X, Zhang, L, Pu, Y, Sun, M, Zhao, Y, Zhang, D, et al. Global, regional, and national burden of protein–energy malnutrition: a systematic analysis for the global burden of disease study. Nutrients. (2022) 14:2592. doi: 10.3390/nu14132592
2. Suryawan, A, Jalaludin, MY, Poh, BK, Sanusi, R, Tan, VM, Geurts, JM, et al. Malnutrition in early life and its neurodevelopmental and cognitive consequences: a scoping review. Nutr Res Rev. (2022) 35:136–49. doi: 10.1017/S0954422421000159
3. De, P, and Chattopadhyay, N. Effects of malnutrition on child development: evidence from a backward district of India. Clinical Epidemiol Global Health. (2019) 7:439–45. doi: 10.1016/j.cegh.2019.01.014
4. Crichton, M, Craven, D, Mackay, H, Marx, W, De Van Der Schueren, M, and Marshall, S. A systematic review, meta-analysis and meta-regression of the prevalence of protein-energy malnutrition: associations with geographical region and sex. Age Ageing. (2019) 48:38–48. doi: 10.1093/ageing/afy144
6. Young, H. Nutrition in Africa’s dry lands: A conceptual framework for addressing acute malnutrition. US: Feinstein International Center, Tufts University (2020).
7. Dipasquale, V, Cucinotta, U, and Romano, C. Acute malnutrition in children: pathophysiology, clinical effects and treatment. Nutrients. (2020) 12:2413. doi: 10.3390/nu12082413
8. Rice, AL, Sacco, L, Hyder, A, and Black, RE. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Organ. (2000) 78:1207–21.
9. World Health Organization (WHO). Malnutrition key fact sheet. (2024). Available at: https://www.googleadservices.com/pagead (Accessed on 09 March 2022)
10. World Health Organization. WHO issues new guideline to tackle acute malnutrition in children under five. (2023). Available at: https://www.who.int/news/item/20-11-2023-who-issues-new-guideline-to-tackle-acute-malnutrition-in-children-under-five (Accessed June 2022).
11. United Nations Children's Fund (UNICEF) for every child. Global hunger crisis pushing one child into severe malnutrition every minute in 15 crisis-hit countries. (2022). Available at: https://www.unicef.org/sudan/press-releases/global-hunger-crisis-pushing-one-child-severe-malnutrition-every-minute-15-crisis (Accessed on 30 June 2022).
12. WHO. Situation report greater horn of Africa food insecurity and health - grade 3 emergency. WHO (2022). Available at: https://cdn.who.int/media/docs/default-source/documents/emergencies/ghoa-food-insecurity-and-health-sitrep-n12-(nov-dec-2023).pdf?sfvrsn=f860e4ec_1&download=true (Accessed June 30, 2022).
13. UNICEF Ethiopia. Annual Report. (2022). Available at: https://www.unicef.org/ethiopia/media/7801/file/UNICEF%20Ethiopia%20Annual%20Report%202022.pdf (Accessed on 23 March 2022)
14. Ethiopian Public Health Institute National Information Platform for Nutrition (NIPN). The national nutrition program (2016–2020) progress analysis: Evidence for the upcoming food and nutrition strategy development. Addis Ababa, (2020). Available from https://nipn.ephi.gov.et/sites/default/files/2023-01/NNPII_Progress_Analysis_Final_0.pdf (Accessed on 30 June 2023).
15. Federal Democratic Republic of Ethiopia. Seqota declaration. Implementation plan (2016–2030), summary Programme approach document. (2016). Available from https://www.exemplars.health/-/media/files/egh/resources/stunting/ethiopia/seqota-declaration-implementation-plan-(20162030).pdf (Accessed on 28 June 2022)
16. World Food Program. Ethiopia country strategic plan (2020–2025). Progress towards Sustainable Development Goal 2 and 17. Page 5–6. 5th revision. (2019). Available from https://www.wfp.org/operations/et02-ethiopia-country-strategic-plan-2020-2025 (Accessed June 30, 2022).
17. Federal ministry of Health of Ethiopia. Ethiopian public health. The demographic and health survey program ICF Institute. Mini demographic and health survey 2019. (2021). Available from https://dhsprogram.com/pubs/pdf/FR363/FR363.pdf (Access on 19 June 2022)
18. UNICEF. Ethiopia humanitarian situation report, no.3. (2022). Available from https://reliefweb.int/report/ethiopia/unicef-ethiopia-humanitarian-situation-report-no-3-march-202 (Accessed on 28 June 2022).
19. Gebre, A, Reddy, PS, Mulugeta, A, Sedik, Y, and Kahssay, M. Prevalence of malnutrition and associated factors among under-five children in pastoral communities of Afar regional state, Northeast Ethiopia: a community-based cross-sectional study. J nutrition and metabolism. (2019) 2019:1–13. doi: 10.1155/2019/9187609
20. WHO/UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: Joint statement by the World Health Organization and the United Nations Children's fund, (2009). Available at: https://iris.who.int/bitstream/handle/10665/44129/9789241598163_eng.pdf (Accessed August, 2022).
21. Dahal, K, Yadav, DK, Baral, D, and Yadav, BK. Determinants of severe acute malnutrition among under 5 children in Satar community of Jhapa. Nepal PloS one. (2021) 16:e0245151. doi: 10.1371/journal.pone.0245151
22. World Health Organization. Malaria surveillance, monitoring and evaluation: A reference manual. (2018). Available from https://www.who.int/publications/i/item/9789241565578 (Accessed on 21 June 2022).
23. Begna, G, Bikila, H, Biru, B, Diriba, D, Tolera, C, Dessalegn, RE, et al. Determinants of severe acute malnutrition among children less than five years visiting health centers in Leqa Dulacha District, east Wallaga zone, Oromia region, Ethiopia: a case control study. Health Sci Reports. (2024) 7:e1939. doi: 10.1002/hsr2.1939
24. Dodos, J, Altare, C, Bechir, M, Myatt, M, Pedro, B, Bellet, F, et al. Individual and household risk factors of severe acute malnutrition among under-five children in Mao, Chad: a matched case-control study. Arch Public Health. (2018) 76:1–9. doi: 10.1186/s13690-018-0281-5
25. Ghimire, U, Aryal, BK, Gupta, AK, and Sapkota, S. Severe acute malnutrition and its associated factors among children under-five years: a facility-based cross-sectional study. BMC Pediatr. (2020) 20:1–9. doi: 10.1186/s12887-020-02154-1
26. World Health Organization. Indicators for assessing infant and young child feeding practices: Part one: Definitions: Conclusions of a consensus meeting held 6–8 November 2007 in Washington DC, USA. US: World Health Organization (2008).
27. Coates, J, Swindale, A, and Bilinsky, P. Household food insecurity access scale (HFIAS) for measurement of food access: Indicator guide. Washington DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development (FANTA) (2007).
28. Biset, G, Woday, A, Mihret, S, and Tsihay, M. Full immunization coverage and associated factors among children age 12-23 months in Ethiopia: systematic review and meta-analysis of observational studies. Hum Vaccin Immunother. (2021) 17:2326–35. doi: 10.1080/21645515.2020.1870392
29. Fekadu, GA, Terefe, MW, and Alemie, GA. Prevalence of pneumonia among under-five children in Este town and the surrounding rural Kebeles, Northwest Ethiopia: a community based cross sectional study. Sci J Public Heal. (2014) 2:150–5. doi: 10.11648/j.sjph.20140203.12
30. WHO. Diarrheal disease. (2017). Available from https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (Accessed on 27 June 2022).
31. Bitew, ZW, Ayele, EG, Worku, T, Alebel, A, Alemu, A, Worku, F, et al. Determinants of mortality among under-five children admitted with severe acute malnutrition in Addis Ababa. Ethiopia Nutrit J. (2021) 20:1–5. doi: 10.1186/s12937-021-00750-0
32. Kassaw, A, Amare, D, Birhanu, M, Tesfaw, A, Zeleke, S, Arage, G, et al. Survival and predictors of mortality among severe acute malnourished under-five children admitted at Felege-Hiwot comprehensive specialized hospital, northwest, Ethiopia: a retrospective cohort study. BMC Pediatr. (2021) 21:1. doi: 10.1186/s12887-021-02651-x
33. Nass, SS, Nass, NS, Iliyasu, Z, Suleiman, B, Yahaya, S, Habibu, B, et al. Determinants of mortality among severely malnourished children in northern Nigeria. Health Services Res Managerial Epidemiol. (2021) 8:23333928211064089. doi: 10.1177/23333928211064089
34. Nyeko, R, Calbi, V, Ssegujja, BO, and Ayot, GF. Treatment outcome among children under-five years hospitalized with severe acute malnutrition in St. Mary’s hospital Lacor, northern Uganda. BMC Nutrition. (2016) 2:1–7. doi: 10.1186/s40795-016-0058-6
35. Abate, HK, Kidane, SZ, Feyessa, YM, and Gebrehawariat, EG. Mortality in children with severe acute malnutrition. Clinical nutrition ESPEN. (2019) 33:98–104. doi: 10.1016/j.clnesp.2019.07.001
36. Prost, A, Nair, N, Copas, A, Pradhan, H, Saville, N, Tripathy, P, et al. Mortality and recovery following moderate and severe acute malnutrition in children aged 6–18 months in rural Jharkhand and Odisha, eastern India: a cohort study. PLoS Med. (2019) 16:e1002934. doi: 10.1371/journal.pmed.1002934
37. WHO. Regional Office for South-East Asia. Guidelines for the inpatient treatment of severely malnourished child. WHO (2003). Available from https://iris.who.int/bitstream/handle/10665/205172/B0003.pdf;sequence=1. (Accessed June 22, 2022).
38. Ethiopia-Federal Ministry of Health. Protocol for management of severe acute malnutrition. (2007). Available from https://www.ennonline.net/attachments/897/ethiopia-sam-guideline-march-2007.pdf (Accessed on 28 June 2022)
39. Geberselassie, SB, Abebe, SM, Melsew, YA, Mutuku, SM, and Wassie, MM. Prevalence of stunting and its associated factors among children 6-59 months of age in Libo-Kemekem district, Northwest Ethiopia; a community-based cross-sectional study. PLoS One. (2018) 13:e0195361. doi: 10.1371/journal.pone.0195361
40. Tadesse, A, Hailu, D, and Bosha, T. Nutritional status and associated factors among pastoralist children aged 6–23 months in Benna Tsemay Woreda, south Omo zone, southern Ethiopia. Int J Nutr Food Sci. (2018) 7:11–23. doi: 10.11648/j.ijnfs.20180701.13
41. Islam, MM, Alam, M, Tariquzaman, M, Kabir, MA, Pervin, R, Begum, M, et al. Predictors of the number of under-five malnourished children in Bangladesh: application of the generalized poisson regression model. BMC Public Health. (2013) 13:1–8. doi: 10.1186/1471-2458-13-11
42. Chowdhury, F, Shahid, AS, Ghosh, PK, Rahman, M, Hassan, MZ, Akhtar, Z, et al. Viral etiology of pneumonia among severely malnourished under-five children in an urban hospital, Bangladesh. PLoS One. (2020) 15:e0228329. doi: 10.1371/journal.pone.0228329
43. Ahmed, AT, Abas, AH, Elmi, A, and Omer, A. Determinants of severe acute malnutrition among children aged 6–36 months in Kalafo district (riverine context) of Ethiopia. Sci Rep. (2022) 12:5198. doi: 10.1038/s41598-022-09184-y
44. Abera, L, Dejene, T, and Laelago, T. Prevalence of malnutrition and associated factors in children aged 6–59 months among rural dwellers of damot gale district, south Ethiopia: community based cross sectional study. International journal for equity in health. (2017) 16:1–8.
45. Awoke, A, Ayana, M, and Gualu, T. Determinants of severe acute malnutrition among under five children in rural Enebsie Sarmidr District, east Gojjam zone, north West Ethiopia, 2016. BMC nutrition. (2018) 4:1–8. doi: 10.1186/s40795-018-0211-5
46. Popat, CN, Chaudhari, AI, Mazumdar, VS, and Patel, SV. A cross sectional study to measure the prevalence of malnutrition and factors associated with malnutrition among under five children of an urban slum of Vadodara city. J Res Medical Dental Sci. (2014) 2:59–64. doi: 10.5455/jrmds.20142313
47. Wuneh, MT, and Guracho, YD. Nutritional status and feeding practice of children 6-59 months old, Metekele zone of Benishangul-Gumuz region, Northwest Ethiopia. Sci J Clin Med. (2018) 12:120–9. doi: 10.11648/j.sjcm.20170606.15
48. Ma’alin, A, Birhanu, D, Melaku, S, Tolossa, D, Mohammed, Y, and Gebremicheal, K. Magnitude and factors associated with malnutrition in children 6–59 months of age in Shinille Woreda, Ethiopian Somali regional state: a cross-sectional study. BMC. Nutrition. (2016) 2:1–2. doi: 10.1186/s40795-016-0079-1
49. Nzalal, SH, Siyizal, S, Babaniyi, O, Songolo, P, Muula, AS, and Radatsikira, E. Demographic, cultural, and environmental factors associated with frequency and severity of malnutrition among Zambian children less than five years of age. J Public Health and Epidemiol. (2011). 3:362–70. doi: 10.5897/JPHE.9000037
Keywords: malnutrition, severe acute malnutrition, outbreak, investigation, risk factors, Ethiopia
Citation: Gashe AD, Woldemichael DZ, Worku FA, Mahmud KA and Endries AY (2024) Investigating a severe acute malnutrition outbreak in Dubti District, Awsiresu Zone, Afar Region, Northeast Ethiopia (2022). Front. Public Health. 12:1475104. doi: 10.3389/fpubh.2024.1475104
Edited by:
Mojisola Olanike Kehinde, Landmark University, NigeriaReviewed by:
Olutosin Ademola Otekunrin, University of Ibadan, NigeriaToluwalase Awe, Landmark University, Nigeria
Copyright © 2024 Gashe, Woldemichael, Worku, Mahmud and Endries. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abiyie Demelash Gashe, YWJpeWllZGVtZWxhc2hAZ21haWwuY29t
 Abiyie Demelash Gashe
Abiyie Demelash Gashe Dawit Zenebe Woldemichael2
Dawit Zenebe Woldemichael2