- 1The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2The Department of Geriatrics, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 3Faculty of Science, The University of Melbourne, Parkville, VIC, Australia
Objective: The correlation between surrogate insulin resistance (IR) indices and sarcopenic obesity (SO) remains uncertain. This study aimed to assess the association between six IR surrogates—triglyceride-glucose (TyG), TyG-waist circumference (TyG-WC), TyG-waist-to-height ratio (TyG-WHtR), triglyceride-to-high-density lipoprotein-cholesterol ratio (TG/HDL), metabolic score for insulin resistance (METS-IR), and Chinese visceral adiposity index (CVAI)—and SO risk in a middle-aged and older population in China.
Methods: The study employed longitudinal data obtained from the China Health and Retirement Longitudinal Study (CHARLS) between 2011 and 2015, involving 6,395 participants. We used multivariate logistic regression models to examine the link between six surrogates and SO. Nonlinear relationships were evaluated using restricted cubic spline analysis, and subgroup analyses were conducted for validation. Receiver operating characteristic (ROC) curves were used to assess predictive capabilities.
Results: Over the course of a 4-year follow-up period, 319 participants (5.0%) developed SO. In the fully adjusted model, all six surrogates were significantly associated with SO. The adjusted odds ratios (ORs) with a 95% confidence interval (95% CI) per standard deviation increase were 1.21 (1.08–1.36) for TyG, 1.56 (1.39–1.75) for TyG-WC, 2.04 (1.81–2.31) for TyG-WHtR, 1.11 (1.01–1.21) for TG/HDL, 1.67 (1.50–1.87) for METS-IR, and 1.74 (1.55–1.97) for CVAI. Notably, TyG-WC, TyG-WHtR, TG/HDL, METS-IR, and CVAI exhibited nonlinear correlations with SO. Conversely, TG/HDL did not exhibit a significant association during subgroup analysis. Furthermore, TyG-WHtR had a significantly larger area under the receiver operating characteristic curve than other indices.
Conclusion: The results indicated that TyG, TyG-WC, TyG-WHtR, METS-IR, and CVAI were significantly and positively associated with SO incidence. Meanwhile, TyG-WC, TyG-WHtR, METS-IR, and CVAI showed nonlinear relationships with SO. Specifically, TyG-WHtR may be the most appropriate indicator for predicting SO among middle-aged and older Chinese adults.
1 Introduction
As its name suggests, the clinical condition known as sarcopenic obesity (SO) arises from the concurrent presence of sarcopenia and obesity. As individuals age, there is often an increase in adipose tissue and a reduction in muscle mass, which can accelerate the progression of SO. SO has a greater negative impact on human health than either obesity or sarcopenia in isolation. Previous studies have shown that SO is associated with an increased risk of developing various metabolic diseases, including diabetes (1), hypertension (2), and metabolic syndrome (3). Furthermore, individuals with SO are prone to develop frailty, disability, and even mortality (4). The prevalence of SO is estimated to be between 2.75 and 20% (5). The current reported prevalence of SO varies considerably, which may be related to the different diagnostic criteria used. Due to the increasing trend of population aging, the prevalence of SO is anticipated to increase. Therefore, early detection and prevention of SO may prove to be an effective strategy for reducing the burden of disease and alleviating global public health pressures.
Insulin resistance (IR) is defined as an impaired ability of insulin to assist in the absorption and utilization of glucose, resulting in compensatory hyperinsulinemia as the body attempts to maintain glucose homeostasis. Individuals with insulin resistance often present with metabolic disturbances, including hyperglycemia and hyperlipidemia. These conditions accelerate muscle breakdown and exacerbate fat accumulation, thereby promoting the development of SO (6, 7). Various methodologies have been established to evaluate IR, with the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) emerging as a prevalent technique. Prior research has provided empirical evidence corroborating that increased HOMA-IR values are significantly and independently associated with an increased risk of SO (8–11), which highlights the potential of HOMA-IR as a strong prognostic biomarker. Nevertheless, the requirement for fasting insulin levels limits the applicability of HOMA-IR in clinical situations. In recent years, alternative indices of IR that do not require insulin measurement have received increasing attention and have been demonstrated to be reliable tools for the assessment of IR. These include the triglyceride-glucose (TyG) index (12), the ratio of triglyceride-to-high-density lipoprotein-cholesterol (TG/HDL) (13), the TyG index adjusted for waist circumference (TyG-WC) (14), the TyG index adjusted for waist-to-height ratio (TyG-WHtR) (14), the metabolic score for IR (METS-IR) (15), and the Chinese visceral adiposity index (CVAI) (16).
Recent research suggests that the TyG index may serve as a valuable predictor of SO (17). Nevertheless, there is a lack of research examining the correlation between IR surrogate indicators and SO, and it remains unclear whether a nonlinear relationship exists between these indicators and SO. To address this gap, this study used the national longitudinal survey of middle-aged and older adults database to investigate the correlations between six IR surrogate indicators and SO risk and to evaluate and compare the diagnostic value of different IR surrogate indicators for new-onset SO.
2 Materials and methods
2.1 Study design and population
The China Health and Retirement Longitudinal Study (CHARLS) is a nationally representative longitudinal study that focuses on the aging process among Chinese community members aged 45 years and older. The results of the study are available to the public for free.1 The baseline survey of the CHARLS was initiated in 2011 (Wave 1), and a total of 17,708 participants were enrolled across 150 counties or districts and 450 villages encompassing 28 provinces throughout China. Following the baseline survey, biennial follow-up surveys were conducted, with Waves 2 in 2013, 3 in 2015, and 4 in 2018. The database contains a comprehensive array of information, including demographic characteristics, health status, physical measurements, family structures, financial status, and other pertinent information. Blood samples were collected during Waves 1 and 3 in order to supplement the research. The methodologies regarding sampling, questionnaire construction, and clinical assessments have been documented in previous reports (18). Prior to their participation, all individuals provided written informed consent, and the study received ethical approval from the Institutional Review Board of Peking University (IRB00001052-11015).
The initial sample of this investigation consisted of 17,708 individuals from the baseline survey (Wave 1). To maintain the integrity and relevance of the study cohort, a set of exclusion criteria were meticulously applied. Individuals were deemed ineligible for the study if they lacked baseline measurements for any six IR indices (n = 7,958), if their age data was incomplete, or if they were under 45 years of age (n = 339). Moreover, participants with a confirmed diagnosis of SO at baseline or with incomplete information regarding SO were excluded (n = 508). Further exclusions were made for those lacking SO data in the subsequent Waves 2 and 3 (n = 1,988), for those with atypical Body mass index (BMI) and waist measurements (n = 178), and for those without comprehensive demographic, health behavior, and chronic disease information (n = 342). Following the application of these exclusionary criteria, the final dataset used for analysis consisted of 6,395 participants (Supplementary Figure S1).
2.2 Evaluation of six surrogate insulin resistance indices
Venous blood samples were collected from each participant following an overnight fast, conducted by professional medical personnel from the Chinese Center for Disease Control and Prevention. Thereafter, the samples were then subjected to centrifugation before being transported to the laboratory at Capital Medical University for further analysis. The blood lipids and glucose levels were determined through the use of enzymatic colorimetric assays. Anthropometric measurements, including body weight and height, were recorded with precision using an Omron HN-286 scale and a Seca 213 stadiometer, respectively, with accuracy to the nearest 0.1 unit. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared. At the conclusion of a gentle exhalation, waist circumference was measured at the umbilical level. The surrogate IR indices were calculated using specific formulas based on the established methodologies of previous studies (12–16), and these six formulas are specified as follows [Equations 1-6]:
Males:
Females:
2.3 Assessment of sarcopenic obesity
Sarcopenic obesity (SO) is defined as the coexistence of both sarcopenia and obesity (19, 20). In this study, obesity was categorized according to the modified Asia-Pacific criteria, with a BMI of 25 kg/m2 or higher being an indicator (21). It has been recognized that the BMI alone has limitations, particularly in individuals of varying heights, where taller individuals may not be obese despite having a higher BMI, whereas shorter individuals may appear overweight. To address this issue, we combined BMI with appendicular skeletal muscle mass (ASM) to provide a more comprehensive assessment. The diagnosis of sarcopenia is based on the criteria developed by the Foundation for the National Institutes of Health (FNIH) (22). To quantify ASM, we employed an established anthropometric formula specific to the Chinese population, which has shown high consistency with dual-energy X-ray absorptiometry (DXA) measurements (23, 24). The calculation formula for ASM is as follows (23):
where sex code is 1 for males and 2 for females. This threshold for “sarcopenia,” i.e., adjusted skeletal muscle mass based on body mass index (BMI) (ASM/BMI), is less than 0.512 for females and 0.789 for males (22). This combined approach mitigates the potential misclassification due to height-related variability.
2.4 Covariates
The selection of covariates in our study was informed by both empirical evidence from preceding scholarly studies and the informed judgment of clinical professionals (25–28). The covariates were classified into the following categories: (1) categorical variables: gender, marriage, residence, educational attainment, smoking habits, alcohol consumption, and chronic diseases, including hypertension, diabetes mellitus, dyslipidemia, cancer, pulmonary disease, heart disease, stroke, liver disease, renal disease, and depression. (2) Continuous variables: age, duration of sleep, BMI, waist circumference (WC), C-reactive protein (CRP), serum high-density lipoprotein cholesterol (HDL-c), serum low-density lipoprotein cholesterol (LDL-c), triglycerides (TG), total serum cholesterol (TC), and serum creatinine.
2.5 Statistical analysis
The characteristics of the participants at the baseline stage were subjected to analysis with the aim of identifying those who were newly diagnosed with SO. Continuous variables with a normal distribution were expressed as mean ± standard deviation (SD), while variables with an irregular distribution were depicted as median and interquartile range (IQR). Categorical variables were presented as counts, while percentages (%) were presented for categorical variables. For continuous variables, comparative analyses across groups were performed using Student’s t-tests or Mann–Whitney U tests and chi-square tests for categorical data.
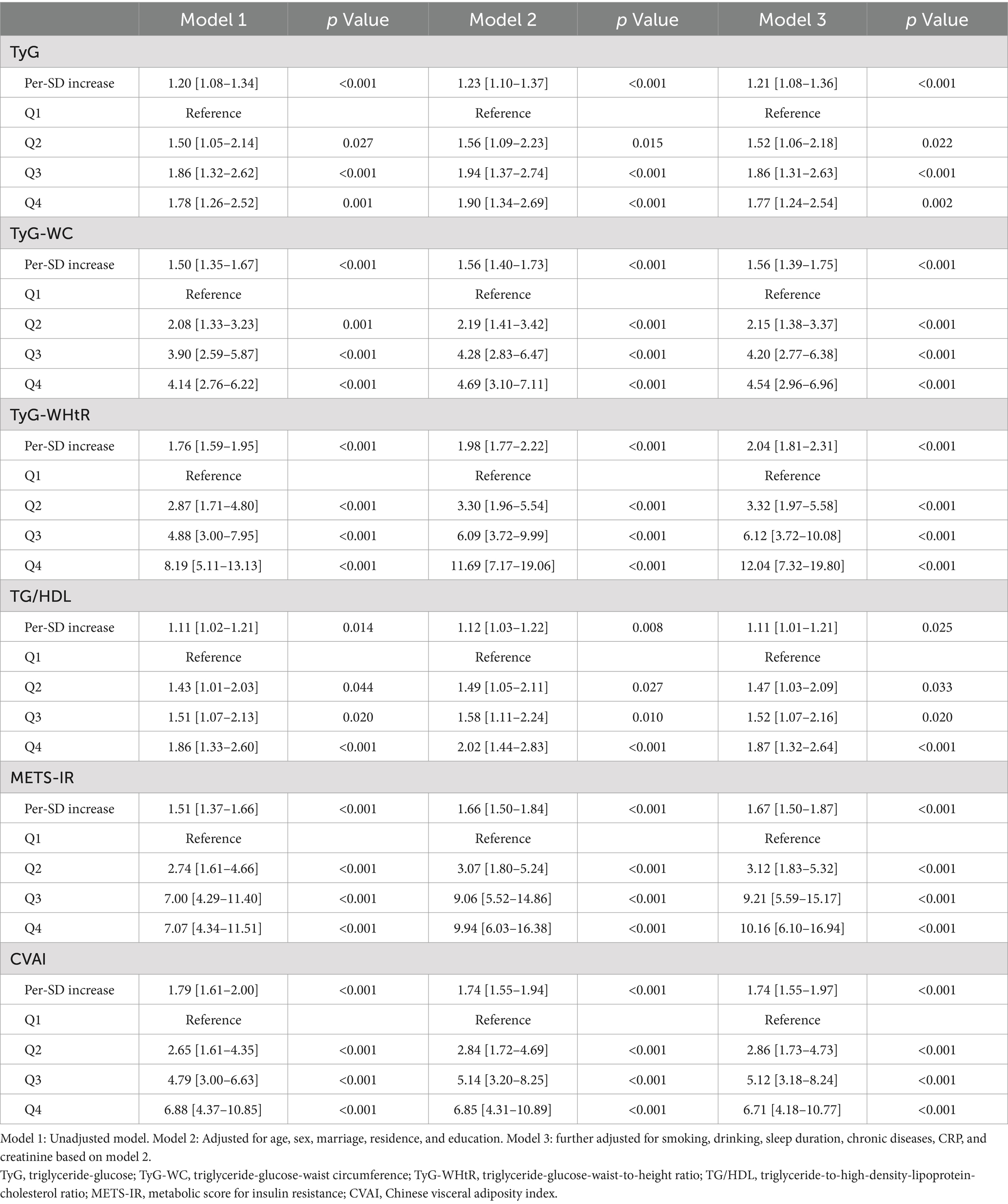
Prior to developing logistic regression models, we computed the generalized variance inflation factor to investigate the possibility of multicollinearity between six IR surrogates and other factors. Due to the significant multicollinearity among the six surrogate IR indices, as detailed in Supplementary Table S1, BMI, WC, TC, TG, LDL, and HDL were excluded from the final logistic regression models. To investigate the correlation between the baseline surrogate IR indices and the likelihood of incident SO, univariate and multivariate logistic regression analyses were employed. Three additional models constructed were as follows: Model 1 (without adjustment), Model 2 (with adjustments for demographics, including age, gender, marital status, living situation, and educational attainment), and Model 3 (with adjustments for additional lifestyle factors and health indicators such as smoking, drinking, sleep duration, chronic diseases, CRP, and creatinine based on Model 2). Moreover, an investigation was conducted to examine the dose–response correlation between the per-SD increases in the surrogate IR indices and SO incidence. Nonlinear relationships were examined using adjusted cubic splines, and inflection points were identified using a recursive algorithm. Afterward, two-piecewise logistic regression models were then formulated around these inflection points. The most suitable models were identified using log-likelihood ratio tests to elucidate the link between IR surrogates and SO.
Subgroup and interaction analyses were conducted to investigate the association between IR surrogates and SO risk with stratification by gender and age. The receiver operating characteristic (ROC) curve was employed to assess the prediction accuracy of six IR surrogates for SO, and differences in the area under the curve (AUC) values were compared using z-tests.
All statistical analyses were executed using SPSS version 25.0 (IBM SPSS, Armonk, NY, United States) and R version 4.2.3 (R Development Core Team, Vienna, Austria). A two-tailed test with a significance level of p < 0.05 was used to determine whether the observed differences in the statistics were statistically significant.
3 Results
3.1 Baseline characteristics
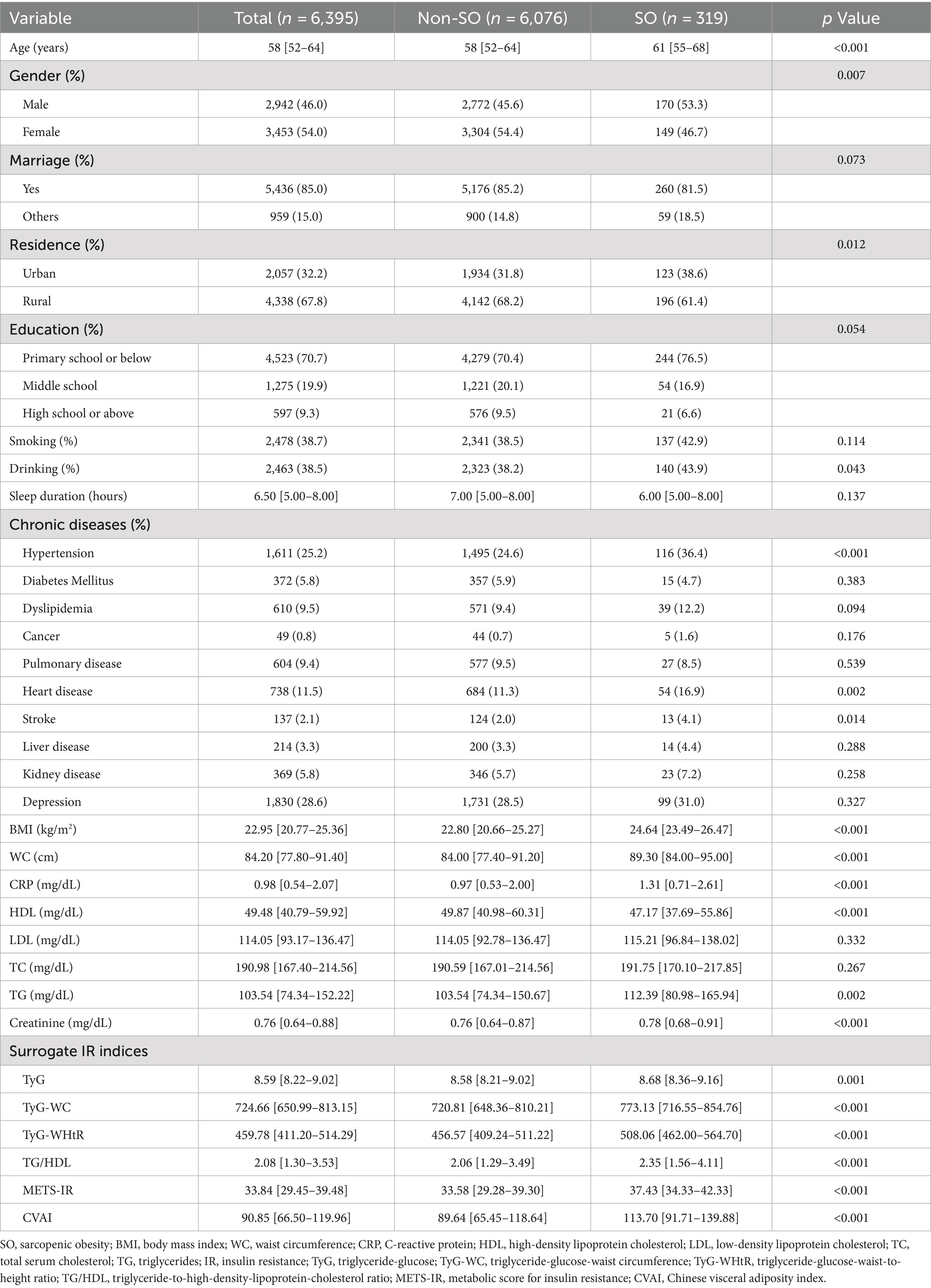
A total of 6,395 individuals, initially unaffected by SO, were continuously monitored till 2015. The baseline characteristics of the cohort are detailed in Table 1, which are differentiated by the frequency of SO throughout the follow-up period. During the four-year observation period, 319 individuals (5.0%) developed new-onset SO. Individuals with SO were more likely to be older, male, urban dwellers, and to consume alcohol, as well as to have increased levels of BMI, WC, CRP, TC, TG, and creatinine, along with decreased HDL levels. This group also experienced a higher prevalence of comorbidities such as hypertension, cardiovascular disease, and stroke. Furthermore, increased values of all six IR surrogates were observed in these participants.
3.2 Association of baseline surrogate IR indices with onset risk of SO based on logistic regression
Supplementary Figure S2 shows the correlation between the quartiles of six surrogates and the prevalence of SO. It can be observed that the incidence of SO increases with higher levels of IR surrogates. Table 2 shows the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for SO across varying quartiles as well as for a per-SD increase in the levels of six surrogate IR indices. Using the adjustment potential confounders as per Model 3, a per-SD increase increment in each index was linked to adjusted ORs of 1.21 (1.08–1.36) for TyG, 1.56 (1.39–1.75) for TyG-WC, 2.04 (1.81–2.31) for TyG-WHtR, 1.11 (1.01–1.21) for TG/HDL, 1.67 (1.50–1.87) for METS-IR, and 1.74 (1.55–1.97) for CVAI. A comprehensive account of the relationship between the indices’ quartiles and SO risk, including the impact of each surrogate IR index, is provided in Table 2 and shown in Supplementary Figure S2.
3.3 Nonlinear relationship between baseline surrogate IR indices and onset risk of SO
The dose–response relationship between IR surrogates and SO is illustrated and examined using restricted cubic splines, as shown in Figure 1. After adjusting for numerous confounding variables in Model 3, the TyG index showed a linear correlation with the emergence of SO (P nonlinear = 0.119). Conversely, the TyG-WC, TyG-WHtR, TG/HDL, METS-IR, and CVAI indices revealed notable nonlinear correlations with SO (P nonlinear <0.001; P nonlinear <0.001; P nonlinear = 0.003; P nonlinear <0.001; P nonlinear <0.001).
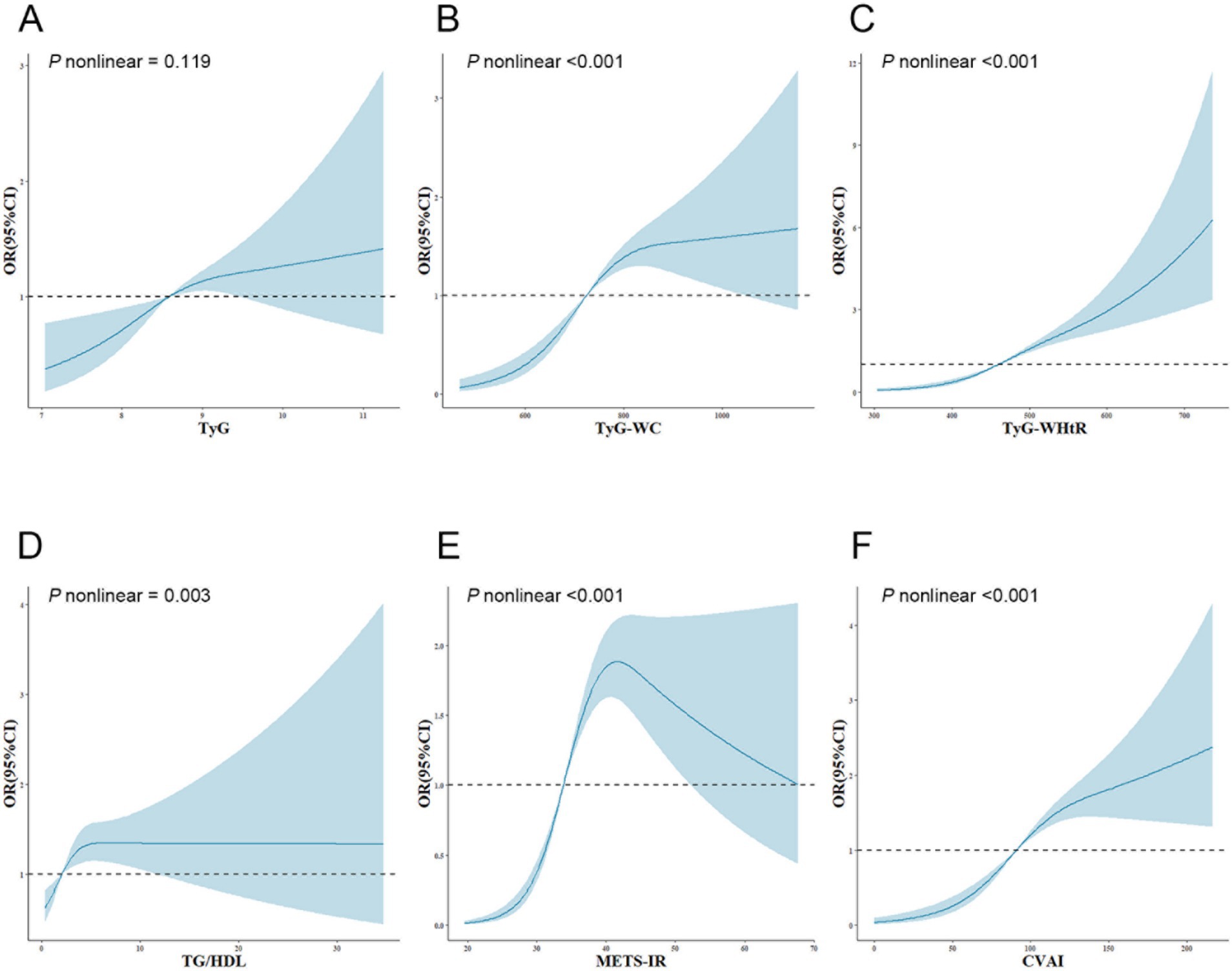
Figure 1. Adjusted cubic spline model of the relationship between six surrogate IR indices and SO risk. All models were adjusted for various factors, including age, sex, marriage, residence, education, smoking, drinking, sleep duration, chronic diseases, CRP, and creatinine. The solid line and blue area represent the estimated adjusted OR and 95% CI, respectively. The horizontal dotted line represents the odds ratio of 1.0. (A) TyG index, (B) TyG-WC, (C) TyG-WHtR, (D) TG/HDL, (E) METS-IR, (F) CVAI. TyG, triglyceride-glucose; TyG-WC, triglyceride-glucose-waist circumference; TyG-WHtR, triglyceride-glucose-waist-to-height ratio; TG/HDL, triglyceride-to-high-density-lipoprotein-cholesterol ratio; METS-IR, metabolic score for insulin resistance; CVAI, Chinese visceral adiposity index; OR, odds ratio; 95%CI, 95% confidence interval.
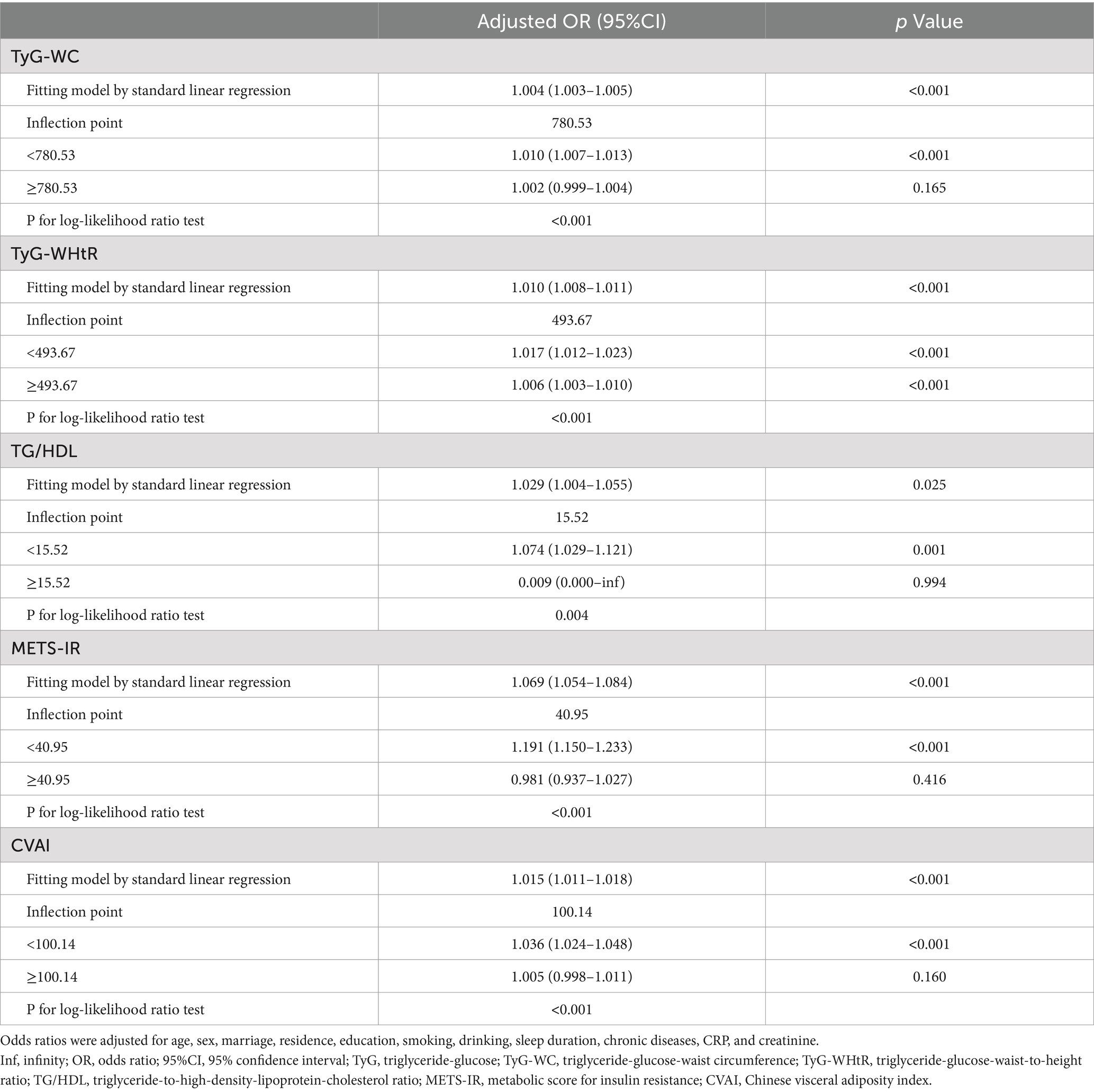
Subsequently, a threshold effect analysis was conducted to identify the inflection points in the connections between TyG-WC, TyG-WHtR, TG/HDL, METS-IR, and CVAI and the risk of SO. A two-piecewise logistic regression model was employed to assess the ORs and CIs on either side of these demarcations, as detailed in Table 3. The inflection points for TyG-WC, TG/HDL, METS-IR, and CVAI were 780.53, 15.52, 40.95, and 100.14, respectively. When TyG-WC, TG/HDL, METS-IR, and CVAI were below their respective inflection points, a significant positive association with SO risk was observed, with ORs of 1.010 (95% CI: 1.007–1.013, p < 0.001), 1.074 (95% CI: 1.029–1.121, p = 0.001), 1.191 (95% CI: 1.150–1.233, p < 0.001), and 1.036 (95% CI: 1.024–1.048, p < 0.001). Following the inflection points, the correlations were not statistically significant, with ORs of 1.002 (95% CI: 0.999–1.004, p = 0.165), 0.009 (95% CI: 0.000 to infinity, p = 0.994), 0.981 (95% CI: 0.937–1.027, p = 0.416), and 1.005 (95% CI: 0.998–1.011, p = 0.160). For TyG-WHtR, the OR was 1.017 (95% CI: 1.012–1.023, p < 0.001) before and 1.006 (95% CI: 1.003–1.010, p < 0.001) after the inflection point of 493.67.
3.4 Subgroup analysis according to gender and age
Figure 2 shows the results of the subgroup analysis, stratified by gender and age, regarding the correlation between six IR surrogates and the incidence of SO. In the various subgroups, significant positive correlations were observed between SO risk and TyG-WC, TyG-WHtR, METS-IR, and CVAI, as demonstrated. In contrast, no discernible correlation was observed between TG/HDL and SO risk in any of the subgroups, and no interactions with age or gender were identified. It is noteworthy that the correlation between TyG-WC, TyG-WHtR, METS-IR, CVAI, and SO was more robust in older individuals than in those of middle age. Female participants demonstrated an especially high correlation between CVAI and SO. In the course of this investigation, age was identified as an important interacting factor influencing the relationship between TyG-WC (p for interaction = 0.001), TyG-WHtR (p for interaction = 0.001), METS-IR (p for interaction = 0.005), and CVAI (p for interaction = 0.010) with SO. Similarly, gender was identified as a significant influencing factor influencing the relationship between CVAI and SO (p for interaction = 0.020).
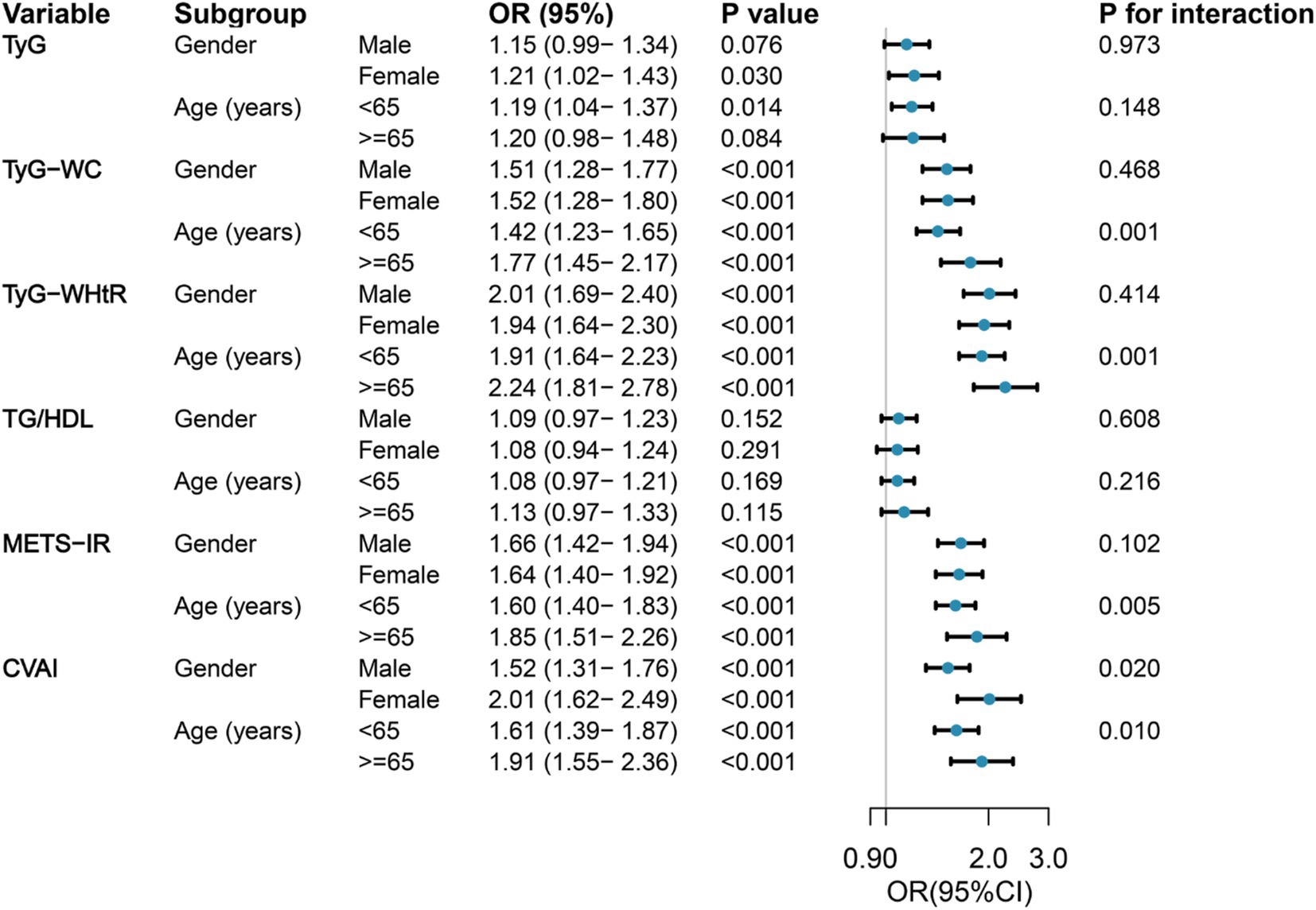
Figure 2. Forest maps of the association between six surrogate IR indices and the risk of incident SO by age and gender. Odds ratios were adjusted for age, sex, marriage, residence, education, smoking, drinking, sleep duration, chronic diseases, CRP, and creatinine. OR, odds ratio; 95%CI, 95% confidence interval; TyG, triglyceride-glucose; TyG-WC, triglyceride-glucose-waist circumference; TyG-WHtR, triglyceride-glucose-waist-to-height ratio; TG/HDL, triglyceride-to-high-density lipoprotein-cholesterol ratio; METS-IR, metabolic score for insulin resistance; CVAI, Chinese visceral adiposity index.
3.5 Predictive performance of six surrogate IR indices for SO
The evaluation of the effectiveness of these IR surrogate indices in predicting SO is shown in Figure 3 and Supplementary Table S2. The TyG-WHtR index exhibited the highest prominent area under the curve (AUC) value of 0.684 (p < 0.001), surpassing the predictive capacities of other indices such as CVAI, METS-IR, TyG-WC, TG/HDL, and TyG. It was noteworthy that the optimal threshold value for TyG-WHtR for identifying SO in the middle-aged and older Chinese population was established at 458.778, with a sensitivity of 0.771 and a specificity of 0.510.
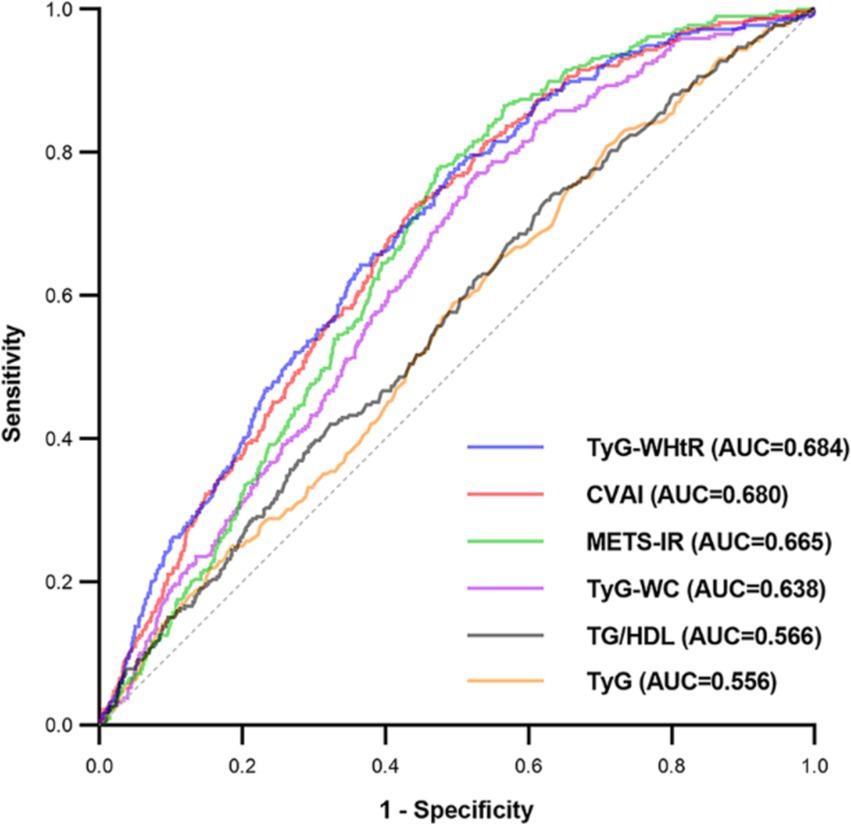
Figure 3. Receiver operating characteristic curves of surrogate IR indices for predicting SO. AUC, area under the curve; TyG, triglyceride-glucose; TyG-WC, triglyceride-glucose-waist circumference; TyG-WHtR, triglyceride-glucose-waist-to-height ratio; TG/HDL, triglyceride-to-high-density-lipoprotein-cholesterol ratio; METS-IR, metabolic score for insulin resistance; CVAI, Chinese visceral adiposity index.
4 Discussion
In this nationwide cohort study, we identified significant positive correlations between the TyG, TyG-WC, TyG-WHtR, METS-IR, and CVAI indices, as well as the incidence of SO in middle-aged and older Chinese adults. The TyG index demonstrated a linear association with SO, whereas the TyG-WC, TyG-WHtR, METS-IR, and CVAI demonstrated nonlinear relationships. In terms of predictive SO, TyG-WHtR demonstrated optimal performance.
Surrogate indices of IR have been demonstrated to be effective tools for assessing IR levels in multiple studies (15, 29–33). However, research on surrogate IR indices with SO is scarce. Kim et al. conducted analyses on 3,821 older Korean people and found that, even after adjustment for multiple variables, a high TyG index was still associated with a higher risk of SO in both men and women (17). In contrast, in our study, the association between the TyG index and SO risk differed between men and women and was positively associated with SO in women (OR = 1.21, 95% CI: 1.02–1.43, p = 0.030) but not in men (OR = 1.15, 95% CI: 0.99–1.34, p = 0.076). The factors contributing to the gender-specific association between TyG and SO may include the following: First, the types of studies differed, as the research of Kim et al. was a cross-sectional study, whereas our study was a longitudinal cohort study. Second, the incidence of SO varies across diverse study populations. Since Kim et al. focused on older adults, a group with a high incidence of SO, their study was more likely to yield significant results. Our study, on the other hand, included a wide range of middle-aged and older people. Besides, the two studies adjusted for different covariates, which could also influence the study results.
Additionally, this study has broadened the investigation into the correlations between additional surrogate IR indices and SO risk. We created three models that adjusted for 20 potential confounding factors (age, gender, marital status, residence, education, smoking, alcohol consumption, sleep duration, comorbidities, and baseline CRP and creatinine). Our results indicated a significant positive connection between IR surrogates and SO risk. More importantly, the results of our study indicated that TyG-WHtR was the most suitable predictor for SO, and recent research has reported the critical role of TyG-WHtR in metabolic disorders and cardiovascular diseases. Xuan et al.’s population-based retrospective cohort study demonstrated that TyG-WHtR was deemed as the most optimal predictor for diabetes, compared to TyG and TyG-WC (34). Miao et al. demonstrated in a cross-sectional study involving 16,834 participants that TyG-WHtR outperformed TyG and other related parameters in identifying high cardiovascular risk (35). A systematic review and meta-analysis of 31 studies involving 123,231 individuals revealed that WHtR had the largest AUC for diabetes compared to WC and BMI (36). This could be attributed to the critical role of WHtR in metabolic disease.
Furthermore, this study demonstrated the nonlinear relationship between surrogate IR indices and SO risk and further refined and elucidated the specific trends of these indices in relation to SO risk through implementing two-piecewise logistic regression analysis. For TyG-WHtR values below 493.67, each increment in unit was associated with a rise of 1.7% in the likelihood of SO. However, once the value reached 493.67, the rate of risk increase slowed, with only a 0.6% increase in risk per unit increase. There was an approximately inverted U-shaped curve relationship between METS-IR and the risk of SO. In instances where METS-IR fell below 40.95, the likelihood of SO increased by 19.1% for every unit increase in METS-IR. In contrast, when METS-IR exceeded 40.95, there was no statistically significant change in the risk of SO for each unit increase in METS-IR. The TyG-WC, TG/HDL, and CVAI demonstrated saturation effects with SO risk by increasing it below specific inflection points. However, once they exceeded the inflection points, further increases did not result in an increase in SO risk. Consequently, the outcomes of this study provide a theoretical foundation for reducing surrogate IR indices in clinical practice, particularly when they are below the inflection points. Furthermore, the research provides specific recommendations for medical professionals to help patients with different surrogate IR index levels reduce their likelihood of developing SO.
Numerous studies have indicated that age and gender hold significant influence on the occurrence of SO (37). The subgroup analysis revealed that individuals aged 65 and above had a greater impact of TyG-WC, TyG-WHtR, METS-IR, and CVAI on SO risk. Previous studies have indicated that older individuals with diabetes tend to exhibit diminished muscle function and reduced muscle mass in comparison to their non-diabetic counterparts (38). However, the administration of insulin sensitizers has been shown to mitigate this condition (39). It is therefore possible that the increased correlation between IR and SO risk observed in the older population may be attributed to aging and its adverse effects on muscle and metabolic functions. Additionally, our study revealed a stronger correlation between CVAI and SO in females, possibly attributable to the influence of hormonal factors. A significant decline in estrogen levels associated with female menopause has been linked to impaired skeletal muscle function and lipid metabolism disorder, which ultimately leads to the development of SO (40, 41).
The primary strength of this study is the use of data from CHARLS, a nationally representative survey. This database is compliant with the highest international standards for data collection, which ensures comprehensive representativeness and high data quality. The findings regarding the association between IR surrogates and SO among the middle-aged and older population in China are both reliable and generalizable. Furthermore, this study not only comprehensively examined the correlation between IR surrogates and SO risk but also examined the nonlinear relationships using longitudinal data. Additionally, the study assessed the predictive capability of each indicator for SO risk, thereby deepening our understanding and application of IR surrogates in evaluating SO risk among middle-aged and older individuals. However, it is noteworthy that our study also possesses certain limitations. First, a validated formula specific to the Chinese population was employed to estimate the skeletal muscle mass in this investigation. Although this formula has previously demonstrated excellent consistency with DXA, it should not be considered a replacement for DXA or bioelectrical impedance for body composition measurement. Second, the follow-up period was restricted to 4 years due to the database containing only physical examination information from 2011 to 2015. To further reinforce the stability of the conclusions, extending the follow-up period is particularly necessary. Finally, although our study adjusted for numerous confounding factors, there remain unmeasured confounders, such as physical activity, nutritional status, and dietary intake, which may have affected the outcome.
5 Conclusion
This study provides compelling evidence that, among middle-aged and older Chinese individuals, the concentration of IR surrogates is positively correlated with the incidence of SO. It is notable that reducing these indicators, especially when they fall below the inflection points, might lower the risk of SO in clinical settings. In order to predict SO, TyG-WHtR might be the best indicator to use. Further study is necessary to confirm these findings spanning diverse ethnic communities and employing intervention strategies.
Data availability statement
The datasets analyzed for this study can be found in the China Health and Retirement Longitudinal Study, https://charls.pku.edu.cn/.
Ethics statement
The studies involving humans were approved by the Ethical Review Committee of Peking University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CX: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. LH: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. YT: Methodology, Software, Writing – original draft. CG: Formal analysis, Writing – original draft. HL: Methodology, Software, Writing – original draft. CaL: Methodology, Software, Writing – original draft. ChL: Methodology, Writing – review & editing. HT: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the internally funded project of the Second Affiliated Hospital of Nanchang University (Number 2023efyB01).
Acknowledgments
We sincerely thank the China Health and Retirement Longitudinal Study (CHARLS) team for providing public data for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1472456/full#supplementary-material
Footnotes
References
1. Chuan, F, Chen, S, Ye, X, Kang, S, Mei, M, Tian, W, et al. Sarcopenic obesity predicts negative health outcomes among older patients with type 2 diabetes: the ageing and body composition of diabetes (ABCD) cohort study. Clin Nutr. (2022) 41:2740–8. doi: 10.1016/j.clnu.2022.10.023
2. Jurdana, M, and Žiberna, L. Sarcopenic obesity and hypertension in elderly patients: a narrative review of pathophysiology and management strategies. Ann Ist Super Sanita. (2023) 59:231–9. doi: 10.4415/ann_23_03_10
3. Poggiogalle, E, Lubrano, C, Sergi, G, Coin, A, Gnessi, L, Mariani, S, et al. Sarcopenic obesity and metabolic syndrome in adult Caucasian subjects. J Nutr Health Aging. (2016) 20:958–63. doi: 10.1007/s12603-015-0638-1
4. Hirani, V, Naganathan, V, Blyth, F, Le Couteur, DG, Seibel, MJ, Waite, LM, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. (2017) 46:413–20. doi: 10.1093/ageing/afw214
5. Donini, LM, Busetto, L, Bauer, JM, Bischoff, S, Boirie, Y, Cederholm, T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. (2020) 39:2368–88. doi: 10.1016/j.clnu.2019.11.024
6. Batsis, JA, and Villareal, DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. (2018) 14:513–37. doi: 10.1038/s41574-018-0062-9
7. Park, MJ, and Choi, KM. Interplay of skeletal muscle and adipose tissue: sarcopenic obesity. Metabolism. (2023) 144:155577. doi: 10.1016/j.metabol.2023.155577
8. Levine, ME, and Crimmins, EM. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring). (2012) 20:2101–6. doi: 10.1038/oby.2012.20
9. Kim, TN, Park, MS, Lim, KI, Choi, HY, Yang, SJ, Yoo, HJ, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean Sarcopenic Obesity Study. Clin Endocrinol. (2013) 78:525–32. doi: 10.1111/j.1365-2265.2012.04433.x
10. Kwon, SS, Lee, SG, Lee, YH, Lim, JB, and Kim, JH. Homeostasis model assessment of insulin resistance in a general adult population in Korea: additive association of sarcopenia and obesity with insulin resistance. Clin Endocrinol. (2017) 86:44–51. doi: 10.1111/cen.13233
11. Han, T, Yuan, T, Liang, X, Chen, N, Song, J, Zhao, X, et al. Sarcopenic obesity with normal body size may have higher insulin resistance in elderly patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. (2022) 15:1197–206. doi: 10.2147/dmso.S360942
12. Salvatori, B, Linder, T, Eppel, D, Morettini, M, Burattini, L, Göbl, C, et al. TyGIS: improved triglyceride-glucose index for the assessment of insulin sensitivity during pregnancy. Cardiovasc Diabetol. (2022) 21:215. doi: 10.1186/s12933-022-01649-8
13. Wu, Z, Zhou, D, Liu, Y, Li, Z, Wang, J, Han, Z, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. (2021) 20:134. doi: 10.1186/s12933-021-01330-6
14. Dang, K, Wang, X, Hu, J, Zhang, Y, Cheng, L, Qi, X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc Diabetol. (2024) 23:8. doi: 10.1186/s12933-023-02115-9
15. Bello-Chavolla, OY, Almeda-Valdes, P, Gomez-Velasco, D, Viveros-Ruiz, T, Cruz-Bautista, I, Romo-Romo, A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. (2018) 178:533–44. doi: 10.1530/eje-17-0883
16. Chen, J, Li, YT, Niu, Z, He, Z, Xie, YJ, Hernandez, J, et al. Association of visceral obesity indices with incident diabetic retinopathy in patients with diabetes: prospective cohort study. JMIR Public Health Surveill. (2024) 10:e48120. doi: 10.2196/48120
17. Kim, B, Kim, G, Lee, Y, Taniguchi, K, Isobe, T, and Oh, S. Triglyceride-glucose index as a potential indicator of sarcopenic obesity in older people. Nutrients. (2023) 15:15. doi: 10.3390/nu15030555
18. Zhao, Y, Hu, Y, Smith, JP, Strauss, J, and Yang, G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. (2014) 43:61–8. doi: 10.1093/ije/dys203
19. Barazzoni, R, Bischoff, SC, Boirie, Y, Busetto, L, Cederholm, T, Dicker, D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. (2018) 37:1787–93. doi: 10.1016/j.clnu.2018.04.018
20. Lee, JH, Park, HM, and Lee, YJ. Using dietary macronutrient patterns to predict sarcopenic obesity in older adults: a representative Korean Nationwide Population-based Study. Nutrients. (2021) 13:13. doi: 10.3390/nu13114031
21. Kim, B, Tsujimoto, T, So, R, and Tanaka, K. Changes in lower extremity muscle mass and muscle strength after weight loss in obese men: a prospective study. Obes Res Clin Pract. (2015) 9:365–73. doi: 10.1016/j.orcp.2014.12.002
22. Studenski, SA, Peters, KW, Alley, DE, Cawthon, PM, McLean, RR, Harris, TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. (2014) 69:547–58. doi: 10.1093/gerona/glu010
23. Wen, X, Wang, M, Jiang, CM, and Zhang, YM. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr. (2011) 20:551–6.
24. Yang, M, Hu, X, Wang, H, Zhang, L, Hao, Q, and Dong, B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. (2017) 8:251–8. doi: 10.1002/jcsm.12163
25. Batsis, JA, Mackenzie, TA, Jones, JD, Lopez-Jimenez, F, and Bartels, SJ. Sarcopenia, sarcopenic obesity and inflammation: results from the 1999-2004 National Health and Nutrition Examination Survey. Clin Nutr. (2016) 35:1472–83. doi: 10.1016/j.clnu.2016.03.028
26. Baek, SJ, Nam, GE, Han, KD, Choi, SW, Jung, SW, Bok, AR, et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008-2010 Korea National Health and Nutrition Examination Survey. J Endocrinol Investig. (2014) 37:247–60. doi: 10.1007/s40618-013-0011-3
27. Yin, T, Zhang, JX, Wang, FX, Zhao, JH, Zhao, Y, Liu, L, et al. The association between sarcopenic obesity and hypertension, diabetes, and abnormal lipid metabolism in Chinese adults. Diabetes Metab Syndr Obes. (2021) 14:1963–73. doi: 10.2147/dmso.S308387
28. Jiang, M, Ren, X, Han, L, and Zheng, X. Associations between sarcopenic obesity and risk of cardiovascular disease: a population-based cohort study among middle-aged and older adults using the CHARLS. Clin Nutr. (2024) 43:796–802. doi: 10.1016/j.clnu.2024.02.002
29. Vasques, AC, Novaes, FS, de Oliveira, MS, Souza, JR, Yamanaka, A, Pareja, JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. (2011) 93:e98–e100. doi: 10.1016/j.diabres.2011.05.030
30. Guerrero-Romero, F, Simental-Mendía, LE, González-Ortiz, M, Martínez-Abundis, E, Ramos-Zavala, MG, Hernández-González, SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
31. Lim, J, Kim, J, Koo, SH, and Kwon, GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007-2010 Korean National Health and nutrition examination survey. PLoS One. (2019) 14:e0212963. doi: 10.1371/journal.pone.0212963
32. Abbasi, F, and Reaven, GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. (2011) 60:1673–6. doi: 10.1016/j.metabol.2011.04.006
33. Han, M, Qin, P, Li, Q, Qie, R, Liu, L, Zhao, Y, et al. Chinese visceral adiposity index: a reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev. (2021) 37:e3370. doi: 10.1002/dmrr.3370
34. Xuan, W, Liu, D, Zhong, J, Luo, H, and Zhang, X. Impacts of triglyceride glucose-waist to height ratio on diabetes incidence: a secondary analysis of a population-based longitudinal data. Front Endocrinol (Lausanne). (2022) 13:949831. doi: 10.3389/fendo.2022.949831
35. Miao, H, Zhou, Z, Yang, S, and Zhang, Y. The association of triglyceride-glucose index and related parameters with hypertension and cardiovascular risk: a cross-sectional study. Hypertens Res. (2024) 47:877–86. doi: 10.1038/s41440-023-01502-9
36. Ashwell, M, Gunn, P, and Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. (2012) 13:275–86. doi: 10.1111/j.1467-789X.2011.00952.x
37. Lu, L, He, X, Song, Y, Zhuang, M, Wu, X, and Chen, N. Prevalence and risk factors of sarcopenia without obesity and sarcopenic obesity among Chinese community older people in suburban area of Shanghai: a cross-sectional study. Front Aging Neurosci. (2022) 14:1034542. doi: 10.3389/fnagi.2022.1034542
38. Park, SW, Goodpaster, BH, Lee, JS, Kuller, LH, Boudreau, R, de Rekeneire, N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. (2009) 32:1993–7. doi: 10.2337/dc09-0264
39. Lee, CG, Boyko, EJ, Barrett-Connor, E, Miljkovic, I, Hoffman, AR, Everson-Rose, SA, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. (2011) 34:2381–6. doi: 10.2337/dc11-1032
40. Lu, L, and Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: the role and mechanisms of estrogen. J Endocrinol. (2023):259. doi: 10.1530/joe-23-0116
Keywords: sarcopenic obesity, China Health and Retirement Longitudinal Study, surrogate insulin resistance indices, TyG-waist-to-height ratio, Chinese middle-aged and older adults
Citation: Xu C, He L, Tu Y, Guo C, Lai H, Liao C, Lin C and Tu H (2024) Longitudinal analysis of insulin resistance and sarcopenic obesity in Chinese middle-aged and older adults: evidence from CHARLS. Front. Public Health. 12:1472456. doi: 10.3389/fpubh.2024.1472456
Edited by:
Giuseppe Caminiti, Università telematica San Raffaele, ItalyReviewed by:
Marcelo Coertjens, Federal University of Piauí, BrazilGiuseppe Marazzi, IRCCS San Raffaele, Italy
Copyright © 2024 Xu, He, Tu, Guo, Lai, Liao, Lin and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaijun Tu, bmRlZnkxMDA2MUBuY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Chunyan Xu
Chunyan Xu Ling He1,2†
Ling He1,2† Huaijun Tu
Huaijun Tu