- 1School of Public Health, Sun Yat-sen University, Guangzhou, China
- 2Department of Clinical Research, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, China
- 4State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-sen University Cancer Center, Guangzhou, China
- 5Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 6Department of Psychology, Faculty of Social Sciences, University of Macau, Taipa, Macao SAR, China
- 7Centre for Cognitive and Brain Sciences, Institute of Collaborative Innovation, University of Macau, Taipa, Macao SAR, China
- 8Clinical Research Design Division, Clinical Research Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 9Department of Family Medicine, Shenzhen University Medical School, Shenzhen University, Shenzhen, China
Background: Despite advancements in cancer treatment, understanding the long-term mental health implications for nasopharyngeal carcinoma (NPC) survivors remains an underexplored area. This study aims to examine the prevalence of mental disorders and their correlations with age at diagnosis and time since diagnosis among NPC survivors.
Methods: A total of 1872 NPC patients were surveyed from September 2020 to June 2021 in this cross-sectional survey. Logistic regression models were used to analyze the associations of age at diagnosis and time since NPC diagnosis with the risk of mental disorders. Additionally, the potential nonlinear trend between these factors was examined using restricted cubic splines. Analyses were conducted both overall and stratified by gender. Gender interaction was also examined.
Results: The prevalences of depression, anxiety, and sleep disorders were 32.4, 33.2, and 61.5%, respectively. Age at NPC diagnosis was significantly associated with an elevated risk of depression (adjusted OR (aOR): 1.75 for 30–39 years old; 2.33 for 50–59 years old; 2.59 for ≥60 years old) and sleep disorders (aOR: 2.41 for 40–49 years old; 1.95 for 50–59 years old; 2.26, for ≥60 years old), compared to patients diagnosed with NPC at age < 30 years. Conversely, the risk of depression, anxiety, and sleep disorders exhibited negative associations with the time since diagnosis, compared to patients <3 months. Notably, significant nonlinear associations were observed between time since diagnosis and the risk of depression, anxiety, and sleep disorders, which showed an initial increase, with the highest risk occurring at approximately 3.0 (ORmax: 2.7), 1.5 (ORmax: 2.1), and 4.0 (ORmax: 1.9) months since NPC diagnosis, followed by a gradual recovery to a lower risk level at around 12 months. No gender interactions were observed.
Conclusion: The prevalence of mental disorders is notable among NPC survivors, showing a positive correlation with age at diagnosis while displaying a negative correlation with time since diagnosis, thus indicating the need for psychological support, especially within the initial several months following NPC diagnosis.
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial cancer originating from the nasopharynx epithelium (1). Epidemiological assessments reported approximately 133,000 newly diagnosed cases of NPC in 2020, with more than 85.2% occurring in Asia, notably in Southern China in regions such as Guangdong and Guangxi provinces (2–6). Over the past two decades, advancements in early detection and treatment modalities have significantly enhanced the prognosis for NPC patients, with the five-year overall survival rate increasing to 94.6% for early-stage NPC patients while ranging from 78.3 to 88.9% for those in stages III-IV (7, 8), thus leading to a considerable population of patients transitioning into long-term survivorship.
NPC patients have been found to encounter various physical and mental health challenges at diagnosis, during treatment, and in the post-treatment period. For instance, the rates of depression, anxiety, and sleep disorders have been reported to significantly increase before, during, and after radiotherapy (9, 10). In addition, after radiotherapy, there could be a significant increase in the prevalence of anxiety, depression, and poor sleep quality compared to pre-radiotherapy levels (19.6% vs. 3.9%; 39.2% vs. 3.9%; 64.7% vs. 37.3%, respectively) (11). These mental problems contribute to emotional instability and maladaptive coping behaviors such as hopelessness and anxious preoccupation, ultimately diminishing health-related quality of life, and therapeutic adherence (12). Moreover, psychological distress can adversely reduce the survival prognosis of NPC patients, as it may compromise the effectiveness of treatment and increase the likelihood of disease progression (13, 14). Severe mental disorders may also increase the risk of suicidal ideation and behaviors (15, 16), thereby increasing healthcare costs due to elevated utilization of mental and primary healthcare services among NPC patients (17, 18).
As the number of long-term survivors among NPC patients grows, it becomes imperative to pinpoint when mental disorders pose the most significant risk to allow for timely and targeted psychological interventions. Psychological support and intervention could be highly beneficial for the mental health of cancer patients, helping to reduce their fear of cancer recurrence, improve mood, enhance pain perception and improve the emotional function aspect of health-related quality of life (19–21). However, the trajectory of mental disorders with both age at diagnosis and time since diagnosis remains unclear, hindering the development of tailored interventions.
Previous studies on NPC have predominantly focused on psychological issues at the time of diagnosis and during treatment phases (9, 10). These studies provide essential insights into the immediate psychological impact of NPC diagnosis and treatment; however, there is a notable gap in understanding the relationship between psychological distress and the time since NPC diagnosis. Limited research has explored how psychological well-being evolves, and distinct stages post-diagnosis may present unique psychological challenges and support needs. Addressing this gap is crucial for developing effective, long-term mental health interventions tailored to the evolving needs of NPC patients.
To bridge this knowledge gap, this study aims to investigate the prevalence of mental disorders (specifically depression, anxiety, and sleep disorders) and their associations with age at diagnosis and time since diagnosis among NPC survivors in South China, which is one of the most representative endemic regions for NPC worldwide. Findings will provide essential insights into identifying high-risk individuals and determining the optimal timing for interventions.
Methods
Study design and participants
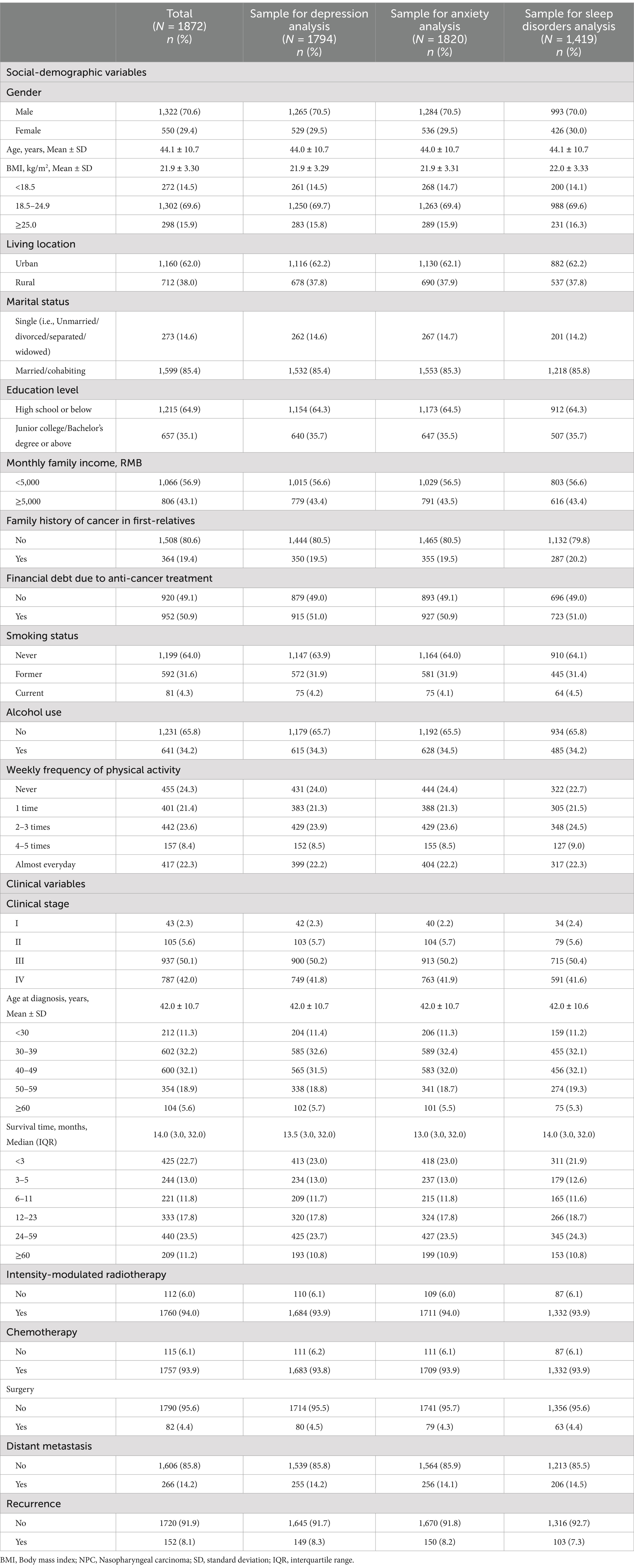
This cross-sectional survey was conducted at our Cancer Center in southern China, which is one of the most representative NPC endemic areas of the world. Eligible patients were aged 18 years or older, pathologically confirmed with NPC, without cognitive impairment, and capable of self-administering questionnaires. Upon obtaining informed consent, eligible patients completed a structured questionnaire with the assistance of field workers. Patients were assured that their data would only be accessed by the researchers. A total of 2,268 patients were surveyed between September 2020 and June 2021, with 1872 of them completing the questionnaires and included in this study. We did not handle missing data and conducted analyses using available data directly. Consequently, there were differences in sample sizes for each mental disorder.
Measurements
Depression and anxiety
Depression and anxiety were assessed using the Chinese version of the Hospital Anxiety and Depression Scale (HADS) (22), which comprises two 7-item subscales dedicated to measuring anxiety and depression, respectively. Each item is scored on a four-point Likert scale ranging from 0 to 3, resulting in a total score ranging from 0 to 21 for each subscale, whereby a higher score indicates a greater severity of anxiety or depression. In this study, Cronbach’s α coefficients were 0.81 for the anxiety subscale and 0.75 for the depression subscale, indicating satisfactory reliability. Depression and anxiety cases were defined as individuals with a subscale score of 8 or higher (22).
Sleep disorders
The Pittsburgh Sleep Quality Index (PSQI) was utilized to assess sleep disorders experienced over the past month (23), and its Chinese version has shown favorable overall psychometric properties (24). Comprising 18 items, the PSQI evaluates patients’ experiences across seven components: sleep latency, sleep duration, habitual sleep efficiency, subjective sleep quality, use of sleep medication, sleep disturbances, and daytime dysfunction. Each component is rated on a scale from 0 (no difficulty) to 3 (severe difficulty). A global score ranging from 0 to 21 is derived by summing the scores of the seven components, with higher scores indicating poorer sleep quality. Sleep disorders are defined as individuals with PSQI global scores exceeding five (23, 24). In this study, the Cronbach’s α coefficients for the seven components were 0.76, indicating satisfactory internal consistency.
Demographic and clinical covariates
The sociodemographic characteristics collected through the questionnaire surveys included gender, age at pathological diagnosis, body weight, height, living location, marital status, education level, monthly family income, family history of cancers among first-degree relatives, smoking status, alcohol use, financial debt resulting from anti-cancer treatment, and weekly physical activity level in the past year. Body mass index (BMI) was computed as weight (in kilogram [kg]) divided by the square of height (in meter [m]), then categorized into three groups according to World Health Organization guidelines: underweight (BMI <18.5 kg/m2), normal weight (BMI ranging from 18.5 to 24.9 kg/m2), and overweight or obesity (BMI ≥25.0 kg/m2). Age at diagnosis, clinical stage, treatment modalities, recurrence, and metastatic status variables were extracted from the patient’s medical records. Time since NPC diagnosis was calculated as the interval in months from the date of NPC pathological diagnosis to the date of survey completion.
Statistical methods
Continuous variables are presented as means with standard deviations (SD) or as medians with interquartile ranges (IQR) when appropriate. Categorical variables are shown as frequencies with proportions. The prevalences of depression, anxiety, sleep disorders, and their comorbidity were estimated and then further stratified by gender, along with their corresponding 95% confidence intervals (CIs).
Binary logistic regression models were applied to investigate the associations between age at diagnosis and time since NPC diagnosis with depression, anxiety, and sleep disorders. Adjustments were made for various factors, including gender, BMI, living location, marital status, educational level, monthly family income, family history of cancer in first-degree relatives, smoking status, alcohol use, financial debt due to anti-cancer treatment, physical activity level, clinical stage, treatment modalities, distant metastasis status, and recurrence status. Both age at diagnosis and time since diagnosis were considered as both continuous and categorical variables. Odds ratios (ORs) with corresponding 95% CIs were calculated. Gender interaction was also examined. Nonlinear trends of mental disorders with age at diagnosis and time since diagnosis were evaluated using restricted cubic spline regression models (25), with the median value as the reference. Analyses were conducted both overall and stratified by gender. No missing data handling method.
All statistical analyses were performed using the R software (version 4.3.3), and a two-sided p < 0.05 was considered statistically significant.
Ethics
This study was approved by the institutional review board of our Cancer Center (IRB NO: B2020-203), and written informed consent was obtained from all participants. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit platform1 with the approval RDD number RDDA2024544536.
Results
Patients’ characteristics
The mean age of the patients was 44.1 years (SD: 10.7; range: 18.0–80.0). We observed that 70.6% were male, and 69.6% had normal weight. A family history of cancer in first-degree relatives was reported by 19.4% of patients, and 50.9% faced financial debt due to anti-cancer treatment. Furthermore, 35.9% had a history of smoking, 34.2% had a history of alcohol use, and 22.3% reported no physical activity in the past year.
Among the 1872 patients, 92.1% were diagnosed in clinical stages III-IV, and 64.3% were diagnosed with NPC between the ages of 30 and 49. Only 11.2% of patients survived more than 5 years. The median time since NPC diagnosis was 14.0 months (IQR: 3.0, 32.0). In addition, approximately 94.0% received intensity-modulated radiotherapy, 93.9% underwent chemotherapy, and 4.4% underwent surgery (Table 1). The associations between sociodemographic and clinical variables with the three mental disorders are shown in Supplementary Table 1.
Prevalence of mental disorders and comorbidity
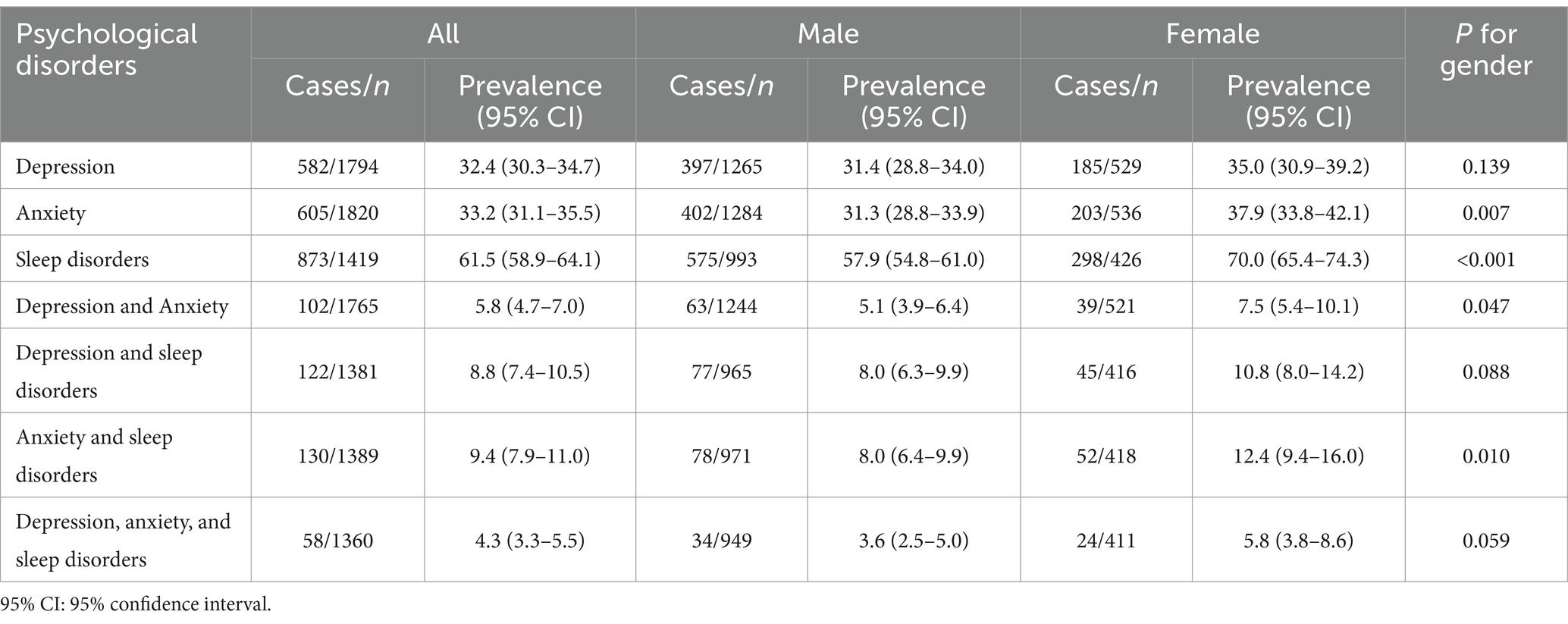
The overall prevalences of depression, anxiety, and sleep disorders were 32.4% (95% CI: 30.3–34.7%), 33.2% (95% CI: 31.1–35.5%), and 61.5% (95% CI: 58.9–64.1%), respectively. The prevalences of anxiety (37.9% vs. 31.3%, p = 0.007) and sleep disorders (70.0% vs. 57.9%, p < 0.001) were significantly higher in females compared to males. The comorbidity prevalences involving depression and anxiety, depression and sleep disorders, as well as anxiety and sleep disorders, were 5.8% (95% CI: 4.7–7.0%), 8.8% (95% CI: 7.4–10.5%), and 9.4% (95% CI: 7.9–11.0%), respectively. The comorbidity prevalence involving all three mental disorders was 4.3% (95% CI: 3.3–5.5%) (Table 2).
The prevalence trends of depression, anxiety, and sleep disorders with age at NPC diagnosis and time since NPC diagnosis are shown in Supplementary Figures 1, 2, and the scores of depression, anxiety, and sleep disorders, along with seven components stratified by gender, are shown in Supplementary Table 2. Overall, it can be observed that female patients reported significantly higher scores compared to male patients.
Associations between age at diagnosis and mental disorders
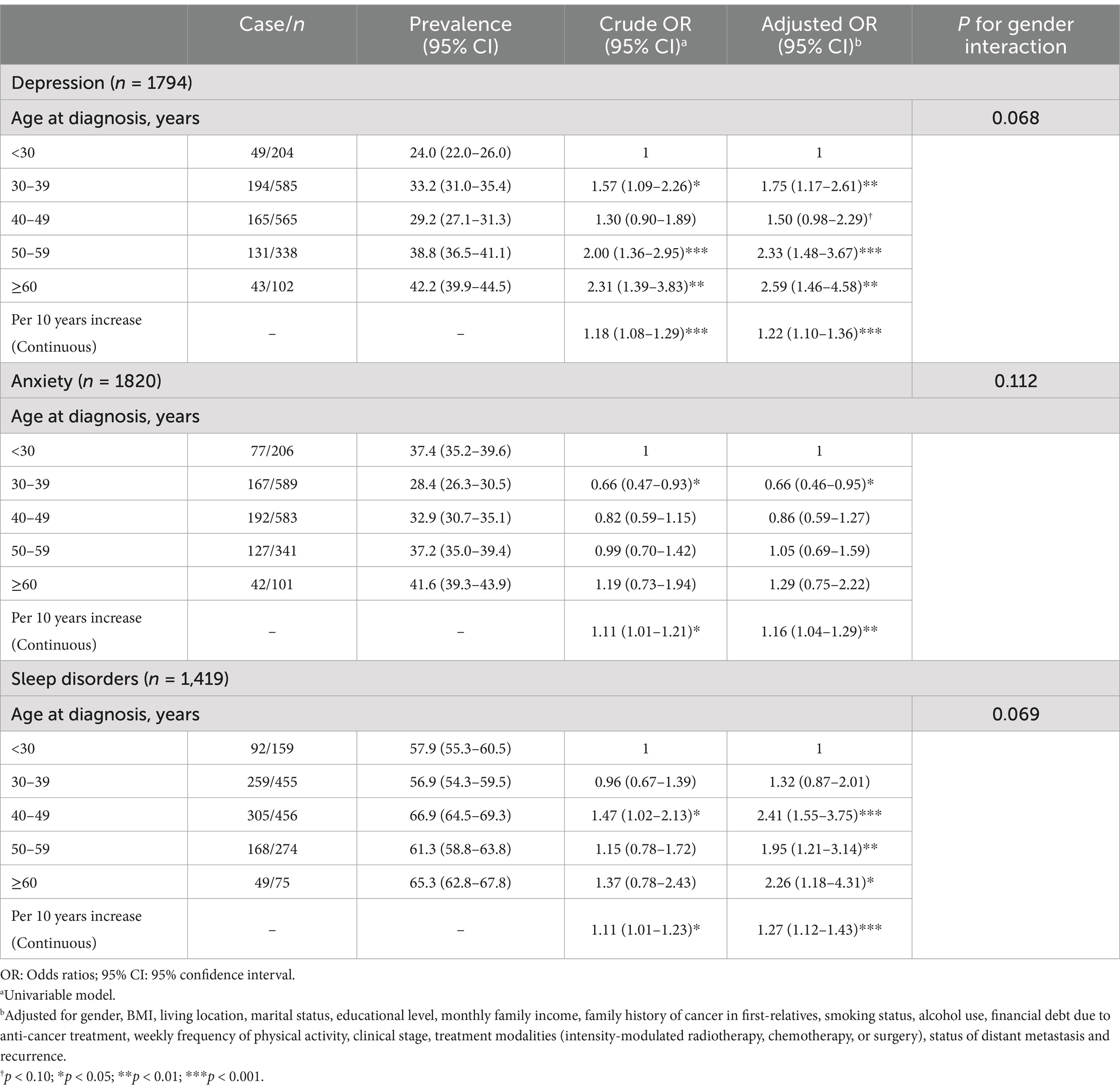
The risk of depression and sleep disorders was significantly associated with age at diagnosis. Compared to patients diagnosed with NPC at age < 30 years, the adjusted ORs (aORs) for depression were 1.75 (95%CI: 1.17–2.61; p = 0.007) in the 30–39 years group, 2.33 (95% CI: 1.48–3.67; p < 0.001) in the 50–59 years group, and 2.59 (95% CI: 1.46–4.58; p = 0.001) in the ≥60 years group. The aORs for sleep disorders were 2.41 (95% CI: 1.55–3.75; p < 0.001) in the 40–49 years group, 1.95 (95% CI: 1.21–3.14; p = 0.006) in the 50–59 years group, and 2.26 (95% CI: 1.18–4.31; p = 0.013) in ≥60 years group. Moreover, for every 10-year increase in age at diagnosis, the aORs were 1.22 (95% CI: 1.10–1.36; p < 0.001) for depression, 1.16 (95% CI: 1.04–1.29; p = 0.008) for anxiety, and 1.27 (95% CI: 1.12–1.43; p < 0.001) for sleep disorders. No significant interaction was observed with gender (Table 3). The results stratified by gender are presented in Supplementary Table 3.
Overall, a nonlinear trend between age at diagnosis and the risk of anxiety was observed (Pnonlinear = 0.032), and the other nonlinear trends were statistically non-significant overall and by gender (all Pnonlinear > 0.05; Figure 1).
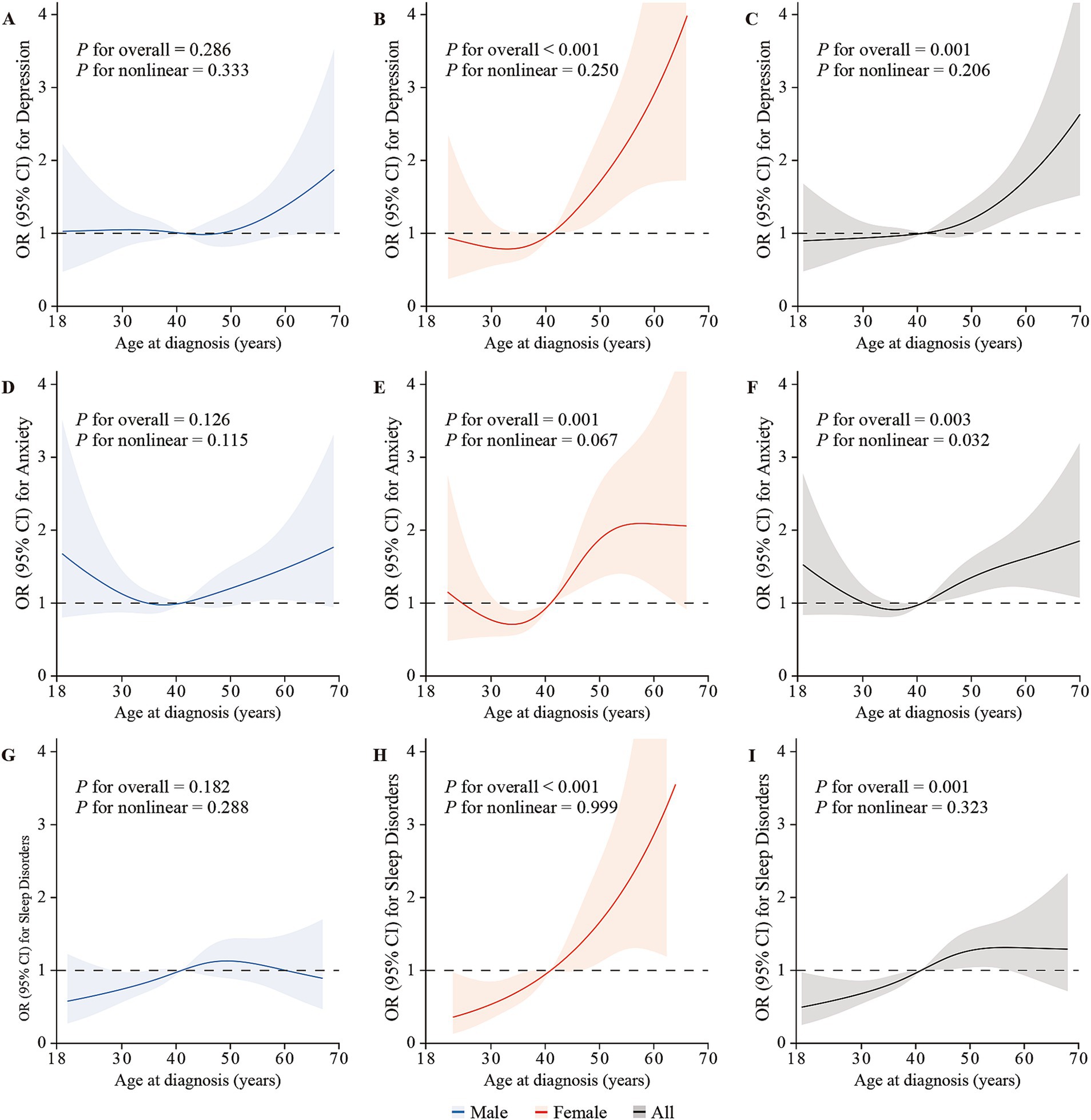
Figure 1. The restricted cubic splines depicting the associations between age at diagnosis and the risk of depression, anxiety, and sleep disorders via logistic regression in males (A,D,G), females (B,E,H), and overall (C,F,I), adjusted for gender (only for overall analysis), age, BMI, living location, marital status, educational level, monthly family income, family history of cancer in first-relatives, financial debt due to anti-cancer treatment, smoking status, alcohol use, weekly frequency of physical activity, clinical stage, intensity-modulated radiotherapy, chemotherapy, surgery, distant metastasis, and recurrence. The lines represent the adjusted odds ratios with shaded bands indicating 95% confidence intervals. The median age at diagnosis (44 years) is set as the reference value. Knots were positioned at the 5th, 35th, 65th, and 95th percentiles of age at NPC diagnosis.
Association between time since NPC diagnosis and mental disorders
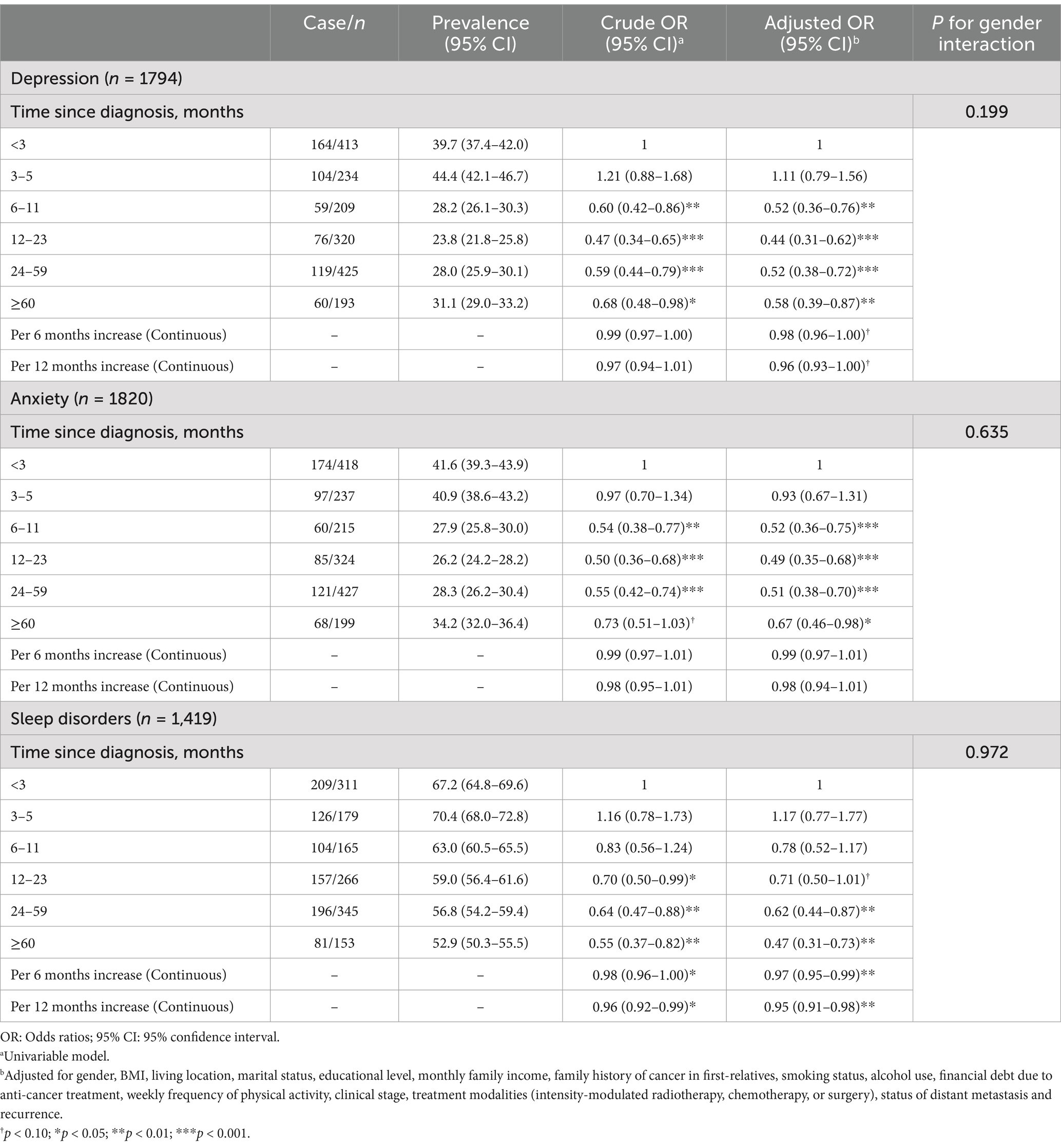
Significantly negative associations were observed between time since NPC diagnosis and depression, anxiety, and sleep disorders. The aORs for depression were 0.52 (95% CI: 0.36–0.76; p = 0.001) for 6–11 months, 0.44 (95% CI: 0.31–0.62; p < 0.001) for 12–23 months, 0.52 (95% CI: 0.38–0.72; p < 0.001) for 24–59 months, and 0.58 (95% CI: 0.39–0.87; p = 0.008) for ≥60 months. The aORs for anxiety were 0.52 (95% CI: 0.36–0.75; p < 0.001) for 6–11 months, 0.49 (95% CI: 0.35–0.68; p < 0.001) for 12–23 months, 0.51 (95% CI: 0.38–0.70; p < 0.001) for 24–59 months, and 0.67 (95% CI: 0.46–0.98; p = 0.039) for ≥60 months. Regarding sleep disorders, the aORs were 0.62 (95% CI: 0.44–0.87; p = 0.006) for 24–59 months, and 0.47 (95% CI: 0.31–0.73; p = 0.001) for ≥60 months. Additionally, the aOR for every 12-month increase since diagnosis was 0.95 (95% CI: 0.91–0.98; p = 0.005) for sleep disorders. No interaction with gender was observed (Table 4). Similar associations were observed by gender (Supplementary Table 4).
A significant nonlinear trend of time since diagnosis with the risk of depression, anxiety, and sleep disorders was observed (all Pnonlinear < 0.05; Figure 2). The highest odds of depression, anxiety, and sleep disorders were observed at around 3.0 months (ORmax: 2.7), 1.5 months (ORmax: 2.1) and 4.0 (ORmax: 1.9) months since NPC diagnosis, respectively, and then gradually recovered to a low-risk level at around 12 months.
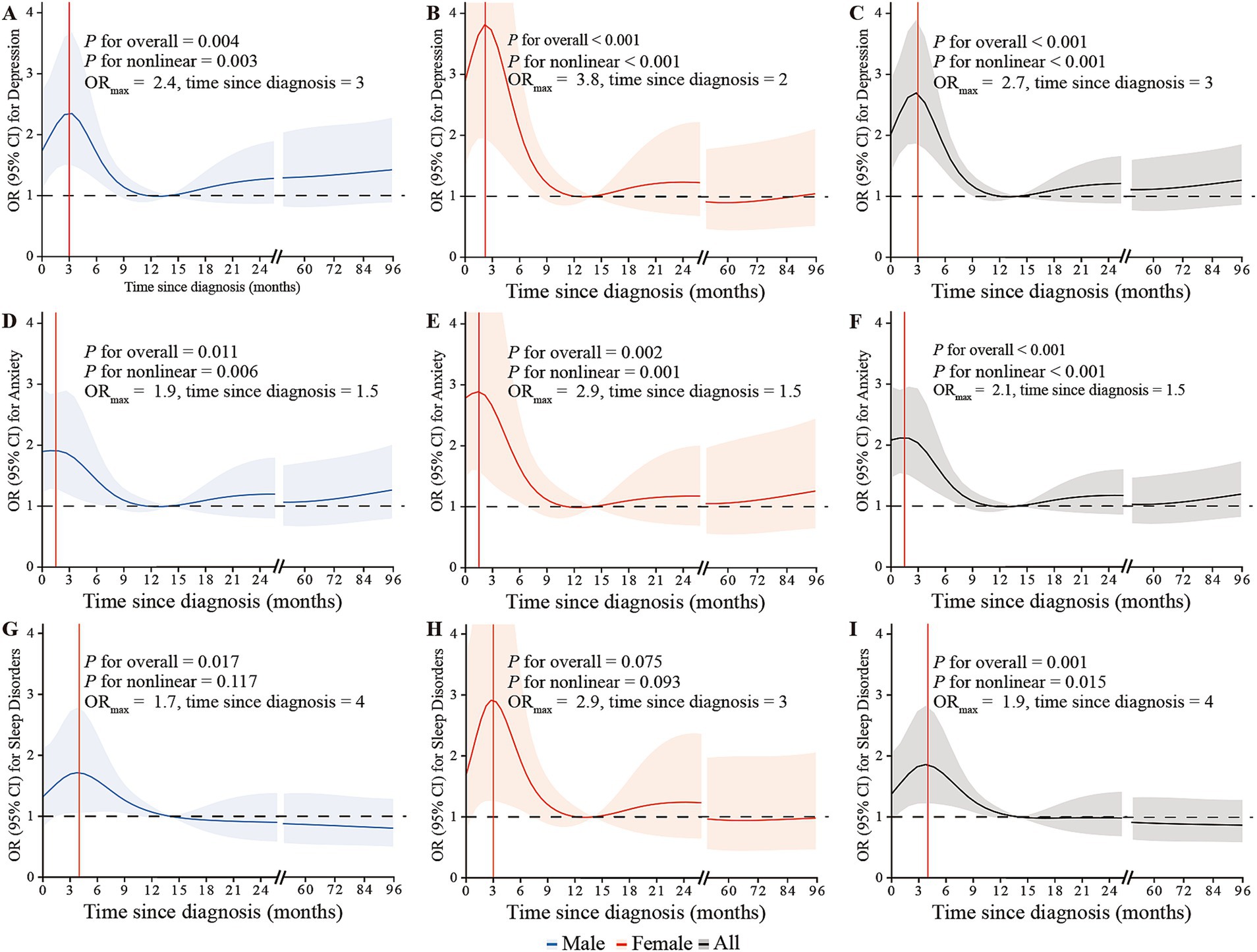
Figure 2. The restricted cubic splines depicting the associations between time since NPC diagnosis and the risk of depression, anxiety, and sleep disorders through logistic regression in males (A,D,G), females (B,E,H), and overall (C,F,I), adjusted for gender, age, BMI, living location, marital status, educational level, monthly family income, family history of cancer in first-relatives, financial debt due to anti-cancer treatment, smoking status, alcohol use, weekly frequency of physical activity, clinical stage, intensity-modulated radiotherapy, chemotherapy, surgery, distant metastasis, and recurrence. The lines represent the adjusted odds ratios with shaded bands indicating 95% confidence intervals. The reference value is set at the median time since diagnosis (14 months). Knots are positioned at the 5th, 23rd, 41st, 59th, 77th, and 95th percentiles of time since NPC diagnosis.
Discussion
This study represents the first comprehensive estimation of mental disorder prevalence among NPC patients in South China, one of the most significant NPC endemic regions globally, and also examines the associations between age at diagnosis and time since NPC diagnosis with depression, anxiety and sleep disorders. In this extensive cross-sectional study based on a large dataset, we discovered significantly high prevalences of depression, anxiety and sleep disorders among NPC patients, with common occurrences of comorbid mental disorders. The risk of these mental disorders showed a linear increase with age at diagnosis, except for anxiety. Additionally, a significant nonlinear trend was observed in the relationship between time since diagnosis and the risk of mental disorders. Specifically, the highest risk occurred within the first 4 months (ranging from 1.5 to 4.0 months) since NPC diagnosis, followed by a gradual decline to a general level around 12 months. Collectively, this study highlights the importance of raising awareness among healthcare professionals, family caregivers, and society as a whole regarding mental health issues in NPC survivors.
Similar to previous studies (11, 26), our findings showed that females were at a higher risk of mental disorders than males in NPC patients. This might be because females tend to be in a weaker social position, showing more vulnerability and a lower ability to cope with adverse events such as a cancer diagnosis (27). Therefore, it is crucial to pay more attention to females’ mental health issues during the diagnosis and treatment period of NPC. Our study also reveals that high physical activity and socioeconomic status were associated with low mental disorders among NPC patients, which aligns with prior studies (28). Previous studies in other cancer populations also suggested that higher levels of physical activity can alleviate symptoms of anxiety and depression (29, 30). These findings highlight the significance of incorporating physical activity programs as a part of comprehensive approaches to improve mental health in NPC patients.
In addition, our findings reveal that NPC patients diagnosed at an older age were more susceptible to mental disorders. However, reports regarding the associations between age at diagnosis and the risk of mental disorders vary among different cancer patients. Some studies on various cancer types (i.e., lung cancer, gynecological cancer, breast cancer, and colorectal cancer) have indicated that depression is more prevalent among younger patients (26, 31). Conversely, in patients with testicular germ cell tumors, a younger age at diagnosis and a shorter time since diagnosis were significantly associated with a higher risk of anxiety (32). Studies encompassing multiple cancer types have reported an association between younger age and poor sleep quality, as measured by self-report scales and structured clinical interviews (33–35). However, this association differs from that observed in NPC patients. A previous study in NPC patients reported similar results to our findings, indicating a positive association between poor sleep quality and older age before treatment (36). This difference in the association between depression, anxiety, and age at NPC diagnosis compared to other cancer types may be attributed in part to the specificity of NPC, including its favorable prognosis and age-specific mortality trends. It has been documented that age-specific mortalities of NPC in China start to increase rapidly from the ages of 35–39 years as of 2013 (6). Moreover, a younger age has been identified as an independent predictor of successful return to work, which is associated with a lower level of psychosocial burden and financial toxicity (37). Thus, older NPC patients, particularly females, should receive increased clinical attention and timely psychological support following NPC diagnosis.
We found nonlinear associations between the risk of mental disorders and time since NPC diagnosis, characterized by a sharp increase in risk within the first 3–4 months, followed by a gradual decrease to a low and stable level around 12 months after NPC diagnosis. A comparable trend between mental disorders and time since NPC diagnosis was documented in a longitudinal study, which indicated that the impact of NPC and its treatment on anxiety and depressive symptoms peaked at around 3 months after diagnosis (38). After cancer diagnosis, patients may experience a feeling of anticipatory grief, which is a forewarning or expecting the impending death (39, 40). As mentioned earlier, NPC patients have a favorable prognosis, and along with an increased understanding of NPC, their anticipatory grief may gradually lessen. The observed trend between mental disorders and time since NPC diagnosis may be partially attributed to variations in treatment stages. In our study, among patients with less than 3 months since NPC diagnosis, 18.6% were at the pre-treatment stage, 77.9% were undergoing treatment, and only 3.5% had completed anti-cancer treatment. For patients with 3–5 months since NPC diagnosis, 60.2% were undergoing treatment, and 39.8% had completed anti-cancer treatment. In patients with more than 6 months since NPC diagnosis, approximately 85% had completed anti-cancer treatment. The psychological well-being of NPC patients is influenced by the treatment stage. A nationwide population-based study reported that anxiety was more likely to be reported at the beginning of radiotherapy, while the proportion of depression increased after the start of treatment and peaked at 1 month post-treatment initiation, declining thereafter in NPC patients (41). The results from a prospective study revealed a significant increase in the proportion of NPC patients experiencing distress during the treatment period (42). One possible explanation is that NPC patients commonly endure severe acute side effects during treatment, which may further heighten the risk of mental disorders (43, 44). Another contributing factor could be the amplified financial burden resulting from treatment toxicity (28). Similarly, patients’ sleep quality correlates with the treatment stage. The severity of apnea and hypopnea events, as well as snoring, typically diminish in the majority of NPC patients 6 months after treatment (45). These findings suggest the importance of monitoring the mental health of NPC patients within the first year post-diagnosis, particularly during the initial 4 months. It is crucial to consider this monitoring in conjunction with the timing of reexaminations after diagnosis and treatment. This period typically encompasses the initiation of treatment and the subsequent follow-up after the first treatment. Providing timely psychological support during this high-risk period is highly warranted. It is recommended that oncologists acquire fundamental psychological knowledge to effectively conduct psychosocial assessments, enabling them to screen for mental health risks in cancer patients and provide timely interventions during this period. Additionally, collaboration with other relevant healthcare disciplines is vital. Cancer centers with the capacity should establish a referral system for psychiatric services, either within their facilities or in collaboration with external organizations.
However, several limitations of this study should be acknowledged. Firstly, the cross-sectional design prevented the assessment of longitudinal trends in depression, anxiety, and sleep disorders along with age at diagnosis and time since diagnosis. Therefore, prospective cohort studies are warranted to address this limitation. Secondly, although our study had a large sample size, the generalizability and representativeness of our findings should be cautious given that the sample was exclusively drawn from one cancer center in South China. The studies involving multiple centers from different regions are highly warranted. Thirdly, we did not evaluate the influence of mental disorders present before NPC diagnosis on our findings due to data limitations. Fourthly, the assessment of mental health conditions (i.e., depression, anxiety, and sleep disorders) relied on epidemiological screening instruments rather than structured diagnostic interviews, which may introduce potential misclassification despite the confirmed psychometric properties of these instruments. Lastly, the study was conducted during the COVID-19 pandemic, and the results may have been influenced by the pandemic.
Conclusion
This study revealed a high level of mental disorders in NPC patients. The odds of these mental disorders increase linearly with age at diagnosis but display a nonlinear association with the time since diagnosis. The risk peaked around the first 3–4 months after NPC diagnosis, then gradually declined to a lower-risk level around 12 months. These findings are highly warranted for further confirm by large-scale longitudinal studies. The timely psychological support following the NPC diagnosis is of critical importance for improving the mental health of this population.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: the authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit platform (www.researchdata.org.cn) with the approval RDD number RDDA2024544536.
Ethics statement
The studies involving humans were approved by the ethics committee of Sun Yat-sen University Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
W-XW: Formal analysis, Writing – original draft, Writing – review & editing. Y-SW: Formal analysis, Writing – review & editing, Data curation. L-PQ: Data curation, Writing – review & editing. AW: Writing – review & editing. Y-YZ: Writing – review & editing. W-JG: Writing – review & editing. S-SG: Writing – review & editing, Data curation. Y-JH: Data curation, Writing – review & editing. D-HL: Data curation, Writing – review & editing. Q-YC: Data curation, Writing – review & editing. Y-QX: Data curation, Writing – review & editing. J-XZ: Writing – review & editing. H-QM: Writing – review & editing, Data curation. J-BL: Data curation, Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was partially supported by grants from the National Natural Science Foundation of China (No. 81803105), the Natural Science Foundation of Guangdong Province (No. 2018A030310238), the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (No. 22qntd4001), and China Medical Board USA (No. 22-484).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1469001/full#supplementary-material
Footnotes
References
1. Chen, YP, Chan, ATC, Le, QT, Blanchard, P, Sun, Y, and Ma, J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/s0140-6736(19)30956-0
2. WHOIARC. Global Cancer observatory (2020). Available at:https://gco.iarc.fr
3. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Torre, LA, Bray, F, Siegel, RL, Ferlay, J, Lortet-Tieulent, J, and Jemal, A. Global Cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
5. Tang, LL, Chen, WQ, Xue, WQ, He, YQ, Zheng, RS, Zeng, YX, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. (2016) 374:22–30. doi: 10.1016/j.canlet.2016.01.040
6. Wei, KR, Zheng, RS, Zhang, SW, Liang, ZH, Li, ZM, and Chen, WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. (2017) 36:90. doi: 10.1186/s40880-017-0257-9
7. Sun, XS, Liu, SL, Luo, MJ, Li, XY, Chen, QY, Guo, SS, et al. The association between the development of radiation therapy, image technology, and chemotherapy, and the survival of patients with nasopharyngeal carcinoma: a cohort study from 1990 to 2012. Int J Radiat Oncol Biol Phys. (2019) 105:581–90. doi: 10.1016/j.ijrobp.2019.06.2549
8. Wang, ZQ, Mei, Q, Li, JB, Rui, Y, You-Ping, L, Rui, S, et al. The long-term survival of patients with iii-Ivb stage nasopharyngeal carcinoma treated with Imrt with or without Nimotuzumab: a propensity score-matched analysis. BMC Cancer. (2019) 19:1122. doi: 10.1186/s12885-019-6156-5
9. Wang, C, Chen, J, Su, L, Hua, Y, Ye, J, Song, X, et al. The psychological status in patients with nasopharyngeal carcinoma during radiotherapy. Eur Arch Otorrinolaringol. (2022) 279:1035–42. doi: 10.1007/s00405-021-06892-5
10. McDowell, LJ, Rock, K, Xu, W, Chan, B, Waldron, J, Lu, L, et al. Long-term late toxicity, quality of life, and emotional distress in patients with nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. (2018) 102:340–52. doi: 10.1016/j.ijrobp.2018.05.060
11. Mo, YL, Li, L, Qin, L, Zhu, XD, Qu, S, Liang, X, et al. Cognitive function, mood, and sleep quality in patients treated with intensity-modulated radiation therapy for nasopharyngeal Cancer: a prospective study. Psychooncology. (2014) 23:1185–91. doi: 10.1002/pon.3542
12. Ghiggia, A, Castelli, L, Riva, G, Tesio, V, Provenzano, E, Ravera, M, et al. Psychological distress and coping in nasopharyngeal Cancer: an explorative study in Western Europe. Psychol Health Med. (2017) 22:449–61. doi: 10.1080/13548506.2016.1220600
13. Chen, J, Hua, Y, Su, L, Wang, C, Zhang, H, Ye, J, et al. The effect of psychological condition before radiotherapy on prognosis in 390 patients initially treated for nasopharyngeal carcinoma. Support Care Cancer. (2021) 29:5967–72. doi: 10.1007/s00520-021-06130-y
14. Jansen, F, Verdonck-de Leeuw, IM, Cuijpers, P, Leemans, CR, Waterboer, T, Pawlita, M, et al. Depressive symptoms in relation to overall survival in people with head and neck Cancer: a longitudinal cohort study. Psychooncology. (2018) 27:2245–56. doi: 10.1002/pon.4816
15. Heinrich, M, Hofmann, L, Baurecht, H, Kreuzer, PM, Knuttel, H, Leitzmann, MF, et al. Suicide risk and mortality among patients with Cancer. Nat Med. (2022) 28:852–9. doi: 10.1038/s41591-022-01745-y
16. Liu, RT, Steele, SJ, Hamilton, JL, Do, QBP, Furbish, K, Burke, TA, et al. Sleep and suicide: a systematic review and Meta-analysis of longitudinal studies. Clin Psychol Rev. (2020) 81:101895. doi: 10.1016/j.cpr.2020.101895
17. Van Beek, FE, Wijnhoven, LMA, Holtmaat, K, Custers, JAE, Prins, JB, Verdonck-de Leeuw, IM, et al. Psychological problems among Cancer patients in relation to healthcare and societal costs: a systematic review. Psychooncology. (2021) 30:1801–35. doi: 10.1002/pon.5753
18. van Beek, FE, Jansen, F, Baatenburg de Jong, RJ, Langendijk, JA, Leemans, CR, Smit, JH, et al. Psychological problems among head and neck Cancer patients in relation to utilization of healthcare and informal care and costs in the first two years after diagnosis. Curr Oncol. (2022) 29:3200–14. doi: 10.3390/curroncol29050260
19. Tauber, NM, O'Toole, MS, Dinkel, A, Galica, J, Humphris, G, Lebel, S, et al. Effect of psychological intervention on fear of Cancer recurrence: a systematic review and Meta-analysis. J Clin Oncol. (2019) 37:2899–915. doi: 10.1200/JCO.19.00572
20. Goodwin, PJ, Leszcz, M, Ennis, M, Koopmans, J, Vincent, L, Guther, H, et al. The effect of group psychosocial support on survival in metastatic breast Cancer. N Engl J Med. (2001) 345:1719–26. doi: 10.1056/NEJMoa011871
21. Caminiti, C, Annunziata, MA, Verusio, C, Pinto, C, Airoldi, M, Aragona, M, et al. Effectiveness of a psychosocial care quality improvement strategy to address quality of life in patients with Cancer: the Hucare2 stepped-wedge cluster randomized trial. JAMA Netw Open. (2021) 4:e2128667. doi: 10.1001/jamanetworkopen.2021.28667
22. Zigmond, AS, and Snaith, RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
23. Buysse, DJ, Reynolds, CF 3rd, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
24. Tsai, PS, Wang, SY, Wang, MY, Su, CT, Yang, TT, Huang, CJ, et al. Psychometric evaluation of the Chinese version of the Pittsburgh sleep quality index (Cpsqi) in primary insomnia and control subjects. Qual Life Res. (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
25. Frank, E, and Harrell, J. Regression Modeling Strategies. 2nd ed. Switzerland: Springer Cham (2015).
26. Linden, W, Vodermaier, A, Mackenzie, R, and Greig, D. Anxiety and depression after Cancer diagnosis: prevalence rates by Cancer type, gender, and age. J Affect Disord. (2012) 141:343–51. doi: 10.1016/j.jad.2012.03.025
27. Piccinelli, M, and Wilkinson, G. Gender Differences in Depression. Br J Psychiatry. (2018) 177:486–92. doi: 10.1192/bjp.177.6.486
28. Jiang, H, Mou, W, Lyu, J, Jiang, L, Liu, Y, Zeng, Y, et al. Assessment of self-reported financial toxicity among patients with nasopharyngeal carcinoma undergoing radiotherapy: a cross-sectional study in Western China. Front Oncol. (2022) 12:1011052. doi: 10.3389/fonc.2022.1011052
29. Carayol, M, Bernard, P, Boiche, J, Riou, F, Mercier, B, Cousson-Gelie, F, et al. Psychological effect of exercise in women with breast Cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol. (2013) 24:291–300. doi: 10.1093/annonc/mds342
30. Fong, DY, Ho, JW, Hui, BP, Lee, AM, Macfarlane, DJ, Leung, SS, et al. Physical activity for Cancer survivors: Meta-analysis of randomised controlled trials. BMJ. (2012) 344:e70. doi: 10.1136/bmj.e70
31. Walker, J, Hansen, CH, Martin, P, Symeonides, S, Ramessur, R, Murray, G, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with Cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. (2014) 1:343–50. doi: 10.1016/S2215-0366(14)70313-X
32. Vehling, S, Mehnert, A, Hartmann, M, Oing, C, Bokemeyer, C, and Oechsle, K. Anxiety and depression in long-term testicular germ cell tumor survivors. Gen Hosp Psychiatry. (2016) 38:21–5. doi: 10.1016/j.genhosppsych.2015.09.001
33. Davies, AN, Patel, SD, Gregory, A, and Lee, B. Observational study of sleep disturbances in advanced Cancer. BMJ Support Palliat Care. (2017) 7:435–40. doi: 10.1136/bmjspcare-2017-001363
34. Akechi, T, Okuyama, T, Akizuki, N, Shimizu, K, Inagaki, M, Fujimori, M, et al. Associated and predictive factors of sleep disturbance in advanced Cancer patients. Psycho-Oncology. (2007) 16:888–94. doi: 10.1002/pon.1122
35. Palesh, OG, Roscoe, JA, Mustian, KM, Roth, T, Savard, J, Ancoli-Israel, S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with Cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. (2010) 28:292–8. doi: 10.1200/JCO.2009.22.5011
36. Lai, XY, Tang, ZM, Zhu, XD, Li, L, Qin, XY, Lan, JL, et al. Sleep disturbance and related factors in patients with nasopharyngeal carcinoma and their family caregivers prior to the initiation of treatment. Sci Rep. (2018) 8:14263. doi: 10.1038/s41598-018-32587-9
37. So, N, McDowell, LJ, Lu, L, Xu, W, Rock, K, Waldron, J, et al. The prevalence and determinants of return to work in nasopharyngeal carcinoma survivors. Int J Radiat Oncol Biol Phys. (2020) 106:134–45. doi: 10.1016/j.ijrobp.2019.09.008
38. Schroevers, M, Ranchor, AV, and Sanderman, R. Adjustment to Cancer in the 8 years following diagnosis: a longitudinal study comparing Cancer survivors with healthy individuals. Soc Sci Med. (2006) 63:598–610. doi: 10.1016/j.socscimed.2006.02.008
39. Simon, JL . Anticipatory grief: recognition and coping. J Palliat Med. (2008) 11:1280–1. doi: 10.1089/jpm.2008.9824
40. Fulton, R . Anticipatory mourning: a critique of the concept. Mortality. (2003) 8:342–51. doi: 10.1080/13576270310001613392
41. Noh, OK, and Heo, J. Mental disorders in nasopharyngeal carcinoma patients receiving radiation therapy: a Nationwide population-based study. In Vivo. (2021) 35:2901–8. doi: 10.21873/invivo.12580
42. Deng, YT, Zhong, WN, and Jiang, Y. Measurement of distress and its alteration during treatment in patients with nasopharyngeal carcinoma. Head Neck. (2014) 36:1077–86. doi: 10.1002/hed.23412
43. Wu, YS, Lin, PY, Chien, CY, Fang, FM, Chiu, NM, Hung, CF, et al. Anxiety and depression in patients with head and neck Cancer: 6-month follow-up study. Neuropsychiatr Dis Treat. (2016) 12:1029–36. doi: 10.2147/NDT.S103203
44. Hong, JS, Tian, J, Han, QF, and Ni, QY. Quality of life of nasopharyngeal Cancer survivors in China. Curr Oncol. (2015) 22:e142–7. doi: 10.3747/co.22.2323
Keywords: nasopharyngeal carcinoma, depression, anxiety, sleep disorders, age at diagnosis, time since diagnosis
Citation: Wang W-X, Wu Y-S, Qi L-P, Wu AMS, Zhu Y-Y, Gong W-J, Guo S-S, Hua Y-J, Luo D-H, Chen Q-Y, Xiang Y-Q, Zhang J-X, Mai H-Q and Li J-B (2024) Prevalence of mental disorders and their associations with age at diagnosis and time since diagnosis of nasopharyngeal cancer. Front. Public Health. 12:1469001. doi: 10.3389/fpubh.2024.1469001
Edited by:
İsmail Toygar, Mugla University, TürkiyeReviewed by:
Emel Öztürk Turgut, Ege University, TürkiyeFerda Akyüz Özdemir, Mugla University, Türkiye
Copyright © 2024 Wang, Wu, Qi, Wu, Zhu, Gong, Guo, Hua, Luo, Chen, Xiang, Zhang, Mai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Bin Li, bGlqaWJAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
 Wen-Xuan Wang
Wen-Xuan Wang Yi-Shan Wu3,4†
Yi-Shan Wu3,4† Anise M. S. Wu
Anise M. S. Wu Ying-Ying Zhu
Ying-Ying Zhu Shan-Shan Guo
Shan-Shan Guo Yi-Jun Hua
Yi-Jun Hua Dong-Hua Luo
Dong-Hua Luo Qiu-Yan Chen
Qiu-Yan Chen Yan-Qun Xiang
Yan-Qun Xiang Jin-Xin Zhang
Jin-Xin Zhang Hai-Qiang Mai
Hai-Qiang Mai Ji-Bin Li
Ji-Bin Li