- 1International Nursing School, Hainan Medical University, Haikou, Hainan, China
- 2Center for Health Policy and Technology Evaluation, Peking University Health Science Center, Beijing, China
Background: Diabetes structured education programs have been demonstrated to effectively improve glycemic control and self-management behaviors. However, evidence on the health economic evaluation of these programs is limited.
Objectives: To systematically review the health economic evaluation of structured education programs for patients with type 1 and type 2 diabetes mellitus.
Methods: The English databases PUBMED, WEB OF SCIENCE, OVID, COCHRANE LIBRARY, EMBASE, and EBSCO, along with the Chinese databases CNKI, WANFANG, VIP, and SINOMED, were searched from their inception to September 2024. The quality of the literature was assessed using the CHEERS 2022 checklist. A descriptive analysis was performed on the studies included in the review, with all currencies converted to international dollars. An incremental cost-effectiveness ratio of less than one times the per capita GDP was considered highly cost-effective, while a ratio between one and three times the per capita GDP was considered cost-effective.
Results: A total of 28 studies from upper-middle-income and high-income countries were included. The average quality score of the included studies was 18.6, indicating a moderate level of reporting quality. Among these, eleven studies demonstrated that diabetes structured education programs were highly cost-effective and twelve were found to be cost-effective. In contrast, three studies were deemed not cost-effective, and two studies provided uncertain results. The ranges of the incremental cost-effectiveness ratios for short-term, medium-term, and long-term studies were − 520.60 to 65,167.00 dollars, −24,952.22 to 14,465.00 dollars, and −874.00 to 236,991.67 dollars, respectively.
Conclusion: This study confirms the cost-effectiveness of structured education programs for diabetes and highlights their importance for patients with type 2 diabetes who have HbA1c levels exceeding 7% and are receiving non-insulin therapy. Additionally, the potential advantages of incorporating telecommunication technologies into structured diabetes education were emphasized. These findings offer valuable insights and guidance for decision-making in diabetes management and clinical practice, contributing to the optimization of medical resource allocation and the improvement of health status and quality of life for patients.
1 Introduction
Diabetes mellitus is one of the most prevalent and severe chronic illnesses globally, posing a significant threat to public health worldwide (1). According to the International Diabetes Federation, the global prevalence of diabetes mellitus was 10.5% in 2021, amounting to approximately 536.6 million individuals, with the number of adult patients expected to rise to 783.2 million by 2045 (2). Diabetes mellitus can lead to various complications, including cardiovascular issues, kidney disease, eye problems, peripheral nerve damage, and stroke, among others (3). The chronic nature of diabetes and its associated complications places a substantial burden and significant economic strain on individuals, their families, and the entire healthcare system. A systematic review indicated that the average annual cost per person to treat type 2 diabetes ranged from 29.91 dollars to 237.38 dollars in low- and low middle-income countries (4). In 2021, global health expenditure on diabetes was projected to be 966 billion dollars, with adult diabetes expenditure estimated to reach 1,054 billion dollars by 2045 (2). A survey in the United States, found that nearly a quarter of individuals with diabetes reported spending an additional 300 dollars per month to manage their health (5). Consequently, identifying a cost-effective method for managing patients with diabetes is urgent.
Diabetes education is recognized as a fundamental aspect of diabetes care (6). Various effective educational interventions have been implemented to improve diabetes self-care (7). Structured education is one of the most essential forms of diabetes self-management education, and the International Consensus Guidelines on Diabetes highlight the need for structured diabetes self-management education programs for patients at the time of diagnosis (8). The Diabetes Structured Education Programs (DSEP) are defined as a self-management education that is systematic, structured, standardized, and personalized. These programs are developed and tailored by a professional diabetes education team, taking into account the patient’s education level, cultural background, health status, and individual needs (9, 10). Diabetes structured education programs have been implemented in numerous countries and have demonstrated significant clinical effects, such as improvements in HbA1c (11–13). Therefore, DSEP is prioritized and recommended to patients both international and multiple national diabetes guidelines (14–17).
Several studies have indicated that DSEP are cost-effective (18, 19). Jiang et al. evaluated the long-term cost-effectiveness of self-efficacy-centered structured diabetes education for non-insulin-treated type 2 diabetes patients from the perspective of the Chinese healthcare system (18). Using a 50-year simulation with the CORE model, the results indicated that structured education not only reduced patient complications but also increased life expectancy and quality-adjusted life years (QALYs), resulting in a savings of 5,221.97 dollars, with an incremental cost-effectiveness ratio (ICER) indicating dominance. However, the overall cost-effectiveness remains uncertain, particularly in health systems that lack sufficient resources to manage diabetes mellitus effectively (20). Wan et al. conducted a comprehensive search of five authoritative literature databases in Malaysia to systematically assess the cost-effectiveness of various educational interventions in diabetes management, including face-to-face education, structured education, and other models (21). Their analysis found that up to 89% of the literature supports the significant cost-effectiveness of these educational interventions. However, due to the variable overall quality of the included literature and the considerable methodological heterogeneity among the studies, it remains challenging to precisely identify the most cost-effective types of specific educational interventions. Therefore, the cost-effectiveness of structured education programs in diabetes management remains unclear. Additionally, two systematic reviews on diabetes self-management education programs suggested that such education is likely to be cost-effective (22, 23). Teljeur et al. conducted a systematic review in Ireland, which included 16 cost-analysis studies and 21 cost-effectiveness analyses. They concluded that the average cost of self-management education programs was 684 dollars, suggesting a likelihood of cost-effectiveness (23). However, both reviews acknowledged the heterogeneity of the included studies, making it difficult to determine which type of self-management education program was the most cost-effective. Furthermore, these reviews were published in 2017, and new evidence has emerged in recent years. Additionally, the focus of these reviews was not specifically on structured education programs. Therefore, it is essential to conduct a systematic review to analyze the cost-effectiveness of the DSEP.
In summary, both international and national diabetes guidelines emphasize the important of DSEP. While there have been original studies evaluating the economic effects of DSEP, as well as some reviews focusing on diabetes education, recent years have seen the emergence of new evidence. However, a systematic review specifically addressing the economic evaluation of structured education programs for patients with type 1 and type 2 diabetes is still absent. Conducting a systematic review on this topic is essential. Therefore, this study aims to systematically review the existing evidence of health economic evaluation studies concerning DSEP.
2 Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement) guided the reporting of this systematic review (24).
2.1 Data resource and search strategy
Searches were conducted across six English databases (PUBMED, WEB OF SCIENCE, OVID, COCHRANE LIBRARY, EMBASE, and EBSCO) and four Chinese databases (CNKI, WANFANG, VIP, and SINOMED). The search strategy incorporated MeSH terms and entry words, tailored to each database. Additionally, manual searches and an inquiry into the ProQuest digital papers database were performed to obtain grey literature. The search terms included economic evaluation, cost effectiveness analysis (CEA), cost benefit analysis (CBA), cost utility analysis (CUA), cost minimization analysis (CMA), cost consequences analysis (CCA), diabetes mellitus, type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), structured education (SE), structured education program (SEP), educational program, patient education, health education, health promotion, self-management, diabetes management program, diabetes education, and care program. The literature search period extended from the establishment of the database to September 2024.
2.2 Study selection
Two researchers independently selected the literature, resolving any disagreements through discussion or by involving a third researcher. Irrelevant literature was excluded by reviewing the title and abstract, with followed by a thorough reading of the full text to finalize inclusion based on the eligibility criteria. The PICOST (Population, Intervention, Comparison, Outcome, Study design, Time) framework guided the study selection process. Studies were included if they met the following criteria (i) population: patients with T1DM, T2DM, or both; (ii) interventions: any form of structured education program for individuals with diabetes; (iii) comparisons: any comparators such as usual care, standard care, or routine education; (iv) outcomes: cost, effectiveness indicators (e.g., HbA1c levels, complications, morbidity, mortality, life expectancy), cost-effectiveness indicators (e.g., ICER), cost-utility indicators (e.g., QALYs, disability-adjusted life years, health utility values, incremental cost-utility ratio (ICUR)), and cost–benefit indicators (e.g., net cost, incremental cost–benefit ratio); (v) study design: any research design related to health economic evaluation, including randomized controlled trials, prospective studies, retrospective studies, mixed-method designs, and modeling analyses; (vi) time: short- or long-term economic evaluations. Studies were excluded if they met any of the following criteria: (i) not published in Chinese or English; (ii) full text not available or if they were repeated publications; (iii) reviews, case reports, comments, protocols, and animal studies; (iv) incomplete content such as partial economic evaluations, those conducting only cost analysis, or lacking health outcome data; (v) patients who were pregnant or preparing for pregnancy; (vi) absence of a control group.
2.3 Data extraction
The retrieved data were imported into NoteExpress 3.9.0 software to eliminate duplicate literature. Two investigators independently screened, extracted, and analyzed the literature based on predefined inclusion and exclusion criteria. Any disagreements were resolved through discussion with a third researcher. A self-designed structured Excel form was utilized for data collection. For the selected studies, the extracted data included: (i) basic information (e.g., first author, title, publication year, country, income level), (ii) study characteristics (e.g., sample size, population, intervention, comparator, study design, data sources), and (iii) economic evaluation content (e.g., cost, perspective, time horizon, type of economic evaluation, discount rate, currency, health outcome, sensitivity analysis, quality score). If a study analyzed both short-term and long-term economic effects of DSEP, the long-term cost-effectiveness analysis was prioritized for reporting in this systematic review as it is more representative.
2.4 Quality assessment
The quality of the included studies was assessed using the 28-item checklist from the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement (25, 26). The CHEERS 2022 checklist is applicable for the standardized review of any type of health economic evaluation research reports, as well as for theoretical and empirical studies based on mathematical models. This checklist comprises seven main categories with 28 questions addressing various aspects of economic evaluations. Each question was scored as follows: 1 point for fully reported, 0.5 points for partially reported, and 0 points for not reported or not applicable (27). The total score, which ranges from 0 to 28, reflects the quality of study reporting, with higher scores indicating better quality. Quality assessments were conducted independently and cross-checked by two researchers, with any disagreements resolved through discussion with a third researcher.
2.5 Data analysis
To promote comparisons between studies, all currencies were converted into international dollars using the currency exchange rates provided by the World Bank (28). If a study did not report the year of the currency, the default was the publication year of the article. Additionally, according to the cost-effectiveness threshold method of per capita GDP recommended by WHO (29), ICER <1 times GDP per capita, with high cost effectiveness; 1 times per capita GDP < ICER <3 times per capita GDP, with cost effectiveness; ICER ≥3 times per capita GDP does not have effectiveness. Given the significant heterogeneity among the included studies, a descriptive analysis was employed to qualitatively evaluate the health economic evaluation studies.
3 Results
3.1 Study selection
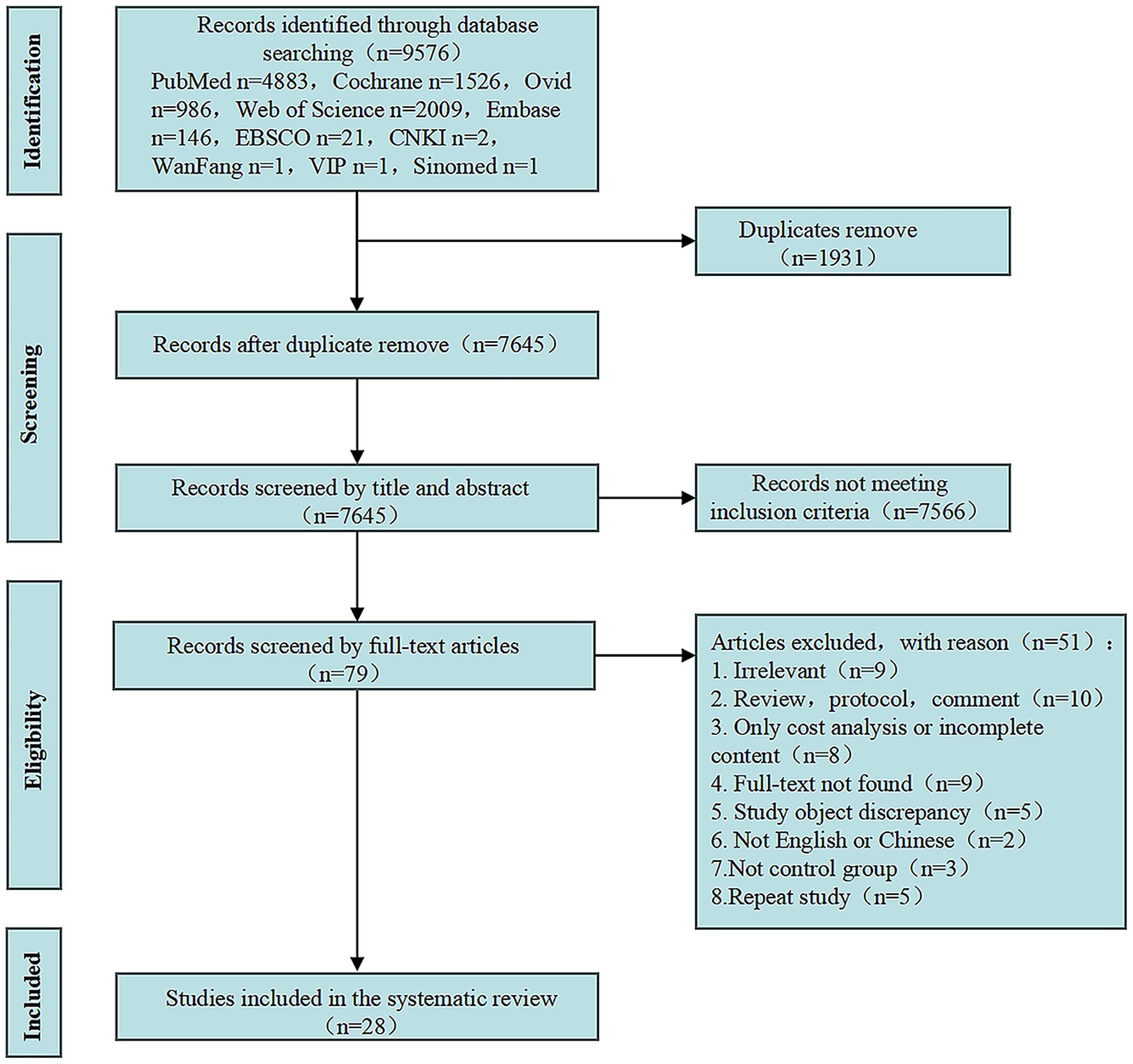
A total of 9,576 relevant studies were retrieved from ten electronic literature databases. Following a review of the abstracts and full texts, 28 studies that met the inclusion criteria were selected. Specific details are presented in the PRISMA flow diagram (Figure 1).
3.2 Quality assessment
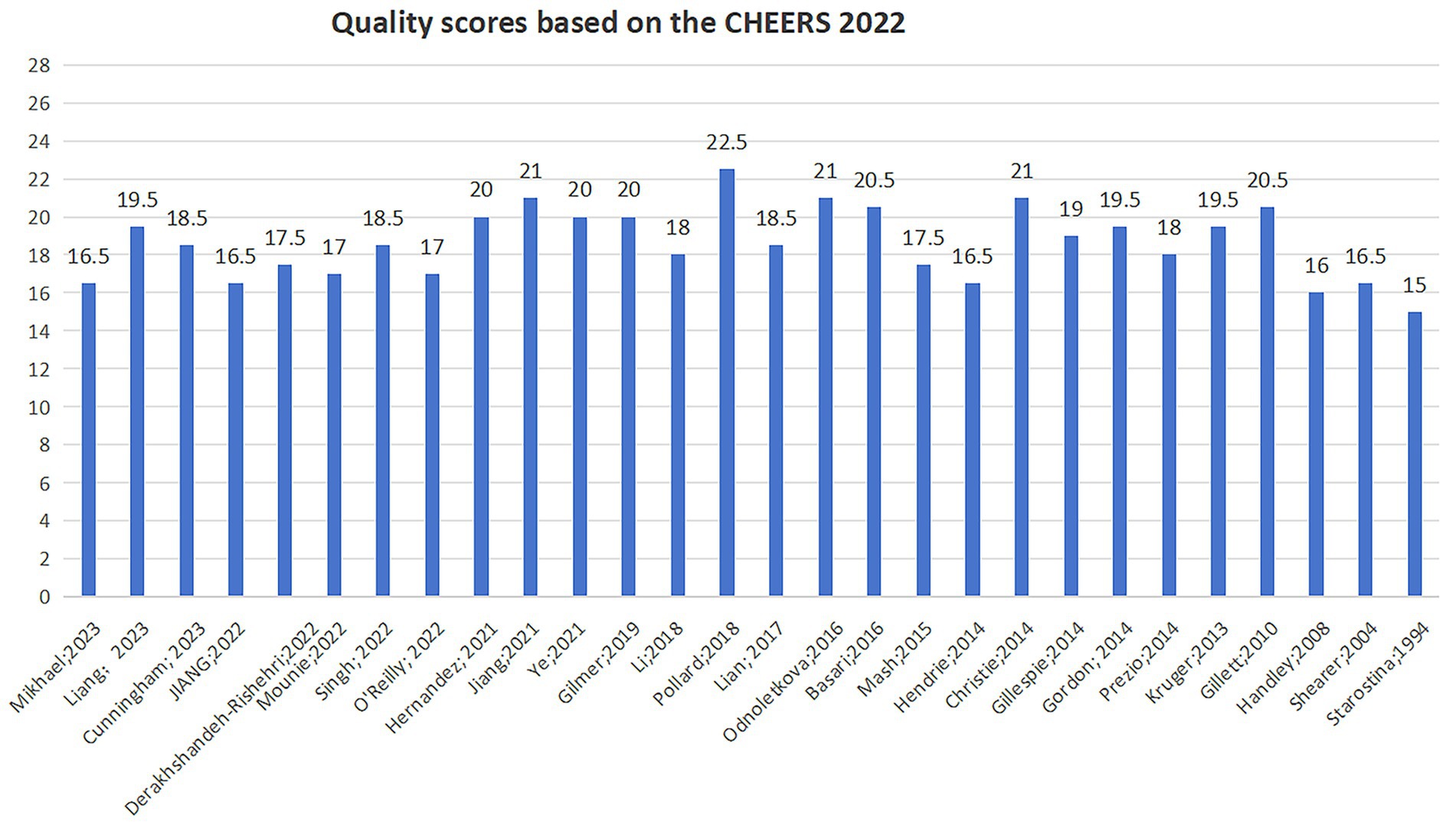
The quality assessment results of the included studies are presented in Figure 2. The scores for reporting quality ranged from 15.0 to 22.5, with a mean score of 18.6, indicating an overall moderate quality of reporting. The studies demonstrated stronger reporting in the title, abstract, background, results, and discussion sections, but weaker reporting in the research methods section. Regarding the health economic analysis plan (HEAP), only one study fully reported that the analysis was conducted according to a pre-developed plan (30). Four studies did not report the selected economic perspective (31–34), while 24 studies did report the perspective; however, most did not justify their choice. All included studies reported the research time horizon, but only one study provided the rationale for it (33). Concerning the research discount rate, only 16 studies reported this information (18, 19, 30, 31, 33, 35–45), and among these, 9 studies explained the reasons for their chosen discount rate (18, 19, 31, 33, 36, 38, 39, 42, 44). All studies reported the currency used, but 6 did not specify the currency reference year (31, 38, 44, 46–48). Among the 17 modeling studies, only (10) described whether the model was internally or externally validated (18, 30, 32, 33, 35, 37, 41–43, 45). Most studies mentioned their sources of funding; however, they did not adequately report the funding methods or the role of the funder in the study.
3.3 Research characteristics
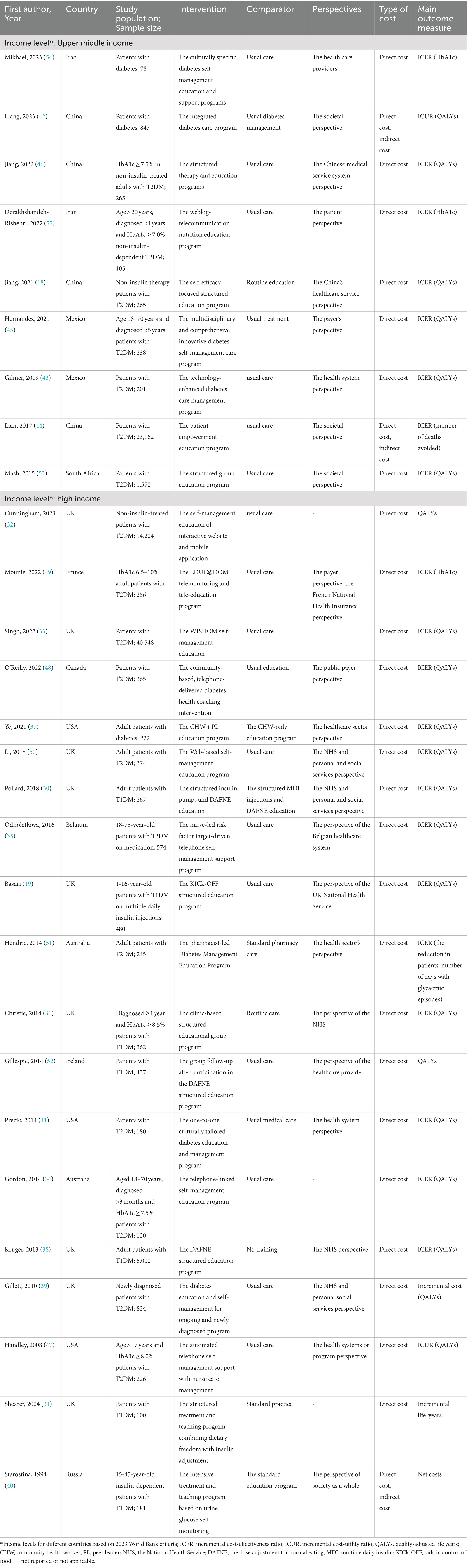
The characteristics of the studies are summarized in Table 1. The 28 included studies, published between 1994 and 2023, comprised one study in Chinese (46) and 27 in English. These studies originated from 13 countries: Australia, Canada, Belgium, France, Ireland, Russia, the UK, the USA, China, Iran, Iraq, Mexico, and South Africa. Among them, 19 studies were from high-income countries (19, 30–41, 47–52) and 9 from upper-middle-income countries (18, 42–46, 53–55). Most studies employed a randomized controlled trial design, while others use cohort design (32, 42, 45), quasi-experimental design (33), and prospective controlled trial (40). Eighteen studies focused on participants with T2DM, seven on participants with T1DM, and three included participants with both types of diabetes. Sample sizes ranged from 78 to 40,548. The most commonly studied populations were non-insulin-treated patients (18, 32, 46, 55), those with moderately controlled diabetes (HbA1c > 7%) (34, 46, 47, 49, 55), newly diagnosed patients (< 1 year) (39, 55), and adults with T2DM. The content and delivery of the structured educational programs varied, with detailed descriptions of the DSEP provided in Table 1. The included studies primarily adopted perspectives from the healthcare systems, societal viewpoints, country-specific healthcare systems, payers, and patients. The types of costs considered varied and include direct medical costs, direct non-medical costs, and indirect costs. However, only three studies addressed both the direct and indirect costs associated with DSEP (40, 42, 44).
One study conducted a CBA (40), seven studies conducted CUA (30, 32, 39, 41, 42, 47, 48), and 20 studies conducted CEA. The included studies assessed DSEP over different durations: seven studies evaluated programs in the short-term (≤ 1 year), five studies in the medium-term (1–5 years), and sixteen studies over the long-term (> 5 years). Seventeen studies used decision analysis models to evaluate the economic outcomes of the DSEP over medium- and long-term periods. Among these, five studies used the Markov models (31, 34–36, 53), four used the United Kingdom Prospective Diabetes Study Outcome Model (32, 33, 42, 43), three used the Sheffield Type 1 Diabetes Policy models (19, 30, 38), two used the CORE Diabetes model (18, 45), one used the Sheffield Type 2 Diabetes Policy model (39), one used the Michigan model (37), one used the Archimedes model (41). The cost discount rates in the included studies ranged from 0 to 6%. Most articles reported the payment thresholds based on GDP per capita, willingness-to-pay, or purchasing power parity, with the exception of three articles that did not provide this information (31, 40, 47). Detailed health economic evaluations of the included studies are presented in Table 2.
3.4 Effectiveness of the programs
In the CEA and CUA studies, the effectiveness indicators employed for health economic evaluation primarily included gained QALYs (18, 19, 30–39, 41–43, 45–48, 50, 52, 53), reduction in HbA1c (49, 54, 55), number of deaths avoided (44), increased life expectancy (18, 33, 35, 38, 43, 45), reduction in blood pressure (42, 54), weight reduction (54), improved lipid levels (54), enhanced fasting blood glucose (54), improvement in diabetes-related distress (50), and fewer days with glycemic episodes (51). Additionally, other studies considered effect indicators such as diabetes complications, hypoglycemia, hyperglycemic episodes, and BMI (19, 35, 44). Diabetes-related distress was assessed using the Problem Areas in Diabetes questionnaire (50). Furthermore, several studies evaluated the health utility values of patients through instruments such as the EuroQol 5 Dimensions scale (31, 35, 39, 46, 48, 50, 52), the Health Utilities Index Mark 2 (19), the Child Health Utility 9 Dimensions (19), the Short Form 36 functional status instrument (51), Short Form-12 Health Survey (47), and the visual analog scale (31). Another CBA study concentrated on calculating the costs and benefits of the DSEP (40).
3.5 Health economic evaluation
A total of 11 studies found that DSEP were highly cost-effective, while 12 studies indicated that they were cost-effective. In contrast, three studies reported that these programs were not cost-effective, and two studies yielded uncertain results. The outcomes of the health economic evaluations from these 28 studies are summarized in Table 2.
3.5.1 Short-term
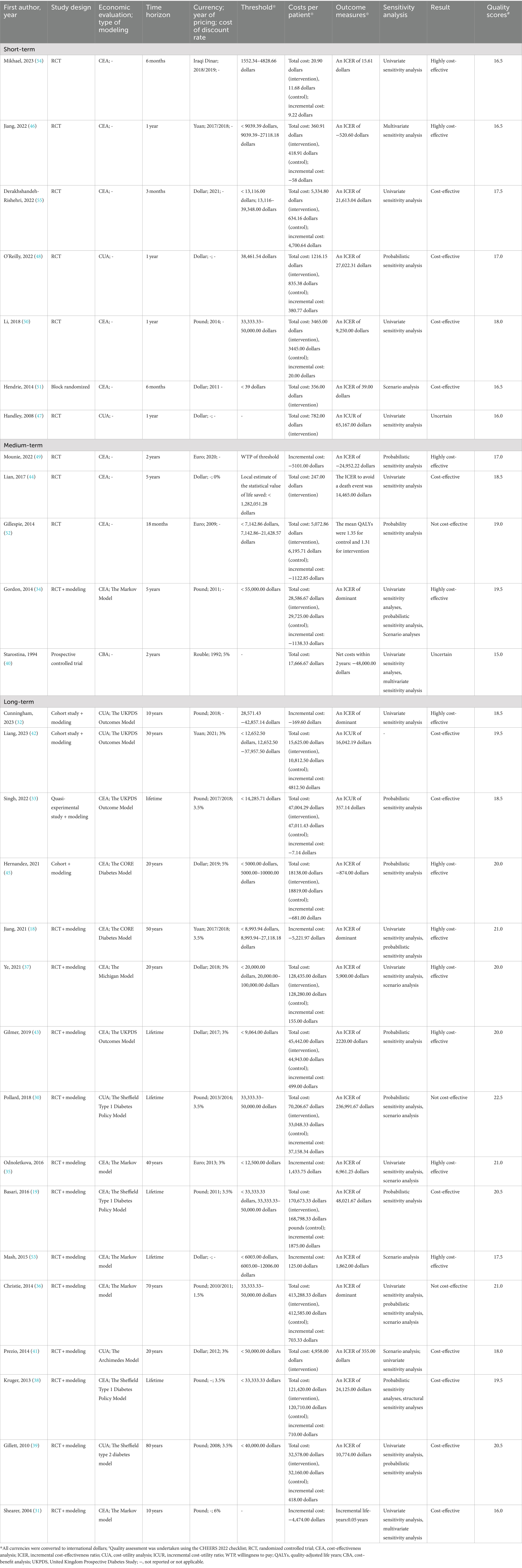
Seven studies evaluated the short-term cost-effectiveness of DSEP, covering periods ranging from of 3 months to 1 year (46–48, 50, 51, 54, 55). The results showed that the total cost of the interventions varied between 20.90 dollars and 5334.80 dollars, with incremental costs ranging from −58 dollars to 4,700.64 dollars. Notably, the structured therapy and education programs conducted in grass-roots areas of China by Jiang et al. (46) and the culturally specific diabetes programs in Iraq by Mikhael et al. (54) demonstrated that DSEP was highly cost effective, with ICER of −520.60 dollars and 15.61 dollars, respectively. Four other studies demonstrated cost-effectiveness, with ICER of 39 dollars, 9,250 dollars, 21,613.04 dollars, and 27,022.31 dollars (48, 50, 51, 55). In these cost-effectiveness studies, all participants were patients with T2DM, most of whom were adults with HbA1c level greater than 7% or non-insulin-treated type 2 diabetes (46, 50, 51, 55). In contrast, Handley et al. (47) evaluated the cost-utility of an automated telephone self-management support and nurse care management program in the USA over one year in patients with T2DM and an HbA1c ≥ 8.0%. This study found an increase of 0.012 QALYs in the intervention group compared to usual care, with a total intervention cost of 782 dollars and an ICUR of 65,167 dollars. However, while the study performed a univariate sensitivity analysis to address result uncertainty in the results, it did not consider cost-effectiveness thresholds for comparative analysis, leaving the cost-effectiveness of the project uncertain. Additionally, the studies by Jiang et al. (46), Derakhshandeh-Rishehri et al. (55), and Handley et al. (47) also assessed the QALYs gained from implementing DSEP, reporting increases of 0.042 years, 0.20 years, and 0.01 years, respectively.
3.5.2 Medium-term
Five studies assessed the medium-term cost-effectiveness of DSEP, with durations ranging from 18 months to 5 years (34, 40, 44, 49, 52). The results indicated that the total cost of the interventions ranged from 247 dollars to 28,586.67 dollars, with incremental costs varying from −5,101 dollars to −1,122.85 dollars. Among these studies, the EDUC@DOM telemonitoring and tele-education program by Mounie et al. (49) and the telephone-linked self-management education program by Gordon et al. (34) both demonstrated that implementing DSEP for adults with T2DM and an average HbA1c greater than 7% was highly cost-effective, with ICER of 24,952.22 dollars and a dominating cost-effectiveness outcome, respectively. Lian et al. (44) found that DSEP was cost-effective in patients with T2DM, incurring a cost of 14,465 dollars to prevent one death event, while also reducing mortality, diabetes complications, and cardiovascular disease. Gillespie et al. (52) conducted a group follow-up after structured education program in Ireland, involving 437 patients with T1DM, and found that the structured education resulted in savings of 1,122.85 dollars. However, the intervention yielded a QALY of 1.31 years, which was 0.04 QALYs less than that of the control group, leading to the conclusion that this intervention was not cost-effective. Additionally, Starostina et al. (40) evaluated the cost–benefit of structured education programs in insulin-dependent patients with T1DM, finding improvements in metabolic control and cost savings in both groups. However, this study did not draw definitive conclusions regarding the cost-effectiveness outcomes, leaving the cost-effectiveness of the two groups uncertain.
3.5.3 Long-term
Sixteen studies utilized health decision analysis models to evaluate the long-term cost-effectiveness of DSEP, with time horizons ranging from 10 years to a lifetime. The findings showed that the total cost of the interventions varied from 4,958 dollars to 413,288.33 dollars, while the incremental costs ranged from −5,221.97 dollars to 37,158.34 dollars. Among these studies, five reported that DSEP resulted in savings of approximately 7.14 dollars to 5,221.97 dollars (18, 31–33, 45); seven studies demonstrated that DSEP was highly cost-effective in patients with T2DM or diabetes, with ICER ranging from −874 dollars to 69,661.25 dollars, with some studies showing dominant cost-effectiveness (18, 32, 35, 37, 43, 45, 53); and seven studies indicated that DSEP was cost-effective, with ICER ranging from 355 dollars to 48,021.67 dollars (19, 31, 33, 38, 39, 41, 42). Most of the cost-effective studies focused on patients with T2DM, with Jiang et al. (18), Cunningham et al. (32), and Odnoletkova et al. (35) specifically examining non-insulin treated type 2 diabetes patients. Additionally, the incremental QALYs ranged from 0 to 0.34 years. However, two studies found no evidence of long-term cost-effectiveness. Pollard et al. conducted a lifetime cost-effectiveness analysis using the Sheffield Type 1 Diabetes Policy Model for structured insulin pumps and DAFNE education in patients with T1DM (30). This study reported an ICER of 236,991.67 dollars, exceeding the threshold of 33,333.33 dollars to 50,000.00 dollars, indicating that the intervention was not cost-effective and was associated with an increase in adverse events in the intervention group. Additionally, Christie et al. (36) used a Markov model to evaluate the 70-year cost-effectiveness of a clinic-based structured educational group program for patients with T1DM with HbA1c levels of ≥ 8.5%. The results showed that the ICER fell below the threshold of 38,767.66 dollars to 58,151.49 dollars; however, the intervention group did not showed improvements in metabolic control or gains in additional QALYs, and thus it was not considered cost-effective. Furthermore, Singh et al. (33), Kruger et al. (38), Jiang et al. (18), Gilmer et al. (43), Hernandez et al. (45), and Odnoletkova et al. (35) also projected life expectancy following the implementation of DSEP. The estimated increases in life expectancy were 0.01, 0.08, 0.2, 0.23, 0.5, and 1.18 years, respectively. The models were also used to simulate the occurrence of long-term complications, demonstrating that DSEP could reduce the incidence of diabetes-related complications, including myocardial infarction, stroke, atrial fibrillation, kidney failure, diabetic cardiovascular disease, diabetic kidney disease, heart failure, ischemic heart disease mortality, foot ulcers, foot amputations, diabetic neuropathy, and diabetic retinopathy (18, 31–33, 35, 38, 41, 45, 53).
3.6 Sensitivity analysis
Various sensitivity analyses were conducted in the included studies, encompassing univariate sensitivity analysis, multivariate sensitivity analysis, probabilistic sensitivity analysis (PSA), scenario analysis, and structural sensitivity analysis. The key indicators considered in these analyses mainly included glycated hemoglobin, discount rate, time horizon, cost of education programs, number of participants, health utility values and relevant thresholds. In the PSA, the probability that DSEP was cost-effective ranged from 14 to 100% (18, 19, 30, 33, 34, 36, 38, 39, 45, 48, 49, 52).
4 Discussion
This systematic review provides new evidence on the cost-effectiveness of structured education programs for individuals with T1DM and T2DM. By analyzing 28 studies from 13 countries, we found that DSEP are likely to be cost-effective in the short, medium and long term, leading to increased quality-adjusted life years, extended life expectancy, and a reduction in complications. These findings align with our initial hypothesis. Moreover, structured education programs for individuals with T2DM demonstrated better cost-effectiveness, particularly among patients on non-insulin therapy with HbA1c levels greater than 7%. However, the evidence for supporting the cost-effectiveness of structured education programs for individuals with T1DM remains limited, highlighting the need for further research to confirm these findings. These results offer valuable guidance for healthcare policymakers. Additionally, the combination of structured diabetes education with telecommunication technology shows promising potential for cost-effectiveness.
The results of this study indicate that, consistent with previous findings, structured education programs for diabetes can be cost-effective (21–23). Specifically, the most cost-effective interventions were the self-efficacy-focused structured education program (18), the multidisciplinary and comprehensive innovative diabetes self-management care program (45), the self-management education delivered via interactive websites and mobile applications (32), and the telephone-linked self-management education program (34). Several factors may contribute to these outcomes: structured education helps patients manage their condition more effectively, reducing medication costs and the risk of complications; the involvement of trained registered nurses at the grassroots level for education and follow-up lowers manpower costs; and the integration of telecommunication technology into structured education promotes long-term follow-up without the need for transportation, saving both time and money (35, 56, 57). However, the short-term cost-effectiveness of DSEP using telecommunication technology, as reported by Handley et al. (47), remains uncertain, and relevant studies are limited. Therefore, further evaluation of the long-term cost-effectiveness of telecommunication technology-based DSEP is necessary to support broader implementation. Additionally, our findings showed that DSEP is cost-effective for a subgroup of patients with type 2 diabetes who have HbA1c levels greater than 7% and are not treated with insulin. Some studies suggest that categorizing diabetes into subgroups based on demographic or disease-related factors can enable more personalized and targeted interventions, benefiting those most likely to see improvements, which has important implications for health policy and resource allocation (58, 59). This suggests that DSEP could serve as a precise and effective educational and management approach for this specific group. However, there is a limited amount of research using raw data in this area. Thus, further investigation is needed to explore the impact of structured education on the application and cost-effectiveness across different subgroups of T2DM.
While structured education programs for diabetes have showed positive outcomes, some studies suggest they may not always be cost-effective or yield conclusive results. These inconsistent findings are often linked to the inability of certain programs to improve blood sugar control, improve quality-adjusted life years, or manage high costs. In the structured educational cost-effectiveness study conducted by Pollard et al. (30), participants with T1DM treated with insulin pumps experienced high intervention costs, largely due to the expense of insulin pump therapy. In a 5-year follow-up study of patients with T1DM, Toresson et al. (60) assessed the costs associated with two different treatment modalities and found that insulin pumps were approximately 3,929 dollars more expensive per year than multiple daily insulin injections. This highlights the need for structured education programs to account for the cost differences between these two treatment modalities and consider the financial feasibility of the intervention for patients, families, and society. Additionally, studies by Starostina et al. (40) and Handley et al. (47) did not establish a cost-effectiveness threshold, which affected the evaluation of their results. Introducing such thresholds is crucial for determining cost-effectiveness and improving the scientific rigor of the assessments. Moreover, we observed that all studies reporting non-cost-effective outcomes involved patients with T1DM (30, 36, 52). This suggests that structured education programs for T1DM may not be cost-effective. However, given the limited evidence available, further investigation is necessary to draw more definitive conclusions.
In this systematic review, the majority of included studies adopted the perspective of the medical service system, with only four studies taking a broader societal perspective (40, 42, 44, 53). The economic burden of diabetes includes not only direct costs but also indirect and intangible costs (61–63). Due to the complexity and uncertainty in defining indirect and intangible costs, most studies focus exclusively on direct costs. It is recommended that future research adopt a societal perspective to evaluate the cost-effectiveness of programs, accounting for both direct and indirect costs, such as labor and productivity losses (e.g., absences from work or school) during illness. This approach would provide a more comprehensive view of the disease’s full economic burden and improve the accuracy of economic evaluations. Furthermore, among the 28 studies reviewed, the health economic studies of DSEP were primarily conducted in high- and middle-income countries, with a notable absence of evaluation from low-income countries. However, the economic burden of diabetes was particularly severe in low-income countries (64). A cross-sectional study showed that fewer than 10% of diabetes patients in low- and middle-income countries received comprehensive, guideline-based treatment (65), reflecting lower coverage of education programs compared to high-income countries. Additionally, several studies have demonstrated that DSEP in low-income countries can effectively control patient metabolism, reduce glycosylated hemoglobin levels, and improve self-management behaviors (66–68). Given the varying economic conditions across countries, future health economic evaluations of DSEP should focus on low-income countries to assess the program’s cost-effectiveness in these settings.
The majority of studies included in this review adhered to the CHEERS 2022 guidelines and achieved moderate levels of reporting quality, indicating that researchers recognize the importance of transparency and reproducibility. However, the quality of reporting in the research methods section remains insufficient. Precise documentation of research methods is essential for enabling other researchers to replicate or validate findings. Notably, only one study reported that its analysis was conducted according to a pre-established plan (30), suggesting that most studies may lack pre-registration or pre-planning, which could introduce bias into study design and execution. In the UK, it was found that only 30% of trials developed a HEAP as standard practice (69), despite health economists calling for HEAP standardization as early as 2008 (70). To improve the quality and transparency of health economics evaluation research, future studies should strengthen compliance with guidelines like CHEERS 2022, thereby improving the overall quality, reliability and applicability of research outcomes.
The findings of this study support the integration of structured educational programs into standard care practices for type 2 diabetes, particularly for patients with HbA1c levels greater than 7% who are not receiving insulin treatment. These results provide valuable guidance for health policymakers and researchers, helping them select the most appropriate and cost-effective DSEP and formulate more effective health policies aimed at improving health management and the quality of life for patients. Regarding future research directions: First, it is crucial to implement structured diabetes education using telecommunication technology in real-world settings to verify its clinical and cost-effectiveness and encourage wider adoption. Second, to deliver tailored educational programs for individuals with T2DM who stand to benefit most, it is important to investigate the effectiveness and cost-effectiveness of structured education across different subgroups, while also identifying their specific health needs and utilization patterns. Third, the cost-effectiveness of structured education for patients with T1DM appears suboptimal, highlighting the need for further research to develop a more cost-effective educational program. Lastly, multi-center studies that take social perspectives into account are recommended, particularly in low-income countries.
This study has several limitations. First, comparing interventions across studies is difficult due to the heterogeneity in the types and designs of DSEP, as well as differences in income levels and cultural backgrounds across countries. However, we have clearly defined DSEP in this context. Additionally, the World Health Organization (29) recommends that the cost-effectiveness of a program or intervention should be assessed based on the specific circumstances of each country or region, given varying levels of economic development. Therefore, we evaluated the cost-effectiveness of the programs strictly based on the threshold and ICER comparisons. Second, different studies adopt varying economic perspectives, mainly focusing on medical services and often overlooking indirect and intangible costs associated with structured education programs, which may impact the overall assessment of cost-effectiveness. Third, although we conducted a comprehensive search, we included only studies published in Chinese and English, which may have excluded relevant research in other languages. Finally, while we assessed the quality of the included studies, future evaluations should consider incorporating additional tools such as GRADE (71) and ECOBIAS (72, 73).
5 Conclusion
In conclusion, DSEP appears to be cost-effective in the long term for patients with T2DM, particularly those with HbA1c levels above 7% who are not receiving insulin therapy. The integration of telecommunication technology further improves both the effectiveness and cost-effectiveness of DSEP. However, the cost-effectiveness of DSEP for patients with T1DM requires further exploration. Given the variability in research quality and economic contexts, it is recommended to conduct multi-center, cross-border social perspective studies, especially in low-income countries, to comprehensively assess the cost-effectiveness of these programs. Such research will provide a scientific basis for policy development, optimize the allocation of medical resources, and ultimately improve the health and quality of life for patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CY: Conceptualization, Formal analysis, Methodology, Resources, Writing – original draft, Data curation. QZ: Data curation, Formal analysis, Resources, Writing – review & editing. WY: Data curation, Formal analysis, Resources, Writing – review & editing. LT: Conceptualization, Supervision, Writing – review & editing. XJ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Project of National Natural Science Foundation of China (grant number: 82304262).
Acknowledgments
We thank the reviewers for their valuable suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1467178/full#supplementary-material
References
1. Zimmet, P, Alberti, KG, and Shaw, J. Global and societal implications of the diabetes epidemic. Nature. (2001) 414:782–7. doi: 10.1038/414782a
2. Sun, H, Saeedi, P, Karuranga, S, Pinkepank, M, Ogurtsova, K, Duncan, BB, et al. Idf diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
3. van Dieren, S, Beulens, JWJ, van der Schouw, YT, Grobbee, DE, and Neal, B. The global burden of diabetes and its complications: an emerging pandemic. European J Cardiovasc Prevent Rehabil. (2010) 17:S3–S08. doi: 10.1097/01.hjr.0000368191.86614.5a
4. Afroz, A, Alramadan, MJ, Hossain, MN, Romero, L, Alam, K, Magliano, DJ, et al. Cost-of-illness of type 2 diabetes mellitus in low and lower-middle income countries: a systematic review. BMC Health Serv Res. (2018) 18:972. doi: 10.1186/s12913-018-3772-8
5. Patel, MR, Anthony Tolentino, D, Smith, A, and Heisler, M. Economic burden, financial stress, and cost-related coping among people with uncontrolled diabetes in the u.s. Prev Med Rep. (2023) 34:102246. doi: 10.1016/j.pmedr.2023.102246
6. Yang, Y, Wu, Y, Lu, Y, Kornelius, E, Lin, Y, Chen, Y, et al. Adherence to self-care behavior and glycemic effects using structured education. J Diabetes Investig. (2015) 6:662–9. doi: 10.1111/jdi.12343
7. Loveman, E, Frampton, GK, and Clegg, AJ. The clinical effectiveness of diabetes education models for type 2 diabetes: a systematic review. Health Technol Assess. (2008) 12:1–116. doi: 10.3310/hta12090
8. Khunti, K, Chatterjee, S, Carey, M, Daly, H, Batista-Ferrer, H, and Davies, MJ. New drug treatments versus structured education programmes for type 2 diabetes: comparing cost-effectiveness. The Lancet. (2016) 4:557–9. doi: 10.1016/S2213-8587(16)30048-1
9. National Institute for Health and Care Excellence . Guidance on the use of patient-education models for diabetes. London: NICE (2003).
10. Chatterjee, S, Davies, MJ, Heller, S, Speight, J, Snoek, FJ, and Khunti, K. Diabetes structured self-management education programmes: a narrative review and current innovations. The Lancet. (2018) 6:130–42. doi: 10.1016/S2213-8587(17)30239-5
11. Pacheco, APF, Sande-Lee, SVD, Sandoval, RDCB, Batista, S, and Marques, JLB. Effects of a structured education program on glycemic control in type 1 diabetes. Arch Endocrinol Metabol. (2017) 61:534–41. doi: 10.1590/2359-3997000000278
12. Alibrahim, A, AlRamadhan, D, Johny, S, Alhashemi, M, Alduwaisan, H, and Al-Hilal, M. The effect of structured diabetes self-management education on type 2 diabetes patients attending a primary health center in Kuwait. Diabetes Res Clin Pract. (2021) 171:108567. doi: 10.1016/j.diabres.2020.108567
13. Walker, GS, Chen, JY, Hopkinson, H, Sainsbury, CAR, and Jones, GC. Structured education using dose adjustment for normal eating (dafne) reduces long-term hba(1c) and hba(1c) variability. Diabetic Med. (2018) 35:745–9. doi: 10.1111/dme.13621
14. Davis, J, Fischl, AH, Beck, J, Browning, L, Carter, A, Condon, JE, et al. 2022 national standards for diabetes self-management education and support. Diabetes Care. (2022) 45:484–94. doi: 10.2337/dc21-2396
15. Chinese, DS . Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition) (part 1). Chinese J Practical Internal Med. (2021) 41:668–95. doi: 10.19538/j.nk2021080106
16. Deakin, TA, Cade, JE, Williams, R, and Greenwood, DC. Structured patient education: the diabetes x-pert programme makes a difference. Diabet Med. (2006) 23:944–54. doi: 10.1111/j.1464-5491.2006.01906.x
17. Sturt, JA, Whitlock, S, Fox, C, Hearnshaw, H, Farmer, AJ, Wakelin, M, et al. Effects of the diabetes manual 1:1 structured education in primary care. Diabet Med. (2008) 25:722–31. doi: 10.1111/j.1464-5491.2008.02451.x
18. Jiang, X, Jiang, H, Tao, L, and Li, M. The cost-effectiveness analysis of self-efficacy-focused structured education program for patients with type 2 diabetes mellitus in mainland China setting. Front Public Health. (2021) 9:767123. doi: 10.3389/fpubh.2021.767123
19. Basarir, H, Brennan, A, Jacques, R, Pollard, D, Stevens, K, Freeman, J, et al. Cost-effectiveness of structured education in children with type-1 diabetes mellitus. Int J Technol Assess Health Care. (2016) 32:203–11. doi: 10.1017/S0266462316000507
20. Brady, EM, Bamuya, C, Beran, D, Correia, J, Crampin, A, Damasceno, A, et al. Extending availability of self-management structured education programmes for people with type 2 diabetes in low-to-middle income countries (extend)-a feasibility study in Mozambique and Malawi. BMJ Open. (2021) 11:e47425. doi: 10.1136/bmjopen-2020-047425
21. Wan Rohimi, WNLH, and Mohd Tahir, NA. The Cost-effectiveness of different types of educational interventions in type ii diabetes mellitus: A systematic review. Front Pharmacol. (2022) 13:953341. doi: 10.3389/fphar.2022.953341
22. Lian, JX, McGhee, SM, Chau, J, Wong, CKH, Lam, CLK, and Wong, WCW. Systematic review on the cost-effectiveness of self-management education programme for type 2 diabetes mellitus. Diabetes Res Clin Pract. (2017) 127:21–34. doi: 10.1016/j.diabres.2017.02.021
23. Teljeur, C, Moran, PS, Walshe, S, Smith, SM, Cianci, F, Murphy, L, et al. Economic evaluation of chronic disease self-management for people with diabetes: a systematic review. Diabet Med. (2017) 34:1040–9. doi: 10.1111/dme.13281
24. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Husereau, D, Drummond, M, Augustovski, F, de Bekker-Grob, E, Briggs, AH, Carswell, C, et al. Consolidated health economic evaluation reporting standards 2022 (cheers 2022) statement: updated reporting guidance for health economic evaluations. Value Health. (2022) 25:3–9. doi: 10.1016/j.jval.2021.11.1351
26. Husereau, D, Drummond, M, Augustovski, F, de Bekker-Grob, E, Briggs, AH, Carswell, C, et al. Consolidated health economic evaluation reporting standards (cheers) 2022 explanation and elaboration: a report of the ispor cheers ii good practices task force. Value Health. (2022) 25:10–31. doi: 10.1016/j.jval.2021.10.008
27. Wei, X, Oxley, S, Sideris, M, Kalra, A, Sun, L, Yang, L, et al. Cost-effectiveness of risk-reducing surgery for breast and ovarian cancer prevention: a systematic review. Cancers (Basel). (2022) 14:6117. doi: 10.3390/cancers14246117
28. Global Economic Monitor (GEM) . (2024). Databank. Available online at: https://databank.worldbank.org/source/global-economic-monitor-(gem)
29. World Health Orgnization . (2017). Who | making choices in health: who guide to cost-effectiveness analysis. Available online at: http://www.who.int/choice/book/en/ (Accessed May 6, 2024)
30. Pollard, DJ, Brennan, A, Dixon, S, Waugh, N, Elliott, J, Heller, S, et al. Cost-effectiveness of insulin pumps compared with multiple daily injections both provided with structured education for adults with type 1 diabetes: a health economic analysis of the relative effectiveness of pumps over structured education (repose) randomised controlled trial. BMJ Open. (2018) 8:e16766. doi: 10.1136/bmjopen-2017-016766
31. Shearer, A, Bagust, A, Sanderson, D, Heller, S, and Roberts, S. Cost-effectiveness of flexible intensive insulin management to enable dietary freedom in people with type 1 diabetes in the Uk. Diabet Med. (2004) 21:460–7. doi: 10.1111/j.1464-5491.2004.01183.x
32. Cunningham, SG, Stoddart, A, Wild, SH, Conway, NJ, Gray, AM, and Wake, DJ. Cost-utility of an online education platform and diabetes personal health record: analysis over ten years. J Diabetes Sci Technol. (2023) 17:715–26. doi: 10.1177/19322968211069172
33. Singh, S, Price, H, Fayers, K, Leal, J, Donoghue, V, Hempenstall, J, et al. The wisdom self-management intervention: a cost-effectiveness analysis to support the transformation of type 2 diabetes care in England. Diabet Med. (2022) 39:e14928. doi: 10.1111/dme.14928
34. Gordon, LG, Bird, D, Oldenburg, B, Friedman, RH, Russell, AW, and Scuffham, PA. A cost-effectiveness analysis of a telephone-linked care intervention for individuals with type 2 diabetes. Diabetes Res Clin Pract. (2014) 104:103–11. doi: 10.1016/j.diabres.2013.12.032
35. Odnoletkova, I, Ramaekers, D, Nobels, F, Goderis, G, Aertgeerts, B, and Annemans, L. Delivering diabetes education through nurse-led telecoaching. Cost-effectiveness analysis. PLoS One. (2016) 11:e163997. doi: 10.1371/journal.pone.0163997
36. Christie, D, Thompson, R, Sawtell, M, Allen, E, Cairns, J, Smith, F, et al. Structured, intensive education maximising engagement, motivation and long-term change for children and young people with diabetes: a cluster randomised controlled trial with integral process and economic evaluation – the cascade study. Health Technol Assess. (2014) 18:1–202. doi: 10.3310/hta18200
37. Ye, W, Kuo, S, Kieffer, EC, Piatt, G, Sinco, B, Palmisano, G, et al. Cost-effectiveness of a diabetes self-management education and support intervention led by community health workers and peer leaders: projections from the racial and ethnic approaches to community health Detroit trial. Diabetes Care. (2021) 44:1108–15. doi: 10.2337/dc20-0307
38. Kruger, J, Brennan, A, Thokala, P, Basarir, H, Jacques, R, Elliott, J, et al. The cost-effectiveness of the dose adjustment for normal eating (dafne) structured education programme: an update using the Sheffield type 1 diabetes policy model. Diabet Med. (2013) 30:1236–44. doi: 10.1111/dme.12270
39. Gillett, M, Dallosso, HM, Dixon, S, Brennan, A, Carey, ME, Campbell, MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (desmond) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ. (2010) 341:c4093. doi: 10.1136/bmj.c4093
40. Starostina, EG, Antsiferov, M, Galstyan, GR, Trautner, C, Jörgens, V, Bott, U, et al. Effectiveness and cost-benefit analysis of intensive treatment and teaching programmes for type 1 (insulin-dependent) diabetes mellitus in Moscow: blood glucose versus urine glucose self-monitoring. Diabetologia. (1994) 37:170–6. doi: 10.1007/s001250050089
41. Prezio, EA, Pagán, JA, Shuval, K, and Culica, D. The community diabetes education (CoDE) program: cost-effectiveness and health outcomes. Am J Prev Med. (2014) 47:771–9. doi: 10.1016/j.amepre.2014.08.016
42. Liang, D, Zhu, W, Huang, J, and Dong, Y. A health economic analysis of an integrated diabetes care program in China: based on real-world evidence. Front Public Health. (2023) 11:1211671. doi: 10.3389/fpubh.2023.1211671
43. Gilmer, T, Burgos, JL, Anzaldo-Campos, MC, and Vargas-Ojeda, A. Cost-effectiveness of a technology-enhanced diabetes care management program in mexico. Value Health Reg Issues. (2019) 20:41–6. doi: 10.1016/j.vhri.2018.12.006
44. Lian, J, McGhee, SM, So, C, Chau, J, Wong, CKH, Wong, WCW, et al. Five-year cost-effectiveness of the patient empowerment programme (pep) for type 2 diabetes mellitus in primary care. Diabetes Obes Metab. (2017) 19:1312–6. doi: 10.1111/dom.12919
45. Hernandez-Jimenez, S, Garcia-Ulloa, AC, Anaya, P, Gasca-Pineda, R, Sanchez-Trujillo, LA, Pena Baca, H, et al. Cost-effectiveness of a self-management and comprehensive training intervention in patients with type 2 diabetes up to 5 years of diagnosis in a specialized hospital in mexico City. BMJ Open Diabetes Res Care. (2021) 9:2097. doi: 10.1136/bmjdrc-2020-002097
46. Xinjun, J, Libo, T, and Mingzi, L. The structured education for type 2 diabetes patients without insulin therapy: a cost⁃effectiveness analysis. Chin Nurs Res. (2022) 36:1257–61. doi: 10.12102/j.issn.1009-6493.2022.07.027
47. Handley, MA, Shumway, M, and Schillinger, D. Cost-effectiveness of automated telephone self-management support with nurse care management among patients with diabetes. Ann Fam Med. (2008) 6:512–8. doi: 10.1370/afm.889
48. O'Reilly, DJ, Blackhouse, G, Bowen, JM, Brozic, A, Agema, P, Punthakee, Z, et al. Economic analysis of a diabetes health coaching intervention for adults living with type 2 diabetes: a single-Centre evaluation from a community-based randomized controlled trial. Can J Diabetes. (2022) 46:165–70. doi: 10.1016/j.jcjd.2021.08.003
49. Mounié, M, Costa, N, Gourdy, P, Latorre, C, Schirr-Bonnans, S, Lagarrigue, J, et al. Correction to: cost-effectiveness evaluation of a remote monitoring programme including lifestyle education software in type 2 diabetes: results of the educ@dom study. Diabet Ther. (2022) 13:1131–2. doi: 10.1007/s13300-022-01248-6
50. Li, J, Parrott, S, Sweeting, M, Farmer, A, Ross, J, Dack, C, et al. Cost-effectiveness of facilitated access to a self-management website, compared to usual care, for patients with type 2 diabetes (help-diabetes): randomized controlled trial. J Med Internet Res. (2018) 20:e201. doi: 10.2196/jmir.9256
51. Hendrie, D, Miller, TR, Woodman, RJ, Hoti, K, and Hughes, J. Cost-effectiveness of reducing glycaemic episodes through community pharmacy management of patients with type 2 diabetes mellitus. J Prim Prev. (2014) 35:439–49. doi: 10.1007/s10935-014-0368-x
52. Gillespie, P, O'Shea, E, O’Hara, MC, and Dinneen, SF. Cost effectiveness of group follow-up after structured education for type 1 diabetes: a cluster randomised controlled trial. Trials. (2014) 15:227. doi: 10.1186/1745-6215-15-227
53. Mash, R, Kroukamp, R, Gaziano, T, and Levitt, N. Cost-effectiveness of a diabetes group education program delivered by health promoters with a guiding style in underserved communities in cape town, South Africa. Patient Educ Couns. (2015) 98:622–6. doi: 10.1016/j.pec.2015.01.005
54. Mikhael, E, Ong, S, and Hussain, S. Cost-effectiveness analysis of the culturally developed diabetes self-management education and support program among type 2 diabetes mellitus patients in Iraq. J Pharm Bioallied Sci. (2023) 15:49. doi: 10.4103/jpbs.jpbs_767_21
55. Derakhshandeh-Rishehri, S, Keshavarz, K, Ghodsi, D, Pishdad, G, and Faghih, S. Cost-effectiveness analysis of group vs. weblog telecommunication (web tel) nutrition education program on glycemic indices in patients with non-insulin dependent diabetes mellitus type 2: a randomized controlled trial. Front Nutr. (2022) 9:915847. doi: 10.3389/fnut.2022.915847
56. Lee, JY, and Lee, SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. (2018) 20:492–500. doi: 10.1089/dia.2018.0098
57. Jiang, X, Ming, W, and You, JH. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res. (2019) 21:e13166. doi: 10.2196/13166
58. Ahlqvist, E, Storm, P, Käräjämäki, A, Martinell, M, Dorkhan, M, Carlsson, A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. The Lancet. (2018) 6:361–9. doi: 10.1016/S2213-8587(18)30051-2
59. Seng, JJB, Kwan, YH, Lee, VSY, Tan, CS, Zainudin, SB, Thumboo, J, et al. Differential health care use, diabetes-related complications, and mortality among five unique classes of patients with type 2 diabetes in Singapore: a latent class analysis of 71,125 patients. Diabetes Care. (2020) 43:1048–56. doi: 10.2337/dc19-2519
60. Toresson Grip, E, Svensson, A, Miftaraj, M, Eliasson, B, Franzén, S, Gudbjörnsdottir, S, et al. Real-world costs of continuous insulin pump therapy and multiple daily injections for type 1 diabetes: a population-based and propensity-matched cohort from the swedish national diabetes register. Diabetes Care. (2019) 42:545–52. doi: 10.2337/dc18-1850
61. Bommer, C, Heesemann, E, Sagalova, V, Manne-Goehler, J, Atun, R, Bärnighausen, T, et al. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. The Lancet. (2017) 5:423–30. doi: 10.1016/S2213-8587(17)30097-9
62. Seuring, T, Archangelidi, O, and Suhrcke, M. The economic costs of type 2 diabetes: a global systematic review. PharmacoEconomics. (2015) 33:811–31. doi: 10.1007/s40273-015-0268-9
63. Jalilian, H, Heydari, S, Imani, A, Salimi, M, Mir, N, and Najafipour, F. Economic burden of type 2 diabetes in Iran: a cost-of-illness study. Health Sci Rep. (2023) 6:e1120. doi: 10.1002/hsr2.1120
64. Abegunde, DO, Mathers, CD, Adam, T, Ortegon, M, and Strong, K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. (2007) 370:1929–38. doi: 10.1016/S0140-6736(07)61696-1
65. Flood, D, Seiglie, JA, Dunn, M, Tschida, S, Theilmann, M, Marcus, ME, et al. The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680 102 adults. The Lancet Healthy Longevity. (2021) 2:e340–51. doi: 10.1016/s2666-7568(21)00089-1
66. Chowdhury, HA, Harrison, CL, Siddiquea, BN, Tissera, S, Afroz, A, Ali, L, et al. The effectiveness of diabetes self-management education intervention on glycaemic control and cardiometabolic risk in adults with type 2 diabetes in low- and middle-income countries: a systematic review and meta-analysis. PLoS One. (2024) 19:e297328. doi: 10.1371/journal.pone.0297328
67. Lamptey, R, Robben, MP, Amoakoh-Coleman, M, Boateng, D, Grobbee, DE, Davies, MJ, et al. Structured diabetes self-management education and glycaemic control in low- and middle-income countries: a systematic review. Diabet Med. (2022) 39:e14812. doi: 10.1111/dme.14812
68. Lamptey, R, Davies, MJ, Khunti, K, Schreder, S, Stribling, B, and Hadjiconstantinou, M. Cultural adaptation of a diabetes self-management education and support (dsmes) programme for two low resource urban settings in Ghana, during the covid-19 era. BMC Health Serv Res. (2022) 22:996. doi: 10.1186/s12913-022-08390-8
69. Dritsaki, M, Gray, A, Petrou, S, Dutton, S, Lamb, SE, and Thorn, JC. Current UK practices on health economics analysis plans (heaps): Are we using heaps of them? Pharmacoeconomics. (2018):253–7. doi: 10.1007/s40273-017-0598-x
70. Edwards, RT, Hounsome, B, Linck, P, and Russell, IT. Economic evaluation alongside pragmatic randomised trials: developing a standard operating procedure for clinical trials units. Trials. (2008) 9:64. doi: 10.1186/1745-6215-9-64
71. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
72. Adarkwah, CC, van Gils, PF, Hiligsmann, M, and Evers, SMAA. Risk of bias in model-based economic evaluations: the ecobias checklist. Expert Rev Pharmacoecon Outcomes Res. (2016) 16:513–23. doi: 10.1586/14737167.2015.1103185
Keywords: health economic evaluation, diabetes, structured education, cost-effectiveness analysis, systematic review
Citation: Ye C, Zhou Q, Yang W, Tao L and Jiang X (2024) Health economic evaluation of structured education programs for patients with diabetes: a systematic review. Front. Public Health. 12:1467178. doi: 10.3389/fpubh.2024.1467178
Edited by:
Teerapon Dhippayom, Naresuan University, ThailandReviewed by:
Jun Jie Benjamin Seng, Ministry of Health, SingaporePiyameth Dilokthornsakul, Chiang Mai University, Thailand
Copyright © 2024 Ye, Zhou, Yang, Tao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Libo Tao, dGFvbGlib0Boc2MucGt1LmVkdS5jbg==; Xinjun Jiang, anhpbmp1bkBtdWhuLmVkdS5jbg==
†These authors have contributed equally to this work
 Caihua Ye
Caihua Ye Qiwei Zhou
Qiwei Zhou Wenfei Yang
Wenfei Yang Libo Tao
Libo Tao Xinjun Jiang
Xinjun Jiang