- 1School of Nursing, Hubei University of Chinese Medicine, Wuhan, China
- 2Nursing Department, Wuhan No.1 Hospital, Wuhan, China
- 3Engineering Research Center of TCM Protection Technology and New Product Development for the Elderly Brain Health, Ministry of Education, Wuhan, China
- 4Hubei Shizhen Laboratory, Wuhan, China
Background: Older adults with cognitive impairment can experience poor oral health due to reduced self-care ability, yet the impact of various oral health indicators on the cognitive ability remains unclear. We investigated the relationship between oral health indicators and mild cognitive impairment (MCI) in older adults.
Methods: A cross-sectional study of 234 older adults aged 65 years or over was performed form January to March 2023 at health screening departments of hospitals. This study used the Mini-mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Activities of Daily Living (ADL), Clinical Dementia Rating (CDR), and Hachinski Ischemic Score (HIS) to measure MCI. Two qualified dentists performed clinical oral examinations (number of teeth lost, dental caries, removable dentures, periodontitis). The other oral health status was measured by subjective assessment questionnaires, and the oral health-related quality of life (OHRQoL) was assessed by Geriatric Oral Health Assessment Index (GOHAI).
Results: Of the 234 older adults, 166 had MCI and 68 had normal cognitive ability. The univariate analyses revealed that older adults with poor oral health indicators of dental caries, mastication ability, oral and maxillofacial pain, self-perceived oral health status and OHRQoL had lower cognitive levels. The stepwise logistic regression analysis observed that higher education level (OR = 0.06, 95%CI = 0.007, 0.567) and OHRQoL score (OR = 0.92, 95%CI = 0.878, 0.963) were negatively associated with the presence of MCI. The area under the ROC curve (AUC) of MCI was 0.675 (95% CI: 0.600, 0.749) with a low sensitivity of 41.6% and a moderate specificity of 86.8%.
Conclusion: OHRQoL was found to be associated with MCI, implying that OHRQoL may be important in cognitive decline. The GOHAI scale can be used to more easily assess the oral health of older adults, which is important for the timely detection of poor oral status to delay cognitive decline.
Introduction
Population aging is currently a significant concern facing the world, and with increasing aging, the health problems of older adults constantly highlighted (1). Among the diseases that endanger the health of older adults, dementia has become the fourth leading cause of death after cardiovascular disease, cancer, and stroke (2). China is one of the fastest-aging countries (3), with a prevalence of 5.56% for dementia and 10–20% for mild cognitive impairment (MCI) in adults over 65 years of age (4, 5), which will bring a serious burden to families and society.
MCI is an intermediate state between normal cognition and dementia in which one or more cognitive domains are impaired and do not yet meet the diagnostic criteria for dementia and do not interfere with the ability to perform daily activities (6). The risk of MCI turning into dementia is much higher than normal older adults, with 10–15% of MCI patients developing dementia every year (7). However, to date there is no cure for dementia (8), the stage of MCI provides a critical “window of opportunity” for the prevention and treatment of dementia (9). Early intervention at the MCI stage can effectively delay the progress of dementia, and even restore the older adults to normal cognitive state (10). Therefore, early intervention and risk management for MCI is greatly important and has become a research hot topic at home and abroad, with important academic significance and lucrative socio-economic benefits.
Oral health is a key indicator of overall health, well-being and quality of life, and poor oral health causes millions of people to suffer from devastating pain and increases the financial burden for society (11). The risk of oral diseases increases with age and can affect the cognitive function of older adults (12, 13). Tooth loss, dental caries, mastication ability, and periodontitis are common oral problems. One of the most common oral problems associated with cognitive impairment is tooth loss (14–16). The older adults with tooth loss can lead to nutritional problems such as impaired intake of micronutrients and vitamins, which can lead to affect cognitive function (17). Mastication is a predictor of cognitive impairment, with the ability to masticate decreasing with age and increasing the risk of cognitive impairment (18). The reason is that normal mastication maintains peripheral sensory input, increases blood supply to different areas of the brain, effectively transmits a large amount of sensory information to the brain, and maintains normal learning and memory functions (19). Periodontitis is also associated with cognitive decline, and the inflammatory response it induces can negatively affect the brain (20).
Although the relationships between tooth loss, mastication ability, and cognitive function have been studied, the measurements and results have been heterogeneous. The relationship between other oral health assessment indicators such as dental caries, lateral mastication, and cognitive function are poorly reported. The relationship between OHRQoL and cognitive function is unclear. Moreover, no study has included comprehensive oral health indicators in the analysis of association between the oral health and cognitive function. Information on the association between various aspects of oral health and cognitive function remains insufficient. Accordingly, this study aimed to determine whether there are associations between various aspects of oral health and cognitive function through oral examinations and electronic questionnaire assessments. The innovation of this study lies in the inclusion of comprehensive indicators and the innovative exploration of the subject by using two methods of oral examinations and questionnaire survey.
Methods
Design and participants
The sample size was calculated with reference to the preliminary study and based on the sample size calculation formula (21). The sample size was estimated using the following formula. n = Z2 * p (1 − p)/e2, Z = 1.96, p = prevalence of MCI in Wuhan (87.8%), e = error rate = 0.05; n = 165 patients, with 15% attrition rate, making a total of 190 participants (22). Eligible participants were recruited from health screening departments at two hospitals, part of the Wuhan in China from January to March 2023. The inclusion criteria were age ≥ 65 years, having sufficient visual and auditory discrimination to undergo neuropsychological tests, willingness to participate in the study, and signing the informed consent form. Exclusion criteria were participants with mental disorders and other serious physical illnesses. The study protocol was reviewed and approved by the Medical Ethics Committee of Hubei University of Chinese Medicine (Approved No. of ethic committee: 2019-IEC-003). Participants signed the informed consent form, after due clarification concerning the study and before data collection. This study developed a data web platform specifically to screen and intervene with MCI, including detailed demographic information, neuropsychological tests, and Traditional Chinese Medicine (TCM) technical interventions, enabling direct target population identification while ensuring correct data entry and export.
Assessments of cognitive function
The cognitive function tests were developed according to the MCI diagnostic criteria of the 2018 China Dementia and Cognitive Disorders Diagnostic and Treatment Guidelines (23). To maximize measurement precision, the cognitive function tests comprised the following tests. The participant was diagnosed with “mild cognitive impairment” when all tests were met.
The mini-mental status examination
The MMSE is one of the most widely used cognitive screening tool worldwide and is used to assess the general cognitive function of participants (24). The cut-off values for this tool are related to education level and this study used education-based cut-offs for MMSE in Chinese to exclude definite dementia: illiterate, ≥17 points; primary school, ≥20 points; and junior high school and above, ≥24 points (25). The Cronbach’s α of MMSE is 0.825, which has good reliability.
The Montreal cognitive assessment
The MoCA is a 30-point test given in 10 min to assess various cognitive tasks including: attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation. A total MoCA score of 26 is the cut-off value for MCI and older adults with a score of <26 were included in the study (26). The Cronbach’s α of MoCA is 0.818, which has a good measurement characteristic.
Activities of daily living
This scale is commonly used to assess physical function and is a common indicator of the lives of people with dementia. In this study, the scale was used to exclude people with impairments in daily living skills and participants with scores <16 were included in the study (27). The scale has good reliability and validity, and the Cronbach’s α of ADL is 0.966.
The clinical dementia rating
The CDR scale comprises six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care, which is graded on a 5-point scale. MCI is defined as CDR = 0.5 (28). The reliability of the scale is 0.84.
Hachinski ischemic score
To exclude cognitive decline due to vascular dementia, participants were asked to have a HIS score of ≤4 in the study (29).
Assessments of oral health status
Oral examinations
Two well-trained dentists used probes and mouth mirrors under artificial lights to examine the number of teeth lost, the presence of dental caries, removable dentures, and periodontitis in older adults.
Oral health surveys
Geriatric Oral Health Assessment Index (GOHAI), the GOHAI is a scale developed specifically to assess oral health-related quality of life (OHRQoL), which is a 12-item questionnaire with the following response options: 1 = very often; 2 = fairly often; 3 = occasionally; 4 = hardly ever; and 5 = never, including 3 subscales of physical function, pain or discomfort, and psychosocial function. A higher total GOHAI score indicates a better OHRQoL, with a range of 12 to 60. The total score was divided into 3 levels: high (57–60 points), medium (51–56 points), and low (≤50 points) (30, 31). For this study, instead of other measurements, GOAHI was chosen, as it has been the most commonly used measurement tool for evaluating the OHRQoL of the older adults in the world. The Cronbach’s α of GOHAI is 0.81, which has a good measurement characteristic. In addition, the caregivers obtained oral health conditions such as mastication ability, unilateral mastication, and oral and maxillofacial pain through interviews.
Potential covariate assessment
The following potential confounders were examined. As possible parameters associated with MCI, demographic confounders included age, gender, monthly income, and educational level.
Data analysis
In the descriptive analysis, categorical and continuous variables were shown by frequency, percentage, mean, and deviation. The comparison analysis between the MCI and normal groups was explored using the chi-squared test and fisher’s exact test. If a p value was less than 0.05, it was considered statistical significance. Logistic regression was set up using those variables that were found to have a significant difference between cognitive function groups in the univariate analysis as independent variables and MCI (Yes = 1, No = 0) as dependent variables to analyze the relationship between OHRQoL and MCI. The binary logistic regression analysis was performed using the stepwise forward method to control for potential confounders. The odds ratio (OR) and 95% confidence interval (CI) were calculated. Receiver operating characteristic (ROC) curves were used to determine the ability of GOHAI score in identifying MCI. All analyses were conducted using SPSS version 25.0 (IBM Corp., Armonk, NY, United States).
Results
Comparison of participant characteristics
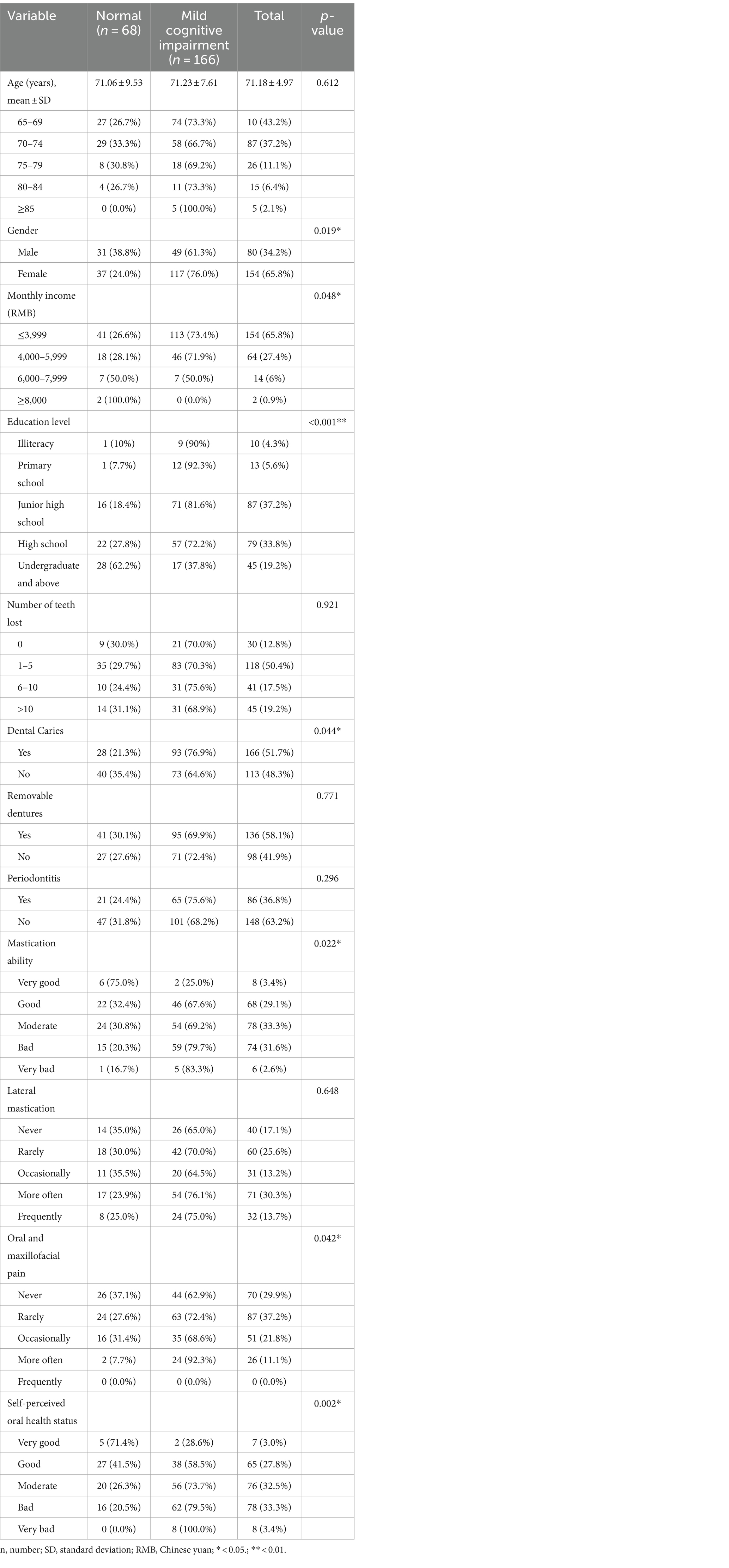
This research included 234 older adults, 80 male and 154 female with a mean age of 71.18 ± 4.97 years. The result showed that 65.8% of older adults had a monthly income of ≤3,999, and the education level was concentrated on junior and high school. Based on the assessment criteria for MCI, more than 70% of people had MCI. Of these, 154 were female and 80 were male, women being more likely to have the condition than men (p = 0.019). In addition, the data showed that there was an association between MCI and different economic levels (p = 0.048) and education levels (p < 0.001). MCI is more common in older adults with low-income (73.4%), and low education level (90.0%), but less common in older adults with high-income levels and high education level. In terms of oral health, dental caries (p = 0.044), mastication ability (p = 0.022), oral and maxillofacial pain (p = 0.042), and self-perceived oral health status (p = 0.002) all differed across cognitive status. MCI is more common in older adults with dental caries (76.9%), masticatory disorders (>70.0%), oral and maxillofacial pain (>60.0%), and bad self-perceived oral health status (>70.0%) (Table 1).
The correlation between GOHAI scale scores and MCI
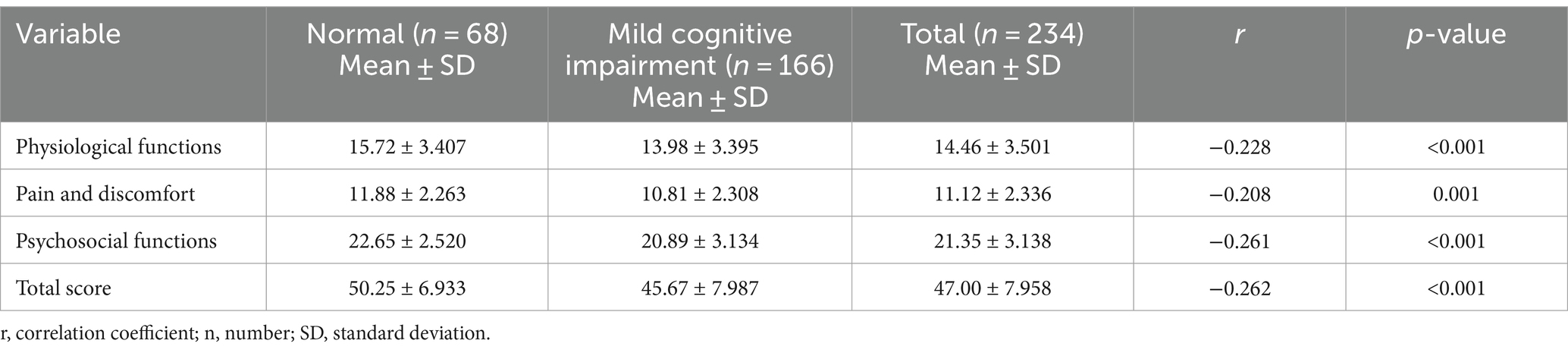
The mean GOHAI assessment index score in this study was 47.00 ± 7.958, which was at a low level of oral health status. The mean score of 14.46 ± 3.501 for the physical function dimension, 11.12 ± 2.336 for the pain and discomfort dimension and 21.35 ± 3.138 for the psychosocial dimension, indicating that OHRQoL of older adults in the region is poor and needs to be improved. We also found a statistically significant weak negative correlation between GOHAI test scores and MCI (p < 0.001). The GOHAI scores were higher in older adults with normal cognition than in those with MCI, suggesting that MCI in older adults is associated with poorer OHRQoL (Table 2).
Oral health-related risk factors for MCI
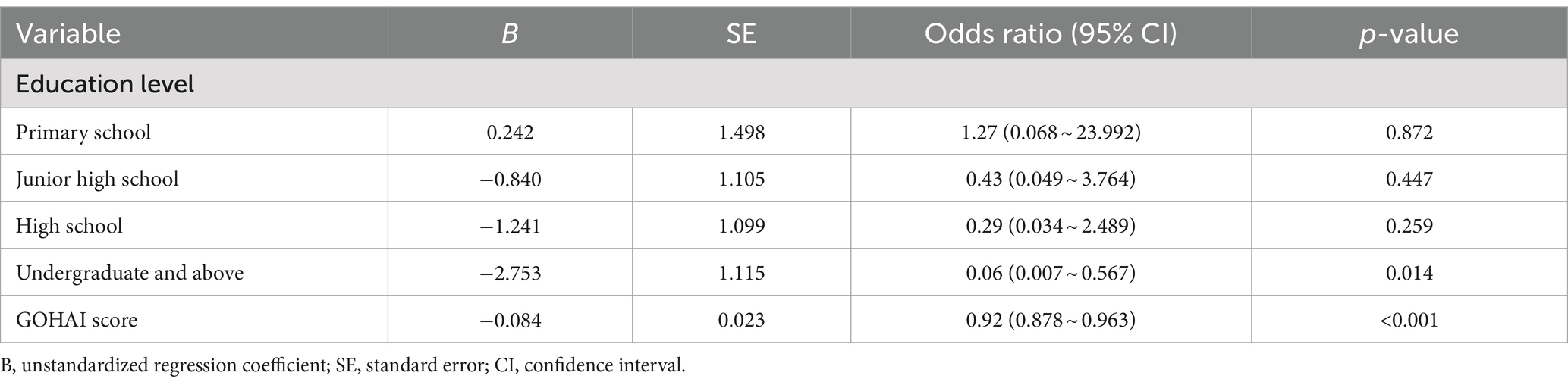
Table 3 describes binary logistic regression analysis for MCI with risk factors. Treating MCI and normal cognition as dependent variables, the variables that were significant for the above analysis (gender, monthly income, education level, dental caries, mastication ability, oral and maxillofacial pain, self-perceived oral health status, and total GOHAI score) were selected as independent variables to enter into a binary logistic regression analysis. Table 3 shows that independent risk factors for MCI were education level (OR = 0.06, 95%CI = 0.007, 0.567) and OHRQoL (OR = 0.92, 95%CI = 0.878, 0.963). The higher education level reduced the risk of MCI, while the lower OHRQoL increased the risk of MCI.
ROC analyses of GOHAI score in identifying MCI
We also carried out the ROC analyses to verify the accuracy and sensitivity of GOHAI in identifying MCI. The best threshold value of GOHAI score in identifying MCI was 43.5. The area under the ROC curve (AUC) of MCI was 0.675 (95%CI: 0.60, 0.75) with a low sensitivity of 41.6% and a moderate specificity of 86.8%.
Discussion
The World Health Organization has listed oral health as one of the top 10 criteria for human health. At present, as the transformation of the medical pattern and the concept of Healthy China continues to grow, oral health has become an important part of people’s pursuit of health. The fourth national oral health epidemiological survey in China showed that the current oral health status of older adults in China is not optimistic, with the serious prevalence of dental caries and periodontal disease (32), and these poor oral health status may increase the risk of cognitive impairment in older adults.
However, research on the relationship between oral health status and MCI is currently limited, which is constrained by the conventional views that “dental disease is not a disease” and “losing teeth is normal aging” (33). This study assessed the relationship between oral health indicators and MCI, the results of the subjective and objective examination revealed that gender, education level, economic level, dental caries, mastication ability, oral and maxillofacial pain, self-perceived oral health status, and the OHRQoL were all correlated with cognitive status in older adults. The regression analysis showed that the GOHAI was negatively associated with cognitive status, and poor OHRQoL in older adults was an independent risk factor for MCI. ROC curves revealed the GOHAI score was determined with 41.6% sensitivity and 86.8% specificity in the prediction of MCI at a cut-off value of 43.5. This is the first study to find that poorer OHRQoL is an independent risk factor for MCI. OHRQoL is a reflection of oral health physiological function, pain and discomfort, and psychosocial function and is closely related to oral physiological dysfunction such as tooth loss, dental caries, and decreased mastication ability, which may be an indicator of true oral health problems. Despite the low sensitivity, the study results provide a certain reference to for clinical study.
Oral diseases are the main influencing factors of OHRQoL, and older adults with poor oral examination results have low OHRQoL (34). Oral diseases, including functional tooth loss, dental caries, and poor mastication function can negatively affect OHRQoL. The low OHRQoL predicts oral problems in older adults and may influence cognitive function through physiological effects (35, 36). Moreover, oral diseases damage the harmony and esthetics of the face, which restricts the social activities of the older adults and can have a detrimental effect on their mental health level. Previous studies have shown that poor OHRQoL can lead to loneliness (37), depression (38), and restriction of mobility and social participation (39). Loneliness (40), depression (41), and reduced social activities (42) have been associated with cognitive decline in older adults. The psychological influence of oral health or the negative psychological impact of OHRQoL on older adults can also affect cognitive function. Therefore, identifying and managing OHRQoL in older adults can help to relieve discomfort, improve oral problems, reduce psychological stress, and also improve cognitive status in older adults with MCI.
In the regression analysis, oral problems such as tooth loss, dental caries, and mastication impairment have not been found to be associated with MCI in older adults, which may be related to factors such as the sample size and the assessment method. Obviously, the impact of poor oral health on the OHRQoL of older adults has a negative effect on cognitive function. Oral health is an easily identifiable and modifiable risk factor. The MCI stage, where older adults retain good mobility and can take care of their oral health, is the best stage for interventions for poor oral health in older adults with cognitive disorders. The OHRQoL assess enables the detection of oral problems, which will help to slow down the decline in cognitive function. The regular objective examination of oral health often has special requirements, and GOHAI is a more comprehensive and subjective evaluation system that reflects the new medical model and view of health. The GOHAI can be used by a variety of researchers to assess the OHRQoL and to improve poor oral status, healthcare behaviors, and habits promptly, which is important for the overall health and quality of life of older adults. Therefore medical workers can adopt targeted interventions to create proper oral awareness and establish proper oral habits in older adults, which may improve their OHRQoL and improve cognitive performance.
There are some limitations in our study. First, participants were recruited through hospitals, which may have selection bias. Second, our study was designed as a cross-sectional study, it was difficult to draw conclusions about the causal relationship between oral health status and MCI. Additionally, Some oral health indicators were assessed by self-report, which may be subject to recall bias. In subsequent studies, there is a need to develop strict inspection measures, refine the classification indicators, expand the sample size and reduce confounding factors to further improve the study design, preferably through longitudinal studies to clarify the causal relationship between various oral health indicators and MCI.
Conclusion
This study intentionally developed a data web platform to screen and intervene older adults with MCI. To improve measurement accuracy and data collation, older adults with MCI were identified through multiple assessments, and their oral health was assessed through subjective and objective examinations. This is the first study to show that OHRQoL is an independent risk factor for MCI and that poor OHRQoL is associated with poor cognitive function in older adults. Therefore medical workers can focus on increasing oral health awareness, establishing proper oral hygiene habits, improving oral problems, and enhancing education about the causes and symptoms of oral problems in older adults, which may improve their OHRQoL and thus maintain and improve cognitive ability. It is also important to pay attention to the psychological impact of poor OHRQoL on older adults, and maintaining the psychological health of older adults also has a role in improving OHRQoL and cognitive ability. However, longitudinal studies with large samples on various oral health indicators are needed in the future.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Hubei University of Chinese Medicine (Approved No. of ethic committee: 2019-IEC-003). The patients/participants provided their written informed consent to participate in this study.
Author contributions
NY: Conceptualization, Writing – original draft, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. BD: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. HH: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – review & editing. YA: Conceptualization, Data curation, Writing – review & editing. XL: Data curation, Investigation, Methodology, Writing – review & editing. SZ: Data curation, Investigation, Writing – original draft. YL: Data curation, Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a National Natural Science Foundation of China (Award number: 81973921).
Acknowledgments
We would like to thank all the participants and special thanks to all experts for support and assistance in the process of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao, J, Wang, L, and Guo, K. Impact of smart health systems on the behavior of older adults under community healthcare. Front Public Health. (2022) 10:1056817. doi: 10.3389/fpubh.2022.1056817
2. Wang, Y, Wang, Q, Li, J, Lu, G, and Liu, Z. Glutamine improves oxidative stress through the Wnt3a/β-catenin signaling pathway in Alzheimer's disease in vitro and in vivo. Biomed Res Int. (2019) 2019:4690280. doi: 10.1155/2019/4690280
3. Wang, X, Liu, M, Li, Y, Guo, C, and Yeh, CH. Community canteen services for the rural elderly: determining impacts on general mental health, nutritional status, satisfaction with life, and social capital. BMC Public Health. (2020) 20:230. doi: 10.1186/s12889-020-8305-9
4. Jia, J, Wang, F, Wei, C, Zhou, A, Jia, X, Li, F, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. (2014) 10:1–9. doi: 10.1016/j.jalz.2013.01.012
5. Jia, J, Zhou, A, Wei, C, Jia, X, Wang, F, Li, F, et al. The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese. Alzheimers Dement. (2014) 10:439–47. doi: 10.1016/j.jalz.2013.09.008
6. Wolk, DA, Sadowsky, C, Safirstein, B, Rinne, JO, Duara, R, Perry, R, et al. Use of Flutemetamol F 18-labeled positron emission tomography and other biomarkers to assess risk of clinical progression in patients with amnestic mild cognitive impairment. JAMA Neurol. (2018) 75:1114–23. doi: 10.1001/jamaneurol.2018.0894
7. Roberts, R, and Knopman, DS. Classification and epidemiology of MCI. Clin Geriatr Med. (2013) 29:753–72. doi: 10.1016/j.cger.2013.07.003
8. Alzheimer's Disease International . World Alzheimer's report 2018: Global impact of Dementia. (2018). Available at: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf (Accessed May 25, 2023).
9. Zhao, X, Wang, L, Ge, C, Liu, X, Chen, M, and Zhang, C. Effect of process-based multi-task cognitive training program on executive function in older adults with mild cognitive impairment: study rationale and protocol Design for a Randomized Controlled Trial. Front Psych. (2020) 11:655. doi: 10.3389/fpsyt.2020.00655
10. Godinho, C, Camozzato, AL, Onyszko, D, and Chaves, ML. Estimation of the risk of conversion of mild cognitive impairment of Alzheimer type to Alzheimer's disease in a south Brazilian population-based elderly cohort: the PALA study. Int Psychogeriatr. (2012) 24:674–81. doi: 10.1017/S1041610211002043
11. World Health Organization . (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/oral-health.Oralhealth (Assessed May 25, 2023).
12. McGrath, C, and Bedi, R. The importance of oral health to older people's quality of life. Gerodontology. (1999) 16:59–63. doi: 10.1111/j.1741-2358.1999.00059.x
13. Noble, JM, Scarmeas, N, and Papapanou, PN. Poor oral health as a chronic, potentially modifiable dementia risk factor: review of the literature. Curr Neurol Neurosci Rep. (2013) 13:384. doi: 10.1007/s11910-013-0384-x
14. Okamoto, N, Morikawa, M, Okamoto, K, Habu, N, Iwamoto, J, Tomioka, K, et al. Relationship of tooth loss to mild memory impairment and cognitive impairment: findings from the Fujiwara-kyo study. Behav Brain Funct. (2010) 6:77. doi: 10.1186/1744-9081-6-77
15. Li, J, Xu, H, Pan, W, and Wu, B. Association between tooth loss and cognitive decline: a 13-year longitudinal study of Chinese older adults. PLoS One. (2017) 12:e0171404. doi: 10.1371/journal.pone.0171404
16. Yoo, JJ, Yoon, JH, Kang, MJ, Kim, M, and Oh, N. The effect of missing teeth on dementia in older people: a nationwide population-based cohort study in South Korea. BMC Oral Health. (2019) 19:61. doi: 10.1186/s12903-019-0750-4
17. Kim, JM, Stewart, R, Prince, M, Kim, SW, Yang, SJ, Shin, IS, et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry. (2007) 22:850–5. doi: 10.1002/gps.1750
18. Zhou, J, Lv, Y, Mao, C, Duan, J, Gao, X, Wang, J, et al. Development and validation of a nomogram for predicting the 6-year risk of cognitive impairment among Chinese older adults. J Am Med Dir Assoc. (2020) 21:864–871.e6. doi: 10.1016/j.jamda.2020.03.032
19. Krishnamoorthy, G, Narayana, AI, and Balkrishanan, D. Mastication as a tool to prevent cognitive dysfunctions. Jpn Dent Sci Rev. (2018) 54:169–73. doi: 10.1016/j.jdsr.2018.06.001
20. Luo, J, Wu, B, Zhao, Q, Guo, Q, Meng, H, Yu, L, et al. Association between tooth loss and cognitive function among 3063 Chinese older adults: a community-based study. PLoS One. (2015) 10:e0120986. doi: 10.1371/journal.pone.0120986
21. Kelsey, J, Whittemore, A, Evans, A, and Thompson, W. Methods of sampling and estimation of sample size. Methods Observat Epidemiol. (1996) 311:340. doi: 10.1093/oso/9780195083774.003.0012
22. Ai, YT, Hu, H, Wang, L, Gao, XL, Wang, ZC, Ren, HR, et al. Current status of cognitive function and risk factors of the older adults in Wuhan. Chin J Gerontol. (2019) 39:2507–10. doi: 10.3969/j.issn.1005-9202.2019.10.065
23. Dementia, C, and Diagnostic, CDTreatment Guidelines Writing GroupCognitive Disorders CommitteeNeurologist BranchChinese Medical Association. 2018 Chinese guidelines for the diagnosis and treatment of dementia and cognitive impairment (V): diagnosis and treatment of mild cognitive impairment. J Chinese Med. (2018) 98:1294–301. doi: 10.3760/cma.j.issn.0376-2491.2018.17.003
24. Folstein, MF, Folstein, SE, and McHugh, PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
25. Zhang, Z, Ren, W, Shao, B, Xu, H, Cheng, J, Wang, Q, et al. Leukoaraiosis is associated with worse short-term functional and cognitive recovery after minor stroke. Neurol Med Chir (Tokyo). (2017) 57:136–43. doi: 10.2176/nmc.oa.2016-0188
26. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
27. Tian, JZ, Xie, HG, Wang, LN, Wang, YH, Wang, HL, Shi, J, et al. Guidelines for the treatment of dementia in Alzheimer's disease in China (2020 edition). J Chinese Geriatr Med. (2021) 40:269–83. doi: 10.3760/cma.j.issn.0254-9026.2021.03.001
28. Morris, JC . The clinical Dementia rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/wnl.43.11.2412-a
29. Moroney, JT, Bagiella, E, Desmond, DW, Hachinski, VC, Mölsä, PK, Gustafson, L, et al. Meta-analysis of the Hachinski ischemic score in pathologically verified dementias. Neurology. (1997) 49:1096–105. doi: 10.1212/wnl.49.4.1096
30. Atchison, KA, and Dolan, TA. Development of the geriatric Oral health assessment index. J Dent Educ. (1990) 54:680–7. doi: 10.1002/j.0022-0337.1990.54.11.tb02481.x
31. Ling, JQ, and Wang, AD. Development of a Chinese version of the geriatric Oral health assessment index (GOHAI). J Chinese Geriatr Dentistry. (2003) 3:5–9.
32. Wang, X . Fourth National Oral Health Epidemiological Sample Survey People's Medical Publishing House (PMPH) (2008).
33. Wang, QQ, Liu, CY, Feng, JY, and Hu, B. Survey of teeth loss status and oral health behaviors of elderly individuals in Binjiang district. China J Mod Med. (2015) 25:73–6.
34. Ortíz-Barrios, LB, Granados-García, V, Cruz-Hervert, P, Moreno-Tamayo, K, Heredia-Ponce, E, and Sánchez-García, S. The impact of poor oral health on the oral health-related quality of life (OHRQoL) in older adults: the oral health status through a latent class analysis. BMC Oral Health. (2019) 19:141. doi: 10.1186/s12903-019-0840-3
35. Swoboda, J, Kiyak, HA, Persson, RE, Persson, GR, Yamaguchi, DK, MacEntee, MI, et al. Predictors of oral health quality of life in older adults. Spec Care Dentist. (2006) 26:137–44. doi: 10.1111/j.1754-4505.2006.tb01714.x
36. Figueredo, OMC, de Oliveira, LFS, Wanderley, RL, Cavalcanti, YW, and Rodrigues Garcia, RCM. Masticatory function in nursing home residents: correlation with the nutritional status and oral health-related quality of life. J Oral Rehabil. (2020) 47:1511–20. doi: 10.1111/joor.13096
37. Rouxel, P, Heilmann, A, Demakakos, P, Aida, J, Tsakos, G, and Watt, RG. Oral health-related quality of life and loneliness among older adults. Eur J Ageing. (2016) 14:101–9. doi: 10.1007/s10433-016-0392-1
38. Rouxel, P, Tsakos, G, Chandola, T, and Watt, RG. Oral health-a neglected aspect of subjective well-being in later life. J Gerontol B Psychol Sci Soc Sci. (2018) 73:382–6. doi: 10.1093/geronb/gbw024
39. Makhija, SK, Gilbert, GH, Clay, OJ, Matthews, JC, Sawyer, P, and Allman, RM. Oral health-related quality of life and life-space mobility in community-dwelling older adults. J Am Geriatr Soc. (2011) 59:512–8. doi: 10.1111/j.1532-5415.2010.03306.x
40. Sutin, AR, Stephan, Y, Luchetti, M, and Terracciano, A. Loneliness and risk of dementia. J Gerontol B Psychol Sci Soc Sci. (2020) 75:1414–22. doi: 10.1093/geronb/gby112
41. Muhammad, T, and Meher, T. Association of late-life depression with cognitive impairment: evidence from a cross-sectional study among older adults in India. BMC Geriatr. (2021) 21:364. doi: 10.1186/s12877-021-02314-7
42. Kelly, ME, Duff, H, Kelly, S, McHugh Power, JE, Brennan, S, Lawlor, BA, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev. (2017) 6:259. doi: 10.1186/s13643-017-0632-2
Keywords: mild cognitive impairment, oral health, oral health-related quality of life, older adults, association
Citation: Ye N, Deng B, Hu H, Ai Y, Liu X, Zhou S and Li Y (2024) The association between oral health and mild cognitive impairment in community-dwelling older adults. Front. Public Health. 12:1464439. doi: 10.3389/fpubh.2024.1464439
Edited by:
Takao Yamasaki, Minkodo Minohara Hospital, JapanReviewed by:
Gerd Faxén Irving, Karolinska Institutet (KI), SwedenSupawadee Naorungroj, Prince of Songkla University, Thailand
Copyright © 2024 Ye, Deng, Hu, Ai, Liu, Zhou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Hu, aHVpaHU5MEBoYnRjbS5lZHUuY24=
 Niansi Ye
Niansi Ye Bei Deng1
Bei Deng1 Yating Ai
Yating Ai Shi Zhou
Shi Zhou