- 1Department of Nursing, Sir Run Run Hospital, Nanjing Medical University, Nanjing, China
- 2Department of Nursing, Changshu No.5 People’s Hospital, Suzhou, China
- 3Department of Nursing, Changshu No.2 People’s Hospital, Suzhou, China
Objective: Hearing and functional mobility impairments are recognized as risk factors for cognitive decline in older adults, yet the causal relationship underlying these associations is not well-understood. This study aims to explore whether engagement in social activities mediates the link between hearing or functional mobility impairment and cognitive decline.
Methods: This cross-sectional study was carried out in two cities in Jiangsu Province, Eastern China. Participants self-reported hearing impairment and social activity engagement, whereas functional mobility impairment was assessed using the Timed Up and Go Test (TUGT). Cognitive function was evaluated through the Modified Mini-Mental State Examination (MMSE). Logistic regression analysis explored factors influencing cognitive function, and mediation analysis was conducted to examine the relationship between hearing or functional mobility impairment and cognitive decline.
Results: The study included 10,217 adults aged 60 and above. Among them, 19.35% reported hearing impairment, while 40.86% failed the Timed Up and Go Test (TUGT). The Modified Mini-Mental State Examination (MMSE) indicated a 30.40% prevalence of cognitive decline. Logistic regression analysis identified significant associations of cognitive function with factors such as gender, age, education level, residency, living arrangement, hyperlipidemia, cerebrovascular disease, alcohol consumption, smoking, Activities of Daily Living (ADLs), Instrumental Activities of Daily Living (IADLs), social activity, hearing, and functional mobility (p < 0.01). Mediation analysis, after adjusting for confounders, showed that social activity engagement partially mediated the impact of functional mobility impairment on cognitive decline (indirect effect: −0.0947, 95% Bootstrapped CI: −0.1228, −0.0695; proportion of total effect: 11.635%, p < 0.01). However, no mediation effect was observed in the relationship between self-reported hearing impairment and cognitive decline.
Conclusion: This study revealed that social activity engagement plays a mediating role in the relationship between functional mobility and cognitive function, but it does not significantly influence the relationship between self-reported hearing impairment and cognitive decline. These findings suggest that social activity engagement could be a crucial factor in preventing cognitive deterioration among older adults with functional mobility impairments.
1 Introduction
With the acceleration of global aging, the older adult population in China is expanding. The data from National Bureau of Statistics in 2023 shows that individuals aged 60 and above now number 280.04 million, comprising 19.8% of China’s total population, signaling a shift toward a moderately aged society. Aging correlates with various non-communicable diseases, such as hearing loss, functional disability, and cognitive decline (1–3). These conditions can potentially progress to more severe cognitive issues like dementia, leading to decreased quality of life and increased early mortality (4–6). Consequently, the identification of risk factors that could prevent or delay cognitive impairment is essential for health care systems.
Numerous studies, encompassing epidemiological research, systematic reviews, and meta-analyses, have established a significant association between age-related hearing loss and cognitive decline (7–12). The underlying etiological mechanisms linking the two are extensively researched but remain inconclusive. Various theoretical models have been suggested, such as the cognitive load hypothesis, common cause hypothesis, cascade hypothesis, and the overdiagnosis or harbinger hypothesis (13, 14). Under the cascade hypothesis, some studies indicate that the link between hearing impairment and cognitive decline is mediated by social engagement (15–19), while others report contrasting findings (7, 14, 20). Consequently, a definitive conclusion has not yet been reached.
Physical fitness often declines significantly with age, with extensive research underscoring reductions in muscular strength, balance, gait speed, functional mobility, and cardiorespiratory capacity in individuals over 60 years old (2). This deterioration in physical performance is consistently associated with diminished cognitive performance and accelerated cognitive decline, though the exact nature and extent of these relationships are still not fully understood (21–24). A prospective cohort study in China identified social isolation as a potential mediator in the link between physical mobility and cognitive function (25). However, the mediating role of social activity in the relationship between functional mobility impairment and cognitive decline has been less explored.
Social engagement is a critical determinant of wellbeing across all life stages (26). Substantial evidence indicated that reduced social participation was related with increased incidence of dementia (27, 28). Considering the established associations between hearing impairment, functional mobility impairment, and cognitive decline, enhancing social engagement in older adults could be a viable approach to mitigating cognitive decline. Thus, this study aimed to investigate the relationship between self-reported hearing impairment, functional mobility impairment, and cognitive decline, and to assess whether social activity plays a mediating role in these relationships.
2 Methods
2.1 Participants and basic information
The Sir Run Run Hospital is conducting a cohort study focused on monitoring older adult disability among individuals aged 60 and older. This research involved participants from diverse settings, such as communities, outpatient clinics, hospital inpatient departments, and nursing homes. Eligible participants were required to be fully conscious, capable of effective communication, and willing to participate in research activities. The present analysis utilizes baseline data collected from February 1st, 2022, to April 30th, 2023, in two cities (Nanjing and Suzhou) in Jiangsu Province. The Ethics Committee of Sir Run Run Hospital, affiliated with Nanjing Medical University, reviewed and approved our study (No. 2021-SR-047). Our study was conducted in strict compliance with the Declaration of Helsinki, and all participants signed a written informed consent form. A total of 10,217 individuals from this cohort are included in the current analysis. As part of the study protocol, all participants underwent a comprehensive standardized geriatric assessment.
Participants provided information on sociodemographic factors such as education level, marital status, alcohol consumption, and smoking habits, along with medical details including chronic conditions. Based on prior research (12, 29), our study collected extensive baseline information, including a variety of potential confounding factors. These included sex (female, male), age (as a continuous variable in years), education level (≤6 years, 7–9 years, 10–12 years, >12 years), marital status (married, single), residency (urban, rural), living arrangement (alone without a caregiver, alone with a caregiver, in a nursing home, living with family), and health-related factors such as hypertension (yes, no), diabetes (yes, no), hyperlipidemia (yes, no), cerebrovascular disease (yes, no), cardiovascular disease (yes, no), polypharmacy (none, 1–2 medications, ≥3 medications), alcohol consumption (never, used to, always), smoking status (never, used to, always), Activities of Daily Living (ADLs) (total dependence, extensive assistance, moderate assistance, limited assistance, independence), and Instrumental Activities of Daily Living (IADLs) as a continuous variable.
2.2 Self-reported hearing impairment
Hearing impairment was determined based on self-reported hearing difficulties. Participants responded to the question, ‘If someone is speaking in a normal voice in the room, would you be able to hear it?’ Those who answered ‘Yes’ were classified as having no self-reported hearing impairment, whereas a ‘No’ response indicated self-reported hearing impairment. Additionally, participants who reported using hearing aids were categorized as having self-reported hearing impairment.
2.3 Cognitive impairment
Cognitive function in this study was evaluated using the modified Chinese version of the Mini-Mental State Examination (MMSE) (30). The MMSE is extensively utilized for dementia screening, cognitive function assessment, and as a standard for determining endpoint outcomes in clinical trials. It also serves as a benchmark in the diagnostic performance and validity studies of various single-domain tests (31, 32). This scale comprises 30 items across five dimensions: orientation, registration, attention and calculation, recall, and language. Scores range from 0 to 30, with higher scores reflecting better cognitive function. The education-adjusted MMSE demonstrates enhanced diagnostic accuracy for dementia, marked by high sensitivity and specificity (33). For this study, cognitive impairment was identified using cutoff scores of 21 and 25 on the MMSE. Specifically, a score below 21 indicated cognitive impairment for participants with less than 6 years of education, and a score below 25 signified impairment for those with more than 6 years of education.
2.4 Functional mobility impairment
Functional mobility in this study was assessed using the Timed Up and Go Test (TUGT). The TUGT is a rapid and practical method for evaluating mobility, gait, balance, and fall risk (34). It involves timing how long it takes for a person to rise from a chair, walk three meters, turn, return to the chair, and sit down again. In our analysis, a cutoff time of 12 s was employed to categorize TUGT results into two groups: pass and fail.
2.5 Social activity
Social activity refers to social interactions between people, where individuals use certain tools to transmit information and exchange ideas to achieve a specific purpose. Social activities encompass various collective events aimed at maintaining interdependent and interconnected social relationships formed through interactions within a social group. For assessing social activity engagement, participants were inquired about their frequency of participation in various social activities. These activities encompassed gatherings, board games, attending events at community senior centers, or any other activities involving communication and interaction. The specific questions posed was, ‘How often do you join in social activities?’ Available response options ranged from none, 1–3 days per week, 4–6 days per week, to daily.
2.6 Statistical analyses
Data analysis and processing in this study were conducted using IBM SPSS Statistics version 26. Categorical variables were represented as counts and percentages, while non-normally distributed continuous variables were described using medians and interquartile ranges (IQR). Group differences were evaluated using the Mann–Whitney U test for non-normally distributed continuous variables and the chi-square test for categorical variables. The risk factors for cognitive decline were analyzed using binomial logistic regression. Additionally, mediation effect analysis was carried out using the SPSS macro-PROCESS program (Model 4) developed by Hayes. A p-value of less than 0.05 was deemed to indicate statistical significance.
3 Results
3.1 Characteristics of subjects
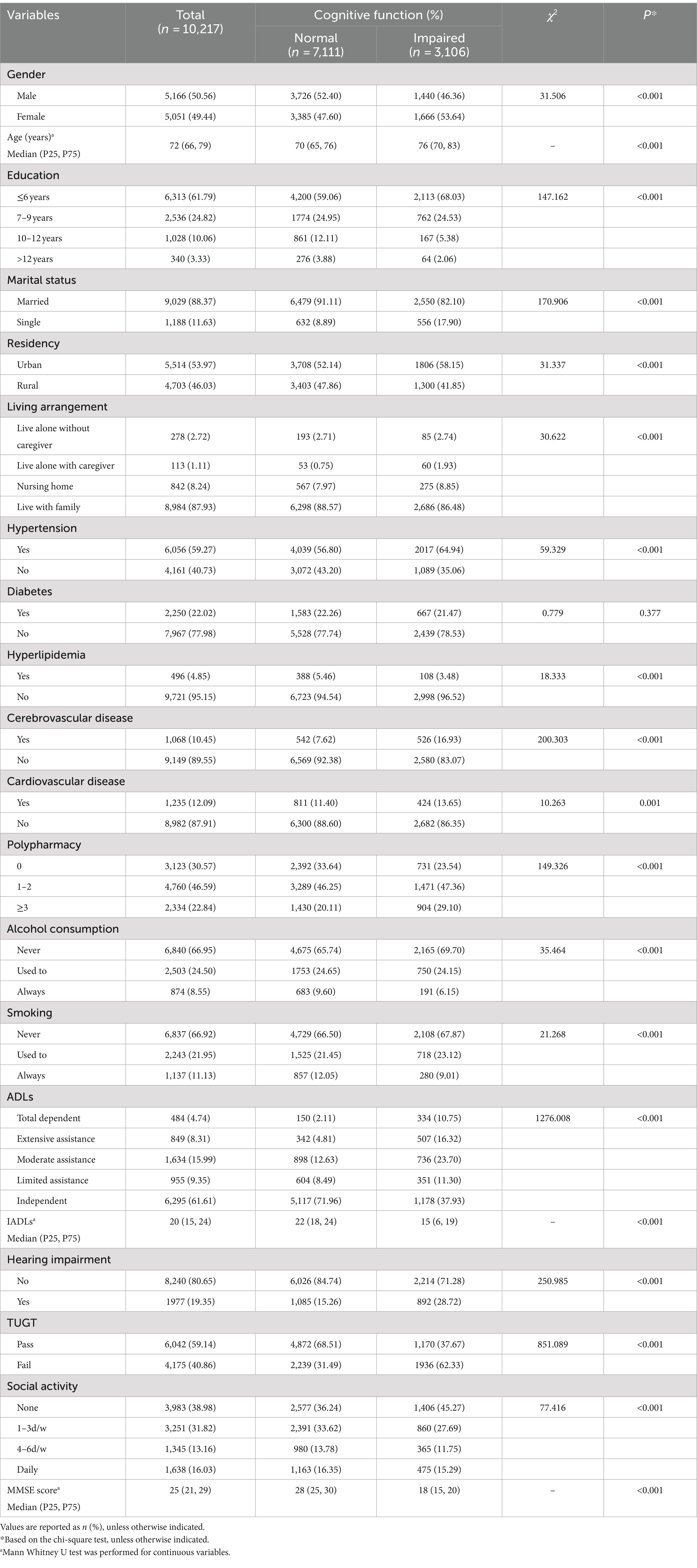
The analysis included 10,217 participants, with a median age of 72 years (interquartile range: 66–79), and 50.56% (5,166) of them being male. Of these participants, 30.40% (3,106) were identified with cognitive impairment, 19.35% (1,977) reported hearing impairment, and 40.86% (4,175) failed the Timed Up and Go Test (TUGT). Regarding social activity, 38.98% (3,983) reported infrequent social engagement, 31.82% (3,251) participated 1 to 3 days per week, 13.16% (1,345) engaged 4–6 days per week, and 16.03% (1,638) were involved in daily social activities. Demographic and clinical characteristics were presented in Table 1. Significant differences were observed between the two cognitive function groups (p < 0.001) in terms of gender, age, education level, marital status, residency, living arrangement, hypertension, hyperlipidemia, cerebrovascular disease, cardiovascular disease, polypharmacy, alcohol consumption, smoking, ADLs, IADLs, self-reported hearing impairment, TUGT, and social activity engagement.
3.2 Logistic regression analysis
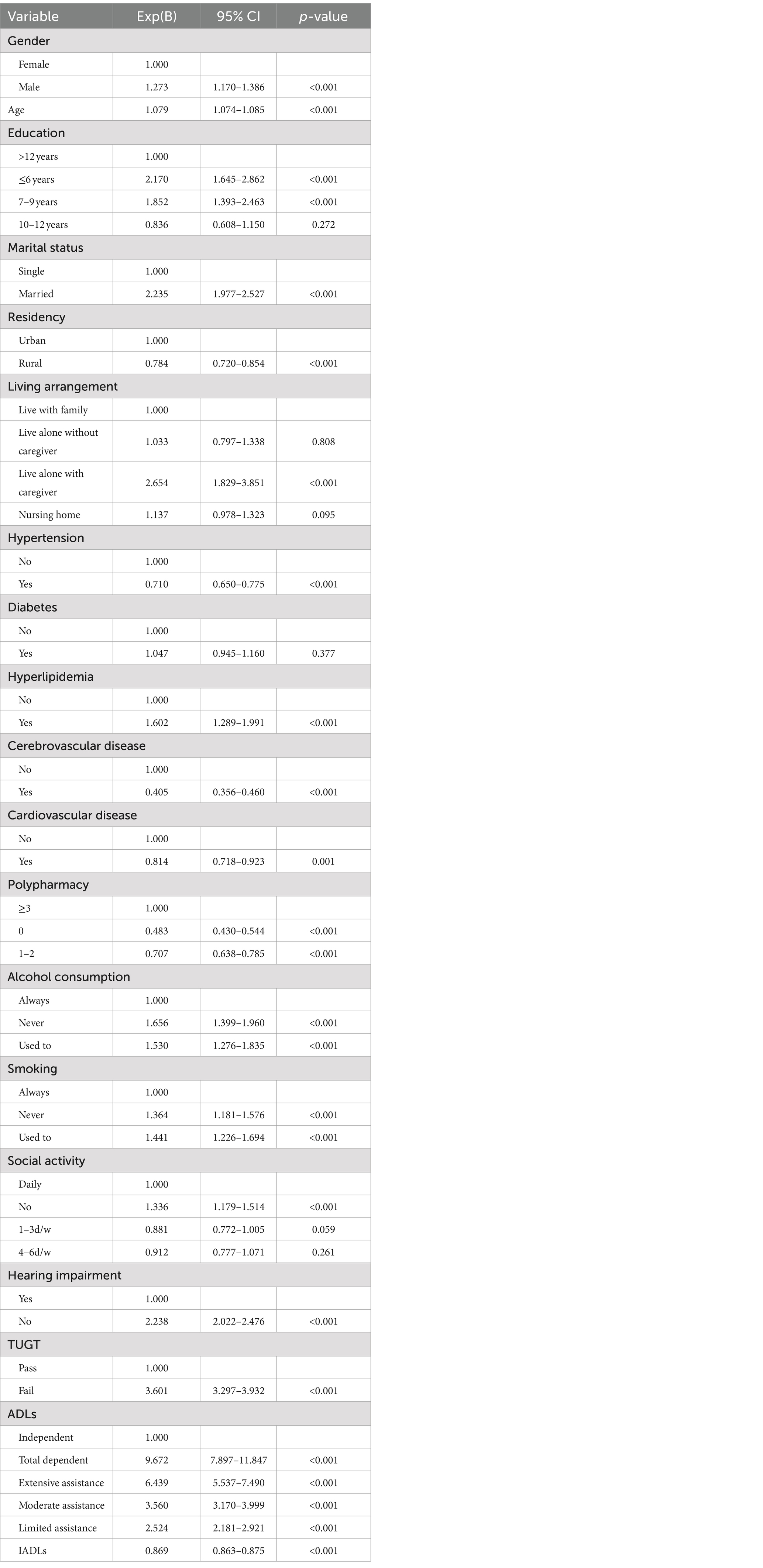
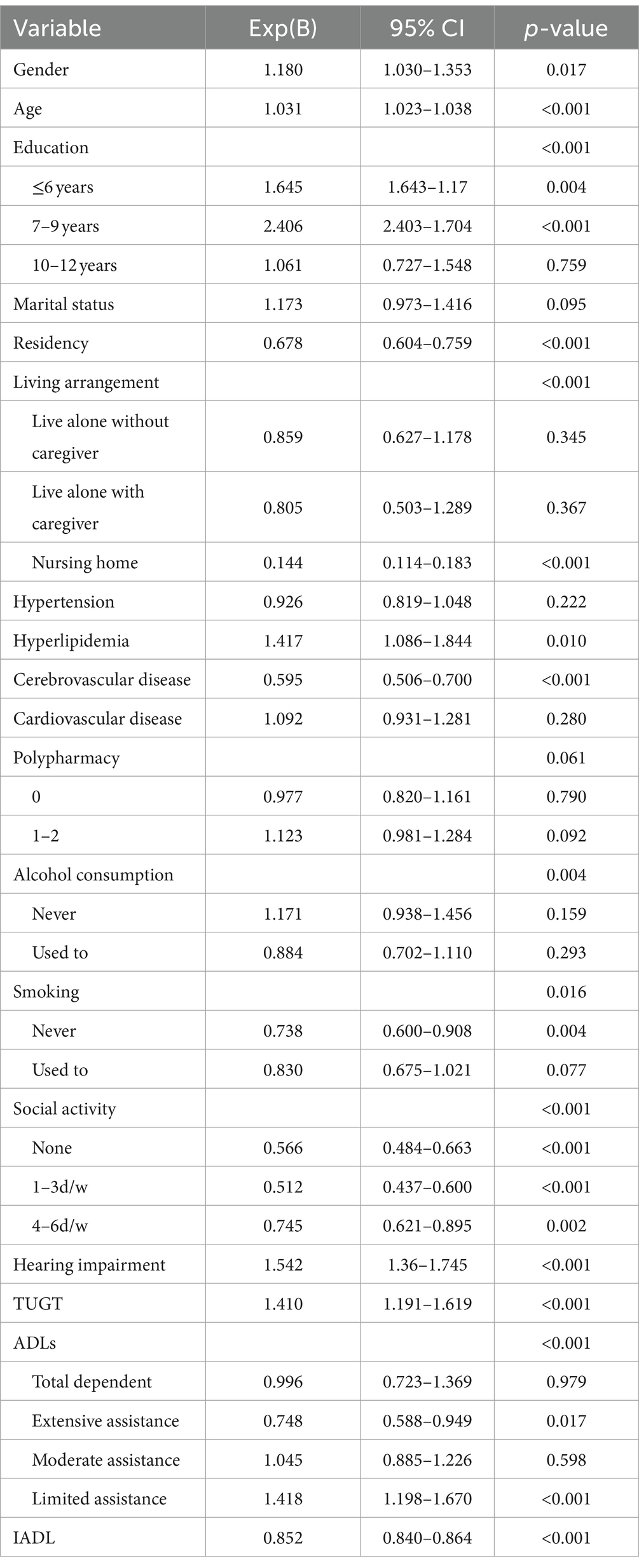
Table 2 displays the outcomes of univariate logistic regression analysis concerning the risk factors for cognitive impairment. Notably, diabetes was the only factor not significantly linked to cognitive decline. In the multivariate logistic regression analysis, factors such as gender, age, education, residency, living arrangement, hyperlipidemia, cerebrovascular disease, alcohol consumption, smoking habits, ADLs, IADLs, social activity engagement, self-reported hearing impairment, and functional mobility (assessed by TUGT) emerged as independent variables associated with cognitive decline, as detailed in Table 3.
3.3 Mediation analysis
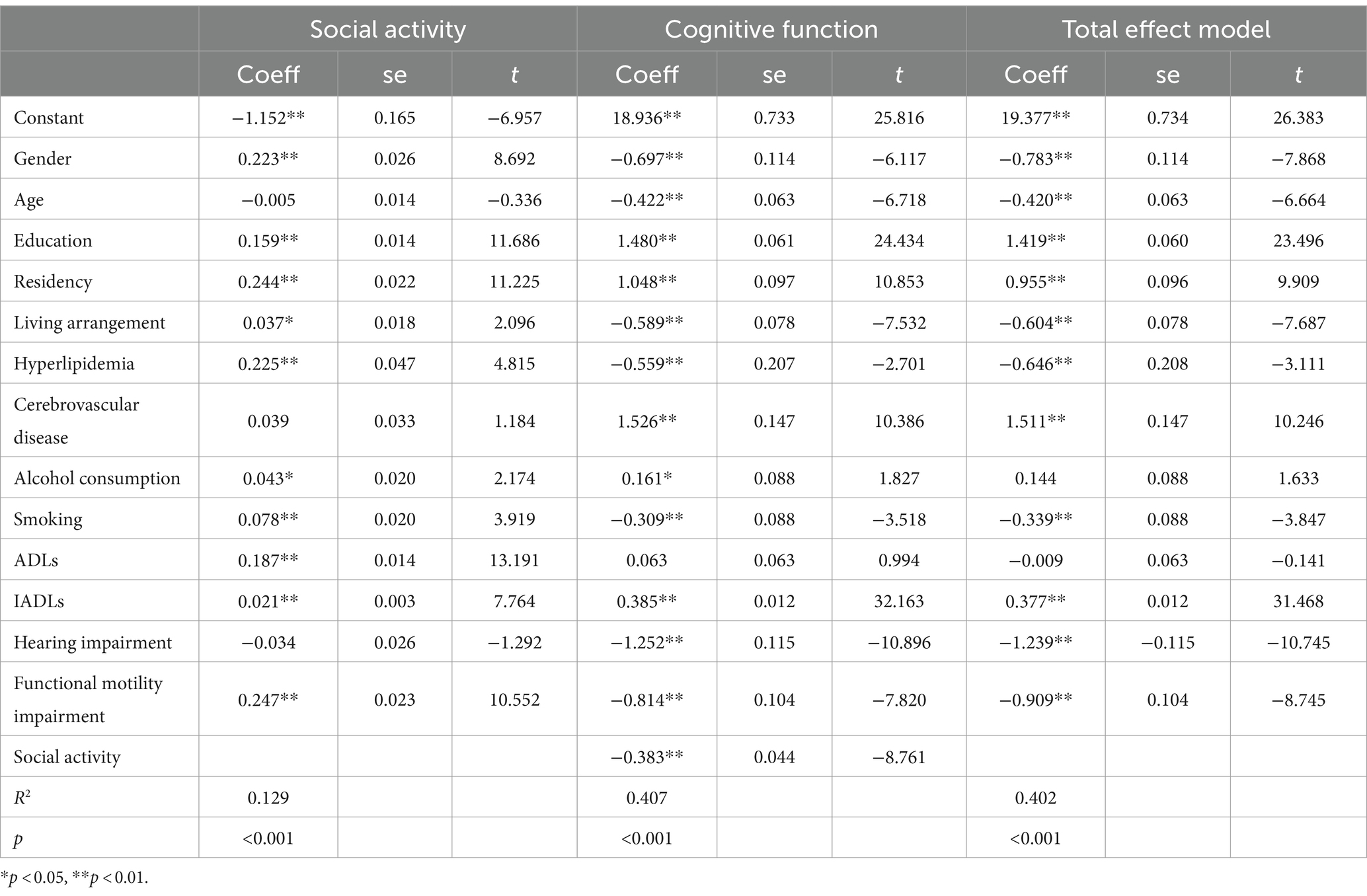
The mediation analysis examining the impact of social activity on hearing, functional mobility, and cognitive function is presented in Tables 4, 5 and Figure 1. The analysis revealed that social activity significantly mediated the relationship between functional mobility and cognitive function, but it did not mediate the association between self-reported hearing impairment and cognitive function. Both the total and direct effects were significant (p < 0.001). In Path 1, after controlling for variables such as gender, age, education, residency, living arrangement, hyperlipidemia, cerebrovascular disease, alcohol consumption, smoking, ADLs, IADLs, and functional mobility, self-reported hearing impairment was significantly associated with cognitive decline. However, the mediation effect of social activity in this pathway was not significant. Conversely, Path 2 demonstrated that the impact of functional mobility impairment on cognitive decline was significantly mediated by social activity, after adjusting for other covariates, with an effect value of −0.0947 (95% Bootstrapped CI: −0.1228 to −0.0695).
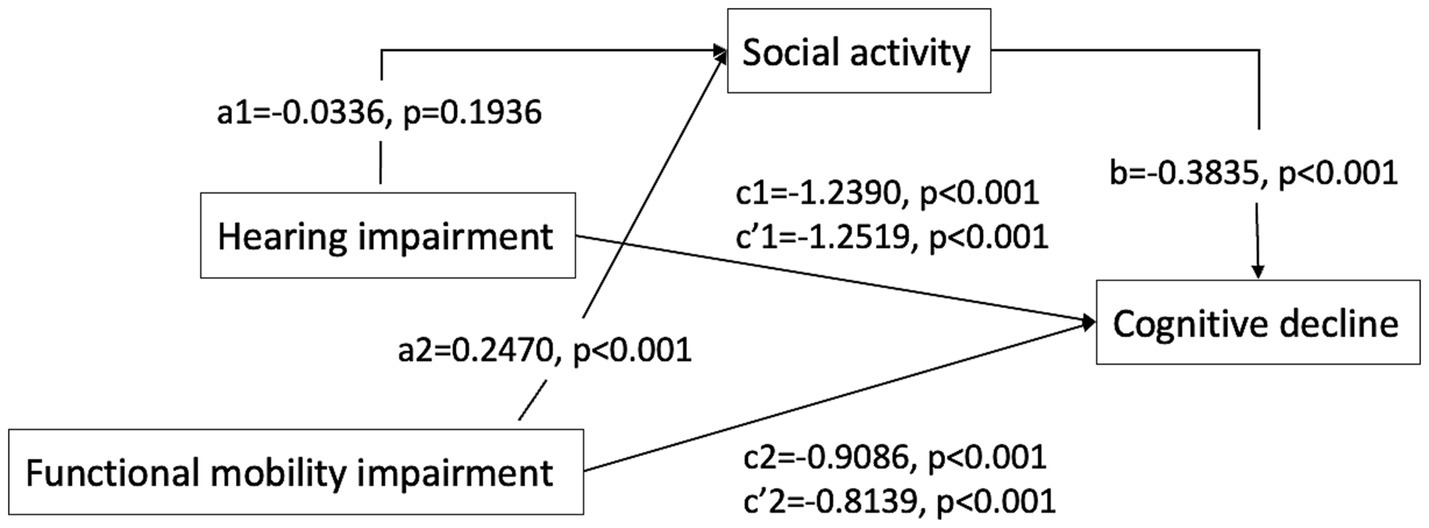
Figure 1. Path diagram of the mediation analysis of social activity on the relationship between hearing impairment/functional mobility impairment and cognitive decline. c, total effect; c’, direct effect; a*b, mediate effect Path1: the direct effect of hearing impairment on cognitive decline was c’1 [−1.2519, p < 0.001]. The indirect effect of hearing impairment on cognitive decline through social activity was a1*b (0.0129, 95% CI [−0.0056, 0.0333]), total effect was c1 [−1.2390, p < 0.001], suggesting that social activity not significantly mediates the relationship between hearing impairment and cognitive decline. Path2: the direct effect of functional mobility impairment on cognitive decline was c’2 [−0.8139, p < 0.001]. The indirect effect of functional mobility impairment on cognitive decline through social activity was a2*b (−0.0947, 95% CI [−0.1228, −0.0695]), total effect was c2 [−0.9086, p < 0.001], suggesting that social activity partially mediates the relationship between functional mobility impairment and cognitive decline.
4 Discussion
The findings of this study indicate that cognitive function in the older adult is significantly influenced by various factors, including gender, age, education level, residency, living arrangement, hyperlipidemia, cerebrovascular disease, alcohol consumption, smoking, ADLs, IADLs, as well as hearing, functional mobility, and social activity engagement. Collectively, these factors contribute to 40.22% of the observed cognitive decline. Moreover, social activity engagement was found to partially mediate the relationship between functional mobility impairment and cognitive decline. However, it did not significantly impact the relationship between self-reported hearing impairment and cognitive decline.
4.1 Risk factors for cognitive decline
The risk factors for cognitive decline in older adults identified in this study aligned closely with those reported in previous research. The 2020 Lancet Commission report highlighted 12 potentially modifiable risk factors for dementia, including lower education levels, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, limited social contact, alcohol consumption, traumatic brain injury, and air pollution. Addressing these factors could potentially prevent or delay up to 40% of dementia cases (12). However, much of the evidence in that report was based on studies from high-income countries, suggesting possible variations across different cultural and environmental contexts. Consequently, this study incorporated significant risk factors identified here as control variables in the mediation analysis to assess the impact of social activity on hearing and functional mobility in relation to cognitive decline.
4.2 Relationship between social activity engagement and cognition
This study has established that social activity engagement serves as both an independent risk factor for cognitive decline and a mediator between functional mobility and cognitive status, aligning with prior research. A 28-year follow-up study in the UK involving 10,308 participants demonstrated that increased social contact at age 60 correlated with a reduced dementia risk over 15 years (HR for one standard deviation increase in social contact frequency 0.9, 95% CI 0.8–1.0) (35). Similarly, a Japanese longitudinal cohort study with 13,984 adults aged over 65, spanning an average of 10 years, employed a five-point social contact scale encompassing marital status, family support exchange, friend contact, community group participation, and paid work. This study found a linear association between higher scores on the scale and decreased dementia risk, with individuals scoring highest being 46% less likely to develop dementia compared to those with the lowest scores (36). Beyond dementia risk, social contact also directly influenced other aspects of mental and physical health, including mortality rates. Research indicated that stronger social connections were linked to improved health outcomes and increased longevity (37, 38). These findings collectively suggest that enhanced social contact in late middle age could modestly reduce dementia risk, even after adjusting for socio-economic and other lifestyle factors.
4.3 Relationship among hearing impairment, social activity, and cognitive decline
Hearing loss currently affects approximately 20% of the global population, which equates to over 1.5 billion people (39). It is a prominent and extensively researched risk factor for cognitive decline. Consistent with previous findings, our study confirmed the significant association between self-reported hearing impairment and cognitive decline. However, the impact of hearing on cognition might extend beyond social activity engagement. Prior research has shown that hearing loss can increase social isolation and loneliness in older adults, often due to challenges in communication and engagement, resulting in reduced participation in social activities (40, 41). The connection between social isolation or loneliness and dementia is well-documented (17). Thus, hearing impairment may lead to decreased social interaction, which in turn can contribute to cognitive impairment (15, 42). Our study presents findings that diverge from some established hypotheses regarding the relationship between hearing impairment and cognitive decline, particularly the cascade hypothesis which posits that hearing impairment decreases cognitive levels by reducing social activity. Notably, only 19.35% of our participants reported hearing impairment, a figure significantly lower than the prevalences documented in other epidemiological studies (43, 44). This discrepancy might be due to the reliance on self-reported data for defining hearing impairment in our study, which could differ from objective measurements like audiometry. Cultural aspects related to aging and attitudes toward well-being in late adulthood, particularly in China, might also influence these findings. With its dense older adult population, close-knit family structures, collectivist culture, isolation and loneliness among older Chinese adults is potentially lower than other countries (45, 46), which diminishing the impact of hearing impairment on socialization. Given these factors, this study do not support the cascade hypothesis, as we did not observe a direct pathway from hearing impairment to cognitive decline mediated by decreased social activity. The relationship between auditory and cognitive function may be more complex and influenced by various confounding factors.
4.4 Relationship among functional mobility, social activity, and cognitive decline
Physical function impairment is a common issue among older adults, with estimates suggesting that up to 50% of individuals over 80 years old may experience some form of motor impairment (47). Research has indicated that physical impairments can both precede and be predictive of the onset of mild cognitive impairment (48). However, these studies often focus on adults with specific mobility dysfunctions or motor disabilities, frequently linked to neurological conditions like Alzheimer’s or Parkinson’s disease (21, 49). Such conditions can disrupt social connectedness due to challenges such as limited transportation or inaccessible physical environments (50), which may not accurately represent the broader older adult population. In this study, we evaluated functional mobility as a variable to investigate its relationship with cognitive performance in the older adult.
Functional mobility refers to an individual’s physiological capacity to move independently and safely in various environments, essential for performing daily activities and maintaining participation in daily life (51). Impaired functional mobility was associated with increased risks of falls, loss of independence, and institutionalization (52). The Timed Up-and-Go (TUG) test, an objective tool for assessing basic functional mobility, was utilized in our analysis (53). The results indicated that functional mobility was an independent risk factor for cognitive decline in older adults. Moreover, older adults with impaired functional mobility and lower levels of social activity were more likely to experience cognitive decline compared to their more socially active counterparts. This finding aligned with other studies focusing on the impact of physical performance on cognition (54, 55). Higher levels of functional mobility are linked to a lower likelihood of cognitive decline. The mediating role of social activity engagement is significant, as older adult individuals with limited mobility often avoid social activities, leading to reduced cognitive stimulation and potentially contributing to cognitive decline.
The study’s findings shed light on the mechanisms of cognitive decline in older adults, emphasizing the mediating role of social activity engagement between functional mobility impairment and cognitive deterioration. This highlights the complexity of cognitive aging and the need for tailored interventions. Promoting social engagement through public initiatives, such as community activities and volunteer programs, could be an effective strategy to delay cognitive decline in older adults, especially for older adult with mobility impairment, as these interventions might foster cognitive resilience by offering mental stimulation, emotional support, and a sense of purpose. This is significant for improving the quality of life for older adults and reducing the caregiving burden on both families and the nation.
4.5 Strengths and limitations
Our study boasts significant strengths, such as its large sample size of older adults and an exhaustive baseline survey, allowing for the adjustment of numerous pertinent confounding factors. Nonetheless, it is important to recognize its limitations. The use of a cross-sectional study design constrains our capacity to establish causal relationships between the variables. Furthermore, the reliance on self-reported data for hearing impairment could lead to measurement bias and may not align with objective assessments. Additionally, employing convenience sampling for participant recruitment might have introduced selection bias, favoring respondents who were more accessible or more inclined to engage in the study. Lastly, the study was conducted in an eastern province of China. While the healthcare institutions were selected randomly, geographic and cultural differences could influence the results.
5 Conclusion
Our study determined that factors such as age, education level, residency, living arrangement, hyperlipidemia, cerebrovascular disease, alcohol consumption, smoking, ADLs, IADLs, as well as hearing, functional mobility, and social activity engagement, were associated with cognitive decline in Chinese older adults. Notably, social activity engagement emerged as a mediating factor in the link between functional mobility impairment and cognitive decline, but it did not mediate the relationship between self-reported hearing impairment and cognitive decline. These results underscored the importance of social activity engagement in potentially preventing cognitive decline in older adults with functional mobility impairments. The study emphasized the need to promote social engagement as a viable approach to mitigate cognitive deterioration in this demographic.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Sir Run Run Hospital, affiliated with Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HW: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing – original draft. DC: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft. DH: Data curation, Methodology, Software, Validation, Visualization, Writing – original draft. FT: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. MD: Conceptualization, Methodology, Software, Visualization, Writing – review & editing. SZ: Formal analysis, Methodology, Software, Validation, Writing – review & editing. LJ: Conceptualization, Funding acquisition, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Key Research and Development Program of China (No. 2020YFC2008504).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Davis, A, McMahon, CM, Pichora-Fuller, KM, Russ, S, Lin, F, Olusanya, BO, et al. Aging and hearing Health: the life-course approach. Gerontologist. (2016) 56:S256–67. doi: 10.1093/geront/gnw033
2. Larsson, L, Degens, H, Li, M, Salviati, L, Lee, Y, Thompson, W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. (2019) 99:427–511. doi: 10.1152/physrev.00061.2017
3. Salthouse, TA . Trajectories of normal cognitive aging. Psychol Aging. (2019) 34:17–24. doi: 10.1037/pag0000288
4. Ashby-Mitchell, K, Jagger, C, Fouweather, T, and Anstey, KJ. Life expectancy with and without cognitive impairment in seven Latin American and Caribbean countries. PLoS One. (2015) 10:e0121867. doi: 10.1371/journal.pone.0121867
5. Bennett, JE, Stevens, GA, Mathers, CD, Bonita, R, Rehm, J, Kruk, ME, et al. NCD countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet. (2018) 392:1072–88. doi: 10.1016/S0140-6736(18)31992-5
6. Foreman, KJ, Marquez, N, Dolgert, A, Fukutaki, K, Fullman, N, McGaughey, M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. (2018) 392:2052–90. doi: 10.1016/S0140-6736(18)31694-5
7. Alattar, AA, Bergstrom, J, Laughlin, GA, Kritz-Silverstein, D, Richard, EL, Reas, ET, et al. Hearing impairment and cognitive decline in older, community-dwelling adults. J Gerontol A. (2020) 75:567–73. doi: 10.1093/gerona/glz035
8. Fu, X, Eikelboom, RH, Tian, R, Liu, B, Wang, S, and Jayakody, DMP. The relationship of age-related hearing loss with cognitive decline and dementia in a Sinitic language-speaking adult population: a systematic review and Meta-analysis. Innov Aging. (2023) 7:igac078. doi: 10.1093/geroni/igac078
9. Gao, J, Armstrong, NM, Deal, JA, Lin, FR, and He, P. Hearing loss and cognitive function among Chinese older adults: the role of participation in leisure activities. BMC Geriatr. (2020) 20:215. doi: 10.1186/s12877-020-01615-7
10. Jafari, Z, Kolb, BE, and Mohajerani, MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. (2019) 56:100963. doi: 10.1016/j.arr.2019.100963
11. Lin F. R.Yaffe, K, Xia, J, Xue, QL, Harris, TB, Purchase-Helzner, E, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. (2013) 173:293–9. doi: 10.1001/jamainternmed.2013.1868
12. Livingston, G, Huntley, J, Sommerlad, A, Ames, D, Ballard, C, Banerjee, S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
13. Slade, K, Plack, CJ, and Nuttall, HE. The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci. (2020) 43:810–21. doi: 10.1016/j.tins.2020.07.005
14. Uchida, Y, Sugiura, S, Nishita, Y, Saji, N, Sone, M, and Ueda, H. Age-related hearing loss and cognitive decline—the potential mechanisms linking the two. Auris Nasus Larynx. (2019) 46:1–9. doi: 10.1016/j.anl.2018.08.010
15. Bennett, DA, Schneider, JA, Tang, Y, Arnold, SE, and Wilson, RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. (2006) 5:406–12. doi: 10.1016/S1474-4422(06)70417-3
16. Lin, FR, and Albert, M. Hearing loss and dementia – who is listening? Aging Ment Health. (2014) 18:671–3. doi: 10.1080/13607863.2014.915924
17. Maharani, A, Pendleton, N, and Leroi, I. Hearing impairment, loneliness, social isolation, and cognitive function: longitudinal analysis using English longitudinal Study on ageing. Am J Geriatr Psychiatry. (2019) 27:1348–56. doi: 10.1016/j.jagp.2019.07.010
18. Ogawa, T, Uchida, Y, Nishita, Y, Tange, C, Sugiura, S, Ueda, H, et al. Hearing-impaired elderly people have smaller social networks: a population-based aging study. Arch Gerontol Geriatr. (2019) 83:75–80. doi: 10.1016/j.archger.2019.03.004
19. Rafnsson, SB, Orrell, M, d’Orsi, E, Hogervorst, E, and Steptoe, A. Loneliness, social integration, and incident dementia over 6 years: prospective findings from the English longitudinal Study of ageing. J Gerontol B. (2020) 75:114–24. doi: 10.1093/geronb/gbx087
20. Dawes, P, Emsley, R, Cruickshanks, KJ, Moore, DR, Fortnum, H, Edmondson-Jones, M, et al. Hearing loss and cognition: the role of hearing aids, social isolation and depression. PLoS One. (2015) 10:e0119616. doi: 10.1371/journal.pone.0119616
21. Albers, MW, Gilmore, GC, Kaye, J, Murphy, C, Wingfield, A, Bennett, DA, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. (2015) 11:70–98. doi: 10.1016/j.jalz.2014.04.514
22. Blankevoort, CG, Scherder, EJA, Wieling, MB, Hortobágyi, T, Brouwer, WH, Geuze, RH, et al. Physical predictors of cognitive performance in healthy older adults: a cross-sectional analysis. PLoS One. (2013) 8:e70799. doi: 10.1371/journal.pone.0070799
23. Liu, J, Cui, K, Chen, Q, Li, Z, Fu, J, Gong, X, et al. Association of walking speed with cognitive function in Chinese older adults: a nationally representative cohort study. Front Aging Neurosci. (2022) 14:1003896. doi: 10.3389/fnagi.2022.1003896
24. Sprague, BN, Phillips, CB, and Ross, LA. Age-varying relationships between physical function and cognition in older adulthood. J Gerontol B. (2019) 74:772–84. doi: 10.1093/geronb/gbx126
25. Zhao, D, Chai, S, Gao, T, Li, J, and Zhou, C. Physical mobility, social isolation and cognitive function: are there really gender differences? Am J Geriatr Psychiatry. (2023) 31:726–36. doi: 10.1016/j.jagp.2023.04.002
26. Cherry, KE, Walker, EJ, Brown, JS, Volaufova, J, LaMotte, LR, Welsh, DA, et al. Social engagement and Health in younger, older, and oldest-old adults in the Louisiana healthy aging Study. J Appl Gerontol. (2013) 32:51–75. doi: 10.1177/0733464811409034
27. Kuiper, JS, Zuidersma, M, Oude Voshaar, RC, Zuidema, SU, van den Heuvel, ER, Stolk, RP, et al. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. (2015) 22:39–57. doi: 10.1016/j.arr.2015.04.006
28. World Health Organization . (2019). Risk reduction of cognitive decline and dementia. Geneva: WHO guidelines.
29. He, CYY, Zhou, Z, Kan, MMP, Chan, DHY, Wong, ACT, Mok, KHY, et al. Modifiable risk factors for mild cognitive impairment among cognitively normal community-dwelling older adults: a systematic review and meta-analysis. Ageing Res Rev. (2024) 99:102350. doi: 10.1016/j.arr.2024.102350
30. Katzman, R, Zhang, M, Ouang-Ya-Qu, W, Wang, Z, Liu, WT, Yu, E, et al. A Chinese version of the mini-mental state examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. (1988) 41:971–8. doi: 10.1016/0895-4356(88)90034-0
31. Mitchell, AJ, and Malladi, S. Screening and case-finding tools for the detection of dementia. Part II: evidence-based Meta-analysis of single-domain tests. Am J Geriatr Psychiatry. (2010) 18:783–800. doi: 10.1097/JGP.0b013e3181cdecd6
32. Tsoi, KKF, Chan, JYC, Hirai, HW, Wong, SYS, and Kwok, TCY. Cognitive tests to detect dementia: a systematic review and Meta-analysis. JAMA Intern Med. (2015) 175:1450–8. doi: 10.1001/jamainternmed.2015.2152
33. Tangalos, EG, Smith, GE, Ivnik, RJ, Petersen, RC, Kokmen, E, Kurland, LT, et al. The Mini-mental state examination in general medical practice: clinical utility and acceptance. Mayo Clin Proc. (1996) 71:829–37. doi: 10.4065/71.9.829
34. Alexandre, TS, Meira, DM, Rico, NC, and Mizuta, SK. Accuracy of timed up and go test for screening risk of falls among community-dwelling elderly. Braz J Phys Ther. (2012) 16:381–8. doi: 10.1590/S1413-35552012005000041
35. Sommerlad, A, Sabia, S, Singh-Manoux, A, Lewis, G, and Livingston, G. Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLoS Med. (2019) 16:e1002862. doi: 10.1371/journal.pmed.1002862
36. Saito, T, Murata, C, Saito, M, Takeda, T, and Kondo, K. Influence of social relationship domains and their combinations on incident dementia: a prospective cohort study. J Epidemiol Community Health. (2018) 72:7–12. doi: 10.1136/jech-2017-209811
37. Fiorillo, D, Lavadera, GL, and Nappo, N. Social participation and self-rated psychological health: a longitudinal study on BHPS. SSM Population Health. (2017) 3:266–74. doi: 10.1016/j.ssmph.2017.02.003
38. Holt-Lunstad, J, Robles, TF, and Sbarra, DA. Advancing social connection as a public health priority in the United States. Am Psychol. (2017) 72:517–30. doi: 10.1037/amp0000103
39. Chadha, S, Kamenov, K, and Cieza, A. The world report on hearing, 2021. Bull World Health Organ. (2021) 99:242–242A. doi: 10.2471/BLT.21.285643
40. Dalton, DS, Cruickshanks, KJ, Klein, BEK, Klein, R, Wiley, TL, and Nondahl, DM. The impact of hearing loss on quality of life in older adults. Gerontologist. (2003) 43:661–8. doi: 10.1093/geront/43.5.661
41. Shukla, A, Harper, M, Pedersen, E, Goman, A, Suen, JJ, Price, C, et al. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngol Head Neck Surg. (2020) 162:622–33. doi: 10.1177/0194599820910377
42. Strawbridge, WJ, Wallhagen, MI, Shema, SJ, and Kaplan, GA. Negative consequences of hearing impairment in old age. Gerontologist. (2000) 40:320–6. doi: 10.1093/geront/40.3.320
43. Gong, R, Hu, X, Gong, C, Long, M, Han, R, Zhou, L, et al. Hearing loss prevalence and risk factors among older adults in China. Int J Audiol. (2018) 57:354–9. doi: 10.1080/14992027.2017.1423404
44. Wang, J, Liu, D, Tian, E, Guo, Z-Q, Chen, J-Y, Kong, W-J, et al. Hearing impairment with cognitive decline increases all-cause mortality risk in Chinese adults aged 65 years or older: a population-based longitudinal Study. Front Aging Neurosci. (2022) 14:865821. doi: 10.3389/fnagi.2022.865821
45. Barreto, M, Victor, C, Hammond, C, Eccles, A, Richins, MT, and Qualter, P. Loneliness around the world: age, gender, and cultural differences in loneliness. Pers Individ Dif. (2021) 169:110066. doi: 10.1016/j.paid.2020.110066
46. Gao, Q, Prina, AM, Prince, M, Acosta, D, Luisa Sosa, A, Guerra, M, et al. Loneliness among older adults in Latin America, China, and India: prevalence, correlates and association with mortality. Int J Public Health. (2021) 66:604449. doi: 10.3389/ijph.2021.604449
47. Buchman, AS, and Bennett, DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother. (2011) 11:665–76. doi: 10.1586/ern.11.57
48. Buracchio, T, Dodge, HH, Howieson, D, Wasserman, D, and Kaye, J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. (2010) 67:980–6. doi: 10.1001/archneurol.2010.159
49. Barclay, L, McDonald, R, and Lentin, P. Social and community participation following spinal cord injury: a critical review. Int J Rehabil Res. (2015) 38:1–19. doi: 10.1097/MRR.0000000000000085
50. Hall, JP, Kurth, NK, and Goddard, KS. Assessing factors associated with social connectedness in adults with mobility disabilities. Disabil Health J. (2022) 15:101206. doi: 10.1016/j.dhjo.2021.101206
51. Bouça-Machado, R, Maetzler, W, and Ferreira, JJ. What is functional mobility applied to Parkinson’s disease? J Parkinsons Dis. (2018) 8:121–30. doi: 10.3233/JPD-171233
52. Lin, S-I, Lee, H-C, Chang, K-C, Yang, Y-C, and Tsauo, J-Y. Functional mobility and its contributing factors for older adults in different cities in Taiwan. J Formos Med Assoc. (2017) 116:72–9. doi: 10.1016/j.jfma.2016.01.011
53. Karakaya, MG, Bilgin, SÇ, Ekici, G, Köse, N, and Otman, AS. Functional mobility, depressive symptoms, level of Independence, and quality of life of the elderly living at home and in the nursing home. J Am Med Dir Assoc. (2009) 10:662–6. doi: 10.1016/j.jamda.2009.06.002
54. Lee, S, Han, J, Jin, Y, Lee, I, Hong, H, and Kang, H. Poor physical fitness is independently associated with mild cognitive impairment in elderly Koreans. Biol Sport. (2016) 33:57–62. doi: 10.5604/20831862.1185889
Keywords: self-reported hearing impairment, functional mobility impairment, social activity, cognitive function, aging
Citation: Wang H, Chen D, Hu D, Tian F, Dai M, Zhang S and Jin L (2024) Risk factors for cognitive decline in older Chinese adults: the impact of social activity on the relationship between hearing, functional mobility, and cognition. Front. Public Health. 12:1460941. doi: 10.3389/fpubh.2024.1460941
Edited by:
Celeste Annemarie De Jager Loots, Imperial College London, United KingdomCopyright © 2024 Wang, Chen, Hu, Tian, Dai, Zhang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyu Jin, amlubGl5dTIwMDgwMTAxQDE2My5jb20=
†These authors have contributed equally to this work
 Hao Wang1†
Hao Wang1† Liyu Jin
Liyu Jin