- 1Department of Medicine and Surgery, University of Perugia, Perugia, Italy
- 2Department of Medicine and Surgery, School of Midwifery, University of Perugia, Perugia, Italy
- 3Center for Research in Perinatal and Reproductive Medicine, University of Perugia, Perugia, Italy
Maternal immunization is a valuable tool for protecting mother and unborn child from vaccine-preventable diseases. However, the implementation of strategies for vaccinating pregnant women has only recently gained traction. This work is aimed at providing an overview of European vaccination strategies and gathering evidence on interventions enhancing vaccination knowledge, attitudes, and behaviors (KAB) in pregnant women. To summarize current pregnancy vaccination strategies in Europe, we consulted literature, institutional national health system websites, and the ECDC Vaccine Scheduler. The review of evidence on interventions targeting pregnant women’s vaccination KAB was performed by searching primary studies on PubMed and Web of Science. The 27 EU member states offer various vaccinations in pregnancy, but only 10 recommend all of these: tetanus, pertussis, diphtheria, influenza, and COVID-19, albeit with different administration schedules. The literature review included 7 studies, 3 from Italy and 4 from other European countries (UK, Netherlands, Greece, Poland, and Ukraine). They were conducted in various settings such as childbirth preparation courses, prenatal visits, and online platforms, and all included educational interventions providing information on vaccine safety and efficacy during pregnancy. Knowledge about vaccines and vaccine-preventable diseases, generally low in the pre-intervention period, increased post-intervention, with a rise in awareness of the risks associated with infectious diseases and the recommended vaccines, a reduction in vaccine-related misinformation, and a greater propensity to vaccinate both newborns and themselves. Furthermore, there was a significant increase in adherence to recommended vaccinations, particularly among those with higher educational levels. However, vaccine hesitancy persisted, influenced by factors such as fear of adverse events and the lack of recommendations from healthcare providers. Variations in pregnancy vaccination strategies across Europe emphasize the importance of establishing a unified framework to optimize maternal and fetal health outcomes through evidence-based policies. Educational interventions may positively impact pregnant women’s KAB, therefore promoting vaccination uptake.
1 Introduction
Throughout pregnancy, the immune system undergoes significant modulation alongside physiological adaptations aimed at maintaining maternal homeostasis and facilitating optimal fetal development. These alterations make women more vulnerable to both viral and bacterial infections (1–3), consequently heightening the likelihood of severe complications for the mother and the potential transmission of pathogens to the developing fetus (4–6).
Due to the immaturity of their immune system in the first months of life, neonates are notably susceptible to the onset of potentially severe or fatal infections until they reach the age suitable for vaccination and complete the vaccination cycle (7).
Vaccinating pregnant women has been identified as an optimal strategy for safeguarding the health of the mother, fetus, and infant, resulting in a triple benefit. This intervention affords pregnant women, protection against vaccine-preventable diseases (VPDs) such as influenza, diphtheria, tetanus, pertussis, and COVID-19 (8, 9). Furthermore, a vaccine against Respiratory Syncytial Virus (RSV) has been recently approved in pregnant women for the protection of infants from lower respiratory tract diseases (10).
Therefore, vaccination in pregnancy is widely recognized as an essential component of the comprehensive antenatal care package aimed at enhancing maternal and child health (11, 12).
In this light, many European countries followed the guidance provided by the World Health Organization (WHO) (13–15) and the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) (16, 17), routinely advocate for maternal immunization to prevent influenza, diphtheria, pertussis, tetanus, and COVID-19, often through fully subsidized vaccine offerings, as evidenced by a comprehensive review of vaccination policies specific to pregnant women in Europe published in 2021 (18). These vaccines have been demonstrated safe, immunogenic, and effective (19). Nevertheless, vaccine coverage in Europe among pregnant women exhibits substantial discrepancies in terms of both monitoring and data (20). The 2018 ECDC report indicated that only nine European Union Member States (21), reduced to four in the most recent 2023 report (22), monitored pregnant women’s adherence to seasonal influenza vaccination. The highest influenza vaccination rates were observed in Northern Ireland (58.6%) and England (44.9%) during the 2016–2017 influenza season, while Ireland reached 62% in 2017–2018 (21). A wide variability in influenza vaccination coverage, ranging from 1.7 to 61%, was indeed shown in 2020–2021 (22). Significant variability was evident also in respect to other vaccinations, such as pertussis, with high vaccination coverage in Spain, Denmark, and Belgium (88.5, 69, and 64.3%, respectively), in stark contrast to the low ones observed in the Czech Republic and Slovenia (1.6 and 6.5%) in 2023 (23).
Regarding SARS-CoV-2 during the 2023–2024 season, only Ireland (19.6%) and Spain (7.8%) have published official data (24), emphasizing the considerable efforts still required, not only to achieve adequate vaccination coverage in this at-risk population but also to ensure effective monitoring.
The substantial variability in vaccination coverages and their unsatisfactory level can be partly attributed to “vaccine hesitancy” (25), which is defined by the WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) (26) as the inclination to postpone or decline vaccination despite its availability and is currently recognized as one of the top ten threats to global health (27, 28).
Several studies have explored the factors that influence vaccine hesitancy in pregnancy. These investigations have consistently identified some elements in the literature, namely vaccine-specific factors, such as fear of adverse events and lack of confidence in vaccine safety, and lack of recommendation from healthcare professionals. Disease-related perceptions as well as previous vaccination behavior have also been shown to have an impact on vaccine uptake (9, 29, 30).
This evidence underscores the imperative need to address the determinants influencing maternal immunization, including knowledge, attitudes, and beliefs about maternal and childhood vaccines, through educational interventions (19, 31–34). Such measures are crucial to promoting behavioral changes in pregnant women and their families, enhancing adherence to vaccination protocols, and thus reducing vaccine hesitancy in pregnancy (35, 36).
This review aims to provide an updated overview of pregnant women’s vaccination policies across Europe and of current evidence regarding educational interventions aimed at promoting knowledge, attitudes, and behaviors related to recommended vaccinations for pregnant women in the European context. Based on the identified issues and problems the paper seeks to explore potential avenues for optimizing maternal and fetal health outcomes within diverse European settings.
2 Materials and methods
To procure a contemporaneous assessment of extant vaccination strategies tailored for pregnant women in Europe, we consulted the “Vaccine Scheduler” of the ECDC (37). Additionally, we examined the recommendations provided by national health systems, as available on their institutional websites, or reported in the comprehensive review of pregnancy vaccination policies in Europe published in 2021 (18).
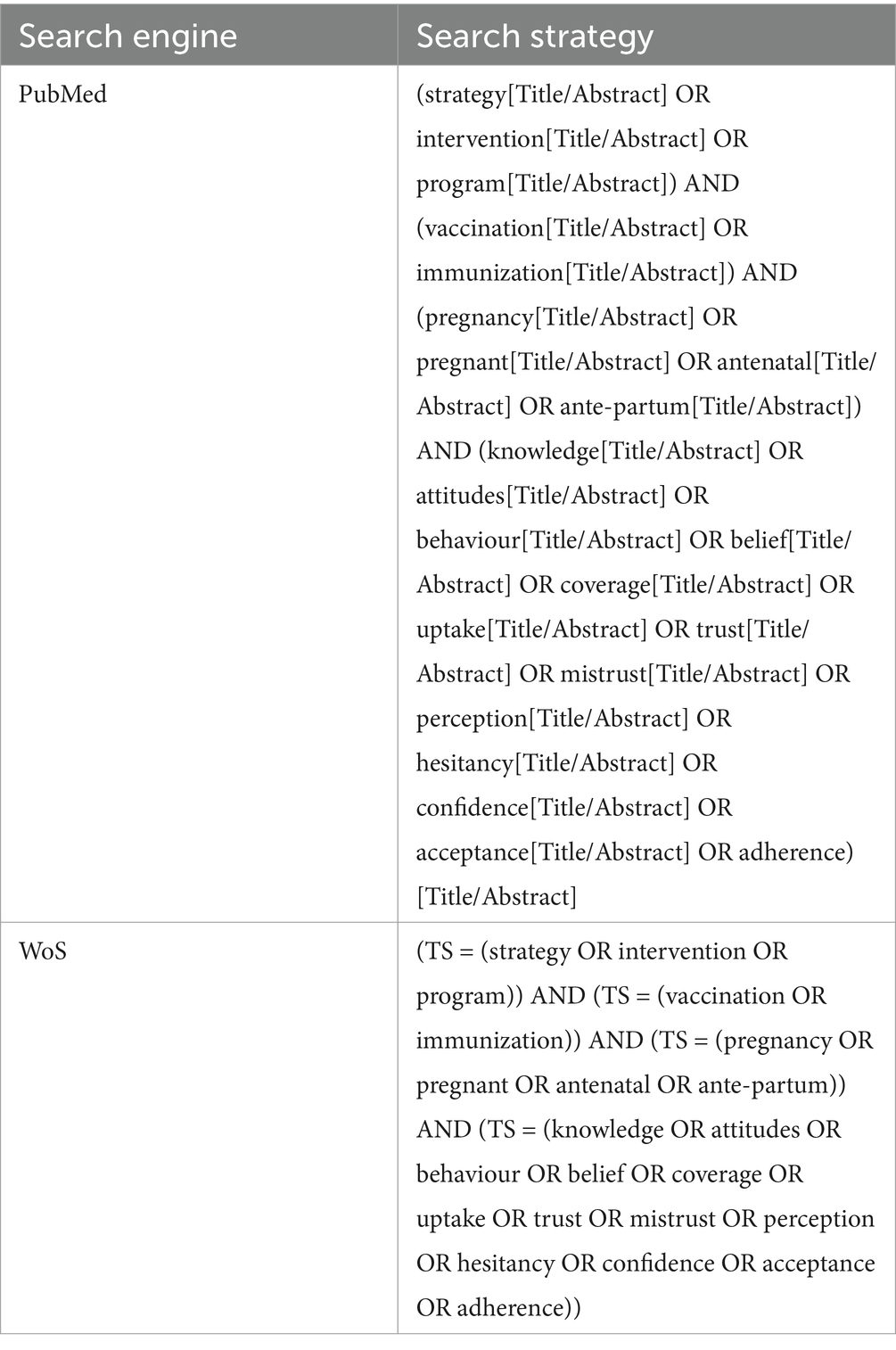
Moreover, a review focusing on educational interventions aimed at promoting knowledge, attitudes, and behaviors regarding recommended vaccinations among pregnant women, namely influenza, diphtheria, tetanus, pertussis, and COVID-19, was conducted. Educational interventions have been considered in various formats, including, for example, expert-led information sessions, digital campaigns, and distribution of themed information materials. The primary objective of the search was to identify studies that assessed the impact of these interventions on pregnant women’s knowledge, attitudes, and behaviors toward vaccination recommended in pregnancy. To achieve this objective, we employed a search string and adhered to the PICOS criteria, although we did not intend to conduct a systematic review. The evidence retrieval was conducted by consulting two databases (MEDLINE/PubMed, and Web of Science) up to 21 May 2023. Search terms related to pregnancy, vaccination, immunization, knowledge, attitudes, and behaviors regarding vaccination were included. Only language filters were applied to include articles in English, French, and Italian.
The entire search strategy is reported in Table 1.
The inclusion criteria for studies were based on the PICOS framework (38), as described below: (P) Population: European pregnant women during any trimester of pregnancy; (I) Intervention: any intervention involving education, training, or vaccination awareness initiatives; (C) Comparison: not applicable; (O) Outcome: knowledge, attitudes, and behaviors of women toward vaccinations; (S) Study design: primary studies with experimental or quasi-experimental designs, including randomized and non-randomized trials, and observational studies.
The PICOPortal platform (39) was used for screening and for identifying duplicates. Records underwent initial screening by two reviewers, with a third reviewer resolving equivocal cases. The full texts of selected articles were independently reviewed by two reviewers for eligibility.
Within the scope of this narrative review, a qualitative synthesis was conducted. Information about the study setting, the study population, the sample size, the type of intervention, and the tools used to assess the impact of the intervention were extracted by each study by a researcher and cross-checked by a second one. Data about pregnant women’s knowledge, attitudes, and behaviors were also collected from each study and reported descriptively highlighting any significant difference due to the intervention. We employed the NIH quality assessment tools, specifically the “Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group” and the “Quality Assessment of Controlled Intervention Studies” to evaluate the quality of the included studies (40). The former tool evaluates pre-post studies by examining 12 aspects such as the clarity of study objectives, the inclusion of pre-specified outcome measures, the appropriateness of statistical analysis, and the consideration of potential confounding factors. Three distinct categories were identified based on the scoring: 0–4 as poor, 5–8 as fair, and 9–12 as good. The second tool assesses controlled intervention studies based on 14 key criteria such as randomization, allocation concealment, blinding, completeness of outcome data, selective reporting, and other sources of bias. Also in this case, three quality categories were identified based on the scoring: 0–4 as poor, 5–9 as fair, and 10–14 as good.
3 Results
3.1 Overview of vaccination policies in Europe
Despite the diversity of vaccination programs, several European countries implement tailored vaccination policies for pregnant women (18), following guidelines outlined by the WHO (13–15). Nevertheless, strategies exhibit variability across European Countries (17, 32).
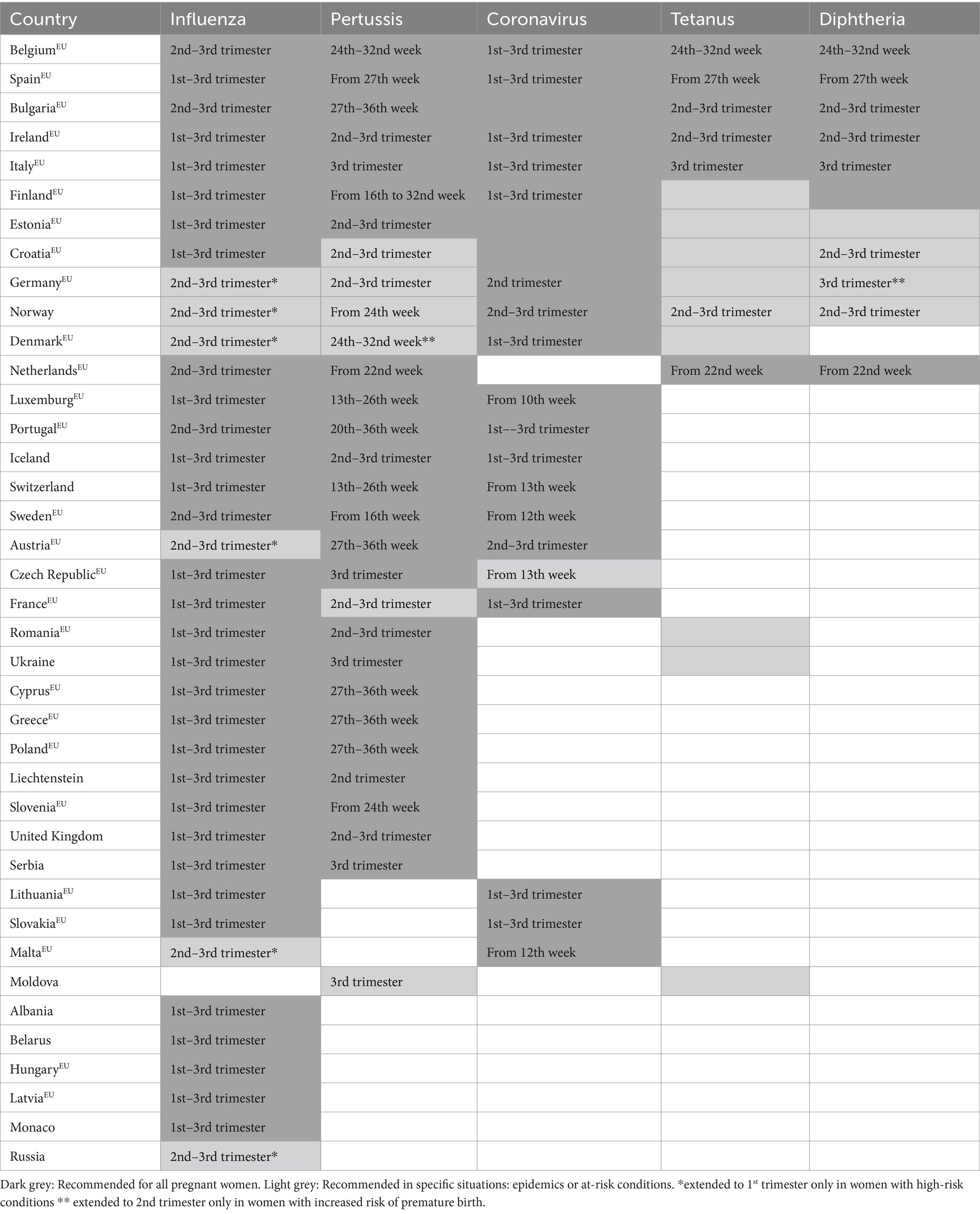
An examination of the most recent directives from 39 states, including European Union member states, revealed that 97% (38) of such states advocate for the administration of the influenza vaccine during the gestational period. Furthermore, 77% (30) endorse vaccination against pertussis, with 38% (15) advocating for the tetanus vaccine, 28% (11) for the diphtheria vaccine, and 56% (22) for vaccination against COVID-19. Lastly, 26% (10) endorse the entirety of the aforementioned vaccinations for women in a pregnant state (Table 2) (18, 37).
Thirty-eight European countries advocate for administering the influenza vaccine to pregnant women, though with different timings (18, 37). Notably, Belgium, Bulgaria, the Netherlands, Portugal, and Sweden recommend influenza vaccine in the 2nd–3rd trimester. Austria, Denmark, Germany, Malta, Norway, and Russia also stipulate that influenza vaccination is advisable for pregnant women in the 2nd to the 3rd trimester (18, 41–45), but extend their recommendation to include vaccination from the onset of the 1st trimester in pregnant women with high-risk conditions or during epidemics (18, 37). Twenty-seven out of the 38 countries (Albania, Belarus, Croatia, Cyprus, Czech Republic, Estonia, Finland, France, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxemburg, Monaco, Poland, Romania, Serbia, Slovakia, Slovenia, Spain, Switzerland, Ukraine, and the United Kingdom), recommend influenza vaccination between the 1st and 3rd trimester (18, 37).
Pertussis vaccination is also advised during pregnancy in numerous European countries, with notable variations in the timing and condition of recommendation. Luxembourg and Switzerland recommend vaccination between the 13th and 26th weeks, Sweden and Finland from the 16th week, Portugal between the 20th and 36th week, Denmark and Belgium between the 24th and 32nd week, the Netherlands from the 22nd week, Slovenia and Norway from the 24th week and Austria, Bulgaria, Cyprus, Czech Republic, Germany, Greece, Italy, Poland, Serbia, Spain and Ukraine from the 27th week (18, 37, 46–54). In Denmark, as well as in Germany, vaccination is extended at the beginning of the 2nd trimester if premature labor is expected (18, 37, 52). Estonia, Iceland, Ireland, and the United Kingdom recommend vaccination between the 2nd and 3rd trimester, as well as Romania if more than 10 years have elapsed after the last dose (18, 37, 55, 56). In Liechtenstein, pertussis vaccination is advocated during the 2nd trimester (18). Few countries recommend the vaccination in response to prevailing epidemiological trends, such as Moldova (recommended in the 3rd trimester during epidemics or high-risk conditions), France (recommended in the 2nd–3rd trimester in the epidemic territory), Croatia (recommended in the 2nd–3rd trimester in light of the ongoing pertussis epidemic) (37).
As far as diphtheria vaccination is concerned, in Bulgaria and Ireland it is recommended between the 2nd and the 3rd trimester of pregnancy, along with tetanus vaccination (18, 37). In the Netherlands, the diphtheria vaccination is advised from the 22nd week of pregnancy, in Belgium between the 24th and 32nd week, in Spain and Italy in the 3rd trimester, ideally from the 27th week and at the 28th week, respectively (18, 37). In these countries, tetanus vaccination is also recommended in the same time window (18, 37, 57–61). In Finland, vaccination against diphtheria is recommended for all pregnant women, preferably at the end of pregnancy (18). In Germany, vaccination against diphtheria is advocated at the beginning of the 3rd trimester, and extended at 2nd in women at risk of pre-term birth (41), while in Estonia it is recommended for women presenting specific risk conditions (18); furthermore, in these countries, as well as in Finland, Denmark, Moldova, Romania, and Ukraine, tetanus vaccination is recommended for pregnant women who are either unvaccinated or incompletely vaccinated, as well as for pregnant women following exposure to potential tetanus risks (18). In Norway, the consideration for administering the diphtheria vaccine arises if clinically warranted; it is prudent to defer vaccination until the 2nd–3rd trimester rather than administering it during the initial trimester (18). Additionally, Norway recommends tetanus vaccination between the 2nd and the 3rd trimesters, specifically during epidemics or for individuals with risk conditions (18).
Croatia temporarily advises diphtheria and tetanus vaccination for all pregnant women during the 2nd–3rd trimester, along with vaccination for all close contacts of newborns (37).
COVID-19 vaccination is recommended for pregnant women across all trimesters in 14 European countries (Belgium, Bulgaria, Croatia, Denmark, Estonia, Finland, France, Iceland, Ireland, Italy, Lithuania, Portugal, Slovakia, Spain) (18, 37). On the contrary, in Luxembourg, it is suggested starting from the 10th week of pregnancy (62), while in Malta and Sweden, the recommendation begins from the 12th week (63, 64). In the Czech Republic, COVID-19 vaccination during pregnancy is deemed particularly appropriate for women exhibiting high-risk conditions predisposing them to infection or severe manifestations of COVID-19; the vaccination protocol stipulates that inoculation during pregnancy should be scheduled after the completion of the 12th week of gestation, hence commencing anytime from the onset of the 13th week of pregnancy (65), as well as in Switzerland (66). Austria and Norway recommend COVID-19 vaccination between the 2nd and 3rd trimesters (54, 67), while Germany during the 2nd (68). Bulgaria, Estonia, and Croatia recommend COVID-19 vaccination generally for all pregnant women (69–71).
A summary of the main vaccinations offered during pregnancy in Europe is provided in Table 2.
The heterogeneous landscape of vaccination policies across European nations underscores the complex interplay between epidemiological variables, healthcare infrastructure, and regulatory paradigms. Tailored vaccination initiatives, informed by WHO directives, are progressively being enacted to address the unique requirements of the pregnant women cohort. Ranging from trimester-specific recommendations to individualized strategies in response to epidemic circumstances, national protocols underscore the necessity for adaptive vaccination approaches. Considering the heterogeneity observed in pregnancy vaccination initiatives across European nations, it becomes imperative to delineate a cohesive framework aimed at ensuring optimal maternal and fetal health outcomes via evidence-informed and collaborative policy formulations.
3.2 Evidence on interventions aimed at promoting pregnant women’s knowledge, attitudes, and behaviors in respect to vaccination
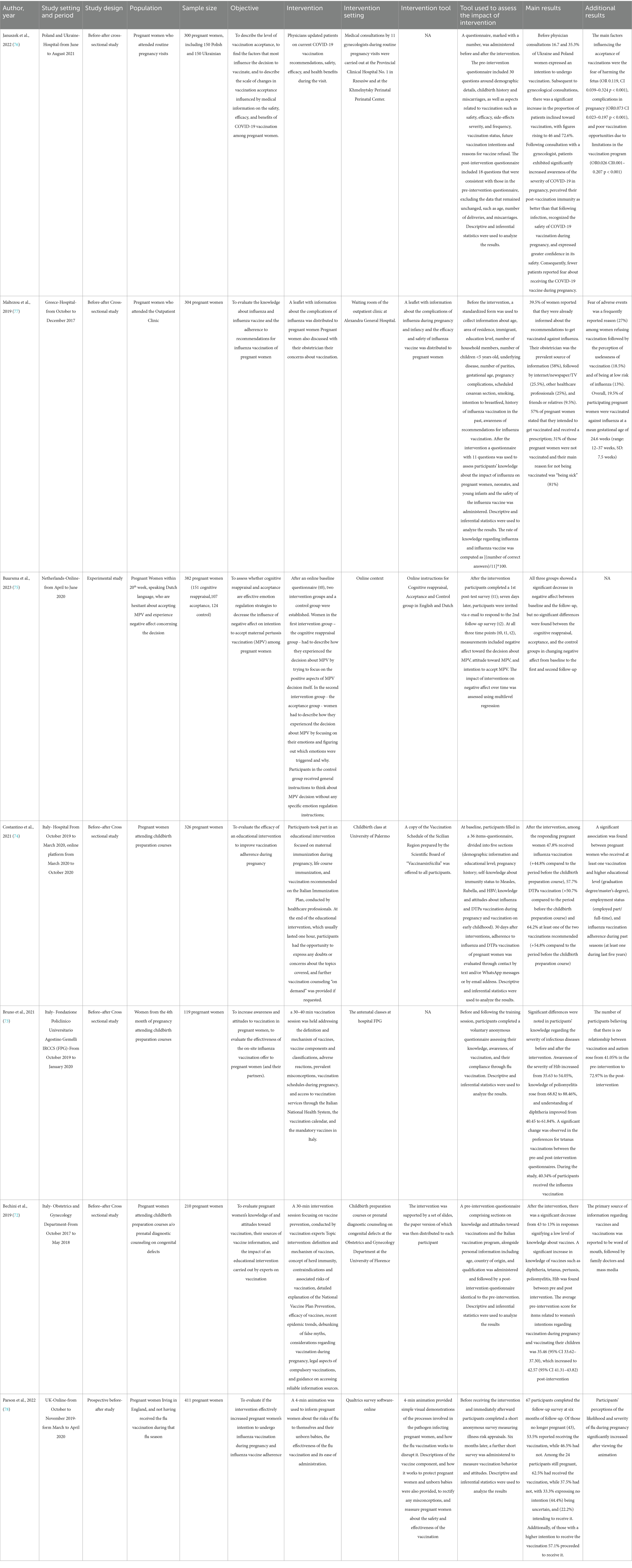
The initial search across MEDLINE/PubMed and Web of Science resulted in the identification of 3,186 studies. Following the removal of 1,470 duplicates and the exclusion of 1,406 studies based on the screening of titles and abstracts, a thorough full-text evaluation of the remaining 310 studies was conducted to assess their eligibility. Ultimately seven studies were included in the review, comprising three conducted in Italy (72–74), one in the Netherlands (75), one in Poland and Ukraine (76), one in Greece (77) and one in the UK (78). They encompassed a variety of research designs, including five before-after cross-sectional (72–74, 76, 77), one prospective (78), and one experimental (75) study. Four studies were conducted within hospital settings (72, 73, 76, 77). In particular, in the Italian studies, the Department of Obstetrics and Gynecology (72) and the Department of Women’s and Children’s Health and Public Health (73) organized and conducted antenatal courses; in Poland and Ukraine (76), as well as in Greece (77), the Perinatal Center and the Outpatient Clinic of the hospital carried out the perinatal visits. On the other hand, researchers in the Netherlands and in UK used online platforms for their studies (75, 78). Another Italian study adopted a hybrid approach combining hospital and online modalities due to the COVID-19 pandemic.
The recruited population across the studies comprised pregnant women participating in antenatal classes, those engaged in prenatal diagnostic consultations for congenital anomalies (72), or those attending routine prenatal visits (76, 77). The participants in the two studies conducted online were, in one case, pregnant women who signed up to the Qualtrics online panel to express interest in taking part in research activities (78), and, in the other case, pregnant women recruited through advertisement on social media (75). Sample sizes ranged from 119 (73) to 2,012 women (75), and included women between 18 and 40 years old (Table 3).
3.2.1 Methodological quality assessment (risk of bias)
One of the included quasi-experimental studies reported a score of 5 out of 12 (64), three a score of 6 out of 12 (59, 60, 65), and two a score of 7 out of 12 (61, 63), showing all fair quality. The only experimental study included in the review (62) reported a score of 7 out of 14 being of fair quality too.
3.2.2 Intervention characteristics
The educational interventions carried out exhibited heterogeneity across the studies. In five studies (72–74, 76, 77), interventions involved participant engagement with healthcare professionals. Among these, three (72–74) were conducted during antenatal classes held at varying frequencies, featuring educational sessions about vaccination and vaccines lasting 30–60 min and facilitated by highly qualified healthcare practitioners, with expertise in vaccinology. Since April 2020, one of these antenatal classes has been delivered online through digital platforms due to the COVID-19 pandemic (74).
Two interventions (76, 77) were integrated during routine prenatal visits. In the study conducted in Poland and Ukraine (76), participants were briefed on the safety, efficacy, and health benefits associated with COVID-19 vaccination by gynecologists, In the study conducted in Greece (77) participants were provided with an informational leaflet on influenza and influenza vaccination while in the waiting room of the clinic (77), followed by consultations with midwives (77).
In the study carried out in the Netherlands (75) pregnant women were randomly assigned to one of the 3 online groups (cognitive reappraisal intervention group, acceptance intervention group, and control group) to evaluate the influence of negative affect on intention to accept maternal pertussis vaccination (MPV). The cognitive reappraisal group was instructed to describe their experience relating to the decision regarding MPV, with specific attention to its positive aspects. The acceptance group received instructions to describe their emotional experience related to the MPV decision, trying to identify the emotions triggered and their causes. Finally, the control group received general instructions to reflect on the decision regarding MPV, without a specific focus on emotion regulation.
In another study (78), carried out online, the intervention comprised a 4-min animated video designed to inform pregnant women about the risks posed by influenza to both themselves and their unborn babies, as well as to elucidate the efficacy of the flu vaccine and its ease of administration.
3.2.3 Tools for assessing the impact of intervention
In all the studies, questionnaires were used to evaluate the impact of the interventions. One Italian study (73) used a pre-and post-intervention questionnaire adapted from a validated tool (79) to assess knowledge, awareness of vaccination, and compliance to influenza vaccination. In another Italian study (72), a pre-and post-intervention non-validated questionnaire was employed, encompassing demographic details (age, country of origin, and educational attainment) alongside inquiries about participants’ knowledge and attitudes toward vaccinations, as well as their awareness of the Italian vaccination schedule. The pre-post intervention questionnaires in both studies (72, 73) included questions about participants’ knowledge and attitudes toward vaccinations; however, the specific focus and detail of these questions differed between studies. In the third Italian study (74), the pre-intervention survey was performed through a questionnaire validated in a preliminary pilot study, while the post-intervention assessment was performed by text message and/or WhatsApp message or e-mail contact and was aimed to evaluate adherence to flu vaccination and/or diphtheria–tetanus–pertussis acellularis (DTPa), as well as the main reasons for refusing vaccination.
Also, the studies conducted in Poland and Ukraine (76) and the UK (78) adopted a pre-post-intervention non-validated questionnaire survey, measuring safety, efficacy, side-effects severity, and frequency of vaccinations (76) and illness risk appraisal (78) respectively; both studies explored vaccination attitudes, one conducting the assessment immediately following the educational intervention (76) and the other six months after the intervention (78). In the investigation undertaken in Greece (77), a standardized non-validated questionnaire with 11 questions was employed to assess pregnant women’s understanding of influenza and their compliance with influenza vaccination after the educational intervention. The study undertaken in the Netherlands employed a survey administered at baseline, alongside two subsequent post-intervention surveys, to assess the impact of negative affect on the intention to accept MPV (75).
3.2.4 Results
3.2.4.1 Effects on knowledge
Pregnant women’s knowledge about vaccines and vaccine-preventable diseases was assessed in six (72–74, 76–78) of the included studies.
The evidence showed that the main sources of vaccination information were obstetricians (58%) (77), independent research (52.9%) (73), word of mouth (friends, family members, etc.) (9.5–50%) (72, 77), traditional mass media (TV, radio, and newspapers, internet) (19.5–35.7%) (72–74, 77), health professionals, particularly family doctors (25–45.7%) (72, 74, 77). Specialists such as pediatricians and gynecologists were consulted less frequently (16.2–21.4%) (72). Additionally, within a study carried out in Italy (73), post-intervention questionnaires revealed that 64.6% of respondents (51/79) deemed the prenatal course highly beneficial for information acquisition, showing a significant increase compared to the pre-intervention questionnaire results (30.3%, 27/89 respondents).
The level of knowledge regarding the recommendation for influenza vaccination during pregnancy exhibits considerable variability among pregnant women. In a study conducted in Italy (74), in the pre-intervention, approximately 70% of the interviewees were aware of the recommendation for influenza vaccination during pregnancy, but only 23.9% demonstrated awareness that influenza vaccination during pregnancy could be administered throughout all trimesters of gestation. Furthermore, 58.6% were aware of the recommendation of DTPa vaccination during pregnancy, but 54.6% did not know the correct timing for vaccination during pregnancy, while only 32.8% knew about the necessity of receiving a DTPa vaccine booster in each pregnancy. In a study conducted in Greece (77), in the post-intervention, 39.5% of the participants reported being already informed about the recommendations for influenza vaccination. The same study found that the average knowledge score on influenza and influenza vaccination, after the intervention, was 87% (77). However, neither the Italian nor the Greek studies evaluated the impact of the intervention on knowledge through a pre-post comparison (74, 77).
Furthermore, regarding information on vaccine-preventable diseases, in the study carried out in Poland and Ukraine (76), only 28.1% of the participants in the pre-intervention declared having received information regarding COVID-19 vaccination from their healthcare provider.
The evidence shows a low level of general knowledge about vaccinations against infectious diseases in the pre-intervention, as demonstrated by 43% of responses indicating poor or insufficient level of knowledge (72); following the educational intervention there was a notable 30% decrease in responses indicating a low level of knowledge in the vaccination field (72).
In terms of understanding the risks associated with infectious diseases, the findings indicate that, before the educational intervention, only 36.5% of participants were aware of the possible complications resulting from pertussis in newborns, and as many as 42.9% were uninformed about the potential repercussions of severe complications of influenza on both the mother and the fetus, as well as the newborn (74).
Moreover, it was revealed that 35.63% of respondents in the pre-intervention questionnaire, perceived influenza as quite serious, while almost 54% of the women in the post-intervention questionnaires shared this perception (73), with a notable increase. A significant increase in participants’ perception of the severity of influenza during pregnancy was also found following the educational intervention conducted in the British study (78).
The data showed that before the intervention, a notable proportion of women (40.5%) regarded diphtheria infection as very severe (73). Following the intervention, there was a significant increase in the proportion of women (61.8%) who perceived the infections as highly severe (73). Furthermore, after medical consultation, participants exhibited significantly heightened awareness regarding the severe clinical manifestations of COVID-19 infection (76).
Regarding vaccine safety, during the pre-intervention of one of the Italian studies (72), 15% of participants reported direct or indirect personal experiences with one or more post-vaccination adverse effects, including severe conditions such as autism, meningitis, deafness, polio, and acute leukemia. However, following the intervention, there was a reduction in this percentage, suggesting that the instances reported in the pre-intervention survey were possibly influenced by unsubstantiated beliefs or misinformation rather than genuine personal experiences.
Two studies conducted in Italy (72, 73) revealed a significant rise in the percentage of individuals who disregarded the existence of a causal association between vaccines and autism after the intervention, escalating from 43.8% (72) and 41% (73) during the pre-intervention to 84% (72) and 73% (73) during the post-intervention.
After the educational intervention, there was a significative increase in the proportion of individuals expressing a lack of concern regarding the adverse effects associated with vaccination (pre-intervention 33.3%, post-intervention 57.2%), believing that vaccines have mild side effects (pre-intervention 77.5%, post-intervention 97.40%) (73), and holding the belief that administering multiple vaccines simultaneously does not pose harm to the health of their offspring (pre-intervention 15.2%, post-intervention 70.1%) (72).
Noteworthy is the significant increase also in general knowledge regarding recommended pediatric vaccines, including diphtheria, tetanus, pertussis, poliomyelitis, and HIb, following the intervention (72).
In conclusion, these studies revealed a significant impact of educational interventions on pregnant women’s knowledge about vaccines and vaccine-preventable diseases. These interventions led to increased awareness of vaccination recommendations, decreased misinformation, and improved understanding of the severity of vaccine-preventable diseases.
3.2.4.2 Effects on attitudes
Six (72, 73, 75–78) out of the seven studies included in the analysis provided insights into the attitudes of pregnant women toward vaccination for themselves.
In an Italian study (72), the mean score quantifying the inclination to vaccinate during pregnancy was 35.46 (95% CI: 33.6–37.3) before the intervention and 42.57 (95% CI: 41.3–43.8) after the intervention. Considering that the score was calculated assigning a value of “0” to responses indicating opposition to vaccination, a value of “1” to neutral or hesitant responses, and a value of “3” to responses showing a support to vaccination, the results showed a shift toward a greater support to vaccination (72).
In another study conducted in Italy, an examination of the expressed preferences for vaccinations against individual infectious diseases revealed a significant surge in the inclination toward tetanus vaccination, with an increase from 80.77 to 91.14% (73).
Following the educational intervention, a notable increase was discerned in the responses concerning women’s intentions to undergo several vaccinations for themselves, including diphtheria and pertussis (72, 73).
A significant increase in the inclination to undergo influenza vaccination during pregnancy was highlighted in the study conducted in the UK (78) at the first follow-up assessment after the educational intervention. Moreover, within this study, both the probability of contracting influenza during pregnancy and the intention to receive the influenza vaccine emerged as significant positive predictors of influenza vaccination (78). Among the cohort of 411 participants in this study (78), 67 individuals completed the second follow-up. Within this subset, 57.1% of the participants who exhibited an increased intention to undergo vaccination (with a score of ≥6 out of 10) during the initial follow-up, subsequently received the vaccine (78).
In the investigation conducted in Greece (77), 57% of the participants expressed the intent to receive the vaccine and were accordingly prescribed it. However, despite the expressed intention and prescription, a substantial portion, comprising 31% of the individuals, did not proceed with vaccination. The predominant reason cited for non-adherence was “being sick,” as reported by 81% of women who had not been vaccinated.
A significant escalation in the intention to receive vaccination is evidenced also in the study conducted in Poland and Ukraine (76). Before medical consultations, 35.3% of patients in Poland and 16.7% of patients in Ukraine indicated their plans to undergo COVID-19 vaccination. Following medical consultations, the percentage of patients expressing willingness to receive vaccination surged to 72.6% in Poland and 46% in Ukraine. The data also showed that participants with higher education exhibited significantly greater level of vaccination acceptance compared to women with lower one (76). The investigation additionally underscored that heightened resistance to vaccination and incidence of patient-perceived post-vaccination complications corresponded with the diminished likelihood of altering the decision regarding COVID-19 vaccination after medical consultation (76). Predictors of reduced likelihood of vaccination included apprehension regarding fetal harm, perceived post-vaccination complications, and limitations in vaccinations program offered (76).
The study carried out in the Netherlands (75) demonstrated that an elevated magnitude of negative affects is markedly linked to a diminished inclination to embrace pertussis vaccination. Furthermore, within this study, all 3 groups, cognitive reappraisal intervention group, acceptance intervention group and control group, exhibited a noteworthy decrease in negative affect, with no notable disparities observed among them (75). Furthermore examining the written responses provided by participants across all groups, the adoption of emotional acceptance emerges as a promising approach in alleviating the influence of negative affect on the intention to accept pertussis vaccination (75).
In conclusion, the studies results revealed a notable shift toward greater acceptance and intention to vaccinate among pregnant women, influenced by educational interventions, medical consultations and emotional regulation strategies.
3.2.4.3 Effects on behavior
Following the educational intervention, a notable increase in adherence to influenza vaccination was observed across four studies (73, 74, 77, 78).
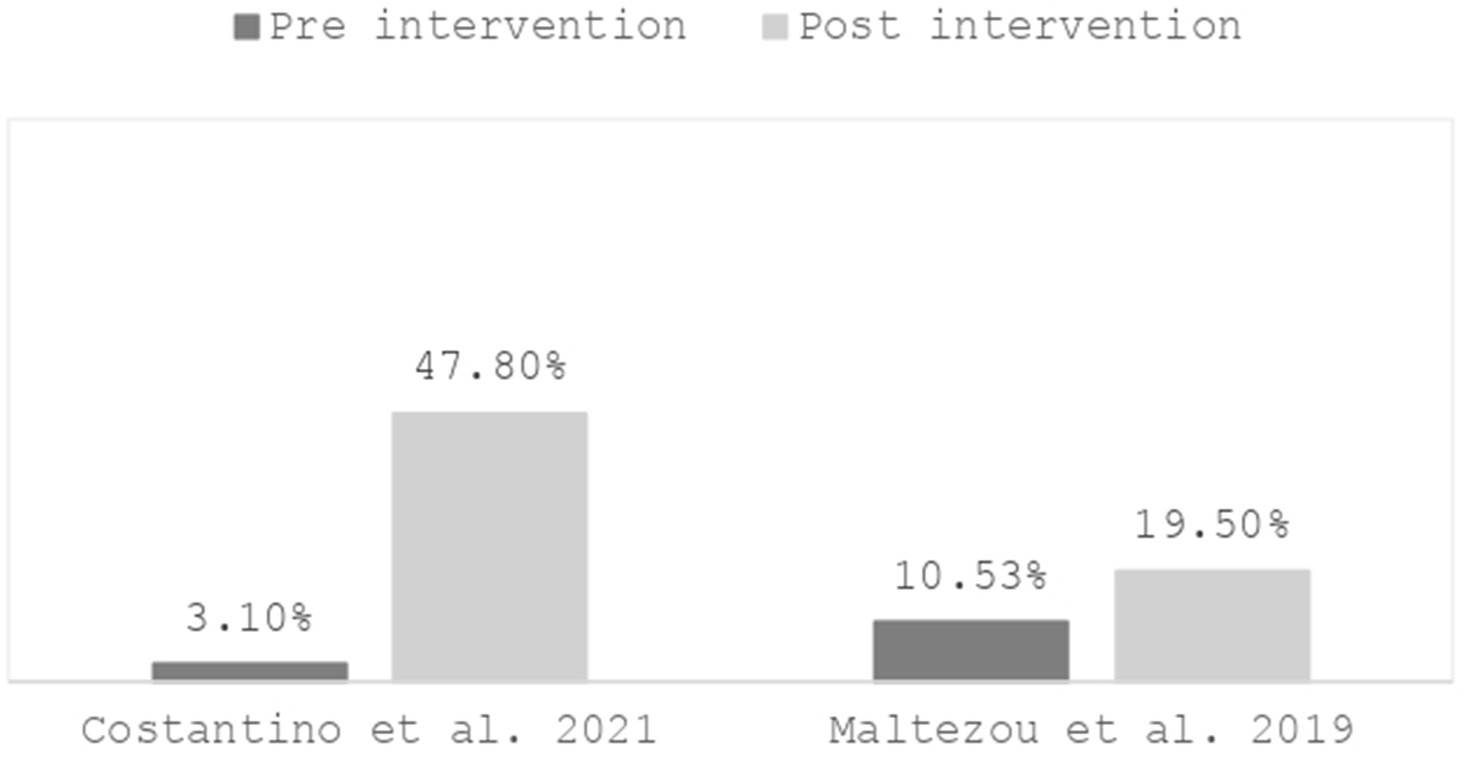
In two studies, conducted, respectively, in Italy (74) and Greece (77), 47.8% of respondents in the follow-up (74) and 19.5% of participants (77) reported having been vaccinated post-intervention, compared to 3.1% (74) and 10.53% (77) in the pre-intervention, indicating a significant increase (Figure 1).
In two studies conducted in Italy (73) and the UK (78), respectively, 40.34% of participants (73) and 57% of respondents (78) reported receiving influenza vaccination after the educational intervention.
The empirical findings suggest that after the implementation of the educational intervention, a significant augmentation in adherence to DTPa vaccination was observed, with rates escalating from 7.4 to 57.7% (74).
Factors influencing vaccination behavior were also addressed in the included studies. In two of them (74, 77) a significant association was also found between adherence to recommended vaccinations and a higher level of education. Indeed, findings from a study conducted in Italy emphasized that individuals with a higher level of education (bachelor’s/master’s degree) exhibited notably greater adherence to recommended vaccinations in comparison to counterparts with lower educational attainment (high school/primary-secondary school diploma) (adjusted OR = 3.12; 95% CI 1.25–4.67) (74). The aforementioned findings are corroborated by those from the investigation undertaken in Greece, wherein a demonstrably significant correlation was established between higher educational attainment (college-university level) and heightened compliance with vaccination protocols (77).
Evidence also indicated that a thorough understanding of influenza and influenza vaccine, and prior influenza vaccination history, were significantly associated with an increased likelihood of receiving influenza vaccination during pregnancy [respectively OR from 1.69 (74) to 17.8 (77), and from 3.6 (77) to 4.12 (74)], in contrast to individuals lacking adequate knowledge regarding influenza and the flu vaccine, as well as those who have not received vaccinations in preceding years.
Despite the implementation of educational interventions, various factors contributed to women’s reluctance to undergo vaccination during pregnancy, as evidenced by findings from three studies (74, 76, 77).
In the study conducted in Poland and Ukraine (76), participants cited concern about fetal harms and post-vaccination complications/adverse reactions, with fear being a key emotional driver influencing their decision to avoid the COVID-19 vaccine. These concerns decreased significantly after the intervention.
Additionally, in two separate studies (74, 77), post-intervention data revealed that 47.6% (74) and 27% (77) of participants who cited reasons for refusing influenza vaccination identified fear of adverse events as the main deterrent. In a study conducted in Italy (74) the secondary predominant reason for vaccine refusal was the absence of recommendations from gynecologists/obstetricians, highlighting the pivotal role of healthcare professionals in addressing vaccination hesitancy. Additionally, the belief that influenza vaccination is unnecessary and that the risk of contracting the flu is low has been cited as additional reason for vaccine refusal (77).
In conclusion, the educational intervention led to a significant increase in vaccination adherence across several studies. Higher education levels were associated with greater adherence to recommended vaccination regimens. However, despite these positive outcomes, vaccine hesitancy persists among pregnant women, emphasizing the continued need for interventions and the crucial role of healthcare professionals in addressing concerns.
4 Discussion
The primary objective of this investigation was to provide an examination of the latest national vaccination policies for pregnant women in European countries and to ascertain the effects of educational interventions targeted at pregnant women on their knowledge, attitudes, and behaviors regarding vaccination within the European setting.
In each country, vaccination policies may be shaped by disparities in the incidence of vaccine-preventable diseases, vaccination adherence rates, costs, and criteria used to issue recommendations and assess potential reimbursement (80, 81). Vaccine characteristics, such as efficacy or effectiveness and safety, are critical in shaping vaccination policies, as they directly influence public health outcomes and disease prevention strategies (81). Equally important is vaccine acceptability, which affects public uptake and the success of vaccination programs. If a vaccine is not widely accepted, its impact may be limited despite its efficacy (81). Additionally, vaccination policies must consider alternative interventions, such as public health campaigns or treatments, to ensure a balanced approach to disease prevention (81). The complex interaction between these factors could be reflected in the diversity in vaccination policies between European countries (18, 37, 62–65, 82–84). Despite this, following WHO guidelines (13–15), tailored vaccination programs are increasingly being implemented. From trimester-specific recommendations to personalized strategies during epidemics, national protocols highlight flexible vaccination approaches in pregnancy.
However, given the European decreasing confidence in vaccines (85), it would be useful to establish cohesive and harmonized pregnancy vaccination strategies across European countries to promote optimal outcomes in terms of maternal and fetal health. A viable approach to harmonize vaccination recommendations across Europe, while accounting for national variations, would involve the establishment of a transparent and common, yet adaptable, European framework to identify a core set of priority recommended vaccines while allowing individual countries to integrate additional vaccines according to their specific epidemiological circumstances. In this light, ongoing and systematic monitoring would facilitate timely adjustments to the core set of recommended vaccines, ensuring it remains responsive to evolving epidemiological conditions, also in relation to specific cases. Furthermore, ensuring that information regarding vaccination schedules and local updates is readily accessible and understandable to both healthcare professionals and the public is crucial to guarantee the equity and continuity of vaccination offer, particularly for individuals traveling between countries. Transparency and standardization in decision-making processes, coupled with a thorough and regular assessment of vaccination policies are imperative to allow harmonization.
In this context, governments assume a central role in structuring and implementing evidence-based vaccination policies and strategies tailored to pregnant women and capable of responding to any specific epidemiological situation, such as a potential high circulation of the pathogen, but also to integrate with existing vaccination recommendations in the general population.
In order to enhance vaccine uptake it is of utmost importance to also address knowledge and attitudes as foundations of individual behaviors. Our review encompassed seven studies addressing these aspects through educational interventions in pregnant women. Comparability across studies was restricted owing to variations in the contexts and nature of interventions implemented, as well as the criteria and methodologies used for evaluating results. Furthermore, the generalizability of the results can be influenced by the specific context of each country. For example, countries such as the United Kingdom, Greece, Poland, and Ukraine have similar vaccination policies for pregnant women, including recommending pertussis and influenza vaccines, as highlighted in our research (18, 37). In these countries, educational interventions have been implemented (76–78) specifically to raise awareness of influenza and pertussis vaccination. Therefore, given the existing vaccination awareness promoted by national policies, one might hypothesize that an educational intervention developed in one of these countries could have similar effectiveness when implemented in another. However, substantial heterogeneity in vaccination policies across countries, coupled with variations in national health cultures and health systems, complicates the prediction of the effectiveness of educational interventions developed within one national context when applied in another. This highlights the need for a more nuanced assessment of the adaptability and effectiveness of such interventions in accordance with the unique conditions of each country. Nevertheless, we contend that a favorable inference can be derived from the findings of the studies we reviewed, albeit challenges remain also in particular with respect to the reproducibility of interventions and methodology to assess their impact.
A relevant aspect that emerged from the collected evidence is concerning primary sources of information for pregnant women that mostly encompass obstetricians and healthcare practitioners (72–74, 77). In this respect, the absence of recommendations from gynecologists/obstetricians emerged as a pivotal determinant influencing vaccine refusal from one study conducted in Italy (74). In a recent Italian survey, about one-third of gynecologists expressed safety concerns about administering the influenza vaccine during the first trimester whereas Tdap vaccination is recommended in the third trimester with less safety concern (86). Furthermore, most participating gynecologists had themselves low influenza and Tdap vaccination rates, which might have affected their confidence in recommending vaccines (86, 87). Indeed, gynecologists/obstetricians are regarded as trusted healthcare professionals during pregnancy in Italy (85), therefore their advice was shown to play a crucial role in influencing decisions regarding vaccination uptake (88). This also aligns with the evidence of the fundamental role of healthcare professionals in combating vaccination hesitancy (29, 89–92). Nonetheless, albeit vaccinations should be addressed during antenatal care, it is not certain that this is done constantly and in a standardized way. The increasing prevalence of healthcare workers declining vaccination for themselves and abstaining from recommending it to their patients (93–96) may contribute to patient vaccine refusal and the observed low rates of vaccination acceptance, as also suggested in the discussion of one of the considered studies (76). A recent systematic review of the literature on vaccine hesitancy and vaccination coverage among healthcare workers in Europe has highlighted significant variability across countries and among vaccines (97). Vaccine hesitancy varies by country, with rates of 8% among all healthcare workers in Italy and up to 40% among physicians in France. Variations are also higher in respect to COVID-19 vaccines. Eventually, despite methodological differences across studies, physicians consistently exhibited lower levels of vaccine hesitancy compared to nurses, alongside higher vaccination rates for several vaccines, including COVID-19, influenza, diphtheria, tetanus, and pertussis (97). Contributing factors to vaccine hesitancy and vaccination refusal among healthcare professionals include concerns about adverse side effects, influence from individuals in personal networks who refuse vaccination, and diminished trust in vaccines, paralleling trends observed in the general public (97). It is anyhow worth noting that not all healthcare practitioners are experts in vaccinology, and their vaccine hesitancy may stem from uncertainties or even doubts regarding potential risks, public controversies, misinformation, as well as interactions with hesitant patients (97, 98). Hence, the training and implementation of tailored educational interventions on vaccination also for healthcare professionals are deemed imperative because awareness and knowledge were also found to increase healthcare professionals’ willingness to recommend vaccination (93).
Moreover, the execution of educational interventions facilitated by healthcare professionals specially trained may serve to alleviate misinformation concerning vaccines, which may stem from traditional (99) and social (100) mass media or word-of-mouth sources (101). Indeed, mass media have the potential to exert negative effects on vaccine-hesitant populations or instead, they could be used as a vital tool for disseminating vaccination culture (99, 102), despite assertions in existing literature indicating that women place greater trust in information provided by healthcare professionals compared to that disseminated through mass media or informal communication channels (89). For this reason, an effective strategy could be represented by educational intervention, carried out through social media but by healthcare professionals. Three studies (74, 75, 78), examined in the review, exemplify a commendable utilization of media for enhancing vaccination awareness among pregnant women, employing online platforms and the internet as vehicles for educational interventions and subsequent evaluation of outcomes, showing an effective approach toward addressing vaccination awareness. In one of the included studies (75), social media platforms were leveraged for participant recruitment, thus allowing the target population to be easily reached, as prospective parents demonstrate regular activity on social media and those uncertain about their decision about vaccination tend to look for information online.
Even if vaccination refusal is usually multifactorial (103), the deficiency of information regarding the safety and efficacy of vaccines commonly catalyzes vaccination refusal (104). The results of our review showed a notable deficiency in knowledge and awareness concerning the vaccination field, specifically recommended vaccines during pregnancy (72, 74, 76, 77), vaccine-preventable diseases, and their severity for both pregnant women and offspring (72–74, 76, 78) before any educational intervention, consistent with extant literature (29, 91, 105–107).
Conversely, following the implementation of educational interventions, there was a discernible increase in comprehension within these domains, leading to an escalation in the inclination to receive vaccinations during pregnancy (72), consequently resulting in a significant enhancement in adherence to recommended vaccination recommendations (73, 76, 77). Nevertheless, caution should be paid in the interpretation of these results because it is expected that pregnant women’s knowledge about recommended vaccination increases with the increase in gestation week. Unfortunately, the specific week of pregnancy during which knowledge was assessed was not explicitly stated, except indirectly in the case of two Italian studies that reported that the most of participants were in the third trimester (73, 74).
Nevertheless, in this respect a standardized and validated curriculum should be developed to lead educational interventions and make them more comparable. This curriculum should be evidence-based and encompass vaccine-preventable diseases characteristics, recommendations for vaccination in pregnancy, and vaccines efficacy, effectiveness and safety. The curriculum could be adopted by trainers in the field as well as by all healthcare professionals engaged in prenatal care, including gynecologists, obstetricians, midwives, and nurses. A particular attention should be paid to adapt the curriculum to pregnant women’s needs and capabilities. In fact, our data also showed a general lower likelihood of vaccination during pregnancy in individuals with a low degree of education (74, 76, 77), in accordance with existing literature (108, 109). Thus, it is advisable to customize educational interventions to align with the educational and socio-demographic context of the target population, given that these variables may exert influence on vaccination decisions.
The educational intervention ought to comprehensively address not only the potential adverse effects of vaccination, debunking associated misconceptions and contrasting negative affect, i.e., fear, discomfort, anticipated regret (75), and perception of complications and damage after administration (76), but also underscore the risks associated with vaccine refusal for both the pregnant woman and her offspring, which may lead to significant complications.
The multi-component approach, incorporating educational interventions and vaccination administered by trained personnel, alongside healthcare professional training and continuous education, has exhibited superior effectiveness in enhancing maternal attitudes toward recommended vaccines during pregnancy (94–96, 98). Moreover, it has proven efficacious in augmenting vaccination adherence rates among both prenatal and postnatal women (94–96, 98). Furthermore, new methodologies, including reminder and active call systems (94, 95), as well as the utilization of digital modalities such as text, video, or audio messages, and internet-based interventions (e.g., websites, mobile applications, or social media platforms), have underscored their effectiveness in a context significantly influenced by the recent COVID-19 pandemic. This context is also marked by heightened vaccine hesitancy, alongside an overall increase in the complexity of vaccination schedules, heightened expectations from caregivers, and lifestyle changes (100).
The findings of our work should be read considering some limitations. First of all, the search strategy adopted to look for both vaccination policies in European countries and the evidence on educational intervention might have failed in identifying all relevant information also considering that some recommendations could be issued in local languages thus being difficult to find and report. Another aspect to be considered is that vaccination policies could be implemented differently between and within countries. Regarding the evidence on the impact of educational interventions, it should be noted that, because all studies relied on questionnaires, whether validated or not, the potential for social desirability bias could not be ruled out. Notably, the studies included in our review did not employ tools designed to specifically measure social desirability bias. However, the use of anonymized questionnaires in these studies may have helped mitigating this bias. Additionally, in one instance (75), being a randomized experimental study, the process of randomization may have contributed to controlling for this bias. As a matter of fact, all studies included in our work were judged of fair quality and this calls for other research in the field to better disentangle the potential impact of educational interventions also considering different contexts.
5 Conclusion
In conclusion, there is considerable variability across European countries regarding vaccination policies during pregnancy. Tailored vaccination policies and recommendations, aligned with WHO guidelines, reflect the diverse epidemiological contexts and healthcare systems of individual countries.
Educational interventions carried out to promote pregnant vaccination by increasing knowledge and changing attitudes varied in approach and context so far. Nonetheless, they collectively demonstrated significant impacts on pregnant women’s vaccination-related knowledge, attitudes, and behaviors in Europe. From antenatal classes to online platforms and informational leaflets, these interventions led to increased awareness of vaccination recommendations, reduced misinformation, and improved understanding of the severity of vaccine-preventable diseases. Indeed, pre-intervention assessments revealed gaps in knowledge and concerns about vaccine safety, but post-intervention, there was a notable improvement, leading to enhanced adherence to recommended vaccination protocols.
Healthcare professionals emerged as the most trusted source of vaccination information, highlighting their crucial role in addressing vaccine hesitancy.
Attitudes emerged as a significant predictor of intention to vaccinate, with positive attitudes associated with stronger intentions. Emotional regulation strategies also played a role in increasing vaccination acceptance.
Behaviorally, there was a significant increase in adherence to influenza and DTPa vaccination post-intervention, particularly among those with higher education levels. However, vaccine hesitancy persisted among some, driven by concerns about adverse events and a lack of recommendations from healthcare professionals.
Overall, the findings of this investigation underscore the importance of strengthening the process behind the development of evidence-based vaccination policies and the need for specific educational interventions to increase vaccination acceptance and optimize maternal and fetal health outcomes in the European context. Further research and collaborative efforts are warranted to address barriers and facilitators to vaccination uptake among pregnant women.
Author contributions
SP: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. RC: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. VB: Data curation, Investigation, Writing – review & editing. VC: Data curation, Investigation, Writing – review & editing. BM: Data curation, Investigation, Writing – review & editing. ES: Data curation, Investigation, Writing – review & editing. EB: Writing – review & editing. CW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ander, SE, Diamond, MS, and Coyne, CB. Immune responses at the maternal-fetal interface. Sci Immunol. (2019) 4:eaat6114. doi: 10.1126/sciimmunol.aat6114
2. Mor, G, and Cardenas, I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. (2010) 63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x
3. Robinson, DP, and Klein, SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. (2012) 62:263–71. doi: 10.1016/j.yhbeh.2012.02.023
4. Megli, CJ, and Coyne, CB. Infections at the maternal-fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol. (2022) 20:67–82. doi: 10.1038/s41579-021-00610-y
5. Vojtek, I, Dieussaert, I, Doherty, TM, Franck, V, Hanssens, L, Miller, J, et al. Maternal immunization: where are we now and how to move forward? Ann Med. (2018) 50:193–208. doi: 10.1080/07853890.2017.1421320
6. Kourtis, AP, Read, JS, and Jamieson, DJ. Pregnancy and infection. N Engl J Med. (2014) 370:2211–8. doi: 10.1056/NEJMra1213566
7. Faucette, AN, Pawlitz, MD, Pei, B, Yao, F, and Chen, K. Immunization of pregnant women: future of early infant protection. Hum Vaccin Immunother. (2015) 11:2549–55. doi: 10.1080/21645515.2015.1070984
8. Etti, M, Calvert, A, Galiza, E, Lim, S, Khalil, A, Le Doare, K, et al. Maternal vaccination: a review of current evidence and recommendations. Am J Obstet Gynecol. (2022) 226:459–74. doi: 10.1016/j.ajog.2021.10.041
9. Mackin, DW, and Walker, SP. The historical aspects of vaccination in pregnancy. Best Pract Res Clin Obstet Gynaecol. (2021) 76:13–22. doi: 10.1016/j.bpobgyn.2020.09.005
10. Willemsen, JE, Borghans, JAM, Bont, LJ, and Drylewicz, J. Disagreement FDA and EMA on RSV maternal vaccination: possible consequence for global mortality. Pediatr Infect Dis J. (2024) 43:e1:–e2. doi: 10.1097/INF.0000000000004173
11. Gidengil, C, Goetz, MB, Newberry, S, Maglione, M, Hall, O, Larkin, J, et al. Safety of vaccines used for routine immunization in the United States: an updated systematic review and meta-analysis. Vaccine. (2021) 39:3696–716. doi: 10.1016/j.vaccine.2021.03.079
12. Marshall, H, McMillan, M, Andrews, RM, Macartney, K, and Edwards, K. Vaccines in pregnancy: the dual benefit for pregnant women and infants. Hum Vaccin Immunother. (2016) 12:848–56. doi: 10.1080/21645515.2015.1127485
13. WHO . Pertussis vaccines: WHO position paper, august 2015—recommendations. Vaccine. (2016) 34:1423–5. doi: 10.1016/j.vaccine.2015.10.136
14. WHO . (2014). Europe WHORO for European Vaccine Action Plan 2015-2020. Available online at: https://iris.who.int/handle/10665/340400 (Accessed October 17, 2024).
15. WHO . (2021) The European Immunization Agenda 2030. Available online at: https://www.who.int/europe/initiatives/the-european-immunization-agenda-2030 (Accessed October 17, 2024).
16. ACIP . (2023). Vaccine Recommendations|CDC. Available online at: https://www.cdc.gov/vaccines/hcp/acip-recs/index.html (Accessed October 17, 2024).
17. CDC . (2024). Centers for Disease Control and Prevention. Vaccination Considerations for People Pregnant or Breastfeeding. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html (Accessed October 17, 2024).
18. Maltezou, HC, Effraimidou, E, Cassimos, DC, Medic, S, Topalidou, M, Konstantinidis, T, et al. Vaccination programs for pregnant women in Europe, 2021. Vaccine. (2021) 39:6137–43. doi: 10.1016/j.vaccine.2021.08.074
19. Qiu, X, Bailey, H, and Thorne, C. Barriers and facilitators associated with vaccine acceptance and uptake among pregnant women in high income countries: a mini-review. Front Immunol. (2021) 12:626717. doi: 10.3389/fimmu.2021.626717
20. Corbeau, M, Mulliez, A, Chenaf, C, Eschalier, B, Lesens, O, and Vorilhon, P. Trends of influenza vaccination coverage in pregnant women: a ten-year analysis from a French healthcare database. Sci Rep. (2022) 12:7153. doi: 10.1038/s41598-022-11308-3
21. European Centre for Disease Prevention and Control . (2018). Seasonal influenza vaccination and antiviral use in EU/EEA Member States – Overview of vaccine recommendations for 2017–2018 and vaccination coverage rates for 2015–2016 and 2016–2017 influenza seasons. Stockholm: ECDC. doi: 10.2900/721517
22. European Centre for Disease Prevention and Control . (2023). Seasonal influenza vaccination recommendations and coverage rates in EU/EEA member states: An overview of vaccination recommendations for 2021–22 and coverage rates for the 2018–19 to 2020–21 influenza seasons. LU: Publications Office. Available online at: https://data.europa.eu/doi/10.2900/335933 (Accessed October 17, 2024).
23. European Centre for Disease Prevention and Control . (2024). Increase of pertussis cases in the EU/EEA: 8 may 2024. LU: Publications Office. Available online at: https://data.europa.eu/doi/10.2900/831122 (Accessed October 17, 2024).
24. ECDC . (2024). Interim COVID-19 vaccination coverage in the EU/EEA during the 2023–24 season campaigns. Available online at: https://www.ecdc.europa.eu/en/publications-data/interim-covid-19-vaccination-coverage-eueea-during-2023-24-season-campaigns (Accessed October 17, 2024).
25. Stoeckel, F, Carter, C, Lyons, BA, and Reifler, J. Association of vaccine hesitancy and immunization coverage rates in the European Union. Vaccine. (2021) 39:3935–9. doi: 10.1016/j.vaccine.2021.05.062
26. NE, MDSAGE Working Group on Vaccine Hesitancy . Vaccine hesitancy: definition, scope and determinants. Vaccine. (2015) 33:4161–4. doi: 10.1016/j.vaccine.2015.04.036
27. World Health Organization . (2019) WHO Names Top Ten Threats to Global Health. Available online at: https://www.wiredhealthresources.net/wired_archive/WHO_Names_Ten_Threats_to_Global_Health.html (Accessed October 17, 2024).
28. Castillo, E, Patey, A, and MacDonald, N. Vaccination in pregnancy: challenges and evidence-based solutions. Best Pract Res Clin Obstet Gynaecol. (2021) 76:83–95. doi: 10.1016/j.bpobgyn.2021.03.008
29. Kilich, E, Dada, S, Francis, MR, Tazare, J, Chico, RM, Paterson, P, et al. Factors that influence vaccination decision-making among pregnant women: a systematic review and meta-analysis. PLoS One. (2020) 15:e0234827. doi: 10.1371/journal.pone.0234827
30. Hoare, J, Mendelson, M, and Frenkel, L. COVID-19 vaccine hesitancy and anti-vaxxers - supporting healthcare workers to navigate the unvaccinated: reflections from clinical practice. S Afr Med J. (2022) 112:13514. doi: 10.7196/SAMJ.2022.v112i1.16208
31. Razai, MS, Mansour, R, Ravindran, P, Freeman, S, Mason-Apps, C, Morris, J, et al. Facilitators and barriers to vaccination uptake in pregnancy: a qualitative systematic review. PLoS One. (2024) 19:e0298407. doi: 10.1371/journal.pone.0298407
32. Bisset, KA, and Paterson, P. Strategies for increasing uptake of vaccination in pregnancy in high-income countries: a systematic review. Vaccine. (2018) 36:2751–9. doi: 10.1016/j.vaccine.2018.04.013
33. Brillo, E, Tosto, V, and Buonomo, E. Interventions to increase uptake of influenza vaccination in pregnancy: a systematic review and meta-analysis. Int J Gynaecol Obstet. (2023) 162:39–50. doi: 10.1002/ijgo.14714
34. Properzi, S, Sepioni, MS, Carestia, R, Cervelli, G, and de Waure, C. Promoting vaccinations in pregnancy: results of a systematic literature review of Italian initiatives. Vaccine. (2024) 12:235. doi: 10.3390/vaccines12030235
35. Arriola, CS, Suntarattiwong, P, Dawood, FS, Soto, G, Das, P, Hunt, DR, et al. What do pregnant women think about influenza disease and vaccination practices in selected countries. Hum Vaccin Immunother. (2021) 17:2176–84. doi: 10.1080/21645515.2020.1851536
36. Yuen, CYS, and Tarrant, M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. (2014) 32:4602–13. doi: 10.1016/j.vaccine.2014.06.067
37. Vaccine Scheduler. Available online at: https://vaccine-schedule.ecdc.europa.eu/ (Accessed October 17, 2024).
38. EQUATOR Network . (2024) Enhancing the QUAlity and Transparency of Health Research. Available online at: https://www.equator-network.org/ (Accessed October 17, 2024).
39. PICO Portal . Synthesize evidence at rapid speed. Available online at: https://picoportal.org/ (Accessed October 17, 2024).
40. NHLBI and NIH . (2021) Study Quality Assessment Tools. Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (Accessed October 17, 2024).
41. Robert Koch Institute . (2022) Empfehlungen der Ständigen Impfkommission (STIKO) beim Robert Koch-Institut 2023. Available online at: https://edoc.rki.de/handle/176904/10636 (Accessed October 17, 2024).
42. Impfen schützt einfach . (2024) Influenza. Available online at: https://impfen.gv.at/impfungen/influenza (Accessed October 17, 2024).
43. Danish Health Authority . (2024) Pregnant women are offered vaccinations against influenza and covid-19. Available online at: http://www.sst.dk/en/english/Vaccination-against-influenza-and-covid-19/Pregnant-women (Accessed October 17, 2024).
44. Helsenorge . (2023). Flu shot in Norway. Available online at: https://www.helsenorge.no/en/vaksiner-og-vaksinasjon/influenza-vaccine/ (Accessed October 17, 2024).
45. RIVM . (2024) Flu vaccine. Available online at: https://www.rivm.nl/en/flu-and-flu-vaccine/vaccine (Accessed October 17, 2024).
46. Folkhälsomyndigheten . (2024) Vaccinationer till vuxna. Available online at: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/vaccinationer-till-vuxna/ (Accessed October 17, 2024).
47. Folkhälsomyndigheten . (2022). Information about vaccinations for people who are pregnant. Available online at: https://www.folkhalsomyndigheten.se/publikationer-och-material/publikationsarkiv/i/information-about-vaccinations-for-people-who-are-pregnant/ (Accessed October 17, 2024).
48. University of Zurich . (2024) Pertussis: What Is It and What Do Pregnant Women Need to Know?. Available online at: https://reisemedizin.uzh.ch/en/blog/pertussis (Accessed October 17, 2024).
49. Impfservice Wien . (2024) Whooping cough (pertussis). Available online at: https://impfservice.wien/en/whooping-cough-pertussis/ (Accessed October 17, 2024).
50. RIVM . (2020) 22-week vaccination (maternal whooping cough vaccination). Available online at: https://www.rivm.nl/en/whooping-cough/22-week-vaccination (Accessed October 17, 2024).
51. Finnish Institute for Health and Welfare (THL), Finland . (2024) THL recommends whooping cough vaccine in pregnancy – The reason is an increase in cases, which may be dangerous for babies - THL. Available online at: https://thl.fi/en/-/thl-recommends-whooping-cough-vaccine-in-pregnancy-the-reason-is-an-increase-in-cases-which-may-be-dangerous-for-babies (Accessed October 17, 2024).
52. Statens Sero Institute . (2023) Gratis kighostevaccination til gravide. Available online at: https://www.ssi.dk/vaccinationer/risikogrupper/gratis-kighostevaccination-til-gravide (Accessed October 17, 2024).
53. Nijz . (2022) Cepljenje nosečnic proti oslovskemu kašlju. Available online at: https://nijz.si/publikacije/cepljenje-nosecnic-proti-oslovskemu-kaslju/ (Accessed October 17, 2024).
54. Helsenorge . (2023) Recommended vaccines during pregnancy. Available online at: https://www.helsenorge.no/en/vaksiner-og-vaksinasjon/vaksiner-i-svangerskapet/ (Accessed October 17, 2024).
55. Ísland.is . (2024) Pertussis (whooping cough) diagnosed in Iceland-First cases since 2019. Available online at: https://island.is/en/news/kighosti-greinist-a-islandi-fyrsta-tilfelli-sidan-2019 (Accessed October 17, 2024).
56. NHS . (2020) Whooping cough vaccination in pregnancy. Available online at: https://www.nhs.uk/pregnancy/keeping-well/whooping-cough-vaccination/ (Accessed October 17, 2024).
57. HSE . (2022) Whooping cough vaccine. Available online at: https://www.hse.ie/eng/health/immunisation/pubinfo/pregvaccs/pertussis/pertussis.html (Accessed October 17, 2024).
58. Sciensano . (n.d.) Whooping cough. Available online at: https://www.sciensano.be/en/health-topics/whooping-cough (Accessed October 17, 2024).
59. RIVM . (2024) Whooping cough injection for pregnant women (22 week injection). Available online at: https://rijksvaccinatieprogramma.nl/kinkhoestprik (Accessed October 17, 2024).
60. Della, Salute M. (2023) Donne in età fertile e in gravidanza. Available online at: https://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4809&area=vaccinazioni&menu=fasce (Accessed October 17, 2024).
61. Sanidad . (2024) Ministerio de Sanidad-Áreas-Salud pública - Prevención de la salud - Vacunaciones-Programa vacunación - Embarazadas. Available online at: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/programasDeVacunacion/embarazadas/mujeres/embarazadas.htm (Accessed October 17, 2024).
62. infoVAXX . (2023). The Luxembourg government. Available online at: http://covid19.public.lu/en/vaccination/infovaxx.html (Accessed October 17, 2024).
63. UNHCR . (2022) COVID-19 Information for Malta–UNHCR Malta. Available online at: https://www.unhcr.org/mt/news/covid-19-information-for-malta (Accessed October 17, 2024).
64. Folkhalsomyndigheten . (2023) Vaccination against flu and COVID-19- The Public Health Agency of Sweden. Available online at: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/vaccinations/vaccination-against-flu-and-covid-19/ (Accessed October 17, 2024).
65. Vakcinace . (2021) Očkování proti onemocnění covid-19 u těhotných a kojících žen. Available online at: https://www.vakcinace.eu/doporuceni-a-stanoviska/ockovani-proti-onemocneni-covid-19-u-tehotnych-a-kojicich-zen (Accessed October 17, 2024).
66. FOPH . (2024) COVID-19: Vaccination. Available online at: https://www.bag.admin.ch/bag/en/home/krankheiten/krankheiten-im-ueberblick/coronavirus/covid-19/impfen.html#-1009560172 (Accessed October 17, 2024).
67. Impfservice Wien . (2024) COVID-19 FAQs. Available online at: https://impfservice.wien/en/covid-19/covid-19-faqs/ (Accessed October 17, 2024).
68. Statement of the Standing Commission on Vaccination (STIKO) at the Robert Koch Institute . (2023) Decision on the implementation of the COVID-19 vaccination into the general recommendations of the STIKO. Available at: https://www.rki.de/EN/Content/infections/Vaccination/recommandations/implementation_covid-19_vaccination.pdf?__blob=publicationFile
69. Godine . (2023) Privremene preporuke za cijepljenje protiv bolesti COVID-19 – jesen 2023. Available online at: https://www.hzjz.hr/sluzba-epidemiologija-zarazne-bolesti/privremene-preporuke-za-cijepljenje-protiv-bolesti-covid-19-jesen-2023-godine/ (Accessed October 17, 2024).
70. ПлюсМен . (2024). A specialized site for immunizations in Bulgaria. Available online at: https://plusmen.bg/ (Accessed October 17, 2024).
71. ENS Seisukohad . (2021). ENS positions. Available online at: https://www.ens.ee/ens-seisukohad (Accessed October 17, 2024).
72. Bechini, A, Moscadelli, A, Pieralli, F, Sartor, G, Seravalli, V, Panatto, D, et al. Impact assessment of an education course on vaccinations in a population of pregnant women: a pilot study. J Prev Med Hyg. (2019) 60:E5–E11. doi: 10.15167/2421-4248/jpmh2019.60.1.1093
73. Bruno, S, Carducci, B, Quaranta, G, Beccia, V, Pilla, AD, Milia, DIL, et al. Enhancement of vaccination attitude and flu vaccination coverage among pregnant women attending birthing preparation course. Vaccine. (2021) 9:1–10. doi: 10.3390/vaccines9020183
74. Costantino, C, Mazzucco, W, Bonaccorso, N, Cimino, L, Conforto, A, Sciortino, M, et al. Educational interventions on pregnancy vaccinations during childbirth classes improves vaccine coverages among pregnant women in Palermo’s province. Vaccines. (2021) 9:1455. doi: 10.3390/vaccines9121455
75. Buursma, P, Anraad, C, van Empelen, P, Ruiter, RAC, and van Keulen, HM. The effect of emotion regulation strategies on decision-making about the maternal pertussis vaccination among pregnant women in the Netherlands: an experimental study. Patient Educ Couns. (2023) 107:107566. doi: 10.1016/j.pec.2022.11.008
76. Januszek, S, Siwiec, N, Januszek, R, Kluz, M, Lebed, R, Toś, P, et al. Approach of pregnant women from Poland and the Ukraine to COVID-19 vaccination-the role of medical consultation. Vaccines. (2022) 10:255. doi: 10.3390/vaccines10020255
77. Maltezou, HC, Pelopidas Koutroumanis, P, Kritikopoulou, C, Theodoridou, K, Katerelos, P, Tsiaousi, I, et al. Knowledge about influenza and adherence to the recommendations for influenza vaccination of pregnant women after an educational intervention in Greece. Hum Vaccin Immunother. (2019) 15:1070–4. doi: 10.1080/21645515.2019.1568158
78. Parsons, J, Grimley, C, and Newby, K. Effectiveness of a digital intervention in increasing flu vaccination-related risk appraisal, intention to vaccinate and vaccination behaviour among pregnant women. Health Educ Behav. (2022) 49:1033–41. doi: 10.1177/10901981221077935
79. Gualano, MR, Bert, F, Voglino, G, Buttinelli, E, D’Errico, MM, De Waure, C, et al. Attitudes towards compulsory vaccination in Italy: results from the NAVIDAD multicentre study. Vaccine. (2018) 36:3368–74. doi: 10.1016/j.vaccine.2018.04.029
80. Cassimos, DC, Effraimidou, E, Medic, S, Konstantinidis, T, Theodoridou, M, and Maltezou, HC. Vaccination programs for adults in Europe, 2019. Vaccines. (2020) 8:34. doi: 10.3390/vaccines8010034
81. Burchett, HED, Mounier-Jack, S, Griffiths, UK, and Mills, AJ. National decision-making on adopting new vaccines: a systematic review. Health Policy Plan. (2012):ii62. doi: 10.1093/heapol/czr049
82. RIVM . (2024) Vaccinating against corona during pregnancy. Available online at: https://www.rivm.nl/corona/coronaprik/zwangerschap (Accessed October 17, 2024).
83. CDC . (2024). Pregnancy and vaccination. Vaccine Safety for Moms-To-Be. Available online at: https://www.cdc.gov/vaccines-pregnancy/moms-to-be/index.html (Accessed October 17, 2024).
84. CDC . (2024). Pregnancy and vaccination. Guidelines for vaccinating pregnant persons. Available online at: https://www.cdc.gov/vaccines-pregnancy/hcp/vaccination-guidelines/index.html (Accessed October 17, 2024).
85. European Commission . (2022) State of Vaccine Confidence in the EU. Available online at: https://health.ec.europa.eu/publications/state-vaccine-confidence-eu-2022_en (Accessed October 17, 2024).
86. Scatigna, M, Appetiti, A, Pasanisi, M, D’Eugenio, S, Fabiani, L, and Giuliani, AR. Experience and attitudes on vaccinations recommended during pregnancy: survey on an Italian sample of women and consultant gynecologists. Hum Vaccin Immunother. (2022) 18:1–8. doi: 10.1080/21645515.2021.1894061
87. Vilca, LM, Cesari, E, Tura, AM, Di Stefano, A, Vidiri, A, Cavaliere, AF, et al. Barriers and facilitators regarding influenza and pertussis maternal vaccination uptake: a multi-center survey of pregnant women in Italy. Eur J Obstet Gynecol Reprod Biol. (2020) 247:10–5. doi: 10.1016/j.ejogrb.2020.02.007
88. Filip, G, Sala, A, Modolo, V, Arnoldo, L, Brunelli, L, and Driul, L. Vaccination: adherence and hesitancy among pregnant women for COVID-19, pertussis, and influenza vaccines. Vaccine. (2024) 12:427. doi: 10.3390/vaccines12040427
89. Krishnaswamy, S, Cheng, AC, Wallace, EM, Buttery, J, and Giles, ML. Understanding the barriers to uptake of antenatal vaccination by women from culturally and linguistically diverse backgrounds: a cross-sectional study. Hum Vaccin Immunother. (2018) 14:1591–8. doi: 10.1080/21645515.2018.1445455
90. Lotter, K, Regan, AK, Thomas, T, Effler, PV, and Mak, DB. Antenatal influenza and pertussis vaccine uptake among aboriginal mothers in Western Australia. Aust N Z J Obstet Gynaecol. (2018) 58:417–24. doi: 10.1111/ajo.12739
91. O’Shea, A, Cleary, B, McEntee, E, Barrett, T, O’Carroll, A, Drew, R, et al. To vaccinate or not to vaccinate? Women’s perception of vaccination in pregnancy: a qualitative study. BJGP Open. (2018) 2:bjgpopen18X101457. doi: 10.3399/bjgpopen18X101457
92. Brillo, E, Ciampoletti, M, and Tosto, V. Exploring Tdap and influenza vaccine uptake and its determinants in pregnancy: A cross-sectional study. Ann Ig. (2022) 34:358–74. doi: 10.7416/ai.2022.2503
93. Paterson, P, Meurice, F, Stanberry, LR, Glismann, S, Rosenthal, SL, and Larson, HJ. Vaccine hesitancy and healthcare providers. Vaccine. (2016) 34:6700–6. doi: 10.1016/j.vaccine.2016.10.042
94. Karnaki, P, Baka, A, Petralias, A, Veloudaki, A, Zota, D, Linos, A, et al. Immunization related behaviour among healthcare workers in Europe: results of the HProImmune survey. Cent Eur J Public Health. (2019) 27:204–11. doi: 10.21101/cejph.a5514
95. Gomez, S, and Godoy, P. Vaccine hesitancy in healthcare professionals and health sciences students of the last courses. Vacunas. (2024) 25:54–63. doi: 10.1016/j.vacune.2024.02.015
96. Verger, P, Botelho-Nevers, E, Garrison, A, Gagnon, D, Gagneur, A, Gagneux-Brunon, A, et al. Vaccine hesitancy in health-care providers in Western countries: a narrative review. Expert Rev Vaccines. (2022) 21:909–27. doi: 10.1080/14760584.2022.2056026
97. Kaur, M, and Coppeta, LOlesen OF. Vaccine hesitancy among healthcare Workers in Europe: a systematic review. Vaccines. (2023) 11:1657. doi: 10.3390/vaccines11111657
98. Manca, T . “One of the greatest medical success stories:” physicians and nurses’ small stories about vaccine knowledge and anxieties. Soc Sci Med. (2018) 196:182–9. doi: 10.1016/j.socscimed.2017.11.027
99. Catalan-Matamoros, D, and Peñafiel-Saiz, C. How is communication of vaccines in traditional media: a systematic review. Perspect Public Health. (2019) 139:34–43. doi: 10.1177/1757913918780142
100. Wilson, SL, and Wiysonge, C. Social media and vaccine hesitancy. BMJ Glob Health. (2020) 5:e004206. doi: 10.1136/bmjgh-2020-004206
101. Erbil Aydoğdu, MB, and Zengin, AY. Vaccine rejection reasons and the role of digital media and word of mouth: Do income and education level matter?. YSBD. (2023) 13:354–67.
102. Wakefield, MA, Loken, B, and Hornik, RC. Use of mass media campaigns to change health behaviour. Lancet. (2010) 376:1261–71. doi: 10.1016/S0140-6736(10)60809-4
103. Mitchell, SL, Schulkin, J, and Power, ML. Vaccine hesitancy in pregnant women: a narrative review. Vaccine. (2023) 41:4220–7. doi: 10.1016/j.vaccine.2023.05.047
104. O’Leary, ST, Riley, LE, Lindley, MC, Allison, MA, Albert, AP, Fisher, A, et al. Obstetrician–gynecologists’ strategies to address vaccine refusal among pregnant women. Obstet Gynecol. (2019) 133:40–7. doi: 10.1097/AOG.0000000000003005
105. Gauld, NJ, Braganza, CS, Babalola, OO, Huynh, TT, and Hook, SM. Reasons for use and non-use of the pertussis vaccine during pregnancy: an interview study. J Prim Health Care. (2016) 8:344–50. doi: 10.1071/HC15049
106. O’Grady, KAF, Dunbar, M, Medlin, LG, Hall, KK, Toombs, M, Meiklejohn, J, et al. Uptake of influenza vaccination in pregnancy amongst Australian aboriginal and Torres Strait islander women: a mixed-methods pilot study. BMC Res Notes. (2015) 8:169. doi: 10.1186/s13104-015-1147-3
107. Larson Williams, A, McCloskey, L, Mwale, M, Mwananyanda, L, Murray, K, Herman, AR, et al. “When you are injected, the baby is protected:” assessing the acceptability of a maternal Tdap vaccine based on mothers’ knowledge, attitudes, and beliefs of pertussis and vaccinations in Lusaka. Zambia Vaccine. (2018) 36:3048–53. doi: 10.1016/j.vaccine.2018.03.081
108. Rand, CM, and Olson-Chen, C. Maternal vaccination and vaccine hesitancy. Pediatr Clin N Am. (2023) 70:259–69. doi: 10.1016/j.pcl.2022.11.004
Keywords: vaccination, vaccine policies, knowledge, behavior, pregnancy
Citation: Properzi S, Carestia R, Birettoni V, Calesso V, Marinelli B, Scapicchi E, Brillo E and de Waure C (2024) Vaccination of pregnant women: an overview of European policies and strategies to promote it. Front. Public Health. 12:1455318. doi: 10.3389/fpubh.2024.1455318
Edited by:
Silvio Tafuri, University of Bari Aldo Moro, ItalyReviewed by:
Patrícia Soares, Instituto Nacional de Saúde Dr Ricardo Jorge, PortugalAna Afonso, NOVA University of Lisbon, Portugal
Copyright © 2024 Properzi, Carestia, Birettoni, Calesso, Marinelli, Scapicchi, Brillo and de Waure. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Properzi, c2FyYS5wcm9wZXJ6aUBzcGVjaWFsaXp6YW5kaS51bmlwZy5pdA==
 S. Properzi
S. Properzi R. Carestia
R. Carestia V. Birettoni
V. Birettoni V. Calesso2
V. Calesso2 E. Brillo
E. Brillo C. de Waure
C. de Waure