- 1Department of Nursing, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Nursing School of Zunyi Medical University, Zunyi, Guizhou, China
- 3Department of Obstetrics, Tongzi People's Hospital, Zunyi, Guizhou, China
- 4Department of Reproductive Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 5Department of Geriatric Medicine, The Third Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
Background: Depression symptoms are a growing concern for adolescent girls with PCOS around the world. However, relatively small samples have given varying reports of its prevalence and risk factors in previous studies. Therefore, there is an urgent need for further research on the prevalence and associated factors of depression among adolescent girls with PCOS.
Methods: A cross-sectional study was performed from October 2021 to May 2022 using a questionnaire and examination of the medical records of a convenience sample of 335 adolescent girls with PCOS. The Chinese version of the Children’s Depression Scale (CDI) was used to investigate depression symptoms. A multivariate logistic regression model was used to determine factors that were significantly associated with depression symptoms.
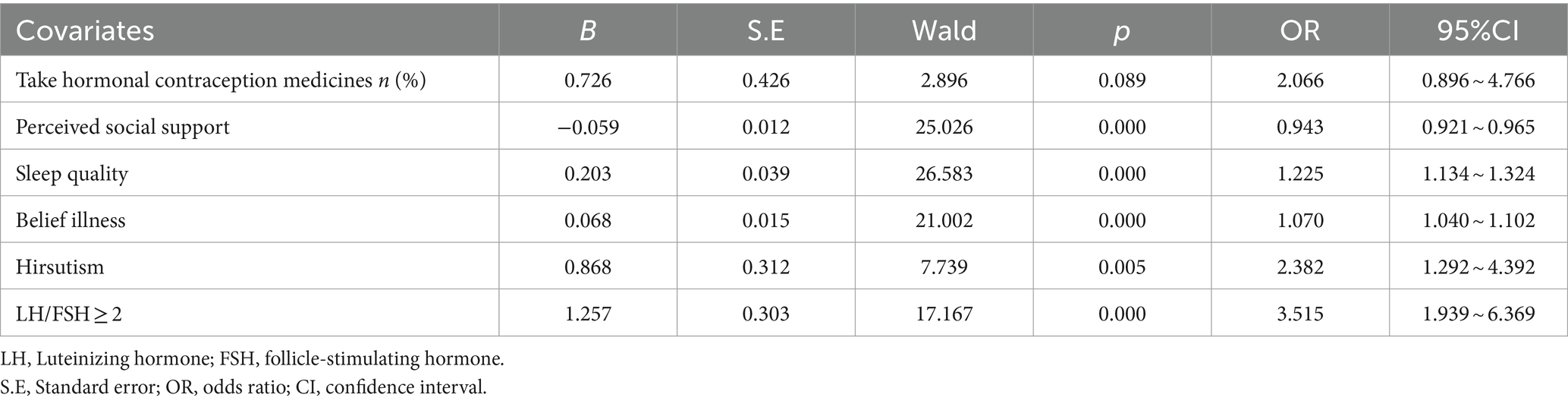
Results: The prevalence of depression symptoms was 36.12% among adolescent girls with PCOS. A multivariate logistic regression model identified significant factors as perceived social support (95% CI: 0.921 ~ 0.965%, p = 0.000), sleep quality (95% CI: 1.134 ~ 1.324%, p = 0.000), belief illness (95% CI, 1.040 ~ 1.102%, p = 0.000), hirsutism (95% CI, 1.292 ~ 4.392%, p = 0.005), and LH/FSH ≥ 2 (95% CI, 1.939 ~ 6.369%, p = 0.000).
Conclusion: Depression symptoms are an important problem among adolescent girls with PCOS in China. A comprehensive approach that encompasses social support, structured health education for the disease, and evaluation of the psychological status of PCOS girls with hirsutism (and) or LH/FSH ≥ 2 in time is important to minimize depression symptoms and improve psychological health among adolescent girls with PCOS.
1 Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine metabolic disorder among women of reproductive age; the prevalence in adolescent girls is from 5.6 to 11.04% worldwide (1), and 10.26% of Chinese adolescent girls (2). Symptoms of PCOS include irregular menstruation, infertility, obesity, acne, hyperandrogenemia, and ovarian polycystic degeneration (3). PCOS also increases an individual’s risk for cardiovascular disease, type 2 diabetes, and endometrial cancer (4).
Simultaneously, PCOS is significantly related to mental illness. A previous study reported that adolescent girls with PCOS are 2.4 times more likely to suffer from depression than normal girls of the same age (5, 6). As a serious mental illness, depression presents with a depressed mood, decreased energy, loss of interest, feelings of guilt or low self-worth, disturbed sleep or appetite, and poor concentration (7). Depression has negative physical, psychological, social, and behavioral effects. In the adolescent population, more than 50% of suicides are related to depression (8, 9), and it also interferes with the successful transition from adolescence to adulthood: approximately 25% of adults suffer from depression during adolescence (10). As for PCOS, life management is recommended by international evidence-based guidelines as the ideal treatment. Patients with PCOS can participate in better life management by improving their psychological status. Therefore, depression is a significant health issue that must be addressed in the management of adolescents with PCOS (11).
As early as 2013, the PCOS Endocrinology Society’s clinical practice guidelines recommended identifying depression in adolescents with PCOS (5), and 2020 international evidence-based guidelines for adolescents with PCOS emphasize the need to manage depression (6). A number of previous studies showed the prevalence of depression symptoms in adolescent girls with PCOS.
However, in those studies, not only did the prevalence rate vary greatly, ranging from 12 to 60%, but the sample size was very limited; the largest number was 153 (12–15). As for the risk factors for depression among adolescents with PCOS, there were also very limited and inconsistent studies. According to Elsenbruch et al., (16) independent predictors of depression among adolescent girls were irregular or absent menstrual periods, infertility, the associated changes in appearance, and possible disturbances in sexual attitudes. Hopkins found that lower control was a predictor of greater depression among adolescent girls with PCOS (12). In the other two studies, there was no significant relationship between obesity, body mass index, acne, hirsutism, and insulin (INS) resistance and depression disorders in the PCOS group (17, 18).
Assessing the prevalence and associated factors of depression among adolescent girls with PCOS is important for early identification and intervention to reduce the effect of depression on adolescent girls with PCOS. Therefore, there is an urgent need for further research on the prevalence and associated factors of depression among adolescent girls with PCOS based on a large sample. Here, we enrolled 335 adolescent girls with PCOS and conducted an observational study to evaluate the prevalence of depression and comprehensively analyze the risk factors.
2 Methods
2.1 Design, setting, and participants
A hospital-based cross-sectional study design was used. Adolescent girls with PCOS were recruited from the gynecology clinic department of the affiliated hospital of Zunyi Medical University. This study was carried out from October 2021 to May 2022. The inclusion criteria include: (I) aged 10–19 years old (19); (II) Rotterdam diagnostic criteria recommended by the PCOS China Diagnosis and Treatment Guide in 2018 (20); (III) able to self-report (verbally understandable and articulate); and (IV) participants and their families volunteer to participate in the study. The exclusion criteria are as follows: (I) have other mental disorders and cognitive impairment; (II) have other serious diseases of important organs, and (III) have other diseases that lead to elevated androgen levels and ovulation disorders.
2.2 Sample size
A single population proportion formula was used to determine the sample size by taking the prevalence of depression among adolescent girls with PCOS as 39.2%, as assessed by the CDI (14), with a 5% margin of error, 95% confidence, and a 10% non-response rate. The final sample size was 312.
2.3 Data collection procedures
The researchers identified potentially eligible adolescent girls with PCOS who visited the gynecology clinic. If the adolescent girls with PCOS fulfilled the inclusion criteria, they and their parent(s) were briefed on the nature of the study and invited to participate after obtaining verbal consent from both the adolescent girls with PCOS and their parent(s).
Then two researchers begin to collect data on adolescent girls with PCOS according to the following three steps: the first step is a validated online self-administered questionnaire consisting of socio-demographic information, duration of PCOS, exercise habits, perceived social support, sleep quality, and belief illness was provided to adolescent girls with PCOS, and who needed to fill out the questionnaire independently. In the second step, researchers collected the height, weight, hirsutism, and acne of adolescent girls with PCOS in the gynecological clinic. In the third step, researchers collected laboratory indicators such as thyroid-stimulating hormone (TSH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), total testosterone (TES), and INS from their medical records.
2.4 Instruments
2.4.1 Depression symptoms
Our main outcome of interest was the provisional diagnosis of depression symptoms, which was assessed using the Chinese version of the CDI (21). The Chinese version of the CDI questionnaire has 27 items and 5 subscales of low self-esteem, negative affect, lack of pleasure, poor performance, and interpersonal problems. For each item, the response options range from 0 to 3, with 0 indicating the absence of symptoms, 1 indicating mild symptoms, and 2 indicating definite symptoms. The total scale score ranges from 0 to 54, with higher scores reflective of higher levels of depressive symptomatology, and a score of 19 indicated the presence of depressive symptoms. The total Cronbach’s alpha value was 0.85 in the reported study, and the test–retest reliability was 0.75.
2.4.2 Perceived social support
Perceived social support was measured with the 12-item Perceived Social Support Scale (PSSS) (22). The scale yields three subscale scores for family, friends, and significant others. Respondents answered each question using a 7-point Likert scale ranging from 1 = “very strongly disagree” to 7 = “very strongly agree.” Higher scores indicate that subjects perceive more social support and are categorized as low (12–36), moderate (37–60), or high (61–84). The total Cronbach’s alpha value was 0.927 in the reported study.
2.4.3 Sleep quality
Sleep quality was measured using an 8-item of Athens Insomnia Scale (AIS) (23). For each item, the response options range from 0 to 3, with 0 indicating no problem at all and 3 indicating serious problems. The total scale score ranges from 0 to 24, with higher scores reflecting lower levels of sleep quality; a score of 6 indicates poor sleep quality. For internal consistency, Cronbach’s alpha was approximately 0.90, and the mean item-total correlation coefficient was approximately 0.70.
2.4.4 Belief illness
Belief illness was measured using a 9-item Brief Illness Perception Questionnaire (BIPQ) (24).For each item, the response options range from 0 to 10, and the total scale score ranges from 0 to 80, with higher scores reflective of higher levels of negative perception of disease. The questionnaire had an internal consistency coefficient of 0.831, and the test–retest reliability was 0.931 in the reported study.
2.4.5 Hirsutism
The modified Ferriman–Gallwey scoring system was used for the diagnosis of hirsutism (25). These areas included the upper lip, lower back, lower abdomen, and thigh. Values between 0 (no excess hair growth) and 4 (extremely disturbing hair growth) in patients may be evaluated. The total scale score ranges from 0 to 16, and patients with FG scores of 3 or higher are regarded as hirsute.
2.4.6 Acne
Acne was graded using the Rosenfield scoring system (26), which may be classified as mild, moderate, or severe based on the number, extent, and nature of the skin lesions and consists of six items with values between 0 (= no acne) and 5 (= cystic acne). The total scale score ranges from 0 to 5, with higher scores indicating more severe disease.
2.5 Statistical analysis
Data entry and analysis were performed using EpiData 3.1 and SPSS - version 21. Descriptive statistics were presented as percentages (n, %) and mean ± SD for continuous variables. We analyzed the differences in characteristics of PCOS girls with or without depression symptoms using bivariate logistic analysis, in which independent t-tests or chi-square tests were conducted. All independent variables with a p-value of <0.05 in the bivariate logistic analysis were candidates to enter the multivariable logistic regression model to control the confounding effect. A multivariable logistic regression model was used to identify factors associated with depression symptoms among PCOS girls, with a p-value of <0.05 with 95% CI was considered statistically significant.
3 Results
A total of 335 adolescent girls with PCOS were recruited for this study through convenience sampling, and all of the data were collected. This left an effective response rate of 100%.
3.1 Participant characteristics
Of the 335 adolescent girls with PCOS who were enrolled in the assessment, we have reported that 121 adolescent girls with PCOS were screened positive for depression symptoms with CDI at a cutoff point of 19, thus resulting in a prevalence of 36.12%.
Of the 335 adolescent girls with PCOS, the mean age was 17.66 ± 1.657 years old (ranging from 12 to 19 years old). The majority (60.60%) of the adolescent girls with PCOS included in this study were from rural, 179 (53.43%) had an education level of senior high school, the mean duration of the disease diagnosis was 6.2 ± 5.7 years, 232 of the adolescent girls with PCOS (69.25%) had no regular exercise, the mean BMI was 23.257 ± 4.426, 109 (31.0%) and 80 (23.89%) had hirsutism and acne, respectively. Of all adolescent girls with PCOS, 51.94% had the LH/FSH ≥ 2. Analysis of participant characteristics is shown in Table 1.
3.2 Factors associated with depression symptoms in adolescent girls with PCOS
In the univariate analysis, six factors were found to be significantly associated with depression symptoms in adolescent girls with PCOS, including having hormonal contraception medicines (p = 0.001), perceived social support (p<0.001), belief illness (p = 0.001), sleep quality (p = 0.001), hirsutism (p = 0.002), and LH/FSH ≥ 2 (p<0.001) (Table 1).
The multivariable logistic regression model analyses identified five statistically significant factors associated with being more likely to develop depression symptoms in adolescent girls with PCOS: perceived social support (OR = 0.944, 95% CI =0.917 ~ 0.971), sleep quality (OR = 1.246, 95% CI =1.131 ~ 1.374), belief illness (OR = 1.082, 95% CI =1.042 ~ 1.123), Hirsutism (OR = 2.530, 95% CI =1.190 ~ 5.377), and LH/FSH ≥ 2 (OR = 2.858, 95% CI =1.400 ~ 5.836) (Table 2).
Our study used the Box–Tidwell method to verify whether the continuous independent variable and the dependent variable logit conversion values were linear. The linear test model included six variables: hormonal contraception medicines, sleep quality, perceived social support, hirsutism, belief illness, and LH/FSH ≥ 2). After the Bonferroni correction, the significance level was 0.005. The line test results showed a linear relationship between all continuous independent variables and the dependent variable logit conversion values. Finally, the resulting logistic model was statistically significant (x2 = 114.098, p < 0.001). A total of five variables associated with depressive symptoms in PCOS patients were statistically significant in the model, including hirsutism (OR = 2.530, 95%CI: 1.190–5.377, p = 0.016), LH/FSH ≥ 2 (OR = 2.858, 95%CI: 1.400–5.836, p = 0.004), perceived social support (OR = 0.944, 95%CI: 0.917–0.971, p<0.001), sleep quality (OR = 1.246, 95%CI: 1.131–1.374, p<0.001), and belief illness (OR = 1.082, 95%CI: 1.042–1.123, p<0.001).
4 Discussion
The prevalence of symptoms of depression was 36.12% among adolescent girls with PCOS in China. Our research finds that six factors (such as social support, sleep quality, belief illness, hirsutism, and LH/FSH ≥ 2) play a critical role in depression symptoms among adolescent girls with PCOS.
To our knowledge, this was the first study with respect to the depression symptoms of adolescent girls with PCOS in China. In the present study, the 36.12% prevalence rate of depression symptoms is in line with a study conducted in Turkey at 39.2% (14), and the reasons for the phenomenon may be related to using the same CDI and the participants were all from the gynecology clinic department of a general hospital. However, the current finding is lower than two small sample studies conducted in America (n = 47 and n = 23), which found the prevalence of depression among adolescent girls with PCOS to be 60 and 56.5%, respectively (12, 13). On the contrary, the present study’s finding is higher than a study conducted in New Zealand, which found the prevalence of depression symptoms among adolescent girls with PCOS to be only 12% (15). The variations might be due to differences in environmental factors, sample size, a tool for assessing depression, and multiple sources of participants.
According to this study, adolescent girls with PCOS who have lower levels of social support have a higher risk of developing depression. The finding corroborates the result of a previous study which was conducted in America (12). This may be explained as follows: PCOS is a common chronic disease, but public awareness and attention to this disease are insufficient. As a result, adolescent girls with PCOS are more likely to have a sense of isolation, increasing the risk of depression.
In the present study, we found that sleep quality is associated with depression symptoms among adolescent girls with PCOS. A systematic review and meta-analysis of cohort studies reported that there is an association between sleep disruption and depressive symptoms in children and youths (27). Another systematic review of the literature regarding sleep duration and mood in adolescents indicated that less sleep was associated with a 55% increase in the likelihood of mood deficits (28).
Different mechanisms have been proposed to explain the association between sleep problems and depression, such as a disruption of circadian rhythm, activation of inflammatory pathways, and altered neuroplasticity and learning (29), a reduction in prefrontal activity, and a decrease in functional connectivity between the prefrontal cortex and limbic regions (30), and the loss of a person’s ability to shift attention away from repeated negative thoughts (31).
Our study demonstrated that belief illness was associated with depression symptoms among adolescent girls with PCOS. Similar to our findings, perceived stress of the disease was an independent risk factor for depression in women with PCOS (32). Many adolescent girls with PCOS reported being uncertain about many aspects of PCOS, and a study indicated that PCOS patients with higher negative perceptions of the disease and its clinical manifestations were more pronounced (33).
Body image is a multidimensional matter that includes the perception of people regarding self-appearance and related thoughts and feelings about it. Hirsutism, acne, and obesity as important influence factors of body image are common symptoms among women with PCOS. Our study found there was a positive association between hirsutism and depression symptoms in adolescent girls with PCOS. Many studies also found that hirsutism was an independent risk factor for depression symptoms in women with PCOS (33–37). The possible reason could be, first hirsutism is characterized by excessive terminal hair growth in a male—pattern distribution in female patients, including the face, chest, back, upper arms, thighs, and abdomen. The unwanted facial and bodily hair often led to the feeling of “not being a female,” which may be theorized to contribute to the psychological burden in this population of relatively young women. Second hirsutism is one of the most important manifestations of high androgen levels, which has a close relationship with depression (38, 39). Contrary to previous findings, which showed that there was a clear association between higher depression scores and elevated BMI among adolescent girls with PCOS (15). In the present study, we found no significant relationship between BMI and depression. This might be a result of the different sample sizes; in the present study, the sample size is 335, which is three times more than in the previous study.
In the present study, we found that LH/FSH ≥ 2 is significantly correlated with depression symptoms in adolescent girls with PCOS. This is consistent with the study by Feng et al., who reported that LH/FSH ≥ 2 is an independent risk factor for depression in patients with PCOS. The mechanism for this phenomenon is with the increase of LH/FSH ratio the incidence of menstrual disorders increased (40). Studies reported that there was a positive association between menstrual disorders and depression symptoms (41, 42).
5 Limitations
Some limitations must be considered in the interpretation of these findings. First, although in our study we enrolled 335 adolescent girls with PCOS, which was relatively a larger sample size compared to the previous studies, it is not possible to generalize the results of the study considering that the sample was recruited in a single hospital. Second, it was not randomized but a convenience sample. The other limitations might be that the design of the study was cross-sectional, and temporality and causality between factors could not be assured.
6 Conclusion
In summary, approximately 36.12% prevalence of depression symptoms was observed among adolescent girls with PCOS in China. Moreover, our research finds that many factors (such as social support, sleep quality, belief illness, hirsutism, and LH/FSH ≥ 2) play a critical role in depression symptoms among adolescent girls with PCOS. Our results suggest the importance of a comprehensive approach at both individual and institutional levels to reduce depression symptoms among adolescent girls with PCOS. More specifically, a holistic strategy that encompasses social support (such as emotional and informational support from parents, peers, and medical staff), structure health education for the disease, and evaluation of the psychological status of PCOS girls with hirsutism (and) or LH/FSH ≥ 2 in time.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Zunyi Medical University: Zunhe Lun Review [2021] 1–093. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LW: Conceptualization, Formal analysis, Supervision, Validation, Writing – original draft, Writing – review & editing. SS: Data curation, Investigation, Writing – review & editing. TX: Data curation, Investigation, Writing – review & editing. MW: Conceptualization, Formal analysis, Visualization, Writing – review & editing. RD: Data curation, Investigation, Writing – review & editing. HT: Data curation, Investigation, Writing – review & editing. MZ: Conceptualization, Formal analysis, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Zunyi Science and Technology Planning Project [Zun Shi Ke He HZ Zi (2021) No. 21] and the Health Commission of Guizhou Province (gzwkj2023-243).
Acknowledgments
The authors are grateful to the adolescent girls with PCOS who took time to participate in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Naz, M, Tehrani, FR, Majd, HA, Ahmadi, F, Ozgoli, G, Fakari, FR, et al. The prevalence of polycystic ovary syndrome in adolescents: a systematic review and meta-analysis. Int J Reprod Biomed. (2019) 17:533–42. doi: 10.18502/ijrm.v17i8.4818
2. Wu, Q, Gao, J, Bai, D, Yang, Z, and Liao, Q. The prevalence of polycystic ovarian syndrome in chinese women: a meta-analysis. Ann Palliat Med. (2021) 10:74–87. doi: 10.21037/apm-20-1893
3. Ding, T, Hardiman, PJ, Petersen, I, Wang, FF, Qu, F, and Baio, G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. (2017) 8:96351–8. doi: 10.18632/oncotarget.19180
4. Wang, JY, Wang, Q, Cui, CC, Zhu, C, Li, H, and Zhang, CL. Research progress on the effect of polycystic ovarian syndrome on the development of the offspring. Chin J Reprod Contracep. (2020) 5:423–6. doi: 10.3760/cma.j.cn101441-20190516-00197
5. Legro, RS, Arslanian, SA, Ehrmann, DA, Hoeger, KM, Murad, MH, Pasquali, R, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2013) 98:4565–92. doi: 10.1210/jc.2013-2350
6. Peña, AS, Witchel, SF, Hoeger, KM, Oberfield, SE, Vogiatzi, MG, Misso, M, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med. (2020) 18:72. doi: 10.1186/s12916-020-01516-x
7. Marcus, M, Yasamy, M, Ommeren, M, and Saxena, S. Depression: a global public health concern[J]. (2012). doi: 10.1037/e517532013-004
8. Furukawa, TA. Adolescent depression: from symptoms to individualised treatment? Lancet Psychiatry. (2020) 7:295–6. doi: 10.1016/S2215-0366(20)30080-8
10. Kiviruusu, O, Strandholm, T, Karlsson, L, and Marttunen, M. Outcome of depressive mood disorder among adolescent outpatients in an eight-year follow-up. J Affect Disord. (2020) 266:520–7. doi: 10.1016/j.jad.2020.01.174
11. Ding, R, Zhou, H, Yan, X, Liu, Y, Guo, Y, Tan, H, et al. Development and validation of a prediction model for depression in adolescents with polycystic ovary syndrome: a study protocol. Front Psych. (2022) 13:984653. doi: 10.3389/fpsyt.2022.984653
12. Hopkins, CS, Kimble, LP, Hodges, HF, Koci, AF, and Mills, BB. A mixed-methods study of coping and depression in adolescent girls with polycystic ovary syndrome. J Am Assoc Nurse Pract. (2019) 31:189–97. doi: 10.1097/JXX.0000000000000125
13. Benson, J, Severn, C, Hudnut-Beumler, J, Simon, SL, Abramson, N, Shomaker, LB, et al. Depression in girls with obesity and polycystic ovary syndrome and/or type 2 diabetes. Can J Diabetes. (2020) 44:507–13. doi: 10.1016/j.jcjd.2020.05.015
14. Almis, H, Orhon, FŞ, Bolu, S, and Almis, BH. Self-concept, depression, and anxiety levels of adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. (2021) 34:311–6. doi: 10.1016/j.jpag.2020.12.011
15. Milsom, SR, Nair, SM, Ogilvie, CM, Stewart, JM, and Merry, SN. Polycystic ovary syndrome and depression in New Zealand adolescents. J Pediatr Adolesc Gynecol. (2013) 26:142–7. doi: 10.1016/j.jpag.2012.11.013
16. Elsenbruch, S, Hahn, S, Kowalsky, D, Offner, AH, Schedlowski, M, Mann, K, et al. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2003) 88:5801–7. doi: 10.1210/jc.2003-030562
17. Sari, SA, Celik, N, and Uzun, CA. Body perception, self-esteem, and comorbid psychiatric disorders in adolescents diagnosed with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. (2020) 33:691–6. doi: 10.1016/j.jpag.2020.08.018
18. Çoban, ÖG, Tulacı, ÖD, Adanır, AS, and Önder, A. Psychiatric disorders, self-esteem, and quality of life in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. (2019) 32:600–4. doi: 10.1016/j.jpag.2019.07.008
19. Peña, AS, and Metz, M. What is adolescent polycystic ovary syndrome? J Paediatr Child Health. (2018) 54:351–5. doi: 10.1111/jpc.13821
20. Group REAP. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
21. Liu, Z, Li, J, Wang, Y, Miao, M, and Zhong, J. Structural verification and measurement invariance of chinese version of children’s depression inventory. Chin J Clin Psychol. (2019) 27:1172–6. doi: 10.16128/j.cnki.1005-3611.2019.06.019
22. Qin, Y, and Luyao, Z. The applicability of the perceived social support scale in adolescents. Jiaoyu Guancha. (2022) 2:29–32. doi: 10.16070/j.cnki.cn45-1388/g4s.2022.02.009
23. Soldatos, CR, Dikeos, DG, and Paparrigopoulos, TJ. Athens insomnia scale: validation of an instrument based on icd-10 criteria. J Psychosom Res. (2000) 48:555–60. doi: 10.1016/s0022-3999(00)00095-7
24. Sun, W, Lou, Q, and Yuan, Y. Application of the chinese version of brief illness perception questionnaire in patients with somatoform disorder. J Chongqing Med Univ. (2015) 40:1138–42.
25. Xiaomiao, Z, Yabo, Y, Yang, H, Du, T, Yan, T, Xiaoli, C, et al. Simplified analysis of modified ferriman-gallwey scoring system in evaluation of chinese women-a prospective follow-up study in new terminal hair among pregnant women. J Sun Yat-Sen Univ. (2017) 38:699–704. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2017.0112
27. Marino, C, Andrade, B, Campisi, SC, Wong, M, Zhao, H, Jing, X, et al. Association between disturbed sleep and depression in children and youths: a systematic review and meta-analysis of cohort studies. JAMA Netw Open. (2021) 4:e212373. doi: 10.1001/jamanetworkopen.2021.2373
28. Short, MA, Booth, SA, Omar, O, Ostlundh, L, and Arora, T. The relationship between sleep duration and mood in adolescents: a systematic review and meta-analysis. Sleep Med Rev. (2020) 52:101311. doi: 10.1016/j.smrv.2020.101311
29. Riemann, D, Krone, LB, Wulff, K, and Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology. (2020) 45:74–89. doi: 10.1038/s41386-019-0411-y
30. Yoo, SS, Gujar, N, Hu, P, Jolesz, FA, and Walker, MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. (2007) 17:R877–8. doi: 10.1016/j.cub.2007.08.007
31. Nota, JA, and Coles, ME. Shorter sleep duration and longer sleep onset latency are related to difficulty disengaging attention from negative emotional images in individuals with elevated transdiagnostic repetitive negative thinking. J Behav Ther Exp Psychiatry. (2018) 58:114–22. doi: 10.1016/j.jbtep.2017.10.003
32. Mirghafourvand, M, Charandabi, SM, Lak, TB, and Aliasghari, F. Predictors of depression in iranian women with polycystic ovarian syndrome. Community Ment Health J. (2018) 54:1274–83. doi: 10.1007/s10597-017-0188-6
33. Veltman-Verhulst, SM, Boivin, J, Eijkemans, MJ, and Fauser, BJ. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update. (2012) 18:638–51. doi: 10.1093/humupd/dms029
34. Pasch, L, He, SY, Huddleston, H, Cedars, MI, Beshay, A, Zane, LT, et al. Clinician vs self-ratings of hirsutism in patients with polycystic ovarian syndrome: associations with quality of life and depression. JAMA Dermatol. (2016) 152:783–8. doi: 10.1001/jamadermatol.2016.0358
35. Sioma Markowska, UMO. Polycystic ovary syndrome and theperception of body image by women. Gin Pol Med Project. (2021) 1:24–8.
36. Khomami, MB, Tehrani, FR, Hashemi, S, Farahmand, M, and Azizi, F. Of pcos symptoms, hirsutism has the most significant impact on the quality of life of iranian women. PLoS One. (2015) 10:e123608. doi: 10.1371/journal.pone.0123608
37. Naqvi, SH, Moore, A, Bevilacqua, K, Lathief, S, Williams, J, Naqvi, N, et al. Predictors of depression in women with polycystic ovary syndrome. Arch Womens Ment Health. (2015) 18:95–101. doi: 10.1007/s00737-014-0458-z
38. Rasgon, NL, Rao, RC, Hwang, S, Altshuler, LL, Elman, S, Zuckerbrow-Miller, J, et al. Depression in women with polycystic ovary syndrome: clinical and biochemical correlates. J Affect Disord. (2003) 74:299–304. doi: 10.1016/s0165-0327(02)00117-9
39. Weber, B, Lewicka, S, Deuschle, M, Colla, M, and Heuser, I. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology. (2000) 25:765–71. doi: 10.1016/s0306-4530(00)00023-8
40. Jing, F, Fangyuan, G, and Jie, X. Related factors for complicating depression in patients with polycystic ovarian syndrome. Chin J Endocrinol. (2018) 12:247–50. doi: 10.3760/cma.j.issn.1674-6090.2018.03.017
41. Wang, T, Zhou, Y, and Luo, Y. Anxiety, depresson status of adolescent females and prediction of related influencing factors in Hunan province. Chin J Dis Cont Preven. (2019) 8:971–6. doi: 10.16462/j.cnki.zhjbkz.2019.08.017
42. Jung, EK, Kim, SW, Ock, SM, Jung, KI, and Song, CH. Prevalence and related factors of irregular menstrual cycles in korean women: the 5th korean national health and nutrition examination survey (knhanes-v, 2010-2012). J Psychosom Obstet Gynaecol. (2018) 39:196–202. doi: 10.1080/0167482X.2017.1321631
Keywords: depression symptoms, risk factors, polycystic ovary syndrome, adolescent girls, prevalence
Citation: Wang L, Su S, Xiong T, Wang M, Ding R, Tan H and Zhu M (2024) Prevalence and associated risk factors for depression symptoms in adolescent girls with polycystic ovary syndrome: a hospital-based cross-sectional study. Front. Public Health. 12:1454415. doi: 10.3389/fpubh.2024.1454415
Edited by:
Wing Fai Yeung, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Duong Le Dinh, Hue University of Medicine and Pharmacy, VietnamUmit Aydogan, University of Health Sciences, Türkiye
Copyright © 2024 Wang, Su, Xiong, Wang, Ding, Tan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minglan Zhu, MTExNTY0MDUyOEBxcS5jb20=
 Lianhong Wang
Lianhong Wang Sihui Su3
Sihui Su3 Rui Ding
Rui Ding Huiwen Tan
Huiwen Tan