- 1Department of Medical Laboratory Sciences, College of Medicine and Health Science, Mizan-Tepi University, Mizan Teferi, Ethiopia
- 2Department of Medicine, College of Medicine and Health Science, Mizan-Tepi University, Mizan Teferi, Ethiopia
Background: Intestinal parasitic infections remain very common, particularly in areas with a high prevalence of immune-compromised patients, such as HIV/AIDS patients. The purpose of this study was to determine the prevalence of intestinal parasites and associated factors in people living with HIV/AIDS at an ART clinic in Mizan-Tepi University Teaching Hospital, southwest Ethiopia.
Method: A cross-sectional survey was conducted from July to September 2021. A total of 191 adult people living with HIV/AIDS participated in this study. Data on socio-demographic, clinical, and other risk factors were collected using a structured questionnaire. Stool samples were collected and processed using a direct wet mount, formol-ether concentration, and modified Ziehl-Nelson staining techniques. The data were analyzed using the Statistical Package for Social Sciences Version 25 software.
Results: Among 67 adult individuals living with HIV/AIDS, the prevalence of intestinal parasites was 35.1%. Specifically, 31.5% (45/143) of patients on antiretroviral therapy (ART) and 45.8% (22/48) of ART-naïve patients were infected. The distribution of intestinal parasites was as follows: protozoa were found in 14.7% of ART-treated patients and 22.9% of ART-naïve patients; helminths in 15.4% of ART-treated patients and 16.7% of ART-naïve patients; and opportunistic parasites in 1.4% of ART-treated patients and 6.25% of ART-naïve patients. Significant associations with a higher prevalence of intestinal parasites were observed for a CD4 count <200 cells/mm3 (Adjusted Odds Ratio [AOR] = 3.77; 95% Confidence Interval [CI]: 1.01–13.15; p = 0.04), consumption of unwashed raw vegetables (AOR = 3.29; 95% CI: 1.23–8.86; p = 0.02), and residing in rural areas (AOR = 2.34; 95% CI: 1.27–4.32; p = 0.01).
Conclusion: The findings indicate that a significant proportion of adults living with HIV/AIDS are affected by intestinal parasites, with a notably higher prevalence among ART-naïve patients compared to those on ART. Factors such as a low CD4 count, consumption of unwashed raw vegetables, and rural residence are associated with increased risk of intestinal parasite infections. These results underscore the importance of improving hygiene practices and access to healthcare, particularly in rural areas, to reduce the burden of parasitic infections among individuals living with HIV/AIDS.
Introduction
Parasitic infections, particularly intestinal parasites, are among the most widespread human infections worldwide. They are the main problem in developing countries, as they have been noticeably heightened with the coexistence of a large burden of Human Immunodeficiency Virus/ Acquired Immunodeficiency Syndrome (HIV/AIDS) and malnutrition in the area. Globally, approximately 38 million people were living with HIV in 2020, with sub-Saharan Africa accounting for nearly 67% of these cases. In Ethiopia, about 620,000 people are estimated to be living with HIV/AIDS, making it one of the countries with a significant burden of the disease. The coexistence of HIV/AIDS with parasitic infections and malnutrition further exacerbates health outcomes in these regions. For instance, it is estimated that more than 60% of the world’s population is infected with intestinal parasites, which significantly contribute to morbidity due to intestinal infections (1–4).
The gastrointestinal problem resulting from opportunistic parasitic infection in people living with HIV/AIDS has dramatically decreased in countries where antiretroviral agents are widely available. However, in most African countries where patients have low access to Antiretroviral Therapy (ART), intestinal pathogens still represent a frequent cause of diarrhea, wasting, and weight loss. Evidence shows that Human Immunodeficiency Virus (HIV)-infected patients are the most vulnerable risk group for acquiring parasitic infections, and about 85% of acquired immunodeficiency syndrome (AIDS) patients die as a result of AIDS-related infections rather than the HIV infection itself. These opportunistic infections most commonly occur in the later stages of HIV infection, when the number of Cluster of Differentiation 4 T cells (CD4 T cells) has declined mostly below 200/mm3 (5–8).
Intestinal parasitic infections are transmitted to humans through soil contaminated by human feces, mostly in areas where sanitation is poor. The infection leads to anemia, vitamin A deficiency, stunted growth, malnutrition, intestinal obstruction, and impaired development. The most commonly reported intestinal parasites are Ascaris lumbricoides, hookworms, Trichuris trichiuria, Giardia lamblia, Entamoeba histolytica, and Schistosoma species are the most common intestinal parasites. Among those opportunistic pathogens, Isospora belli, Cryptosporidium parvum, Cyclospora cayetanenis, and Microsporidia species are increasingly reported as causes of enteritis and as opportunistic pathogens in immune-compromised individuals (9–12).
Despite ART improving the quality of life and reducing the occurrence of opportunistic infections due to immunosuppression, malnutrition, poor waste management, ignorance (illiteracy), unhygienic sources of drinking water, and depleted CD4 counts among people living with HIV/AIDS are the most common determinants of parasitic infection (8, 11, 12). As Ethiopia is one of the developing countries in the world, it is categorized under countries with a high prevalence of parasitic infections. For instance, according to the studies, the average prevalence of parasitic infections was 39.6% (13–16), and the estimated pooled prevalence in HIV/AIDS patients was 39.2% (17). The severity and magnitude of IP in people living with HIV require attention and study, especially in countries like Ethiopia where there is high HIV/AIDS and parasite prevalence. For this reason, this study was conducted to determine the magnitude of intestinal parasites and risk factors among people living with HIV/AIDS at the ART clinic of Mizan-Tepi University (MTU) Teaching Hospital, southwestern Ethiopia. The finding will help to sensitize local and national responsible bodies as a prerequisite for updating strategies in the prevention and control of intestinal parasitic infection among people living with HIV/AIDS.
Methodology
Study area and period
This study was conducted at MTU teaching hospital from July to September 30, 2021. In the southwest direction, the hospital is located 591 km from Addis Ababa, the capital city of Ethiopia. It is the second teaching hospital with more than 139 beds in the southwestern part of the country, which provides services to approximately 5 million people from four catchment zones such as BENCH SHEKO, KEFA, SHEKA, and MAJANG as referral centers. Up to 350 clients visit the hospital daily for different services and more than 750 HIV-positive patients were taking ART services.
Study design and subjects
A cross-sectional descriptive study design was conducted to determine the prevalence of intestinal parasites among HIV-positive patients who were attending the ART clinic at the MTU teaching hospital in southwest Ethiopia.
Inclusion and exclusion criteria
Inclusion criteria
Adults aged 18 years and older.
Individuals diagnosed with HIV/AIDS.
Patients attending the ART clinic at MTU Teaching Hospital during the study period.
Individuals who provided informed consent to participate in the study.
Exclusion criteria
Individuals who were unwilling or unable to provide informed consent.
Patients who were unable or unwilling to provide stool samples.
Operational definitions
Prevalence of intestinal parasites
The percentage of patients with HIV/AIDS irrespective of their ART status at the ART clinic of MTU University Teaching Hospital who test positive for one or more intestinal parasites during a specified study period.
ART patients
Individuals who have been diagnosed with HIV and have started ART. This includes anyone currently undergoing ART treatment.
ART naïve patients
Individuals who have been newly diagnosed with HIV and have not yet started ART. These are typically people screened at voluntary counseling and testing (VCT) centers who are ready to begin ART.
People living with HIV/AIDS
This term encompasses all individuals who have been diagnosed with HIV, regardless of whether they have started ART or are newly diagnosed and ready to start ART.
Sample size and sampling technique
The required sample size was calculated using a single population proportion formula by assuming that confidence interval of 95%, a margin error of 5%, z statistic for confidence level (z = 1.96 at 95% CI), and the prevalence of intestinal parasites among people with HIV (p) as 13.9% (18), which is reported in previous study in Ethiopia, n = sample size was calculated as the followig.
By assuming a 5% non-respondent rate, the final sample size for this study was 191.
Data collection
Socio-demographic and clinical data collection
During the implementation of the data collection, the study subjects were asked about their willingness to participate in the study. Two trained BSc nurses who were working at the ART clinic were recruited for the data collection. A structured questionnaire (face to face interview) was administered to collect socio-demographic (age, sex, residence, marital status, and educational status) and other risk factors data, such as hand washing practice, consuming raw food, owning domestic animals, the availability and usage of latrines, shoe wearing habits, and the source and treatment of water for drinking. Clinical information on the level of CD4+ T cell count, viral load, and ART status was obtained from patients’ medical records.
Laboratory sample collection and processing
A laboratory technologist collected a single fresh stool sample from people living with HIV/AIDS attending at ART clinic of MTU Teaching Hospital. All the patients were oriented and provided with appropriate leak-proof stool cups to bring a 4-5 g of stool specimens. Each sample was labeled with a specific code number and taken to the parasitology laboratory of the MTU teaching hospital for parasitological analysis.
Sample processing
The stools were examined both macroscopically and microscopically. Macroscopically, the stool samples were inspected for appearance; while microscopically they were examined using direct iodine mount smear preparation, formol-ether concentration, and the modified Ziehl-Neelson technique (19–21). During the formol-ether concentration technique, 1 g of stool was mixed with 7 mL of formalin-saline in a clear conical centrifuge tube. After collecting the sieved suspension in another conical test tube, 3 mL of diethyl ether was added, mixed for 1 min, and then centrifuged for 2 min at approximately 3,000 rpm. The supernatant (i.e., the ether, fecal debris, and formol water) was discarded, and the remaining small sediment was mixed well by shaking. About a drop of the sediments was transferred to a slide, to which a drop of iodine solution was added and covered with a cover slide. Finally, the preparations were examined under the light microscope with the 10X and 40X objectives (19–21). A modified Ziehl-Neelsen staining technique was used to detect Cryptosporidium parvum and Isospora belli. Hence, thin smears were prepared directly from sediment obtained by the formol ether concentration technique and then allowed to air dry and fixed with methanol for 3 min. Then the smears were flooded with carbon fuchsine for 15 min. The slide was washed with tap water and discolored with 1% acid-alcohol for 15 s. The slide was then rinsed in tap water and flooded with 0.5% methylene blue for 30 s. Wash off the slides with water, allow the smear to dry, and observe under a light microscope with 100X objectives (20, 21).
Data analysis
The data were manually checked for completeness and entered into Statistical Package for the Social Sciences (SPSS) Version 25 software for analysis. Descriptive statistics, such as frequency distributions in relation different variables were determined. Binary logistic regression analyses were also performed to assess the association of different variables with intestinal parasite infection. Those variable with p-value >0.25 in the bivariate logistic regression were fitted to multivariable logistic regression. Statistical test results were declared significant when the 𝑃- value was <0.05 and rough and adjusted odds ratios with 95% confidence intervals (CIs) were reported.
Results
Socio-demographics of the study subjects
A total of 191 people living with HIV/AIDS were participated in the study. Among these, 113 (59.2%) were females, and 89 (46.6%) were between the ages of 35 to 50 years. Of the total study participants, 103 (53.9%) were urban residents and 133 (69.6%) were married. O the other hand 65 (34.0%) of the study participants had completed their primary school, and 44 (23.0%) were working as housewives (Table 1).
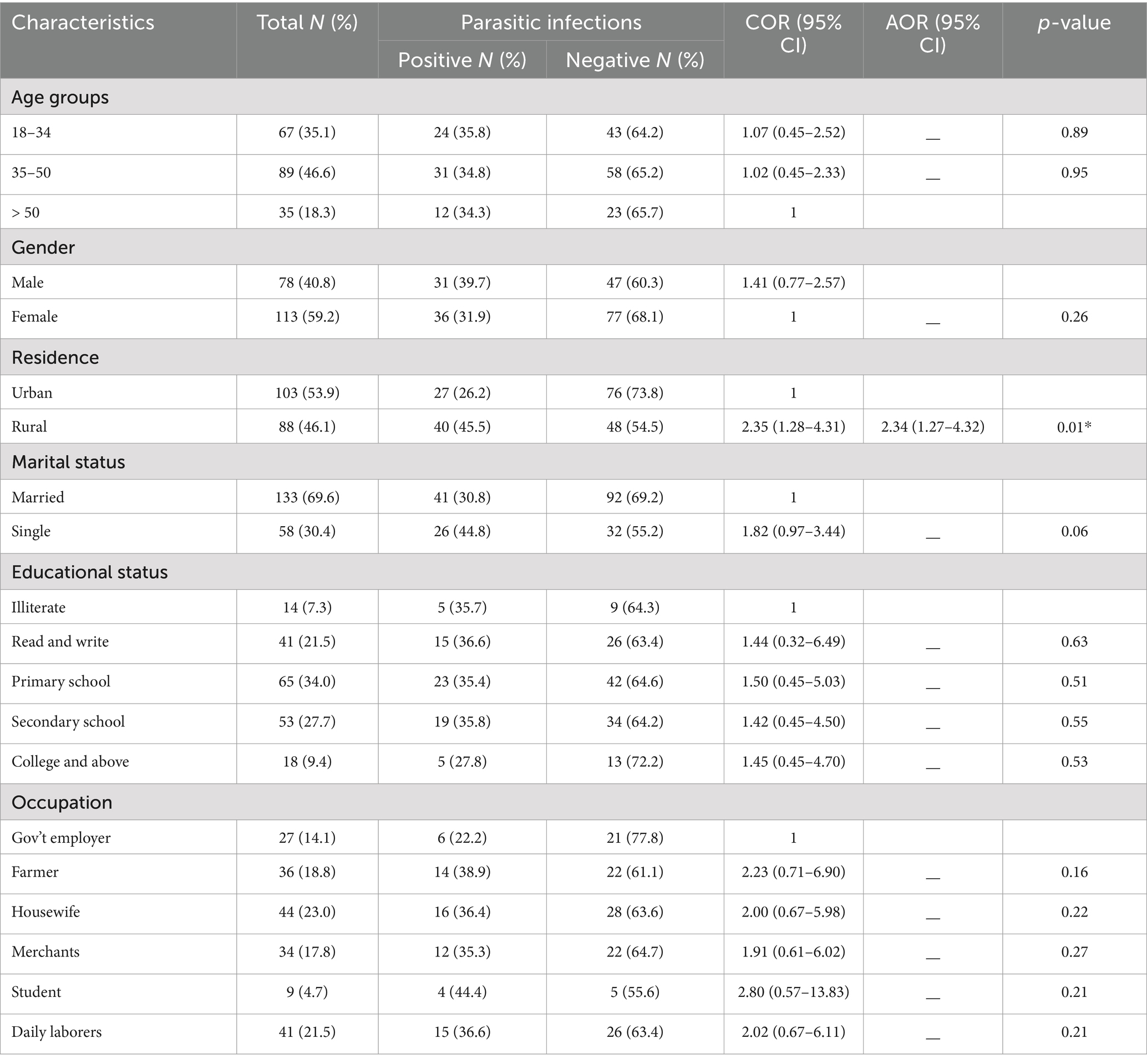
Table 1. Prevalence of intestinal parasites with socio-demographic characteristics of people living with HIV/AIDS (N = 191).
Intestinal parasites in ART and ART naïve group
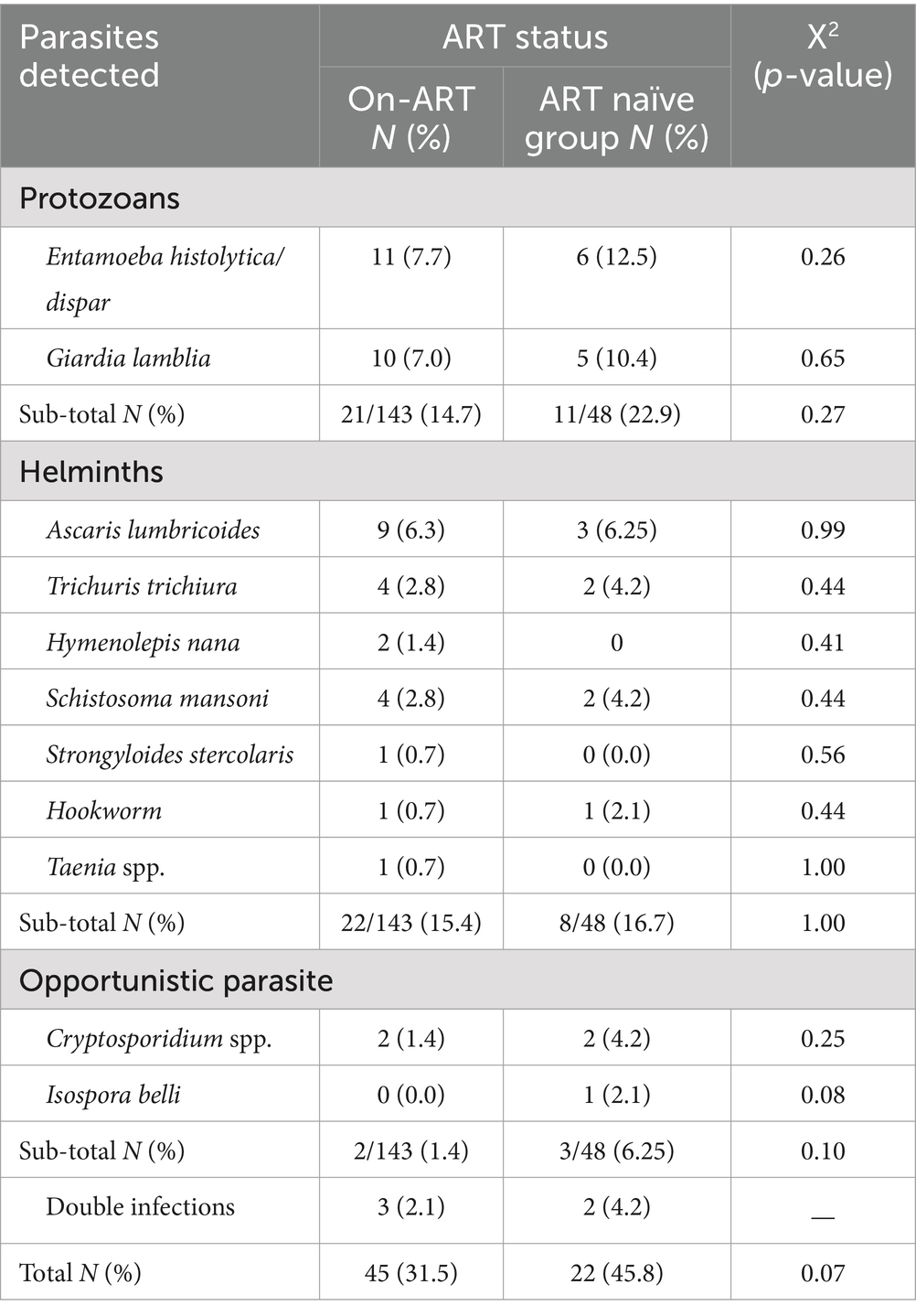
In this study, a total of 191 stool samples were tested for the detection of intestinal parasites (IPs). Of these, 143 (75.0%) were taken from people living with HIV/AIDS who were on ART, and the rest, 48 (25.0%), were taken from the ART naïve group. The overall prevalence of IPs was 67 (35.1%). The prevalence of IPs among people living with HIV/AIDS who were on ART was 45 (31.5%) and 22 (45.8%) among the ART naïve group. The distribution of intestinal parasites showed no statistically significant differences between the ART and ART naïve groups (X2 = 3.256; P-value >0.05). The prevalence of protozoa, helminths, and opportunistic parasites were 21/143 (14.7%), 22/143 (15.4%), and 2/143 (1.4%) among ART-started patients, and 11/48 (22.9%), 8/48 (16.7%), and 3/48 (6.25%) among ART naïve patients, respectively. Entamoeba histolytica/dispar was the most prevalent parasite detected from protozoan parasites in both ART and ART naïve patients, which was, 7.7 and 12.5%, respectively. At the same time, Ascaris lumbricoides was the most prevalent parasite detected from helminths in both ART and ART naïve patients, which was 6.3 and 6.25%, respectively. From opportunistic parasites, the prevalence of Cryptosporidium spp. among ART and ART naïve patients was 1.4 and 4.2%, respectively, however, Isospora belli (2.1%) was detected only in the ART naïve group (Table 2).
Intestinal parasites regarding socio-demographics of the study participants
In this study, analysis with binary logistic regression showed that the place of residence of the study participants was identified as the only socio-demographic determinant of intestinal parasite infections among people living with HIV/AIDS. Intestinal parasites were detected in 40 (45.5%) of rural residences, which was 2.34 times (AOR = 2.34; 95% CI: 1.27–4.32; p-value = 0.01) greater than those of urban residences. The other socio-demographic characteristics, such as gender, age, marital status, and educational status, were not associated with intestinal parasite infection (Table 1).
Intestinal parasites regarding CD4 level, viral load, and ART status
In this study, from clinical findings, having CD4 counts <200 cells/mm3 was the only factor significantly associated with intestinal parasitic infection. As shown in Table 3, people living with HIV/AIDS whose CD4 counts <200 cells/mm3 was 3.77 times (AOR = 3.77; 95% CI: 1.01–13.15; p-value = 0.04) more likely to get an infection with intestinal parasites than those with CD4 counts >500 cells/mm3 (Table 3).
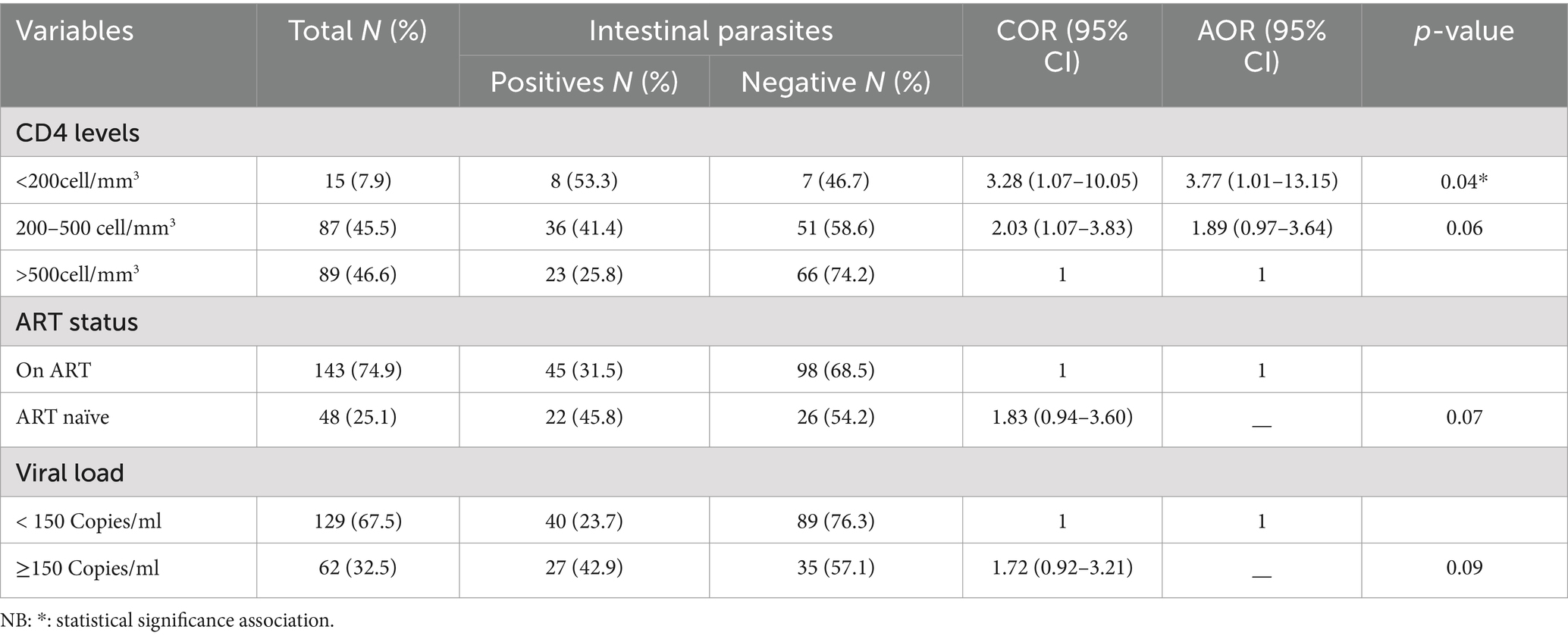
Table 3. Association of intestinal parasite prevalence with CD4 count, viral load, and ART status in people living with HIV/AIDS.
Intestinal parasites regarding hygienic practices of the study participants
In this study, among the studied variables related to the environmental and hygienic practices of study participants, consuming unwashed raw vegetables was significantly associated with intestinal parasitic infection. The odds of having an intestinal parasitic infection in people eating unwashed raw vegetables were 3.29 times (AOR = 3.29; 95% CI: 1.23–8.86; p-value = 0.02) higher than those not consuming unwashed raw vegetables (Table 4).
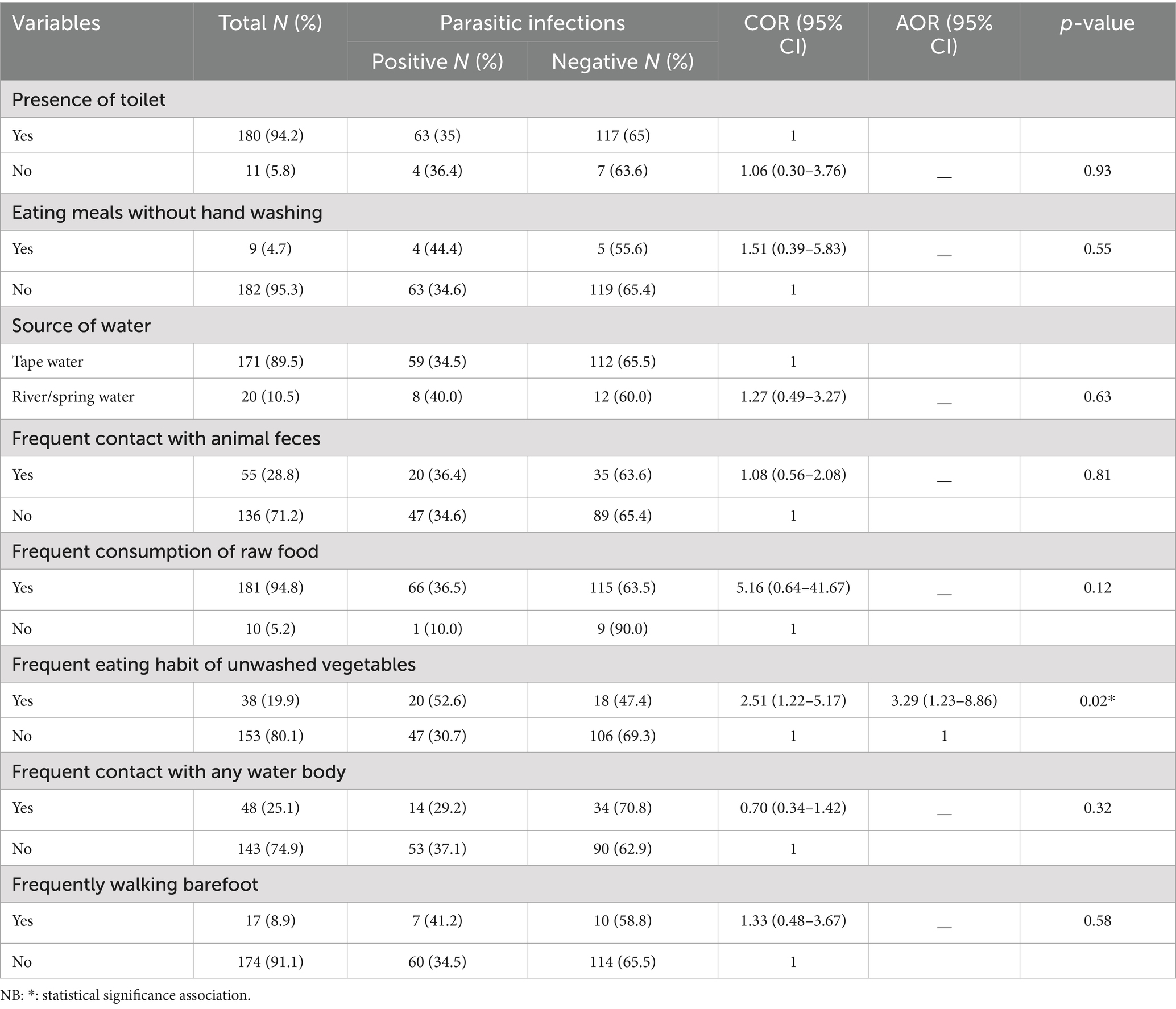
Table 4. Association of intestinal parasite prevalence with environmental conditions and hygienic practices among HIV-positive patients (N = 191).
Discussion
This hospital-based cross-sectional study was conducted to assess the prevalence of intestinal parasites among people living with HIV/AIDS in the southwest district of Ethiopia. The observed prevalence of intestinal parasites among HIV/AIDS-infected individuals was found to be 35.1%, indicating high rates of parasitic infections in this population. The high prevalence of intestinal parasites among HIV-infected individuals is also reported in various African regions, particularly sub-Saharan Africa. For instance, a study conducted in Nigeria reported 68.2% (22), Gabon 42.6% (23), Cameroon 57.5% (24), and Kenya 50.9% (25). Additionally, a similar study from Ethiopia reported rates of 73.3% in Nekemte (26) and 45.3% in Gondar (27), underscoring the widespread nature of these infections across the region. These trends suggest that intestinal parasitic infections continue to be a significant burden among HIV-infected populations, especially in areas where ART access is limited and sanitation practices are inadequate.
In contrast, lower prevalence rates have been reported in other areas. For instance, a study conducted Mozambique reported 26.4% (28), India 27.6% (29), and Colombia 29.2% (30). This difference might be attributed to the widespread availability of ART and improved public health measures, including better sanitation and hygiene practices, in these settings. The lower prevalence in such countries highlights the critical role that ART and robust healthcare systems play in reducing the burden of opportunistic infections.
Moreover, in this study, the prevalence of intestinal parasites in the ART and ART naïve groups was (31.5%) and (45.8%) respectively, which was in line with a study finding in Northern Ethiopia, in which a higher proportion of intestinal parasites was observed in the ART naïve groups (27, 31). This consistent trend across multiple studies underscores the protective effect of ART in mitigating the risk of parasitic infections by improving immune function. However, the persistent high prevalence even among ART-treated patients in our study suggests that other factors, such as environmental conditions, hygiene practices, and possibly suboptimal ART adherence or effectiveness, also play a critical role in the transmission and maintenance of these infections (32).
Although there were no statistically significant differences in overall parasite species detected between the ART and ART-naive groups, in the present study, Entamoeba histolytica/dispar and Giardia lamblia were the most prevalent parasites detected both in the ART and ART-naïve groups. However, the prevalence of Cryptosporidium spp. in ART and ART naïve groups was 1.4 and 4.2%, respectively. This finding is in line with other study findings in Ethiopia (18, 27, 31) and Colombia (30), in which a higher predominance of opportunistic intestinal parasites was reported. The higher proportion of opportunistic intestinal parasites in ART naïve groups indicates that ART may also contribute to the reduction of opportunistic intestinal parasite infections.
In this study, intestinal parasitic infection occurrence was significantly higher in patients with a CD4 count of less than 200 cells/mm3, which is in line with other study findings from different parts of Ethiopia (33–36). Another study in Ethiopia reported that a CD4 count of less than 500 cells/mm3 was significantly associated with opportunistic intestinal parasitic infections (37). The low level of CD4 count leads to weakened immune responses and increases their chances of getting infections that the body would normally fight off very easily. Immuno-deficient patients are more vulnerable to acquiring intestinal parasites and are unable to clear the infection once it is established (36, 38). Some studies reported that a baseline CD4 count of ≥500 cells/μl was significantly associated with viral load reductions or suppression (39–41).
In this study a significant association between the consumption of unwashed raw vegetables and a higher prevalence of intestinal parasites were identified. This association consuming of contaminated food with intestinal parasitic infections is a known associated factor as reported in several other studies (42, 43). For instance, a study conducted in Brazil showed that HIV-infected individuals who consumed raw vegetables were twice as likely to be infected with intestinal parasites compared to their counterpart (44). In areas where there is contamination open field with human excreta, the risk of contamination is particularly high. Public health interventions that emphasis on educating HIV-infected individuals about safe food handling practices, as well as improving the quality of water used for washing vegetables, are essential in reducing the burden of intestinal parasitic infections.
Furthermore, the study also identified that a significant association between living in rural area with a higher prevalence of intestinal parasites. This study finding is in agreement with other studies from low-resource areas, where rural populations often have limited access to clean water, sanitation, and healthcare services (45). For instance, a study in rural Uganda, reported a similar association, contributing to the higher prevalence of intestinal parasitic infections to the lack of infrastructure and health education in rural communities (46). The disparity in the prevalence of parasitic infections in different geographic areas underscores the need for targeted interventions that address the specific challenges faced by rural populations, such as improving access to healthcare, sanitation, and clean water.
In the present study, only saline and iodine wet mounts, formaldehyde-ether concentrations, and modified acid-fast staining were used to detect intestinal parasites. Thus, the added yield of intestinal parasites may be an underestimate as we have not used other methods like molecular techniques and immunofluorescent techniques, which are sensitive to parasites. Additionally, patients may have received deworming and/or been diagnosed with parasites and treated as well before.
Conclusion
The study revealed a high prevalence of intestinal parasite infections among adults living with HIV/AIDS, with a higher infection rate observed in ART-naïve patients compared to those on ART. The findings indicate that lower CD4 counts, consumption of unwashed raw vegetables, and residing in rural areas are significant risk factors for intestinal parasitosis in this population. These results underscore the need for targeted interventions, including improving ART access, promoting better hygiene practices, especially regarding food safety, and focusing on vulnerable populations in rural areas. Regular screening for intestinal parasites in HIV-infected individuals, particularly those with low CD4 counts, is also recommended to reduce morbidity and improve patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Mizan-Tepi university/Ethical Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MeA: Conceptualization, Data curation, Methodology, Supervision, Visualization, Writing – review & editing. YH: Conceptualization, Data curation, Methodology, Supervision, Visualization, Writing – review & editing. TD: Conceptualization, Data curation, Methodology, Supervision, Visualization, Writing – review & editing. MiA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
First, we would like to thank Mizan-Tepi University, Institute of Research and Community Support Coordinating Office. Thanks to all data collectors for their willingness during data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Faria, CP, Zanini, GM, Dias, GS, da Silva, S, de Freitas, MB, Almendra, R, et al. Geospatial distribution of intestinal parasitic infections in Rio de Janeiro (Brazil) and its association with social determinants. PLoS Negl Trop Dis. (2017) 11:e0005445. doi: 10.1371/journal.pntd.0005445
2. Speich, B, Croll, D, Fürst, T, Utzinger, J, and Keiser, J. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect Dis. (2016) 16:87–99. doi: 10.1016/S1473-3099(15)00349-7
3. Teklemariam, Z, Abate, D, Mitiku, H, and Dessie, Y. Prevalence of intestinal parasitic infection among HIV positive persons who are naïve and on antiretroviral treatment in Hiwot Fana specialized university hospital, eastern Ethiopia. ISRN AIDS. (2013) 2013:1–6. doi: 10.1155/2013/324329
4. WHO . Global Health Observatory data: HIV/AIDS. Geneva, Switzerland: World Health Organization (2021).
5. Oguntibeju, OO . Prevalence of intestinal parasites in HIV –positive /AIDS patients in sout Africa. Malaysian. J Med Sci. (2006) 13:68–73.
6. Nelson, M., and Gantz,. Manual of clinical problems in infectious disease. 3rd ed. Philadelphia: Lippincott Williams and Wilkins, (1998); p 435–437, 440-44.
7. Engels, D, and Zhou, X-N. Neglected tropical diseases: an effective global response to local poverty-related disease priorities. Infect Dis Poverty. (2020) 9:10. doi: 10.1186/s40249-020-0630-9
8. Ochola, EA, Karanja, DMS, and Elliott, SJ. The impact of neglected tropical diseases (NTDs) on health and wellbeing in sub-Saharan Africa (SSA): a case study of Kenya. PLoS Negl Trop Dis. (2021) 15:e0009131. doi: 10.1371/journal.pntd.0009131
9. World Health Organization . Working to overcome the global impact of neglected tropical diseases. Geneva: WHO (2010).
10. World Health Organization . Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases: summary? World Health Organization (2013) Available at: https://apps.who.int/iris/handle/10665/80245.
11. Deribe, K, Meribo, K, Gebre, T, Hailu, A, Ali, A, Aseffa, A, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit Vectors. (2012) 5:240. doi: 10.1186/1756-3305-5-240
12. Hotez, PJ, and Kamath, A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. (2009) 3:e412. doi: 10.1371/journal.pntd.0000412
13. Alemu, G, Abossie, A, and Yohannes, Z. Current status of intestinal parasitic infections and associated factors among primary school children in Birbir town, southern Ethiopia. BMC Infect Dis. (2019) 19:270. doi: 10.1186/s12879-019-3879-5
14. Eyayu, T, Kiros, T, Workineh, L, Sema, M, Damtie, S, Hailemichael, W, et al. Prevalence of intestinal parasitic infections and associated factors among patients attending at Sanja primary hospital, Northwest Ethiopia: an institutional-based cross-sectional study. PLoS One. (2021) 16:e0247075. doi: 10.1371/journal.pone.0247075
15. Gebretsadik, D, Tesfaye, M, Adamu, A, and Zewde, G. Prevalence of intestinal parasitic infection and its associated factors among school children in two primary schools in Harbu town, north East Ethiopia: cross-sectional study. Pediatric Health Med Therap. (2020) 11:179. doi: 10.2147/PHMT.S252061
16. Tigabu, A, Taye, S, Aynalem, M, and Adane, K. Prevalence and associated factors of intestinal parasitic infections among patients attending Shahura health center, Northwest Ethiopia. BMC Res Notes. (2019) 12:333. doi: 10.1186/s13104-019-4377-y
17. Wondmieneh, A, Gedefaw, G, Alemnew, B, Getie, A, Bimerew, M, and Demis, A. Intestinal parasitic infections and associated factors among people living with HIV/AIDS in Ethiopia: a systematic review and meta-analysis. PLoS One. (2020) 15:e0244887. doi: 10.1371/journal.pone.0244887
18. Gebretsadik, D, Haileslasie, H, and Feleke, DG. Intestinal parasitosis among HIV/AIDS patients who are on anti-retroviral therapy in Kombolcha, north central, Ethiopia: a cross-sectional study. BMC Res Notes. (2018) 11:613. doi: 10.1186/s13104-018-3726-6
19. Ritchie, LS . An ether sedimentation technique for routine stool examinations. Washington, D.C., USA: Bulletin of the US Army Medical Department (1948); 8:326.
20. Henriksen, SA, and Pohlenz, JF. Staining of Cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. (1981) 22:594–6. doi: 10.1186/BF03548684
21. Cheesbrough, M. Medical laboratory manual for tropical countries, 1. 2nd edition. Cambridge, United Kingdom: Cambridge University Press (1992):208–210.
22. Obateru, OA, Bojuwoye, BJ, Olokoba, AB, Fadeyi, A, Fowotade, A, and Olokoba, LB. Prevalence of intestinal parasites in newly diagnosed HIV/AIDS patients in Ilorin, Nigeria. Alexandria J Med. (2017) 53:111–6, ISSN 2090-5068,. doi: 10.1016/j.ajme.2016.04.001
23. Lengongo, JVK, Ngondza, BP, Ditombi, BM, M’Bondoukwé, NP, Ngomo, JMN, Delis, AM, et al. Prevalence and associated factors of intestinal parasite infection by HIV infection status among asymptomatic adults in rural Gabon. Afri Health Sci. (2020) 20:1024–34. doi: 10.4314/ahs.v20i3.5
24. Vouking, MZ, Enoka, P, Tamo, CV, and Tadenfok, CN. Prevalence of intestinal parasites among HIV patients at the Yaoundé central hospital, Cameroon. Pan Afr Med J. (2014) 18:136. doi: 10.11604/pamj.2014.18.136.3052
25. Kipyegen, CK, Shivairo, RS, and Odhiambo, RO. Prevalence of intestinal parasites among HIV patients in Baringo, Kenya. Pan Afr Med J. (2012) 13:37.
26. Gebrewahid, T, Gebrekirstos, G, Teweldemedhin, M, Gebreyesus, H, Awala, A, and Tadla, K. Intestinal parasitosis in relation to CD4 count and anemia among ART initiated patients in St. Mary Aksum general hospital, Tigray, Ethiopia. BMC Infect Dis. (2019) 19:350. doi: 10.1186/s12879-019-3989-0
27. Gebrecherkos, T, Kebede, H, and Gelagay, AA. Intestinal parasites among HIV/AIDS patients attending University of Gondar Hospital, Northwest Ethiopia. J Health Dev. (2019) 33:65–72. Available at: https://www.ajol.info/index.php/ejhd/article/view/188835
28. Cerveja, BZ, Tucuzo, RM, Madureira, AC, Nhacupe, N, Langa, IA, Buene, T, et al. Prevalence of intestinal parasites among HIV infected and HIV uninfected patients treated at the 1° De Maio health Centre in Maputo. Mozambique EC Microbiol. (2017) 9:231–40.
29. Chhangte, MZ, Koticha, A, Ingole, N, and Mehta, P. Prevalence of intestinal parasites in HIV sero-positive patients attending an integrated counseling and testing Centre. J Evolution Med Dent Sci. (2020) 9:919–23. doi: 10.14260/jemds/2020/198
30. Botero, JH, Villegas-Arbeláez, E, Giraldo, S, UránVelásquez, J, Arias-Agudelo, L, Alzate-Angel, JC, et al. Prevalence of intestinal parasites in a cohort of HIV-infected patients from Antioquia, Colombia. Biomedica. (2021) 41:153–64. doi: 10.7705/biomedica.5992
31. Missaye, A, Dagnew, M, Alemu, A, and Alemu, A. Prevalence of intestinal parasites and associated risk factors among HIV/AIDS patients with pre-ART and on-ART attending Dessie hospital ART clinic, Northeast Ethiopia. AIDS Res Ther. (2013) 10:7. doi: 10.1186/1742-6405-10-7
32. Teke Apalata, T, Aviwe, B, Oladimeji, O, and Abaver, DT. Prevalence of intestinal parasites in HIV/AIDS-infected patients attending clinics in selected areas of the eastern cape. Microbiol Res. (2022) 13:574–83. doi: 10.3390/microbiolres13030040
33. Alemayehu, E, Gedefie, A, Adamu, A, Mohammed, J, Kassanew, B, Kebede, B, et al. Intestinal parasitic infections among HIV-infected patients on antiretroviral therapy attending Debretabor general hospital, northern Ethiopia: a cross-sectional study. HIV AIDS (Auckl). (2020) 12:647–55. doi: 10.2147/HIV.S275358
34. Bayleyegn, B, Woldu, B, Yalew, A, Kasew, D, and Asrie, F. Prevalence of intestinal parasitic infection and associated factors among HAART initiated children attending at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. HIV AIDS (Auckl). (2021) 13:81–90. doi: 10.2147/HIV.S287659
35. Miressa, R, and Dufera, M. Prevalence and predisposing factors of intestinal parasitic infections among HIV positive patients visiting Nekemte specialized hospital, Western Ethiopia. HIV AIDS (Auckl). (2021) 13:505–12. doi: 10.2147/HIV.S304294
36. Gupta, K, Bala, M, Deb, M, Muralidhar, S, and Sharma, DK. Prevalence of intestinal parasitic infections in HIV-infected individuals and their relationship with immune status. Indian J Med Microbiol. (2013) 31:161–5. doi: 10.4103/0255-0857.115247
37. Alemu, G, Alelign, D, and Abossie, A. Prevalence of opportunistic intestinal parasites and associated factors among HIV patients while receiving ART at Arba Minch Hospital in South Ethiopia: a cross-sectional study. Ethiop J Health Sci. (2018) 28:147. doi: 10.4314/ejhs.v28i2.6
38. Evering, T, and Weiss, L. The immunology of parasite infections in immunocompromised hosts. Parasite Immunol. (2006) 28:549–65. doi: 10.1111/j.1365-3024.2006.00886.x
39. Abdullahi, SB, Ibrahim, OR, Okeji, AB, Yandoma, RI, Bashir, I, Haladu, S, et al. Viral suppression among HIV-positive patients on antiretroviral therapy in northwestern Nigeria: an eleven-year review of tertiary care Centre records, January 2009–December 2019. BMC Infect Dis. (2021) 21:1031. doi: 10.1186/s12879-021-06722-3
40. Wakooko, P, Gavamukulya, Y, and Wandabwa, JN. Viral load suppression and associated factors among HIV patients on antiretroviral treatment in Bulambuli District, eastern Uganda: a retrospective cohort study. Infect Dis (Auckl). (2020) 13:1178633720970632. doi: 10.1177/1178633720970632
41. Pius, A, Josephine, NN, Erick, S, Winifred, A, Rita, M, Silverjoseph, O, et al. Influence of intensified adherence counseling on viral load suppression of people receiving antiretroviral therapy at a health Centre IV in southwestern Uganda: a qualitative study. AIDS Res Ther. (2021) 18:45. doi: 10.1186/s12981-021-00372-w
42. Silva, CV, Ferreira, MS, Borges, AS, et al. Intestinal parasitic infections in HIV/AIDS patients: experience at a teaching hospital in Central Brazil. Scand J Infect Dis. (2005) 37:211–5. doi: 10.1080/00365540410020875
43. Awole, M, Gebre-Selassie, S, Kassa, T, and Kibru, G. Prevalence of intestinal parasites in HIV-infected adult patients in southwestern Ethiopia. Ethiop J Health Dev. (2003) 17:71–8. doi: 10.4314/ejhd.v17i1.9783
44. Barcelos, NB, Silva, LDF, Dias, RFG, Filho, HRD, and Rodrigues, RM. Opportunistic and non-opportunistic intestinal parasites in HIV/AIDS patients in relation to their clinical and epidemiological status in a specialized medical service in Goiás, Brazil. Rev Inst Med Trop Sao Paulo. (2018) 60. doi: 10.1590/1678-9946201860013
45. Navaneethan, U, and Giannella, RA. Mechanisms of infectious diarrhea. Nat Clin Pract Gastroenterol Hepatol. (2008) 5:637–47. doi: 10.1038/ncpgasthep1264
46. Fuhrimann, S, Winkler, MS, Kabatereine, NB, Tukahebwa, EM, Halage, AA, Rutebemberwa, E, et al. Risk of intestinal parasitic infections in people with different exposures to wastewater and fecal sludge in Kampala, Uganda: a cross-sectional study. PLoS Negl Trop Dis. (2016) 10. doi: 10.1371/journal.pntd.0004469
Keywords: prevalence, intestinal parasite, people with HIV/AIDS, Ethiopia, ART clinic
Citation: Abayneh M, Habtemariam Y, Duguma T and Abera M (2024) Prevalence of intestinal parasites and associated factors among patients with HIV/AIDS at the anti-retroviral treatment clinic of Mizan-Tepi University Teaching Hospital, Southwest Ethiopia. Front. Public Health. 12:1451757. doi: 10.3389/fpubh.2024.1451757
Edited by:
William Harold Witola, University of Illinois at Urbana–Champaign, United StatesReviewed by:
Cristina Carranza-Rodríguez, Complejo Hospitalario Universitario Insular-Materno Infantil, SpainGete Berihun, Wollo University, Ethiopia
Copyright © 2024 Abayneh, Habtemariam, Duguma, and Abera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mitiku Abera, bWl0aWt1YWJlcmFkQGdtYWlsLmNvbQ==
†ORCID: Tadesse Duguma, https://orcid.org/0000-0001-7372-7175
 Mengistu Abayneh
Mengistu Abayneh Yosef Habtemariam2
Yosef Habtemariam2 Mitiku Abera
Mitiku Abera