- 1Department of Interdisciplinary Medicine (DIM), University of Bari Aldo Moro, Piazza Giulio Cesare, Bari, Italy
- 2Centre for Environmental Sciences, Hasselt University, Diepenbeek, Belgium
- 3Department of Public Health and Primary Care, Environment and Health Unit, Leuven University, Leuven, Belgium
- 4Local Healthcare Authority of Bari, ASL Bari, Bari, Italy
- 5Department of Translational Biomedicine and Neuroscience "DiBraiN", University of Bari "Aldo Moro", Bari, Italy
- 6Department of Obstetrics and Gynecology, "Miulli" General Hospital, Bari, Italy
- 7Obstetrics and Gynecology Unit, Vito Fazzi Hospital, Lecce, Italy
- 8Department of Environmental Protection, Faculty of Geology, Geophysics and Environmental Protection, AGH University of Krakow, Al. Mickiewicza, Krakow, Poland
- 9Unit of Statistics and Epidemiology, Local Health Authority of Taranto, Taranto, Italy
- 10Department of Eye and Vision Sciences, University of Liverpool, Liverpool, United Kingdom
Background: Growing evidence indicates an association between ambient air pollution and decreased human reproductive potential. This study aims to systematically review the association between air pollutants and female ovarian reserve.
Methods: The literature was searched in six electronic databases through June 2024. Screening the 136 articles retrieved for inclusion criteria resulted in the selection of 15 human observational studies that evaluated the effect of environmental pollutants on ovarian reserve markers. The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO, registration code: CRD42023474218).
Results: The study design of the selected studies was found to be cross-sectional (2 of 15), retrospective cohort (10 of 15), prospective cohort (2 of 15), and case–control (1 of 15). The study population was distributed as follows: Asians (53%, eight studies), Americans (33%, five studies), and Europeans (14%, two studies). The main findings showed a higher body of evidence for the environmental pollutants PM2.5, PM10, and NO2, while a low body of evidence for PM1, O3, SO2, and a very low body of evidence for benzene, formaldehyde, and benzo(a)pyrene, yet consistently showing significant inverse association data. The overall methodological quality of the selected studies was rated moderated across the 14 domains of the National Institutes of Health (NIH) toolkit.
Conclusion: The data suggest that increased exposure to air pollutants seems to be associated with reduced ovarian reserve, with the most substantial evidence for pollutants such as PM2.5, PM10, and NO2. However, more evidence is needed to draw conclusions about causality.
Introduction
Public health data on air pollution from the global burden of disease (GBD) estimates 213 million disability-adjusted life years (DALYs)—equal to 0.84% of the global DALY—6.67 million deaths in 2019 (1). Much of the scientific community has supported the association between air pollution and the risk of cardiovascular (2–4), respiratory (5, 6), endocrine (7), reproductive (8), and all-cause mortality to date (9, 10). Until now, there is substantial consistency around biological mechanisms involving inflammation, oxidative stress, endocrine disruption, and epigenetic changes.
Some early studies pointed to air pollution exposure being associated with reduced fertility and a range of adverse pregnancy outcomes, such as miscarriage, preterm delivery, and stillbirth, regardless of having natural pregnancies or undergoing assisted reproductive technologies (2, 11–14). The underlying mechanism of female fertility decline due to air pollutants remains unclear. At the same time, limited evidence speculates that impaired ovarian reserve caused by oxidative stress and inflammatory response caused by air pollution May be a critical path.
The number and quality of the ovarian follicle pool are commonly referred to as ovarian reserve, which indicates a woman’s reproductive potential or fertility (15). After puberty, follicle development begins under gonadotropin stimulation (16), and the entire development process mainly includes the development of a small number of primordial follicles to the antral stage and the selection of an antral follicle for growth to the preovulatory stage during each menstrual cycle (16).
In clinical settings, a common practice is to use hormonal and ultrasound markers as proxies of ovarian reserve (17, 18). In this context, ultrasound antral follicle count (AFC), serum levels of follicle-stimulating hormone (FSH), anti-Müllerian hormone (AMH), inhibin B, and E2 have been proposed as potential markers of fertility, among which AMH is considered the most sensitive and specific available marker (19, 20). Indeed, AFC results tend to have operator skill-dependent variability, whereas serum AMH is the best predictor of ovarian reserve for its high representativeness of small AFC (19, 20). Also, previous studies found that AMH levels remain stable during the menstrual cycle and can be detected on any day of the period (21).
Animal studies have documented that exposure to particulate matter 2.5 (PM2.5) is associated with decreased levels of reproductive hormones and the number of antral and primordial ovarian follicles in mice. Gai and colleagues showed that PM2.5 reduced AMH levels and increased interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) levels in mouse ovarian tissue (22). A significant reduction in the proportion of primordial follicles was observed by Ogliari and colleagues in mice exposed to diesel exhaust with doses equal to the average daily levels of PM2.5 (fine particles in ambient air 2.5 μm or less in size) reported by the World Health Organization (23).
However, till today, the body of evidence on the association between exposure to air pollutants and markers of ovarian reserve in women lacks a synthesis of evidence. Therefore, to fill this gap, this study aimed to systematically investigate the association between major environmental air pollutants and female fertility in childbearing females.
Methods
Search strategy, study selection, and data extraction
A computer search of the literature on databases, namely, MEDLINE and the Cochrane Library, identified no previous systematic reviews on exposure to environmental pollutants and ovarian reserve in women. The present systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, adhering to the PRISMA 27-item checklist (Page et al., 2021). An a priori protocol for search strategy and inclusion criteria was established and registered, with no particular changes to the information provided at the time of registration on the International Prospective Register of Systematic Reviews (PROSPERO), an international prospective registry of systematic reviews (CRD42023474218). We performed separate searches in the US National Library of Medicine (PubMed), Medical Literature Analysis and Retrieval System Online (MEDLINE), EMBASE, Scopus, Ovid, and Google Scholar to find human observational studies that evaluated the effect of major environmental pollutants [PM1, PM2.5, PM10, NO2, O3, SO2, CO, polycyclic aromatic hydrocarbons (PAHs), black carbon, 1,3-butadiene, benzene, diesel PM, formaldehyde, methylene chloride, and tetrachloroethylene] on female fertility expressed by recognized markers such as AMH, poor ovarian reserve (POR), and antral follicle count (AFC). Therefore, the primary objective was to assess the amount, consistency, and direction of the association between any of these pollutants and markers of ovarian reserve. We also considered the gray literature using the vast archive of preprints1 the study selection phase and the database2 to access abstracts of significant conferences and other unreviewed material.
The following criteria were applied to include various studies in the analysis: (1) human observational study; (2) reporting of the effect of environmental pollutants on women’s fertility as expressed by recognized ovarian reserve markers; and (3) studies that involved women of childbearing age. Animal studies, conference abstracts, reviews, letters, editorials, nonclinical trial studies, and studies involving children and/or adolescents were excluded.
The search strategy used in PubMed and MEDLINE and adapted to the other four electronic sources included keywords such as antral follicle count, ovarian reserve, PM, black carbon, and air pollutant(s) combined through the use of Boolean indicators such as AND and OR (Table 1). The search strategy used the Boolean indicator NOT to exclude opinion articles, letters, reviews, and meta-analyses. The literature search had no time restrictions, and articles were retrieved until June 2024. Two researchers (RZ and FC) searched the articles—separately and in duplicate—reviewed the titles and abstracts of the retrieved articles, checked the full texts, and selected the articles for inclusion in the study. Interrater reliability (IRR) was used to estimate intercoder agreement and then κ statistic to measure accuracy and precision. According to PRISMA concepts and quality assessment steps, a κ coefficient of at least 0.9 was obtained in all data extraction steps (24).
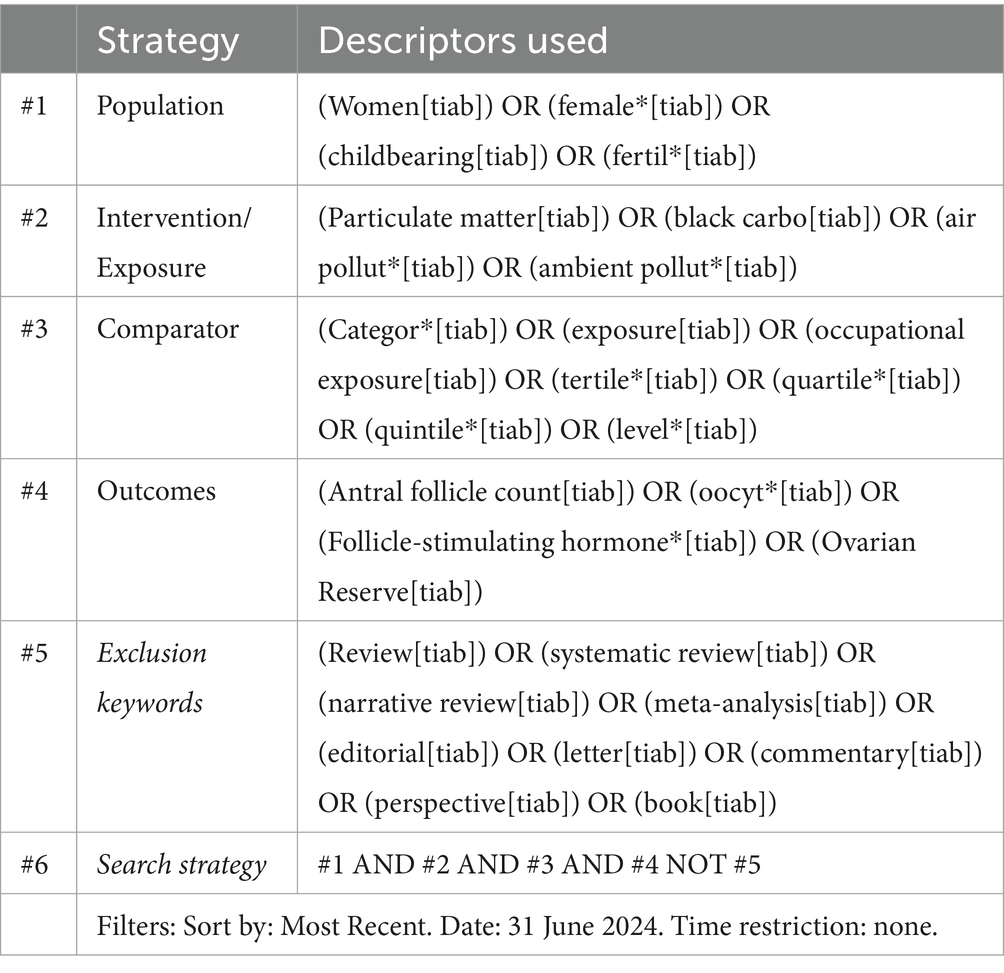
Table 1. Search strategy used in the US national library of medicine (PubMed) and medical literature analysis and retrieval system online (MEDLINE) and adapted to the other sources, according to selected descriptors.
Quality assessment within and across studies and overall quality assessment
The methodological quality of the included studies was independently assessed by two researchers (RZ and FC) using the National Institutes of Health Quality Assessment Toolkits for Observational Cohort and Cross-Sectional Studies (25, 26). According to the criteria given in the toolkit, the ratings—high (good), fair (moderate), or poor—were assigned to the studies. This toolkit contains 14 questions assessing several aspects associated with the risk of bias, types I and II errors, transparency, and confounding factors: study question, population, participation rate, inclusion criteria, sample size justification, time of exposure/outcome measurement, timing, exposure levels, defined exposure, blinded assessors, repeated exposure, defined outcomes, loss to follow-up, and confounding factors. Items 6, 7, and 13 did not refer to cross-sectional studies; the maximum possible scores for cross-sectional and prospective studies were 8 and 14, respectively. Disagreements on the methodological quality of the included studies (e.g., interpretation of toolkit domains, appropriateness, and response type) between the two investigators were resolved through discussion until a consensus was reached with a third investigator (RS). A modified version of the grading system, namely, Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (27) was used to assess the quality of evidence of the studies included in this systematic review. The following factors were considered: strength of association between air pollutants exposure and related female fertility, methodological quality/study design, consistency, bias, precision, size, and (where possible) dose–response gradient of effect estimates in the evidence base. Evidence was graded as very low, low, moderate, and high, as in the GRADE grading system.
Results
The first systematic search of the literature yielded 321 entries. After excluding the duplicates, 136 were classified as potentially relevant and selected for the title and abstract analysis. Then, 99 were excluded for not meeting the characteristics of the approach or the review goal. After reviewing the full text of the remaining records, only 15 met the inclusion criteria and were included in the systematic review (28–42). The PRISMA flowchart illustrating the number of studies at each stage of the review is shown in Figure 1. The final study base included 15 observational studies reporting on the effect of environmental pollutants on markers of ovarian reserve in childbearing females.
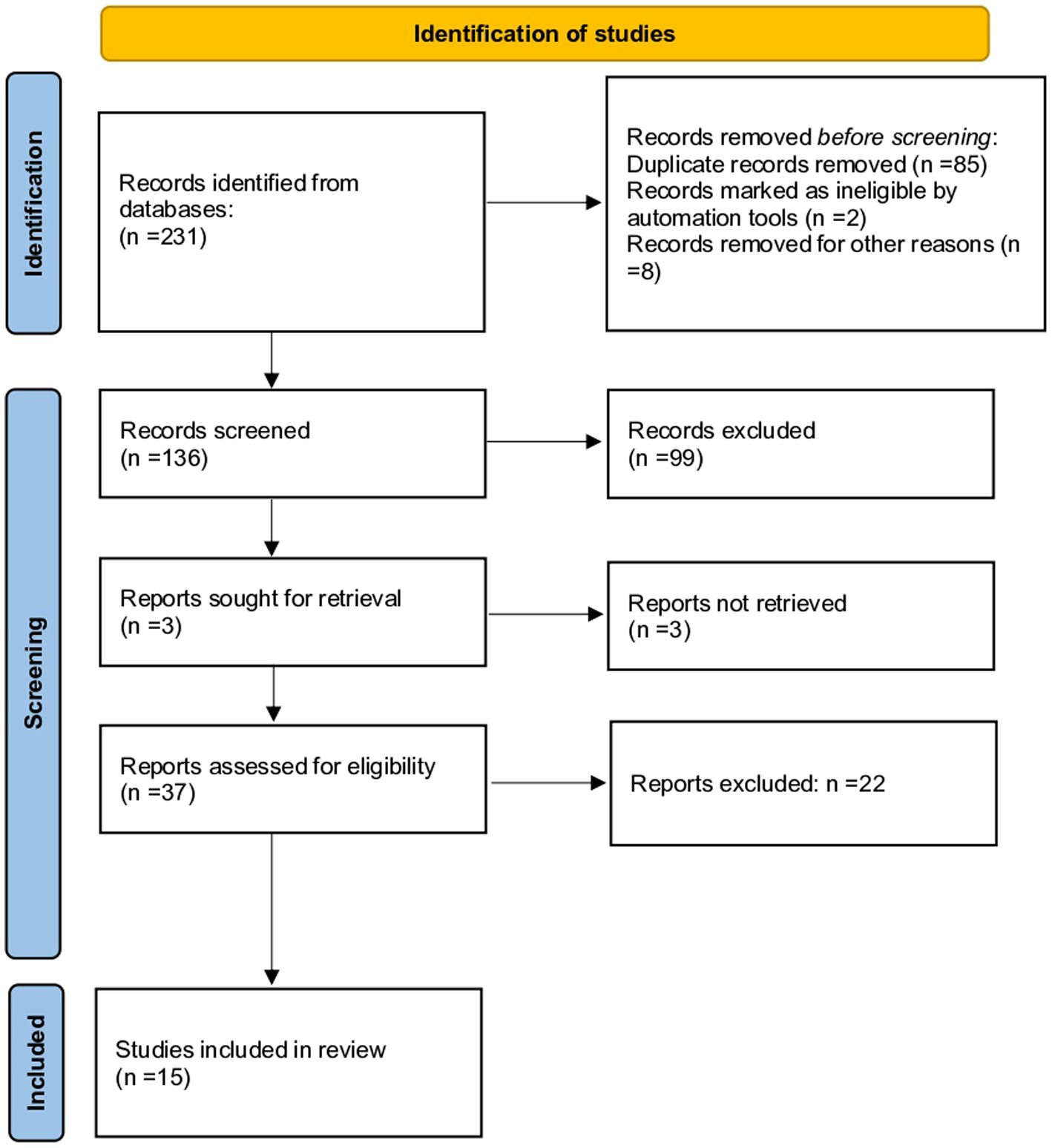
Figure 1. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart illustrating the number of studies at each stage of the review.
The details of the study design, sample size (N), country, author(s) and year of publication, exclusion criteria, population age, exposure pollutant(s), outcome(s) of ovarian reserve, significant findings, and covariates considered for adjust models are provided in Table 2.
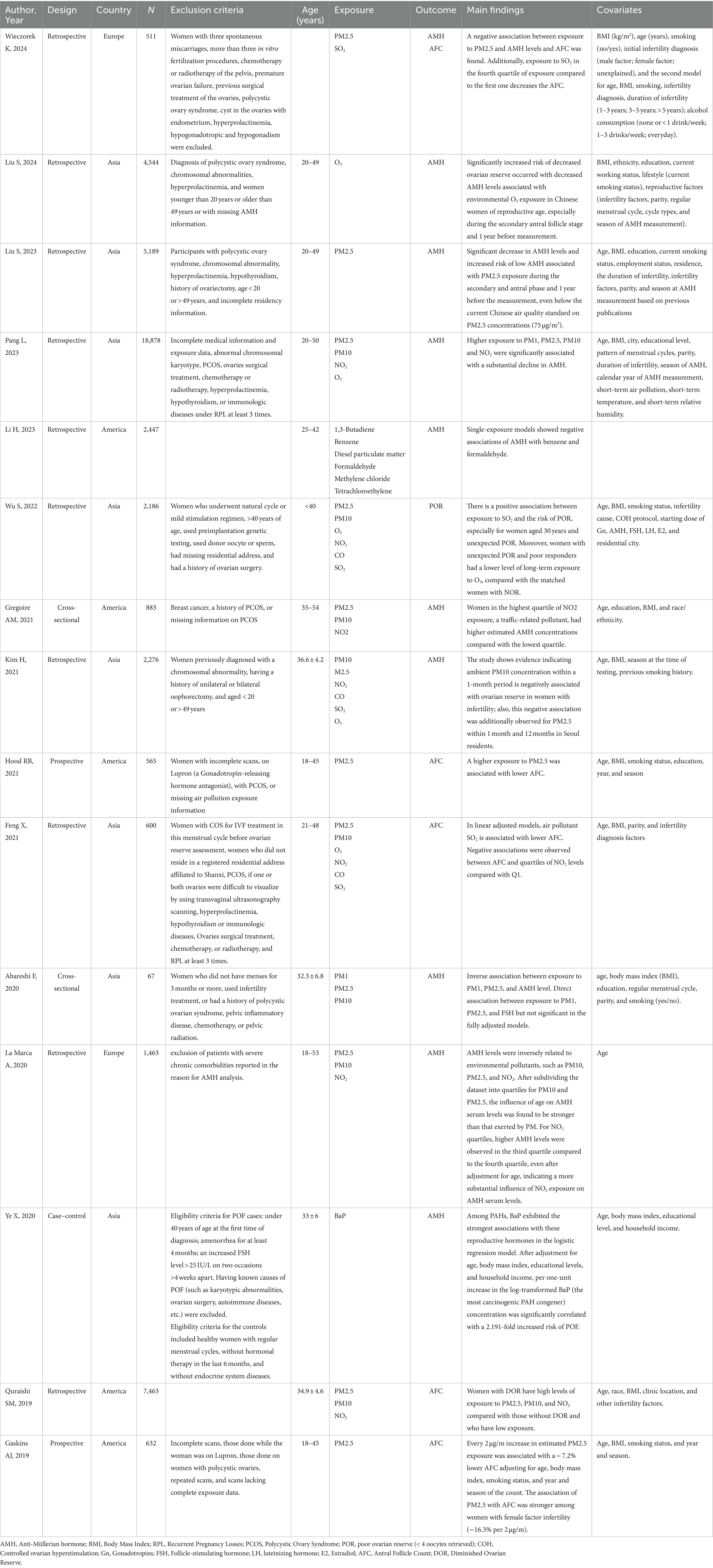
Table 2. Description of selected studies exploring the association between air pollutants and markers of ovarian reserve in childbearing women, N = 12.
The study design of selected studies was found to be cross-sectional (2 of 15), retrospective cohort (10 of 15) (28–32, 34, 36, 38, 40–42), prospective cohort (2 of 15) (33, 39), and case–control (1 of 15) (37). The study population was distributed as follows: Asians (53%, eight studies), Americans (33%, five studies), and Europeans (14%, two studies).
Data extraction from the selected studies resulted in a total of 14 entries of environmental pollutants (PM, PM2.5, PM10, NO2, O3, CO, SO2, 1,3-butadiene, benzene, diesel PM, formaldehyde, methylene chloride, tetrachloroethylene, and benzo(a)pyrene). A majority of 65% of those resulting air pollutants, that is, PM1, PM2.5, PM10, NO2, O3, benzene, formaldehyde, SO2, and benzo(a)pyrene reported on a meaningful association with marker(s) of ovarian reserve. Indeed, no significant association resulted in CO, 1,3-butadiene, diesel PM, methylene chloride, and tetrachloroethylene about ovarian reserve. For ovarian reserve markers, the majority of the selected studies considered AMH (62%) as an outcome, followed by AFC (31%), and POR (7%).
Table 3 summarizes findings on different environmental pollutants associated with female ovarian reserve items. To focus on the evidence surrounding each environmental pollutant about ovarian reserve, the available retrieved literature will be elucidated as follows.
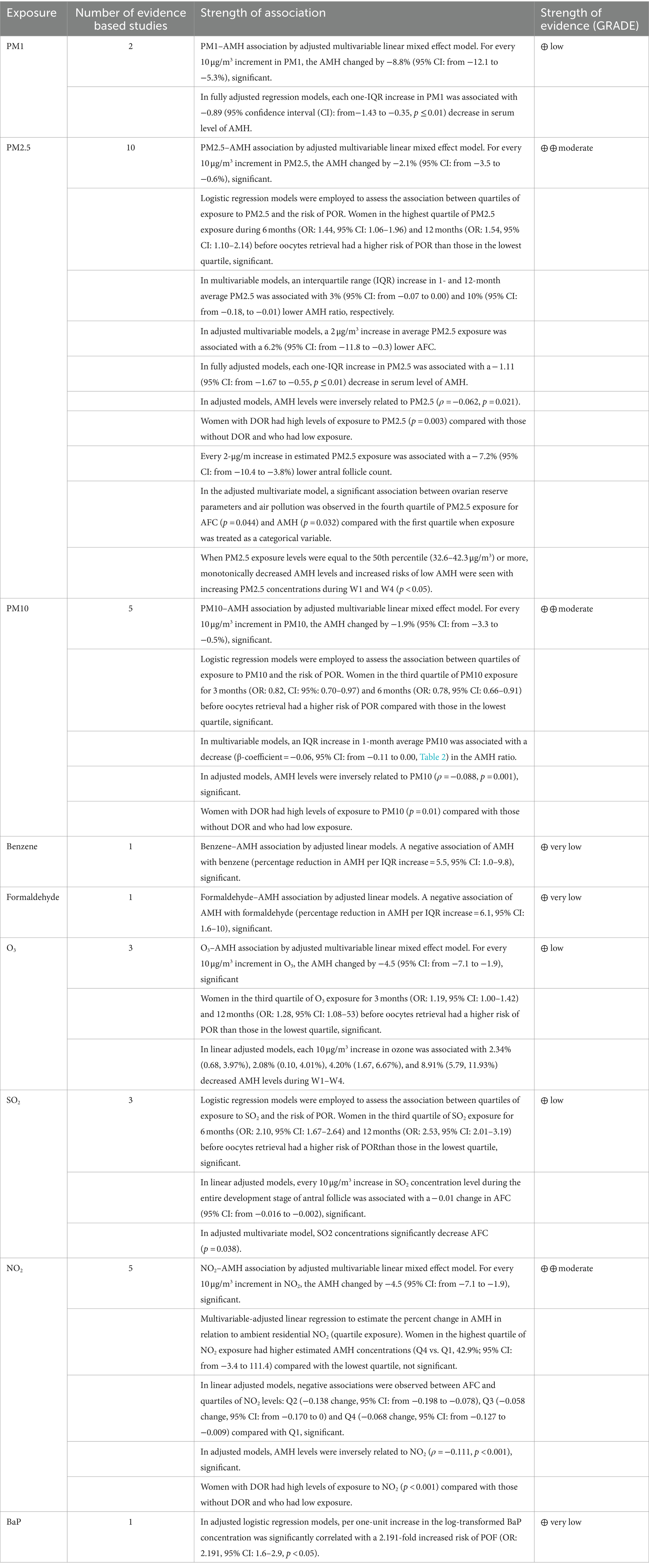
Table 3. Summary of findings on different environmental pollutants associated with ovarian reserve markers in childbearing females.
PM2.5 and ovarian reserve
For the pollutant PM2.5, 10 studies (28, 30, 32, 33, 35, 36, 38, 39, 41, 42) were retrieved, providing moderate strength of evidence. Six of these studies considered AMH as an outcome: Pang and colleagues (28) indicated that for every increase of 10 μg/m3 in ambient PM2.5, AMH changed by −2.1% (95% confidence interval [CI]: from −3.5 to −0.6); Kim and colleagues (32) indicated that an increase in the interquartile range (IQR) in mean PM2.5 at 1 and 12 months was associated with a 3% (95% CI: from −0.07 to 0.00) and 10% (95% CI: from −0.18 to −0.01) (28, 30, 32, 33, 35, 36, 38, 39); Abareshi and colleagues (35) showed that each increase of an IQR of ambient PM2.5 was associated with a decrease in serum AMH level of −1.11 (95% CI: from −1.67 to −0.55); La Marca and colleagues (36) showed AMH levels weakly inversely correlated with PM2.5 (ρ = −0.062, p = 0.021) in the adjusted models; Wieczorek and colleagues (42) found a negative association between PM2.5 exposure and AMH and AFC levels; and finally, Liu and colleagues (41) demonstrated a significant drop in AMH levels and increased risk of low AMH associated with PM2.5 exposure during the secondary and antral phases and 1 year before the measurement, even below the current Chinese air quality standard on PM2.5 concentrations (75 μg/m3). The other four studies considered AFC or decreased/poor ovarian reserve (DOR/POR) as an outcome: Wu and colleagues (30) used logistic regression models to assess the association between quartiles of PM2.5 exposure and POR risk finding that women in the highest quartile of PM2.5 exposure during 6 months (OR: 1.44, 95% CI: 1.06–1.96) and 12 months (OR: 1.54, 95% CI: 1.10–2.14) before oocytes retrieval had a higher risk of POR than those in the lowest quartile; Hood and colleagues (33) demonstrated a 2 μg/m3 increase in mean PM2.5 exposure to be associated with an AFC reduction of −6.2% per 2 μg/m3 (1 standard deviation (SD) increase; 95% CI: from −11.8 to −0.3) in multivariable adjusted models; Quraishi and colleagues (38) showed that women with DOR had high levels of PM2.5 exposure (p = 0.003) compared with those without DOR and with low exposure; finally, Gaskins and colleagues (39) showed that each 2 μg/m increase in estimated PM2.5 exposure was associated with a reduction of −7.2% (95% CI: = from −10.4 to −3.8%) lower AFC count.
PM10 and ovarian reserve
For the pollutant PM10, a total of five studies were retrieved (28, 30, 32, 36, 38), providing moderate strength of evidence. Three of these studies considered AMH as an outcome: Kim and colleagues (32) found an inverse PM10–AMH association by multivariable linear mixed-effects adjusted model. For each 10 μg/m3 increase in PM10, AMH varied by −1.9% significantly (95% CI: from −3.3 to −0.5%); Pang and colleagues (28) developed multivariable models showing an increase in the IQR of mean PM10 at 1 month was associated with a decrease (β-coefficient = −0.06, 95% CI: from −0.11 to 0.00) of AMH ratio; Quraishi and colleagues (38) found AMH levels inversely correlated with PM10 (ρ = −0.088, p = 0.001), and the findings were significant for women with a reduced ovarian reserve in adjusted models. The other two studies considered POR and DOR as an outcome: La Marca and colleagues (36) used logistic regression models to assess the association between PM10 exposure quartiles and the risk of POR. Women in the third quartile of PM10 exposure for 3 months (OR: 0.82, 95% CI: 0.70–0.97) and 6 months (OR: 0.78, 95% CI: 0.66–0.91) before oocytes retrieval had a higher risk of POR than those in the lowest quartile; Wu and colleagues (30) showed that women with decreased ovarian reserve (DOR) had high levels of PM10 exposure (p = 0.01) compared with those without DOR and with low exposure.
NO2 and ovarian reserve
For the pollutant NO2, a total of five studies were retrieved (28, 31, 34, 36, 38), providing moderate strength of evidence. Three of these studies considered AMH as an outcome: Feng and colleagues (34) reported a significant inverse NO2−AMH association by a multivariable linear mixed-effect adjusted model. Here, for each 10 μg/m3 increase in NO2, AMH changed by −4.5% (95% CI: from −7.1 to −1.9) significantly; Gregoire and colleagues (31) performed a multivariable-adjusted linear regression to estimate the percent change in AMH in relation to residential ambient NO2 (exposure quartile) and found that women in the highest quartile of NO2 exposure had higher estimated AMH concentrations (Q4 vs. Q1, 42.9%; 95% CI: from −3.4 to 111.4) than the lowest quartile; however, the data lacked statistical significance; Pang and colleagues (28) demonstrated that in the adjusted models, AMH levels were inversely, statistically related to NO2 (ρ = −0.111, p < 0.001). The other two studies considered AFC and DOR as an outcome: La Marca and colleagues (36) performed adjusted linear models observing negative, statistically significant associations between AFC and quartiles of NO2 levels: Q2 (−0.138 change, 95% CI: from −0.198 to −0.078), Q3 (−0.058 change, 95% CI: from −0.170 to 0) and Q4 (−0.068 change, 95% CI: from −0.127 to −0.009) compared with Q1; Quraishi and colleagues (38) showed that women with DOR had high levels of NO2 exposure (p < 0.001) compared with those without DOR and with low exposure, and the difference was statistically significant.
PM1 and ovarian reserve
For the pollutant PM1, only two studies were retrieved (28, 35), providing an overall low strength of evidence. Abareshi and colleagues (35) studied the PM1 − AMH association by multivariable linear mixed-effects adjusted model, finding that for each 10 μg/m3 increase in PM1, AMH varied significantly by −8.8% (95% CI: from −12.1 to −5.3%). Pang and colleagues (28) implemented fully adjusted regression models, finding that each IQR increase in ambient PM1 was associated with a − 0.89 (95% CI: from −1.43 to −0.35, p ≤ 0.01) decrease in serum AMH level.
SO2 and ovarian reserve
For the pollutant SO2, only three studies were retrieved (30, 34), providing an overall low strength of evidence. Feng and colleagues (34) employed logistic regression models to assess the association between quartiles of SO2 exposure and the risk of POR, finding that women in the third quartile of SO2 exposure during 6 months (OR: 2.10, 95% CI: 1.67–2.64) and 12 months (OR: 2.53, 95% CI: from 2.01 to 3.19) before oocytes retrieval had a higher risk of POR than those in the lowest quartile. Wieczorek and colleagues (42), in adjusted multivariate models, found SO2 concentrations significantly decreased AFC (p = 0.038). Wu and colleagues (2) employed adjusted linear models showing a 10 μg/m3 increase in SO2 concentration level during the entire antral follicle development phase to be statistically associated with a − 0.01 change in AFC (95% CI: form −0.016 to −0.002).
O3 and ovarian reserve
For the pollutant O3, only three studies were retrieved (28, 30), providing an overall low strength of evidence. Pang and colleagues (28) studied the O3−AMH association by multivariable linear mixed-effect adjusted model, finding that for each 10 μg/m3 increase in O3, AMH varied significantly by −4.5 (95% CI, from −7.1 to −1.9). In adjusted linear models, Liu and colleagues (40) showed that each 10 μg/m3 increase in ozone was associated with a decrease in AMH levels of 2.34% (0.68, 3.97%), 2.08% (0.10, 4.01%), 4.20% (1.67, 6.67%), and 8.91% (5.79, 11.93%) during W1–W4. Wu and colleagues (2) showed that women in the third quartile of O3 exposure during 3 months (OR: 1.19, 95% CI: 1.00–1.42) and 12 months (OR: 1.28, 95% CI: 1.08–1.53) before oocytes retrieval had a higher risk of POR than those in the lowest quartile.
Benzene and ovarian reserve
For the pollutant benzene, only one report was retrieved (29), providing an overall very low strength of evidence. Li and colleagues studied the benzene–AMH association using adjusted linear models, finding a negative, significant association of AMH with benzene [percent reduction in AMH per increase in interquartile range (IQR) = 5.5, 95% CI: 1.0–9.8].
Formaldehyde and ovarian reserve
For the pollutant formaldehyde, only one report was retrieved (29), providing an overall very low strength of evidence. Li and colleagues studied the formaldehyde–AMH association using adjusted linear models and found a negative and statistically significant association of AMH with formaldehyde (percent reduction in AMH per increase in the IQR = 6.1, 95% CI = 1.6–10).
Benzo(a)pyrene and ovarian reserve
For the pollutant benzo(a)pyrene (BaP), only one report was retrieved (37), providing an overall very low strength of evidence. Ye and colleagues ran adjusted logistic regression models, finding each one-unit increase in log-transformed BaP concentration to be significantly related to a 2.191-fold increased risk of premature ovarian failure (POF; OR: 2.191, 95% CI: 1.6–2.9, p < 0.05).
Quality assessment and risk of bias
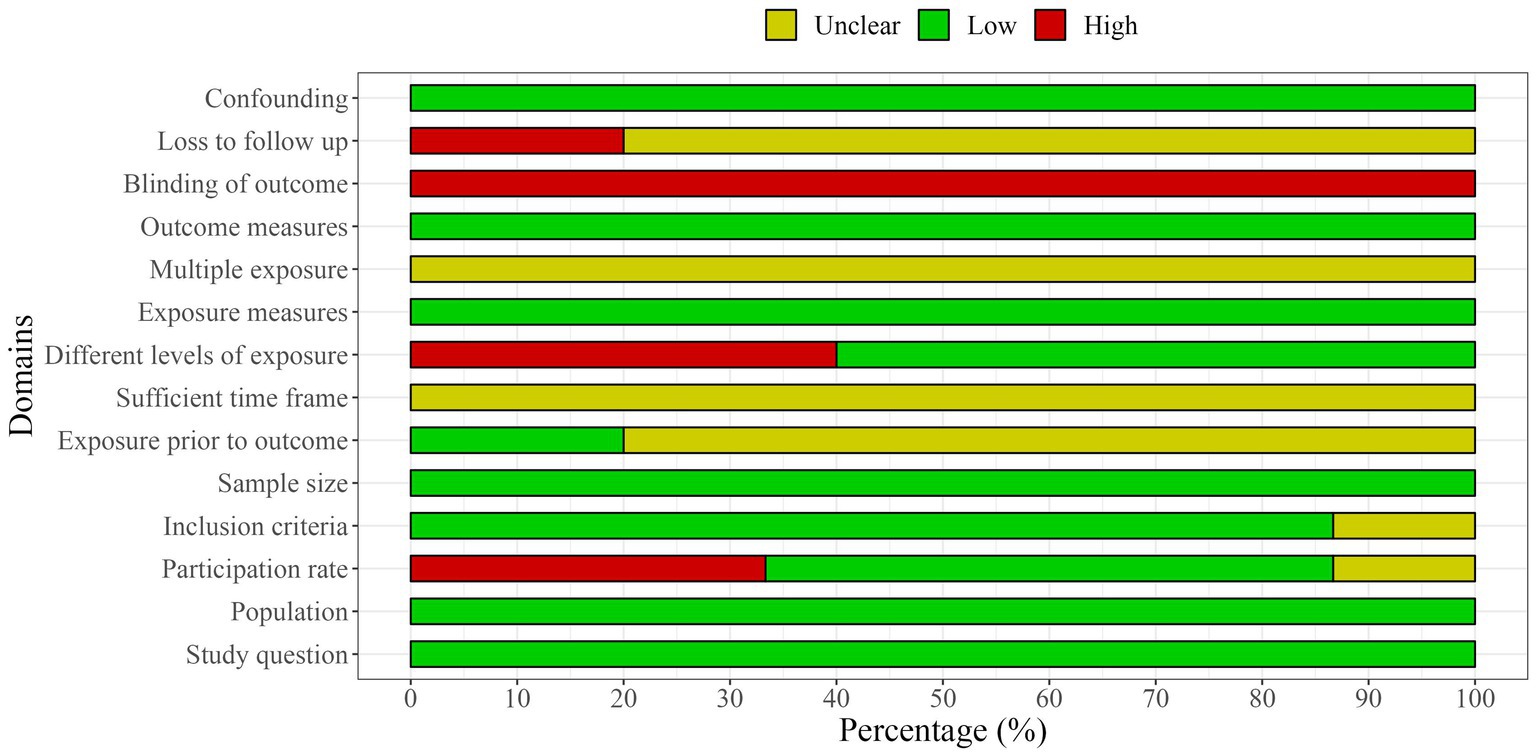
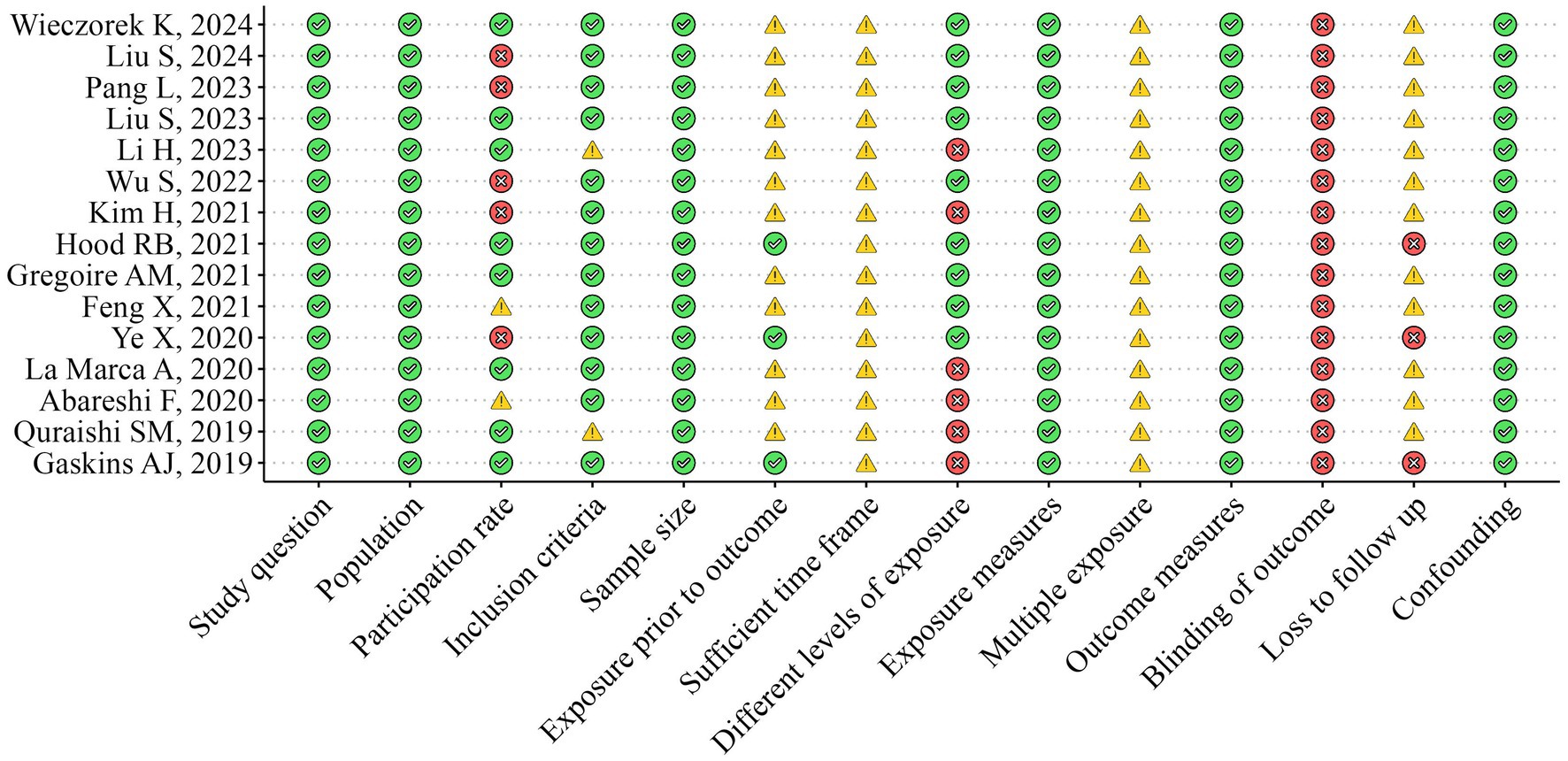
The methodological quality of the included studies was independently assessed by two researchers (RZ and FC) using the National Institutes of Health Quality Assessment Toolkits for Observational Cohort and Cross-Sectional Studies (25, 26) (Figures 2, 3). The overall methodological quality of the selected studies was rated moderate across the 14 toolkit domains. In particular, the risk was rated low for domains such as study question, population, exposure measures, outcome measures, sample size, and confounding factors across studies. Some concerns arose in some studies for domains such as inclusion criteria, participation rate, and multiple exposure. In contrast, a medium to high risk of bias was found for the domain participation rate, blinding of the outcome, and loss to follow-up across selected studies.
Discussion
This review aimed to systematically explore the association between major environmental pollutants and markers of ovarian reserve as proxies of female fertility. After retrieving 15 original reports from the literature screening process, we found a cluster of exposure items reporting on PM1, PM2.5, PM10, NO2, O3, SO2, CO, PAHs, 1,3-butadiene, benzene, diesel PM, formaldehyde, methylene chloride, and tetrachloroethylene as environmental pollutants in relation to ovarian reserve markers as AMH, AFC, and indices of poor or reduced ovarian reserve. The main findings showed a higher body of evidence for the environmental pollutants PM2.5, PM10, and NO2, while a low body of evidence for PM1, O3, SO3, and a very low body of evidence for benzene, formaldehyde, and benzo(a)pyrene, yet consistently showing significant inverse association data.
Although the mechanisms underlying the adverse health effects of exposure to air pollution have not yet been established, inflammation and oxidative stress have been suggested to be the key pathways. Indeed, folliculogenesis has been described to be impaired by increased oxidative stress and cell apoptosis induced by ambient polluted air (43).
For the inflammatory pathway, it has been reported that PM2.5 exposure can support the enhancement of inflammatory fluid markers, as indicated by changes in IL-6 and TNF-levels (44), as well as morphological changes in ovarian tissue, such as mitochondrial structural changes, vascular congestion, and hemorrhage, triggered by the inflammation itself (39). Therefore, this response might result in ovarian damage and reduced fertility. Along these lines, findings on animal models showed that IL-6 and TNF-α concentrations and the number of apoptotic cells were increased in ovarian tissue and histological structures of the ovary showing signs of hemorrhage and vascular congestion in mice exposed to PM2.5 compared with the control group (22).
For oxidative stress, reactive oxygen species (ROS) and mitochondrial DNA (mtDNA) have been shown to impact cellular aging in the human body, including in the female reproductive tract (45). Some studies have suggested that an excess of ROS May hurt ovarian aging. ROS are highly reactive oxygen-containing compounds, such as superoxide anions, hydrogen peroxide, and hydroxyl radicals. ROS are formed endogenously by oxygen metabolism during cellular processes. Usually, cells can eliminate excess ROS; however, when produced in excess, these compounds cause oxidative stress and cellular damage. High concentrations of ROS in cells lead to mitochondrial and nuclear DNA damage and apoptosis. These types of damage have been shown to affect ovarian follicle development and ovulation negatively.
Further explaining the findings, three other paths have been described by the scientific community so far, which include vitamin D3 metabolism, vitamin A metabolism, and bile acid biosynthesis. Indeed, vitamins D and A have long been implicated in human reproduction. Vitamin D signaling is directly involved in the expression of AMH, which is produced by ovarian granulosa cells and is known for its role in regulating follicular recruitment and selection. Therefore, vitamin D deficiency in females May contribute to impaired ovarian physiology through altered AMH signaling (46). Given that enzymes known to be involved in retinoid synthesis are found in the ovary, it is plausible that vitamin A deficiency May lead to deterioration in oocyte quality. Emerging evidence also suggests that air pollution May directly (through reduced ultraviolet B [UVB] exposure) and indirectly (through reduced time spent outdoors) decrease skin production of vitamin D3 (47) and reduce levels of the vitamin A precursor, β-carotene (a potent antioxidant), in the body (48).
Last, as little evidence has linked exposure to PAHs to ovarian reserve, it is useful to point out that PAHs are ubiquitous environmental pollutants worldwide and generated mainly during incomplete combustion of organic materials, including anthropogenic combustion sources (vehicle emissions, cigarette smoke, waste incineration, and so on) and natural combustion sources (volcanic activities, forest fires, etc.) (49, 50). Inhalation, ingestion, and skin contact are the main routes of exposure to PAHs. BaP, as the most carcinogenic PAH congener, has been described to retard follicular development in the ovary and decrease follicle viability, probably through activation of Aryl hydrocarbon Receptor (AhR) signaling (51). Here, reports on animal models also found that exposure to traffic-related air pollution correlated with a reduction in the number of antral follicles (23, 52, 53), yet further human research is needed to fill the gap.
Limitations
First, the ascertainment of exposure was heterogeneous among the studies. Most studies assessed air quality using a specific air monitoring station, while others estimated exposure based on proximity to the potential source. In addition, the reference levels of each pollutant could vary between studies. These factors, together with the small number of articles found, make a quantitative approach to this problem difficult.
Conclusion
Increased exposure to air pollutants might be associated with reduced female ovarian reserve, and while the evidence is more substantial for pollutants such as PM2.5, PM10, and NO2, more evidence is needed to allow conclusions about causality to be drawn. In light of these findings, global action is required for all significant modern pollutants. Global efforts May act synergistically with other international environmental policy programs to avoid any of the risks associated with this topic, such as birth rate cuts or the use of assisted reproductive technologies. A rapid and large-scale transition from all fossil fuels to clean, renewable energy is a win-win strategy to prevent pollution while mitigating climate change, thereby achieving a double benefit for the planet’s health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RZ: Conceptualization, Writing – original draft, Writing – review & editing. FC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Writing – review & editing. TN: Project administration, Supervision, Validation, Visualization, Writing – review & editing. LL: Investigation, Writing – review & editing. IB: Project administration, Supervision, Validation, Writing – review & editing. FM: Supervision, Validation, Visualization, Writing – review & editing. GC: Supervision, Validation, Visualization, Writing – review & editing. AG: Software, Supervision, Validation, Writing – review & editing. OG: Data curation, Formal analysis, Project administration, Writing – original draft, Writing – review & editing. RS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project receives funding and support from MISTRAL—a toolkit for dynaMic health Impact analysiS to predicT disability-Related costs in the Aging population based on three case studies of steeL—industry areas in Europe through the European Union’s Horizon Europe under the grant agreement number 101095119.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1425876/full#supplementary-material
Footnotes
References
1. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49.
2. Wu, S, Zhang, Y, Wu, X, Hao, G, Ren, H, Qiu, J, et al. Association between exposure to ambient air pollutants and the outcomes of in vitro fertilization treatment: a multicenter retrospective study. Environ Int. (2021) 153:106544. doi: 10.1016/j.envint.2021.106544
3. de Bont, J, Jaganathan, S, Dahlquist, M, Persson, Å, Stafoggia, M, and Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J Intern Med. (2022) 291:779–800. doi: 10.1111/joim.13467
4. Murro, I, Lisco, G, Di Noia, C, Lampignano, L, Zupo, R, Giagulli, VA, et al. Endocrine disruptors and obesity: An overview. Endocr Metab Immune Disord Drug Targets. (2022) 22:798–806. doi: 10.2174/1871530322666220328122300
5. Guan, W-J, Zheng, X-Y, Chung, KF, and Zhong, N-S. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. (2016) 388:1939–51. doi: 10.1016/S0140-6736(16)31597-5
6. Gordon, SB, Bruce, NG, Grigg, J, Hibberd, PL, Kurmi, OP, Lam, K-BH, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet. Respir Med. (2014) 2:823–60. doi: 10.1016/S2213-2600(14)70168-7
7. Mousavi, SE, Delgado-Saborit, JM, Adivi, A, Pauwels, S, and Godderis, L. Air pollution and endocrine disruptors induce human microbiome imbalances: a systematic review of recent evidence and possible biological mechanisms. Sci Total Environ. (2022) 816:151654. doi: 10.1016/j.scitotenv.2021.151654
8. Veras, MM, Caldini, EG, Dolhnikoff, M, and Saldiva, PHN. Air pollution and effects on reproductive-system functions globally with particular emphasis on the Brazilian population. J Toxicol Environ Health B Crit Rev. (2010) 13:1–15. doi: 10.1080/10937401003673800
9. Li, T, Zhang, Y, Wang, J, Xu, D, Yin, Z, Chen, H, et al. All-cause mortality risk associated with long-term exposure to ambient PM in China: a cohort study. Lancet Public Health. (2018) 3:e470–7. doi: 10.1016/S2468-2667(18)30144-0
10. Chen, J, and Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int. (2020) 143:105974. doi: 10.1016/j.envint.2020.105974
11. Chu, C, Zhu, Y, Liu, C, Chen, R, Yan, Y, Ren, Y, et al. Ambient fine particulate matter air pollution and the risk of preterm birth: a multicenter birth cohort study in China. Environ Pollut. (2021) 287:117629. doi: 10.1016/j.envpol.2021.117629
12. Li, Q, Zheng, D, Wang, Y, Li, R, Wu, H, Xu, S, et al. Association between exposure to airborne particulate matter less than 2.5 μm and human fecundity in China. Environ Int. (2021) 146:106231
13. Xue, T, Zhu, T, Geng, G, and Zhang, Q. Association between pregnancy loss and ambient PM using survey data in Africa: a longitudinal case–control study, 1998-2016. Lancet Planet Health. (2019) 3:e219–ee225. doi: 10.1016/S2542-5196(19)30047-6
14. Zhang, Y, Wang, J, Chen, L, Yang, H, Zhang, B, Wang, Q, et al. Ambient PM and clinically recognized early pregnancy loss: a case–control study with spatiotemporal exposure predictions. Environ Int. (2019) 126:422–9. doi: 10.1016/j.envint.2019.02.062
15. Donnez, J, and Dolmans, M-M. Fertility preservation in women. N Engl J Med. (2017) 377:1657–65. doi: 10.1056/NEJMra1614676
16. McGee, EA, and Hsueh, AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. (2000) 21:200–14. doi: 10.1210/edrv.21.2.0394
17. Broekmans, FJ, Kwee, J, Hendriks, DJ, Mol, BW, and Lambalk, CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. (2006) 12:685–718. doi: 10.1093/humupd/dml034
18. Sharara, FI, Scott, RT Jr, and Seifer, DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol. (1998) 179:804–12. doi: 10.1016/S0002-9378(98)70087-0
19. Fanchin, R, Schonäuer, LM, Righini, C, Guibourdenche, J, Frydman, R, and Taieb, J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. (2003) 18:323–7. doi: 10.1093/humrep/deg042
20. Kaya, C, Pabuccu, R, and Satıroglu, H. Serum anti-Müllerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle are predictive of the fertilization, implantation, and pregnancy in polycystic ovary syndrome patients undergoing assisted reproduction. Fertil Steril. (2010) 94:2202–7. doi: 10.1016/j.fertnstert.2009.12.002
21. Tsepelidis, S, Devreker, F, Demeestere, I, Flahaut, A, Gervy, C, and Englert, Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. (2007) 22:1837–40. doi: 10.1093/humrep/dem101
22. Gai, H-F, An, J-X, Qian, X-Y, Wei, Y-J, Williams, JP, and Gao, G-L. Ovarian damages produced by aerosolized fine particulate matter (PM) pollution in mice: possible protective medications and mechanisms. Chin Med J. (2017) 130:1400–10. doi: 10.4103/0366-6999.207472
23. Ogliari, KS, Lichtenfels, AJ, MRR, DM, Ferreira, AT, Dolhnikoff, M, and PHN, S. Intrauterine exposure to diesel exhaust diminishes adult ovarian reserve. Fertil Steril. (2013) 99:1681–1688.e2. doi: 10.1016/j.fertnstert.2013.01.103
24. Belur, J, Tompson, L, Thornton, A, and Simon, M. Interrater reliability in systematic review methodology: exploring variation in coder decision-making. Sociol Methods Res. (2021) 50:837–65. doi: 10.1177/0049124118799372
25. Koren-Hakim, T, Gumieiro, DN, and Drevet, S. Quality of the selected observational study was assessed using the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies. Criteria1. Was the research question or objective in this paper clearly stated? Criteria 2. Was the study population clearly specified and defined? Criteria 3. Was the participation rate of eligible persons at least 50%? Criteria 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion. Available at: https://pdfs.semanticscholar.org/dd03/e83e07f3ac68d55542dcd5cfcb6c84a02ea0.pdf (Accessed June 25, 2024).
26. Schwingshackl, L, Schünemann, HJ, and Meerpohl, JJ. Improving the trustworthiness of findings from nutrition evidence syntheses: assessing risk of bias and rating the certainty of evidence. Eur J Nutr. (2020) 60:2893–903. doi: 10.1007/s00394-020-02464-1
27. Piggott, T, Morgan, RL, Cuello-Garcia, CA, Santesso, N, Mustafa, RA, Meerpohl, JJ, et al. Grading of recommendations assessment, development, and evaluations (GRADE) notes: extremely serious, GRADE’s terminology for rating down by three levels. J Clin Epidemiol. (2020) 120:116–20. doi: 10.1016/j.jclinepi.2019.11.019
28. Pang, L, Yu, W, Lv, J, Dou, Y, Zhao, H, Li, S, et al. Air pollution exposure and ovarian reserve impairment in Shandong province, China: the effects of particulate matter size and exposure window. Environ Res. (2023) 218:115056. doi: 10.1016/j.envres.2022.115056
29. Li, H, Hart, JE, Mahalingaiah, S, Nethery, RC, James, P, Bertone-Johnson, E, et al. Environmental exposures and anti-Müllerian hormone: a mixture analysis in the nurses’ health study II. Epidemiology. (2023) 34:150–61. doi: 10.1097/EDE.0000000000001547
30. Wu, S, Hao, G, Zhang, Y, Chen, X, Ren, H, Fan, Y, et al. Poor ovarian response is associated with air pollutants: a multicentre study in China. EBioMedicine. (2022) 81:104084. doi: 10.1016/j.ebiom.2022.104084
31. Gregoire, AM, Upson, K, Niehoff, NM, Chin, HB, Kaufman, JD, Weinberg, CR, et al. Outdoor air pollution and anti-Müllerian hormone concentrations in the sister study. Environ Epidemiol. (2021) 5:e163. doi: 10.1097/EE9.0000000000000163
32. Kim, H, Choe, S-A, Kim, O-J, Kim, S-Y, Kim, S, Im, C, et al. Outdoor air pollution and diminished ovarian reserve among infertile Korean women. Environ Health Prev Med. (2021) 26:20. doi: 10.1186/s12199-021-00942-4
33. Hood, RB, James, P, Fong, KC, Mínguez-Alarcón, L, Coull, BA, Schwartz, J, et al. The influence of fine particulate matter on the association between residential greenness and ovarian reserve. Environ Res. (2021) 197:111162. doi: 10.1016/j.envres.2021.111162
34. Feng, X, Luo, J, Wang, X, Xie, W, Jiao, J, Wu, X, et al. Association of exposure to ambient air pollution with ovarian reserve among women in Shanxi province of North China. Environ Pollut. (2021) 278:116868. doi: 10.1016/j.envpol.2021.116868
35. Abareshi, F, Sharifi, Z, Hekmatshoar, R, Fallahi, M, Lari Najafi, M, Ahmadi Asour, A, et al. Association of exposure to air pollution and green space with ovarian reserve hormones levels. Environ Res. (2020) 184:109342. doi: 10.1016/j.envres.2020.109342
36. La Marca, A, Spaggiari, G, Domenici, D, Grassi, R, Casonati, A, Baraldi, E, et al. Elevated levels of nitrous dioxide are associated with lower AMH levels: a real-world analysis. Hum Reprod. (2020) 35:2589–97. doi: 10.1093/humrep/deaa214
37. Ye, X, Pan, W, Li, C, Ma, X, Yin, S, Zhou, J, et al. Exposure to polycyclic aromatic hydrocarbons and risk for premature ovarian failure and reproductive hormones imbalance. J Environ Sci. (2020) 91:1–9. doi: 10.1016/j.jes.2019.12.015
38. Quraishi, SM, Lin, PC, Richter, KS, Hinckley, MD, Yee, B, Neal-Perry, G, et al. Ambient air pollution exposure and Fecundability in women undergoing in vitro fertilization. Environ Epidemiol. (2019) 3:e036. doi: 10.1097/EE9.0000000000000036
39. Gaskins, AJ, Mínguez-Alarcón, L, Fong, KC, Abdelmessih, S, Coull, BA, Chavarro, JE, et al. Exposure to fine particulate matter and ovarian reserve among women from a fertility clinic. Epidemiology. (2019) 30:486–91. doi: 10.1097/EDE.0000000000001029
40. Liu, S, Liu, L, Ye, X, Fu, M, Wang, W, Zi, Y, et al. Ambient ozone and ovarian reserve in Chinese women of reproductive age: identifying susceptible exposure windows. J Hazard Mater. (2024) 461:132579. doi: 10.1016/j.jhazmat.2023.132579
41. Liu, S, Zhao, J, Ye, X, Fu, M, Zhang, K, Wang, H, et al. Fine particulate matter and its constituent on ovarian reserve: identifying susceptible windows of exposure. Sci Total Environ. (2023) 904:166744. doi: 10.1016/j.scitotenv.2023.166744
42. Wieczorek, K, Szczęsna, D, Radwan, M, Radwan, P, Polańska, K, Kilanowicz, A, et al. Exposure to air pollution and ovarian reserve parameters. Sci Rep. (2024) 14:461. doi: 10.1038/s41598-023-50753-6
43. Dechanet, C, Anahory, T, Mathieu Daude, JC, Quantin, X, Reyftmann, L, Hamamah, S, et al. Effects of cigarette smoking on reproduction. Hum Reprod Update. (2011) 17:76–95. doi: 10.1093/humupd/dmq033
44. Hajat, A, Allison, M, Diez-Roux, AV, Jenny, NS, Jorgensen, NW, Szpiro, AA, et al. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the multi-ethnic study of atherosclerosis (MESA). Epidemiology. (2015) 26:310–20. doi: 10.1097/EDE.0000000000000267
45. Rizzo, A, Roscino, MT, Binetti, F, and Sciorsci, RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. (2012) 47:344–52. doi: 10.1111/j.1439-0531.2011.01891.x
46. Luk, J, Torrealday, S, Neal Perry, G, and Pal, L. Relevance of vitamin D in reproduction. Hum Reprod. (2012) 27:3015–27. doi: 10.1093/humrep/des248
47. Mousavi, SE, Amini, H, Heydarpour, P, Amini Chermahini, F, and Godderis, L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: evidence and potential mechanisms. Environ Int. (2019) 122:67–90. doi: 10.1016/j.envint.2018.11.052
48. Bernard, N, Saintot, M, Astre, C, and Gerber, M. Personal exposure to nitrogen dioxide pollution and effect on plasma antioxidants. Arch Environ Health. (1998) 53:122–8. doi: 10.1080/00039896.1998.10545973
49. Shen, G, Wang, W, Yang, Y, Ding, J, Xue, M, Min, Y, et al. Emissions of PAHs from indoor crop residue burning in a typical rural stove: emission factors, size distributions, and gas-particle partitioning. Environ Sci Technol. (2011) 45:1206–12. doi: 10.1021/es102151w
50. Wolska, L, Mechlińska, A, Rogowska, J, and Namieśnik, J. Sources and fate of PAHs and PCBs in the marine environment. Crit Rev Environ Sci Technol. (2012) 42:1172–89. doi: 10.1080/10643389.2011.556546
51. Sadeu, JC, and Foster, WG. Effect of in vitro exposure to benzo[a]pyrene, a component of cigarette smoke, on folliculogenesis, steroidogenesis and oocyte nuclear maturation. Reprod Toxicol. (2011) 31:402–8. doi: 10.1016/j.reprotox.2010.12.006
52. Carré, J, Gatimel, N, Moreau, J, Parinaud, J, and Léandri, R. Does air pollution play a role in infertility?: a systematic review. Environ Health. (2017) 16:82. doi: 10.1186/s12940-017-0291-8
Keywords: air pollutants, fine particulate matter, ovarian reserve, fertility, systematic review
Citation: Zupo R, Castellana F, Nawrot TS, Lampignano L, Bortone I, Murgia F, Campobasso G, Gruszecka Kosowska A, Giannico OV and Sardone R (2024) Air pollutants and ovarian reserve: a systematic review of the evidence. Front. Public Health. 12:1425876. doi: 10.3389/fpubh.2024.1425876
Edited by:
Hong Lu, Peking University, ChinaReviewed by:
Octavio Jiménez-Garza, University of Guanajuato, MexicoLihua Ren, Peking University, China
Copyright © 2024 Zupo, Castellana, Nawrot, Lampignano, Bortone, Murgia, Campobasso, Gruszecka Kosowska, Giannico and Sardone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodolfo Sardone, cm9kb2xmby5zYXJkb25lQGFzbC50YXJhbnRvLml0
 Roberta Zupo
Roberta Zupo Fabio Castellana
Fabio Castellana Tim S. Nawrot
Tim S. Nawrot Luisa Lampignano
Luisa Lampignano Ilaria Bortone
Ilaria Bortone Ferdinando Murgia6
Ferdinando Murgia6 Gianluca Campobasso
Gianluca Campobasso Agnieskza Gruszecka Kosowska
Agnieskza Gruszecka Kosowska Orazio Valerio Giannico
Orazio Valerio Giannico Rodolfo Sardone
Rodolfo Sardone