- 1Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, MA, United States
- 2Harvard Medical School, Boston, MA, United States
Background: Vaccine clinical trials should strive to recruit a racially, socioeconomically, and ethnically diverse range of participants to ensure appropriate representation that matches population characteristics. Yet, full inclusion in research is often limited.
Methods: A single-center retrospective study was conducted of adults enrolled at Brigham and Women’s Hospital (Boston, MA) between July 2020 and December 2021. Demographic characteristics, including age, race, ethnicity, ZIP code, and sex assigned at birth, were analyzed from both HIV and COVID-19 vaccine trials during the study period, acknowledging the limitations to representation under these parameters. We compared the educational attainment of vaccine trial participants to residents of the Massachusetts metropolitan area, geocoded participants’ addresses to their census block group, and linked them to reported median household income levels from publicly available data for 2020. Frequency and quartile analyses were carried out, and spatial analyses were performed using ArcGIS Online web-based mapping software (Esri).
Results: A total of 1030 participants from four COVID-19 vaccine trials (n = 916 participants) and six HIV vaccine trials (n = 114 participants) were included in the analysis. The median age was 49 years (IQR 33–63) and 28 years (IQR 24–34) for the COVID-19 and HIV vaccine trials, respectively. Participants identifying as White were the majority group represented for both the COVID-19 (n = 598, 65.3%) and HIV vaccine trials (n = 83, 72.8%). Fewer than 25% of participants identified as Hispanic or Latin. Based on ZIP code of residence, the median household income for COVID-19 vaccine clinical trial participants (n = 846) was 102,088 USD (IQR = 81,442–126,094). For HIV vaccine clinical trial participants (n = 109), the median household income was 101,266 USD (IQR 75,052–108,832).
Conclusion: We described the characteristics of participants enrolled for HIV and COVID-19 vaccine trials at a single center and found similitude in geographical distribution, median incomes, and proportion of underrepresented individuals between the two types of vaccine candidate trials. Further outreach efforts are needed to ensure the inclusion of individuals from lower educational and socioeconomic brackets. In addition, continued and sustained efforts are necessary to ensure inclusion of individuals from diverse racial and ethnic backgrounds.
Background
The COVID-19 pandemic parallels the early days of the HIV epidemic, with both diseases disproportionally impacting marginalized communities (1). Both began with efforts to identify the causative pathogen but differed between the time to authorization and rollout of effective preventive vaccines (2). Emergency Use Authorization (EUA) for several vaccines against COVID-19 was granted by the US Food and Drug Administration (FDA) less than 1 year after identifying the virus, while for HIV, there are no approved vaccines despite global efforts over the past four decades. Even with the striking impact of COVID-19 on individuals with stark health disparities, including those from communities of color and those that experience multiple forms of social disadvantage, these individuals were not initially well represented in the COVID-19 vaccine trials (3, 4). When sponsors presented the preliminary enrollment reports, Black people comprised only 5% of clinical trial participants despite representing 13% of the total US population, and Latin, 1%, even though they account for 18% of the US population (5).
In this way, the COVID-19 pandemic was reminiscent of the early days of the HIV epidemic, with poor inclusion of communities with intersectional identities in prevention and curative treatment trials and persistent failures to address these determinants of health (6). Few studies analyze sex or gender unless it is the primary focus (7), and the intersection of sex and gender with exposure to gender-affirming therapy and cultural impacts linked to gender remains poorly studied (8). Furthermore, disparities persist in including individuals from racial and ethnic backgrounds with these intersecting identities in early-phase HIV vaccine clinical trials (9).
Mindful of these findings, the National Institute of Allergy and Infectious Diseases (NIAID) quickly formed the COVID-19 Prevention Network (CoVPN), comprising the HIV Vaccine Trials Network (HVTN), the HIV Prevention Trials Network (HPTN), the AIDS Clinical Trials Group, and the Infectious Disease Clinical Research Consortium (IDCRC), to develop and assess novel COVID-19 vaccines in early 2020 (10, 11). Accelerated review of grants, contracts, and agreements was followed with a common Data and Safety Monitoring Board providing safety oversight across all US Government-funded COVID-19 vaccine trials (12). Among the goals of the CoVPN was clear accountability regarding how to maintain diversity and inclusion of participants from historically marginalized areas, with a specific focus on settings other than optimal urban settings where clinical research has traditionally occurred (13–15).
Given that our site is in an academic medical center and has been enrolling participants in HIV vaccine trials for over two decades, we evaluated our enrollment metrics to determine if the COVID-19 vaccine trials conducted at our site had similar representation as compared to contemporaneous HIV vaccine candidate trials. This study analyzed enrollment data for preventative HIV and COVID-19 vaccine trials from 2020 through 2021. Considering our history of outreach and engagement with marginalized communities for HVTN studies, we hypothesized that the CoVPN studies would have enrollment diversity analogous to HVTN studies.
Methods
Study design
This is a single-center retrospective study of healthy adults screened and enrolled at Brigham and Women’s Hospital in Boston, Massachusetts, between July 2020 and December 2021. Participants were included if they enrolled in a COVID-19 or HIV candidate vaccine trial; participants were excluded if they transferred from other clinical trial sites.
Study population
Adult participants aged 18 years or older at the time of enrolling in the vaccine clinical trials, with a valid address for linkage to population-level data, were included in this study.
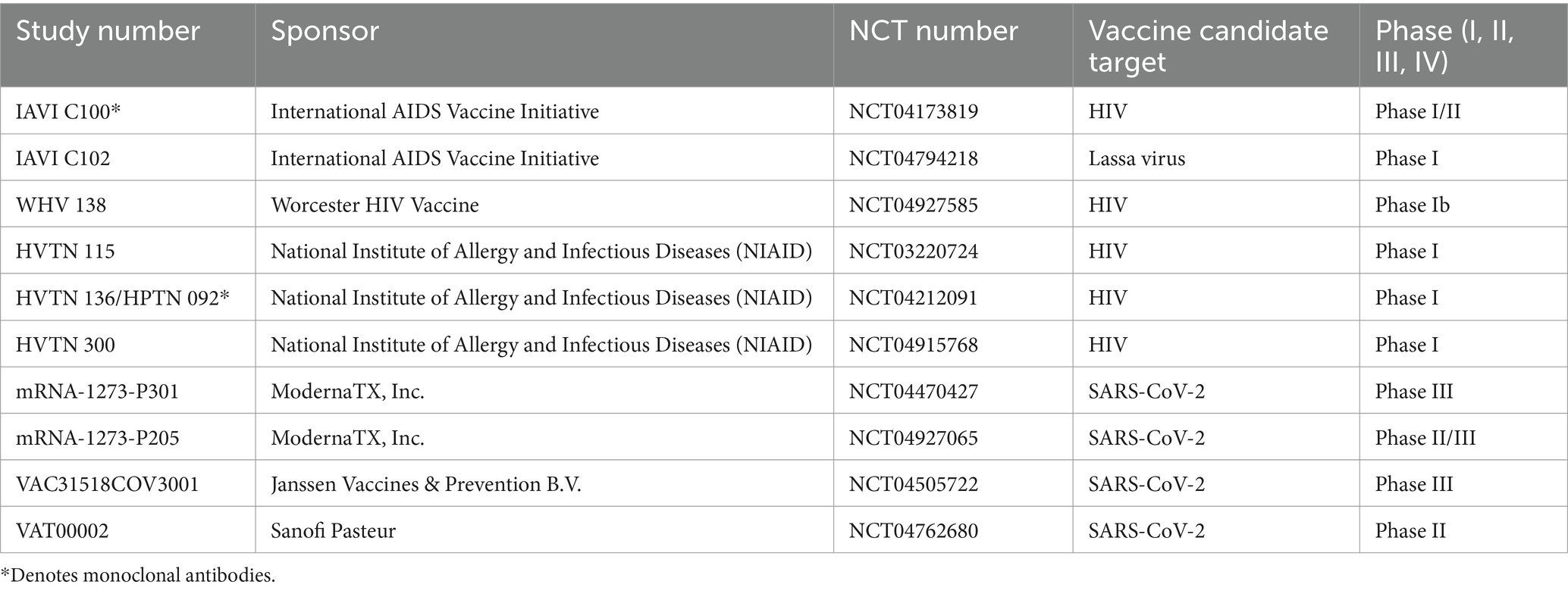
A total of 10 studies were selected to determine if we are accessing different communities through previous outreach and engagement efforts, with five in Phase I, one in Phase I/II, one in Phase II, one in Phase II/III, and two in Phase III (Table 1).
Data collection and management
Variables collected included age, race, ethnicity, ZIP code, and sex assigned at birth from case report forms (CRF) as self-reported by the participants. Sexual orientation, highest level of education, occupation, and household characteristics were extracted from CRFs when available by study. All variables were extracted and tabulated in Microsoft Excel.
Race and ethnicity categorizations were defined using the US Office of Management and Budget guidelines for federal data: American Indian or Alaskan Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, and White. Hispanic identity was either recorded as a racial category or as ethnicity (16). To reconcile differences between studies and acknowledge the diversity within the participants’ identities, we recorded all participants who did not identify as Latin or Hispanic separately in a subgroup from those of Hispanic or Latin descent. Participants who selected two races or more were recorded as “Multiracial.”
Statistical analysis
Descriptive summary statistics of participants’ demographic characteristics were calculated. Vaccine trial participants were geocoded to their census block group by linkage to their residential address. Incomplete and out-of-state addresses were excluded. Address data were obtained from metropolitan areas in Massachusetts exclusively and matched to the reported median household income level from the 2020 American Community Survey (ACS) 5-Year Estimates to account for demographic changes over time and because they are based on larger sample size, and thus more reliable, than the 1- and 3-year ACS estimates (17). These were then analyzed by frequency and quartile. Spatial analyses were performed using ArcGIS Online (https://www.arcgis.com/index.html, accessed 10 July, 2022).
Results
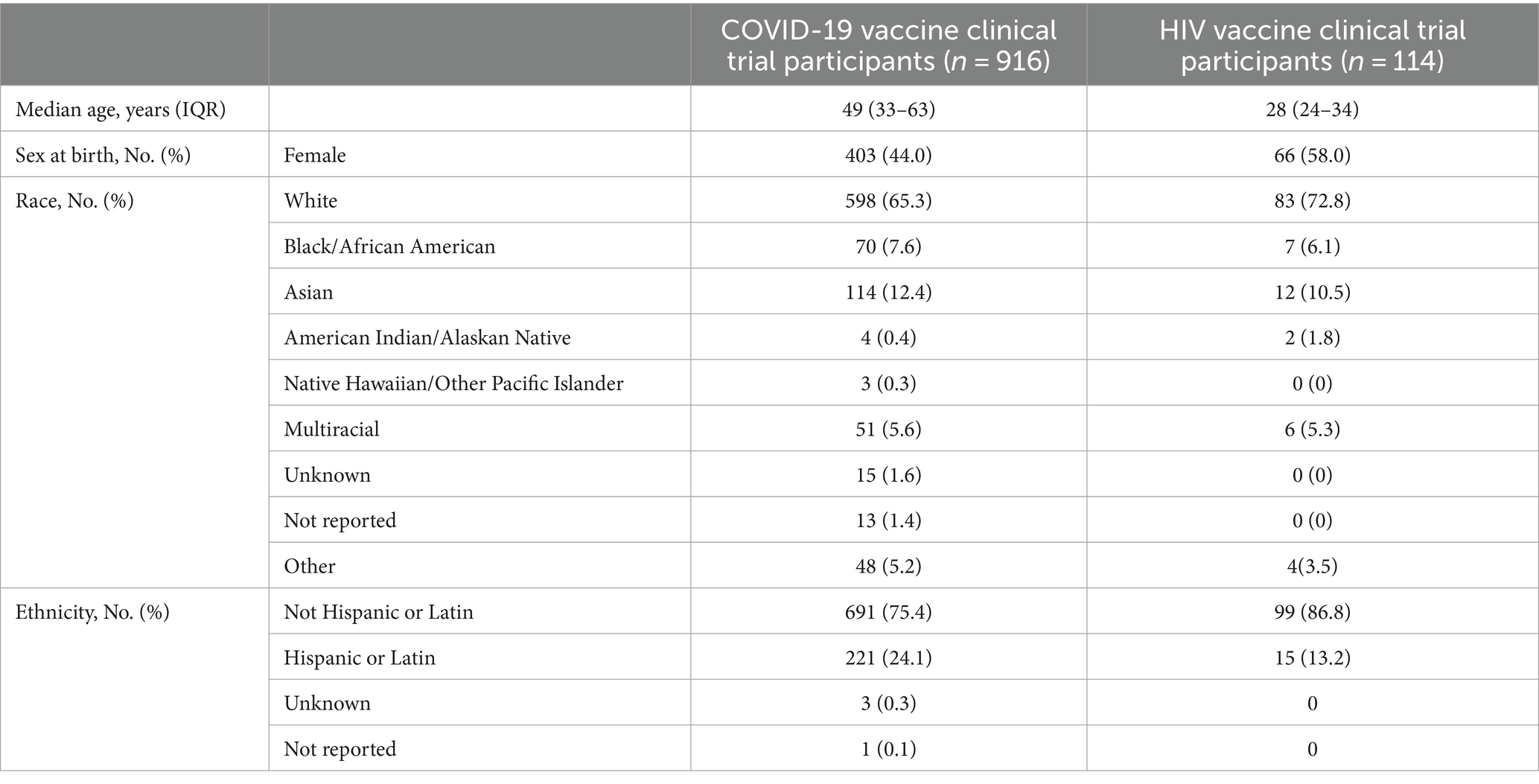
For the study period, four COVID-19 vaccine clinical trials (n = 916 total participants) and six HIV vaccine trials (n = 114 total participants) were included (Figure 1). In the COVID-19 vaccine clinical studies, the age of participants ranged from 18 to 92 years [median = 49; interquartile range (IQR) = 33–63 years; Table 2]. About 44% of participants identified as female. Most identified as White (65.3%), and those identifying as Black/African American (7.6%) were present at slightly higher numbers than Multiracial (5.6%). Participants identifying as Hispanic compromised 24.1% of all participants.

Figure 1. Consort diagram describing the process of how many participants in the vaccine clinical trials were eligible for inclusion.
The candidate HIV vaccine trial participants’ demographic characteristics are summarized in Table 2. Studies selected with kindred eligibility, follow-up, and operational features were Worcester HIV Vaccine (WHV), International AIDS Vaccine Initiative (IAVI), and HVTN studies, and two, namely IAVI C100 and HVTN 136/HPTN 092, were monoclonal antibody trials. The age of participants ranged from 18 to 50 years (median = 28; IQR = 24–34 years). A higher percentage of participants identified as female (58%), White (72.8%), and Not Hispanic (86.8%) compared to the COVID-19 vaccine clinical studies. Black/African American-identifying participants and Multiracial participants were represented at a similar frequency.
Other covariate data collected during screening and enrollment for some HIV vaccine studies were educational attainment and sexual orientation (Table 3). Out of 79 participants, only five (6.3%) had not accessed higher education. About 77% attained an undergraduate degree; 38% had at least some graduate schooling. In contrast, according to the ACS, of people living in Massachusetts, approximately 4.2% possessed middle school graduation as their highest educational attainment, 28.2% possessed some high school or high school graduation, 15.3% some college with no degree, 7.7% an associate degree, 24.5% a bachelor’s degree, and 20% a graduate or professional degree. Regarding sexual orientation in our studies, 57% identified as part of the lesbian, gay, bisexual, transgender, queer, and more (LGBTQ+) community.

Table 3. Educational attainment and sexual orientation for HIV vaccine studies conducted at Brigham and Women’s Hospital.
Subsequently, we mapped participants’ ZIP codes of residence to visually show the geographical range of participants and the density of participants living within commuting distance of our center (Figures 2, 3). Figure 2 corresponds to the COVID-19 vaccine clinical trials participants, and Figure 3 corresponds to the HIV vaccine clinical trial participants. Participants for the COVID-19 vaccine trials represented a wider geographical spread as compared to participants in the HIV vaccine trials, although there were also more participants in the COVID-19 vaccine trials.

Figure 2. Geographical distribution for COVID-19 vaccine clinical trial participants and associated median household income.

Figure 3. Geographical distribution for HIV vaccine clinical trial participants and associated median household income.
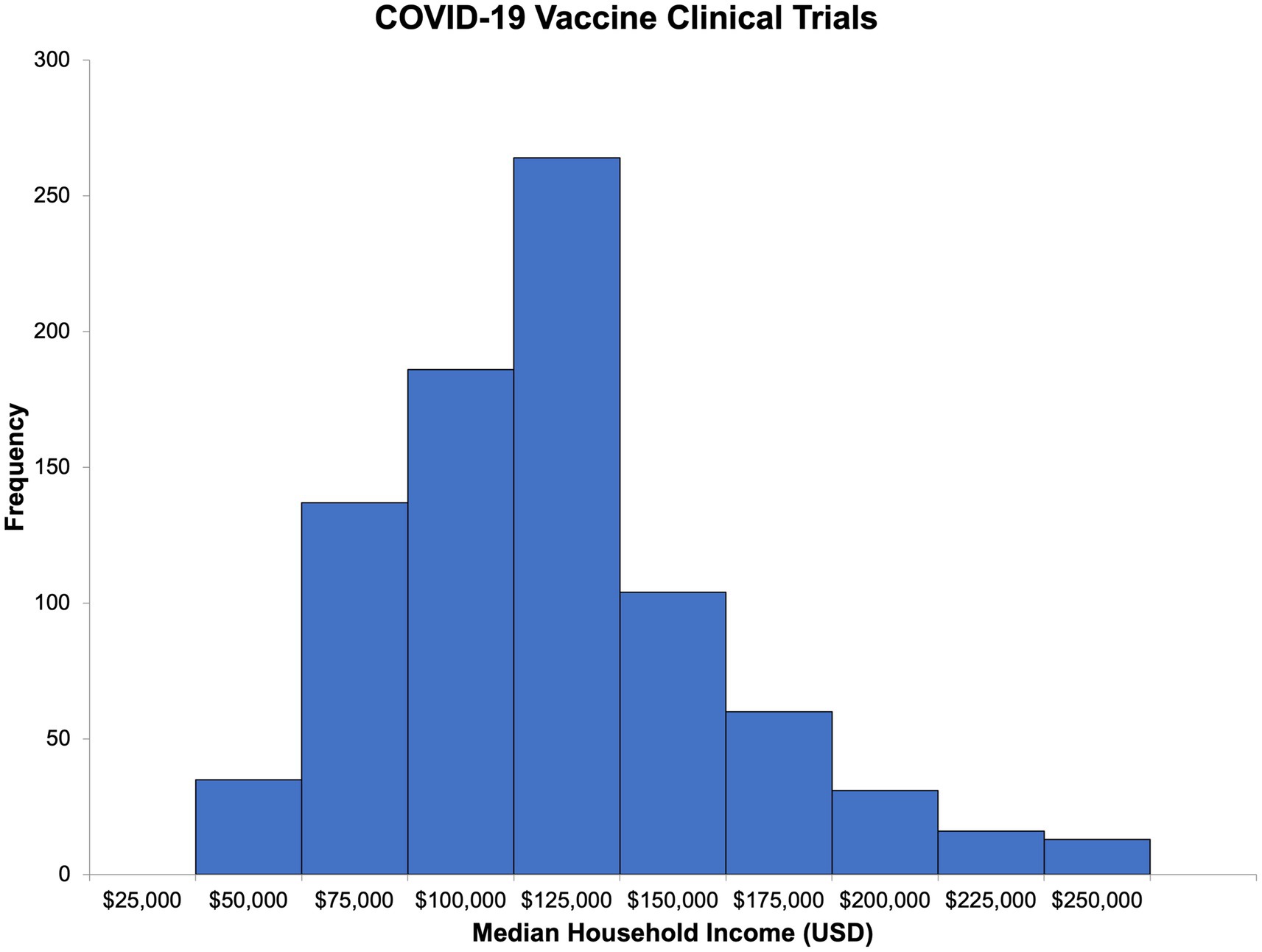
Using publicly available census-level ZIP code income data for Massachusetts residents, we were able to estimate the median household income and its distribution for participants in both types of trials (Figures 4, 5). The median household income for the ZIP code reported by COVID-19 vaccine clinical trial participants (n = 846) was 102,088 USD (IQR = 81,442–126,094, range 25,077–250,000; Supplementary Table S1). For HIV vaccine clinical trial participants (n = 109), the median household income of the reported ZIP code was 101,266 USD (IQR = 75,052–108,832, range 25,077–250,000 USD; Supplementary Table S2). Comparatively, in 2020, the median household income for Massachusetts residents was 84,385 USD, slightly lower than that of HIV and COVID-19 vaccine clinical trial participants, and interestingly, the same ZIP code, 02130, was the most commonly reported among participants for both.
Discussion
Our study characterizes demographics of participants enrolled at our site in COVID-19 and HIV candidate vaccine trials during the peak of the COVID-19 pandemic, accentuating the enrollment characteristics of racial/ethnic minorities, females, and older individuals in vaccine clinical trials. The use of demographic characteristics to assess the success of community engagement practices on enrollment revealed that the local communities that participated in COVID-19 and HIV vaccine clinical trials appear to be only minimally different.
We found that the HIV vaccine trials enrolled a high percentage of individuals from the LGBTQ+ community. HIV itself may be selectively motivating, and the HVTN and our clinical site has longstanding dedicated initiatives and outreach to these communities, which likely influenced the success of recruiting a sexual orientation and gender-diverse community at risk. Consistent with these findings, other research has indicated that sexual minority people are more likely to utilize some health services, such as those focused on HIV prevention, than heterosexual people (18).
Of great concern, however, is the lower research participation among Black and Latin sexual minority men (18, 19). Startlingly, among many racial/ethnic minorities, awareness of trials is a significant barrier to clinical trial participation, with many reporting they are simply not asked to participate (20), and longstanding bias compounded by providers’ perception of underrepresented populations’ reluctance can lower the likelihood that a provider offer trials as a treatment option (21). Other studies have shown that undocumented immigrants often experience additional immigration-related barriers and low healthcare utilization that may limit trial participation, and immigration status measured inconsistently through the use of proxy measures (22, 23).
The findings from this study align with broader literature that has demonstrated underrepresentation in clinical research is associated with low levels of education and inadequacy in health literacy. Moreover, our study identified a disproportionate enrollment compared to the Massachusetts average with respect to socioeconomic status. Similar to outcomes reported in other scholarly work, we found that individuals who had higher levels of education (some college/completed college/graduate school) were more likely to have actively participated in a clinical trial (24).
With 7.2% of the total participants commuting from Jamaica Plain, a neighborhood of Boston in Suffolk County, Massachusetts, adjacent to our academic medical center, commuting distance may have facilitated their enrollment. Surprisingly, Jamaica Plain’s corresponding ZIP code had a median household income of 102,088 USD in 2020, the same as the median household income for COVID-19 vaccine clinical trial participants. Overall, the estimated median household income was higher for the ZIP codes reported by COVID-19 vaccine clinical trial participants, which may be due to the higher age range of the participants. In the HIV vaccine studies, volunteers could only be enrolled within the age range of 18–50 years rather than 18 years and older, as in the COVID-19 vaccine studies, suggesting this finding was due to eligibility criteria and study design, not failure in our recruitment efforts.
Albeit individual-level income, a known social determinant of health, was not collected in our studies, using ZIP code-level median household income as a surrogate to approximate socioeconomic status can aid in identifying inequities in access, socioeconomic barriers, implicit bias, and outcomes when no direct income data is available (25). In future studies, we propose that income ranges could be placed in intake forms at the time of enrollment to allow a more detailed analysis of socioeconomic status in recruitment/retention and maps overlaying high deprivation index and high diversity areas with existing hospitals, existing major HIV trial centers, and commuting distance to the closest HIV trial center to identify biases in the sociodemographic of populations living within commuting distance.
Apart from providing a detailed reference for our single center, we hope our work will encourage further collaborative efforts with nearby hospitals, community clinics, and community centers closer to underrepresented populations to lessen the socioeconomic burdens of clinical trial participation. Rather than allowing rapid digitization without considering community engagement processes, resulting in deepening health inequities, implementing mobile data collection, online recruitment, and strategic approaches for those traditionally hard-to-reach can more broadly engage these communities (26–28). After all, providing community benefits will strengthen community capacity and agency.
Our data suggest a need to address not only the difficulties that come with categorized race reporting but the urgent need to harmonize data collection methods. Inferring racial makeup using definitions that do not capture the increasing diversity of the US population calls for updated definitions, as there is no consensus about how to define race and ethnicity (29, 30). Shifting demographics demonstrate that non-white US populations are projected to become a majority population by 2044. Determining the optimal way to record race and ethnicity data when compiling descriptive statistics is vital in improving trials’ precision and equitable application in practice.
There are a few limitations identified in this study. First, the data available were limited due to the retrospective design and, therefore, relied on data captured within the participants’ clinical trial documentation and electronic health records. Another concern is that self-reported race/ethnicity was inconsistent across trials, particularly for Hispanic and Multiracial populations, and thus, estimates of Hispanic enrollment may be inaccurate. Third, individual-level data concerning socioeconomic status are absent; thus, ZIP codes and associated median incomes are an approximation. We attempted to account for this with the use of aggregate-level data. Finally, this study was conducted at a single academic medical center in an urban setting. Therefore, the generalizability of the findings may be limited, and future research is warranted within non-urban settings and across multiple centers.
Conclusion
In this retrospective study, we describe the characteristics of participants enrolled for HIV and COVID-19 vaccine trials at a single center in Boston, Massachusetts. We describe similarities in geographical distribution, median incomes, and proportion of underrepresented individuals between the two types of trials. We found that the HIV vaccine trials successfully recruited individuals from sexual orientation and gender-diverse backgrounds. Our findings demonstrate that further outreach efforts are needed to ensure the inclusion of individuals from lower educational and socioeconomic brackets. In addition, continued and sustained efforts are needed to ensure inclusion of individuals from diverse racial and ethnic backgrounds. We illustrate the importance, benefits, and contribution of sustained community relationships to provide direction and evidence-based support for the inclusion of historically marginalized subgroups in clinical trials of vaccines for COVID-19, HIV, and other emerging pathogens.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Confidential data that includes potentially identifiable data. Requests to access these datasets should be directed to YWNzaGVybWFuQGJ3aC5oYXJ2YXJkLmVkdQ==.
Ethics statement
The studies involving humans were approved by Brigham and Women’s Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because IRB determined informed consent was not required since there was no intervention or interaction performed. This was a retrospective review only.
Author contributions
DL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. EK: Data curation, Resources, Writing – review & editing. OO: Data curation, Writing – review & editing. JP: Data curation, Methodology, Writing – review & editing. UK: Data curation, Investigation, Writing – review & editing. NM: Data curation, Investigation, Writing – review & editing. AA: Data curation, Investigation, Writing – review & editing. AT: Data curation, Investigation, Writing – review & editing. LB: Resources, Supervision, Writing – review & editing. AS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Writing – review & editing. SW: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. LB has research support from the NIH (including the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, Grant 1UL1TR002541-01), the Wellcome Trust, and the Bill & Melinda Gates Foundation.
Acknowledgments
We would like to thank the participants who have enrolled in the vaccine clinical trials and provided their time and effort to advance scientific discovery.
Conflict of interest
LB, SW, and AS are involved in HIV, COVID, and other vaccine clinical trials conducted in collaboration with the NIH, HIV Vaccine Trials Network, COVID Vaccine Prevention Network, International AIDS Vaccine Initiative, Crucell/Janssen, Moderna, Military HIV Research Program, the Bill & Melinda Gates Foundation, and the Ragon Institute. SW has received grant and research support from Johnson & Johnson, Moderna, Pfizer, Sanofi Pasteur, VIR, and Worcester HIV Vaccine. AS has received grant and research support from Merck.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1411970/full#supplementary-material
References
1. Owen, WF, Carmona, R, and Pomeroy, C. Failing another National Stress Test on health disparities. JAMA. (2020) 323:1905–6. doi: 10.1001/jama.2020.6547
2. Vasan, S, and Pitisuttithum, P. Vaccine development lessons between HIV and COVID-19. Lancet Infect Dis. (2021) 21:759–61. doi: 10.1016/S1473-3099(21)00274-7
3. Turner, BE, Steinberg, JR, Weeks, BT, Rodriguez, F, and Cullen, MR. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health Am. (2022) 11:100252. doi: 10.1016/j.lana.2022.100252
4. Nephew, LD. Accountability in clinical trial diversity: the buck stops where? EClinicalMedicine. (2021) 36:100906. doi: 10.1016/j.eclinm.2021.100906
5. Varma, S, Vora, K, Fox, K, Berkhout, S, and Benmarhnia, T. Why calls to diversify trial populations fall short. Med. (2021) 2:25–8. doi: 10.1016/j.medj.2020.12.012
6. Andrasik, M, Broder, G, Oseso, L, Wallace, S, Rentas, F, and Corey, L. Stigma, implicit Bias, and Long-lasting prevention interventions to end the domestic HIV/AIDS epidemic. Am J Public Health. (2020) 110:67–8. doi: 10.2105/AJPH.2019.305454
7. Scully, EP. Sex, gender and infectious disease. Nat Microbiol. (2022) 7:359–60. doi: 10.1038/s41564-022-01064-5
8. Mauvais-Jarvis, F, Bairey Merz, N, Barnes, PJ, Brinton, RD, Carrero, JJ, DeMeo, DL, et al. Sex and gender: modifiers of health, disease, and medicine [published correction appears in lancet. 2020;396(10252):668]. Lancet. (2020) 396:565–82. doi: 10.1016/S0140-6736(20)31561-0
9. Huamani, KF, Metch, B, Broder, G, and Andrasik, M. A demographic analysis of racial/ethnic minority enrollment into HVTN preventive early phase HIV vaccine clinical trials conducted in the United States, 2002-2016. Public Health Rep. (2019) 134:72–80. doi: 10.1177/0033354918814260
10. Calder, T, Tong, T, Hu, DJ, Kim, JH, Kotloff, KL, Koup, RA, et al. Leveraging lessons learned from the COVID-19 pandemic for HIV. Commun Med (Lond). (2022) 2:110. doi: 10.1038/s43856-022-00175-8
11. Mena Lora, AJ, Long, JE, Huang, Y, Baden, LR, el Sahly, HM, Follmann, D, et al. Rapid development of an integrated network infrastructure to conduct phase 3 COVID-19 vaccine trials. JAMA Netw Open. (2023) 6:e2251974. doi: 10.1001/jamanetworkopen.2022.51974
12. Corey, L. Behind the scenes heroes: the COVID-19 vaccine data and safety monitoring board. J Infect Dis. (2021) 224:1993–4. doi: 10.1093/infdis/jiab267
13. Khalil, L, Leary, M, Rouphael, N, Ofotokun, I, Rebolledo, PA, and Wiley, Z. Racial and ethnic diversity in SARS-CoV-2 vaccine clinical trials conducted in the United States. Vaccines (Basel). (2022) 10:290. doi: 10.3390/vaccines10020290
14. Knepper, TC, and McLeod, HL. When will clinical trials finally reflect diversity? Nature. (2018) 557:157–9. doi: 10.1038/d41586-018-05049-5
15. Flores, LE, Frontera, WR, Andrasik, MP, del Rio, C, Mondríguez-González, A, Price, SA, et al. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Netw Open. (2021) 4:e2037640. doi: 10.1001/jamanetworkopen.2020.37640
16. National Institutes of Health; US Office of budget and management. Revisions to the standards for the classification of Federal Data on race and ethnicity. (2020). Available at: https://orwh.od.nih.gov/toolkit/other-relevant-federal-policies/OMB-standards (Accessed June 2022).
17. US Census Bureau. 5-year estimates subject tables: Survey program: American community survey. (2020). Available at: https://data.census.gov/table?q=median+household+income+massachusetts&g=040XX00US25,25$8600000&tid=ACSST5Y2020.S1901 (Accessed July 2022).
18. Raifman, J, Dean, LT, Montgomery, MC, Almonte, A, Arrington-Sanders, R, Stein, MD, et al. Racial and ethnic disparities in HIV pre-exposure prophylaxis awareness among men who have sex with men. AIDS Behav. (2019) 23:2706–9. doi: 10.1007/s10461-019-02462-3
19. Fields, EL, Hussen, SA, and Malebranche, DJ. Mind the gap: HIV prevention among young black men who have sex with men. Curr HIV/AIDS Rep. (2020) 17:632–42. doi: 10.1007/s11904-020-00532-z
20. Bass, SB, D'Avanzo, P, Alhajji, M, Ventriglia, N, Trainor, A, Maurer, L, et al. Exploring the engagement of racial and ethnic minorities in HIV treatment and vaccine clinical trials: a scoping review of literature and implications for future research. AIDS Patient Care STDs. (2020) 34:399–416. doi: 10.1089/apc.2020.0008
21. FitzGerald, C, and Hurst, S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. (2017) 18:19. doi: 10.1186/s12910-017-0179-8
22. Harkness, A, Rogers, BG, Balise, R, Mayo, D, Weinstein, ER, Safren, SA, et al. Who Aren't we reaching? Young sexual minority Men's non-participation in an HIV-prevention and mental health clinical trial. AIDS Behav. (2021) 25:2195–209. doi: 10.1007/s10461-020-03148-x
23. Ornelas, IJ, Yamanis, TJ, and Ruiz, RA. The health of undocumented Latinx immigrants: what we know and future directions. Annu Rev Public Health. (2020) 41:289–308. doi: 10.1146/annurev-publhealth-040119-094211
24. Castillo-Mancilla, JR, Cohn, SE, Krishnan, S, Cespedes, M, Floris-Moore, M, Schulte, G, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials. (2014) 15:14–26. doi: 10.1310/hct1501-14
25. Berkowitz, SA, Traore, CY, Singer, DE, and Atlas, SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. (2015) 50:398–417. doi: 10.1111/1475-6773.12229
26. Tan, RKJ, Wu, D, Day, S, Zhao, Y, Larson, HJ, Sylvia, S, et al. Digital approaches to enhancing community engagement in clinical trials. NPJ Digit Med. (2022) 5:37. doi: 10.1038/s41746-022-00581-1
27. Beyrer, C, Malone, J, Baral, S, Wang, Z, Rio, CD, Mayer, KH, et al. Comparing recruitment strategies to engage hard-to-reach men who have sex with men living with HIV with unsuppressed viral loads in four US cities: results from HPTN 078. J Int AIDS Soc. (2021) 24:e25798. doi: 10.1002/jia2.25798
28. Bradford-Rogers, J, Lopez-Matos, J, Cain, D, Lopez, D, and Starks, TJ. Comparing the efficiency of online and field-based outreach for the recruitment of black and Latino sexual minority men into an HIV prevention implementation trial. Prev Sci. (2022) 23:900–6. doi: 10.1007/s11121-022-01367-3
29. Vyas, DA, Eisenstein, LG, and Jones, DS. Hidden in plain sight - reconsidering the use of race correction in clinical algorithms. N Engl J Med. (2020) 383:874–82. doi: 10.1056/NEJMms2004740
Keywords: clinical trial enrollment, diversity, representation, vaccine, HIV, SARS-CoV-2
Citation: Lezo Ramirez D, Koleske E, Ometoruwa O, Park Chang JB, Kanwal U, Morreale N, Avila Paz AA, Tong A, Baden LR, Sherman AC and Walsh SR (2024) Evaluating enrollment and representation in COVID-19 and HIV vaccine clinical trials. Front. Public Health. 12:1411970. doi: 10.3389/fpubh.2024.1411970
Edited by:
Elham Hatef, Johns Hopkins Medicine, United StatesReviewed by:
Madhura Rane, The City University of New York, United StatesJacques L. Tamuzi, Stellenbosch University, South Africa
Copyright © 2024 Lezo Ramirez, Koleske, Ometoruwa, Park Chang, Kanwal, Morreale, Avila Paz, Tong, Baden, Sherman and Walsh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy C. Sherman, YWNzaGVybWFuQGJ3aC5oYXJ2YXJkLmVkdQ==
†These authors share senior authorship
 Daisy Lezo Ramirez
Daisy Lezo Ramirez Emily Koleske
Emily Koleske Omolola Ometoruwa1
Omolola Ometoruwa1 Andres Alberto Avila Paz
Andres Alberto Avila Paz Amy C. Sherman
Amy C. Sherman Stephen R. Walsh
Stephen R. Walsh