- 1Department of Pediatrics, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 2Child Healthcare Department, Anhui Hospital Affiliated to Children’s Hospital of Fudan University/Anhui Provincial Children’s Hospital, Hefei, Anhui, China
- 3Key Laboratory of Population Health Across Life Cycle (Anhui Medical University), Ministry of Education of the People’s Republic of China, Hefei, Anhui, China
The phthalic acid esters (PAEs) are one class of the most abundant and frequently studied pseudo-persistent organic pollutants. Noninvasive urine is an effective substrate for evaluating PAE exposure, but repeated sampling is needed to overcome this bias. This adds much work to on-site collection and the cost of detection increases exponentially. Therefore, the aim of this study was to conduct a scope review to describe the detection methods and validity of the use of other noninvasive matrices, such as nails and hair, for assessing long-term exposure to PAEs. The PubMed, Web of Science and China National Knowledge Infrastructure (CNKI), electronic databases were searched from 1 January 2000 to 3 April 2024, and 12 studies were included. Nine and three studies used hair and nails, respectively, as noninvasive matrices for detecting PAE exposure. Five articles compared the results of nail or hair and urine tests for validity of the assessment of PAE exposure. The preprocessing and detection methods for these noninvasive samples are also described. The results of this review suggest that, compared with nails, hair may be more suitable as a noninvasive alternative matrix for assessing long-term exposure to PAEs. However, sample handling procedures such as the extraction and purification of compounds from hair are not uniform in various studies; therefore, further exploration and optimization of this process, and additional research evidence to evaluate its effectiveness, are needed to provide a scientific basis for the promotion and application of hair detection methods for assessing long-term PAE exposure levels.
1 Introduction
Rapid agricultural and industrial development has resulted in significant exposure to potentially harmful chemicals, including pseudo-persistent organic pollutants such as phthalic acid esters (PAEs), bisphenols and organophosphates. These pseudo-persistent environmental pollutants have a relatively short biological half-life in the human body (1–4), but widespread environmental pollution and continued human exposure make them easy to detect in human biological samples. Take the PAEs, one class of the most abundant and frequently studied pseudo-persistent organic pollutant, as an example, people including pregnant women and children are generally exposed to these substances worldwide (5). It subsequently interferes with endocrine (6, 7) and immune functions (8, 9) and has significant effects on reproduction (10), neurodevelopment (1, 11), cardiovascular health (12) and even cancer development (9).
PAEs can be classified into low-molecular weight (LMW) phthalates and high-molecular weight (HMW) phthalates based on their molecular weight (13). LMW phthalates, such as dimethyl phthalate (DMP), dibutyl phthalate (DBP), and diethyl phthalate (DEP), are commonly used in nail polish, perfume, cosmetics, and pharmaceutical coatings. HMW phthalates, such as di(2-ethylhexyl) phthalate (DEHP), diisononyl phthalate (DiNP), di(noctyl) phthalate (DOP), and diisodecyl phthalate (DiDP), are mainly used in medical equipment, toys, buildings, food packaging and so on (13, 14). PAEs enter the circulatory system of the body mainly through oral ingestion, inhalation and skin absorption (15). According to the original study of the health effects of PAEs, blood is an ideal sample for assessing exposure to chemical pollutants. However, since it is an invasive sample, complications such as hematoma and pain may occur during collection (16), and it is difficult to collect in some special populations; thus, there are certain difficulties in practical application. Therefore, noninvasive surrogate matrices are necessary for assessing PAE exposure. In addition, PAE metabolites, whose half-life is usually approximately 12 h (1), are mainly excreted through the urine shortly after exposure. Therefore, urine is the best biological substrate for the assessment of exposure to these pseudo-persistent organic pollutants (17). There is a good correlation between urinary PAE metabolite concentrations and blood PAE metabolite concentrations (18). However, new problems arise. Although single random urine collection is convenient, it can lead to exposure misclassification bias, especially when large lifestyle and physical condition changes occur, such as pregnancy. Researchers have proposed collecting urine at multiple time points to overcome misclassification bias (19). There is evidence that even at just 9 months of gestation, multiple urine samples were collected to assess exposure, but the time variability was high (19). Moreover, the duration of repeated measurements is not uniform, and different measurement intervals may affect the study results and increase the amount of on-site sampling and related testing costs. Therefore, more economical, convenient and effective noninvasive matrices for assessing long-term PAE exposure need to be explored.
It has been suggested that nails are bioindicators that reflect long-term chemical exposure. In previous studies, nails were used more to detect metallic chemicals such as lead, mercury, zinc, copper, and iron (20). Several studies have shown that nails can be used to detect PAEs, and the concentration stability of PAEs in nails is much greater than that in urine within a certain period of time (21).
Hair is also a stable matrix. It starts in hair follicles, each of which has a capillary system at the root. During the growth phase of the hair shaft, chemicals in the serum bind to the hair and migrate into the hair (22). Thus, substances in the serum would theoretically be present in hair, making it a suitable matrix for assessing chemical exposure. Currently, hair is widely used to assess human exposure to metals, drugs, dioxins, polychlorinated biphenyls, and pesticides (23, 24), but research assessing PAE exposure is rare. In animal experiments, eight metabolites of DiNP were detected in the hair of rats chronically exposed to different doses of DiNP for 30 days. The levels of eight metabolites in hair showed a dose-dependent relationship with increasing exposure level. The results suggest that hair analysis is a better tool for assessing high-dose and long-term exposure (25). However, studies on the use of hair and nails as noninvasive matrices for assessing PAE exposure in the population are rare.
Therefore, the aim of this study was to systematically and comprehensively collect relevant published studies and conduct a scoping review to determine the detection methods and validity of long-term exposure to PAEs in nails and hair. The findings will provide clues as to whether noninvasive substrates such as hair and nails can be used to accurately assess long-term exposure to persistent organic pollutants.
2 Materials and methods
We conducted a scope-based review according to the five steps described by Arksey and O’Malley’s framework (26).
2.1 Defining the research question
Can hair and nails be used as noninvasive matrices for assessing long-term exposure to PAEs? If it is,
1. How do the results of PAE exposure assessed using nail/hair compare to the results assessed using blood/urine?
2. What sample handling procedures, such as extraction and purification, are used to detect long-term exposure to PAEs in hair and nails?
2.2 Search criteria
We conducted a systematic literature search for articles published from 1 January 2000 to 3 April 2024, using databases such as PubMed, the Web of Science, and the China National Knowledge Infrastructure (CNKI). The search terms used were subject headings and free words. The following terms were used in combination: “phthalates” (PAEs, “phthalate esters,” “phthalic acid esters”) and “detection” (test, monitor, determination, method, approach) “nail” “hair.” We also conducted a search of other relevant studies by reference.
2.3 Screening the target literature
According to the prespecified PECOS (Population, Exposure, Comparison, Outcome, Study Design) criteria (Supplementary Table S1), two authors (Li-wen Chen, and Xin Chen) identified eligible articles as follows: (1) published methodological studies for the detection of PAEs and their metabolites in human nails/hair, and (2) studies that simultaneously described the results of PAE exposure assessed by nail/hair vs. urine/blood; all observational studies (such as cross-sectional surveys, cohort studies, or case–control studies) were eligible.
Articles that met the following criteria were excluded: (1) if the data were from the same population or overlapped, only the article with the largest sample size was included; (2) the study was a review, meta-analysis, report, letter, comment, etc.; and (3) the subjects were animals, water, soil, etc., not humans.
2.4 Extraction of data
Hua-yan Mo and Chun-han Shan used a unified information extraction table for data extraction according to the purpose of the research. The following data were extracted: author, publication date, biological matrix, sample size, outcome indicators, etc. When there was disagreement, three or more authors discussed and voted according to the PECOS criteria, and opinions with a turnout of more than 50% were retained.
2.5 Summary of results
We report this review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines-Scope Review Extension (27). We present a narrative synthesis of the methods used to assess long-term exposure to PAEs using nails and hair and their validity.
3 Results
3.1 Included studies
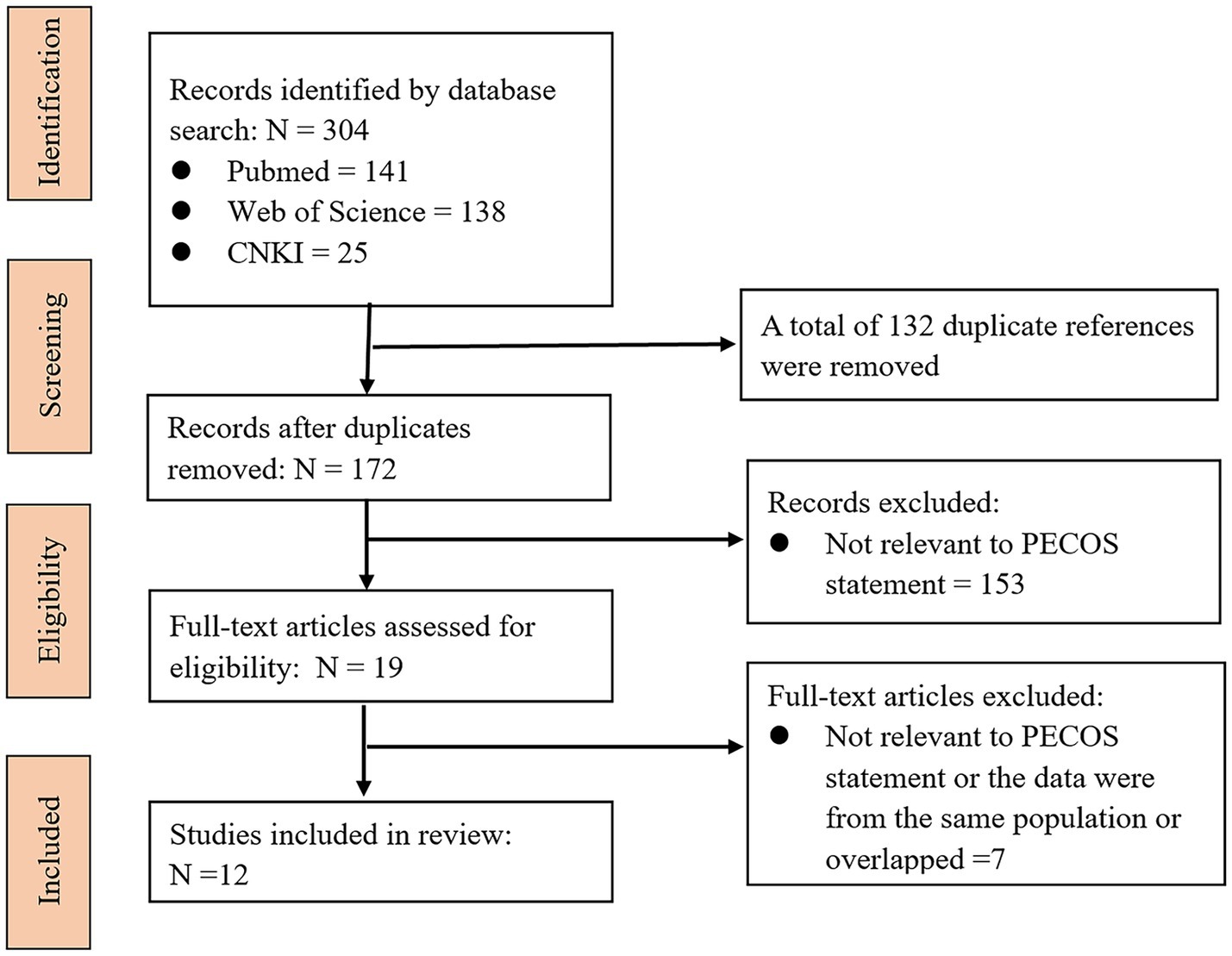
Through the systematic search strategy, 304 articles were initially identified. We excluded 132 duplicate articles based on the same unique identifier in PubMed. Then, according to the PECOS statement, 153 articles were excluded after browsing the titles and abstracts, and 7 additional articles were excluded after screening the full texts. Therefore, 12 eligible articles were ultimately included. The literature screening process is shown in Figure 1. For ease of reading, the PAEs and their metabolite name abbreviations that appear in this article are listed in Table 1.
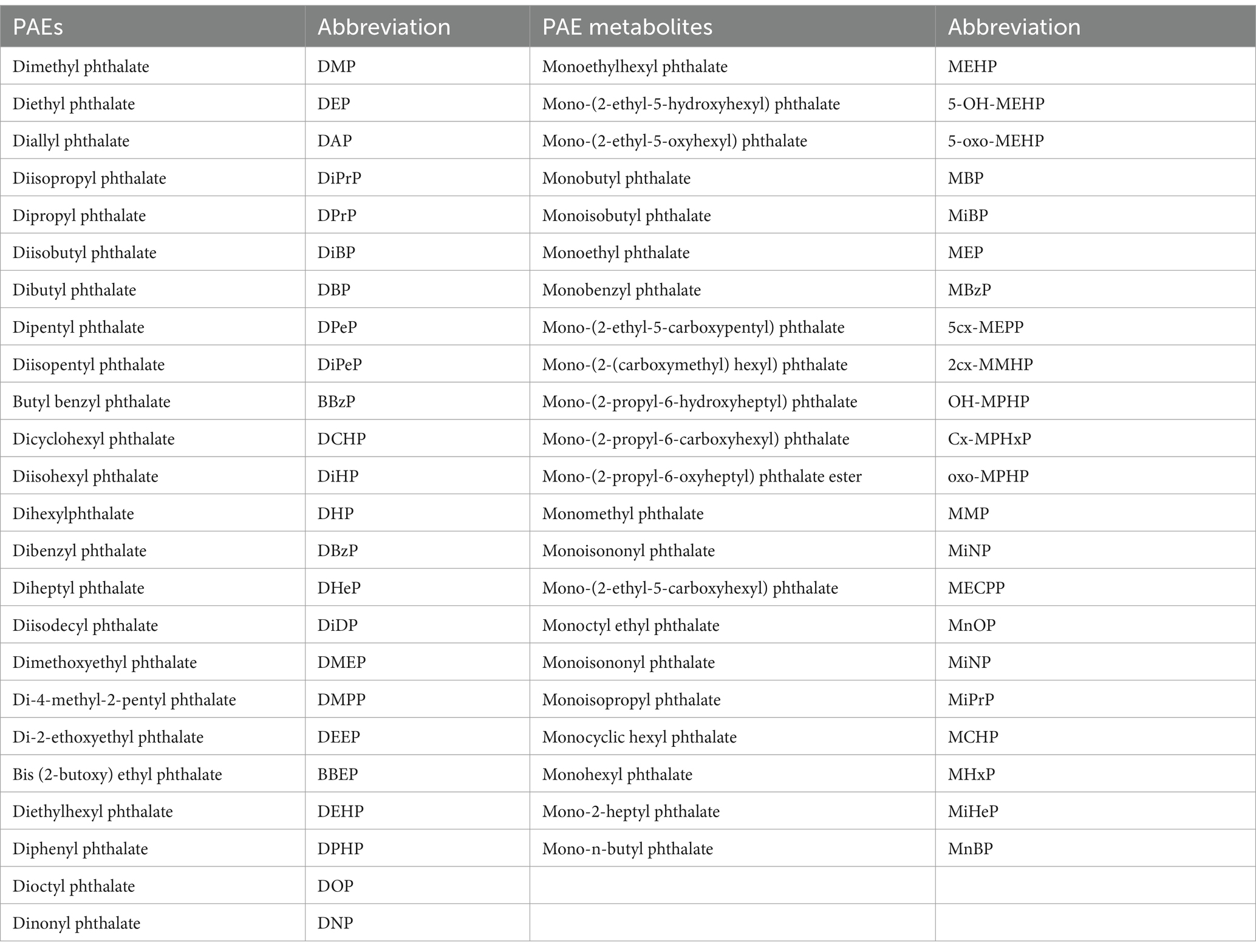
Table 1. PAE and PAE metabolites and their abbreviations detected in nail, hair, and urine samples from 12 studies.
3.2 Overview of the characteristics of the included studies
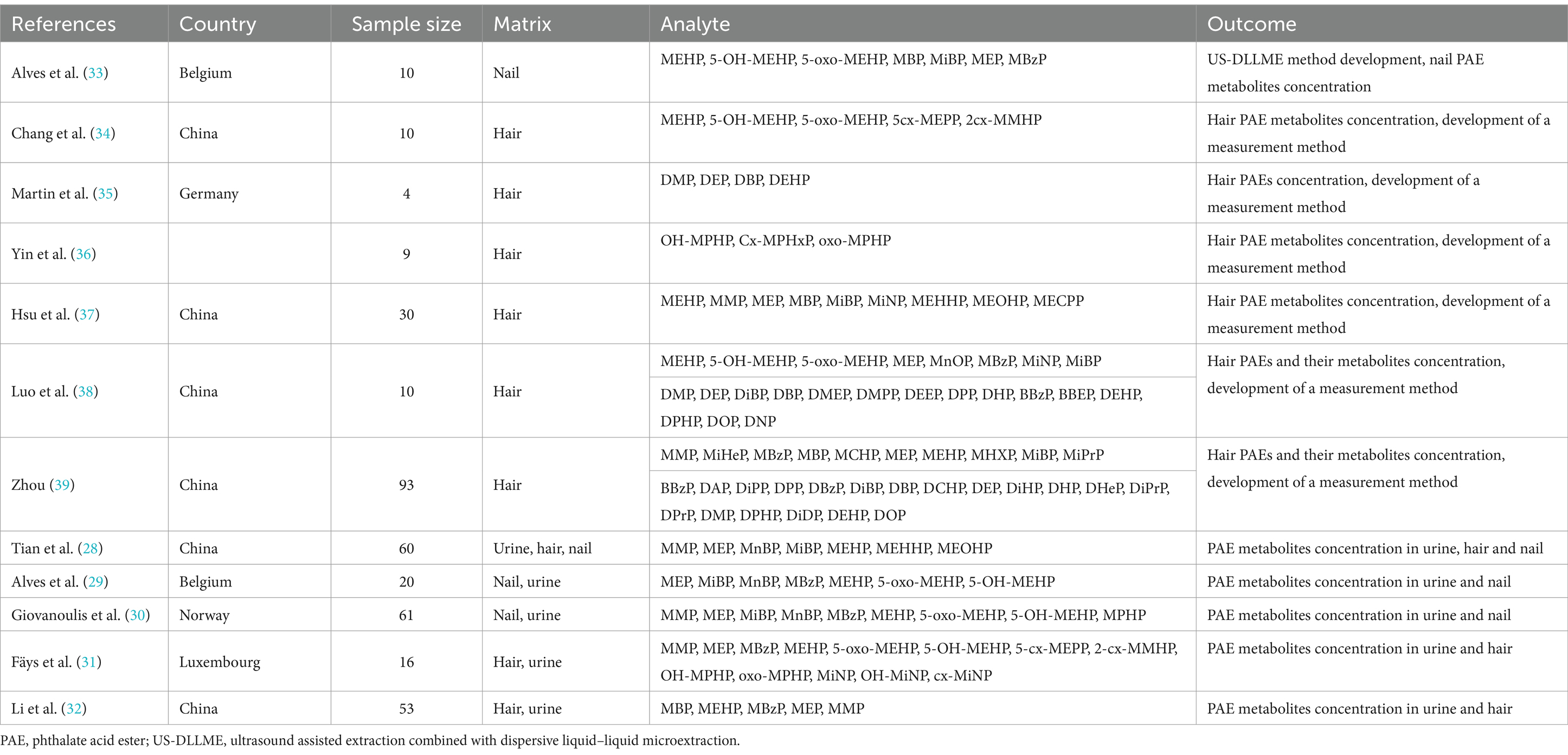
Eight studies used hair as a noninvasive matrix for detecting PAE exposure, three studies used nails as a noninvasive matrix, and one study used both hair and nails as detection samples (28). Two articles have compared the results of nail and urine tests as noninvasive matrices for the assessment of PAE exposure (29, 30). Two articles compared the evaluation results of hair with those of urine (31, 32). One article reported PAE metabolite concentrations in hair, urine, and nails (28). No articles comparing the assessment results of nails/hair with blood were found. Information on the included studies is provided in Table 2.
3.3 Sample collection and storage
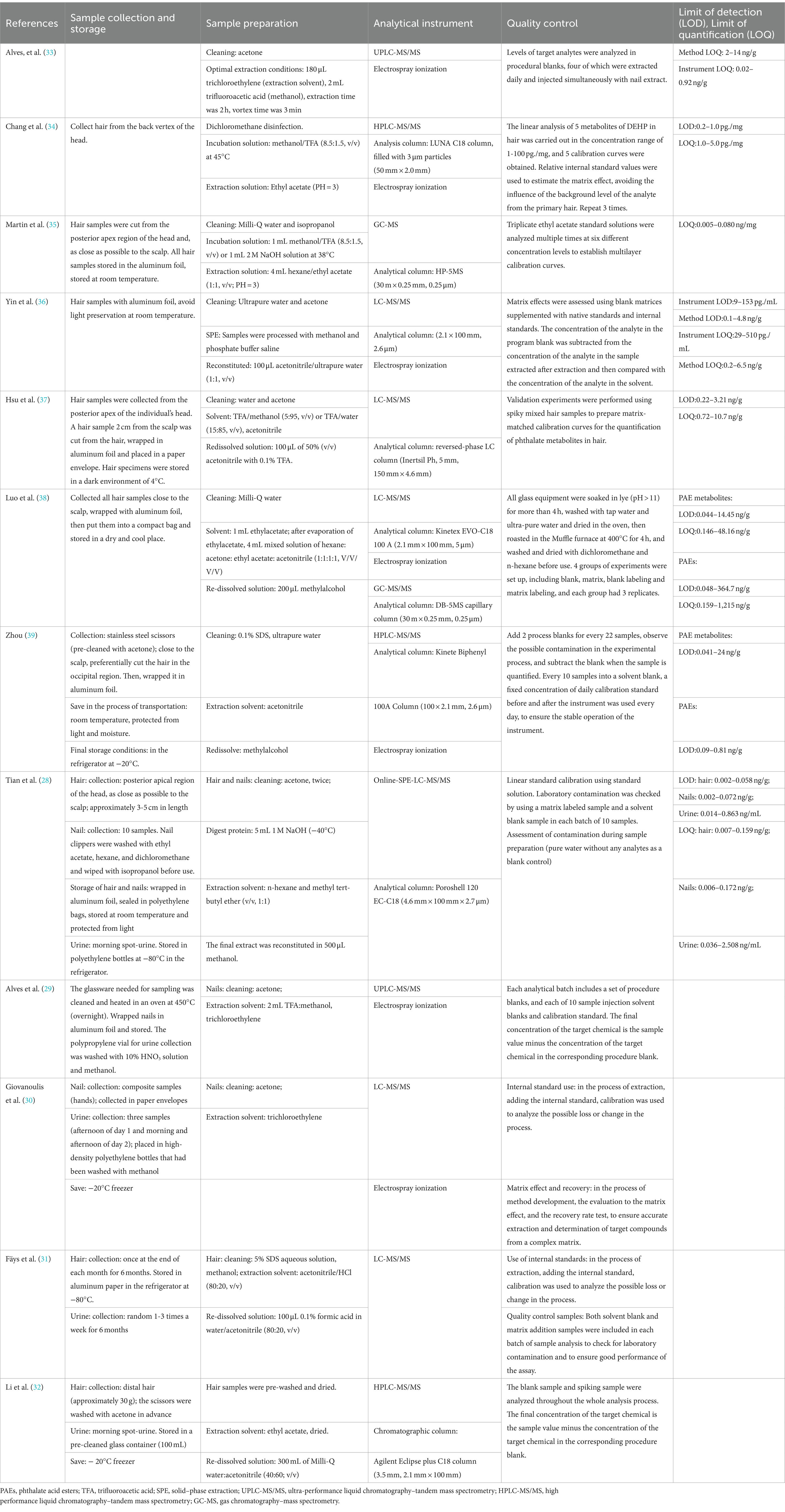
Nail collection did not emphasize which finger the nail was to be collected, and the preservation methods used were inconsistent. Although both Tian et al. (28) and Alves et al. (29) described nails that were wrapped in aluminum foil and then stored, Tian et al. (28) also emphasized storage conditions at room temperature and protected them from light. Two other studies involving nails did not describe how the nails were preserved (30, 33). The hair collection area was mostly the posterior apex/occipital part of the head (28, 34, 35, 37, 39), and hair was collected mainly near the scalp (28, 35, 37–39). Most researchers described hair samples as needing to be wrapped in aluminum foil. However, there were differences in the final storage conditions, some were recommended to be stored at room temperature and away from light (28, 36), some were recommended to be stored at 4°C (37), some were placed in a −20°C refrigerator (32, 39), and some were stored in a − 80°C refrigerator (31). Detailed information is provided in Table 3.
3.4 Preparation of samples
Effective cleaning and extraction techniques are important analytical methods for the detection of PAEs in biological samples. Before the nails and hair were used for analysis, they were first washed to remove contaminants such as dust from the surface. In the included studies, acetone was the solvent used for nail cleaning (28–30, 33). Compared with nails, hair washing solvents were significantly more diverse, and there was no uniform method. In the included articles, there were three main hair sample washing solvents: inorganic solvents (35–38), organic solvents (28, 31, 35–37), and surfactants (31, 39). The inorganic solvent was mainly ultrapure water (including Milli-Q water), the most commonly used organic solvent was acetone, followed by isopropyl alcohol and methanol, and the surfactant was an aqueous SDS solution. Detailed information can be found in Table 3. After cleaning, the nail/hair sample was ground to a powder for further manipulation. Most of the included articles did not specify what solvent was used to promote hair and nail dissolution prior to extraction. Martin et al. (35) reported that hair samples were incubated overnight with 1 mL of NaOH solution or 1 mL of methanol/trifluoroacetic acid (8.5:1.5, v/v) at 38°C prior to extraction.
The present study revealed that the handling procedures for nails and hair mainly included solid–phase extraction (SPE) (36, 37) and liquid–liquid extraction (LLE). Among the considered methods, trifluoroacetic acid/methanol is the most common organic solvent, followed by methanol, trifluoroacetic acid/water, acetonitrile, etc. The extraction solvent was different in different studies; for example, Alves et al. (33) found that the best extraction solution was trichloroethylene after experiments. Chang et al. (34) used ethyl acetate for LLE. Martin et al. used a mixed solution of n-hexane/ethyl acetate (1:1,v/v) (30) to perform LLE on preliminarily treated samples (35). Luo et al. (38) reported that a mixture of n-hexane:acetone:ethyl acetate:acetonitrile (1:1:1:1, V/V/V/V) was the most suitable extraction solvent after experimental adjustment. However, Xu suggested that acetonitrile is the best extraction solvent after experiments (39). See Table 3 for further details.
3.5 Analytical instrument
The use of analytical instruments is also crucial for the detection of PAEs in nails/hair. We found that the separation of PAEs and their metabolites in nails or hair was mainly carried out by chromatography. Chromatography is a separation technique that is coupled with a detector, such as ultraviolet or mass spectrometry (MS). Of the studies using hair samples for detection, four used only liquid chromatography–tandem mass spectrometry (LC-MS) for analysis (34, 36, 37, 39). One study used only gas chromatography–mass spectrometry (GC-MS) for analysis (35). However, in the study by Luo et al. (38), GC-MS/MS was used to detect PAEs in hair, and LC-MS/MS was used to detect PAE metabolites. LC-MS/MS was used for studies in which nails were used as the matrix (33). The details can be found in Table 3.
3.6 Comparison of PAE metabolite concentrations between hair/nail and urine samples
In a small sample study conducted by Alves et al. (29) in Belgium, ∑(MnBP, MiBP) and MEP were the major PAE metabolites detected in both urine and nails. They also performed correlation analysis and found that there was a significant correlation between different metabolites in nails and urine. The levels of MEHP in the nails were strongly correlated with the levels of ∑ (MnBP, MiBP; r = 0.73, p < 0.01) and MBzP (r = 0.52, p < 0.05) in the urine. There was a moderate correlation between 5-OH-MEHP and ∑ (MnBP, MiBP; r = 0.62, p < 0.01) and between 5-OH-MEHP and MEP (r = 0.56, p < 0.05). However, no significant correlation was observed for the same metabolites measured in either matrix. A study in Norway revealed that MEP was one of the main metabolites detected in nails and urine, and MnBP and MiBP were also found at relatively high concentrations in urine and nails (30). Except for MEP (r = 0.56-0.68, p < 0.001), no correlation was found between PAE metabolite concentrations in the nail and urine. Tian et al. (28) reported that the most common PAE metabolite in nails is MMP, followed by MiBP and MnBP.
Fäys et al. (31) reported that the three most common metabolites in hair were MEP, MEHP and MMP; MBP, MMP and MEHP in Li et al.’s study (32); and MMP, MEHP and MEP in Tian et al.’s study (28). The major metabolites detected in the urine of the three studies all contained MEP. Fäys et al. (31) showed that the MEP concentration in hair was significantly correlated with the MEP concentration in urine, but no correlation was found for other PAE metabolites. Tian et al. (28) reported no or only weakly significant correlations between PAE metabolites in nail, hair, and urine samples. The correlation coefficients of the PAE metabolite concentrations measured in these five studies for the same substances in different matrices are shown in Table 4.
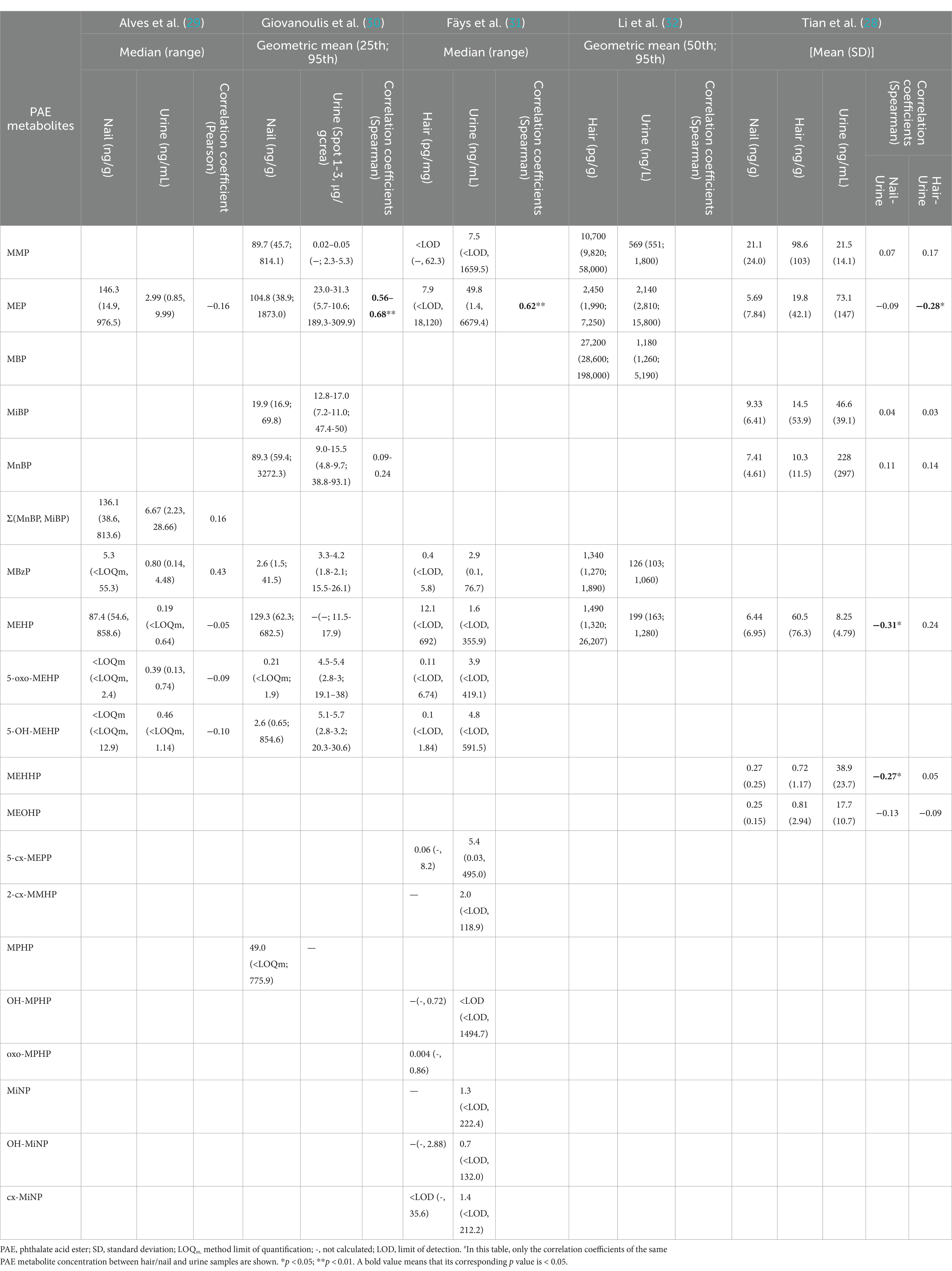
Table 4. Comparison and correlation coefficient# of PAE metabolite concentrations between hair/nail and urine samples.
4 Discussion
Overall, nails and hair may be a noninvasive matrices for assessing long-term PAE exposure levels. However, the evidence is insufficient, especially regarding the use of nail assessment in PAE exposure studies. The main solvents used for hair cleaning were water, acetone, isopropanol, and 0.1% SDS. In addition, sample handling procedures such as the extraction and purification of compounds from hair were not the same among the studies.
At present, six phthalates, namely, DMP, DEP, DBP, DOP, DEHP, and BBP, have been identified as priority control pollutants by the United States Environmental Protection Agency (USEPA). These compounds or their primary and secondary metabolites were mostly examined in these studies. Although nails and hair have been used as biological matrices for detecting PAE exposure, urinalysis is still the most common method.
After Alves et al. developed a method to analyze long-term exposure to PAEs in nails, few studies have used nails to assess long-term exposure to PAEs, and the results were mainly from their team. Their findings indicated that major PAE metabolites in nails and urine were similar, and specific PAE metabolites in nails and urine were significant correlated, suggesting that nails can be used as a noninvasive alternative matrix to assess long-term PAE exposure in humans (21, 29). Nonetheless, it has been argued that the use of nails is not suitable for reflecting intra-PAE exposure in humans. The theoretical concentrations of nail DiBP and DnBP estimated from pharmacokinetic models were much lower than the actual concentrations (40). Research by Giovanoulis et al. showed that participants who frequently used hand care products had higher concentrations of MnBP and MEP in their nails. After adjusting for other confounding factors, the use of more than 5 care products per day was significantly positively associated with the concentration of MEP in the nails (30). Therefore, PAEs or their metabolites in hand care products may increase the concentration of PAE metabolites in nails through direct penetration into nails. Nails are more likely to reflect external exposure than internal exposure (40). In addition, the study by Giovanoulis et al. did not find a correlation between nails and urinary MnBP or MiBP (30). The sample size of this study (N = 61) is currently the largest among studies exploring the feasibility of using nails to assess long-term exposure to PAEs. Theoretically, PAE incorporation into the nail occurs primarily through diffusion through blood, with the blood supply depositing PAEs or their metabolites into the germinal matrix and nail bed on the lower side of the nail plate, resulting in incorporation during nail formation (41). However, there are no studies on the comparison and correlation between nail and blood PAE metabolite concentrations. This finding needs to be further explored to determine the value of nail assessment of long-term PAEs exposure.
The use of hair as a noninvasive alternative matrix substrate for assessing long-term PAE exposure in humans has been studied more than the use of nails, but hair is more commonly used to monitor PAE exposure in vulnerable populations, such as newborns (42). This may be because the metabolic pathways reflected in hair are different from those in urine. For example, the metabolite profiles of DPHP in urine and hair are not the same, suggesting that metabolites often measured in urine may not be directly suitable for detection in hair (43). Second, because the steps of cleaning and extraction are not reasonable, PAEs and their metabolites may not be detected. Current studies have shown that most PAE metabolite concentrations in hair have no significant or weak correlation with PAE metabolite concentrations in urine (28, 31). Contaminants from external sources, such as chemicals in food and care products, may have an impact on these processes and thus affect the concentration of PAE metabolites in hair (44). In addition, only the study by Fays et al. evaluated the correlation of hair PAE metabolite concentrations with mean urinary PAE metabolite concentrations over time (31). Therefore, some scholars believe that to use hair analysis as a biological monitoring method for human exposure, it is necessary to clarify the absorption pathway of PAEs and their metabolic processes in hair, determine the relationship between exposure dose and PAE content in hair, and determine the relationship between PAE metabolite concentrations in hair and concentrations in urine (35). However, further research is needed.
Several researchers have studied methods for the detection of PAEs and their metabolites using hair as a matrix. Due to the use of shampoo and other products and air exposure, to better assess internal exposure to PAEs, it is necessary to wash hair before testing to reduce the impact of exogenous exposure (45). However, there is no unified scheme for the cleaning solvent used. The solvents used for investigator cleaning in our included studies included water, acetone, isopropanol, and 0.1% SDS. Zhou et al. (39) reported that the use of 0.1% SDS and ultrapure water to wash hair can better reduce the matrix effect and achieve a higher internal standard recovery rate. This finding is similar to the results of Martin et al. (46), who reported that the concentration of organic matter in hair after washing with organic solvents was lower than that after washing with water and surfactants. Similarly, a study by Zheng et al. (47) revealed that hair shafts of hair that had been washed with warm Milli-Q water appeared smooth under scanning electron microscopy, indicating effective removal of external contamination. The use of organic solvents as washing solvents may require consideration: During the washing process, impurities may be removed from the hair surface, and compounds of interest may also be extracted from the hair matrix, which will have an impact on the final analysis results (48). Therefore, water and 0.1% SDS may be more suitable washing solvents. Various studies have also used different approaches for sample handling procedures such as the extraction and purification of compounds from hair. Although some scholars have also explored the optimal extraction conditions during this study (38, 39), there is still a lack of data comparing with the use of other matrices to assess PAE exposure. These questions need to be explored further.
This study has important implications for our recent development of biological sample detection methods for the long-term assessment of pseudo-persistent organic pollutant exposure. However, there are some shortcomings that need to be carefully considered. For instance, this article only takes PAEs of the nonpersistent organic pollutants as an example. Different organic compounds have different chemical structures and volatilities, so the sample handling and detection methods may be different. For example, compared with the HPLC-MS/MS method, the GC-MS method is more suitable for detecting more volatile chemicals, and in the pretreatment derivatization is needed. In addition, the metabolic process involved in the detection of chemicals in the hair and nails, needs to be fully understood. This is important for selecting the chemicals to be tested. For example, PAE parent chemicals are very common in air pollution, and the use of products such as nail polish and shampoo inevitably causes hair and nail pollution. By washing hair and nails with organic solvents before grinding and measuring PAE metabolites, contamination problems can be avoided to some extent. However, it is not clear whether the organic solvent cleaning process can lead to the loss of chemicals in hair or nails, and thus the level of exposure is underestimated. The team is using neonatal hair and nails to develop tests to better avoid exogenous contamination, and looks forward to adding new evidence to the findings of this study.
5 Conclusion
The results of this review suggest that hair may be a noninvasive matrix for assessing long-term exposure to PAEs compared with nails. However, due to the lack of correlation between the concentration of PAE metabolites in hair and the average concentration of PAE metabolites in urine samples collected continuously over a period of time, the use of hair to assess long-term PAE exposure still needs further validation. Water and 0.1% SDS may be more suitable as washing solvents for the treatment of hair. However, handling procedures such as the extraction and purification of compounds from hair are not uniform in various studies; therefore, further exploration and optimization of this process and additional research evidence to evaluate its effectiveness are needed to provide a scientific basis for the promotion and application of hair detection methods for assessing long-term exposure levels of pesudo-persistent organic pollutant PAE.
Author contributions
L-wC: Writing – original draft, Methodology, Formal analysis, Data curation. XC: Writing – original draft, Formal analysis, Data curation. H-yM: Writing – original draft, Data curation. C-hS: Writing – original draft, Data curation. R-pZ: Writing – review & editing. HG: Writing – review & editing, Funding acquisition. F-bT: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research reported in this publication was supported by the Research Project for Outstanding Young People in Universities of Anhui Province (no. 2023AH030118), the National Natural Science Foundation of China (no. 82103856), and funds of the MOE Key Laboratory of Population Health Across Life Cycle (no. JK20204).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1411588/full#supplementary-material
References
1. Chang, WH, Herianto, S, Lee, CC, Hung, H, and Chen, HL. The effects of phthalate ester exposure on human health: a review. Sci Total Environ. (2021) 786:147371. doi: 10.1016/j.scitotenv.2021.147371
2. Teeguarden, JG, Twaddle, NC, Churchwell, MI, Yang, X, Fisher, JW, Seryak, LM, et al. 24-hour human urine and serum profiles of bisphenol a: evidence against sublingual absorption following ingestion in soup. Toxicol Appl Pharmacol. (2015) 288:131–42. doi: 10.1016/j.taap.2015.01.009
3. Sasso, AF, Pirow, R, Andra, SS, Church, R, Nachman, RM, Linke, S, et al. Pharmacokinetics of bisphenol a in humans following dermal administration. Environ Int. (2020) 144:106031. doi: 10.1016/j.envint.2020.106031
4. Egeghy, PP, Cohen, HE, Tulve, NS, Melnyk, LJ, Morgan, MK, Fortmann, RC, et al. Review of pesticide urinary biomarker measurements from selected US EPA children's observational exposure studies. Int J Environ Res Public Health. (2011) 8:1727–54. doi: 10.3390/ijerph8051727
5. Wang, Y, Zhu, H, and Kannan, K. A review of biomonitoring of phthalate exposures. Toxics. (2019) 7:21. doi: 10.3390/toxics7020021
6. Kim, DH, Park, CG, Kim, SH, and Kim, YJ. The effects of mono-(2-Ethylhexyl) phthalate (MEHP) on human Estrogen receptor (hER) and androgen receptor (hAR) by YES/YAS in vitro assay. Molecules. (2019) 24:1558. doi: 10.3390/molecules24081558
7. Gao, H, Wu, W, Xu, Y, Jin, Z, Bao, H, Zhu, P, et al. Effects of prenatal phthalate exposure on thyroid hormone concentrations beginning at the embryonic stage. Sci Rep. (2017) 7:13106. doi: 10.1038/s41598-017-13672-x
8. Huang, RG, Li, XB, Wang, YY, Wu, H, Li, KD, Jin, X, et al. Endocrine-disrupting chemicals and autoimmune diseases. Environ Res. (2023) 231:116222. doi: 10.1016/j.envres.2023.116222
9. Thompson, PA, Khatami, M, Baglole, CJ, Sun, J, Harris, SA, Moon, EY, et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis. (2015) 36:S232–53. doi: 10.1093/carcin/bgv038
10. Yu, H . Sources of phthalate esters in food and their hazards to human body (in Chinese). Modern Food. (2021) 29:41–3. doi: 10.16736/j.cnki.cn41-1434/ts.2021.19.011
11. Nidens, N, Vogel, M, Korner, A, and Kiess, W. Prenatal exposure to phthalate esters and its impact on child development. Best Pract Res Clin Endocrinol Metab. (2021) 35:101478. doi: 10.1016/j.beem.2020.101478
12. Mariana, M, Feiteiro, J, Verde, I, and Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: a review. Environ Int. (2016) 94:758–76. doi: 10.1016/j.envint.2016.07.004
13. Zhang, YJ, Guo, JL, Xue, JC, Bai, CL, and Guo, Y. Phthalate metabolites: characterization, toxicities, global distribution, and exposure assessment. Environ Pollut. (2021) 291:118106. doi: 10.1016/j.envpol.2021.118106
14. Sathyanarayana, S . Phthalates and children's health. Curr Prob Pediatr. (2008) 38:34–49. doi: 10.1016/j.cppeds.2007.11.001
15. Swan, SH . Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. (2008) 108:177–84. doi: 10.1016/j.envres.2008.08.007
16. Rockett, JC, Buck, GM, Lynch, CD, and Perreault, SD. The value of home-based collection of biospecimens in reproductive epidemiology. Environ Health Perspect. (2004) 112:94–104. doi: 10.1289/ehp.6264
17. Calafat, AM, and McKee, RH. Integrating biomonitoring exposure data into the risk assessment process: phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environ Health Perspect. (2006) 114:1783–9. doi: 10.1289/ehp.9059
18. Frederiksen, H, Jorgensen, N, and Andersson, AM. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J Anal Toxicol. (2010) 34:400–10. doi: 10.1093/jat/34.7.400
19. Gao, H, Zhu, YD, Xu, YY, Zhang, YW, Yao, HY, Sheng, J, et al. Season-dependent concentrations of urinary phthalate metabolites among Chinese pregnant women: repeated measures analysis. Environ Int. (2017) 104:110–7. doi: 10.1016/j.envint.2017.03.021
20. Alves, A, Kucharska, A, Erratico, C, Xu, F, Den Hond, E, Koppen, G, et al. Human biomonitoring of emerging pollutants through non-invasive matrices: state of the art and future potential. Anal Bioanal Chem. (2014) 406:4063–88. doi: 10.1007/s00216-014-7748-1
21. Alves, A, Koppen, G, Vanermen, G, Covaci, A, and Voorspoels, S. Long-term exposure assessment to phthalates: how do nail analyses compare to commonly used measurements in urine. J Chromatogr B. (2016) 1036-1037:124–35. doi: 10.1016/j.jchromb.2016.09.039
22. Pragst, F, and Balikova, MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. (2006) 370:17–49. doi: 10.1016/j.cca.2006.02.019
23. Ren, M, Jia, X, Shi, J, Yan, L, Li, Z, Lan, C, et al. Simultaneous analysis of typical halogenated endocrine disrupting chemicals and metal(loid)s in human hair. Sci Total Environ. (2020) 718:137300. doi: 10.1016/j.scitotenv.2020.137300
24. Iglesias-Gonzalez, A, Hardy, EM, and Appenzeller, B. Cumulative exposure to organic pollutants of French children assessed by hair analysis. Environ Int. (2020) 134:105332. doi: 10.1016/j.envint.2019.105332
25. Hsu, JY, Ho, HH, and Liao, PC. The potential use of diisononyl phthalate metabolites hair as biomarkers to assess long-term exposure demonstrated by a rat model. Chemosphere. (2015) 118:219–28. doi: 10.1016/j.chemosphere.2014.09.025
26. Arksey, H, and O'Malley, L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 26:19–32. doi: 10.1080/1364557032000119616
27. Tricco, AC, Lillie, E, Zarin, W, O'Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
28. Tian, X, Huang, K, Liu, Y, Jiang, K, Liu, R, Cui, J, et al. Distribution of phthalate metabolites, benzophenone-type ultraviolet filters, parabens, triclosan and triclocarban in paired human hair, nail and urine samples. Environ Pollut. (2023) 333:122083. doi: 10.1016/j.envpol.2023.122083
29. Alves, A, Covaci, A, and Voorspoels, S. Are nails a valuable non-invasive alternative for estimating human exposure to phthalate esters? Environ Res. (2016) 151:184–94. doi: 10.1016/j.envres.2016.07.023
30. Giovanoulis, G, Alves, A, Papadopoulou, E, Cousins, AP, Schütze, A, Koch, HM, et al. Evaluation of exposure to phthalate esters and DINCH in urine and nails from a Norwegian study population. Environ Res. (2016) 151:80–90. doi: 10.1016/j.envres.2016.07.025
31. Fäys, F, Hardy, EM, Palazzi, P, Haan, S, Beausoleil, C, and Appenzeller, B. Biomonitoring of fast-elimination endocrine disruptors—results from a 6-month follow up on human volunteers with repeated urine and hair collection. Sci Total Environ. (2021) 778:146330. doi: 10.1016/j.scitotenv.2021.146330
32. Li, N, Ying, GG, Hong, H, Tsang, E, and Deng, WJ. Plasticizer contamination in the urine and hair of preschool children, airborne particles in kindergartens, and drinking water in Hong Kong. Environ Pollut. (2021) 271:116394. doi: 10.1016/j.envpol.2020.116394
33. Alves, A, Vanermen, G, Covaci, A, and Voorspoels, S. Ultrasound assisted extraction combined with dispersive liquid-liquid microextraction (US-DLLME)-a fast new approach to measure phthalate metabolites in nails. Anal Bioanal Chem. (2016) 408:6169–80. doi: 10.1007/s00216-016-9727-1
34. Chang, YJ, Lin, KL, and Chang, YZ. Determination of Di-(2-ethylhexyl)phthalate (DEHP) metabolites in human hair using liquid chromatography-tandem mass spectrometry. Clin Chim Acta. (2013) 420:155–9. doi: 10.1016/j.cca.2012.10.009
35. Martín, J, Möder, M, Gaudl, A, Alonso, E, and Reemtsma, T. Multi-class method for biomonitoring of hair samples using gas chromatography-mass spectrometry. Anal Bioanal Chem. (2015) 407:8725–34. doi: 10.1007/s00216-015-9026-2
36. Yin, S, Been, F, Liu, W, and Covaci, A. Hair as an alternative matrix to monitor human exposure to plasticizers—development of a liquid chromatography—tandem mass spectrometry method. J Chromatogr B. (2019) 1104:94–101. doi: 10.1016/j.jchromb.2018.09.031
37. Hsu, JF, Chang, WC, Ho, WY, and Liao, PC. Exploration of long-term exposure markers for phthalate esters in human hair using liquid chromatography-tandem mass spectrometry. Anal Chim Acta. (2022) 1200:339610. doi: 10.1016/j.aca.2022.339610
38. Luo, ZN, Qin, RX, Zhang, SY, Mo, L, Li, H, Tang, B, et al. The establishment of a new method for the detection of emerging organic contaminants in hair (in Chinese). Environ Chem. (2023) 42:1509–23. doi: 10.7524/j.issn.0254-6108.2022091405
39. Zhou, Y. Analytical methods and population exposure characterisation of typical plasticisers and flame retardants in hair samples (in Chinese). (2023).
40. Bui, TT, Alves, A, Palm-Cousins, A, Voorspoels, S, Covaci, A, and Cousins, IT. Estimating uptake of phthalate ester metabolites into the human nail plate using pharmacokinetic modelling. Environ Int. (2017) 100:148–55. doi: 10.1016/j.envint.2017.01.007
41. Cappelle, D, Yegles, M, Neels, H, van Nuijs, ALN, De Doncker, M, Maudens, K, et al. Nail analysis for the detection of drugs of abuse and pharmaceuticals: a review. Forensic Toxicol. (2015) 33:12–36. doi: 10.1007/s11419-014-0258-1
42. Cleys, P, Panneel, L, Bombeke, J, Dumitrascu, C, Malarvannan, G, Poma, G, et al. Hair as an alternative matrix to assess exposure of premature neonates to phthalate and alternative plasticizers in the neonatal intensive care unit. Environ Res. (2023) 236:116712. doi: 10.1016/j.envres.2023.116712
43. Shih, CL, Wu, HY, Liao, PM, Hsu, JY, Tsao, CY, Zgoda, VG, et al. Profiling and comparison of toxicant metabolites in hair and urine using a mass spectrometry-based metabolomic data processing method. Anal Chim Acta. (2019) 1052:84–95. doi: 10.1016/j.aca.2018.11.009
44. Katsikantami, I, Tzatzarakis, MN, Karzi, V, Stavroulaki, A, Xezonaki, P, Vakonaki, E, et al. Biomonitoring of bisphenols a and S and phthalate metabolites in hair from pregnant women in Crete. Sci Total Environ. (2020) 712:135651. doi: 10.1016/j.scitotenv.2019.135651
45. Zhang, S, Yan, X, Tang, B, Luo, W, Chen, S, Luo, X, et al. Human hair as a noninvasive matrix to assess exposure to micro-organic contaminants: state of the art review. Sci Total Environ. (2023) 892:164341. doi: 10.1016/j.scitotenv.2023.164341
46. Martin, J, Santos, JL, Aparicio, I, and Alonso, E. Analytical method for biomonitoring of endocrine-disrupting compounds (bisphenol a, parabens, perfluoroalkyl compounds and a brominated flame retardant) in human hair by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. (2016) 945:95–101. doi: 10.1016/j.aca.2016.10.004
47. Zheng, J, Yan, X, Chen, SJ, Peng, XW, Hu, GC, Chen, KH, et al. Polychlorinated biphenyls in human hair at an e-waste site in China: composition profiles and chiral signatures in comparison to dust. Environ Int. (2013) 54:128–33. doi: 10.1016/j.envint.2013.01.018
Keywords: phthalate acid esters, nail, hair, noninvasive matrices, long-term exposure
Citation: Chen L-w, Chen X, Mo H-y, Shan C-h, Zhu R-p, Gao H and Tao F-b (2024) Exploring noninvasive matrices for assessing long-term exposure to phthalates: a scoping review. Front. Public Health. 12:1411588. doi: 10.3389/fpubh.2024.1411588
Edited by:
Malarvannan Govindan, University of Antwerp, BelgiumReviewed by:
Noelia Caballero-Casero, University of Cordoba, SpainChong Liu, Huazhong University of Science and Technology, China
Copyright © 2024 Chen, Chen, Mo, Shan, Zhu, Gao and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruo-ping Zhu, enJwMDkyMkAxMjYuY29t Hui Gao, Z2gyMDE5MDEzMEAxNjMuY29t
†These authors have contributed equally to this work
 Li-wen Chen
Li-wen Chen Xin Chen1
†
Xin Chen1
†
 Hui Gao
Hui Gao