- 1Department of Sports Medicine and Sportology, Graduate School of Medicine, Juntendo University, Tokyo, Japan
- 2Sportology Center, Graduate School of Medicine, Juntendo University, Tokyo, Japan
- 3Department of Metabolism and Endocrinology, Graduate School of Medicine, Juntendo University, Tokyo, Japan
- 4Graduate School of Health and Sports Science, Juntendo University, Chiba, Japan
- 5Juntendo Advanced Research Institute for Health Science, Tokyo, Japan
- 6Faculty of International Liberal Arts, Juntendo University, Tokyo, Japan
Introduction: Exercise is a crucial method for preventing geriatric depression. In this cross-sectional study, we investigated the associations between exercise habits in adolescence and old age and geriatric depressive symptoms.
Methods: This study used baseline data from the Bunkyo Health Study, a prospective observational cohort study investigating the preventive effects of physical activity on causative diseases requiring long-term care. This analysis included 1,629 older adults (687 men and 942 women) aged 65–84 years who participated in the Bunkyo Health Study. Participants were divided into four groups according to their exercise habits in adolescence and old age: never exercised (none-none; NN), exercised only in old age (none-active; NA), exercised only in adolescence (active-none; AN), and exercised in adolescence and old age (active-active; AA). Geriatric depressive symptoms were defined as the short version of the Geriatric Depression Scale score ≥ 5, including depression tendency. Multivariate-adjusted logistic regression models were used to estimate the odds ratios (ORs) and associated 95% confidence intervals in each group for the prevalence of geriatric depressive symptoms compared with the NN group.
Results: The ORs for geriatric depressive symptoms were notably lower in the AN, NA, and AA groups than in the NN group in both older men and older women.
Conclusion: These results indicate that older adults with exercise habits in adolescence and/or in old age exhibit a lower prevalence of geriatric depressive symptoms.
1 Introduction
Geriatric depression causes mood symptoms and decreases the quality of life (QOL) of older adults (1). Its prevalence ranges from 1 to 4% in older adults, with a higher prevalence in community-dwelling women (2). Although it is not sufficient to diagnose major depression, a previous study found that approximately 10–15% of older adults have clinical depressive symptoms (3). Geriatric depression is associated with suicidal ideation and a high rate of progression to dementia (4). Geriatric depression is associated with a higher severity of physical symptoms than depression in younger adults (5), and the presence of residual symptoms can increase recurrence rates (6). Thus, the prevention of geriatric depression is essential for enhancing the QOL of older adults.
Exercise is crucial for preventing depression in geriatric patients. Exercise in old age is well-established to prevent geriatric depression (7, 8). Additionally, exercise in adolescence may play a pivotal role in preventing geriatric depression. Regular adolescent exercise has been found to enhance cognitive function and promote neuroplasticity (9), potentially strengthening an individual’s resilience to depression later in life (10). Therefore, it can be inferred that a combination of exercise habits in both age periods (e.g., adolescence and old age) may have an additive effect in the prevention of geriatric depression, which remains to be seen.
Thus, in this study, we aimed to examine the associations between exercise habits in adolescence and old age and geriatric depressive symptoms. We hypothesized that exercise habits in both adolescence and old age could be effective in decreasing the prevalence of geriatric depressive symptoms.
2 Materials and methods
2.1 Study design and participants
This cross-sectional study used baseline data from the Bunkyo Health Study, a prospective observational cohort study investigating the preventive effects of physical activity on causative diseases requiring long-term care (11). The Bunkyo Health Study recruited older individuals aged 65–84 years living in Bunkyo-Ku, an urban area in Tokyo, Japan; 1,629 participants (687 men and 942 women) completed a two-day study examination at the Sportology Center between October 15, 2015, and October 1, 2018. Briefly, we evaluated cognitive function and performed a screening test for geriatric depressive symptoms using a questionnaire, assessed muscle strength and physical performance, performed brain lesion evaluations using magnetic resonance imaging, body composition and bone mineral density assessment using dual-energy X-ray absorptiometry, blood sample collection, and a 75-g oral glucose tolerance test (75-g oral glucose tolerance tests [OGTT]) after overnight fasting.
The study protocol was approved by the Ethics Committee of Juntendo University in September 2015 (first approval no. 2015061 and the latest revised version no. M15-0057-M08). The study was conducted in accordance with the principles of the Declaration of Helsinki. All participants were given a clear explanation of the study’s details. Following this, they provided written informed consent, indicating their voluntary agreement to participate.
2.2 Definition of geriatric depressive symptoms
The short version of the Geriatric Depression Scale (GDS-15) was used to assess depressive symptoms in older adults (12). GDS-15 scores range from 0 to 15, which can be divided to four groups: (1) Normal, 0–4 points; (2) Mild depressive symptoms, 5–8 points; (3) Moderate depressive symptoms, 9–11 points; (4) Severe depressive symptoms, 12–15 points. In this study, 5 or higher GDS-15 scores were defined as geriatric depressive symptoms, including depressive tendencies (13). The GDS-15 has been reported to be a reliable and valid screening tool for major depressive disorder across gender, ethnicity, and chronic illness status (13, 14).
2.3 Definition of exercise habits
Exercise habit data were collected by self-report using the Bunkyo Health Study baseline questionnaire. All participants were interviewed using the following questions: “Did you participate in sports club activities when you were in junior high school or high school?” and “Do you currently have exercise habits?” We then asked those who answered “yes” about their engagement in specific sports, club activities, current exercise types, and frequency. Additionally, current physical activity levels were evaluated using the International Physical Activity Questionnaire. We defined those who participated in sports club activities during junior high or high school as exercising in adolescence. For participants with only a junior high school education, their specific duration of exercise habits in adolescence was 3 years. However, for those with a high school or higher education, the duration may have been 3 or 6 years, reflecting exercise during junior high, high school, or both periods. Based on the definition of exercise habits in the National Health and Nutrition Exercise Survey in Japan, those who exercised more than twice a week for at least 30 min per session were defined as having exercise habits in old age. We also asked, “Did you have exercise habits at each age from your 20s to 50s?” Those who responded “yes” were defined as having exercise habits at each age range from their 20s to 50s.
2.4 Other measurements
In the upright position, height was measured within 0.1 cm using a stadiometer (YS-201-P; YAGAMI Inc., Nagoya, Japan). Body mass was measured by 0.1 kg using an electronic scale (InBody770; Biospace, Seoul, Korea). Body mass index (BMI) was calculated as body weight divided by height squared in meters. Self-administered questionnaires were used to determine age (years), years of education (years), smoking status (current or past) and living alone. Current or past smokers answered questions about the amount and years of smoking to calculate the Brinkman index: the number of cigarettes smoked per day multiplied by the number of years of smoking. Dietary intake was assessed using a Brief self-administered Diet History Questionnaire (BDHQ) to measure alcohol intake. The BDHQ has been validated in a previous study (15). Physical activity levels were evaluated using the International Physical Activity Questionnaire (IPAQ).
Medical history and medication information were recorded by a physician during the interviews using a semi-structured questionnaire. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or current use of antihypertensive medications. Blood samples were collected in the morning after an overnight fast to perform appropriate biochemical tests. Blood glucose and hemoglobin A1C levels were measured at the Commissioned Clinical Laboratory Center (SRL Inc., Tokyo, Japan). Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL and a 2-h blood glucose level ≥ 200 mg/dL after a 75-g oral glucose tolerance test, hemoglobin A1C ≥6.5%, or currently taking diabetes medication. Dyslipidemia was defined as low-density lipoprotein (LDL) cholesterol ≥140 mg/dL, high-density lipoprotein (HDL) cholesterol <40 mg/dL, triglycerides ≥150 mg/dL, or the current use of lipid-lowering agents. We combined hypertension, diabetes mellitus and dyslipidemia as cardiovascular risk factors. Serum Brain-derived neurotrophic factor levels (BDNF) were measured using a multiplex assay (MILLIPLEX MAP Human Myokine Magnetic Bead Panel; Merck, Darmstadt, Germany). Hippocampus volume (Vol) was assessed by Magnetic Resonance Imaging using a 0.3 T clinical MR scanner (AIRIS Vento, Hitachi, Tokyo, Japan). Hippocampus Vol was corrected by intracranial volume (ICV). Mild cognitive impairment was defined as a score ≤ 22 on the Japanese version of the Montreal Cognitive Assessment (MoCA-J).
2.5 Statistical analysis
All participants were divided into four groups according to their exercise habits in adolescence and old age as follows: (1) never exercised (none-none; NN), (2) exercised only in old age (none-active; NA), (3) exercised only in adolescence (active-none; AN), and (4) exercised in adolescence and old age (active-active; AA). Continuous variables were summarized by presenting the median and interquartile range of patients who contributed values. Categorical variables were summarized by presenting the frequency and proportion of patients in each category. Characteristics were compared using the Kruskal–Wallis and chi-squared tests for continuous and categorical variables, respectively. The NN group was used as the reference group, and logistic regression analysis was performed to estimate the odds ratio (ORs) and 95% confidence interval (CIs) for the prevalence of geriatric depressive symptoms in each group. Model 1 was adjusted for the following potential covariates: age (continuous variables), years of education (continuous variables), BMI (continuous variables), Brinkman index (continuous variables), and alcohol intake (continuous variables). Model 2 was adjusted for model 1 covariables plus diabetes mellitus (yes or no), cerebrovascular disease (yes or no), and mild cognitive impairment (yes or no). Model 3 was adjusted for model 2 covariables plus living alone (yes or no). Subsequently, multiple regression analysis was performed to determine the independent contribution of exercise habits in each age and potential covariates to the GDS-15 score; differences among the four groups were compared using analysis of covariance (ANCOVA), adjusted for the potential confounders as follows: age (continuous variable), years of education (continuous variables), BMI (continuous variable), Brinkman index (continuous variable), alcohol intake (continuous variables), former and current smoking status (yes or no), diabetes mellitus (yes or no), cerebrovascular disease (yes or no), mild cognitive impairment (yes or no) and living alone (yes or no). We adjusted for multiple comparisons using post-hoc Bonferroni correction. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 28.0 (IBM Corp., Armonk, NY, USA). All tests were two-sided, with a 5% significance level.
3 Results
3.1 Demographic and baseline characteristics
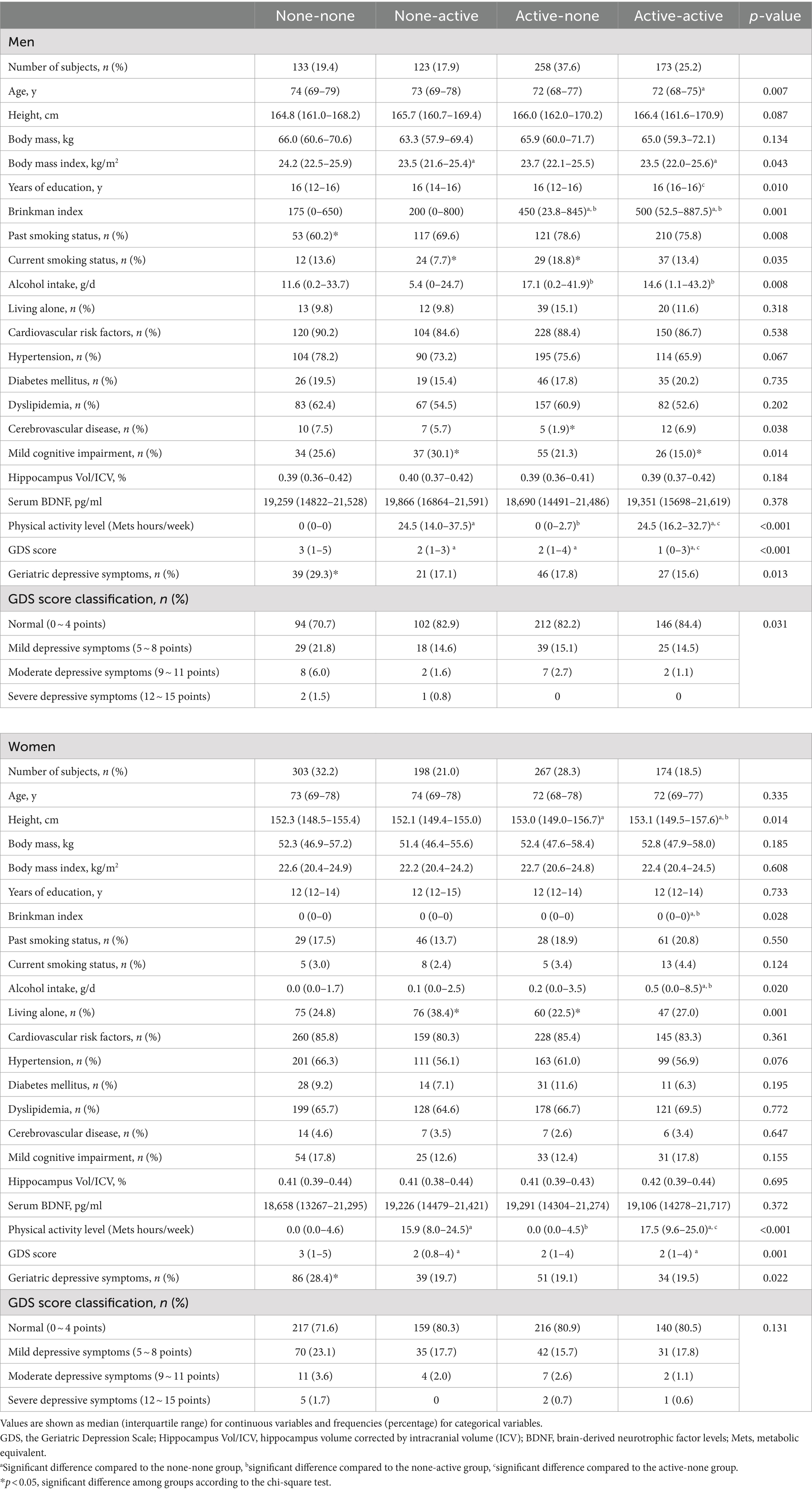
The characteristics of the participants in the four groups, classified by the combination of exercise habits in adolescence and old age, are shown in Table 1. Among men, the participants were significantly younger in the AA group than in the NN group, and the BMI in the NA and AA groups was significantly lower than that in the NN group. The Brinkman indices of the AN and AA groups were significantly higher than those of the NN and NA groups. Alcohol intake was significantly higher in the AN and AA groups than in the NA group. The GDS-15 scores of the NA, AN, and AA groups were significantly lower than those of the NN group. Moreover, it was significantly lower in the AA group than in the AN group. Among participants with depressive symptoms, mild depressive symptoms were the most common, while severe depressive symptoms were the least frequent in both men and women. Among all categories of depressive symptoms, the NN group had the highest prevalence. The prevalence of geriatric depressive symptoms in men in the four groups was 29.3, 17.1, 17.8, and 15.6%, respectively. The prevalence of geriatric depressive symptoms was highest in the NN group. Among the women, age was comparable among the four groups. The Brinkman index and alcohol intake were significantly higher in the AA group than in the NN and NA groups. The GDS-15 scores in the NA and AA groups were significantly lower than those in the NN group, and the prevalence of geriatric depressive symptoms in women in the four groups was 28.4, 19.7, 19.1, and 19.5%, respectively. The prevalence of geriatric depressive symptoms was highest in the NN group.
3.2 Association between exercise habits among the four groups and the ORs of geriatric depressive symptoms
The ORs for geriatric depressive symptoms in the NA, AN, and AA groups compared with the NN group are shown in Table 2. In men, the ORs for geriatric depressive symptoms were significantly lower in the NA, AN, and AA groups than in the NN group after adjusting for model 3 (NA; OR: 0.48, 95% CI: 0.26–0.90; AN; OR: 0.51, 95%CI: 0.31–0.86; AA; OR: 0.45, 95%CI: 0.25–0.81). In women, the ORs for geriatric depressive symptoms were significantly lower in the NA, AN, and AA groups than in the NN group after adjusting for model 3 (NA; OR: 0.63, 95% CI: 0.40–0.97; AN; OR: 0.59, 95%CI: 0.40–0.89; AA; OR: 0.63, 95%CI: 0.40–0.99).
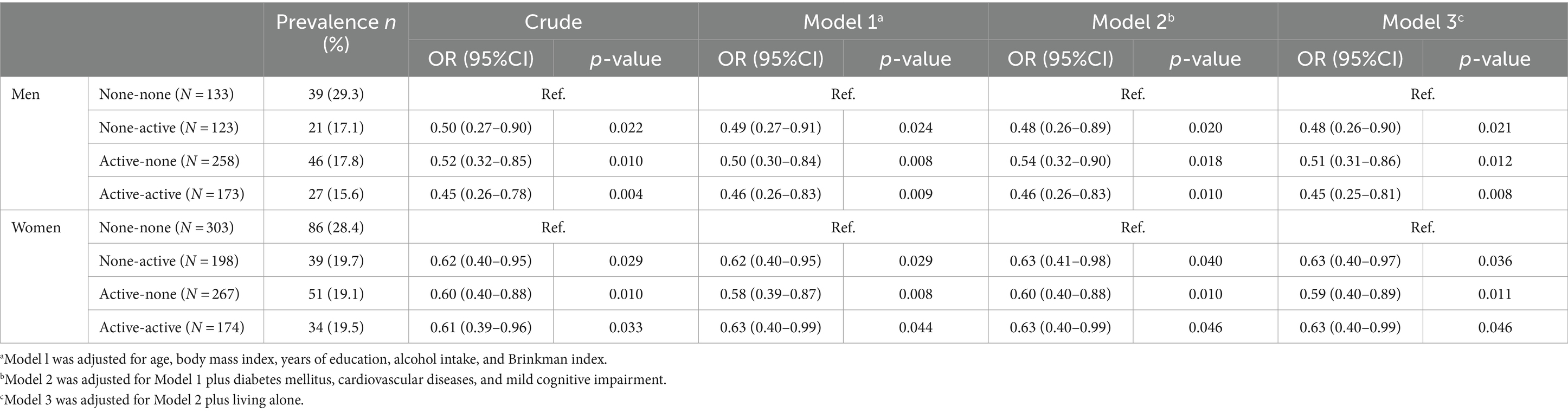
Table 2. Association between the combination of exercise habits and prevalence of geriatric depressive symptoms.
3.3 Comparison of the GDS-15 scores among four groups
Differences in the GDS-15 scores among the four groups were compared using ANCOVA after adjusting for potential confounders (Figure 1). In men, the GDS-15 scores were significantly higher in the NN group than in the other three groups (NA: p = 0.003; AN: p = 0.010; AA: p < 0.001). However, in women, the GDS-15 scores were significantly higher in the NN group than in the NA (p = 0.004) and AA groups (p = 0.007).
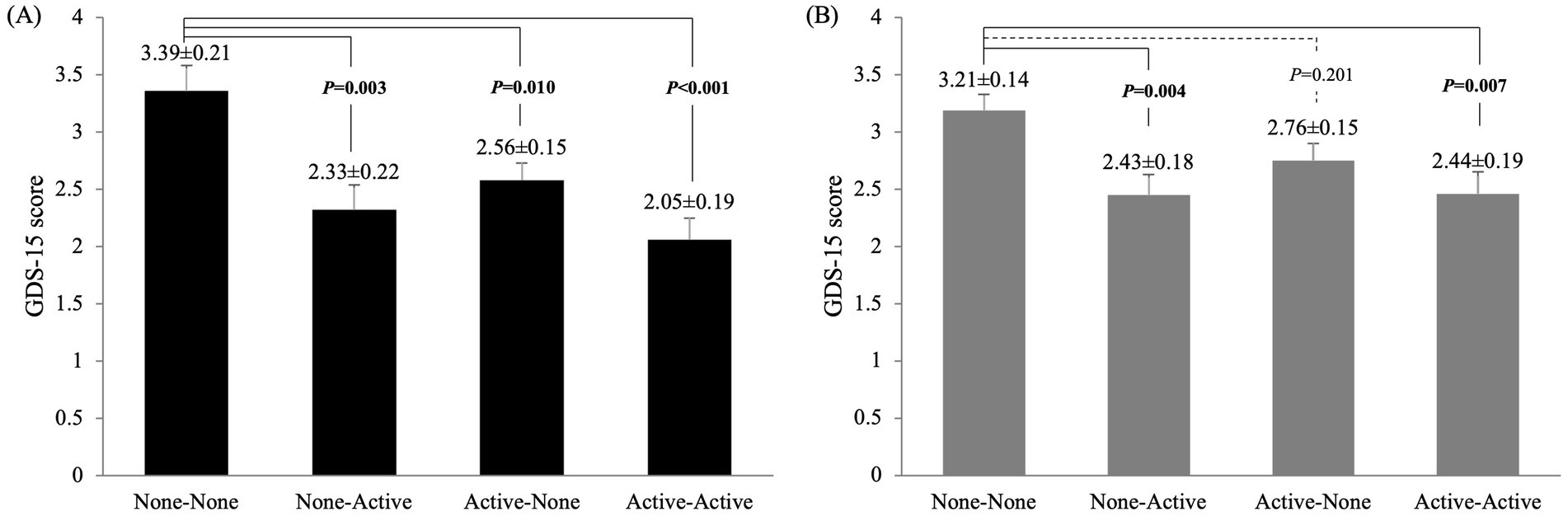
Figure 1. Comparison of GDS-15 scores among the four groups in (A) men and (B) women: no exercise in either period (none-none), exercise only in adolescence (active-none), exercise only in old age (none-active), and exercise in both periods (active-active). Values are presented as means ± SE. Adjusted variables: age, years of education, alcohol intake, Brinkman index, diabetes mellitus, cardiovascular disease, mild cognitive impairment and living alone.
4 Discussion
In this study, we investigated the association between exercise habits in adolescence, old age, or both and the prevalence of geriatric depressive symptoms. The prevalence of geriatric depressive symptoms in the NA, AN, and AA groups was significantly lower than that in the NN group for both men and women.
We found that exercise habits in adolescence and old age were associated with a lower prevalence of geriatric depressive symptoms in both men and women. These results partially supported our hypothesis that exercise habits in both age groups might protect against geriatric depressive symptoms. Previous research has demonstrated that regular exercise in old age can benefit geriatric depression (16–18) and that regular exercise in adolescence is associated with a lower prevalence of depression in adolescence (19), which may affect the early onset of geriatric depression. Therefore, our findings are consistent with these results. Regular exercise can promote positive emotions and self-efficacy in adolescence (20, 21) and old age (22). Increased self-efficacy in individuals with exercise habits may promote greater well-being and reduce depressive symptoms later in life (23). However, our study did not find a synergistic effect of exercise habits on geriatric depressive symptoms in adolescence or old age. The reasons for this are unclear, and further research is required.
Conversely, the logistic regression analysis indicated an increased risk of geriatric depressive symptoms only in the NN group. We enquired about exercise habits from the 20s to the 50s and drew trajectories of exercise habits for depressed and non-depressed individuals (Supplementary Figure S1). We found that the proportion of people with exercise habits in the geriatric depressive symptoms group was lower than that in the non-geriatric depressive symptoms group at almost all ages, both for men and women. This diminished consistency in exercise habits over the lifespan mirrors the pattern observed in the NN group (Supplementary Figure S2), suggesting that this led to higher ORs for geriatric depressive symptoms in the NN group. Since physical activity is reduced in depressed individuals (24), it is not clear whether the absence of an exercise habit is associated with geriatric depression or whether people prone to geriatric depression simply do not like exercise. Therefore, further longitudinal studies and analyses, including the effects of genetic polymorphisms favoring exercise and the genes responsible for geriatric depression, are needed to clarify this causal relationship.
We found that exercise habits in adolescence and old age had different effects on GDS-15 scores in men and women. In men, the GDS-15 scores were significantly higher in the NN group than in the other three groups. In women, the GDS-15 scores were significantly lower in the NA and AA groups than in the NN group; however, there was no significant difference between the AN and NN groups, which was generally consistent with the results of the logistic analysis, except for the AN group in women.
The present study had some limitations. First, the participants were all older adults living in urban areas (Bunkyo-Ku, Tokyo, Japan), which may have introduced a selection bias. Education level positively correlates with cognitive function in older adults (25, 26) and can moderate the adverse effects of geriatric depression (27). In the present cohort study, participants from similar studies in Japan had a higher level of education than general older adults (11, 28, 29). Second, self-reported exercise habits may have been affected by recall bias, as participants were asked to recall their exercise habits up to 50–60 years prior. “Bukatsudo” is a traditional sports club activity commonly performed in junior and senior high schools in Japan as part of the educational curriculum (30). Students who participate in “Bukatsudo” generally engage in three to three and a half hours of daily practice 6 days a week, regardless of vacations (31). Therefore, the recall bias was likely limited. Third, this study did not account for the specific number of years participants engaged in exercise. However, it has been reported that once a student joins a club, they typically continue participating in the same activities for 3 years (30). Another potential limitation is that all study participants had at least a junior high school education, and the impact of exercise habits on mental health may differ for individuals with a lower educational level. Therefore, future studies should aim to include participants with more diverse educational backgrounds and conduct analyses that incorporate detailed information on adolescent exercise habits to better understand their long-term effects on mental health.
In conclusion, the present study showed that older adults with exercise habits in adolescence and/or old age had a lower prevalence of geriatric depressive symptoms. Thus, it is suggested that having an exercise habit in adolescence or old age may be beneficial for good mental health in old age.
Previous presentation
The data of this study have been presented via poster previously by Huicong Shi at the 9th Asian Conference for Frailty and sarcopenia on October 28, 2023, in Singapore. The title of this talk was “Effect of exercise habits in adolescence and older age on Geriatric depression: the Bunkyo Health Study.”
Data availability statement
Some or all datasets generated and/or analyzed during the current study are not publicly available because participant consent for public data sharing was not obtained at the time of data collection; however, they can be obtained from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by the study protocol was approved by the Ethics Committee of Juntendo University in September 2015 (first approval no. 2015061 and the latest revised version no. M15-0057-M08). The study was conducted in accordance with the principles of the Declaration of Helsinki. All participants were given a clear explanation of the study’s details. Following this, they provided written informed consent, indicating their voluntary agreement to participate. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HS: Formal analysis, Investigation, Methodology, Writing – original draft, Visualization, Writing – review & editing. HT: Methodology, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Supervision, Validation, Writing – original draft. HO: Investigation, Writing – review & editing, Methodology. HK: Data curation, Funding acquisition, Investigation, Writing – review & editing. YS: Data curation, Investigation, Writing – review & editing. AA: Investigation, Writing – review & editing. SK: Investigation, Writing – review & editing, Data curation. HN: Data curation, Investigation, Writing – review & editing. NI: Investigation, Writing – review & editing. TT: Investigation, Writing – review & editing. YY: Investigation, Writing – review & editing. RK: Project administration, Writing – review & editing, Funding acquisition. HW: Funding acquisition, Writing – review & editing, Project administration. YT: Supervision, Validation, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (S1411006); JSPS KAKENHI Grant Numbers JP18H03184 and JP20K23261; the Mizuno Sports Promotion Foundation; and the Mitsui Life Social Welfare Foundation. Y.T. and R.K. received research support from Curves Japan Co. Ltd. Y.T. received research support from LOTTE Co., Ltd. and Imasen Electric Industrial Co., Ltd. The funders Curves Japan Co. Ltd., LOTTE Co., Ltd. and Imasen Electric Industrial Co., Ltd were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We are grateful to all participants of the Bunkyo Health Study. We would like to express our gratitude to all the members of the Sportology Center, Juntendo University for their invaluable advice and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1405666/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Exercise participation rate (%) by ages for the non-geriatric depressive symptoms group and geriatric depressive symptoms group. (A) Men; (B) women. *p < 0.05, significant difference between non-geriatric depressive symptoms group and geriatric depressive symptoms group, **p < 0.01, significant difference between non-geriatric depressive symptoms group and geriatric depressive symptoms group.
SUPPLEMENTARY FIGURE S2 | Exercise participation rate (%) by four exercise groups: no exercise in either period (none-none), exercise only in adolescence (active-none), exercise only in old age (none-active), and exercise in both periods (active-active). (A) Men; (B) women. †p < 0.05 for significant differences compared to the None-None group, #p < 0.05 for significant differences compared to the none-active group, §p < 0.05 for significant differences compared to the active-none group for the Chi-squared tests.
References
1. Noël, PH, Williams, JW, Unützer, J, Worchel, J, Lee, S, Cornell, J, et al. Depression and comorbid illness in elderly primary care patients: impact on multiple domains of health status and well-being. Ann Fam Med. (2004) 2:555–62. doi: 10.1370/afm.143
2. Blazer, DG . Depression in late life: review and commentary. J Gerontol Ser A Biol Med Sci. (2003) 58:M249–65. doi: 10.1093/gerona/58.3.M249
3. Kok, RM, and Reynolds, CF 3rd. Management of Depression in older adults: a review. JAMA. (2017) 317:2114–22. doi: 10.1001/jama.2017.5706
4. Taylor, WD . Depression in the elderly. N Engl J Med. (2014) 371:1228–36. doi: 10.1056/NEJMcp1402180
5. Hegeman, JM, Kok, RM, Van Der Mast, RC, and Giltay, EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry. (2012) 200:275–81. doi: 10.1192/bjp.bp.111.095950
6. Kiosses, DN, and Alexopoulos, GS. The prognostic significance of subsyndromal symptoms emerging after remission of late-life depression. Psychol Med. (2013) 43:341–50. doi: 10.1017/S0033291712000967
7. Blake, H, Mo, P, Malik, S, and Thomas, S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. (2009) 23:873–87. doi: 10.1177/0269215509337449
8. Mammen, G, and Faulkner, G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. (2013) 45:649–57. doi: 10.1016/j.amepre.2013.08.001
9. Hötting, K, and Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. (2013) 37:2243–57. doi: 10.1016/j.neubiorev.2013.04.005
10. Pittenger, C, and Duman, RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. (2008) 33:88–109. doi: 10.1038/sj.npp.1301574
11. Someya, Y, Tamura, Y, Kaga, H, Nojiri, S, Shimada, K, Daida, H, et al. Skeletal muscle function and need for long-term care of urban elderly people in Japan (the Bunkyo health study): a prospective cohort study. BMJ Open. (2019) 9:e031584. doi: 10.1136/bmjopen-2019-031584
12. Sheikh, JI, and Yesavage, JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol J Aging Mental Health. (1986) 5:165–73.
13. Nyunt, MSZ, Fones, C, Niti, M, and Ng, T-P. Criterion-based validity and reliability of the geriatric depression screening scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Health. (2009) 13:376–82. doi: 10.1080/13607860902861027
14. Mitchell, AJ, Bird, V, Rizzo, M, and Meader, N. Diagnostic validity and added value of the geriatric depression scale for depression in primary care: a meta-analysis of GDS30 and GDS15. J Affect Disord. (2010) 125:10–7. doi: 10.1016/j.jad.2009.08.019
15. Kobayashi, S, Honda, S, Murakami, K, Sasaki, S, Okubo, H, Hirota, N, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. (2012) 22:151–9. doi: 10.2188/jea.JE20110075
16. Blumenthal, JA, Babyak, MA, Moore, KA, Craighead, WE, Herman, S, Khatri, P, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. (1999) 159:2349–56. doi: 10.1001/archinte.159.19.2349
17. Mura, G, and Carta, MG. Physical activity in depressed elderly. A systematic review. Clin Pract Epidemiol Ment Health. (2013) 9:125–35. doi: 10.2174/1745017901309010125
18. Strawbridge, WJ, Deleger, S, Roberts, RE, and Kaplan, GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. (2002) 156:328–34. doi: 10.1093/aje/kwf047
19. Craft, LL, and Perna, FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. (2004) 6:104–11. doi: 10.4088/PCC.v06n0301
20. Biddle, SJ, and Asare, M. Physical activity and mental health in children and adolescents: a review of reviews. Br J Sports Med. (2011) 45:886–95. doi: 10.1136/bjsports-2011-090185
21. Steptoe, A, and Butler, N. Sports participation and emotional wellbeing in adolescents. Lancet. (1996) 347:1789–92. doi: 10.1016/S0140-6736(96)91616-5
22. Mcauley, E, Mailey, EL, Mullen, SP, Szabo, AN, Wójcicki, TR, White, SM, et al. Growth trajectories of exercise self-efficacy in older adults: influence of measures and initial status. Health Psychol. (2011) 30:75–83. doi: 10.1037/a0021567
23. Maciejewski, PK, Prigerson, HG, and Mazure, CM. Self-efficacy as a mediator between stressful life events and depressive symptoms. Differences based on history of prior depression. Br J Psychiatry. (2000) 176:373–8. doi: 10.1192/bjp.176.4.373
24. Roshanaei-Moghaddam, B, Katon, WJ, and Russo, J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. (2009) 31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002
25. Bennett, DA, Wilson, R, Schneider, J, Evans, D, De Leon, CM, Arnold, S, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. (2003) 60:1909–15. doi: 10.1212/01.WNL.0000069923.64550.9F
26. Lövdén, M, Fratiglioni, L, Glymour, MM, Lindenberger, U, and Tucker-Drob, EM. Education and cognitive functioning across the life span. Psychol Sci Public Interest. (2020) 21:6–41. doi: 10.1177/1529100620920576
27. Lee, J, Park, H, and Chey, J. Education as a protective factor moderating the effect of depression on memory impairment in elderly women. Psychiatry Investig. (2018) 15:70–7. doi: 10.4306/pi.2018.15.1.70
28. Narazaki, K, Nofuji, Y, Honda, T, Matsuo, E, Yonemoto, K, and Kumagai, S. Normative data for the Montreal cognitive assessment in a Japanese community-dwelling older population. Neuroepidemiology. (2013) 40:23–9. doi: 10.1159/000339753
29. Sakuma, N, Ura, C, Miyamae, F, Inagaki, H, Ito, K, Niikawa, H, et al. Distribution of Mini-mental state examination scores among urban community-dwelling older adults in Japan. Int J Geriatr Psychiatry. (2017) 32:718–25. doi: 10.1002/gps.4513
30. Cave, P . " Bukatsudō": the educational role of Japanese school clubs. J Jap Stud. (2004) 30:383–415. doi: 10.1353/jjs.2004.0041
Keywords: physical activity, teenage, older adults, GDS-15, geriatric depression, prevalence, sport
Citation: Shi H, Tabata H, Otsuka H, Kaga H, Someya Y, Abudurezake A, Kakehi S, Naito H, Ito N, Tajima T, Yoshizawa Y, Kawamori R, Watada H and Tamura Y (2024) Exercise habits in adolescence and old age are positively associated with geriatric depressive symptoms: the Bunkyo Health Study. Front. Public Health. 12:1405666. doi: 10.3389/fpubh.2024.1405666
Edited by:
Daniela Stackeová, College of Physical Education and Sport Palestra, CzechiaReviewed by:
Ermilo Canton-Martinez, Universidad Autónoma de Baja California, MexicoQi Gao, National Centre for Infectious Diseases (NCID), Singapore
Copyright © 2024 Shi, Tabata, Otsuka, Kaga, Someya, Abudurezake, Kakehi, Naito, Ito, Tajima, Yoshizawa, Kawamori, Watada and Tamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroki Tabata, aC50YWJhdGEubXBAanVudGVuZG8uYWMuanA=; Yoshifumi Tamura, eXMtdGFtdXJAanVudGVuZG8uYWMuanA=
 Huicong Shi1,2
Huicong Shi1,2 Hiroki Tabata
Hiroki Tabata Hideyoshi Kaga
Hideyoshi Kaga Yuki Someya
Yuki Someya Abulaiti Abudurezake
Abulaiti Abudurezake Hitoshi Naito
Hitoshi Naito Yasuyo Yoshizawa
Yasuyo Yoshizawa Hirotaka Watada
Hirotaka Watada Yoshifumi Tamura
Yoshifumi Tamura