- Department of Nephrology, First Medical Center of Chinese PLA General Hospital, State Key Laboratory of Kidney Diseases, National Clinical Research Center for Kidney Diseases, Beijing Key Laboratory of Kidney Diseases Research, Beijing, China
Background: Population aging is a pivotal trend observed globally, and the exposure to heavy metals can exacerbate the aging process and lead to kidney damage. However, the impact of combined heavy metal exposure on renal function among older individuals remains elusive. Our study employs machine learning techniques to delve into the effects and underlying mechanisms of mixed exposure to heavy metals on the renal function of the aging population.
Methods: This study extracted comprehensive data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2015 and 2020. A total of 3,175 participants aged 60 years and above, with complete information on six metals – lead, cadmium, manganese, cobalt, mercury, and selenium, along with relevant covariates, were included in the study. To assess the impact of single or mixed metal exposure on the renal function of older adult individuals, various statistical techniques were employed: multiple logistic regression, weighted quantitative sum (WQS) regression, Bayesian kernel machine regression (BKMR), and mediation effects analysis.
Results: Multiple logistic regression revealed that selenium and manganese were protective factors for chronic kidney disease (CKD). Cobalt was a risk factor for CKD. High concentrations of lead, cadmium, and cobalt were risk factors for urinary albumin creatinine ratio (ACR). WQS analyses revealed that mixed metal exposure was positively correlated with estimated glomerular filtration rate (eGFR) but negatively correlated with CKD. Selenium and manganese can neutralize the effects of other metals on eGFR. Mixed metal exposure was positively correlated with ACR, with lead and cadmium having a substantial effect. Mediation analysis showed that uric acid (UA) had a mediating effect of 9.7% and −19.7% in the association between mixed metals exposure and proteinuria and CKD, respectively.
Conclusion: The impact of heavy metals on renal function in the older adult differs from that of adolescents and adults. This study suggests that elevated levels of mixed metals exposure are linked to proteinuria and CKD, with UA serving as a mediating factor.
1 Introduction
Population aging is a global concern. Globally, the population aged ≥60 years is estimated to be 9.01 million (12% of the total population); this number is expected to increase to 2 billion by 2050 because of the rise in life expectancy (1). By 2040, the number of Americans aged ≥65 years will reach 80.8 million, accounting for approximately 21.6% of the total population. Among them, the population aged ≥85 years will reach 14.4 million by 2040, an increase of 123% from 6.5 million in 2017 (2). In Europe, by 2060, the population aged ≥65 years will account for 28% of the European population (3). In China, by 2050, the population aged ≥65 years will increase to 400 million, accounting for 26.9% of the total population, of which the population aged ≥80 years will reach 150 million (4). During the aging process, the kidneys undergo progressive functional decline and macroscopic and microscopic histological changes, especially after 70 years of age (5). Despite significant structural and physiological changes, healthy older individuals seem to retain normal kidney function. As their renal reserve is substantially decreased, their kidneys are more susceptible to physiological, pathological, and toxicological challenges (6). However, there is relatively little research on the effect of environmental toxin exposure on the kidney health of older adult people.
Heavy metals are prevalent in various environmental media, such as air, soil, drinking water, and food; kidneys are important targets of heavy metal attacks (7). Older people may be exposed to toxic pollutants more frequently than in previous decades because of the extension of life expectancy and the increase in environmental pollution levels. They may also be exposed to higher levels of toxic pollutants, which increases the likelihood of kidney damage (8). Previous studies have found that metals and their combinations, including arsenic (As), lead (Pb), mercury (Hg), and cadmium (Cd), may affect kidney function in adolescents (9). Another study found that plasma levels of manganese (Mn), iron (Fe), and zinc (Zn) can prevent chronic kidney disease (CKD) in older people aged ≥90 years in long-lived areas (10). In individuals over 60 years of age, urinary copper (Cu) concentration is strongly positively correlated with ACR (11). The effect of heavy metals on renal function in older people might be significantly different from that in adults. However, research focusing on the effects of heavy metals on the renal function of older adult people is scarce. Furthermore, the role of metals in diseases often depends on their cooperation and interaction. A single metal is insufficient to comprehensively elucidate the occurrence and development of diseases. Therefore, it is worth exploring the effect of mixed metal exposure on the renal function of older adult people. In this study, we investigated the effects of six metals (Pb, Cd, Mn, cobalt (Co), Hg, and selenium (Se)) on renal function due to their known potential impacts on human health. These metals are commonly found in the environment and can be introduced into the body through various pathways, such as ingestion, inhalation, or skin contact (12). Exposure to these metals has been associated with a range of adverse health effects, including kidney damage, neurological problems, and developmental issues (13, 14). The potential pathophysiological mechanisms between metal exposure and renal health can be complex and multifaceted. Here are some possible pathways: oxidative stress, inflammation, direct toxicity, enzyme inhibition (15–17).
Moreover, the accumulation of uric acid (UA) in the body can lead to the occurrence of hyperuricemia or CKD (18). Moreover, there is a positive correlation between blood metal mixture and gout related results (19). However, there have been no studies reporting whether the effects of metal mixed exposure on the kidneys are mediated by UA. The aim of this study is to use cross-sectional survey data to elucidate the association between metal mixed exposure and kidney injury in the older adult, as well as the mediating role of UA.
2 Materials and methods
2.1 Study population
The National Health and Nutrition Examination Survey (NHANES) is an ongoing nationwide cross-sectional survey with data available at the Centers for Disease Control and Prevention in the United States. This research protocol was approved by the Research Ethics Review Committee of the National Center for Health Statistics in the United States. All participants provided written consent during recruitment. We included data from older adult participants aged ≥60 years between March 2015 and March 2020 (N = 5,323). Next, we excluded dialysis participants (N = 37) and individuals lacking information on all six metals (N = 1,458). We also excluded individuals with missing data on serum creatinine (Scr), urinary albumin creatinine ratio (ACR), age, body mass index (BMI), alcohol consumption, and UA (N = 653). Finally, we enrolled 3,175 patients, of whom 639 were diagnosed with CKD (Figure 1).
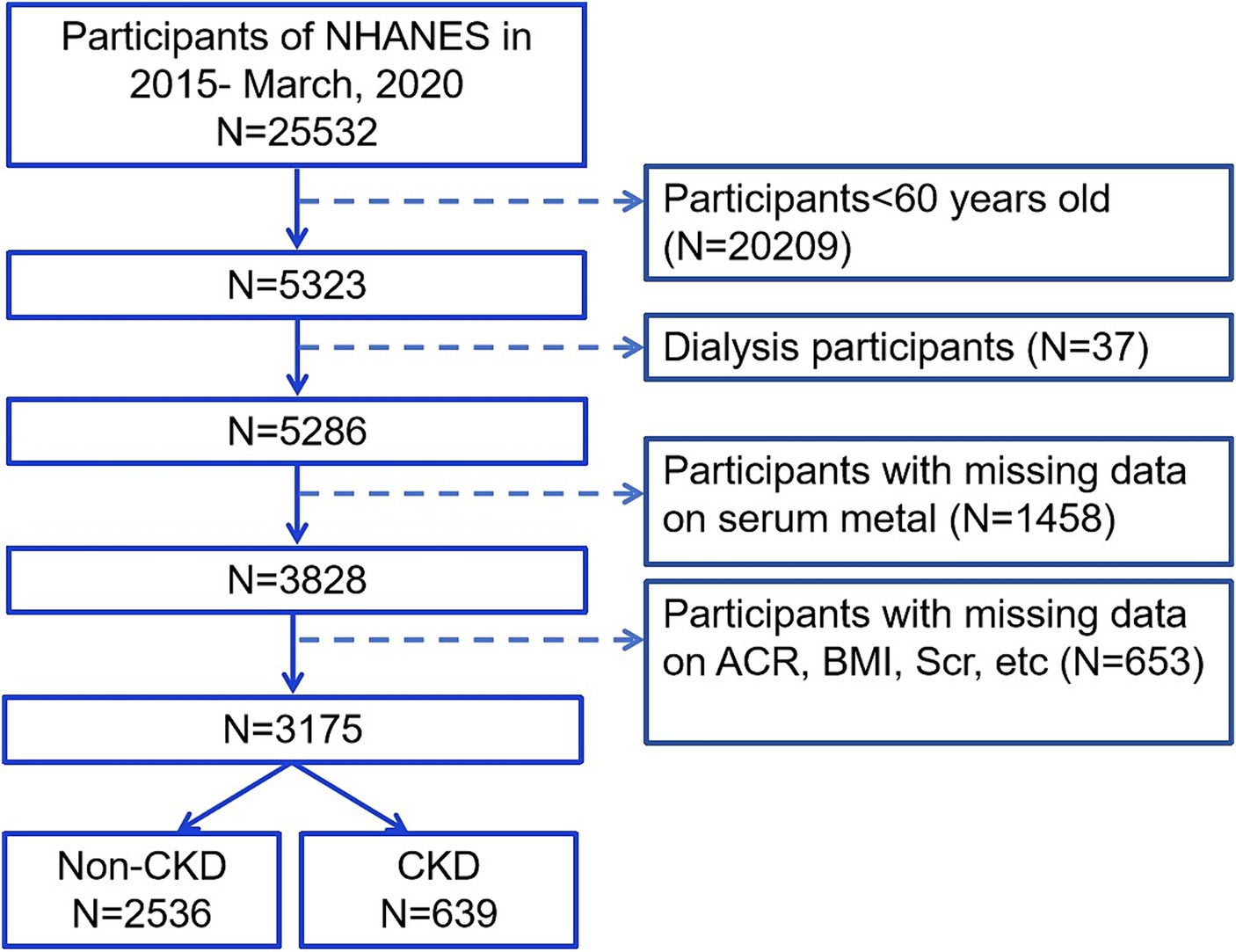
Figure 1. Flow chart of the inclusion subjects. Scr, serum creatinine; ACR, urinary albumin creatinine ratio; BMI, body mass index; CKD, chronic kidney disease.
2.2 Renal function assessment
We used estimated glomerular filtration rate (eGFR) and ACR to evaluate renal function. eGFR is based on the Chronic Kidney Disease Epidemiological Collaboration equation (20). The CKD-EPI equation, expressed as a single equation, is GFR = 141 × min(Scr/κ,1) ɑ × max(Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, ɑ is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1. We conducted continuous and binary result analyses on eGFR and ACR. As a binary result, eGFR below 60 mL/min/1.73 m2 was used to define CKD, and proteinuria was defined based on ACR ≥ 30 mg/g (21).
2.3 Measurement of blood chromium (Cr), cobalt (co), Pb, cd, Mn, selenium (se), and hg
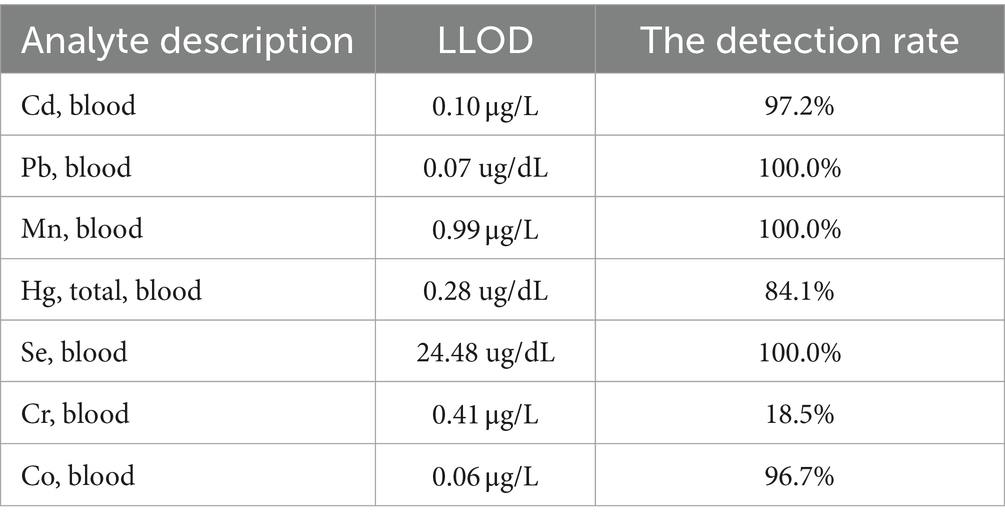
All measurements of metal exposure were based on whole blood samples. All measurements were conducted at the National Center for Environmental Health and Centers for Disease Control and Prevention in Atlanta, Georgia. Cr and Co concentrations were measured using inductively coupled plasma mass spectrometry. However, Cr was not used in the analysis as Cr levels were below the minimum detection limit in 81.5% of the participants. Moreover, after the dilution sample preparation step, mass spectrometry was used to measure the concentrations of Pb, Cd, total Hg, Mn, and Se in whole blood. The detection rate and LLOD of the seven metals were presented in Table 1.
2.4 Statistical analysis
2.4.1 Baseline statistical methods
All analyses were conducted using R (version 4.3.1). The demographic characteristics were compared between CKD status groups through chi-square test, t-test, and Mann–Whitney U test for categorical variables, normal continuous variables, and nonnormal continuous variables. Metal concentrations were categorized into four quartiles (Q1, Q2, Q3, and Q4) as categorical variables. Multivariate logistic regression was used to estimate the odds ratio (OR) and corresponding 95% confidence interval (CI) of metals related to CKD risk and ACR. All analyses were adjusted for age, gender, race/ethnicity, education, drinking status, BMI, smoking status, hypertension, and diabetes history.
2.4.2 WQS
Weighted quantile sum (WQS) regression was used to explore the overall effect of metals on CKD and ACR, as it performs well in characterizing environmental mixtures. The R package gWQS ver 3.0.5 was used to calculate the WQS index, which comprises the weighted sum of individual metal concentrations based on experience. The WQS index (ranging from 0 to 1) represents the mixed exposure level of metals, and the components of interest are determined by non-negligible weights. The final result was explained as the synchronous effect of adding one quartile to the mixed metal on CKD and ACR.
2.4.3 BKMR
Considering the potential nonlinear and nonadditive relationships among metals, Bayesian kernel machine regression (BKMR) was used to evaluate the mixed effects of all metals and the dose–response relationship between a single metal and CKD and ACR risks when fixing other metal concentrations (22). We mainly used the BKMR model to explore and visualize the following three exposure-response functions. (1) The univariate exposure-response function of single heavy metal exposure on CKD and ACR risk while fixing the other five metals at their corresponding median concentrations; (2) cumulative effects of metal mixtures at different quantiles on CKD and ACR risk compared to setting all mixtures at median concentrations; and (3) the posterior inclusion probability (PIP) of each metal in the mixture to determine the metal with the most significant effect on CKD risk.
We implemented a BKMR variable selection model with 10,000 iterations using the Markov Chain Monte Carlo algorithm. We then studied the convergence of the model by visually examining the trajectory map. The BKMR model was analyzed using the R package bkmr ver 0.2.2.
2.4.4 Mediation analysis
Finally, mediation analysis allows us to calculate how many mediated effects are produced by other factors. In addition to providing statistical evidence for mechanistic analyses, this strategy is ideal for revealing pathways. The direct effect indicates the association between mixed metals and eGFR or ACR. The indirect effect indicates the association is mediated by other factors. The mediation ratio indicates the percentage of mediated effects. The WQS is a method for evaluating a mixture-outcome association by calculating a summary score for the mixture. If we consider the mixed metal exposure as a score, it becomes straightforward to treat it as any other variable. There are studies adopting similar methodologies, such as Wu et al. investigating the mediator between volatile organic compound co-exposure and kidney stones (23). Mediation analysis was performed using the R package mediation ver 4.5.0.
3 Results
3.1 Demographic characteristics
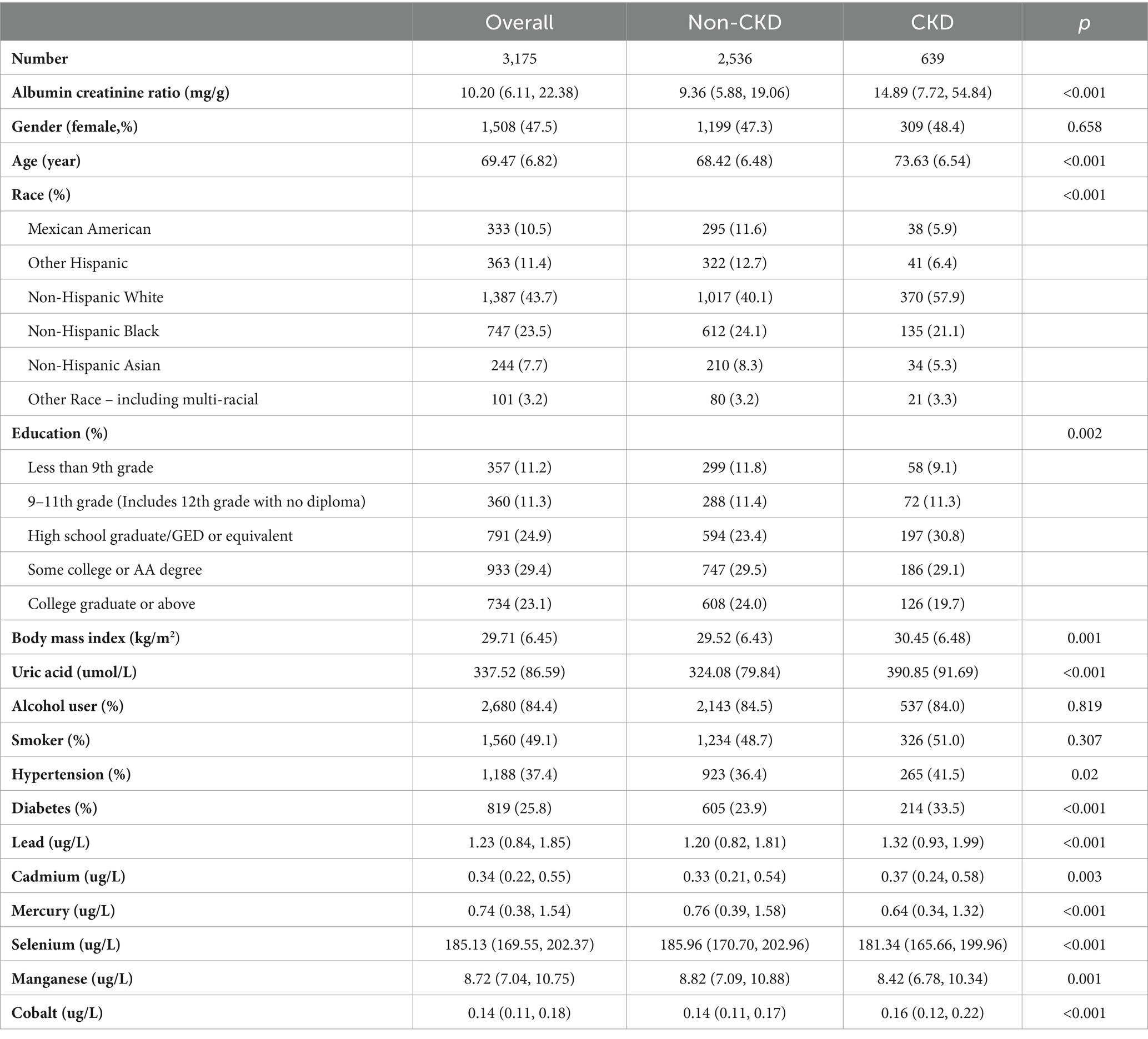
Of 3,175 older adult people, 639 were diagnosed with CKD (Table 2). The demographic characteristics of study participants with or without CKD. There were significant differences in age, race, BMI, education level, ACR, uric acid, hypertension, and diabetes history between the CKD and non-CKD groups.
3.2 Distribution and correlation of metals in the blood
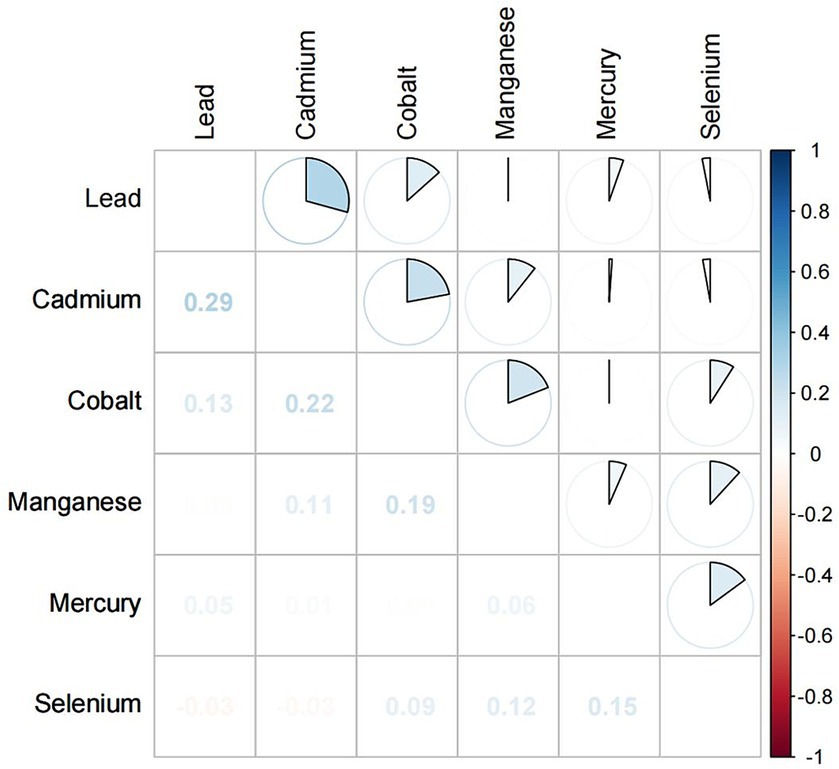
There were significant differences in the serum concentrations of Pb, Cd, Hg, Se, Mn, and Co between the CKD and non-CKD groups. The CKD group had significantly higher concentrations of Pb, Cd, and Co than the non-CKD group. Spearman correlation analysis showed that the correlation coefficients of Cd and Pb, Cd and Co were 0.29 and 0.22, respectively (Figure 2).
3.3 Multiple logistic regression analysis
Multiple logistic regression analysis revealed significant correlation between the concentrations of Se (Q3 vs. Q1; OR, 0.68; 95% CI, 0.51–0.91; p = 0.010), Mn (Q4 vs. Q1; OR, 0.62; 95% CI, 0.45–0.83; p = 0.002), and Co (Q4 vs. Q1; OR, 1.58; 95% CI, 1.19–2.12; p = 0.002) and the incidence of CKD (Figure 3A). Meanwhile, high concentrations of Pb (Q4 vs. Q1; OR, 1.70; 95% CI, 1.27–2.27; p < 0.001), Cd (Q4 vs. Q1; OR, 1.39; 95% CI, 1.04–1.87; p = 0.029), and Co (Q3 vs. Q1; OR, 1.33; 95% CI, 1.02–1.74; p = 0.037) were identified as risk factors for proteinuria (Figure 3B). Continuous outcome results also be provided in Figures 3C,D.
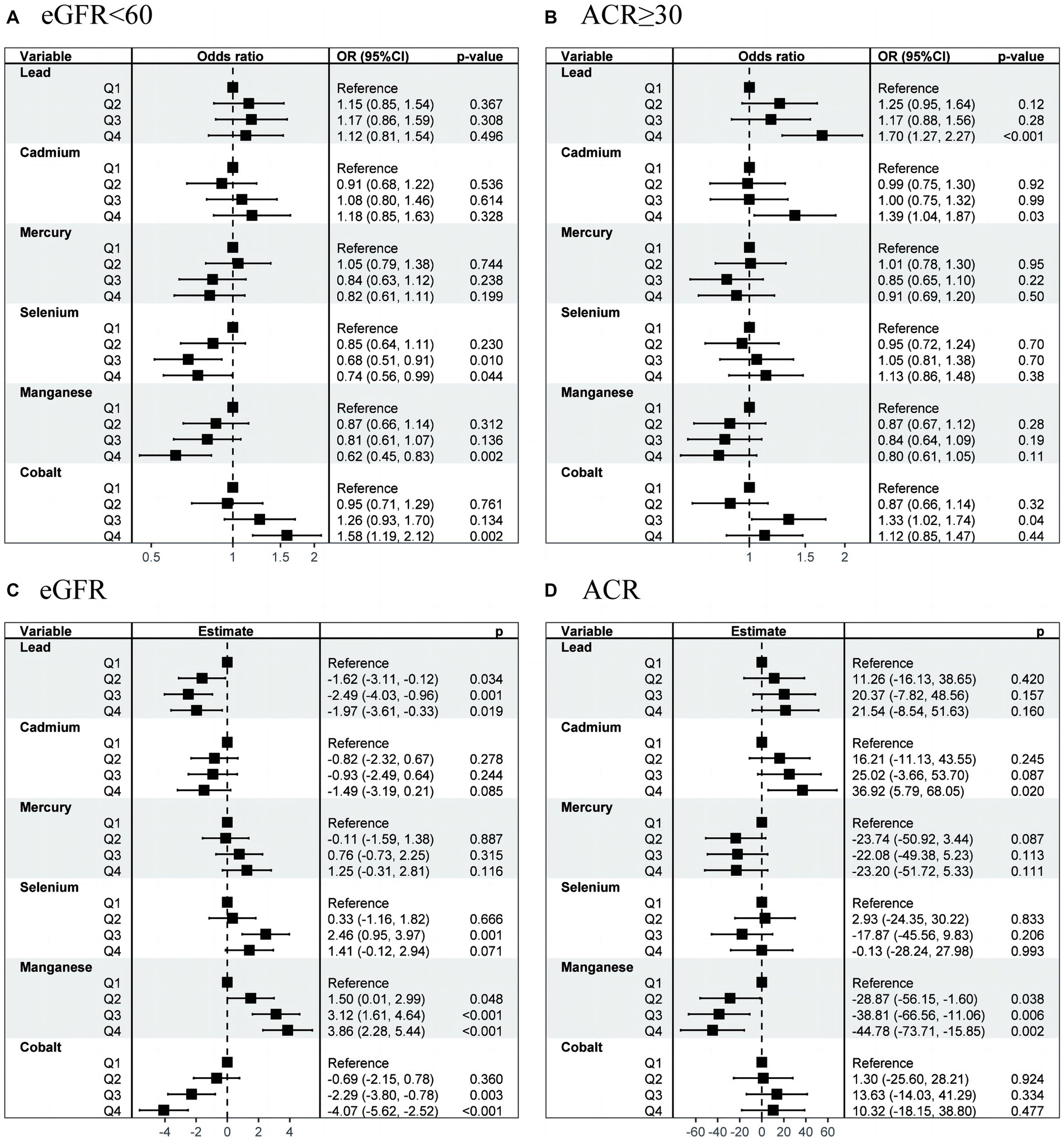
Figure 3. OR (95% CI) in eGFR, CKD, ACR, and proteinuria associated with single metals levels. OR, odds ratio; CI, confidence interval; CKD, chronic kidney disease.
3.4 WQS analysis
We preliminarily studied the effects of mixed serum heavy metal exposure on eGFR, ACR continuous variables, and CKD and proteinuria binary variables (Figure 4). Mixed metal exposure was positively correlated with eGFR but negatively correlated with CKD. Se, Hg, and Mn had significant effects on eGFR and CKD. Mixed metal exposure was positively correlated with ACR and proteinuria, and the weights of Pb, Cd, and Co metals were relatively high, consistent with logistic regression results.
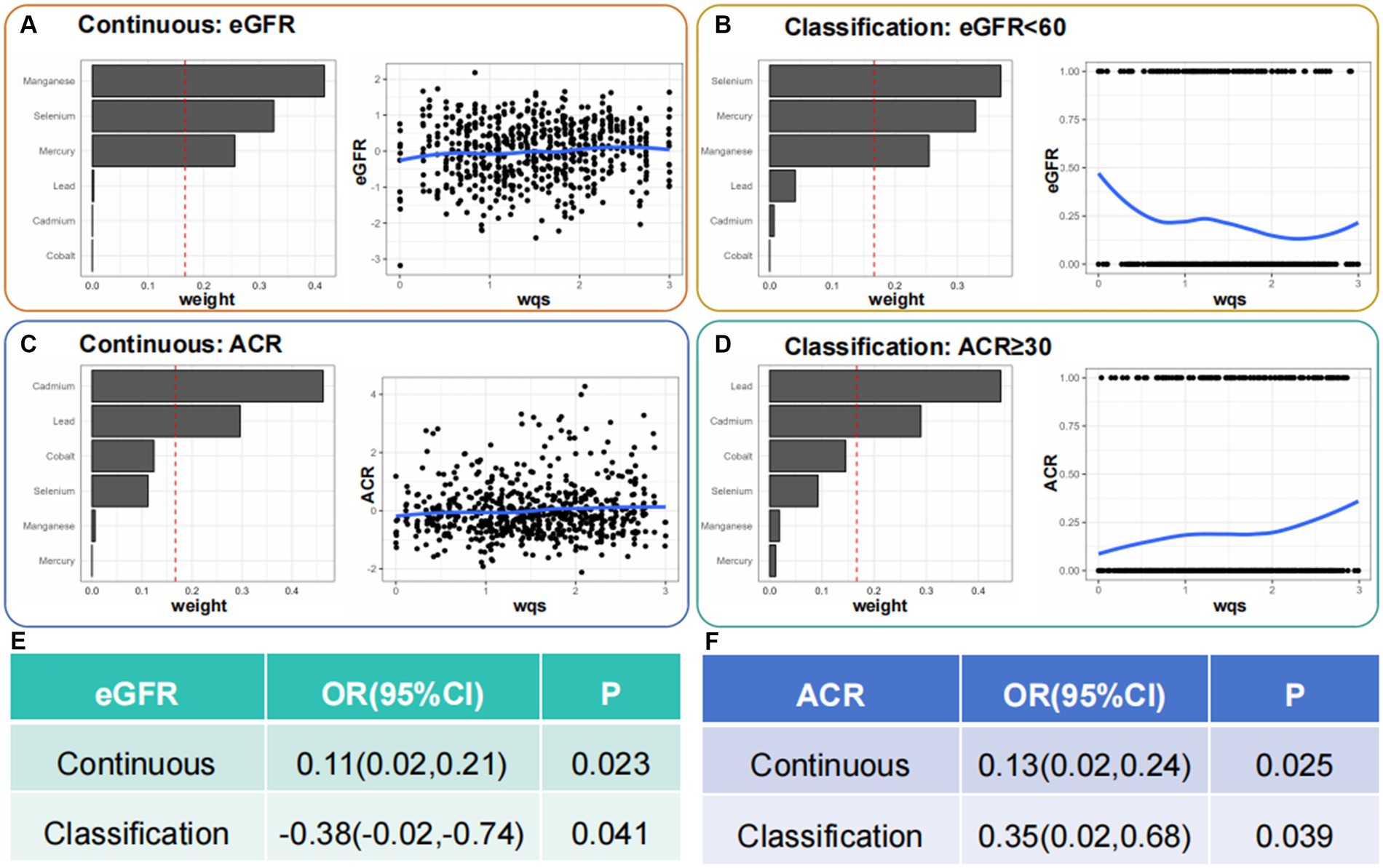
Figure 4. Identification of blood heavy metals in the mixture using the WQS model. WQS, weighted quantile sum.
3.5 BKMR analysis
We further investigated the relationship between the co-exposure to six blood heavy metals and eGFR, CKD, ACR, and proteinuria using the BKMR model (Figures 5, 6). The Figure 5 shows a negative correlation between mixed exposure to six metals and CKD. Co, Se, and Mn had the highest weight proportion in mixed metal exposure. The exposure level of other blood heavy metals was set at the median to evaluate the effect of a single metal exposure reaction function. Co was positively correlated with CKD, Mn was negatively correlated with CKD, and Se was positively U-shaped correlated with CKD. Mixed exposure to six metals was positively correlated with proteinuria in the older adult participants, with Cd, Pb, and Se weighing the highest. Figure 6 shows the correlation between each metal and ACR.
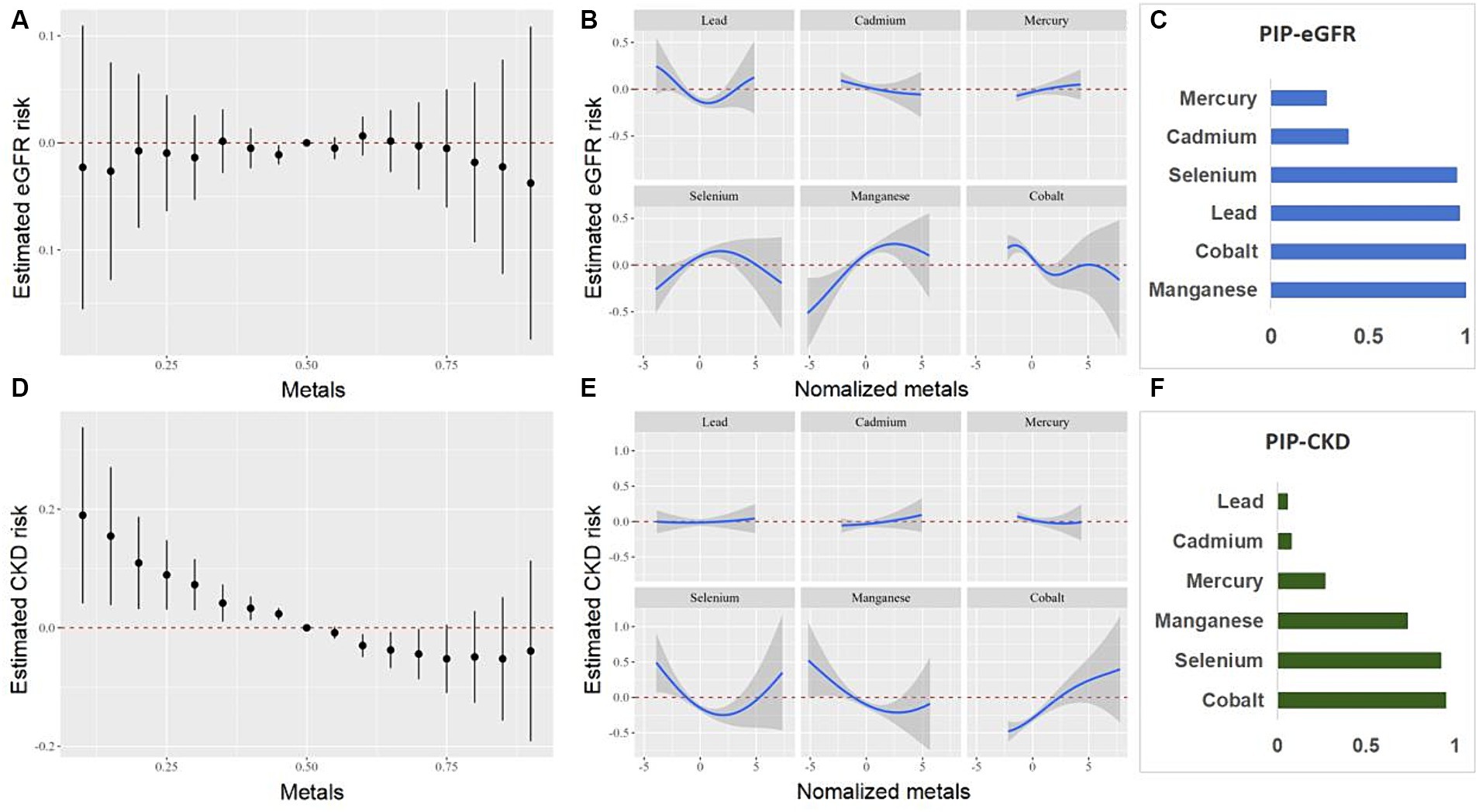
Figure 5. Combined effects of the metals as a mixture on eGFR and CKD in older adult people. eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease.
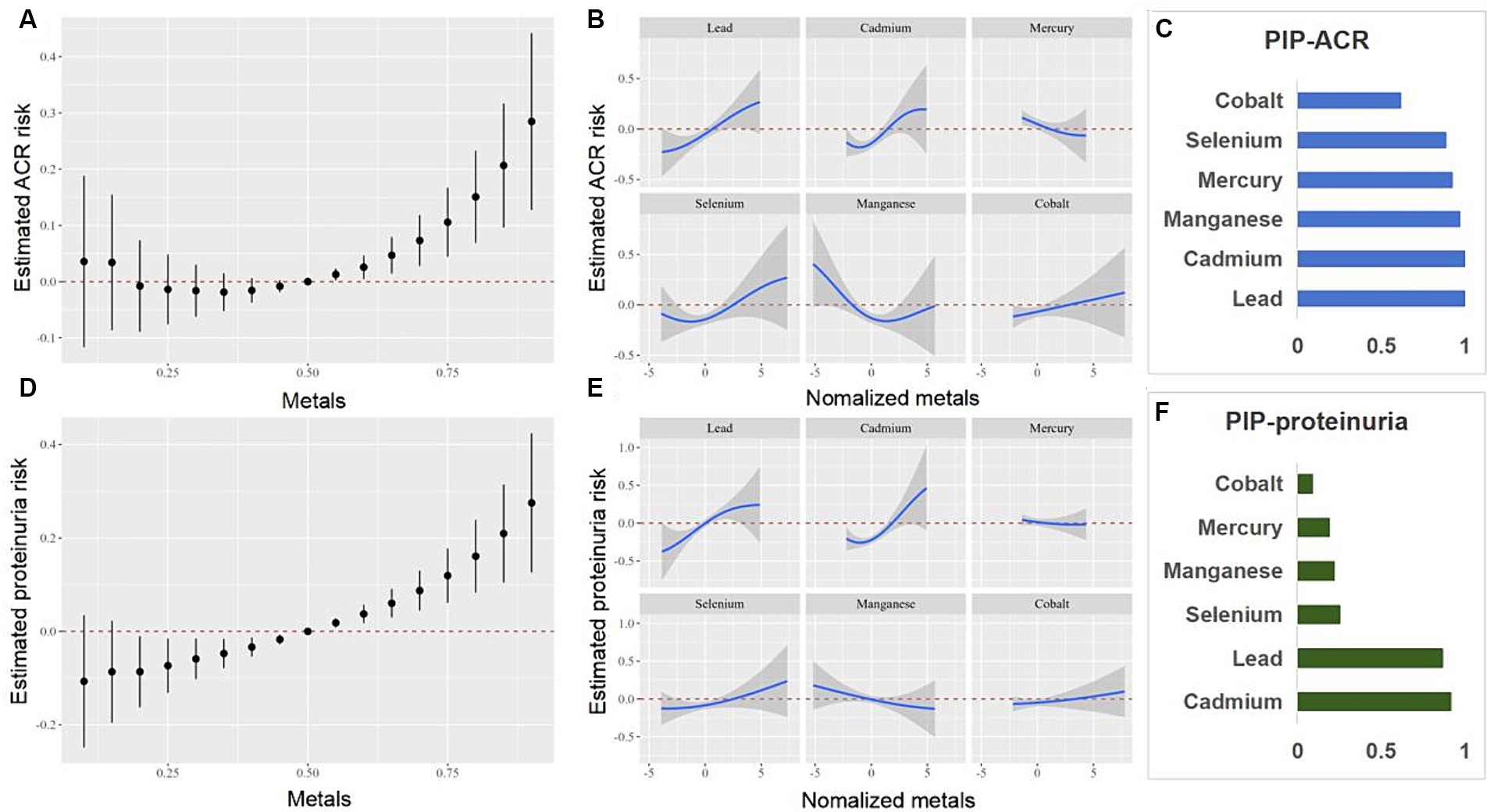
Figure 6. Combined effects of the metals as a mixture on ACR and proteinuria in older adult people. ACR, urinary albumin creatinine ratio.
3.6 Mediation of UA
We have performed mediation analyses to assess whether UA mediates the association between metals and the occurrence of proteinuria and CKD. The model and pathway for the mediation analysis are shown in Figure 7. After adjusting for all potential confounders, the results showed approximately 9.7% ([95% CI, 3.5–29.1%]; p = 0.003) of the effect of mixed metals exposure on proteinuria was mediated by UA. Approximately −19.7% ([95% CI, −64.2 – −1.9%]; p = 0.03) of the effect of mixed metals exposure on CKD was mediated by UA. The mediating effect of UA between mixed metals and eGFR and ACR is also presented in Figure 7.
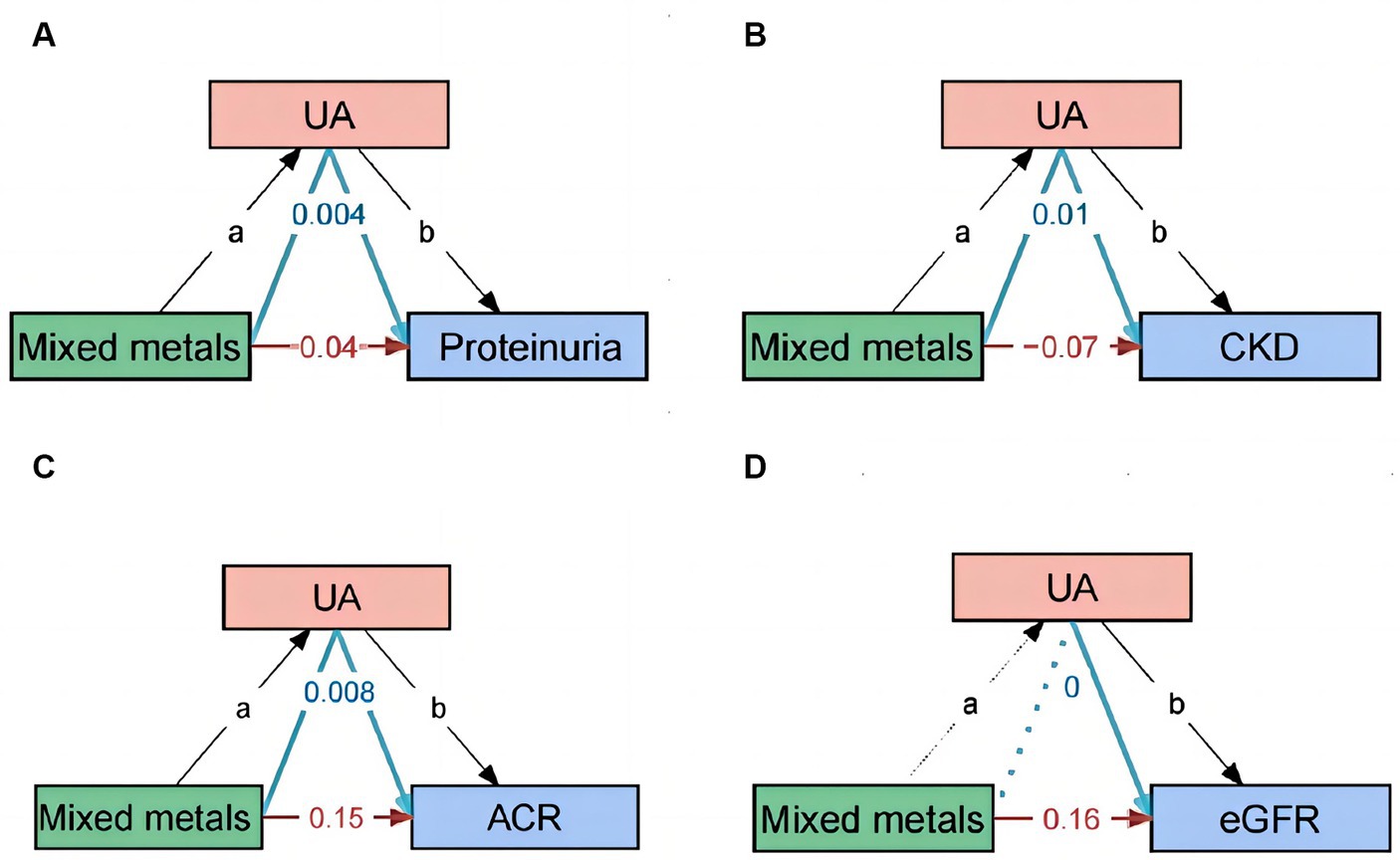
Figure 7. Path diagram of the mediation analysis model. In the mediation analysis, mixed metals are defined as the exposure factor; proteinuria, ACR, eGFR, and CKD are defined as the outcome; and UA is defined as the mediator. CKD, chronic kidney disease; UA, uric acid.
4 Discussion
The study firstly found that mixed exposure to six metals was positively correlated with proteinuria in older adult participants, with UA acting as a mediating factor. Se and Mn were identified as protective factors against CKD, while Co was determined to be a risk factor for the development of CKD. However, this is a cross-sectional study, which cannot conclude the casual relationship between metals and renal function. Longitudinal studies should further evaluate whether the mixed effects of metals and other nephrotoxic substances may be a risk factor for renal injury in older adult people.
Cd, Pb, and Hg are toxic metals generated both naturally and through human activities; these metals can chemically pollute products that enter the human food chain (24, 25). Cd, Pb, and Hg are associated with an increased risk of various age-related chronic diseases, including cardiovascular disease, CKD, and osteoporosis (26–28). Hg, Cd, and Pb ions in the blood bind to thiol-containing biomolecules such as albumin, glutathione, and cysteine to some extent, leading to kidney damage (29). Inflammation is also an important mechanism by which cadmium and other substances cause kidney damage (30, 31). Even low levels of Cd in the kidneys during renal biopsy can induce mild tubular atrophy, and a positive correlation has been reported between renal Hg and renal arteriosclerosis (32, 33). A study from South Korea found no association between Pb, Hg, and Cd levels in the blood of adolescents and eGFR (34). However, a study from China found that both single and mixed exposure to Cd and Pb in adults is associated with renal dysfunction (35). A study from the United States also found that, in adults, excessive exposure to Pb, as well as any level of Cd and total Hg, can have adverse effects on kidney function and health (36). Therefore, the effects of Cd, Pb, and Hg on renal function may vary among age groups. The present study found that Pb, Hg, and Cd were not associated with CKD in the older adult participants, whereas Pb and Cd were associated with proteinuria in the older adult participants. Co—an essential element and a well-known component of cobalamin (vitamin B ₁₂)—has recently been proven to be a mimic of hypoxia and a stimulant for reactive oxygen species production, but it is toxic at high concentrations (37). This study found a close correlation between Co and proteinuria.
There are many studies about the effects and mechanisms of Se and Mn on renal function and proteinuria in participants. Among all human organs, the kidneys and thyroid have the highest Se content (38). Se can enhance antioxidant capacity, primarily by increasing the activity of antioxidant enzymes, such as glutathione peroxidase (39). A placebo-controlled study targeting the older adult population in Sweden demonstrated that low Se status is associated with age-related decline in kidney function. In this study, dietary supplements were administered for 4 years with a Se content of 200 μg. Compared with the functional indicators of the placebo group, there was a significant improvement in renal function with Se and coenzyme Q10 (40). A study on the Se status and CKD of 5,381 middle-aged and older adult people suggests that adequate Se intake may have a positive effect on CKD (41). Our study found that Se has a protective effect on proteinuria and CKD. Mn is crucial for the normal operation of various metabolic enzymes and cofactors. It is also an essential trace element for maintaining normal bodily functions, a cofactor of many enzymes, crucial for substance and energy metabolism, immune function, and blood glucose regulation, and has antioxidant effects (42). Studying plasma levels of Mn, Fe, and Zn can prevent the risk of CKD in older adult people (≥ 90 years) in long-lived areas (10). This study also found that Mn was negatively correlated with the incidence rate of CKD. Another study showed that blood Cu levels are significantly associated with CKD risk, showing a positive dose–response relationship in the Chinese older adult population. Exposure to Mn can antagonize the toxicity of Cu on renal function (43).
The analyses of mixtures, rather than of single metals, may provide a real-world perspective on the relationship between metals and kidney function (44). Previous studies have found that mixed exposure to three nephrotoxic metals (Cd, Pb, and Hg) in the blood is unrelated to eGFR and urinary protein in adolescents (9). Another study reported that exposure to coexisting heavy metal mixtures (Co, Cr, Hg, and Pb) is associated with indicators of poor kidney function in adults (45). Our study found that there is a positive correlation between mixed exposure to six metals and ACR, with higher concentrations leading to greater proteinuria risk, among which Pb and Cr have a larger weight. Moreover, in the univariate analysis, Pb and Cd were identified as risk factors for proteinuria. However, mixed exposure to six metals was positively correlated with CKD, possibly because Pb, Hg, and Cd were not associated with CKD in older adult participants. Although Co was negatively correlated with CKD, Se and Mn accounted for a significant proportion, which could neutralize the kidney damage caused by Co.
Increased serum levels of UA have been associated with the onset and development of CKD, through several molecular pathogenetic mechanisms, such as inflammation and oxidative stress (46). Multiple studies have found a close correlation between heavy metals and high UA levels (19, 47). Our study reveals for the first time that heavy metals can not only directly affect the kidneys, but also regulate renal function by mediating UA levels.
5 Conclusion
To our knowledge, the study is the first to investigate the relationship between mixed exposure to six metals (Pb, Cd, Hg, Se, Mn, and Co) and renal function measured in the blood of older adult people, and UA serving as a mediating factor. Our results indicate that both single and mixed metal exposure may affect renal function, although potential reverse causal relationships cannot be ruled out because of the cross-sectional study design. Our results show that the heavy metals in the blood (Pb, Cd, Hg, and Co) are associated with renal function damage, whereas Se and Mn have a protective effect on renal function in older adult people. When exposed to a metal mixture, Se and Mn may counteract the renal damage caused by other metal ions. Longitudinal studies should further evaluate whether the mixed effects of metals and other nephrotoxic substances may be a risk factor for renal injury in older adult people.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NHANES – National Health and Nutrition Examination Survey Homepage (cdc.gov).
Ethics statement
The studies involving humans were approved by the data comes from publicly available databases (NHANES). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SP: Investigation, Writing – original draft, Formal analysis. YN: Methodology, Writing – original draft. SD: Writing – review & editing, Investigation. DZ: Methodology, Writing – original draft. QW: Investigation, Writing – original draft. ZD: Conceptualization, Funding acquisition, Writing – review & editing. GC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. XC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Beijing Natural Science Foundation (Grant nos. 7244305, 7242032, L232122, L222133), the National Key R&D Projects (32141005), Key Projects of the Ministry of Science and Technology for International Scientific and Technological Innovation Cooperation (2018YFE0126600), National Natural Science Foundation of China (No. 62250001), Science & Technology Project of Beijing, China (No. Z221100007422121).
Acknowledgments
The authors thank NHANES providing the data for our research and all the participants and researchers for their participation. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. 2016 UN. World Population Ageing 1950–2050. Ii. Magnitude and Speed of Population Ageing. United Nations, Geneva, Switzerland.
3. Martin, NBD, and Gil, J. Ageing as developmental decay: insights from P16 (Ink4a.). Trends Mol Med. (2014) 20:667–74. doi: 10.1016/j.molmed.2014.09.008
4. Fang, EF, Scheibye-Knudsen, M, Jahn, HJ, Li, J, Ling, L, Guo, HW, et al. A research agenda for aging in China in the 21st century. Ageing Res Rev. (2015) 24:197–205. doi: 10.1016/j.arr.2015.08.003
5. Fang, YD, Gong, AY, Haller, ST, Dworkin, LD, Liu, ZS, and Gong, RJ. The ageing kidney: molecular mechanisms and clinical implications. Ageing Res Rev. (2020) 63:63. doi: 10.1016/j.arr.2020.101151
6. Bridges, CC, and Zalups, RK. The aging kidney and the nephrotoxic effects of mercury. J Toxicol Environ Health B Crit Rev. (2017) 20:55–80. doi: 10.1080/10937404.2016.1243501
7. Clemens, S, and Ma, JF. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol. (2016) 67:489–512. doi: 10.1146/annurev-arplant-043015-112301
8. Mishra, M, Nichols, L, Dave, AA, Pittman, EH, Cheek, JP, Caroland, AJV, et al. Molecular mechanisms of cellular injury and role of toxic heavy metals in chronic kidney disease. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms231911105
9. Sanders, AP, Mazzella, MJ, Malin, AJ, Hair, GM, Busgang, SA, Saland, JM, et al. Combined exposure to Lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in Nhanes 2009–2014. Environ Int. (2019) 131:131. doi: 10.1016/j.envint.2019.104993
10. Shen, Y, Yin, ZX, Lv, YB, Luo, JS, Shi, WH, Fang, JL, et al. Plasma element levels and risk of chronic kidney disease in elderly populations (≥ 90 years old). Chemosphere. (2020) 254:254. doi: 10.1016/j.chemosphere.2020.126809
11. Li, A, Zhao, J, Liu, L, Mei, Y, Zhou, Q, Zhao, M, et al. Association of Metals and Metalloids with urinary albumin/creatinine ratio: evidence from a cross-sectional study among elderly in Beijing. Front Public Health. (2022) 10:832079. doi: 10.3389/fpubh.2022.832079
12. Farkhondeh, T, Naseri, K, Esform, A, Aramjoo, H, and Naghizadeh, A. Drinking water heavy metal toxicity and chronic kidney diseases: a systematic review. Rev Environ Health. (2021) 36:359–66. doi: 10.1515/reveh-2020-0110
13. Aaseth, J, Alexander, J, Alehagen, U, Tinkov, A, Skalny, A, Larsson, A, et al. The aging kidney-as influenced by heavy metal exposure and selenium supplementation. Biomol Ther. (2021) 11. doi: 10.3390/biom11081078
14. Satarug, S, Garrett, SH, Sens, MA, and Sens, DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. (2010) 118:182–90. doi: 10.1289/ehp.0901234
15. Makhdoumi, P, Karimi, H, and Khazaei, M. Review on metal-based nanoparticles: role of reactive oxygen species in renal toxicity. Chem Res Toxicol. (2020) 33:2503–14. doi: 10.1021/acs.chemrestox.9b00438
16. Sabath, E, and Robles-Osorio, ML. Renal health and the environment: heavy metal nephrotoxicity. Nefrologia. (2012) 32:279–86. doi: 10.3265/Nefrologia.pre2012.Jan.10928
17. Yang, H, and Shu, Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci. (2015) 16:1484–94. doi: 10.3390/ijms16011484
18. Zhang, M, Cui, R, Zhou, Y, Ma, Y, Jin, Y, Gou, X, et al. Uric acid accumulation in the kidney triggers mast cell degranulation and aggravates renal oxidative stress. Toxicology. (2023) 483:153387. doi: 10.1016/j.tox.2022.153387
19. Xu, J, Zhu, X, Hui, R, Xing, Y, Wang, J, Shi, S, et al. Associations of metal exposure with hyperuricemia and gout in general adults. Front Endocrinol. (2022) 13:1052784. doi: 10.3389/fendo.2022.1052784
20. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YP, Castro, AF, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
21. Eknoyan, G, and Levin, NW. K/Doqi clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification – foreword. Am J Kidney Dis. (2002) 39:S14–6. doi: 10.1053/ajkd.2002.30939
22. Bobb, JF, Valeri, L, Claus Henn, B, Christiani, DC, Wright, RO, Mazumdar, M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. (2015) 16:493–508. doi: 10.1093/biostatistics/kxu058
23. Wu, M, Liu, M, Zhang, Y, Wu, J, Gao, M, Huang, F, et al. Serum Hdl partially mediates the association between exposure to volatile organic compounds and kidney stones: a nationally representative cross-sectional study from Nhanes. Sci Total Environ. (2024) 907:167915. doi: 10.1016/j.scitotenv.2023.167915
24. Almerud, P, Zamaratskaia, G, Lindroos, AK, Bjermo, H, Andersson, EM, Lundh, T, et al. Cadmium, Total mercury, and Lead in blood and associations with diet, sociodemographic factors, and smoking in Swedish adolescents. Environ Res. (2021) 197:110991. doi: 10.1016/j.envres.2021.110991
25. Rebelo, FM, and Caldas, ED. Arsenic, Lead, mercury and cadmium: toxicity, levels in breast Milk and the risks for breastfed infants. Environ Res. (2016) 151:671–88. doi: 10.1016/j.envres.2016.08.027
26. García-Esquinas, E, Navas-Acien, A, Pérez-Gómez, B, and Artalejo, FR. Association of Lead and Cadmium Exposure with frailty in us older adults. Environ Res. (2015) 137:424–31. doi: 10.1016/j.envres.2015.01.013
27. Yimthiang, S, Pouyfung, P, Khamphaya, T, Kuraeiad, S, Wongrith, P, Vesey, DA, et al. Effects of environmental exposure to cadmium and Lead on the risks of diabetes and kidney dysfunction. Int J Environ Res Public Health. (2022) 19. doi: 10.3390/ijerph19042259
28. Nan, Y, Yang, J, Yang, J, Wei, L, and Bai, Y. Associations between individual and combined metal exposures in whole blood and kidney function in U.S. adults aged 40 years and older. Biol Trace Elem Res. (2024) 202:850–65. doi: 10.1007/s12011-023-03722-z
29. Rooney, JP. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology. (2007) 234:145–56. doi: 10.1016/j.tox.2007.02.016
30. Li, A, Mei, Y, Zhao, M, Xu, J, Zhao, J, Zhou, Q, et al. Do urinary metals associate with the homeostasis of inflammatory mediators? Results from the perspective of inflammatory signaling in middle-aged and older adults. Environ Int. (2022) 163:107237. doi: 10.1016/j.envint.2022.107237
31. Hong, H, Lin, X, Xu, Y, Tong, T, Zhang, J, He, H, et al. Cadmium induces Ferroptosis mediated inflammation by activating Gpx4/ager/P65 Axis in pancreatic Β-cells. Sci Total Environ. (2022) 849:157819. doi: 10.1016/j.scitotenv.2022.157819
32. Barregard, L, Fabricius-Lagging, E, Lundh, T, Mölne, J, Wallin, M, Olausson, M, et al. Cadmium, mercury, and Lead in kidney cortex of living kidney donors: impact of different exposure sources. Environ Res. (2010) 110:47–54. doi: 10.1016/j.envres.2009.10.010
33. Barregard, L, Sallsten, G, Lundh, T, and Mölne, J. Low-level exposure to Lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ Res. (2022) 211:113119. doi: 10.1016/j.envres.2022.113119
34. Joo, SH, Seo, S, Cho, MH, and Kim, KS. Environmental exposure to Lead, mercury, and cadmium is not associated with abnormal kidney function in Korean adolescents. Pediatr Nephrol. (2022) 37:625–31. doi: 10.1007/s00467-021-05215-4
35. Chen, X, Zhu, G, Wang, Z, Zhou, H, He, P, Liu, Y, et al. The association between Lead and cadmium co-exposure and renal dysfunction. Ecotoxicol Environ Saf. (2019) 173:429–35. doi: 10.1016/j.ecoenv.2019.01.121
36. Jain, RB. Co-exposures to toxic metals cadmium, Lead, and mercury and their impact on unhealthy kidney function. Environ Sci Pollut Res Int. (2019) 26:30112–8. doi: 10.1007/s11356-019-06182-y
37. Edel, J, Pozzi, G, Sabbioni, E, Pietra, R, and Devos, S. Metabolic and toxicological studies on cobalt. Sci Total Environ. (1994) 150:233–44. doi: 10.1016/0048-9697(94)90159-7
38. Zachara, BA, Pawluk, H, Bloch-Boguslawska, E, Sliwka, KM, Korenkiewicz, J, Skok, Z, et al. Tissue level, distribution, and Total body selenium content in healthy and diseased humans in Poland. Arch Environ Health. (2001) 56:461–6. doi: 10.1080/00039890109604483
39. Kornhauser, C, Garcia-Ramirez, JR, Wrobel, K, Pérez-Luque, EL, Garay-Sevilla, ME, and Wrobel, K. Serum selenium and glutathione peroxidase concentrations in type 2 diabetes mellitus patients. Prim Care Diabetes. (2008) 2:81–5. doi: 10.1016/j.pcd.2008.02.003
40. Alehagen, U, Aaseth, J, Alexander, J, Brismar, K, and Larsson, A. Selenium and coenzyme Q10 supplementation improves renal function in elderly deficient in selenium: observational results and results from a subgroup analysis of a prospective randomised double-blind placebo-controlled trial. Nutrients. (2020) 12. doi: 10.3390/nu12123780
41. Xie, C, Zeng, M, Shi, Z, Li, S, Jiang, K, and Zhao, Y. Association between selenium status and chronic kidney disease in middle-aged and older Chinese based on Chns data. Nutrients. (2022) 14. doi: 10.3390/nu14132695
43. Guo, F, Lin, Y, Meng, L, Peng, L, Zhang, H, Zhang, X, et al. Association of Copper Exposure with prevalence of chronic kidney disease in older adults. Clin Nutr. (2022) 41:2720–8. doi: 10.1016/j.clnu.2022.10.016
44. Zhou, TT, Hu, B, Meng, XL, Sun, L, Li, HB, Xu, PR, et al. The associations between urinary metals and metal mixtures and kidney function in Chinese community-dwelling older adults with diabetes mellitus. Ecotoxicol Environ Saf. (2021) 226:112829. doi: 10.1016/j.ecoenv.2021.112829
45. Luo, J, and Hendryx, M. Metal mixtures and kidney function: an application of machine learning to Nhanes data. Environ Res. (2020) 191:110126. doi: 10.1016/j.envres.2020.110126
46. Roumeliotis, S, Roumeliotis, A, Dounousi, E, Eleftheriadis, T, and Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: a review. Nutrients. (2019) 11. doi: 10.3390/nu11081911
Keywords: kidney function, uric acid, aging, heavy metals, machine learning
Citation: Pan S, Niu Y, Duan S, Zhao D, Wang Q, Dong Z, Cai G and Chen X (2024) Uric acid mediates the relationship between mixed heavy metal exposure and renal function in older adult people. Front. Public Health. 12:1403878. doi: 10.3389/fpubh.2024.1403878
Edited by:
Jing Xu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Jia Xin Zhao, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaYayuan Mei, Capital Medical University, China
Copyright © 2024 Pan, Niu, Duan, Zhao, Wang, Dong, Cai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangmei Chen, eG1jaGVuMzAxQDEyNi5jb20=; Guangyan Cai, Y2FpZ3Vhbmd5YW5Ac2luYS5jb20=; Zheyi Dong, c2hlbmdkYWkyNkAxNjMuY29t
†These authors share first authorship
 Sai Pan
Sai Pan Yue Niu†
Yue Niu† Qian Wang
Qian Wang Zheyi Dong
Zheyi Dong Guangyan Cai
Guangyan Cai Xiangmei Chen
Xiangmei Chen