- 1Pfizer Vaccines Emerging Markets, Medical Affairs, Paris, France
- 2Pfizer Vaccines Emerging Markets, Medical Affairs, New York, NY, United States
- 3P95 Epidemiology & Pharmacovigilance, Leuven, Belgium
Background: Most publications on invasive pneumococcal disease (IPD) serotype distribution are from about 20 countries (Australia, Canada, China, European Union members, Japan, New Zealand, South Korea, and USA). Here, we reviewed the literature among underrepresented countries in the Americas (AMRO), Africa (AFRO), Eastern Mediterranean (EMRO), South-East Asia (SEARO), and Western Pacific (WPRO) WHO regions.
Methods: We performed a systematic review of the most recent IPD serotype surveillance publications (from 01/01/2010 to 31/12/2021, Medline/Embase) in those WHO regions. Selection criteria were delineated by contemporality, within-country geographical scope, and number of samples. Reported serotype distributions for each country were stratified by age group, pneumococcal conjugate vaccine (PCV) serotype category (considering undifferentiated serotypes), and PCV program period (pre-PCV, intermediate, or PCVhv [higher valency PCV formulation]). Pre-PCV period pooled data estimated PCV serotype category distribution by age group across WHO regions, while for the PCVhv period, country-level dataset tables were prepared.
Results: Of 2,793 publications screened, 107 were included (58 pediatric, 11 adult, 37 all ages, and one comprising every age group). One-third of eligible countries (51/135) published serotype distribution, ranging from 30 to 43% by WHO region. Considering number of samples per WHO region, a few countries prevailed: AMRO (Brazil), AFRO (South Africa, Malawi, and Burkina Faso), and WPRO (Taiwan). In the pre-PCV period, PCV13 formulation serotypes predominated: ranging from 74 to 85% in children and 58–86% in adults in the different WHO regions. The PCVhv period represented half of the most recent IPD surveillance by countries (26/51). Undifferentiated serotypes represented >20% of IPD from most countries (34/51).
Conclusion: Ubiquity of undifferentiated serotypes among the publications could constrain estimates of PCV program impact and of serotype coverage for newer PCVhv formulations; consequently, we recommend that countries favor techniques that identify serotypes specifically and, rather than reporting PCV formulation serotype distributions, provide serotype results individually.
Systematic review registration: The protocol has been prospectively registered at PROSPERO, identifier: CRD42021278501. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=278501.
Introduction
Streptococcus pneumoniae infection can lead to invasive pneumococcal disease (IPD) where the pneumococcus invades otherwise sterile body sites such as the bloodstream or the central nervous system, associated with clinical presentations like sepsis, pneumonia, or meningitis. Childhood immunization against S. pneumoniae is recommended by the World Health Organization (WHO) (1). Pneumococcal conjugate vaccines (PCVs) are licensed and available in childhood national immunization programs (NIPs) of up to 160 countries. These pediatric PCV NIPs have helped to prevent pneumococcal disease and save lives around the world (2, 3).
Timely surveillance of predominant IPD serotypes is essential for evaluation of the status of a PCV NIP, where changes in vaccine-serotype (VT) IPD serotype distribution, in the background of lower IPD incidence after PCV introduction, can help to substantiate serotype-specific disease burden declines through both direct effects on vaccinated children and indirect effects on unvaccinated children and adults (4, 5). The proportion of non-vaccine serotype (NVT) nasopharyngeal carriage and transmission might increase (6, 7), which can be attributed to serotype replacement due to the uncovering of minor serotypes obscured by the previously dominant vaccine serotypes in the nasopharynx or by the phenomenon of “capsular switch” (i.e., a change in the expressed capsular serotype), and NVT nasopharyngeal carriage consequently could lead to an increase in NVT IPD. Extensive IPD serotype distribution information is available from countries mostly located in North America, the European Union, and in Asia (namely, Japan, South Korea, and China, as well as New Zealand and Australia). Elsewhere, recently published publications with serotype distribution surveillance are limited, although low- and middle-income countries represent a substantial proportion of the IPD burden worldwide (8). For instance, between 2012 and 2017, four largely populated countries — Democratic Republic of the Congo, India, Nigeria, and Pakistan — accounted for half of the global pneumococcal disease deaths (9). Absent or incomplete serotype distributions may hamper informed evaluations of pneumococcal disease burden.
The objective of this systematic review was to describe, among countries that tend to be underrepresented in the international IPD surveillance literature, the most up to date serotype distributions based on criteria of contemporality, geographic scope, and sample size.
Methods
We conducted a systematic literature review of observational studies as well as the control arm of interventional studies that reported an IPD serotype distribution from 135 countries of the WHO regions of Africa, Americas (except Canada and USA), Eastern Mediterranean, South-East Asia, and the Western Pacific Regions (except Australia, China, Japan, New Zealand, and South Korea). The protocol has been prospectively registered at PROSPERO [CRD42021278501]. We searched MEDLINE via PubMed, EMBASE, LILACS, and CABI Direct (Global Health) for relevant articles published between 1 January 2010 and 31 December 2021. Search terms were as follows: (((((streptococcus pneumoniae) OR (pneumococ*)) AND (serotyp*)) AND ((((((((invasive pneumococcal disease) OR (“IPD”)) OR ((((bacteremia) OR (bacteremic) OR (invasive)) AND (pneumonia)) OR (pleural effusion)) OR (empyema)) OR (peritonitis)) OR (osteoarticular infection)) OR (hemolytic uremic syndrome)) OR (arthritis)) OR (endocarditis))) AND ((“2010/01/01”[Date – Publication]: “3,000”[Date – Publication]))) NOT ((immunogenicity) OR (immune response)). The systematic literature review was performed using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (10). Reference lists from eligible articles were also searched to identify other relevant studies.
Inclusion criteria
Publications describing pneumococcal studies had to meet the following inclusion criteria by (a) population and (b) outcome: (a) persons of any age and gender participating in studies performed in one of the selected countries; and (b) serotype distribution of laboratory-confirmed IPD, as established by microbiological culture, molecular detection assay, and/or antigen-based testing.
All S. pneumoniae serotypes were captured as reported by the investigators. Publications in the following languages were included: English, French, Spanish, Portuguese, or Dutch.
Exclusion criteria
Publications from countries in the WHO European Region (EURO), Canada, the United States of America, Japan, South Korea, China (including Hong Kong), New Zealand, or Australia were excluded. Publications that only included complicated pneumococcal pneumonia (e.g., empyema) were also excluded.
Study selection process and data extraction
Two researchers screened publications obtained through the electronic searches based on title and abstract, using DistillerSR [Version 2.35. Evidence Partners. https://www.evidencepartners.com], and they conducted a full-text review to make a final selection of eligible publications for data extraction.
Selection of the most recent publications per country was based on an algorithm using three criteria: contemporality, scope, and size. This selection process was performed separately for the three age groups (pediatric, adult, and all ages).
• Contemporality: all publications with a study period that began in 2010 or later, or that included the year 2015, were included. If for a given country and age group, no publication was available in this period, then the most recently available publication was included according to the selection criteria of “Scope” and “Size.”
• Scope: national (country-wide) studies were prioritized over regional (i.e., region of a country), which were prioritized over sub-regional.
• Size: total number of cases: “100+” prioritized over “51–99,” which was prioritized over “25–50.”
Note that any single publication that included at least 25 cases in total with a study period that began in 2010 or later, or that included the year 2015, would be included.
A single reviewer extracted the data. Quality control of data extraction was performed by a second reviewer through re-extraction of 10% of the publications. Uncertainties were settled through discussion with the entire study team. The reasons for exclusion were documented in accordance with PRISMA guidelines (10) and included: (a) inappropriate outcome, (b) inappropriate period, (c) inappropriate design, (d) small study size, (e) data not available, and (f) duplicate publication.
Study variables for data extraction were country (i.e., location where the study was performed), study period, study design, age range of the population, geographical setting (e.g., national, regional, or subregional), clinical presentation, sample source, number of samples, PCV vaccination program during the study period, laboratory methods for detection and serotyping (classical Quellung serotyping or molecular methods that include PCR and whole genome sequencing), and serotype distribution (reported as serotype proportions or serotype-specific incidence rates).
Quality assessment
Quality assessment of the selected publications was done using an adaption of the Newcastle-Ottawa Scale (NOS) for assessing the quality of cohort and case–control studies (11) and the National Institute of Health (NIH) checklist for before-after and cross-sectional studies (12). The quality of the extracted publications was scored as “good”, “fair”, or “poor”.
Analysis
As detailed in Table 1, each reported serotype was categorized in one of three manners: as “serotype clearly identifiable” that could be unambiguously included in a pneumococcal conjugate vaccine serotype formulation category (i.e., PCV13, PCV20non13, or NonPCV20); as “undifferentiated”; or as “serotyping not done”.

Table 1. Definitions for pneumococcal conjugate vaccine (PCV) formulation serotype coverage estimates.
Serotype 15C was included in the PCV20non13 serotype formulation category because serotypes 15B and 15C were often described together as 15B/15C. The serotype distribution was reported as proportions (%) calculated by the number of samples with “serotype clearly identifiable” (i.e., a specific serotype or serogroup) divided by the total number of samples that had been serotyped; consequently, the denominator included “undifferentiated” samples. In contrast, samples categorized as “serotyping not done” were not included in the denominator.
We classified serotype distribution by age range into three age groups. The “pediatric” age group included cases whose age was in the range between 0 to <18 years. The “adult” age group included cases whose age was ≥18 years of age. The third group, labeled “all ages”, included publication reporting that did not differentiate between pediatric and adult cases.
We classified PCV program periods as “pre-PCV”, “intermediate”, or “higher valency PCV” (PCVhv, PCV10 or PCV13). In a pre-PCV period, serotype data was collected before an NIP started (even if PCV was available in the private market), whereas for a PCVhv period a single higher valency PCV was used in the NIP. Nonetheless, certain PCV programs could not be unambiguously identified as pre-PCV or PCVhv, and these periods were classified as “intermediate”. For instance, an intermediate period could correspond to a PCV7 period, before PCVhv was introduced. Publications that reported across years representing a mix of PCV program periods that could not be distinguished, but included PCVhv, were classified as “multiple”.
The analysis was descriptive. We determined from each publication the serotype classification (e.g., serotype clearly identifiable, undifferentiated, or serotyping not done), and we then calculated serotype proportions by country (classified by WHO region), by years of surveillance, by age group, and by PCV program period. IPD serotype results were stratified by PCV program during the study surveillance period, regardless of any subsequent changes to the PCV program after the closing year of the study. From the pre-PCV period, we pooled IPD serotype proportions across the same country (classified by WHO region), age group, and PCV program period. In the pre-PCV period, we identified through pooling the top five ranking nonPCV20 serotypes (by WHO region and age group), and this analysis was performed in R version 4.1.0 to avoid skewing by smaller-sized studies. In the intermediate and PCVhv periods, there was heterogeneity across the countries—by the vaccine used (PCV10 or PCV13), by the transition history of any preceding PCVs introduced in the national program, by the PCV uptake, and by the maturity of the program since introduction. Consequently, for intermediate or PCVhv period results, we presented the distribution of individual serotypes in tabular format by country, publication by publication.
Results
Study selection
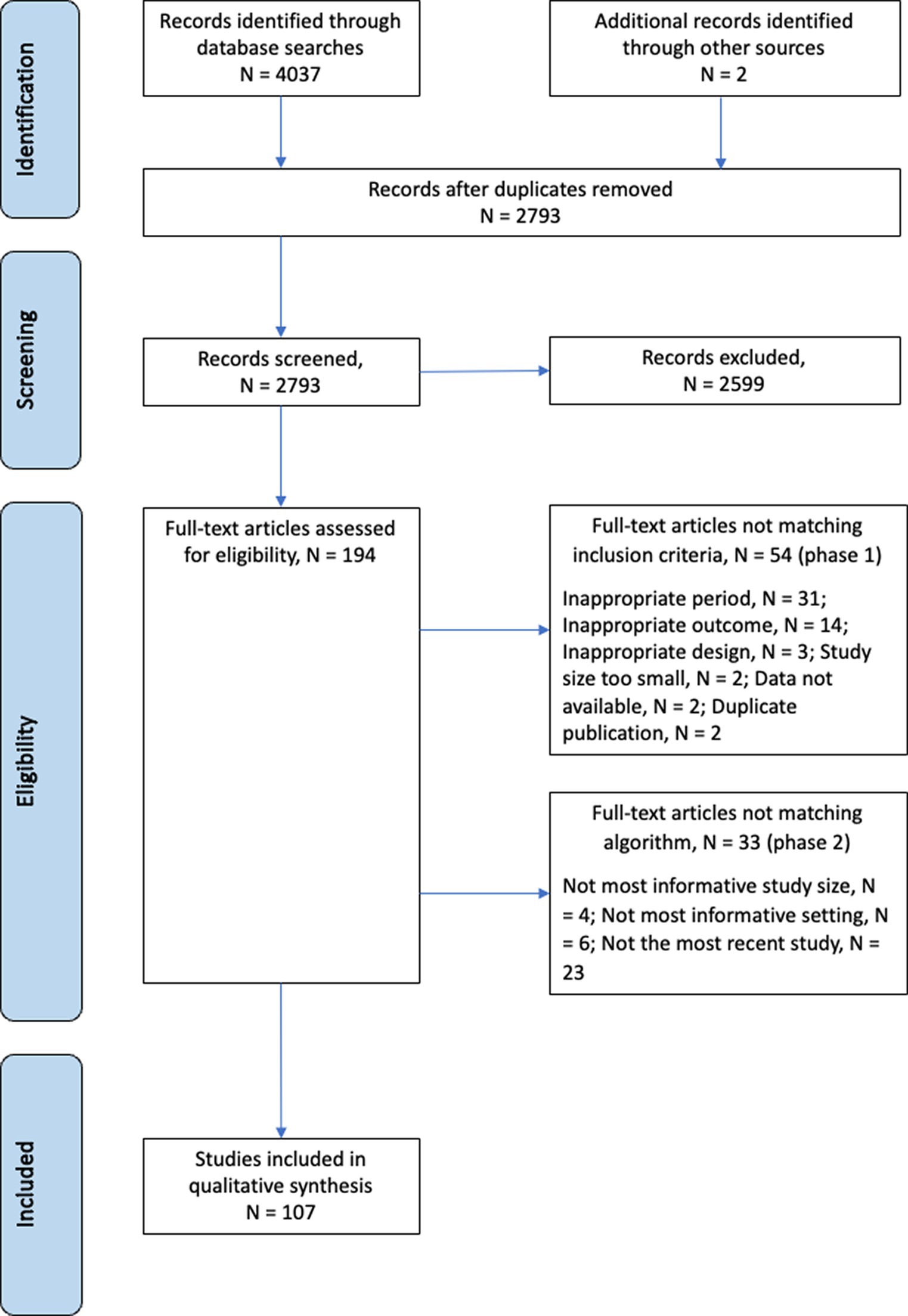
We identified 2,793 publications during the selection process (Figure 1). Of the 2,793 publications, 194 were assessed for eligibility by full-text review: 54 met the exclusion criteria and 33 did not adhere to the contemporality, scope, or size criteria of the selection algorithm. In total, 107 publications were retained. Only four of 107 publications reported incidences at the serotype level (13–16).
See Supplementary Table S1 for the 107 included publications and Supplementary Table S2 for the 87 publications excluded after full text review. The quality assessment scored 43 publications as “good”, 51 as “fair”, and 13 as “poor” (Supplementary Table S1, final column). In general, for publications that scored good or fair, the risk of bias was deemed acceptable. Although studies with a poor score were perceived to have a high risk of bias, they were kept when they provided the only available data for a given country.
Full tables of individual serotypes are available in the Supplement (Supplementary Tables S3–S5).
Distribution of publications
These publications were distributed by WHO region (Figure 2) as follows: 26 publications from AMRO (13, 17–42), 32 publications from AFRO (14, 15, 43–71), 17 publications from EMRO (16, 72–87), 13 publications from SEARO (88–100), and 19 publications from WPRO (100–119).
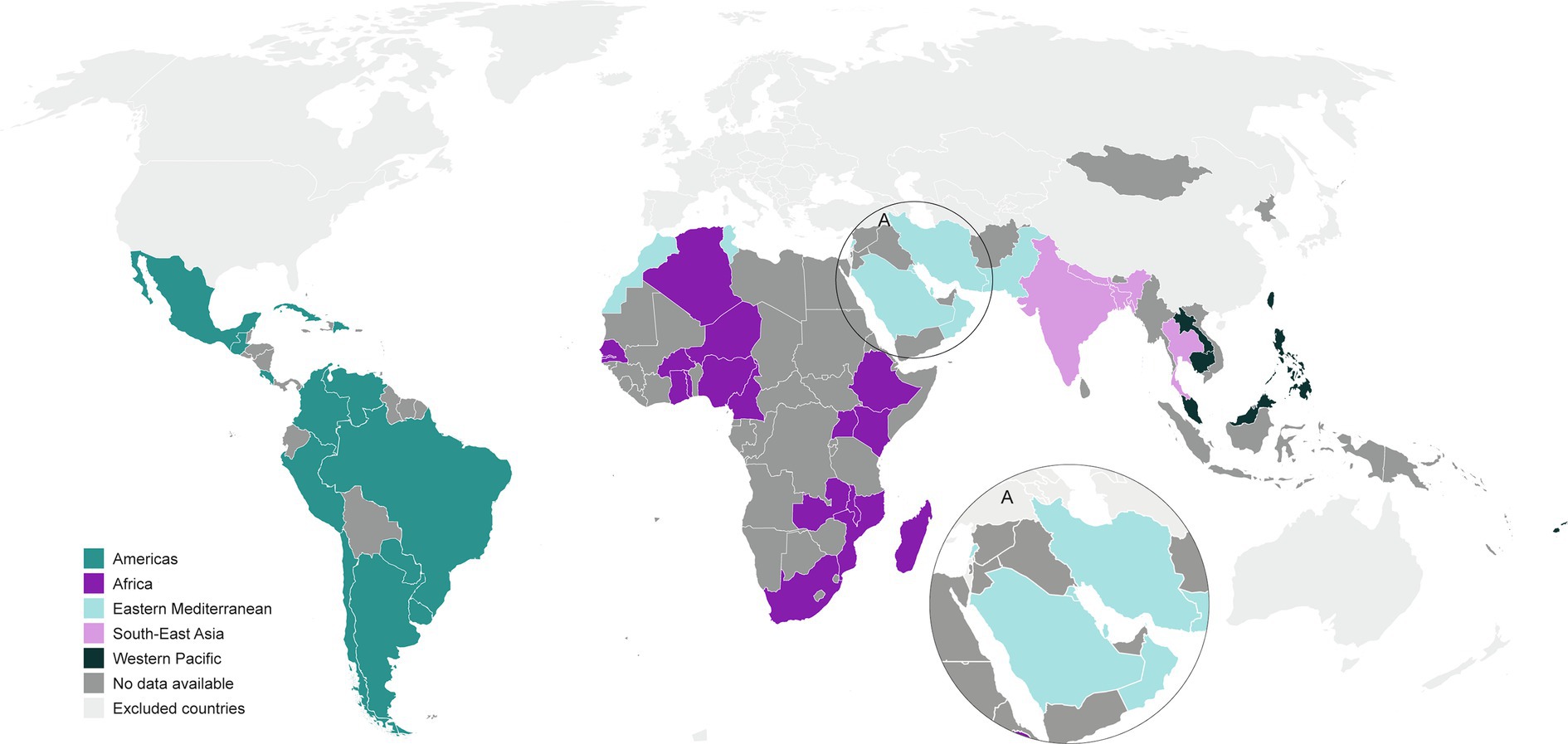
Figure 2. Global map of countries providing the most recent IPD serotype surveillance publications (from 2010 to 2021) in these WHO regions.
Among the 135 eligible countries across the five WHO regions, we identified publications from 51 countries. By proportion of countries per region, the publications represented 30 to 43% of countries for each WHO region: 39% (13 of 33) from AMRO (excluding Canada and USA); 38% (18 of 47) from AFRO; 43% (9 of 21) from EMRO; 36% (4 of 11) from SEARO; and 30% (7 of 23) from WPRO (excluding Japan, South Korea, China/Hong Kong, New Zealand, and Australia).
When looking at the age groups, 58 publications reported on pediatric, 11 on adult, and 37 on all-ages populations, while one publication reported separately on each of the three age groups (81). Further stratification within the pediatric group showed that one publication reported on 0–2-year-olds (28), 11 publications reported on 0–5-year-olds, including the publication reporting on 0–2-year-olds, while the remaining 47 publications reported on 0–18-year-olds.
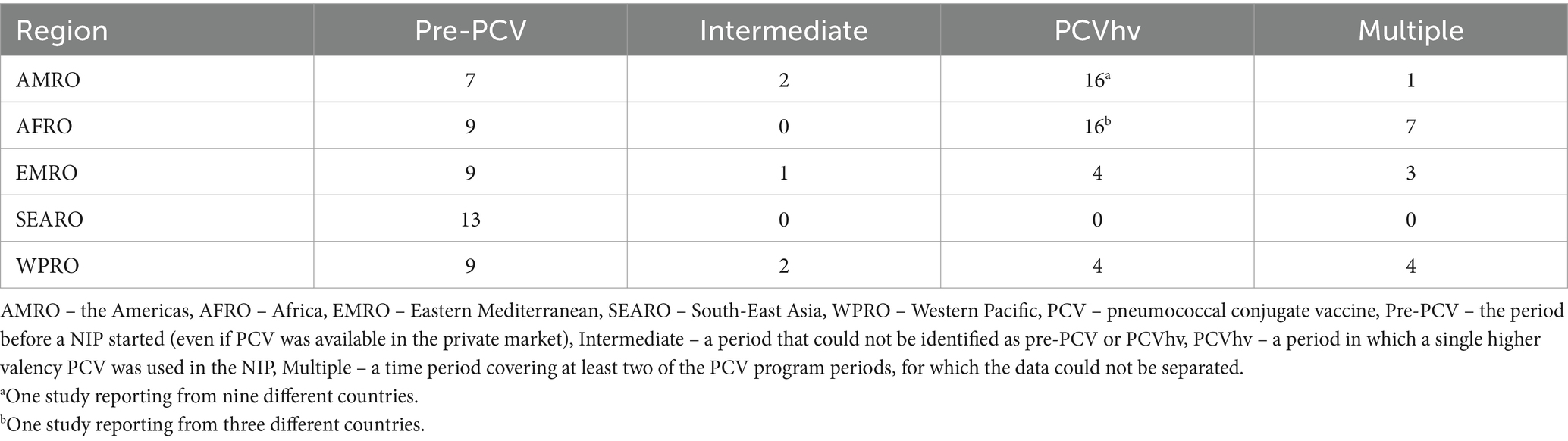
In terms of the PCV program period, 47 publications reported from the pre-PCV period, five publications from the intermediate period and 40 publications from the PCVhv period. Fifteen publications that reported across a mix of PCV program periods that could not be distinguished but included PCVhv were classified as “multiple”. The publication breakdown by WHO region is shown in Table 2.
In the 26 PCVhv period country programs, 11 countries were based on PCV10 and 15 countries on PCV13. The use of PCV10 was reported in AMRO (Brazil, Chile, Colombia, and Paraguay), AFRO (Ethiopia, Kenya, Madagascar, Mozambique and Zambia), and EMRO (Morocco and Pakistan), but not in WPRO. PCV13 was used in AMRO (Argentina, Dominican Republic, Mexico, and Uruguay), AFRO (Burkina Faso, Cameroon, Gambia, Ghana, Niger, South Africa, and Togo), EMRO (Kuwait, and Oman), and WPRO (Singapore, and Taiwan).
Serotype distributions and pre-PCV period
Study duration in the pre-PCV period ranged widely, between studies that began surveillance in 1995 (Gambia, Malawi), 1996 (Guatemala), or 1997 (Singapore) and other studies covering a more recent period, such as 2016–2019 (India) or 2017–2019 (Iran).
In the pre-PCV period, the pooled distribution of serotype categories is presented by WHO region (Figure 3). The proportion of PCV13 serotypes was consistently large across regions, ranging from a potential serotype coverage of 74 to 85% in the pediatric age group, from 58 to 86% in adults, and from 54 to 76% in the all-ages population. The proportion of PCV20non13 serotypes ranged from 1 to 5% in the pediatric age group, from 3 to 10% in adults, and from 1 to 10% in the all-ages population. Finally, the proportion of nonPCV20 serotypes ranged from 4 to 14% in the pediatric age group, from 5 to 26% in adults, and from 1 to 12% in the all-ages population. A substantial proportion of serotype distribution data fell in the “undifferentiated” category, ranging from 1.2% (India) to 55.4% (Togo).
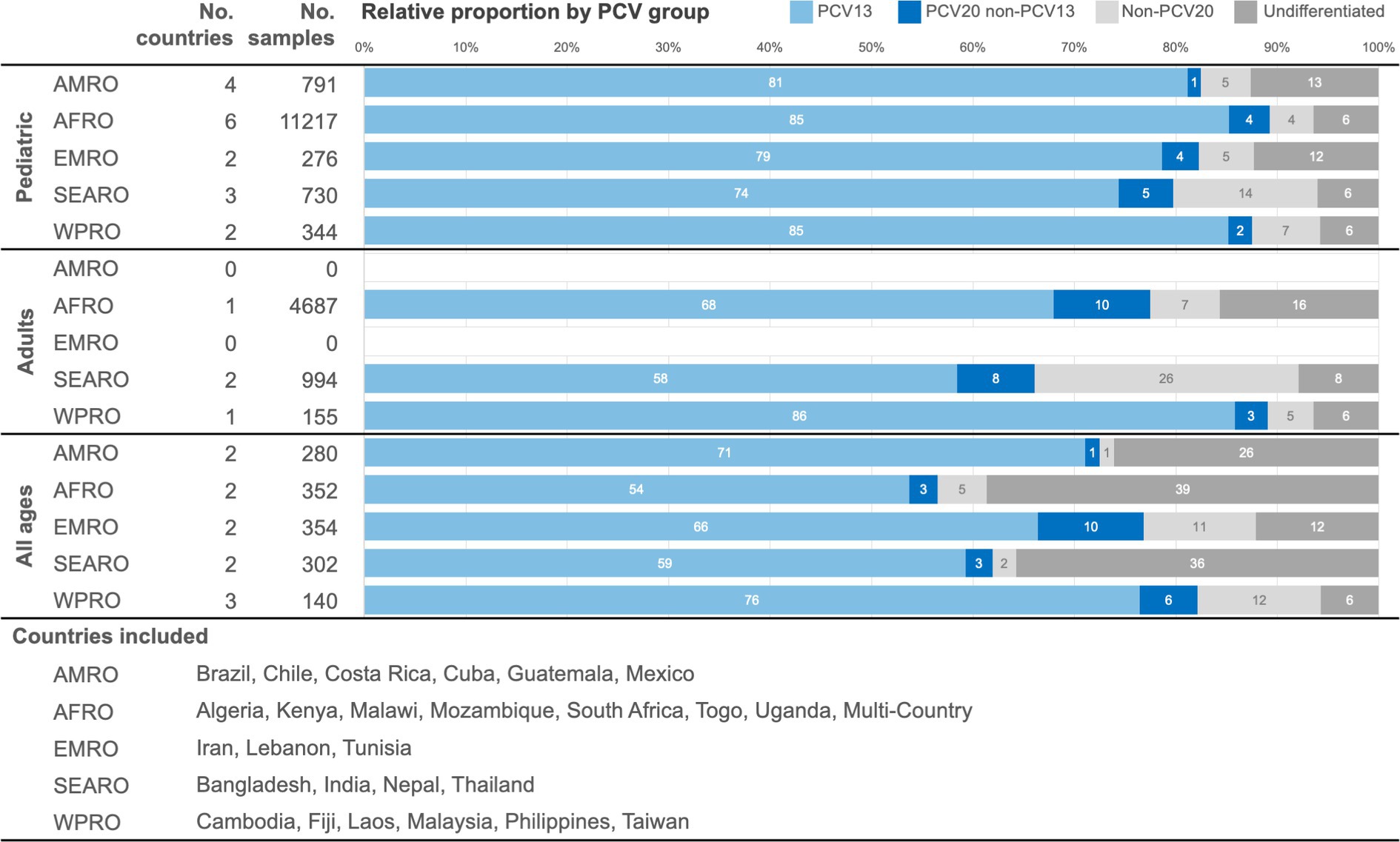
Figure 3. Pooled distribution by PCV formulation serotype coverage categories in the pre-PCV period by age, presented by WHO region. AMRO – the Americas, AFRO – Africa, EMRO – Eastern Mediterranean, SEARO – South-East Asia, WPRO – Western Pacific, PCV – pneumococcal conjugate vaccine.
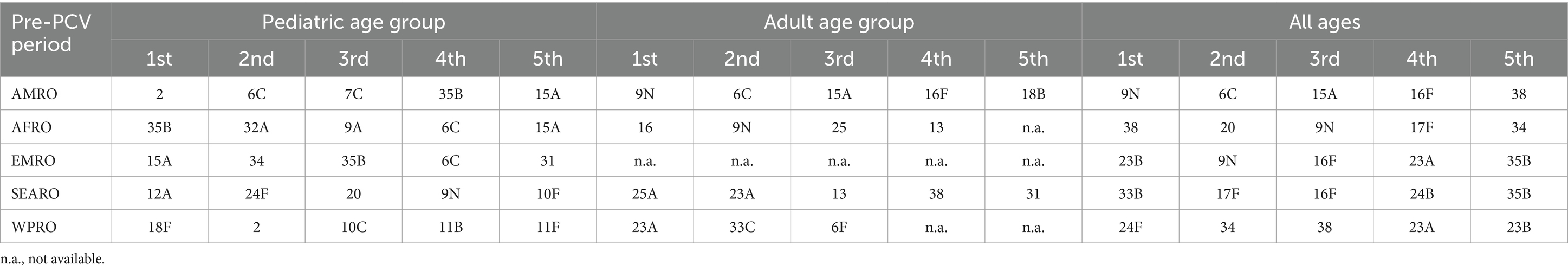
The top five nonPCV20 serotypes from the pre-PCV period pooled data differed by WHO region and by age group (Table 3). Overall, the most frequently identified nonPCV20 serotypes in the pre-PCV period were 6C, 9N, 15A, 23A, and 13. In detail, the results for the nonPCV20 serotypes by pooled proportion of all serotypes or by pooled proportion of nonPCV20 serotypes for all regions combined were as follows: 6C (1.3% of all, 16.9% of nonPCV20), 9N (1.2% of all, 16.0% of nonPCV20), 15A (1.0% of all, 12.6% of nonPCV20), 23A (1.0% of all, 12.3% of nonPCV20), and 13 (0.6% of all, 7.9% of nonPCV20).
Serotype distributions, intermediate, and PCVhv periods
For the intermediate and PCVhv periods, the serotype distributions are presented by publication in a country-level dataset table in Table 4. A substantial proportion of the serotype distribution from the intermediate and PCVhv period in AFRO and EMRO fell in the “undifferentiated” category. Higher numbers of publications and countries in the intermediate or PCVhv period with a proportion > 20% of “undifferentiated” serotypes were found in AFRO, [58.3% (14 of 24) of publications and 80.0% (12 of 15) of countries] and in EMRO, (50.0% (5 of 10) of publications and 33.3% (2 of 6) of countries), compared to the other WHO regions [14.3% (5 of 35) of publications and 30.8% (4 of 13) of countries].
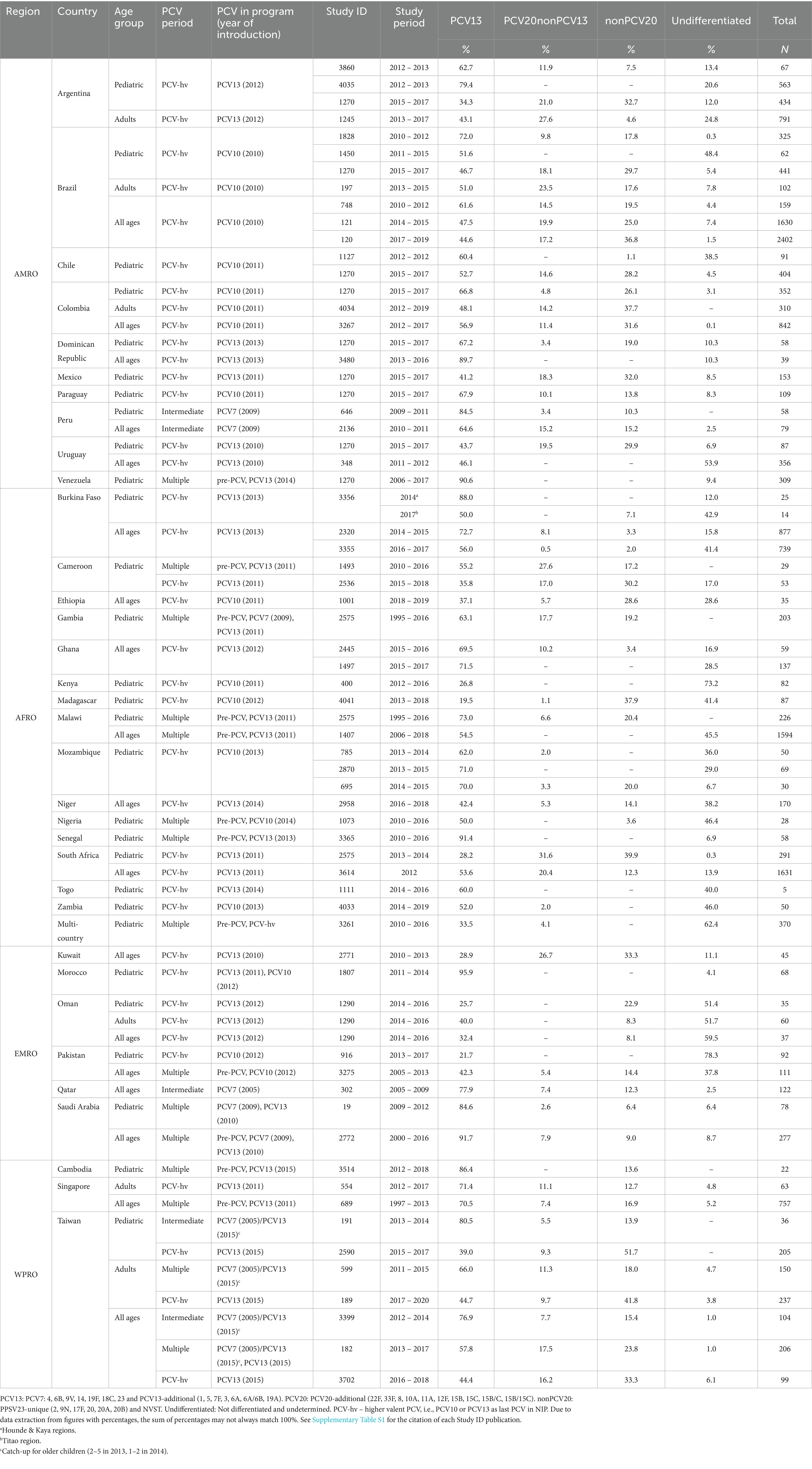
Table 4. Distribution by PCV formulation serotype coverage categories in the intermediate and PCVhv periods.
For the PCVhv period, most publications reported between 40 to 300 isolates by study, although reported sample size varied from less than 10 to greater than 6,000. From the pediatric age group, EMRO surveillance is limited to 205 pediatric samples in our selection, while SEARO did not report any results. Adult surveillance is even more scarce, limited to 6 publications, based on only 63 samples from SEARO, 60 samples from EMRO, and no data from AFRO.
A disbalance per region existed in the number of samples available, and a few countries predominated in each region. AMRO contributed more than 10,000 samples, and Brazil contributed 5,100 samples or half of the AMRO samples, with the remaining half distributed between nine other countries. AFRO contributed nearly 7,000 samples. South Africa (1922 samples), Malawi (1820 samples), and Burkina Faso (1,655 samples), each contributed individually about one-quarter of AFRO samples, with the remaining quarter (1,515 samples) distributed between 12 other countries. WPRO contributed nearly 2000 samples, while the main contributor was Taiwan (1,037 samples, or 55% of the total). EMRO contributed 925 samples (evenly distributed across six countries).
The range of proportions of PCV13 serotypes in the PCVhv period varied slightly by age group: pediatric, between 20% (Madagascar) and 96% (Morocco); adults, from 40% (Oman) to 71% (Singapore); and in all ages group, from 29% (Kuwait) to 90% (Dominican Republic). The PCV20non13 serotype proportion values differed by country, ranging from 1% (Madagascar) to 32% (South Africa) in pediatric, 10% (Taiwan) to 28% (Argentina) in adults, and 1% (Burkina Faso) to 27% (Kuwait) in all ages. Across the countries, particular nonPCV20 serotypes predominated according to the age group: pediatric (23A, 6C, 15A, 23B, and 16F), adults (23A, 15A, 9N, 6C, and 29), and in all ages (6C, 9N, 15A, 23A and 20). The range of nonPCV20 serotypes differed by country, from 1% (Chile) to 52% (Taiwan) in the pediatric age group, from 5% (Argentina) to 42% (Taiwan) in adults, and from 2% (Burkina Faso) to 37% (Brazil) in the all-ages group.
Discussion
The global map shows that there is still an IPD serotype distribution world to conquer (Figure 2). Our overview of the published studies available, within the WHO regions of Africa, Americas, Eastern Mediterranean, South-East Asia, and Western Pacific, revealed that published IPD serotype distributions were lacking from two-thirds of the 135 eligible countries; depending on the region, 57 to 70% of countries lacked published IPD serotype distributions in the literature. Furthermore, half of the published studies provided IPD serotyping results that would not be considered as current, given that they represented only the pre-PCV or intermediate periods. Even in WHO regions that appear to have IPD serotype results available, such as AMRO, AFRO, and WPRO, often the region is overrepresented by a few countries, while many countries in the same WHO region are not represented in the IPD surveillance literature. This does not seem to be unique to the WHO regions under review, as even for Europe national data is unavailable or incomplete (8).
The 74 to 85% proportion of pediatric PCV13 serotypes in the pre-PCV period pooled data estimations is in line with findings from other systematic reviews and multicenter studies that described in the pre-PCV10 or pre-PCV13 periods the pediatric serotype distributions in Latin America [73 to 88% (20)], Africa [81% (120)], or South-East Asia [70 to 80% (121)], while the proportion reported from these studies was even higher in East Asia and South-East Asia [92 to 93% in Taiwan and Singapore (122)]. The high proportion of IPD caused by PCV13 serotypes in the pre-PCV period would support the 2019 WHO recommendation to include PCVs in childhood NIPs worldwide (1). All of the four countries most affected by pneumococcal deaths, for instance, introduced a higher valent PCV into state or national immunization programs (Pakistan, PCV10 in 2012; the Democratic Republic of the Congo, PCV13 in 2013; Nigeria, PCV10 in 2014; and India, PCV13 in 2017). While serotype distribution results were available for Nigeria [pediatric (63)] and Pakistan [pediatric (82) and all ages (83)], there were no publications available on PCVhv period serotype distribution in the Democratic Republic of the Congo or India.
The PCVhv period represents half of the recently available study data (26 of 51 countries, 40 of 107 publications). From the pediatric groups, in particular, PCVhv period surveillance was available from all regions except SEARO that only published IPD serotype distribution results for the pre-PCV period. The proportion of PCV13 serotypes in the PCVhv period varied widely by country, in contrast to the consistently large proportions of PCV13 serotypes in the pediatric age group during the pre-PCV period data estimations pooled by region. We believe that the difference between countries is explained by the length of surveillance of these PCVhv programs. Most of our included publications only report from the initial years of an NIP, that is, the first 1 to 6 years after a PCV10 or PCV13 program implementation. Nonetheless, in publications from our review from the same country that reported successive PCV13 periods in the same population, the proportion of pediatric PCV13 serotypes gradually declined over time, for instance, falling from 88 to 56% in Burkina Faso (2011–2017) (45), from 54 to 36% in Cameroon (2011–2018) (48), and from 90 to 25% in Taiwan (2010–2017) (117) (Table 4). Furthermore, with the surveillance periods of more than 8 years after implementation that are typical of surveillance from Europe or the USA, PCV13 serotypes ultimately accounted for 23 and 22%, respectively, of all IPD in the latest periods (123, 124).
From the country-level dataset tables within the WHO regions of Africa, Americas, Eastern Mediterranean, South-East Asia, and Western Pacific, PCV20non13 serotype proportions during the PCVhv period in the pediatric age group ranged from 1 to 32%. These values overlap with those reported from a global systematic literature review that included 26 studies from the Americas and 64 from Europe, also looking at pediatric age groups, where PCV20non13 serotypes accounted for 28% of IPD (125).
Beyond the PCV20 serotypes, nonPCV20 serotypes in the PCVhv period ranged from 1 to 52% in the pediatric age group, from 5 to 42% in the adult age group, and from 1 to 27% in the all-ages group. Like the pre-PCV period pooled data estimations, the most prevalent serotypes varied by region and by age group, although serotypes 6C, 15A, and 23A were among the most frequent in varying order across regions or age groups.
Strikingly, the proportion of undifferentiated serotype cases from the PCVhv period ranges from 0 to 78%, depending on the WHO region. Moreover, the studies where undifferentiated serotypes represented greater than 20% of the reported distribution were substantial in AFRO (58% of publications, 80% of countries) and in EMRO (50% of publications, 33% of countries). There could be two explanations for these high proportions. First, investigators occasionally reported the IPD serotyping outcomes only by PCV formulation, for instance as PCV13 or nonPCV13, in which case nonPCV13 would qualify as “undifferentiated” because individual PCV20non13 or nonPCV20 serotypes are not “clearly identifiable”. Note that this reporting style is not limited to publications from AFRO and EMRO (6, 126). Second, there may be economic limitations: the cost of Quellung reagents for testing may be prohibitive, while the application of PCR primers may not precisely identify a serotype.
By contrast to the pediatric age group, PCVhv period country-level results for adults are not available in certain regions, such as in AFRO, while the only a few adult IPD serotype results were available from EMRO (<100) or WPRO (<450). Although the evidence required for policymaking on childhood immunization programs explains the pediatric focus, our findings suggest a lack of surveillance systems or serotyping capacity dedicated to adult IPD in AFRO, EMRO, and WPRO.
A major strength of our review is that it identified 51 countries from five WHO regions to provide an analysis of the most recently available serotype distributions by PCV program period and by age group. We followed a systematic process (based on pre-established criteria of contemporality, geographic scope, and sample size) that allowed us to include the most recent publications from all countries, including occasionally, if no more recent publications were available, publications that were published earlier than the contemporality criteria or with sizes as low as 25 samples.
This review has limitations. First, this is not a systematic review of all publications from those regions and countries, as we focused this overview on the most recently available publications of serotype distribution in countries from these WHO regions. Second, two-thirds of the 135 countries that were reviewed across the five WHO regions lacked IPD serotype publications. Third, a few countries in each region had the largest data sets, dominating the published literature in the PCVhv period, such as Brazil for AMRO, Burkina Faso, Malawi, and South Africa for AFRO, and Taiwan for WPRO. Consequently, PCVhv period results were either lacking from many countries or among other countries in the same region based on fewer samples. Fourth, about half of the countries with published results provided surveillance from a PCVhv period; consequently, the most recently available published information from the other countries represented in the surveillance literature was restricted to the pre-PCV or intermediate period. A fifth limitation is the lack of incidence outcomes that prevents us from determining the absolute disease burden. A final limitation is the high proportion of undifferentiated serotype reporting, as explained above, that is due to surveillance settings where the applied serotyping method did not allow the IPD serotype to be clearly identifiable.
Conclusion
The WHO recommends that, rather than awaiting local surveillance results, countries with no serotype distribution surveillance in place take into consideration in the decision to introduce a PCV program the information from neighboring countries that have similar disease burden and socioeconomic or demographic patterns, which could include “sustained, high-quality sentinel and population-based surveillance for pneumococcal disease” and periodic cross-sectional surveys of nasopharyngeal carriage to provide “an indication of potential indirect effects of vaccination” (1). Nonetheless, once an NIP is in place, individual countries may expect national surveillance results to take evidence-based decisions about PCV program impact and about the possible transition to higher valency PCVs. Although about half of the recently published IPD surveillance in the five WHO regions covered by this review is from a higher valency PCV program period, the ubiquity of undifferentiated serotype reporting could hinder accurate estimates of serotype coverage for future higher valency formulations. Our review points to the need to enhance serotype reporting worldwide, first, by providing results by each individual serotype rather than based only on a calculated PCV formulation serotype coverage estimate and, second, by favoring serotyping methods that avoid “undifferentiated” serotype outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MAF: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Validation, Writing – original draft. DD: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. MS: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. MB: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. GH: Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft. GC: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Pfizer.
Acknowledgments
The authors would like to thank Omar Okasha (P95) for help with the initial stages of the project, Madeleine Crowe, Ana Goios, Alejandra Gonzalez, Francesca Lemme, and Ingrid Sepúlveda-Pachón (all at P95) for help with data extraction and analyses, data visualization, and editorial review—that was funded by Pfizer.
Conflict of interest
MAF, DD, and GC are employees of Pfizer and may hold stock or stock options. MS, MB, and GH are employees of P95, which received funding from Pfizer in connection with the development of this manuscript.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1402795/full#supplementary-material
References
1. World Health Organization (WHO). Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Wkly Epidemiol Rec. (2019) 94:85–104. Available at: https://iris.who.int/handle/10665/310970
2. Horn, EK, Wasserman, MD, Hall-Murray, C, Sings, HL, Chapman, R, and Farkouh, RA. Public health impact of pneumococcal conjugate vaccination: a review of measurement challenges. Expert Rev Vaccines. (2021) 20:1291–309. doi: 10.1080/14760584.2021.1971521
3. Chapman, R, Sutton, K, Dillon-Murphy, D, Patel, S, Hilton, B, Farkouh, R, et al. Ten year public health impact of 13-valent pneumococcal conjugate vaccination in infants: a modelling analysis. Vaccine. (2020) 38:7138–45. doi: 10.1016/j.vaccine.2020.08.068
4. Richter, SS, Heilmann, KP, Dohrn, CL, Riahi, F, Diekema, DJ, and Doern, GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.). Emerg Infect Dis. (2013) 19:1074–83. doi: 10.3201/eid1907.121830
5. Miller, E, Andrews, NJ, Waight, PA, Slack, MP, and George, RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. (2011) 11:760–8. doi: 10.1016/S1473-3099(11)70090-1
6. Ladhani, SN, Collins, S, Djennad, A, Sheppard, CL, Borrow, R, Fry, NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. (2018) 18:441–51. doi: 10.1016/S1473-3099(18)30052-5
7. Lewnard, JA, and Hanage, WP. Making sense of differences in pneumococcal serotype replacement. Lancet Infect Dis. (2019) 19:e213–20. doi: 10.1016/S1473-3099(18)30660-1
8. Deloria Knoll, M, Bennett, JC, Garcia Quesada, M, Kagucia, EW, Peterson, ME, Feikin, DR, et al. Global landscape review of serotype-specific invasive pneumococcal disease surveillance among countries using PCV10/13: the pneumococcal serotype replacement and distribution estimation (PSERENADE) Project. Microorganisms. (2021) 9:742. doi: 10.3390/microorganisms9040742
9. Wahl, B, O’Brien, KL, Greenbaum, A, Majumder, A, Liu, L, Chu, Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. (2018) 6:e744–57. doi: 10.1016/S2214-109X(18)30247-X
10. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
11. Wells, GA, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 3, 2021).
12. National Heart Lung and Blood Institute. Study quality assessment tools. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
13. García Gabarrot, G, López Vega, M, Pérez Giffoni, G, Hernández, S, Cardinal, P, Félix, V, et al. Effect of pneumococcal conjugate vaccination in Uruguay, a middle-income country. PLoS One. (2014) 9:e112337. doi: 10.1371/journal.pone.0112337
14. Hammitt, LL, Etyang, AO, Morpeth, SC, Ojal, J, Mutuku, A, Mturi, N, et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. Lancet. (2019) 393:2146–54. doi: 10.1016/S0140-6736(18)33005-8
15. Bar-Zeev, N, Swarthout, TD, Everett, DB, Alaerts, M, Msefula, J, Brown, C, et al. Impact and effectiveness of 13-valent pneumococcal conjugate vaccine on population incidence of vaccine and non-vaccine serotype invasive pneumococcal disease in Blantyre, Malawi, 2006–18: prospective observational time-series and case-control studies. Lancet Glob Health. (2021) 9:e989–98. doi: 10.1016/S2214-109X(21)00165-0
16. Diawara, I, Zerouali, K, Katfy, K, Zaki, B, Belabbes, H, Najib, J, et al. Invasive pneumococcal disease among children younger than 5 years of age before and after introduction of pneumococcal conjugate vaccine in Casablanca, Morocco. Int J Infect Dis. (2015) 40:95–101. doi: 10.1016/j.ijid.2015.09.019
17. Zintgraff, J, Fossati, S, Pereira, CS, Veliz, O, Regueira, M, Moscoloni, MA, et al. Distribution of PCV13 and PPSV23 Streptococcus pneumoniae serotypes in Argentinean adults with invasive disease, 2013–2017. Rev Argent Microbiol. (2020) 52:189–94. doi: 10.1016/j.ram.2019.11.004
18. Pérez, G, Mastroianni, A, Parra, A, Casimir, L, Reijtman, V, Lopardo, H, et al. Infecciones invasivas con bacteriemia por Streptococcus pneumoniae en niños: ¿qué pasó en los últimos 5 años? Med Infant. (2014) 21:318–23. Available at: http://www.medicinainfantil.org.ar/images/stories/volumen/2014/xxi_4_318.pdf
19. Gagetti, P, Lo, SW, Hawkins, PA, Gladstone, RA, Regueira, M, Faccone, D, et al. Population genetic structure, serotype distribution and antibiotic resistance of Streptococcus pneumoniae causing invasive disease in children in Argentina. Microb Genom. (2021) 7:000636. doi: 10.1099/mgen.0.000636
20. Agudelo, CI, Castañeda-Orjuela, C, Brandileone, MCDC, Echániz-Aviles, G, Almeida, SCG, Carnalla-Barajas, MN, et al. The direct effect of pneumococcal conjugate vaccines on invasive pneumococcal disease in children in the Latin American and Caribbean region (SIREVA 2006–17): a multicentre, retrospective observational study. Lancet Infect Dis. (2021) 21:405–17. doi: 10.1016/S1473-3099(20)30489-8
21. Brandileone, MC, Almeida, SCG, Bokermann, S, Minamisava, R, Berezin, EN, Harrison, LH, et al. Dynamics of antimicrobial resistance of Streptococcus pneumoniae following PCV10 introduction in Brazil: Nationwide surveillance from 2007 to 2019. Vaccine. (2021) 39:3207–15. doi: 10.1016/j.vaccine.2021.02.063
22. Brandileone, MC, Almeida, SCG, Minamisava, R, and Andrade, AL. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine. (2018) 36:2559–66. doi: 10.1016/j.vaccine.2018.04.010
23. Christophe, BL, Mott, M, da Cunha, G, Caierão, J, d’Azevedo, P, and Dias, C. Characterisation of Streptococcus pneumoniae isolates from invasive disease in adults following the introduction of PCV10 in Brazil. J Med Microbiol. (2018) 67:687–94. doi: 10.1099/jmm.0.000717
24. Dullius, CR, Zani, L, and Chatkin, JM. Theoretical pneumococcal vaccine coverage: analysis of serotypes isolated from inpatients at a tertiary care hospital. J Bras Pneumol. (2018) 44:361–6. doi: 10.1590/s1806-37562017000000056
25. Mott, M, Caierão, J, Rosa da Cunha, G, Rodrigues Perez, LR, Matusiak, R, Pilger de Oliveira, KR, et al. Susceptibility profiles and correlation with pneumococcal serotypes soon after implementation of the 10-valent pneumococcal conjugate vaccine in Brazil. Int J Infect Dis. (2014) 20:47–51. doi: 10.1016/j.ijid.2013.11.009
26. Berezin, EN, Jarovsky, D, Cardoso, MRA, and Mantese, OC. Invasive pneumococcal disease among hospitalized children in Brazil before and after the introduction of a pneumococcal conjugate vaccine. Vaccine. (2020) 38:1740–5. doi: 10.1016/j.vaccine.2019.12.038
27. Domingues, CMAS, Verani, JR, Montenegro Renoiner, EI, de Cunto Brandileone, MC, Flannery, B, de Oliveira, LH, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. The lancet. Respir Med. (2014) 2:464–71. doi: 10.1016/S2213-2600(14)70060-8
28. Valenzuela, MT, Seoane, M, Canals, A, Pidal, P, Hormazábal, JC, Araya, P, et al. Laboratory surveillance of Streptococcus pneumoniae from invasive disease, Chile 2007–2012. Rev Chilena Infectol. (2014) 31:651–8. doi: 10.4067/S0716-10182014000600002
29. Rioseco, ZML, Riquelme, OR, Riquelme, OM, Inzunza, PC, Riquelme, DJ, and Sanhueza, RA. Neumonía neumocócica bacteriémica en adultos en hospital regional de Chile. Rev Méd Chile. (2018) 146:839–45. doi: 10.4067/s0034-98872018000700839
30. Parra, EL, Ramos, V, Sanabria, O, and Moreno, J. Serotype and genotype distribution among invasive Streptococcus pneumoniae isolates in Colombia, 2005–2010. PLoS One. (2014) 9:e84993. doi: 10.1371/journal.pone.0084993
31. Severiche-Bueno, DF, Severiche-Bueno, DF, Bastidas, A, Caceres, EL, Silva, E, Lozada, J, et al. Burden of invasive pneumococcal disease (IPD) over a 10-year period in Bogotá, Colombia. Int J Infect Dis. (2021) 105:32–9. doi: 10.1016/j.ijid.2021.02.031
32. Narváez, PO, Gomez-Duque, S, Alarcon, JE, Ramirez-Valbuena, PC, Serrano-Mayorga, CC, Lozada-Arcinegas, J, et al. Invasive pneumococcal disease burden in hospitalized adults in Bogota, Colombia. BMC Infect Dis. (2021) 21:1059. doi: 10.1186/s12879-021-06769-2
33. Arguedas, A, Abdelnour, A, Soley, C, Jimenez, E, Jimenez, AL, Ramcharran, D, et al. Prospective epidemiologic surveillance of invasive pneumococcal disease and pneumonia in children in San José, Costa Rica. Vaccine. (2012) 30:2342–8. doi: 10.1016/j.vaccine.2012.01.047
34. Toraño-Peraza, G, Pías-Solis, L, Abreu-Capote, M, Rodríguez-Ortega, M, Dickinson-Meneses, F, and Varcárcel-Sánchez, M. Serotypes and antimicrobial resistance of meningeal isolates of Streptococcus pneumonia. Cuba, 2007–2012. VacciMonitor. (2014) 23:117–23. Available at: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-028X2014000300006&lng=en
35. Morera Álvarez, O, Madruga Jiménez, D, Fonseca Hernández, M, and Martínez, UA. Enfermedad neumocócica invasiva en menores de cinco años en Cienfuegos (2009–2015). MediSur. (2019) 17:494–504. Available at: http://www.medisur.sld.cu/index.php/medisur/article/view/4050
36. Toraño Peraza, G, Suárez Aspaza, D, Abreu Capote, M, Barreto Núnez, B, Toledo Romaní, E, and Linares Pérez, N. Serotipos de Streptococcus pneumoniae responsables de enfermedad invasiva en niños cubanos. Rev Cuba Pediatr. (2017) 89:172–80. Available at: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0034-75312017000500017&lng=es
37. Tomczyk, S, Lessa, FC, Sánchez, J, Peña, C, Fernández, J, Gloria Carvalho, M, et al. Effectiveness of 13-pneumococcal conjugate vaccine (PCV13) against invasive pneumococcal disease in children in the Dominican Republic. BMC Infect Dis. (2018) 18:152. doi: 10.1186/s12879-018-3047-3
38. Gaensbauer, JT, Asturias, EJ, Soto, M, Holt, E, Olson, D, and Halsey, NA. Pediatric invasive pneumococcal disease in Guatemala City: importance of serotype 2. Pediatr Infect Dis J. (2016) 35:e139–43. doi: 10.1097/INF.0000000000001067
39. Echaniz-Aviles, G, Soto-Nogueron, A, Miranda-Novales, G, Carnalla-Barajas, MN, Velazquez-Meza, ME, Solórzano-Santos, F, et al. Streptococcus pneumoniae serotypes identified in Mexican children with invasive disease before and after the introduction of PCV7 (1993–2012). Arch Med Res. (2015) 46:149–53. doi: 10.1016/j.arcmed.2015.02.004
40. Luna-Muschi, A, Castillo-Tokumori, F, Deza, MP, Mercado, EH, Egoavil, M, Sedano, K, et al. Invasive pneumococcal disease in hospitalised children from Lima, Peru before and after introduction of the 7-valent conjugated vaccine. Epidemiol Infect. (2019) 147:e91. doi: 10.1017/S0950268819000037
41. Hawkins, P, Mercado, E, Chochua, S, Castillo, ME, Reyes, I, Chaparro, E, et al. Key features of invasive pneumococcal isolates recovered in Lima, Peru determined through whole genome sequencing. Int J Med Microbiol. (2017) 307:415–21. doi: 10.1016/j.ijmm.2017.07.008
42. Pírez, MC, Mota, MI, Giachetto, G, Sánchez Varela, M, Galazka, J, Gutierrez, S, et al. Pneumococcal meningitis before and after universal vaccination with pneumococcal conjugate vaccines 7/13, impact on pediatric hospitalization in public and nonpublic institutions, in Uruguay. Pediatr Infect Dis J. (2017) 36:1000–1. doi: 10.1097/INF.0000000000001671
43. Ziane, H, Manageiro, V, Ferreira, E, Moura, IB, Bektache, S, Tazir, M, et al. Serotypes and antibiotic susceptibility of Streptococcus pneumoniae isolates from invasive pneumococcal disease and asymptomatic carriage in a pre-vaccination period, in Algeria. Front Microbiol. (2016) 7:803. doi: 10.3389/fmicb.2016.00803
44. Kambiré, D, Soeters, HM, Ouédraogo-Traoré, R, Medah, I, Sangaré, L, Yaméogo, I, et al. Early impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis—Burkina Faso, 2014–2015. J Infect. (2018) 76:270–9. doi: 10.1016/j.jinf.2017.12.002
45. Soeters, HM, Kambiré, D, Sawadogo, G, Ouédraogo-Traoré, R, Bicaba, B, Medah, I, et al. Impact of 13-Valent pneumococcal conjugate vaccine on pneumococcal meningitis, Burkina Faso, 2016–2017. J Infect Dis. (2019) 220:S253–62. doi: 10.1093/infdis/jiz301
46. Soeters, HM, Kambiré, D, Sawadogo, G, Ouédraogo-Traoré, R, Bicaba, B, Medah, I, et al. Evaluation of pneumococcal meningitis clusters in Burkina Faso and implications for potential reactive vaccination. Vaccine. (2020) 38:5726–33. doi: 10.1016/j.vaccine.2020.06.002
47. Boula, A, Senghore, M, Ngoh, R, Tassadjo, F, Fonkoua, MC, Nzouankeu, A, et al. Hospital-based surveillance provides insights into the etiology of pediatric bacterial meningitis in Yaoundé, Cameroon, in the post-vaccine era. Clin Infect Dis. (2019) 69:S148–55. doi: 10.1093/cid/ciz506
48. Libwea, JN, Fletcher, MA, Ndombo, PK, Boula, A, Ashukem, NT, Baleba, MN, et al. Impact of 13-valent pneumococcal conjugate vaccine on laboratory-confirmed pneumococcal meningitis and purulent meningitis among children <5 years in Cameroon, 2011–2018. PLoS One. (2021) 16:e0250010. doi: 10.1371/journal.pone.0250010
49. Sharew, B, Moges, F, Yismaw, G, Mihret, A, Abebe, W, Fentaw, S, et al. Serotype distribution of Streptococcus pneumoniae isolates causing invasive and non-invasive infections using whole-genome sequencing in Ethiopia. Infect Drug Resist. (2021) 14:787–94. doi: 10.2147/IDR.S293578
50. Lo, SW, Gladstone, RA, van Tonder, AJ, Lees, JA, du Plessis, M, Benisty, R, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. (2019) 19:759–69. doi: 10.1016/S1473-3099(19)30297-X
51. Bozio, CH, Abdul-Karim, A, Abenyeri, J, Abubakari, B, Ofosu, W, Zoya, J, et al. Continued occurrence of serotype 1 pneumococcal meningitis in two regions located in the meningitis belt in Ghana five years after introduction of 13-valent pneumococcal conjugate vaccine. PLoS One. (2018) 13:e0203205. doi: 10.1371/journal.pone.0203205
52. Kwambana-Adams, BA, Asiedu-Bekoe, F, Sarkodie, B, Afreh, OK, Kuma, GK, Owusu-Okyere, G, et al. An outbreak of pneumococcal meningitis among older children (≥5 years) and adults after the implementation of an infant vaccination programme with the 13-valent pneumococcal conjugate vaccine in Ghana. BMC Infect Dis. (2016) 16:575. doi: 10.1186/s12879-016-1914-3
53. Brueggemann, AB, Muroki, BM, Kulohoma, BW, Karani, A, Wanjiru, E, Morpeth, S, et al. Population genetic structure of Streptococcus pneumoniae in Kilifi, Kenya, prior to the introduction of pneumococcal conjugate vaccine. PLoS One. (2013) 8:e81539. doi: 10.1371/journal.pone.0081539
54. Raboba, JL, Rahajamanana, VL, Andriatahirintsoa, EPR, Razafindrakoto, AC, Andrianarivelo, AM, Nimpa Mengouo, M, et al. Decline in vaccine-type Streptococcus pneumoniae serotypes following pneumococcal conjugate vaccine introduction in Madagascar. J Infect Dis. (2021) 224:S285–92. doi: 10.1093/infdis/jiab226
55. Cornick, JE, Everett, DB, Broughton, C, Denis, BB, Banda, DL, Carrol, ED, et al. Invasive Streptococcus pneumoniae in children, Malawi, 2004–2006. Emerg Infect Dis. (2011) 17:1107–9. doi: 10.3201/eid/1706.101404
56. Massora, S, Lessa, FC, Moiane, B, Pimenta, FC, Mucavele, H, Chaúque, A, et al. Invasive disease potential of Streptococcus pneumoniae serotypes before and after 10-valent pneumococcal conjugate vaccine introduction in a rural area, southern Mozambique. Vaccine. (2019) 37:7470–7. doi: 10.1016/j.vaccine.2019.09.079
57. Nhantumbo, AA, Gudo, ES, Caierão, J, Munguambe, AM, Comé, CE, Zimba, TF, et al. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae in children with acute bacterial meningitis in Mozambique: implications for a national immunization strategy. BMC Microbiol. (2016) 16:134. doi: 10.1186/s12866-016-0747-y
58. Sigaúque, B, Verani, JR, Massora, S, Vubil, D, Quintó, L, Acácio, S, et al. Burden of invasive pneumococcal disease among children in rural Mozambique: 2001–2012. PLoS One. (2018) 13:e0190687. doi: 10.1371/journal.pone.0190687
59. Nhantumbo, AA, Weldegebriel, G, Katsande, R, De Gouveia, L, Comé, CE, Cuco, AZ, et al. Surveillance of impact of PCV-10 vaccine on pneumococcal meningitis in Mozambique, 2013–2015. PLoS One. (2017) 12:e0177746. doi: 10.1371/journal.pone.0177746
60. Gessner, BD, Sanou, O, Drabo, A, Tamekloe, TA, Yaro, S, Tall, H, et al. Pneumococcal serotype distribution among meningitis cases from Togo and Burkina Faso during 2007–2009. Vaccine. (2012) 30:G41–5. doi: 10.1016/j.vaccine.2012.10.052
61. Senghore, M, Tientcheu, PE, Worwui, AK, Jarju, S, Okoi, C, Suso, SMS, et al. Phylogeography and resistome of pneumococcal meningitis in west africa before and after vaccine introduction. Microbial. Genomics. (2021) 7:000506. doi: 10.1099/mgen.0.000506
62. Ousmane, S, Kobayashi, M, Seidou, I, Issaka, B, Sharpley, S, Farrar, JL, et al. Characterization of pneumococcal meningitis before and after introduction of 13-valent pneumococcal conjugate vaccine in Niger, 2010–2018. Vaccine. (2020) 38:3922–9. doi: 10.1016/j.vaccine.2020.04.009
63. Tagbo, BN, Bancroft, RE, Fajolu, I, Abdulkadir, MB, Bashir, MF, Okunola, OP, et al. Pediatric bacterial meningitis surveillance in Nigeria from 2010 to 2016, prior to and during the phased introduction of the 10-Valent pneumococcal conjugate vaccine. Clin Infect Dis. (2019) 69:S81–8. doi: 10.1093/cid/ciz474
64. Sonko, MA, Dube, FS, Okoi, CB, Diop, A, Thiongane, A, Senghore, M, et al. Changes in the molecular epidemiology of pediatric bacterial meningitis in Senegal after pneumococcal conjugate vaccine introduction. Clin Infect Dis. (2019) 69:S156–63. doi: 10.1093/cid/ciz517
65. Cohen, C, Naidoo, N, Meiring, S, de Gouveia, L, von Mollendorf, C, Walaza, S, et al. Streptococcus pneumoniae serotypes and mortality in adults and adolescents in South Africa: analysis of National Surveillance Data, 2003 – 2008. PLoS One. (2015) 10:e0140185. doi: 10.1371/journal.pone.0140185
66. von Gottberg, A, Cohen, C, de Gouveia, L, Meiring, S, Quan, V, Whitelaw, A, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003–2008. Vaccine. (2013) 31:4200–8. doi: 10.1016/j.vaccine.2013.04.077
67. Von Gottberg, A, De Gouveia, L, Tempia, S, Quan, V, Meiring, S, Von Mollendorf, C, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. (2014) 371:1889–99. doi: 10.1056/NEJMoa1401914
68. Tsolenyanu, E, Bancroft, RE, Sesay, AK, Senghore, M, Fiawoo, M, Akolly, D, et al. Etiology of pediatric bacterial meningitis pre- and post-PCV13 introduction among children under 5 years old in Lomé, Togo. Clin Infect Dis. (2019) 69:S97–s104. doi: 10.1093/cid/ciz473
69. Moïsi, JC, Makawa, MS, Tall, H, Agbenoko, K, Njanpop-Lafourcade, BM, Tamekloe, S, et al. Burden of pneumococcal disease in northern Togo before the introduction of pneumococcal conjugate vaccine. PLoS One. (2017) 12:e0170412. doi: 10.1371/journal.pone.0170412
70. Kisakye, A, Makumbi, I, Nansera, D, Lewis, R, Braka, F, Wobudeya, E, et al. Surveillance for Streptococcus pneumoniae meningitis in children aged <5 years: implications for immunization in Uganda. Clin Infect Dis. (2009) 48:S153–61. doi: 10.1086/596495
71. Yamba, K, Mpabalwani, E, Nakazwe, R, Mulendele, E, Weldegebriel, G, Mwenda, JM, et al. The burden of invasive bacterial disease and the impact of 10-Valent pneumococcal conjugate vaccine in children <5 years hospitalized for meningitis in Lusaka, Zambia, 2010–2019. J Infect Dis. (2021) 224:S275–84. doi: 10.1093/infdis/jiab193
72. Habibi Ghahfarokhi, S, Mosadegh, M, Ahmadi, A, Pourmand, MR, Azarsa, M, Rahbar, M, et al. Serotype distribution and antibiotic susceptibility of Streptococcus pneumoniae isolates in Tehran, Iran: a surveillance study. Infect Drug Resist. (2020) 13:333–40. doi: 10.2147/IDR.S234295
73. Houri, H, Tabatabaei, SR, Saee, Y, Fallah, F, Rahbar, M, and Karimi, A. Distribution of capsular types and drug resistance patterns of invasive pediatric Streptococcus pneumoniae isolates in Teheran, Iran. Int J Infect Dis. (2017) 57:21–6. doi: 10.1016/j.ijid.2017.01.020
74. Mousavi, SF, Nobari, S, Rahmati Ghezelgeh, F, Lyriai, H, Jalali, P, Shahcheraghi, F, et al. Serotyping of Streptococcus pneumoniae isolated from Tehran by multiplex PCR: are serotypes of clinical and carrier isolates identical? Iran J Microbiol. (2013) 5:220–6. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3895558/
75. Abdoli, S, Safamanesh, S, Khosrojerdi, M, and Azimian, A. Molecular detection and serotyping of Streptococcus pneumoniae in children with suspected meningitis in Northeast Iran. Iran J Med Sci. (2020) 45:125–33. doi: 10.30476/IJMS.2019.45423
76. Tabatabaei, SR, Karimi, A, Rahbar, M, Shirvani, F, Azimi, L, Shirdoost, M, et al. Profile of streptococcus pneumoniae serotypes of children with invasive disease in Tehran, Iran. An implication for vaccine coverage. Iran J Pediatr. (2021) 31:e106086. doi: 10.5812/ijp.106086
77. Mokaddas, E, and Albert, MJ. Serotype distribution and penicillin-non-susceptibility of Streptococcus pneumoniae causing invasive diseases in Kuwait: a 10-year study of impact of pneumococcal conjugate vaccines. Expert Rev Vaccines. (2016) 15:1337–45. doi: 10.1080/14760584.2016.1198698
78. Hanna-Wakim, R, Chehab, H, Mahfouz, I, Nassar, F, Baroud, M, Shehab, M, et al. Epidemiologic characteristics, serotypes, and antimicrobial susceptibilities of invasive Streptococcus pneumoniae isolates in a nationwide surveillance study in Lebanon. Vaccine. (2012) 30:G11–7. doi: 10.1016/j.vaccine.2012.07.020
79. Moghnieh, R, Tamim, H, Awad, L, Abdallah, D, Sleiman, R, Jisr, T, et al. Epidemiology of invasive and non-invasive pneumococcal infections in hospitalised adult patients in a Lebanese medical Centre, 2006–2015. J Infect Public Health. (2020) 13:2092–100. doi: 10.1016/j.jiph.2019.03.003
80. Elmdaghri, N, Benbachir, M, Belabbes, H, Zaki, B, and Benzaid, H. Changing epidemiology of pediatric Streptococcus pneumoniae isolates before vaccine introduction in Casablanca (Morocco). Vaccine. (2012) 30:G46–50. doi: 10.1016/j.vaccine.2012.10.044
81. Al-Jardani, A, Al Rashdi, A, Al Jaaidi, A, Al Bulushi, M, Al Mahrouqi, S, Al-Abri, S, et al. Serotype distribution and antibiotic resistance among invasive Streptococcus pneumoniae from Oman post 13-valent vaccine introduction. Int J Infect Dis. (2019) 85:135–40. doi: 10.1016/j.ijid.2019.05.027
82. Riaz, A, Mohiuddin, S, Husain, S, Yousafzai, MT, Sajid, M, Kabir, F, et al. Effectiveness of 10-valent pneumococcal conjugate vaccine against vaccine-type invasive pneumococcal disease in Pakistan. Int J Infect Dis. (2019) 80:28–33. doi: 10.1016/j.ijid.2018.12.007
83. Shakoor, S, Kabir, F, Khowaja, AR, Qureshi, SM, Jehan, F, Qamar, F, et al. Pneumococcal serotypes and serogroups causing invasive disease in Pakistan, 2005–2013. PLoS One. (2014) 9:e98796. doi: 10.1371/journal.pone.0098796
84. Elshafie, S, and Taj-Aldeen, SJ. Emerging resistant serotypes of invasive Streptococcus pneumoniae. Infect Drug Resist. (2016) 9:153–60. doi: 10.2147/IDR.S102410
85. al-Sheikh, YA, K Gowda, L, Mohammed Ali, MM, John, J, Khaled Homoud Mohammed, D, and Chikkabidare Shashidhar, P. Distribution of serotypes and antibiotic susceptibility patterns among invasive pneumococcal diseases in Saudi Arabia. Ann. Lab Med. (2014) 34:210–5. doi: 10.3343/alm.2014.34.3.210
86. Mokaddas, EM, Shibl, AM, Elgouhary, A, and Elsobky, M. Effect of the introduction of pneumococcal conjugate vaccines on serotype prevalence in Kuwait and Saudi Arabia. Vaccine. (2018) 36:6442–8. doi: 10.1016/j.vaccine.2018.07.067
87. Haddad-Boubaker, S, Lakhal, M, Fathallah, C, Mhimdi, S, Bouafsoun, A, Kechrid, A, et al. Epidemiological study of bacterial meningitis in Tunisian children, beyond neonatal age, using molecular methods: 2014–2017. Afr Health Sci. (2020) 20:1124–32. doi: 10.4314/ahs.v20i3.14
88. Baqui, AH, McCollum, ED, Mahmud, A, Roy, A, Chowdhury, NH, Rafiqullah, I, et al. Population-based incidence and serotype distribution of invasive pneumococcal disease prior to introduction of conjugate pneumococcal vaccine in Bangladesh. PLoS One. (2020) 15:e0228799. doi: 10.1371/journal.pone.0228799
89. Arjun, R, Ratheesh, RS, Mohan, V, Uduman, S, Jalaludeen, S, Prabhakaran, A, et al. Susceptibility and serotypes of Streptococcus pneumoniae isolates in invasive pneumococcal disease: a study from Kerala, South India. Infez Med. (2020) 28:558–64. Available at: https://www.infezmed.it/index.php/article?Anno=2020&numero=4&ArticoloDaVisualizzare=Vol_28_4_2020_558
90. Gopi, T, Ranjith, J, Anandan, S, and Balaji, V. Epidemiological characterisation of Streptococcus pneumoniae from India using multilocus sequence typing. Indian J Med Microbiol. (2016) 34:17–21. doi: 10.4103/0255-0857.174113
91. Jain, S, Das, BK, Mahajan, N, Kapil, A, Chaudhry, R, Sood, S, et al. Molecular capsular typing and multi locus sequence typing of invasive, non-invasive and commensal Streptococcus pneumoniae isolates from North India. Indian J Med Microbiol. (2020) 38:78–86. doi: 10.4103/ijmm.IJMM_20_111
92. Manoharan, A, Manchanda, V, Balasubramanian, S, Lalwani, S, Modak, M, Bai, S, et al. Invasive pneumococcal disease in children aged younger than 5 years in India: a surveillance study. Lancet Infect Dis. (2017) 17:305–12. doi: 10.1016/S1473-3099(16)30466-2
93. Ganaie, F, Govindan, V, Nagraj, G, and Ravikumar, KL. Serotype distribution and antimicrobial resistance of invasive Spneumoniae among Indian children before the introduction of pneumococcal conjugate vaccine. J Pediatr Infect Dis. (2016) 11:118–25. doi: 10.1055/s-0036-1597650
94. Jayaraman, R, Varghese, R, Kumar, JL, Neeravi, A, Shanmugasundaram, D, Ralph, R, et al. Invasive pneumococcal disease in Indian adults: 11 years’ experience. J Microbiol Immunol Infect. (2019) 52:736–42. doi: 10.1016/j.jmii.2018.03.004
95. Jayaraman, Y, Veeraraghavan, B, Chethrapilly Purushothaman, GK, Sukumar, B, Kangusamy, B, Kapoor, AN, et al. Burden of bacterial meningitis in India: preliminary data from a hospital based sentinel surveillance network. PLoS One. (2018) 13:e0197198. doi: 10.1371/journal.pone.0197198
96. Jayaraman, Y, Veeraraghavan, B, Girish Kumar, CP, Sukumar, B, Rajkumar, P, Kangusamy, B, et al. Hospital-based sentinel surveillance for bacterial meningitis in under-five children prior to the introduction of the PCV13 in India. Vaccine. (2021) 39:3737–44. doi: 10.1016/j.vaccine.2021.05.041
97. Chhetri, UD, Shrestha, S, Pradhan, R, Shrestha, A, Adhikari, N, Thorson, S, et al. Clinical profile of invasive pneumococcal diseases in Patan hospital, Nepal. Kathmandu Univ Med J (KUMJ). (2011) 9:45–9. doi: 10.3126/kumj.v9i1.6262
98. Phongsamart, W, Srifeungfung, S, Chatsuwan, T, Nunthapisud, P, Treerauthaweeraphong, V, Rungnobhakhun, P, et al. Changing trends in serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae causing invasive diseases in Central Thailand, 2009–2012. Hum Vaccin Immunother. (2014) 10:1866–73. doi: 10.4161/hv.28675
99. Srifeungfung, S, Phongsamart, W, Tribuddharat, C, Chatsuwan, T, Rungnobhakhun, P, Sapcharoen, S, et al. Serotype distribution and antibiotic susceptibility of invasive Streptococcus pneumoniae isolates in patients aged 50 years or older in Thailand. Hum Vaccin Immunother. (2014) 10:40–4. doi: 10.4161/hv.26418
100. Kim, SH, Chung, DR, Song, JH, Baek, JY, Thamlikitkul, V, Wang, H, et al. Changes in serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates from adult patients in Asia: emergence of drug-resistant non-vaccine serotypes. Vaccine. (2020) 38:6065–73. doi: 10.1016/j.vaccine.2019.09.065
101. Inghammar, M, By, Y, Farris, C, Phe, T, Borand, L, Kerleguer, A, et al. Serotype distribution of clinical streptococcus pneumoniae isolates before the introduction of the 13-valent pneumococcal conjugate vaccine in Cambodia. Am J Trop Med Hyg. (2018) 98:791–6. doi: 10.4269/ajtmh.17-0692
102. Turner, P, Leab, P, Ly, S, Sao, S, Miliya, T, Heffelfinger, JD, et al. Impact of 13-valent pneumococcal conjugate vaccine on colonization and invasive disease in cambodian children. Clin Infect Dis. (2020) 70:1580–8. doi: 10.1093/cid/ciz481
103. Russell, FM, Carapetis, JR, Tikoduadua, L, Paeds, D, Chandra, R, Seduadua, A, et al. Invasive pneumococcal disease in Fiji: clinical syndromes, epidemiology, and the potential impact of pneumococcal conjugate vaccine. Pediatr Infect Dis J. (2010) 29:870–2. doi: 10.1097/INF.0b013e3181ec7ae2
104. Moore, CE, Sengduangphachanh, A, Thaojaikong, T, Sirisouk, J, Foster, D, Phetsouvanh, R, et al. Enhanced determination of Streptococcus pneumoniae serotypes associated with invasive disease in Laos by using a real-time polymerase chain reaction serotyping assay with cerebrospinal fluid. Am J Trop Med Hyg. (2010) 83:451–7. doi: 10.4269/ajtmh.2010.10-0225
105. Arushothy, R, Ahmad, N, Amran, F, Hashim, R, Samsudin, N, and Azih, CRC. Pneumococcal serotype distribution and antibiotic susceptibility in Malaysia: a four-year study (2014–2017) on invasive paediatric isolates. Int J Infect Dis. (2019) 80:129–33. doi: 10.1016/j.ijid.2018.12.009
106. Yasin, RM, Zin, NM, Hussin, A, Nawi, SH, Hanapiah, SM, Wahab, ZA, et al. Current trend of pneumococcal serotypes distribution and antibiotic susceptibility pattern in Malaysian hospitals. Vaccine. (2011) 29:5688–93. doi: 10.1016/j.vaccine.2011.06.004
107. Capeding, MR, Bravo, L, Santos, J, Kilgore, PE, Kim, SA, Balter, I, et al. Prospective surveillance study of invasive pneumococcal disease among urban children in the Philippines. Pediatr Infect Dis J. (2013) 32:e383–9. doi: 10.1097/INF.0b013e318298dfd5
108. Martinez-Vega, R, Jauneikaite, E, Thoon, KC, Chua, HY, Huishi Chua, A, Khong, WX, et al. Risk factor profiles and clinical outcomes for children and adults with pneumococcal infections in Singapore: a need to expand vaccination policy? PLoS One. (2019) 14:e0220951. doi: 10.1371/journal.pone.0220951
109. Chen, CH, Su, LH, Li, HC, Hsu, MH, Wu, TL, Chen, CL, et al. Evaluation of the impact of 13-valent pneumococcal conjugate vaccine immunization in children by surveillance of culture-confirmed pneumococcal disease: a prospective clinical microbiological study. Vaccine. (2019) 37:5147–52. doi: 10.1016/j.vaccine.2019.07.073
110. Chen, YY, Hsieh, YC, Gong, YN, Liao, WC, Li, SW, Chang, IY, et al. Genomic insight into the spread of Meropenem-resistant Streptococcus pneumoniae Spain(23F)-ST81, Taiwan. Emerg Infect Dis. (2020) 26:711–20. doi: 10.3201/eid2604.190717
111. Chien, YC, Lee, YL, Liu, PY, Lu, MC, Shao, PL, Lu, PL, et al. National surveillance of antimicrobial susceptibilities to dalbavancin, telavancin, tedizolid, eravacycline, omadacycline and other comparator antibiotics and serotype distribution of invasive Streptococcus pneumoniae isolates in adults: results from the surveillance of multicenter antimicrobial resistance in Taiwan (SMART) programme in 2017–2020. J Glob Antimicrob Resist. (2021) 26:308–16. doi: 10.1016/j.jgar.2021.07.005
112. Cho, YC, Chiu, NC, Lu, CY, Huang, DT, Huang, FY, Chang, LY, et al. Redistribution of Streptococcus pneumoniae serotypes after Nationwide 13-valent pneumococcal conjugate vaccine program in children in northern Taiwan. Pediatr Infect Dis J. (2017) 36:e334–40. doi: 10.1097/INF.0000000000001664
113. Lee, HY, Wu, TL, Su, LH, Li, HC, Janapatla, RP, Chen, CL, et al. Invasive pneumococcal disease caused by ceftriaxone-resistant Streptococcus pneumoniae in Taiwan. J Microbiol Immunol Infect. (2018) 51:500–9. doi: 10.1016/j.jmii.2016.12.004
114. Lee, MC, and Kuo, KC. The clinical implication of serotype distribution and drug resistance of invasive pneumococcal disease in children: a single center study in southern Taiwan during 2010–2016. J Microbiol Immunol Infect. (2019) 52:937–46. doi: 10.1016/j.jmii.2019.04.006
115. Lee, MC, Kuo, KC, Lee, CH, Hsieh, YC, Tsai, MH, Huang, CT, et al. The antimicrobial susceptibility in adult invasive pneumococcal disease in the era of pneumococcus vaccination: a hospital-based observational study in Taiwan. J Microbiol Immunol Infect. (2020) 53:836–44. doi: 10.1016/j.jmii.2020.01.003
116. Chi, HC, Hsieh, YC, Tsai, MH, Lee, CH, Kuo, KC, Huang, CT, et al. Impact of pneumococcal conjugate vaccine in children on the serotypic epidemiology of adult invasive pneumococcal diseases in Taiwan. J Microbiol Immunol Infect. (2018) 51:332–6. doi: 10.1016/j.jmii.2016.08.009
117. Lu, CY, Chiang, CS, Chiu, CH, Wang, ET, Chen, YY, Yao, SM, et al. Successful control of Streptococcus pneumoniae 19A replacement with a catch-up primary vaccination program in Taiwan. Clin Infect Dis. (2019) 69:1581–7. doi: 10.1093/cid/ciy1127
118. Su, LH, Kuo, AJ, Chia, JH, Li, HC, Wu, TL, Feng, Y, et al. Evolving pneumococcal serotypes and sequence types in relation to high antibiotic stress and conditional pneumococcal immunization. Sci Rep. (2015) 5:15843. doi: 10.1038/srep15843
119. Wu, CJ, Lai, JF, Huang, IW, Shiau, YR, Wang, HY, and Lauderdale, TL. Serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae in pre- and post- PCV7/13 eras, Taiwan, 2002–2018. Front Microbiol. (2020) 11:557404. doi: 10.3389/fmicb.2020.557404
120. Iroh Tam, PY, Thielen, BK, Obaro, SK, Brearley, AM, Kaizer, AM, Chu, H, et al. Childhood pneumococcal disease in Africa – a systematic review and meta-analysis of incidence, serotype distribution, and antimicrobial susceptibility. Vaccine. (2017) 35:1817–27. doi: 10.1016/j.vaccine.2017.02.045
121. Kolhapure, S, Yewale, V, Agrawal, A, Krishnappa, P, and Soumahoro, L. Invasive pneumococcal disease burden and PCV coverage in children under five in Southeast Asia: implications for India. J Infect Dev Ctries. (2021) 15:749–60. doi: 10.3855/jidc.12166
122. Tai, SS. Streptococcus pneumoniae serotype distribution and pneumococcal conjugate vaccine serotype coverage among pediatric patients in east and Southeast Asia, 2000–2014: a pooled data analysis. Vaccines (Basel). (2016) 4:4. doi: 10.3390/vaccines4010004
123. Hanquet, G, Krizova, P, Dalby, T, Ladhani, SN, Nuorti, JP, Danis, K, et al. Serotype replacement after introduction of 10-Valent and 13-Valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis. (2022) 28:137–8. doi: 10.3201/eid2801.210734
124. Kobayashi, M, Farrar, JL, Gierke, R, Leidner, AJ, Campos-Outcalt, D, Morgan, RL, et al. Use of 15-Valent pneumococcal conjugate vaccine among U.S. children: updated recommendations of the advisory committee on immunization practices – United States, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1174–81. doi: 10.15585/mmwr.mm7137a3
125. Meroc, E, Fletcher, MA, Hanquet, G, Slack, MPE, Baay, M, Hayford, K, et al. Systematic literature review of the epidemiological characteristics of pneumococcal disease caused by the additional serotypes covered by the 20-Valent pneumococcal conjugate vaccine. Microorganisms. (2023) 11:1816. doi: 10.3390/microorganisms11071816
126. Moore, MR, Link-Gelles, R, Schaffner, W, Lynfield, R, Lexau, C, Bennett, NM, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. (2015) 15:301–9. doi: 10.1016/S1473-3099(14)71081-3
Keywords: pneumococcal conjugate vaccines, IPD (invasive pneumococcal disease), serotype, undifferentiated, surveillance, World Health Organization, age groups, immunization programs
Citation: Fletcher MA, Daigle D, Siapka M, Baay M, Hanquet G and del Carmen Morales G (2024) Serotype distribution of invasive pneumococcal disease from countries of the WHO Africa, Americas, Eastern Mediterranean, South-East Asia, and Western Pacific regions: a systematic literature review from 2010 to 2021. Front. Public Health. 12:1402795. doi: 10.3389/fpubh.2024.1402795
Edited by:
Catarina Silva Costa, University of Lisbon, PortugalReviewed by:
Willem René Miellet, University Medical Center Utrecht, NetherlandsDavid William Cleary, University of Birmingham, United Kingdom
Copyright © 2024 Fletcher, Daigle, Siapka, Baay, Hanquet and del Carmen Morales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark A. Fletcher, bWFyay5hLmZsZXRjaGVyQHBmaXplci5jb20=
†Present address: Mariana Siapka, Impact Epilysis, Thessaloniki, Greece
 Mark A. Fletcher
Mark A. Fletcher Derek Daigle
Derek Daigle Mariana Siapka
Mariana Siapka Marc Baay
Marc Baay Germaine Hanquet3
Germaine Hanquet3