- 1Key Laboratory of Physical Fitness and Exercise, Ministry of Education, Beijing Sport University, Beijing, China
- 2Department of Strength and Conditioning Assessment and Monitoring, Beijing Sport University, Beijing, China
- 3School of Physical Education, Xihua University, Chengdu, China
- 4China Institute of Sport and Health Science, Beijing Sport University, Beijing, China
- 5School of Sport Sciences, Beijing Sport University, Beijing, China
Background: A growing body of studies have examined the effect of exercise in people with multiple sclerosis (MS), while findings of available studies were conflicting. This meta-analysis aimed to explore the effects of exercise on balance, walking ability, walking endurance, fatigue, and quality of life in people with MS.
Methods: We searched PubMed, Web of Science, Scopus, and Cochrane databases, through March 1, 2024. Inclusion criteria were: (1) RCTs; (2) included an intervention and control group; (3) had people with MS as study subjects; (4) had balance, walking ability, walking endurance, fatigue, or quality of life as the outcome measures. Exclusion criteria were: (1) non-English publications; (2) animal model publications; (3) review articles; and (4) conference articles. A meta-analysis was conducted to calculate weighted mean difference (WMD) and 95% confidence interval (CI). Cochrane risk assessment tool and Physiotherapy Evidence Database (PEDro) scale were used to evaluate the methodological quality of the included studies.
Results: Forty studies with a total of 56 exercise groups (n = 1,300) and 40 control groups (n = 827) were eligible for meta-analysis. Exercise significantly improved BBS (WMD, 3.77; 95% CI, 3.01 to 4.53, P < 0.00001), TUG (WMD, −1.33; 95% CI, −1.57 to −1.08, P < 0.00001), MSWS-12 (WMD, −2.57; 95% CI, −3.99 to −1.15, P = 0.0004), 6MWT (WMD, 25.56; 95% CI, 16.34 to 34.79, P < 0.00001), fatigue (WMD, −4.34; 95% CI, −5.83 to −2.84, P < 0.00001), and MSQOL-54 in people with MS (WMD, 11.80; 95% CI, 5.70 to 17.90, P = 0.0002) in people with MS. Subgroup analyses showed that aerobic exercise, resistance exercise, and multicomponent training were all effective in improving fatigue in people with MS, with resistance exercise being the most effective intervention type. In addition, a younger age was associated with a larger improvement in fatigue. Furthermore, aerobic exercise and multicomponent training were all effective in improving quality of life in people with MS, with aerobic exercise being the most effective intervention type.
Conclusion: Exercise had beneficial effects in improving balance, walking ability, walking endurance, fatigue, and quality of life in people with MS. Resistance exercise and aerobic exercise are the most effective interventions for improving fatigue and quality of life in people with MS, respectively. The effect of exercise on improving fatigue was associated with the age of the participants, with the younger age of the participants, the greater the improvement in fatigue.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=371056, identifier: CRD42022371056.
Introduction
Multiple sclerosis (MS) is a disabling neurological disease common in young and middle-aged adults with a mean age of onset of 29 years (1, 2). The manifestations of people with MS include physical symptoms such as muscle weakness, muscle spasms, decreased mobility and balance, and increased sensitivity to pain, with psychiatric episodes and fatigue leading to severe disability and deterioration of physical condition, mobility, cognition, and quality of life (3–7). In fact, 50–80% of people with MS, even in its mild stages, will result in impaired walking performance, further reducing their quality of life as the disease progresses (8).
People with MS usually use pharmacologic strategies that down-regulate immune activation to halt disease progression, prevent relapse, or partially reverse disability (9). However, pharmacologic treatments are often accompanied by adverse effects such as infection, headache, and diarrhea (10). In recent years, exercise has been found to be beneficial in improving aerobic capacity, muscle strength, flexibility, balance, fatigue, and cognitive function in people with MS (11).
A growing body of studies have examined the effect of exercise in people with MS, while findings of available studies were conflicting. Kubsik et al. (12) showed that exercise not only contributes to the physical abilities of people with MS, but also to their mood and attitude toward exercise. In addition, Grazioli et al. (13) reported that multicomponent training was effective in improving quality of life, walking ability, and balance, as well as reducing depression, fatigue, and disease severity in people with MS. Furthermore, Feys et al. (14) showed that running improved aerobic capacity, functional mobility, spatial memory, fatigue, and quality of life in people with MS. However, a meta-analysis showed no significant differences in step count and moderate to vigorous physical activity among individuals with MS, both within and between groups receiving physical activity interventions (14). To the best of our knowledge, Arntzen et al. (15) included only eight randomized controlled trials (RCTs) and the number of included studies was quite small, and the authors included one study in which participants in the control group also received exercise intervention. Another study evaluated the effects of Pilates on balance in people with MS, which included only seven RCTs (16). However, the authors included studies in which control group participants also received exercise interventions such as home exercises (two studies), relaxation exercises (one study), aerobic exercises (one study), and traditional exercises (one study), which may have had some impact on their findings. Therefore, we conducted a comprehensive systematic review and meta-analysis of RCTs to explore the effects of exercise on balance, walking ability, walking endurance, fatigue, and quality of life in people with MS.
Methods
This systematic review and meta-analysis was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA, 2020) guidelines (17) and the implementing PRISMA in exercise, rehabilitation, sport medicine, and sports science (PERSiST) guidance (18). The protocol was registered with PROSPERO (CRD42022371056).
Search strategy
We searched the PubMed, Web of Science, Scopus, and Cochrane databases for RCTs relating to the effect of exercise on balance, gait, fatigue, and quality of life in patients with MS from the inception dates to March 1, 2024 (Supplementary Table 1). We also manually searched references listed in the identified systematic reviews and meta-analyses, in addition to the reference lists of identified studies included in the screening. Two authors (L.D. and H.X.) independently completed the article screening using a standardized form.
Inclusion and exclusion criteria
Inclusion criteria were: (1) RCTs; (2) included an intervention and control group; (3) had people with MS as study subjects; (4) had balance, walking ability, walking endurance, fatigue, or quality of life as the outcome measures. Exclusion criteria were: (1) non-English publications; (2) animal model publications; (3) review articles; and (4) conference articles.
Data extraction
Two authors (L.D. and H.X.) independently performed the data extraction, mainly including: (1) study characteristics (surname of the first author, year of publication, and sample size); (2) intervention characteristics (intensity, duration, and frequency); (3) participant characteristics (gender, disease stage, and disease duration); (4) treatment effects [mean and standard deviation (SD) values reflecting changes in balance, walking ability, walking endurance, fatigue, and quality of life from baseline to post intervention].
Methodological quality assessment
The methodological quality for the included studies was independently assessed by two authors (L.D. and H.X.) based on the Cochrane risk of bias tool (RoB2) (19) and Physiotherapy Evidence Database (PEDro) scale (20, 21). If there was disagreement between the two authors, a third author (LY) would join the discussion until the three reach a consensus. RoB2 was assessed mainly from seven items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. PEDro scale is an 11-item scale used to evaluate the quality of the RCTs of the physical therapy studies, where studies scoring <4, 4–5, 6–8, and >9 points are considered poor quality, average, good, and excellent, respectively (21).
Statistical analysis
We extracted the mean and SD values reflecting changes in timed up and go test (TUG), Berg balance scale (BBS), multiple sclerosis walking scale-12 (MSWS-12), 6-minute walk test (6MWT), fatigue severity scale (FSS), modified fatigue impact scale (MFIS), and multiple sclerosis quality of life-54 (MSQOL-54) from baseline to post-intervention from each study for pooling effects. Weighted mean difference (WMD) and 95% confidence interval (CI) were used to estimate the effects of exercise on balance, walking ability, walking endurance, fatigue, and quality of life in people with MS. For studies reporting standard error (SE) or 95% confidence interval (CI), SD was calculated using the previously described formula. Otherwise, PlotDigitizer online software (www.plotdigitizer.com) was used (22). The I2 static was used to assess heterogeneity, where I2 <25% indicates no significant heterogeneity, 25% <I2 <50% indicates low heterogeneity, 50% <I2 <75% indicates moderate heterogeneity, and I2 > 75% indicates high heterogeneity (23). If there was a high heterogeneity (I2 > 60%), meta-regression analysis, subgroup analysis, and sensitivity analysis were used to interpret the results (19).
For subgroup analyses, we examined the effects of intervention type (aerobic exercise, resistance exercise, and multicomponent exercise), participants' age (young, <45 years old; and middle-aged and older adult, ≥45 years old), and type of fatigue detection (FSS and MFIS) on fatigue and intervention type (aerobic exercise and multicomponent exercise) on quality of life in people with MS. Meta-regressions were conducted based on the participants' age, disease duration, duration of intervention, session duration, and weekly time. The analysis result, funnel plot, and forest plot were generated using RevMan 5.2 software. Statistical significance was considered for outcomes with a P < 0.05.
Results
Study selection
As shown in Figure 1, 5,432 records were initially identified from the databases and 11 records from other sources. Three thousand nine hundred and twenty-five studies remained after excluding duplicates and 130 potentially eligible studies remained after the title and abstract screening. Ninety studies were excluded by reading the full text: (1) wrong publication type (e.g., reviews, conference abstracts, n = 42); (2) the experimental group combined with other interventions (n = 22); (3) studied irrelevant outcome (n = 15); (4) recruited non-multiple sclerosis participants (n = 11). Finally, 40 studies (24–63) were considered eligible for systematic review and meta-analysis.
Characteristics of the included studies
The main characteristics of participants and interventions were shown in Table 1. Among the included studies, there were 1,300 people with MS in the 56 exercise groups and 827 people with MS in the 40 control groups. Six studies involved women, 1 study involved men, and 30 studies involved both men and women. The mean age of the participants ranged from 16.3 to 61.6 years. Thirty-seven studies (24–28, 30–32, 34–49, 51–63) involved participants with mean age <60 years, and three studies (29, 33, 50) involved participants with mean age ≥60 year. Most interventions specified aerobic exercise (n = 16) (24–26, 30, 33, 34, 36, 38, 39, 45, 46, 51, 53, 56, 58, 63), balance training (n = 10) (29, 37, 43, 47, 50, 52, 54, 55, 59, 61), resistance exercise (n = 6) (27, 28, 32, 41, 44, 49), or other types of exercise [such as multicomponent training (n = 5) (31, 35, 42, 48, 62) water sports (n = 2) (40, 57); interval training (n = 1) (60)]. Of the 40 studies, 26 studies provided data for balance, which was tested by BBS (20 studies) (24, 29, 34, 36–40, 42–44, 46, 49, 50, 52–54, 58, 59, 61) and TUG (17 studies) (25–27, 33, 34, 37, 38, 42–44, 50, 51, 54–56, 59, 61, 62). In addition, 17 studies provided data for gait, which was tested by MSWS-12 (walking ability, eight studies) (29, 30, 34, 47, 50, 55, 60, 61) and 6MWT (walking endurance, 14 studies) (25, 28, 30, 39, 40, 42, 45, 48, 51, 53, 54, 56, 60, 61). Furthermore, 17 studies provided data for fatigue, which was tested by FSS (nine studies) (24, 26, 28, 42, 44, 51–54) and MFIS (eight studies) (30, 32, 33, 39, 41, 43, 50, 57). Moreover, six studies provided data for quality of life (24, 31, 45, 48, 57, 63), which was tested by MSQOL-54.
Meta-analysis results
Effects of exercise on balance in people with MS
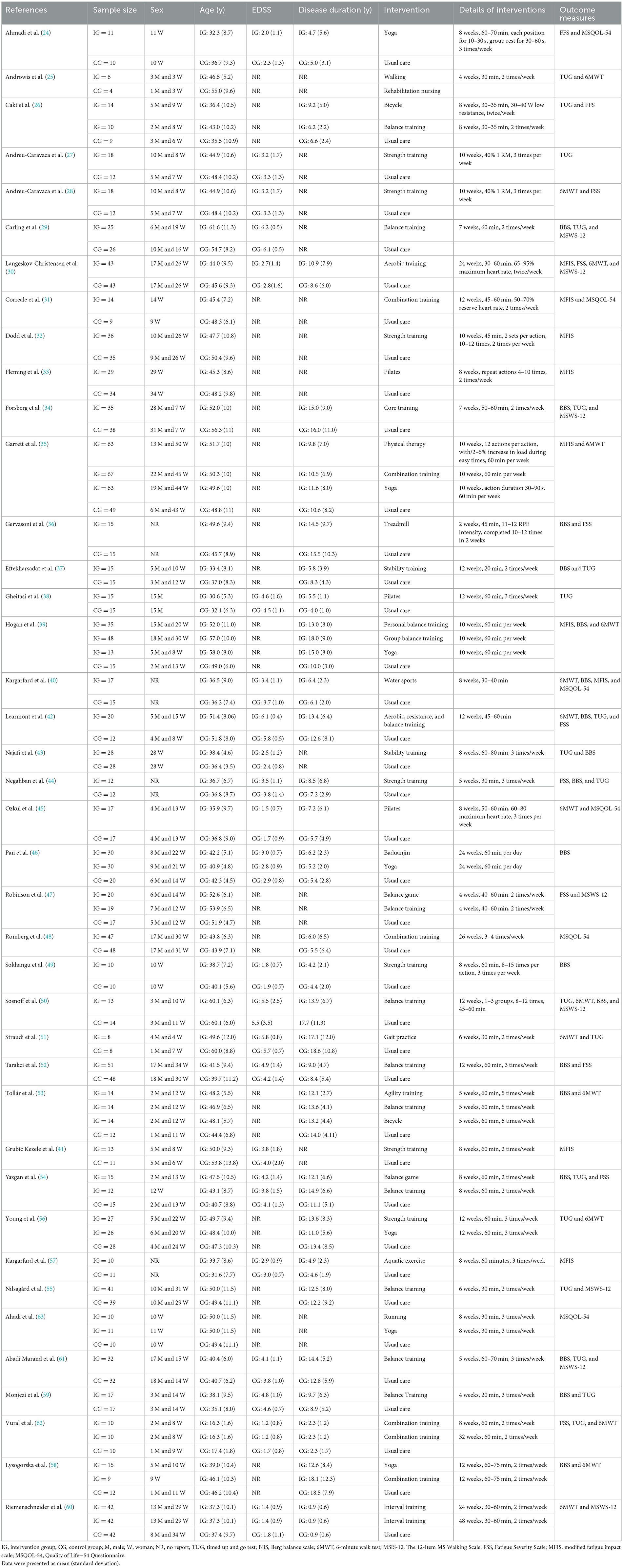
The balance of people with MS was detected by BBS and TUG, with 20 studies providing BBS data and 20 studies providing TUG data. Our results showed that exercise had a significant effect on improving BBS (WMD, 3.77; 95% CI, 3.01 to 4.53, P < 0.00001, I2 = 50%, Figure 2) and TUG (WMD, −1.33; 95% CI, −1.57 to −1.08, P < 0.00001, I2 = 34%, Figure 3) in people with MS.
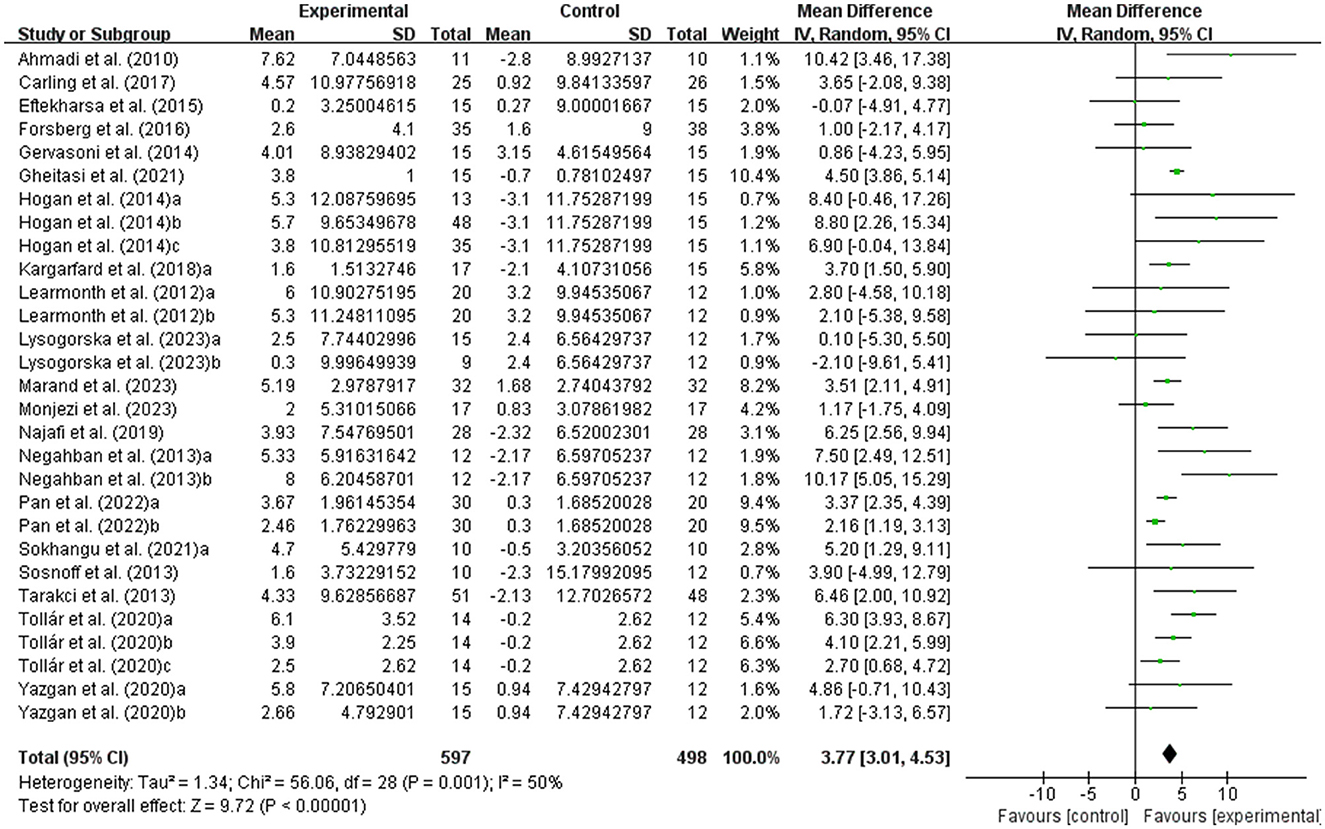
Figure 2. Meta-analysis results of the effects of exercise on Berg balance scale (BBS) in people with MS. Exercise had a significant effect on improving BBS in people with MS (P < 0.00001).
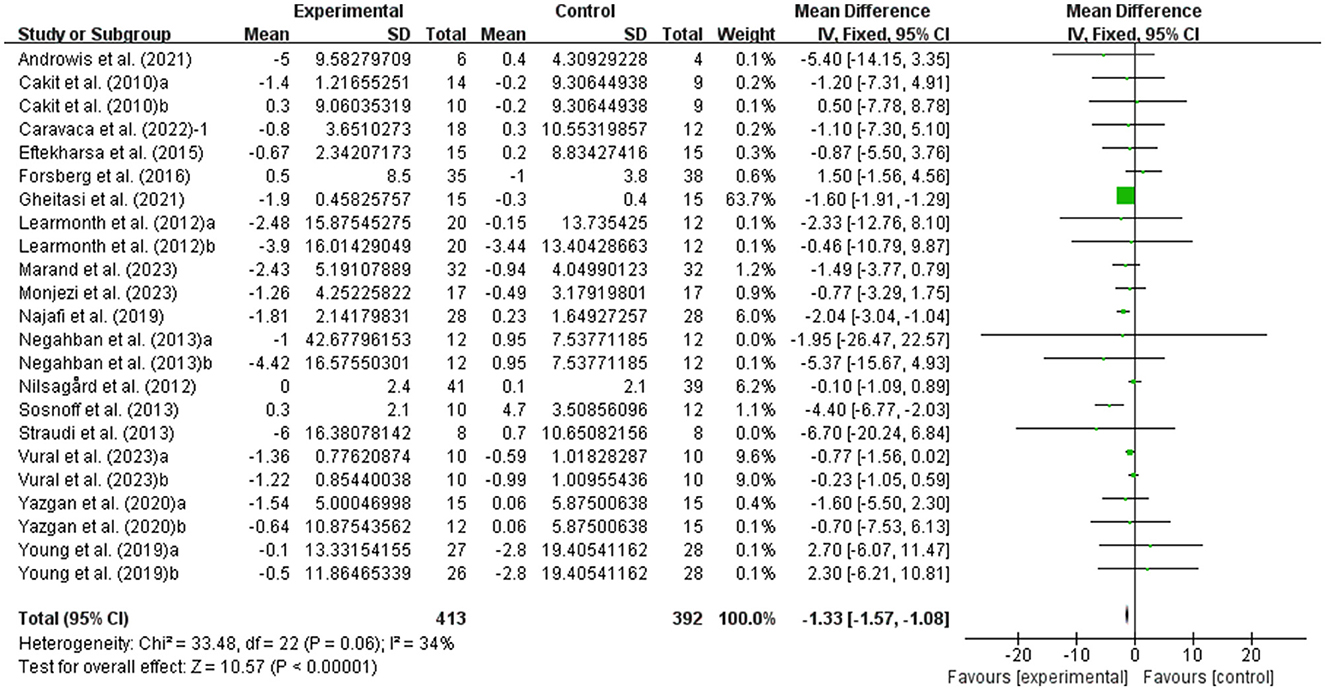
Figure 3. Meta-analysis results of the effect of exercise on timed up and go test (TUG) in people with MS. Exercise had a significant effect on improving TUG in people with MS (P < 0.00001).
Effects of exercise on walking ability and walking endurance in people with MS
MSWS-12 was used to test walking ability and 6MWT was used to test walking endurance of people with MS. It was found that exercise had a significant effect on improving MSWS-12 (WMD, −2.57; 95% CI, −3.99 to −1.15, P = 0.0004, I2 = 19%, Figure 4) and 6MWT (WMD, 25.56; 95% CI, 16.34 to 34.79, P < 0.00001, I2 = 47%, Figure 5) in people with MS.
Figure 4. Meta-analysis results of the effects of exercise on walking ability in people with MS. Exercise had a significant effect on improving walking ability in people with MS (P = 0.0004).
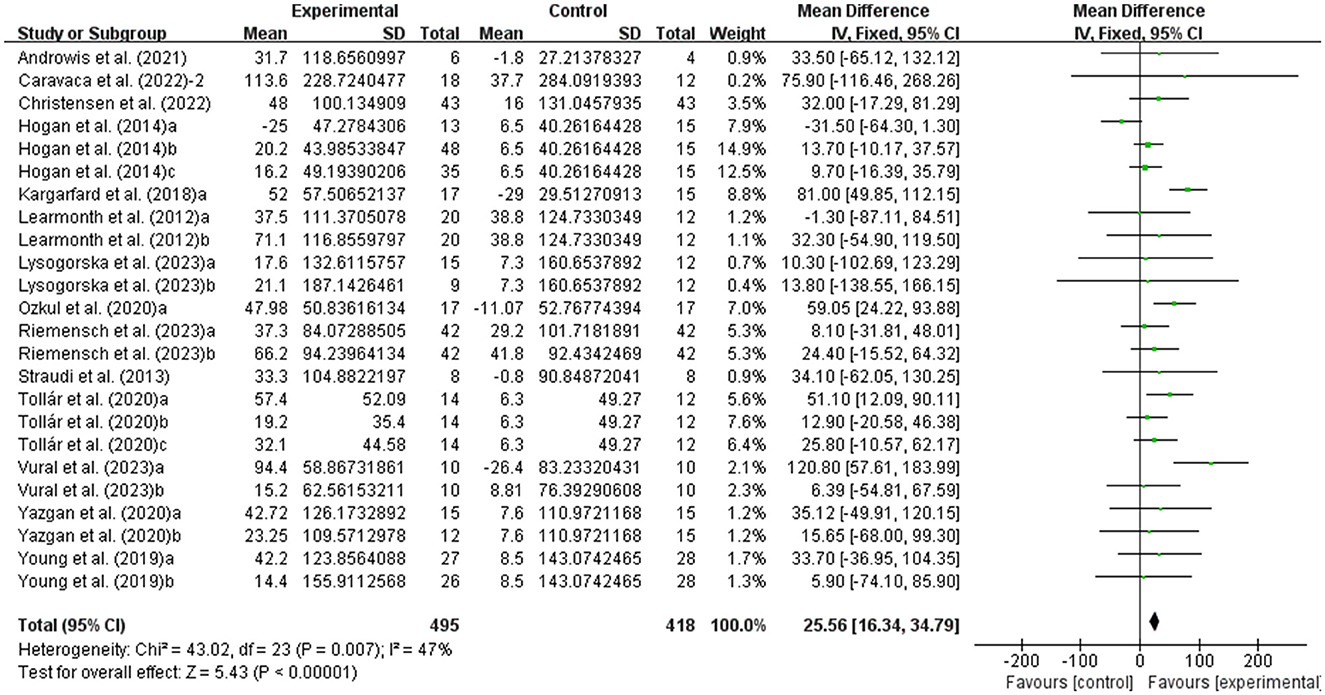
Figure 5. Meta-analysis results of the effects of exercise on walking endurance in people with MS. Exercise had a significant effect on improving walking endurance in people with MS (P < 0.00001).
Effects of exercise on fatigue in people with MS
The fatigue of people with MS was detected by FSS and MFIS. As shown in Figure 6, exercise had a significant effect on improving fatigue in people with MS (WMD, −4.34; 95% CI, −5.83 to −2.84, P < 0.00001, I2 = 79%).
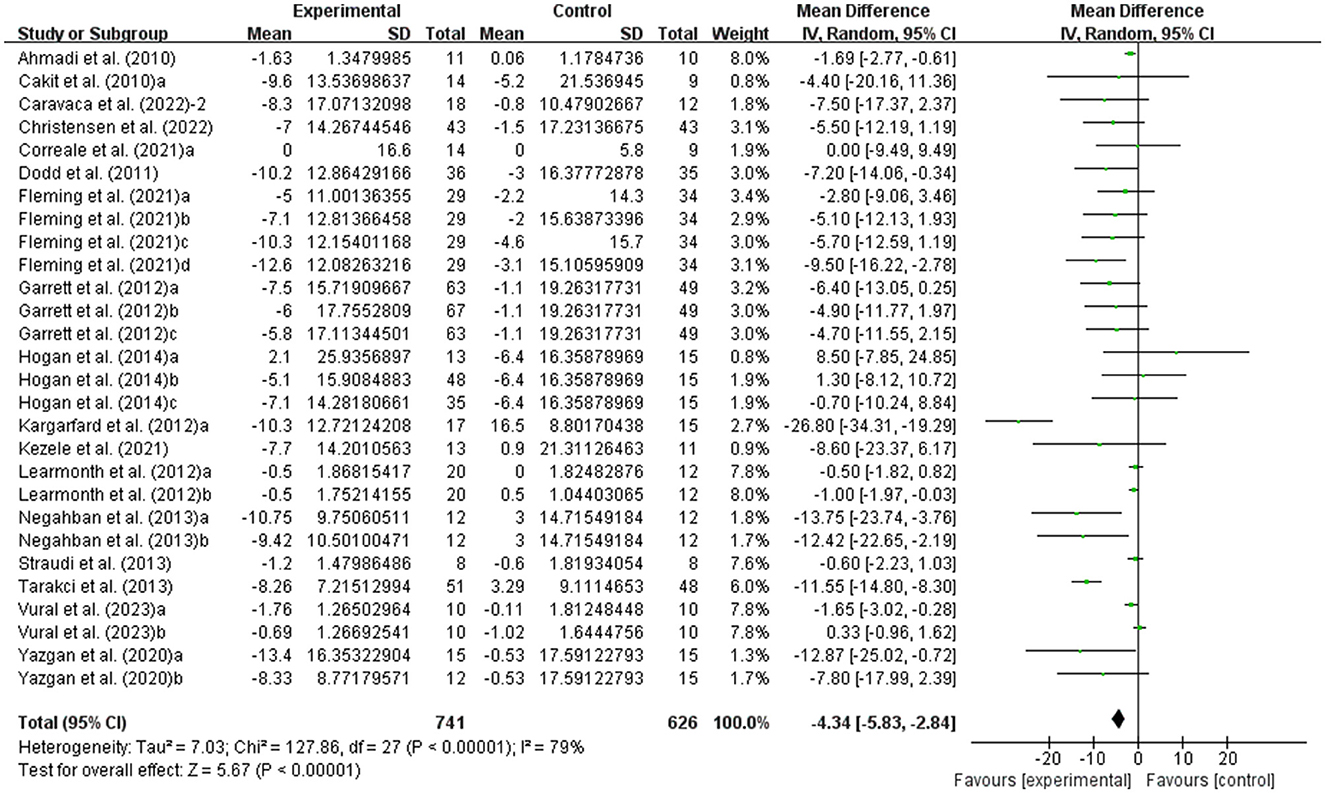
Figure 6. Results of the effects of exercise on fatigue in people with MS. Exercise had a significant effect on improving fatigue in people with MS (P < 0.00001).
Effects of exercise on quality of life in people with MS
The fatigue of people with MS was detected by MSQOL-54. As shown in Figure 7, exercise had a significant effect on improving MSQOL-54 in people with MS (WMD, 11.80; 95% CI, 5.70 to 17.90, P = 0.0002, I2 = 66%).
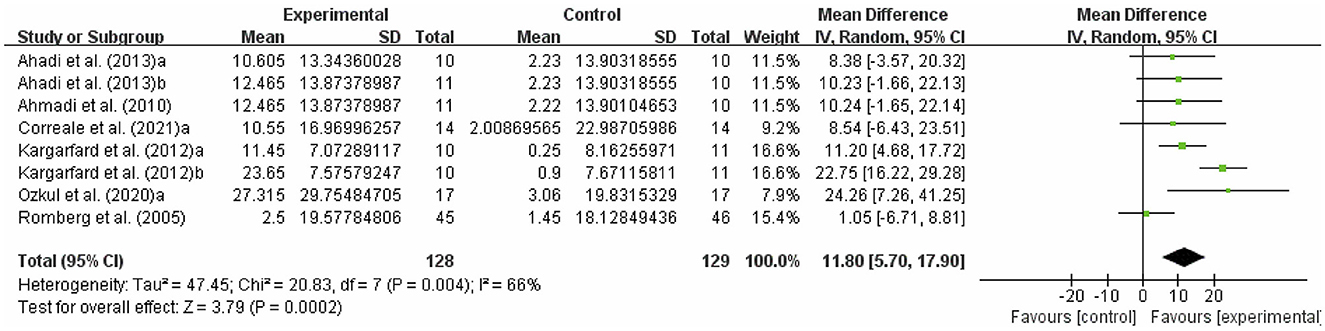
Figure 7. Results of the effects of exercise on quality of life in people with MS. Exercise had a significant effect on improving quality of life in people with MS (P = 0.0002).
Our meta-analysis results showed high heterogeneity in fatigue (I2 = 78%) and quality of life (I2 = 66%), to explain the heterogeneity between included studies and find modifiable factors of exercise, meta-regression analysis, subgroup analysis, and sensitivity analysis were further performed.
Meta-regression analysis
Meta-regression analyses were performed on intervention characteristics (duration of intervention, weekly time, and session duration) and participant characteristics (age and disease duration). There was no significant association between age (P = 0.782), duration of intervention (P = 0.124), weekly time (P = 0.730), session duration (P = 0.124), or disease duration (P = 0.559) and fatigue (Supplementary Figure 1). In addition, no significant associations were observed between duration of intervention (P = 0.086), weekly time (P = 0.583), session duration (P = 0.878), age (P = 0.172), or disease duration (P = 0.289) and quality of life (Supplementary Figure 2).
Subgroup analysis
Fatigue
We conducted three different subgroup analyses by participants' age, type of fatigue detection, and type of intervention. Subgroup analysis indicated that a younger age was associated with larger improvement in fatigue (young, age <45, WMD, −6.67; 95% CI, −9.57 to −3.60, P < 0.0001, I2 = 91%; middle-aged and older adult, age ≥ 45, WMD, −1.76; 95% CI, −3.29 to −0.24, P = 0.02, I2 = 22%, Supplementary Figure 3).
Stratifying the analysis by type of fatigue detection, the improvement in fatigue scores remained significant in FSS (WMD, −2.75; 95% CI, −4.27 to −1.24, P = 0.0004, I2 = 81%) and MFIS (WMD, −5.84; 95% CI, −9.28 to −2.40, P = 0.0009, I2 = 65%, Supplementary Figure 4).
In addition, aerobic exercise (WMD, −7.07; 95% CI, −11.25 to −2.88, P = 0.0009, I2 = 81%), resistance exercise (WMD, −8.03; 95% CI, −11.84 to −4.22, P < 0.0001, I2 = 0%), and multicomponent training (WMD, −2.54; 95% CI, −4.44 to −0.65, P = 0.009, I2 = 80%) were effective in improving fatigue in people with MS, with resistance exercise being the most effective intervention type (Supplementary Figure 5).
Quality of life
We conducted a subgroup analysis by type of intervention. Aerobic exercise (WMD, 11.68; 95% CI, 5.31 to 18.05, P = 0.0003, I2 = 0%) and multicomponent training (WMD, 7.28; 95% CI, 2.77 to 11.79, P = 0.002, I2 = 24%) were effective in improving quality of life in people with MS, with aerobic exercise being the most effective intervention type (Supplementary Figure 6).
Sensitivity analysis
Sensitivity analyses showed that there is no change in the direction or level of compatibility of the overall effect of exercise on fatigue (Supplementary Figure 7) and quality of life (Supplementary Figure 8) in people with MS when any of the included studies are omitted.
Risk of bias
The quality of included studies was assessed by the Cochrane Collaboration tool in terms of selection bias, performance bias, attrition bias, reporting bias, detection bias, and other bias (Supplementary Figure 9). The results of PEDro scale showed that of the 40 included studies, 39 were of good quality and one was of fair quality (Supplementary Table 2).
Publication bias
Possible publication bias was assessed by examining the funnel plot (Supplementary Figure 10). Visual inspection of the funnel plot suggested the absence of funnel plot asymmetry. The results of the egger's test indicated that the small sample size studies were not enough to affect the final results (TUG, P = 0.575; BBS, P = 0.705; 6MWT, P = 0.586; MSWS-12, P = 0.137; quality of life, P = 0.791; Supplementary Table 3), with the exception of fatigue (P = 0.002). Therefore, we performed the Dsuval and Tweedie's trim and fill procedure, and the results indicated that no evidence of publication bias was found for fatigue.
Discussion
In this systematic review and meta-analysis, exercise significantly improved balance, walking ability, walking endurance, fatigue, and quality of life in people with MS. Subgroup analyses showed that a younger age was associated with larger improvement in fatigue. In addition, resistance exercise and aerobic exercise were the most effective interventions for improving fatigue and quality of life, respectively.
Loss of balance and walking ability are two of the primary impairments of MS that leads to increased fatigue perception and disease severity, and loss of autonomy (13). Imbalance, gait dysfunction and falls are common in people with MS, with the overwhelming majority having abnormal postural control and gait even early in the course of the disease. It has been reported that 50–80% people with MS have balance and gait dysfunction and over 50% fall at least once each year (64). Exercise has been shown to improve physical function and psychological rehabilitation in people with MS, and to help reduce the risk of falls (65, 66). Our study showed that exercise significantly improved balance function (TUG and BBS) in people with MS, which was consistent with a previous study (13), showing that the combination of resistance and aerobic exercise training is effective in improving balance in people with MS and supports functional and psychological therapeutic effects through exercise. In addition, a meta-analysis showed that yoga was the best intervention to improve static and dynamic balance, and aquatic training was the best intervention to improve walking ability in people with MS (67). The mechanisms by which exercise improves balance may be that exercise improves neurological control of muscles, increases unconscious deliberate muscle responses to dynamic joint stabilization signals, and enhances core area muscle strength to strengthen body stability.
Our results showed that exercise significantly improved walking ability (MSWS-12) and walking endurance (6MWT) in people with MS, which was in agreement with previous studies, showing that aerobic exercise, aquatic exercise, virtual reality training, and assisted gait training significantly improved walking ability (67–69), as well as that Pilates, aerobic exercise, resistance exercise, high-intensity training, and intermittent walking training significantly improved walking endurance in people with MS (28, 68, 70–72). Furthermore, fast-velocity concentric resistance training may have a greater effect on walking endurance with greater neural adaptations in a shorter period of time (28). A meta-analysis showed that walking training programs significantly improved functional ability (mobility, walking endurance, and gait speed), possibly due to improved walking economy (68). The mechanisms by which exercise improves walking ability and walking endurance in people with MS may be improvements in maximal oxygen uptake, muscular strength, and fitness. The increase in muscle strength is due to improved firing and synchronization of motor units and improved synergistic coordination of agonists and antagonists (73). Moreover, another mechanism may be increased bilateral symmetry, which reduces the amount of time the lower limbs are supported on the ground (74).
Early fatigue in people with MS presents with common symptoms such as decreased endurance and muscle strength (75). Statistically, fatigue affects approximately two-thirds of people with MS (76). Current evidence suggests that pharmacological interventions are largely ineffective and that exercise significantly reduces fatigue in people with MS (77, 78). Our results showed that exercise significantly improved fatigue in people with MS, which was consistent with the results of Taul-Madsen et al. (79), showing that aerobic exercise is effective in reducing perceived fatigue in people with MS. The mechanism by which exercise improves fatigue may be an improvement in cardiorespiratory fitness, which increases available energy reserve and reduces fatigue. In addition, exercise may induce upregulation of neuroendocrine growth factor secretion, which increases neuronal plasticity and thus may improve compensatory cortical activation (80, 81). Furthermore, exercise-induced upregulation of anti-inflammatory cytokines may have beneficial effects on fatigue (82–84).
Resistance exercise has been reported to be an effective intervention to ameliorate physical and generalized fatigue and result in significant changes in muscle strength and postural stability (85). Subgroup analysis showed that aerobic exercise, resistance exercise, and multicomponent training were effective in improving fatigue in people with MS, with resistance exercise being the most effective intervention type, which may be due to the fact that resistance exercise is well-tolerated in people with MS, restores the ability to respond quickly to stimuli, and improves autonomy when walking (13). Previous studies have shown that motor and cognitive function deteriorate with age in adult people with MS and that older people with MS exhibit worse cognitive performance (86–89). Therefore, we conducted a subgroup analysis based on the participants' age and the results showed that a younger age was associated with larger improvement in fatigue. Horton et al. (90) showed that with age, people with MS develop a sedentary lifestyle, which increases the risk of secondary disease. Although exercise is an effective therapy, dyskinesia is common in older adult patients. Increased fatigue is a severe barrier when exercise energy expenditure is relatively high, and older patients can lose confidence in their ability to exercise and may feel at risk of injury, especially when exercise equipment is involved (91–94).
In addition, exercise significantly improved the quality of life in people with MS, which was consistent with a previous study, showing that exercise seems to be the most effective way to improve the quality of life in people with MS by increasing strength and balance, thereby reducing the risk of falls (94). Previous studies have shown that multicomponent training is well-tolerated and can effective in improving the quality of life in people with MS (13), and that group exercise is an effective intervention for people with MS to cope with fatigue, with the Baduanjin playing a more prominent role in improving the quality of life through respiration and psychology (46). Improvements in quality of life may be related to exercise-induced increases in fitness, mobility, balance, muscle strength, and sleep quality (53, 95). Subgroup analysis showed that aerobic exercise and multicomponent training were effective in improving quality of life, with aerobic exercise being the most effective intervention type, which was in agreement with previous studies, showing that aerobic exercise increases aerobic capacity and improves physical and mental health, thereby enhancing functional independence and fatigue resistance in people with MS (96). In addition, aerobic exercise may stimulate the activity of the sympathetic nervous system and activate the activity of the parasympathetic nervous system, which leads to the release of acetylcholine, resulting in a sedative effect (97).
Strengths and limitations of this systematic review
In this systematic review and meta-analysis, we included studies on the effect of exercise on balance, walking ability, walking endurance, fatigue, and quality of life in people with MS, and excluded studies where participants in the control group received exercise interventions, which can better reflect the effect of exercise interventions. Our findings provide an alternative treatment strategy for people with MS, clinically recommending engagement in resistance exercise and aerobic exercise, respectively, to alleviate fatigue and enhance quality of life.
However, this study has some limitations that should be noted. First, the heterogeneity between each of the original studies is unavoidable (the proportion of male and female participants from different regions, the age of subjects, etc.), which may affect the scientific validity of the meta-analysis. Second, many of the included studies had small sample sizes, which may have had some impact on the results. Finally, it was not possible to exclude a placebo effect, as blinding could not be performed during the exercise intervention. Future reviews could reduce the heterogeneity between included studies by restricting the inclusion criteria more strictly.
Conclusion
This meta-analysis revealed that exercise had beneficial effects in improving balance, walking ability, walking endurance, fatigue, and quality of life in people with MS. The effect of exercise on improving fatigue was associated with the age of the participants, with the younger the age, the greater the improvement in fatigue. To improve fatigue and quality of life, this meta-analysis provides clinicians with evidence to recommended that people with MS participate in resistance exercise and aerobic exercise, respectively.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. HX: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. SZ: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. YZ: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. XT: Data curation, Formal analysis, Investigation, Writing – review & editing. YL: Data curation, Formal analysis, Investigation, Writing – review & editing. XH: Data curation, Funding acquisition, Investigation, Project administration, Resources, Software, Writing – review & editing. LY: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key R&D Program of China (2022YFC3600201) and the Chinese Universities Scientific Fund (2021QN001 and 2022QN015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1387658/full#supplementary-material
References
1. Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. (2017) 389:1336–46. doi: 10.1016/S0140-6736(16)30959-X
2. Correale J, Gaitán MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. (2017) 140:527–46. doi: 10.1093/brain/aww258
3. Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. (2017) 389:1357–66. doi: 10.1016/S0140-6736(16)31320-4
4. Freiha J, Riachi N, Chalah MA, Zoghaib R, Ayache SS, Ahdab R. Paroxysmal symptoms in multiple sclerosis-a review of the literature. J Clin Med. (2020) 9:103100. doi: 10.3390/jcm9103100
5. Pilutti LA, Platta ME, Motl RW, Latimer-Cheung AE. The safety of exercise training in multiple sclerosis: a systematic review. J Neurol Sci. (2014) 343:3–7. doi: 10.1016/j.jns.2014.05.016
6. Mitchell AJ, Benito-León J, González JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. Lancet Neurol. (2005) 4:556–66. doi: 10.1016/S1474-4422(05)70166-6
7. Tallner A, Waschbisch A, Hentschke C, Pfeifer K, Mäurer M. Mental health in multiple sclerosis patients without limitation of physical function: the role of physical activity. Int J Mol Sci. (2015) 16:14901–11. doi: 10.3390/ijms160714901
8. Comber L, Galvin R, Coote S. Gait deficits in people with multiple sclerosis: a systematic review and meta-analysis. Gait Posture. (2017) 51:25–35. doi: 10.1016/j.gaitpost.2016.09.026
9. Torkildsen Ø, Myhr KM, Bø L. Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur J Neurol. (2016) 23:18–27. doi: 10.1111/ene.12883
10. McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. J Am Med Assoc. (2021) 325:765–79. doi: 10.1001/jama.2020.26858
11. Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. (2017) 17:185. doi: 10.1186/s12883-017-0960-9
12. Kubsik AM, Klimkiewicz P, Klimkiewicz R, Janczewska K, Woldańska-Okońska M. Rehabilitation in multiple sclerosis patients. Adv Clin Exp Med. (2017) 26:861–70. doi: 10.17219/acem/62329
13. Grazioli E, Tranchita E, Borriello G, Cerulli C, Minganti C, Parisi A. The effects of concurrent resistance and aerobic exercise training on functional status in patients with multiple sclerosis. Curr Sports Med Rep. (2019) 18:452–7. doi: 10.1249/JSR.0000000000000661
14. Feys P, Moumdjian L, Van Halewyck F, Wens I, Eijnde BO, Van Wijmeersch B, et al. Effects of an individual 12-week community-located “start-to-run” program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult Scler. (2019) 25:92–103. doi: 10.1177/1352458517740211
15. Arntzen EC, Bidhendi-Yarandi R, Sivertsen M, Knutsen K, Dahl SSH, Hartvedt MG, et al. The effect of exercise and physical activity-interventions on step count and intensity level in individuals with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Front Sports Act Living. (2023) 5:1162278. doi: 10.3389/fspor.2023.1162278
16. Arik MI, Kiloatar H, Saracoglu I. Do Pilates exercises improve balance in patients with multiple sclerosis? A systematic review and meta-analysis. Mult Scler Relat Disord. (2022) 57:103410. doi: 10.1016/j.msard.2021.103410
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol. (2021) 74:790–9. doi: 10.1136/bmj.n71
18. Ardern CL, Büttner F, Andrade R, Weir A, Ashe MC, Holden S, et al. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: the PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br J Sports Med. (2022) 56:175–95. doi: 10.1136/bjsports-2021-103987
19. Tao X, Chen Y, Zhen K, Ren S, Lv Y, Yu L. Effect of continuous aerobic exercise on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Front Physiol. (2023) 14:1043108. doi: 10.3389/fphys.2023.1043108
20. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. (2009) 55:129–33. doi: 10.1016/S0004-9514(09)70043-1
21. Zhang S, Zhen K, Su Q, Chen Y, Lv Y, Yu L. The effect of aerobic exercise on cognitive function in people with Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2022) 19:2315700. doi: 10.3390/ijerph192315700
22. You Q, Yu L, Li G, He H, Lv Y. Effects of different intensities and durations of aerobic exercise on vascular endothelial function in middle-aged and elderly people: a meta-analysis. Front Physiol. (2021) 12:803102. doi: 10.3389/fphys.2021.803102
23. Zhen K, Zhang S, Tao X, Li G, Lv Y, Yu L, et al. systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson's disease. NPJ Parkinsons Dis. (2022) 8:146. doi: 10.1038/s41531-022-00418-4
24. Ahmadi A, Nikbakh M, Arastoo A, Habibi AH. The effects of a yoga intervention on balance, speed and endurance of walking, fatigue and quality of life in people with multiple sclerosis. J Hum Kinet. (2010) 23:71–8. doi: 10.2478/v10078-010-0009-2
25. Androwis GJ, Sandroff BM, Niewrzol P, Fakhoury F, Wylie GR, Yue G, et al. pilot randomized controlled trial of robotic exoskeleton-assisted exercise rehabilitation in multiple sclerosis. Mult Scler Relat Disord. (2021) 51:102936. doi: 10.1016/j.msard.2021.102936
26. Cakt BD, Nacir B, Genç H, Saraçoglu M, Karagöz A, Erdem HR, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil. (2010) 89:446–57. doi: 10.1097/PHM.0b013e3181d3e71f
27. Andreu-Caravaca L, Ramos-Campo DJ, Chung LH, Manonelles P, Boas JPV, Rubio-Arias J. Fast-velocity resistance training improves force development and mobility in multiple sclerosis. Int J Sports Med. (2022) 43:593–9. doi: 10.1055/a-1710-1492
28. Andreu-Caravaca L, Ramos-Campo DJ, Chung LH, Manonelles P, Abellán-Aynés O, Rubio-Arias J. Effects of fast-velocity concentric resistance training in people with multiple sclerosis: a randomized controlled trial. Acta Neurol Scand. (2022) 146:652–61. doi: 10.1111/ane.13704
29. Carling A, Forsberg A, Gunnarsson M, Nilsagård Y. CoDuSe group exercise programme improves balance and reduces falls in people with multiple sclerosis: a multi-centre, randomized, controlled pilot study. Mult Scler. (2017) 23:1394–404. doi: 10.1177/1352458516677591
30. Langeskov-Christensen M, Hvid LG, Jensen HB, Nielsen HH, Petersen T, Stenager E, et al. Efficacy of high-intensity aerobic exercise on common multiple sclerosis symptoms. Acta Neurol Scand. (2022) 145:229–38. doi: 10.1111/ane.13540
31. Correale L, Buzzachera CF, Liberali G, Codrons E, Mallucci G, Vandoni M, et al. Effects of combined endurance and resistance training in women with multiple sclerosis: a randomized controlled study. Front Neurol. (2021) 12:698460. doi: 10.3389/fneur.2021.698460
32. Dodd KJ, Taylor NF, Shields N, Prasad D, McDonald E, Gillon A. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Mult Scler. (2011) 17:1362–74. doi: 10.1177/1352458511409084
33. Fleming KM, Coote SB, Herring MP. Home-based Pilates for symptoms of anxiety, depression and fatigue among persons with multiple sclerosis: an 8-week randomized controlled trial. Mult Scler. (2021) 27:2267–79. doi: 10.1177/13524585211009216
34. Forsberg A, von Koch L, Nilsagård Y. Effects on balance and walking with the CoDuSe balance exercise program in people with multiple sclerosis: a multicenter randomized controlled trial. Mult Scler Int. (2016) 2016:7076265. doi: 10.1155/2016/7076265
35. Garrett M, Hogan N, Larkin A, Saunders J, Jakeman P, Coote S. Exercise in the community for people with minimal gait impairment due to MS: an assessor-blind randomized controlled trial. Mult Scler. (2013) 19:782–9. doi: 10.1177/1352458512461966
36. Gervasoni E, Cattaneo D, Jonsdottir J. Effect of treadmill training on fatigue in multiple sclerosis: a pilot study. Int J Rehabil Res. (2014) 37:54–60. doi: 10.1097/MRR.0000000000000034
37. Eftekharsadat B, Babaei-Ghazani A, Mohammadzadeh M, Talebi M, Eslamian F, Azari E. Effect of virtual reality-based balance training in multiple sclerosis. Neurol Res. (2015) 37:539–44. doi: 10.1179/1743132815Y.0000000013
38. Gheitasi M, Bayattork M, Andersen LL, Imani S, Daneshfar A. Effect of twelve weeks pilates training on functional balance of male patients with multiple sclerosis: randomized controlled trial. J Bodyw Mov Ther. (2021) 25:41–5. doi: 10.1016/j.jbmt.2020.11.003
39. Hogan N, Kehoe M, Larkin A, Coote S. The effect of community exercise interventions for people with MS who use bilateral support for gait. Mult Scler Int. (2014) 2014:109142. doi: 10.1155/2014/109142
40. Kargarfard M, Shariat A, Ingle L, Cleland JA, Kargarfard M. Randomized controlled trial to examine the impact of aquatic exercise training on functional capacity, balance, and perceptions of fatigue in female patients with multiple sclerosis. Arch Phys Med Rehabil. (2018) 99:234–41. doi: 10.1016/j.apmr.2017.06.015
41. Grubić Kezele T, Trope Z, Ahel V, RuŽić N, Omrčen H, Ðudarić L, et al. Upper-lower limb and breathing exercise program for improving sleep quality and psychological status in multiple sclerosis: a pilot randomized controlled trial. Brain Impair. (2023) 24:86–102. doi: 10.1017/BrImp.2021.17
42. Learmonth YC, Paul L, Miller L, Mattison P, McFadyen AK. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil. (2012) 26:579–93. doi: 10.1177/0269215511423946
43. Najafi B, Rajabi R, Seidi F, Maemodan FG. Effect of combined training protocol on postural control and motor functions of individuals with multiple sclerosis. J Adv Med Biomed Res. (2019) 122:43. doi: 10.30699/jambs.27.122.43
44. Negahban H, Rezaie S, Goharpey S. Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil. (2013) 27:1126–36. doi: 10.1177/0269215513491586
45. Ozkul C, Guclu-Gunduz A, Eldemir K, Apaydin Y, Yazici G, Irkec C. Combined exercise training improves cognitive functions in multiple sclerosis patients with cognitive impairment: a single-blinded randomized controlled trial. Mult Scler Relat Disord. (2020) 45:102419. doi: 10.1016/j.msard.2020.102419
46. Pan Y, Huang Y, Zhang H, Tang Y, Wang C. The effects of Baduanjin and yoga exercise programs on physical and mental health in patients with Multiple Sclerosis: a randomized controlled trial. Complement Ther Med. (2022) 70:102862. doi: 10.1016/j.ctim.2022.102862
47. Robinson J, Dixon J, Macsween A, van Schaik P, Martin D. The effects of exergaming on balance, gait, technology acceptance and flow experience in people with multiple sclerosis: a randomized controlled trial. BMC Sports Sci Med Rehabil. (2015) 7:8. doi: 10.1186/s13102-015-0001-1
48. Romberg A, Virtanen A, Ruutiainen J. Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol. (2005) 252:839–45. doi: 10.1007/s00415-005-0759-2
49. Sokhangu MK, Rahnama N, Etemadifar M, Rafeii M, Saberi A. Effect of neuromuscular exercises on strength, proprioceptive receptors, and balance in females with multiple sclerosis. Int J Prev Med. (2021) 12:5. doi: 10.4103/ijpvm.IJPVM_525_18
50. Sosnoff JJ, Finlayson M, McAuley E, Morrison S, Motl RW. Home-based exercise program and fall-risk reduction in older adults with multiple sclerosis: phase 1 randomized controlled trial. Clin Rehabil. (2014) 28:254–63. doi: 10.1177/0269215513501092
51. Straudi S, Benedetti MG, Venturini E, Manca M, Foti C, Basaglia N. Does robot-assisted gait training ameliorate gait abnormalities in multiple sclerosis? A pilot randomized-control trial. NeuroRehabilitation. (2013) 33:555–63. doi: 10.3233/NRE-130990
52. Tarakci E, Yeldan I, Huseyinsinoglu BE, Zenginler Y, Eraksoy M. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: a randomized controlled trial. Clin Rehabil. (2013) 27:813–22. doi: 10.1177/0269215513481047
53. Tollár J, Nagy F, Tóth BE, Török K, Szita K, Csutorás B, et al. Exercise effects on multiple sclerosis quality of life and clinical-motor symptoms. Med Sci Sports Exerc. (2020) 52:1007–14. doi: 10.1249/MSS.0000000000002228
54. Yazgan YZ, Tarakci E, Tarakci D, Ozdincler AR, Kurtuncu M. Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord. (2020) 39:101902. doi: 10.1016/j.msard.2019.101902
55. Nilsagård YE, Forsberg AS, von Koch L. Balance exercise for persons with multiple sclerosis using Wii games: a randomised, controlled multi-centre study. Mult Scler. (2013) 19:209–16. doi: 10.1177/1352458512450088
56. Young HJ, Mehta TS, Herman C, Wang F, Rimmer JH. The effects of M2M and adapted yoga on physical and psychosocial outcomes in people with multiple sclerosis. Arch Phys Med Rehabil. (2019) 100:391–400. doi: 10.1016/j.apmr.2018.06.032
57. Kargarfard M, Etemadifar M, Baker P, Mehrabi M, Hayatbakhsh R. Effect of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil. (2012) 93:1701–8. doi: 10.1016/j.apmr.2012.05.006
58. Lysogorskaia E, Ivanov T, Mendalieva A, Ulmasbaeva E, Youshko M, Brylev L. Yoga vs. physical therapy in multiple sclerosis: results of randomized controlled trial and the training protocol. Ann Neurosci. (2023) 30:242–50. doi: 10.1177/09727531231161994
59. Monjezi S, Molhemi F, Shaterzadeh-Yazdi MJ, Salehi R, Mehravar M, Kashipazha D, et al. Perturbation-based Balance Training to improve postural responses and falls in people with multiple sclerosis: a randomized controlled trial. Disabil Rehabil. (2023) 45:3649–55. doi: 10.1080/09638288.2022.2138570
60. Riemenschneider M, Hvid LG, Petersen T, Stenager E, Dalgas U. Exercise therapy in early multiple sclerosis improves physical function but not cognition: secondary analyses from a randomized controlled trial. Neurorehabil Neural Repair. (2023) 37:288–97. doi: 10.1177/15459683231159659
61. Abadi Marand L, Noorizadeh Dehkordi S, Roohi-Azizi M, Dadgoo M. Effect of dynamic neuromuscular stabilization on balance, trunk function, falling, and spasticity in people with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil. (2023) 104:90–101. doi: 10.1016/j.apmr.2022.09.015
62. Vural P, Zenginler Yazgan Y, Tarakci E, Guler S, Saltik S. The effects of online exercise training on physical functions and quality of life in patients with pediatric-onset multiple sclerosis. Mult Scler Relat Disord. (2023) 74:104710. doi: 10.1016/j.msard.2023.104710
63. Ahadi F, Tabatabaee SM, Rajabpour M, Ghadamgahi A, Kaljahi MP. Effect of 8-week aerobic exercise and yoga training on depression, anxiety, and quality of life among multiple sclerosis patients. Iran Rehabil J. (2013) 11:75–80.
64. Cameron MH, Nilsagard Y. Balance, gait, and falls in multiple sclerosis. Handb Clin Neurol. (2018) 159:237–50. doi: 10.1016/B978-0-444-63916-5.00015-X
65. Gunn H, Markevics S, Haas B, Marsden J, Freeman J. Systematic review: the effectiveness of interventions to reduce falls and improve balance in adults with multiple sclerosis. Arch Phys Med Rehabil. (2015) 96:1898–912. doi: 10.1016/j.apmr.2015.05.018
66. Molhemi F, Monjezi S, Mehravar M, Shaterzadeh-Yazdi MJ, Salehi R, Hesam S, et al. Effects of virtual reality vs. conventional balance training on balance and falls in people with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil. (2021) 102:290–9. doi: 10.1016/j.apmr.2020.09.395
67. Hao Z, Zhang X, Chen P. Effects of different exercise therapies on balance function and functional walking ability in multiple sclerosis disease patients—a network meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2022) 19:35. doi: 10.37766/inplasy2022.6.0035
68. Andreu-Caravaca L, Ramos-Campo DJ, Chung LH, Rubio-Arias J. Dosage and effectiveness of aerobic training on cardiorespiratory fitness, functional capacity, balance, and fatigue in people with multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2021) 102:1826–39. doi: 10.1016/j.apmr.2021.01.078
69. Sconza C, Negrini F, Di Matteo B, Borboni A, Boccia G, Petrikonis I, et al. Robot-assisted gait training in patients with multiple sclerosis: a randomized controlled crossover trial. Medicina. (2021) 57:70713. doi: 10.3390/medicina57070713
70. Abasiyanik Z, Ertekin Ö, Kahraman T, Yigit P, Özakbaş S. The effects of Clinical Pilates training on walking, balance, fall risk, respiratory, and cognitive functions in persons with multiple sclerosis: a randomized controlled trial. Explore. (2020) 16:12–20. doi: 10.1016/j.explore.2019.07.010
71. Bae M, Kasser SL. High intensity exercise training on functional outcomes in persons with multiple sclerosis: a systematic review. Mult Scler Relat Disord. (2023) 75:104748. doi: 10.1016/j.msard.2023.104748
72. Karpatkin H, Rachwani J, Rhodes R, Rodriguez L, Rodriguez R, Rubeo A, et al. The effect of intermittent vs. continuous walking on distance to fatigue in persons with multiple sclerosis. Disabil Rehabil. (2022) 44:8429–35. doi: 10.1080/09638288.2021.2018055
73. Shepherd RB, Carr JH. Neurological rehabilitation. Disabil Rehabil. (2013) 28:811–2. doi: 10.1080/09638280500534705
74. Jensen HB, Mamoei S, Ravnborg M, Dalgas U, Stenager E. Distribution-based estimates of minimum clinically important difference in cognition, arm function and lower body function after slow release-fampridine treatment of patients with multiple sclerosis. Mult Scler Relat Disord. (2016) 7:58–60. doi: 10.1016/j.msard.2016.03.007
75. Petajan JH, White AT. Recommendations for physical activity in patients with multiple sclerosis. Sports Med. (1999) 27:179–91. doi: 10.2165/00007256-199927030-00004
76. Harrison AM, Safari R, Mercer T, Picariello F, van der Linden ML, White C, et al. Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis. Mult Scler Oct. (2021) 27:1657–78. doi: 10.1177/1352458521996002
77. Razazian N, Kazeminia M, Moayedi H, Daneshkhah A, Shohaimi S, Mohammadi M, et al. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol. (2020) 20:93. doi: 10.1186/s12883-020-01654-y
78. Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Mult Scler Int. (2014) 2014:798285. doi: 10.1155/2014/798285
79. Taul-Madsen L, Connolly L, Dennett R, Freeman J, Dalgas U, Hvid LG. Is aerobic or resistance training the most effective exercise modality for improving lower extremity physical function and perceived fatigue in people with multiple sclerosis? A systematic review and meta-analysis. Arch Phys Med Rehabil. (2021) 102:2032–48. doi: 10.1016/j.apmr.2021.03.026
80. Gold SM, Schulz KH, Hartmann S, Mladek M, Lang UE, Hellweg R, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. (2003) 138:99–105. doi: 10.1016/S0165-5728(03)00121-8
81. Prakash RS, Snook EM, Erickson KI, Colcombe SJ, Voss MW, Motl RW, et al. Cardiorespiratory fitness: a predictor of cortical plasticity in multiple sclerosis. NeuroImage. (2007) 34:1238–44. doi: 10.1016/j.neuroimage.2006.10.003
82. Schulz KH, Gold SM, Witte J, Bartsch K, Lang UE, Hellweg R, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci. (2004) 225:11–8. doi: 10.1016/j.jns.2004.06.009
83. Heesen C, Gold SM, Hartmann S, Mladek M, Reer R, Braumann KM, et al. Endocrine and cytokine responses to standardized physical stress in multiple sclerosis. Brain Behav Immun. (2003) 17:473–81. doi: 10.1016/S0889-1591(03)00077-1
84. Castellano V, Patel DI, White LJ. Cytokine responses to acute and chronic exercise in multiple sclerosis. J Appl Physiol. (2008) 104:1697–702. doi: 10.1152/japplphysiol.00954.2007
85. Torres-Costoso A, Martínez-Vizcaíno V, Reina-Gutiérrez S, Álvarez-Bueno C, Guzmán-Pavón MJ, Pozuelo-Carrascosa DP, et al. Effect of exercise on fatigue in multiple sclerosis: a network meta-analysis comparing different types of exercise. Arch Phys Med Rehabil. (2022) 103:970–87.e18. doi: 10.1016/j.apmr.2021.08.008
86. Roy S, Frndak S, Drake AS, Irwin L, Zivadinov R, Weinstock-Guttman B, et al. Differential effects of aging on motor and cognitive functioning in multiple sclerosis. Mult Scler. (2017) 23:1385–93. doi: 10.1177/1352458516679036
87. Bollaert RE, Motl RW. Physical and cognitive functions, physical activity, and sedentary behavior in older adults with multiple sclerosis. J Geriatr Phys Ther. (2019) 42:304–12. doi: 10.1519/JPT.0000000000000163
88. Baird JF, Cederberg KLJ, Sikes EM, Jeng B, Sasaki JE, Sandroff BM, et al. Changes in cognitive performance with age in adults with multiple sclerosis. Cogn Behav Neurol. (2019) 32:201–7. doi: 10.1097/WNN.0000000000000200
89. Branco M, Ruano L, Portaccio E, Goretti B, Niccolai C, Patti F, et al. Aging with multiple sclerosis: prevalence and profile of cognitive impairment. Neurol Sci. (2019) 40:1651–7. doi: 10.1007/s10072-019-03875-7
90. Horton S, Macdonald DJ, Erickson K. MS exercise, and the potential for older adults. Eur Rev Aging Phys Act. (2010) 62:9. doi: 10.1007/s11556-010-0062-9
91. Morris KS, McAuley E, Motl RW. Self-efficacy and environmental correlates of physical activity among older women and women with multiple sclerosis. Health Educ Res. (2008) 23:744–52. doi: 10.1093/her/cym067
92. Motl RW, Snook EM, Schapiro RT. Symptoms and physical activity behavior in individuals with multiple sclerosis. Res Nurs Health. (2008) 31:466–75. doi: 10.1002/nur.20274
93. Snook EM, Motl RW. Physical activity behaviors in individuals with multiple sclerosis: roles of overall and specific symptoms, and self-efficacy. J Pain Symptom Manage. (2008) 36:46–53. doi: 10.1016/j.jpainsymman.2007.09.007
94. Motl RW, Snook EM, Mcauley E, Scott JA, Hinkle ML. Demographic correlates of physical activity in individuals with multiple sclerosis. Disabil Rehabil. (2009) 29:1301–4. doi: 10.1080/09638280601055873
95. Al-Sharman A, Khalil H, El-Salem K, Aldughmi M, Aburub A. The effects of aerobic exercise on sleep quality measures and sleep-related biomarkers in individuals with Multiple Sclerosis: a pilot randomised controlled trial. NeuroRehabilitation. (2019) 45:107–15. doi: 10.3233/NRE-192748
96. Reina-Gutiérrez S, Cavero-Redondo I, Martínez-Vizcaíno V, Núñez de Arenas-Arroyo S, López-Muñoz P, Álvarez-Bueno C, et al. The type of exercise most beneficial for quality of life in people with multiple sclerosis: a network meta-analysis. Ann Phys Rehabil Med. (2022) 65:101578. doi: 10.1016/j.rehab.2021.101578
Keywords: exercise, multiple sclerosis, balance, walking ability, walking endurance, fatigue, quality of life
Citation: Du L, Xi H, Zhang S, Zhou Y, Tao X, Lv Y, Hou X and Yu L (2024) Effects of exercise in people with multiple sclerosis: a systematic review and meta-analysis. Front. Public Health 12:1387658. doi: 10.3389/fpubh.2024.1387658
Received: 18 February 2024; Accepted: 26 March 2024;
Published: 10 April 2024.
Edited by:
Feng Jiang, Shanghai Jiao Tong University, ChinaReviewed by:
Ylva Nilsagård, University Research Health Care Center, SwedenMehmet Özkeskin, Ege University, Türkiye
Lin Luo, Guizhou Normal University, China
Cagla Ozkul, Gazi University, Türkiye
Copyright © 2024 Du, Xi, Zhang, Zhou, Tao, Lv, Hou and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laikang Yu, eXVsYWlrYW5nQDEyNi5jb20=; Xiao Hou, aG91eGlhbzAzMjdAYnN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Liwen Du1,2†
Liwen Du1,2† Xifeng Tao
Xifeng Tao Yuanyuan Lv
Yuanyuan Lv Xiao Hou
Xiao Hou Laikang Yu
Laikang Yu