- 1College of Health Sciences, Woldia University, Woldia, Ethiopia
- 2Faculties of Social Science, Geography department, Bahir Dare University, Bahir Dare, Ethiopia
Background: Despite the effectiveness of antiretroviral therapy in reducing mortality from opportunistic infections among people living with HIV (PLHIV), tuberculosis (TB) continues to be a significant cause of death, accounting for over one-third of all deaths in this population. In Ethiopia, there is a lack of comprehensive and aggregated data on the national level for TB-associated mortality during co-infection with HIV. Therefore, this systematic review and meta-analysis aimed to estimate TB-associated mortality and identify risk factors for PLHIV in Ethiopia.
Methods: We conducted an extensive systematic review of the literature using the Preferred Reporting of Systematic Review and Meta-Analysis (PRISMA) guidelines. More than seven international electronic databases were used to extract 1,196 published articles from Scopus, PubMed, MEDLINE, Web of Science, HINARY, Google Scholar, African Journal Online, and manual searching. The pooled mortality proportion of active TB was estimated using a weighted inverse variance random-effects meta-regression using STATA version-17. The heterogeneity of the articles was evaluated using Cochran’s Q test and I2 statistic test. Subgroup analysis, sensitivity analysis, and Egger’s regression were conducted to investigate publication bias. This systematic review is registered in Prospero with specific No. CRD42024509131.
Results: Overall, 22 individual studies were included in the final meta-analysis reports. During the review, a total of 9,856 cases of TB and HIV co-infection were screened and 1,296 deaths were reported. In the final meta-analysis, the pooled TB-associated mortality for PLHIV in Ethiopia was found to be 16.2% (95% CI: 13.0–19.2, I2 = 92.9%, p = 0.001). The subgroup analysis revealed that the Amhara region had a higher proportion of TB-associated mortality, which was reported to be 21.1% (95% CI: 18.1–28.0, I2 = 84.4%, p = 0.001), compared to studies conducted in Harari and Addis Ababa regions, which had the proportions of 10% (95% CI: 6–13.1%, I2 = 83.38%, p = 0.001) and 8% (95% CI: 1.1–15, I2 = 87.6%, p = 0.001), respectively. During the random-effects meta-regression, factors associated with co-infection of mortality in TB and HIV were identified, including WHO clinical stages III & IV (OR = 3.01, 95% CI: 1.9–4.7), missed co-trimoxazole preventive therapy (CPT) (OR = 1.89, 95% CI: 1.05–3.4), and missed isoniazid preventive therapy (IPT) (OR = 1.8, 95% CI: 1.46–2.3).
Conclusion: In Ethiopia, the mortality rate among individuals co-infected with TB/HIV is notably high, with nearly one-fifth (16%) of individuals succumbing during co-infection; this rate is considered to be higher compared to other African countries. Risk factors for death during co-infection were identified; the included studies examined advanced WHO clinical stages IV and III, hemoglobin levels (≤10 mg/dL), missed isoniazid preventive therapy (IPT), and missed cotrimoxazole preventive therapy (CPT) as predictors. To reduce premature deaths, healthcare providers must prioritize active TB screening, ensure timely diagnosis, and provide nutritional counseling in each consecutive visit.
Systematic review registration: Trial registration number in Prospero =CRD42024509131 https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=509131.
Introduction
People living with the human Immune deficiency virus (PLHIV) are more susceptible to tuberculosis (TB), which is a leading cause of mortality (1, 2). There is a strong synergy between HIV infection and TB, as PLHIV are at high risk of dying from TB, and HIV infection is the biggest risk factor for active TB incidence due to declining cellular immunity and increased endogenous reactivation of latent TB bacilli in the lungs (3, 4).
Globally in 2020, 1.2 million TB deaths from HIV-negative people and 208,000 deaths from HIV-positive individuals were reported (5). TB infection remains the leading cause of morbidity and mortality for PLHIV worldwide (6). In Sub-Saharan Africa (SSA) countries, the incidence of new TB-associated cases and new mortality cases were reported to be 2,017 cases/100,000 patients per year (7) and 25 cases/100 patients per year, respectively (8–10). Global TB reports of 2022 indicated that the African continent accounted for 1.5 million TB deaths, with 14.30% of those being co-infected deaths among PLHIV (1).
Previous studies have identified several factors contributing to mortality during TB/HIV co-infection, including missed isoniazid preventive therapy (IPT), poor adherence to antiretroviral therapy (ART), acute malnutrition, late diagnosis, the influence of HIV on clinical presentation and diagnosis, immune reconstitution inflammatory syndrome (IRIS), interruptions in drug treatment, and loss to follow-up (LTFU), presence of multiple comorbidities, hepatotoxicity, low body mass index (BMI), and poor medication adherence, were prominent predictors for premature death among PLHIV (11–13). However, having a CD4 count of ≤200 cells/mL serves as a proxy indicator for premature death during twine infection (during TB and HIV co-infection) (6, 14). The finding of a previous retrospective cohort study involving 4,210 co-infected patients in Ethiopia revealed that more than 72.1% of death cases occurred when the CD4 count was ≤200 cells/mL (15).
Reducing mortality during co-infection can be achieved by implementing effective public health policies, such as comprehensive case screening and timely treatment, along with nutritional counseling (3, 16), and concurrent administration of IPT with highly active antiretroviral therapy (HAART) could decrease active TB occurrences by ≥90% (17). However, perceived stigma during health care provision was attributed to 63.7% incidence of common mental health disorders, indirectly contributing to loss to follow-up and premature death (16, 18, 19). In Ethiopia, deaths from the co-infection varied both at the population level and across each region, with 23.01 cases per 100 person-years in Tigray for adults (20), 17.15 cases per 100 person-years for children in Southern Ethiopia (21), and 10.9 cases per 100 person-years in Amhara region (17).
In Ethiopia, despite improvements in ART coverage, implementation of IPT, and collaborative efforts in TB/HIV management, the rates of co-infection and associated mortality remain high. Limited systematic review and meta-analysis studies exist on the mortality for TB/HIV-co-infected population in Ethiopia, and there is a lack of aggregated data on TB-associated mortality during co-infection; this meta-analysis aimed to estimate the pooled TB-associated mortality and identify risk factors for people living with HIV in Ethiopia. This systematic review and meta-analysis report provide evidence on TB mortality rates in both adults and children, regional estimates of mortality rates, and associated risk factors for vulnerable populations.
Methods
Study setting and periods
This systematic review and meta-analysis study was conducted from 1 January 2013 to 30 December 2022 over a 10-year-period in Ethiopia. The study covered nine regions in Ethiopia, namely, Tigray, Afar, Amhara, Oromia, SNNR, Somalia, Gambella, Benishangul Gumuz, and Harari, and two city administrative areas (11).
Protocol registration and searching strategy
Based on the information provided, the protocol of the study can be accessed through the following web address: https://www.crd.york.ac.uk/prospero/#recordDetails. The protocol registration number for the study is CRD42024509131. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA-2020) guideline has been utilized to report the findings of this study (Supplementary Table S1) (22).
Eligibility criteria
Inclusion criteria
This systematic review and meta-analysis report included articles with defined outcomes of any type of TB-associated death among PLHIV based on the following inclusion criteria: (1) scientific papers reporting co-infections of TB and HIV leading to death among PLHIV in Ethiopia; (2) articles containing defined estimates of death, including either incidence proportion or prevalence proportion of TB and HIV co-infection in their final reports; (3) observational studies (including cross-sectional, cohort, and case control studies) published in English, without time restrictions; and (4) study subjects including either young adults or adolescents or adult population living with HIV in Ethiopia. Only studies that met the inclusion criteria were eligible for this meta-analysis.
Exclusion criteria
Studies that reported lacking abstracts and/or full-text, anonymous reports, editorials, and qualitative studies were excluded from the analysis. Furthermore, before the analysis, unfitted articles without a journal name and/or author, articles that lacked the year of publication, and those with citations but without abstracts and/or full text available were excluded from the final meta-analysis.
Outcome ascertainment
This systematic review and meta-analysis had two main objectives. The first objective was the estimation of the proportion of death during TB and HIV co-infection (TB can be PTB or EXPTB) among HIV-infected population after HIV care was started in Ethiopia. The second objective was identifying risk factors for death during co-infection treatment, which was achieved by collecting significant predictors reported in the included studies with their adjusted odds ratio and their respective 95% CIs extracted and then calculated during the meta-analysis.
Information sources for articles searching
Articles were retrieved from seven databases, namely PubMed (N = 784), Scopus (N = 68), MEDLINE (N = 43), Web of Science (N = 39), HINARY (N = 161), Google Scholar (N = 70), and African Journal Online (N = 22), without time restriction for inclusion. However, the search was limited by studies conducted in Ethiopia and articles published only in the English language.
Data searching
The search strategy employed included specified eligible criteria and the following MeSH terms: (1) mortality, (2) incidence, (3) tuberculosis, (4) HIV infection, (5) individuals, (6) children, (7) adults, and (8) Ethiopia; the included articles were extracted. The authors employed the following search terms to retrieve relevant studies from databases: “Epidemiology” OR “prevalence” OR “Incidence” AND “Death,” OR “Mortality,” OR” Case fatality” AND “Tuberculosis,” OR “Pulmonary TB,” OR “Disseminated TB,” OR “TB lymphadenitis,” AND “HIV,” OR “AIDS,” AND “Children” OR “Pediatrics” OR “Infant” OR “Adult” OR “Population” AND “Ethiopia.” The search process involved three authors (FK, DT, and TK) who selected the most pertinent studies based on predefined criteria.
Quality assessment and appraisal procedures
Four authors (FK, BB, SA, and AA) independently extracted the data and evaluated the quality of each study by determining the eligibility of the titles and abstracts of the studies after removing duplicates. Any disagreement or uncertainty that arose during the article extraction process was resolved by discussion. These reviewers assessed the full-text of the studies; if one or more of them believed a study could be significant, it qualified for inclusion after the study was carefully examined for its titles, abstracts, and full text. Three authors (MW, NS, and BB) used a Microsoft Excel spreadsheet to extract the specifics of each study. After through discussion with a third-party reviewer (FK and AA), the disagreement during data extraction was resolved by consensus. All eligible studies were approved with the agreement of all authors, and any differences were worked out through discussion until a consensus was reached. Following the agreement, information about principal investigators, year of publication, study period, study setting, study population, and sample size was retrieved from the identified articles. The authors of the study conducted a comprehensive assessment of the risk of bias in all included studies. This assessment was performed by SA, TK, and BB and involved evaluating and screening the studies. The Joanna Briggs Institute of Critical Appraisal (JBI) checklist was utilized to evaluate the quality of the studies, and the findings from this assessment were incorporated into the final meta-analysis. To gather the relevant published article citations, the authors employed Endnote version 8 and exported and collected all potentially suitable citations, ensuring that any duplication was removed during the selection and screening processes. A team of five independent reviewers (FK, BB, NS, MW, and TK) first reviewed the abstracts of the publications to determine their suitability. Subsequently, they evaluated the full-text articles. The quality of the included articles was assessed using the JBI checklist for cohort-study design evaluation criteria, which consists of 11 critical appraisal criteria for each eligible article (Supplementary Table S3). During the process of screening articles for eligibility, any disagreements regarding the critical appraisal were resolved through discussion among the assigned reviewers.
Data synthesis and analysis procedures
After cleaning and modifying the extracted data on a Microsoft Excel spreadsheet, we employed STATA version 17 for further descriptive statistics, fixed effects regression, and random-effect regression to present the results of pooled TB-associated deaths for PLHIV (23). The I2 statistics and Cochran’s Q test were utilized to detect the degree of heterogeneity and elaborate on heterogeneity among studies, respectively (24). The source of heterogeneity among the included studies was further examined using the subgroup analysis and sensitivity test (23). The estimated risk factors obtained from each eligible study were combined and determined as a single estimated risk factor after performing a random effect meta-analysis regression analysis for TB-associated mortality among PLHIV in Ethiopia (23).
Publication of biases and sensitivity analysis
The publication biases were assessed by inspecting funnel plots of the shape of the graph and quantitatively using Egger’s weighted regression test of a p value of <0.1 (23, 25, 26). The subgroup analysis was also conducted based on predefined themes such as study facilities (health center and hospital setup, study regions, and study population) for identifying further sources of heterogeneity and final policy implication based on the findings.
Results
Study screening process
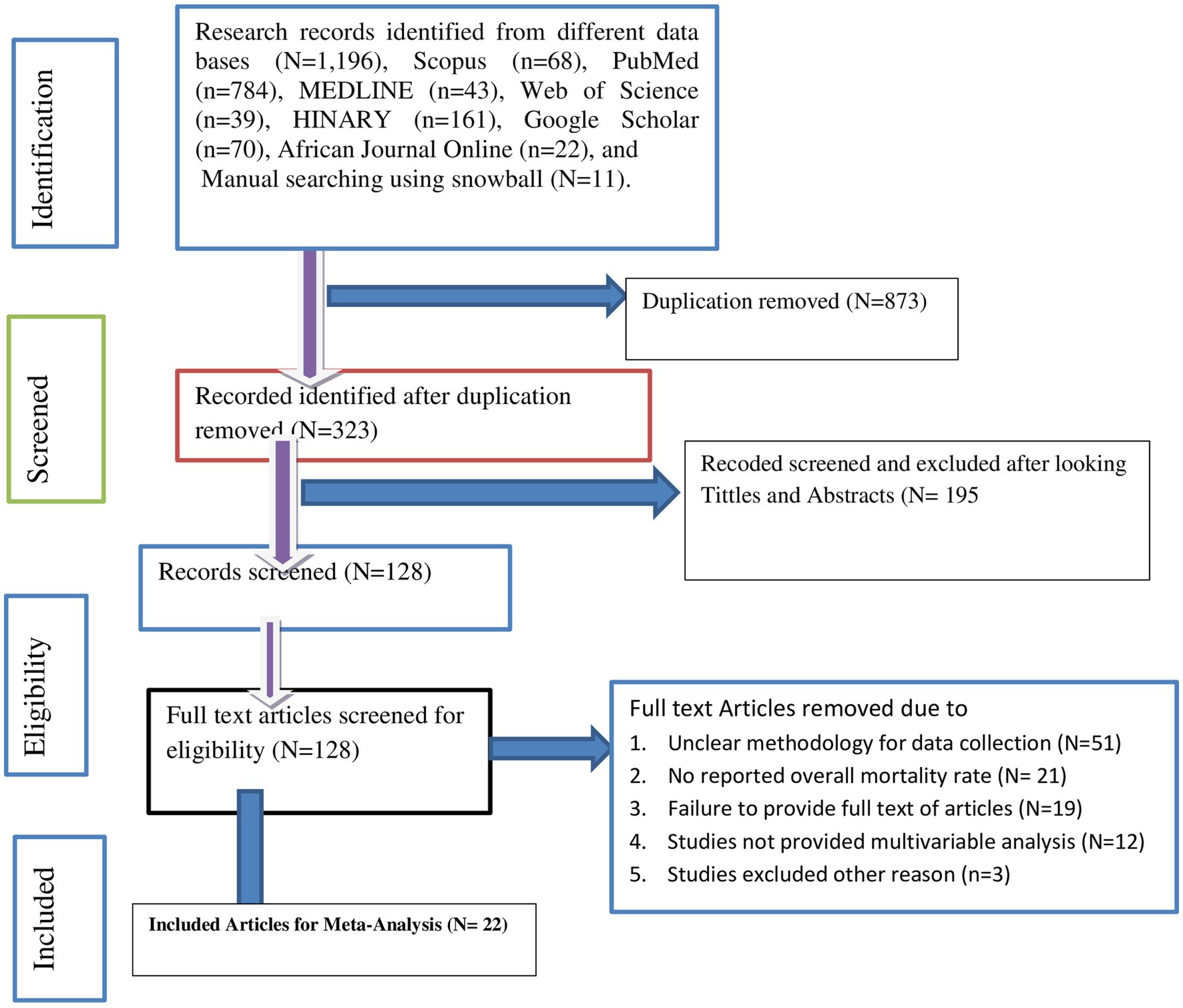
A total of 1,196 published articles were extracted from seven international electronic databases: Scopus (N = 68), PubMed (N = 784), MEDLINE (N = 43), Web of Science (N = 39), HINARY (N = 161), Google Scholar (N = 70), and African Journal Online (N = 22). Additionally, manual searching was employed, resulting in the identification of 11 articles using snowball techniques. After removing 873 duplicates, we assessed 323 articles. Among these articles, 195 were excluded based on titles and abstracts. We then screened the full text of the remaining 128 articles and excluded 51 due to unclear methodology, 21 for not reporting the overall mortality rate, 19 because the full text was inaccessible, 12 for not having reported multivariable analysis, and three articles for other reasons. Finally, 22 individual studies met the inclusion criteria for the meta-analysis shown in the PRISMA flow diagrams (5, 7, 27–44) (Figure 1).
Description reports of included studies
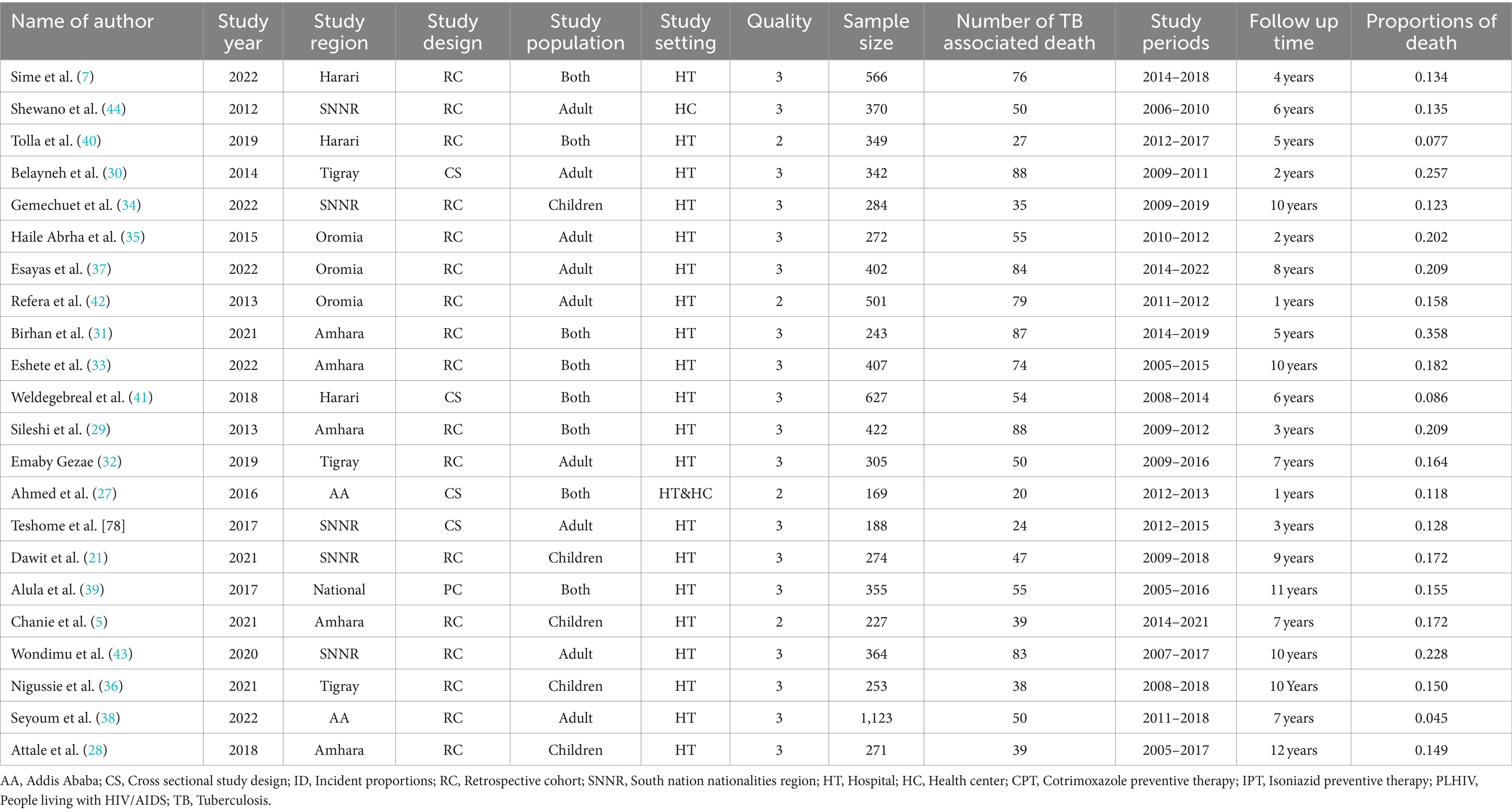
The included studies were extracted from six Ethiopian regions. Accordingly, five were from Amhara, five were from SNNR, three were from Oromia, three were from Tigray, three were from Harari, two were from Addis Ababa, and one was from a national-level (5, 7, 27–44) study. The smallest sample size was 169 from the Amhara region (28), and the largest number of participants was 1,123 from Addis Ababa (38).
Among the included studies, only nine (40.1%) were conducted on the adult population, whereas the remaining 8 (36.6%) and 5 (22.8%) studies involved children and both adult and children populations, respectively. More than half of the included studies, i.e., 81.5%, employed cohort study designs, and the remaining used cross-sectional designs with the study period ranging from 1 year (42) to 12 years (28) (Table 1).
Tuberculosis associated mortality
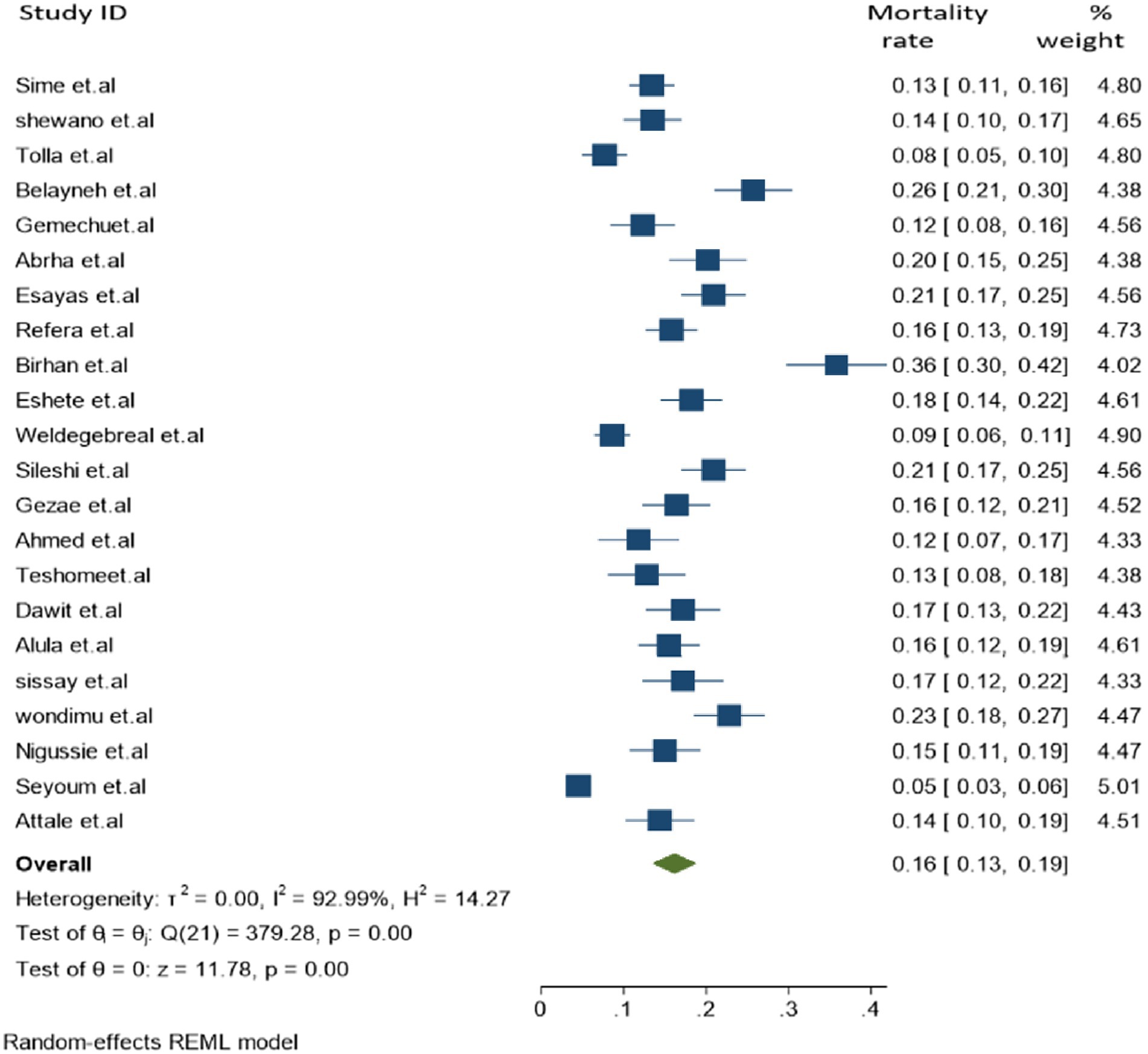
In the final meta-analysis, a total of 22 individual studies were included and a total of 9,856 cases of TB and HIV co-infection were screened, which reported 1,296 co-infected deaths. The overall pooled proportion of TB-associated mortality among people living with HIV (PLHIV) was found to be 16.2% [95% CI: 13.0–19.2, (I2 = 92.99%, p = 0.001)] (Figure 2).
Subgroup analysis
In the subgroup analysis, TB-associated mortality rates varied across each region, with the estimated rates being 21.1% (95% CI: 18.1–28.0, I2 = 84.4%) in Amhara, followed 19% (95% CI: 15–22, I2 = 56.95%) in Oromia, 19% (95% CI: 12.01–25, I2 = 6.64%) in Tigray, 16% (95% CI: 12.01–20, I2 = 76.44%) in SNNR, and 10% (95% CI: 6–13, I2 = 81.6%) in Harari, which had the lowest mortality rate compared to other regions (Figure 3).
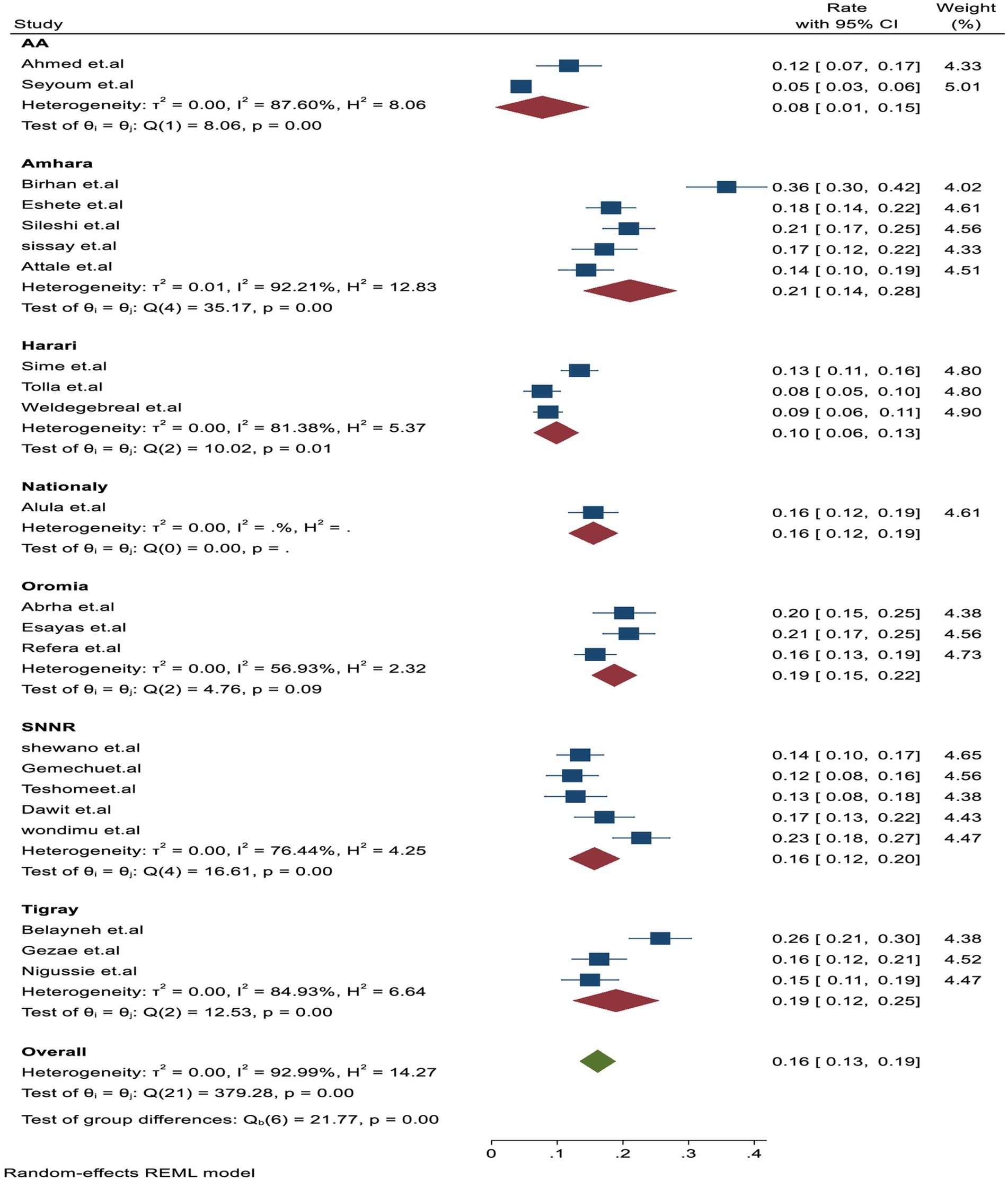
Figure 3. Subgroup analysis by study regions for tuberculosis-associated death of PLHIV in Ethiopia.
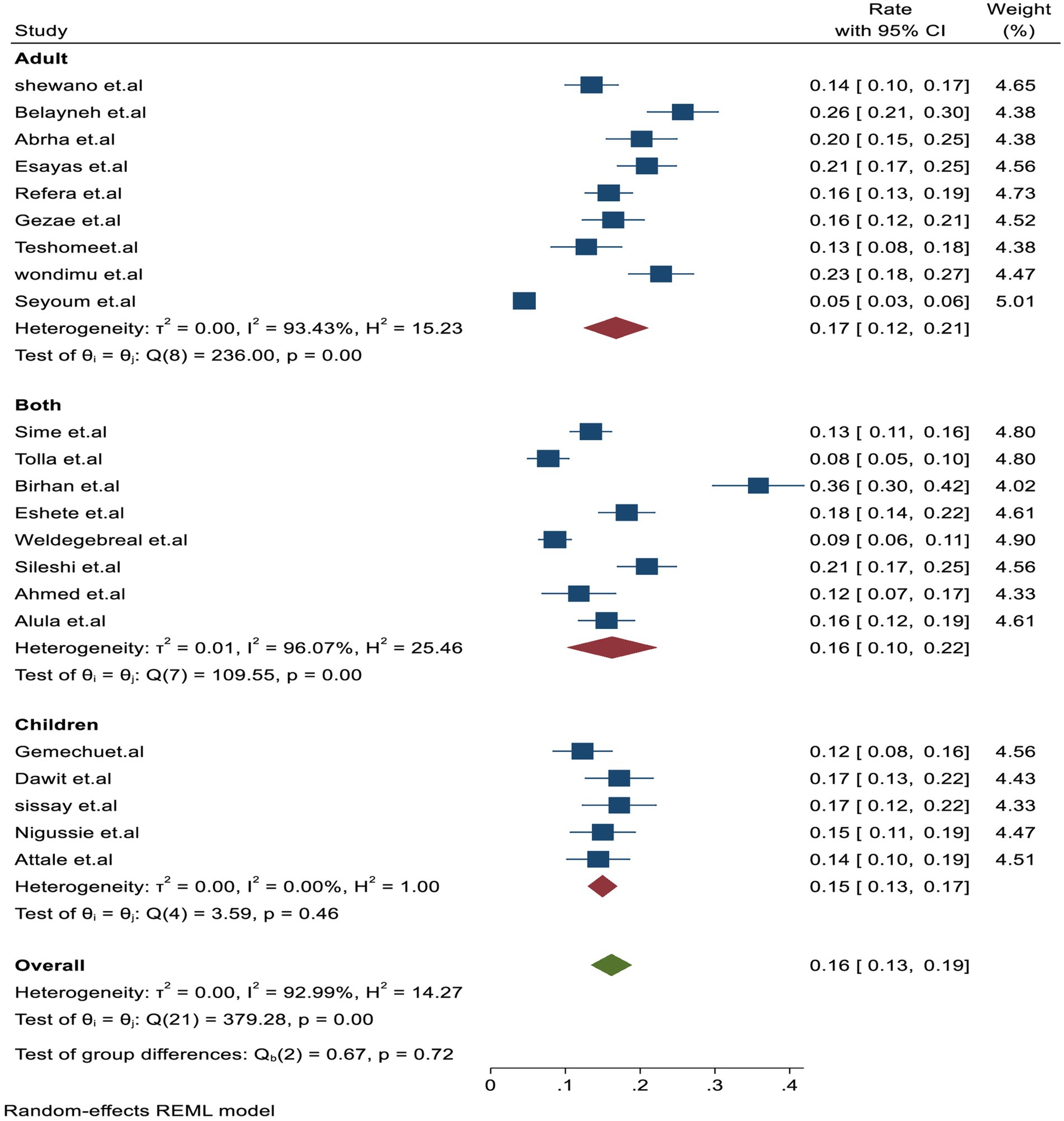
Similarly, the subgroup analysis based on the study subjects was higher in adults with 17% (95% CI: 12–21.01, I2 = 93.4%) than in studies that included both adult and children participants with 16% (95% CI: 10–20.01, I2 = 96.4%) and children alone with 15% (95% CI: 13–17, I2 = 0.001%), respectively (Figure 4). In addition, based on the study setting, a higher proportion of TB-associated mortality of 17% (95% CI: 14–19, I2 = 93.8%) was observed among studies conducted in hospital settings compared to those conducted in health centers, which reported 14% (95% CI: 10.0–17.01, I2 = 0.001), and in hospital setups, which reported 12% (95% CI: 7–17, I2 = 0.001%).
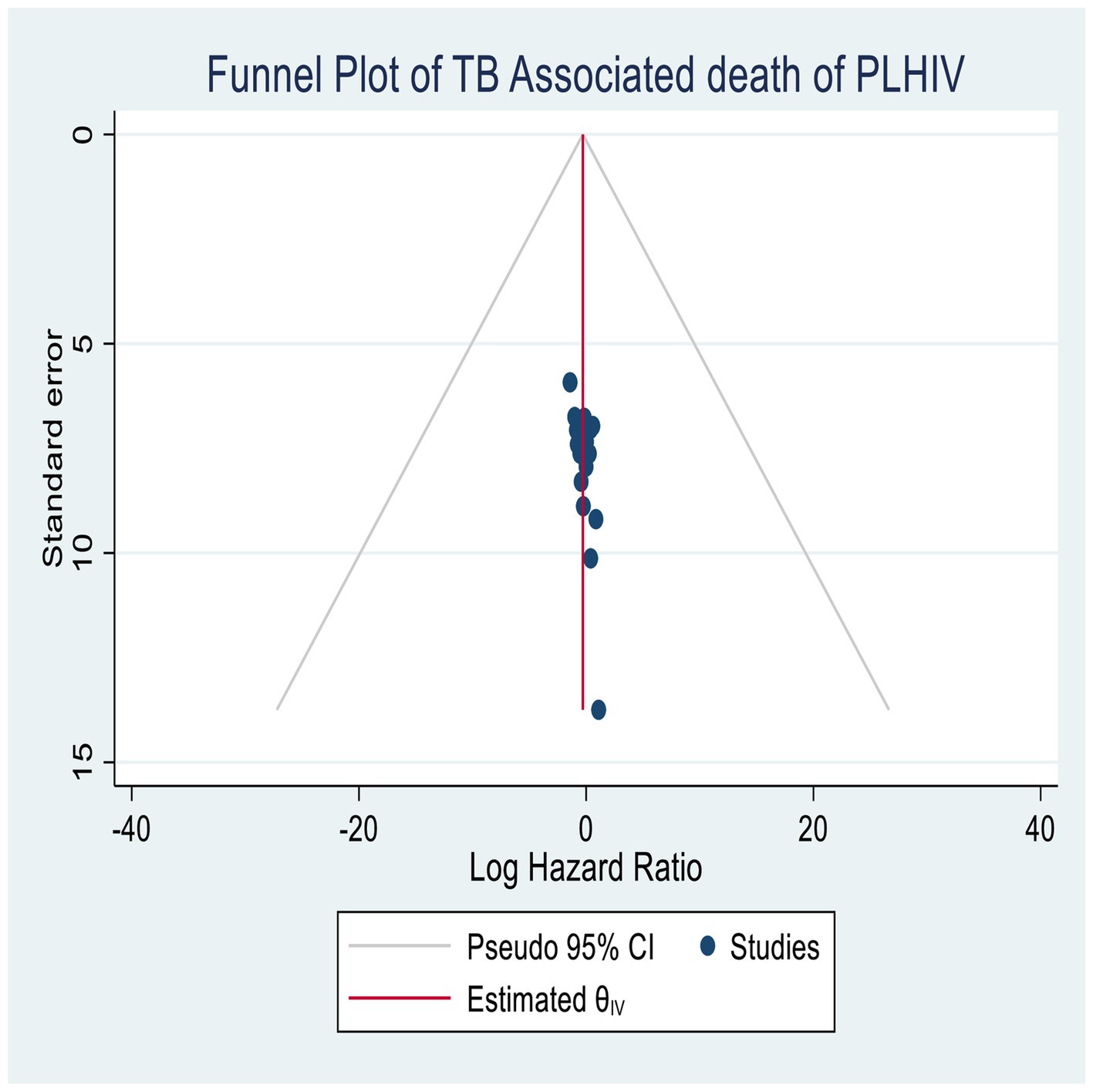
Publication bias
The publication bias was assessed using funnel plots, as well as quantitative methods such as Begg’s and Egger’s tests (45, 46). The final publication result indicated no evidence of publication bias, and all studies were included in the plots, suggesting no significant publication biases in both graphical and quantitative estimation (Figure 5).
Sensitivity analysis
The sensitivity analysis showed that all studies were within the confidence interval bounds of the meta-regression, as shown in Egger’s (p = 0.01) and Begg’s tests (p = 0.01) with no significant publication bias.
Predictors for TB-associated death
In this systematic review, we categorized adjusted odds ratios from primary studies into themes to identify the risk factors for tuberculosis (TB)-associated mortality of PLHIV. Thus, TB-associated mortality was assessed mainly in four categorical themes, including WHO advanced clinical stages (III and IV), declining CD4 count (≤200 cell/mm2), missed IPT, and missed CPT. Accordingly, missed IPT, missed CPT, and WHO advanced clinical stages (III and IV) themes were significantly associated with mortality during co-infection (Table 2).
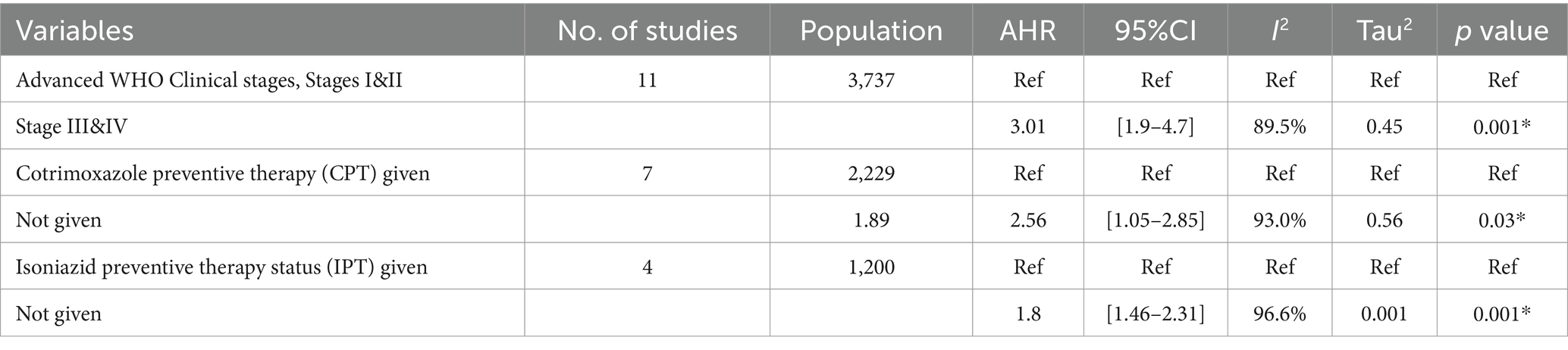
Table 2. Random effect meta-regression of factors associated with TB-associated death of PLHIV in Ethiopia.
Effects of advanced clinical stages on TB-associated death
We analyzed 11 studies (7, 20, 30, 31, 33, 36, 37, 39, 42, 47, 48) that examined the association between WHO clinical stages (III and IV) and TB-associated mortality among 3,737 participants. The results indicated that participants in the advanced WHO clinical stages III and IV had an increased risk of TB-associated death compared with their counter groups [OR = 3.01 (95% CI: 1.9–4.7)] with (Tau2 = 0.45, I2 = 89.5%, p = 0.001) (Figure 6).
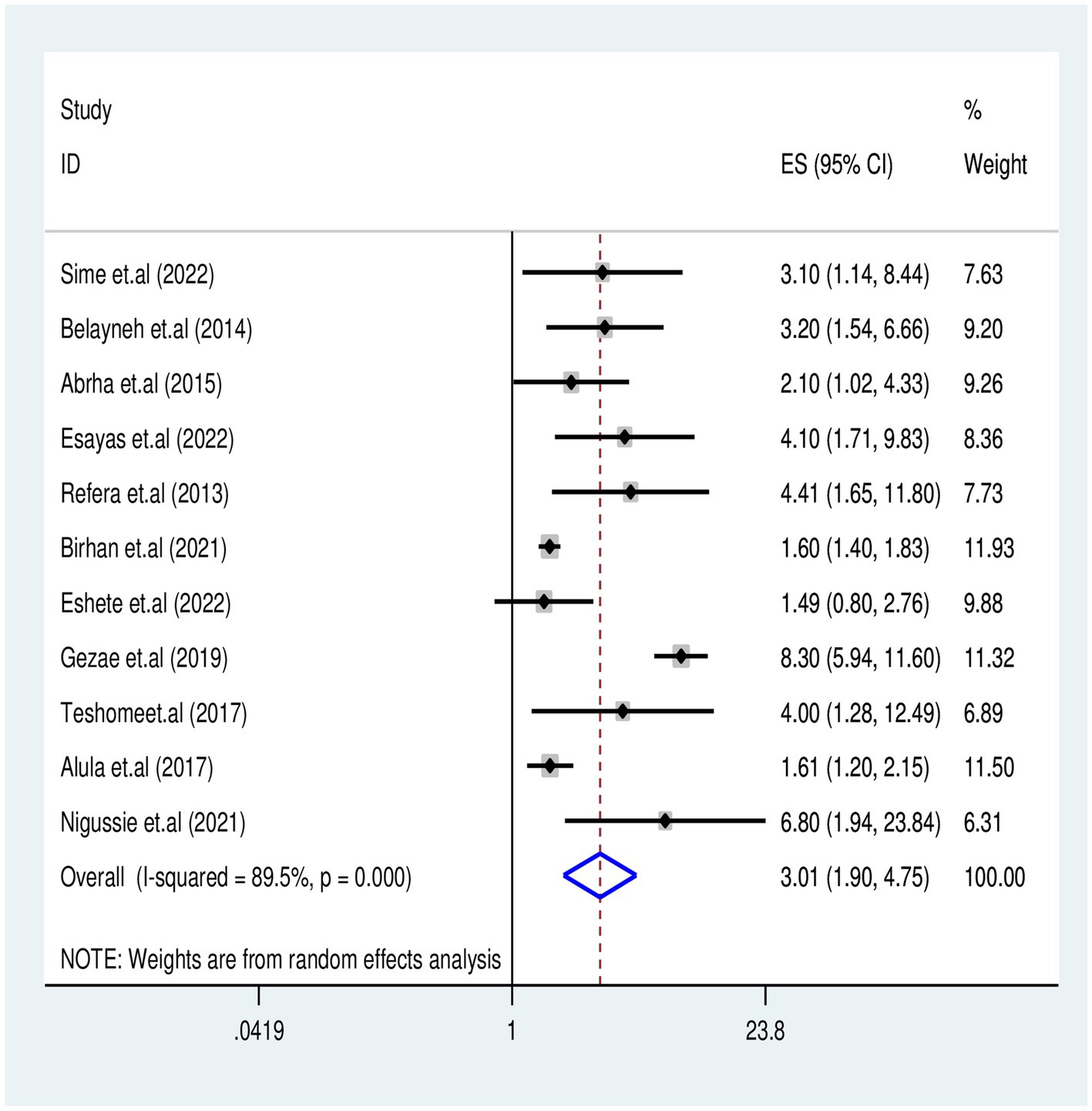
Figure 6. Association of WHO Clinical Stages III&IV with TB associated mortality for PLHIV in Ethiopia.
Effects of missed IPT on TB-associated death
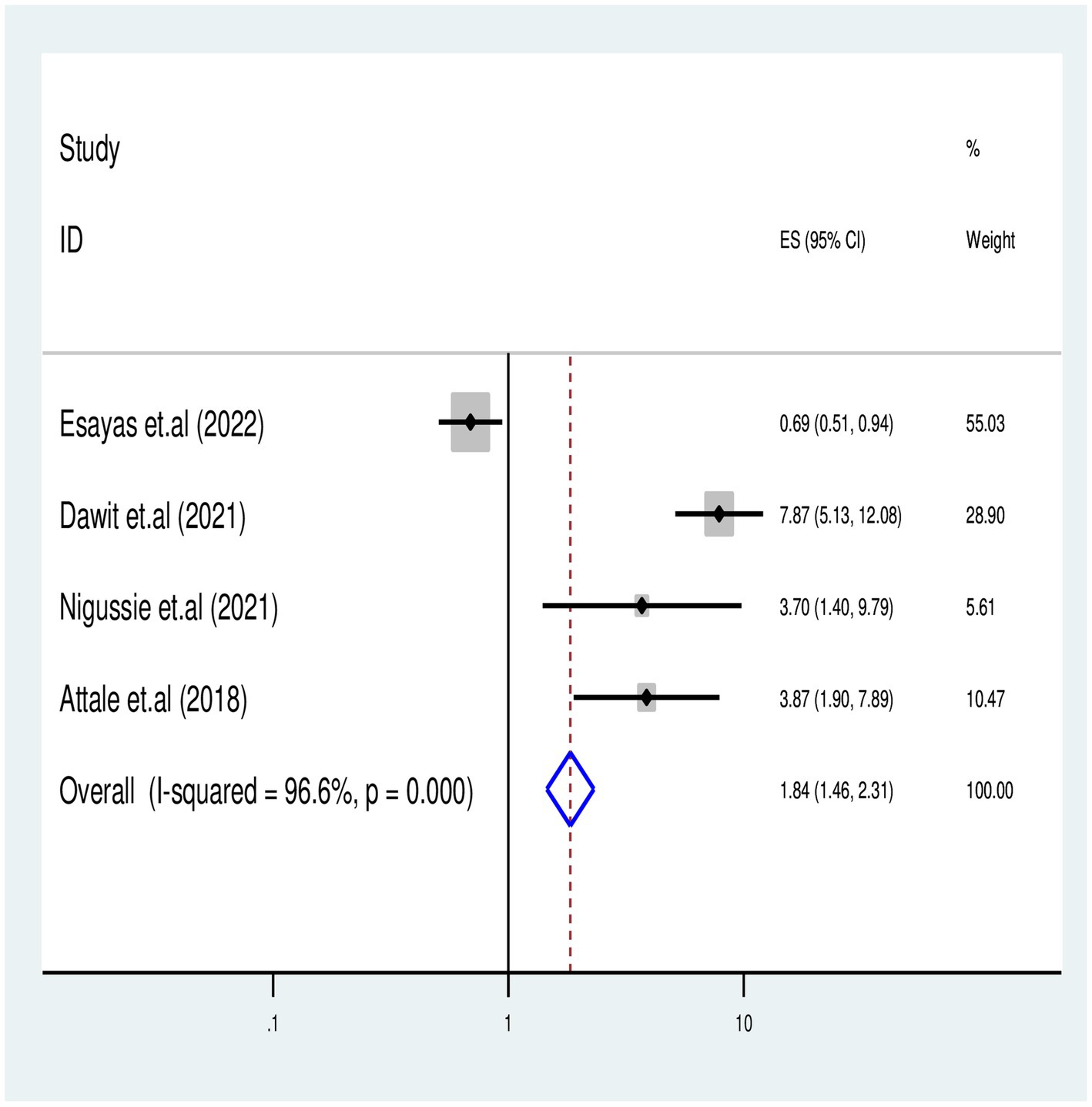
We identified four published articles investigating the association between missed isoniazid preventive therapy (IPT) and TB-associated death. Three of these studies reported a significant risk of TB-associated death with missing IPT, while one study found no significant association. A meta-regression analysis involved 1,200 study participants, and the meta-analysis revealed that PLHIV who missed IPT had a 2-fold risk of TB-associated death compared to those who received IPT [OR = 1.8 (95% CI: 1.46–2.31) with (I2 = 96.6%, p = 0.001)] (Figure 7).
Effects of missed CPT on TB-associated death
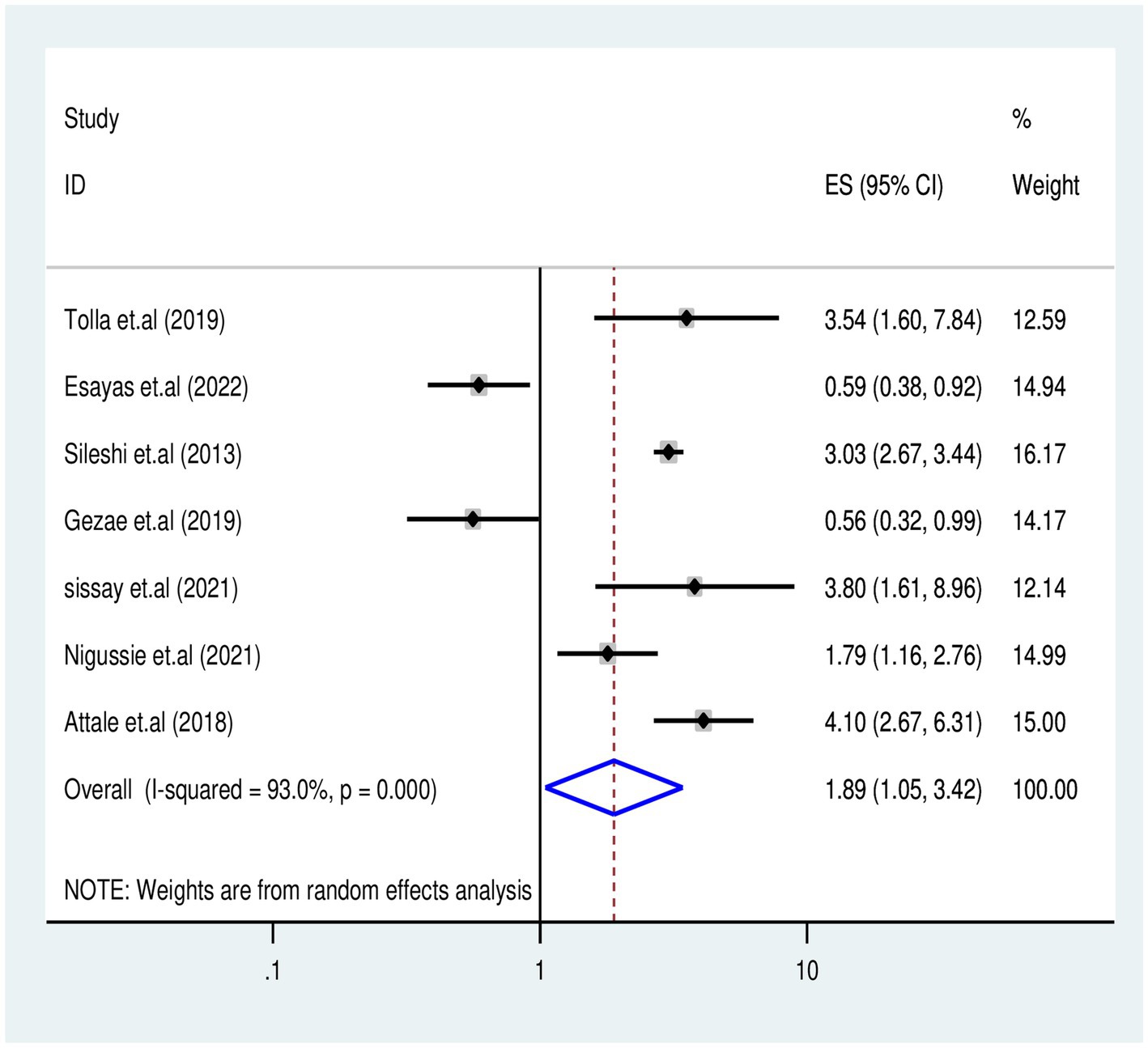
The association between missed CPT and TB-associated death was examined in five studies (5, 20, 29, 36, 37, 40) involving 2,229 participants. Five studies reported a significant association with missed CPT, and the final meta-regression analysis revealed that missed CPT doubled (OR: 1.89, 95% CI: 1.05–3.42) the risk of mortality (I2 = 93%, p = 0.035) (Figure 8).
Effects of declining CD4 count on TB-associated death
A total of six studies (7, 30, 37, 40, 42, 43) investigated the impact of declining CD4 count on TB-associated mortality among PLHIV. The final meta-regression analysis revealed no significant association between CD4 count ≤200 cells/mL and increased mortality risk (OR = 2.18, 95% CI: 0.97–3.08; I2 = 82.8%, p = 0.064).
Discussion
In the final report of this systematic review and meta-analysis, we included 22 published studies, encompassing a total of 9,856 cases of TB and HIV co-infection. Among these cases, a total of 1,296 deaths were reported. The highest mortality was observed in the Tigray region (30) and the lowest mortality in Addis Ababa city (27). The overall pooled proportion report of TB-associated death in Ethiopia was estimated at 16.2% (95% CI: 13.0–19.2) (I2 = 92.99%, p = 0.001). This finding is significantly higher than the previous meta-analysis that reported 4.3% in South Africa (19), 9.2% in Amsterdam (49), and 11.1% in England (50), and 2.8% in the Netherlands (51). The possible reason for the estimated difference is poor screening and diagnosis of TB by healthcare providers during successive follow-ups for PLHIV, poor adherence to treatment guidelines, low completion rate for IPT among patients during successive follow-ups, and reports of higher mortality compared with the developed nations (3). Conversely, the estimated proportions of TB-associated mortality are lower than those reported elsewhere in systematic review reports: 17.7% in Geneva (6) and 40.1% in London (52). This difference may be attributed to differences in the included studies and recruitment study periods. Furthermore, the possible reason for the estimated difference between the two countries might be the effect of poor screening and diagnostic ability of TB by healthcare providers among suspected PLHIV in Ethiopia compared to the highly sophisticated diagnosing equipment for TB detection available in countries such as Geneva and London.
In this report, the observed heterogeneity among studies during the meta-analysis report was assessed by using a subgroup analysis, including predetermined themes of study region, population, and follow-up time. Accordingly, the Amhara region had the highest proportion of TB-associated mortality at 21.1% (95% CI: 18.1–28.0, I2 = 84.4%, p = 0.001) compared to Addis Ababa, which had 8% (95% CI: 1.1–15, I2 = 87.6%, p = 0.001). This difference may be attributed to the ongoing civil war in the Amhara region since July 2021, leading to the loss of life and displacement of more than 1.4 million population who ended up living in slums and destitution, including PLHIV, where, tuberculosis fatality and transmission dynamics are high (53, 54).
In this review, factors associated with TB-associated mortality for PLHIV were identified from aggregated studies; patients who missed CPT experienced a two-fold increase in mortality risk compared to those who received CPT during follow-up periods (AHR = 1.89, 95% CI: 1.05–3.42, I2 = 93%, p = 0.03). This finding is consistent with the findings of previous primary studies (3, 5, 20, 29, 36, 37, 40) and meta-analyses reported by universities in Gondar (55) and Debre Markos (56). This consistency in findings may be attributed to cotrimoxazole, which is an effective drug against the incidence of lethal opportunistic infections, including TB, which can increase immunosuppression and mortality risk.
This systematic review found that PLHIV co-infected with TB and in advanced WHO clinical stages (III and IV) had 3.01 times increased risk of death compared to those in stages I and II (AHR = 3.01, 95% CI: 1.9–4.7). This finding is consistent with previous reports from universities in Debre Markos (57), Gondar (10), and Addis Ababa (58). This association might be related to PLHIV who developed TB commonly at the advanced WHO clinic stages, leading to a higher risk of having a reduction in CD4 count and decreased cellular immunity. This decline accelerated the existence of rapid viral replication and indirectly contributed to the increased likelihood of premature mortality among PLHIV.
The final report of this meta-analysis revealed that PLHIV who missed IPT had a significant risk of mortality during TB co-infection disease courses. Accordingly, TB-associated mortality showed a two-fold increase for those who missed IPT as compared to those who completed IPT during successive follow-ups of care (AHR = 1.8: 95% CI 1.46–2.32). This finding aligns with the findings of a previous meta-analysis conducted in Ethiopia (10, 57) and is supported by previous premier studies (59–61). In line with the above finding, the concomitant use of IPT with ART administration has been shown to reduce or demote new cases of TB incidence and associated deaths by over 90% among PLHIV (10). However, factors such as IRIS, inadequate screening by a caregiver, low IPT completion, and poor comprehensive care during successive follow-ups have been associated with poor treatment outcomes.
In contrast to previous systematic review findings (6, 14, 15, 52, 55) and primary studies (6, 7, 14, 15, 30, 37, 40, 42, 43, 52, 55), this meta-regression found no significant association between declined CD4 count (≤200 cells/mL), age of patients, duration of follow-up, comorbidity status, and body mass index with the risk of death during TB and HIV co-infection treatment (AHR = 2.18, 95% CI: 0.97–3.08, I2 = 82.8%, p = 0.064). This lack of association might be related to the methodological differences, heterogeneity of included studies and populations, sample size limitations, publication bias, and unaccounted factors, and further experimental studies are highly needed to understand this relationship better.
Strengths and limitations of the study
The strengths of this study included a comprehensive search and involvement of multiple authors. In addition, it included 22 studies with 8,315 TB-HIV co-infected cases, providing valuable mortality screening data for national-level representation. However, it is important to consider the limitation that the majority of the studies employed a retrospective nature of data collection and thus were limited to specific regions in Ethiopia, which may impact the generalizability of the results.
Conclusion and recommendations
In Ethiopia, the mortality rate among individuals co-infected with TB and HIV is notably high, with nearly one-fifth of individuals (16%) succumbing during co-infection, which is higher compared to other African countries. Risk factors for death during co-infection were identified; the included studies examined advanced WHO clinical stages IV and III, hemoglobin levels (≤10 mg/dL), missed isoniazid preventive therapy (IPT), and missed cotrimoxazole preventive therapy (CPT) as predictors. To reduce premature deaths, healthcare providers must prioritize active TB screening, ensure timely diagnosis, and provide nutritional counseling in each consecutive visit.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the corresponding author.
Author contributions
FKB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TKB: Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. SAM: Formal analysis, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. AAK: Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MWA: Conceptualization, Funding acquisition, Investigation, Resources, Software, Writing – original draft, Writing – review & editing. NS: Data curation, Funding acquisition, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. BBA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Resources, Software, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express our sincere gratitude to the Department of Epidemiology of Woldia University for initiating and conducting this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1386113/full#supplementary-material
SUPPLEMENTARY TABLE S1 | PRISMA CHEKLIST 2020.
SUPPLEMENTARY TABLE S2 | Final meta dataset.
SUPPLEMENTARY TABLE S3 | JBI critical appraisal checklists.
References
1. WHO (2022). Global tuberculosis report 2022. Available at: https://iris.who.int/bitstream/handle/10665/363752/9789240061729-eng.pdf?sequence=1
2. WHO (2023). Global tuberculosis report 2023. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports
3. Kebede, F, Kebede, B, Kebede, T, and Agmasu, M. Effect of isoniazid preventive therapy on the incidence of tuberculosis among seropositive children attending HIV/AIDS Care in two General Hospitals, Northwest Ethiopia. J Trop Med. (2021) 2021:9996953. doi: 10.1155/2021/9996953
4. Kebede, F, Tarekegn, H, Molla, M, Jara, D, and Abate, A. Incidence and predictors of pulmonary tuberculosis among children who received antiretroviral therapy (ART), Northwest Ethiopia: a multicenter historical cohorts study 2009-2019. J Trop Med. (2022) 2022:9925693. doi: 10.1155/2022/9925693
5. Chanie, ES, Gelaye, GA, Tadesse, TY, feleke, DG, Admas, WT, Molla Alemu, E, et al. Estimation of lifetime survival and predictors of mortality among TB with HIV co-infected children after test and treat strategies launched in northwest, Ethiopia, 2021; a multicentre historical follow-up study. PLoS One. (2021) 16:e0258964. doi: 10.1371/journal.pone.0258964
6. Ford, N, Matteelli, A, Shubber, Z, Hermans, S, Meintjes, G, Grinsztejn, B, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc. (2016) 19:20714. doi: 10.7448/IAS.19.1.20714
7. Sime, T, Oljira, L, Diriba, A, Firdisa, G, and Gezimu, W. Effect of active tuberculosis on the survival of HIV-infected adult patients who initiated antiretroviral therapy at public hospitals of eastern Ethiopia: a retrospective cohort study. PLoS One. (2022) 17:e0277021. doi: 10.1371/journal.pone.0277021
8. Schutz, C, Barr, D, Andrade, BB, Shey, M, Ward, A, Janssen, S, et al. Clinical, microbiologic, and immunologic determinants of mortality in hospitalized patients with HIV-associated tuberculosis: a prospective cohort study. PLoS Med. (2019) 16:e1002840. doi: 10.1371/journal.pmed.1002840
9. Marley, G, Zou, X, Nie, J, Cheng, W, Xie, Y, Liao, H, et al. Improving cascade outcomes for active TB: a global systematic review and meta-analysis of TB interventions. PLoS Med. (2023) 20:e1004091. doi: 10.1371/journal.pmed.1004091
10. Wondmeneh, TG, and Mekonnen, AT. The incidence rate of tuberculosis and its associated factors among HIV-positive persons in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. (2023) 23:613. doi: 10.1186/s12879-023-08533-0
11. Alebel, A, Engeda, EH, Kelkay, MM, Petrucka, P, Kibret, GD, Wagnew, F, et al. Mortality rate among HIV-positive children on ART in Northwest Ethiopia: a historical cohort study. BMC Public Health. (2020) 20:1303. doi: 10.1186/s12889-020-09418-6
12. Gebremedhin, A, Gebremariam, S, Haile, F, Weldearegawi, B, and Decotelli, C. Predictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle hospital, northern Ethiopia: a retrospective cohort study. BMC Public Health. (2013) 13:1047. doi: 10.1186/1471-2458-13-1047
13. Ayalaw, SG, Alene, KA, and Adane, AA. Incidence and predictors of tuberculosis among HIV positive children at University of Gondar Referral Hospital, Northwest Ethiopia: A Retrospective Follow-Up Study. Int Sch Res Notices. (2015) 2015:307810. doi: 10.1155/2015/307810
14. Belay, GM, and Wubneh, CA. Childhood tuberculosis treatment outcome and its association with HIV co-infection in Ethiopia: a systematic review and meta-analysis. Trop Med Health. (2020) 48:7. doi: 10.1186/s41182-020-00195-x
15. Kassa, A, Teka, A, Shewaamare, A, and Jerene, D. Incidence of tuberculosis and early mortality in a large cohort of HIV infected patients receiving antiretroviral therapy in a tertiary hospital in Addis Ababa, Ethiopia. Trans Roy Soc Trop Med Hyg. (2012) 106:363–70. doi: 10.1016/j.trstmh.2012.03.002
16. Deribew, A, Tesfaye, M, Hailmichael, Y, Apers, L, Abebe, G, Duchateau, L, et al. Common mental disorders in TB/HIV co-infected patients in Ethiopia. BMC Infect Dis. (2010) 10:201. doi: 10.1186/1471-2334-10-201
17. Tsegaye, D, Wude, S, Kebede, T, Adane, S, Shumet, T, and Kebede, F. Epidemiological survival pattern, risk factors, and estimated time to develop tuberculosis after test and treat strategies declared for children living with human immune deficiency virus. Indian J Tuberc. (2023) 70:S89–99. doi: 10.1016/j.ijtb.2023.05.008
18. Zhang, J, Kern-Allely, S, Yu, T, and Price, RK. HIV and tuberculosis co-infection in East Asia and the Pacific from 1990 to 2017: results from the global burden of disease study 2017. J Thorac Dis. (2019) 11:3822–35. doi: 10.21037/jtd-23-1960
19. Kufa, T, Mabuto, T, Muchiri, E, Charalambous, S, Rosillon, D, Churchyard, G, et al. Incidence of HIV-associated tuberculosis among individuals taking combination antiretroviral therapy: a systematic review and Meta-analysis. PLoS One. (2014) 9:e111209. doi: 10.1371/journal.pone.0111209
20. Gezae, KE, Abebe, HT, Gebretsadik, LGE, and Gebremeskel, AK. Predictors of accelerated mortality of Tb/Hiv co-infected patients on art in mekelle, ethiopia: an 8 years retrospective follow-up study. Ann Biostat Biometric Appl. (2019) 3:2641–6336. doi: 10.33552/ABBA.2019.03.000572
21. Dawit, Z, Abebe, S, Dessu, S, Mesele, M, Sahile, S, and Ajema, D. Incidence and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at public hospitals in southern Ethiopia. PLoS One. (2021) 16:e0253449. doi: 10.1371/journal.pone.0253449
22. PRISMA Checklist. PRISMA_2020_expanded_checklist for systemic reviwe and meta-analysis studies reporting systematic review. BMJ. (2021) 372:n16. doi: 10.1136/bmj.n71
23. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
24. Thompson, SG, and Higgins, JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. (2002) 21:1559–73. doi: 10.1002/sim.1187
25. Borenstein, M, Hedges, LV, Higgins, JP, and Rothstein, HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
26. Duval, S, and Tweedie, R. A nonparametric “Trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. (2000) 95:89–98. doi: 10.1080/01621459.2000.10473905
27. Ali, SA, Mavundla, TR, Fantu, R, and Awoke, T. Outcomes of TB treatment in HIV co-infected TB patients in Ethiopia: a cross-sectional analytic study. BMC Infect Dis. (2016) 16:640. doi: 10.1186/s12879-016-1967-3
28. Atalell, KA, Birhan Tebeje, N, and Ekubagewargies, DT. Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. A retrospective follow-up study. PLoS One. (2018) 13:e0197145. doi: 10.1371/journal.pone.0197145
29. Balewgizie Sileshi, ND, Girma, B, Melese, M, and Suarez, P. Predictors of mortality among TB-HIV co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect Dis. (2013) 13:297. doi: 10.1186/1471-2334-13-297
30. Belayneh, M, Giday, K, and Lemma, H. Treatment outcome of human immunodeficiency virus and tuberculosis co-infected patients in public hospitals of eastern and southern zone of Tigray region, Ethiopia. Braz J Infect Dis. (2015) 19:47–51. doi: 10.1016/j.bjid.2014.09.002
31. Birhan, H, Derebe, K, Muche, S, and Melese, B. Statistical analysis on determinant factors associated with time to death of HIV/TB co-infected patients under HAART at Debre Tabor referral hospital: An application of accelerated failure time-shared frailty models. HIV/AIDS. (2021) 13:775–87. doi: 10.2147/HIV.S319745
32. Gebremeske, KE, et al. Predictors of time to death among TB/HIV co-infected adults on ART at two governmental hospitals in Mekelle, Ethiopia, 2009-2016: a retrospective cohort study. Ann Infect Dis Epidemiol. (2020) 5:1049
33. Gebreyes, D. A survival analysis of prognostic determinant factors of time-to-death of HIV/TB co-infected patients under HAART followed-up in a public hospital in Ethiopia. HIV AIDS Rev. (2023) 22:110–30. doi: 10.5114/hivar.2022.121399
34. Gemechu, J, Gebremichael, B, Tesfaye, T, Seyum, A, and Erkalo, D. Predictors of mortality among TB-HIV co-infected children attending anti-retroviral therapy clinics of selected public hospitals in southern, Ethiopia: retrospective cohort study. Arch Belg Sante Publ. (2022) 80:11. doi: 10.1186/s13690-021-00713-1
35. Hailay Abrha, BT. Survival experience and its predictors among TB/HIV co-infected patients in Southwest Ethiopia. Epidemiology. (2015) 5:11. doi: 10.4172/2161-1165.100019
36. Jemberu Nigussie, MK, and Grima, B. Predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus in region, North Ethiopia, retrospective follow-up study. Biomed J Sci & Tech Res. (2021) 39:1–9.
37. Lelisho, ME, Wotale, TW, Tareke, SA, Alemu, BD, Hassen, SS, Yemane, DM, et al. Survival rate and predictors of mortality among TB/HIV co-infected adult patients: retrospective cohort study. Sci Rep. (2022) 12:18360. doi: 10.1038/s41598-022-23316-4
38. Seyoum, E, Demissie, M, Worku, A, Mulu, A, Berhane, Y, and Abdissa, A. Increased mortality in HIV infected individuals with tuberculosis: A retrospective cohort study, Addis Ababa, Ethiopia. HIV/AIDS. (2022) 14:143–54. doi: 10.2147/HIV.S354436
39. Teklu, AM, Nega, A, Mamuye, AT, Sitotaw, Y, Kassa, D, Mesfin, G, et al. Factors associated with mortality of TB/HIV co-infected patients in Ethiopia. Ethiop J Health Sci. (2017) 27:29–38. doi: 10.4314/ejhs.v27i1.4s
40. Tola, A, Mishore, KM, Ayele, Y, Mekuria, AN, and Legese, N. Treatment outcome of tuberculosis and associated factors among TB-HIV co-infected patients at public hospitals of Harar town, eastern Ethiopia. A five-year retrospective study. BMC Public Health. (2019) 19:1658. doi: 10.1186/s12889-019-7980-x
41. Weldegebreal, F, Mitiku, H, and Teklemariam, Z. Treatment outcome of tuberculosis among human immunodeficiency virus positive patients in eastern Ethiopia: a retrospective study. Pan Afr Med J. (2018) 30:32. doi: 10.11604/pamj.2018.30.32.12554
42. Wencheko, HRE. Survival of HIV-TB co-infected adult patients under ART in ambo referral hospital, Ethiopia. Ethiop J Health Dev. (2013) 27:88–93.
43. Wondimu, W, Dube, L, and Kabeta, T. Factors affecting survival rates among adult TB/HIV co-infected patients in Mizan Tepi university teaching hospital, south West Ethiopia. HIV/AIDS. (2020) 12:157–64. doi: 10.2147/HIV.S242756
44. Shaweno, D, and Worku, A. Tuberculosis treatment survival of HIV positive TB patients on directly observed treatment short-course in southern Ethiopia: a retrospective cohort study. BMC Res Notes. (2012) 5:682. doi: 10.1186/1756-0500-5-704
45. Shi, L, and Lin, L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore). (2019) 98:e15987. doi: 10.1097/MD.0000000000015987
46. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
47. Teshome Kefale, A, and Anagaw, YK. Outcome of tuberculosis treatment and its predictors among HIV infected patients in Southwest Ethiopia. Int J Gen Med. (2017) 10:161–9. doi: 10.2147/IJGM.S135305
48. Abrha, H, Tsehayneh, B, Massa, D, Tesfay, A, and Kahsay, H. Survival experience and its predictors among TB/HIV co-infected patients in Southwest Ethiopia. Epidemiology. (2015) 5:2. doi: 10.4172/2161-1165.1000191
49. Straetemans, M, Glaziou, P, Bierrenbach, AL, Sismanidis, C, and van der Werf, MJ. Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS One. (2011) 6:e20755. doi: 10.1371/journal.pone.0020755
50. Odone, A, Amadasi, S, White, RG, Cohen, T, Grant, AD, and Houben, RMGJ. The impact of antiretroviral therapy on mortality in HIV positive people during tuberculosis treatment: a systematic review and Meta-analysis. PLoS One. (2014) 9:e112017. doi: 10.1371/journal.pone.0112017
51. Straetemans, M, Bierrenbach, AL, Nagelkerke, N, Glaziou, P, and van der Werf, MJ. The effect of tuberculosis on mortality in HIV positive people: a meta-analysis. PLoS One. (2010) 5:e15241. doi: 10.1371/journal.pone.0015241
52. Gupta, RK, Lucas, SB, Fielding, KL, and Lawn, SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: A systematic review and meta-analysis. AIDS. (2015) 29:1987–2002. doi: 10.1097/QAD.0000000000000802
53. Kebede, MJF. Exploring multi-level risk factors and post-war burdens of trachomatous trichiasis among displaced population in Raya kobo districts, implication for urgent action. Int J Ophthalmol. (2023) 16:1299–308. doi: 10.18240/ijo.2023.08.17
54. Mohamed, SOO, and Ahmed, EM. Prevalence and determinants of antenatal tetanus vaccination in Sudan: a cross-sectional analysis of the multiple Indicator cluster survey. Trop Med Health. (2022) 50:7. doi: 10.1186/s41182-022-00398-4
55. Azanaw, MM, Derseh, NM, Yetemegn, GS, and Angaw, DA. Incidence and predictors of tuberculosis among HIV patients after initiation of antiretroviral treatment in Ethiopia: a systematic review and meta-analysis. Trop Med Health. (2021) 49:18. doi: 10.1186/s41182-021-00306-2
56. Alebel, A, Demant, D, Petrucka, P, and Sibbritt, D. Effects of undernutrition on mortality and morbidity among adults living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. (2021) 21:1. doi: 10.1186/s12879-020-05706-z
57. Tesfaye, B, Alebel, A, Gebrie, A, Zegeye, A, Tesema, C, and Kassie, B. The twin epidemics: prevalence of TB/HIV co-infection and its associated factors in Ethiopia; a systematic review and meta-analysis. PLoS One. (2018) 13:e0203986. doi: 10.1371/journal.pone.0203986
58. Abay, SM, Deribe, K, Reda, AA, Biadgilign, S, Datiko, D, Assefa, T, et al. The effect of early initiation of antiretroviral therapy in TB/HIV-Coinfected patients: a systematic review and Meta-analysis. J Int Assoc Provid AIDS Care. (2015) 14:560–70. doi: 10.1177/2325957415599210
59. Kebede, F, Kebede, T, Kebede, B, Abate, A, Jara, D, Negese, B, et al. Time to develop and predictors for incidence of tuberculosis among children receiving antiretroviral therapy. Tuberc Res Treat. (2021) 2021:6686019. doi: 10.1155/2021/6686019
60. Alemu, YM, Andargie, G, and Gebeye, E. High incidence of tuberculosis in the absence of isoniazid and Cotrimoxazole preventive therapy in children living with HIV in northern Ethiopia: a retrospective follow-up study. PLoS One. (2016) 11:e0152941. doi: 10.1371/journal.pone.0152941
61. Endalamaw, A, Engeda, EH, and Tezera, N. Incidence of tuberculosis in children on antiretroviral therapy: a retrospective cohort study. BMC Res Notes. (2018) 11:745. doi: 10.1186/s13104-018-3846-z
62. Getaneh, T, Negesse, A, Dessie, G, and Desta, M. The impact of tuberculosis co-infection on virological failure among adults living with HIV in Ethiopia: a systematic review and meta-analysis. J Clin Tubercul Other Mycobacter Dis. (2022) 27:100310. doi: 10.1016/j.jctube.2022.100310
63. Assefa, DG, Zeleke, ED, Bekele, D, Ejigu, DA, Molla, W, Woldesenbet, TT, et al. Isoniazid preventive therapy for prevention of tuberculosis among people living with HIV in Ethiopia: a systematic review of implementation and impacts. Int J Environ Res Public Health. (2023) 20. doi: 10.3390/ijerph20010621
Keywords: mortality, tuberculosis, HIV, antiretroviral, children, adult, Ethiopia, population
Citation: Bizuneh FK, Bizuneh TK, Masresha SA, Kidie AA, Arage MW, Sirage N and Abate BB (2024) Tuberculosis-associated mortality and risk factors for HIV-infected population in Ethiopia: a systematic review and meta-analysis. Front. Public Health. 12:1386113. doi: 10.3389/fpubh.2024.1386113
Edited by:
Hai-Feng Pan, Anhui Medical University, ChinaReviewed by:
Lusiana Rusdi Idrus, University of Groningen, NetherlandsWorku Misganaw Kebede, Debre Berhan University, Ethiopia
Copyright © 2024 Bizuneh, Bizuneh, Masresha, Kidie, Arage, Sirage and Abate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fassikaw Kebede Bizuneh, ZmFzc2lrYXcxMjNAZ21haWwuY29t; ZmFzc2lrYXcxMjNAZG11LmVkdS5ldA==
 Fassikaw Kebede Bizuneh
Fassikaw Kebede Bizuneh Tsehay Kebede Bizuneh2
Tsehay Kebede Bizuneh2 Seteamlak Adane Masresha
Seteamlak Adane Masresha Atitegeb Abera Kidie
Atitegeb Abera Kidie Nurye Sirage
Nurye Sirage Biruk Beletew Abate
Biruk Beletew Abate