
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 17 May 2024
Sec. Environmental Health and Exposome
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1363362
 Hecheng Li1
Hecheng Li1 Guoliang Li2
Guoliang Li2 Mushi Yi1
Mushi Yi1 Jiazhen Zhou2
Jiazhen Zhou2 Yaotang Deng2,3
Yaotang Deng2,3 Yiqi Huang3
Yiqi Huang3 Shuirong He4
Shuirong He4 Xiaojing Meng1
Xiaojing Meng1 Lili Liu1,2*
Lili Liu1,2*Background: Heavy metal exposure is an important cause of reduced bone mineral density (BMD). Epidemiological studies focusing on the effects of mixed heavy metal exposure on BMD in middle-aged and older people are scarce. In single-metal studies, men and women have shown distinct responses of BMD to environmental metal exposure. This study therefore aimed to elucidate the association between mixed heavy metal exposure and BMD and to investigate whether it is sex-specific.
Methods: Data from the 2017–2020 National Health and Nutrition Examination Survey were selected for this cross-sectional study. The study used three statistical methods, i.e., linear regression, Bayesian kernel machine regression (BKMR) modeling, and weighted quartiles (WQS) regression, to explore the association between the urinary concentrations of 11 metals (barium, cadmium, cobalt, cesium, manganese, molybdenum, lead, antimony, tin, thallium, and Tungsten), either individually or as a mixture, and total femoral BMD.
Results: A total of 1,031 participants were included in this study. Femoral BMD was found to be higher in men than women. A significant negative correlation between the urinary concentrations of the 10 metals and femoral BMD was found in the overall cohort. Further gender sub-stratified analyses showed that in men, urinary metal concentrations were negatively correlated with femoral BMD, with cobalt and barium playing a significant and non-linear role in this effect. In women, although urinary metal concentrations negatively modulated femoral BMD, none of the correlations was statistically significant. Antimony showed sex-specific differences in its effect.
Conclusion: The urinary concentrations of 10 mixed heavy metals were negatively correlated with femoral BMD in middle-aged and older participants, and this effect showed gender differences. These findings emphasize the differing role of mixed metal exposure in the process of BMD reduction between the sexes but require further validation by prospective studies.
Osteoporosis, a chronic systemic skeletal disease characterized by an insidious decrease in bone mineral density (BMD) and increased bone fragility, has become a widespread public health problem (1, 2). Bone loss affects 40 million people in the U.S. and is largely concentrated in the middle-aged and older population, severely impacting people’s quality of life (3, 4). Studies have shown that BMD declines significantly in people over 50 years of age, and prevalence rates are projected to continue to rise in the coming decades, adding a significant healthcare burden to society, with the cost of treating osteoporosis and fracture-related diseases expected to reach $25 billion by 2025 (5, 6). BMD adequately reflects bone strength and is a commonly used indicator for assessing bone health status, with femoral BMD being the most widely used in epidemiological studies (7, 8). Identifying risk factors for decreased BMD is an important measure to prevent the development of osteoporosis.
Heavy metals are prevalent in a variety of environmental media and can enter the body in a multitude of ways, including through food, drinking water, smoking, and occupational exposure (9). Exposure to heavy metals is known to be a risk factor for chronic bone diseases such as fractures and osteoporosis (10, 11). In vitro and in vivo studies have found that metal exposure affects bone tissue through distinct genetic, nutritional, and metabolic mechanisms (12, 13). For example, cadmium (Cd) can contribute to an increased risk of fractures and osteoporosis by promoting bone marrow mesenchymal stem cell (BMSC) senescence and mitochondrial dysfunction (14, 15). Environmental lead (Pb) is absorbed into the body through a variety of pathways, with bone trabeculae and cortex being the main targets for Pb accumulation. BMD decreases with the accumulation of Pb in the body, which in turn leads to the development of bone-related diseases (16). Chronic manganese (Mn) exposure was found to increase the risk of osteoporosis in a cohort of retired older adults, and this effect was particularly significant in women (17). However, because humans are inevitably exposed to multiple metals at the same time, exposure to any single metal cannot fully account for the onset and progression of disease; both synergistic and antagonistic interactions between metals play a role. Therefore, it is crucial to explore the effects of mixed exposure to multiple metals on BMD. Noticeably, sex-specific associations of heavy metals with liver fibrosis, kidney function and cognitive function were observed in previous studies (18–20).
Therefore, the present study, based on the most recent data from the National Health and Nutrition Examination Survey (NHANES), investigated the effects of the urinary concentrations of 11 metals, individually and in combination, on femoral BMD in the middle-aged and older adult population, and the potential gender specificity of this effect.
NHANES is a series of cross-sectional studies conducted by the U.S. Centers for Disease Control and Prevention to assess the health status of the national non-institutionalized U.S. population. The data are released following a two-year cycle, but because 2019–2020 was affected by the prevalence of coronavirus disease 2019, the 2017–2020 data were combined into one nationally representative dataset in this study, with a total of 15,560 individuals included. Initially, we excluded persons with missing urinary metal data (N = 11,624). Then, we excluded people ≤50 years of age and those with missing total femoral BMD data (N = 12,015). Finally, participants with incomplete baseline information were excluded (N = 8,751), leaving 1,031 participants with data for final analysis. All participants provided written informed consent to take part in the study. The National Ethical Review Board for Health Statistics Research approved the investigation protocol.
The main factors in the NHANES 2017–2020 database that may affect BMD were considered as covariates for inclusion in this study. The covariates primarily included age (years), sex (male or female), race/ethnicity (Mexican-American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other), education level (less than 9th grade, grades 9–11, high school graduate, some college, or college graduate), marital status (married/cohabiting, widowed/divorced/separated, or never married), and intensity of work (strenuous, moderate, or none). Alcohol consumption status was categorized as never or ever based on ever having been exposed to alcoholic beverages. Smoking status was categorized as yes or no on the basis of tobacco use equivalent to at least 100 cigarettes in life. Body mass index (BMI) was categorized as <25, 25–30, or > 30 kg/m2. Diabetes mellitus was defined according to previous diagnosis (yes, borderline, or no). Hypertension was defined according to previous diagnosis (yes or no). Household economic status was categorized as ≤1.30, 1.30–1.85, or ≥ 1.85 based on the monthly household poverty level index, these categories were chosen because they represented commonly used percentages of the poverty guidelines (i.e., 130 percent and 185 percent of the guidelines), by federal programs, in determining eligibility.
Total femoral BMD was estimated by dual-energy X-ray absorptiometry (DXA) (21). The BMD measurements were performed by professionally trained and certified radiologists. The femur scans provide bone measurements for the total femur. Participants who self-reported using contrast media in the past 7 days, were pregnant, or weighed more than 450 pounds were not allowed to participate in the DXA examination. Detailed information is recorded in the NHANES database.
Data on the detection of 11 metals in urine were obtained from NHANES 2017–2020. Initial determinations of Ba, Cd, cobalt (Co), Cs, Mn, Mo, Pb, antimony (Sb), tin (Sn), thallium (Tl), and Tungsten (W) were made in spot urine using inductively coupled plasma mass spectrometry. Due to the different detection methods used in 2017–2018 and 2019–2020, the higher value of those given by the two methods was officially adopted as the limit of detection (LOD) for each metal to make the combined datasets compatible. The LOD divided by the square root of two was used to replace values below the LOD according to the NHANES standard. The percentage of persons below LOD for each metal is shown in the Supplementary Table S1. For >50% below LOD (Mn) also investigated as binary variables, coded 0 when below the LOD and 1 when above the LOD (22). To reduce the effect of urine dilution, the metal concentrations were corrected for urinary creatinine and expressed as micrograms per gram of creatinine.
Data on the participants’ baseline characteristics are expressed as the mean (standard deviation) for continuous variables and frequency (percentage) for categorical variables. Differences between groups were compared using Student’s t-test or Kruskal-Wallis H test for continuous variables, and the χ2 test for categorical variables. Raw data for the urinary metal concentrations are presented as medians (upper quartile, lower quartile), and the logarithms of the urinary metal data were used in the statistical and analytical processes. Pearson correlation analysis was used to determine the correlation between the concentrations of the 10 urinary metals and the total BMD. The correlation between each urinary metal concentration and total BMD was assessed by weighted multiple linear regression modeling, incorporating different covariates for adjustment (model 1: unadjusted; model 2: adjusted for age, race, marital status, and BMI; model 3: adjusted for all covariates). Weighted quartiles (WQS) regression was used to assess the effect of multiple urinary metal concentrations on total BMD, with the contribution to the overall effect calculated through the corresponding weighted response for each metal. The WQS regression type was set to linear, and the data were randomly divided into training and validation datasets at a ratio of 4:6, with the number of replication results for analysis specified as 1,000. The Bayesian kernel machine regression (BKMR) model was used to assess the interaction effects and non-linear correlations of exposure to metals with respect to the study outcomes, after adjusting for potential confounders. The effects of all metals on total BMD were predicted by calculating the specific percentile exposure levels compared with the median exposure levels. All analyses were further stratified by gender and adjusted to include all covariates except gender. The analyses were performed using the statistical software packages SPSS 21.0 and R software version 4.3.1. p-values <0.05 were considered as statistically significant.
Table 1 shows the basic characteristics of the participants, stratified by sex. A total of 1,031 older people were included in this study, including 556 men and 475 women. The average age of the men was 64.43 years and that of the women was 63.53 years. The differences between men and women in marital status, BMI, smoking, alcohol consumption, diabetes mellitus, and intensity of work were statistically significant (p < 0.05). A greater proportion of men are physically overweight, while women are predominantly obese. The higher prevalence of diabetes mellitus in men than in women may be related to the higher frequency of unhealthy behaviors, such as smoking and alcohol consumption, among men. The mean femoral BMD was higher in men than women, at 0.998 g/cm2 and 0.862 g/cm2, respectively. The medians and quartiles of the participants’ urinary metal concentrations are presented in Table 2, stratified by gender; no difference in the distribution of Mn between men and women, and statistically significant differences were found between men and women for other 10 urinary concentration of metals (p < 0.05).
As shown in Table 3, the univariate linear regression analyses revealed that 10 metals other than Mn (β = −0.004, 95%CI = −0.0256 – 0.0176) were associated with BMD in total participants, whereas Cs (β = −0.0276, 95% CI = −0.0543 − −0.001), Mo (β = −0.0287, 95% CI = −0.0504 − −0.007), Sb (β = −0.0217, 95% CI = −0.0416 − −0.0018), and W (β = −0.0219, 95% CI = −0.0378 − −0.0061) were correlated with femoral BMD only in women. After adjusting for covariates, femoral BMD was negatively and linearly correlated with Ba (β = −0.014, 95% CI = −0.0246 − −0.0034), Cd (β = −0.0198, 95% CI = −0.0366 − −0.003), Co (β = −0.0201, 95% CI = −0.0337 − −0.0064), and Sb (β = 0.0167, 95% CI = −0.001 − −0.0324) in men, whereas no metal was found to be linearly correlated with femoral BMD in women. Mn was more than 50% below the LOD, and Mn was excluded to ensure the efficacy of the mixed-exposure test.
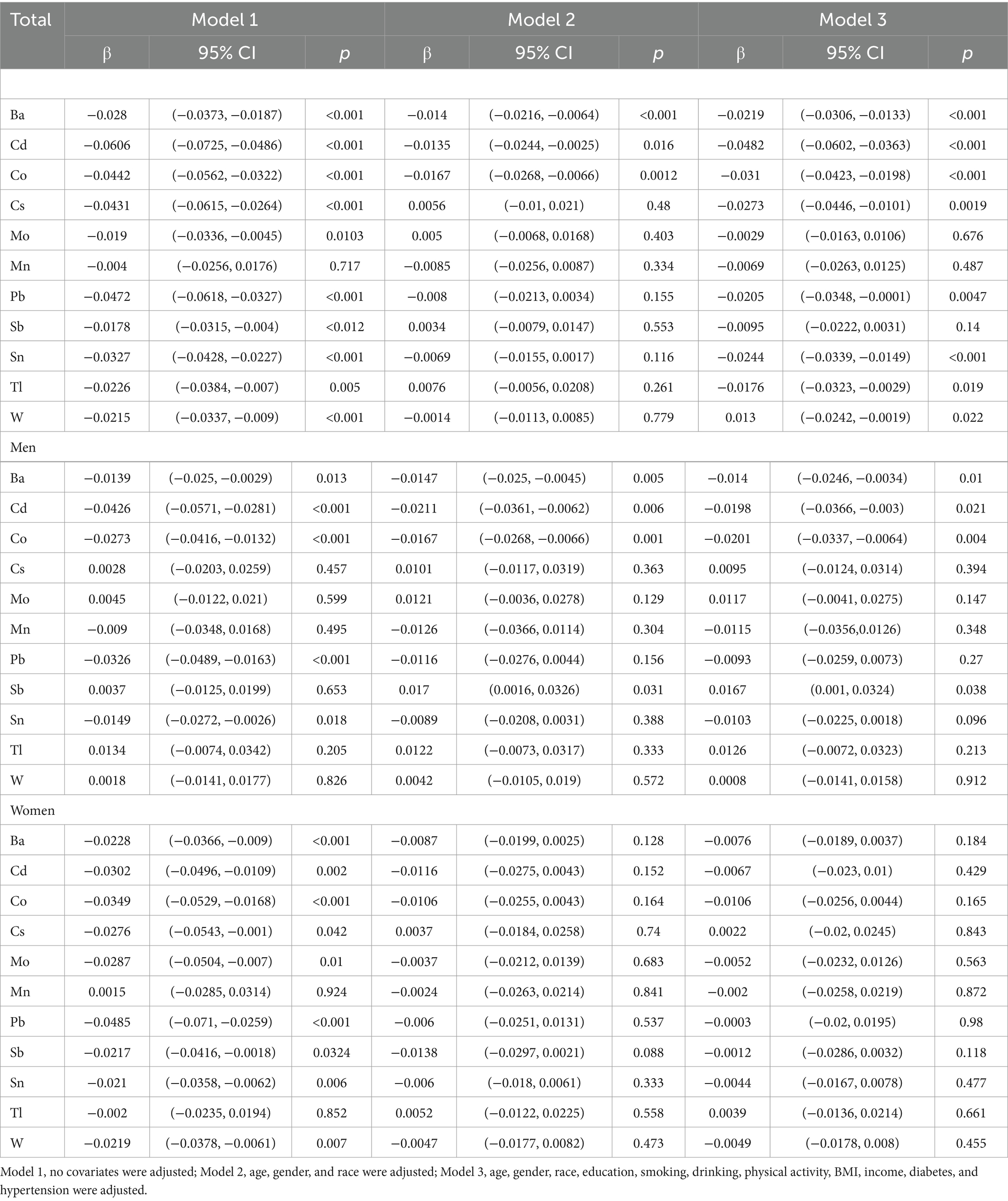
Table 3. Multiple linear regression analysis of urinary metal concentrations and femoral BMD in NHANES 2017–2020 (n = 1,031).
Pearson’s correlation analysis showed a weak negative correlation between all 10 urinary metals and total femoral BMD. Further sex-stratified analyses revealed that Cd (r: −0.24) was more strongly correlated with femoral BMD than others in men, whereas femoral BMD in women had a strongest correlation with Pb (r: −0.19) (Figure 1).
The combined effect of mixed exposure to the 10 heavy metals on femoral BMD was assessed by fitting a BKMR model. Overall, mixed exposure to the 10 metals was positively correlated with femoral BMD below the 50th percentile, but this effect shifted to a significant negative correlation above the 50th percentile. Gender subgroup analyses showed that mixed metal exposure levels above the 50th percentile had a negative correlation with femoral BMD in men, but a non-significant negative correlation in women (Figure 2A).
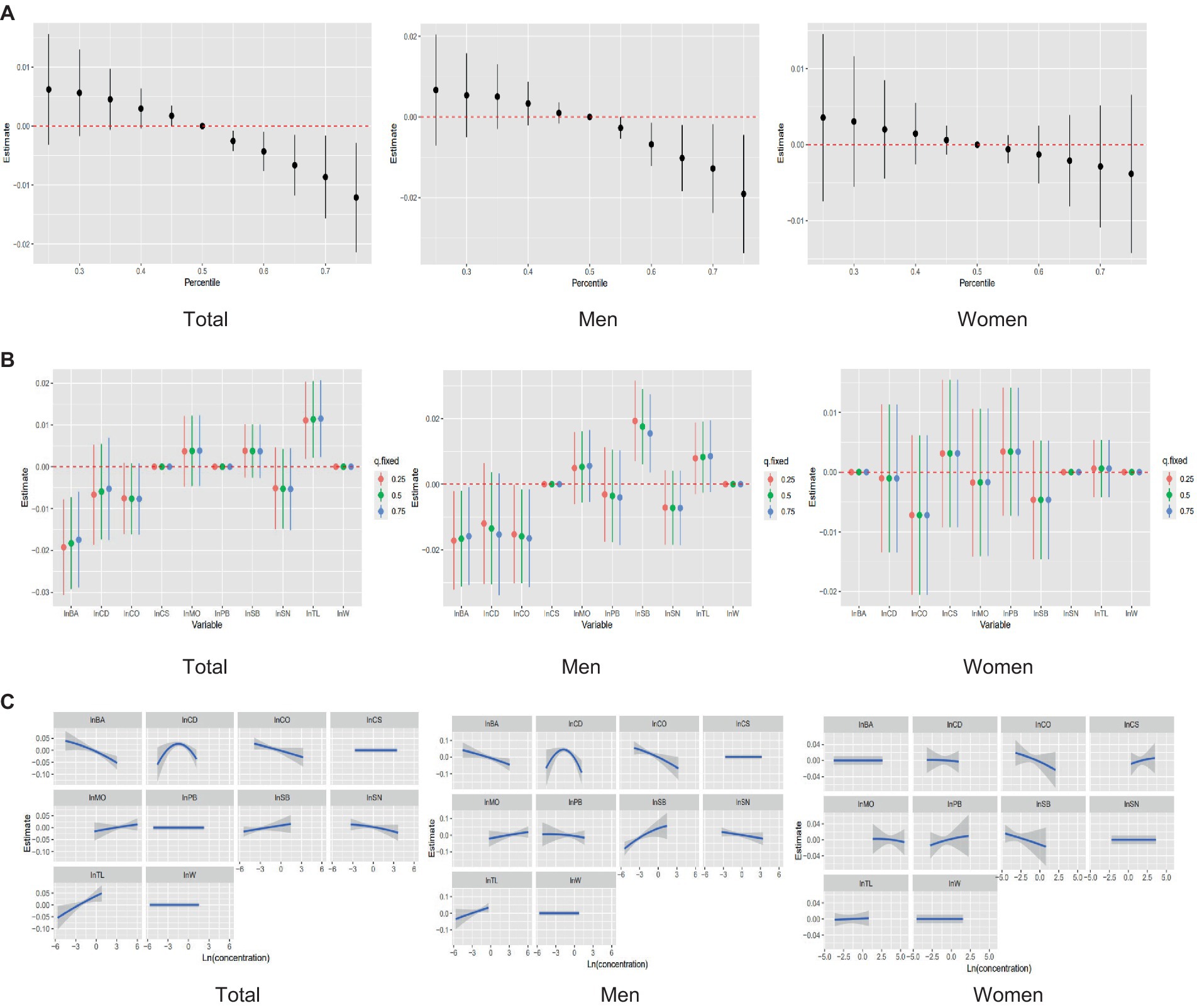
Figure 2. Analysis of the relationship between mixed metal exposure and femoral BMD using the BKMR model. (A) The overall effect of a mixture of whole, male, and female individuals on femoral BMD. (B) Univariate effects for whole, male, and female. (C) Univariate exposure–response relationship for each metal concentration on estimates of femoral BMD.
The concentrations of the 10 metals were fixed at the 25th, 50th, or 75th percentile to evaluate the effect of each metal on femoral BMD. In the overall analysis, both Ba, Cd and Co were found to have a negative effect on femoral BMD, whereas the effect of Tl was positive. In the sex-stratified analyses, Ba and Co showed negative correlations with femoral BMD, but these correlations were only significant in men. Cd had a stronger negative correlation with femoral BMD in men than in women. Sb was a protective factor in men, being positively correlated with femoral BMD, but was negatively correlated with femoral BMD in women (Figure 2B).
Finally, the non-linear relationship between each metal and femoral BMD was predicted when the concentrations of the other metals were fixed at the 50th percentile. In each subgroup, elevated urinary concentrations of Ba and Co were accompanied by a decrease in femoral BMD; the relationship between Cd concentration and femoral BMD had an inverted U-shape in men. Sb is sex-specific in its correlation with femoral BMD, and a positive correlation is shown in males, while females had a negative correlation (Figure 2C).
WQS regression was performed to analyze the negative effect of the urinary concentrations of the 10 metals on femoral BMD, calculating the percentage weight that each metal accounted for in this effect. The analysis including all participants showed that Co (23.0%) and Sn (20.9%) had the greatest weights of influence on femoral BMD. When the analysis was performed on the gender subgroups, femoral BMD in men was most influenced by Co (50.0%), while the metal with the greatest weight of influence on femoral BMD in women was Sb (28.2%) (Figure 3).
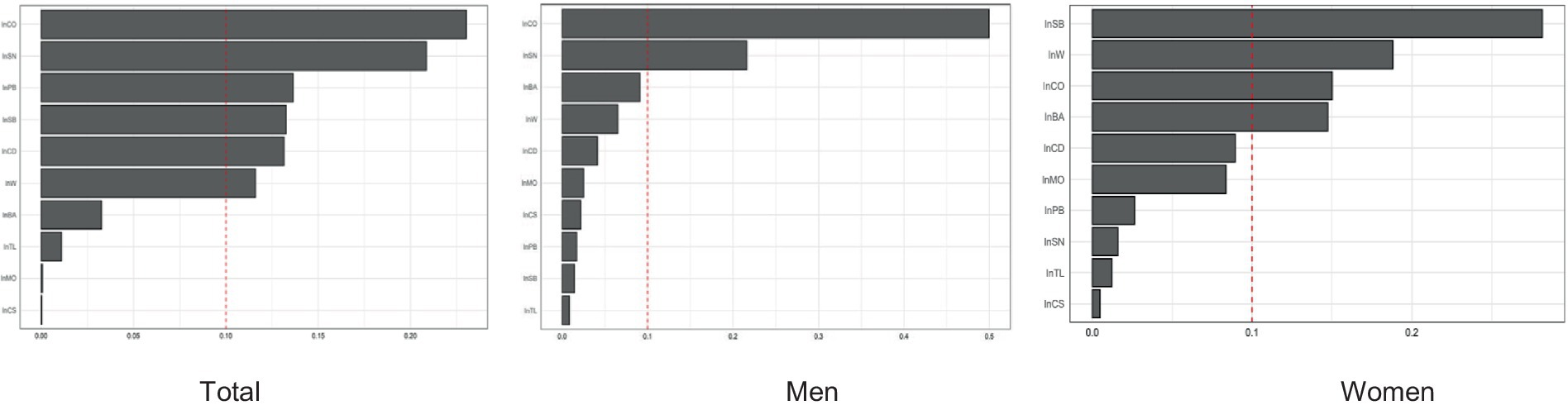
Figure 3. The WSQ regression estimated weights of each of the 11 metals associated with femoral BMD.
In this study using a nationally representative dataset, various methods with distinct strengths and limitations—multiple linear regression, WQS regression, and BKMR modeling—were combined to assess the effects of urinary metals (Ba, Cd, Co, Cs, Mo, Pb, Sb, Sn, Tl, and W) on total femoral BMD in a middle-aged and older population, with further analysis using gender subgroups. The BKMR model found a significant negative correlation between the concentrations of the 10 urinary metals and femoral BMD in these middle-aged and older adults, including a non-linear relationship of femoral BMD with both Ba and Co. Interestingly, the same trends were obtained in the subgroup of male participants, with the urinary concentrations of Co and Ba accounting for the largest weight in the effect of mixed metal exposure, and Cd also showing a tendency to cause a decrease in BMD, which was not significant. Further analysis of the weighting of the negative effect of urinary metal concentrations on femoral BMD in men showed that Co accounted for the largest proportion of the overall effect among the mixed metals. In women, mixed exposure to the 10 metals showed a trend toward reducing femoral BMD, but no correlations were statistically significant in this analysis. Sb showed a gender difference, being positively correlated with femoral BMD in men but having the opposite effect in women.
The three statistical methods applied in this study yielded consistent results, indicating that Co played a significant role in the reduction of femoral BMD induced by mixed metal exposure, and that the response was more pronounced in male than in female participants. In a previous population-based study, urinary Co concentrations were found to be strongly associated with elevated fasting blood glucose and insulin levels in men, but not in women (23). This suggests that men may be more sensitive to the effects of Co, a toxic metal that has been reported in both eukaryotic and prokaryotic cells to induce the production of reactive oxygen species, superoxide, and free radicals, which damage DNA and inhibit its repair (24). In recent years, Co has been widely used in hip and knee arthroplasty because of its good mechanical properties, which has led to concerns about Co inducing adverse biological reactions such as hypersensitivity and tissue destruction in bone (25, 26). Animal experiments revealed that Co induced lysosomal damage and tissue protease leakage in bone tissue, leading to the activation of the inflammatory vesicle NLRP3, which in turn secreted inflammatory factors such as IL-1β and ultimately induced inflammatory osteolysis (27). In a study of the dose–response relationship between Co and bone homeostasis, in vivo toxicity was not observed at Co concentrations of 0.1–5 ppm, although Co was found to cause upregulation of anti-inflammatory, osteogenic, and pro-angiogenic factors at 1 ppm (28). Bone resorption was activated with increasing Co exposure, and an inflammatory response inhibited osteogenic differentiation and caused apoptosis of BMSCs.
The heavy metal Ba is being used in an ever-expanding range of industrial applications; however, it is gradually being discovered that Ba affects metabolic, neurological, and behavioral functions and can cause kidney and cardiovascular disease (29). In general population monometallic exposure studies, urinary Ba concentrations were negatively correlated with total BMD and lumbar spine BMD, and Ba was also found to have a combined effect with Pb and Cd in causing a decrease in BMD, possibly by inducing adverse effects such as oxidative stress (30). In the present study, a non-linear relationship, with an overall trend of negative correlation, between the Ba concentration and femoral BMD was found when the other urinary metals were fixed at median concentrations. Meanwhile, a weak interaction of Ba with other metals was found, with the overall femoral BMD concentrations decreasing as the concentrations of Ba and one other metal increased. Bone tissue is the main target organ for Ba accumulation, which can be attributed to the fact that the radius of Ba ions is similar to that of calcium ions in similar chemical environments (31). With the continuous development of new nanomaterials in recent years, it has been found that Ba-containing nanocomposites can be used as fracture-healing materials, and these have shown beneficial effects on bone tissue healing, such as promotion of bone regeneration and osteoconduction (32). Therefore, the mechanism of Ba-induced BMD reduction is still unclear and needs further examination.
Sb has unique flame retardancy, corrosion resistance, and antioxidant properties. Sb and its compounds are widely used in the production of semiconductors, flame retardants, and pharmaceuticals, and have become emerging environmental pollutants around the world (33). Recently, an increasing number of scientists have paid attention to the relationship between Sb and human health, and whether Sb is harmful to human health is controversial. Chronic exposure to diantimony trioxide impairs particle clearance and promotes the development of lung tumors, adrenal tumors, and lymphomas, as demonstrated by rodent experiments (34). Elsewhere, in vitro experiments revealed that exposure to high levels of Sb causes dose-dependent apoptosis in human bronchial epithelial cells (35). Antimony could damage mitochondria through ROS-dependent oxidative stress pathways, and low-dose antimony (0.8uM) could inhibit the level of mitophagy by directly decreasing the PINK1/Parkin pathway (36). In a metabolism-related study, high levels of Sb were found to cause redox imbalance and promote bone loss (37). It might be a promising agent to treat some cancers in an appropriate dose. The polyoxometalate SbW9 inhibits proliferation and induces apoptosis in non-small cell lung cancer cells via the PTEN-dependent AKT signalling pathway (38). In the present study, the Sb concentration was found to affect femoral BMD in a sex-specific manner, being positively correlated with femoral BMD in men and negatively correlated with femoral BMD in women. However, there are few studies on the association between Sb and bone damage, and further in vivo and in vitro experiments are needed to provide evidential support for the association between Sb and BMD.
The bone mass of an individual peaks in early adulthood and then decreases with age (39). Bone mass is higher in men than women throughout this process, which is consistent with the finding of this study that male femoral BMD was higher than that of women. Estrogen plays an important role in the maintenance of bone homeostasis by promoting anti-apoptosis mechanisms in osteoblasts and osteoclasts and pro-apoptosis mechanisms in osteoclasts (40). Numerous epidemiological studies have found that bone mass in women is significantly affected by estrogen, and that starting around the age of 50 years, women experience an accelerated rate of bone loss due to estrogen deficiency (41). As a result, there are guidelines that recommend screening for osteoporosis using clinical risk assessment tools for menopausal women younger than 65 years, and a variety of preventive and therapeutic medications have been approved by the U.S. Food and Drug Administration (42). The female group in this study, with a mean age of 64 years, were likely to already be receiving anti-osteoporosis treatments. This effective protection may be the reason why no significant effect of mixed metal exposure on femoral BMD was found in women. Alternatively, this absence of a significant correlation may be due to the fact that the effect of estrogen deficiency on BMD greatly outweighs the effect of metal exposure (43).
The present study has the following strengths. First, the data were obtained from the US NHANES database, which is nationally representative. Urinary metals were chosen in this study to reflect bodily metal-exposure levels, and it is well known that urinary concentrations can be a better index of long-term exposure to specific metals than blood concentrations. Second, this study used the BKMR model to investigate the overall trend of the effects of the 10 urinary metals on femoral BMD, as well as WQS regression to further explore the contribution of each metal to the effect of mixed exposure and to avoid bias caused by the covariance of two or more metals. Besides the common heavy metals Cd and Pb, which are linked with an increased risk of osteoporosis, Co, Ba, and Sb were found to affect the femoral BMD in the participants in this study, indicating the need for exposure assessment of these metals. However, the study also has some limitations. The data collected in the most recent round of the NHANES cross-sectional survey were analyzed in a comprehensive manner in this study, but due to the potential influences of drug use, lifestyle behaviors, and government interventions on BMD, more data and experiments are needed to further support the results. Additionally, this study was cross-sectional, making causal inferences difficult and necessitating further support from cohort studies and mixed metal exposure experiments. Finally, a 24-h urine sample does provide a better assessment of an individual’s level of environmental exposure than spot urine sample.
In conclusion, BKMR modeling and WQS regression were applied to investigate the combined effects of multiple metals on femoral BMD in middle-aged and older U.S. citizens. All 10 metals investigated in this study were correlated with reduced femoral BMD, and there were differences between the effects in men and women. Urinary Co, Ba, and Sb appear to play the most important roles in the association of femoral BMD with the combined effects of these metals. Further confirmation of our findings is required in cohort studies and mechanistic experiments.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The studies involving humans were approved by National Center for Health Statistics Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HL: Writing – original draft, Conceptualization, Data curation, Formal analysis, Resources. GL: Writing – review & editing, Conceptualization. MY: Data curation, Formal analysis, Resources, Writing – review & editing. JZ: Writing – review & editing, Methodology. YD: Methodology, Writing – review & editing. YH: Data curation, Resources, Writing – review & editing. SH: Data curation, Resources, Writing – review & editing. XM: Supervision, Writing – review & editing. LL: Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Project of National Natural Science Foundation of China (No. 81972990), and Medical Science and Technology Foundation of Guangdong Province (No. A2021421), and Natural Science Foundation of Guangzhou Municipality (202201011000).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1363362/full#supplementary-material
1. Compston, JE, McClung, MR, and Leslie, WD. Osteoporosis. Lancet. (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
2. Cauley, JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. (2013) 68:1243–51. doi: 10.1093/gerona/glt093
3. Wright, NC, Looker, AC, Saag, KG, Curtis, JR, Delzell, ES, Randall, S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. (2014) 29:2520–6. doi: 10.1002/jbmr.2269
4. Brauer, CA, Coca-Perraillon, M, Cutler, DM, and Rosen, AB. Incidence and mortality of hip fractures in the United States. JAMA. (2009) 302:1573–9. doi: 10.1001/jama.2009.1462
5. Kanis, JA, Johnell, O, Oden, A, Sernbo, I, Redlund-Johnell, I, Dawson, A, et al. Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int. (2000) 11:669–74. doi: 10.1007/s001980070064
6. Burge, R, Dawson-Hughes, B, Solomon, DH, Wong, JB, King, A, and Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. (2007) 22:465–75. doi: 10.1359/jbmr.061113
7. Xue, S, Kemal, O, Lu, M, Lix, LM, Leslie, WD, and Yang, S. Age at attainment of peak bone mineral density and its associated factors: the National Health and nutrition examination survey 2005-2014. Bone. (2020) 131:115163. doi: 10.1016/j.bone.2019.115163
8. Cai, S, Fan, J, Zhu, L, Ye, J, Rao, X, Fan, C, et al. Bone mineral density and osteoporosis in relation to all-cause and cause-specific mortality in NHANES: a population-based cohort study. Bone. (2020) 141:115597. doi: 10.1016/j.bone.2020.115597
9. Wu, X, Cobbina, SJ, Mao, G, Xu, H, Zhang, Z, and Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. (2016) 23:8244–59. doi: 10.1007/s11356-016-6333-x
10. Feng, X, Zan, G, Wei, Y, Ge, X, Cai, H, Long, T, et al. Relationship of multiple metals mixture and osteoporosis in older Chinese women: an aging and longevity study. Environ Pollut. (2023) 317:120699. doi: 10.1016/j.envpol.2022.120699
11. Jalili, C, Kazemi, M, Taheri, E, Mohammadi, H, Boozari, B, Hadi, A, et al. Exposure to heavy metals and the risk of osteopenia or osteoporosis: a systematic review and meta-analysis. Osteoporos Int. (2020) 31:1671–82. doi: 10.1007/s00198-020-05429-6
12. Callaway, DA, and Jiang, JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. (2015) 33:359–70. doi: 10.1007/s00774-015-0656-4
13. Greenblatt, MB, Tsai, JN, and Wein, MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. (2017) 63:464–74. doi: 10.1373/clinchem.2016.259085
14. Luo, H, Gu, R, Ouyang, H, Wang, L, Shi, S, Ji, Y, et al. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-κB pathway and mitochondrial dysfunction. Environ Pollut. (2021) 290:118043. doi: 10.1016/j.envpol.2021.118043
15. Hu, R, Luo, H, Ji, Y, Wang, Z, Zheng, P, Ouyang, H, et al. Activation of NLRP3 signaling contributes to cadmium-induced bone defects, associated with autophagic flux obstruction. Sci Total Environ. (2023) 893:164787. doi: 10.1016/j.scitotenv.2023.164787
16. Wang, WJ, Wu, CC, Jung, WT, and Lin, CY. The associations among lead exposure, bone mineral density, and FRAX score: NHANES, 2013 to 2014. Bone. (2019) 128:115045. doi: 10.1016/j.bone.2019.115045
17. Li, D, Ge, X, Liu, Z, Huang, L, Zhou, Y, Liu, P, et al. Association between long-term occupational manganese exposure and bone quality among retired workers. Environ Sci Pollut Res Int. (2020) 27:482–9. doi: 10.1007/s11356-019-06694-7
18. Wan, H, Jiang, Y, Yang, J, Ma, Q, Liu, L, Peng, L, et al. Sex-specific associations of the urinary fourteen-metal mixture with NAFLD and liver fibrosis among US adults: a nationally representative study. Ecotoxicol Environ Saf. (2022) 248:114306. doi: 10.1016/j.ecoenv.2022.114306
19. Song, S, Liu, N, Wang, G, Wang, Y, Zhang, X, Zhao, X, et al. Sex specificity in the mixed effects of blood heavy metals and cognitive function on elderly: evidence from NHANES. Nutrients. (2023) 15:2874. doi: 10.3390/nu15132874
20. Zhang, S, Tang, H, and Zhou, M. Sex-specific associations between nine metal mixtures in urine and urine flow rate in US adults: NHANES 2009-2018. Front. Public Health. (2023) 11:1241971. doi: 10.3389/fpubh.2023.1241971
22. Cosemans, C, van Larebeke, N, Janssen, BG, Martens, DS, Baeyens, W, Bruckers, L, et al. Glyphosate and AMPA exposure in relation to markers of biological aging in an adult population-based study. Int J Hyg Environ Health. (2022) 240:113895. doi: 10.1016/j.ijheh.2021.113895
23. Yang, J, Lu, Y, Bai, Y, and Cheng, Z. Sex-specific and dose-response relationships of urinary cobalt and molybdenum levels with glucose levels and insulin resistance in U.S. adults. J Environ Sci (China). (2023) 124:42–9. doi: 10.1016/j.jes.2021.10.023
24. Jomova, K, and Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology. (2011) 283:65–87. doi: 10.1016/j.tox.2011.03.001
25. Cheung, AC, Banerjee, S, Cherian, JJ, Wong, F, Butany, J, Gilbert, C, et al. Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition part 1 - history, mechanism, measurements, and pathophysiology. Bone Joint J. (2016) 98-B:6–13. doi: 10.1302/0301-620X.98B1.36374
26. Vasconcelos, DM, Santos, SG, Lamghari, M, and Barbosa, MA. The two faces of metal ions: from implants rejection to tissue repair/regeneration. Biomaterials. (2016) 84:262–75. doi: 10.1016/j.biomaterials.2016.01.046
27. Samelko, L, Landgraeber, S, McAllister, K, Jacobs, J, and Hallab, NJ. Cobalt alloy implant debris induces inflammation and bone loss primarily through danger Signaling, not TLR4 activation: implications for DAMP-ening implant related inflammation. PLoS One. (2016) 11:e0160141. doi: 10.1371/journal.pone.0160141
28. Liu, G, Wang, X, Zhou, X, Zhang, L, Mi, J, Shan, Z, et al. Modulating the cobalt dose range to manipulate multisystem cooperation in bone environment: a strategy to resolve the controversies about cobalt use for orthopedic applications. Theranostics. (2020) 10:1074–89. doi: 10.7150/thno.37931
29. Kravchenko, J, Darrah, TH, Miller, RK, Lyerly, HK, and Vengosh, A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Health. (2014) 36:797–814. doi: 10.1007/s10653-014-9622-7
30. Tang, P, Liao, Q, Huang, H, Chen, Q, Liang, J, Tang, Y, et al. Effects of urinary barium exposure on bone mineral density in general population. Environ Sci Pollut Res Int. (2023) 30:106038–46. doi: 10.1007/s11356-023-29791-0
31. Panahifar, A, Chapman, LD, Weber, L, Samadi, N, and Cooper, DML. Biodistribution of strontium and barium in the developing and mature skeleton of rats. J Bone Miner Metab. (2019) 37:385–98. doi: 10.1007/s00774-018-0936-x
32. Mabrouk, M, Ibrahim Fouad, G, Beherei, HH, and Das, DB. Barium oxide doped magnesium silicate Nanopowders for bone fracture healing: preparation, characterization, antibacterial and in vivo animal studies. Pharmaceutics. (2022) 14:1582. doi: 10.3390/pharmaceutics14081582
33. Nishad, PA, and Bhaskarapillai, A. Antimony, a pollutant of emerging concern: a review on industrial sources and remediation technologies. Chemosphere. (2021) 277:130252. doi: 10.1016/j.chemosphere.2021.130252
34. Boreiko, CJ, and Rossman, TG. Antimony and its compounds: health impacts related to pulmonary toxicity, cancer, and genotoxicity. Toxicol Appl Pharmacol. (2020) 403:115156. doi: 10.1016/j.taap.2020.115156
35. Zhao, X, Jin, Y, Yang, L, Hou, Z, Liu, Y, Sun, T, et al. Promotion of SIRT1 protein degradation and lower SIRT1 gene expression via reactive oxygen species is involved in Sb-induced apoptosis in BEAS-2b cells. Toxicol Lett. (2018) 296:73–81. doi: 10.1016/j.toxlet.2018.07.047
36. Lou, Y, Ma, C, Liu, Z, Shi, J, Zheng, G, Zhang, C, et al. Antimony exposure promotes bladder tumor cell growth by inhibiting PINK1-parkin-mediated mitophagy. Ecotoxicol Environ Saf. (2021) 221:112420. doi: 10.1016/j.ecoenv.2021.112420
37. Galvez-Fernandez, M, Rodriguez-Hernandez, Z, Grau-Perez, M, Chaves, FJ, Garcia-Garcia, AB, Amigo, N, et al. Metabolomic patterns, redox-related genes and metals, and bone fragility endpoints in the Hortega study. Free Radic Biol Med. (2023) 194:52–61. doi: 10.1016/j.freeradbiomed.2022.11.007
38. Sun, HB, Xu, L, Wang, ZX, Zheng, Y, Zhao, Y, Yin, YY, et al. Polyoxometalate SbW9 regulates proliferation and apoptosis of NSCLC cells via PTEN-dependent AKT signaling pathway retraction of: Eur rev med Pharmacol Sci. 2019 Sep;23(18):7959-7967. Eur Rev Med Pharmacol Sci. (2021) 25:2825. doi: 10.26355/eurrev_202104_25525
39. Rizzoli, R, Bianchi, ML, Garabédian, M, McKay, HA, and Moreno, LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. (2010) 46:294–305. doi: 10.1016/j.bone.2009.10.005
40. Väänänen, HK, and Härkönen, PL. Estrogen and bone metabolism. Maturitas. (1996) 23:S65–9. doi: 10.1016/0378-5122(96)01015-8
41. Lu, L, and Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: the role and mechanisms of estrogen. J Endocrinol. (2023) 259:e230116. doi: 10.1530/JOE-23-0116
42. US Preventive Services Task Force Curry, SJ, Krist, AH, Owens, DK, Barry, MJ, Caughey, AB, et al. Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. JAMA. (2018) 319:2521–31. doi: 10.1001/jama.2018.7498
Keywords: urinary metal mixture, bone mineral density, NHANES, middle-aged and older population, WQS regression, BKMR
Citation: Li H, Li G, Yi M, Zhou J, Deng Y, Huang Y, He S, Meng X and Liu L (2024) Sex-specific associations of urinary mixed-metal concentrations with femoral bone mineral density among older people: an NHANES (2017–2020) analysis. Front. Public Health. 12:1363362. doi: 10.3389/fpubh.2024.1363362
Received: 02 January 2024; Accepted: 06 May 2024;
Published: 17 May 2024.
Edited by:
Malarvannan Govindan, University of Antwerp, BelgiumReviewed by:
Charlotte Cosemans, University of Hasselt, BelgiumCopyright © 2024 Li, Li, Yi, Zhou, Deng, Huang, He, Meng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Liu, NjE5MDI4OTcxQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.