
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 12 February 2024
Sec. Environmental Health and Exposome
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1354149
Introduction: Earlier research has indicated that being exposed to polychlorinated dibenzo-p-dioxins (PCDDs) in the workplace can heighten the likelihood of cancer-related deaths. Nevertheless, there is limited information available regarding the connection between PCDD exposure and the risk of cancer mortality in the general population (i.e., individuals not exposed to these substances through their occupation).
Methods: The National Health and Nutrition Examination Survey (NHANES) detected PCDDs in the general population, and the death data were recently updated as of December 31, 2019. We conducted Cox regression analysis and controlled for covariates including age, gender, ethnicity, educational attainment, physical activity, alcohol intake, NHANES survey period, BMI category, cotinine concentration, and household earnings.
Results: After accounting for confounding factors, the findings indicated that for each incremental rise of 1 log unit in 1,2,3,4,6,7,8,9-octachlorodibenzo-p-dioxin, there was a 76% rise in the likelihood of death from any cause, with a p value of 0.003. An increase of 1 log unit in the concentration of 1,2,3,4,6,7,8-heptachlorodibenzofuran could potentially lead to a 90% higher risk of cancer mortality, as indicated by a p value of 0.034 and a 95% confidence interval of 0.05–2.43. As the concentrations of 1,2,3,4,6,7,8-heptachlorodibenzofuran increased, the dose–response curve indicated a proportional rise in the risk of cancer mortality, accompanied by a linear p value of 0.044. The sensitivity analysis demonstrated that our findings were resilient.
Discussion: In the general population, an elevated risk of cancer mortality was observed in PCDDs due to the presence of 1,2,3,4,6,7,8-heptachlorodibenzofuran. Mechanistic research is required to further confirm it.
PCDDs, also known as polychlorinated dibenzo-p-dioxins, are aromatic compounds that are widely found in the environment and are difficult to degrade. PCDDs can originate from various sources, such as incineration facilities for solid waste, mills involved in pulp and paper production, fish that have been contaminated, and soil. The accumulation of PCDDs in the human body can occur through the food chain and biological enrichment, resulting in detrimental impacts on human health due to their extended half-life. Earlier research has indicated that PCDDs can lead to a range of adverse health effects in humans, such as reproductive harm (1), hypertension symptoms (2), coronary heart disease, diabetes, nervous system impairment, disruption of endocrine, immune, and reproductive systems (3), and the development of tumors (4, 5). Nevertheless, limited research has been conducted on the correlation between PCDDs and the mortality rate of cancer.
According to previous studies, occupational exposure to PCDDs can heighten the likelihood of dying from cancer (6–9). According to Li et al. (10), among workers of a municipal solid waste incinerator plant the level of PCDDs in serum due to occupational exposure is 28.0 pg/g lipid. Compared with occupational exposure, non occupational exposure has a lower level of PCDD. Nevertheless, the concentration of serum PCDDs in individuals not occupationally exposed (i.e., the general population) is 8.0 pg/g lipid for males and 9.0 pg/g lipid for females, as reported by Chen et al. in 2005. Whether relatively low PCDD exposure level can increase the risk of cancer mortality needs more research evidence. In a previous study, it was found that PCDDs were linked to an increased risk of cancer death over a period of 4.63 years. The hazard ratio (HR) and its corresponding 95% confidence interval were reported as 1.17 (0.89–1.53), but these findings did not reach statistical significance (11). The short duration of the follow-up period could be the cause of these insignificant findings. Hence, further investigation with long follow-up time is required to determine the of PCDDs non-exposure among the general population and its association with cancer mortality.
In this research, an extended period of observation was utilized to investigate the correlation between PCDDs and the death rate caused by cancer. We examine the correlation between the collective exposure levels of PCDDs and individual PCDD exposure levels, as well as the risk of cancer mortality, by employing a combination of statistical techniques that incorporate exposure models and single exposure models.
The NHANES is a survey conducted by the NCHS that aims to be representative of the entire nation, focusing on health and nutrition. In this study, we used a sample of adults collected from three survey cycles of NHANES from 1999 to 2004 to investigate the association between PCDDs exposures and cancer mortality. More details can be found on the NHANES official website below.1 The inclusion criteria included subjects who underwent PCDDs measurement and those who had mortality data (N = 3,497). The exclusion criteria was participant who were less than 40 years old (N = 505). Ultimately, 2,992 individuals were included in our analysis. A Cox proportional hazards model was generated to estimate the association of PCDDs and genetic factors with all-cause and cancer mortality and to assess the hazard ratio (HR) and 95% confidence interval (CI) with covariants. The restricted cubic spline (RCS) method was performed to investigate the association between PCDDs and cancer mortality. We performed sensitivity analysis guarantees the reliability of the findings. The survey was authorized by the NCHS Research Ethics Review Board. All study participants submitted written informed consent.
High-resolution mass spectrometry (HRMS) was used to detect PCDDs in serum through high-resolution gas chromatography and isotope dilution (ID-HRMS). The specific methods are as follows. The serum samples were supplemented with the internal standard, and then the PCDDs were separated using either C18 solid-phase extraction or liquid–liquid extraction methods. Subsequently, multicolumn automatic purification and enrichment procedures were employed. A Hewlett-Packard 6,890 gas chromatograph was utilized to chromatograph the PCDDs, and the quantification of the chosen PCDDs was done through ID-HRMS using selective ion monitoring (SIM) and in EI mode using either a Micromass AutoSpec ULTIMA or Finnigan MAT95 mass spectrometer. To ensure quality control, the following procedure is implemented: each PCDD concentration is calibrated and calculated using an individual standard curve. Each detection and analysis run was carried out by a blinded method. The makeup of the examined samples was not known, and for detection, the method blank, quality control samples, and serum samples to be tested were utilized. Simultaneously, the overall mass and lipids of every sample were measured and adjusted to determine the PCDD concentration following lipid correction. Table 1 displays the detection rates of the PCDDs, represented as medians and quartiles. Because the distributions of the PCDDs were all skewed, these data were all log 10-transformed in the subsequent statistical analysis.
We obtained relevant mortality data from the NCHS, which can match the study subjects of the public database of consecutive NHANES from 1999 to 2004. These documents provide survey registry mortality data updated as of December 31, 2019. Data on deaths related to NCHS include information on the origin and reason for the deceased’s passing. The guidelines for all causes of death adhered to the 10th edition of the International Classification of Statistics, Diseases, Injuries, and Causes of Death (ICD-10). In accordance with the 10th edition of the International Classification of Diseases (ICD-10), we established the definitions for mortality from any cause and mortality specifically related to cancer (codes c00-c97), based on a prior investigation conducted by Duan et al. (12).
To adjust the results of the Cox regression and RCS analyses, the provided baseline information was utilized as a covariate. The variables that are considered are age, gender, ethnicity, body mass index (BMI) category, and NHANES period. Some variables considered to be possibly related to cancer mortality were also included in covariates, including alcohol consumption, serum cotinine (reflecting smoking), and activity level. Questionnaires are used to gather the aforementioned details, including but not limited to age, gender, race, education level, poverty income ratio (PIR), alcohol consumption, and activity level. BMI levels were calculated by measuring height and weight. Serum cotinine is obtained by detecting cotinine in serum. The intensity of physical activity was obtained through a questionnaire survey and was divided into three categories: none, moderate, and vigorous. Drinking alcohol status was based on the following question: “Have you had at least 12 drinks of any type of alcoholic beverage?” We divided the answers into “No” and “Yes.”
When the data were continuous variables, the baseline information was presented as the average ± deviation from the norm. Percentages are used to present categorical variables. For continuous variables, if they were normality, the t-test were used; if they do not conform to normality, the Wilcoxon test were used. For categorical variables, chi-square test was used. We used Cox proportional hazards models with time in study as the underlying time metric to calculate the hazard ratios and corresponding 95% confidence intervals for cancer mortality in relation to blood PCDDs concentrations. We examined the proportional hazards assumption by creating a cross product of follow-up time and PCDDs levels. Likelihood ratio tests comparing models with and without this variable were not significant, suggesting no departure from the proportional hazards assumption. Continuous PCDD refers to the increased risk of all-cause and cancer mortality for each additional unit of PCDD. The RCS method was used to examine the correlation between PCDDs and cancer mortality in a dose–response relationship. Restricted cubic spline analysis with 3 knots (10th, 50th, and 90th percentiles) was used to examine the nonlinear association of blood PCDDs levels with cancer mortality within the values between the first and 95th percentile to minimize the influence of potential outliers. Nonlinearity was tested using the likelihood ratio test. To ensure the reliability of the findings, sensitivity analyses were performed. One analysis is to remove the PIR variable in the model. The other is to add educational status as a variable in the model. R software (version 4.04) was used to conduct all statistical analyses in this study. Statistical significance was determined if the p value, which was less than 0.05, indicated a two-sided outcome.
The study included a total of 2,992 participants, comprising of 1,461 males and 1,531 females (Table 2). The mean follow-up time was 138.3 months. In total, 1,002 people died, of whom 230 died from cancer. The racial distribution did not differ between males and females (p = 0.835). In women, the occurrence rate of PIR below 1 was greater compared to men, with a p-value less than 0.001. Men had a higher level of smoking exposure, with 36.5% of them having a cotinine frequency greater than 10, compared to 23.5% in women. BMI classification between men and women also has a different distribution. The male vigorous group accounted for 52.9% more than the female group, which had a significant difference (p < 0.001), among the exercise variables. The proportion of males in the drinking group was significantly higher than that of females, with 57.4 and 40.6%, respectively (p < 0.001).
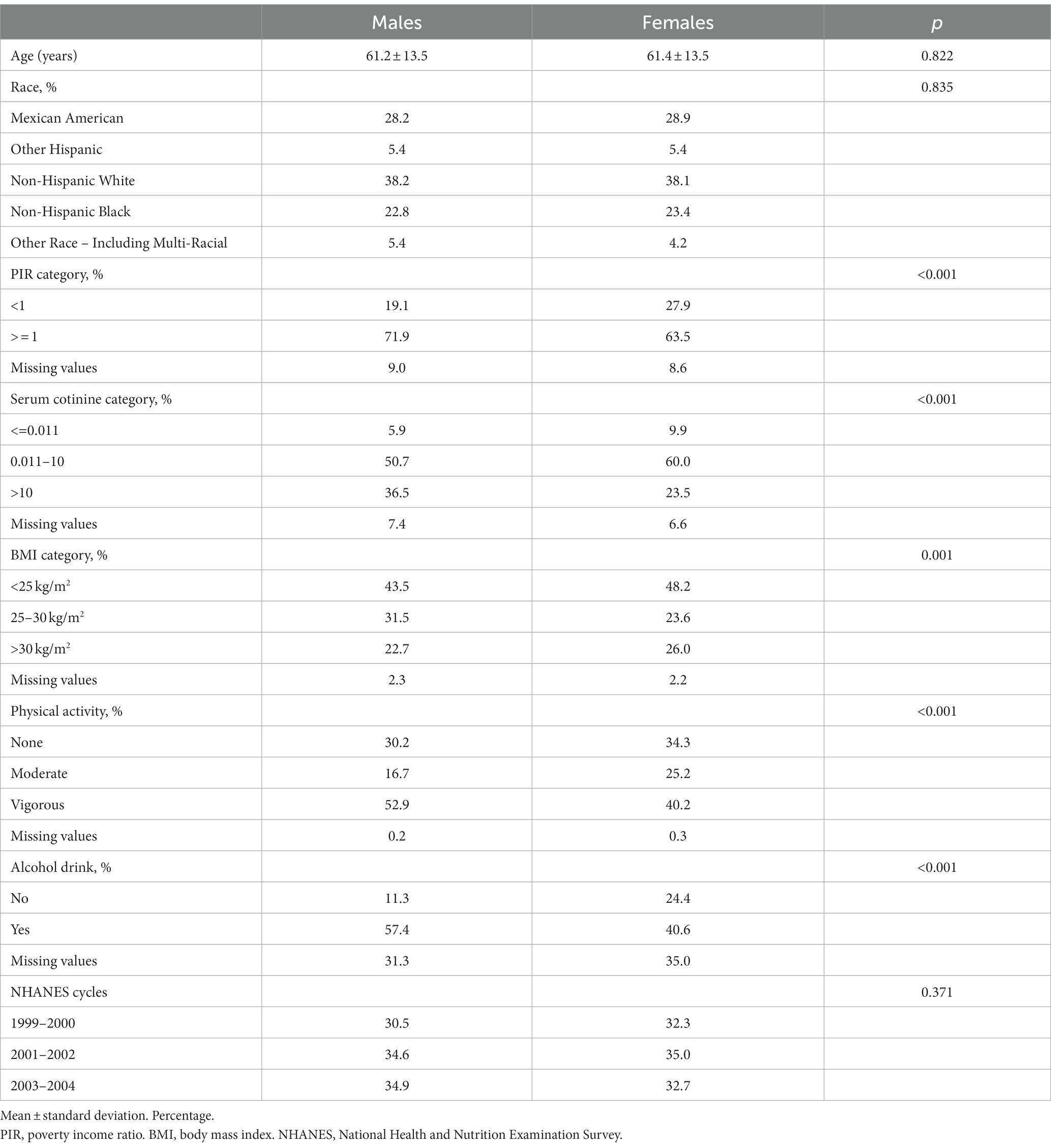
Table 2. Comparison of selected demographic and anthropometric characteristics between males (n = 1,461) and females (n = 1,531) in the NHANES (1999–2004).
In the examination of overall death rate, we discovered that there was no notable distinction in the correlation between the five PCDDs and overall death rate in the findings of model 1 (Table 3). In the second model, there was a correlation observed between a rise of 1 log unit in 1,2,3,4,6,7,8,9-octachlorodibenzo-p-dioxin and all-cause mortality (HR = 1.79, 95% CI 1.22, 2.53, p = 0.003). There was no significant correlation found between the remaining four PCDDs and all-cause mortality. In our examination of cancer death rates, we discovered that there was no notable distinction in the correlation between the five PCDDs and cancer mortality in model 1. In the second model, it was discovered that a rise of 1 log unit in the presence of 1,2,3,4,6,7,8-heptachlorodibenzofuran led to an increased likelihood of cancer-related deaths (HR = 1.98, 95% CI 1.05, 3.43, p = 0.034). The remaining four PCDDs were not statistically associated with cancer mortality.
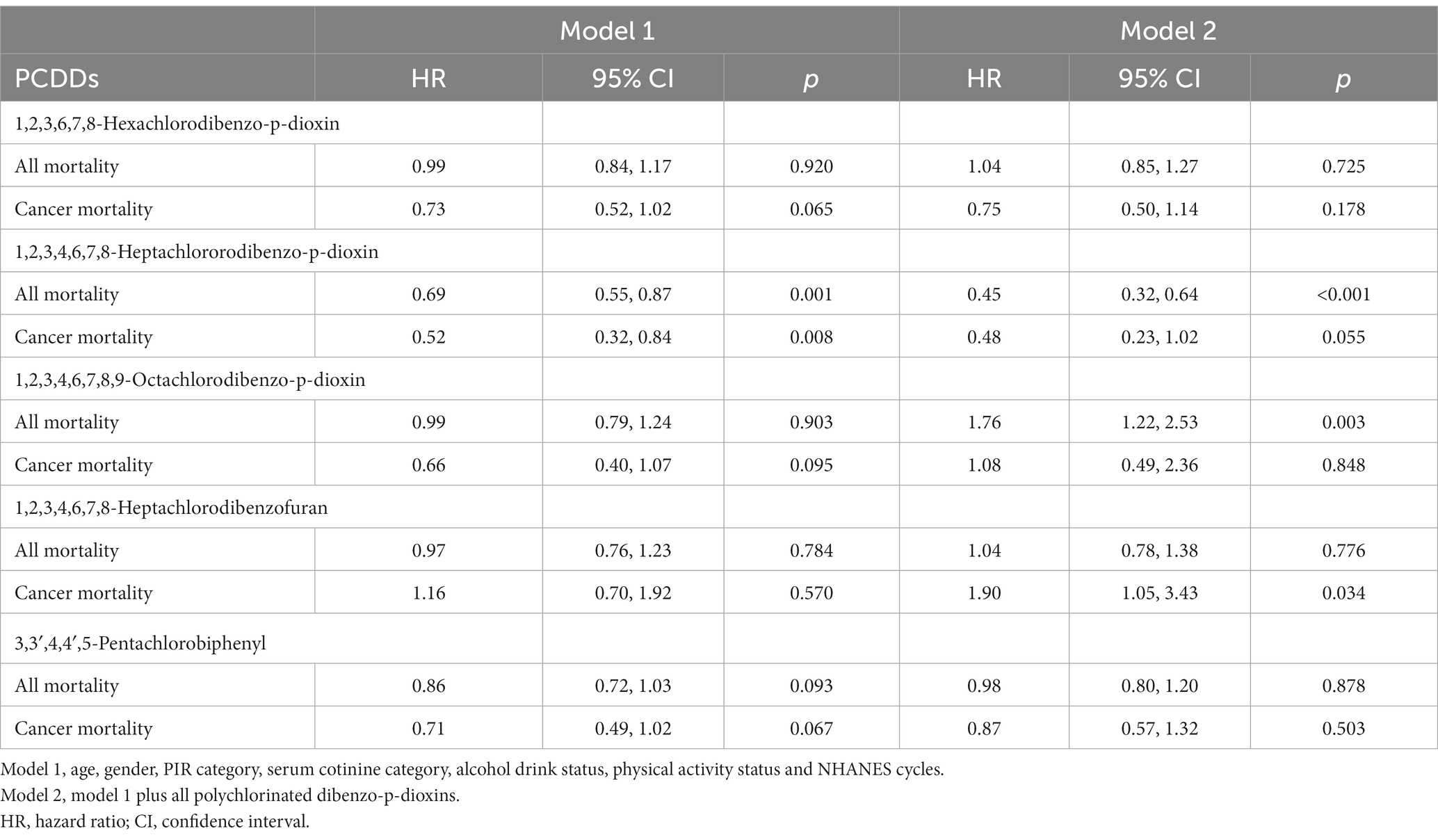
Table 3. The association of polychlorinated dibenzo-p-dioxins (PCDDs) concentration with all-cause mortality, cardiovascular mortality and cancer mortality in NHANES 1999–2004.
Upon analyzing the quartiles of PCDDs, it was determined that there was no heightened risk associated with all-cause mortality for any of the five PCDDs (refer to Table 4). In the findings of the association analysis concerning cancer mortality (Table 5), it was noted that the HR for the uppermost quantile of 1,2,3,4,6,7,8-heptachlorodibenzofuran, in comparison to the lowest quantile, was 2.00 (1.06, 3.79). The HR for the highest quantile of 1,2,3,4,6,7,8-heptachlorodibenzofuran, compared to the lowest quantile, was 2.23 (1.13, 4.40). The other four PCDDs were not statistically associated with cancer mortality risk.
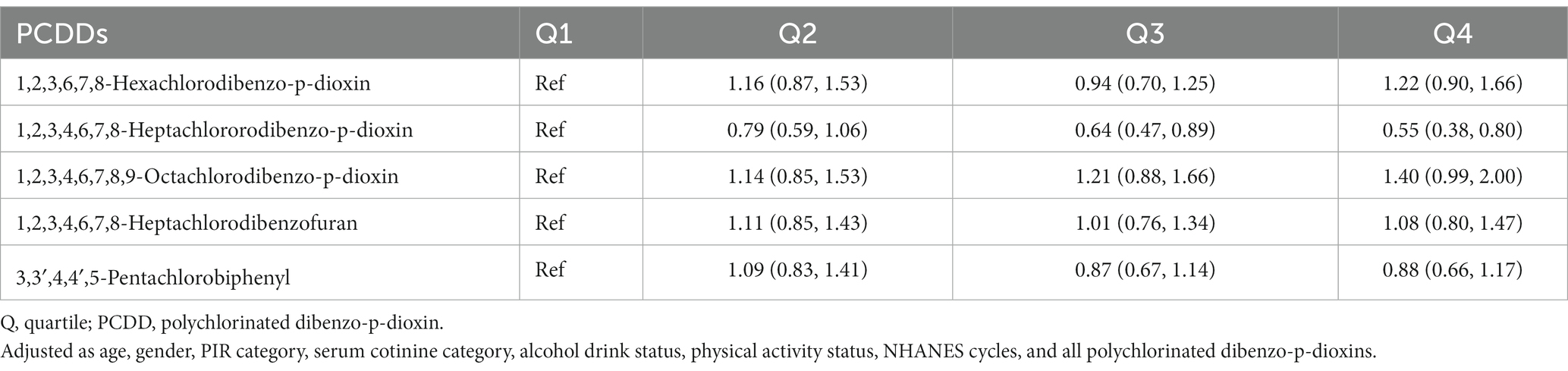
Table 4. The association of quartiles polychlorinated dibenzo-p-dioxins concentration with all-cause mortality in NHANES 1999–2004.
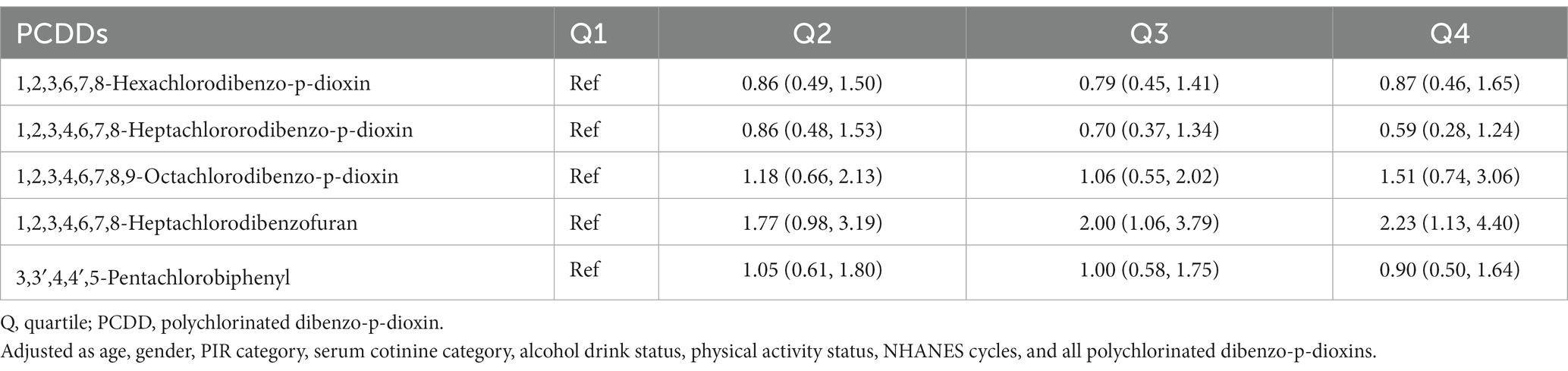
Table 5. The association of quartiles polychlorinated dibenzo-p-dioxins (PCDDs) concentration with cancer mortality in NHANES 1999–2004.
In addition, we examined the correlation between cancer mortality risk and the dosage of 1,2,3,4,6,7,8-heptachlorodibenzofuran using an RCS model (Figure 1). The findings indicated a direct correlation between the compound 1,2,3,4,6,7,8-heptachlorodibenzofuran and the risk of cancer-related deaths, with a Plinear value of 0.044. The above two variables did not exhibit a nonlinear relationship, with a Pnon-linear value of 0.262. Furthermore, we carried out a sensitivity analysis as well. By either eliminating the covariate PIR variable or enhancing the covariate education level, the findings remained consistent, indicating the robustness of our results (Tables 6, 7).
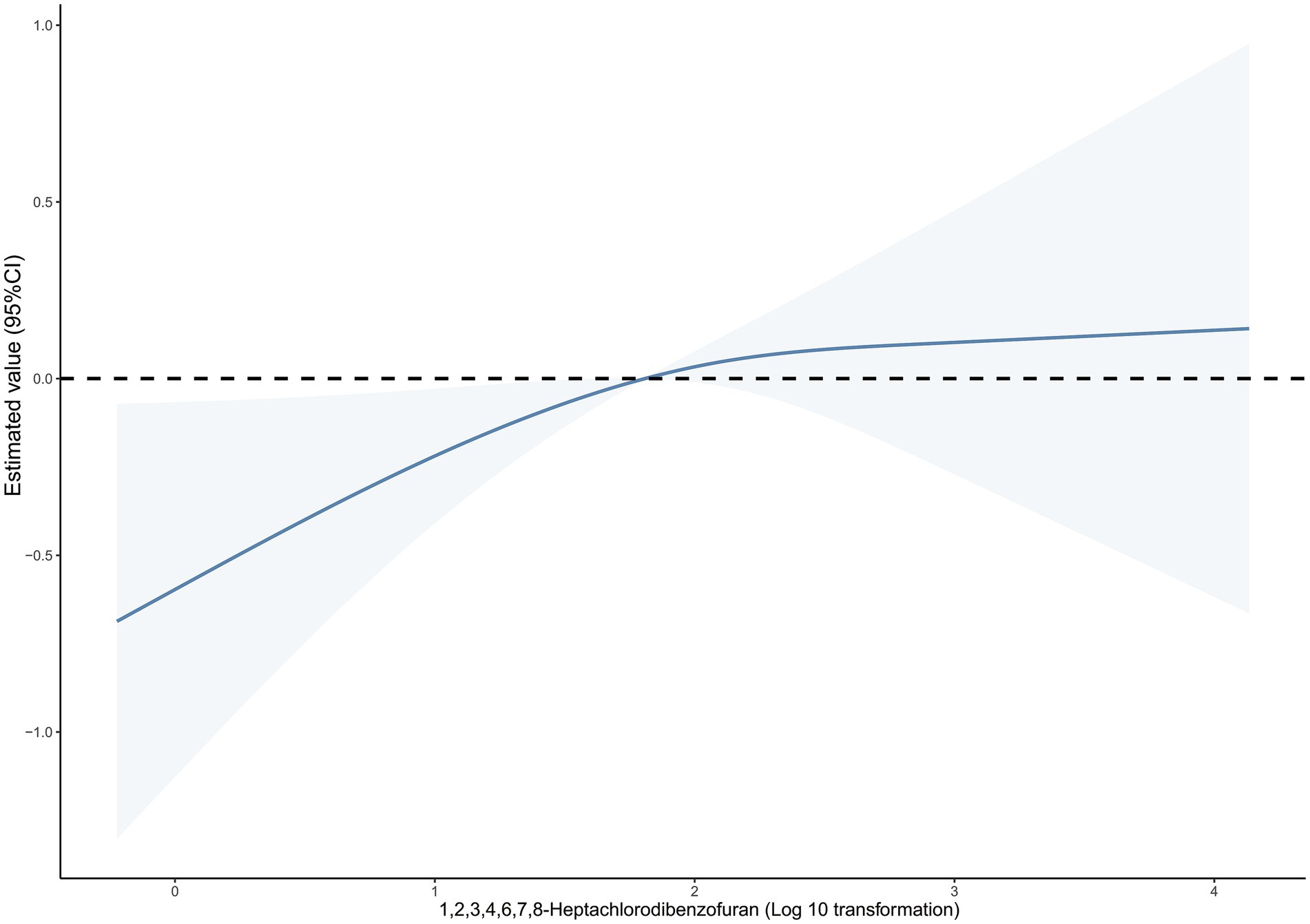
Figure 1. Dose–response relationship between blood 1,2,3,4,6,7,8-Heptachlorodibenzofuran levels and cancer mortality. The solid line and dashed line represent the estimated values and their 95% confidence intervals.
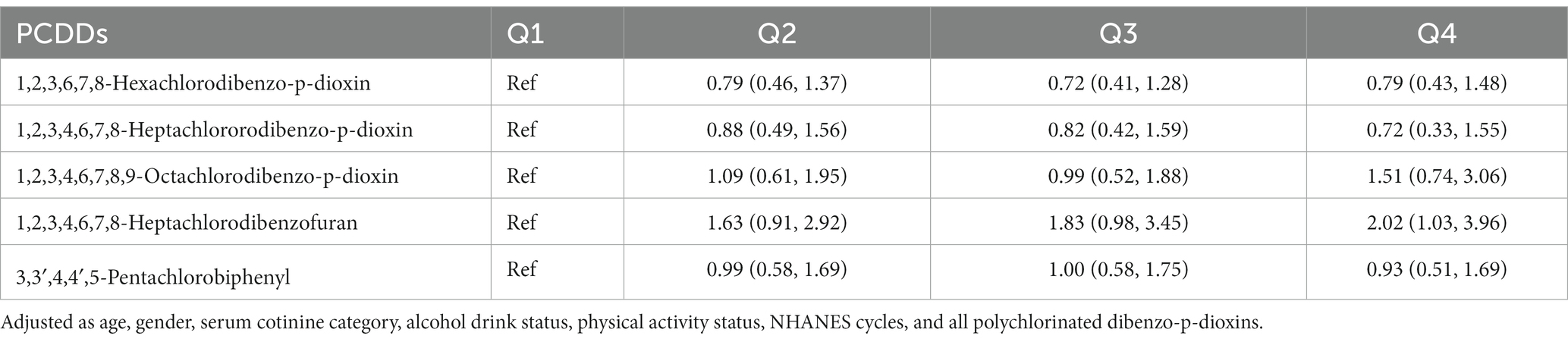
Table 6. The association of quartiles polychlorinated dibenzo-p-dioxins (PCDDs) concentration with cancer mortality in NHANES 1999–2004.
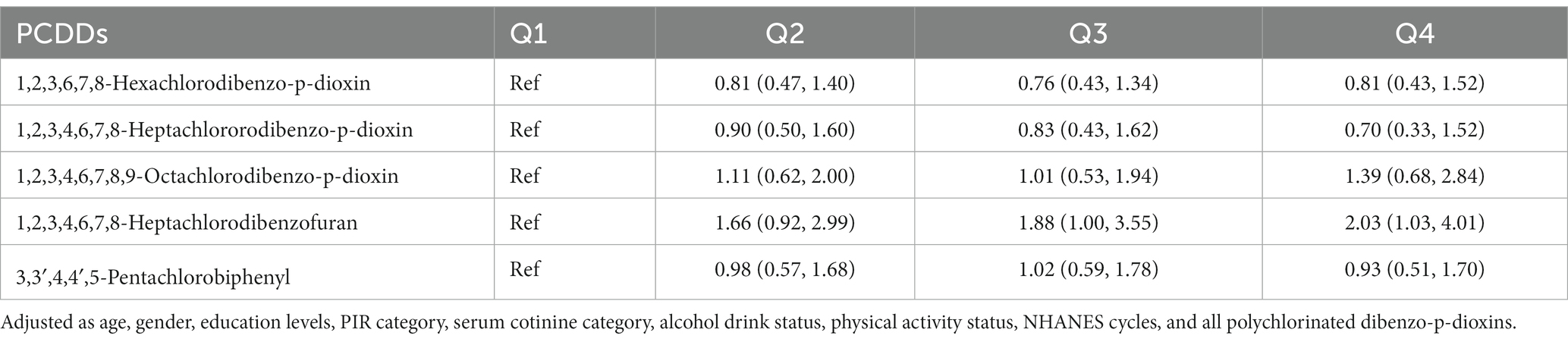
Table 7. The association of quartiles polychlorinated dibenzo-p-dioxins (PCDDs) concentration with cancer mortality in NHANES 1999–2004.
The findings of the current research revealed that higher levels of exposure to 1,2,3,4,6,7,8-heptachlorodibenzofuran, a form of PCDD, were linked to an elevated likelihood of cancer fatality. The risk of cancer mortality increased by 90% with the addition of each extra 1,2,3,4,6,7,8-heptachlorodibenzofuran. In addition, 1,2,3,4,6,7,8-heptachlorodibenzofuran was linearly associated with cancer mortality.
In the general population, our research discovered a correlation between exposure to PCDD and the likelihood of cancer-related deaths. In previous studies, the cancer mortality of environmental chemicals in occupational populations has been widely studied. Occupationally exposed individuals have a higher concentration of chemicals in their bodies compared to the general population. According to Leroyer et al.’s study (13), there was no observed link between cancer mortality and the exposure to various chemicals in the workplace. In the study conducted by Zhivin and colleagues (14), the mortality risk of kidney and bladder cancer due to exposure to hydrazine and other fuels in the population exposed to these substances at work was observed, however, no statistically significant distinction was found. The findings from these occupational exposure categories did not establish a connection between environmental chemicals and the risk of death, indicating that not all occupational exposures to environmental chemicals are linked to mortality risk. There is chemical specificity between environmental chemicals and mortality risk. An elevated amount of dioxin exposure is linked to a rise in mortality rates from bladder cancer (15). Furthermore, a meta-analysis indicated that the exposure to dioxin was linked to a heightened likelihood of cancer fatality (16). Furthermore, being exposed to PCDDs in the workplace can lead to a rise in cancer-related deaths (7, 17). These findings additionally demonstrate that there exists a distinct chemical specificity between environmental substances and the risk of mortality. Previous research in the field of mortality risk among the overall populace has indicated a strong correlation between the element cadmium and tumor fatality (18, 19). It is suggested that PCDD may also have similar risks. In contrast, Lin et al. discovered that PCDD did not have any connection with the likelihood of tumor death among the overall populace (11). Upon enlarging the sample size and extending the duration of follow-up, we discovered a correlation between PCDD and a heightened likelihood of tumor fatality. Different results may be discovered with a substantial sample size and an extended duration of follow-up, as recommended.
This study found that nonoccupational exposure to PCDD can cause an increase in tumor mortality, which may have certain public health significance. Currently, numerous elements contribute to the heightened likelihood of tumor fatality, encompassing cadmium of metallic nature (20), PM2.5 particles (21), and phthalate substances (22). Nevertheless, these factors alone are insufficient to account for tumor mortality, implying that unidentified environmental substances impact tumor mortality. The findings of our research indicate that even a small amount of PCDD can lead to a higher likelihood of tumor-related deaths, making it the first study to establish this connection. Reducing the exposure to PCDD may potentially decrease the risk of tumor mortality. PCDD can be found in various sources that humans are exposed to, such as emissions from industries, outdated pesticide supplies, heating in households, recycling of electronic waste, and the incineration and combustion of household waste (23). To decrease human PCDD exposure, another approach is to minimize the origin of PCDD exposure. Hence, minimizing the contact with chemicals might lead to a decrease in the presence of chemicals within the body. Hence, considering public health, implementing drug intervention and minimizing chemical contact are two approaches to decrease the level of chemicals in the body, which could potentially lower the chances of tumor fatality among the population.
This study has the following advantages. In the initial investigation using the cohort study approach, we discovered, for the very first instance, that among individuals not occupationally exposed, there exists a direct association between combined exposure to PCDDs and tumor mortality. Furthermore, we observed a noteworthy 90% rise in the likelihood of risk. Furthermore, our research revealed a strong correlation between tumor mortality and the presence of 1,2,3,4,6,7,8-heptachlorodibenzofuran in PCDD. This study also has limitations. Firstly, in this study, the origin of PCDD exposure cannot be disclosed initially. Clarifying the exposure source of PCDD can reduce its harm. Secondly, the PCDD exposure level in this study was based on a single determination, which could introduce bias. Therefore, it is necessary to conduct multiple determinations in future studies to establish a reliable and consistent exposure level. Thirdly, varying individuals may possess distinct PCDD metabolism, potentially resulting in diverse health consequences stemming from identical levels of PCDD exposure. This requires adding variables affecting the metabolic level of PCDD in subsequent studies to reduce such bias. Fourthly, it remains uncertain if the findings of this research can be universally applied worldwide. People in different regions have different exposure levels to PCDDs and different life spans. Hence, additional areas and larger sample sizes should be examined. Fifthly, occupational exposure is one of the important reasons affecting PCDD, but due to the lack of occupational information data in NHANES, we can not adjust this variable in the statistical analysis of the present study. This may affect our results, which needs attention in the follow-up study. Sixthly, this study was conducted by sampling, and there was also sampling bias existing. In the discussion part, we gave the biological evidence of potential PCDD causing tumor, which may reduce such bias, but the sampling bias should not be ignored in the future study. Seventhly, previous studies have found that PCDD residues can be partially detected in eggs, mussels, milk and cheese produced around the areas where PCDD industry comes from (24–27). Therefore, the intake of eggs, mussels, milk and cheese produced may be a confounding factor of PCDD, which needs to be collected as a covariate in subsequent studies. Eighthly, dietary exposure is the main source of non occupational exposure to human to exposure to PCDD. Mussels, as a kind of seafood, and PCDD is accumulated in it (25). Eating seafood can reduce the total cause of death, although there is no statistical difference (28). These evidences suggest that the dietary exposure, such as consumption of mussels, may lead to biased results.
This research revealed that PCDD may elevate the likelihood of cancer-related deaths among the overall populace.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The consent form was signed by the survey participants, and the participants consented to storing specimens of their blood for future research. The CDC/NCHS Ethics Review Board approved the NHANES study and gave approval for public dissemination.
LZ: Writing – original draft. XZ: Data curation, Writing – review & editing. ZG: Formal analysis, Writing – original draft. CZ: Validation, Writing – review & editing. DQ: Methodology, Writing – review & editing. QY: Conceptualization, Supervision, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gaspari, L, Paris, F, Kalfa, N, Soyer-Gobillard, MO, Sultan, C, and Hamamah, S. Experimental evidence of 2,3,7,8-Tetrachlordibenzo-p-dioxin (TCDD) transgenerational effects on reproductive health. Int J Mol Sci. (2021) 22:9091. doi: 10.3390/ijms22169091
2. Pelclova, D, Urban, P, Preiss, J, Lukas, E, Fenclova, Z, Navratil, T, et al. Adverse health effects in humans exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Rev Environ Health. (2006) 21:119–38. doi: 10.1515/reveh.2006.21.2.119
3. Dai, Q, Xu, X, Eskenazi, B, Asante, KA, Chen, A, Fobil, J, et al. Severe dioxin-like compound (DLC) contamination in e-waste recycling areas: an under-recognized threat to local health. Environ Int. (2020) 139:105731. doi: 10.1016/j.envint.2020.105731
4. Leng, L, Chen, X, Li, CP, Luo, XY, and Tang, NJ. 2,3,7,8-Tetrachlorodibezo-p-dioxin exposure and prostate cancer: a meta-analysis of cohort studies. Public Health. (2014) 128:207–13. doi: 10.1016/j.puhe.2013.10.006
5. van Gerwen, M, Vasan, V, Genden, E, and Saul, SR. Human 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure and thyroid cancer risk. Toxicology. (2023) 488:153474. doi: 10.1016/j.tox.2023.153474
6. Fingerhut, MA, Halperin, WE, Marlow, DA, Piacitelli, LA, Honchar, PA, Sweeney, MH, et al. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med. (1991) 324:212–8. doi: 10.1056/NEJM199101243240402
7. Flesch-Janys, D, Berger, J, Gurn, P, Manz, A, Nagel, S, Waltsgott, H, et al. Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. Am J Epidemiol. (1995) 142:1165–75. doi: 10.1093/oxfordjournals.aje.a117575
8. Manz, A, Berger, J, Dwyer, JH, Flesch-Janys, D, Nagel, S, and Waltsgott, H. Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet. (1991) 338:959–64. doi: 10.1016/0140-6736(91)91835-i
9. Warner, M, Mocarelli, P, Samuels, S, Needham, L, Brambilla, P, and Eskenazi, B. Dioxin exposure and cancer risk in the Seveso Women’s health study. Environ Health Perspect. (2011) 119:1700–5. doi: 10.1289/ehp.1103720
10. Li, M, Tang, B, Zheng, J, Ma, S, Zhuang, X, Wang, M, et al. PCDD/fs in paired hair and serum of workers from a municipal solid waste incinerator plant in South China: concentrations, correlations, and source identification. Environ Int. (2020) 144:106064. doi: 10.1016/j.envint.2020.106064
11. Lin, YS, Caffrey, JL, Hsu, PC, Chang, MH, Faramawi, MF, and Lin, JW. Environmental exposure to dioxin-like compounds and the mortality risk in the U.S. population. Int J Hyg Environ Health. (2012) 215:541–6. doi: 10.1016/j.ijheh.2012.02.006
12. Duan, W, Xu, C, Liu, Q, Xu, J, Weng, Z, Zhang, X, et al. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: a population-based cohort study. Environ Pollut. (2020) 263:114630. doi: 10.1016/j.envpol.2020.114630
13. Leroyer, A, Gomajee, H, Leroy, R, Mazzuca, M, Leleu, B, and Nisse, C. Cancer mortality and chemical exposure in a retrospective zinc and lead smelter cohort: a 48-year follow-up. Int J Hyg Environ Health. (2022) 242:113955. doi: 10.1016/j.ijheh.2022.113955
14. Zhivin, S, Laurier, D, Caer-Lorho, S, Acker, A, and Guseva, CI. Impact of chemical exposure on cancer mortality in a French cohort of uranium processing workers. Am J Ind Med. (2013) 56:1262–71. doi: 10.1002/ajim.22231
15. Mossanen, M, Kibel, AS, and Goldman, RH. Exploring exposure to agent Orange and increased mortality due to bladder cancer. Urol Oncol. (2017) 35:627–32. doi: 10.1016/j.urolonc.2017.07.030
16. Xu, J, Ye, Y, Huang, F, Chen, H, Wu, H, Huang, J, et al. Corrigendum: association between dioxin and cancer incidence and mortality: a meta-analysis. Sci Rep. (2017) 7:41665. doi: 10.1038/srep41665
17. Wang, L, Weng, S, Wen, S, Shi, T, Sun, G, Zeng, Y, et al. Polychlorinated dibenzo-p-dioxins and dibenzofurans and their association with cancer mortality among workers in one automobile foundry factory. Sci Total Environ. (2013) 443:104–11. doi: 10.1016/j.scitotenv.2012.10.073
18. Fan, Y, Tao, C, Li, Z, Huang, Y, Yan, W, Zhao, S, et al. Association of Endocrine-Disrupting Chemicals with all-cause and cause-specific mortality in the U.S.: a prospective cohort study. Environ Sci Technol. (2023) 57:2877–86. doi: 10.1021/acs.est.2c07611
19. Li, Z, Fan, Y, Tao, C, Yan, W, Huang, Y, Qian, H, et al. Association between exposure to cadmium and risk of all-cause and cause-specific mortality in the general US adults: a prospective cohort study. Chemosphere. (2022) 307:136060. doi: 10.1016/j.chemosphere.2022.136060
20. Larsson, SC, and Wolk, A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: systematic review and meta-analysis of cohort studies. Int J Epidemiol. (2016) 45:782–91. doi: 10.1093/ije/dyv086
21. Yu, P, Xu, R, Li, S, Coelho, M, Saldiva, PHN, Sim, MR, et al. Associations between long-term exposure to PM(2.5) and site-specific cancer mortality: a nationwide study in Brazil between 2010 and 2018. Environ Pollut. (2022) 302:119070. doi: 10.1016/j.envpol.2022.119070
22. Ahern, TP, Broe, A, Lash, TL, Cronin-Fenton, DP, Ulrichsen, SP, Christiansen, PM, et al. Phthalate exposure and breast Cancer incidence: a Danish Nationwide cohort study. J Clin Oncol. (2019) 37:1800–9. doi: 10.1200/JCO.18.02202
23. Ssebugere, P, Sillanpaa, M, Matovu, H, and Mubiru, E. Human and environmental exposure to PCDD/fs and dioxin-like PCBs in Africa: a review. Chemosphere. (2019) 223:483–93. doi: 10.1016/j.chemosphere.2019.02.065
24. Giannico, OV, Baldacci, S, Basile, FC, Pellegrino, A, Desiante, F, Franco, E, et al. PCDD/fs and PCBs in hen eggs from a contaminated area in Italy: a 9 years spatio-temporal monitoring study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2023) 40:294–304. doi: 10.1080/19440049.2022.2157051
25. Giannico, OV, Baldacci, S, Desiante, F, Basile, FC, Franco, E, Fragnelli, GR, et al. PCDD/fs and PCBs in Mytilus galloprovincialis from a contaminated area in Italy: the role of mussel size, temperature and meteorological factors. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2022) 39:1123–35. doi: 10.1080/19440049.2022.2059108
26. Giannico, OV, Fragnelli, GR, Baldacci, S, Desiante, F, Pellegrino, A, Basile, FC, et al. Dioxins and PCBs contamination in milk and dairy products from province of Taranto (Puglia region, southern Italy): a six years spatio-temporal monitoring study. Ann Ist Super Sanita. (2021) 57:233–8. doi: 10.4415/ANN_21_03_06
27. Giannico, OV, Desiante, F, Basile, FC, Franco, E, Baldacci, S, Fragnelli, GR, et al. Dioxins and PCBs contamination in mussels from Taranto (Ionian Sea, Southern Italy): a seven years spatio-temporal monitoring study. Ann Ist Super Sanita. (2020) 56:452–61. doi: 10.4415/ANN_20_04_07
Keywords: polychlorinated dibenzo-p-dioxins, NHANES, cancer mortality, general population, restricted cubic spline (RCS)
Citation: Zheng L, Zhang X, Gao Z, Zhong C, Qiu D and Yan Q (2024) The association between polychlorinated dibenzo-p-dioxin exposure and cancer mortality in the general population: a cohort study. Front. Public Health. 12:1354149. doi: 10.3389/fpubh.2024.1354149
Received: 12 December 2023; Accepted: 02 February 2024;
Published: 12 February 2024.
Edited by:
Renata Sisto, National Institute for Insurance Against Accidents at Work (INAIL), ItalyReviewed by:
Orazio Valerio Giannico, Local Health Authority of Taranto, ItalyCopyright © 2024 Zheng, Zhang, Gao, Zhong, Qiu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Yan, eXEzODgwNzUyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.