
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 20 June 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1353415
This article is part of the Research Topic Public Risk Perception in Public Health Policies View all 25 articles
 Wei-Hua Hu1,2,3
Wei-Hua Hu1,2,3 Huan-Le Cai4
Huan-Le Cai4 Huan-Chang Yan4
Huan-Chang Yan4 Han Wang4
Han Wang4 Hui-Min Sun1,2,3
Hui-Min Sun1,2,3 Yong-Yue Wei1,2,3
Yong-Yue Wei1,2,3 Yuan-Tao Hao1,2,3*
Yuan-Tao Hao1,2,3*Background: The protective effectiveness provided by naturally acquired immunity against SARS-CoV-2 reinfection remain controversial.
Objective: To systematically evaluate the protective effect of natural immunity against subsequent SARS-CoV-2 infection with different variants.
Methods: We searched for related studies published in seven databases before March 5, 2023. Eligible studies included in the analysis reported the risk of subsequent infection for groups with or without a prior SARS-CoV-2 infection. The primary outcome was the overall pooled incidence rate ratio (IRR) of SARS-CoV-2 reinfection/infection between the two groups. We also focused on the protective effectiveness of natural immunity against reinfection/infection with different SARS-CoV-2 variants. We used a random-effects model to pool the data, and obtained the bias-adjusted results using the trim-and-fill method. Meta-regression and subgroup analyses were conducted to explore the sources of heterogeneity. Sensitivity analysis was performed by excluding included studies one by one to evaluate the stability of the results.
Results: We identified 40 eligible articles including more than 20 million individuals without the history of SARS-CoV-2 vaccination. The bias-adjusted efficacy of naturally acquired antibodies against reinfection was estimated at 65% (pooled IRR = 0.35, 95% CI = 0.26–0.47), with higher efficacy against symptomatic COVID-19 cases (pooled IRR = 0.15, 95% CI = 0.08–0.26) than asymptomatic infection (pooled IRR = 0.40, 95% CI = 0.29–0.54). Meta-regression revealed that SARS-CoV-2 variant was a statistically significant effect modifier, which explaining 46.40% of the variation in IRRs. For different SARS-CoV-2 variant, the pooled IRRs for the Alpha (pooled IRR = 0.11, 95% CI = 0.06–0.19), Delta (pooled IRR = 0.19, 95% CI = 0.15–0.24) and Omicron (pooled IRR = 0.61, 95% CI = 0.42–0.87) variant were higher and higher. In other subgroup analyses, the pooled IRRs of SARS-CoV-2 infection were statistically various in different countries, publication year and the inclusion end time of population, with a significant difference (p = 0.02, p < 0.010 and p < 0.010), respectively. The risk of subsequent infection in the seropositive population appeared to increase slowly over time. Despite the heterogeneity in included studies, sensitivity analyses showed stable results.
Conclusion: Previous SARS-CoV-2 infection provides protection against pre-omicron reinfection, but less against omicron. Ongoing viral mutation requires attention and prevention strategies, such as vaccine catch-up, in conjunction with multiple factors.
• The efficacy of naturally immunity against reinfection was estimated at 65% (IRR = 0.35, 95% confidence interval (CI) = 0.26–0.47).
• For different SARS-CoV-2 variant, the pooled IRRs for the Alpha (IRR = 0.11), Delta (IRR = 0.19) and Omicron (IRR = 0.61) variant means a progressively lower protective effectiveness.
SARS-CoV-2 has evolved into many variants since its initial outbreak in 2019, and the WHO has identified the Alpha, Beta, Gamma, Delta, and Omicron variants as variations of concern (VOCs). The Beta and Delta variants are distinguished by specific combinations of unique mutations, which can potentially lead to structural and functional abnormalities (1). Studies have demonstrated that these variants are associated with a higher risk compared to the Alpha and Gamma variants, as shown by a higher hospitalization rate, severity of illness, and mortality (2). Moreover, the Omicron variant emerged in late November 2021 and possesses a significantly higher number of mutations in the Spike protein compared to the afore-mentioned VOCs, surpassing them by 3–4 times (3). Consequently, the highly contagious Omicron variant quickly became the dominant strain and widespread around the world (4, 5). This, in conjunction with the gradual relaxation of strict COVID-19 control measures, led to a SARS-CoV-2 infection peak at the end of 2022 (6).
To date, the vast majority of the world’s population has been infected with SARS-CoV-2 at least once, and the issue of reinfection has become a concern. Although most people have received a COVID-19 booster vaccination, the ability of vaccines to protect against infection of Omicron is still controversial due to its great number of mutations in the spike protein, which led to antigen escape (7). Besides, studies have shown that the neutralization titer induced by previous vaccination would drop significantly after 6 months of vaccination (8) and could not be detected after 1 year (9). In such cases, the immunity built up after natural infection may be a key aspect to fight against reinfection.
With the emergence of new variants of SARS-CoV-2, there has been a significant increase in reinfection rates. For example, a meta-analysis revealed an overall reinfection rate of 0.97% [95% confidence interval (CI) = 0.71–1.27%]. However, studies providing specific data on the Alpha wave showed a reinfection rate of 0.57% (95% CI: 0.28–0.94%), which rose to 1.25% (95% CI: 0.97–1.55%) with the Delta strain, and peaked to 3.31% (95% CI: 1.15–6.53%) during the first 3 months of the Omicron wave (10). These findings suggest that the Omicron variant has a strong ability to evade immunity from previous infections (11). Correspondingly, the protection of the immunity acquired by natural infection against reinfection gradually declined with the evolution of the variants. Studies have indicated an estimated protective effect of over 82% against Alpha, Beta, and Delta variants reinfection (12, 13), whereas the protection against reinfection of the Omicron variant from previous infection was significantly reduced to 45.3%. Moreover, it will continue to decline over time (12, 14), which would last for about 5–12 months (15).
The objective of this meta-analysis was to systematically evaluate the protective effect of natural immunity against SARS-CoV-2 reinfection (both symptomatic and asymptomatic) and its trend over time. We also conducted subgroup analysis to explore divergences of natural immunity in different variants, study population, and age groups. Compared with previous relevant studies, the present study included the most recent studies up to March 5, 2023, and in particular included more studies on Omicron; and evaluated evidence from cohort studies that included only unvaccinated populations to focus on the impact of natural immunity.
We systematically searched for the relevant literature published before 5 Mar 2023 in seven databases, including four peer-reviewed databases (PubMed, Embase, Web of Science and Scopus) and three preprint platforms (medRxiv, bioRxiv, and Europe PMC). Key search terms included the following: SARS-CoV-2, natural infection, protection and reinfection. The full search strategy was described in Supplementary Table S1. A secondary reference search on all eligible studies and relevant review articles was also conducted (10, 13, 16–21). We used EndNote X8.2 (Thomson Research Soft, Stanford, CA, United States) to manage records, screen, and exclude duplicates. This study was followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, Supplementary Table S2) (22), and had been registered at PROSPERO (Registration number: CRD42023405080).
Inclusion and exclusion criteria were shown in Table 1. All retrieved publications were independently assessed by two investigators according to the below criteria, and any inconsistencies were resolved by agreement in consultation with a third investigator.
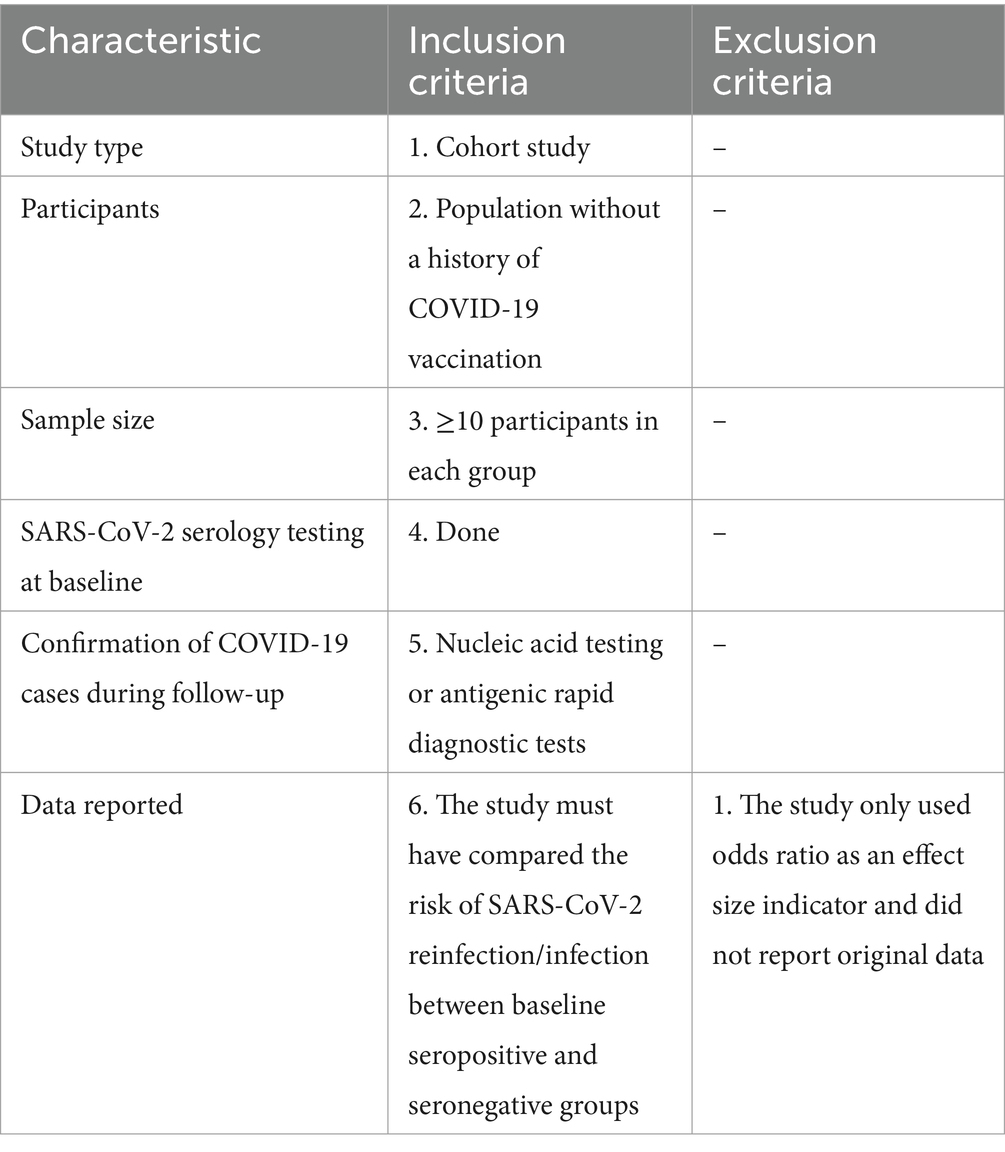
Table 1. Inclusion and exclusion criteria for this systematic review and meta-analysis about protective effectiveness of previous infection against subsequent SARS-COV-2 infection in the world from 2020 to 2022.
A standardized electronic data collection form will be used to extract the following data from included studies: (1) literature information (i.g., study title, first author, title, publication or preprint date), (2) study details (e.g., study location, study population, demographic characteristics of the study population, SARS-CoV-2 variant, sample sizes, the date of study start and end, follow up time, effect measure, the type of target antibodies, the reinfection/infection cases in baseline seropositive or seronegative groups, the definition of reinfection, whether researchers attempted to adjust for any potential covariates, IRRs and 95% CI). We calculated the IRR by constructing a 2 × 2 contingency table for those study in which the IRR was not reported directly. We used the Newcastle–Ottawa quality assessment scale to evaluate the risk of bias of the included cohort studies. A score of 0–3 stars was considered a low-quality study, a score of 4–6 stars was considered a moderate-quality study, and a score of 7–9 stars was considered a high-quality study. Data extraction and quality assessment was conducted independently by two investigators and checked by a third investigator, and disagreements were resolved through discussion.
We performed a meta-analysis to estimate the pooled incidence rate ratio (IRR) and its 95% CI for estimating the risk of subsequent infection between the baseline seropositive and seronegative groups. The primary outcome was the risk of SARS-CoV-2 reinfection/infection between the two groups, while the second outcome was the risk of symptomatic and asymptomatic SARS-CoV-2 reinfection/infection between the two groups. A suitable model (Fixed-effects or random-effects model) was used to pool the rates across studies separately, based on the heterogeneity between estimates which was evaluated by using the I-squared (I2) (23). Fixed-effects models would be used if I2 ≤ 50%, which represents low to moderate heterogeneity, and random-effects models would be used if I2 ≥ 50%, representing substantial heterogeneity. We performed meta-regression to explore between-study heterogeneity.
Subgroup analyses of the primary outcome were performed in the following groups: SARS-CoV-2 variant (Alpha, Delta, and Omicron), definition of reinfection (two positive SARS-CoV-2 PCR test results at least 60 or 90 days apart), population (HCWs or general population), age (<60 years old or ≥60 years old, <55 years old or ≥55 years old), country, publication year (2020, 2021, or 2022), inclusion end time of population (every 6 months from 2020 to 2022), and study quality (moderate or high). The classification criteria for each subgroup are described in the Supplementary Table S3. Bubble plots were used to explore trends in the immune protection acquired from natural infection with COVID-19. We used funnel plots and Begg’s test to examine the potential for publication bias. If the results are suggestive of publication bias, we will further provide bias-adjusted results using trim-and-fill, a non-parametric method based on examining the funnel plot’s asymmetry. We conducted sensitivity analysis with the one-study-at-a-time method adopted for assessing the reliability of the results. All statistical analyses were conducted using meta libraries in R 4.0.5.
A total of 9,537 relevant records were identified, of which 1,119 duplicate records were removed. Eight thousand, four hundred eighteen article titles and abstracts were screened and 117 underwent full-text review. Finally, 40 unique articles reporting data for 52 studies were included in this meta-analysis (Figure 1). After a secondary reference search of all eligible studies and relevant review articles, no new studies were included. The 40 eligible articles included more than 20 million COVID-19 unvaccinated individuals without the history of COVID-19 vaccination. The sample sizes of the included studies ranged from 209 to 8,901,064 (median: 15075). Among the 40 unique articles, 11 studies were conducted in the United States, 9 in the United Kingdom, four in Switzerland, three in Qatar, two each in Sweden, Nicaragua, Italy and Israel, and one each in Austria, Bangladesh, Denmark, France and India. The mean/median ages of the enrolled participants were mostly less than 60 years old, with only two studies reporting median age over 60 years old. The study populations mainly included the general population, HCWs, care home residents and staffs, and hemodialysis patients. The included studies initiated between January 2020 and September 2021, and the length of the follow-up time ranged from 1.47 to 24.07 months. Different studies have used different window periods between positive PCR tests and baseline seropositive or previous RNA-positive results in defining reinfection. This is due to the fact that most studies were initiated in the early stages of the COVID-19 pandemic, when the persistence of SARS-CoV-2 RNA was not clearly understood. Of the included studies, 23 defined reinfection as two positive SARS-CoV-2 PCR test results at least 90 days apart, and 4 defined reinfection as two positive SARS-CoV-2 PCR test results at least 60 days apart, 1 study each defined reinfection as two positive SARS-CoV-2 PCR tests separated by a period of 270 or 28 days, and the remainder of the studies did not report a specific definition of reinfection. The quality score of study according to the NOS ranged from 4 to 9, with 14 studies of high quality, 26 studies of moderate quality, and none of low quality (Supplementary Table S4). The main characteristics of 40 eligible studies were summarized in Table 2.
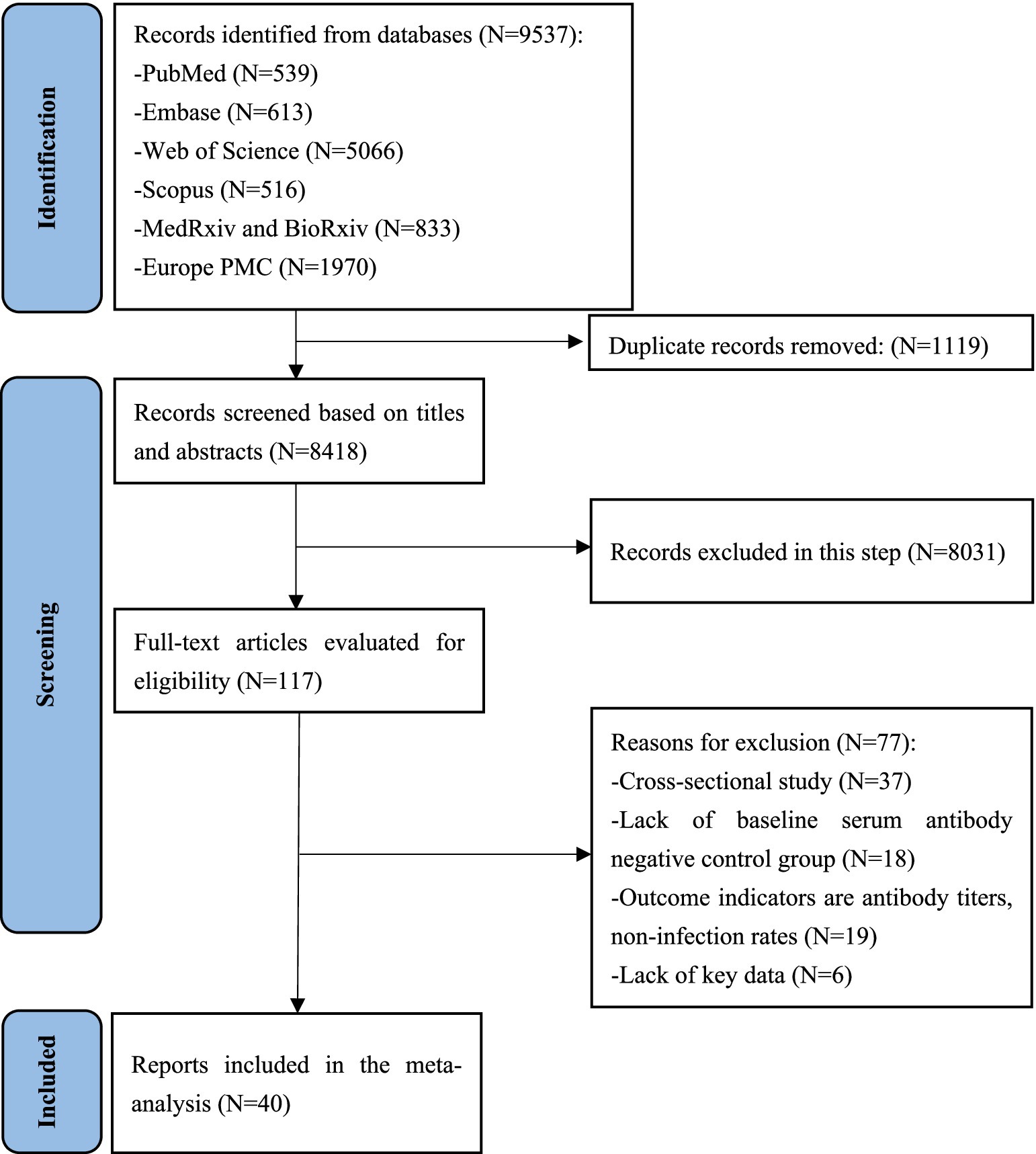
Figure 1. PRISMA flow diagram for this systematic review and meta-analysis about protective effectiveness of previous infection against subsequent SARS-COV-2 infection in the world from 2020 to 2022.
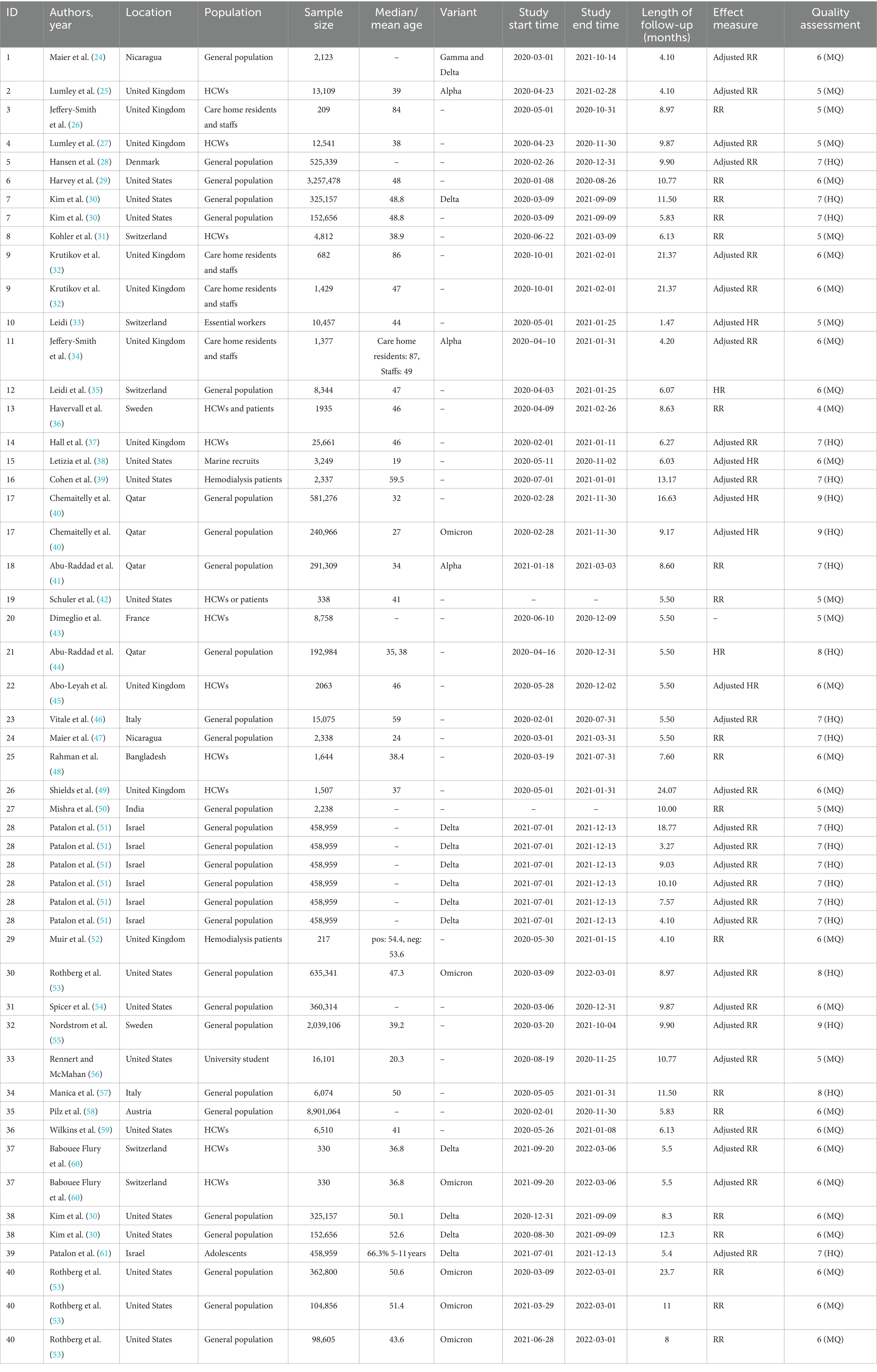
Table 2. Description of included studies in this systematic review and meta-analysis about protective effectiveness of previous infection against subsequent SARS-COV-2 infection in the world from 2020 to 2022.
The asymmetry in funnel plot and the result of Begg’s test suggested a possible publication bias in the included studies (p < 0.05), so we adopted the trim-and-fill method. The funnel plot for publication bias before and after trimming and filling were shown in Supplementary Figure S3. The pooled results for the protection of naturally acquired antibodies against future SARS-CoV-2 infection after using the trim-and-fill method were shown in Figure 2, while the original results without the trim-and-fill method were shown in Supplementary Figure S1. Adopting random effect meta-analysis models, we observed significant protection against SARS-CoV-2 reinfection in the seropositive population compared with seronegative individuals (pooled IRR = 0.35, 95% CI = 0.26–0.47). The original pooled IRRs without the trim-and-fill method was 0.19 (95% CI = 0.15–0.23). In the sensitivity analysis for the original result, the pooled IRRs of remaining studies ranges from 0.15–0.24 after removing any one of the studies, which suggested the good reliability of the pooled IRR (Supplementary Figure S2).

Figure 2. Forest plot of the pooled incidence rate ratio for SARS-CoV-2 infection comparing baseline seropositive and seronegative individuals (trim-and-fill method).
For secondary outcome, 12 studies reported the protection of the antibodies induced by a previous infection against future symptomatic between baseline seropositive and seronegative groups while there were 10 studies for asymptomatic reinfections. Natural infections of SARS-CoV-2 provided a lower level of protection against asymptomatic infection (pooled IRR = 0.40, 95% CI = 0.29–0.54) than symptomatic COVID-19 cases (pooled IRR = 0.15, 95% CI = 0.08–0.26) (Figure 3).
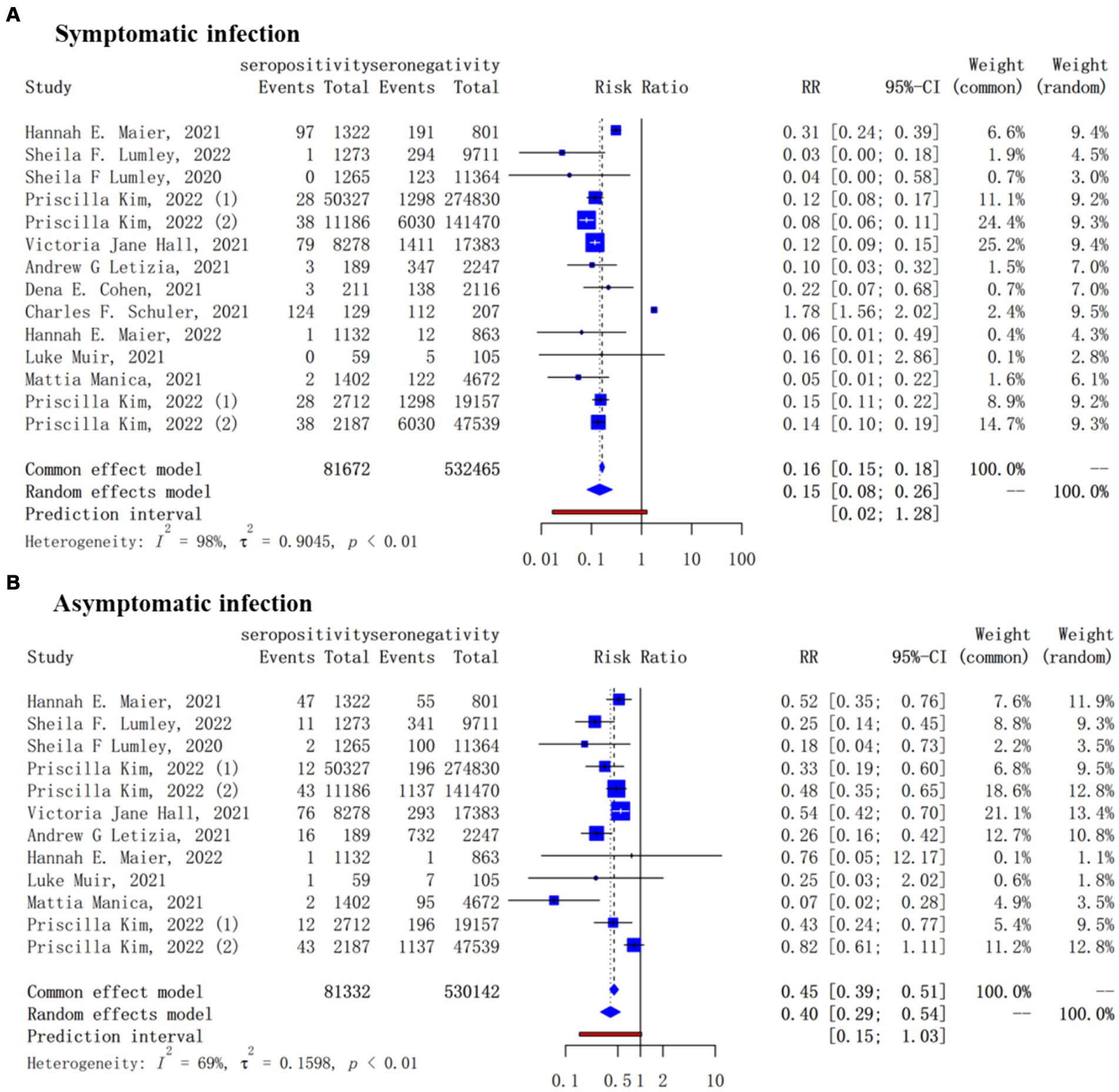
Figure 3. Forest plot of the protection provided by naturally acquired antibodies against future symptomatic (A) and asymptomatic (B) COVID-19 between baseline seropositive and seronegative individuals.
Meta-regression revealed that SARS-CoV-2 variant was a statistically significant effect modifier, which explaining 46.40% of the variation in IRRs. The subgroup analysis for different SARS-CoV-2 variant showed that the pooled IRRs for the Alpha (pooled IRR = 0.11, 95% CI = 0.06–0.19), Delta (pooled IRR = 0.19, 95% CI = 0.15–0.24) and Omicron (pooled IRR = 0.61, 95% CI = 0.42–0.87) variant were higher and higher, that is, the protection of natural infection for reinfection against these variants was progressively lower (Figure 4).
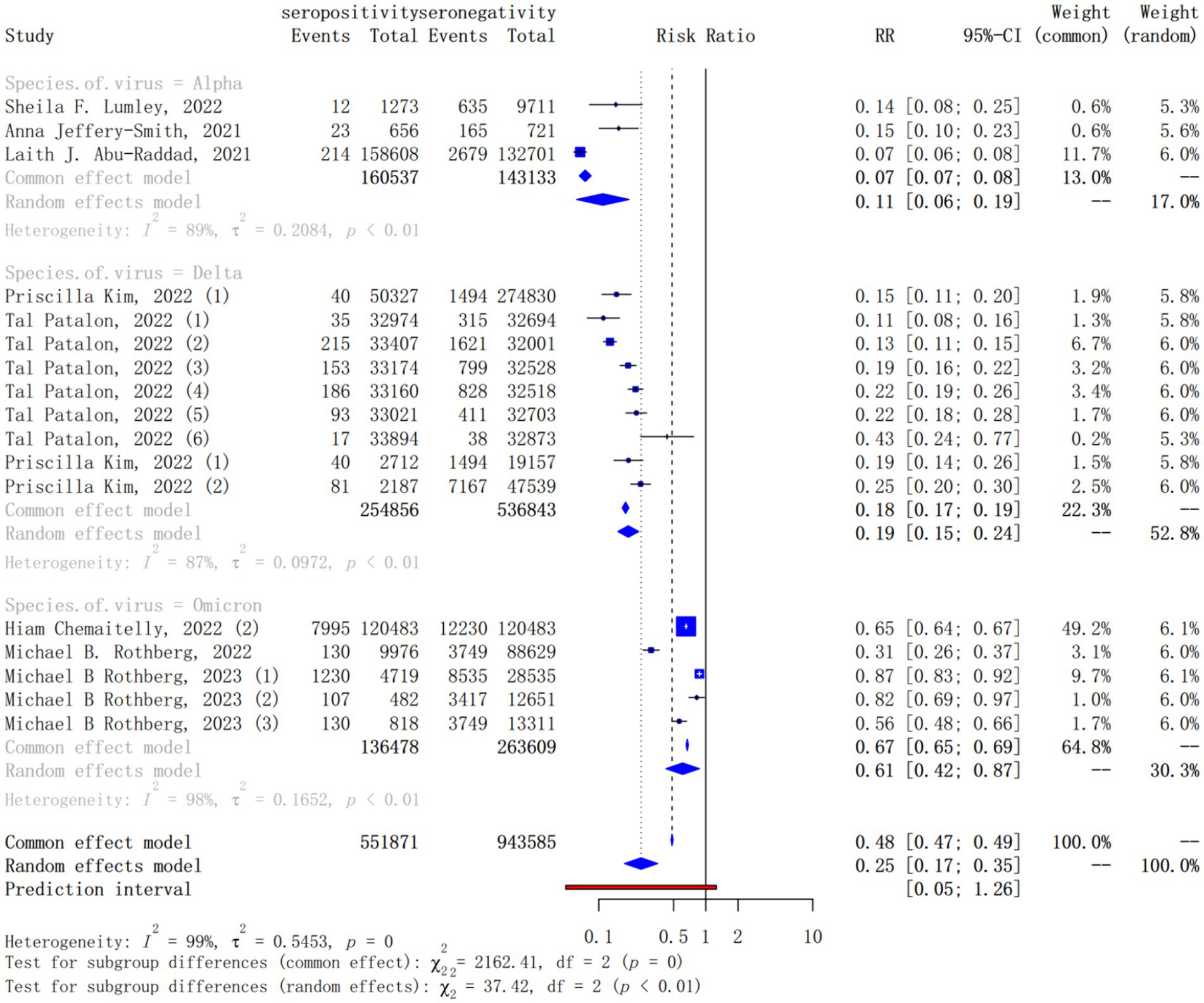
Figure 4. Forest plot of the pooled incidence rate ratio for different SARS-CoV-2 variant infection comparing baseline seropositive with seronegative individuals.
In other subgroup analyses, statistically significant differences were observed in the subgroup analysis of the country (the pooled IRR = 0.20, 95% CI = 0.16–0.25, p = 0.02, Supplementary Figure S6), the publication year (the pooled IRR = 0.19, 95% CI = 0.15–0.23, p < 0.010, Supplementary Figure S8–1) and the inclusion end time of population (the pooled IRR = 0.20, 95% CI = 0.16–0.24, p < 0.010, Supplementary Figure S8–2). In different countries, Nicaragua was found a lower level of protection against reinfection (pooled IRR = 0.31, 95% CI = 0.07–1.43), while Italy was found a higher level of protection against reinfection (pooled IRR = 0.07, 95% CI = 0.04–0.14). For studies published from 2020 to 2023, the pooled IRR was on the rise. It is 0.09 (95% CI = 0.02–0.35) for studies published in 2020, 0.15 (95% CI = 0.11–0.22) in 2021, 0.19 (95% CI = 0.15–0.23) in 2022 and 0.74 (95% CI = 0.57–0.97) in 2023. However, no significant differences were observed in the subgroup analysis of the definition of reinfection (the pooled IRR = 0.21, 95% CI = 0.17–0.27, p = 0.06, Supplementary Figure S10), the population type (the pooled IRR = 0.20, 95% CI = 0.16–0.26, p = 0.40, Supplementary Figure S4) and the study quality score (the pooled IRR = 0.19, 95% CI = 0.15–0.23, p = 0.82, Supplementary Figure S7). In addition, the pooled IRRs of reinfection was higher in participants aged less than 60 years than those greater than 60 years (0.19, 95% CI = 0.15–0.25 vs. 0.07, 95% CI = 0.03–0.18), differences (p < 0.04) between the two age groups were significant (Supplementary Figure S5–1). However, given that there were only two studies with a median age of over 60, the results may not be representative. Therefore, we also used the median age of 55 years as the basis of grouping for exploratory analysis. We found the difference of the pooled IRRs in participants aged less than 55 years than those greater than 55 years (0.19, 95% CI = 0.14–0.25 vs. 0.13, 95% CI = 0.04–0.42) was not statistical (Supplementary Figure S5–2).
Most studies that reported the mean/median follow-up times were included in the bubble plot to explore the changing trends of the protection provided by naturally acquired antibodies after a prior COVID-19 infection, the protection appeared to decrease slowly over time (Supplementary Figure S9).
This systematic review and meta-analysis, including 40 studies and over 20 million unvaccinated individuals, provides a synthesis of the evidence that natural immunity from primary infection can prevent SARS-CoV-2 reinfection (IRR = 0.35), especially symptomatic reinfection (IRR = 0.15). Meanwhile, the protective efficacy declined during Omicron wave and varied by study location and publication year. These findings suggests that people after primary infection should still be vaccinated and use personal protections to reduce the risk of reinfection.
A high protective efficacy of natural infection against SARS-CoV-2 reinfection has been reported in the available systematic reviews (10, 13, 62–64), but our estimate (65%) is much lower than others (>80%). On one side, the original estimated efficacy in our primary analysis was 81% (Supplementary Figure S2) and in line with the previous estimates, but the conservative estimate was obtained with a non-parametric “trim-and-fill” method to reduce publication bias (65). On the other side, evidence in South Africa suggests increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron (66), and we included the most recent studies during Omicron epidemic which may lead to a lower protection effect due to the omicron’s immune escape ability. Therefore, SARS-CoV-2 reinfection should be highlighted for the further prevention strategies over time.
In our study, protection against symptomatic reinfection is substantial with an estimate corresponding with the previous reviews (18, 64), while the effect on asymptomatic reinfection (60%) was weaker than on symptomatic reinfection (85%). The findings might be biased by the inadequate detection of all asymptomatic infections in those studies based on surveillance. Nevertheless, it is similar to the SIREN (SARS-CoV-2 Immunity and Reinfection Evaluation) study with the best methods, that the protective efficacy of primary infection was 93 and 52% against symptomatic and asymptomatic reinfection, respectively (37). Also, Deng et al. (16) found reinfection cases were more likely to present with mild symptoms than primary infection ones. In contrast, the meta-analysis performed by Bowe et al. (67) showed that reinfection can further increase risks of death, hospitalization, and sequelae in the acute and post-acute phase, regardless of vaccination status. Still, strategies for reinfection prevention remains to be carefully considered and evaluated.
Furthermore, the efficacy of natural infection against reinfection by the Alpha, Delta, and Omicron variants was estimated at 89, 81, and 49%, respectively. In spite of the limited number of variant-specific studies, similar pattern was observed in the sub-group analysis for the study publication year and the inclusion end time of population, that the efficacy of natural infection was lower during the period of omicron outbreak than during pre-omicron outbreak. Our findings are identical to a previous meta-analysis (10), suggesting an increase of reinfection risk as the omicron variant emerged. The low efficacy against the omicron variant might result from its unique mutations on pre-existing antibodies (68), as well as antibody neutralization (69). Accordingly, the risk of reinfection was lower among the vaccinated population than among the unvaccinated during the omicron wave, strengthening the need of multiple dose vaccination after primary infection (10). However, in addition to focusing on the rate of reinfection with a specific variant, it is equally important to assess the prevalence of long-COVID and the overall health impact on individuals following reinfection. For instance, studies have indicated that the prevalence of long-COVID is significantly lower among individuals infected with the Omicron variant compared to those infected with previous variants such as Alpha and Delta (70). Moreover, among patients with long-COVID, it was not Omicron-infected but Alpha-infected patients who had a higher prevalence of central neurological symptoms (71). Hence, it is crucial to consider multiple factors comprehensively when developing a vaccination strategy.
Due to the unavailability of data and the complexity of the study, the present study was not focused on the protective effect of natural infection with a particular SARS-CoV-2 variant on reinfection with the same variant, but rather on the protective effect of a previously naturally infection on subsequent reinfections, and if there was a difference in its protective effect on reinfections with different variants. This review currently includes 40 relevant studies published up to March 2023 for extraction 52 study data (Table 2). Of the 17 study data that reported the type of reinfection variant, 3 data focused on the protective effect of natural infection on reinfection of Alpha variant (17.65%), 9 data focused on the protective effect of natural infection on reinfection of Delta variant (52.94%), and 5 data focused on the protective effect of natural infection on reinfection of omicron variant (29.41%). The remaining 35 data were from studies that did not report a specific reinfection variant of interest, and it is highly likely that there is a mishmash of reinfection with multiple variants. Therefore, only these 17 data focusing on reinfection with a single variant were included in the subgroup analysis of viral variants in this paper. The virus has evolved over time, and the majority of the current population is infected with Omicron. However, there is a paucity of studies on the protective effect of previous infection with Omicron on reinfection with Omicron and its subsequent variants, which has not been considered at this time in this review study, and may therefore lead to an underestimation of the overall protective effect of previous infection on reinfection. In view of this, we will continue to follow up the study and plan to update the results at an appropriate time, such as in 6 months or a year later, depending on subsequent SARS-CoV-2 infections.
Here, we found poor protective effect of prior infection against SARS-CoV-2 reinfection in Nicaragua but a higher protective effect in Italy, which may be due to the lower oxford policy stringency index in the former, that is, the looser prevention and control policy; and the higher index in the latter, meaning a stricter prevention and control policy. Distinctively, our study shows a low protective efficacy of natural infection among people over 60 years old, contrast to the previous findings (13, 55, 62). It may be because the median age of only 2 studies is greater than 60, the results obtained are not representative. However, there were four studies with a median age greater than 55 and we found there was no statistical difference in the protective effect of natural infection between people over and below 55 years old.
In China, the vaccine immunity of most people has been reduced to a very low level, and the current immunity to reinfection with SARS-CoV-2 mainly relies on the natural immunity generated during the Omicron epidemic at the end of last year. Therefore, this study is very in line with China’s current national conditions and will help provide a scientific basis for preventing re-infection in the Chinese population.
However, this study was subject to limitations. Firstly, the I2 value and Cochran’s Q test suggests high heterogeneity between the studies in our analyses, due to the various regions, periods and populations (72). Under this circumstance, we had to accept the existence of the heterogeneity. Therefore, we used the random effects model instead of the fixed effects model to estimate the combined effect value in our meta-analysis. The greater uncertainty brought by heterogeneity to our estimate has been reflected in the method of estimation and calculation of the confidence interval under the random effects model. To explore the sources of heterogeneity and their impact on the results, we have conducted meta-regression and subgroup analyses. The meta-regression results of this study showed that the SARS-CoV-2 variant that the studies focused on and the year of publication of the studies were important sources of high heterogeneity. As the fact that the dominant strains of SARS-CoV-2 differed from year to year, we believe that the heterogeneity among studies due to different years of publication is essentially due to the different endemic strains of SARS-CoV-2 represented behind the different years, which explaining 46.40% of the variation in IRRs. Therefore, this review next focused on the protective effects of natural infection with SARS-CoV-2 against reinfection with different variants through subgroup analysis, which indicated the protective effects of natural infection against reinfection Alpha to Omicron gradually decreases. Compared to the overall protective effect of natural infection against reinfection, we believe that the subgroup results of the sub-variant are of greater interest and are the highlight of this study. To evaluate the stability of the results of this review, we performed a sensitivity analysis by excluding the included literature one by one. The results showed that there was no significant change in the results of the meta-analysis of the remaining studies after excluding any of them. This suggests that the included studies had stable results despite their heterogeneity. Secondly, the estimated efficacy against asymptomatic reinfection might be underestimated, for the inadequate detection. Lastly, publication bias was detected in the included studies but we used trim-and-fill method to reduce its potential effect.
Our findings indicate that individuals who have previously been infected with SARS-CoV-2 possess significant protection against reinfection from pre-omicron variants. However, when it comes to the omicron variant, the level of protection against reinfection is notably diminished. This will require continued attention to viral mutation in the future and careful consideration of strategies to prevent reinfection, such as vaccine catch-up, in conjunction with other factors, such as the reinfection rate, the prevalence of long-COVID and the overall health impact on individuals following reinfection.
W-HH: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. H-LC: Writing – original draft, Data curation. H-CY: Writing – original draft, Data curation. HW: Writing – original draft, Data curation. H-MS: Writing – original draft, Data curation. Y-YW: Writing – review & editing, Methodology. Y-TH: Writing – review & editing, Supervision, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China [grant numbers 81973150 and 82009Y6112] and the Basic and Applied Basic Research Foundation of Guangdong Province [grant number 2021A1515011591]. Y-TH gratefully acknowledges the support of K. C. Wong Education Foundation. The study sponsor has no role in study design, data analysis and interpretation of data, the writing of manuscript, or the decision to submit the paper for publication.
We thank Cai Li for her methodological guidance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1353415/full#supplementary-material
IRR, incidence rate ratio; 95% CI, 95% confidence interval; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; I2, I-squared
1. Perez-Gomez, R. The development of SARS-CoV-2 variants: the gene makes the disease. J Dev Biol. (2021) 9:58. doi: 10.3390/jdb9040058
2. Mohammad, T, Choudhury, A, Habib, I, Asrani, P, Mathur, Y, Umair, M, et al. Genomic variations in the structural proteins of SARS-CoV-2 and their deleterious impact on pathogenesis: a comparative genomics approach. Front Cell Infect Microbiol. (2021) 11:765039. doi: 10.3389/fcimb.2021.765039
3. He, X, Hong, W, Pan, X, Lu, G, and Wei, X. SARS-CoV-2 omicron variant: characteristics and prevention. MedComm. (2020) 2:838–45. doi: 10.1002/mco2.110
4. Muleme, M, McNamara, BJ, Ampt, FH, Baptista, M, Dittmer, J, Osborne, A, et al. Severity of COVID-19 among residents in aged care facilities in Victoria, Australia: a retrospective cohort study comparing the delta and omicron epidemic periods. J Am Med Dir Assoc. (2023) 24:434–440.e5. doi: 10.1016/j.jamda.2023.01.006
5. Liu, C, Lu, J, Li, P, Feng, S, Guo, Y, Li, K, et al. A comparative study on epidemiological characteristics, transmissibility, and pathogenicity of three COVID-19 outbreaks caused by different variants. Int J Infect Dis. (2023) 134:78–87. doi: 10.1016/j.ijid.2023.01.039
6. Yao, Y, Yang, Y, Wu, Q, Liu, M, Bao, W, Wang, Q, et al. A survey and antibody test following the surge of SARS-CoV-2 omicron infection in China. medRxiv. (2023):2023–2. doi: 10.1101/2023.02.28.23286535
7. Rubin, R. COVID-19 vaccines vs. variants-determining how much immunity is enough. JAMA. (2021) 325:1241–3. doi: 10.1001/jama.2021.3370
8. Cheng, ZJ, Huang, H, Zheng, P, Xue, M, Ma, J, Zhan, Z, et al. Humoral immune response of BBIBP COVID-19 vaccination before and after the booster immunization. Allergy. (2022) 77:2404–14. doi: 10.1111/all.15271
9. Zhang, H, Hua, Q, Xu, N, Zhang, X, Chen, B, Ma, X, et al. Evaluation of antibody kinetics and durability in health subjects vaccinated with inactivated COVID-19 vaccine (CoronaVac): a cross-sectional and cohort study in Zhejiang, China. Elife. (2023) 12:e84056. doi: 10.7554/eLife.84056.sa0
10. Flacco, ME, Acuti Martellucci, C, Baccolini, V, De Vito, C, Renzi, E, Villari, P, et al. Risk of reinfection and disease after SARS-CoV-2 primary infection: meta-analysis. Eur J Clin Investig. (2022) 52:e13845. doi: 10.1111/eci.13845
11. Lusvarghi, S, Pollett, SD, Neerukonda, SN, Wang, W, Wang, R, Vassell, R, et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster-elicited serum but evades most convalescent serum and therapeutic antibodies. Sci Transl Med. (2022) 14:eabn8543. doi: 10.1126/scitranslmed.abn8543
12. Stein, C, Nassereldine, H, Sorensen, RJD, Amlag, JO, Bisignano, C, Byrne, S, et al. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. (2023) 401:833–42. doi: 10.1016/S0140-6736(22)02465-5
13. Chen, Q, Zhu, K, Liu, X, Zhuang, C, Huang, X, Huang, Y, et al. The protection of naturally acquired antibodies against subsequent SARS-CoV-2 infection: a systematic review and Meta-analysis. Emerg Microbes Infect. (2022) 11:793–803. doi: 10.1080/22221751.2022.2046446
14. Bobrovitz, N, Ware, H, Ma, X, Li, Z, Hosseini, R, Cao, C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. (2023) 23:556–67. doi: 10.1016/S1473-3099(22)00801-5
15. Milne, G, Hames, T, Scotton, C, Gent, N, Johnsen, A, Anderson, RM, et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med. (2021) 9:1450–66. doi: 10.1016/S2213-2600(21)00407-0
16. Deng, J, Ma, Y, Liu, Q, Du, M, Liu, M, and Liu, J. Severity and outcomes of SARS-CoV-2 reinfection compared with primary infection: a systematic review and meta-analysis. Int J Environ Res Public Health. (2023) 20:3335. doi: 10.3390/ijerph20043335
17. Diani, S, Leonardi, E, Cavezzi, A, Ferrari, S, Iacono, O, Limoli, A, et al. SARS-CoV-2-the role of natural immunity: a narrative review. J Clin Med. (2022) 11:6272. doi: 10.3390/jcm11216272
18. Mao, Y, Wang, W, Ma, J, Wu, S, and Sun, F. Reinfection rates among patients previously infected by SARS-CoV-2: systematic review and meta-analysis. Chinese Med J. (2021) 135:145–52. doi: 10.1097/CM9.0000000000001892
19. Pilz, S, Theiler-Schwetz, V, Trummer, C, Krause, R, and Ioannidis, JPA. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. (2022) 209:112911. doi: 10.1016/j.envres.2022.112911
20. Shenai, MB, Rahme, R, and Noorchashm, H. Equivalency of protection from natural immunity in COVID-19 recovered versus fully vaccinated persons: a systematic review and pooled analysis. Cureus. (2021) 13:e19102. doi: 10.7759/cureus.19102
21. Soleimanian, S, Alyasin, S, Sepahi, N, Ghahramani, Z, Kanannejad, Z, Yaghobi, R, et al. An update on protective effectiveness of immune responses after recovery from COVID-19. Front Immunol. (2022) 13:884879. doi: 10.3389/fimmu.2022.884879
22. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
23. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Maier, HE, Balmaseda, A, Ojeda, S, Cerpas, C, Sanchez, N, Plazaola, M, et al. An immune correlate of SARS-CoV-2 infection and severity of reinfections. medRxiv. (2021) 24:2021.11.23.21266767. doi: 10.1101/2021.11.23.21266767
25. Lumley, SF, Rodger, G, Constantinides, B, Sanderson, N, Chau, KK, Street, TL, et al. An observational cohort study on the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin Infect Dis. (2022) 74:1208–19. doi: 10.1093/cid/ciab608
26. Jeffery-Smith, A, Iyanger, N, Williams, SV, Chow, JY, Aiano, F, Hoschler, K, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill. (2021) 26:2100092. doi: 10.2807/1560-7917.ES.2021.26.5.2100092
27. Lumley, SF, O'Donnell, D, Stoesser, NE, Matthews, PC, Howarth, A, Hatch, SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. (2021) 384:533–40. doi: 10.1056/NEJMoa2034545
28. Hansen, CH, Michlmayr, D, Gubbels, SM, Molbak, K, and Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. (2021) 397:1204–12. doi: 10.1016/S0140-6736(21)00575-4
29. Harvey, RA, Rassen, JA, Kabelac, CA, Turenne, W, Leonard, S, Klesh, R, et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. (2021) 181:672–9. doi: 10.1001/jamainternmed.2021.0366
30. Kim, P, Gordon, SM, Sheehan, MM, and Rothberg, MB. Duration of severe acute respiratory syndrome coronavirus 2 natural immunity and protection against the Delta variant: a retrospective cohort study. Clin Infect Dis. (2022) 75:e185–90. doi: 10.1093/cid/ciab999
31. Kohler, P, Gusewell, S, Seneghini, M, Egger, T, Leal, O, Brucher, A, et al. Impact of baseline SARS-CoV-2 antibody status on syndromic surveillance and the risk of subsequent COVID-19-a prospective multicenter cohort study. BMC Med. (2021) 19:270. doi: 10.1186/s12916-021-02144-9
32. Krutikov, M, Palmer, T, Tut, G, Fuller, C, Shrotri, M, Williams, H, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study. Lancet Healthy Longev. (2021) 2:e362–70. doi: 10.1016/S2666-7568(21)00093-3
33. Leidi, A. Occupational risk of SARS-CoV-2 infection and reinfection during the second pandemic surge: a cohort study. Occup Environ Med. (2021) 116–119. doi: 10.1136/oemed-2021-107924
34. Jeffery-Smith, A, Rowland, TAJ, Patel, M, Whitaker, H, Iyanger, N, Williams, SV, et al. Reinfection with new variants of SARS-CoV-2 after natural infection: a prospective observational cohort in 13 care homes in England. Lancet Healthy Longev. (2021) 2:e811–9. doi: 10.1016/S2666-7568(21)00253-1
35. Leidi, A, Koegler, F, Dumont, R, Dubos, R, Zaballa, ME, Piumatti, G, et al. Risk of reinfection after seroconversion to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a population-based propensity-score matched cohort study. Clin Infect Dis. (2022) 74:622–9. doi: 10.1093/cid/ciab495
36. Havervall, S, Ng, H, Jernbom Falk, A, Greilert-Norin, N, Manberg, A, Marking, U, et al. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. J Intern Med. (2022) 291:72–80. doi: 10.1111/joim.13387
37. Hall, VJ, Foulkes, S, Charlett, A, Atti, A, Monk, EJM, Simmons, R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. (2021) 397:1459–69. doi: 10.1016/S0140-6736(21)00675-9
38. Letizia, AG, Ge, Y, Vangeti, S, Goforth, C, Weir, DL, Kuzmina, NA, et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study. Lancet Respir Med. (2021) 9:712–20. doi: 10.1016/S2213-2600(21)00158-2
39. Cohen, DE, Sibbel, S, Marlowe, G, Bludorn, K, Miller, D, Kelley, T, et al. Antibody status, disease history, and incidence of SARS-CoV-2 infection among patients on chronic Dialysis. J Am Soc Nephrol. (2021) 32:1880–6. doi: 10.1681/ASN.2021030387
40. Chemaitelly, H, Nagelkerke, N, Ayoub, HH, Coyle, P, Tang, P, Yassine, HM, et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J Travel Med. (2022) 29:taac109. doi: 10.1093/jtm/taac109
41. Abu-Raddad, LJ, Chemaitelly, H, Ayoub, HH, Coyle, P, Malek, JA, Ahmed, AA, et al. Introduction and expansion of the SARS-CoV-2 B.1.1.7 variant and reinfections in Qatar: a nationally representative cohort study. PLoS Med. (2021) 18:e1003879. doi: 10.1371/journal.pmed.1003879
42. Schuler, CF, Gherasim, C, O'Shea, K, Manthei, DM, Chen, J, Zettel, C, et al. Mild SARS-CoV-2 illness is not associated with reinfections and provides persistent spike, nucleocapsid, and virus-neutralizing antibodies. Microbiol Spectr. (2021) 9:e0008721. doi: 10.1128/Spectrum.00087-21
43. Dimeglio, C, Herin, F, Miedougé, M, Martin-Blondel, G, Soulat, J-M, and Izopet, J. Protection of healthcare workers against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection. Clin Infect Dis. (2021) 73:1323–4. doi: 10.1093/cid/ciab069
44. Abu-Raddad, LJ, Chemaitelly, H, Coyle, P, Malek, JA, Ahmed, AA, Mohamoud, YA, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine. (2021) 35:100861. doi: 10.1016/j.eclinm.2021.100861
45. Abo-Leyah, H, Gallant, S, Cassidy, D, Giam, YH, Killick, J, Marshall, B, et al. The protective effect of SARS-CoV-2 antibodies in Scottish healthcare workers. ERJ Open Res. (2021) 7:00080–2021. doi: 10.1183/23120541.00080-2021
46. Vitale, J, Mumoli, N, Clerici, P, De Paschale, M, Evangelista, I, Cei, M, et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy. Italy JAMA Intern Med. (2021) 181:1407–8. doi: 10.1001/jamainternmed.2021.2959
47. Maier, HE, Kuan, G, Saborio, S, Carrillo, FAB, Plazaola, M, Barilla, C, et al. Clinical Spectrum of severe acute respiratory syndrome coronavirus 2 infection and protection from symptomatic reinfection. Clin Infect Dis. (2022) 75:e257–66. doi: 10.1093/cid/ciab717
48. Rahman, S, Rahman, MM, Miah, M, Begum, MN, Sarmin, M, Mahfuz, M, et al. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci Rep. (2022) 12:1438. doi: 10.1038/s41598-022-05325-5
49. Shields, AM, Faustini, SE, Kristunas, CA, Cook, AM, Backhouse, C, Dunbar, L, et al. COVID-19: seroprevalence and vaccine responses in UK dental care professionals. Journal of dental research. (2021) 100.11:1220–1227. doi: 10.1177/00220345211020270
50. Mishra, BK, Bhattacharya, D, Kshatri, JS, and Pati, S. Natural immunity against COVID-19 significantly reduces the risk of reinfection: findings from a cohort of sero-survey participants. medRxiv. (2021) 2021–07. doi: 10.1101/2021.07.19.21260302
51. Patalon, T, Saciuk, Y, Hadad, HO, Perez, G, Peretz, A, Ben-Tov, A, et al. Dynamics of naturally acquired immunity against severe acute respiratory syndrome coronavirus 2 in children and adolescents. The Journal of Pediatrics. (2023) 257:113371. doi: 10.1016/j.jpeds.2023.02.016
52. Muir, L, Jaffer, A, Rees-Spear, C, Gopalan, V, Chang, FY, Fernando, R, et al. Neutralizing antibody responses after SARS-CoV-2 infection in end-stage kidney disease and protection against reinfection. Kidney Int Rep. (2021) 6:1799–809. doi: 10.1016/j.ekir.2021.03.902
53. Rothberg, MB, Kim, P, Shrestha, NK, Kojima, L, and Tereshchenko, LG. Protection against the omicron variant offered by previous SARS-CoV-2 infection: a retrospective cohort study. Clin Infect Dis. (2022) 76:e142–7. doi: 10.1093/cid/ciac604
54. Spicer, KB, Glick, C, Cavanaugh, AM, and Thoroughman, D. Protective immunity after natural infection with severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) - Kentucky, USA 2020. Int J Infect Dis. (2022) 114:21–8. doi: 10.1016/j.ijid.2021.10.010
55. Nordstrom, P, Ballin, M, and Nordstrom, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. (2022) 22:781–90. doi: 10.1016/S1473-3099(22)00143-8
56. Rennert, L, and McMahan, C. Risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection in a university student population. Clin Infect Dis. (2022) 74:719–22. doi: 10.1093/cid/ciab454
57. Manica, M, Pancheri, S, Poletti, P, Giovanazzi, G, Guzzetta, G, Trentini, F, et al. Risk of symptomatic infection during a second coronavirus disease 2019 wave in severe acute respiratory syndrome coronavirus 2-seropositive individuals. Clin Infect Dis. (2022) 74:893–6. doi: 10.1093/cid/ciab556
58. Pilz, S, Chakeri, A, Loannidis, JPA, Richter, L, Theiler-Schwetz, V, Trummer, C, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Investig. (2021) 51:e13520. doi: 10.1111/eci.13520
59. Wilkins, JT, Hirschhorn, LR, Gray, EL, Wallia, A, Carnethon, M, Zembower, TR, et al. Serologic status and SARS-CoV-2 infection over 6 months of follow up in healthcare Workers in Chicago: a cohort study. Infect Control Hosp Epidemiol. (2022) 43:1207–15. doi: 10.1017/ice.2021.367
60. Babouee Flury, B, Güsewell, S, Egger, T, Leal, O, Brucher, A, Lemmenmeier, E, et al. Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and omicron waves in Switzerland-a multicentre cohort study. PLoS Med. (2022) 19:e1004125. doi: 10.1371/journal.pmed.1004125
61. Patalon, T, Saciuk, Y, Perez, G, Peretz, A, Ben-Tov, A, and Gazit, S. Dynamics of naturally acquired immunity against severe acute respiratory syndrome coronavirus 2 in children and adolescents. J Pediatr. (2023) 257:113371.
62. Murchu, EO, Byrne, P, Carty, PG, De Gascun, C, Keogan, M, O'Neill, M, et al. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev Med Virol. (2022) 32:e2260. doi: 10.1002/rmv.2260
63. Chivese, T, Matizanadzo, JT, Musa, OAH, Hindy, G, Furuya-Kanamori, L, Islam, N, et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery - a comprehensive systematic review and meta-analysis. Pathog Glob Health. (2022) 116:269–81. doi: 10.1080/20477724.2022.2029301
64. Helfand, M, Fiordalisi, C, Wiedrick, J, Ramsey, KL, Armstrong, C, Gean, E, et al. Risk for reinfection after SARS-CoV-2: a living, rapid review for American College of Physicians Practice Points on the role of the antibody response in conferring immunity following SARS-CoV-2 infection. Ann Intern Med. (2022) 175:547–55. doi: 10.7326/M21-4245
65. Duval, S, and Tweedie, R. A non-parametric "trim and fill" method of accounting for publication Bias in Meta-analysis. J Am Stat Assoc. (2000) 95:89–98.
66. Pulliam, JRC, van Schalkwyk, C, Govender, N, von Gottberg, A, Cohen, C, Groome, MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of omicron in South Africa. Science. (2022) 376:eabn4947. doi: 10.1126/science.abn4947
67. Bowe, B, Xie, Y, and Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. (2022) 28:2398–405. doi: 10.1038/s41591-022-02051-3
68. Kannan, SR, Spratt, AN, Sharma, K, Chand, HS, Byrareddy, SN, and Singh, K. Omicron SARS-CoV-2 variant: unique features and their impact on pre-existing antibodies. J Autoimmun. (2022) 126:102779. doi: 10.1016/j.jaut.2021.102779
69. Planas, D, Saunders, N, Maes, P, Guivel-Benhassine, F, Planchais, C, Buchrieser, J, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature. (2022) 602:671–5. doi: 10.1038/s41586-021-04389-z
70. Fernández-de-Las-Peñas, C, Notarte, KI, Peligro, PJ, Velasco, JV, Ocampo, MJ, Henry, BM, et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. (2022) 14:2629. doi: 10.3390/v14122629
71. Canas, LS, Molteni, E, Deng, J, Sudre, CH, Murray, B, Kerfoot, E, et al. Profiling post-COVID-19 condition across different variants of SARS-CoV-2: a prospective longitudinal study in unvaccinated wild-type, unvaccinated alpha-variant, and vaccinated delta-variant populations. Lancet Digit Health. (2023) 5:e421–34. doi: 10.1016/S2589-7500(23)00056-0
Keywords: SARS-CoV-2, variant, naturally infection, reinfection, protective effectiveness
Citation: Hu W-H, Cai H-L, Yan H-C, Wang H, Sun H-M, Wei Y-Y and Hao Y-T (2024) Protective effectiveness of previous infection against subsequent SARS-Cov-2 infection: systematic review and meta-analysis. Front. Public Health. 12:1353415. doi: 10.3389/fpubh.2024.1353415
Received: 10 December 2023; Accepted: 04 June 2024;
Published: 20 June 2024.
Edited by:
Ibrahim A. Elshaer, King Faisal University, Saudi ArabiaReviewed by:
Kin Israel Notarte, Johns Hopkins University, United StatesCopyright © 2024 Hu, Cai, Yan, Wang, Sun, Wei and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Tao Hao, aGFveXRAYmptdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.