
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 21 February 2024
Sec. Environmental Health and Exposome
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1352570
Introduction: Glyphosate, a widely utilized herbicide globally, has been linked to various health issues, including cancer, birth abnormalities, and reproductive issues. Additionally, there is growing experimental support indicating potential harm to skeletal muscles. Despite this, the impact of glyphosate on human muscle health remains unclear.
Methods: We examined information gathered from the 2013-2014 National Health and Nutrition Examination Survey (NHANES), which included 1466 adults aged 18 or older. Our primary aim was to investigate the relationship between glyphosate exposure and hand grip strength, as well as its influence on lean muscle mass.
Results and discussion: Our investigation uncovered a detrimental correlation between glyphosate exposure and all measures of grip strength, except for the second test of the first hand. Specifically, we observed a statistically significant adverse association between glyphosate exposure and combined grip strength, which is calculated as the sum of the highest readings from both hands (ß coefficient of −2.000, S.E. = 0.891, p = 0.040). We did not observe a significant correlation between glyphosate levels, lean muscle mass, and the likelihood of reaching maximum grip strength meeting sarcopenia criteria. Additionally, we observed an interaction between age and glyphosate, as well as between body mass index (BMI) and glyphosate, concerning the association with combined grip strength. In this comprehensive analysis of NHANES data, our study reveals a potential association between glyphosate exposure and hand grip strength in the adult population. Our findings suggest the need for deeper exploration into the health effects of glyphosate exposure and its impact on muscle strength, shedding light on possible public health concerns.
Glyphosate, which has been the active component in herbicides since 1974, works as a chemical that disrupts the shikimate pathway, a metabolic pathway used by plants to synthesize essential aromatic amino acid (1). Glyphosate-based herbicides (GBH) are a combination of glyphosate and surfactants that amplify its permeation into plants and augment its efficacy (2). Glyphosate and GBH are widely used due to their exceptional efficacy in managing weed proliferation, rendering them the most extensively utilized herbicides worldwide (3). Individuals may potentially come into contact with these two chemicals through various routes, including skin, inhalation, and oral consumption (4). Although glyphosate was once thought to be safe in animals, increasing apprehension has arisen in recent years regarding possible negative health effects associated with glyphosate and GBH. Numerous investigations have established connections between glyphosate exposure and a range of health concerns, such as cancer, birth abnormalities, endocrine, and reproductive issues (5, 6). As a result of these findings, the International Agency for Research on Cancer classified glyphosate as a probable human carcinogen (7).
Glyphosate and GBH have been found to have demonstrated varying impacts on different types of cells, depending on the concentration levels used during testing (1). Moreover, research has shown that these herbicides can cause cytotoxicity and genotoxicity in human cell cultures in a dose-dependent manner, even at environmentally relevant concentrations (8). Experimental research has examined the influence of glyphosate and GBH on skeletal muscle. Glyphosate exposure was found to decrease energy reserves (9, 10), alter acetylcholinesterase enzyme activity (11), change muscle morphology and functioning (12), and reduce muscle strength (13). However, results from these studies were inconsistent (14, 15).
While experimental research has yielded evidence suggesting that glyphosate and GBH could have adverse impacts on skeletal muscle, their connection in humans remains inadequately explored. It’s worth highlighting that there is a lack of studies examining the potential link between glyphosate exposure and the well-being of skeletal muscles in the general human population representing a country. Dynamometry is a reliable, valid, and responsive method for measuring muscle strength (16, 17). To assess lean muscle mass, dual-energy X-ray absorptiometry (DXA) is widely recognized as the gold standard for measuring body composition. Lean body mass excluding bone mineral content is a useful measure for evaluating lean muscle mass (18). To address this knowledge gap, we analyzed data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2013 and 2014. The dataset offers information on urinary glyphosate levels, hand grip strength tests, and lean muscle mass measured by DXA. Our study solely examined the adult population since hand grip strength and lean body mass can significantly differ among adults and children. Furthermore, adults may have a higher incidence of underlying medical conditions such as diabetes and chronic kidney disease that must be accounted for in the analysis (19, 20). Limiting our study to adults allowed us to better control for these variables and gain a clearer understanding of the effects of glyphosate. Our study aimed to enhance our comprehension of the association between glyphosate levels and muscle health in the general adult population by examining the relationship between urinary glyphosate levels, hand grip strength, and lean muscle mass.
The NHANES is a biennial nationwide survey that recruits a representative sample of the general population in the United States. Detailed information on the survey methodology and consent forms can be found on the NHANES website (21). In the current study, we utilized the NHANES 2013–2014 database and constrained population to individuals who were 18 years or older, possessed available measurements of glyphosate exposure, and pertinent demographic data. In addition, we excluded those who lacked measurements of hand grip strength or lean muscle mass. Our final study sample comprised 1,466 subjects, and a flow chart detailing the algorithm can be observed in Figure 1.
In the NHANES 2013–2014 study, urinary glyphosate levels were evaluated in a subgroup consisting of one-third of participants aged 6 years and above. Our analysis focused on data collected from individuals who were 18 years of age or older. The techniques used for measuring glyphosate levels have been documented in prior publications (22). For glyphosate levels that fell below the limits of detection (LOD), NHANES provided an imputed value, which was calculated as LOD divided by the square root of 2. The analytical methodology employed in the study is available on the NHANES website (23).
NHANES 2013–2014 evaluated the strength of participants’ hand grips, aged 6 and above while excluding those who had undergone hand or wrist surgery within the past 3 months or were unable to grip the dynamometer with both hands, through the use of a dynamometer. We collected data from individuals aged 18 and over in this study. Each participant proceeded to squeeze the dynamometer as forcefully as feasible with one hand, followed by the other hand. Three repetitions were conducted for each hand, with a 60-s rest interval between measurements of the same hand. Combined grip strength was calculated as the sum of the highest readings obtained from three attempts on each hand. Hand strength is an essential diagnostic measure to identify sarcopenia, a condition characterized by weak muscles. Grip strength cutoff points of less than 30 kg in men and less than 20 kg in women are indicative of low muscle strength (24). For further instructions, please refer to the NHANES website (25).
The NHANES DXA scan offers a comprehensive assessment of body composition and is performed on the entire body. Individuals between the ages of 8 and 59 years were eligible, except for those who were pregnant, had recently received radiographic contrast material, or exceeded the weight or height limit of the DXA table. This study included data from individuals aged 18 and over to assess the relationship between glyphosate exposure and muscle mass in adults. We used lean body mass, which excludes bone mineral content, as a measure to evaluate lean muscle mass. The examination protocol details can be found in the NHANES website (26).
According to the NHANES website, proficient personnel at all study sites employed uniform procedures to collect data. During the household interview, data on sociodemographic factors like age, gender, and race/ethnicity were collected. After analyzing the responses to the smoking questionnaire, participants were sorted into one of three categories: active smokers, exposed to environmental tobacco smoke (ETS), or non-smokers (27). The alcohol consumption questionnaire determined whether a participant had consumed at least 12 alcoholic beverages in the past year, and the responses were then divided into two categories. Total energy and total protein intake calculations involved averaging data from 2 days of dietary intake questionnaires. Physical activity was assessed by adding up the scores of different activities and multiplying them by their corresponding metabolic equivalent of task scores, as recommended by the NHANES website (28). For this study, chronic kidney disease was characterized as an estimated glomerular filtration rate below 60 mL/min per 1.73 square meters (29). Diabetes mellitus was defined as a fasting serum glucose level ≥126 mg/dL, or a glycated hemoglobin ≥6.5% or the self-reported current use of anti-hyperglycemic medications. Additionally, potential confounders such as body mass index (BMI), urinary creatinine, and diabetes mellitus were considered in this study.
The investigation displayed glyphosate concentrations in units of μg/L or μg/g creatinine. Comparisons of geometric means between groups were carried out through the two-tailed Student’s t-test and one-way analysis of variance. The sampling weights used were in accordance with protocols specified on the NHANES website (30). To evaluate the association between urinary glyphosate levels, hand grip strength, and lean body mass, a complex sample of the general linear model was employed. To investigate whether there is clinical significance in understanding the relationship between glyphosate and muscle strength, we employed a complex sample of the logistic regression analysis to explore a potential link between glyphosate concentrations and the presence of sarcopenia. Sarcopenia was defined as having a maximal grip strength assessment of less than 30 kg in males and less than 20 kg in females. For the purpose of covariate adjustment, two distinct models were utilized. In Model 1, age, gender, ethnicity, BMI, smoking, alcohol consumption, household income, and urinary creatinine were adjusted. Model 2, in addition to the adjustments made in Model 1, also took into account total energy intake, total protein intake, physical activity, chronic renal failure, and diabetes mellitus. Instead of being adjusted for hydration, urinary creatinine was treated as an independent variable based on previous research (31). The analysis utilized the natural logarithm (ln) of glyphosate and urinary creatinine owing to their non-Gaussian distributions. The statistical examination was carried out using SPSS version 20 (SPSS Inc., Chicago, Illinois, United States), and the significance level was set at p < 0.05 to determine statistical significance.
The study sample had a mean age of 48.16 years (SD = 18.31). Among the participants, 80.2% had detectable concentrations of glyphosate, with a mean of 0.55 μg/L (SD = 0.54). Table 1 presents the geometric means of glyphosate for different subgroups, indicating elevated urinary glyphosate levels among men, older individuals, non-Hispanic Black participants, and those with a higher BMI. Additionally, after adjusting for creatinine, glyphosate levels were higher in women, older individuals, non-Hispanic white participants, and non-smokers.
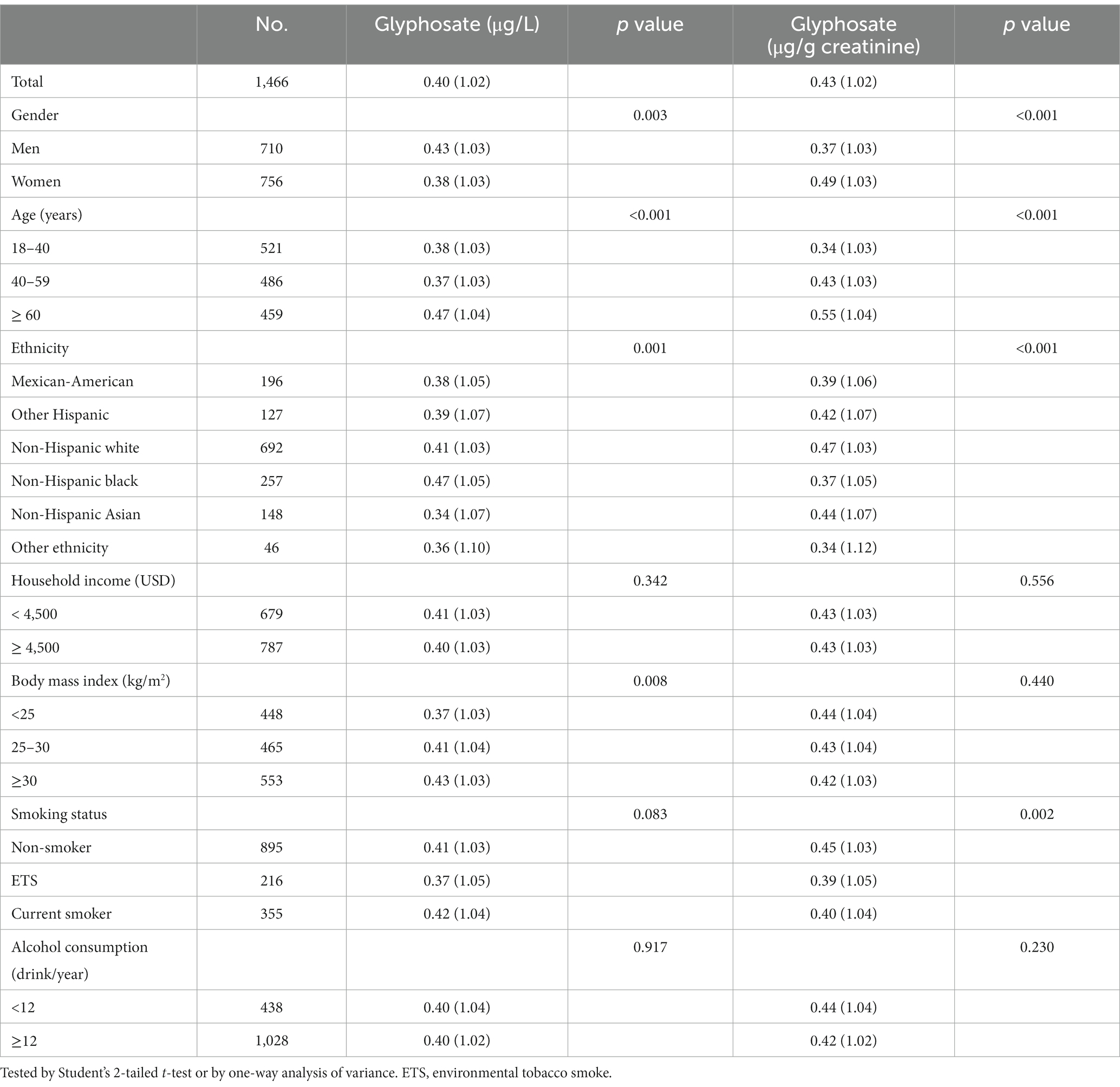
Table 1. The geometric means (S.E.) of urinary glyphosate levels in different demographic subgroups.
Table 2 presents the linear regression coefficients of grip strength with a one-unit increase in ln-urinary glyphosate. Except for test 2 of hand 1, all other grip strength measurements were negatively correlated with ln-glyphosate levels, with ß coefficients of −2.000 (S.E. = 0.891, p = 0.040) for combined grip strength. Figure 2 shows an overview of grip strength across urinary glyphosate tertiles in multiple linear regression models. The results indicate that average hand 1, average hand 2, and combined grip strength do not significantly decrease with increasing glyphosate tertiles. However, both average hand 2 and combined grip strength at the highest glyphosate tertile showed a significant reduction compared to the lowest tertile (p = 0.015 for average hand 2 strength and p = 0.028 for combined grip strength, respectively).
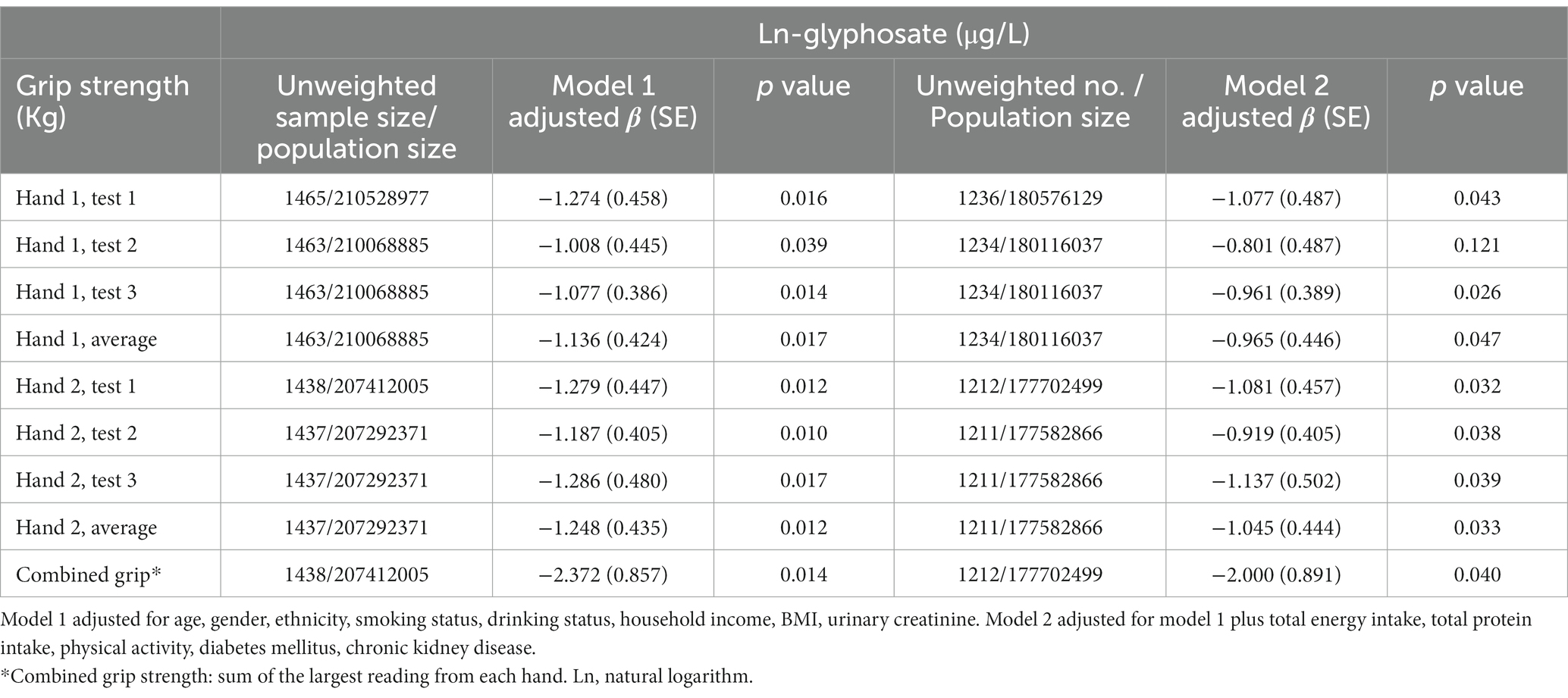
Table 2. Linear regression coefficients (S.E.) of grip strength with a unit increase in ln-urinary glyphosate in multiple linear regression models, with results weighted for sampling strategy.
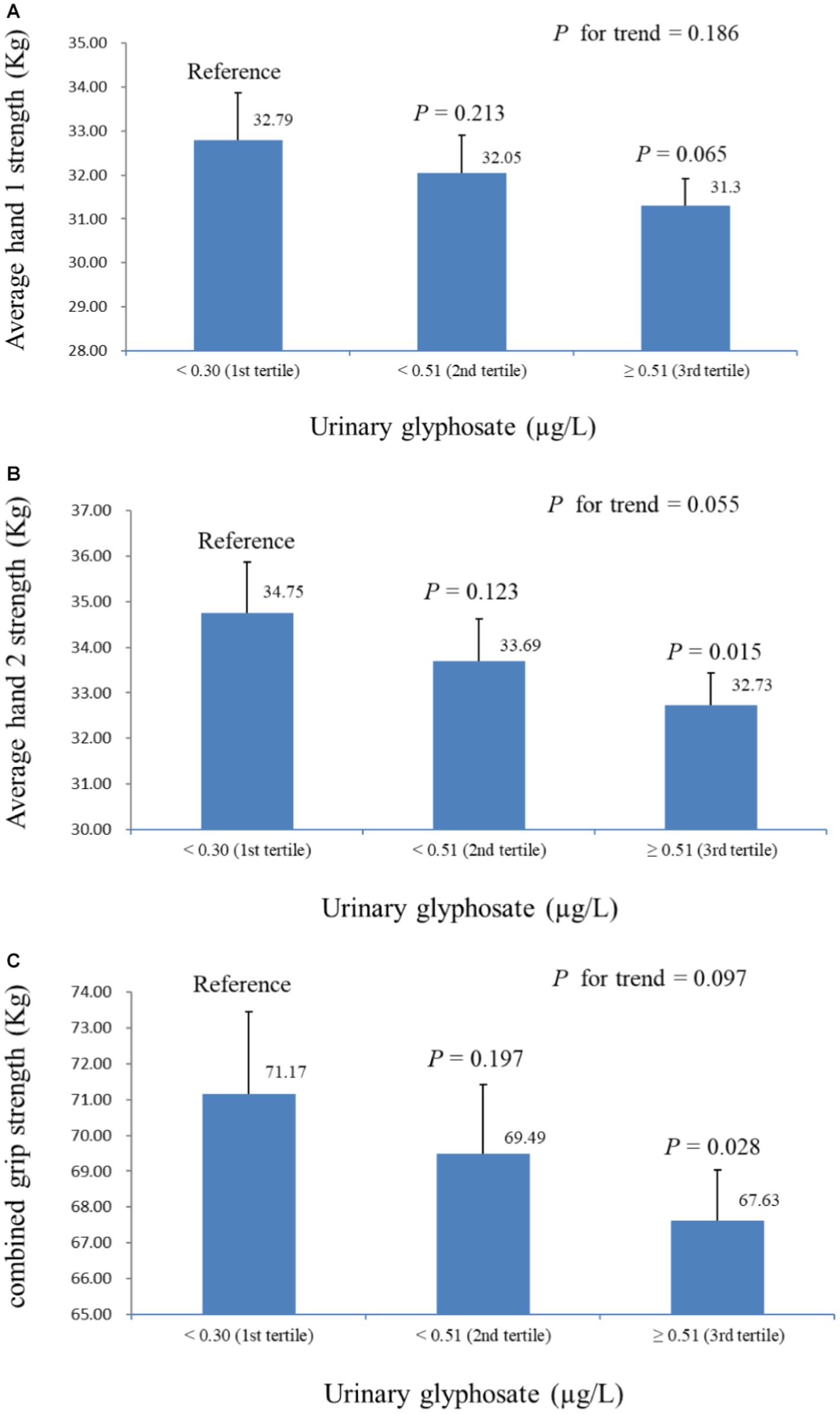
Figure 2. Hand grip strength across tertiles of urine glyphosate in multiple linear regression models (adjusted for model 2), with results weighted for sample strategy. (A) Average hand 1 strength. (B) Average hand 2 strength. (C) Combined grip strength.
Table 3 displays the linear regression coefficients of lean muscle mass with a one-unit increase in ln-urinary glyphosate. There is no significant association between glyphosate levels and lean muscle mass (p = 0.063 for right arm and p = 0.143 for left arm, respectively). In Table 4, the odds ratios of maximal grip strength fulfilling the criteria of sarcopenia with a one-unit increase in ln-glyphosate in logistic regression models are presented. However, glyphosate levels are not statistically significant in relation to the risk of sarcopenia, as defined by maximal hand grip strength. Table 5 depicts the inverse association between glyphosate and the combined grip strength across various subgroups of the research participants. This association was significant in men, individuals aged ≥60, non-Hispanic white, those with lower income, and those with a BMI between 25 and 30. Furthermore, we observed an interaction between age and glyphosate (p for interaction = 0.001), as well as between BMI and glyphosate (p for interaction = 0.004), concerning the association with combined grip strength.
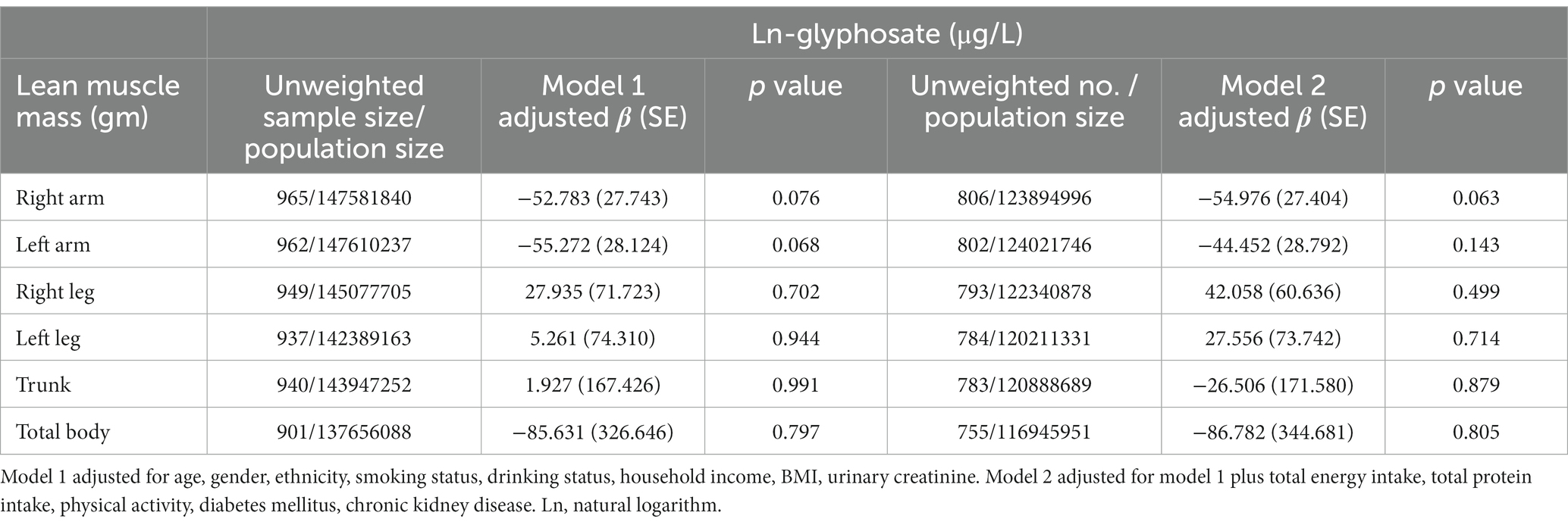
Table 3. Linear regression coefficients (S.E.) of lean muscle mass with a unit increase in ln-urinary glyphosate in multiple linear regression models, with results weighted for sampling strategy.
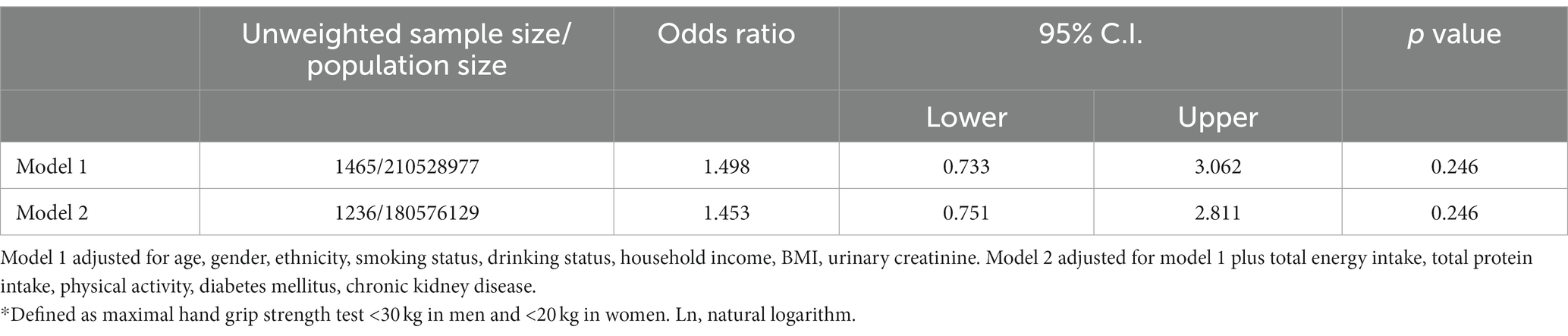
Table 4. Odds ratios (95% confidence interval [C.I.]) of maximal grip strength fulfill the criteria of sarcopenia* with one unit increase in ln-glyphosate (μg/L) in logistic regression models, with results weighted for sampling strategy.
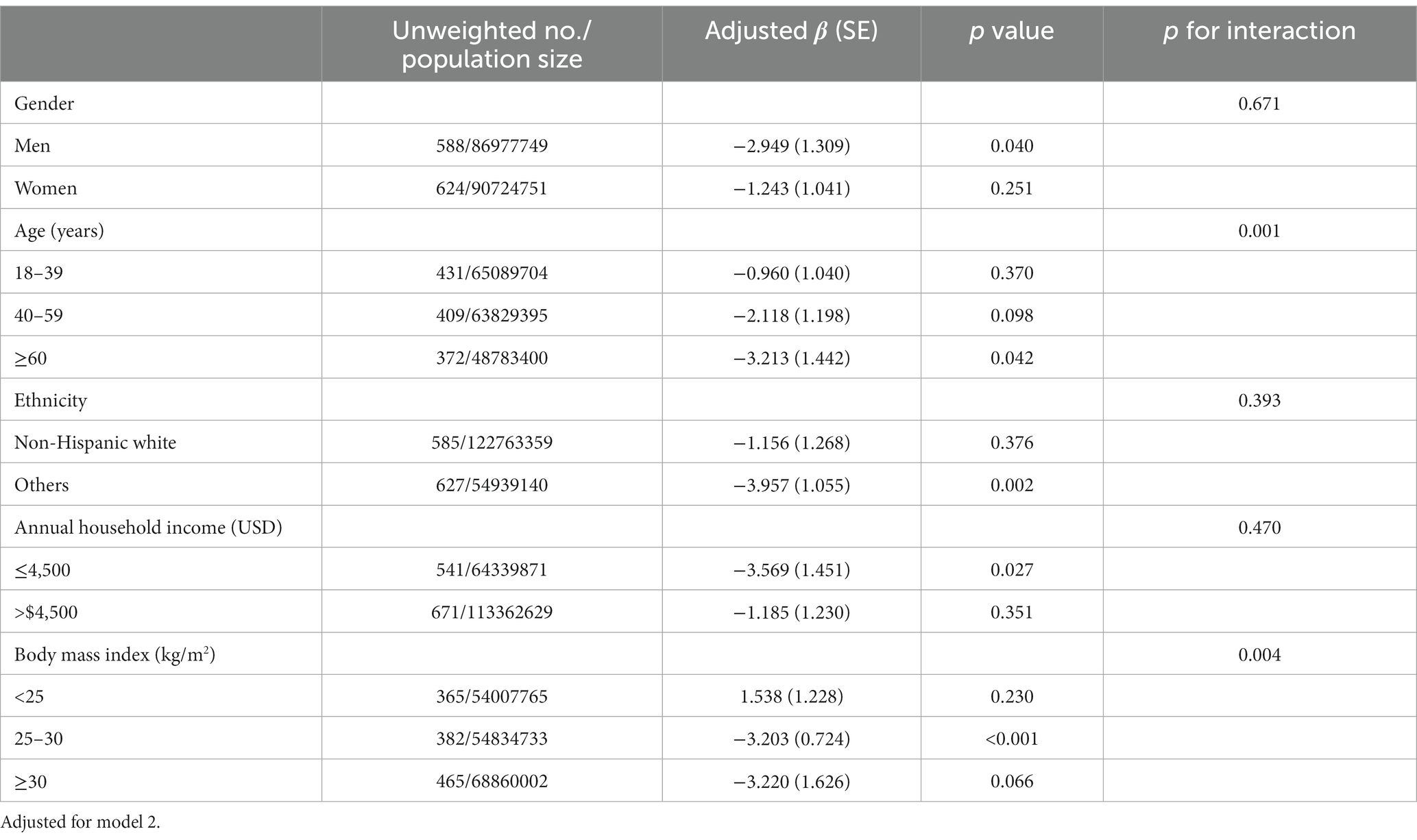
Table 5. Linear regression coefficients (standard error) of combined grip strength* per unit increase in ln glyphosate (μg/L) in different subgroups, with results weighted for sampling strategy.
Our research utilized a sample that represents the wider adult population of the United States and has brought to light a substantial negative correlation between levels of glyphosate in urine and grip strength. However, our discoveries do not find an association between glyphosate levels, lean muscle mass, and the likelihood of reaching maximum grip strength meeting sarcopenia criteria. Our finding offers preliminary clues of a possible connection between glyphosate and muscle strength among adults in the general population. The significance of this research lies in the extensive and thorough dataset obtained from the NHANES, as well as the incorporation of a diverse cross-section of American adults.
Within this study, a remarkable 80.2% of the participants were identified as having measurable levels of glyphosate, with a mean concentration of 0.40 μg/L (4). France had a higher prevalence, with traces of glyphosate in the urine of 99.8% of the population, and an average concentration of 1.19 μg/L (32). Portugal surveyed adults from the general public and found an average glyphosate concentration of 0.1 μg/L, and a detection rate of 73% (33). A comprehensive review of 19 studies concluded that the usual glyphosate levels in urine samples from the general population were generally below 4 μg/L (34). Considering that glyphosate has a reported half-life of elimination of 5.5–10 h (35), the high detection rate implies that there are unknown and unavoidable sources of exposure to glyphosate during daily activities, which have not been evaluated by any global regulatory agency. Glyphosate residues have been detected in various food samples, including fruits, nuts, cereals, and vegetables (36). Recent research suggests that individuals with heightened glyphosate exposure may have consistently consumed foods contaminated with herbicides (37). The current study disclosed a significant elevation in glyphosate levels when adjusted for urinary creatinine among women, older individuals, non-Hispanic whites, and non-smokers. It is plausible that these subgroups had a greater consumption of such contaminated foods.
Numerous experiments have been undertaken to explore the impacts of glyphosate and GBH on skeletal muscle. A decrease in muscle strength during glyphosate exposure can be attributed to an impairment in energy metabolism. For instance, a study found that exposure to 1 or 10 mg/L concentration of glyphosate led to a notable reduction in the energy reserves in the muscles of Odontesthes bonariensis (10). Another study indicated that exposure to 18 μg/L concentration of glyphosate resulted in elevated energy expenditure, and a reduction in the levels of glycogen and triglycerides in the muscle of bullfrog tadpoles (9). Researchers have also investigated the effects of glyphosate treatment on muscle morphology and morphometry. In one study, the administration of water containing 0.5% glyphosate to pregnant C57BL/6 mice during both pregnancy and lactation periods did not reveal statistically significant differences in the morphology of muscle fibers and connective tissue (15). However, in another study focusing on the male offspring of these mice, a decrease in neuromuscular junctions, along with an increase in fibrosis, was observed in the soleus muscle (12). Additionally, one study examined the effect of chronic oral glyphosate (10 μg/kg for 30 days) on muscle strength in rats. The muscle contraction power decreased to 41% of the control values (13). In summary, glyphosate exposure may reduce energy reserves, alter muscle morphology and function, and reduce muscle strength. However, these studies have yielded inconsistent results, and further research is necessary.
It has been known that a decrease in neuromuscular function and a loss of motor neurons will reduce muscle fiber size and performance (38). Researchers have also explored the impact of GBH on neuromuscular junctions. In one study, exposure to 0.5 mg/L concentration of GBH inhibited muscle acetylcholinesterase enzyme activity and increased oxidative stress levels in Cyprinus carpio (11). However, another study involving male zebrafish showed that the activity of acetylcholinesterase remained unchanged in the muscles exposed to 5 or 10 mg/L of glyphosate during the first 96 h. Instead, there was an observed increase in the expression of lipid peroxidation levels (14). Numerous animal reports have also explored the impacts of glyphosate or GBH on the nervous system. In addition to their effects during early developmental stages (39), exposure in adulthood can induce significant alterations in both the structure and function of the nervous system (40, 41).
Most studies examining the effects of glyphosate on human health have primarily focused on the consequences of intoxication (42–44). Current epidemiological research suggests that exposure below tolerable levels is unlikely to result in adverse health effects. Nevertheless, the impact on skeletal muscles falls outside the scope of past investigations (45, 46). While there have been no previous epidemiological reports investigating the effects of glyphosate/GBH exposure on skeletal muscle, emerging research has indicated that glyphosate exposure may result in neurotoxic effects. In one occupational study, a positive correlation was found between the use of glyphosate and olfactory impairment (47), whereas another study reported a positive link between glyphosate exposure and macular degeneration (48). However, a prospective study involving Chinese farmers did not uncover any significant association between glyphosate exposure and an elevated risk of health issues, including abnormalities in nerve conduction (49, 50). Utilizing NHANES data, the current research identified an inverse relationship between glyphosate levels and hand grip strength. Additionally, the maximal grip strength threshold used in the study (maximal hand grip strength test <30 kg in men and < 20 kg in women) may not have been sensitive enough to detect differences in grip strength between individuals with varying glyphosate levels. If a causal link between glyphosate levels and hand grip strength is established, it could potentially lead to adverse effects on skeletal muscle among American adults exposed to glyphosate and GBH. Evidence suggests that glyphosate and GBH may have detrimental effects on skeletal muscle through various mechanisms, including neurotoxicity, interference with acetylcholinesterase enzyme activity, depletion of energy reserves, alteration of muscle morphology and function. Another possible explanation is that glyphosate exposure may indirectly affect hand grip strength by disrupting the gut microbiome. Glyphosate has been demonstrated to disrupt the composition of the gut microbiome in both animals and humans (51, 52), and some studies have suggested that changes in the gut microbiome can affect muscle function (53). Disruption of the gut microbiome could potentially lead to inflammation and oxidative stress in skeletal muscle, which could impair muscle function and reduce grip strength.
Our study revealed a detrimental interaction between age and glyphosate—specifically, as age increased, the negative impact of glyphosate on combined grip strength intensified. Existing literature suggests that age could play a role in influencing the sensitivity and vulnerability of the nervous system to glyphosate. Older individuals may exhibit lower levels of neurogenesis, neuroplasticity, and neurorepair, coupled with higher levels of oxidative stress and inflammation. These age-related factors may exacerbate the neurotoxic effects of glyphosate (54, 55). A similar correlation was observed in our study within skeletal muscle. Additionally, we noted that this association was more stronger in individuals with higher BMI. Several potential explanations exist for this finding. For example, research has shown that glyphosate can disrupt the gut microbiome, leading to increased inflammation and oxidative stress in the body (51, 56). This may have a greater impact on individuals with higher BMI, who are already at a higher risk of developing inflammation and oxidative stress-related diseases. As of our current understanding, this study represents the first instance in which specific demographic subgroups have been identified as potentially susceptible to the deleterious effects of glyphosate exposure on hand grip strength. Additional investigation is necessary to gain a complete understanding of the potential mechanisms underlying the observed distinctions.
It is crucial to acknowledge the study’s limitations when interpreting the results. Firstly, the study’s sample size was limited to data on glyphosate, grip strength, and DXA exams from NHANES 2013–2014, potentially constrained the feasibility of conducting a thorough analysis. Furthermore, NHANES is a valuable resource for assessing the health of the U.S. population, but it has inherent limitations as a cross-sectional study, such as the absence of detailed occupational exposure data and limited information on exposure routes and durations. Thirdly, urine glyphosate level can provide reliable estimates of actual internal human exposure that can be compared to appropriate reference values. However, the analytical methods used to measure urine glyphosate levels vary widely in terms of sensitivity, accuracy, and specificity, and that the sampling and storage conditions may affect the stability and representativeness of the samples (57). More research is needed to validate and standardize the measurement of urine glyphosate level and to elucidate the mechanisms and effects of glyphosate exposure on human health. Fourthly, the study did not consider the potential impact of other pollutants that may have been simultaneously exposed alongside glyphosate or could have affected the results. Fifthly, the age limitation of the DXA scans (18–60 years) compared to the broader age range examined in the overall study population (18 years and older) may be a potential source of ambiguity in the interpretation of the observed differences between lean mass and glyphosate. Future research should consider a more inclusive age range for relevant measurements to increase the robustness and applicability of the findings. Lastly, the study exclusively focused on adult individuals in the United States, which restricts the generalizability of the findings to other age groups and geographical regions.
After conducting an analysis of a representative sample of U.S. adults, our study has unveiled significant evidence pointing to an inverse connection between urinary glyphosate levels and hand grip strength. Furthermore, our findings hint at a potential negative correlation between glyphosate levels and lean body mass in both arms, although this relationship was only marginally significant. While additional research is needed to ascertain the clinical significance and causative factors behind these findings, our results underscore the importance of continuous investigation into the potential harmful effects of glyphosate on adult skeletal muscle. Such studies have the potential to inform public health policies regarding glyphosate usage, thereby contributing to the protection of human health.
The datasets analyzed during the current study are available at the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm (Accessed March 5, 2023).
The studies involving humans were approved by Ethics Committee of the En Chu Kong Hospital (ECKH_W11212). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Y-WF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. CW: Formal analysis, Methodology, Software, Supervision, Validation, Writing – original draft. C-YL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Software, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the National Science and Technology Council of Taiwan (NSTC 110-2314-B-385-001-MY3) and the Shin Kong Wu Ho-Su Memorial Hospital (2021SKHADR003).
We sincerely thank everyone who has contributed to the NHANES, including all participants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Peillex, C, and Pelletier, M. The impact and toxicity of glyphosate and glyphosate-based herbicides on health and immunity. J Immunotoxicol. (2020) 17:163–74. doi: 10.1080/1547691x.2020.1804492
2. Lacroix, R, and Kurrasch, DM. Glyphosate toxicity: in vivo, in vitro, and epidemiological evidence. Toxicol Sci. (2023) 192:131–40. doi: 10.1093/toxsci/kfad018
3. Benbrook, CM. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur. (2016) 28:3. doi: 10.1186/s12302-016-0070-0
4. Ospina, M, Schütze, A, Morales-Agudelo, P, Vidal, M, Wong, L-Y, and Calafat, AM. Exposure to glyphosate in the United States: data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int. (2022) 170:107620. doi: 10.1016/j.envint.2022.107620
5. de Araujo, JS, Delgado, IF, and Paumgartten, FJ. Glyphosate and adverse pregnancy outcomes, a systematic review of observational studies. BMC Public Health. (2016) 16:472. doi: 10.1186/s12889-016-3153-3
6. Muñoz, JP, Bleak, TC, and Calaf, GM. Glyphosate and the key characteristics of an endocrine disruptor: a review. Chemosphere. (2021) 270:128619. doi: 10.1016/j.chemosphere.2020.128619
7. Barnor, K, Caton, J, and Miljkovic, D. The role of funding on research and science: the impact of glyphosate herbicides on health and the environment. J Policy Model. (2023) 45:103–20. doi: 10.1016/j.jpolmod.2023.01.001
8. Vanlaeys, A, Dubuisson, F, Seralini, GE, and Travert, C. Formulants of glyphosate-based herbicides have more deleterious impact than glyphosate on Tm4 Sertoli cells. Toxicol In Vitro. (2018) 52:14–22. doi: 10.1016/j.tiv.2018.01.002
9. Dornelles, MF, and Oliveira, GT. Toxicity of atrazine, glyphosate, and Quinclorac in bullfrog tadpoles exposed to concentrations below legal limits. Environ Sci Pollut Res Int. (2016) 23:1610–20. doi: 10.1007/s11356-015-5388-4
10. Menéndez-Helman, RJ, Miranda, LA, dos Santos, AM, and Salibián, A. Subcellular energy balance of Odontesthes bonariensis exposed to a glyphosate-based herbicide. Ecotoxicol Environ Saf. (2015) 114:157–63. doi: 10.1016/j.ecoenv.2015.01.014
11. Cattaneo, R, Clasen, B, Loro, VL, de Menezes, CC, Pretto, A, Baldisserotto, B, et al. Toxicological responses of Cyprinus Carpio exposed to a commercial formulation containing glyphosate. Bull Environ Contam Toxicol. (2011) 87:597–602. doi: 10.1007/s00128-011-0396-7
12. Barbosa, A, Zazula, MF, Oliveira, MC, Teleken, JL, Costa, RM, Bonfleur, ML, et al. Maternal exposure to glyphosate-based herbicide promotes changes in the muscle structure of C57bl/6 mice offspring. Anat Rec. (2022) 305:3307–16. doi: 10.1002/ar.24922
13. Nozdrenko, D, Abramchuk, O, Prylutska, S, Vygovska, O, Soroca, V, Bogutska, K, et al. Analysis of biomechanical parameters of muscle soleus contraction and blood biochemical parameters in rat with chronic glyphosate intoxication and therapeutic use of C(60) fullerene. Int J Mol Sci. (2021) 22:94977. doi: 10.3390/ijms22094977
14. Lopes, FM, Caldas, SS, Primel, EG, and da Rosa, CE. Glyphosate adversely affects Danio rerio males: acetylcholinesterase modulation and oxidative stress. Zebrafish. (2017) 14:97–105. doi: 10.1089/zeb.2016.1341
15. Barbosa, A, Oliveira, MC, Kuhn-Fraga, C, Ribeiro, LFC, Balbo, SL, and Torrejais, MM. Study of muscle fibers of the extensor digitorium longus and soleus muscles of C57bl/6 females exposed to glyphosate during pregnancy and lactation. Einstein (São Paulo). (2021) 19:eAO5657. doi: 10.31744/einstein_journal/2021AO5657
16. Kim, JK, Park, MG, and Shin, SJ. What is the minimum clinically important difference in grip strength? Clin Orthop Relat Res. (2014) 472:2536–41. doi: 10.1007/s11999-014-3666-y
17. Bohannon, RW, Wang, YC, Yen, SC, and Grogan, KA. Handgrip strength: a comparison of values obtained from the Nhanes and Nih toolbox studies. Am J Occup Ther. (2019) 73:7302205080p1–9. doi: 10.5014/ajot.2019.029538
18. Scafoglieri, A, and Clarys, JP. Dual energy X-ray absorptiometry: gold standard for muscle mass? J Cachexia Sarcopenia Muscle. (2018) 9:786–7. doi: 10.1002/jcsm.12308
19. Perry, BD, Caldow, MK, Brennan-Speranza, TC, Sbaraglia, M, Jerums, G, Garnham, A, et al. Muscle atrophy in patients with type 2 diabetes mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc Immunol Rev. (2016) 22:94–109.
20. Cheng, T-C, Huang, S-H, Kao, C-L, and Hsu, P-C. Muscle wasting in chronic kidney disease: mechanism and clinical implications & Mdash; a narrative review. Int J Mol Sci. (2022) 23:6047. doi: 10.3390/ijms23116047
21. CDC. Nhanes 2013–2014; (2016). Available at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013. (Accessed October 03, 2022)
22. Schütze, A, Morales-Agudelo, P, Vidal, M, Calafat, AM, and Ospina, M. Quantification of glyphosate and other organophosphorus compounds in human urine via ion chromatography isotope dilution tandem mass spectrometry. Chemosphere. (2021) 274:129427. doi: 10.1016/j.chemosphere.2020.129427
23. CDC. 2013–2014 Data Documentation, Codebook, and Frequencies: Glyphosate (Glyp) Centers for Disease Control and Prevention, National Center for Health Statistics. (2022). Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/SSGLYP_H.htm. (Accessed Feburary 11, 2023)
24. Cruz-Jentoft, AJ, Baeyens, JP, Bauer, JM, Boirie, Y, Cederholm, T, Landi, F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
25. CDC. 2013–2014 Data Documentation, Codebook, and Frequencies Muscle Strength - Grip Test Center of Disease Control; (2016). Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/MGX_H.htm. (Accessed March 29, 2023).
26. CDC. 2013-2014 Data Documentation, Codebook, and Frequencies: Dual-Energy X-ray Absorptiometry - Whole Body: Center of Disease Control (2020). Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DXX_H.htm. (Accessed April 05, 2023)
27. CDC. National Health and Nutrition Examination Survey (NHANES): Smoking Center of Disease Control and Prevention (2016). Available at: http://wwwn.cdc.gov/nchs/nhanes/search/DataPage.aspx?Component=Questionnaire&CycleBeginYear=2013. (Accessed October 03, 2022)
28. CDC. 2013-2014 Data Documentation, Codebook, and Frequencies Physical Activity: Center of Disease Control; (2017). Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/PAQ_H.htm#Appendix_1.__Suggested_MET_Scores. (Accessed March 30)
29. Lees, JS, Rutherford, E, Stevens, KI, Chen, DC, Scherzer, R, Estrella, MM, et al. Assessment of cystatin C level for risk stratification in adults with chronic kidney disease. JAMA Netw Open. (2022) 5:e2238300. doi: 10.1001/jamanetworkopen.2022.38300
30. CDC. Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES): National Center for Health Statistics; (2005). Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. (Accessed October 3, 2023).
31. O'Brien, KM, Upson, K, Cook, NR, and Weinberg, CR. Environmental Chemicals in Urine and Blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. (2016) 124:220–7. doi: 10.1289/ehp.1509693
32. Grau, D, Grau, N, Gascuel, Q, Paroissin, C, Stratonovitch, C, Lairon, D, et al. Quantifiable urine glyphosate levels detected in 99% of the French population, with higher values in men, in younger people, and in farmers. Environ Sci Pollut Res. (2022) 29:32882–93. doi: 10.1007/s11356-021-18110-0
33. Nova, P, Calheiros, CSC, and Silva, M. Glyphosate in Portuguese adults – a pilot study. Environ Toxicol Pharmacol. (2020) 80:103462. doi: 10.1016/j.etap.2020.103462
34. Gillezeau, C, van Gerwen, M, Shaffer, RM, Rana, I, Zhang, L, Sheppard, L, et al. The evidence of human exposure to glyphosate: a review. Environ Health. (2019) 18:2. doi: 10.1186/s12940-018-0435-5
35. Zoller, O, Rhyn, P, Zarn, JA, and Dudler, V. Urine glyphosate level as a quantitative biomarker of Oral exposure. Int J Hyg Environ Health. (2020) 228:113526. doi: 10.1016/j.ijheh.2020.113526
36. Soares, D, Silva, L, Duarte, S, Pena, A, and Pereira, A. Glyphosate use, toxicity and occurrence in food. Food Secur. (2021) 10:2785. doi: 10.3390/foods10112785
37. Ashley-Martin, J, Huang, R, MacPherson, S, Brion, O, Owen, J, Gaudreau, E, et al. Urinary concentrations and determinants of glyphosate and Glufosinate in pregnant Canadian participants in the Mirec study. Environ Res. (2023) 217:114842. doi: 10.1016/j.envres.2022.114842
38. Aagaard, P, Suetta, C, Caserotti, P, Magnusson, SP, and Kjaer, M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. (2010) 20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x
39. Ait-Bali, Y, Ba-M'hamed, S, Gambarotta, G, Sassoè-Pognetto, M, Giustetto, M, and Bennis, M. Pre- and postnatal exposure to glyphosate-based herbicide causes behavioral and cognitive impairments in adult mice: evidence of cortical ad hippocampal dysfunction. Arch Toxicol. (2020) 94:1703–23. doi: 10.1007/s00204-020-02677-7
40. Costas-Ferreira, C, Durán, R, and Faro, LRF. Toxic effects of glyphosate on the nervous system: a systematic review. Int J Mol Sci. (2022) 23:4605. doi: 10.3390/ijms23094605
41. Baier, CJ, Gallegos, CE, Raisman-Vozari, R, and Minetti, A. Behavioral impairments following repeated intranasal glyphosate-based herbicide Administration in Mice. Neurotoxicol Teratol. (2017) 64:63–72. doi: 10.1016/j.ntt.2017.10.004
42. Cellier, M, Anthony, N, Bruneau, C, and Descatha, A. Determination of glyphosate and Ampa in blood can predict the severity of acute glyphosate herbicide poisoning. Lab Med. (2022) 53:394–8. doi: 10.1093/labmed/lmac002
43. Dou, JR, Zhou, X, Miao, RF, Yang, Y, Liu, X, Zhang, F, et al. Analysis of clinical characteristics and prognostic factors in 40 cases of acute glyphosate poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2021) 39:676–81. doi: 10.3760/cma.j.cn121094-20201030-00601
44. Lee, HL, Chen, KW, Chi, CH, Huang, JJ, and Tsai, LM. Clinical presentations and prognostic factors of a glyphosate-surfactant herbicide intoxication: a review of 131 cases. Acad Emerg Med. (2000) 7:906–10. doi: 10.1111/j.1553-2712.2000.tb02069.x
45. Agostini, LP, Dettogni, RS, Dos Reis, RS, Stur, E, Dos Santos, EVW, Ventorim, DP, et al. Effects of glyphosate exposure on human health: insights from epidemiological and in vitro studies. Sci Total Environ. (2020) 705:135808. doi: 10.1016/j.scitotenv.2019.135808
46. Berni, I, Menouni, A, Creta, M, El Ghazi, I, Duca, R-C, Godderis, L, et al. Exposure of children to glyphosate in Morocco: urinary levels and predictors of exposure. Environ Res. (2023) 217:114868. doi: 10.1016/j.envres.2022.114868
47. Shrestha, S, Umbach, DM, Beane Freeman, LE, Koutros, S, Alavanja, MCR, Blair, A, et al. Occupational pesticide use and self-reported olfactory impairment in us farmers. Occup Environ Med. (2021) 78:179–91. doi: 10.1136/oemed-2020-106818
48. Montgomery, MP, Postel, E, Umbach, DM, Richards, M, Watson, M, Blair, A, et al. Pesticide use and age-related macular degeneration in the agricultural health study. Environ Health Perspect. (2017) 125:077013. doi: 10.1289/EHP793
49. Zhang, C, Hu, R, Huang, J, Huang, X, Shi, G, Li, Y, et al. Health effect of agricultural pesticide use in China: implications for the development of gm crops. Sci Rep. (2016) 6:34918. doi: 10.1038/srep34918
50. Zhang, C, Sun, Y, Hu, R, Huang, J, Huang, X, Li, Y, et al. A comparison of the effects of agricultural pesticide uses on peripheral nerve conduction in China. Sci Rep. (2018) 8:9621. doi: 10.1038/s41598-018-27713-6
51. Hu, J, Lesseur, C, Miao, Y, Manservisi, F, Panzacchi, S, Mandrioli, D, et al. Low-dose exposure of glyphosate-based herbicides disrupt the urine metabolome and its interaction with gut microbiota. Sci Rep. (2021) 11:3265. doi: 10.1038/s41598-021-82552-2
52. Puigbò, P, Leino, LI, Rainio, MJ, Saikkonen, K, Saloniemi, I, and Helander, M. Does glyphosate affect the human microbiota? Life (Basel). (2022) 12:707. doi: 10.3390/life12050707
53. Lustgarten, MS. The role of the gut microbiome on skeletal muscle mass and physical function: 2019 update. Front Physiol. (2019) 10:1435. doi: 10.3389/fphys.2019.01435
54. Winstone, JK, Pathak, KV, Winslow, W, Piras, IS, White, J, Sharma, R, et al. Glyphosate infiltrates the brain and increases pro-inflammatory cytokine Tnfα: implications for neurodegenerative disorders. J Neuroinflammation. (2022) 19:193. doi: 10.1186/s12974-022-02544-5
55. Bloem, BR, Boonstra, TA, Elbaz, A, and Vermeulen, RCH. Glyphosate and neurotoxicity — a call for scientific renewal. Nat Rev Neurol. (2024). doi: 10.1038/s41582-023-00919-7 (Online ahead of print).
56. Aoun, A, Darwish, F, and Hamod, N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and Synbiotics for weight loss. Prev Nutr Food Sci. (2020) 25:113–23. doi: 10.3746/pnf.2020.25.2.113
Keywords: glyphosate exposure, herbicide effects, muscle strength, National Health and Nutrition Examination Survey (NHANES) data analysis, muscle mass assessment, sarcopenia risk factors
Citation: Fang Y-W, Wang C and Lin C-Y (2024) Association between urinary glyphosate levels and hand grip strength in a representative sample of US adults: NHANES 2013–2014. Front. Public Health. 12:1352570. doi: 10.3389/fpubh.2024.1352570
Received: 13 December 2023; Accepted: 01 February 2024;
Published: 21 February 2024.
Edited by:
Ariane Zamoner, Federal University of Santa Catarina, BrazilReviewed by:
Sotirios Maipas, National and Kapodistrian University of Athens, GreeceCopyright © 2024 Fang, Wang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Yu Lin, MDA3MjRAa20uZWNrLm9yZy50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.