
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health, 31 July 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1345328
This article is part of the Research TopicExploring the Dynamics of Emerging and Re-emerging Diseases: From Origins to ImpactView all 9 articles
Introduction: Tuberculosis (TB) remains a leading cause of mortality worldwide. We conducted this systematic review to understand the distribution of bovine and zoonotic tuberculosis in the World Health Organization (WHO)’s Southeast Asia Region (SEAR) and Western Pacific Region (WPR) to inform our understanding of the risk posed by this disease.
Methods: A two-pronged strategy was used by evaluating data from peer-reviewed literature and official reports. A systematic search was conducted using a structured query in four databases (Web of Science, Scopus, Medline, and PubMed) to identify any reports of the occurrence of zoonotic TB. No language and time constraints were used during the search, but non-English language articles were later excluded. The official data were sourced from the World Organization for Animal Health’s (WOAH) World Animal Health Information System (WAHIS) and WHO’s global TB database.
Results: The retrieved records from SEAR and WPR (n = 113) were screened for eligibility, and data about disease occurrence were extracted and tabulated. In SEAR, all of the five studies that conducted Mycobacterium speciation (5/6) in humans were from India, and the reported Mycobacterium species included M. tuberculosis, M. bovis, M. scrofulacium, M. kansasii, M. phlei, M. smegmatis and M. orygis. In WPR, Mycobacterium speciation investigations in humans were conducted in Australia (8), China (2), Japan (2), NewZealand (2) and Malaysia (1), and the reported Mycobacterium species included M. bovis, M. africanum and M. tuberculosis. Seven countries in WHO’s SEAR have officially reported the occurrence of Mycobacterium bovis in their animals: Bangladesh, India, Indonesia, Myanmar, Nepal, Sri Lanka and Thailand. In WPR, the WAHIS information system includes reports of the identification of M. bovis from 11 countries – China, Fiji, Japan, Malaysia, Mongolia, New Zealand, the Philippines, the Republic of Korea, Singapore, Tonga and Viet Nam. In contrast, human zoonotic TB cases in the WHO database were only listed from Australia, Brunei Darussalam and Palau countries.
Discussion: The available data suggests under-reporting of zoonotic TB in the regions. Efforts are required to strengthen zoonotic TB surveillance systems from both animal and human health sides to better understand the impact of zoonotic TB in order to take appropriate action to achieve the goal of ending the TB epidemic.
Tuberculosis (TB) is a global public health emergency and the leading cause of infectious disease-related mortality, after COVID-19 (1). It predominantly affects marginalized populations and is associated with poverty (2). Although the number of TB deaths declined from 2005 to 2019, the COVID-19 pandemic has reversed this trend due to impeded access to TB services (1). In 2021, an estimated 10.6 million people were infected with TB, resulting in 1.6 million deaths (1). In line with the United Nations Sustainable Development Goal of ending the TB epidemic, the WHO developed The End TB strategy to reduce incidence by 80% and deaths due to TB by 90% compared to 2015 levels and eliminate associated socio-economic impacts by 2030 (3). The strategy has been prioritized in WHO’s Southeast Asia and Western Pacific regions (SEAR and WPR), the two most populous WHO regions accounting for more than half of the world’s population, with the development of region-specific strategies (4–7). The global targets are currently off-track as the target to reduce TB incidence by 80% and deaths by 90% compared to 2015 levels by 2030 seems unachievable.
Human TB is primarily caused by Mycobacterium tuberculosis, but zoonotic TB caused by M. bovis also contributes to the disease burden. Approximately 140,000 people globally (including 43,400 from SEAR and 18,000 from WPR) contracted zoonotic TB in 2019, resulting in about 11,400 (including 2,020 from SEAR and 270 from WPR) deaths (8). Zoonotic TB in people predominantly arises from the transmission of M. bovis from cattle, referred to as bovine TB. M. bovis not only impacts human health but also causes economic losses and trade barriers, affecting the livelihoods of marginalized communities. Diagnosing and treating zoonotic TB presents challenges because M. bovis is naturally resistant to pyrazinamide, a standard first-line TB treatment drug, and routine surveillance systems do not identify zoonotic TB cases. Investigating and acting on zoonotic TB is significant for protecting public health, safeguarding economic stability and ensuring global health security. Zoonotic TB poses a direct threat to public health. Humans can contract the disease through direct contact with infected animals or consumption of contaminated animal products, leading to severe health complications. Secondly, zoonotic TB has substantial economic implications. The disease affects livestock, reducing productivity and causing economic losses for farmers and communities dependent on animal husbandry. Additionally, M. bovis is often associated with extra-pulmonary TB. The Survival and ongoing transmission of M. bovis in its animal reservoirs is a serious deterrent for The End TB strategy in human populations, in particular when human–livestock interactions are not uncommon in TB-endemic areas.
In 2017, WHO, the World Organization for Animal Health (WOAH), the Food and Agriculture Organization of the United Nations (FAO), and the International Union Against Tuberculosis and Lung Disease (The Union) launched the ‘Roadmap for Zoonotic TB’. This roadmap recommends using the One Health approach to tackle zoonotic TB in animals and humans and identifies ten priorities to reduce disease transmission at the human-animal interface. The reduction of transmission at this interface is one of the three recommended axes for investment outlined by the roadmap. More recently, WHO, FAO, and WOAH published the Tripartite Zoonoses Guide, promoting operational multisectoral tools and one health approach to address zoonotic diseases (9). Additionally, FAO, United Nations Environment Programme (UNEP), WOAH, and WHO (collectively known as the Quadripartite) have developed the One Health Joint Plan of Action (2022–2026) to address challenges at the human-animal–plant-environment interface on regional, national, and global scales (10).
We conducted this systematic review of scientific literature and official databases of WHO and WOAH to understand the current status of bovine and zoonotic TB in WHO’s SEAR and WPR to provide an updated consolidated evidence base on the burden of zoonotic and bovine TB. This information will be used to assess the level of implementation of the Roadmap.
We used a two-pronged strategy by evaluating the peer-review literature and data from official reports.
We conducted the review for all 11 countries in SEAR: Bangladesh, Bhutan, Democratic People’s Republic of Korea, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, Thailand, and Timor-Leste (11). All 37 countries in WPR were also included. These were: American Samoa, Australia, Brunei Darussalam, Cambodia, China, Cook Islands, Fiji, French Polynesia (France), Guam (USA), Hong Kong SAR (China), Japan, Kiribati, Lao People’s Democratic Republic, Macao SAR (China), Malaysia, Marshall Islands, Micronesia Federated States of, Mongolia, Nauru, New Caledonia (France), New Zealand, Niue, Northern Mariana Islands Commonwealth of the (USA), Palau, Papua New Guinea, Philippines, Pitcairn Island (UK), Republic of Korea, Samoa, Singapore, Solomon Islands, Tokelau (New Zealand), Tonga, Tuvalu, Vanuatu, Viet Nam, Wallis and Futuna (France) (12) (Figure 1).
Following the PRISMA guidelines (Supplementary Tables S1, S2), we conducted a systematic review to investigate the occurrence of bovine TB and zoonotic TB among animals and humans in the selected countries (13). The search was conducted in August 2022 using four databases – Web of Science, Scopus, Medline, and PubMed. The search strategy described in Box 1 was applied, restricting the specified keywords to the title/abstract of the article. No language and time constraints were used during the search, but non-English language articles were later excluded. The search records were imported to Endnote, duplicate entries were removed, and the abstracts and titles were screened for eligibility using the criteria presented in Table 1. Two reviewers (BBS and ND) independently screened and collected the TB reporting data. In addition, data about the host country, Mycobacterium species involved, hosts, and other relevant factors were extracted.
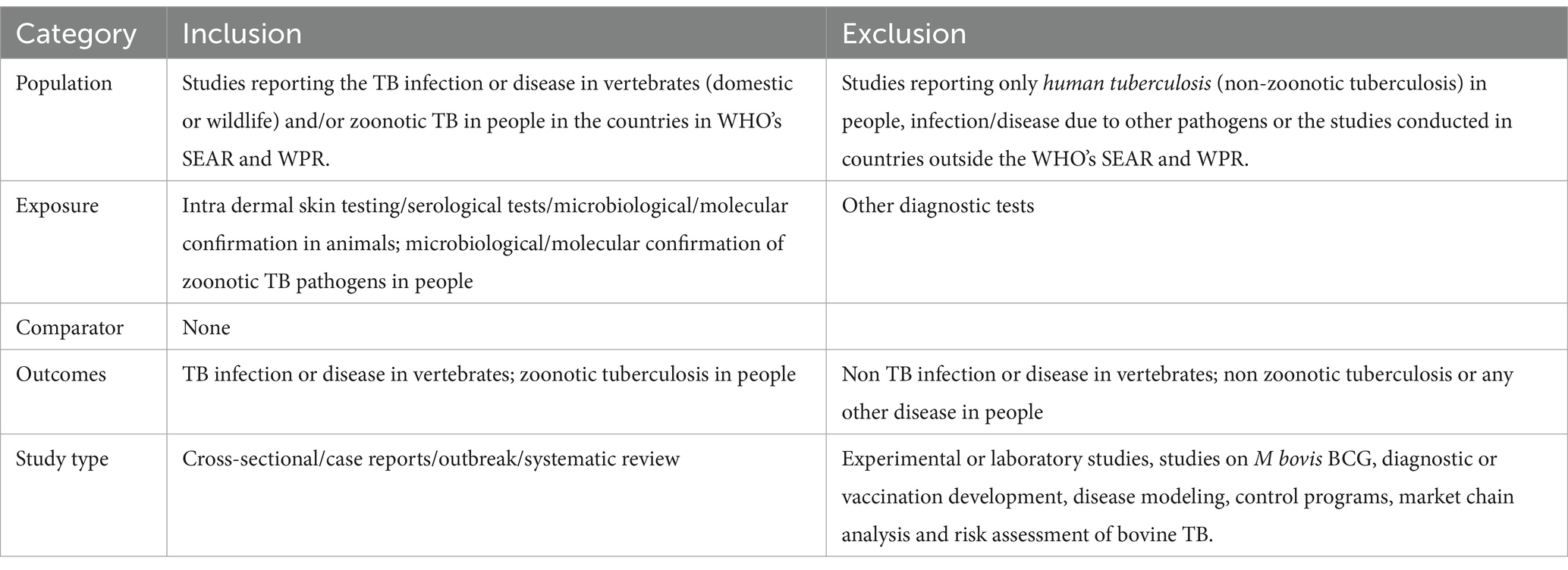
Table 1. Eligibility criteria used to select research articles on zoonotic/bovine TB in the target countries of WHO’s Southeast Asia and Western Pacific regions.
BOX 1 Queries used to search for peer-reviewed articles about the occurrence of zoonotic/bovine TB in the target countries of WHO’s SEAR and WPR.
Official animal disease report data were sourced from WOAH’s World Animal Health Information System – WAHIS (14). Data on human cases of zoonotic TB were sourced from WHO’s global TB database. These were only available for 2018 (15).
Detailed information about the number of studies selected at each stage in SEAR and WPR is provided in Figures 2, 3. Data extracted from 113 selected studies conducted in both regions (SEAR = 42; WPR = 71) are presented in Supplementary Tables S3, S4. These studies were conducted in India (25), New Zealand (23), Australia (18), China (12), the Republic of Korea (11), Bangladesh (6), Nepal (5), Thailand (5), Japan (3), Malaysia (2), Fiji (1), Lao PDR (1) and Sri Lanka (1) (Table 2). Overall, limited studies have been conducted to assess zoonotic TB in humans in South East Asia and Western Pacific countries (Supplementary Tables S3, S4). No data on the status of zoonotic TB or bovine TB are available from many South East Asia and Western Pacific region countries.
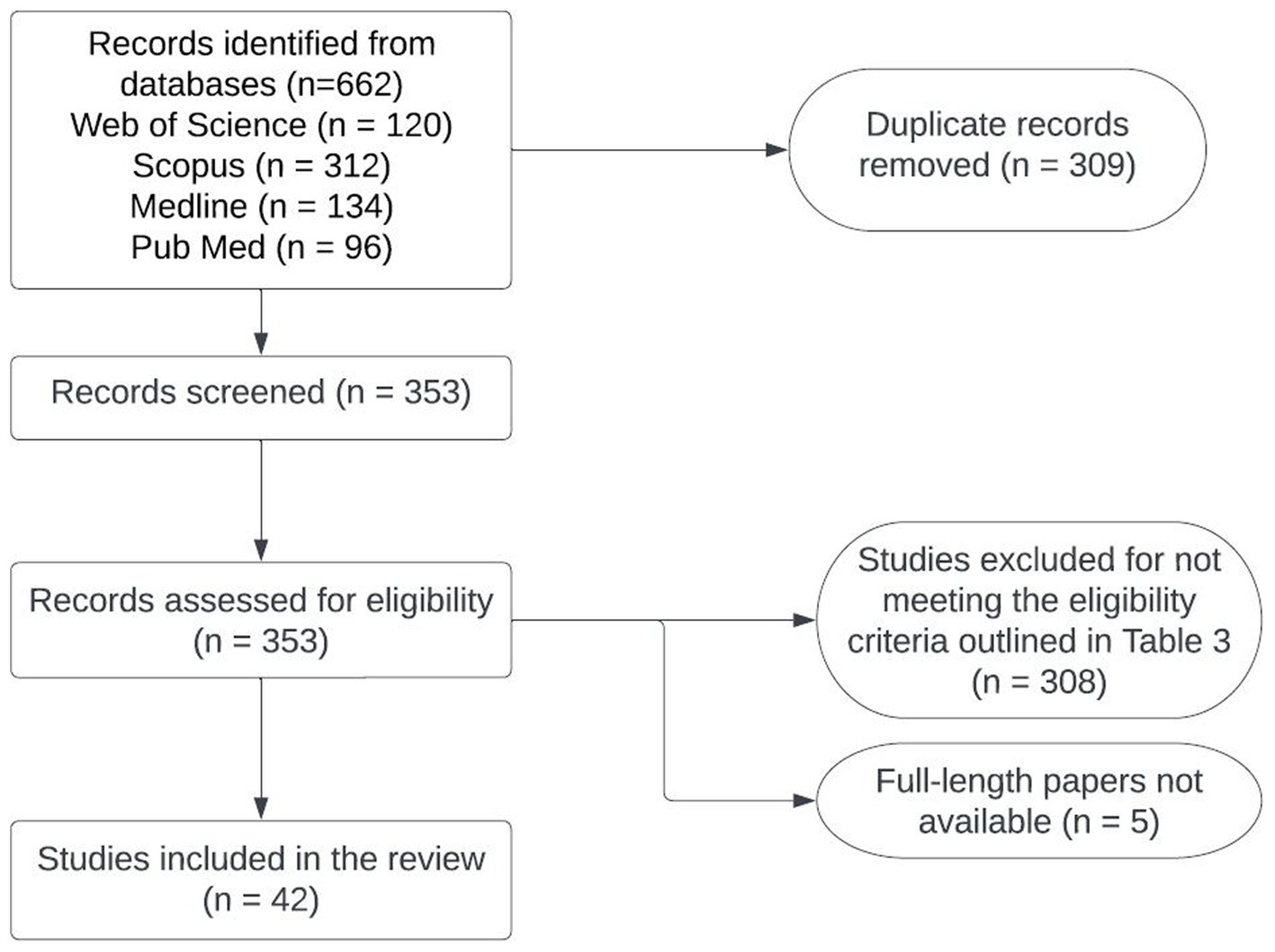
Figure 2. Flowchart of the process used for selecting studies to conduct situation analyses of zoonotic TB in Southeast Asia.
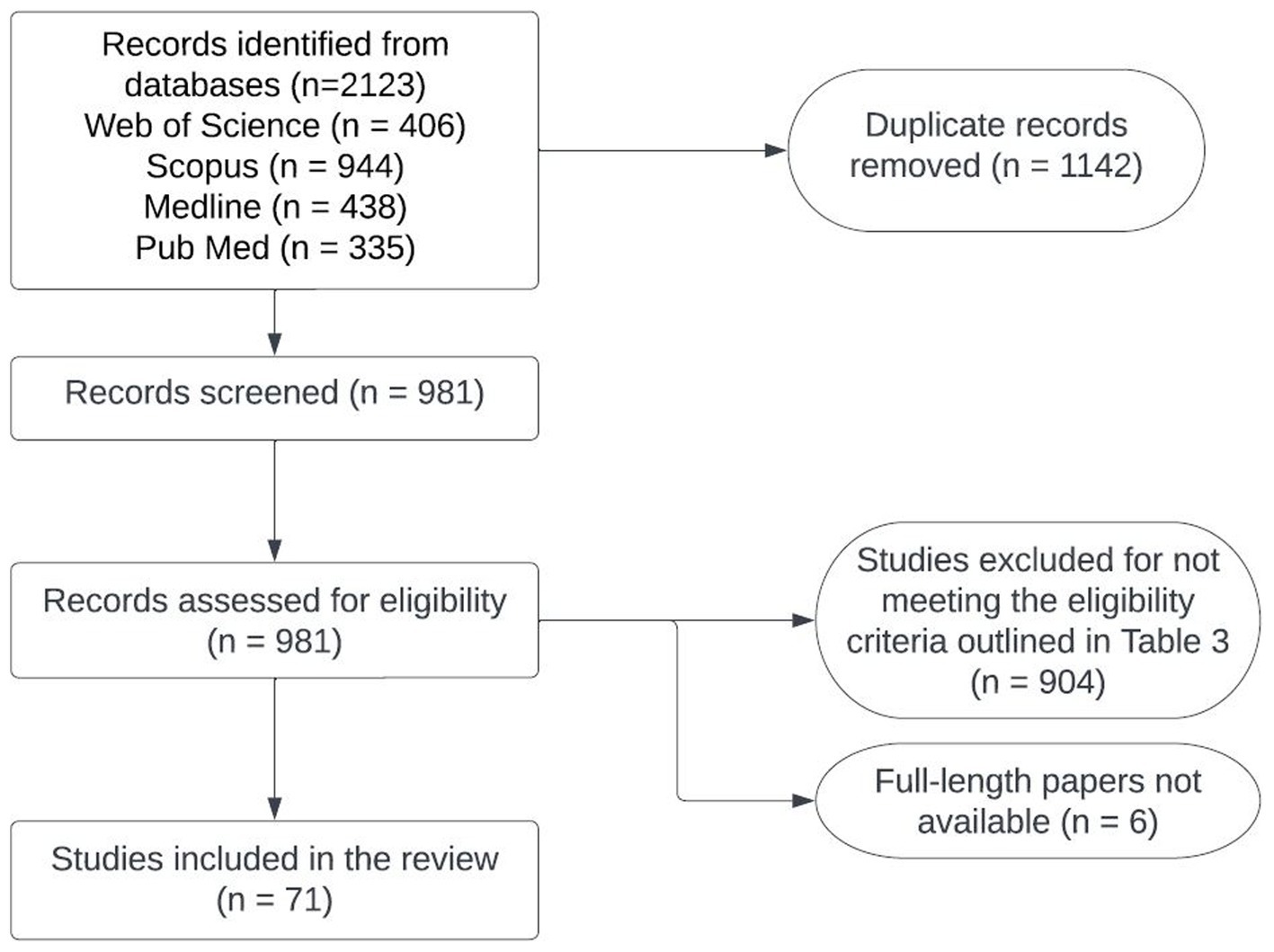
Figure 3. Flowchart of the process used for selecting studies to conduct situation analyses of zoonotic TB in the Western Pacific region.
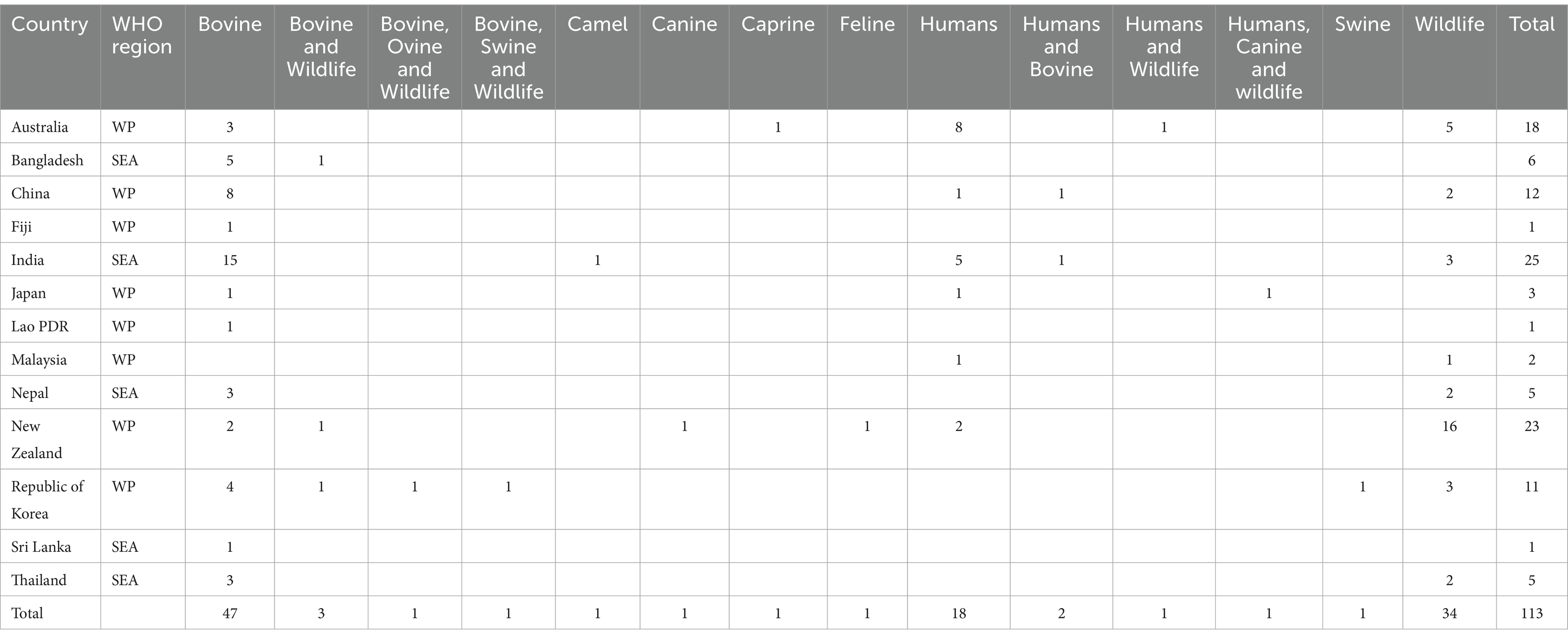
Table 2. Studies conducted on bovine TB or zoonotic TB in different countries in the Southeast Asia and Western Pacific regions.
There were 90.9% (20/22) investigations where Mycobacterium speciation was conducted in humans – 83.3% (5/6/) in SEAR and 93.7% (15/16) in the WPR. In SEAR, all the five studies that conducted Mycobacterium speciation (5/6) in humans were from India, and the reported Mycobacterium species included M. tuberculosis, M. bovis, M. scrofulacium, M. kansasii, M. phlei, M. smegmatis and M. orygis. In the WPR, the Mycobacterium speciation investigations in humans were conducted in Australia (8), China (2), Japan (2), NewZealand (2) and Malaysia (1), and the reported Mycobacterium species included M. bovis, M. africanum and M. tuberculosis. M. bovis-associated zoonotic TB cases were reported from India. Cases of zoonotic TB have also been reported in Australia, New Zealand and China. M. orygis has been reported in human patients in India.
There were 64.9% (61/94) investigations where Mycobacterium speciation was conducted in animals – 56.7% (21/37) in SEAR and 70.1% (40/57) in the WPR. In SEAR, the pathogens reported from bovines and wildlife included M. africanum subtype I (human pathogen), M. bovis, M. orygis, M. tuberculosis, M. fortuitum, M. phlei and M. smegmatis (Supplementary Tables S3, S4). Bovine TB cases associated with M. orygis have been reported from Bangladesh, India, and Nepal (Supplementary Table S3). In the studies with speciation data in South East Asia, M. bovis TB infections were reported from Bangladesh, India, Nepal, Sri Lanka and Thailand (Supplementary Table S3). In WPR, the pathogens reported from bovine and wildlife included M. bovis, M. orygis, M. tuberculosis, M. elephantis, M. pinnipedii, and M. chelonae (Supplementary Table S4). In the studies with speciation data in WPR, M. bovis TB was reported in China, South Korea, and New Zealand. Australia has successfully eradicated bovine TB. A bovine TB case associated with M. orygis has been reported from New Zealand (Supplementary Table S4).
Mycobacterium bovis, M. orygis and M. tuberculosis have been reported from wildlife in SEAR (Supplementary Table S3). M. bovis and M. pinnipedii have been reported from wildlife in the WPR (Supplementary Table S4).
The official TB data from WOAH and WHO are summarized in Supplementary Tables S5, S6, and Table 3. Briefly, bovine TB infection in domestic animals in SEAR was reported from Bangladesh, India, Indonesia, Myanmar, Nepal, Sri Lanka and Thailand during 2005–2018 (Supplementary Table S5). In WPR, bovine TB infection among domestic animals was reported in China, Fiji, Japan, Malaysia, Mongolia, New Zealand, the Philippines, the Republic of Korea, Singapore, Tonga and Viet Nam (Supplementary Table S6). The bovine TB infection in wildlife was reported in New Zealand, Mongolia, and Singapore and reported and/or suspected in Fiji, Tonga and Vietnam (Supplementary Table S6). No bovine TB data were available from other countries. For zoonotic TB, M. bovis was reported in Australia, Brunei Darussalam and Palau in humans in 2018 (Table 3). Zoonotic TB data were missing from many countries in WPR and SEAR (Supplementary Tables S3, S4, and Table 3).
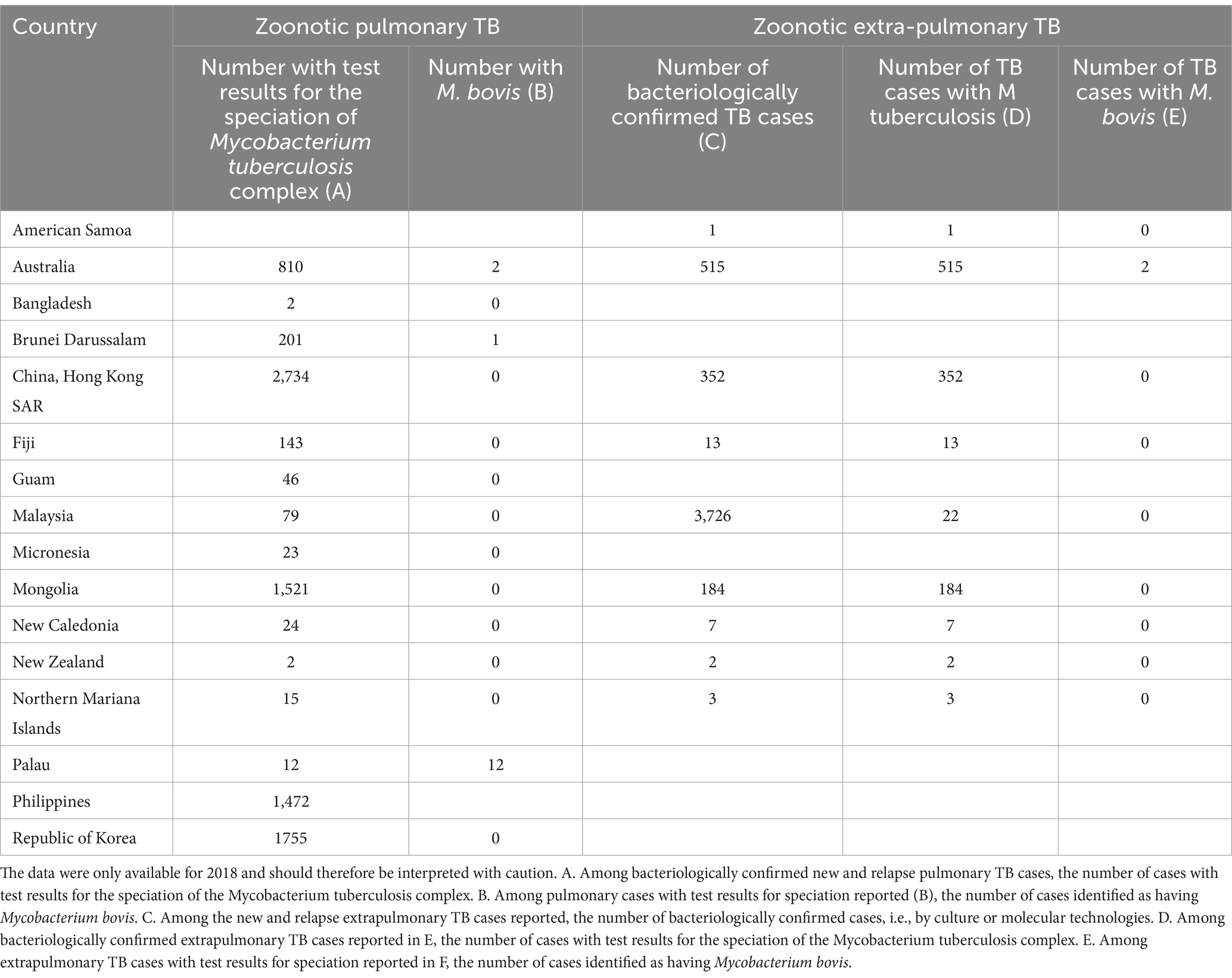
Table 3. WHO compiled zoonotic TB data from the Western Pacific and Southeast regions in 2018 under the global tuberculosis program (https://www.who.int/teams/global-tuberculosis-programme/data).
The Asia-Pacific region, comprising the WHO’s SEAR and WPR, is a major hotspot for TB, with a high burden of cases and deaths. These regions accounted for approximately 63% of the estimated 10.6 million global TB cases in 2021 (1). Four countries in WPR, namely Viet Nam, Lao People’s Democratic Republic (PDR), China, and the Philippines, accounted for 90% of new incident cases and 82% of TB-related deaths in 2019 (16). The COVID-19 pandemic has jeopardized the ongoing TB control efforts. To reinvigorate these efforts, the WHO’s SEAR office has developed a new strategic plan for the 2021–2025 (6).
This systematic review aimed to assess the burden of bovine and zoonotic TB in the region and inform the ongoing control efforts, particularly the Roadmap for zoonotic TB launched by the WHO in 2017. The review aimed to identify countries where bovine and zoonotic TB have been reported and countries with no available information on these forms of TB. The review findings indicate that zoonotic TB is not limited to infections caused by M. bovis. The role of M. orygis in zoonotic TB epidemiology is gaining importance and requires further investigation. M. orygis has been reported from human patients in India, and the pathogen has been reported from bovines in Bangladesh, India, and Nepal in South East Asia. A bovine case of M. orygis transmitted by an immigrant suffering with M. orygis associated TB has also been reported in New Zealand. Currently, the M. orygis appears to be circulating in India and its neighboring countries, but its global spread in the near future cannot be ruled out. Differentiating pathogen species in zoonotic TB infections is important for informing TB control efforts. Detecting M. tuberculosis infections in cattle and the possibility of human-to-bovine transmission can complicate TB eradication efforts.
The true impact of zoonotic TB in SEAR and WPR remains largely unknown. In 2019, there were an estimated 43,400 zoonotic TB cases and 2020 deaths in Southeast Asia, and 18,000 cases and 270 deaths in Western Pacific (8). Having reliable regional, national, and subnational data is crucial for policy development and determining zoonotic TB control priorities. In countries like Indonesia and Myanmar, among the 30 countries defined by WHO between 2021 and 2025 as high-TB burden (8), there is a lack of data on the presence/absence of bovine TB or on the prevalence of infection in cattle populations despite the fact that there are substantial cattle populations and interactions at the human-bovine interface in these countries. Efforts are needed to estimate the true burden of zoonotic TB in these regions.
The One Health approach, integrating zoonotic TB surveillance and control strategies in humans, bovines, and wildlife, has been advocated to end the zoonotic TB (17). This approach recommends estimating the zoonotic TB burden, using novel diagnostic tools, and implementing One Health interventions to improve zoonotic TB response and prevention (17). The approaches such as the estimation of zDALYs (zoonotic disability adjusted life years), including animal loss equivalents (ALE), have been recommended for zoonotic diseases (18). Developing prompt bovine and zoonotic TB notification capable surveillance systems, roadmaps for enhanced intersectoral coordination, and the use of both WOAH and WHO joint external evaluation tools is recommended. Encouraging milk pasteurization, mandatory meat inspection and condemnation of infected carcasses are important strategies to be adopted in the animal health sector (19). Environment focused strategies such as having dispersed water sources and the use of hedgerows on boundaries could reduce the risk of bTB transmission (19, 20). However, not much progress using such approaches has been achieved in many countries in the Southeast Asia and Western Pacific regions.
The lack of data reported to WHO for 2018 for zoonotic TB in people indicates that these regions need to establish zoonotic TB surveillance systems, strengthen diagnostic laboratory capacity, and incorporate species differentiation into routine TB diagnostic protocols.
The systematic review utilized multiple sources to compile data on bovine and zoonotic TB infections in the Southeast Asia and Western Pacific regions. However, the search strategy focused on country-level keywords, potentially excluding some studies. Additionally, the exclusion of non-English literature may have resulted in missed studies. It is also possible that studies available in national or subnational databases were not included. Nevertheless, the study’s purpose of highlighting the bovine and zoonotic TB situation in the regions was achieved.
In conclusion, zoonotic TB is not much investigated in SEAR and WPR, emphasizing the need to enhance surveillance and strengthen diagnostic laboratory capacity and capability to achieve the goal of human TB eradication.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
BS: Formal analysis, Writing – original draft. ND: Formal analysis, Methodology, Writing – review & editing. SC: Methodology, Writing – review & editing. AD: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. CM: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This survey was funded by TDR, the Special Programme for Research and Training in Tropical Diseases. TDR can conduct its work thanks to the commitment and support from a variety of funders. These include long-term core contributors from national governments and international institutions, as well as designated funding for specific projects within our current priorities. A full list of TDR donors is available at: https://www.who.int/tdr/about/funding/en/.
The authors thank the World Health Organization and World Organization for Animal Health (formerly OIE) for collecting and sharing the tuberculosis-related data. The contributions of the authors of the published research included in the systematic review are also thankfully acknowledged.
ND was employed by the company One Health Epi Consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AD and CM are currently staff members of the World Health Organization; the authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1345328/full#supplementary-material
1. WHO . (2022). Global tuberculosis report 2022. Geneva. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
2. Zumla, A, Raviglione, M, Hafner, R, and Fordham von Reyn, C. Tuberculosis. N Engl J Med Overseas Ed. (2013) 368:745–55. doi: 10.1056/NEJMra1200894
3. WHO . (2015). The end TB strategy. Geneva. Available at: https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy
4. WHO . (2015). World health organization. The end TB strategy. Available at: https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19
5. WHO . (2016). Regional Office for South-East Asia. Tuberculosis control in the South-East Asia Region: Annual Report 2016. World Health Organization. Available at: https://apps.who.int/iris/handle/10665/205286
6. WHO . (2021). Regional strategic plan towards ending TB in the WHO South-East Asia region: 2021–2025. Available at: https://www.who.int/publications/i/item/9789290228974
7. World Health Organization . (2022). Regional Office for the Western P. Western Pacific regional framework to end TB: 2021–2030: Brief summary. Manila: WHO Regional Office for the Western Pacific. Available at: https://apps.who.int/iris/handle/10665/352375
8. WHO . (2020). World Health Organization global tuberculosis report 2020. World Health Organization. Report No.: 924001313X. Available at: https://www.who.int/publications/i/item/9789240013131
9. FAO, WOAH, WHO . (2019). Taking a multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. Available at: https://www.who.int/initiatives/tripartite-zoonosis-guide.
10. WHO, FAO, WOAH, UNEP . (2022). One health joint plan of action (2022-2026). Working together for the health of humans, animals, plants and environment. Rome. Available from: https://www.who.int/publications/i/item/9789240059139
11. WHO . (2023). World Health Organisation – South-East Asia. Available at: https://www.who.int/southeastasia/about/contact
12. WHO . (2023). World Health Organization – Western Pacific. Available at: https://www.who.int/westernpacific/about/where-we-work
13. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. WOAH . (2023). World animal health information system (WAHIS). Available at: https://www.woah.org/en/what-we-do/animal-health-and-welfare/disease-data-collection
15. WHO . (2023). Global tuberculosis Programme. Available at: https://www.who.int/teams/global-tuberculosis-programme/data
16. Estill, J, Islam, T, Houben, RMGJ, Rudman, J, Ragonnet, R, McBryde, ES, et al. Tuberculosis in the Western Pacific region: estimating the burden of disease and return on investment 2020–2030 in four countries. Lancet Regional Health Western Pacific. (2021) 11:11. doi: 10.1016/j.lanwpc.2021.100147
17. Villa, S, Carugati, M, Rubach, M, Cleaveland, S, Mpagama, S, Khan, S, et al. One health´ approach to end zoonotic TB. Int J Tuberc Lung Dis. (2023) 27:101–5. doi: 10.5588/ijtld.22.0393
18. Torgerson, PR, Rüegg, S, Devleesschauwer, B, Abela-Ridder, B, Havelaar, AH, Shaw, APM, et al. zDALY: an adjusted indicator to estimate the burden of zoonotic diseases. One Health. (2018) 5:40–5. doi: 10.1016/j.onehlt.2017.11.003
19. Macedo Couto, R, Ranzani, OT, and Waldman, EA. Zoonotic tuberculosis in humans: control, surveillance, and the one health approach. Epidemiol Rev. (2019) 41:130–44. doi: 10.1093/epirev/mxz002
Keywords: bovine tuberculosis, Southeast Asia, roadmap for zoonotic tuberculosis, Western Pacific, zoonotic tuberculosis
Citation: Singh BB, Dhand NK, Cadmus S, Dean AS and Merle CS (2024) Systematic review of bovine and zoonotic tuberculosis in the Western Pacific and the Southeast Asia regions of the World Health Organization. Front. Public Health. 12:1345328. doi: 10.3389/fpubh.2024.1345328
Received: 27 November 2023; Accepted: 22 July 2024;
Published: 31 July 2024.
Edited by:
ThankGod Emmanuel Onyiche, University of Maiduguri, NigeriaReviewed by:
Jasini Musa, University of Maiduguri, NigeriaCopyright © 2024 Singh, Dhand, Cadmus, Dean and Merle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Balbir B. Singh, YmJzZGhhbGl3YWxAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.