
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 28 May 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1344089
Background: Despite the Ethiopian government included the Pneumococcal Conjugate Vaccine (PCV) in the national expanded program for immunization in 2011, only 56% of children aged 12–23 months received the full dose of PCV. Despite some studies on PCV uptake in Ethiopia, there was a dearth of information on the geographical distribution and multilevel factors of incomplete PCV uptake. Hence, this study aimed to identify the spatial variations and predictors of incomplete PCV uptake among children aged 12–35 months in Ethiopia.
Methods: The study was based on an in-depth analysis of 2016 Ethiopia Demographic Health Survey data, using a weighted sample of 3,340 women having children aged 12–35 months. Arc-GIS version 10.7 and SaTScan version 9.6 statistical software were used for the spatial analysis. To explore spatial variation and locate spatial clusters of incomplete PCV, the Global Moran's I statistic and Bernoulli-based spatial scan (SaTScan) analysis were carried out, respectively. A multilevel mixed-effect multivariable logistic regression was done by STATA version 16. Adjusted odds ratio (AOR) with its corresponding 95% CI was used as a measure of association, and variables with a p < 0.05 were deemed as significant determinants of incomplete PCV.
Results: The overall prevalence of incomplete PCV in Ethiopia was found to be 54.0% (95% CI: 52.31, 55.69), with significant spatial variation across regions (Moran's I = 0.509, p < 0.001) and nine most likely significant SaTScan clusters. The vast majority of Somali, southeast Afar, and eastern Gambela regions were statistically significant hot spots for incomplete PCV. Lacking ANC visits (AOR = 2.76, 95% CI: 1.91, 4.00), not getting pre-birth Tetanus injections (AOR = 1.84, 95% CI: 1.29, 2.74), home birth (AOR = 1.72, 95% CI: 1.23, 2.34), not having a mobile phone (AOR = 1.64, 95% CI: 1.38, 1.93), and residing in a peripheral region (AOR = 4.63; 95% CI: 2.34, 9.15) were identified as statistically significant predictors of incomplete PCV.
Conclusion: The level of incomplete PCV uptake was found to be high in Ethiopia with a significant spatial variation across regions. Hence, the federal and regional governments should collaborate with NGOs to improve vaccination coverage and design strategies to trace those children with incomplete PCV in peripheral regions. Policymakers and maternal and child health program planners should work together to boost access to maternal health services like antenatal care and skilled delivery services to increase immunization coverage.
Pneumococcal illness is an acute infection caused by the Streptococcus pneumoniae bacteria, which can cause serious invasive diseases such as pneumonia, meningitis, and septicemia as well as milder but more common illnesses such as sinusitis and otitis media (ear infections) in children (1). In 2015, these infections were responsible for 294,000 of the 5.83 million global deaths among children under the age of five (2). Streptococcus pneumoniae, the causal agent, frequently colonizes the human nasopharynx and is primarily transmitted through respiratory droplets (3, 4). Infants and young children are its main reservoirs, with greater carriage rates among children in low- and middle-income countries (LMICs) (3).
Pneumonia is a type of acute respiratory tract infection that affects the lungs and their parenchyma. It is the single most common infectious cause of death among children globally, killing 740, 180 children under the age of five in 2019, making up 14% of all deaths in children under the age of five but 22% of the deaths in children aged one to five (5). Sub-Saharan Africa carries the greatest (50%) burden of worldwide under-five mortality from pneumonia (6). A considerable number of children in Ethiopia had not been immunized (7). As a result, infant mortality in Ethiopia is among the highest in the world, and many of these deaths were thought to be caused by vaccine-preventable diseases (8). Pneumonia is the top cause of death among children under the age of five in Ethiopia. It is estimated that 3,370,000 children are affected by pneumonia each year, accounting for 20% of all causes of death and killing over 40,000 children under the age of five (9).
Vaccines are considered one of the most safe and cost-efficient interventions to combat childhood illness and death (10). Vaccination currently averts 2–3 million deaths worldwide each year (11). Pneumococcal conjugate vaccine (PCV) is the most effective intervention to prevent pneumonia in children (3, 12, 13). A double-blind, randomized controlled experiment showed that 13-valent PCV provided 78% protection against pneumococcus serotype 6B (BHN418) acquisition (14). Even though various vaccines have been manufactured to minimize or eliminate the burden of infections caused by S. pneumonia, the WHO presently recommends two types of vaccines, namely the unconjugated 23-valent polysaccharide vaccine (PPSV) and the 10- or 13-valent conjugated polysaccharide vaccine (PCV) (3, 15). Based on the national epidemiology of S. pneumoniae serotypes and cost, the WHO recommended that nations utilize either 10- or 13-valent vaccines in their national immunization programs (3). The 10-valent pneumococcal conjugate vaccine (PCV10) is made up of capsular polysaccharides isolated from ten serotypes and is conjugated to either protein D, tetanus toxoid, or diphtheria toxoid (3).
The Global Vaccine Action Plan (GVAP) suggests all countries should plan to reach 90% national coverage of all vaccines at the end of 2020. However, as per the WHO report, worldwide immunization coverage was only 85% in 2017, with 19.9 million children missing out on basic life-saving vaccines (11). By the end of 2022, PCV had been adopted in 155 WHO Member States, and global third dose (PCV3) coverage was predicted to be 60%. There is significant regional variation, with the WHO European Region expected to have 83% coverage, whereas the WHO Western Pacific Region has only 23% (11).
The Ethiopian government included the PCV10 in the national infant immunization program in November 2011 (16). It was given to children in three doses, during the sixth, tenth, and fourteenth weeks of life. However, when it was first introduced in Ethiopia, all children under the age of one had to be vaccinated as part of a catch-up program (16–18). However, the 2016 Ethiopian Demographic Health Survey (2016 EDHS) report showed that only 56% of children aged 12–23 months received the full dose of PCV (19). This result lags far behind the EPI and the GVAP, which set national vaccine coverage goals of 90% by 2020 (11). According to the reports, there is a significant disparity in immunization coverage between regions due to different challenges and possibilities. Access to health services, family economic status, place of delivery, antenatal care (ANC) visits, maternal education, knowledge of immunization, access to mass media, place of residence, and religion were also identified as factors influencing childhood vaccination (7, 20–22).
Although numerous studies on vaccine uptake have been undertaken in Ethiopia, they have primarily focused on overall immunization, with little emphasis placed on the spatial distribution and multilevel determinants of incomplete PCV uptake. Hence, this study aimed to identify the spatial variations as well as the individual and community-level predictors of missing PCV among children aged 12–35 months in Ethiopia. By incorporating spatial analysis into the assessment of PCV coverage, policymakers can make data-driven decisions to enhance vaccine delivery mechanisms, address disparities in coverage, and ultimately improve vaccination rates nationwide. This approach not only supports the achievement of vaccination targets but also contributes to the overarching goal of reducing pneumococcal-related morbidity and mortality, thereby advancing public health outcomes in Ethiopia.
Ethiopia is the second most populous country in Africa, located in the Horn of Africa, at 30-150 N latitude and 330-480 E longitude. The country is administratively segmented into 9 geographical regions (Tigray, Afar, Amhara, Oromiya, Somali, Benishangul Gumuz, Southern Nations Nationalities and Peoples (SNNP), Gambela, and Harari) and two administrative cities (Addis Ababa and Dire Dawa)(19). This study was based on a secondary analysis of the women's (IR) file of 2016 EDHS, which was carried out between January 18, to June 27, 2016.
The study population consisted of women having children aged 12–35 months who resided in the selected clusters and had complete information on vaccination status for PCV. Women whose geographical locations were not available through the global positioning system (GPS) were excluded from the spatial analysis.
To select study participants, the 2016 EDHS used a stratified, two-stage cluster sampling approach, with each region divided into urban and rural areas. First, 645 enumeration areas (EAs) (202 and 443 from urban and rural areas, respectively) were selected using a probability proportional to EA size. Then, in each of the selected EAs, a household listing operation was run from September to December 2015, and the resulting lists were used as a sampling frame for the selection of households. The newly formed household listing was then used to select a fixed number of 28 households per cluster with an equal probability of systematic selection. Finally, a total of 3,340 women with children aged 12–35 months were included in the analysis. The detailed methodology is available in the 2016 EDHS final report (19). Data were collected by using the Woman's Questionnaire, which contains background characteristics, birth history, maternal health service characteristics, vaccination status, and childhood illnesses.
The outcome variable was having incomplete PCV schedules among children aged 12–35 months. The status of each dose of PCV was assessed, and if a child received all three doses of PCV (PCV1= h54_1, PCV2= h55_1, and PCV3 = h56_1), it was classified as ‘complete=0”, otherwise considered as “incomplete=1”. Written vaccination records (including the infant's immunization card and other health cards) and verbal reports from mothers were used to assess the vaccination status.
Following a review of relevant and related studies, individual and community-level determinants of incomplete PCV uptake were identified (Table 1).
Using STATA version 16, the data was extracted, recoded, and cleaned. In addition, ArcGIS version 10.7, and SaTScan version 9.6 were used for spatial analysis. A weighting was done to account for the unequal likelihood of selection among strata caused by the non-proportional distribution of samples to different areas. This helps to restore the statistical representativeness of the survey, which is essential for reliable estimation (26). Descriptive statistics such as frequency and percentage of different variables were estimated and displayed using texts, and tables.
The weighted proportions of the overall PCV uptake (Complete vs. Incomplete/missed) were computed in Microsoft Excel 2016 (CSV format) and then imported into ArcGIS 10.7. The CSV file was then combined with the geographic coordinates located in the shape file using the unique identification code of each cluster.
Global spatial autocorrelation was carried out using the Global Moran's I statistic (Moran's I) to determine whether the overall pattern of incomplete PCV uptake is clustered, dispersed, or random throughout the study areas. A Moran's I value close to 1 indicates a significant positive autocorrelation (the outcome of interest is clustered/non-random) and close to −1 indicates negative spatial autocorrelation (the outcome is dispersed) across enumeration areas (EAs). Moran's I value near zero implies that the spatial distribution was random (27, 28). In the current analysis, a statistically significant Moran's I (p < 0.001) leads to the rejection of the null hypothesis (the outcome is randomly distributed) and indicates the presence of spatial autocorrelation (28).
Getis-Ord Gi* statistics were calculated for each location to detect significant hot and cold spots for children with incomplete PCV. To determine the statistical significance of clustering, the Z-score was obtained, and the p-value was determined. Statistical output with a high GI suggests a “hot spot,” whereas one with a low GI indicates a “cold spot” (29, 30).
Spatial interpolation was done by using the Ordinary Kriging technique to forecast the status of incomplete/missed PCV in unsampled areas by using evidence from the sampled EAs. The ordinary Kriging spatial interpolation approach was chosen from among several interpolation techniques because it has a low residual and mean square error (31, 32).
Using Kuldorff's SaTScan version 9.6 statistical software, the Bernoulli-based model was employed to detect the statistically significant spatial clusters with incomplete PCV uptake. A Bernoulli-based model was used in which events at particular places were analyzed, whether children were fully vaccinated (Control) or not (Case). Scan statistics scanned the space gradually to determine the number of observed and expected observations inside the window at each cluster. The scanning window with the maximum likelihood was the most likely and high-performing cluster for being a case, and the level of significance was determined at a p-value < 0.05.
To account for the hierarchical nature of EDHS data, a multilevel multivariable logistic regression analysis was carried out to identify community and individual-level predictors of incomplete PCV uptake. Running a multilevel analysis upon such hierarchical data allows for minimal biased parameter estimates (33, 34).
Initially, a multilevel bivariable logistic regression analysis was run to examine the effect of each explanatory variable on the outcome variable, and variables with p < 0.25 were included in the multilevel multivariable logistic regression. The Variance Inflation Factor (VIF) was used to detect multicollinearity among covariates at a cutoff point of 10 and found none (the VIF ranged from 1.01 to 2.27 with a mean of 1.54). Finally, a multilevel multivariable logistic regression analysis was performed to find significant predictors of incomplete PCV uptake, and statistically significant variables at p < 0.05 were reported along with their 95% confidence intervals.
Four distinct models were fitted: the first (model 1) was without any covariate (empty model), the second (model 2) with only individual-level factors, the third (model 3) with merely community-level variables, and the fourth (Model 4/full model) with both individual- and community-level variables. Intra-class correlation coefficient (ICC), median odds ratio (MOR), and proportional change in variance (PCV) metrics were computed for measures of variation.
ICC is a measure of the degree of heterogeneity of not having full PCV between clusters, and it was determined using:
; where Var(b) is the estimated variance each model and Var(w) is a predicted individual variance component, which is π2/3 ≈3.29.
The PCV was used to evaluate the contribution of individual- and/or community-level factors to the overall variation in the null model, and it was determined by:
, where, Va is the variance of the initial model (null model), and Vb=variance of the subsequent models (models 2, 3, and 4).
MOR measured the unexplained heterogeneity in the odds of incomplete PCV uptake from one cluster to another one and is determined by using:
Deviance = −2 * Log Likelihood (LL), Schwarz's Bayesian Information Criterion (BIC), and Akaike's Information Criterion (AIC) were used to compare models. Finally, the best-fitted model was determined to be the one with the highest likelihood ratio test and the lowest deviance.
A weighted sample of 3,340 women having children aged 12–35 months was included in the current study. The mean (±SD) age of women was 28.96 (±6.59) years. On the other hand, the mean (±SD) age of the children was 21.98 (±6.89), with the majority (57.0%) of them falling between the ages of 12–23 months. The vast majority of the respondents (90.9%) were from major central regions. Nearly two-thirds of the mothers (63.9%) and one-fourth (24.7%) had no formal education and were in the poorest wealth quintile, respectively. The highest proportions of children with incomplete PCV uptake were reported among women from peripheral regions (65.0%), and who had no formal education (61.1%) (Table 2).
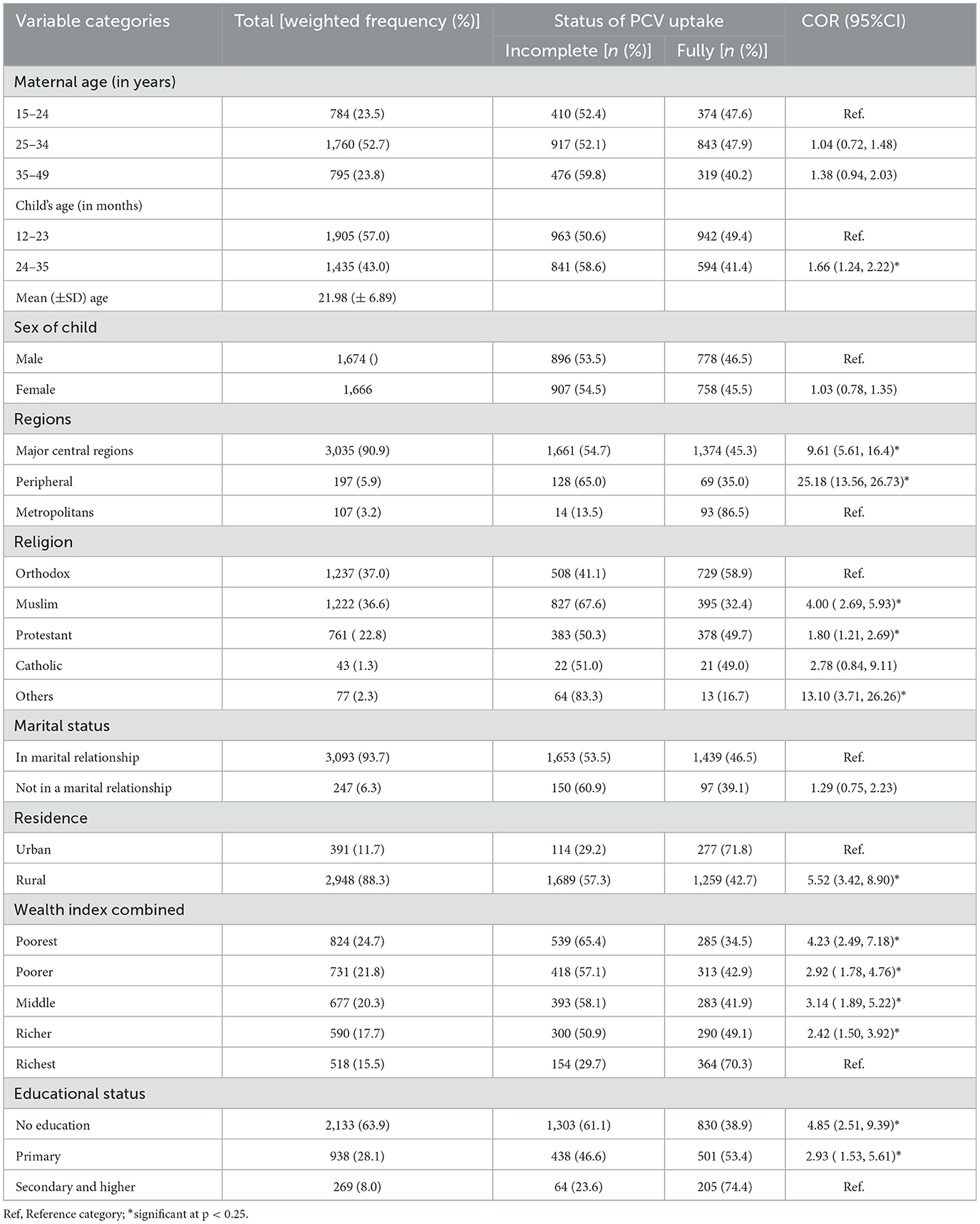
Table 2. The background characteristics of study participants across the uptake of PCV in Ethiopia, EDHS 2016.
The majority (46.3%) of women were multiparous. In terms of maternal health service utilization, 37.4% and 66.7% of respondents, respectively, did not get Antenatal care (ANC) and gave birth at home during their most recent pregnancy. A large proportion of women, 72.7%, 80.9%, and 92.8%, were never exposed to radio, television, and newspapers, respectively. Only 17.5% of women owned a mobile phone and 4.2% were enrolled in health insurance schemes. Distance and money were reported impediments to medical care in 58.7% and 61.3% of women, respectively. The highest proportions of children with incomplete PCV uptake were reported among women who had no ANC visit (72.5%), no pre-birth Tetanus toxoid injection, and who gave birth at home (63.1%) (Table 3).
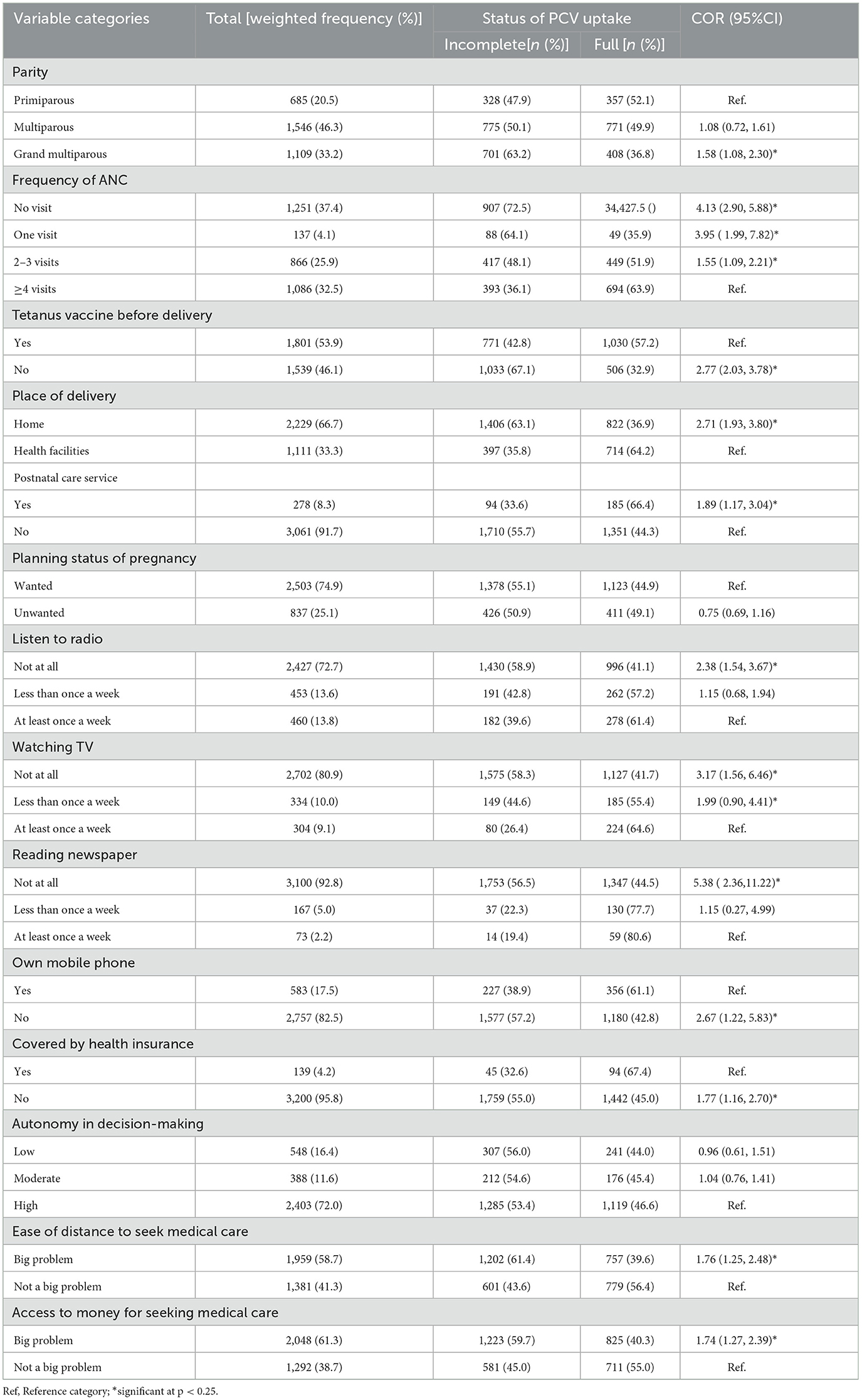
Table 3. Obstetric and health service-related characteristics of study participants across the uptake of PCV in Ethiopia, EDHS 2016.
Regarding the vaccination status, more than half, 54.0% (95% CI: 52.31, 55.69) of children were not fully vaccinated for the Pneumococcal conjugate vaccine (PCV). Afar region accounted for the highest proportion of children who were not fully vaccinated for PCV (84.5%), followed by Somali (72.2%) and Oromia (65.2%) regions. Addis Ababa, Tigray, and Diredawa regions, on the other hand, had the greatest proportions of children who were fully vaccinated for PCV, at 91.5%, 77.2%, and 75.3%, respectively (Figure 1).
The proportion of children with incomplete PCV uptake was higher in Somalia, the western border of Afar, northern and southern Amhara, northern Gambella, and SNNPR. In contrast, Tigray, Benishangul, central Addis Ababa, and the eastern border of Amhara had a lower proportion of children with incomplete PCV (Figure 2).
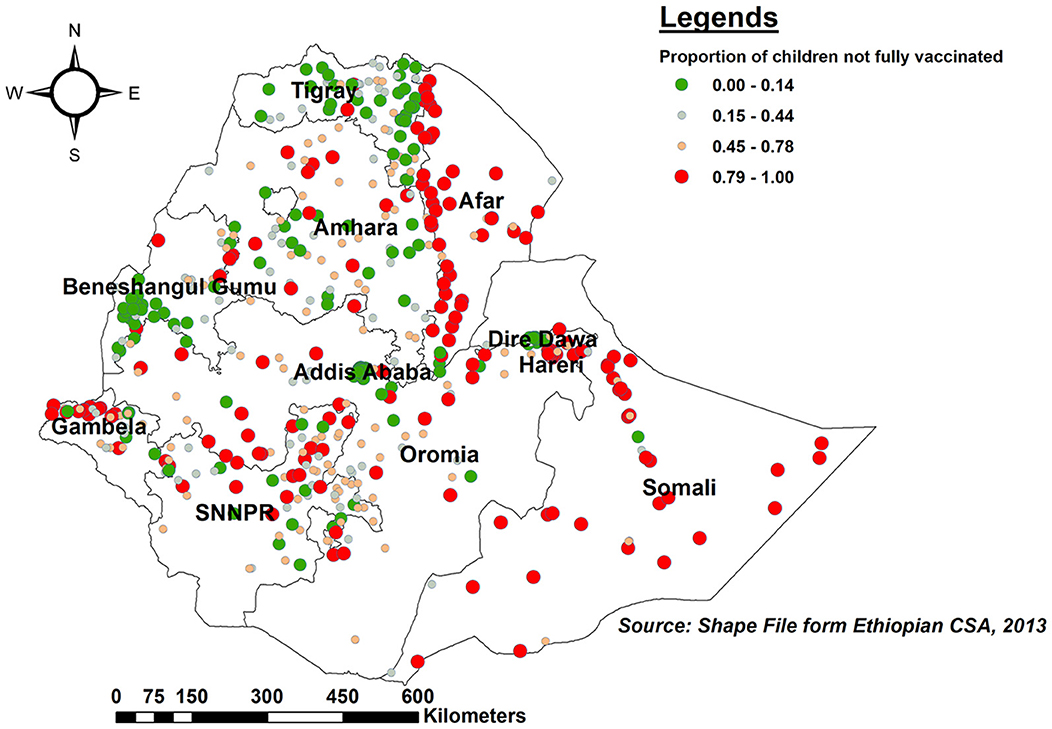
Figure 2. Spatial distribution of incomplete PCV uptake among children aged 12–35 months in Ethiopia, EDHS 2016.
The global spatial autocorrelation result revealed that incomplete PCV uptake was non-random Ethiopia (Global Moran's I = 0.509, p < 0.001) (Figure 3). The incremental spatial autocorrelation across a series of distances was displayed by a line graph with a corresponding z-score to establish the average nearest neighbor and minimum and maximum distance band. A total of 10 distance bands were uncovered with an initial distance of 122,990.0 meters, with the first maximum peak (clustering) identified at 153,314.99 meters (Figure 4).
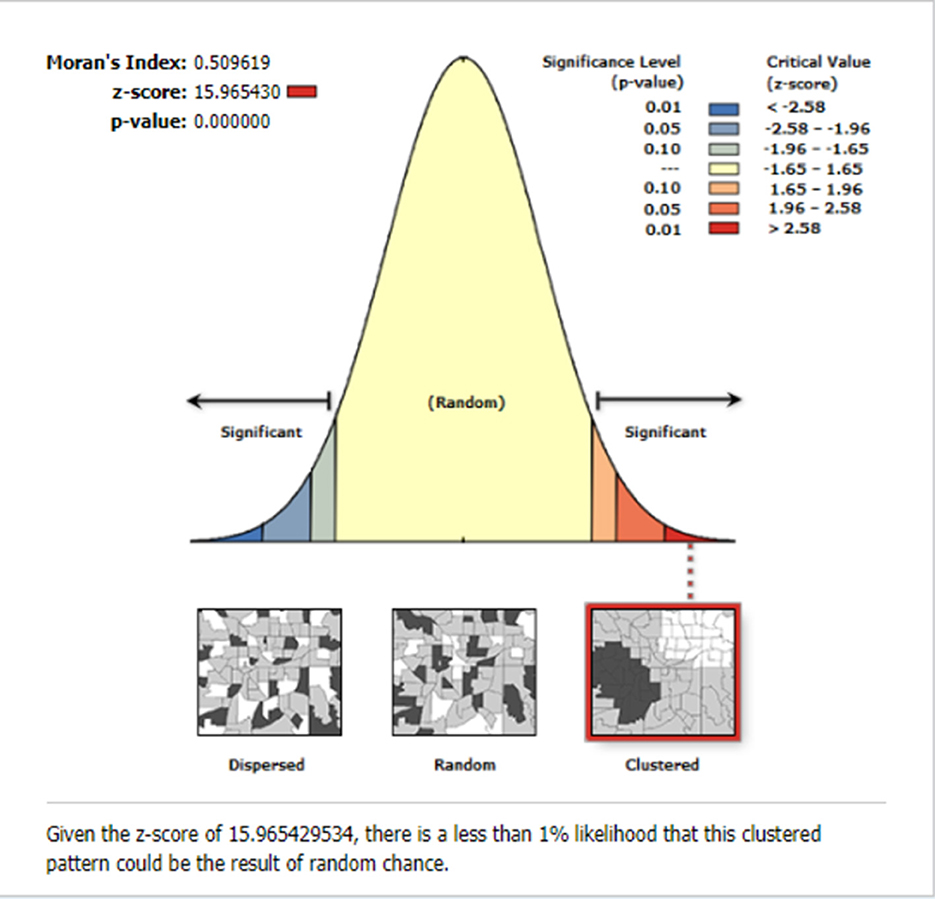
Figure 3. The global spatial autocorrelation of incomplete PCV uptake among children aged 12–35 months in Ethiopia, EDHS 2016.
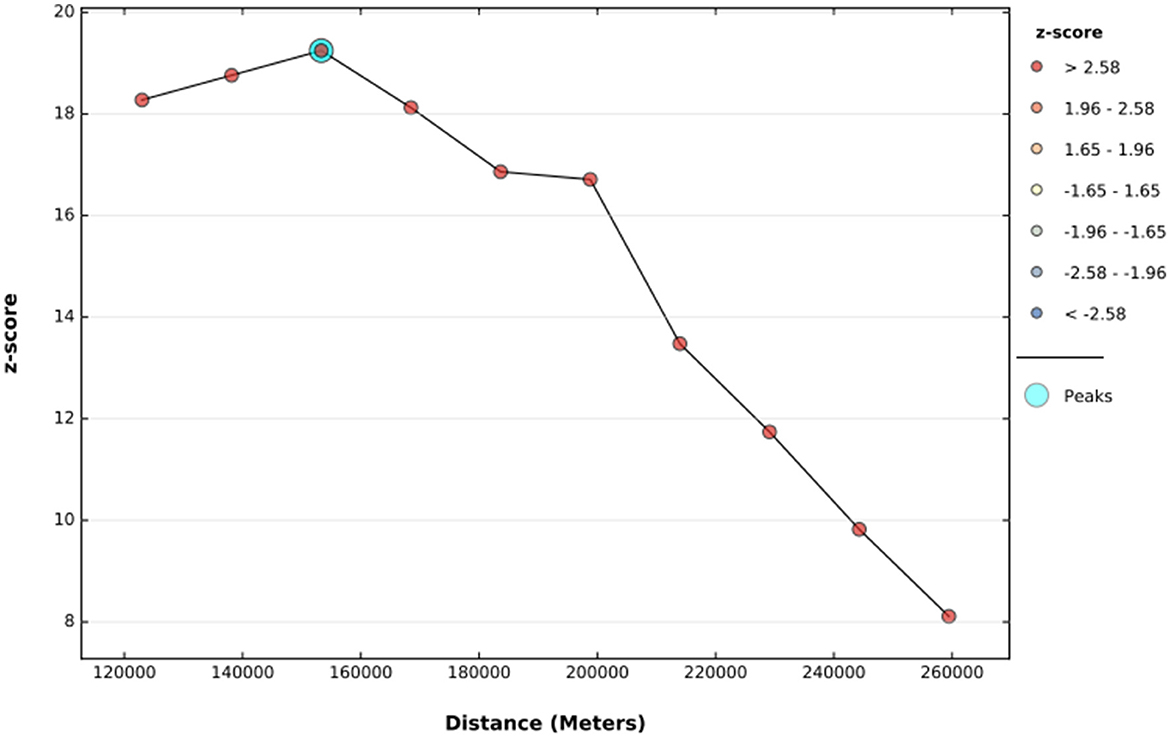
Figure 4. The incremental autocorrelation of incomplete PCV uptake among children aged 12–35 months in Ethiopia, EDHS 2016.
As per hot spot and cold spot analysis, the vast majority of Somali, southeast Afar, and eastern Gambela regions were hot spots (areas with a higher proportion of children with an incomplete PCV schedule), as indicated by red-colored points. Central Tigray, southwest Benishangul-Gumuz, Central Addis Ababa, and Dire Dawa, on the other hand, were cold spot areas (areas with lower rates of incomplete PCV uptake) (Figure 5).
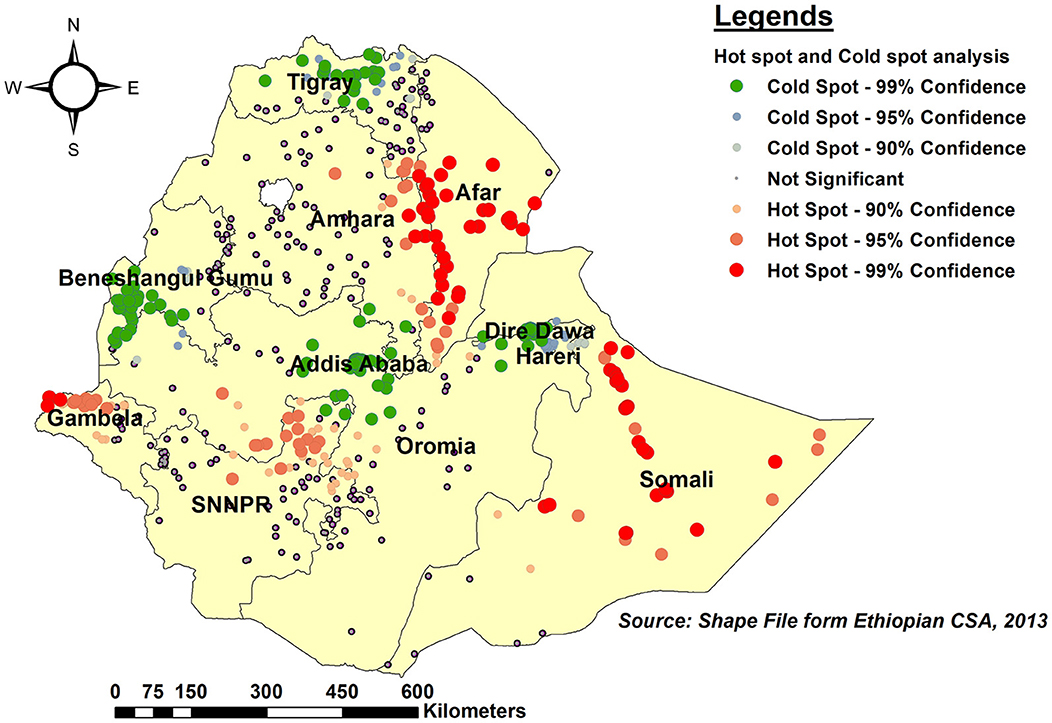
Figure 5. Hot spot and Cold spot analysis of incomplete PCV uptake among children aged 12–35 months in Ethiopia, EDHS 2016.
As per the kriging interpolation results, the entire Somali region, the vast majority of Afar, and the western border of Gambella had a higher predicted proportion of incomplete PCV uptake. However, the predicted proportion of incomplete PCV uptake was lower in eastern and western Tgiray, Central Addis Abeba, southwest of Benishangul-Gumuz, and the northern part of Dire Dawa (Figure 6).
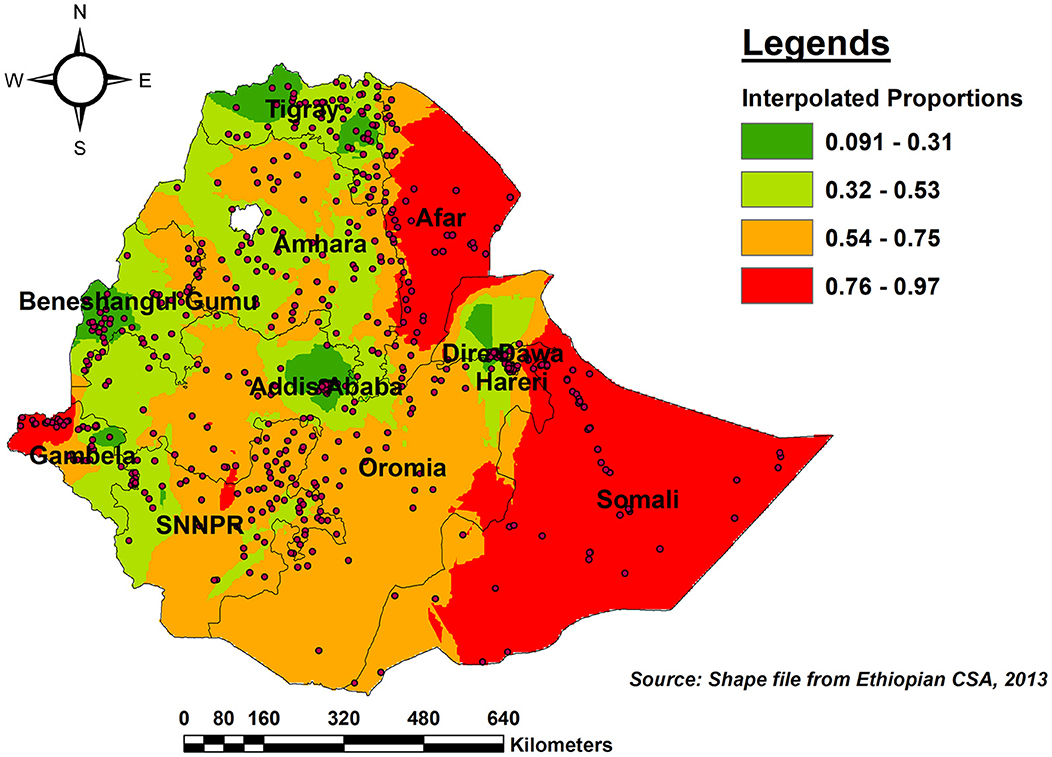
Figure 6. Ordinary Kriging interpolation of the spatial distribution of incomplete PCV uptake among children aged 12–35 months in Ethiopia, EDHS 2016.
The SaTScan spatial analysis detected a total of nine statistically significant SaTScan cluster areas with a high proportion of children who were not fully vaccinated for PCV, which means that the prevalence of incomplete vaccination for PCV is higher inside the SaTScan circular window compared to outside the SaTScan window. The most likely primary SaTScan cluster of areas with a higher rate of incomplete PCV was detected in the Somali region at geographical coordinates of (6.023458 N, 44.807507 E) with (LLR = 33.79, RR=1.76, p < 0.01). This revealed that children within the spatial window had 1.76 times higher odds of receiving incomplete PCV as compared to those children outside the spatial window (Supplementary File).
The ICC in the null model revealed that 42.2% of the variability in incomplete PCV uptake was explained by cluster variations (ICC = 0.422, p < 0.001). In addition, variation at the individual and community levels accounted for 32.7% (ICC = 0.0.327, p < 0.001) and 39.0% (ICC = 0.390, p < 0.001) of the variation in the probabilities of having incomplete PCV, respectively. The median odds ratio (MOR) indicates the amount to which the risk of having incomplete PCV is determined by clusters and is hence suitable for assessing contextual factors. In the current study, a MOR of 4.43 in the null model revealed that if we randomly selected a child from two different clusters, the child from the cluster with a higher rate of incomplete PCV uptake had 4.43 times higher odds of not being fully vaccinated as compared to the child from the cluster with a lower uptake. Furthermore, a proportionate change in variance (PCV) of 55.5% suggests that individual and community-level factors together accounted for 55.5% of the variability seen in the null model. As we moved from model 1 (the empty model) to model 4 (the full model), the values of AIC, BIC, and Deviance declined, indicating that the final model fitted throughout the study had appropriate goodness of fit. Finally, the fourth model with the lowest deviation (3,597.2) was selected as the best model fit for the study (Table 4).
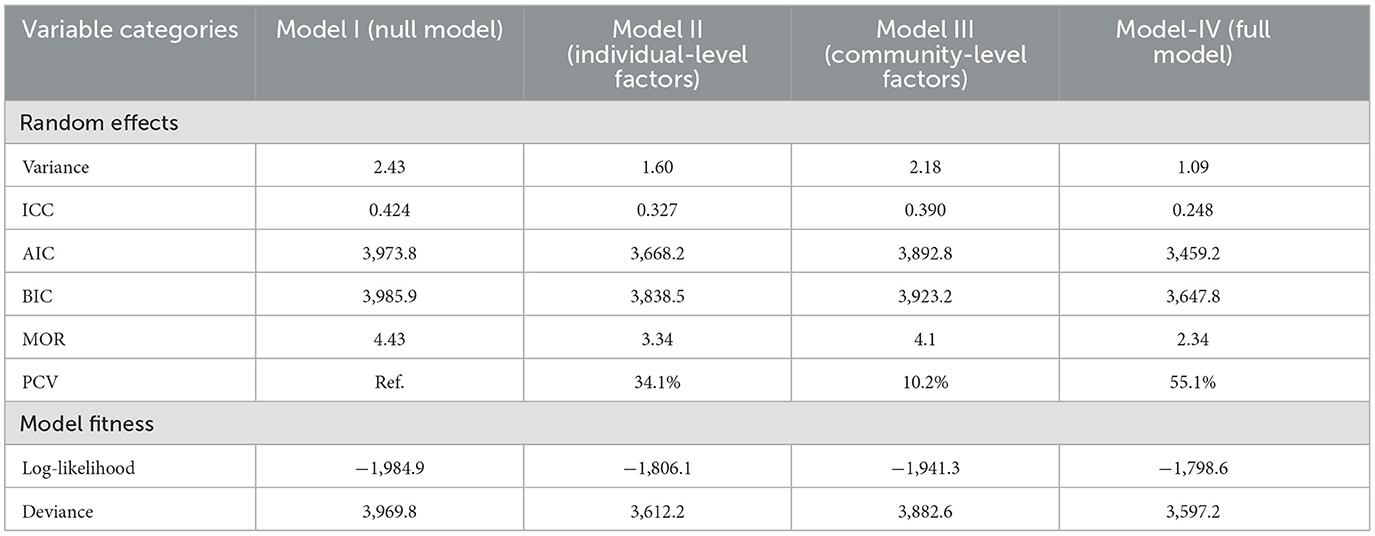
Table 4. Measures of variation (random intercept models) and model fit statistics of incomplete PCV uptake among children aged 12–35 months in Ethiopia, EDHS 2016.
In the mixed-effect multilevel multivariable logistic regression analysis, living in peripheral regions, religion, not having ANC visits, home birth, never listening to the radio, and not owning a mobile phone were found to be significantly associated with incomplete PCV uptake. As compared to children from metropolitan regions, the odds of incomplete PCV uptake were 4.63 (AOR= 4.63; 95% CI: 2.34, 9.15) and 3.16 (AOR= 3.16; 95% CI: 2.06, 7.38) times higher for children from peripheral and major central regions, respectively. Children from Traditional and Muslim religious families had a 7.41(AOR = 7.41, 95% CI: 2.8, 13.18) and 2.62 (AOR = 2.62, 95% CI: 1.96, 3.51) times higher likelihood of not being fully vaccinated than children from Orthodox families, respectively. Children from mothers who did not receive ANC during their last pregnancy were 2.76 times more likely not to be fully vaccinated for PCV than children from mothers who received four or more ANC visits (AOR = 2.76, 95% CI: 1.91, 4.00). Children born to mothers who did not receive the Tetanus injection before childbirth were 1.84 times more likely to have an incomplete PCV schedule than children born to mothers who received at least a single shot (AOR = 1.84, 95% CI: 1.29, 2.74). Similarly, the odds of incomplete PCV vaccinations were 1.72 times higher among children delivered at home vs. their counterparts delivered in health facilities (AOR = 1.72, 95% CI: 1.23, 2.34). Children born to mothers who do not own a mobile phone were 1.64 (AOR = 1.64, 95% CI: 1.38, 1.93) times more likely to have incomplete PCV than their counterparts (Table 5).
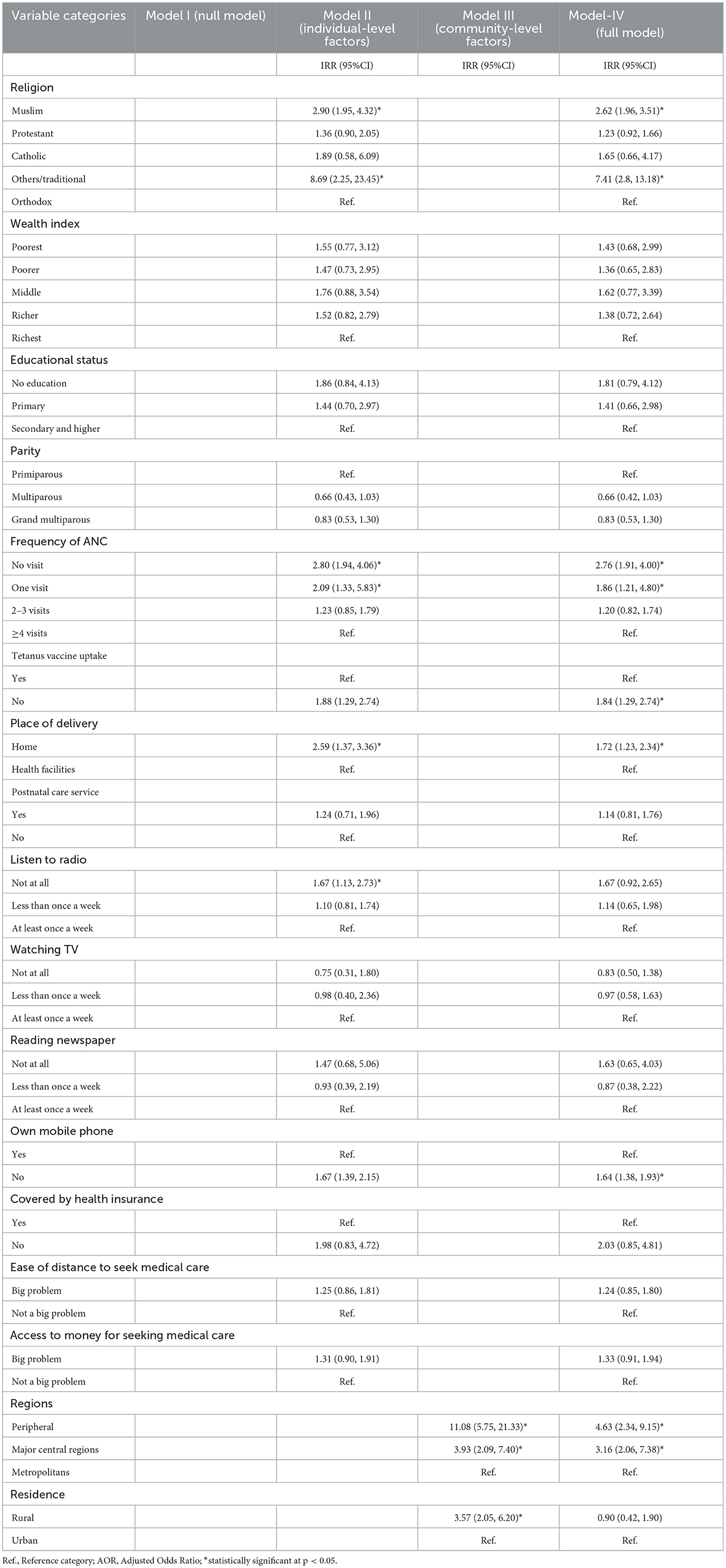
Table 5. Results of a multilevel mixed-effect multivariable logistic regression to identify the determinants of failure to take and/or missing PCV in Ethiopia, EDHS 2016.
In this study, the proportion of children in Ethiopia who did not receive the complete schedule of Pneumococcal conjugate vaccination (PCV) was found to be 54.0% (95% CI: 52.31, 55.69), which was higher than a similar study conducted in Ethiopia among children aged 12–23 months (50.9%) (17). This result lags far behind the EPI and the Global Vaccine Action Plan (GVAP), which set national vaccine coverage goals of 90% by 2020 (11). Global spatial Moran's investigation found that the proportion of children who missed the full PCV schedule varied significantly across regions d (Global Moran's I = 0.509, p < 0.0001). As per hot spot analysis, statistically significant hotspot areas for missing complete PCV were the vast majority of Somali, southeast Afar, and eastern Gambela regions. These regions were known for having a low coverage of routine vaccination (17, 35–37). This could be owing to geographical and logistics challenges, as these regions are known for their harsh climate and limited healthcare infrastructures, making it difficult to reach remote areas with vaccination programs, which could impede vaccine delivery (38, 39). Furthermore, due to a shortage of healthcare providers, access to up-to-date information about vaccines and their advantages may be limited, potentially leading to noncompliance with recommended immunization schedules. This high level of incomplete childhood PCV coverage may indicate that not only the country but also regions with high levels of childhood PCV coverage are nonetheless at high risk for pneumococcal infections in children due to low herd immunity (40).
According to the multilevel analysis, the likelihood of missing the full PCV schedule was attributable to both individual and community-level characteristics. Accordingly, living in peripheral regions, not having ANC visits, not having pre-birth Tetanus injection, home birth, and not owning a mobile phone were found to be significantly associated with incomplete PCV uptake.
Children from the peripheral regions (Afar, Somali, and Gambella) had a higher chance of not being fully vaccinated for PCV. This was supported by studies conducted in Ethiopia (7, 35, 41). This could be owing to difficulty in accessing health services, inadequate knowledge of childhood immunization, socio-cultural, and logistical difficulties, as well as differences in socio-cultural and religious backgrounds (41). Furthermore, because the majority of people in these regions live pastoral or partially nomadic lives (39), women may find it difficult to adhere to recommended vaccination schedules as their places of residency shift over time. As a result, the federal and regional governments should work together to build mobile vaccination, plan vaccination campaigns, and work with local organizations and non-governmental organizations to minimize incomplete PCV uptake.
Failure to take complete PCV was associated with a lack of maternal health services, like ANC, skilled delivery service, and pre-birth tetanus injection. Lack of ANC was found to be a significant predictor of an incomplete PCV schedule. This was supported by studies conducted in Afghanistan (42), Indonesia (43), Nigeria (44), Malawi (45), Benin (46), and Ethiopia (17, 47, 48). ANC is a crucial entrance point into the healthcare system for pregnant women and their families to receive a broad range of health promotion and prevention services (49). ANC allows healthcare providers to educate expecting mothers about the importance, timing, and place of childhood vaccination. Without ANC, women may not have this essential information, leading to noncompliance with routine child health services like immunization. The positive relationship between no ANC and incomplete PCV coverage highlights the need for Ethiopia's Ministry of Health to improve ANC provision for pregnant women. Likewise failure to receive a pre-birth tetanus injection was also associated with incomplete vaccination, which was supported by studies conducted in Myanmar (50), Indonesia (51), and Ethiopia (52). Similarly home birth also significantly contributes to incomplete PCV uptake. Studies conducted in Indonesia (43), Myanmar (50), Pakistan, Benin (46), Senegal (53), and Ethiopia (48) were in tandem with the current finding. This could be owing to limited access to healthcare services, as the majority of women who give birth at home may come from remote areas to health facilities, resulting in lower vaccination rates. Furthermore, when women give birth outside of healthcare institutions, they frequently do not receive counseling or information on the need for child immunizations which could result in noncompliance. Thus, the Ethiopian Federal Ministry of Health needs to focus on improving the provision of maternal health services in order to enhance immunization coverage of newly introduced vaccines. This can be achieved through targeted outreach efforts, improving healthcare infrastructure, and increasing community engagement and awareness.
The odds of an incomplete PCV schedule were higher among children from mothers without mobile phones. Studies conducted in Bangladesh (54), and Ethiopia (55). Mobile phones contribute to vaccine compliance by delivering SMS reminders for vaccination appointments and offering information about vaccine schedules, the importance of vaccines, and their benefits (56). Community health workers or healthcare practitioners in Ethiopia continue to use mobile phones for exchanging health-related communications, especially vaccine-related information (57). As a result, women or caretakers without a mobile phone may miss timely reminders on when and where to take their child for vaccination, resulting in missed appointments.
The study has the following strengths. To begin, the findings relied on an analysis of nationally representative data with a high response rate, which made the findings more generalizable. The findings of Hot Spot, SaTScan, and multilevel mixed-effect logistic regression analysis can help government and program planners develop geographic, individual, and community-focused public health initiatives that tackle the level of incomplete PCV. However, the finding should be seen in light of its limitations. First of all, as EDHS data is cross-sectional, it is difficult to establish a temporal/causal relationship between the outcome and explanatory variables. In addition, since the data was collected through retrospective interviews of women, the findings may be exposed due to recall bias.
The level of incomplete PCV schedule was found to be high in Ethiopia with a significant spatial variation across regions. The vast majority of Somali, southeast Afar, and eastern Gambella regions were statistically significant hot spots for incomplete PCV. Lacking ANC visits, not getting pre-birth Tetanus injections, home birth, not possessing a mobile phone, and residing in a peripheral region were identified as statistically significant predictors of incomplete PCV. Hence, the federal and regional governments should collaborate with NGOs to improve vaccination coverage and design strategies to trace those children with incomplete PCV in peripheral regions. In addition, it's imperative that policymakers and maternal-child health program planners implement together territorial strategies by enhancing access to maternal and child health services to increase in a few months of coverage vaccines.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Wachemo University Ethics Committee, Department of Public Health. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
AH: Conceptualization, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SH: Methodology, Software, Writing – review & editing. LT: Methodology, Software, Writing – review & editing. BW: Formal analysis, Methodology, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to acknowledge the Demographic Health Survey program office for allowing us to access all the relevant DHS data for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1344089/full#supplementary-material
Supplementary File. The most likely SaTScan clusters of areas with a high prevalence of incomplete PCV uptake among children aged 12–35 months in Ethiopia, EDHS 2016.
AIC, Akaike's information criterion; ANC, Antenatal Care; AOR, Adjusted Odds Ratio; BIC, Bayesian Information Criterion; COR, Crude Odds Ratio; CSA, Central Statistical Agency; EDHS, Ethiopian Demographic and Health Survey; EPI, Expanded Program for Immunization; GVAP, Global Vaccine Action Plan; ICC, Intraclass Correlation Coefficient; LMICs, Low and Middle-Income Countries; MOR, Median Odds Ratio; PCV, Pneumococcal Conjugate Vaccine; SaTScan, Spatial scan statistical; VIF, Variance Inflation Factor; WHO, World Health Organization.
1. Centre for Disease Control. (2022). Pneumococcal Disease. (2022). Available online at: https://www.cdc.gov/pneumococcal/index.html (accessed September 10, 2023).
2. Wahl B, O'Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae. type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Global Health. (2018) 6:e744-e757. doi: 10.1016/S2214-109X(18)30247-X
3. mondiale de la Santé O World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper–February 2019– Vaccins antipneumococciques conjugués chez les nourrissons et les enfants de moins de 5 ans: note de synthèse de l'OMS–février 2019. Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire. (2019) 94:85–103. Available online at: https://iris.who.int/bitstream/handle/10665/310968/WER9408.pdf?sequence=1
4. Johnston C, Campo N, Bergé MJ, Polard P, Claverys J-P. Streptococcus pneumoniae, le transformiste. Trends Microbiol. (2014) 22:113–9. doi: 10.1016/j.tim.2014.01.002
5. WHO. Pneumonia in Children. (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed November 11, 2022).
6. Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. (2013) 381:1380–90. doi: 10.1016/S0140-6736(12)61901-1
7. Asmare Atalell K, Asmare Techane M, Adugna Wubneh C, Tezera Assimamaw N, Mulualem Belay G, Tarik Tamir T, et al. Spatiotemporal distributions of immunization coverage in Ethiopia from 2000 to 2019. Vaccine. (2022) 40:1413–20. doi: 10.1016/j.vaccine.2022.01.053
8. Deribew A, Tessema GA, Deribe K, Melaku YA, Lakew Y, Amare AT, et al. Trends, causes, and risk factors of mortality among children under 5 in Ethiopia, 1990–2013: findings from the global burden of disease study 2013. Popul Health Metrics. (2016) 14:42. doi: 10.1186/s12963-016-0112-2
9. UNICEF. Steep Drop in Pneumonia Deaths in Last Decade, but Much Further to Go. (2014). Available online at: https://ethiopia.un.org/en/13646-unicef-steep-drop-pneumonia-deaths-last-decade-much-further-go#:~:text=In%20Ethiopia%2C%20pneumonia%20is%20a,under-five%20children%20every%20year (accessed November 14, 2014).
11. WHO. Immunization Coverage, Key Facts. (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed July 18, 2023).
12. Ayieko P, Griffiths UK, Ndiritu M, Moisi J, Mugoya IK, Kamau T, et al. Assessment of health benefits and cost-effectiveness of 10-valent and 13-valent pneumococcal conjugate vaccination in Kenyan children. PLoS ONE. (2013) 8:e67324. doi: 10.1371/journal.pone.0067324
13. Mezones-Holguin E, Canelo-Aybar C, Clark AD, Janusz CB, Jaúregui B, Escobedo-Palza S, et al. Cost-effectiveness analysis of 10- and 13-valent pneumococcal conjugate vaccines in Peru. Vaccine. (2015) 33:39. doi: 10.1016/j.vaccine.2014.12.039
14. German EL, Solórzano C, Sunny S, Dunne F, Gritzfeld JF, Mitsi E, et al. Protective effect of PCV vaccine against experimental pneumococcal challenge in adults is primarily mediated by controlling colonisation density. Vaccine. (2019) 37:3953–6. doi: 10.1016/j.vaccine.2019.05.080
15. Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. (2010) 201:32–41. doi: 10.1086/648593
16. Minstery of Health. Ethiopia Expanded Program on Immunization (EPI). Available online at: https://www.moh.gov.et/en/Expanded_Program_on_Immunization_%28EPI%29?language_content_entity=en (accessed September 08, 2023).
17. Wondimu A, Cao Q, Wilschut JC, Postma MJ. Factors associated with the uptake of newly introduced childhood vaccinations in Ethiopia: the cases of rotavirus and pneumococcal conjugate vaccines. BMC Public Health. (2019) 19:1–10. doi: 10.1186/s12889-019-8002-8
18. Kebede TT, Svensson M, Addissie A, Trollfors B, Andersson R. Cost-effectiveness of childhood pneumococcal vaccination program in Ethiopia: results from a quasi-experimental evaluation. BMC Public Health. (2019) 19:1078. doi: 10.1186/s12889-019-7423-8
19. Central Statistical Agency (CSA) [Ethiopia] and ICF (2016). Ethiopia Demographic and Health Survey. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF. (2016).
20. Adokiya MN, Baguune B, Ndago JA. Evaluation of immunization coverage and its associated factors among children 12–23 months of age in Techiman Municipality, Ghana, 2016. Archives of Public Health. (2017) 75:1–10. doi: 10.1186/s13690-017-0196-6
21. Animaw W, Taye W, Merdekios B, Tilahun M, Ayele G. Expanded program of immunization coverage and associated factors among children age 12–23 months in Arba Minch town and Zuria District, Southern Ethiopia, 2013. BMC Public Health. (2014) 14:1–10. doi: 10.1186/1471-2458-14-464
22. Yenit M, Assegid S, Abrha H. Factors associated with incomplete childhood vaccination among children 12–23 months of age in Machakel Woreda, East Gojjam Zone: aCase-controlStudy. J Pregn Child Health. (2015) 2:180. doi: 10.4172/2376-127X.1000180
23. Habte A, Tamene A, Bogale B. Women empowerment domains and unmet need for contraception among married and cohabiting fecund women in Sub-Saharan Africa: a multilevel analysis based on gender role framework. PLoS ONE. (2023) 18:e0291110. doi: 10.1371/journal.pone.0291110
24. Teshale AB, Amare T. Exploring spatial variations and the individual and contextual factors of uptake of measles-containing second dose vaccine among children aged 24 to 35 months in Ethiopia. PLoS ONE. (2023) 18:e0280083. doi: 10.1371/journal.pone.0280083
25. Teshale AB, Tesema GA. Magnitude and associated factors of unintended pregnancy in Ethiopia: a multilevel analysis using 2016 EDHS data. BMC Pregn Childbirth. (2020) 20:1–8. doi: 10.1186/s12884-020-03024-5
26. Hermalin AI, Entwisle B, Khadr Z. Reweighting DHS data to serve multiple perspectives. Stud Family Plann. (1996) 1996:88–97. doi: 10.2307/2138136
27. Kissling WD, Carl G. Spatial autocorrelation and the selection of simultaneous autoregressive models. Global Ecol Biogeog. (2008) 17:59–71. doi: 10.1111/j.1466-8238.2007.00334.x
28. Getis A. Reflections on spatial autocorrelation. Reg Sci Urban Econ. (2007) 37:491–6. doi: 10.1016/j.regsciurbeco.2007.04.005
29. Manepalli U, Bham GH, Kandada S. Evaluation of hotspots identification using kernel density estimation (K) and Getis-Ord (Gi*) on I-630. In: 3rd International Conference on Road Safety and Simulation. Indianapolis IN: National Academy of Sciences Indianapolis Indiana, United States. (2011).
30. Songchitruksa P, Zeng X, Getis–Ord spatial statistics to identify hot spots by using incident management data. Transp Res Rec. (2010) 2165:42–51. doi: 10.3141/2165-05
31. Myers DE. Spatial interpolation: an overview. Geoderma. (1994) 62:17–28. doi: 10.1016/0016-7061(94)90025-6
32. Wu C, Wu J, Luo Y, Zhang H, Teng Y, DeGloria SD. Spatial interpolation of severely skewed data with several peak values by the approach integrating kriging and triangular irregular network interpolation. Environm Earth Sci. (2011) 63:1093–103. doi: 10.1007/s12665-010-0784-z
34. Leyland AH, Groenewegen PP. Multilevel Modelling for Public Health and Health Services Research: Health in Context. Cham: Springer Nature. (2020).
35. Geweniger A, Abbas KM. Childhood vaccination coverage and equity impact in Ethiopia by socioeconomic geographic maternal child characteristics. Vaccine. (2020) 38:3627–38. doi: 10.1016/j.vaccine.2020.03.040
36. Tamirat KS, Sisay MM. Full immunization coverage and its associated factors among children aged 12–23 months in Ethiopia: further analysis from the 2016 Ethiopia demographic and health survey. BMC Public Health. (2019) 19:1–7. doi: 10.1186/s12889-019-7356-2
37. Bobo FT, Hayen A. Decomposition of socioeconomic inequalities in child vaccination in Ethiopia: results from the 2011 and 2016 demographic and health surveys. BMJ Open. (2020) 10:e039617. doi: 10.1136/bmjopen-2020-039617
38. Wolka S, Alemu MD, Gobana M, Bati GT, Gerawork H, Abebaw Z. Mobile health and nutrition team service implementation in Somali and Afar Regions of Ethiopia: a qualitative implementation science study. J Multidiscipl Healthc. (2022) 2022:2881–9. doi: 10.2147/JMDH.S388104
39. Pati RN, Basha SY. Socio-Cultural Determinants of Maternal Health Seeking Behaviour Among Young Women in Remote Pastoral Communities of South Omo, Afar and Somali Regions of Ethiopia. International Journal of Advanced Multidisciplinary Scientific Research.
40. Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand J Infect Dis. (2011) 43:683–9. doi: 10.3109/00365548.2011.582247
41. Tilahun B, Mekonnen Z, Sharkey A, Shahabuddin A, Feletto M, Zelalem M, et al. What we know and don't know about the immunization program of Ethiopia: a scoping review of the literature. BMC Public Health. (2020) 20:1365. doi: 10.1186/s12889-020-09304-1
42. Boyce T, Gudorf A, de Kat C, Muscat M, Butler R, Habersaat KB. Towards equity in immunisation. Eurosurveillance. (2019) 24. doi: 10.2807/1560-7917.ES.2019.24.2.1800204
43. Efendi F, Pradiptasiwi DR, Krisnana I, Kusumaningrum T, Kurniati A, Sampurna MTA, et al. Factors associated with complete immunizations coverage among Indonesian children aged 12–23 months. Children Youth Serv Rev. (2020) 108:1–44. doi: 10.1016/j.childyouth.2019.104651
44. Adedire EB, Ajayi I, Fawole OI, Ajumobi O, Kasasa S, Wasswa P, et al. Immunisation coverage and its determinants among children aged 12-23 months in Atakumosa-west district, Osun state Nigeria: a cross-sectional study. BMC Public Health. (2016) 16:905. doi: 10.1186/s12889-016-3531-x
45. Ntenda PAM. Factors associated with non- and under- vaccination among children aged 12e23 months in Malawi. A multinomial analysis of the population-based sample. Pediatrics Neonatol. (2019) 60:5. doi: 10.1016/j.pedneo.2019.03.005
46. Budu E, Seidu A-A, Agbaglo E, Armah-Ansah EK, Dickson KS, Hormenu T, et al. Maternal healthcare utilization and full immunization coverage among 12–23 months children in Benin: a cross sectional study using population-based data. Arch Public Health. (2021) 79:1–12. doi: 10.1186/s13690-021-00554-y
47. Yismaw AE, Assimamaw NT, Bayu NH, Mekonen SS. Incomplete childhood vaccination and associated factors among children aged 12–23 months in Gondar city administration, northwest, Ethiopia 2018. BMC Res Notes. (2019) 12:241. doi: 10.1186/s13104-019-4276-2
48. Desalew A, Semahegn A, Birhanu S, Tesfaye G. Incomplete vaccination and its predictors among children in Ethiopia: a systematic review and meta-analysis. Global Pediat Health. (2020) 7:2333794X20968681. doi: 10.1177/2333794X20968681
49. World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization. (2016).
50. Nozaki I, Hachiya M, Kitamura T. Factors influencing basic vaccination coverage in Myanmar: secondary analysis of 2015 Myanmar demographic and health survey data. BMC Public Health. (2019) 19:1–8. doi: 10.1186/s12889-019-6548-0
51. Hardhantyo M, Chuang Y-C. Urban-rural differences in factors associated with incomplete basic immunization among children in Indonesia: a nationwide multilevel study. Pediatr Neonatol. (2021) 62:80–9. doi: 10.1016/j.pedneo.2020.09.004
52. Ali Y, Mekonnen FA, Lakew AM, Wolde HF. Poor maternal health service utilization associated with incomplete vaccination among children aged 12-23 months in Ethiopia. Hum Vaccin Immunother. (2020) 16:1202–7. doi: 10.1080/21645515.2019.1670124
53. Mbengue MAS, Sarr M, Faye A, Badiane O, Camara FBN, Mboup S, et al. Determinants of complete immunization among senegalese children aged 12–23 months: evidence from the demographic and health survey. BMC Public Health. (2017) 17:630. doi: 10.1186/s12889-017-4493-3
54. Ahmed N, Ishtiak ASM, Rozars MFK, Bonna AS, Alam KMP, Hossan ME, et al. Factors associated with low childhood immunization coverage among Rohingya refugee parents in Cox's Bazar, Bangladesh. PLoS ONE. (2023) 18:e0283881. doi: 10.1371/journal.pone.0283881
55. Mekonnen ZA, Gelaye KA, Were M, Tilahun B. Effect of mobile phone text message reminders on the completion and timely receipt of routine childhood vaccinations: superiority randomized controlled trial in Northwest Ethiopia. JMIR mHealth uHealth. (2021) 9:e27603. doi: 10.2196/27603
56. Oliver-Williams C, Brown E, Devereux S, Fairhead C, Holeman I. Using mobile phones to improve vaccination uptake in 21 low-and middle-income countries: systematic review. JMIR mHealth uHealth. (2017) 5:e7792. doi: 10.2196/mhealth.7792
Keywords: incomplete immunization, children, PCV, predictors, spatial, Ethiopia
Citation: Hailegebireal AH, Hailegebreal S, Tirore LL and Wolde BB (2024) Spatial variation and predictors of incomplete pneumococcal conjugate vaccine (PCV) uptake among children aged 12–35 months in Ethiopia: spatial and multilevel analyses. Front. Public Health 12:1344089. doi: 10.3389/fpubh.2024.1344089
Received: 28 November 2023; Accepted: 13 May 2024;
Published: 28 May 2024.
Edited by:
Zhimin Tao, Jiangsu University, ChinaReviewed by:
Erica De Vita, University of Pisa, ItalyCopyright © 2024 Hailegebireal, Hailegebreal, Tirore and Wolde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aklilu Habte Hailegebireal, YWtsaWx1aGFidGU1N0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.