- 1Directorate-General for Health Prevention, Ministry of Health, Rome, Italy
- 2Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy
- 3Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
Public health genomics (PHG) aims to integrate advances in genomic sciences into healthcare for the benefit of the general population. As in many countries, there are various research initiatives in this field in Italy, but a clear picture of the national research portfolio has never been sketched. Thus, we aimed to provide an overview of current PHG research projects at the national or international level by consultation with Italian institutional and academic experts. We included 68 PHG projects: the majority were international projects in which Italian researchers participated (n = 43), mainly funded by the European Commission, while the remainder were national initiatives (N = 25), mainly funded by central government. Funding varied considerably, from € 50,000 to € 80,803,177. Three main research themes were identified: governance (N = 20); precision medicine (PM; N = 46); and precision public health (N = 2). We found that research activities are preferentially aimed at the clinical application of PM, while other efforts deal with the governance of the complex translation of genomic innovation into clinical and public health practice. To align such activities with national and international priorities, the development of an updated research agenda for PHG is needed.
1 Introduction
Public health genomics (PHG) is an attempt to responsibly and effectively translate the rapid increases in genome-based knowledge and technologies into population health benefits (1). Recent decreases in sequencing costs and the marked progress in data science present many opportunities for the incorporation of genomic information into new strategies for clinical and public health practice. Precision medicine (PM) focuses on identifying the most effective medical intervention for patients based on their genetic and biochemical profile, as well as specific environmental and lifestyle factors (2). Impressive advances have been made in cancer, where next generation sequencing has enabled the prediction of cancer susceptibility, sensitivity to therapy, prognosis and residual disease (3). Moreover, the use of population level data on genomics and other health determinants is expected to improve health outcomes at the population level, paving the way for a field called precision public health (PPH) (4). Some emerging applications of PPH are the development of more intensive screening programs for people at greater risk of cancer, and the detection and investigation of infectious disease outbreaks (5).
To realize the full potential of these promising approaches, many countries are fostering research initiatives aimed at driving the development of public policies, guidelines and health programs for the implementation of evidence-based genomic applications (6–8). Italy is no exception, although research activities seem to have begun in a disorganized way. The task of assuring coordination of national efforts in PHG has recently been entrusted to the Inter-institutional Committee for PHG (IC), a national board established in 2022, which includes representatives from the Italian Ministry of Health (MoH), the Italian Institute of Health (ISS), the National Agency for Regional Healthcare Services (AGENAS), the Italian Medicine Agency (AIFA), and the Italian Regions, in addition to academic experts in the field (9).
In particular, the IC is responsible for supervising PHG activities throughout the country and evaluating their alignment with European priorities. For its first assignment, the IC aimed to investigate the current state of development of PHG research in Italy. Since no comprehensive repository for national PHG projects is available, a mapping exercise was performed. The aim of this paper is to describe the methods and the results of an overview of the current PHG research portfolio in Italy and to discuss its main policy implications.
2 Methods
2.1 Subject of investigation and inclusion criteria
This overview included any research project involving PHG that started or was ongoing in Italy during 2022 (January 1st–December 31st). Both national projects and international projects in which Italian researchers participated were eligible for inclusion, whereas projects sponsored only at a regional level (i.e., involving one or more Italian Regions without a national commitment) were excluded. For the purposes of this study, PHG was defined as “a multidisciplinary field concerned with the responsible and effective translation of genomic science and technologies into clinical and public health practice” (10).
2.2 Data collection
We retrieved candidate projects by email consultation with the Italian IC, which comprised 39 expert representatives from the following institutions: I. Italian MoH (N = 26, from seven different Directorates-General); II. ISS (N = 2); III. AGENAS (N = 2); IV. AIFA (N = 2); V. Italian Regions (N = 4); VI. Italian universities (N = 3, leaders in the field of PHG from three different universities).
The experts were provided with the details of the overview during an online meeting and were asked to participate in the design of the project tracker tool (PTT). A draft of the PTT was e-mailed to the experts and their feedback was used to improve it. The final PTT consisted of an Excel data sheet collecting the following information for each project: title; start/end date; objectives; funder; lead institution; number of implementing partners; number of countries involved; funding; referring website; involvement of the IC (Supplementary Table S1).
In May 2022, we officially started data collection by emailing the experts with the PTT and the instructions for completion. To ensure the completeness of the results, we asked the experts to continuously update the PTT with newly funded research projects as they became available over the year. Data collection was closed at the end of December 2022. Reminders were issued during IC meetings and were also sent twice by e-mail during the year.
2.3 Project selection and data synthesis
For each project selected, unclear or missing information was clarified or retrieved by exploring official websites (Supplementary Table S2). Two researchers removed duplicates and screened the projects according to the inclusion criteria. Projects that clearly did not meet the eligibility criteria were excluded.
A descriptive analysis of the projects included was performed, using frequencies, percentages and ranges. Moreover, according to their primary aim, projects were mapped to three thematic categories and six sub-categories, as follows: (I) governance, further divided into (Ia) networking and coordination for innovation, (Ib) data and infrastructure, (Ic) adoption of health technology; (II) PM, further divided into (IIa) cancer and (IIb) non-oncological diseases; and (III) PPH, including only one sub-category, namely (IIIa) surveillance of infectious diseases. Topic attribution was cross-checked by three reviewers.
3 Results
3.1 Project selection
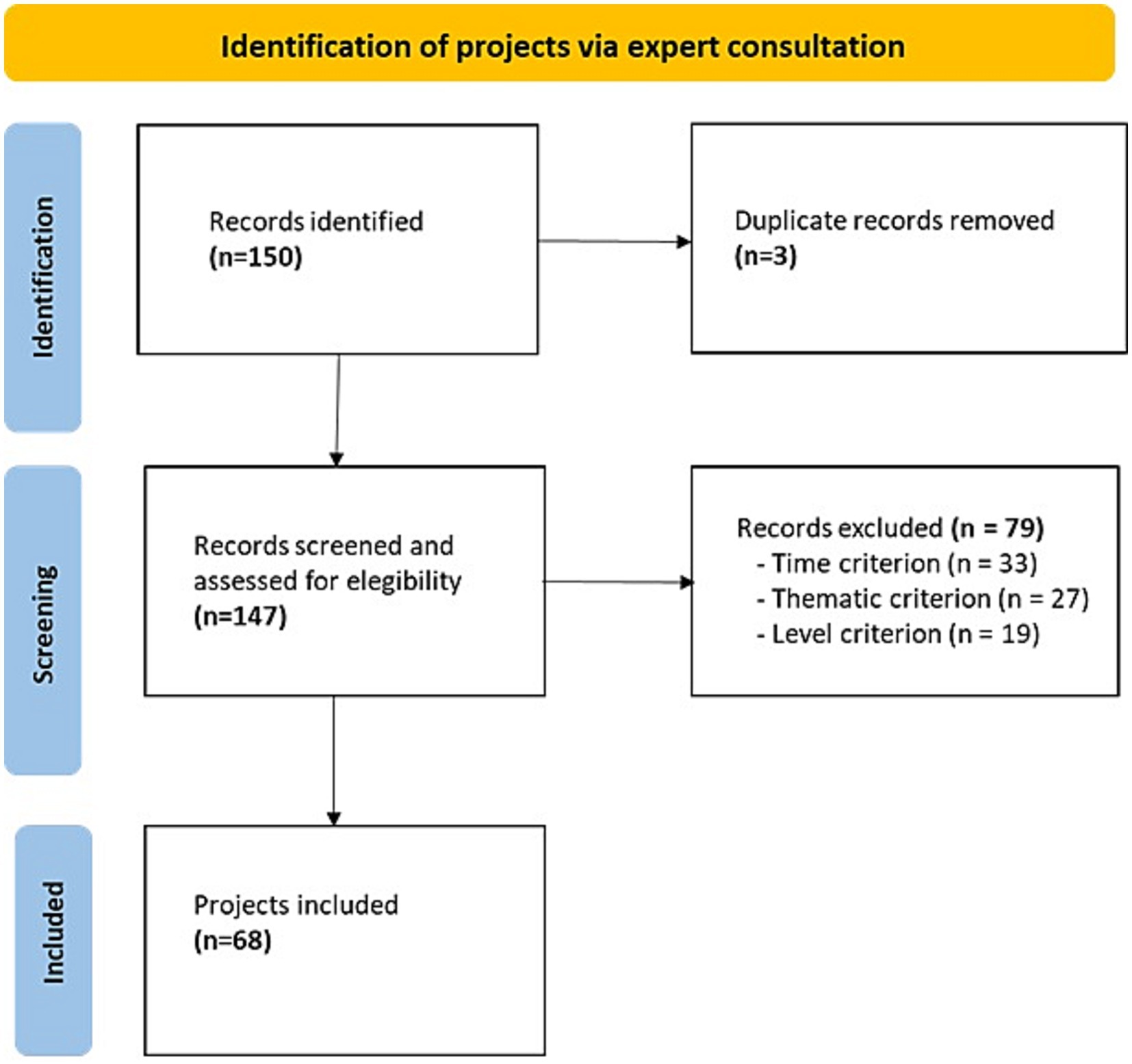
After removal of duplicates, a total of 147 projects resulted from the consultation with IC members (Figure 1). Screening by inclusion criteria selected 68 projects (11–70) (Supplementary Table S2). The reasons for exclusion were: projects not in progress during the year 2022 (N = 33; concluded before 2022 or yet to start); projects off-topic (N = 20; mainly basic research) or topic not clear due to lack of information (N = 7); and projects sponsored only at the regional level (N = 19).
3.2 Project characteristics
3.2.1 General features
The characteristics of the 68 projects included in the study are extremely variable. Thus, with regard to timing, they were launched between the years 2017 and 2022, and are expected to conclude between 2022 and 2029 (Supplementary Table S2), with a duration of 1 to 10 years (median: 3, interquartile range: 3–4.5). The number of countries involved ranges from 1 (Italy alone) to 20 (median: 4, interquartile range: 1–6), while the number of partners involved ranges from 1 (one Italian participant) to 84 (large multi-country projects; median: 6, interquartile range: 4–15). The total amount of funding ranged from € 50,000 to € 80,803,177 (median: € 871,820 interquartile range: € 471,000 – € 4,000,000).
3.2.2 Funders and coordinators
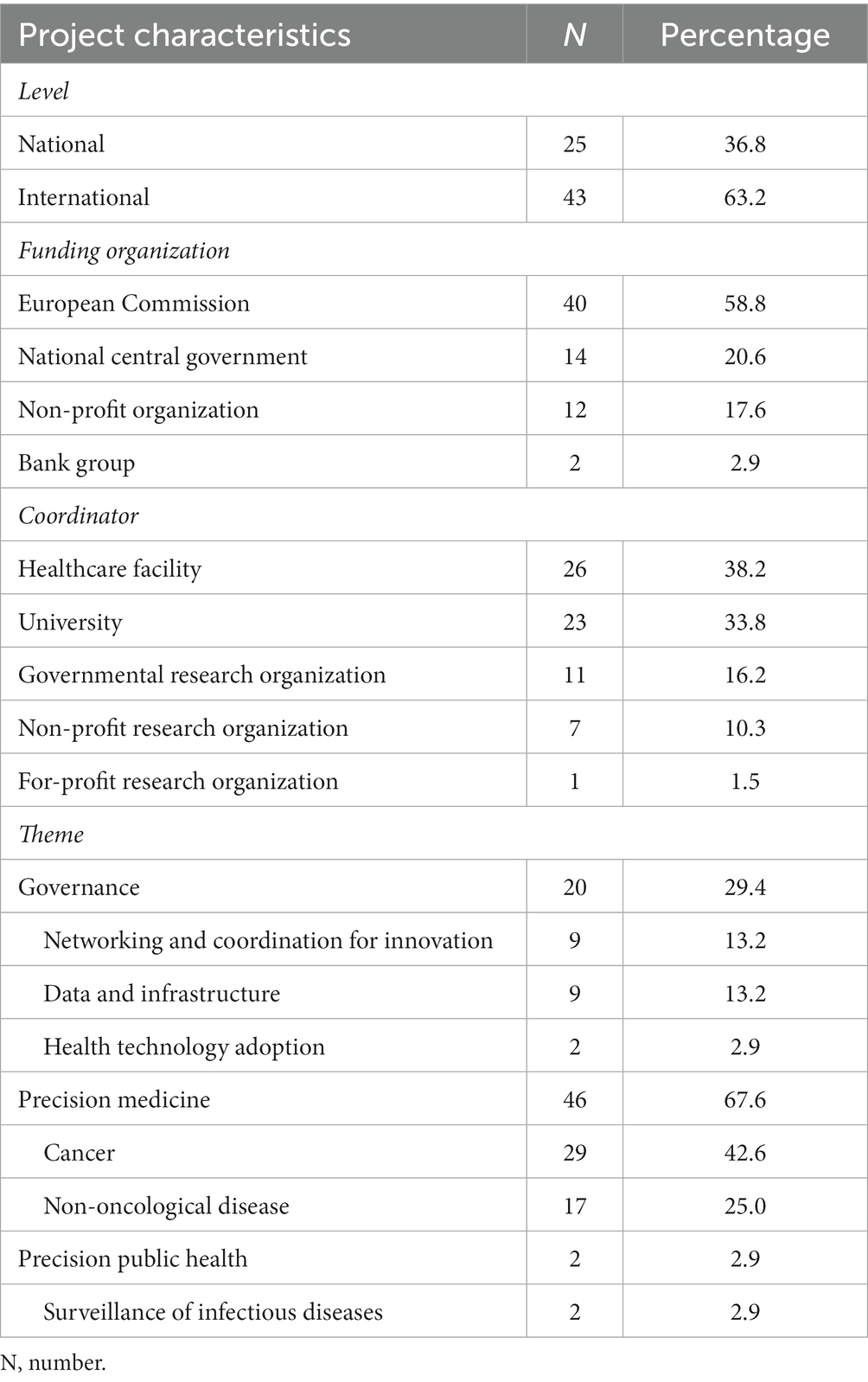
There are markedly more international (N = 43) than national (N = 25) projects (Table 1; Supplementary Table S2). with almost all the international ones being funded by the European Commission (EC; N = 40), while the main funder of national projects is central government (N = 14). A small number of national projects are funded by bank groups (N = 2), while the remaining national and international projects are financed by non-profit organizations (N = 12).
Overall, the retrieved projects are mostly coordinated by healthcare facilities (N = 26) and universities (N = 23; Table 1; Supplementary Table S2), with the remainder being managed by research organizations, either governmental (N = 11) or non-governmental (N = 8), most of the latter being non-profit (N = 7). Of the international projects, one quarter are coordinated by an Italian institution (N = 17). Finally, almost all of the retrieved projects count among its funders or executors at least one of the Institutions represented in the IC (N = 63).
3.2.3 Theme
The projects were mapped to three thematic categories, namely (I) governance (N = 20); (II) PM (N = 46); and (III) PPH (N = 2; Table 1; Supplementary Table S2).
(I) Governance
The 20 projects in the governance category aim to ensure a coordinated approach to the long-term implementation of genomics for the personalization of healthcare (Supplementary Table S2). They can be further classified into three sub-categories (Table 1).
(Ia) Networking and coordination for innovation
The first sub-category, “Networking and coordination for innovation,” includes nine EU-funded projects aimed at fostering collaboration across European countries and beyond, with special regard to the development of training programs for researchers and healthcare professionals, the identification of recommendations for the harmonization of research and implementation initiatives, and the engagement of relevant stakeholders (including citizens, patients, healthcare professionals, policy makers and private companies; Supplementary Table S2). In Italy, an important leading role is played by the Catholic University of the Sacred Heart of Rome, which coordinates three of these projects funded through EU Horizon programs. The first is the ExACT (European network staff eXchange for integrAting precision health in the health Care SysTems) project, which involves eight member states (MS) plus the United Kingdom (UK), Canada and the United States, working together since 2019 to train a new generation of precision health professionals through a five-year secondment plan (16). The second is PROPHET (A PeRsOnalized Prevention roadmap for the future HEalThcare), a four-year project involving 12 EU MS plus the UK, launched in 2022 to develop a strategic roadmap for the implementation of innovative, sustainable and effective personalized programs to prevent common chronic diseases (27). Finally, the four-year IC2PerMed (Integrating China in the International Consortium for Personalized Medicine) project was launched in 2020 with the specific aim of fostering EU-China cooperation in the field of personalized medicine. This project is managed by the International Consortium for Personalized Medicine, a MS-driven initiative of over 40 international ministries and funding agencies (20).
(Ib) Data and infrastructure
The second sub-category, “Data and infrastructure,” includes nine projects aimed at developing infrastructure, tools and regulatory frameworks for the collection and use of genomic data (Supplementary Table S2). The key driver of this sub-category is the 2018 One Million Genomes Initiative (1 + MG), which is committed to creating a European data infrastructure for genomic and clinical data to support research, personalized healthcare and health policy formation (71). In fact, Italy is involved in both EU-funded projects launched to realize 1 + MG initiatives, coordinated by ELIXIR, a European intergovernmental organization. The first is the three-year B1MG (Beyond 1 Million Genomes), launched in 2020 to provide coordination and support to the 1 + MG initiative by defining the infrastructure, data standards and legal guidance for cross-border access to genomic data (11). The second is the 2022 GDI (Genomic Data Infrastructure) project, which will implement the recommendations of B1MG to create and deploy the technical capacity for accessing genomic data by 2027 (17). Since the end of 2021, Italy has further supported the fulfillment of these European goals through a dedicated two-year national project financed by the MoH-National Centre for Disease Prevention and Control and coordinated by Sapienza University of Rome, entitled “Italian Genomic Strategy” (30). Moreover, the ten-year project “Health Big Data,” dedicated to the deployment of an IT platform for sharing genomic and clinical data between the national Scientific Institutes for Research, Hospitalization and Healthcare, has been funded by the Italian Ministry of Economy and Finance since 2019 and is coordinated by a national oncology network named Alliance Against Cancer (19).
(Ic) Health technology adoption
Finally, the third sub-category, “Health technology adoption,” embraces two projects, one Italian and one European, that focus on guiding genomic-technology acquisition and use through health technology assessment (HTA) and procurement, respectively (Supplementary Table S2). The Italian project was financed at the end of 2019 by the MoH for a period of two years to design a comprehensive national path for the HTA of genetic and genomic tests, including the three phases of priority setting, assessment and appraisal (14); the European OncNGS (NGS diagnostics in 21st century oncology: the best, for all, at all times) project, launched in 2020 with the participation of eight buyers from five EU MS, coordinated by researchers in Belgium, will prepare a pre-commercial procurement procedure to provide the best next-generation sequencing diagnostic technologies for all solid-tumor and lymphoma patients by 2026 (25).
(II) Precision medicine
PM is the largest category and comprises 46 projects aimed at assessing the use of genomic information to provide a more precise approach to diagnosis, prognosis and treatment of disease (Supplementary Table S2). In particular, the majority of these projects aim to identify molecular biomarkers that predict the course of a disease and the response to treatment. While customizing care using a combination of clinical and genomic factors is not unusual among the projects in this study, very few also consider lifestyle and environmental factors. PM projects can be further classified according to the disease of interest (Table 1).
(IIa) Cancer
Over half of the PM projects focus on cancer (N = 29), especially hematological cancers and gastrointestinal cancers (Figure 2A; Supplementary Table S2). Among the international projects coordinated by Italy, one example is the three-year project IMAGene (Epigenomic and machine learning models to predict pancreatic cancer), supported by ERA PerMed, a funding scheme for personalized medicine research projects cofounded by the EC (49). IMAGene, which involves participants from six MS and is coordinated by the European Institute of Oncology in Milan, commenced in 2022 to develop, implement and test a comprehensive Cancer Risk Prediction Algorithm for the early detection of pancreatic cancer in high-risk asymptomatic subjects, based on germline mutations, DNA methylation profiling and magnetic resonance imaging. Among the national projects, one that has attracted many participants is the 2019 GerSom (Germline Somatic Panel) project, which is funded by the MoH for a period of three years under the supervision of Alliance Against Cancer (47). It aims to improve the management of patients with ovarian, breast and colorectal cancer through the validation of a genomic panel for somatic and germline variants, which allows the diagnosis of both genetic risk and sensitivity to new drugs.
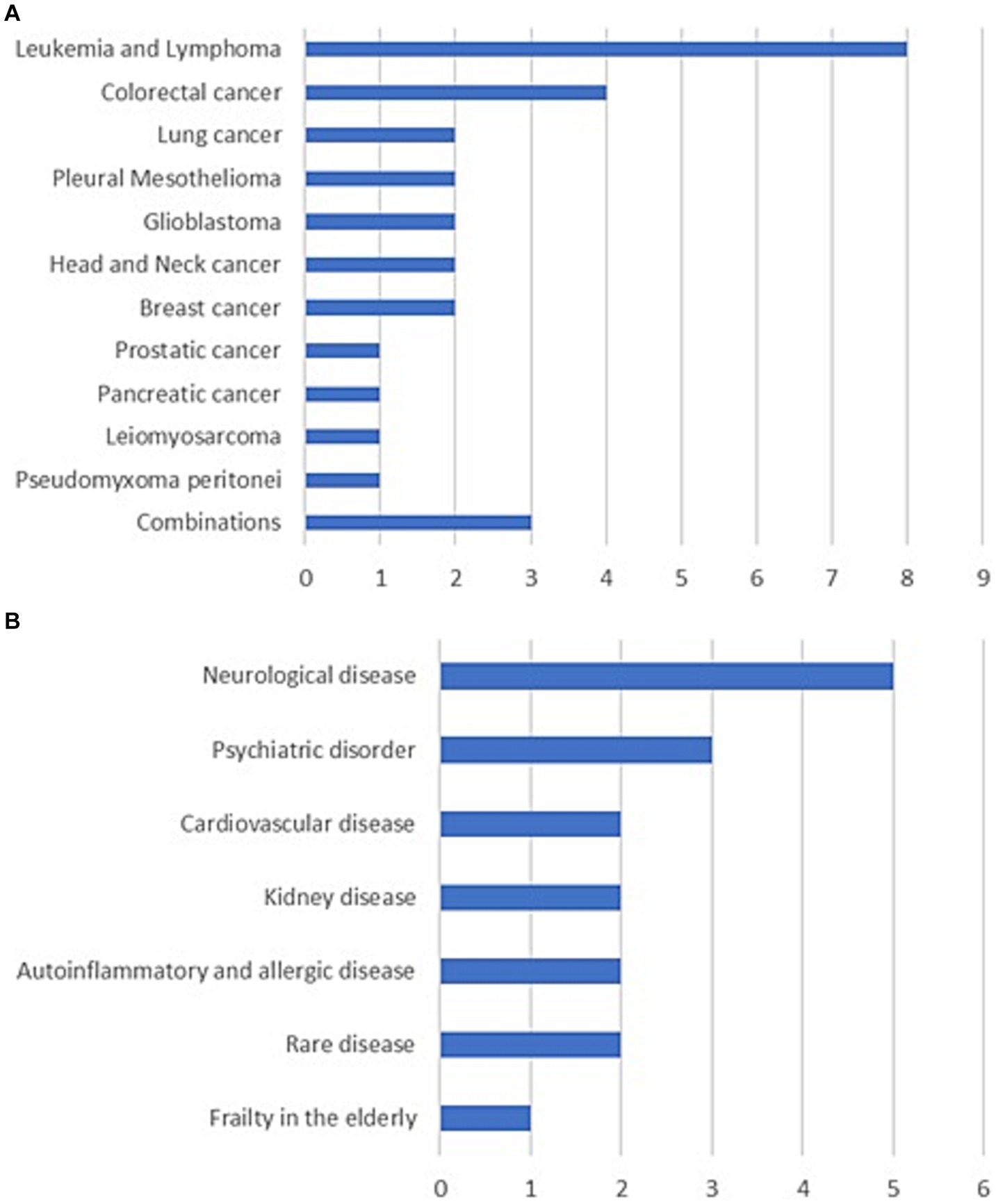
Figure 2. Number of precision medicine projects by disease. (A) Cancer. (B) Non-oncological diseases.
(IIb) Non-oncological diseases
Of the projects on non-oncological diseases (N = 17), the largest group addresses neurological diseases (N = 5), especially multiple sclerosis (Figure 2B; Supplementary Table S2). Among the international projects coordinated by Italy, there is another three-year ERA PerMed project named FindingMS (An integrated approach to predict disease activity in the early phases of multiple sclerosis), launched in 2019 with the participation of three MS and coordinated by the San Raffaele Research Hospital of Milan (45). It aims to design a predictive algorithm of disease activity for multiple sclerosis based on genetic and environmental factors, which will enable personalized treatment from the early phases of the disease. At the national level, a notable example is the 2022 NEUDIG (Unveiling the hidden side of NEUrodevelopmental DIsorder Genetics) project, funded by the Ministry of University and Research for a period of three years and coordinated by the University of Genova (38). Its purpose is to strengthen the molecular diagnosis of neurodevelopmental disorders by non-conventional genomic, transcriptomic, and functional analyses.
(III) Precision public health
Only two projects mapped to the PPH category, i.e., the use of genomic technologies to improve public health policy and practice (Supplementary Table S2). They are two national projects, funded by the Italian MoH-National Centre for Disease Prevention and Control in 2020 and 2022 for a period of two years each. They both focus on the same thematic sub-category, i.e., the molecular surveillance of infectious viral diseases (Table 1): while the first project, “Molecular characterization of SARS-CoV-2 in Italy,” coordinated by the ISS, is aimed at monitoring the SARS-CoV-2 virus in the country, by both time and geographical area, through genomic analysis (70), the other, i.e., SURVEID (SURVeillance of Emerging Infectious Diseases), coordinated by the Experimental Zooprophylactic Institute of Lombardia and Emilia-Romagna, plans to test a metagenomics next generation sequencing diagnostic platform for the surveillance of emerging viral disease threats (30).
4 Discussion
This paper highlights the main features of current PHG research in Italy by the collection and analysis of national and international research projects on the topic. Overall, PHG research in Italy seems to be mainly funded by the EC and is managed by healthcare facilities and universities. Notably, three main research themes for the integration of genomics into healthcare were identified: governance, PM and PPH. The predominant theme appears to be PM, mainly in its narrowest sense, as most PM projects aim to explore the use of molecular biomarkers to guide clinical decision making, with a particular focus on therapeutic choices for cancer patients (72). By contrast, only a few PM projects embrace a broader approach, as they combine the assessment of genomic and environmental or lifestyle determinants to guide the management of patients, more often in the field of non-oncological diseases (73). While PM, in its various facets, dominates the current research portfolio, PPH is clearly the least active research area. According to our results, in Italy the integration of genomics into public health strategies has probably been boosted by the Covid-19 pandemic, as the two PPH projects retrieved relate to the genomic surveillance of viral infectious diseases, including Covid-19. In the meantime, considerable efforts seem to be underway to guarantee the governance and sustainability of the long-term implementation of PM, the third key research theme emerging from the overview. In particular, major investments in governance are being made to foster partnership and collaboration across European countries to tackle two main implementation challenges: (i) ensuring the alignment of research activities and workforce education; and (ii) enabling the sharing and use of large-scale genomic data.
To the best of our knowledge, this overview is the first attempt to portray the Italian PHG research portfolio over a specific period of time, with the ultimate aim of informing relevant national decision makers. Nevertheless, it has to be considered that, although most of the projects identified will take months and even years to conclude, the growing number of PHG research projects funded each year may rapidly change the scenario we outline here. Thus, it would be useful to regularly update the database to monitor any potential change in research investment. To this end, designing a national or international repository for PHG research projects would surely be appropriate, as this would reduce duplicated effort, improve transparency, highlight opportunities for funding and monitor progress in this quickly evolving area.
Moreover, it would be interesting to understand whether the currently funded research activities align with national and international reference standards in the field. Unfortunately, we are not aware of any updated benchmark recommendations for PHG research, either nationally or at the European level. Although the “National plan for the innovation of the Health System based on omics sciences,” published in 2017, identified seven research opportunities for the integration of omic sciences into the National Health Service, these are likely to be outdated. The opportunities identified in 2017 were: (I) Big data and computational medicine; (II) Health literacy of citizens and healthcare professionals; (III) Drug repositioning and pharmacogenomics; (IV) Primary prevention of chronic disease; (V) Secondary prevention of breast cancer; (VI) Early detection of cancer; and (VII) Undiagnosed rare diseases (74). More recently, given the need to update the plan, the National Health Council issued its own recommendations for new priorities to be addressed (75). With regard to genomics research, the main indication is to further investigate the complex interactions between genetic and non-genetic factors in the pathogenesis of disease and to include non-genetic factors in risk-assessment algorithms. This resolution is consistent with our results, as the role of environmental and lifestyle factors appears to be neglected in ongoing Italian research.
At the European level, we found no official documents on shared research priorities for PHG. Some very general directions are provided by a policy briefing recently published in the context of the 1MG project (76). The first aim of the document is to set out policy recommendations for the implementation of genomics in healthcare, some of which also concern the field of research. It is recommended that close cooperation between clinical, research and industrial partners be established to ensure that the latest advances in science and technology are captured as they arise, and that research and clinical outcomes are coordinated. A further recommendation is to implement a data management plan to facilitate sharing of genomic and health information for clinical and research purposes at regional, national and international levels. According to our results, these two proposals are already being pursued in the research projects currently ongoing in Italy.
The main limitation of our work is in the comprehensiveness of the overview, which is affected by two conditions. First, we included only projects reported by members of the IC. Indeed, nearly all projects benefit from the involvement of at least one of the consulted institutions, as a funder or executor. However, since the IC includes the main national governmental and academic institutions involved in PHG, it is expected to account for most of the ongoing activities. Nevertheless, it is worth mentioning that a government funding organization not included in the IC emerged from the overview, namely the Ministry of Research (MoR). Indeed, the commitment of the MoR to PM is being strengthened by its recent decision to assign part of the research funds provided by the National Recovery and Resilience Plan to a nationwide research partnership, called HEAL ITALIA, which aims to create a Health Extended ALliance for Innovative Therapies, Advanced Lab-research, and Integrated Approaches of Precision Medicine (77). Thus, although it is not purely a healthcare institution, the future involvement of the MoR in the IC could be considered. Second, as stated in the Methods, we included only projects financed on either an international or a national scale. In fact, the Italian national healthcare system is decentralized to 21 regional healthcare systems, with different degrees of autonomy, and just four Regions participate in the IC on behalf of all the others, making the search for regional projects flawed by a potential selection bias. For this reason, we tried to avoid the tangle of regionally funded projects by focusing on centrally funded efforts in PHG research.
As for the accuracy of the evidence provided, we confirmed all data by an internet search of official websites. Nevertheless, we cannot exclude the possibility that in some cases a lack of clear information on the projects’ aims, together with their cross-cutting nature, may have affected their inclusion or assignment to particular thematic categories, even though both the project selection and data collection were performed by at least two authors. Lastly, it should be noted that the present overview made no attempt to assess the quality of the projects retrieved or whether they met their objectives, as this was not our goal.
In conclusion, we have provided an overview of the national research portfolio in PHG in Italy, with the primary aim of informing policy makers and fostering the coordination of national efforts for the implementation of evidence-based genomic applications. We found that research investments, mainly supported by the EC, are preferentially aimed at the clinical application of PM, but significant endeavors are also underway on the governance of the complex translation of genomic innovation into clinical and public health practice. Nevertheless, this is only the first step on a challenging path toward a coordinated and sustainable research agenda. First of all, this overview should be regularly updated to keep up with the systematic launch of new research projects. Then, updated research plans should be developed to align national activities with national and international priorities and avoid unaddressed needs and waste of resources.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EP: Writing – original draft, Conceptualization, Data curation. VB: Writing – review & editing Data curation. CI: Data curation, Writing – review & editing. PM: Investigation, Writing – review & editing. CM: Project administration, Writing – review & editing. PV: Supervision, Writing – review & editing. DG: Conceptualization, Writing – review & editing. FV: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the members of the Italian Inter-institutional Committee for Public Health Genomics for their participation in the study. Moreover, we would like to acknowledge the support received from the Italian project Strategia Genomica italiana: istituzione di una cabina di regia a supporto dell’iniziativa europea 1 + Million Genomes (1 + MG) e Beyond 1 + MG (B1MG) e del Coordinamento Interistituzionale per la Genomica in Sanità Pubblica (Italian Genomic Strategy: institution of a steering committee in support of the 1+ Million Genomes initiative, the Beyond 1 + MG project and the Italian Inter-institutional Committee for Public Health Genomics) funded by the Italian Ministry of Health - National Center for Disease Prevention and Control.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1343509/full#supplementary-material
References
1. Burke, W, Khoury, MJ, Stewart, A, and Zimmern, RLBellagio Group. The path from genome-based research to population health: development of an international public health genomics network. Genet Med. (2006) 8:451–8. doi: 10.1097/01.gim.0000228213.72256.8c
2. Carrasco-Ramiro, F, Peiró-Pastor, R, and Aguado, B. Human genomics projects and precision medicine. Gene Ther. (2017) 24:551–61. doi: 10.1038/gt.2017.77
3. Nakagawa, H, and Fujita, M. Whole genome sequencing analysis for cancer genomics and precision medicine. Cancer Sci. (2018) 109:513–22. doi: 10.1111/cas.13505
4. Khoury, M, Bowen, M, Clyne, M, Dotson, W, Gwinn, M, Green, R, et al. From public health genomics to precision public health: a 20-year journey. Genet Med. (2018) 20:574–82. doi: 10.1038/gim.2017.211
5. Khoury, MJ, Iademarco, MF, and Riley, WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med. (2016) 50:398–401. doi: 10.1016/j.amepre.2015.08.031
6. Onstwedder, SM, Jansen, ME, Leonardo Alves, T, Cornel, MC, and Rigter, T. Pursuing Public Health Benefit Within National Genomic Initiatives: Learning from Different Policies. Front Genet. (2022) 13:799. doi: 10.3389/fgene.2022.865799
7. Khoury, MJ, Bowen, S, Dotson, WD, Drzymalla, E, Green, RF, Goldstein, R, et al. Health equity in the implementation of genomics and precision medicine: A public health imperative. Genet Med. (2022) 24:1630–9. doi: 10.1016/j.gim.2022.04.009
8. Burns, BL, Bilkey, GA, Coles, EP, Bowman, FL, Beilby, JP, Pachter, NS, et al. Healthcare system priorities for successful integration of genomics: An Australian focus. Front Public Health. (2019) 7:425346. doi: 10.3389/FPUBH.2019.00041/BIBTEX
9. Italian Ministry of Health. Directorate General for Health Prevention. Directorial Decree 24 October 2022. Italy: Institution of the national Inter-Institutional Committee for Public Health Genomics (2022).
10. Molster, CM, Bowman, FL, Bilkey, GA, Cho, AS, Burns, BL, Nowak, KJ, et al. The evolution of public health genomics: Exploring its past, present, and future. Front Public Health. (2018) 6:397690. doi: 10.3389/FPUBH.2018.00247/BIBTEX
11. Beyond 1M Genomes | B1MG | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/951724 [Accessed October 11, 2023]
12. CAN.HEAL: Building the EU Cancer and Public Health Genomics platform. Available at: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/how-to-participate/org-details/999999999/project/101080009/program/43332642/details [Accessed October 11, 2023]
13. Innovative strategies for cancer prevention with focus on sex hormone signaling and chronic inflammation | CANCERPREV | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/859860 [Accessed October 11, 2023]
14. Definizione e promozione di programmi per l’implementazione delle azioni centrali di supporto al “Piano per l’innovazione del sistema sanitario basata sulle scienze omiche” | CCM - Network. Available at: https://www.ccm-network.it/progetto.jsp?id=node/2047&idP=740 [Accessed October 11, 2023]
15. EOSC4Cancer: A European-wide foundation to accelerate Data-driven Cancer Research. Available at: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/how-to-participate/org-details/984108263/project/101058427/program/43108390/details [Accessed October 11, 2023]
16. European network staff eXchange for integrAting precision health in the health Care sysTems | ExACT | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/823995 [Accessed October 11, 2023]
17. GDI: Genomic Data Infrastructure. Available at: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/how-to-participate/org-details/999999999/project/101081813/program/43152860/details [Accessed October 11, 2023]
18. Genomics and Personalized Medicine for all though Artificial Intelligence in Haematological Diseases | GenoMed4ALL | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/101017549 [Accessed October 11, 2023]
19. Health Big Data I Alleanza contro il cancro. Available at: https://www.alleanzacontroilcancro.it/en/progetti/italia/health-big-data/ [Accessed October 11, 2023]
20. Integrating China in the International Consortium for Personalised Medicine | IC2PerMed | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/874694 [Accessed October 11, 2023]
21. Secretariat for the International Consortium for Personalised Medicine (ICPerMed) | ICPerMed Secretariat | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/964197 [Accessed October 11, 2023]
22. Italian Multiple Sclerosis Foundation. Research on multiple sclerosis funded by the Italian Multiple Sclerosis FoundationFoundation. (2022). Available at: https://www.aism.it/sites/default/files/Compendio_Ricerca_2022_0.pdf [Accessed October 11, 2023]
23. International consortium for integrative genomics prediction | INTERVENE | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/101016775 [Accessed October 11, 2023]
24. IndividualizedPaediatricCure: Cloud-based virtual-patient models for precision paediatric oncology | iPC | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/826121 [Accessed October 11, 2023]
25. NGS diagnostics in 21st century oncology: the best, for all, at all times | oncNGS | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/874467 [Accessed October 11, 2023]
26. PERsonalised MedicIne Trials | PERMIT | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/874825 [Accessed October 11, 2023]
27. A PeRsOnalized Prevention roadmap for the future HEalThcare | PROPHET | Project | Fact sheet | HORIZON | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/101057721 [Accessed October 11, 2023]
28. Interregional Coordination for a fast and deep uptake of personalised health | REGIONS4PERMED | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/825812 [Accessed October 11, 2023]
29. Widening Sino-EU policy and research cooperation in Personalised Medicine | SINO-EU-PerMed | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/874556 [Accessed October 11, 2023]
30. CCM - Network. Available at: https://www.ccm-network.it/home.jsp [Accessed October 11, 2023]
31. Identification of the Molecular Mechanisms of non-response to Treatments, Relapses and Remission in Autoimmune, Inflammatory, and Allergic Conditions | 3TR | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/831434 [Accessed October 11, 2023]
32. Risultati promettenti dallo studio sui tumori al seno tripli negativi | Edo ed Elvo Tempia: per la lotta contro i tumori. Available at: https://www.fondazionetempia.org/risultati-promettenti-dallo-studio-sui-tumori-al-seno-tripli-negativi/ [Accessed October 11, 2023]
33. A Functional Precision Medicine Platform In Adult Leukemia | IFAB International Foundation. Available at: https://www.ifabfoundation.org/ifab-activities/projects/a-functional-precision-medicine-platform-in-adult-leukemia/ [Accessed October 11, 2023]
34. A genomic-driven diagnosis to deliver bespoke therapeutic strategies in HER2-low breast cancer patients | Fondazione AIRC per la Ricerca sul Cancro ETS. Available at: https://www.airc.it/ricercatori/i-nostri-ricercatori/caterina-marchi%C3%B2 [Accessed October 11, 2023]
35. Accelerator Award: Portfolio of funded projects and outputs | Cancer Research UK. Available at: https://www.cancerresearchuk.org/funding-for-researchers/accelerator-award/portfolio-funded-projects-outputs [Accessed October 11, 2023]
36. Actionable targets in clonal progression and systemic spreading of myeloid neoplasms | Fondazione AIRC per la Ricerca sul Cancro ETS. Available at: https://www.airc.it/ricercatori/i-nostri-ricercatori/alessandro-vannucchi [Accessed October 11, 2023]
37. Identification of markers for personal phenotyping in Acne Inversa | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/1st-joint-transnational-call-for-proposals-2018/identification-of-markers-for-personal-phenotyping-in-acne-inversa [Accessed October 11, 2023]
38. Italian Ministry of University and Research Project Portal. Available at: https://prin.mur.gov.it/ [Accessed October 11, 2023]
39. Defining stratification of patients with C3 Glomerulopathies /Immune complex –mediated glomerular diseases for better diagnosis and tailored treatment | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/multidisciplinary-research-projects-on-personalised-medicine-2013-pre-clinical-research-big-data-and-ict-implementation-and-user2019s-perspective/defining-stratification-of-patients-with-c3-glomerulopathies-immune-complex-2013mediated-glomerular-diseases-for-better-diagnosis-and-tailored-treatment [Accessed October 11, 2023]
40. Tumore della prostata, diventate protagonisti della ricerca | Edo ed Elvo Tempia: per la lotta contro i tumori. Available at: https://www.fondazionetempia.org/tumore-della-prostata-diventate-protagonisti-della-ricerca/ [Accessed October 11, 2023]
41. Epigenetic modeling/remodeling of cancer metastases and tumor immune contexture to improve efficacy of immunotherapy | Fondazione AIRC per la Ricerca sul Cancro ETS. Available at: https://www.airc.it/ricercatori/i-nostri-ricercatori/michele-maio [Accessed October 11, 2023]
42. Plasma extracellular vesicles (EVs): the key for precision medicine in Glioblastoma | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/multidisciplinary-research-projects-on-personalised-medicine-2013-pre-clinical-research-big-data-and-ict-implementation-and-user2019s-perspective/plasma-extracellular-vesicles-evs-the-key-for-precision-medicine-in-glioblastoma [Accessed October 11, 2023]
43. Italian Ministry of Health Research Project Portal. Available at: https://areapubblica.cbim.it/areapubblica/areaprogetti [Accessed October 11, 2022]
44. Gut OncoMicrobiome Signatures (GOMS) associated with cancer incidence, prognosis and prediction of treatment response. | ONCOBIOME | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/825410 [Accessed October 11, 2023]
45. An integrated approach to predict disease activity in the early phases of Multiple Sclerosis | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/1st-joint-transnational-call-for-proposals-2018/an-integrated-approach-to-predict-disease-activity-in-the-early-phases-of-multiple-sclerosis [Accessed October 11, 2023]
46. AIFA. FRAIL-BRAIN: Biological markers of frailty in the physiological and pathological aging brain: correlations with pharmacological frailty. Study protocol. (2018). https://www.aifa.gov.it/documents/20142/516919/Bando-AIFA-2016-19.07.2018.pdf [Accessed October 11, 2023]
47. GerSom | Alleanza contro il cancro. Available at: https://www.alleanzacontroilcancro.it/en/progetti/italia/gersom/ [Accessed October 11, 2023]
48. Rethinking personalized cancer therapy: targeting minimal residual disease in high-risk lymphoma patients | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/multidisciplinary-research-projects-on-personalised-medicine-2013-pre-clinical-research-big-data-and-ict-implementation-and-user2019s-perspective/rethinking-personalized-cancer-therapy-targeting-minimal-residual-disease-in-high-risk-lymphoma-patients [Accessed October 11, 2023]
49. Epigenomic and machine learning models to predict pancreatic cancer: development of a new algorithm to integrate clinical, omics, DNA methylation biomarkers and environmental data for early detection of pancreatic cancer in high-risk individuals | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/joint-transnational-call-for-proposals-2021-for-201cmultidisciplinary-research-projects-on-personalised-medicine-2013-development-of-clinical-support-tools-for-personalised-medicine-implementation201d/epigenomic-and-machine-learning-models-to-predict-pancreatic-cancer-development-of-a-new-algorithm-to-integrate-clinical-omics-dna-methylation-biomarkers-and-environmental-data-for-early-detection-of-pancreatic-cancer-in-high-risk-individuals [Accessed October 11, 2023]
50. To the NEXT level of risk prediction in patients with Long QT Syndrome | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/sc1-bhc-04-2018/1st-ejp-rd-joint-transnational-call-for-rare-diseases-research-project-jtc-2019/to-the-next-level-of-risk-prediction-in-patients-with-long-qt-syndrome [Accessed October 11, 2023]
51. Stratification of heart failure patients for cardiac recovery upon cardiac unloading by left ventricular assist device therapy: addressing the molecular, epigenetic, and proteomic changes associated with reverse cardiac remodelling | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/1st-joint-transnational-call-for-proposals-2018/stratification-of-heart-failure-patients-for-cardiac-recovery-upon-cardiac-unloading-by-left-ventricular-assist-device-therapy-addressing-the-molecular-epigenetic-and-proteomic-changes-associated-with-reverse-cardiac-remodelling [Accessed October 11, 2023]
52. Metabolic vulnerabilities for personalized therapeutic approaches in acute myeloid leukemia | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/personalised-medicine-multidisciplinary-research-towards-implementation/metabolic-vulnerabilities-for-personalized-therapeutic-approaches-in-acute-myeloid-leukemia [Accessed October 11, 2023]
53. Methylation based liquid biopsy to predict molecular residual disease and risk of recurrence in colon cancer patients | Fondazione AIRC per la Ricerca sul Cancro ETS. Available at: https://www.airc.it/ricercatori/i-nostri-ricercatori/federica-di-nicolantonio [Accessed October 11, 2023]
54. A Machine learning approach to Identify patients with Resected non-small-cell lung cAnCer with high risk of reLapsE | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/joint-transnational-call-for-proposals-2021-for-201cmultidisciplinary-research-projects-on-personalised-medicine-2013-development-of-clinical-support-tools-for-personalised-medicine-implementation201d/a-machine-learning-approach-to-identify-patients-with-resected-non-small-cell-lung-cancer-with-high-risk-of-relapse [Accessed October 11, 2023]
55. Multiple manifestations of genetic and non-genetic factors in Multiple Sclerosis disentangled with a multi-omics approach to accelerate personalised medicine | MultipleMS | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/733161 [Accessed October 11, 2023]
56. PARP1 and immune checkpoint inhibition after chemotherapy induction in leiomyosarcoma: a model to unleash immunoresponse | Fondazione AIRC per la Ricerca sul Cancro ETS. Available at: https://www.airc.it/ricercatori/i-nostri-ricercatori/giovanni-grignani [Accessed October 11, 2023]
57. Personalized Mitochondrial Medicine (PerMiM): Optimizing diagnostics and treatment for patients with mitochondrial diseases | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/personalised-medicine-multidisciplinary-research-towards-implementation/personalized-mitochondrial-medicine-permim-optimizing-diagnostics-and-treatment-for-patients-with-mitochondrial-diseases [Accessed October 11, 2023]
58. Implementation of personalised management in nephrotic syndrome | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/joint-transnational-call-for-proposals-2021-for-201cmultidisciplinary-research-projects-on-personalised-medicine-2013-development-of-clinical-support-tools-for-personalised-medicine-implementation201d/implementation-of-personalised-management-in-nephrotic-syndrome [Accessed October 11, 2023]
59. Integrative Personal Omics Profiles in Glioblastoma Recurrence and Therapy Resistance | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/1st-joint-transnational-call-for-proposals-2018/integrative-personal-omics-profiles-in-glioblastoma-recurrence-and-therapy-resistance [Accessed October 11, 2023]
60. Personalized medicine. Advancing chemical and genomic strategies for relapsed/refractory T-ALL | Fondazione Ginema. Available at: https://www.gimema.it/fondo-per-le-idee-2019-progetti-finanziati/ [Accessed October 11, 2023]
61. Personalization of Long term Treatment in Bipolar Disorder |ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/1st-joint-transnational-call-for-proposals-2018/personalization-of-long-term-treatment-in-bipolar-disorder [Accessed October 11, 2023]
62. AI for new signatures and models for tailored organ preservation approaches in laryngeal and hypopharyngeal cancer | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/multidisciplinary-research-projects-on-personalised-medicine-2013-pre-clinical-research-big-data-and-ict-implementation-and-user2019s-perspective/ai-for-new-signatures-and-models-for-tailored-organ-preservation-approaches-in-laryngeal-and-hypopharyngeal-cancer [Accessed October 11, 2023]
63. Toward PrecisiOn Medicine for the Prediction of Treatment response in major depressive disorder through stratification of combined clinical and -omics signatures | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/multidisciplinary-research-projects-on-personalised-medicine-2013-pre-clinical-research-big-data-and-ict-implementation-and-user2019s-perspective/toward-precision-medicine-for-the-prediction-of-treatment-response-in-major-depressive-disorder-through-stratification-of-combined-clinical-and-omics-signatures [Accessed October 11, 2023]
64. Optimizing response to Li treatment through personalized evaluation of individuals with bipolar I disorder: the R-LiNK initiative | R-LiNK | Project | Fact sheet | H2020 | CORDIS | European Commission. Available at: https://cordis.europa.eu/project/id/754907 [Accessed October 11, 2023]
65. Strategies to overcome acquired resistance to targeted therapies in colorectal cancer | Fondazione AIRC per la Ricerca sul Cancro ETS. Available at: https://www.airc.it/ricercatori/i-nostri-ricercatori/sabrina-arena [Accessed October 11, 2023]
66. Supporting Personalized Treatment Decisions in Head and Neck Cancer through Big Data | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/personalised-medicine-multidisciplinary-research-towards-implementation/supporting-personalized-treatment-decisions-in-head-and-neck-cancer-through-big-data [Accessed October 11, 2023]
67. Subpopulation heterogeneitY and MicroenvironMental Engagement as predictors for Treatment Resistance in lYmphoma | ERA-LEARN. Available at: https://www.era-learn.eu/network-information/networks/era-permed/joint-transnational-call-for-proposals-2021-for-201cmultidisciplinary-research-projects-on-personalised-medicine-2013-development-of-clinical-support-tools-for-personalised-medicine-implementation201d/subpopulation-heterogeneity-and-microenvironmental-engagement-as-predictors-for-treatment-resistance-in-lymphoma [Accessed October 11, 2023]
68. THRuST | Transcan-2 translational cancer research program. Available at: https://www.transcanfp7.eu/index.php/abstract/thrust.html [Accessed October 11, 2023]
69. TOPMESO | Transcan-2 translational cancer research program. Available at: https://www.transcanfp7.eu/index.php/abstract/topmeso.html [Accessed October 11, 2023]
70. Caratterizzazione molecolare del virus pandemico SARS- CoV-2 in Italia | CCM - Network. Available at: https://www.ccm-network.it/progetto.jsp?id=node/2031&idP=740 [Accessed October 11, 2023]
71. European “1+ Million Genomes” Initiative | Shaping Europe’s digital future. Available at: https://digital-strategy.ec.europa.eu/en/policies/1-million-genomes [Accessed October 11, 2023]
72. Wang, X. New strategies of clinical precision medicine. Clin Transl Med. (2022) 12:e135. doi: 10.1002/CTM2.135
73. Pitini, E, Adamo, G, Gray, M, and Jani, A. Resetting priorities in precision medicine: the role of social prescribing. J R Soc Med. (2020) 113:310–3. doi: 10.1177/0141076820910325
74. Italian Ministry of Health. Piano per l’innovazione del sistema sanitario basata sulle scienze omiche. Italy (2022). Available at: https://www.salute.gov.it/imgs/C_17_notizie_3270_listaFile_itemName_0_file.pdf [Accessed October 11, 2023]
75. Italian Ministry of Health. Le priorità del Piano Nazionale della Genomica. Italy (2022). Available at: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3311_allegato.pdf [Accessed October 11, 2023]
76. Beyond 1M Genomes. Policy Brief. Genomics in Healthcare Key issues for implementation. (2022). Available at: https://b1mg-project.eu/images/pdf/Policy_Brief_Genomics_in_Healthcare_2022.pdf [Accessed October 11, 2023]
77. Italian Ministry of Research. HEAL ITALIA. (2023). Available at: https://www.mur.gov.it/sites/default/files/2023-02/D.D.%20341%20_PE0000019_re181022NF.pdf [Accessed October 23, 2023]
Keywords: public health genomics, research, precision medicine, precision public health, governance, Italy
Citation: Pitini E, Baccolini V, Isonne C, Maran P, Marzuillo C, Villari P, Galeone D and Vaia F (2024) Public health genomics research in Italy: an overview of ongoing projects. Front. Public Health. 12:1343509. doi: 10.3389/fpubh.2024.1343509
Edited by:
Anja Kovanda, University Medical Centre Ljubljana, SloveniaReviewed by:
Jernej Zavrsnik, Health Center dr Adolf Drolc, SloveniaTaneisha Gillyard Cheairs, Meharry Medical College, United States
Copyright © 2024 Pitini, Baccolini, Isonne, Maran, Marzuillo, Villari, Galeone and Vaia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erica Pitini, ZS5waXRpbmlAc2FuaXRhLml0
 Erica Pitini
Erica Pitini Valentina Baccolini
Valentina Baccolini Claudia Isonne
Claudia Isonne Paola Maran1
Paola Maran1 Carolina Marzuillo
Carolina Marzuillo Paolo Villari
Paolo Villari