
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 21 May 2024
Sec. Environmental Health and Exposome
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1340929
Objective: The escalating prevalence of chronic pain poses a substantial socio-economic burden. Chronic pain primarily stems from musculoskeletal and nervous system impairments. Given cadmium's known toxicity to these systems, our study sought to investigate the correlation between blood cadmium levels and chronic pain.
Methods: The cross-sectional study was conducted from the National Health and Nutrition Examination Survey (NHANES, 1999–2004), and comprised US adults who participated in a chronic pain interview. We employed logistic regression models and smooth curve fitting to elucidate the relationship between blood cadmium levels and chronic pain.
Results: Our findings revealed a linear association between blood cadmium levels and chronic pain. Compared to the lower blood cadmium tertile 1 (<0.3 ug/dL), the adjusted odds ratios (ORs) for tertile 2 (0.3–0.4 ug/dL), and tertile 3 (≥0.5 ug/dL), were 1.11 (0.96–1.29) and 1.2 (1.03–1.39), respectively. Sensitivity analyses corroborated these results.
Conclusion: Elevated levels of blood cadmium are associated with a heightened risk of chronic pain among adults in the United States. Mitigating cadmium exposure could potentially decrease the risk of chronic pain, thereby enhancing strategies for chronic pain prevention and management.
Chronic pain is a pervasive, complex, and often debilitating condition that is increasing in prevalence worldwide, resulting in significant economic and social consequences (1). While it is frequently a sequela of injury or disease, it is also recognized as a distinct condition in its own right, not simply a symptom of other diseases (2). Chronic pain is characterized as a persistent discomfort that surpasses the typical duration of tissue healing. According to the International Association for the Study of Pain, this period is generally considered to exceed 3 months, particularly in the absence of other contributing factors (3). Numerous risk factors are implicated in the onset of chronic pain, encompassing a broad spectrum of domains such as sociodemographic characteristics, psychological conditions, clinical manifestations, and biological attributes (4–12). A thorough understanding of these risk factors can help guide specific prevention and management strategies.
Cadmium is a well-known hazardous pollutant that is widely distributed in the environment (13). Exposure to cadmium in the environment poses a range of public health issues. In the general population, cadmium is commonly absorbed through inhalation of cigarette smoke particles or ingestion of contaminated food, such as shellfish, offal, and vegetables (14). According to previous research, cadmium can be harmful to both the musculoskeletal and neurological systems (15–18), potentially leading to chronic pain (19). Cadmium poisoning is known to cause femoral and lumbar pain initially, followed by the spread of pain to other parts of the body (14). Itai-itai disease represents an extreme manifestation of chronic cadmium toxicity (20) with back pain as a distinguishing symptom (21).
As far as we understand, no existing studies have delved into the association between chronic pain and exposure to cadmium. Consequently, we aim to utilize data from the National Health and Nutrition Examination Survey (NHANES) to conduct an in-depth investigation into the relationship between blood cadmium levels and the incidence of chronic pain among adults in the United States. Our research hypothesis posits that individuals with higher blood cadmium levels are more susceptible to chronic pain.
The study population for this research was derived from the National Health and Nutrition Examination Survey (NHANES), a comprehensive study conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The NHANES database includes in-person interviews, laboratory tests, and clinical evaluations. Data from three consecutive NHANES survey cycles (1999–2000, 2001–2002, 2003–2004) that included both chronic pain assessment and blood cadmium level data were selected for this study.
The assessment of chronic pain was conducted for all participants aged 20 years and above through a series of interview questions. Participants who reported experiencing pain persisting for 3 months or more were classified under the chronic pain category, while the remainder were categorized as non-chronic pain sufferers.
For the years 1999–2002, an atomic absorption spectrometer was utilized for the quantification of blood cadmium levels. In contrast, for the years 2003–2004, inductively coupled plasma mass spectrometry was employed.
The study analyzed several demographic factors, including age, sex, race, marital status, body mass index (BMI), poverty income ratio (PIR), and education level. Alcohol consumption was assessed by asking participants about their frequency of drinking over the previous 12 months. Smoking status was determined based on whether an individual had consumed a minimum of 100 cigarettes throughout their lifetime (22). Physical activity was divided into four categories: mainly sitting, walking around, light physical activity, and heavy physical activity. Data on cotinine levels, blood lead levels, arthritis, cancer or malignancy, coronary heart disease, and osteoporosis were available and can be accessed at http://www.cdc.gov/nchs/nhanes/. Determining if someone has diabetes involves three criteria: (1) the person reports having the condition or is taking medication for it; (2) their blood sugar level after fasting is at least 7.0 mmol/L; and (3) their HbA1c level is at least 6.5% (23). To determine if someone has hypertension, two criteria are used: (1) the person reports having the condition or is taking medication for it, and (2) the average of three systolic blood pressure readings is at least 140 mmHg or the average of three diastolic blood pressure readings is at least 90 mmHg (24).
Continuous data were presented either as mean ± standard deviation or median (Q1–Q3) values. A P-value of <0.05 was considered statistically significant (two-tailed). All data analyses were performed using the statistical software packages R 3.3.2 and Free Statistics software version 1.7.1 (25).
The relationship between blood cadmium and chronic pain was investigated using logistic regression models. Blood cadmium levels (ug/dL) were divided into three tertiles (<0.3, 0.3–0.4, and ≥0.5) to evaluate their impact. Three models were constructed: an unadjusted model, a model adjusted for demographic factors, and a model adjusted for demographic factors + alcohol consumption, body mass index, physical activity, cotinine, blood lead, and comorbidities. These potential confounders were determined based on previous studies or the results of univariate logistic regression analyses. Subgroup analyses and smooth curve fitting were performed in this study. Sensitivity analysis was conducted using complete cases. Multiple imputations (5 replications) were used to address missing data.
In this study, 31,126 potential participants were initially identified, but only 13,485 adults aged 20 years and above were included. Figure 1 presents the exclusion criteria for the study. Table 1 displays the characteristics of participants, categorized by their blood cadmium level. The findings suggest a higher propensity for chronic pain among individuals with elevated blood cadmium concentrations.
Univariate analysis (Supplementary Table 1) revealed that several factors, including age, sex, BMI, education level, race, alcohol consumption, smoking status, physical activity, and comorbidities, such as arthritis, diabetes, coronary heart disease, cancer or malignancy, hypertension, and osteoporosis, were related to chronic pain.
To evaluate the association between blood cadmium and chronic pain, multivariable logistic regression analyses were performed. The results showed that blood cadmium was positively related to chronic pain, even after adjusting for potential covariates (Table 2, Model 1, OR: 1.28 [1.18–1.38], P < 0.001; Model 2, OR: 1.1 [1.03–1.23], P = 0.01).
When blood cadmium was analyzed in terms of tertiles, the association between blood cadmium and chronic pain was robust. The adjusted ORs for tertile 2 (0.3–0.4 ug/dL), and tertile 3 (≥0.5 ug/dL) were 1.11 (0.96–1.29) and 1.2 (1.03–1.39), respectively, compared with the lowest blood cadmium tertile 1 (<0.3 ug/dL) (Table 2, Model 2). Furthermore, the smooth curve analysis revealed a linear relationship between blood cadmium and chronic pain after adjusting for Model 2 (Figure 2, only 97.5% of the data is shown).
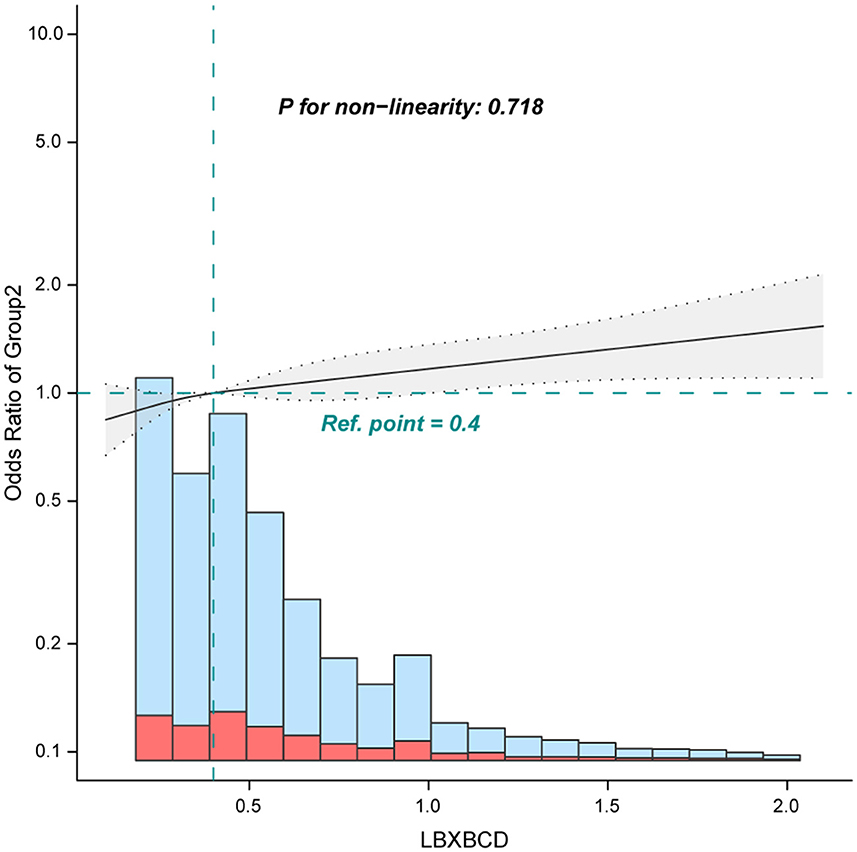
Figure 2. Association between blood cadmium with chronic pain. Adjusted for age, sex, marital status, poverty income ratio, race, education level, body mass index, alcohol consumption, physical activity, osteoporosis, diabetes, hypertension, cancer or malignancy, arthritis, coronary heart disease, cotinine, and blood lead. Only 97.5% of the data is shown.
Stratified analyses were performed to examine potential modifiers in the relationship between blood cadmium levels and chronic pain. Upon stratification by factors such as sex, age (<65 and ≥65 years), BMI (<25 and ≥25 kg/m2), physical activity, education level, and comorbidities (as depicted in Figure 3). However, considering multiple testing, the P-values for the interaction in hypertension (P = 0.02) and diabetes (P = 0.046) subgroups may not reach statistical significance.
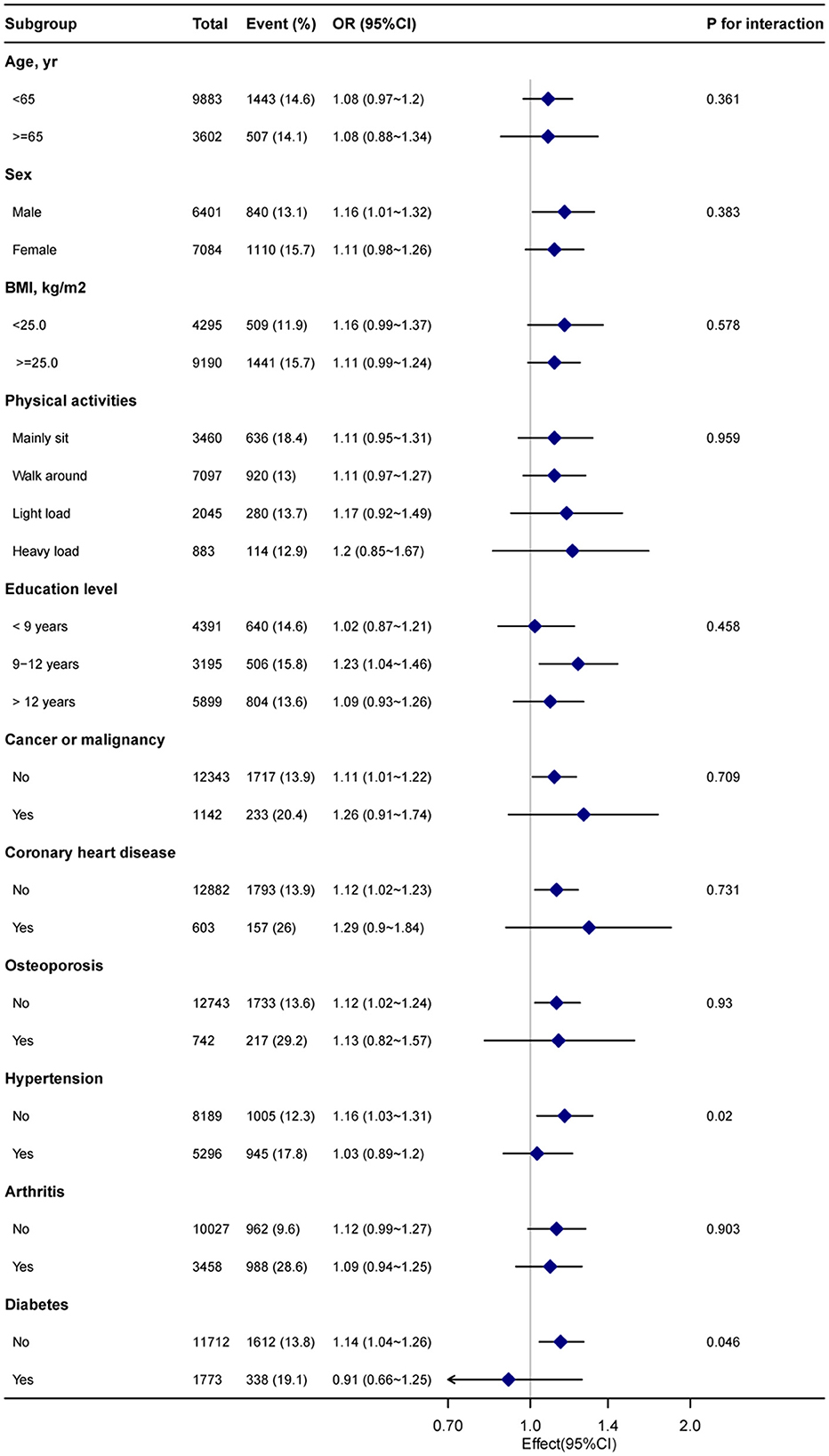
Figure 3. Subgroup analysis of the blood cadmium and chronic pain. Each stratification factor was adjusted for age, sex, poverty income ratio, marital status, race, education level, body mass index, alcohol consumption, physical activity, osteoporosis, diabetes, hypertension, cancer or malignancy, arthritis, coronary heart disease, cotinine, and blood lead.
Finally, the sensitivity analysis confirmed the linear association between blood cadmium and chronic pain even after excluding those with missing data (Supplementary Table 2).
The objective of this investigation was to elucidate the relationship between blood cadmium levels and chronic pain within the adult population of the United States, utilizing the nationally representative NHANES database. Our findings revealed a positive correlation between elevated blood cadmium levels and the prevalence of chronic pain. This association remained consistent across various subgroups and sensitivity analyses, thereby underscoring the robustness of the relationship between blood cadmium concentrations and chronic pain.
Previous studies on blood cadmium did not focus on chronic pain as a disease in its own right, but rather as a comorbidity of other diseases. However, chronic pain should be considered a healthcare issue and recognized as a disease in its own right (26). Cadmium can cause various diseases accompanied by chronic pain, such as Itai-itai disease, which results from long-term exposure to cadmium-contaminated rice fields. Patients experience a gradual extension of back and hip pain to the rest of the body, which persists for several years (17). Chronic pain is also accompanied by long-term back pain, and cadmium has been significantly associated with arthritis and rheumatoid arthritis in NHANES data (27). However, large cohort studies are still required to supplement the evidence of the relationship between blood cadmium and chronic pain.
While the mechanism underlying the relationship between blood cadmium and chronic pain is not fully understood, it can be explained by cadmium's toxic effects on the skeletal and nervous systems. Cadmium exerts an inhibitory effect on bone formation and stimulates bone resorption, influencing the functional activity of osteoblasts and osteoclasts. It also substitutes calcium in hydroxyapatite crystals, which leads to disorders in calcium metabolism, bone osteoporosis, osteomalacia, and demineralization. Consequently, this results in chronic pain localized in the limbs, pelvis, and spine (28–31). Cadmium has been observed to instigate oxidative stress, augmenting H2O2 levels within bone structures, while concurrently diminishing the concentrations of glutathione peroxidase and catalase (32). Furthermore, it stimulates the generation of inflammatory mediators, known as cytokines, within the immune system, leading to significant pain (33). Moreover, cadmium serves as a potent neurotoxic agent, inflicting adverse effects on the peripheral nervous system (34), which leads to oxidative stress (35, 36) and neurogenic damage resulting in neuronal cell death (37). Nerve damage is a major cause of neuropathic pain (19).
Reducing cadmium exposure may help reduce the risk of chronic pain, but it is not easy to achieve. Occupational and environmental exposure to cadmium primarily originates from industrial activities such as battery production, metallurgy, plastic manufacturing, pigments, mining, chemical stabilizers, and metal coatings, as well as contaminated farmland and food (38). Tobacco smoke contains cadmium, which further increases human exposure to cadmium (39). Moreover, cadmium is characterized by an extended biological half-life and a sluggish elimination rate within the human organism, leading to its persistent accumulation (40). Reducing smoking and exposure to secondhand smoke are individual choices that can help reduce cadmium exposure. Identifying food contamination levels and suspected areas and considering public education and awareness programs for cadmium poisoning prevention are also recommended (14).
However, this study has several limitations. Firstly, the pain data in the NHANES database is self-reported through questionnaire surveys, which may be subject to recall bias. However, trained interviewers were used to collect the data, and these methods have been validated and consistent over many years of data collection (41). Secondly, the NHANES database only collected pain data from 1999 to 2004, and there were no more detailed pain data, such as measurements of pain intensity and functional interference. This hinders our ability to examine the intricate details of pain in correlation with blood cadmium levels. Furthermore, the NHANES dataset is collected from the U.S. population, which may lead to regional bias. Finally, retrospective studies have inherent limitations such as missing data for some variables. Nevertheless, multiple imputations were performed and the results were stable. Multivariate logistic regression and subgroup analysis were conducted to reduce residual confounding effects from unknown factors.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HW: Conceptualization, Methodology, Supervision, Writing – review & editing. PM: Data curation, Formal analysis, Investigation, Writing – original draft. HD: Data curation, Formal analysis, Investigation, Writing – original draft. SC: Data curation, Investigation, Writing – original draft. XG: Data curation, Investigation, Writing – original draft. XC: Data curation, Investigation, Writing – original draft. YL: Data curation, Investigation, Writing – original draft. GF: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1340929/full#supplementary-material
1. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. (2016) 9:457–67. doi: 10.2147/JPR.S105892
2. Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. (2019) 123:e273–e83. doi: 10.1016/j.bja.2019.03.023
3. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
4. van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. (2013) 111:13–8. doi: 10.1093/bja/aet123
5. Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. (2005) 2:137–45. doi: 10.1016/S1550-8579(05)80042-7
6. Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. (2008) 136:235–8. doi: 10.1016/j.pain.2008.04.003
7. Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. (2008) 136:21–9. doi: 10.1016/j.pain.2007.06.008
8. Boersma K, Linton SJ. Expectancy, fear and pain in the prediction of chronic pain and disability: a prospective analysis. Eur J Pain. (2006) 10:551–7. doi: 10.1016/j.ejpain.2005.08.004
9. Bär KJ, Wagner G, Koschke M, Boettger S, Boettger MK, Schlösser R, et al. Increased prefrontal activation during pain perception in major depression. Biol Psychiatry. (2007) 62:1281–7. doi: 10.1016/j.biopsych.2007.02.011
10. Darlow B, Fullen BM, Dean S, Hurley DA, Baxter GD, Dowell A. The association between health care professional attitudes and beliefs and the attitudes and beliefs, clinical management, and outcomes of patients with low back pain: a systematic review. Eur J Pain. (2012) 16:3–17. doi: 10.1016/j.ejpain.2011.06.006
11. Hocking LJ, Morris AD, Dominiczak AF, Porteous DJ, Smith BH. Heritability of chronic pain in 2195 extended families. Eur J Pain. (2012) 16:1053–63. doi: 10.1002/j.1532-2149.2011.00095.x
12. Badraoui R, Blouin S, Moreau MF, Gallois Y, Rebai T, Sahnoun Z, et al. Effect of alpha tocopherol acetate in walker 256/b cells-induced oxidative damage in a rat model of breast cancer skeletal metastases. Chem-Biol Interact. (2009) 182:98–105. doi: 10.1016/j.cbi.2009.09.010
13. García-Esquinas E, Téllez-Plaza M, Pastor-Barriuso R, Ortolá R, Olmedo P, Gil F, et al. Blood cadmium and physical function limitations in older adults. Environ Pollut. (2021) 276:116748. doi: 10.1016/j.envpol.2021.116748
14. Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: an update. Caspian J Intern Med. (2017) 8:135–45. doi: 10.22088/cjim.8.3.135
15. Al-Ghafari A, Elmorsy E, Fikry E, Alrowaili M, Carter WG. The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress. PLoS ONE. (2019) 14:e0225341. doi: 10.1371/journal.pone.0225341
16. Pacini S, Fiore MG, Magherini S, Morucci G, Branca JJV, Gulisano M, et al. Could cadmium be responsible for some of the neurological signs and symptoms of myalgic encephalomyelitis/chronic fatigue syndrome. Med Hypotheses. (2012) 79:403–7. doi: 10.1016/j.mehy.2012.06.007
17. Reyes-Hinojosa D, Lozada-Pérez CA, Zamudio Cuevas Y, López-Reyes A, Martínez-Nava G, Fernández-Torres J, et al. Toxicity of cadmium in musculoskeletal diseases. Environ Toxicol Pharmacol. (2019) 72:103219. doi: 10.1016/j.etap.2019.103219
18. Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longevity. (2013) 2013:898034. doi: 10.1155/2013/898034
19. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
20. Umemura T, Wako Y. Pathogenesis of osteomalacia in itai-itai disease. J Toxicol Pathol. (2006) 19:69–74. doi: 10.1293/tox.19.69
21. Inaba T, Kobayashi E, Suwazono Y, Uetani M, Oishi M, Nakagawa H, et al. Estimation of cumulative cadmium intake causing itai–itai disease. Toxicol Lett. (2005) 159:192–201. doi: 10.1016/j.toxlet.2005.05.011
22. Hollingshead NA, Vrany EA, Stewart JC, Hirsh AT. Differences in mexican americans' prevalence of chronic pain and co-occurring analgesic medication and substance use relative to non-hispanic white and black americans: results from nhanes 1999-2004. Pain Med. (2016) 17:1001–9. doi: 10.1093/pm/pnv003
23. Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diab Care. (2020) 43:S14–S31. doi: 10.2337/dc20-S002
24. Zheng Y, Wang J, Wang Y, Xu K, Chen X. The hidden dangers of plant-based diets affecting bone health: a cross-sectional study with u.S. National health and nutrition examination survey (nhanes) data from 2005–2018. Nutrients. (2023) 15:1794. doi: 10.3390/nu15071794
25. Ruan Z, Lu T, Chen Y, Yuan M, Yu H, Liu R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient us adults. JAMA Dermatol. (2022) 158:745–53. doi: 10.1001/jamadermatol.2022.1609
26. Raffaeli W, Arnaudo E. Pain as a disease: an overview. J Pain Res. (2017) 10:2003–8. doi: 10.2147/JPR.S138864
27. Guan T, Wu Z, Xu C, Su G. The association of trace elements with arthritis in us adults: Nhanes 2013-2016. J Trace Elem Med Biol. (2023) 76:127122. doi: 10.1016/j.jtemb.2022.127122
28. Brzóska MM, Moniuszko-Jakoniuk J. Low-level lifetime exposure to cadmium decreases skeletal mineralization and enhances bone loss in aged rats. Bone. (2004) 35:1180–91. doi: 10.1016/j.bone.2004.07.010
29. Nambunmee K, Honda R, Nishijo M, Swaddiwudhipong W, Nakagawa H, Ruangyuttikarn W. Bone resorption acceleration and calcium reabsorption impairment in a thai population with high cadmium exposure. Toxicol Mech Methods. (2010) 20:7–13. doi: 10.3109/15376510903452941
30. Chen X, Wang G, Li X, Gan C, Zhu G, Jin T, et al. Environmental level of cadmium exposure stimulates osteoclasts formation in male rats. Food Chem Toxicol. (2013) 60:530–5. doi: 10.1016/j.fct.2013.08.017
31. Charkiewicz AE, Omeljaniuk WJ, Nowak K, Garley M, Nikliński J. Cadmium toxicity and health effects—a brief summary. Molecules. (2023) 28:6620. doi: 10.3390/molecules28186620
32. Brzóska MM, Rogalska J, Kupraszewicz E. The involvement of oxidative stress in the mechanisms of damaging cadmium action in bone tissue: a study in a rat model of moderate and relatively high human exposure. Toxicol Appl Pharmacol. (2011) 250:327–35. doi: 10.1016/j.taap.2010.11.012
33. Mahdi AA, Fatima G. A quest for better understanding of biochemical changes in fibromyalgia syndrome. Indian J Clin Biochem. (2014) 29:1–2. doi: 10.1007/s12291-013-0395-z
34. Viaene MK, Roels HA, Leenders J, De Groof M, Swerts LJ, Lison D, et al. Cadmium: a possible etiological factor in peripheral polyneuropathy. Neurotoxicology. (1999) 20:7–16.
35. Chen L, Liu L, Huang S. Cadmium activates the mitogen-activated protein kinase (mapk) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2a and 5. Free Radic Biol Med. (2008) 45:1035–44. doi: 10.1016/j.freeradbiomed.2008.07.011
36. Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, et al. Cadmium induction of reactive oxygen species activates the mtor pathway, leading to neuronal cell death. Free Radicals Biol Med. (2011) 50:624–32. doi: 10.1016/j.freeradbiomed.2010.12.032
37. Son J, Lee S-E, Park B-S, Jung J, Park HS, Bang JY, et al. Biomarker discovery and proteomic evaluation of cadmium toxicity on a collembolan species, paronychiurus kimi (lee). Proteomics. (2011) 11:2294–307. doi: 10.1002/pmic.200900690
38. Anetor JI. Rising environmental cadmium levels in developing countries: threat to genome stability and health. Niger J Physiol Sci. (2012) 27:103–15. doi: 10.4172/2161-0525.1000140
39. Li H, Wallin M, Barregard L, Sallsten G, Lundh T, Ohlsson C, et al. Smoking-induced risk of osteoporosis is partly mediated by cadmium from tobacco smoke: the mros sweden study. J Bone Miner Res. (2020) 35:1424–9. doi: 10.1002/jbmr.4014
40. Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. (2010) 118:182–90. doi: 10.1289/ehp.0901234
Keywords: blood cadmium, chronic pain, risk factor, NHANES, cross-sectional study
Citation: Mi P, Dong H, Chen S, Gao X, Cao X, Liu Y, Wang H and Fan G (2024) Blood cadmium level as a risk factor for chronic pain: NHANES database 1999–2004. Front. Public Health 12:1340929. doi: 10.3389/fpubh.2024.1340929
Received: 19 November 2023; Accepted: 08 May 2024;
Published: 21 May 2024.
Edited by:
Dimirios Nikolopoulos, University of West Attica, GreeceReviewed by:
Giuseppe De Palma, University of Brescia, ItalyCopyright © 2024 Mi, Dong, Chen, Gao, Cao, Liu, Wang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijie Wang, d2FuZ19odWlqaWVAb3V0bG9vay5jb20=; Guofeng Fan, MjQwMzA4MTE4MUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.