
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 16 February 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1337646
 Shu-Farn Tey1,2
Shu-Farn Tey1,2 Ya-Wen Tsai3,4
Ya-Wen Tsai3,4 Jheng-Yan Wu5,6
Jheng-Yan Wu5,6 Ting-Hui Liu7
Ting-Hui Liu7 Min-Hsiang Chuang8
Min-Hsiang Chuang8 Wan-Hsuan Hsu8
Wan-Hsuan Hsu8 Po-Yu Huang8
Po-Yu Huang8 Chih-Cheng Lai9,10*
Chih-Cheng Lai9,10* Chi-Kuei Hsu11,12*
Chi-Kuei Hsu11,12*Background: The outcomes of older adult people acquiring SARS-CoV-2 reinfection was unclear. This study aimed to compare the outcomes of older adult patients with COVID-19 reinfection and those with primary infection.
Methods: This retrospective cohort study used electronic medical records from the TriNetX Research Network. Older adult patients (aged ≥65 years) with COVID-19 between January 1, 2022, and December 31, 2022, were included in the study. The patients were subsequently categorized into reinfection or primary infection groups, according to whether they manifested two distinct COVID-19 episodes with an intervening period of more than 90 days. Propensity score matching was performed for covariate adjustment between the reinfection and primary infection groups. The primary outcome was a composite outcome, including emergency department visits, hospitalization, intensive care unit admission, mechanical ventilation use, and mortality, following primary infection and reinfection.
Results: After matching, 31,899 patients were identified in both the reinfection and primary infection groups. The risk of primary composite outcomes was 7.15% (n = 2,281) in the reinfection group and 7.53% (n = 2,403) in the primary infection group. No significant difference in the primary outcome was observed between groups (HR, 0.96; 95% CI, 0.91 to 1.02, p = 0.17). In addition, there was no significant differences between the reinfection and primary infection groups in terms of emergency department visit (HR, 1.03; 95% CI, 0.95 to 1.11, p = 0.49), all-cause hospitalization (HR, 0.94; 95% CI, 0.86 to 1.02, p = 0.14), intensive care unit admission (HR, 0.92; 95% CI, 0.67 to 1.28, p = 0.62), mechanical ventilation use (HR,1.35 95% CI, 0.69 to 2.64 p = 0.38), and all-cause mortality (HR, 0.94; 95% CI, 0.74 to 1.20, p = 0.62).
Conclusion: There were no significant differences in clinical outcomes between older adult patients with COVID-19 reinfection and those with primary infection.
Since the first outbreak of SARS-CoV-2 infection at the end of 2019, more than 770 million confirmed cases of COVID-19 have been reported to the World Health Organization (1). To combat this global health threat, the implementation of non-pharmacological interventions and widespread vaccination programs have helped prevent the transmission of SARS-CoV-2 (2, 3). Additionally, the development of novel antiviral and other anti-COVID-19 treatments has been effective in improving patient outcomes (4–6). However, SARS-CoV-2 has been constantly evolving during the pandemic, giving rise to new variants with immune escape characteristics. These variants not only possess the ability to evade neutralizing antibodies but can also overcome immune protection following natural infection. Consequently, the emergence of SARS-CoV-2 reinfection has become a serious threat and raised new global health concerns. Older adult people, especially those with comorbidities, have a higher risk of acquiring SARS-CoV-2 reinfection (7–9). Moreover, age is a significant risk factor for COVID-19 progression (3, 10, 11). However, the outcomes in older adult patients with SARS-CoV-2 reinfection are poorly understood.
A meta-analysis including 52 studies between 2019 and 2022 reported that the overall prevalence of SARS-CoV-2 reinfection was 4.2% (95% confidence interval [CI]: 3.7–4.8%); however, high heterogeneity was observed between studies (I2 = 99.9%) (12). Another meta-analysis involving 11 studies and 11 case reports showed that the pooled SARS-CoV-2 reinfection incidence rate was 0.70 (standard deviation [SD], 0.33) per 10,000 person-days (13). Furthermore, a cohort study utilizing the United States Department of Veterans Affairs’ national healthcare database found that SARS-CoV-2 reinfection was associated with an increased risk of death, hospitalization, and the likelihood of experiencing at least one sequela (14). In contrast, another nationwide study conducted in the United Kingdom showed that reinfection with SARS-CoV-2 presented with milder symptoms and carried a lower risk of COVID-19-related hospitalization and intensive care unit (ICU) admission compared to primary infection (15). To resolve these conflicting findings, this study using a global database – TriNetX research network, was conducted to compare the short-term outcome of SARS-CoV-2 reinfection compared with primary infection among the older adult patients, who are at high risk of COVID-19 progression.
We extracted data from the TriNetX Research Network, a global collaborative clinical research platform that contains real-time electronic medical data collected from 110 million patients from 78 healthcare organizations (HCOs) across four countries. Diagnoses based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) were retrieved from the TriNetX platform. This study was approved by the Institutional Review Board of the Chi Mei Medical Center (No. 11202–002).
Older adult patients (aged ≥65 years) with more than two visits to the HCOs and a COVID-19 diagnosis between January 1, 2022, and December 31, 2022, were included in the study. The ICD-10-CM diagnostic codes (U07.1, J12.81, and J12.82), positive SARS-CoV-2 RNA test (LOINC 94309–2, 94,500–6, 95,406–5, 94,502–2, 94,565–9, 95,608–6, 94,759–8, 94,845–5), and COVID-19 antigen test by immunoassay (LOINC 94558–4, 96,119–3) were used to extract the COVID-19 cohort. Patients were excluded from the analysis if they were admitted to a hospital from 3 days before to 2 days after the COVID diagnosis, or if they died on the same day as their COVID diagnosis. The patients were subsequently categorized into reinfection or primary infection groups according to whether they manifested two distinct COVID-19 episodes with an intervening period of more than 90 days.
A 1:1 propensity score matching (PSM) through the greedy nearest-neighbor algorithm was performed using the TriNetX built-in platform for covariate adjustment. Demographic characteristics (age, sex, and race) and comorbidities (body mass index, primary hypertension [ICD-10-CM E10], neoplasms [C00-D49], hyperlipidemia [E78.5], diabetes mellitus [E08-E13], atherosclerotic heart disease [I25.1], heart failure [I50], transplantation, and immunosuppressive therapy) were used to minimize confounding factors and selection bias, as previous studies (16–18). A standard difference of <0.1 was considered an acceptable balanced match.
The primary composite outcomes were all-cause emergency department (ED) visits, hospitalization, utilization of intensive care (ICU), utilization of mechanical ventilation (MV), or death during the 30-day follow-up period. The secondary outcomes were the individual components of the primary outcomes: all-cause hospitalization, ED visits, intensive care utilization, mechanical ventilation utilization, and death.
All statistical analyses were performed using TriNetX built-in function. Baseline characteristics were delineated as either mean ± standard deviation or specific frequency and proportion. Hazard ratios (HRs) with 95% confidence intervals (CIs) were derived using Cox proportional hazard regression. Kaplan–Meier curves facilitated the determination of cumulative probability, with a value of p of less than 0.05 deemed statistically significant. Moreover, subgroup analyses were performed based on age, sex, comorbidities, vaccination status, and use of antiviral medication.
The initial screening was conducted on August 30, 2023, identifying 295,268 older adult patients with COVID-19 from 78 healthcare organizations across the four countries (Figure 1). Among them, 31,908 had reinfections and 263,360 patients had primary infection. After PSM, 31,899 patients were identified in both the reinfection and primary infection groups.
Before matching, there were no significant differences in age, sex, or race between the reinfection and primary infection groups. However, patients with reinfection had a higher BMI than the primary infection group (29.3 ± 6.2 vs. 28.5 ± 5.9, p < 0.0001) and a higher prevalence of comorbidities, including hypertension, hyperlipidemia, neoplasms, diabetes mellitus, ischemic heart diseases, chronic lower respiratory diseases, overweight/obesity, cerebrovascular diseases, asthma, chronic liver disease, and pulmonary heart disease, as well as a higher percentage receiving immunosuppressants compared to those with primary infection. After PSM, there were no significant differences in the demographic features, including comorbidities, between the groups (Table 1).
Regarding the primary outcomes following the SARS-CoV-2 infections, the risk of composite outcomes, including ED visits, all-cause hospitalization, ICU admission, MV use, and mortality, was 7.15% (n = 2,281) in the reinfection group and 7.53% (n = 31,899) in the primary infection group. There was no significant difference between the groups (HR, 0.96; 95% CI, 0.91 to 1.02, p = 0.17; Table 2). Additionally, there was no significant difference in time-to-event-free survival between the reinfection and primary infection groups using Kaplan–Meier plots (log-rank p = 0.07; Figure 2).
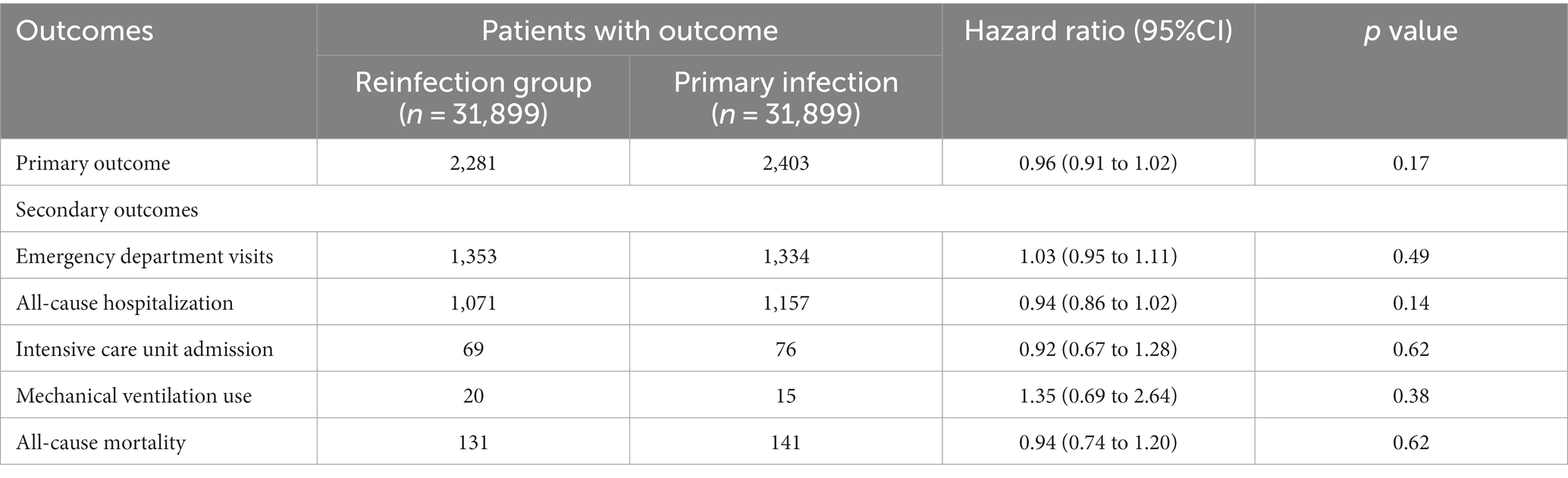
Table 2. The hazard ratio for comparing matched between the reinfection and primary infection cohorts for the outcomes.
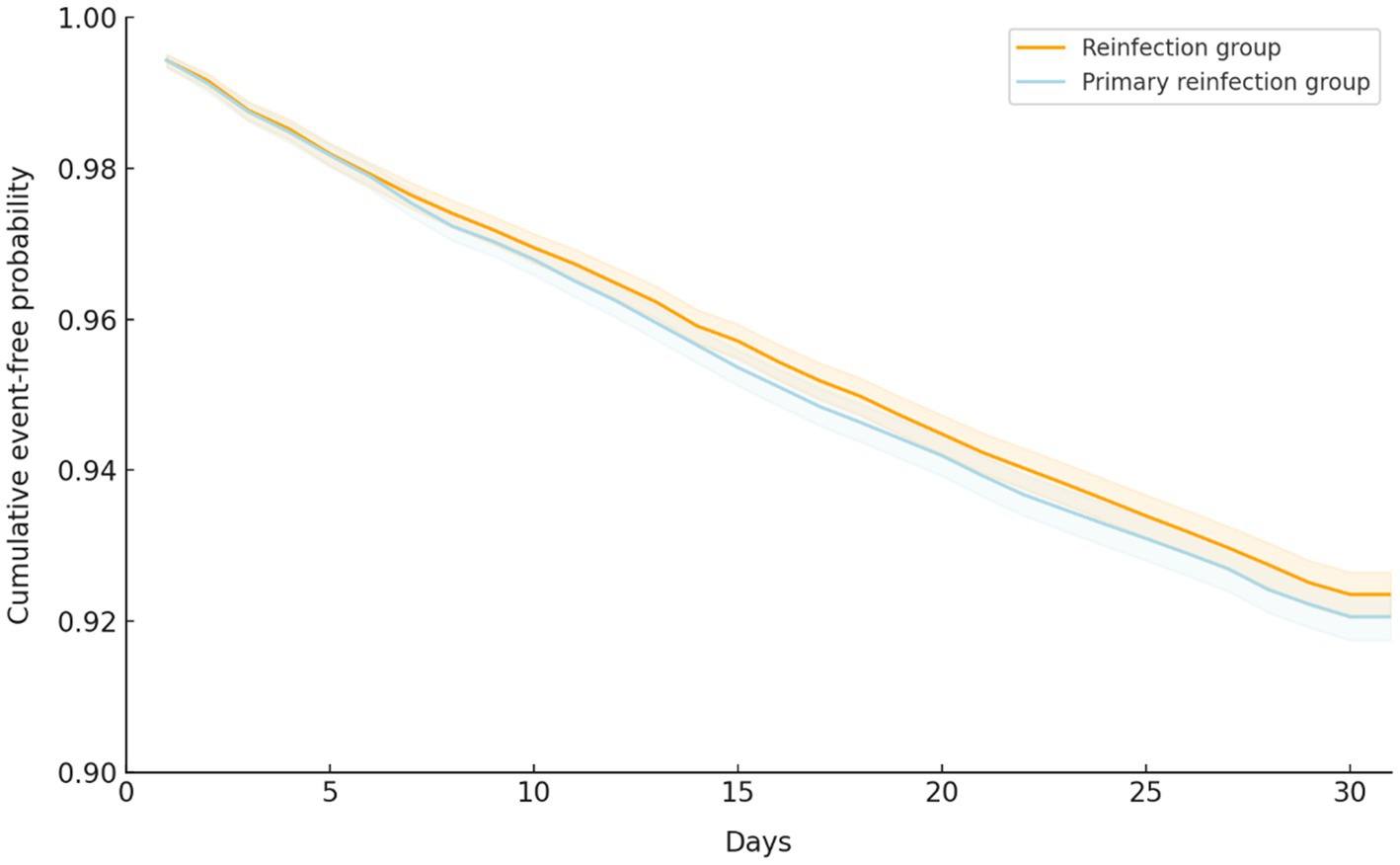
Figure 2. Kaplan–Meier time-to-event free curve for the primary composite outcome of patients with COVID-19 reinfection and primary infection.
Further subgroup analysis revealed a similar trend with no significant differences between the groups in the primary outcomes following the SARS-CoV-2 infections for the various subgroups. This included patients aged 65–74 years (HR, 1.00; 95% CI, 0.92 to 1.08, p = 0.97), those aged ≥75 years (HR, 0.93; 95% CI, 0.86 to 1.02, p = 0.11), males (HR, 0.93; 95% CI, 0.85 to 1.02, p = 0.10), females (HR, 0.99; 95% CI, 0.92 to 1.07, p = 0.76), individuals with obesity (HR, 1.09; 95% CI, 1.00 to 1.19, p = 0.05), those with diabetes mellitus (HR, 0.94; 95% CI, 0.86 to 1.02, p = 0.14), patients with malignancy (HR, 0.97; 95% CI, 0.88 to 1.07, p = 0.11), those receiving 0–1 vaccinations (HR, 0.96; 95% CI, 0.81 to 1.02, p = 0.18), individuals receiving 2 vaccinations (HR, 0.93; 95% CI, 0.86 to 1.31, p = 0.57), and those receiving booster vaccination (HR, 1.09; 95% CI, 0.87 to 1.36, p = 0.57). In contrast, among the subgroup of individuals receiving oral antiviral agents, the reinfection group was associated with a significantly higher risk than the primary infection group (HR, 1.41; 95% CI, 1.23 to 1.76, p < 0.001; Figure 3).

Figure 3. Forest plots of the primary outcome between COVID-19 reinfection and primary infection according to the subgroup.
Regarding secondary outcomes following the SARS-CoV-2 infections, there were no significant differences between the reinfection and primary infection group in terms of ED visit (HR, 1.03; 95% CI, 0.95 to 1.11, p = 0.49), all-cause hospitalization (HR, 0.94; 95% CI, 0.86 to 1.02, p = 0.14), ICU admission (HR, 0.92; 95% CI, 0.67 to 1.28, p = 0.62), MV use (HR,1.35 95% CI, 0.69 to 2.64 p = 0.38), all-cause mortality (HR, 0.94; 95% CI, 0.74 to 1.20, p = 0.62; Table 2).
The alarming surge in COVID-19 cases, which is further exacerbated by reinfection, highlights the need to scrutinize the implications and outcomes of SARS-CoV-2 reinfection, especially among older adults. This study meticulously curated data from the TriNetX research network with the aim of unveiling and comparing the short-term outcomes between reinfection and primary infection among older adult patients who are characteristically at a heightened risk of severe COVID-19 progression.
Our finding from this large retrospective cohort study revealed no significant difference of composite outcome including ED visits, all-cause hospitalization, ICU admission, MV use, and mortality following the SARS-CoV-2 infections between the reinfection and primary infection among the older adult populations (HR, 0.96; 95% CI, 0.91 to 1.02, p = 0.17). In the subgroup analysis, outcomes were similar with respect to patient age, sex, commodity, and number of vaccinations. This could indicate that previous SARS-CoV-2 infections may not dramatically alter the risk of severe outcomes during a subsequent infection within this demographic, which diverges from the conclusions of preceding research outcomes.
Although the reinfection risk is relatively low among the older adult population, a higher risk of disease severity was found to be associated with older adults age and underlying comorbidities, in a previous study in the United Kingdom (15). Therefore, it is important to know the outcome of older adult patients with COVID-19 reinfection. Our study is the first to compare the outcomes between primary and reinfected older adult populations with similar baseline characteristics, after matching. This contributes a nuanced layer to our growing understanding of SARS-CoV-2 reinfection, particularly in the older adults.
Intriguingly, while our data did not indicate a universal increase in risk across all older adult patients with reinfection, the subgroup analysis revealed a peculiar exception for those administered antiviral agents. Previous studies (19, 20) revealed hybrid immunity offers stronger protection than immunity obtained from a previous infection or vaccination alone. Warth et al. revealed that vaccination of a previously infected patient induced a higher concentration of three types of antibodies, including anti-S, anti-N, and Angiotensin I Converting Enzyme 2 Receptor Binding Protein (ACE2-RBD)-blocking antibodies (20). However, the effectiveness against severe, critical, or fatal COVID-19 was inconclusive due to the small number of cases. In our study, the outcomes of the reinfected older adult population were not significantly different from those of the population that received at least two doses of vaccination. This implies that hybrid immunity may effectively prevent infection but does not affect disease severity. Most importantly, the previous antiviral treatment did not offer the benefits for the outcomes after reinfection. There are some speculations, including the use of antiviral agents, which may induce mutations in the virus genome during replication (21, 22), and the other possibility, including the patient who had antiviral treatment, mostly belonged to high risk, significant symptomatic, or severe disease during the primary infection. Whether these signals are a possible underlying comorbidity, variations in immune response, or perhaps allude to an aspect of the viral encounter that escalates the risk, remains a potent avenue for future investigations.
The strength of this study is that it included a large cohort based on the TriNetX platform, and the outcomes of interest were accurately recorded. However, this study has several limitations. First, coding or uncoding errors may have occurred, particularly in the presence of comorbidities. Second, although PSM was used to balance the baseline differences between these two groups of patients, the severity of the comorbidities used could not be evaluated using the claims database. In addition, geographical diversity and variability in healthcare systems and policies across the participating HCOs might have introduced heterogeneity, even though a robust PSM technique was employed to minimize confounding and selection biases. Finally, variable vaccination statuses and immunization schedules, in conjunction with the potential influence of various antiviral and therapeutic strategies, embed additional complexity in the interpretive framework.
This study provided updated information on the outcomes of older adult patients with COVID-19. Overall, the outcomes of reinfection did not differ from those of the primary infection, and this trend remained unchanged across most subgroups, including vaccination status, age, comorbidities, and sex. However, the use of antiviral agents for reinfection is not as effective as for primary infection.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Chi Mei Medical Center, Tainan, Taiwan. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because retrospective study design based on TriNetX platform.
S-FT: Conceptualization, Investigation, Writing – original draft. Y-WT: Data curation, Formal analysis, Investigation, Writing – original draft. J-YW: Data curation, Investigation, Methodology, Writing – original draft. T-HL: Data curation, Formal analysis, Investigation, Writing – original draft. M-HC: Writing – original draft. W-HH: Data curation, Formal analysis, Investigation, Validation, Writing – original draft. P-YH: Data curation, Formal analysis, Investigation, Writing – original draft. C-CL: Conceptualization, Writing – review & editing. C-KH: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization. Available at: https://covid19.who.int/ Accessed on September 2, 2023.
2. Lai, CC, Chen, IT, Chao, CM, Lee, PI, Ko, WC, and Hsueh, PR. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. (2021) 20:1013–25. doi: 10.1080/14760584.2021.1949293
3. Lai, CC, Lee, PI, and Hsueh, PR. How Taiwan has responded to COVID-19 and how COVID-19 has affected Taiwan, 2020-2022. J Microbiol Immunol Infect. (2023) 56:433–41. doi: 10.1016/j.jmii.2023.04.001
4. Lai, CC, Wang, YH, Chen, KH, Chen, CH, and Wang, CY. The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. Viruses. (2022) 14:1706. doi: 10.3390/v14081706
5. Chuang, MH, Wu, JY, Liu, TH, Hsu, WH, Tsai, YW, Huang, PY, et al. Efficacy of nirmatrelvir and ritonavir for post-acute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection. J Med Virol. (2023) 95:e28750. doi: 10.1002/jmv.28750
6. Huang, PY, Liu, TH, Wu, JY, Tsai, YW, and Lai, CC. Clinical efficacy and safety of molnupiravir for nonhospitalized and hospitalized patients with COVID-19: a systematic review and meta-analysis of randomized control trials. J Med Virol. (2023) 95:e28621. doi: 10.1002/jmv.28621
7. Abedi, P, Afshari, P, Ansari, S, Alavi, SM, Dashtpayma, S, and Amiri, H. The prevalence of and factors related to reinfection with COVID-19 in Ahvaz, Iran: a comparative cross-sectional study. Health Sci Rep. (2023) 6:e1420. doi: 10.1002/hsr2.1420
8. Reynolds, SL, Kaufman, HW, Meyer, WA 3rd, Bush, C, Cohen, O, Cronin, K, et al. Risk of and duration of protection from SARS-CoV-2 reinfection assessed with real-world data. PloS One. (2023) 18:e0280584. doi: 10.1371/journal.pone.0280584
9. Li, M, Peng, H, Duan, G, Wang, J, Yu, Z, Zhang, Z, et al. Older age and depressive state are risk factors for re-positivity with SARS-CoV-2 omicron variant. Front Public Health. (2022) 10:1014470. doi: 10.3389/fpubh.2022.1014470
10. Huang, RC, Chiu, CH, Shang, HS, Perng, CL, Chiang, TT, Tsai, CC, et al. Clinical characteristics analysis of COVID-19 patients from the first significant community outbreak by SARS-CoV-2 variant B.1.1.7 in Taiwan as experienced from a single northern medical center. J Microbiol Immunol Infect. (2022) 55:1036–43. doi: 10.1016/j.jmii.2022.08.007
11. Nakakubo, S, Kishida, N, Okuda, K, Kamada, K, Iwama, M, Suzuki, M, et al. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: a registry-based observational study. Lancet Infect Dis. (2023) 23:1244–56. doi: 10.1016/S1473-3099(23)00271-2
12. Ukwishaka, J, Ndayishimiye, Y, Destine, E, Danwang, C, and Kirakoya-Samadoulougou, F. Global prevalence of coronavirus disease 2019 reinfection: a systematic review and meta-analysis. BMC Public Health. (2023) 23:778. doi: 10.1186/s12889-023-15626-7
13. Deng, L, Li, P, Zhang, X, Jiang, Q, Turner, D, Zhou, C, et al. Risk of SARS-CoV-2 reinfection: a systematic review and meta-analysis. Sci Rep. (2022) 12:20763. doi: 10.1038/s41598-022-24220-7
14. Bowe, B, Xie, Y, and Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. (2022) 28:2398–405. doi: 10.1038/s41591-022-02051-3
15. Mensah, AA, Lacy, J, Stowe, J, Seghezzo, G, Sachdeva, R, Simmons, R, et al. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect. (2022) 84:542–50. doi: 10.1016/j.jinf.2022.01.012
16. Hsu, WH, Shiau, BW, Liu, TH, Wu, JY, Tsai, YW, Huang, PY, et al. Clinical effectiveness of nirmatrelvir plus ritonavir in the treatment of COVID-19 in patients with cirrhosis. Expert Rev Anti Infect Ther. (2023) 21:1143–51. doi: 10.1080/14787210.2023.2267846
17. Liu, TH, Huang, PY, Wu, JY, Chuang, MH, Hsu, WH, Tsai, YW, et al. Comparison of post-acute sequelae following hospitalization for COVID-19 and influenza. BMC Med. (2023) 21:480. doi: 10.1186/s12916-023-03200-2
18. Wu, JY, Liu, MY, Liu, TH, Chuang, MH, Hsu, WH, Huang, PY, et al. Association between nirmatrelvir plus ritonavir and the outcomes of non-hospitalized obese patients with COVID-19. Int J Antimicrob Agents. (2023) 62:106984. doi: 10.1016/j.ijantimicag.2023.106984
19. Altarawneh, HN, Chemaitelly, H, Ayoub, HH, Tang, P, Hasan, MR, Yassine, HM, et al. Effects of previous infection, vaccination, and hybrid immunity against symptomatic alpha, Beta, and Delta SARS-CoV-2 infections: an observational study. EBioMedicine. (2023) 95:104734. doi: 10.1016/j.ebiom.2023.104734
20. AbdelWareth, L, Alhousani, F, Abuyadek, R, Donnelly, J, Leinberger-Jabari, A, Atef, S, et al. Natural infection versus hybrid (natural and vaccination) humoral immune response to SARS-CoV-2: a comparative paired analysis. Front Immunol. (2023) 14:1230974. doi: 10.3389/fimmu.2023.1230974
21. Sanderson, T, Hisner, R, Donovan-Banfield, I, Hartman, H, Løchen, A, Peacock, TP, et al. A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes. Nature. (2023) 623:594–600. doi: 10.1038/s41586-023-06649-6
Keywords: COVID-19, outcome, primary infection, reinfection, SARS-CoV-2
Citation: Tey S-F, Tsai Y-W, Wu J-Y, Liu T-H, Chuang M-H, Hsu W-H, Huang P-Y, Lai C-C and Hsu C-K (2024) Comparative outcomes of SARS-CoV-2 primary and reinfection in older adult patients. Front. Public Health. 12:1337646. doi: 10.3389/fpubh.2024.1337646
Received: 13 November 2023; Accepted: 06 February 2024;
Published: 16 February 2024.
Edited by:
Chengming Wang, Auburn University, United StatesReviewed by:
Najiba Abdulrazzaq, Emirates Health Services (EHS), United Arab EmiratesCopyright © 2024 Tey, Tsai, Wu, Liu, Chuang, Hsu, Huang, Lai and Hsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Cheng Lai, ZHRtZWQxNDFAZ21haWwuY29t; Chi-Kuei Hsu, b3NwcmV5aHN1QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.