- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Utah, Salt Lake City, UT, United States
- 2Division of General Internal Medicine, Department of Internal Medicine, University of Utah, Salt Lake City, UT, United States
- 3Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Francisco, San Francisco, CA, United States
- 4Department of Internal Medicine, School of Medicine, University of Utah, Salt Lake City, UT, United States
Background: Social determinants of health (SDoH) have been associated with disparate outcomes among those with metabolic dysfunction-associated steatotic liver disease (MASLD) and its risk factors. To address SDoH among this population, real-time SDoH screening in clinical settings is required, yet optimal screening methods are unclear. We performed a scoping review to describe the current literature on SDoH screening conducted in the clinical setting among individuals with MASLD and MASLD risk factors.
Methods: Through a systematic literature search of MEDLINE, Embase, and CINAHL Complete databases through 7/2023, we identified studies with clinic-based SDoH screening among individuals with or at risk for MASLD that reported pertinent clinical outcomes including change in MASLD risk factors like diabetes and hypertension.
Results: Ten studies (8 manuscripts, 2 abstracts) met inclusion criteria involving 148,151 patients: 89,408 with diabetes and 25,539 with hypertension. Screening was primarily completed in primary care clinics, and a variety of screening tools were used. The most commonly collected SDoH were financial stability, healthcare access, food insecurity and transportation. Associations between clinical outcomes and SDoH varied; overall, higher SDoH burden was associated with poorer outcomes including elevated blood pressure and hemoglobin A1c.
Conclusion: Despite numerous epidemiologic studies showing associations between clinical outcomes and SDoH, and guidelines recommending SDoH screening, few studies describe in-clinic SDoH screening among individuals with MASLD risk factors and none among patients with MASLD. Future research should prioritize real-time, comprehensive assessments of SDoH, particularly among patients at risk for and with MASLD, to mitigate disease progression and reduce MASLD health disparities.
Introduction
Metabolic-dysfunction associated steatotic liver disease (MASLD), the most common chronic liver disease in the United States, (1) disproportionately impacts vulnerable populations including non-White racial/ethnic groups and individuals with lower income and education (2–5). MASLD is strongly associated with obesity, diabetes, hypertension, dyslipidemia and metabolic syndrome (6–8). In fact, it is the hepatic manifestation of metabolic syndrome; the development of liver inflammation and fibrosis in MASLD is a result of nutrition, insulin resistance, and lipotoxicity (6, 9). Metabolic syndrome and its individual components are also more prevalent among vulnerable populations (10–16). There is a growing body of evidence that health disparities including those observed in chronic liver disease, are primarily the result of social determinants of health (SDoH) (17–23), the conditions where people are born, live, learn and work (24). Informative studies include systematic reviews and meta-analyses that show the associations of low socioeconomic status with (1) increased obesity prevalence (15), (2) higher hemoglobin A1c among individuals with diabetes (11), and (3) higher diabetes-related mortality (25). Data from the Centers for Disease Control and Prevention show higher prevalence of diabetes among non-White racial/ethnic groups; (10) based on national data, obesity is associated with lower household income and lower education level (16). Although the direct relationship between SDoH and MASLD is less well-studied, emerging evidence has shown that education (5) and race/ethnicity (4) are associated with MASLD prevalence and severity, neighborhood-level SDoH is associated with mortality, complications and cardiovascular disease, (26) and food insecurity is associated with increased risk of advanced liver fibrosis and all-cause mortality among those with MASLD (Figure 1) (2, 24, 27–30).
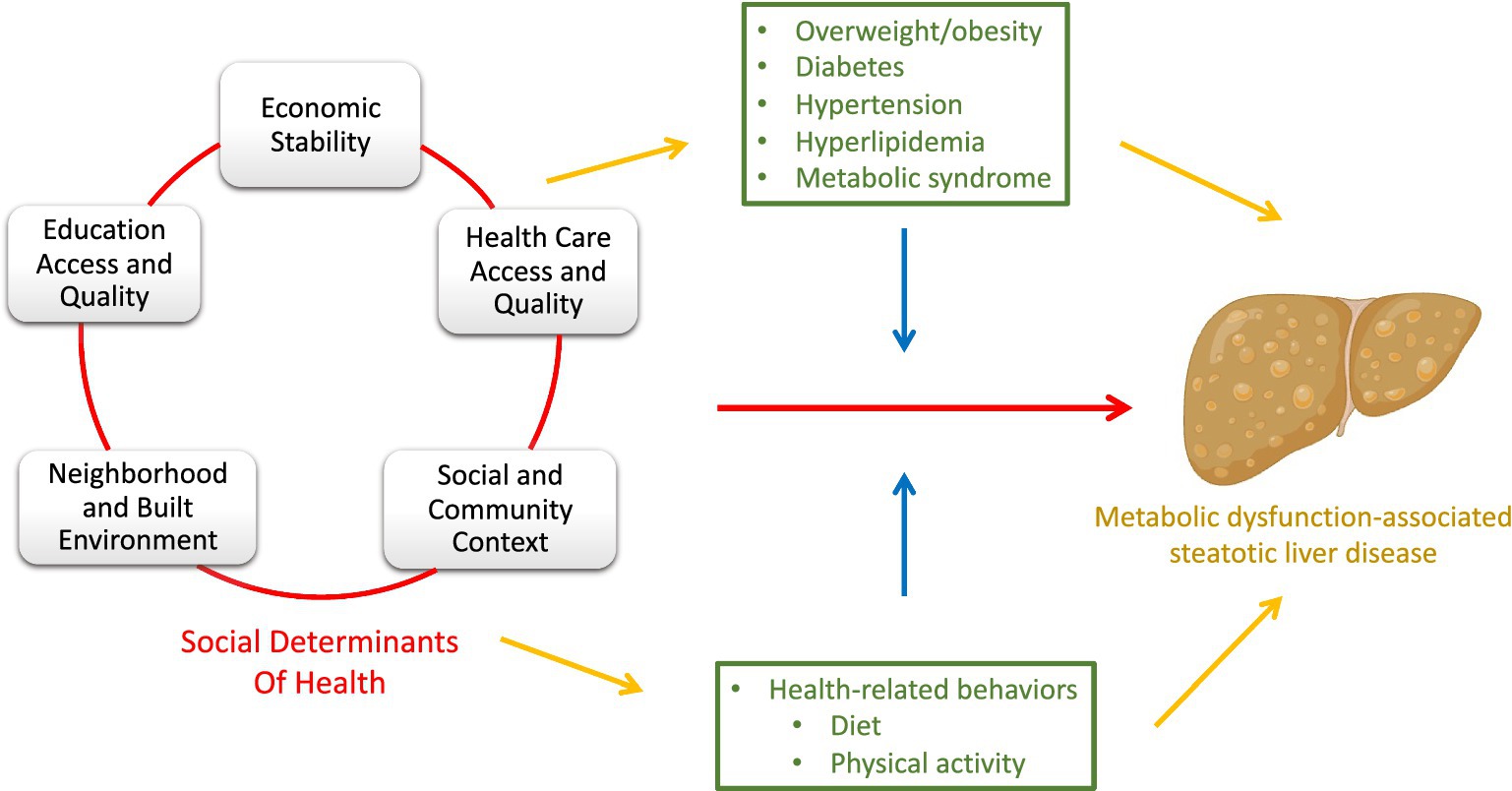
Figure 1. Simplified directed acyclic graph (DAG) depicting the relationship between social determinants of health (SDoH) and metabolic dysfunction-associated steatotic liver disease (MASLD). The DAG includes the direct effect of SDoH on MASLD (red pathway), as well as the indirect effect through mediators (yellow pathway). The blue pathway represents the way in which the covariates modify the effect of SDoH on MASLD (effect modifiers). Clinical conditions related to MASLD, and health-related behaviors are green representing their roles as both mediators (yellow) and effect modifiers (blue). *Made with graphics from Biorender.com.
Based on these data, as well decades of other studies with similar findings, (31–35) societies have recommended SDoH screening in clinical practice (36–38). In the Standards of Care in Diabetes guideline published in 2023, incorporation of SDoH into patient care is recommended to improve diabetes care, specifically for individualized self-management of diabetes and when selecting pharmacologic agents (36). In the most recent American Academy of Family Physicians’ guidelines for hypertension, providers are recommended to screen for SDoH and be conscious of how SDoH impact patient care, and the guidelines state future research assessing the impact of SDoH should be prioritized (37). The 2023 guidelines for Management of Patients with Chronic Coronary Disease developed by multiple cardiology societies recommend “routine assessment by clinicians and the care team for SDoH to inform patient-centered treatment decisions” (38). Although hepatology guidelines do not explicitly recommend SDoH screening, several recent reviews and editorials in top hepatology and liver transplant journals have concluded that SDoH data must be collected systematically to address liver disease disparities and improve patient outcomes overall (3, 39–41).
Despite these recommendations to screen for SDoH, a consensus on how to successfully conduct SDoH screening is lacking and a systematic approach for broad use has not been developed (42). The purpose of this scoping review is to describe if and how SDoH screening is being conducted among patients with MASLD and those at highest risk of MASLD. We aimed to summarize current efforts to screen for and address SDoH within hepatology clinics, primary care clinics, and other outpatient and inpatient settings, specifically among individuals with MASLD and its risk factors.
Materials and methods
We performed a scoping review, which uses a systematic search to bring together literature covering topics with emerging evidence (43, 44). Through a summarization of the body of literature addressing our research question, we aimed to (1) report on current evidence that addresses and informs practice and (2) identify gaps in the research knowledge. We hypothesized that current literature would contain a breadth of studies describing in-clinic SDoH screening for patients with MASLD risk factors, but there would be few, if any, studies among those with MASLD.
Search strategy
The literature search was completed just following the multi-society announcement of the change in nomenclature for non-alcoholic fatty liver disease (NAFLD) to MASLD on June 24, 2023 (45). Therefore, as all studies published up until that point referred to patients with MASLD as NAFLD, our search was conducted using NAFLD and terms with “fatty liver disease” instead of steatotic liver disease.
To identify all relevant articles that describe screening for SDoH among adults with MASLD-related risk factors, we conducted a systematic literature search of MEDLINE (1946 through July 2023), Embase (1988 through July 2023), and CINAHL Complete (1937 through July 2023) databases, with no language restrictions. The search strategy was designed after consultation with a librarian and implemented by the study’s investigators using the search strategy as described in the Supplementary Tables. The search for SDoH was designed using (1) different phrases similar to SDoH like “socioeconomic determinant” and “health structural determinant,” and (2) categories of SDoH as defined by Healthy People 2030, including “economic stability,” “education access,” “health care quality,” etc. (24). Two reviewers (RGK, AB) independently assessed the title and abstract of studies identified in the primary search for inclusion, and the full text of remaining articles were examined to determine whether they met inclusion criteria (46). Bibliographies from the selected articles and review articles on the topic were manually searched for additional studies. Any conflicting decisions were reviewed by RGK and discussed with co-authors as needed.
Study selection
Studies were included if they met the following inclusion criteria: (1) conducted in adults with or at risk for overweight/obesity, diabetes, hypertension, hyperlipidemia or MASLD, (2) collected SDoH data from screening performed in a clinical setting defined as real-time SDoH screening associated with a clinical encounter, and (3) assessed the prevalence or association of SDoH with clinical outcomes including incidence or disease severity. We excluded international and non-English studies, those reporting health-related behaviors (e.g., physical activity, dietary choices, etc. which are impacted by SDoH but are not considered to be SDoH) or built environment, intervention studies, perceived health or quality of life as their clinical outcome, and epidemiologic studies, particularly those conducting retrospective analyses of large national databases.
Data extraction and analysis
Data collected from each study included the following: time period of the study, location, patient population, SDoH screening questions used, setting of screening, clinical outcomes reported, and association of SDoH and clinical outcomes of interest including overweight/obesity, diabetes, hypertension, hyperlipidemia or MASLD.
Results
Search results
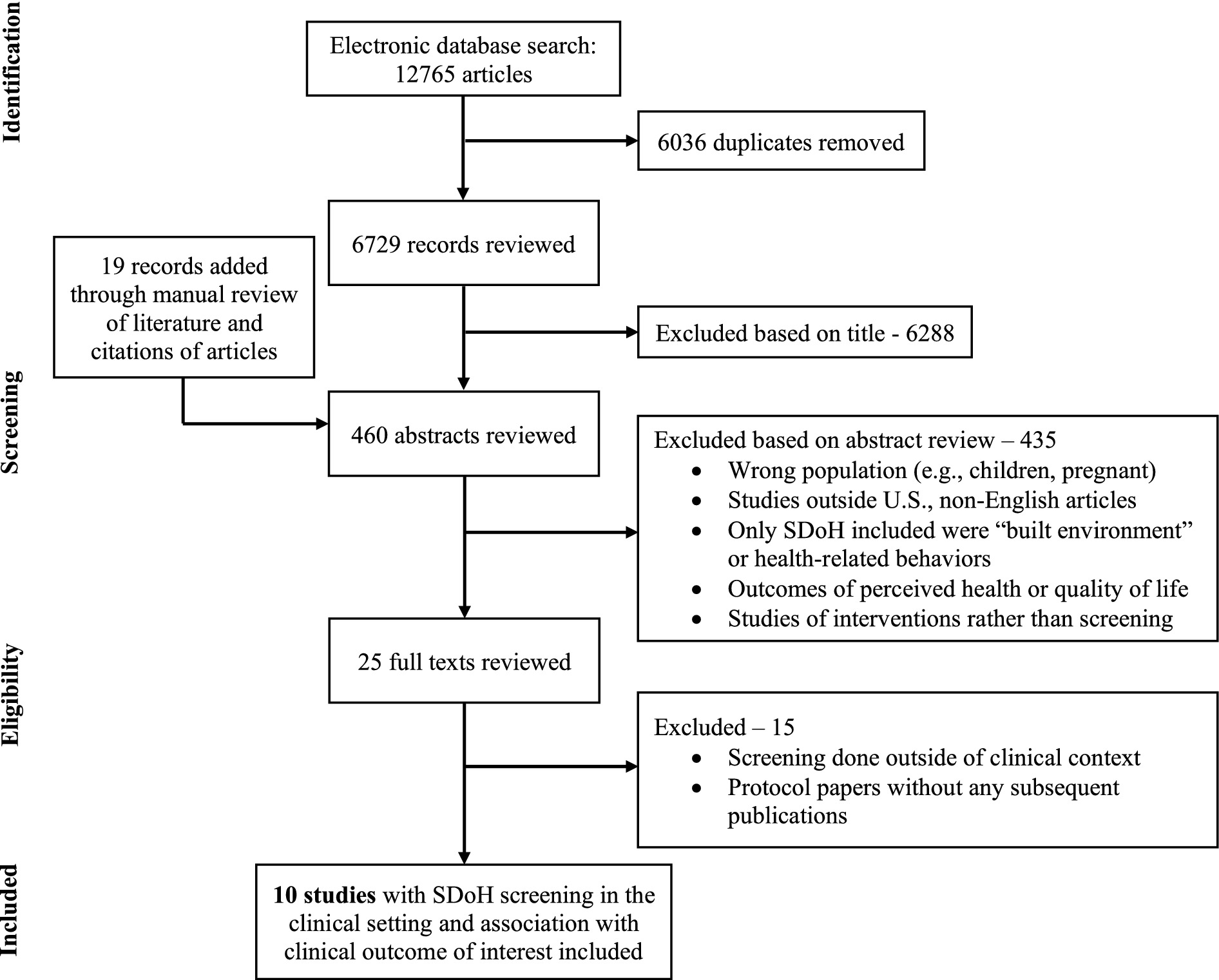
Of the 6,729 unique studies identified using our search criteria, after reviewing 460 abstracts and 25 full texts, 10 studies met our inclusion criteria – 8 manuscripts and 2 conference abstracts (47–56). Figure 2 shows the flow diagram summarizing our study identification and selection. Notably, there were no studies identified among individuals with MASLD.
Characteristics of included studies
The characteristics of these studies are shown in Table 1. Overall, these studies included 148,151 participants, of whom 89,408 (60%) had diabetes and 25,539 (17%) had hypertension. All studies were conducted in primary care clinics; 7 were specifically done in clinics affiliated with an academic medical center, one was performed in community health centers, and another was completed in federally qualified community health center (FQHC) adult and family medicine clinics. Two studies were limited to patients with hypertension and three studies only included individuals with diabetes. The other five studies included all primary care patients, but specifically assessed for the association of SDoH with overweight/obesity or body mass index (BMI), hypertension, diabetes, and/or atherosclerotic cardiovascular disease (ASCVD) risk.
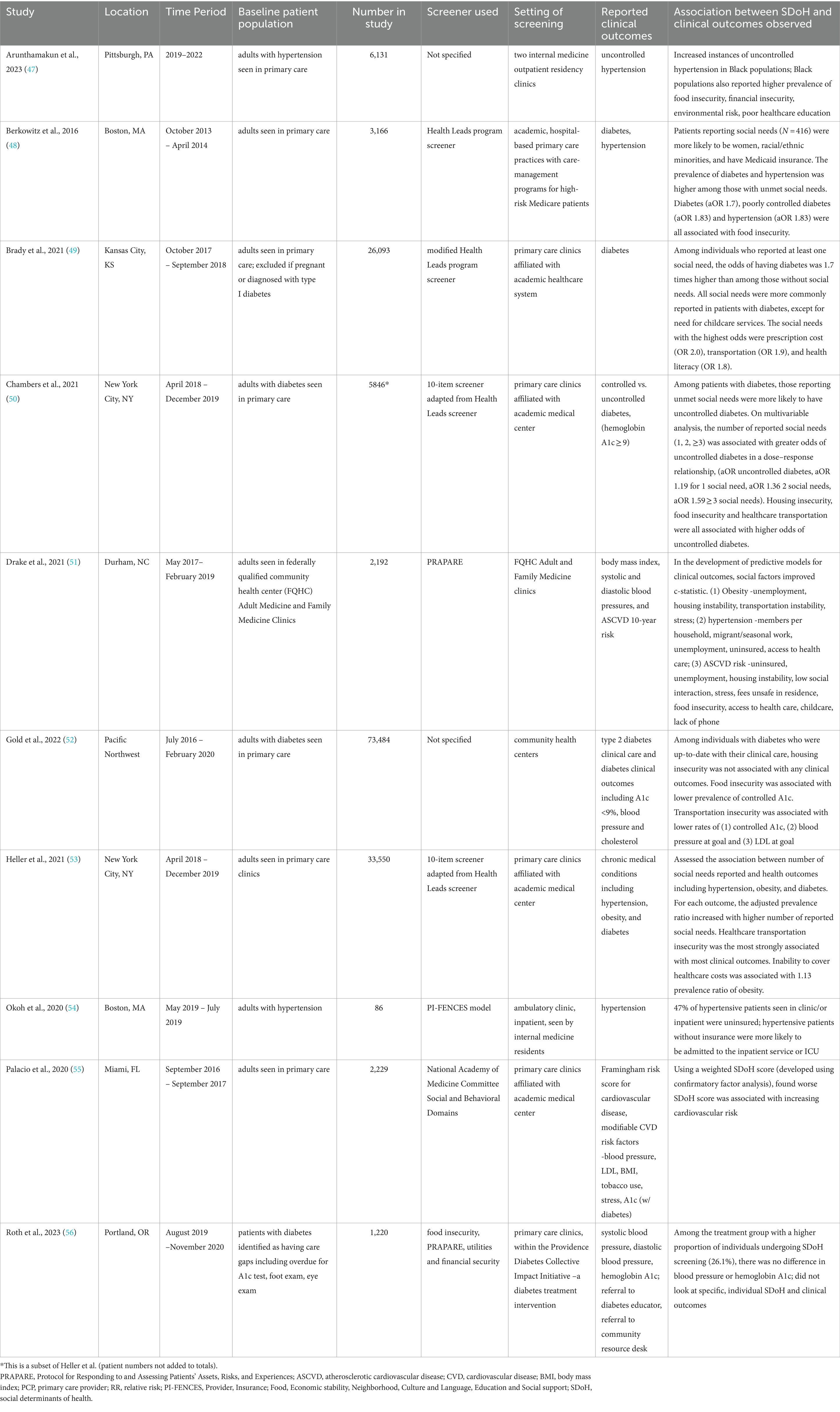
Table 1. Characteristics of included studies describing social determinants of health screening in a clinical setting to populations at risk for metabolic-dysfunction associated steatotic liver disease.
In nine of the 10 studies, only participants who completed SDoH screening were including in analyses. The number eligible for screening was not reported in most, however Palacio et al. reported an SDoH screening response rate of 36% among patients scheduled in primary care clinic (55). In the tenth study by Roth et al., participants were randomized to standard of care primary care clinics or to those included in the Diabetes Collective Impact Initiative. SDoH screening was available in either setting as it was embedded into existing clinical workflows, however remained less than 30% overall. Screening rates were higher among clinics within the Diabetes Collective Impact Initiative, where 26.1% of patients were screened, compared to standard of care primary care clinics with 1.5% screened (56). The study did not describe barriers to SDoH screening or specific differences between clinics in their approaches to SDoH screening.
Methods for SDoH screening
Based on the information provided, each study used different SDoH questions; however, some did use previously developed and validated questionnaires. Four studies used a version of the SDoH screener used in the Health Leads program (48–50, 53). Two studies used the Protocol for Responding to and Assessing Patients’ Assets, Risks, and Experiences (PRAPARE) screening tool (51, 56, 57). One study supplemented this with the validated 2-question screener for food insecurity (58). One study described using the PI-FENCES model, which includes Provider, Insurance, Food, Economic stability, Neighborhood, Culture and Language, Education and Social support, (54) while another asked similar questions based on the National Academy of Medicine Committee Social and Behavioral Domains (59, 60).
Among the studies that included specifics regarding survey administration, the most common approaches were collection via patient interview by the provider (51, 54) or clinic staff; in Palacio et al., patients were sent messages via text or email asking them to access their patient portal to complete the SDoH survey. The two remaining studies described collection of SDoH in clinic but did not provide additional details. In general, details regarding screening methodology and implementation of SDoH screening were limited.
Specific SDoH collected
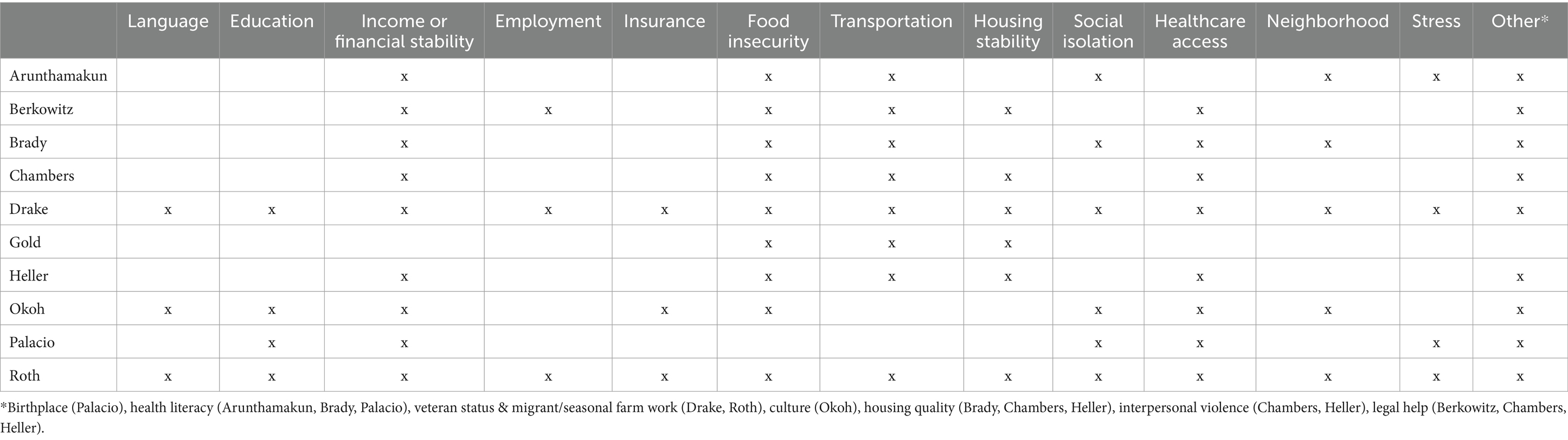
The social determinants collected in each study are summarized in Table 2. There was not a single SDoH factor that was asked in all studies. Most (9/10) studies asked participants about financial stability (income, ability to pay utilities, afford childcare, afford medications, etc.) and food insecurity. Eight of the ten studies asked about transportation insecurity and healthcare access (primary care provider, regular care or delay in care in last 12 months, etc.). Other commonly collected SDoH (among 5–6 studies) included social isolation (participation with groups, talking with friends/family, feeling lonely, etc.), neighborhood and stress.
Prevalence of SDoH
Only some studies included specific values for SDoH prevalence. Drake and colleagues collected SDoH in FQHC adult and family medicine clinics, starting in 2017. Among the 2,192 participants, 58.4% were uninsured, 34.6% obtained less than a high school education, 29% were unemployed and not seeking work, 19.4% were without housing and 16.2% reported food insecurity. Additional values can be found in the study (51). Palacio and colleagues reported the following among their study population: 39% were born outside the US, 21% had less than a high school education, 9% reported health illiteracy, 33% had financial strain, 22% described “quite a lot or very much stress,” a range from 9 to 56% responded yes to questions indicative of social isolation, 2% of participants’ income was at or below the federal poverty level and 31% reported a delay in medical care in the prior 12 months (55). In Berkowitz et al., of the 3,166 participants, 416 reported at least one social need. The prevalence of social needs ranged from 5.9% of people needing legal assistance up to 40.1% reporting food insecurity and 46.5% with healthcare needs including difficulties with insurance coverage and affording medications (48).
Use of SDoH data
Among the 10 studies reviewed, four of the studies described clear action plans for the SDoH data collected from patients. Drake et al. referred patients to community resources or social services based on their identified needs (51). Similarly, Roth et al. referred individuals to their community resource desk staffed by multilingual and multicultural resource specialists employed by community partners to assist with the navigation of community services including nutrition assistance, housing and employment support, and dental care. When appropriate, patients were also enrolled in their Diabetic Transportation Program (56). Berkowitz and colleagues also connected participants with social needs to community resources. In their study, participants with defined social needs worked alongside an advocate who linked them to appropriate resources based on eligibility, desirability and accessibility. The study included details of their resource connections: successful referrals were most frequent for health-related needs like prescription assistance (35% of cases) and adult health insurance (15%), referrals to food pantries and soup kitchens (56%), and utilities like electric, gas and oil discount rates (49%) (48).
Okoh et al. described future directions for use of SDoH data that included the development of a follow up and referral plan for uninsured patients presenting to the inpatient setting, as well as free health screening for families and friends of uninsured individuals, components of their Reducing ReAdmission Secondary to Hypertension proposal (54).
Associations of specific SDoH with clinical outcomes
A variety of outcomes were reported (Table 1). In general, SDoH indicative of increased social risk or social needs were associated with poorer clinical outcomes. Arunthamakun et al. found that Black populations with higher prevalence of hypertension had concurrent increased rates of household-level economic and social disparities (47). Additionally, patients with hypertension without insurance, based on findings by Okoh et al., were more likely to be admitted inpatient or to the intensive care unit likely as a result of limited healthcare access and associated financial strain (54).
Drake et al. reported the prevalence of each SDoH factor among their FQHC population overall as described above, but also specifically among those with (1) obesity, (2) elevated blood pressure, and (3) increased ASCVD risk. They did not observe differences in all SDoH, (e.g., uninsured, less than high school education), however, for the following, a higher prevalence of social risk or social need was identified among those with metabolic dysfunction and increased cardiovascular risk. Overall, 29% were unemployed not seeking work, which was 29% among obese individuals, 33.9% with elevated blood pressure, and 39.3% with high ASCVD risk. Nineteen percent were unhoused, which was 16.6% among those with obesity, 21.4% with elevated blood pressure, and 21.6% in those with high ASCVD risk. Food insecurity overall was 16.2%, increased to 16.4, 20, and 20.4% among those with obesity, high blood pressure, and high ASCVD risk, respectively (51).
Berkowitz et al. and Brady et al. described a higher prevalence of diabetes and hypertension among people with unmet social needs (48, 49). Moreover, Chambers et al. and Gold et al. found that, among patients with diabetes, social needs were associated with poorer control of diabetes based on higher hemoglobin A1c levels (50, 52).
Discussion
In this scoping review of 10 studies on SDoH screening in the clinical setting among 148,151 patients at-risk for MASLD, we made several key observations. First, despite strong evidence and general acceptance that SDoH powerfully impact health outcomes, including MASLD risk factors and MASLD related complications, there are few studies describing real-time SDoH screening in the clinical setting, a critical gap in the current literature. Second, among studies with SDoH screening, specific SDoH factors as well as a higher burden of SDoH were associated with poorer clinical outcomes including (1) higher prevalence of diabetes and hypertension, (2) poorly-controlled hypertension, (3) higher hemoglobin A1c among individuals with diabetes, and (4) higher ASCVD risk score. Third, there was little consistency in the SDoH screening methods; a variety of questions were asked and the specific SDoH factors collected differed across studies. Fourth, only 4 of the included studies described how SDoH data were used; 3 studies connected those with social needs to available community resources.
Numerous retrospective, epidemiologic studies have shown an association between SDoH and overweight/obesity, diabetes, hypertension and hyperlipidemia (61–66). These studies often use national databases that collect data on social factors via surveys or link census data by geographic location and use area or neighborhood deprivation indices. They have described associations between race/ethnicity, education, income, type of insurance, and food insecurity (among several other SDoH) with self-reported medical conditions or international classification of diagnoses codes (2, 11–16, 31, 62, 65, 66). Several studies have been published using various large databases like the National Health and Nutrition Examination Survey, the National Health Interview Survey, and the Behavioral Risk Factor Surveillance System, etc. Specifically, through our literature review, we identified over 110 studies that used data from more than 25 unique epidemiologic databases to establish the link between SDoH and chronic diseases.
Now that these relationships are established, the next step should be to act on these findings in real-time; however, the optimal method to systematically gather SDoH information in the clinical setting is unknown. While our scoping review included studies with SDoH screening in the clinical setting and clinical outcomes, De Marchis and colleagues recently reviewed implementation science studies describing SDoH screening in the clinical setting (42). In their systematic scoping review, they summarized 41 studies describing the implementation of SDoH screening in the clinical setting. They identified steps necessary for success, described remaining challenges of real-time SDoH screening, and defined ongoing critical gaps including the need for practices that maximize screening reach, adoption, and sustainability in clinical settings (42). As more healthcare systems incorporate SDoH screening into clinical practice, the standardization of SDoH screening is critical for collection of comparable and generalizable data. More specifically, in hepatology practice, systematic SDoH screening among patients with MASLD can serve as an example for screening among individuals with other chronic liver diseases and may also be implemented in the transplant setting where disparities are known to be prevalent (67).
Potential next steps include (1) optimization of a single screening tool that can be systematically incorporated into clinical flow among healthcare systems nationally, (2) strategies to incentivize clinicians and healthcare systems to conduct SDoH screening regularly, and (3) normalization of SDoH screening with the general population to improve acceptability. Historically, barriers to collect SDoH data in the clinical setting included time limitations, discomfort from providers and/or patients, and the absence of solutions or resources when social needs are identified (68–72). Among the 10 studies reviewed, 6 studies incorporated SDoH data into the electronic medical record making it easily collected and readily available. Three of the studies included clear action items for providers when social needs were identified. While useful, overall data were limited and insufficient to guide the standardization of SDoH screening among individuals with or at risk for MASLD. In the future, with successful implementation of standardized, sustainable SDoH screening, (1) patients will benefit from individualized and contextualized care plans, (2) social needs may be addressed through referrals to available community resources, (3) higher utilization of community-based resources will demonstrate their value potentially leading to greater financial support and the development of similar interventions, and (4) data collected may inform and promote health policies to address SDoH at the local and national level, (Figure 3) (73).
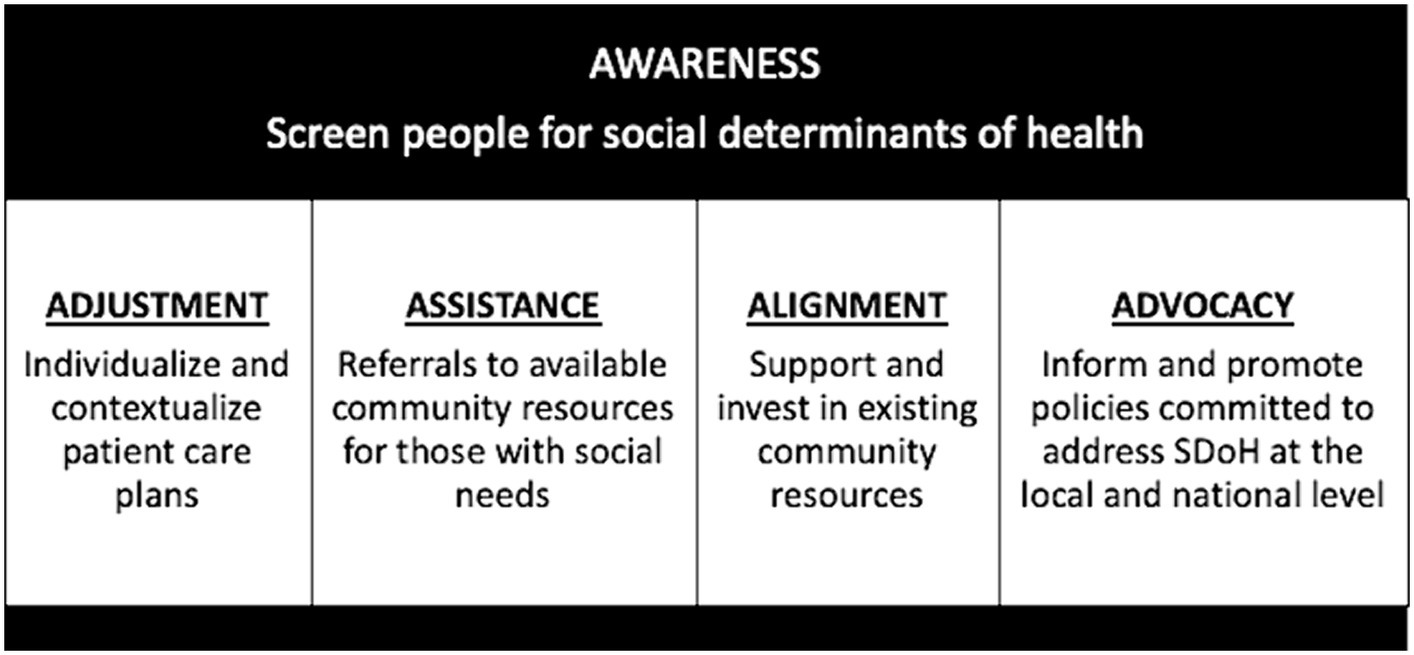
Figure 3. Proposed long-term health systems outcomes that result from standardized, comprehensive social determinants of health screening – Awareness, Adjustment, Assistance, Alignment, and Advocacy.
A strength of this scoping review is the comprehensive and systematic literature search with well-defined inclusion criteria. The limitations of the study include the results of our literature search and the small number of studies that met our inclusion criteria. Only 10 studies were identified, and 2 were only published as conference abstracts with limited data available. This demonstrates an important gap in the literature. Another limitation is the heterogeneity of SDoH screening methods; different screening tools were used that collected different determinants and different approaches were used to administer surveys. Moreover, only 3 studies described what providers did when SDoH were identified. Although we hoped that a review of the literature could inform specific guidelines for how to screen for SDoH, instead it demonstrated the paucity of studies and the work that remains to be done.
In conclusion, prospective SDoH screening in the clinical setting adds to the existing data that SDoH are associated with disparate health outcomes, particularly related to overweight/obesity, diabetes, and hypertension. Real-time screening allows for incorporation of SDoH into patient care, specifically among patients with MASLD and its risk factors. However, barriers to SDoH screening remain, including the lack of consensus regarding which standardized screening tool to use and optimal approaches to achieve feasible, acceptable and sustainable SDoH screening in patient care settings. Future studies should be designed to effectively incorporate standardized SDoH screening into clinical practice to examine specific SDoH and define impactful determinants among individuals with MASLD. These data, along with input from patients, staff and communities, can then be used to develop and implement effective SDoH interventions to improve clinical care and reduce health disparities observed among populations with MASLD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RK: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. AB: Data curation, Writing – review & editing. MC: Methodology, Supervision, Writing – review & editing. JP: Methodology, Supervision, Writing – review & editing. JI: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1332870/full#supplementary-material
Abbreviations
SDoH, social determinants of health; MASLD, metabolic dysfunction-associated steatotic liver disease; NAFLD, non-alcoholic fatty liver disease; FQHC, federally qualified community health center; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease; PRAPARE, Protocol for Responding to and Assessing Patients’ Assets, Risks, and Experiences; CVD, cardiovascular disease; PCP, primary care provider; RR, relative risk; PI-FENCES, Provider, Insurance, Food, Economic stability, Neighborhood, Culture and Language, Education and Social support.
References
1. Asrani, SK, Devarbhavi, H, Eaton, J, and Kamath, PS. Burden of liver diseases in the world. J Hepatol. (2019) 70:151–71. doi: 10.1016/j.jhep.2018.09.014
2. Kardashian, A, and Dodge, JL. Food insecurity is associated with mortality among U.S. adults with nonalcoholic fatty liver disease and advanced fibrosis. Clin Gastroenterol Hepatol. (2021) 20:2790–2799.e4. doi: 10.1016/j.cgh.2021.11.029
3. Kardashian, A, Serper, M, Terrault, N, and Nephew, LD. Health disparities in chronic liver disease. Hepatology. (2023) 77:1382–403. doi: 10.1002/hep.32743
4. Rich, NE, Oji, S, Mufti, AR, Browning, JD, Parikh, ND, Odewole, M, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and Meta-analysis. Clin Gastroenterol Hepatol. (2018) 16:198–210.e2. doi: 10.1016/j.cgh.2017.09.041
5. Koutny, F, Aigner, E, Datz, C, Gensluckner, S, Maieron, A, Mega, A, et al. Relationships between education and non-alcoholic fatty liver disease. Eur J Intern Med. (2023) 118:98–107. doi: 10.1016/j.ejim.2023.07.039
6. Rinella, ME, Neuschwander-Tetri, BA, Siddiqui, MS, Abdelmalek, MF, Caldwell, S, Barb, D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. (2023) 77:1797–835. doi: 10.1097/HEP.0000000000000323
7. Huang, DQ, Wilson, LA, Behling, C, Kleiner, DE, Kowdley, KV, Dasarathy, S, et al. Fibrosis progression rate in biopsy-proven nonalcoholic fatty liver disease among people with Diabetes versus people without Diabetes: a multicenter study. Gastroenterology. (2023) 165:e465:463–472.e5. doi: 10.1053/j.gastro.2023.04.025
8. Shih, CI, Wu, KT, Hsieh, MH, Yang, JF, Chen, YY, Tsai, WL, et al. Severity of fatty liver is highly correlated with the risk of hypertension and diabetes: a cross-sectional and longitudinal cohort study. Hepatol Int. (2024) 18:138–54. doi: 10.1007/s12072-023-10576-z
9. Carr, RM, Oranu, A, and Khungar, V. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol Clin N Am. (2016) 45:639–52. doi: 10.1016/j.gtc.2016.07.003
10. Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services (2020).
11. Bijlsma-Rutte, A, Rutters, F, Elders, PJM, Bot, SDM, and Nijpels, G. Socio-economic status and HbA1c in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. (2018) 34:e3008. doi: 10.1002/dmrr.3008
12. Commodore-Mensah, Y, Turkson-Ocran, RA, Foti, K, Cooper, LA, and Himmelfarb, CD. Associations between social determinants and hypertension, stage 2 hypertension, and controlled blood pressure among men and women in the United States. Am J Hypertens. (2021) 34:707–17. doi: 10.1093/ajh/hpab011
13. Frank, AT, Zhao, B, Jose, PO, Azar, KM, Fortmann, SP, and Palaniappan, LP. Racial/ethnic differences in dyslipidemia patterns. Circulation. (2014) 129:570–9. doi: 10.1161/CIRCULATIONAHA.113.005757
14. Moore, JX, Chaudhary, N, and Akinyemiju, T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and nutrition examination survey, 1988-2012. Prev Chronic Dis. (2017) 14:E24. doi: 10.5888/pcd14.160287
15. Newton, S, Braithwaite, D, and Akinyemiju, TF. Socio-economic status over the life course and obesity: systematic review and meta-analysis. PLoS One. (2017) 12:e0177151. doi: 10.1371/journal.pone.0177151
16. Ogden, CL, Fakhouri, TH, Carroll, MD, Hales, CM, Fryar, CD, Li, X, et al. Prevalence of obesity among adults, by household income and education -United States, 2011-2014. MMWR Morb Mortal Wkly Rep. (2017) 66:1369–73. doi: 10.15585/mmwr.mm6650a1
17. Link, BG, and Phelan, J. Social conditions as fundamental causes of disease. J Health Soc Behav. (1995) 35:80–94. doi: 10.2307/2626958
18. Braveman, P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health. (2006) 27:167–94. doi: 10.1146/annurev.publhealth.27.021405.102103
19. Chetty, R, Stepner, M, Abraham, S, Lin, S, Scuderi, B, Turner, N, et al. The Association between income and life expectancy in the United States, 2001-2014. JAMA. (2016) 315:1750–66. doi: 10.1001/jama.2016.4226
20. Galea, S, Tracy, M, Hoggatt, KJ, Dimaggio, C, and Karpati, A. Estimated deaths attributable to social factors in the United States. Am J Public Health. (2011) 101:1456–65. doi: 10.2105/AJPH.2010.300086
21. McGinnis, JM, and Foege, WH. Actual causes of death in the United States. JAMA. (1993) 270:2207–12. doi: 10.1001/jama.1993.03510180077038
22. Stringhini, S, Sabia, S, Shipley, M, Brunner, E, Nabi, H, Kivimaki, M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. (2010) 303:1159–66. doi: 10.1001/jama.2010.297
23. Zimmerman, FJ, and Anderson, NW. Trends in health equity in the United States by race/ethnicity, sex, and income, 1993-2017. JAMA Netw Open. (2019) 2:e196386. doi: 10.1001/jamanetworkopen.2019.6386
24. Healthy People (2020). U.S. department of health and human services, office of disease prevention and health promotion. Healthy People 2030 Available at:https://health.gov/healthypeople/objectives-and-data/social-determinants-health.In.
25. Saydah, S, and Lochner, K. Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep. (2010) 125:377–88. doi: 10.1177/003335491012500306
26. Chen, VL, Song, MW, Suresh, D, Wadhwani, SI, and Perumalswami, P. Effects of social determinants of health on mortality and incident liver-related events and cardiovascular disease in steatotic liver disease. Aliment Pharmacol Ther. (2023) 58:537–45. doi: 10.1111/apt.17631
27. Golovaty, I, Tien, PC, Price, JC, Sheira, L, Seligman, H, and Weiser, SD. Food insecurity may be an independent risk factor associated with nonalcoholic fatty liver disease among low-income adults in the United States. J Nutr. (2020) 150:91–8. doi: 10.1093/jn/nxz212
28. Tamargo, JA, Sherman, KE, Campa, A, Martinez, SS, Li, T, Hernandez, J, et al. Food insecurity is associated with magnetic resonance-determined nonalcoholic fatty liver and liver fibrosis in low-income, middle-aged adults with and without HIV. Am J Clin Nutr. (2021) 113:593–601. doi: 10.1093/ajcn/nqaa362
29. Tutunchi, H, Saghafi-Asl, M, Ebrahimi-Mameghani, M, and Ostadrahimi, A. Food insecurity and lipid profile abnormalities are associated with an increased risk of nonalcoholic fatty liver disease (NAFLD): a case-control study. Ecol Food Nutr. (2021) 60:508–24. doi: 10.1080/03670244.2021.1875453
30. Song, M, Goyal, T, Miller, M, Wijarnpreecha, K, and Chen, V. Abstracts. Hepatology. (2022) 76:S1–S1564. doi: 10.1002/hep.32697
31. Hill-Briggs, F, Adler, NE, Berkowitz, SA, Chin, MH, Gary-Webb, TL, Navas-Acien, A, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. (2020) 44:258–79. doi: 10.2337/dci20-0053
32. Levi, R, Bleich, SN, and Seligman, HK. Food insecurity and Diabetes: overview of intersections and potential dual solutions. Diabetes Care. (2023) 46:1599–608. doi: 10.2337/dci23-0002
33. Salinas-Roca, B, Rubio-Pique, L, Carrillo-Alvarez, E, and Franco-Alcaine, G. Impact of health and social factors on the Cardiometabolic risk in people with food insecurity: a systematic review. Int J Environ Res Public Health. (2022) 19:14447. doi: 10.3390/ijerph192114447
34. Goldblatt, PB, Moore, ME, and Stunkard, AJ. Social factors in obesity. JAMA. (1965) 192:1039–44. doi: 10.1001/jama.1965.03080250017004
35. McLaren, L. Socioeconomic status and obesity. Epidemiol Rev. (2007) 29:29–48. doi: 10.1093/epirev/mxm001
36. ElSayed, NA, Aleppo, G, Aroda, VR, Bannuru, RR, Brown, FM, Bruemmer, D, et al. Summary of revisions: standards of Care in Diabetes-2023. Diabetes Care. (2023) 46:S5–9. doi: 10.2337/dc23-Srev
37. Coles, S, Fisher, L, Lin, K, Lyon, C, Vosooney, A, and Bird, M. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Am Fam Physician. (2022) 6:106
38. Virani, SS, Newby, LK, Arnold, SV, Bittner, V, Brewer, LC, Demeter, SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation. (2023) 148:e9–e119. doi: 10.1161/CIR.0000000000001168
39. Jones, PD, Lai, JC, Bajaj, JS, and Kanwal, F. Actionable solutions to achieve health equity in chronic liver disease. Clin Gastroenterol Hepatol. (2023) 21:1992–2000. doi: 10.1016/j.cgh.2023.03.043
40. Kardashian, A, Wilder, J, Terrault, NA, and Price, JC. Addressing social determinants of liver disease during the COVID-19 pandemic and beyond: a call to action. Hepatology. (2021) 73:811–20. doi: 10.1002/hep.31605
41. Ge, J, Lai, JC, and Wadhwani, SI. Novel approaches are needed to study social determinants of health in liver transplantation. Liver Transpl. (2023) 29:241–3. doi: 10.1002/lt.26554
42. Marchis, EH, Aceves, BA, Brown, EM, Loomba, V, Molina, MF, and Gottlieb, LM. Assessing implementation of social screening within US health care settings: a systematic scoping review. J Am Board Fam Med. (2023) 36:626–49. doi: 10.3122/jabfm.2022.220401R1
43. Peters, MD, Godfrey, CM, Khalil, H, McInerney, P, Parker, D, and Soares, CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. (2015) 13:141–6. doi: 10.1097/XEB.0000000000000050
44. Munn, Z, Peters, MDJ, Stern, C, Tufanaru, C, McArthur, A, and Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143. doi: 10.1186/s12874-018-0611-x
45. American Association for the Study of Liver Diseases, Latin American Association for the Study of the Liver, European Association for the Study of the Liver. A call for unity: the path towards a more precise and patient-centric nomenclature for NAFLD. Hepatology. (2023) 78:3–5. doi: 10.1097/HEP.0000000000000412
46. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
47. Arunthamakun, N, Appiah-Kubi, E, Amoateng, R, Johnston, A, and Gadani, M. Association of Social Determinants of health in patients with hypertension in the outpatient setting. J Am Coll Cardiol. (2023) 81:1752. doi: 10.1016/S0735-1097(23)02196-4
48. Berkowitz, SA, Hulberg, AC, Hong, C, Stowell, BJ, Tirozzi, KJ, Traore, CY, et al. Addressing basic resource needs to improve primary care quality: a community collaboration programme. BMJ Qual Saf. (2016) 25:164–72. doi: 10.1136/bmjqs-2015-004521
49. Brady, E, Bridges, K, Murray, M, Cheng, H, Liu, B, He, J, et al. Relationship between a comprehensive social determinants of health screening and type 2 diabetes mellitus. Prev Med Rep. (2021) 23:101465. doi: 10.1016/j.pmedr.2021.101465
50. Chambers, EC, McAuliff, KE, Heller, CG, Fiori, K, and Hollingsworth, N. Toward understanding social needs among primary care patients with uncontrolled Diabetes. J Prim Care Community Health. (2021) 12:215013272098504. doi: 10.1177/2150132720985044
51. Drake, C, Lian, T, Trogdon, JG, Edelman, D, Eisenson, H, Weinberger, M, et al. Evaluating the association of social needs assessment data with cardiometabolic health status in a federally qualified community health center patient population. BMC Cardiovasc Disord. (2021) 21:342. doi: 10.1186/s12872-021-02149-5
52. Gold, R, Kaufmann, J, Gottlieb, LM, Weiner, SJ, Hoopes, M, Gemelas, JC, et al. Cross-sectional associations: social risks and Diabetes care quality, outcomes. Am J Prev Med. (2022) 63:392–402. doi: 10.1016/j.amepre.2022.03.011
53. Heller, CG, Rehm, CD, Parsons, AH, Chambers, EC, Hollingsworth, NH, and Fiori, KP. The association between social needs and chronic conditions in a large, urban primary care population. Prev Med. (2021) 153:106752. doi: 10.1016/j.ypmed.2021.106752
54. Okoh, AK, Meledathu, S, Jackson, D, Gold, J, Engell, C, Bustillo, J, et al. Abstract P 466: health and healthcare disparities in the Management of Systemic Hypertension and Cardiovascular Disease: the critical role of insurance status. Circulation. (2020) 141:466. doi: 10.1161/circ.141.suppl_1.P466
55. Palacio, A, Mansi, R, Seo, D, Suarez, M, Garay, S, Medina, H, et al. Social determinants of health score: does it help identify those at higher cardiovascular risk? Am J Manag Care. (2020) 26:e312–8. doi: 10.37765/ajmc.2020.88504
56. Roth, SE, Gronowski, B, Jones, KG, Smith, RA, Smith, SK, Vartanian, KB, et al. Evaluation of an integrated intervention to address clinical care and social needs among patients with type 2 Diabetes. J Gen Intern Med. (2023) 38:38–44. doi: 10.1007/s11606-022-07920-8
57. National Association of Community Health Centers, Inc., Association of Asian Pacific Community Health Organizations, & Oregon Primary Care Association. (2016). PRAPARE protocol for responding to and assessing patient assets, risks, and experiences. Available at:https://prapare.org/wp-content/uploads/2023/01/PRAPARE-English.pdf.
58. Cook, JT, Frank, DA, Casey, PH, Rose-Jacobs, R, Black, MM, Chilton, M, et al. A brief indicator of household energy security: associations with food security, child health, and child development in US infants and toddlers. Pediatrics. (2008) 122:e867–75. doi: 10.1542/peds.2008-0286
59. Institute of Medicine. Capturing social and behavioral domains in electronic health records: phase 1. Washington, DC: The National Academies Press (2014).
60. Institute of Medicine. Capturing social and behavioral domains and measures in electronic health records: phase 2. Washington, DC: The National Academies Press (2014).
61. Alawode, O, Humble, S, and Herrick, CJ. Food insecurity, SNAP participation and glycemic control in low-income adults with predominantly type 2 diabetes: a cross-sectional analysis using NHANES 2007-2018 data. BMJ Open Diabetes Res Care. (2023) 11:11. doi: 10.1136/bmjdrc-2022-003205
62. Jain, V, Al Rifai, M, Khan, SU, Kalra, A, Rodriguez, F, Samad, Z, et al. Association between social vulnerability index and cardiovascular disease: a behavioral risk factor surveillance system study. J Am Heart Assoc. (2022) 11:e024414. doi: 10.1161/JAHA.121.024414
63. Kurani, SS, Heien, HC, Sangaralingham, LR, Inselman, JW, Shah, ND, Golden, SH, et al. Association of Area-Level Socioeconomic Deprivation with Hypoglycemic and Hyperglycemic Crises in US adults with Diabetes. JAMA Netw Open. (2022) 5:e2143597. doi: 10.1001/jamanetworkopen.2021.43597
64. Maldonado, A, Hoffman, RM, Baquero, B, Sewell, DK, Laroche, HH, Afifi, R, et al. Identifying the social determinants of treated hypertension in new and established Latino destination states. J Immigr Minor Health. (2023) 25:50–61. doi: 10.1007/s10903-022-01376-y
65. Shah, MK, Gandrakota, N, Gujral, UP, Islam, N, Narayan, KMV, and Ali, MK. Cardiometabolic risk in Asian Americans by social determinants of health: serial cross-sectional analyses of the NHIS, 1999-2003 to 2014-2018. J Gen Intern Med. (2023) 38:571–81. doi: 10.1007/s11606-022-07933-3
66. Shah, NS, Huang, X, Petito, LC, Bancks, MP, Ning, H, Cameron, NA, et al. Social and psychosocial determinants of racial and ethnic differences in cardiovascular health in the United States population. Circulation. (2023) 147:190–200. doi: 10.1161/CIRCULATIONAHA.122.061991
67. Nephew, LD, and Serper, M. Racial, gender, and socioeconomic disparities in liver transplantation. Liver Transpl. (2021) 27:900–12. doi: 10.1002/lt.25996
68. Brewster, AL, Fraze, TK, Gottlieb, LM, Frehn, J, Murray, GF, and Lewis, VA. The role of value-based payment in promoting innovation to address social risks: a cross-sectional study of social risk screening by US physicians. Milbank Q. (2020) 98:1114–33. doi: 10.1111/1468-0009.12480
69. Cartier, Y, and Gottlieb, L. The prevalence of social care in US health care settings depends on how and whom you ask. BMC Health Serv Res. (2020) 20:481. doi: 10.1186/s12913-020-05338-8
70. Eder, M, Henninger, M, Durbin, S, Iacocca, MO, Martin, A, Gottlieb, LM, et al. Screening and interventions for social risk factors: technical brief to support the US preventive services task force. JAMA. (2021) 326:1416–28. doi: 10.1001/jama.2021.12825
71. Gottlieb, L, Tobey, R, Cantor, J, Hessler, D, and Adler, NE. Integrating social and medical data to improve population health: opportunities and barriers. Health Aff. (2016) 35:2116–23. doi: 10.1377/hlthaff.2016.0723
72. Quinones-Rivera, A, Wing, HE, Barr-Walker, J, Yee, M, Harrison, JM, and Gottlieb, LM. Provider impacts of socioeconomic risk screening and referral programs: a scoping review. J Am Board Fam Med. (2021) 34:820–31. doi: 10.3122/jabfm.2021.04.210039
Keywords: metabolic dysfunction-associated steatotic liver disease, SDoH screening, obesity, diabetes, hypertension
Citation: Kim RG, Ballantyne A, Conroy MB, Price JC and Inadomi JM (2024) Screening for social determinants of health among populations at risk for MASLD: a scoping review. Front. Public Health. 12:1332870. doi: 10.3389/fpubh.2024.1332870
Edited by:
Cyrille Delpierre, INSERM Public Health, FranceReviewed by:
Malgorzata Wojcik, Jagiellonian University Medical College, PolandSina Azadnajafabad, University of Leeds, United Kingdom
Cosmin Mihai Vesa, University of Oradea, Romania
Copyright © 2024 Kim, Ballantyne, Conroy, Price and Inadomi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca G. Kim, UmViZWNjYS5nLmtpbUBoc2MudXRhaC5lZHU=
 Rebecca G. Kim
Rebecca G. Kim April Ballantyne
April Ballantyne Molly B. Conroy2
Molly B. Conroy2