- 1Pantang Hospital, Accra, Ghana
- 2Faculty of Psychiatry, Ghana College of Physicians and Surgeons, Accra, Ghana
- 3Dodowa Health Research Centre, Research and Development Division, Ghana Health Service, Dodowa, Ghana
- 4Faculty of Public Health, Ghana College of Physicians and Surgeons, Accra, Ghana
- 5Department of Epidemiology and Disease Control, School of Public Health, University of Ghana, Legon, Ghana
- 6Centre for Evidence Synthesis and Policy, University of Ghana, Accra, Ghana
- 7Institutional Care Division, Ghana Health Service, Accra, Ghana
- 8Nossal Institute for Global Health, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, VIC, Australia
- 9Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, United Kingdom
Introduction: In sub-Saharan Africa, pregnant and postpartum women with mental health problems are often missed in healthcare systems. To address this, a practical and simple screening tool for maternal mental health should be available to primary healthcare workers. An important step toward having such a tool is to assess the existing tools and their effectiveness in primary care settings.
Methods: We systematically searched PubMed, LILAC, CINAHL, Google Scholar, African Index Medicus, HINARI, and African Journals Online from inception to 31 January 2023, without language restriction. Reference lists of retrieved articles were reviewed and experts in the field were contacted for studies not captured by our searches. All retrieved records were collated in Endnote, de-duplicated, and exported to Rayyan for screening. Study selection and data extraction were done by at least two reviewers using a pre-tested flow chart and data extraction form. Disagreements between reviewers were resolved through discussion. We contacted primary authors for missing or insufficient information and conducted a content analysis of the psychometric properties of the tools.
Results: In total, 1,181 studies were retrieved by our searches, of which 119 studies were included in this review. A total of 74 out of 119 studies (62%) were screened for depression during pregnancy and or the postpartum period. The Edinburg Postpartum Depression Scale (EPDS) and the Patient Health Questionnaire (PHQ-9) were the most commonly used tools. In total, 12 studies reported specificity and sensitivity for tools for measuring depression (EPDS, PHQ-9, and Whooley) and psychological distress [Self Report Questionnaire (SRQ) and Kessler Psychological Distress Scale (KPDS)]. The average sensitivity and specificity of the EPDS reported were 75.5 and 76.5%, respectively, at a cut-off of ≥13. The EPDS appears to be the most acceptable, adaptable, user-friendly, and effective in screening for maternal mental health conditions during pregnancy and postpartum. However, the methodological approach varied for a particular tool, and documentation on the attributes was scanty.
Conclusion: The EPDS was the most commonly used tool and considered as most acceptable, adaptable, user-friendly, and effective. Information on the performance and psychometric properties of the vast majority of screening tools was limited.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022323558, identifier CRD42022323558 (PROSPERO).
Introduction
In 2018, several African countries were considered to be 4 years behind maternal, newborn, and child health targets for the attainment of the sustainable development goals (SDGs) (1). This has been further compromised by the effects of the COVID-19 pandemic (2). Although infant and child health are intricately linked to maternal health and wellbeing, most countries have prioritized the former over maternal health (3, 4). In particular, the mother’s mental health may affect the health status of their children (5–7), and thus addressing the mental wellbeing of mothers should be seen as key to improving both maternal and child health outcomes (8). Undoubtedly, the foundation for quality childhood development must begin with comprehensive programs that address maternal health and wellbeing (9–11). Therefore, in 2022, the WHO launched guidance for the integration of mental health services into primary care as the most viable way of narrowing the mental health gap in LIMICs, placing emphasis on maternal mental health (MMH) (12). However, key challenges for such integration have been the low capacity to detect and manage mental disorders by non-specialized primary care health workers and the lack of cross-culturally validated screening tools (13).
MMH conditions are psychiatric disorders that can occur before pregnancy, during pregnancy (antepartum), or after delivery (postpartum). A significant proportion of women experience these conditions during the perinatal period, which includes both the period during pregnancy and after delivery (14–18). The burden of MMH conditions varies among low-middle- and high-income countries due to multiple factors such as genetic predisposition, previous history of unfavorable birth outcome, and socio-economic status (19). Most common disorders, mainly anxiety and depression, affect an estimated 20% of women in low-income countries (17) and 10% in high-income countries (20, 21) during pregnancy and the postpartum period. Pregnancy increases the vulnerability of women, particularly their physiological and mental health, evidenced by their low capacity to cope with stress and normal daily activities (22). It is estimated that up to a third of women presenting with depressive episodes in the antepartum period have no previous history of depression (22). Pregnant women with pre-existing mental illness are at a higher risk of recurrence or exacerbation during the pregnancy and after delivery (12).
In Sub-Saharan Africa (SSA), several studies have reported a range of MMH conditions, commonly depression (10–70%) and anxiety (10–50%) during pregnancy and postpartum period (18, 23–25). These disorders have been linked with poverty, experience of adverse life events, intimate partner violence, and poor social support to name but a few (24–29). One of three postpartum mood disorders, postpartum depression (PPD) typically begins within 2 weeks after childbirth but can occur anytime within the first 12 months after delivery (30). Signs and symptoms of PPD may include persistent sadness, guilt, insomnia, anxiety, and thoughts of infant harm and self-harm (22, 30). Treatment is vital for PPD because symptoms can linger for years after childbirth if untreated (31).
Common mental disorders (CMDs) such as anxiety, depression, suicide and substance use, and psychosis can be debilitating as they affect thought patterns, feelings, behaviors, and relationships, as well as home and community functions (7, 15, 19, 24–26, 32–39) during pregnancy and postpartum periods (40–44). Globally, PPD is estimated to affect 10–15% of women within 4 weeks to 1 year after delivery (45–47). Evidence suggests PPD increases the risk of suicidal ideations in the mother (48) including potential harm (49–51) and poor health outcomes of the infant (52). Though initially thought to be less common in low- and middle-income countries (LMICs), current research shows that PPD is high in these countries (13, 47). The proportion of women with PPD was estimated to be 900,000 a year in 2015, but only 6% of these women reportedly would seek care (53). The low tendency to seek healthcare could be due to the lack of responsive health systems toward the needs of this vulnerable group (53, 54). The problem in LMICs is exacerbated by context, lack of social support, poverty, and negative life events such as the positive HIV status of the mother (55, 56).
Tools for screening MMH conditions encompass a variety of structured questionnaires or assessment protocols tailored to detecting conditions such as antenatal and PPD, anxiety disorders, and postpartum psychosis among pregnant and postpartum women. Globally, the Edinburg Postnatal Depression Scale, the 9-item-Patient Health Questionnaire (PHQ-9), the Centre for Epidemiological Studies-Depression Score (CES-D), the 20-WHO-Self Report Questionnaire (SRQ-20), the Kessler Psychological Distress Scale (K10), the General Anxiety Disorder and the Hopkins Symptom Check List (HSCL) are the common screening tools for assessing MMH conditions. The same tools are used in LMICs and SSA, with some adapted before use in SSA. Each screening tool inherently incorporates specific ‘screening’ criteria (symptom frequency and duration, cut-off scores) tailored to identify various MMH problems, such as depression and anxiety. Typically, these criteria are based on widely accepted standards, including those from the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the International Classification of Diseases (ICD), as well as adaptations suitable for the cultural and healthcare context of SSA.
Screening for CMDs during the pregnancy and postpartum periods is not routinely done in most SSAs unless there is, perhaps, a personal or family history of a mental disorder (57, 58). Multiple screening tools have been documented, mainly in the context of research, for assessing MMH conditions (59) but their potential for routine use in primary care does not appear to be well-researched. In most African countries, screening tools developed in Western countries are usually translated into the local language and used without necessarily assessing the impact of their psychometric properties in the local context (59, 60) despite the fact that the expression of mental health conditions is influenced by culture and context (61–65). There is paucity of data on the available tools and contexts in which they are used across SSA countries.
There are a number of existing systematic reviews on MMH screening tools and programs (66–71). However, these reviews either focused on the global context (66, 72), or specific country context (68), targeted one or few MMH conditions (chiefly, depression, and anxiety) (66, 67, 69–71, 73–76), or focused on an individual screening tool (69–71, 73–76). SSA is characterized by unique cultural, socioeconomic, and healthcare delivery challenges (77–79), impacting these diagnostic tools that are culturally and context-sensitive. Moreover, the focus of our review on primary care settings aligns with efforts to integrate mental health services into existing healthcare infrastructure in SSA, which is in line with the WHO’s Mental Health Gap Action Programme (mhGAP) (80) and the SDGs.
Rationale
While the antenatal care (ANC) and skilled birth attendance (SBA) coverage have improved, it is largely unknown whether health professionals in primary care facilities have the necessary skills that enable them to screen for mental health conditions of pregnant and postpartum women visiting primary care facilities in SSA countries and what context-sensitive screening tools are available.
An important innovation to improve the responsiveness of primary healthcare systems in SSA with more whole person-centered health systems approaches to MMH is the development of a simple, context-relevant, inexpensive, and user-friendly mental health screening tool for frontline primary healthcare workers. In the first step toward the development of such a tool, it is important to identify tools currently available for detecting MMH conditions, their sensitivity and specificity, and their potential usefulness in the SSAn health systems using a systematic or scoping review.
Our review questions were as follows: What screening tools for MMH have been used and evaluated in SSA in the antepartum and/or postpartum period?; What is known about their sensitivity and specificity, and what is their potential for use or adaptation for use at the primary care level in Sub-Saharan African health systems? The overarching aim of this review was to collate and describe the available evidence on MMH screening tools used in primary care settings in SSA. Specifically, this systematic review documents available tools as reported in the published literature, their effectiveness, and assesses their potential for early detection of MMH distress as part of antenatal and postnatal care in primary healthcare facilities.
Definitions
Mental health, psychiatric disorders, and mental health conditions
Mental Health is a state of wellbeing in which every individual realizes his or her own potential can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to his or her community (81).
A psychiatric disorder is a clinically significant behavioral or psychological syndrome or pattern that occurs in an individual and is associated with distress, disability, or impairment in one or more important areas of functioning.
Mental health conditions are a group of disorders characterized by significant disturbances in a person’s thoughts, emotions, or behaviors that impact their ability to function in daily life. These conditions cause considerable distress or impairment in social, occupational, or other important areas of functioning.
These definitions align with criteria used in diagnostic manuals which provide specific guidelines for diagnosing various mental health conditions (82, 83).
Tools, scales, interviews, and schedules
Psychological tools is a general term for any instrument or method used in psychological assessment and treatment (84–86). It encompasses any instrument or method used to assess, evaluate, or treat psychological conditions. This includes scales, interviews, questionnaires, and various assessment tools. These have been elaborated as follows:
Psychological scales are standardized instruments that measure specific psychological attributes or symptoms using standardized questions and rating systems.
Psychological interviews are used to conduct detailed assessments through structured or semi-structured conversations which gather detailed information about the client’s mental state, life history, and psychological functioning. Interviews can be diagnostic, exploratory, or therapeutic.
Psychological schedules are systematic tools used to organize and structure the administration of assessments or interventions. They outline the sequence and content of questions or tasks to be administered.
Reliability and validity
The concept of reliability and validity (84, 85) provides a foundation for understanding how well psychological tools function and how their results can be interpreted. Reliability refers to the consistency or stability of a measurement tool over time. A reliable tool produces consistent results when used under the same conditions. Validity refers to the extent to which a tool measures what it is intended to measure. A valid tool accurately reflects the construct it aims to assess.
Sensitivity and specificity
Sensitivity refers to the ability of a test to correctly identify individuals who have a particular condition, while specificity refers to the ability of a test to correctly identify individuals who do not have a particular condition (87, 88). Sensitivity and specificity are statistical measures commonly used to assess the performance of a tool or test against a gold standard. Sensitivity and specificity are crucial metrics for evaluating the effectiveness of diagnostic tests, where sensitivity focuses on correctly identifying those with the condition, and specificity focuses on correctly identifying those without it.
Effectiveness of tools
The effectiveness of tools is an overarching concept that integrates elements of reliability and validity, focusing on how well a tool performs its intended function in practical, real-world scenarios (87). The degree to which a tool successfully accomplishes its intended purpose or achieves its objectives in real-world settings. This includes how well it performs in terms of practical utility, impact on outcomes, and the extent to which it improves the target condition or behavior.
Psychological tool development
Psychological tool development involves a systematic process of conceptualization, design, testing, and standardization. These tools are used across various settings for assessment, intervention, screening, and research purposes, depending on their intended application and the context in which they are used.
Review methods
This systematic review was developed in line with an established review methods framework (89). A review protocol was developed and revised after feedback from healthcare providers, policymakers, patient groups, and experts involved in systematic review methods. We did not apply PICOS (P—participants/population; I—intervention; C—control; O—outcomes; and study types) in the strictest sense, rather we used a modified form PCC (P—participants/population, C—concept, and C—context) for defining our criteria for considering studies in this review since our review aimed to systematically explore the literature to identify key concepts, theories, sources of evidence, and gaps in research (90).
Criteria for considering studies for inclusion in this review
Type of studies
Studies that reported a tool used for detecting or screening for any mental health condition in the pregnancy, delivery, and postpartum period in a primary clinical care setting in SSA and describing the psychometric properties of the tool were eligible for inclusion. Additionally, the study design had to be either of the following: a randomized controlled trial (RCT), cohort, case–control, and cross-sectional study or case series. Reviews, commentaries, case studies, or opinions were not eligible for inclusion. We went through the full text and reference lists of included studies to identify further potentially eligible studies missed by our searches. If the study was part of a global review having, for example, SSA as a sub-set or sub-regional focus such as an East/West/Central/Southern African focus, such a review was not included as a whole. Instead, we retrieved the studies conducted in the SSA primary care context for inclusion. If the study reported a country or regional estimate without a well-defined sample (representative sample or sub-sample within the source population), it was considered not eligible for inclusion. For multi-country studies that included studies from SSA and reported separately for each of the countries, data from the SSA context were selected for inclusion.
Population
Our population of interest was women in the pregnancy, delivery, and postpartum period. We defined pregnancy as the period from conception (starting from the last menstrual period) to delivery, lasting on average 40 weeks (22); and the postpartum or postnatal period as the period from birth of the baby to 12 months after delivery (45, 47). There are variations in the definition of the length of the postpartum period in the literature (45, 46, 91) and the choice of 12 months was based on the definition by the International Marce Society for perinatal mental health. We defined a primary care facility to include all places or facilities that provide perinatal care, including formal sector facilities such as hospitals or clinics, and informal facilities such as traditional and faith-based healers; as long as they reported on the use of a tool for detecting MMH condition in the SSA context. Polyclinics of tertiary facilities staffed with non-specialist mental health professionals were considered as primary care and were eligible for inclusion. Pregnant and postpartum women who sought care in a specialized tertiary facility, or by psychiatrists or specially trained (clinical) psychologists were not eligible for inclusion.
Concept
We considered diagnostic tools applied in an SSA context for screening or detecting MMH problems in pregnant and postpartum women who sought care in a primary care facility. The concepts explored included the availability of screening tools for screening MMH conditions, performance of tools (specificity and sensitivity as reported by the primary study investigators), and psychometric properties of the tools such as ease of use/application, preference, how it is understood by the patients, context relevance, type of health professionals who are able to apply it, overall performance of the tool, appropriateness, feasibility, and adaptability for primary care practice in Sub-Saharan African setting. Any other information or characteristics that further described the tools were considered.
Understandability of the screening tool refers to how the participants appreciate or comprehend what the questions on the screening tool are seeking to elicit and their capacity to provide an appropriate response based on the specific requirements of the tool. In addition to the specific questions stated, whether the options or the instruction for selecting an applicable response is appropriate for the specific target group. This is sometimes influenced by the culture and other factors of the participant and the context in which the questionnaire was originally developed. Ease of use of the screening tool refers to the user-friendly nature of the screening tool usually from the point of view of the administrator of the questionnaire. It therefore can be ranked by the participant for a self-administered questionnaire or by the interviewer for an interviewer-administered questionnaire. In some instances, the psychometric properties may be judged by the duration of administration, particularly in primary care settings, where staff may be overburdened by daily consultations. Adaptability of the screening tool refers to the ease or otherwise of altering aspects of the questionnaire to make it relevant to the target population ensuring the purpose of the tool is achieved. This may include changing the wording without losing the content of what it seeks to measure or translation into a locally understood language without losing the meaning of the questions participants are supposed to answer. Commonly, the tools are worded in the English language as they are developed in the global North.
Context
This systematic review considered the detection of MMH conditions in primary care facilities in all countries, cultures, and contexts across SSA. We explored regional differences in the availability and adaptability of tools for screening MMH conditions, grouping the regions into East, West, Central, and Southern Africa. We considered South Sudan as part of East Africa. We were interested in the types of MMH conditions identified in research as well as primary care delivery settings where pregnant and postpartum women received care.
Outcomes
Primary outcomes
• Availability of screening tools used in countries across SSA
• Performance of the tools (specificity, sensitivity, etc.) as reported in the primary studies
Secondary outcomes
• Proportion of MMH conditions identified by the tools across SSA
• Types of MMH conditions identified across countries in SSA
Searches in electronic databases and other sources
We systematically searched the following electronic databases: PubMed, PsycINFO, LILAC, Google Scholar, and CINAHL from inception to 31 March 2023, without language restriction. In addition, we searched, African Index Medicus, HINARI, African Journals Online, IMSEAR, and Maternity and Infant Care (MIC) under MIDIRS and Global Health. The search terms (Supplementary Table S1) and search strategy (Supplementary Table S2) have been reported as Supplemental materials. Gray literature including dissertations, preprint repositories, and conference proceedings were searched; reference lists of retrieved articles were reviewed and experts in this field were contacted for studies that could not be captured by our searches, including unpublished studies.
Managing the search results and selecting studies
All the studies retrieved from the electronic databases and gray literature were exported to Endnote (92), collated, and deduplicated. Then the articles were exported to Rayyan QCRI (93) for screening using a flow chart developed from the eligibility criteria (Supplementary Table S3). Two reviewers independently screened titles and abstracts of all retrieved articles for potentially eligible studies. Then, full texts were obtained for all the potentially eligible studies for further screening for inclusion in the review. Those that did not meet our eligibility criteria at the full-text stage were excluded with reasons for exclusion. Disagreements between screeners on the eligibility of studies were resolved through discussion between the reviewers.
Data collection
Five trained data abstractors collected data using the data extraction form developed and pretested by the review team. Relevant psychometric properties and attributes on each tool that would enable establishing commonalities and differences across the included tools in terms of populations among which they have been used, and whether and in what ways they have been validated. To ensure reliability between reviewers, a series of training exercises was conducted prior to commencing screening. The data abstraction form was piloted on a random sample of 10 included articles and modified as required based on feedback from the team. Specifically, we extracted study characteristics such as the year in which the study was conducted, the year in which the study was published, the country in which the study was conducted, the study design used, and characteristics of participants such as age, level of education, socio-economic status, and occupation; obstetric factors (pregnant or postpartum); mental health conditions (PPD, postpartum anxiety, depression in pregnancy, and anxiety in pregnancy); and elements of the screening tools such as the name of the specific tool, whether the tool was applied to the mothers alone or in combination with others, which tool was used as the reference standard, and which tool was used as the index tool, and specificity and sensitivity, if reported. We extracted additional relevant information or attributes of the tool that could help with decisions to adopt the tool for use such as ease of use, and type of professionals required to administer the tool. We did not extract quantitative data such as the numbers of true positives, false positives, true negatives, and false negatives for calculating test accuracy, as this will be reported separately in a quantitative systematic review with meta-analysis. When necessary, we contacted the authors of the published articles of included studies to see if they could clarify or supplement the published results or provide raw data that we could use. There was a high level of consistency between the data extractors. Disagreements between data extractors were resolved through discussion.
Data synthesis
The synthesis included simple quantitative analysis to generate frequencies, means, and ranges, where necessary. The qualitative synthesis focused on content analysis of mainly the psychometric properties of the tools, considering where the study was conducted (study setting) and the types of participants. Data were synthesized according to the research questions to identify similarities and differences, where relevant, as well as the patterns of the data similar to the process of reviewing heterogeneous studies in a meta-analysis. The reasons for the differences and similarities in results and the pattern of findings were reviewed. The overall synthesized studies were presented as tables under key headings. Where reported, sensitivity and specificity are summarized in Table 1.
Dealing with missing data
We contacted the primary study authors for additional data or insufficient information. Where it was not possible to obtain the missing information, data were synthesized based on those with complete outcome data.
Results
Description of the included studies
We retrieved 1,158 studies from electronic databases and 23 from other sources, making a total of 1,181 studies. After removing 57 duplicates, 1,124 studies were left, of which 946 were excluded following titles and abstracts screening. In total, 178 full-text documents were retrieved, and 62 records were excluded with reasons, leaving 116 papers. Three of these papers (94–96) presented data from multi-country studies (conducted in Ghana and Cote D’Ivoire in each case) which were disaggregated into six studies (Figure 1). The characteristics of the included studies are presented in Supplementary Table S4 and the full results in Supplementary Table S5.
In total, 116 papers from 119 studies met the inclusion criteria. Out of this number, three studies (97–99) were published before 2000; all three were published in the 1990s. A total of 11 (8.9%) (29, 100–109) were published between 2000 and 2010 and 114 (92.7%) were published after 2010 (Supplementary Table S4).
In total, 45 (38%) of the included studies were from East Africa, 41 (34%) from Southern Africa, 28 (24%) from West Africa, and 5 (4%) from Central Africa. Of the 45 included studies from East Africa, 15 came from Ethiopia (54, 110–123), 9 from Malawi (107, 124–131), 7 from Kenya (18, 27, 132–136), 7 from Tanzania (29, 106, 137–139), 3 from Uganda (102, 140, 141), and 2 each from Rwanda (24, 142) and Mozambique (143, 144). Of the (35) included studies from Southern Africa, 32 were from South Africa (25, 28, 31, 145–172), 7 from Zimbabwe (99, 108, 173–177), and 1 each from Zambia (109) and Eswatini (178). Of the 28 included studies from West Africa, 18 were from Nigeria (16, 97, 98, 100, 101, 103, 179–190), 7 from Ghana (94–96, 191–193), and 3 from Cote d’Ivoire (94–96). The five included studies from Central Africa were two each from Congo (105, 194) and DR Congo (195, 196), and one from Angola (104). Figure 2 summarizes the countries from which the included studies came as well as the tools used in the studies.
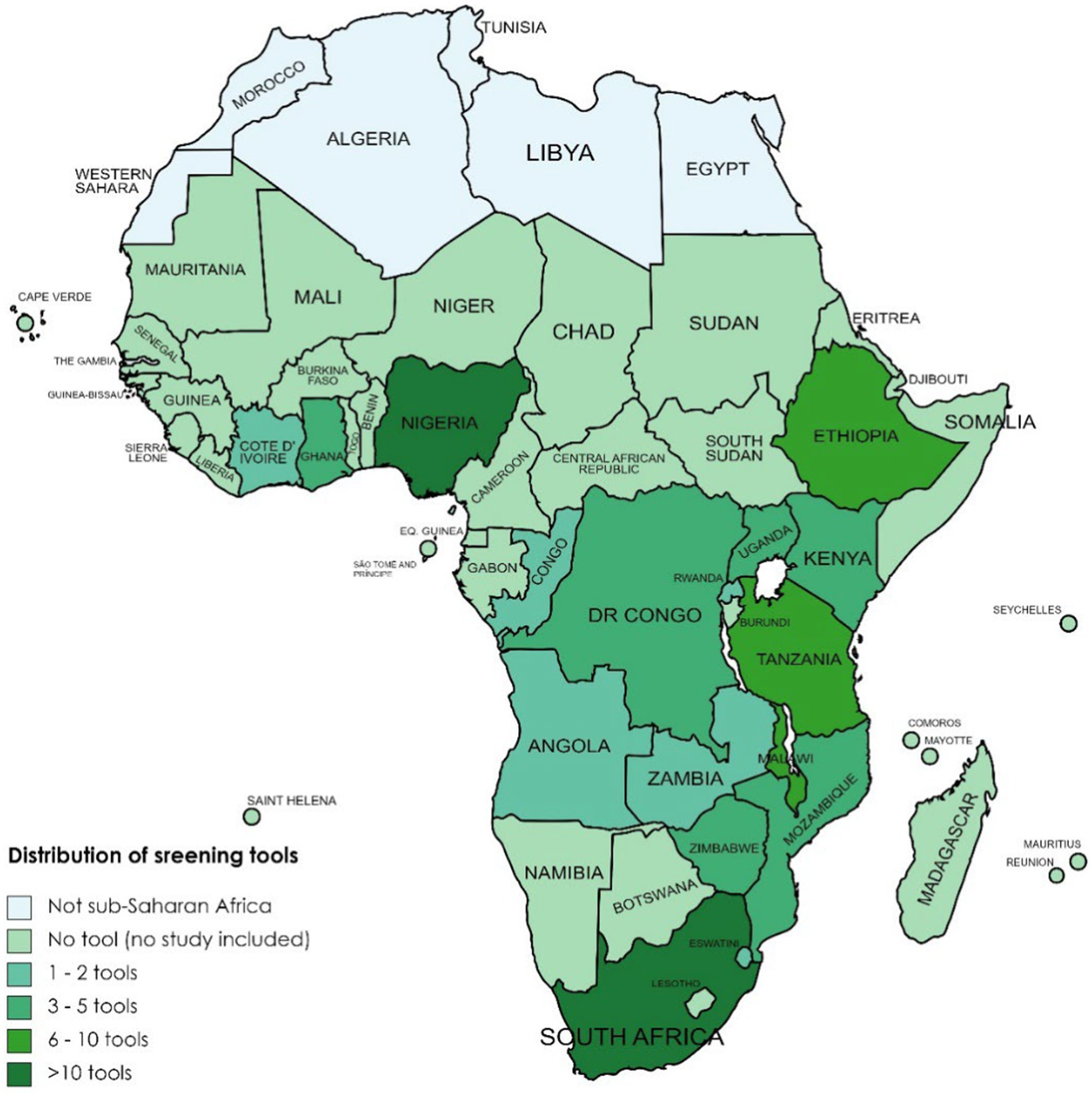
Figure 2. Availability and distribution of screening tools applied across countries in sub-Saharan Africa.
Of the 119 studies, 76 (63.9%) included pregnant women only, 53 (36.1%) included postpartum women only, and 10 studies (24, 25, 131, 132, 143, 144, 161, 165, 185, 186) included both pregnant and postpartum women. In two studies (127, 142), the participants were classified as being in the period from pregnancy to 1 year post-delivery. In total, 8 of the 76 studies (104, 128, 145, 152, 153, 161, 175) involved pregnant women living with HIV, while 4 (27, 133, 153, 190) involved adolescent mothers. In five of the studies involving postpartum women, the study participants were living with HIV (109, 135, 154, 161, 174). In one other study (114), the authors included only pregnant women with a history of intimate partner violence.
Tools available in SSA for screening MMH conditions
There were a total of 47 different tools that were either independently or in combination used to screen for seven different MMH conditions (depression, anxiety, suicidal behavior, post-traumatic stress disorder, substance use disorders, obsessive-compulsive disorders, and stress) across the studies (Figure 3). In terms of conditions most frequently screened for, depression was the lead with 68 studies among pregnant and 58 among postpartum women. It was followed by anxiety (21 studies), suicidal behavior (7 studies), post-traumatic stress disorder (6 studies), substance use conditions (5 studies), obsessive-compulsive disorder (OCD) (1 study), and stress (1 study).

Figure 3. Tools available in countries across Sub-Saharan Africa for screening mental health problems in pregnant and postpartum women in primary care settings.
Of the 47 different tools described in the studies, the most commonly used tool was the EPDS (see Figure 3). It was used in 72 of the 119 studies (60.5%) to screen for PPD in studies across 13 countries in SSA. Three studies (100, 105, 132) were validation studies, where the performance of EPDS was assessed in postnatal women. In two other studies (125, 126), an abbreviated version of the tool comprising only three items was used to screen depression in postnatal women.
The nine-item Patient Health Questionnaire (PHQ-9) mostly used for screening depression in pregnant and postpartum women was the second most commonly used tool (21 studies) across 10 countries. The questionnaire is designed for individuals to assess their own depression level over the previous 2 weeks. It consists of nine items, and respondents are asked to indicate the frequency of their symptoms using a Likert scale, with options ranging from “0 = not at all” to “3 = nearly every day.” In one study (144), a shortened version of the tool (PHQ-2), comprising only two items (“Little interest or pleasure in doing things”; and “Feeling down, depressed, or hopeless”), was used to screen for depression in pregnant and postpartum women.
The WHO’s Self-Reporting Questionnaire (SRQ) was used to screen for various mental health disorders (such as depression, anxiety, and psychological distress) in 10 studies (102, 107, 125, 126, 129, 130, 146, 164, 188) across four countries in SSA. The tool was mostly translated and adapted to the local context or the respective setting. For example, in two of the included studies (129, 130) conducted in Malawi, the tool was translated into the widely spoken local language—Chichewa.
The Mini International Neuropsychiatric Interview (MINI) was used in 10 studies (31, 102, 121, 126, 143, 144, 149, 153, 160, 172) to screen for various mental disorders including depression, suicidal behavior, and perinatal psychological distress across six countries. In three of the studies (121, 143, 144), the tool was used as the gold standard to evaluate the validity of other screening tools, including the PHQ-9. In one study (153), a version of this tool, specially designed for children and adolescents (MINI-KID) was used to screen for suicidality and self-harm in pregnant adolescents living with HIV in South Africa.
The Generalized Anxiety Disorder (GAD) instrument was used in nine of the included studies (94–96, 160, 168, 190) to screen mainly for anxiety disorders in pregnant and postpartum women. Two variations of this tool—GAD-7 and GAD-2—were used in seven (94–96, 190) and two (160, 168) studies, respectively. The GAD-7 is a 7-item tool, while the GAD-2, a shortened form of the 7-item tool, comprises only two items.
The Hopkins Symptoms Checklist (HSCL), which uses 25 items, was used in eight studies (29, 105, 106, 126, 194, 197, 198) to screen mainly antenatal/postnatal depression and anxiety across three countries. In two studies (124, 125), 15 items instead of the original 25 items of the HSCL were used. The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) was reported in six studies (108, 140, 171, 177, 190, 195) across five countries, Beck Depression Inventory (BDI) in six studies (112, 113, 118, 133, 146, 148) in three countries, and WHO’s Alcohol Use Disorders Identification Test (AUDIT) in five studies (115, 118, 133, 143, 167) across four countries. The General Health Questionnaire (GHQ) was used in four studies (97, 98, 104, 189) across two countries to screen majorly for depression.
The following tools were used three times each (MINI plus, CES-D, ASSIST, and Kessler Psychological Distress Scale), and two times each (WHODAS, PDS, Whooley Questions, SAS, PRAQ, and PRAS). The rest of the tools (DSQ-19, PSS-19, SRDS, SSQ, PSES, HDRS, FTND, CAS, OCI-R, RCMAS, CDI-S, CIDI, PAS, GHSQ, SEMI, PSAS, GDAS, Child PTSD Checklist, and PTSD Symptom Checklist) were each used once in the studies included in the review.
Sensitivity and specificity of MH screening tools used in SSA
Sensitivity and specificity are statistical measures commonly used to assess the performance of a tool or test against a gold standard. The sensitivity rate of a screening tool is the proportion of a population such as pregnant women diagnosed as having an MMH condition who truly have that condition. The specificity rate is the proportion of the population diagnosed by the tool as not having the condition who truly do not have that condition. A total of 12 studies reported specificity and sensitivity values for specific screening tools for specific conditions among pregnant or postpartum women. The EPDS, PHQ-9, and the Whooley (for depression) and the SRQ and KPDS (for psychological distress) were the main tools for which reporting of sensitivity and specificity values were done in the papers reviewed. In some cases, the assessment was done for varying screening tool score cut-off points. Generally, if the cut-off point of a test is raised, there are fewer false positives but more false negatives, implying the test is highly specific (measures what it intends to measure to a large extent) but not very sensitive (may miss potential cases). Similarly, if the cut-off point is low, there are fewer false negatives but more false positives indicating the test is highly sensitive but not very specific (Table 1).
The average sensitivity and specificity values for the EPDS were 75.5 and 76.5%, respectively, for four studies (110, 111, 160, 162) at a cut-off point of ≥13; 88 and 74% at a cut-off of ≥12 (175); 88 and 87% at a cut-off point ≥11 for a study (177) and cut-off of 11/12 for a Shona version (108); 78 and 79.5% for two studies at a cut-off point of ≥10 (124, 125); and 87 and 78.5% at a cut-off of ≥8 (99, 133). The PHQ-9 at a cut-off of ≥10 was reported to have a sensitivity of 66% and specificity of 76% (160), while a cut-off of ≥9 gave a 72 and 79% (144) and PHQ-2 at ≥2 gave an average of 74.5 and 70% sensitivity and specificity, respectively, for the two studies (144, 160). The Whooley was reported to have a sensitivity of 75 and 82% and the Whooley +help reported 75 and 82%, respectively, both at a cut-off of 2 by the same study (160).
Psychological distress was measured using the SRQ with a sensitivity of 72% and sensitivity of 96% at a cut-off of ≥10 (99) and 82 and 66%, respectively, at a cut-off of ≥8 (93). The KPDS-10 reported a sensitivity and specificity of 80 and 79% at a cut-off of 11 and KPDS-6 reported 74 and 85% at a cut-off of 8 by the same study (160).
Potential for adaptation and routine use in SSA health systems
Understandability of the tools
Understandability of the tools by those being administered was reported in a total of 15 (96, 108, 115, 121, 124, 127–129, 131, 143, 144, 157, 167, 171, 180) of the 119 studies. Five studies reported difficulty in understanding the EPDS (127, 157), the PHQ-9 (128), and the GAD-7 (96), which was attributed to the low literacy level and construct of the questions due to the culture and context of the study population requiring rewording of the questions to make them relevant.
Ease of use
In total, 12 studies stated they found the tools HAD (97), EPDS (100, 103, 129, 131, 157, 162, 168), AUDIT (133, 143), SRQ (107, 129), and the Whooley (160) easy to use especially with reference to busy primary healthcare settings. A few researchers modified the tools (107, 168), used shorter versions of the original tools (162), or modified the scoring (129).
Adaptability
Related to how the screening tools suspect MMH conditions in SSA, 57 studies identified that the reported adaptation of the tools was predominantly translated into local languages based on the population of interest. Thirty-two studies (56%) involved adaptations of the EPDS, with most focusing on translations into local languages. One study, however, explored culturally relevant terminology for depression (108), incorporating local syndromes (61) and adapting the EPDS for self-administration via mobile technology (149). The EPDS was translated into Yoruba (101, 103, 185), Igbo (100, 182), Afrikaans, Zulu, isiXhosa, Swahili (27, 28, 133, 145, 148, 151, 154, 157, 159, 160, 162, 170), Chichewa (124, 125, 129, 130, 167), and Shona (108, 176). The PHQ-9 was also adapted (94–96, 121, 144, 160, 186) through translation into local languages for administration.
Type of health staff administering the screening tool
In total, 69 out of 119 studies (52%) reported on personnel who administered the questionnaires (screening tools) in their studies. The cadre of health staff ranged from medical doctors and students (100, 102, 156, 166, 177, 193, 197); nurses, midwives, and nursing students (17, 18, 111, 113, 117, 120–122, 127, 128, 141, 148, 151, 163, 164, 166); clinical psychologist or with a background in psychology (27, 160, 166); (community) health staff, mental health officer; background in mental health or social worker (99, 132, 135, 149, 168, 172, 196); participant (self) administration (28, 148, 180); and non-health staff (108, 130). The rest were categorized as research assistants without specification of their cadre and whether or not they were from the health sector.
Discussion
This systematic review set out to identify screening tools currently available for detecting MMH conditions in the SSAn health systems, what is known about their sensitivity and specificity, and their potential usefulness for use at scale to screen for MMH conditions in primary care settings in Sub-Saharan African health system context. As such, it addresses an important gap in the published literature about the implementation and appropriateness of tools within the context of SSA which are developed elsewhere (59–63).
In relation to what screening tools are currently available, there is clearly a large number with 47 (19) mental health screening tools that have been used either independently or in combination to screen for various MMH conditions across countries in SSA. Tools for screening for depression, especially for PPD dominate with the EPDS, developed from a combination of several screening tools – Irritability, Depression and Anxiety Scale (IDA), the Hospital Anxiety and Depression Scale (HAD), and the State of Anxiety and Depression scale (SAD) (50, 199), being the most common tool used for assessing MMH conditions. It was used to assess for depression in pregnant as well as postpartum women. Although the tool was mainly developed for the assessment of PPD, it was found to be useful for assessing depression in pregnant women.
Why is this tool so commonly preferred, given there are multiple other screening tools available for depression as well as for other mental health conditions with approximately 37 least commonly (scales with a frequency of 3 and below) screening tools (e.g., CES-D, PRAS, and HDRS) for assessing MMH conditions? Moreover, why is the predominant focus on maternal depression, even though there are other MMH conditions, such as bipolar affective disorder that also affect pregnant and postpartum women? Why do the findings of this review suggest low prioritization of other mental health conditions in the pregnancy and postpartum period? A possible explanation may be as Cox and Holden (50) indicate that the experiences of clinicians in both developed and developing countries reveal a high incidence of depression among women during the pregnancy and postpartum period causing distress due to the effect on their social interactions and that of the infant. The physiological changes occurring during the peripartum period, the cultural norms surrounding the period, and how this defines what is an acceptable expression of emotions or behavior by the woman may make women more prone to depressive mental health disorders as compared to other kinds of mental health challenges. It may also be that the under-researched nature of MMH is itself limiting the research questions that are being asked in this area and the answers sought. It will be important to conduct a review of the prevalence of MMH conditions and relate that information to what kind of mental health conditions should be screened for in pregnant and postpartum women. Given the variety of tools available and the variety of conditions that can be screened for, it is important to be sure if the predominant focus on postpartum and other kinds of depression in maternal health clients should remain or whether it is time for advocacy to screen more generally for other potential mental health problems.
Beyond what a screening tool is designed to detect, its specificity and sensitivity are also important. Since specificity and sensitivity tend to be inversely related it is not possible to have one perfect test. Generally, it is better that a first-line screening test is highly sensitive so that as many potential cases are identified as possible. The cases identified at screening can then be narrowed down by using tools with higher specificity. The specificity and sensitivity of specific screening tools for MMH were determined by studies that sought to establish the psychometric properties of the tools within the population of interest to validate the tools. For instance, the sensitivity of the EPDS was found to be 86%, signifying the proportion of depressed women who truly had depression and with a specificity of 78% indicating the proportion of women who were not depressed who were truly not depressed at a cut of 12/13 score. It was noted, however, that these figures vary in the studies reviewed because of different factors. First, the cut-off points used by researchers in their studies were not standardized which could have affected the sensitivity and specificity of the tools in identifying the condition of interest. Additionally, it was also deduced that the SSA population has a different socio-demographic characteristic from the population where the tools were developed; therefore, some researchers translated the tools into local languages or revised them to make them culturally acceptable. This is in line with findings from earlier reviews (59, 60) both in Africa and non-African LMIC (200, 201) on screening tools for assessing depression and anxiety among pregnant and postpartum women. These adaptations could have been a contributory factor that affected the outcome of the specificity and sensitivity of the tool in capturing the condition of interest. Findings from this review suggest that some studies did not validate screening tools used within the population and condition of interest because sometimes the researchers use sensitivity and specificity values from the psychometric properties of the tool or rely on values documented from studies among a similar population from another country. This may possibly be because of resource constraints (time and financial) and or lack of skill on the part of the research team. The tools developed elsewhere may be generally appropriate to SSA; however, some components of each sensitivity and specificity values are probably due to the differences in contexts in which the tools were developed and applied.
The EPDS screening tool was the most acceptable (based on versatility and sensitivity) for screening for depression among postpartum women. The tool has been used to screen for depression both in the antenatal and postnatal periods and has been found to be valid in identifying women who may be depressed. However, Murray and Cox (202) proposed a higher cut-off point of 14/15 during the antenatal period after validating the EPDS among a sample of 100 women 28 and 34 weeks of pregnancy living in the United Kingdom. Contrary to this proposition, none of the studies reporting on the performance of the EPDS among pregnant women used this suggested cut-off point. Additionally, the set of questions does not specifically cite the postpartum period making it easy to be used in multiple situations even outside pregnancy and the postpartum period (50). Despite some contextual issues raised about the EPDS by some researchers on African and non-African populations (60, 108, 200, 201, 203, 204), from this systematic review, it appears to be the tool with evidence for acceptability, adaptability, ease of use, and effectiveness in identifying potential women who may be depressed during and after pregnancy. It can only identify depression which requires the use of other tools to complement screening for other relevant mental health conditions such as bipolar disorder and anxiety. This may adversely affect the duration of administration, leading to patient fatigue and worker overload for the primary healthcare staff.
Contextual issues related to screening tools developed in the Global North and used in LMIC (205–207) and other non-native English-speaking populations even when emigrated to English-speaking HICs have been well documented (200, 201, 204, 208). Considering that cultural nuances and language greatly affect the expression of distress and symptoms of psychiatric conditions, translations of screening tools do not adequately capture the assessment of the construct and cross-cultural reliability of these tools (207). Our review of the publications reporting on the screening tools for MMH conditions predominantly translated these tools developed and validated for populations in the HIC into languages specific to the population of interest. However, this practice may not be entirely accurate in capturing the context and nuances of the language and culture of the population. In administering the questionnaires by an interviewer as was reported by most of the papers in this review, the interviewer may have discussions in an attempt to clarify some of the constructs through engagement in their local dialect introducing a potential margin of error. This is similar to the finding from a systematic review of tools for assessing behavioral challenges in LIMCs (206). Indeed, using tools validated decades prior from a population similar to the population of interest may not be ideal, as the population may have changed due to the effects of globalization influencing their culture. These influences may need to be considered in the interpretation of results from such studies as the persistent use of these tools indirectly promotes them as cross-culturally appropriate as argued by other researchers (206).
The answer to our third question related to the potential for using any of the tools identified in primary care settings in SSA is influenced by properties of the tools such as understandability of the tool and ease of use, especially by the cadres of staff who are likely to administer the tools or the clients if they are self-administered, and adaptability to context. Reporting on the properties of specific screening tools used in screening for MMH conditions is not routinely done by researchers. From this systematic review, only 12% reported on the understandability of the questions of the screening tool by research participants, 10% on ease of use by researchers, 48% adaptability, and 52% on a specific cadre of health staff or otherwise who administered the tools with nurses, midwives, and nursing students being the most used staff category. Although these may have been noted during the research process and probably discussed among the research team but not formally documented as part of communicating the research outcome to potential readers of the papers. Not documenting this important information on screening tools for detecting MMH conditions deprives the research community and clinicians of essential guidance in appraising these tools holistically in their usage in research or clinical settings.
There are obviously methodological challenges affecting the outcome of the use of these screening tools (52) as well as the complexity of pregnancy and the postpartum period including the hormonal changes, genetic predisposition to mental health conditions, and the environment further influenced by cultural variations in different populations affecting systems, and structures for support. A key population of women who are most predisposed to mental health conditions specifically those who lose their pregnancy before the age of viability or after viability such as stillbirth or death of the fetus from any cause were significantly missing from the studies included in this review, which reported the use of different screening tools. This may be due to the postnatal period primarily focused on a positive outcome of pregnancy and not the woman regardless of the outcome (live child or otherwise).
An overarching finding from our review is that the EPDS appeared to be the most effective, acceptable, user-friendly, and adaptable screening tool for identifying MMH conditions and depression among pregnant and postpartum women in SSA in primary care settings. An ultra-shorter 5-item and 3-item versions have been proposed to address the challenge of work overload for its usage in routine assessment in primary care. There was, however, a wide variation in methodology in the usage of the tool such as the cut-off points from the original 13 or more, an attempt to diagnose or reclassify depression into severity using different cut-off points as well as documentation of the attributed of the tools in the methodology of the papers.
Limitation of the study
Although the study systematically reviewed the psychometric properties of published papers reporting on a wide range of tools, a significant limitation is the variability in methodological approaches used in the included studies. This variability includes differences in study designs, sample populations, and the cut-off for tools, particularly in validation studies. These have an impact on the outcome and comparability and generalizability of the findings. Additionally, the scanty documentation on the specific attributes of the tools used, such as understandability, ease of use, and adaptability. This lack of detailed and standardized reporting limits the ability to draw robust conclusions about the effectiveness and applicability of the tools across different contexts.
Conclusion
The EPDS, PHQ-9, Whooley Questions, SRQ, and KPDS were predominantly reported to be effective in screening for MMH problems, demonstrating good sensitivity and specificity across various validation studies. Several studies highlighted the EPDS, Whooley Questions, AUDIT, and HAD as user-friendly and easy to administer, even in busy primary healthcare settings. Additionally, the EPDS and PHQ-9 were frequently noted for their adaptability, with the EPDS being widely translated into local languages and adapted for mobile technology to facilitate self-administration. Among the tools reviewed, the EPDS emerged as the most commonly used, effective, acceptable, user-friendly, and adaptable screening tool for depression in pregnant and postpartum women in SSA’s primary healthcare settings.
However, mental health conditions during this period beyond depression include other conditions, which may be prevalent during this critical period that are not screened for. There is a need to expand advocacy and research to include other prevalent mental health conditions, and address the associated morbidity, to improve the well-being of women and their children during this important period.
Recommendations
The findings from this study provide some recommendations for consideration at three key levels: clinical practice, policy, and future research.
For practice
Health authorities and primary care providers in SSA should integrate EPDS into routine antenatal and postnatal care. The EPDS has demonstrated high effectiveness, usability, and adaptability, making it an ideal tool for the early detection of MMH distress. Additionally, the short and ultra-short versions of available tools can be incorporated into routine care to reduce the burden on practitioners.
Comprehensive training programs should be developed for healthcare providers on the use of EPDS and other effective screening tools such as PHQ-9, Whooley questions, SRQ, and KPDS. These tools may be incorporated into pre-service curricula for key health staff, including midwives, nurses, and medical students in administering these tools, as this could enhance the accuracy and efficiency of mental health screenings in primary care settings, facilitating better integration into routine practice.
For policy
Policymakers should develop and implement clear policies supporting the routine use of validated mental health screening tools in maternal healthcare. These policies should mandate the screening of all pregnant women regardless of the outcome to ensure no woman is left behind in having access to mental health support when they are most vulnerable. Targeted policy and strategy will facilitate the systematic integration of these tools into healthcare protocols, promoting consistent and effective mental healthcare for all pregnant and postpartum women.
For future research
Ongoing research and evaluation should be encouraged to continuously assess the effectiveness and acceptability of mental health screening tools in different SSA contexts. The focus should be on expanding research beyond depression to include a range of MMH conditions. Continuous evaluation identifies areas for improvement and adaptation, ensuring that screening tools remain relevant and effective. Expanding research ensures comprehensive MMH care.
Efforts to translate and culturally adapt the EPDS and other effective screening tools into local languages and contexts within SSA should continue. Collaboration between clinicians and researchers is essential in developing and testing these adapted tools. Cultural and linguistic adaptations improve accessibility and acceptability, making the tools more effective in diverse communities. Collaborative efforts ensure the tools are practical and context-appropriate.
The use of MMH screening tools should be standardized among researchers in SSA. Studies conducted outside the original context of the tools should document various field experiences to guide holistic appraisal.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LG: Validation, Software, Data curation, Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. IA: Validation, Supervision, Writing – review & editing, Formal analysis, Data curation, Conceptualization. DO: Validation, Software, Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. EA: Writing – review & editing, Formal analysis, Data curation. MA: Writing – review & editing. LY: Validation, Writing – review & editing, Formal analysis, Data curation. SGA: Writing – review & editing, Project administration, Data curation. SA: Writing – review & editing, Data curation. AC: Writing – review & editing, Data curation. TM: Writing – original draft, Writing – review & editing, Conceptualization. AD-A: Validation, Writing – review & editing, Writing – original draft, Supervision, Methodology, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research reported in this paper received funding from the Joint Health Systems Research Initiative comprising Medical Research Council (MRC), Foreign, Commonwealth & Development Office (FCDO) and Wellcome Trust (grant ref: MR/T023481/2). The views are of the authors only and do not necessarily represent those of the funders.
Acknowledgments
This systematic review was part of capacity building initiative by the Centre for Evidence Synthesis and Policy (CESP), University of Ghana; The Africa Communities of Evidence Synthesis and Translation (ACEST), and the RESPONSE Project that are jointly training experts in evidence synthesis and translation across LMICs. We thank the anonymous peer reviewers for the very useful comments and the editorial base for their handling of the editorial that have helped improved the quality of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of the funders.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1321689/full#supplementary-material
References
1. Bjegovic-mikanovic, V, Broniatowski, R, Byepu, S, and Laaser, U. A gap analysis of mother, new-born, and child health in West Africa with reference to the sustainable development goals 2030. Afr J Reprod Health. (2018) 22:123–34. doi: 10.29063/ajrh2018/v22i4.13
2. Ahmed, T, Rahman, AE, Amole, TG, Galadanci, H, Matjila, M, Soma-pillay, P, et al. The effect of COVID-19 on maternal newborn and child health (MNCH) services in Bangladesh, Nigeria and South Africa: call for a contextualised pandemic response in LMICs. Int J Equity Health. (2021) 20:1–6. doi: 10.1186/s12939-021-01414-5
3. Bhutta, Z, Guerrant, RL, and Nelson, CA III. Neurodevelopment, nutrition, and inflammation: the evolving global child health landscape. Paediatrics. (2017) 139:S12–22. doi: 10.1542/peds.2016-2828D
4. Requejo, J, Strong, K, Agweyu, A, Billah, SM, Boschi-pinto, C, Horiuchi, S, et al. Measuring and monitoring child health and wellbeing: recommendations for tracking progress with a core set of indicators in the sustainable development goals era. Lancet Child Adolesc Health. (2022) 6:345–52. doi: 10.1016/S2352-4642(22)00039-6
5. Agbozo, F, Amardi-Mfoafo, J, Dwase, H, and Ellahi, B. Nutrition knowledge, dietary patterns and anthropometric indices of older persons in four peri-urban communities in Ga west municipality, Ghana. Afr Health Sci. (2018) 18:743–55. doi: 10.4314/ahs.v18i3.33
6. Haithar, S, Kuria, MW, Sheikh, A, Kumar, M, and Vander, SA. Maternal depression and child severe acute malnutrition: a case-control study from Kenya. BMC Pediatr. (2018) 18:1–9. doi: 10.1186/s12887-018-1261-1
7. Ware, SG, Daniel, AI, Bandawe, C, Mulaheya, YP, Nkunika, S, Nkhoma, D, et al. Perceptions and experiences of caregivers of severely malnourished children receiving inpatient care in Malawi: an exploratory study. Malawi Med J. (2018) 30:167–73. doi: 10.4314/mmj.v30i3.7
8. Vythilingum, B, Field, S, Kafaar, Z, Baron, E, Stein, DJ, Sanders, L, et al. Screening and pathways to maternal mental health care in a south African antenatal setting. Arch Womens Ment Health. (2013) 16:371–9. doi: 10.1007/s00737-013-0343-1
9. Richter, L, Daelmans, B, Lombardi, J, Heymann, J, Boo, FL, Behrman, JR, et al. Investing in the foundation of sustainability development: pathways to scale up for early childhood development. Lancet. (2018) 389:103–18. doi: 10.1016/S0140-6736(16)31698-1
10. World Health Organization. Nurturing care for early childhood development: a framework for helping children survive and thrive to transform health and human potential. Geneva: World Health Organization (2018).
11. Manolova, G, Waqas, A, Chowdhary, N, Salisbury, TT, and Dua, T. Integrating perinatal mental healthcare into maternal and perinatal services in low and middle income countries. BMJ. (2023) 381:e073343. doi: 10.1136/bmj-2022-073343
12. World Health Organization. Guide for integration of perinatal mental health in maternal and child health services. Geneva: World Health Organization (2022).
13. Gelaye, B, Rondon, M, Araya, PR, and Williams, MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. (2016) 3:973–82. doi: 10.1016/S2215-0366(16)30284-X
14. Anderson, FM, Hatch, SL, Comacchio, C, and Howard, LM. Prevalence and risk of mental disorders in the perinatal period among migrant women: a systematic review and meta-analysis. Arch Womens Ment Health. (2017) 20:449–62. doi: 10.1007/s00737-017-0723-z
15. Onah, MN, Field, S, Bantjes, J, and Honikman, S. Perinatal suicidal ideation and behaviour: psychiatry and adversity. Arch Womens Ment Health. (2017) 20:321–31. doi: 10.1007/s00737-016-0706-5
16. Agbaje, OS, Anyanwu, JI, Umoke, PIC, Iwuagwu, TE, Iweama, CN, Ozoemena, EL, et al. Depressive and anxiety symptoms and associated factors among postnatal women in Enugu-north Senatorial District, south-East Nigeria: a cross-sectional study. Arch Public Health. (2019) 77:1. doi: 10.1186/s13690-018-0329-6
17. Dadi, AF, Akalu, TY, Baraki, AG, and Wolde, HF. Epidemiology of postnatal depression and its associated factors in Africa: A systematic review and meta-analysis. PLoS One. (2020) 15:1–18. doi: 10.1371/journal.pone.0231940
18. Ongeri, L, Wanga, V, Otieno, P, Mbui, J, Juma, E, Vander, SA, et al. Demographic, psychosocial and clinical factors associated with postpartum depression in Kenyan women. BMC Psychiatry. (2018) 18:1–9. doi: 10.1186/s12888-018-1904-7
19. Burger, M, Hoosain, M, Einspieler, C, Unger, M, and Niehaus, D. Maternal perinatal mental health and infant and toddler neurodevelopment – evidence from low and middle-income countries. A systematic review. J Affect Disord. (2020) 2020:158–72. doi: 10.1016/j.jad.2020.03.023
20. Dennis, C, Lee Falah-Hassani, K, and Shiri, R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. (2017) 210:315–23. doi: 10.1192/bjp.bp.116.187179
21. Woody, CA, Ferrari, AJ, Siskind, DJ, Whiteford, HA, and Harris, MG. Journal of affective disorders review article A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. (2017) 219:86–92. doi: 10.1016/j.jad.2017.05.003
22. McEwan, A. Obstetrics by ten teachers In: PN Baker and LC Kenny, editors. Obsterics by ten teachers. 19th ed. London: Hodder Arnold (2011). 272–80.
23. Bindt, C, Appiah-Poku, J, Te Bonle, M, Schoppen, S, Feldt, T, Barkmann, C, et al. Antepartum depression and anxiety associated with disability in African women: Cross-sectional results from the CDS study in Ghana and Côte d’Ivoire. PLoS One. (2012) 7:1–7. doi: 10.1371/journal.pone.0048396
24. Umuziga, MP, Adejumo, O, and Hynie, M. A cross-sectional study of the prevalence and factors associated with symptoms of perinatal depression and anxiety in Rwanda. BMC Pregnancy Childbirth. (2020) 20:68. doi: 10.1186/s12884-020-2747-z
25. Govender, D, Naidoo, S, and Taylor, M. Antenatal and postpartum depression: prevalence and associated risk factors among adolescents’ in KwaZulu-Natal. South Africa Depress Res Treat. (2020) 2020:1–12. doi: 10.1155/2020/5364521
26. Nakku, JEM, Okello, ES, Kizza, D, Honikman, S, Ssebunnya, J, Ndyanabangi, S, et al. Perinatal mental health care in a rural African district, Uganda: a qualitative study of barriers, facilitators and needs. BMC Health Serv Res. (2016) 16:1–12. doi: 10.1186/s12913-016-1547-7
27. Osok, J, Kigamwa, P, Vander, SA, Huang, KY, and Kumar, M. Depression and its psychosocial risk factors in pregnant Kenyan adolescents: A cross-sectional study in a community health Centre of Nairobi. BMC Psychiatry. (2018) 18:136. doi: 10.1186/s12888-018-1706-y
28. Manikkam, L, and Burns, JK. Antenatal depression and its risk factors: an urban prevalence study in KwaZulu-Natal. S Afr Med J. (2012) 102:940–4. doi: 10.7196/SAMJ.6009
29. Kaaya, SF, Mbwambo, JK, Kilonzo, GP, Van Den Borne, H, Leshabari, MT, Smith Fawzi, MC, et al. Socio-economic and partner relationship factors associated with antenatal depressive morbidity among pregnant women in Dar Es Salaam, Tanzania. Tanzan J Health Res. (2010) 12:1–15. doi: 10.4314/thrb.v12i1.56276
30. Semple, D, and Smyth, R. Reproductive psychiatry and sexuality In: Oxford Handbook of Psychiatry. 3rd edn. Oxford: OUP Oxford (2013). 468–71.
31. Abrahams, Z, Lund, C, Field, S, and Honikman, S. Factors associated with household food insecurity and depression in pregnant south African women from a low socio-economic setting: a cross-sectional study. Soc Psychiatry Psychiatr Epidemiol. (2018) 53:363–72. doi: 10.1007/s00127-018-1497-y
32. Brittain, K, Remien, RH, Philips, T, Zerbe, A, Abrams, EJ, Myer, L, et al. Factors associated with alcohol use prior to and during pregnancy among HIV-infected pregnant women in Cape Town, South Africa. Drug Alcohol Dependence. (2017) 173:69–77. doi: 10.1016/j.drugalcdep.2016.12.017
33. Banik, BK. Improving maternal health. J Health Manag. (2017) 19:4. doi: 10.1177/0972063417727614
34. Chessick, CA, and Dimidjian, S. Screening for bipolar disorder during pregnancy and the postpartum period. Arch Womens Ment Health. (2010) 13:233–48. doi: 10.1007/s00737-010-0151-9
35. Fatori, D, Bordin, IA, Curto, BM, and de Paula, CS. Influence of psychosocial risk factors on the trajectory of mental health problems from childhood to adolescence: A longitudinal study. BMC Psychiatry. (2013) 13:1–6. doi: 10.1186/1471-244X-13-31
36. Kleintjes, S, Lund, C, Flisher, AJ, and Consortium, P. A situational analysis of child and adolesecent mental health services in Ghana, Uganda, South Africa and Zambia. Afr J Psychiatry. (2010) 13:132–9. doi: 10.4314/ajpsy.v13i2.54360
37. Ranøyen, I, Klöckner, CA, Wallander, J, and Jozefiak, T. Associations between internalizing problems in adolescent daughters versus sons and mental health problems in mothers versus fathers (the HUNT study). J Child Fam Stud. (2015) 24:2008–20. doi: 10.1007/s10826-014-0001-x
38. Louw, KA. Substance use in pregnancy: the medical challenge. Obstet Med. (2018) 11:54–66. doi: 10.1177/1753495X17750299
39. Warton, F, Taylor, P, Warton, CMR, Molteno, CD, Wintermark, P, Lindinger, NM, et al. Prenatal methamphetamine exposure is associated with corticostriatal white matter changes in neonates. Metab Brain Dis. (2018) 33:507–22. doi: 10.1007/s11011-017-0135-9
40. Ng’oma, M, Meltzer-Brody, S, Chirwa, E, and Stewart, RC. “Passing through difficult times”: perceptions of perinatal depression and treatment needs in Malawi – A qualitative study to inform the development of a culturally sensitive intervention. PLoS One. (2019) 14:1–20. doi: 10.1371/journal.pone.0217102
41. Boukakiou, R, Glangeaud-Freudenthal, NMC, Falissard, B, Sutter-Dallay, AL, and Gressier, F. Impact of a prenatal episode and diagnosis in women with serious mental illnesses on neonatal complications (prematurity, low birth weight, and hospitalization in neonatal intensive care units). Arch Womens Ment Health. (2019) 22:439–46. doi: 10.1007/s00737-018-0915-1
42. Ngocho, JS, Watt, MH, Minja, L, Knettel, BA, Mmbaga, BT, Williams, P, et al. Depression and anxiety among pregnant women living with HIV in Kilimanjaro region, Tanzania. PLoS One. (2019) 14:1–15. doi: 10.1371/journal.pone.0224515
43. Brankin, L. (2014) A Phenomenological Analysis: Exploring the Lived Experiences of the Adult Daughter’ s Perception of Maternal Mental Illness and the Trans-Generational Impact on Parenting Leslie Brankin A Dissertation Submitted to the Faculty of The Chicago School of Pr.
44. Turner, RE, and Honikman, S. Maternal mental health and the first 1000 days. S Afr Med J. (2016) 106:1164–7. doi: 10.7196/SAMJ.2017.v106i12.12129
45. Semple, D, and Smyth, R. Depressive illness In: D Semple and R Smyth, editors. Oxford handbook of psychiatry. 3rd ed. Oxford: Oxford University Press (2013). 231–300.
46. American Psychiatric Association. Depressive disorders In: Diagnostic and statistical manual of mental disorders. 5th ed. Washington DC: (2013). 186–7.
47. Miller, LJ. Postpartum Depression. J Am Med Assoc. (2002) 287:762–7. doi: 10.1001/jama.287.6.762
48. Knight, M, Bunch, K, Patel, R, Shakespeare, J, Kotnis, R, Kenyon, S, et al. Saving lives, improving mothers’ care Core report-lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2018–20. Oxford: National Perinatal Epidemiology Unit, University of Oxford. (2022).
49. Lindahl, V, Pearson, JL, Colpe, L, and Carolina, N. Review article prevalence of suicidality during pregnancy and the postpartum. Achiv Women’s Mental Health. (2005) 8:77–87. doi: 10.1007/s00737-005-0080-1
50. Cox, J, and Holden, J. (2003). Perinatal mental health; a guide to the Edinburg postnatal depression scale. London: Royal College of Psychiatrist. 1–130 p.
51. Okronipa, HET, Marquis, GS, Lartey, A, Brakohiapa, L, Perez-Escamilla, R, and Mazur, RE. Postnatal depression symptoms are associated with increased diarrhea among infants of HIV-positive Ghanaian mothers. AIDS Behav. (2012) 16:2216–25. doi: 10.1007/s10461-012-0153-x
52. Slomian, J, Honvo, G, Emonts, P, Yves, RJ, and Bruyère, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health. (2019) 15:174550651984404–55. doi: 10.1177/1745506519844044
53. Jones, A. Help seeking in the perinatal period: A review of barriers and facilitators help seeking in the perinatal period: A review of barriers and facilitators. Soc Work Public Health. (2019) 34:596–605. doi: 10.1080/19371918.2019.1635947
54. Azale, T, Fekadu, A, and Hanlon, C. Treatment gap and help-seeking for postpartum depression in a rural African setting. BMC Psychiatry. (2016) 16:196. doi: 10.1186/s12888-016-0892-8
55. Villegas, L, Mckay, K, Lee, DC, and Ross, LE. Postpartum depression among rural women from developed and developing countries: A systematic review. J Rural Health. (2011) 27:278–88. doi: 10.1111/j.1748-0361.2010.00339.x
56. Parsons, CE, Young, KS, Rochat, TJ, Kringelbach, ML, and Stein, A. Postnatal depression and its effects on child development: A review of evidence from low-and middle-income countries. Br Med Bull. (2012) 101:57–79. doi: 10.1093/bmb/ldr047
57. Harris, L, and Lancet, T. Screening for perinatal depression: A missed opportunity. Lancet. (2016) 387:505. doi: 10.1016/S0140-6736(16)00265-8
58. Tefera, TB, Erena, AN, Kuti, KA, and Hussen, MA. Perinatal depression and associated factors among reproductive aged group women at Goba and robe town of bale zone, Oromia region, south East Ethiopia. Matern Health Neonatol Perinatol. (2015) 1:1–9. doi: 10.1186/s40748-015-0013-6
59. Sweetland, AC, Belkin, GS, and Verdeli, H. Measuring depression and anxiety in sub-Saharan Africa. Depress Anxiety. (2014) 31:223–32. doi: 10.1002/da.22142
60. Tsai, AC, Scott, JA, Hung, KJ, Zhu, JQ, and Matthews, LT. Reliability and validity of instruments for assessing perinatal depression in African settings: systematic review and Meta-analysis. PLoS One. (2013) 8:e82521. doi: 10.1371/journal.pone.0082521
61. Lasater, ME, Beebe, M, Warren, NE, Souko, F, Keita, M, Murray, SM, et al. Dusukasi—the heart that cries: an idiom of mental distress among perinatal women in rural Mali. Cult Med Psychiatry. (2018) 42:930–45. doi: 10.1007/s11013-018-9579-6
62. Zoellner, L, Graham, B, Marks, E, Feeny, N, Bentley, J, Franklin, A, et al. Islamic trauma healing: initial feasibility and pilot data. Societies. (2018) 8:1–12. doi: 10.3390/soc8030047
63. Kyei, JJ, Dueck, A, Indart, MJ, and Nyarko, NY. Supernatural belief systems, mental health and perceptions of mental disorders in Ghana. Int J Cult Ment Health. (2014) 7:137–51. doi: 10.1080/17542863.2012.734838
64. Akomolafe, AC. Decolonizing the notion of mental illness and healing in Nigeria, West Africa. Crit Psychol Chang World. (2012):726–40.
65. Hickling, FW. Owning our madness: contributions of Jamaican psychiatry to decolonizing global mental health. Transcult Psychiatry. (2020) 57:19–31. doi: 10.1177/1363461519893142
66. Bhat, A, Nanda, A, Murphy, L, Ball, AL, Fortney, J, and Katon, J. A systematic review of screening for perinatal depression and anxiety in community-based settings. Arch Womens Ment Health. (2022) 25:33–49. doi: 10.1007/s00737-021-01151-2
67. Chorwe-sungani, G, and Chipps, J. A systematic review of screening instruments for depression for use in antenatal services in low resource settings. BMC Psychiatry. (2017) 17:1–10. doi: 10.1186/s12888-017-1273-7
68. Fellmeth, G, Harrison, S, Opondo, C, Nair, M, Kurinczuk, JJ, and Alderdice, F. Validated screening tools to identify common mental disorders in perinatal and postpartum women in India: a systematic review and meta-analysis. BMC Psychiatry. (2021) 21:200. doi: 10.1186/s12888-021-03190-6
69. Gibson, J, McKenzie-McHarg, K, Shakespeare, J, Price, J, and Gray, R. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. (2009) 119:350–64. doi: 10.1111/j.1600-0447.2009.01363.x
70. Levis, B, Negeri, Z, Sun, Y, Benedetti, A, and Thombs, BD. Accuracy of the Edinburgh postnatal depression scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. (2020) 371:m4022–2. doi: 10.1136/bmj.m4022
71. Park, SH, and Kim, JI. Predictive validity of the Edinburgh postnatal depression scale and other tools for screening depression in pregnant and postpartum women: a systematic review and meta-analysis. Arch Gynecol Obstet. (2023) 307:1331–45. doi: 10.1007/s00404-022-06525-0
72. Waqas, A, Koukab, A, Meraj, H, Dua, T, Chowdhary, N, Fatima, B, et al. Screening programs for common maternal mental health disorders among perinatal women: report of the systematic review of evidence. BMC Psychiatry. (2022) 22:54. doi: 10.1186/s12888-022-03694-9
73. Smith, RD, Shing, JSY, Lin, J, Bosanquet, K, Fong, DYT, and Lok, KYW. Meta-analysis of diagnostic properties of the Whooley questions to identify depression in perinatal women. J Affect Disord. (2022) 315:148–55. doi: 10.1016/j.jad.2022.07.026
74. Wang, L, Kroenke, K, Stump, TE, and Monahan, PO. Screening for perinatal depression with the patient health questionnaire depression scale (PHQ-9): A systematic review and meta-analysis. Gen Hosp Psychiatry. (2021) 68:74–82. doi: 10.1016/j.genhosppsych.2020.12.007
75. Thombs, BD, Levis, B, Lyubenova, A, Neupane, D, Negeri, Z, Wu, Y, et al. Overestimation of postpartum depression prevalence based on a 5-item version of the EPDS: systematic review and individual participant data Meta-analysis. Can J Psychiatr. (2020) 65:835–44. doi: 10.1177/0706743720934959
76. Shrestha, SD, Pradhan, R, Tran, TD, Gualano, RC, and Fisher, JRW. Reliability and validity of the Edinburgh postnatal depression scale (EPDS) for detecting perinatal common mental disorders (PCMDs) among women in low-and lower-middle-income countries: a systematic review. BMC Pregnancy Childbirth. (2016) 16:1–19. doi: 10.1186/s12884-016-0859-2
77. Gershman, B, and Rivera, D. Subnational diversity in sub-Saharan Africa: insights from a new dataset. J Dev Econ. (2018) 133:231–63. doi: 10.1016/j.jdeveco.2018.01.003
78. Oleribe, OO, Momoh, J, Uzochukwu, BSC, Mbofana, F, Adebiyi, A, Barbera, T, et al. Identifying key challenges facing healthcare systems in Africa and potential solutions. Int J Gen Med. (2019) 12:395–403. doi: 10.2147/IJGM.S223882
79. Doctor, HV, Nkhana-Salimu, S, and Abdulsalam-Anibilowo, M. Health facility delivery in sub-Saharan Africa: successes, challenges, and implications for the 2030 development agenda. BMC Public Health. (2018) 18:765. doi: 10.1186/s12889-018-5695-z
80. Programme WHO Organization MHGA. (2015). Update of the mental health gap action Programme (mhGAP) guidelines for mental, neurological and substance use disorders.
81. World Health Organization. The world health report 2001: Mental health: New understanding. Geneva: New Hope (2001).
82. World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical description and diagnostic guidelines, vol. 1. Geneva: World Health Organization (1992).
83. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Publishing, Inc (2013).
85. Urbina, S. Essentials of psychological testing. New Jersey: John Wiley & Sons, Inc (2004). 14 p.
86. Murphy, KR, and Davidshofer, CO In: M Jessica and L Jewel, editors. Psychological Testing: Principles and Applications. 6th ed. Hoboken, NJ: Pearson Educational International (2005). 1.
87. Dicker, RC, Coronado, F, and Koo, D. Principles of Epidemiology in Public Health Practice. 3rd ed. Atlanta, GA: Centers for Disease Control and Prevention (2006).
88. Daniel, WW, and Cross, CL. Biostatistics: A foundation for analysis in health sciences. Hoboken, NJ: Wiley (2018).
89. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
90. Peters, DJ, Marnie, C, Tricco, AC, Pollock, D, Munn, Z, Alexander, L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. (2020) 18:2119–26. doi: 10.11124/JBIES-20-00167
91. Matthey, S, Barnett, B, Kavanagh, DJ, and Howie, P. Validation of the Edinburgh postnatal depression scale for men, and comparison of item endorsement with their partners. J Affect Disord. (2001) 64:175–84. doi: 10.1016/S0165-0327(00)00236-6
92. Bramer, WM, Milic, J, and Mast, F. Reviewing retrieved references for inclusion in systematic reviews using endnote. J Med Libr Assoc. (2017) 105:84–7. doi: 10.5195/jmla.2017.111
93. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:1–10.
94. Barthel, D, Barkmann, C, Ehrhardt, S, Schoppen, S, Bindt, C, Appiah-Poku, J, et al. Screening for depression in pregnant women from Côte d’Ivoire and Ghana: psychometric properties of the patient health Questionnaire-9. J Affect Disord. (2015) 187:232–40. doi: 10.1016/j.jad.2015.06.042
95. Barthel, D, Kriston, L, Barkmann, C, Appiah-Poku, J, Te Bonle, M, Esther Doris, KY, et al. Longitudinal course of ante-and postpartum generalized anxiety symptoms and associated factors in west-African women from Ghana and ZAF. J Affect Disord. (2016) 197:125–33. doi: 10.1016/j.jad.2016.03.014
96. Guo, N, Bindt, C, Te Bonle, M, Appiah-Poku, J, Tomori, C, Hinz, R, et al. Mental health related determinants of parenting stress among urban mothers of young children – results from a birth-cohort study in Ghana and Côte d’Ivoire. BMC Psychiatry. (2014) 14:1–12. doi: 10.1186/1471-244X-14-156
97. Abiodun, OA. A validity study of the hospital anxiety and depression scale in general hospital units and a community sample in Nigeria. Br J Psychiatry. (1994) 165:669–72. doi: 10.1192/bjp.165.5.669
98. Aderibigbe, YA, and Gureje, O. The validity of the 28-item general health questionnaire in a Nigerian antenatal clinic. Soc Psychiatry Psychiatr Epidemiol. (1992) 27:280–3. doi: 10.1007/BF00788899
99. Nhiwatiwa, S, Patel, V, and Acuda, W. Predicting postnatal mental disorder with a screening questionnaire: A prospective cohort study from Zimbabwe. J Epidemiol Community Health (1978). (1998) 52:262–6.
100. Uwakwe, R. Affective (depressive) morbidity in puerperal Nigerian women: validation of the Edinburgh postnatal depression scale. Acta Psychiatr Scand. (2003) 107:251–9. doi: 10.1034/j.1600-0447.2003.02477.x
101. Adewuya, AO, Fatoye, FO, Ola, BA, Ijaodola, OR, and Ibigbami, SMO. Sociodemographic and obstetric risk factors for postpartum depressive symptoms in Nigerian women. J Psychiatr Pract. (2005) 11:353–8. doi: 10.1097/00131746-200509000-00009
102. Nakku, JEM, Nakasi, G, and Mirembe, F. Postpartum major depression at six weeks in primary health care: prevalence and associated factors. Afr Health Sci. (2006) 6:207–14. doi: 10.5555/afhs.2006.6.4.207
103. Abiodun, OA. Postnatal depression in primary care populations in Nigeria. Gen Hosp Psychiatry. (2006) 28:133–6. doi: 10.1016/j.genhosppsych.2005.11.002
104. Bernatsky, S, and Souza, R. Care KDJA, 2007 undefined. Mental health in HIV-positive pregnant women: results from Angola. Taylor Francis. (2007) 19:674–6. doi: 10.1080/09540120601012705
105. Bass, JK, Ryder, RW, Lammers, MC, Mukaba, TN, and Bolton, PA. Post-partum depression in Kinshasa, Democratic Republic of Congo: validation of a concept using a mixed-methods cross-cultural approach. Trop Med Int Health. (2008) 13:1534–42. doi: 10.1111/j.1365-3156.2008.02160.x
106. Kaaya, SF, Lee, B, Mbwambo, JK, Smith-Fawzi, MC, and Leshabari, MT. Detecting depressive disorder with a 19-item local instrument in Tanzania. Int J Soc Psychiatry. (2008) 54:21–33. doi: 10.1177/0020764006075024
107. Stewart, RC, Kauye, F, Umar, E, Vokhiwa, M, Bunn, J, Fitzgerald, M, et al. Validation of a Chichewa version of the self-reporting questionnaire (SRQ) as a brief screening measure for maternal depressive disorder in Malawi, Africa. J Affect Disord. (2009) 112:126–34. doi: 10.1016/j.jad.2008.04.001
108. Chibanda, D, Mangezi, W, Tshimanga, M, Woelk, G, Rusakaniko, P, Stranix-Chibanda, L, et al. Validation of the Edinburgh postnatal depression scale among women in a high HIV prevalence area in urban Zimbabwe. Arch Womens Ment Health. (2010) 13:201–6. doi: 10.1007/s00737-009-0073-6
109. Cyimana, A, Andrews, B, Ahmed, Y, and Vwalika, B. HIV/AIDS and postnatal depression at the university teaching hospital, Lusaka, Zambia. Med J Zambia. (2010) 37:78–83.
110. Abebe, A, Tesfaw, G, Mulat, H, Hibdye, G, and Yohannes, K. Postpartum depression and associated factors among mothers in Bahir Dar town, Northwest Ethiopia. Ann General Psychiatry. (2019) 18:1–8. doi: 10.1186/s12991-019-0244-4
111. Adamu, AF, and Adinew, YM. Domestic violence as a risk factor for postpartum depression among Ethiopian women: facility based study. Clin Pract Epidemiol Mental Health. (2018) 14:109–19. doi: 10.2174/1745017901814010109
112. Alenko, A, Dejene, S, and Girma, S. Sociodemographic and obstetric determinants of antenatal depression in Jimma medical center, Southwest Ethiopia: facility based case–control study. Int J Women's Health. (2020) 12:557–65. doi: 10.2147/IJWH.S252385
113. Ayele, TA, Azale, T, Alemu, K, Abdissa, Z, Mulat, H, and Fekadu, A. Prevalence and associated factors of antenatal depression among women attending antenatal Care Service at Gondar University Hospital, Northwest Ethiopia. PLoS One. (2016) 11:e0155125. doi: 10.1371/journal.pone.0155125
114. Ayele, S, Alemayehu, M, Fikadu, E, and Tarekegn, GE. Prevalence and associated factors of depression among pregnant mothers who had intimate partner violence during pregnancy attending antenatal Care at Gondar University Hospital Northwest Ethiopia in 2020. Biomed Res Int. (2021) 2021:1–9. doi: 10.1155/2021/9965289
115. Belete, H, and Misgan, E. Suicidal behaviour in postnatal mothers in northwestern Ethiopia: A cross-sectional study. BMJ Open. (2019) 9:e027449–8. doi: 10.1136/bmjopen-2018-027449
116. Dadi, AF, Mwanri, L, Woodman, RJ, Azale, T, and Miller, ER. Causal mechanisms of postnatal depression among women in Gondar town, Ethiopia: application of a stress-process model with generalized structural equation modeling. Reprod Health. (2020) 17:1–15. doi: 10.1186/s12978-020-00912-z
117. Fantahun, A, Cherie, A, and Deribe, L. Clinical Practice & Epidemiology in prevalence and factors associated with postpartum depression among mothers attending public health centers of Addis Ababa. Ethiopia. (2016) 14:196–206. doi: 10.2174/1745017901814010196
118. Mossie, TB, Sibhatu, AK, Dargie, A, and Ayele, AD. Prevalence of antenatal depressive symptoms and associated factors among pregnant women in Maichew, North Ethiopia: an institution based study. Ethiop J Health Sci. (2017) 27:59–66. doi: 10.4314/ejhs.v27i1.8
119. Necho, M, Belete, A, and Zenebe, Y. The association of intimate partner violence with postpartum depression in women during their first month period of giving delivery in health centers at Dessie town, 2019. Ann General Psychiatry. (2020) 19:1–12. doi: 10.1186/s12991-020-00310-6
120. Tesfaye, Y, and Agenagnew, L. Antenatal depression and associated factors among pregnant women attending antenatal Care Service in Kochi Health Center, Jimma town, Ethiopia. J Pregnancy. (2021) 2021:1–10. doi: 10.1155/2021/5047432
121. Woldetensay, YK, Belachew, T, Tesfaye, M, Spielman, K, Biesalski, HK, Kantelhardt, EJ, et al. Validation of the patient health questionnaire (PHQ-9) as a screening tool for depression in pregnant women: Afaan Oromo version. PLoS One. (2018) 13:1–15. doi: 10.1371/journal.pone.0191782
122. Wubetu, AD, Engidaw, NA, and Gizachew, KD. Prevalence of postpartum depression and associated factors among postnatal care attendees in Debre Berhan, Ethiopia, 2018. BMC Pregnancy Childbirth. (2020) 20:189. doi: 10.1186/s12884-020-02873-4
123. Zelalem, ED, Asaye, MM, and Mihret, MS. Antenatal depression and its correlates on northwestern ethiopian women: community-based cross-sectional study. Pan Afr Med J. (2020) 36:1–13. doi: 10.11604/pamj.2020.36.239.19890
124. Chorwe-Sungani, G, and Chipps, J. Performance of the 3-item screener, the Edinburgh postnatal depression scale, the Hopkins symptoms checklist-15 and the self-reporting questionnaire and pregnancy risk questionnaire, in screening of depression in antenatal clinics in the Blantyre district. Malawi Med J. (2018) 30:184–90. doi: 10.4314/mmj.v30i3.10
125. Chorwe-Sungani, G, and Chipps, J. Validity and utility of instruments for screening of depression in women attending antenatal clinics in Blantyre district in Malawi. S Afr Fam Pract. (2018) 60:114–20. doi: 10.1080/20786190.2018.1432136
126. Chorwe-Sungani, G, and Chipps, J. A cross-sectional study of depression among women attending antenatal clinics in Blantyre district, Malawi. S Afr J Psychiatry. (2018) 24:1181. doi: 10.4102/sajpsychiatry.v24i0.1181
127. Harrington, BJ, Hosseinipour, MC, Maliwichi, M, Phulusa, J, Jumbe, A, Wallie, S, et al. Prevalence and incidence of probable perinatal depression among women enrolled in option B+ antenatal HIV care in Malawi. J Affect Disord. (2018) 239:115–22. doi: 10.1016/j.jad.2018.06.001
128. Harrington, BJ, Pence, BW, Maliwichi, M, Jumbe, AN, Gondwe, NA, Wallie, SD, et al. Probable antenatal depression at antiretroviral initiation and postpartum viral suppression and engagement in care. AIDS. (2018) 32:2827–33. doi: 10.1097/QAD.0000000000002025
129. Stewart, RC, Umar, E, Tomenson, B, and Creed, F. Validation of screening tools for antenatal depression in Malawi-A comparison of the Edinburgh postnatal depression scale and self reporting questionnaire. J Affect Disord. (2013) 150:1041–7. doi: 10.1016/j.jad.2013.05.036
130. Stewart, RC, Umar, E, Tomenson, B, and Creed, F. Validation of the multi-dimensional scale of perceived social support (MSPSS) and the relationship between social support, intimate partner violence and antenatal depression in Malawi. BMC Psychiatry. (2014) 14:1–11. doi: 10.1186/1471-244X-14-180
131. Zafar, S, Jean-Baptiste, R, Rahman, A, Neilson, JP, and Van Den Broek, NR. Non-life threatening maternal morbidity: Cross sectional surveys from Malawi and Pakistan. PLoS One. (2015) 10:e0138026. doi: 10.1371/journal.pone.0138026
132. Green, EP, Tuli, H, Kwobah, E, Menya, D, Chesire, I, and Schmidt, C. Disorders developing and validating a perinatal depression screening tool in Kenya blending Western criteria with local idioms: A mixed methods study. J Affect Disord. (2018) 228:49–59. doi: 10.1016/j.jad.2017.11.027
133. Kimbui, E, Kuria, M, Yator, O, and Kumar, M. A cross-sectional study of depression with comorbid substance use dependency in pregnant adolescents from an informal settlement of Nairobi: drawing implications for treatment and prevention work. Ann General Psychiatry. (2018) 17:53. doi: 10.1186/s12991-018-0222-2
134. Madeghe, BA, Kogi-Makau, W, Ngala, S, and Kumar, M. Nutritional factors associated with maternal depression among pregnant women in urban low-income settlements in Nairobi, Kenya. Food Nutr Bull. (2021) 42:334–46. doi: 10.1177/03795721211025123
135. Yator, O, Mathai, M, Vander Stoep, A, Rao, D, and Kumar, M. Risk factors for postpartum depression in women living with HIV attending prevention of mother-to-child transmission clinic at Kenyatta National Hospital, Nairobi. AIDS Care Psychol Socio Med Aspects AIDS/HIV. (2016) 28:884–9. doi: 10.1080/09540121.2016.1160026
136. Yator, O, Mathai, M, Albert, T, and Kumar, M. Burden of HIV-related stigma and post-partum depression: A Cross-sectional study of patients attending prevention of mother-to-child transmission Clinic at Kenyatta National Hospital in Nairobi. Front Psych. (2020) 11:532557. doi: 10.3389/fpsyt.2020.532557
137. Mahenge, B, Stöckl, H, Mizinduko, M, Mazalale, J, and Jahn, A. Adverse childhood experiences and intimate partner violence during pregnancy and their association to postpartum depression. J Affect Disord. (2017) 229:159–63. doi: 10.1016/j.jad.2017.12.036
138. Mwita, M, Kasongi, D, Bernard, E, Gunda, D, and Mmbaga, B. The magnitude and determinants of antepartum depression among women attending antenatal clinic at a tertiary hospital, in Mwanza Tanzania: A cross-sectional study. Pan Afr Med J. (2021) 38. doi: 10.11604/pamj.2021.38.258.27023
139. Yamamoto, SS, Premji, SS, Nyanza, EC, and Jahanpour, O. Investigating the association between stress. Anxiety and geophagy among pregnant women in Mwanza, Tanzania. Appetite. (2019) 142:104328. doi: 10.1016/j.appet.2019.104328
140. Atuhaire, C, Rukundo, GZ, Brennaman, L, Cumber, SN, and Nambozi, G. Lived experiences of Ugandan women who had recovered from a clinical diagnosis of postpartum depression: a phenomenological study. BMC Pregnancy Childbirth. (2021) 21:826. doi: 10.1186/s12884-021-04287-2
141. Kakyo, TA, Muliira, JK, Mbalinda, SN, Kizza, IB, and Muliira, RS. Factors associated with depressive symptoms among postpartum mothers in a rural district in Uganda. Midwifery. (2012) 28:374–9. doi: 10.1016/j.midw.2011.05.001
142. Umuziga, MP, Adejumo, O, and Hynie, M. Abstract: assessment of common perinatal mental disorders in a selected district Hospital of the Eastern Province in Rwanda. Rwanda J. (2015) 2:96. doi: 10.4314/rj.v2i2.34F
143. Atkins, DL, Cumbe, VFJ, Muanido, A, Manaca, N, Fumo, H, Chiruca, P, et al. Validity and item response theory properties of the alcohol use disorders identification test for primary care alcohol use screening in Mozambique (AUDIT-MZ). J Subst Abus Treat. (2021) 127:108441. doi: 10.1016/j.jsat.2021.108441
144. Cumbe, VFJ, Muanido, A, Manaca, MN, Fumo, H, Chiruca, P, Hicks, L, et al. Validity and item response theory properties of the patient health Questionnaire-9 for primary care depression screening in Mozambique (PHQ-9-MZ). BMC Psychiatry. (2020) 20:1–15. doi: 10.1186/s12888-020-02772-0
145. Brittain, K, Mellins, CA, Phillips, T, Zerbe, A, Abrams, EJ, Myer, L, et al. Social support, stigma and antenatal depression among HIV-infected pregnant women in South Africa. AIDS Behav. (2017) 21:274–82. doi: 10.1007/s10461-016-1389-7
146. MacGinty, RP, Kariuki, SM, Barnett, W, Wedderburn, CJ, Hardy, A, Hoffman, N, et al. Associations of antenatal maternal psychological distress with infant birth and development outcomes: results from a south African birth cohort. Compr Psychiatry. (2020) 96:152128. doi: 10.1016/j.comppsych.2019.152128
147. Mokwena, K, and Masike, I. The need for universal screening for postnatal depression in South Africa: confirmation from a sub-district in pretoria, South Africa. Int J Environ Res Public Health. (2020) 17:1–13. doi: 10.3390/ijerph17196980
148. Stellenberg, EL, and Abrahams, JM. Prevalence of and factors influencing postnatal depression in a rural community in South Africa. Afr J prim health care. Fam Med. (2015) 7:874. doi: 10.4102/phcfm.v7i1.874
149. van Heyningen, T, Honikman, S, Myer, L, Onah, MN, Field, S, and Tomlinson, M. Prevalence and predictors of anxiety disorders amongst low-income pregnant women in urban South Africa: a cross-sectional study. Arch Womens Ment Health. (2017) 20:765–75. doi: 10.1007/s00737-017-0768-z
150. Mandell, LN, Parrish, MS, Rodriguez, VJ, Alcaide, ML, Weiss, SM, Peltzer, K, et al. Blood pressure, depression, and suicidal ideation among pregnant women with HIV. AIDS Behav. (2022) 26:1289–98. doi: 10.1007/s10461-021-03486-4
151. Nydoo, P, Naicker, T, and Moodley, J. Depressive scores in newly diagnosed HIV-infected and HIV-uninfected pregnant women. S Afr J Psychiatry. (2017) 23:1–6. doi: 10.4102/sajpsychiatry.v23i0.1085
152. Wong, M, Myer, L, Zerbe, A, Phillips, T, Petro, G, Mellins, CA, et al. Depression, alcohol use, and stigma in younger versus older HIV-infected pregnant women initiating antiretroviral therapy in Cape Town, South Africa. Arch Womens Ment Health. (2017) 20:149–59. doi: 10.1007/s00737-016-0688-3
153. Roberts, KJ, Smith, C, Cluver, L, Toska, E, Zhou, S, Boyes, M, et al. Adolescent motherhood and HIV in South Africa: examining prevalence of common mental disorder. AIDS Behav. (2022) 26:1197–210. doi: 10.1007/s10461-021-03474-8
154. Peltzer, K, and Shikwane, ME. Prevalence of postnatal depression and associated factors among HIV-positive women in primary care in Nkangala District, South Africa. South Afr J HIV Med. (2011) 12:24–8. doi: 10.4102/sajhivmed.v12i4.168
155. Malemela, RD, and Mashegoane, S. The prevalence of obsessive-compulsive disorder symptoms and their psychological correlates amongst pregnant clinic attendees in the Capricorn district. Afr J Reprod Health. (2019) 23:44–55. doi: 10.29063/ajrh2019/v23i2.5
156. Phukuta, NSJ, and Omole, OB. Prevalence and risk factors associated with postnatal depression in a south African primary care facility. Afr J Prim Health Care Fam Med. (2020) 12:2538. doi: 10.4102/phcfm.v12i1.2538
157. Abrahams, Z, Schneider, M, Field, S, and Honikman, S. Validation of a brief mental health screening tool for pregnant women in a low socio-economic setting. BMC Psychol. (2019) 7:1–11. doi: 10.1186/s40359-019-0355-3
158. Brittain, K, Myer, L, Koen, N, Koopowitz, S, Donald, KA, Barnett, W, et al. Risk factors for antenatal depression and associations with infant birth outcomes: results from a south african birth cohort study. Paediatr Perinat Epidemiol. (2015) 29:505–14. doi: 10.1111/ppe.12216
159. Brittain, K, Mellins, CA, Remien, RH, Phillips, T, Zerbe, A, Abrams, EJ, et al. HIV-status disclosure and depression in the context of unintended pregnancy among south African women. Glob Public Health. (2019) 14:1087–97. doi: 10.1080/17441692.2018.1560485
160. Van Heyningen, T, Honikman, S, Tomlinson, M, Field, S, and Myer, L. Comparison of mental health screening tools for detecting antenatal depression and anxiety disorders in south African women. PLoS One. (2018) 13:1–18. doi: 10.1371/journal.pone.0193697
161. Peltzer, K, Rodriguez, VJ, Lee, TK, and Jones, D. Prevalence of prenatal and postpartum depression and associated factors among HIV-infected women in public primary care in rural South Africa: A longitudinal study. AIDS Care Psychol Socio Med Aspects AIDS/HIV. (2018) 30:1372–9. doi: 10.1080/09540121.2018.1455960
162. Rochat, TJ, Tomlinson, M, Newell, ML, and Stein, A. Detection of antenatal depression in rural HIV-affected populations with short and ultrashort versions of the Edinburgh postnatal depression scale (EPDS). Arch Womens Ment Health. (2013) 16:401–10. doi: 10.1007/s00737-013-0353-z
163. Sorsdahl, K, Petersen Williams, P, Everett-Murphy, K, Vythilingum, B, de Villiers, P, Myers, B, et al. Feasibility and preliminary responses to a screening and brief intervention program for maternal mental disorders within the context of primary care. Community Ment Health J. (2015) 51:962–9. doi: 10.1007/s10597-015-9853-9
164. Spedding, M, Stein, DJ, Naledi, T, Myers, B, Cuijpers, P, and Sorsdahl, KR. A task-sharing intervention for prepartum common mental disorders: feasibility, acceptability and responses in a south African sample. Afr J Prim Health Care Fam Med. (2020) 12:2071–928. doi: 10.4102/phcfm.v12i1.2378
165. Tuthill, EL, Pellowski, JA, Young, SL, and Butler, LM. Perinatal depression among HIV-infected women in KwaZulu-Natal South Africa: prenatal depression predicts lower rates of exclusive breastfeeding. AIDS Behav. (2017) 21:1691–8. doi: 10.1007/s10461-016-1557-9
166. Baron, E, Field, S, Kafaar, Z, and Honikman, S. Patterns of use of a maternal mental health service in a low-resource antenatal setting in South Africa. Health Soc Care Community. (2015) 23:502–12. doi: 10.1111/hsc.12167
167. Bernstein, M, Phillips, T, Zerbe, A, McIntyre, JA, Brittain, K, Petro, G, et al. Intimate partner violence experienced by HIV-infected pregnant women in South Africa: A cross-sectional study. BMJ Open. (2016) 6:e011999–8. doi: 10.1136/bmjopen-2016-011999
168. van Heyningen, T, Myer, L, Tomlinson, M, Field, S, and Honikman, S. The development of an ultra-short, maternal mental health screening tool in South Africa. Global Mental Health. (2019) 6:e24–4. doi: 10.1017/gmh.2019.21
169. Mokhele, I, Nattey, C, Jinga, N, Mongwenyana, C, Fox, MP, and Onoya, D. Prevalence and predictors of postpartum depression by HIV status and timing of HIV diagnosis in Gauteng, South Africa. PLoS One. (2019) 14:1–15. doi: 10.1371/journal.pone.0214849
170. Peltzer, K, Rodriguez, VJ, and Jones, D. Prevalence of prenatal depression and associated factors among hiv-positive women in primary care in Mpumalanga province. South Africa. Sahara J. (2016) 13:60–7. doi: 10.1080/17290376.2016.1189847
171. Rochat, TJ, Tomlinson, M, Bärnighausen, T, Newell, ML, and Stein, A. The prevalence and clinical presentation of antenatal depression in rural South Africa. J Affect Disord. (2011) 135:362–73. doi: 10.1016/j.jad.2011.08.011
172. Garman, EC, Schneider, M, and Lund, C. Perinatal depressive symptoms among low-income south African women at risk of depression: trajectories and predictors. BMC Pregnancy Childbirth. (2019) 19:1–11. doi: 10.1186/s12884-019-2355-y
173. January, J, and Chimbari, MJ. Prevalence and factors associated with postnatal depression among women in two rural districts of Manicaland, Zimbabwe. S Afr J Psychiatry. (2018) 24:24. doi: 10.4102/sajpsychiatry.v24i0.1176
174. Mebrahtu, H, Simms, V, Chingono, R, Mupambireyi, Z, Weiss, HA, Ndlovu, P, et al. Postpartum maternal mental health is associated with cognitive development of HIV-exposed infants in Zimbabwe: a cross-sectional study. AIDS Care Psychol Socio Med Aspects AIDS/HIV. (2018) 30:74–82. doi: 10.1080/09540121.2018.1468015
175. Nyamukoho, E, Mangezi, W, Marimbe, B, Verhey, R, and Chibanda, D. Depression among HIV positive pregnant women in Zimbabwe: A primary health care based cross-sectional study. BMC Pregnancy Childbirth. (2019) 19:53. doi: 10.1186/s12884-019-2193-y
176. January, J, Chivanhu, H, Chiwara, J, Denga, T, Dera, K, Dube, T, et al. Prevalence and the correlates of postnatal depression in an urban high density suburb of Harare. Cent Afr J Med. (2015) 61:1–4.
177. Chibanda, D, Shetty, AK, Tshimanga, M, Woelk, G, Stranix-Chibanda, L, and Rusakaniko, S. Group problem-solving therapy for postnatal depression among HIV-positive and HIV-negative mothers in Zimbabwe. J Int Assoc Provid AIDS Care. (2014) 13:335–41. doi: 10.1177/2325957413495564
178. Dlamini, LP, Mahanya, S, Dlamini, SD, and Shongwe, MC. Prevalence and factors associated with postpartum depression at a primary healthcare facility in Eswatini. S Afr J Psychiatry. (2019) 25:1–7. doi: 10.4102/sajpsychiatry.v25i0.1404
179. Bakare, M, Bello-Mojeed, M, Munir, K, Duduyemi, O, Orovwigho, A, Odetunde, O, et al. Improving access to interventions among mothers screened positive for post-partum depression (PPD) at National Programme on immunization (NPI) clinics in south-western and South-Eastern Nigeria – A service development report. Matters (Zur). (2017) 2017:5. doi: 10.19185/matters.201707000005
180. Odinka, JI, Nwoke, M, Chukwuorji, JC, Egbuagu, K, Mefoh, P, Odinka, PC, et al. Post-partum depression, anxiety and marital satisfaction: A perspective from southeastern Nigeria. S Afr J Psychiatry. (2018) 24:1–8. doi: 10.4102/sajpsychiatry.v24i0.1109
181. Tungchama, FP, Umar, MU, Maigari, TY, Davou, FJ, Armiyau, AY, Goar, S, et al. The role of infant’s gender and other psycho-ocial variables on women with postpartum depression in Jos, Nigeria. Jos J Med. (2017) 10:20–8.
182. Ukaegbe, C, Iteke, O, Bakare, M, and EMJ, AA. Undefined. Postpartum depression among Igbo women in an urban Mission hospital south East Nigeria. Ebonyi Med J. (2012, 2012) 11:1–2.
183. Okeke, EN. Money and my mind: maternal cash transfers and mental health. Health Econ. (2021) 30:2879–904. doi: 10.1002/hec.4398
184. Adeyemo, EO, Oluwole, EO, Kanma-Okafor, OJ, Izuka, OM, and Odeyemi, KA. Prevalence and predictors of postpartum depression among postnatal women in Lagos, Nigeria. Afr Health Sci. (2020) 20:1943–54. doi: 10.4314/ahs.v20i4.53
185. Gureje, O, Oladeji, BD, Montgomery, AA, Bello, T, Kola, L, Ojagbemi, A, et al. Articles effect of a stepped-care intervention delivered by lay health workers on major depressive disorder among primary care patients in Nigeria (STEPCARE): a cluster-randomised controlled trial. Lancet Glob Health. (2019) 7:e951–60. doi: 10.1016/S2214-109X(19)30148-2
186. Gureje, O, Oladeji, BD, Montgomery, AA, Araya, R, Bello, T, Chisholm, D, et al. High- versus low-intensity interventions for perinatal depression delivered by non-specialist primary maternal care providers in Nigeria: cluster randomised controlled trial (the EXPONATE trial). Br J Psychiatry. (2019) 215:528–35. doi: 10.1192/bjp.2019.4
187. Ikeako, LC, Iteke, OC, Ezegwi, HU, and Okeke, T. Prevalence and correlates of postpartum depression among women visiting postnatal clinic in a tertiary health institution in Southeast Nigeria. Orient J Med. (2018) 30:43–51.
188. Adebowale, O, and James, B. The association between intimate partner violence, psychiatric morbidity amongst pregnant women and partner alcohol use in southern Nigeria. Afr J Prim Health Care Fam Med. (2020) 12:1–7. doi: 10.4102/phcfm.v12i1.2226
189. Adekanle, DA, and Adebayo, KO. AOFTJ of, 2015 undefined. Psychiatric morbidity among pregnant women with hypertensive disorders – a comparative study. Trop J Obstet Gynaecol. (2015) 32:90–5.
190. Oladeji, BD, Bello, T, Ayinde, O, Idowu, P, and Gureje, O. Prevalence and correlates of depression among pregnant adolescents in primary maternal care in Nigeria. Arch Womens Ment Health. (2022) 25:441–50. doi: 10.1007/s00737-021-01198-1
191. Acheampong, K, Pan, X, Kaminga, AC, Wen, SW, and Liu, A. Risk of adverse maternal outcomes associated with prenatal exposure to moderate-severe depression compared with mild depression: A fellow-up study. J Psychiatr Res. (2021) 136:32–8. doi: 10.1016/j.jpsychires.2021.01.036
192. Kugbey, N, Ayanore, M, Doegah, P, Chirwa, M, Bartels, SA, Davison, CM, et al. Prevalence and correlates of prenatal depression, anxiety and suicidal behaviurs in the Volta region of Ghana. Int J Environ Res Public Health. (2021) 18:1–13. doi: 10.3390/ijerph18115857
193. Wemakor, A, and Mensah, KA. Association between maternal depression and child stunting in northern Ghana: A cross-sectional study. BMC Public Health. (2016) 16:1–7. doi: 10.1186/s12889-016-3558-z
194. Emerson, JA, Caulfield, LE, Kishimata, EM, Nzanzu, JP, and Doocy, S. Mental health symptoms and their relations with dietary diversity and nutritional status among mothers of young children in eastern Democratic Republic of the Congo. BMC Public Health. (2020) 20:1–9. doi: 10.1186/s12889-019-8092-3
195. Eandjafono, DOL, Essam, BI, ANS, M, Omba, AN, and Mbuyi, TK. Maternal affectivity during pregnancy, motherchild relationship, infant’s health and development in Kinshasa. Pan Afr Med J. (2020) 36:1–15. doi: 10.11604/pamj.2020.36.203.18294
196. Agler, RA, Zivich, PN, Kawende, B, Behets, F, and Yotebieng, M. Postpartum depressive symptoms following implementation of the 10 steps to successful breastfeeding program in Kinshasa, Democratic Republic of Congo: A cohort study. PLoS Med. (2021) 18:e1003465. doi: 10.1371/journal.pmed.1003465
197. Mahenge, B, Likindikoki, S, Stöckl, H, and Mbwambo, J. Intimate partner violence during pregnancy and associated mental health symptoms among pregnant women in Tanzania: A cross-sectional study. BJOG. (2013) 120:940–7. doi: 10.1111/1471-0528.12185
198. Mahenge, B, Stöckl, H, Likindikoki, S, Kaaya, S, and Mbwambo, J. The prevalence of mental health morbidity and its associated factors among women attending a prenatal clinic in Tanzania. Int J Gynecol Obstet. (2015) 130:261–5. doi: 10.1016/j.ijgo.2015.04.032
199. Cox, JL, Holden, JM, and Sagovsky, R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
200. Russell, PSS, Chikkala, SM, Earnest, R, Viswanathan, SA, Russell, S, and Mammen, PM. Diagnostic accuracy and clinical utility of non-English versions of Edinburgh post-Natal depression scale for screening post-natal depression in India: A meta-analysis. World J Psychiatry. (2020) 10:71–80. doi: 10.5498/wjp.v10.i4.71
201. Tran, TD, Tran, T, La, B, Lee, D, Rosenthal, D, and Fisher, J. Screening for perinatal common mental disorders in women in the north of Vietnam: A comparison of three psychometric instruments. J Affect Disord. (2011) 133:281–93. doi: 10.1016/j.jad.2011.03.038
202. Murray, D, Psych, MRC, and Ma, JLC. Screening for depression during pregnancy with the Edinburgh depression scale (EDDS). J Reprod Infant Psychol. (2013) 8:99–107. doi: 10.1080/02646839008403615
203. Weobong, B, Akpalu, B, Doku, V, Owusu-Agyei, S, Hurt, L, Kirkwood, B, et al. The comparative validity of screening scales for postnatal common mental disorder in Kintampo, Ghana. J Affect Disord. (2009) 113:109–17. doi: 10.1016/j.jad.2008.05.009
204. Husain, N, Chaudhry, N, Rhouma, A, Sumra, A, Tomenson, B, and Waheed, W. Validation of the self-reporting questionnaire (SRQ 20) in British Pakistani and white European population in the United Kingdom. J Affect Disord. (2016) 189:392–6. doi: 10.1016/j.jad.2015.08.068
205. De, GAM, Cuijpers, P, Leeflang, M, Sferra, I, Uppendahl, JR, and De Vries, R. A systematic review and meta-analysis of diagnostic test accuracy studies of self-report screening instruments for common mental disorders in Arabic-speaking adults. Global Mental Health. (2021) 8:1–18. doi: 10.1017/gmh.2021.39
206. Maldonado, BN, Chandna, J, and Gladstone, M. A systematic review of tools used to screen and assess for externalising behaviour symptoms in low and middle income settings. Global Mental Health. (2019) 6:E13. doi: 10.1017/gmh.2019.11
207. Chandra, M, and Chandra, BR. Developing and translating screening tools for CMD in older adults in South Asia: cultural and contextual aspects. Int Psychogeriatr. (2022) 34:407–9. doi: 10.1017/S1041610221000387
Keywords: screening tools, diagnostic tools, maternal mental health, mental disorders, mental conditions, pregnant women, postpartum women, primary care
Citation: Gyimah L, Agyepong IA, Owiredu D, Awini E, Yevoo LL, Ashinyo ME, Aye SGEV, Abbas S, Cronin de Chavez A, Mirzoev T and Danso-Appiah A (2024) Tools for screening maternal mental health conditions in primary care settings in sub-Saharan Africa: systematic review. Front. Public Health. 12:1321689. doi: 10.3389/fpubh.2024.1321689
Edited by:
Wulf Rössler, Charité University Medicine Berlin, GermanyReviewed by:
Suresh Munuswamy, Assam Kaziranga University, IndiaAshlesha Bagadia, The Green Oak Initiative, India
Darpan Kaur, Mahatma Gandhi Missions Medical College and Hospital, India
Copyright © 2024 Gyimah, Agyepong, Owiredu, Awini, Yevoo, Ashinyo, Aye, Abbas, Cronin de Chavez, Mirzoev and Danso-Appiah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony Danso-Appiah, YWRhbnNvLWFwcGlhaEB1Zy5lZHUuZ2g=; dGRhcHBpYWhAeWFob28uY28udWs=
 Leveana Gyimah
Leveana Gyimah Irene Akua Agyepong
Irene Akua Agyepong David Owiredu
David Owiredu Elizabeth Awini3
Elizabeth Awini3 Mary Eyram Ashinyo
Mary Eyram Ashinyo Shazra Abbas
Shazra Abbas Tolib Mirzoev
Tolib Mirzoev Anthony Danso-Appiah
Anthony Danso-Appiah