- 1Department of Children Health Care, Dongguan Children's Hospital, Dongguan, Guangdong, China
- 2Department of Occupational and Environmental Health, Guangdong Provincial Engineering Technology Research Center of Environmental Pollution and Health Risk Assessment, School of Public Health, Sun Yat-sen University, Guangzhou, Guangdong, China
- 3The First Affiliated Hospital, University of Science and Technology of China, Hefei, Anhui, China
- 4Key Laboratory of Brain, Cognition and Education Sciences, Ministry of Education; Institute for Brain Research and Rehabilitation, and Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou, Guangdong, China
- 5Department of Maternal and Child Health, Research Center of Children and Adolescent Psychological and Behavioral Development, School of Public Health, Sun Yat-sen University, Guangzhou, Guangdong, China
- 6JNU-HKUST Joint Laboratory for Neuroscience and Innovative Drug Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, China
Objective: To investigate the relationship between maternal folic acid (FA) supplementation during the pre-conceptional and prenatal periods and the subsequent risk of autism spectrum disorder (ASD) in offspring.
Methods: A total of 6,049 toddlers aged 16–30 months were recruited from August 2016 to March 2017 for this cross-sectional study conducted in China. The parents of the enrolled toddlers provided information on maternal supplemental FA, socio-demographic information, and related covariates. Standard diagnostic procedures were implemented to identify toddlers with ASD.
Results: Among the 6,049 children included in the study, consisting of 3,364 boys with an average age of 22.7 ± 4.1 months, a total of 71 children (1.2%) were diagnosed with ASD. Mothers who did not consume FA supplements during the prenatal period were found to have a significantly increased risk of having offspring with ASD, in comparison to those who were exposed to FA supplements (odds ratio [OR] = 2.47). However, we did not find a similar association during the pre-conceptional period. Compared to mothers who consistently used FA supplements from pre-conception to the prenatal period, those who never used FA supplements were statistically significantly associated with a higher risk of ASD in their offspring (OR = 2.88).
Conclusion: This study indicated that providing continuous maternal FA supplementation during the pre-conceptional and prenatal periods may decrease the risk of ASD in offspring. The prenatal period is considered to be the most crucial time for intervention.
1 Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social communication challenges and restricted, repetitive patterns of behaviors, interests, or activities (1). The multifactorial theory of ASD risk has been widely accepted (2), involving the jointly mediated role of environmental factors and genetic predispositions (3, 4). Environmental factors may trigger predisposing hereditary high-risk gene modifications during fetal development through epigenetic mechanisms (3, 5). Emerging evidence suggests that folate, as a modifiable risk factor, may play an important role in the etiology of ASD (6, 7).
Folate, one water-soluble substance belonging to the vitamin B family, participates in various important reactions, such as DNA methylation and replication, during multiple physiological processes (6). Cell folate, as one of the methyl donors, exerts its influence on the developing brain through the synthesis of DNA, neurotransmitters, and myelination (6). Mechanism-related studies have revealed that folate might provide preventive effects for ASD and other neurological diseases, such as neural tube defects (NTDs) (6). Epidemiological studies have already investigated the potential causal association between folate intake and the onset of ASD in offspring, but the results have been with conflicting (8–19) (See Supplementary Table 1). One recent meta-analysis study, which included a total of 10 studies with 23 sub-studies (9,795 ASD cases), found that supplementing with FA during early pregnancy was associated with a lower risk of ASD in offspring (OR = 0.57, 95% CI: 0.41–0.78), while consuming a daily amount of at least 400 μg from dietary sources and supplements was also associated with a reduced risk of offspring ASD (OR = 0.55, 95% CI: 0.36–0.83) (4). However, the results showed high heterogeneity, and meta-regression analyses indicated that countries and supplementary timings may be significant source of heterogeneity. Most of the aforementioned studies were conducted in Western countries, while few studies were performed in China, yielding mixed results (16, 19). A case–control study from China showed that children born to mothers who did not use FA supplementation had an increased risk (1.905, 95%CI, 1.238–2.933) compared to those who used 400 μg FA daily for a duration of 24 weeks [i.e., starting 12 weeks before the last menstrual period (LMP) and continuing for 12 weeks after the LMP] (19). However, Li et al. (16) found no association between FA supplementation during pregnancy preparation, pregnancy or lactation, and the risk of offspring ASD. These findings indicate that the timing of the FA supplement may affect the associations between FA and the risk of ASD in offspring, which is still unclear.
Given this background, we conducted a nationwide cross-sectional study to distinguishing the timing of FA supplementation during the pre-conceptional and prenatal period. Our aim is to investigate the associations between different timing of FA supplementation and the odds of ASD in Chinese toddlers.
2 Methods
2.1 Study population and overall design
We used a cross-sectional study design. From August 2016 to March 2017, we conducted a national validation study of the Chinese version of the Modified Checklist for Autism in Toddlers, Revised with Follow-Up (M-CHAT-R/F) in China. We implemented a convenient cluster-sampling strategy and gathered the sample from seven cities in six provinces across five geographical regions of China. The cities included Beijing City (Northern region), Chongqing City, and Guiyang City in Guizhou Province (Western region), Guangzhou City, and Foshan City in Guangdong Province (Southern region), Wuhan City in Hubei Province (Central region), and Hangzhou City in Zhejiang Province (Eastern region). In each sampling site, we recruited study samples from a variety of sources, including hospital-based, community-based, and school-based populations. Finally, we recruited a total of 7,928 caregivers who agreed to participate in the validation study, along with their children aged 16–30 months. The participants were selected from seven tertiary hospitals, 21 communities, and seven kindergartens. At the same time as the validation study, an additional survey was conducted for all 7,928 caregivers of the toddlers, of which 6,049 agreed to complete questionnaires (The derivation of the study sample was shown in Supplementary Figure 1). The demographic characteristics of participants who completed or did not complete the questionnaires were compared in Supplementary Table 2. All the 6,049 mothers and their children from our validation study were included in this study. The further details of the study could be referred from a published article (20).
We followed the reporting guideline for cross-sectional studies according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (21). We have obtained the ethical approval from the Ethical Review Committee for Biomedical Research at Sun Yat-sen University. All participants provided written informed consent, which informed them of the purpose of the study.
2.2 Procedure of ASD screening and diagnosis
We adopted a two-stage process to confirm the ASD diagnosis of the included population. In the first stage, caregivers completed the M-CHAT-R/F during the well-child visit. The M-CHAT-R/F consists of 20 questions regarding the presence or absence of symptoms related to autism. A total score ranging from 0 to 2 indicates a low risk. Children who failed three out of the 20 items in the M-CHAT-R were considered at risk for autism (22). In the second stage, children who screened positive on M-CHAT-R/F or whose caregiver or physician subsequently expressed concern were referred by their physician for a diagnostic evaluation. Diagnostic evaluations were conducted, which included face-to-face assessments using the Childhood Autism Rating Scale (23), as well as 30 min of parent interviews and interactions with toddlers. These evaluations were carried out by licensed child psychiatrists and trained psychologists. The final diagnosis was made based on a 30-min interaction and the criteria outlined in the Statistical Manual of Mental Disorders, Fifth Edition (1).
2.3 Assessment of FA supplementation
Folic acid supplement consumption during the pre-conceptional and prenatal periods was reported by caregivers. We defined FA supplementation during the pre-conceptional period as taking FA supplements for at least 3 months before becoming aware of pregnancy. The answer was a binary variable encoded as 0 for “no” and 1 for “yes.” FA supplementation during the prenatal period was defined and coded in a similar way.
We further categorized the mothers into three groups: continuously FA users, who took FA supplements during both the pre-conceptional and prenatal periods; ever FA users, who took FA supplements during either the pre-conceptional or prenatal period; and FA nonusers, who never took FA supplements. The details of three groups were shown in Supplementary Figure 2.
2.4 Statistical analysis
T-tests were used to compare the characteristics of continuous variables, while chi-square analysis was performed to compare the characteristics of categorical variables between children with and without ASD. We estimated the odds ratios (ORs) and 95% confidence intervals (CI) of ASD based on the maternal FA supplementation status using multivariable logistic regression. We first adjusted for child age, child sex, whether there was only one child, maternal age, maternal education level, annual household income, research area, and ethnic background. In model 2, we further adjusted for gestational age, complications during pregnancy, depressive symptoms during pregnancy, secondhand smoke exposure during pregnancy, and pre-pregnancy overweight/obesity. All covariates were selected based on their association with ASD or the use of FA supplements (24, 25).
We conducted several sensitivity analyses: (1) we re-ran the models using Firth’s bias-reduced logistic regression (26). Firth’s bias reduction method corrects bias in small-sample logistic regression by adding a penalty term to the maximum likelihood estimation. In situations with limited events or rare outcomes, traditional methods yield imprecise and biased results. Firth’s approach introduces a profiled Jeffreys prior as a penalty, stabilizing estimation and mitigating small-sample bias. This method improves parameter accuracy by adjusting the likelihood function, making it particularly valuable when dealing with sparse data or infrequent events (27). (2) We repeated the primary analysis separately for male offspring, full-term offspring, offspring of mothers without pregnancy complications, and offspring of mothers without overweight/obesity prior to pregnancy. We did not conduct stratified analyses because the number of ASD cases was small.
Statistical analyses were conducted using the statistical software R, version 3.6.1. All tests conducted were bilateral tests, and a p value of less than 0.05 was considered statistically significant.
3 Results
3.1 Demographic characteristics and maternal risk factors during pregnancy
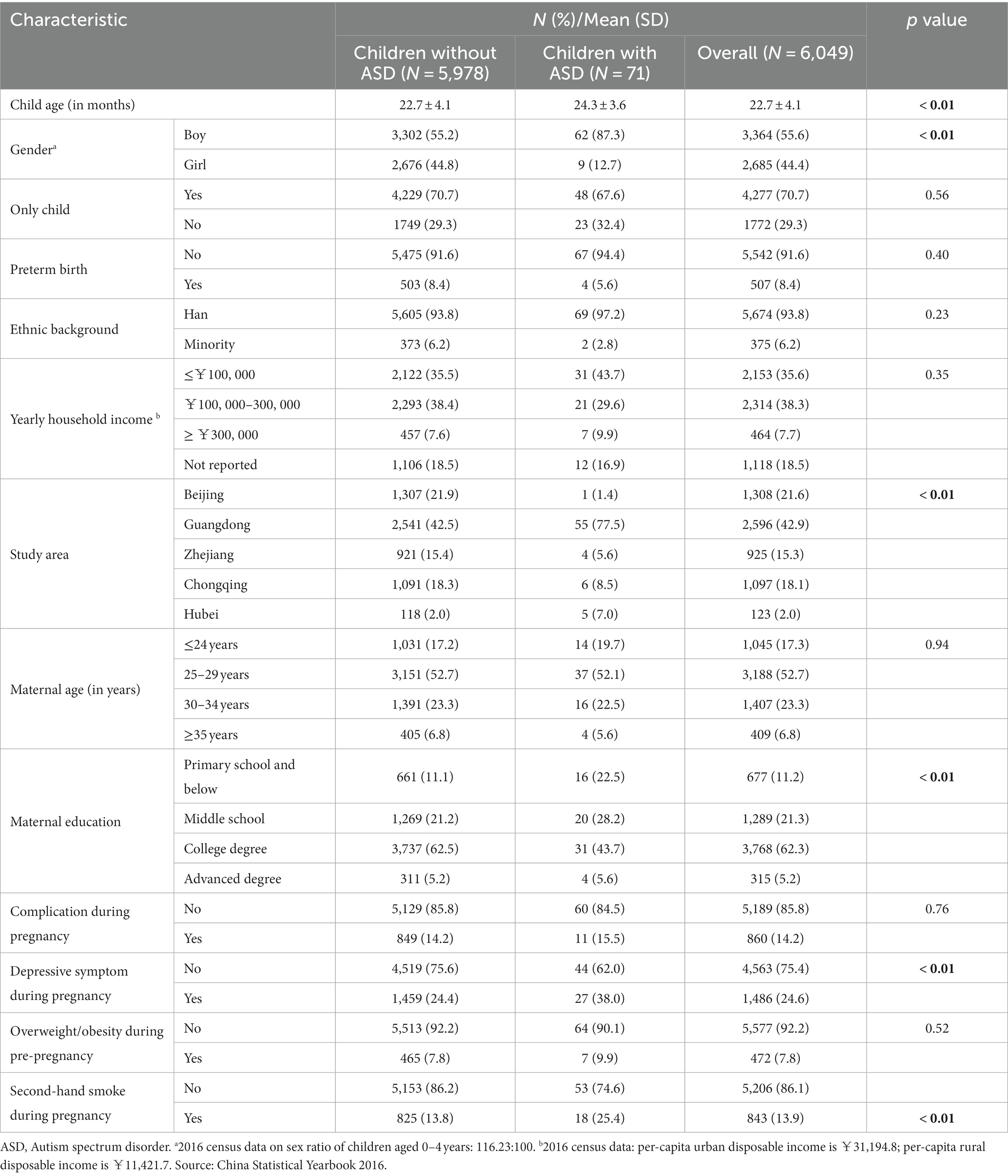
The mean age of the children at the time of screening was 22.7 months (range 16–30 moths), and there were slightly more boys than girls (55.6 vs. 44.4%), as shown in Table 1. Additionally, 70.7% of the children were the only child in their family. Most of them were full-term babies (91.6%) and of Han nationality (93.8%). The mean age of the mothers was 30.4 ± 4.1 years. Most of the mothers were well-educated, with 67.5% having at least an undergraduate university degree. Few mothers reported complications during pregnancy (14.2%), obesity/overweight before pregnancy (7.8%), or second-hand smoke exposure during pregnancy (13.9%). About three-quarters of mothers did not feel depressed during pregnancy. We identified 71 cases of ASD in our survey study sample, which accounted for 1.2% of the total. Male gender, lower levels of maternal education, depressive symptoms during pregnancy, and exposure to second-hand smoke were more frequently observed in cases of ASD in this study (Table 1).
3.2 Prevalence of ASD associated with FA supplements
Figure 1 showed the prevalence of ASD with different timing of FA supplementation. The prevalence of ASD would be higher if mothers did not take FA supplements during the pre-conceptional and prenatal periods (For specific values, see Supplementary Table 3). From the pre-conceptional to prenatal period, the prevalence of ASD in offspring was lowest in mothers who continuously used FA supplements, followed by those who had ever used them. For mothers who did not take FA supplements, the prevalence of their children was highest.
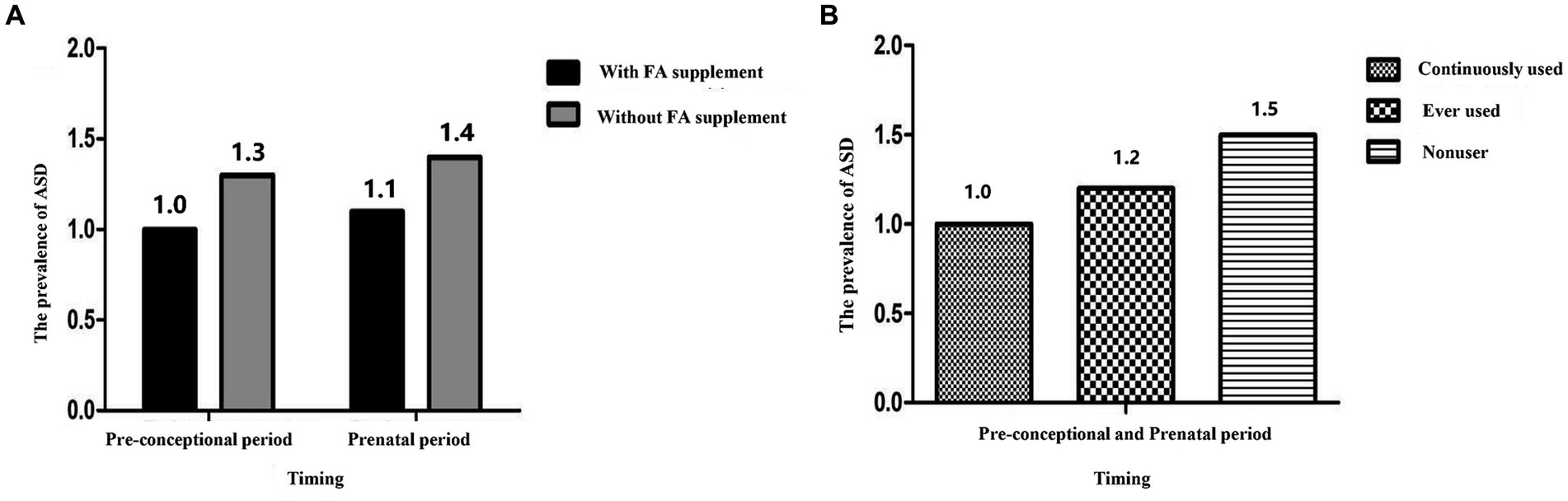
Figure 1. The prevalence of ASD with different timing of FA supplement. (A) The prevalence of ASD with FA supplement during the pre-conceptional and prenatal period; (B) the prevalence of ASD with continuously used, ever used, nonuser. FA, Folic acid; ASD, Autism spectrum disorder.
3.3 FA supplements and the risk of ASD
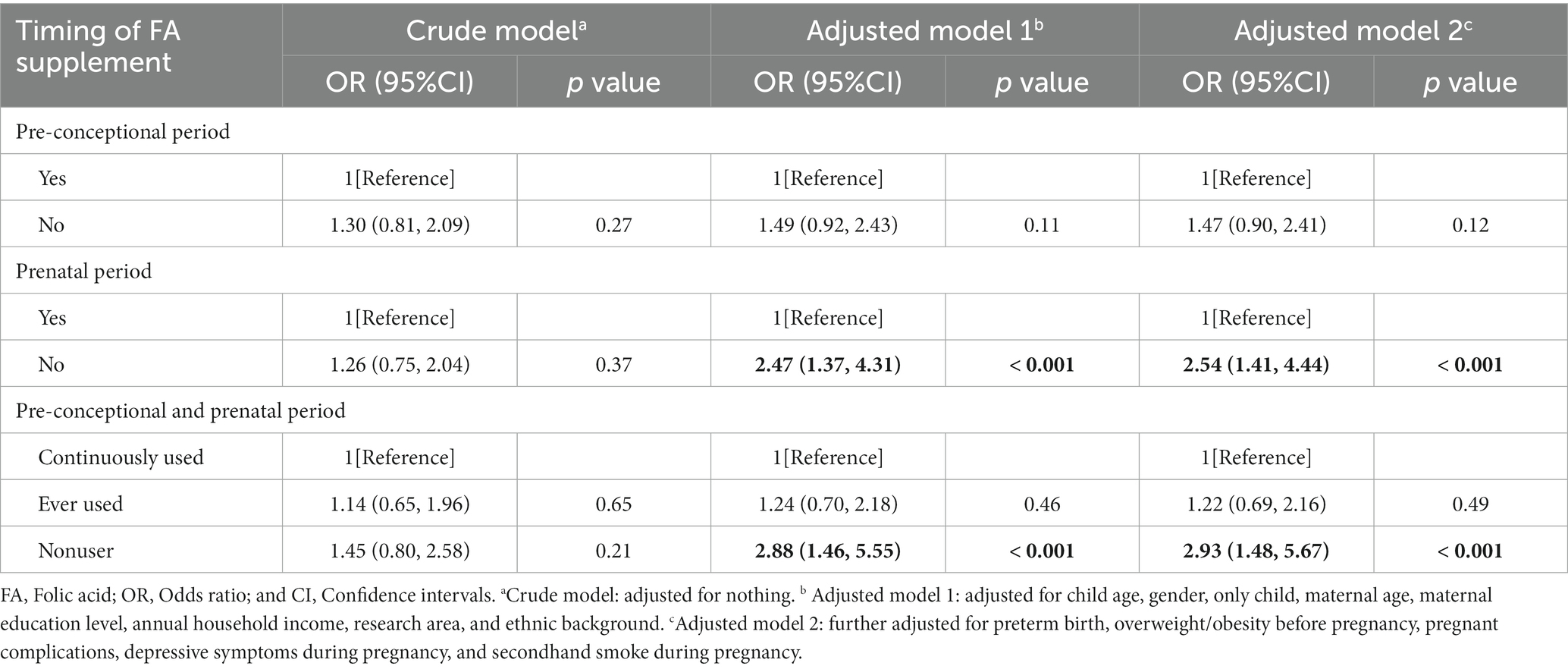
We assessed the potential association between FA supplementation and the development of ASD in various time. During the pre-conceptional period, there appeared to be no detectable association between FA supplementation and the risk of offspring ASD, as indicated by both the crude model and the adjusted model (Table 2). However, during the prenatal period, the adjusted odds ratio (OR) was 2.47 (95% CI, 1.37–4.31) and 2.54 (95% CI, 1.41–4.44) after adjusting for covariates in model 1 and model 2, respectively (Table 2). We further grouped maternal FA supplements into three categories: continuously used, ever used, and nonuser. Compared to the continuously used group, the nonuser group had a higher risk of ASD in the offspring (adjusted OR was estimated at 2.88 [95% CI, 1.46–5.55] and 2.93 [95% CI, 1.48–5.67] in model 1 and model 2, respectively). Interestingly, the risk of offspring ASD was not significant when mothers took FA supplements either during the pre-conceptional or prenatal period. Even after adjustment, the adjusted OR of ASD in the ever-used group compared with the continuously used group was estimated at 1.24 (95% CI, 0.70–2.18) and 1.22 (95% CI, 0.69–2.16) in model 1 and model 2.
3.4 Sensitivity analyses
Considering the small number of ASD cases in our study, we re-run the models using Firth’s bias reduction method. The results were consistent with the primary analysis (see Supplementary Table 4). We also conducted a separate analysis focusing on male offspring, full-term offspring, offspring of mothers without pregnancy complications, and offspring of mothers without overweight/obesity before pregnancy (see Supplementary Tables 5–8). The results were consistent with previous findings, suggesting that maternal exposure to FA supplements during pregnancy was significantly associated with a reduced risk of ASD.
4 Discussion
In the nationwide cross-sectional study conducted in China, we found that mothers who did not take FA supplements during the prenatal period had a higher risk of having offspring with ASD compared to those who did take FA supplements. However, we did not observe any significant association during the pre-conceptional period. However, mothers without FA supplements during both pre-conceptional and prenatal periods showed higher risks of having offspring with ASD compared to those who took continuous FA supplements.
This study indicated that maternal FA supplementation during the pre-conceptional and prenatal periods might have a protective effect on ASD in offspring. Additionally, the prenatal period might be the more critical time window for supplementation. Our results were consistent with several previous studies that found a protective role of prenatal FA supplementation. These studies include the Stockholm Youth Cohort Study (14) and the Norwegian Mother and Child Cohort Study, specifically the Autism Birth Cohort (ABC) sub-study (10). However, a case–control study from China with 374 children diagnosed with ASD and 354 typically developing children found no association during either the pre-conceptional or prenatal period (16). On the other hand, a case–control cohort study of 45,300 Israeli children found that both pre-conceptional and prenatal FA supplements reduce the risk of ASD in the offspring (15). In China, women who are planning a pregnancy are recommended to take FA starting from 3 months before conception until the end of the first trimester (28) in order to prevent NTDs. However, we found that only 46.5% of women continuously took FA supplements during the pre-conceptional and prenatal periods, and approximately one-third of women ever took FA supplements during either the pre-conceptional or prenatal periods. For women who have ever taken FA supplement, we did not find an increased risk of their offspring developing ASD compared to those who consistently took FA supplements. A potential explanation could be that the folate level of Chinese women was insufficient because food fortification with FA has not yet been implemented in China (29). For instance, the median serum folate concentrations in Chinese women, as reported by the Chinese Center for Disease Control and Prevention in 2015, were 8.02 ng/mL, which is significantly lower than the levels found in developed countries like the United States (14.9–16.8 ng/mL) (30) and New Zealand (21.4 ng/mL) (31). Therefore, taking FA supplements at any time during the pre-conceptional or prenatal period might help to achieve adequate serum folate concentrations (29).
In addition, although a previous meta-analysis (4) revealed that consuming FA supplements during periconception or early pregnancy could reduce the risk of ASD in offspring, emerging evidence shows that low plasma/serum folate concentration in the second and third trimesters of pregnancy is also likely associated with increased risks of physical health issues (e.g., low birthweight) (32) and cognitive development (e.g., language development) in offspring (33, 34). Folate plays an essential role in DNA methylation as a provider of methyl groups (6), which is considered crucial for the development of the central nervous system and is implicated in the formation of fundamental brain structures (15). There might be different windows of susceptibility to maternal changes in the folate-dependent 1-carbon pathway. The periods beyond periconception may play important roles in influencing epigenetic changes in the offspring (35). Therefore, our findings suggested that FA supplements should be taken during the pre-conception period for women who are planning to become pregnant. For women with unintended pregnancies, it is recommended to continue taking FA supplements during the prenatal period.
Our findings have important clinical implications for the relevant policy and practice of FA supplementation in China. In 2009, China’s Ministry of Health launched a major public health project to provide women with FA supplements from 3 months before pregnancy to the first trimester in order to prevent NTDs. After the implementation of the FA intervention project, the rate of pregnant women taking FA supplements increased significantly. However, the rate of women planning pregnancy who take FA supplements as recommended is still low, ranging from 10 to 35% (36). Social-economic status, unintended pregnancy, and awareness and belief about FA supplement were the main influencing factors for taking it as recommended (37). Our results highlighted that the critical window for protecting offspring from ASD is the prenatal period. Policy-led interventions, such as health education, are still urgently needed in China to encourage women to insist on taking folic acid supplements from pre-conception to the prenatal period. In addition, healthcare professionals should also suggest and encourage expectant parents to take FA supplements routinely when they are preparing for pregnancy.
This study had one important strength: it used standard procedures for ASD screening and diagnosis to ensure accurate outcome measurements. However, several limitations should be noted. First, the prevalence of ASD was found to be low in this large epidemiological survey. However, the prevalence in this study was similar to the national estimate in China (37). In addition, we have applied relevant statistical analyses, such as Firth’s bias-reduced regression, to address this problem. Second, the use of questionnaires might introduce potential recall bias, but this remains the most effective investigation method in large epidemiological studies. Thirdly, we did not collect information on the dosage of the FA supplement, and we did not measure maternal serum folate levels. However, doctors usually give advice with a fixed dose as a recommendation to women who regularly take FA supplements during the preconception and prenatal period. Further studies are needed to investigate the dose–response associations during the pre-conceptional and prenatal periods. Furthermore, it should be noted that this study employed a cross-sectional design, which limits the ability to establish causality. Moreover, it is important to acknowledge that other nutritional supplements during pregnancy that could confound and interact the results of the current study, such as iron intake (38), vitamin D deficiency (39), or multivitamin supplementation. Additionally, despite we have considered to fit mix-effect models to preclude the random effects, the small sample size of individuals with ASD prevented the models from converging effectively, thus hindering our ability to confirm the presence of unobserved heterogeneity. A larger sample size with more diagnosed ASD will be needed to confirm the stability of results in the future.
5 Conclusion
According to the results of the current investigation, regular maternal use of FA supplements during the pre-conceptional and pre-natal phases may lower the incidence of ASD in offspring. Additionally, it was determined that the most crucial window of time for this intervention occurred during the prenatal period. The importance of maternal prenatal FA supplementation in lowering the risk of ASD in offspring is highlighted by our research. In order to get Chinese women to take FA supplements, urgent policy-led interventions and health promotion initiatives are required.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethical Review Committee for Biomedical Re-search, Sun Yat-sen University (2016-No.013). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. CG: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. MK: Data curation, Investigation, Writing – review & editing. LL: Funding acquisition, Resources, Software, Validation, Writing – review & editing. GX: Writing – review & editing. NP: Resources, Software, Validation, Writing – review & editing. XWe: Writing – review & editing. JJ: Funding acquisition, Project administration, Writing – review & editing. LS: Funding acquisition, Project administration, Writing – review & editing. QY: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. XWa: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Guangdong Basic and Applied Basic Research Foundation (2020A1515111096 and 2022B1515130007), the Key-Area Research and Development Program of Guangdong Province (2019B030335001), and the National Natural Science Foundation of China (81872639, 82103794, and 81973063).
Acknowledgments
We would like to thank all of the participants and their parents for their kind support throughout the course of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1321046/full#supplementary-material
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). New York: American Psychiatric Pub (2013).
2. Lord, C, Brugha, TS, Charman, T, Cusack, J, Dumas, G, Frazier, T, et al. Autism spectrum disorder. Nat Rev Dis Primers. (2020) 6:5. doi: 10.1038/s41572-019-0138-4
3. Saxena, R, Babadi, M, Namvarhaghighi, H, and Roullet, FI. Role of environmental factors and epigenetics in autism spectrum disorders. Prog Mol Biol Transl Sci. (2020). 173:35–60. doi: 10.1016/bs.pmbts.2020.05.002
4. Liu, X, Zou, M, Sun, C, Wu, L, and Chen, WX. Prenatal folic acid supplements and Offspring's autism Spectrum disorder: a Meta-analysis and Meta-regression. J Autism Dev Disord. (2022) 52:522–39. doi: 10.1007/s10803-021-04951-8
5. Oldenburg, KS, O'Shea, TM, and Fry, RC. Genetic and epigenetic factors and early life inflammation as predictors of neurodevelopmental outcomes. Semin Fetal Neonatal Med. (2020) 25:101115. doi: 10.1016/j.siny.2020.101115
6. Hoxha, B, Hoxha, M, Domi, E, Gervasoni, J, Persichilli, S, Malaj, V, et al. Folic acid and autism: a systematic review of the current State of knowledge. Cell. (2021) 10:1976. doi: 10.3390/cells10081976
7. Raghavan, R, Selhub, J, Paul, L, Ji, Y, Wang, G, Hong, X, et al. A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am J Clin Nutr. (2020) 112:1304–17. doi: 10.1093/ajcn/nqaa208
8. DeSoto, MC, and Hitlan, RT. Synthetic folic acid supplementation during pregnancy may increase the risk of developing autism. J Pediatr Biochem. (2012) 2:251–61. doi: 10.1055/s-0036-1586421
9. Schmidt, RJ, Tancredi, DJ, Ozonoff, S, Hansen, RL, Hartiala, J, Allayee, H, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood autism risks from genetics and environment) case-control study. Am J Clin Nutr. (2012) 96:80–9. doi: 10.3945/ajcn.110.004416
10. Nilsen, RM, Suren, P, Gunnes, N, Alsaker, ER, Bresnahan, M, Hirtz, D, et al. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr Perinat Epidemiol. (2013) 27:553–63. doi: 10.1111/ppe.12077
11. Suren, P, Roth, C, Bresnahan, M, Haugen, M, Hornig, M, Hirtz, D, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. (2013) 309:570–7. doi: 10.1001/jama.2012.155925
12. Steenweg-de Graaff, J, Ghassabian, A, Jaddoe, VW, Tiemeier, H, and Roza, SJ. Folate concentrations during pregnancy and autistic traits in the offspring. The generation R study. Eur J Pub Health. (2015) 25:431–3. doi: 10.1093/eurpub/cku126
13. Virk, J, Liew, Z, Olsen, J, Nohr, EA, Catov, JM, and Ritz, B. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. (2016) 20:710–8. doi: 10.1177/1362361315604076
14. DeVilbiss, EA, Magnusson, C, Gardner, RM, Rai, D, Newschaffer, CJ, Lyall, K, et al. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: population based cohort study. BMJ. (2017) 359:j4273. doi: 10.1136/bmj.j4273
15. Levine, SZ, Kodesh, A, Viktorin, A, Smith, L, Uher, R, Reichenberg, A, et al. Association of Maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism Spectrum disorder in offspring. JAMA Psychiatry. (2018) 75:176–84. doi: 10.1001/jamapsychiatry.2017.4050
16. Li, YM, Shen, YD, Li, YJ, Xun, GL, Liu, H, Wu, RR, et al. Maternal dietary patterns, supplements intake and autism spectrum disorders: a preliminary case-control study. Medicine (Baltimore). (2018) 97:e13902. doi: 10.1097/MD.0000000000013902
17. Raghavan, R, Riley, AW, Volk, H, Caruso, D, Hironaka, L, Sices, L, et al. Maternal multivitamin intake, plasma folate and vitamin B12 levels and autism Spectrum disorder risk in offspring. Paediatr Perinat Epidemiol. (2018) 32:100–11. doi: 10.1111/ppe.12414
18. Schmidt, RJ, Iosif, AM, Guerrero Angel, E, and Ozonoff, S. Association of Maternal Prenatal Vitamin use with Risk for autism Spectrum disorder recurrence in young siblings. JAMA Psychiatry. (2019) 76:391–8. doi: 10.1001/jamapsychiatry.2018.3901
19. Tan, M, Yang, T, Zhu, J, Li, Q, Lai, X, Li, Y, et al. Maternal folic acid and micronutrient supplementation is associated with vitamin levels and symptoms in children with autism spectrum disorders. Reprod Toxicol. (2020) 91:109–15. doi: 10.1016/j.reprotox.2019.11.009
20. Guo, C, Luo, M, Wang, X, Huang, S, Meng, Z, Shao, J, et al. Reliability and validity of the Chinese version of modified checklist for autism in toddlers, revised, with follow-up (M-CHAT-R/F). J Autism Dev Disord. (2019) 49:185–96. doi: 10.1007/s10803-018-3682-y
21. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
22. Robins, DL, Casagrande, K, Barton, M, Chen, CM, Dumont-Mathieu, T, and Fein, D. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics. (2014) 133:37–45. doi: 10.1542/peds.2013-1813
23. Schopler, E, Reichler, RJ, and Renner, BR. The Childhood Autism Rating Scale (CARS). Los Angeles, CA: Western Psychological Services (1988).
24. Modabbernia, A, Velthorst, E, and Reichenberg, A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. (2017) 8:13. doi: 10.1186/s13229-017-0121-4
25. Kim, JY, Son, MJ, Son, CY, Radua, J, Eisenhut, M, Gressier, F, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. (2019) 6:590–600. doi: 10.1016/S2215-0366(19)30181-6
26. Heinze, G, Ploner, M, and Jiricka, L (eds.) (2020). Firth's Bias-reduced logistic regression [R package logistf version 1.24].
27. Puhr, R, Heinze, G, Nold, M, Lusa, L, and Geroldinger, A. Firth's logistic regression with rare events: accurate effect estimates and predictions? Stat Med. (2017) 36:2302–17. doi: 10.1002/sim.7273
28. Working Group of folic acid supplementation in China, The folic acid supplementation working group, birth defects prevention and molecular genetics branch, China maternal and child health association. Guideline for the prevention of neural tube defects by periconceptional folic acid supplementation. Chin J Reprod Health. (2017) 28:401–10.
29. Jiang, S, Wang, J, Duan, Y, Pang, X, Bi, Y, Zhang, H, et al. Folic Acid Status and Associated Factors for Pregnant Chinese Women - China, 2015. China CDC Wkly. (2021) 3:226–231. doi: 10.46234/ccdcw2021.065
30. Marchetta, CM, and Hamner, HC. Blood folate concentrations among women of childbearing age by race/ethnicity and acculturation, NHANES 2001-2010. Matern Child Nutr. (2016) 12:39–50. doi: 10.1111/mcn.12134
31. Bulloch, RE, McCowan, LME, Thompson, JMD, Houghton, LA, and Wall, CR. Plasma folate and its association with folic acid supplementation, socio-demographic and lifestyle factors among New Zealand pregnant women. Br J Nutr. (2019) 122:910–8. doi: 10.1017/S0007114519001788
32. Zhu, X, Wei, L, Cao, D, Liu, C, Tian, J, Long, Y, et al. Low serum folate status in the second trimester increase the risk of low birthweight in Chinese women. J Obstet Gynaecol Res. (2018) 44:2037–44. doi: 10.1111/jog.13757
33. Caffrey, A, McNulty, H, Irwin, R, Cassidy, T, McLaughlin, M, Lees-Murdock, D, et al. Impact of folic acid supplementation during pregnancy on cognitive performance of children at age 11 years: preliminary results from the FASSTT offspring study. Proc Nutr Soc. (2018) 77. (OCE3):E98. doi: 10.1017/S0029665118001027
34. Huang, X, Ye, Y, Li, Y, Zhang, Y, Zhang, Y, Jiang, Y, et al. Maternal folate levels during pregnancy and children's neuropsychological development at 2 years of age. Eur J Clin Nutr. (2020) 74:1585–93. doi: 10.1038/s41430-020-0612-9
35. Caffrey, A, Irwin, RE, McNulty, H, Strain, JJ, Lees-Murdock, DJ, McNulty, BA, et al. Gene-specific DNA methylation in newborns in response to folic acid supplementation during the second and third trimesters of pregnancy: epigenetic analysis from a randomized controlled trial. Am J Clin Nutr. (2018) 107:566–75. doi: 10.1093/ajcn/nqx069
36. Song, QF, Wang, JM, Gao, SH, Liu, XH, Li, ZW, and Zahng, L. Study advance on correct taking rate of folic acid in pregnant women and its influencing factors. Chin J Reprod Health. (2019) 30:487–9.
37. Song, QF, Wang, JM, Gao, SH, Liu, XH, Li, ZW, Zahng, L, et al. Prevalence of autism Spectrum disorder in China: a Nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. (2020) 36:961–71. doi: 10.1007/s12264-020-00530-6
38. De Giacomo, A, Medicamento, S, Pedaci, C, Giambersio, D, Giannico, OV, Petruzzelli, MG, et al. Peripheral Iron levels in autism Spectrum disorders vs. other neurodevelopmental disorders: preliminary data. Int J Environ Res Public Health. (2022) 19:4006. doi: 10.3390/ijerph19074006
Keywords: autism spectrum disorder, folic acid, pre-conceptional period, prenatal period, environmental factor
Citation: Jiang Y, Guo C, Kuang M, Lin L, Xu G, Pan N, Weng X, Jing J, Shi L, Yi Q and Wang X (2024) Examining associations of folic acid supplements administered to mothers during pre-conceptional and prenatal periods with autism spectrum disorders in their offspring: insights from a multi-center study in China. Front. Public Health. 12:1321046. doi: 10.3389/fpubh.2024.1321046
Edited by:
Roberta Zupo, University of Bari Aldo Moro, ItalyReviewed by:
Orazio Valerio Giannico, Local Health Authority of Taranto, ItalyGopal Yadav, Ministry of Health and Population (Nepal), Nepal
Copyright © 2024 Jiang, Guo, Kuang, Lin, Xu, Pan, Weng, Jing, Shi, Yi and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wang, d3hpbjNAc2NudS5lZHUuY24=; Quanying Yi, WVFZXzgwMTEyOEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yan Jiang1†
Yan Jiang1† Lizi Lin
Lizi Lin Guifeng Xu
Guifeng Xu Xuchu Weng
Xuchu Weng Jin Jing
Jin Jing Xin Wang
Xin Wang