- 1KITE Research Institute, Toronto Rehabilitation Institute—University Health Network, Toronto, ON, Canada
- 2Telerehab Centre for Acquired Brain Injury, Toronto Rehabilitation Institute—University Centre, University Health Network, Toronto, ON, Canada
- 3Krembil Brain Institute, University of Toronto, Toronto Western Hospital, University Health Network, Toronto, ON, Canada
- 4University of Toronto, Centre for Mental Health and Senior Scientist, University Health Network, Toronto, ON, Canada
- 5University of Toronto Scarborough, Neuro-Rehab Program, Toronto Rehabilitation Institute—University Centre, University Health Network, Toronto, ON, Canada
- 6Krembil Research Institute, University of Toronto, University Health Network, Toronto, ON, Canada
- 7Medical Psychiatry and Psychiatry and Psychosocial Oncology, University Health Network, Toronto, ON, Canada
- 8Department of Psychiatry, Division of Neurosciences and Clinical Translation, University of Toronto, Toronto, ON, Canada
Post-acute sequelae of SARS-COV-2 (PASC) is growing in prevalence, and involves symptoms originating from the central neurological, cardiovascular, respiratory, gastrointestinal, autonomic nervous, or immune systems. There are non-specific symptoms such as fatigue, headaches, and brain fog, which cannot be ascribed to a single system. PASC places a notable strain on our healthcare system, which is already laden with a large number of acute-COVID-19 patients. Furthermore, it impedes social, academic and vocational functioning, and impacts family life, relationships, and work/financial life. The treatment for PASC needs to target this non-specific etiology and wide-ranging sequelae. In conditions similar to PASC, such as “chemo brain,” and prolonged symptoms of concussion, the non-specific symptoms have shown to be effectively managed through education and strategies for self-management and Mindfulness interventions. However, such interventions have yet to be empirically evaluated in PASC to our knowledge. In response to this gap, we have developed a virtual education intervention synthesized by psychiatrists and clinical psychologists for the current study. We will undertake a two-phase randomized controlled trial to determine the feasibility (Phase 1; N = 90) and efficacy (Phase 2; sample sized based on phase 1 results) of the novel 8 week Education and Self-Management Strategies group compared to a mindfulness skills program, both delivered virtually. Main outcomes include confidence/ability to self-manage symptoms, quality of life, and healthcare utilization. This study stands to mitigate the deleterious intrusiveness of symptoms on everyday life in patients with PASC, and may also help to reduce the impact of PASC on the healthcare system.
Clinical trial registration:https://classic.clinicaltrials.gov/ct2/show/NCT05268523; identifier NCT05268523.
Introduction
Long-COVID: diagnosis and epidemiology
The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). While the word “acute” implies a brief illness (2), many patients experience persisting symptoms (3). These ongoing and sometimes debilitating symptoms were initially brought to public attention by online social support groups that quickly proliferated early in the pandemic (4); these groups coined the terms “Long-Hauler and ‘Long-COVID.” Since then, prolonged symptoms have been additionally referred to as post-COVID syndrome, long-haul COVID-19, and post-acute sequelae of SARS-COV-2 (PASC) (4). The labels initially referred to patients with symptoms lasting more than one month following diagnosis of SARS-CoV-2 (5–8), and now typically refer to symptoms lasting three or more months (9–11). Here, we use the term PASC because it is explicit in its focus on long-lasting symptoms of COVID-19 without etiological assumptions of symptoms.
Like the acute illness, enduring symptoms of COVID-19 are often multi-faceted (12–15). Origins of symptoms may be central neurological (e.g., cognitive impairment, depression/anxiety, loss of sense of smell/taste) (14, 16), cardiovascular (e.g., chest pain/tightness, tachycardia) (17, 18), respiratory (e.g., dyspnea, cough) (14, 19–23), gastrointestinal (e.g., nausea, abdominal pain, altered gut microbiota) (24–26) and/or musculoskeletal (e.g., joint/muscle pain) (27). Symptoms may also have their origins in the autonomic nervous (28) or immune systems (inflammatory and neuroinflammatory) (29). While some symptoms can be ascribed to specific etiology, there are also symptoms where the pathophysiology and causes are uncertain (30).
The exact prevalence of PASC remains uncertain, but given the vast numbers of patients sustaining SARS-CoV-2 – nearly 500 million at the time of writing (31) – even the smallest estimates are concerning, especially as protracted sequelae occur even in mild acute illness and in young and fit patients. One of the largest meta-analyzes to date, pooling 54 studies and almost 1.2 million patients’ data from around the world, found that 6.2% of patients experienced symptoms lasting beyond 4 weeks (32). Other smaller-scale studies have reported prevalence rates varying between 10 and 65% (33–35).
Not surprisingly, the societal impact of PASC is significant. For instance, SARS-CoV-2 is estimated to share a similar mortality rate to that of seasonal influenza, causing an estimated doubling proportion of deaths annually (36). Moreover, the increased healthcare utilization places a notable strain on a system already burdened by large numbers of patients and healthcare workers with acute COVID-19 (28, 37). A retrospective cohort study in Canada found that the mean days in hospital per-person-year increased by 47–53%, 8 weeks or more after a COVID-19 infection (38). Moreover, the array of cognitive, physical and emotional sequelae of PASC impede social, academic and vocational functioning with deleterious financial consequences (39). A large-scale survey of >3,000 patients with PASC (40) found 71% of respondents endorsing compromise to family life and relationships, with 31% endorsing reduced ability to care for children and dependents. Work-related impacts were endorsed by 81% of people, and 36% endorsed financial impact. Similarly, Davis and colleagues (14) in a survey of 3,762 participants with PASC found that 45% needed a reduced work schedule, and 22% were not working due to illness at 6 months post-diagnosis.
Given the above consequences, a treatment for PASC is needed to reduce its burden on patients and society. However, the wide-ranging non-specific and specific nature of symptoms has made this a significant treatment challenge (41).
Identifying treatment options: overlap of PASC with related disorders
One path for treating PASC is to identify other disorders with an overlapping clinical presentation that have treatments with demonstrated efficacy (42). PASC shares a likeness with several conditions in which a discrete organic event is followed by a cluster of prolonged and non-specific symptoms. These include “chemo brain” (43–47), post-viral syndromes such as post influenza, SARS (48), Middle East Respiratory Syndrome (49), myalgic encephalomyelitis/chronic fatigue syndromes (50), and the prolonged symptoms of concussion (51). In all of these disorders, symptoms can include fatigue, sleep disturbance, brain fog, headache and mood disturbance, for example, which are some of the more common non-specific PASC sequelae.
Of particular relevance to PASC is the idea of symptom interplay and exacerbation. This has been described in the concussion literature from a “network perspective,” whereby non-specific symptoms amplify and reinforce one another (52). For example, fatigue may cause or exacerbate cognitive symptoms, which may cause or escalate mood symptoms, which may in turn worsen sleep (53).
The diagnostic challenges of non-specific and trans-diagnostic symptoms in post-viral syndromes, chemo brain and prolonged symptoms of concussion have led to treatment approaches that do not necessitate a mapping between symptom and cause at the individual patient level. One such approach is education and strategies for symptom self-management (e.g., educating about the disorder and expectations regarding symptom recovery; providing strategies for managing one’s own symptoms), which has shown efficacy for managing non-specific and specific symptoms associated with post-viral syndromes, and chemo-brain (54). Education has also been found to be prophylactic against the development of prolonged symptoms of concussion (55). Not surprisingly, education and strategies have been advocated for managing prolonged COVID-19 symptoms (56), but remain to be empirically tested in this population. Another approach with efficacy for non-specific and specific symptoms is Mindfulness (referring to increasing one’s awareness of the present moment) (57), and which can enhance an individual’s internal locus of control for optimizing health (58). Mindfulness treatments have been shown to improve: fatigue in patients with cancer (59–61); anxiety, depression and quality of life in patients with myalgic encephalomyelitis/chronic fatigue syndromes (62); chronic pain, depression relapse, addiction (57); and, symptoms of fatigue and depression in concussion (54, 63). Mindfulness interventions have also shown to improve prolonged symptoms of concussion (63–65). In the context of PASC, however, Mindfulness has limitations for certain physical symptoms such as shortness of breath, lung function, or exercise intolerance (66).
Limited research into current approaches to treatment of PASC
While a number of clinical recommendations have been made for PASC patients, there is a paucity of treatment research to date. The recommended pharmacological approach is symptom-based; this has mainly included the repurposing of drugs that are employed for similar conditions and symptoms (5, 67). For example, medications for chemotherapy and brain fog [e.g., methylphenidate, donepezil, modafinil, and memantine (68)] are under consideration for brain fog in PASC (69). However, currently, no drugs have been found to effectively target subjective cognitive symptoms of PASC, and studies investigating their efficacy are still limited to case-studies (70).
A number of recommendations have been made to promote self-education and strategy use. The National Institute for Health and Care Excellence (NICE) guidelines recommend directing patients to online resources and apps to optimize self-management, for example referring to behavioral guidelines published by the WHO (71) and a PASC self-management web-page created by the Provincial Health Services Authority in Canada (72). As well, a recent guide published for primary care physicians advocated educating patients with PASC with concrete strategies and recommendations for managing their respiratory, cognitive, neurological, and somatic symptoms (73). To date, there are no published multidisciplinary education, strategy and self-management approaches for PASC.
Aims and hypotheses
In sum, while management of acute COVID is being studied extensively, there is no candidate treatment that targets, let alone alleviates, all or most symptoms of PASC across patients. In response to this gap in care, we developed an education and strategies intervention inspired by findings in related populations that have shown efficacy. This intervention synthesized by psychiatrists and clinical psychologists provides practical recommendations to manage both the specific and non-specific symptoms of PASC. The current study aims to (i) demonstrate the feasibility and efficacy of a novel education and strategies intervention for self-management of PASC symptoms that can be delivered cost-effectively, (ii) and to disseminate an intervention package to aid symptom self-management free-of-charge to licensed therapists for delivery to their patients with PASC. The intervention package will be fully manualized, with (i) videos of presentations from clinician-scientists (cardiology, neurology, respirology, rheumatology, psychology) with experience in PASC that includes frequently asked questions from patients and answers from clinicians; and (ii) PowerPoint presentations plus scripts to be delivered by the facilitator.
The treatment under development employs remote delivery, which has shown to be cost-effective (74, 75), and can tackle the scale and geographic distance of patients from treatment centers. Remote delivery also prevents infection risk, employing a group-based approach which has shown to be cost-effective (76), and may be psychologically optimal given the proliferation of online groups for individuals with PASC, suggesting a need for mutual support. Importantly, group-based approaches for related disorders have been proven effective and feasible (77) as have telerehabilitation and education approaches (78).
We will undertake a two-phase randomized controlled trial (RCT) to determine feasibility and effect size (Phase 1) and efficacy (Phase 2) for a novel 8-week Education and Self-Management Strategies group compared to a mindfulness skills program. Phase 2 is a full-scale quantitative-only RCT, with refinements and power analysis based on Phase 1 results, and an additional third no-treatment control arm. Outcomes of interest include confidence and ability to self-manage symptoms, quality of life, healthcare utilization. Hypotheses: Both interventions will be associated with enhanced mood, anxiety, quality of life and management of dyspnea symptoms. The education and self-management strategies program will additionally increase confidence to self-manage symptoms, decrease intrusiveness of Long-COVID symptoms in everyday life, and decrease medical visits, more so than the mindfulness group.
Methods
Participants
Individuals (N = 90; >18 years) with previously diagnosed COVID-19 and experiencing symptoms lasting for more than 3 months (i.e., persisting symptoms) are currently being recruited for Phase 1. Sample size is based on paired tests from our prior concussion study (79) (N = 61; under review) that used the same primary outcome. With effect size 0.59 and standard alpha and beta values, 26 pairs are needed. Factoring (i) 20% attrition (conservative given 7% attrition in a similar concussion study [79; under review] and 9% in the pilot group for the present study), and (ii) a further 25% for 1-month follow-up, we will recruit N = 45 for education of self-management strategies group and N = 45 for mindfulness skills group. Key inclusion criteria are: positive COVID-19 diagnosis a minimum of 3 months prior to study participation; proof of COVID-19 diagnosis through PCR, rapid antigen or serology test; self-report experiencing 3 or more persisting symptoms from 2 or more categories of mood, cognitive and somatic domains; age > 18; and English speaking. Key exclusion criteria include past acute ventilator support; past or present major neurological/psychiatric conditions including dementia, mild cognitive impairment, psychotic illness, mania, chronic fatigue syndrome, fibromyalgia, chronic Lyme disease, and/or traumatic brain injury.
Study design
The Phase 1 trial is a block randomized, controlled, parallel group, two arm, assessor-blinded trial with a 1:1 allocation. Participants will be randomly assigned to either the Education and Self-Management Strategies or the Mindfulness Skills groups. The allocation sequence is determined using the random function in Microsoft Excel by the study coordinators prior to recruitment. The study coordinators will screen, consent, enroll, and assign participants to their study identity numbers. Qualitative assessment of N = 20 patients from the Education and Self-Management group will be purposively (i.e., subjectively) sampled to attain a heterogeneous subset of patients. Qualitative information will be combined with quantitative to permit a more thorough understanding of the outcomes and to aid in further program refinement before undertaking the Phase 2 full-scale RCT. This study design is summarized in COSORT flow diagram presented in Figure 1.

Figure 1. CONSORT study flow diagram of the present protocol; CONSORT, Consolidated Standards of Reporting.
Phase 2 will be a full-scaled trial with an additional third arm (1:1:1 allocation) as a non-treatment, standard-of-care group, with refinements and power analysis based on Phase 1 results.
Procedures
For both groups, 8 weekly sessions will be delivered remotely on Microsoft Teams by licensed therapists (e.g., Psychologist, Occupational Therapist) with mental health experience; discipline experts will also deliver content for the Education and Self Management Strategies Group. Sessions will last 1.5 h, with 10–15 patients per group (factoring in attrition).
Education and Self-Management group protocol
All 8 sessions include education and independently deployable practical strategies (e.g., energy conservation, mindfulness and Cognitive Behavioral Therapy) and principles of self-compassion; self-care, cued diaphragmatic breathing, and phased return to physical activity that will be practiced during and between sessions. While the literature reveals that PASC symptoms can have specific aetiologies, the diagnosis of the etiology of a given symptom in a given patient may be uncertain. Moreover, the presence of some PASC symptoms may cause or exacerbate other symptoms (e.g., fatigue may result in or worsen attentional difficulties). Therefore, the education and strategies we provide are intended to be useful whether or not we have certainty of the underlying symptom cause in any given patient. Restated, the focus is on providing education and strategies for ameliorating symptoms, without making any assumptions about the cause of symptoms in any individual patient.
Benefits of this type of protocol (i.e., strategies and education) include non-invasiveness and no financial cost to patients. This is important given growing concern that in the absence of treatment for the wide-ranging symptoms of PASC, pernicious treatments (80–83) may fill the void.
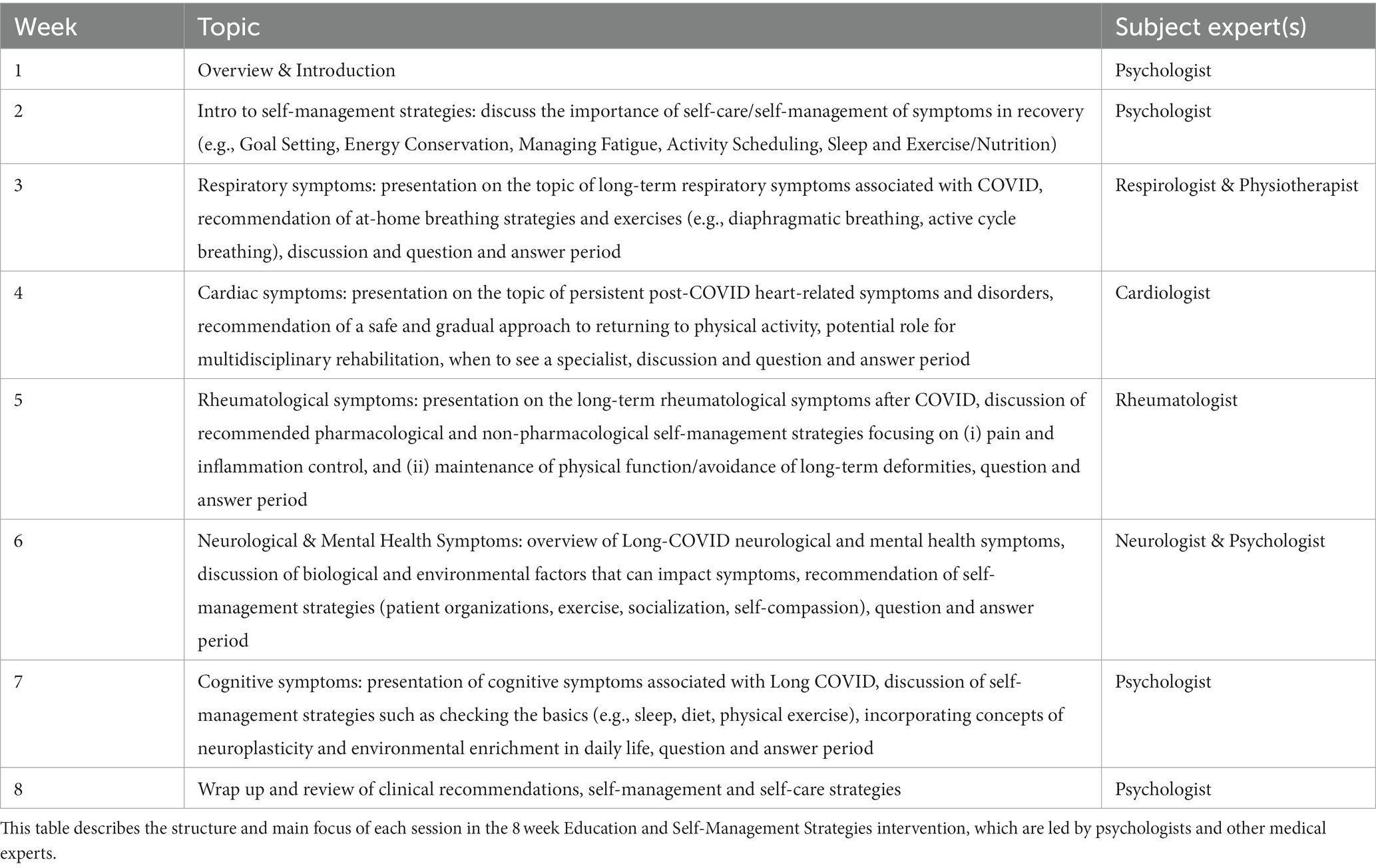
Each session will end with a question-and-answer period. The content presented in each week is outlined in Table 1. During weeks 3–6, medical experts will deliver a 20–25-min presentation on their respective area of expertise and its relationship to Long-COVID, and provide self-management strategies.
Mindfulness skills group protocol
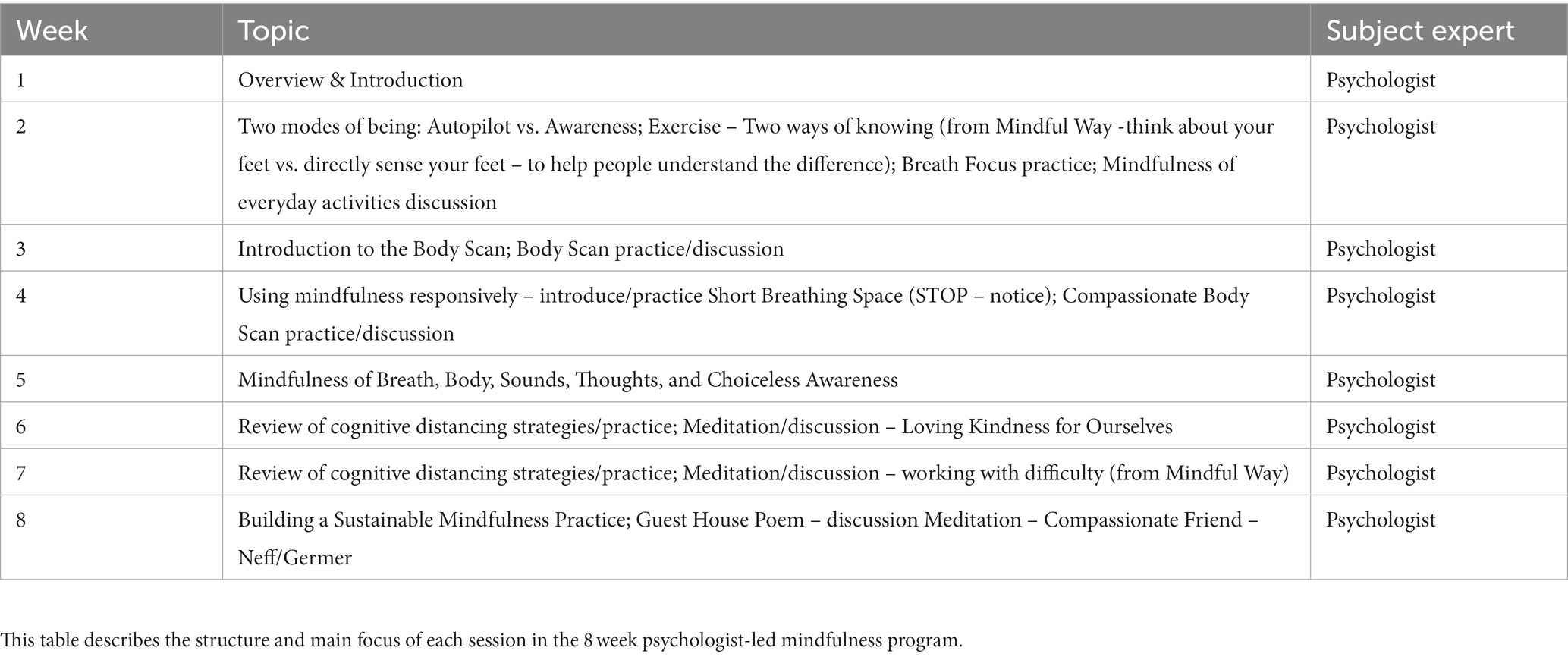
The Mindfulness Skills intervention is an 8-week program designed to provide an introduction to basic mindfulness skills. Each session begins with a brief breath focus practice followed by discussion of the participants’ experience from the previous week. Each session also includes some didactics on a new related mindfulness skill that is introduced and practiced, followed by a question and answer period. Topics covered include breath focus practices, modes of being: autopilot vs. awareness, body scan practice, cognitive distancing strategies, and building a sustainable mindfulness practice. The content covered each week is outlined in Table 2.
Outcome measures
After providing informed consent to participate in the study and prior to commencing the intervention groups, participants will be asked to complete an online background questionnaire in order to assess potential moderators. This background questionnaire collects demographic information (e.g., age race, sex, gender, highest education, occupation), previous and current medical and psychiatric history, current symptoms and healthcare supports.
Feasibility measures (Education and Self-Management group only; Objective 1)
(i) Recruitment (percentage of eligible participants that consented); retention (percentage of consented participants that completed the study); and adherence rates (percentage of sessions attended and outcome measures completed)
(ii) Standard Session Feedback Form: this 5-item patient reported outcome measure (PROM) was created by our group, and uses a 5 point Likert scale to measure the utility of content/strategies, amount of content, barriers to application, and any changes/benefits noticed from applying the strategies.
(iii) Exit interview: a semi-structured, qualitative interview comprising open-ended questions followed by probes. The interview probes pros/cons of intervention design and impact of the intervention on health and symptom self-management. Probes are based on the Workgroup for Intervention Development and Evaluation Research (WIDER) recommendations regarding content, format, delivery, timing issues and personnel (84). The interview will also contain probes for impact on health, health-related actions, including self-management of Long-COVID symptoms and health care visits.
Efficacy measures (Objective 2)
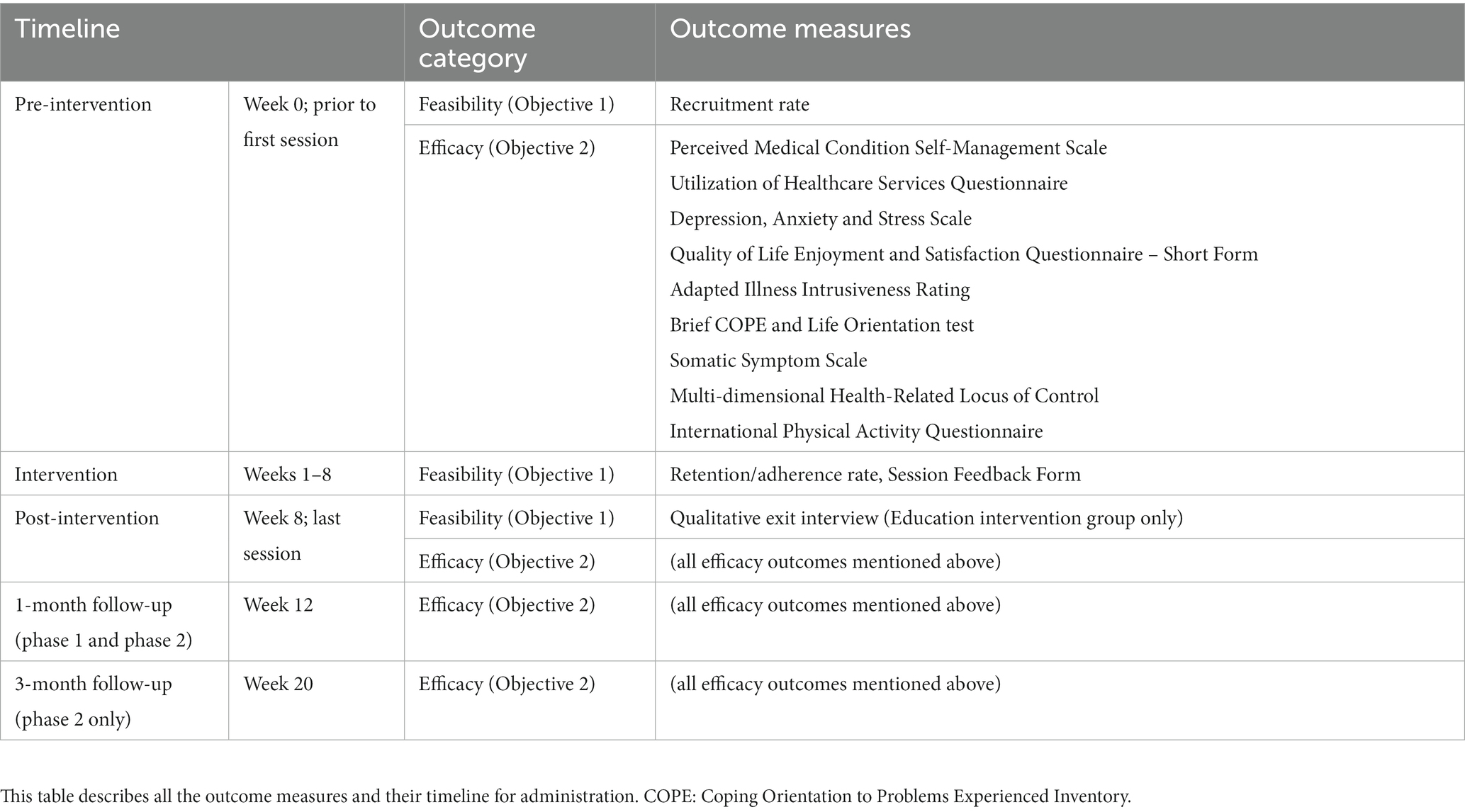
All primary and secondary outcomes are PROMs. Table 3 summarizes the timeline of the outcome measure collection.
(i) Primary outcome:
a. Perceived Medical Condition Self-Management Scale (85) (PMCSMS). An 8 item tool using a 6-point scale to measure confidence to self-manage symptoms, with strong validity and reliability (85, 86), and associated with high treatment adherence and beneficial outcomes (85).
(ii) Secondary outcomes:
a. Utilization of Healthcare Services Questionnaire. This tool was created by our group to measure past and planned healthcare-related visits.
b. Depression, Anxiety and Stress Scale (DASS-21 (87)). A 21-item tool that uses a 4-point scale to measure stress, depression and anxiety symptoms.
c. Quality of Life Enjoyment and Satisfaction Questionnaire – Short Form (Q-LES-Q-SF (88)). A 16-time tool that uses a 5-point scale to measure quality of life and degree of enjoyment and satisfaction experienced by participants in various areas of daily living.
d. Adapted Illness Intrusiveness Rating (AIIR (89, 90)). A 13-item scale using a 7-point scale to measure intrusiveness of symptoms in daily life.
e. Life Orientation Test – Revised (LOT-R) (91). A 10-item tool using a 5-point scale to measure dispositional optimism.
f. International Physical Activity Questionnaire (IPAC) (92). An 8-item tool to measure engagement in physical activity.
(iii) Control/Moderator variables
a. Brief COPE (93). A 28-item tool using a 4-point scale to measure ways of coping with stress in life.
b. Somatic Symptom Scale – 8 (SSS-8 (94)). An 8-item tool using a 5-point scale to measure of somatic symptom burden.
c. Multi-dimensional Health-Related Locus of Control (MHLC (95)). An 18-item tool to measure the locus of control for health-related behaviors.
Statistical procedures
Feasibility and qualitative analyzes
Descriptive statistics will be used to summarize feasibility outcomes. Qualitative data are included to permit a deeper understanding of quantitative findings in this new population. A research assistant supervised by study investigator will use content analysis and constant comparison to identify, expand or merge themes in transcripts of recorded interviews, and develop a codebook of themes and exemplar quotes. We will tabulate themes by sex and within groups by sampling characteristics. This will identify common views about Education and Self-Management Strategies group design and its impact, yielding insight on how to further refine the intervention. By virtual meeting, the Research Team will interpret findings, which we will use to refine intervention design prior to the ensuing Phase 2 RCT.
Efficacy analyzes
To assess pre-to post-treatment and follow-up changes for the primary outcome (PMCSMS), we will undertake repeated measures group (Education vs. Mindfulness) by time (pre-, post-, and follow-up-education/mindfulness/waitlist group). ANCOVAs controlling for acute COVID-19 severity and time post-diagnosis. Secondary outcomes will also be examined in the same manner.
Hierarchical regression analyzes will be performed to examine impact of potential moderator variables on pre-to-post treatment improvement on the PMCSMS. In the first block, the moderators will be age, acute COVID severity, and time-post diagnosis. Sex and gender will be included in the second block, and Brief COPE, AIIR and MHLC will be added in the final block. Analyzes of repeated measures data will be performed using the Mixed Models for Repeated Measures (MMRM) methodology (96). We will employ a comparison of bias-corrected covariance estimators for MMRM analysis in longitudinal data with dropouts in order to accommodate the possibility of missing assessments or of assessments at different times post-diagnoses and to provide an effective framework for hierarchical regression analyzes and further exploratory analyzes (96).
Discussion
The overall aim of this research is to create and disseminate an intervention package for the self-management of PASC symptoms. This brief, cost-effective and scalable intervention can be available to any licensed therapist with mental health expertise and enable treatment of patients regardless of geographic location or mobility restrictions and will enable treatment without concern for infection risk to patients or therapist. The current RCT protocol will determine if the Education and Self-Management Strategies group offers better (or at least non-inferior) symptom management outcomes compared with the Mindfulness and waitlist groups. The inability to attribute many symptoms to a natural cause may be anxiety-provoking for patients, which emphasizes the importance of education to understand the range of possible non-specific and specific symptoms, and mindfulness for accepting symptoms non-judgementally/agnostically and destigmatizing discomfort by alleviating secondary maladaptive attributions.
The increasing strain of PASC on healthcare resources may deleteriously affect the management and care of other patient populations (38, 97). Literature on the challenges of self-managing symptoms of Long COVID is limited at this time, however we can learn from research on other chronic conditions (e.g., chronic obstructive pulmonary disease, rheumatic diseases, kidney disease) that likely present similar challenges and limitations that individuals may encounter in their recovery journey (80, 98–100). Firstly, the heterogeneity of symptoms and their unpredictable nature make it challenging to develop universally effective strategies. What works for one person may not necessarily benefit another due to the diverse manifestations of Long COVID. Additionally, the limited understanding of the underlying mechanisms and long-term effects poses a challenge in tailoring targeted interventions. The fluctuating and persistent nature of symptoms can also create difficulties in establishing a consistent and manageable routine. Furthermore, the emotional toll of prolonged illness, including anxiety and depression, can impact one’s ability to effectively self-manage (98, 99). Access to healthcare resources and professional guidance is another limitation, as not all individuals may have equal opportunities to consult with specialists or access tailored medical advice (98–100). The proposed intervention, which provides accessible, remotely delivered education and teaching strategies pertaining to the diverse symptom domains of Long COVID, was designed to mitigate some of these challenges.
Hence, the study stands to reduce the suffering and improve the quality of life of patients with PASC. Recognizing the unique and diverse nature of symptoms, the strategies and education intervention protocol incorporates a combination of educational modules, teaching of practical strategies, interactive question and answer period and peer support, to empower participants with knowledge, skills, and a robust support system. Educational presentations are tailored to provide insights into the varied symptoms and their management, fostering a proactive approach to healthcare. A question-and-answer period ensures that individuals receive guidance specific to their experiences, promoting a targeted and effective self-management strategy. Peer support, on the other hand, creates a community where shared experiences foster mutual understanding and encouragement. The ultimate goal is to enhance the overall quality of life for participants by mitigating symptom burden and intrusiveness, improving coping mechanisms, and fostering confidence to self-manage. We anticipate that a well-rounded approach to self-management will not only alleviate symptoms but also positively impact mental health, thereby contributing to an improved quality of life. Moreover, the intervention may also help to reduce the impact of PASC on the healthcare system by empowering participants to manage their condition independently and helping patients to discern when to seek out physician support for symptoms and when to self-manage. This, in turn, may alleviate the burden on healthcare resources while offering individuals a sense of control and agency over their health. Through these interventions, our study aspires to bridge existing gaps in Long COVID care and contribute meaningful insights to the broader field of chronic illness management.
Ethics and dissemination
This trial has already received ethics approval. If preliminary efficacy is observed in Phase 1 of the study, we will conduct a larger scale RCT at the University Health Network (UHN) in which we will provide clinical care through study participation in research. Control arm patients will receive treatment after the control phase. For the larger scale study, subject experts will create presentations that can be delivered by non-experts. We will manualize the entire protocol (comprising a clinician guide, PowerPoint presentations, FAQs), which will be made available to licensed therapists with mental health expertise. For rapid dissemination, the current protocol will be published online early in the study. Feasibility and preliminary efficacy results from the pilot will be submitted for publication as soon as the current pilot is complete. Full-scale results of the planned larger-scale RCT will be published first on bioRxiv (for more rapid sharing of information) and subsequently in peer- reviewed journals. Vehicles for knowledge uptake include: (i) local (Telerehab Centre at UHN; COVID-19 rehab clinic at UHN; CANCOV); Sunnybrook Health Sciences Centre, (ii) study team networks within Canada and abroad (e.g., ECHO COVID; International Neuropsychological Society).
Ethics statement
This trial received ethical approval from the University Health Network Research Ethics Board (case #21-5038.0), and written informed consent was provided by the participants for participation.
Author contributions
JR and NJ were involved in composing and revising the manuscript. BC, RG, SA, MM, L-AM, LR, DS, and DG were the original developers of the protocol, providing expertise, critical input, and editing throughout the writing process. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Canadian Institutes of Health Research Operating Grant - Emerging COVID-19 Research Gaps and Priorities funding opportunity (Funding Reference Number GA4 177750) and Cass Family Grants for Catalyzing Access and Change. Additionally, we would like to acknowledge the donor funding received by The Walter and Maria Schroeder Foundation and The Joseph & Antoinette Sorbara Foundation in support of this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pollard, CA , Morran, MP , and Nestor-Kalinoski, AL . The COVID-19 pandemic: a global health crisis. Physiol Genomics. (2020) 52:549–57. doi: 10.1152/physiolgenomics.00089.2020
2. Hu, B , Guo, H , Zhou, P , and Shi, ZL . Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
3. Chen, C , Haupert, SR , Zimmermann, L , Shi, X , Fritsche, LG , and Mukherjee, B . Global prevalence of post-acute sequelae of COVID-19 (PASC) or long COVID: a meta-analysis and systematic review. J Infect Dis. (2021). 226:1593–1607. doi: 10.1101/2021.11.15.21266377
4. Callard, F , and Perego, E . How and why patients made long Covid. Soc Sci Med. (2021) 268:113426. doi: 10.1016/j.socscimed.2020.113426
5. Yong, SJ . Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). (2021) 53:737–54. doi: 10.1080/23744235.2021.1924397
6. Datta, SD , Talwar, A , and Lee, JT . A proposed framework and timeline of the Spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. (2020) 324:2251–2. doi: 10.1001/jama.2020.22717
7. Mendelson, M , Nel, J , Blumberg, L , Madhi, SA , Dryden, M , Stevens, W, et al. Long-COVID: an evolving problem with an extensive impact. S Afr Med J. (2020) 111:10–2. doi: 10.7196/SAMJ.2020.v111i11.15433
8. Sivan, M , and Taylor, S . NICE guideline on long covid. BMJ. (2020) 371:m4938. doi: 10.1136/bmj.m4938
9. Venkatesan, P . NICE guideline on long COVID. Lancet Respir Med. (2021) 9:129. doi: 10.1016/S2213-2600(21)00031-X
10. Soriano, JB , Murthy, S , Marshall, JC , Relan, P , and Diaz, JVWHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. (2022) 22:e102–7. doi: 10.1016/S1473-3099(21)00703-9
11. Demko, ZO , Yu, T , Mullapudi, SK , Heslin, MGV , Dorsey, CA , Payton, CB, et al. Post-acute sequelae of SARS-CoV-2 (PASC) impact quality of life at 6, 12 and 18 months post-infection. medRxiv. (2022). doi: 10.1101/2022.08.08.22278543
12. Nalbandian, A , Sehgal, K , Gupta, A , Madhavan, MV , McGroder, C , Stevens, JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
13. Taquet, M , Geddes, JR , Husain, M , Luciano, S , and Harrison, PJ . 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8:416–27. doi: 10.1016/S2215-0366(21)00084-5
14. Davis, HE , Assaf, GS , McCorkell, L , Wei, H , Low, RJ , Re'em, Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
15. Wang, Z , and Yang, L . Post-acute sequelae of SARS-CoV-2 infection: a neglected public health issue. Front Public Health. (2022) 10:908757. doi: 10.3389/fpubh.2022.1007528
16. Spudich, S , and Nath, A . Nervous system consequences of COVID-19. Science. (2022) 375:267–9. doi: 10.1126/science.abm2052
17. Xie, Y , Xu, E , Bowe, B , and al-Aly, Z . Long-term cardiovascular outcomes of COVID-19. Nat Med. (2022) 28:583–90. doi: 10.1038/s41591-022-01689-3
18. Larsen, NW , Stiles, LE , Shaik, R , Schneider, L , Muppidi, S , Tsui, CT, et al. Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults. Front Neurol. (2022) 13:1012668. doi: 10.3389/fneur.2022.1012668
19. Bull-Otterson, L , Baca, S , Saydah, S , Boehmer, TK , Adjei, S , Gray, S, et al. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and≥ 65 years—United States, march 2020–November 2021. Morb Mortal Wkly Rep. (2022) 71:713–7. doi: 10.15585/mmwr.mm7121e1
20. Yu, JZ , Granberg, T , Shams, R , Petersson, S , Sköld, M , Nyrén, S, et al. Lung perfusion disturbances in nonhospitalized post-COVID with dyspnea-a magnetic resonance imaging feasibility study. J Intern Med. (2022) 292:941–56. doi: 10.1111/joim.13558
21. Cho, JL , Villacreses, R , Nagpal, P , Guo, J , Pezzulo, AA , Thurman, AL, et al. Quantitative chest CT assessment of small airways disease in post-acute SARS-CoV-2 infection. Radiology. (2022) 304:185–92. doi: 10.1148/radiol.212170
22. Vijayakumar, B , Boustani, K , Ogger, PP , Papadaki, A , Tonkin, J , Orton, CM, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. (2022) 55:542–556.e5. doi: 10.1016/j.immuni.2022.01.017
23. Littlefield, KM , Watson, RO , Schneider, JM , Neff, CP , Yamada, E , Zhang, M, et al. SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLoS Pathog. (2022) 18:e1010359. doi: 10.1371/journal.ppat.1010359
24. Meringer, H , and Mehandru, S . Gastrointestinal post-acute COVID-19 syndrome. Nat Rev Gastroenterol Hepatol. (2022) 19:345–6. doi: 10.1038/s41575-022-00611-z
25. Yeoh, YK , Zuo, T , Lui, GCY , Zhang, F , Liu, Q , Li, AYL, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. (2021) 70:698–706. doi: 10.1136/gutjnl-2020-323020
26. Liu, Q , Mak, JWY , Su, Q , Yeoh, YK , Lui, GCY , Ng, SSS, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. (2022) 71:544–52. doi: 10.1136/gutjnl-2021-325989
27. Tarantino, U , Visconti, VV , Bonanni, R , Gatti, A , Marcozzi, M , Calabrò, D, et al. Osteosarcopenia and long-COVID: a dangerous combination. Ther Adv Musculoskelet Dis. (2022) 14:1759720X2211304. doi: 10.1177/1759720X221130485
28. Orrù, G , Bertelloni, D , Diolaiuti, F , Mucci, F , di Giuseppe, M , Biella, M, et al. Long-COVID syndrome? A study on the persistence of neurological, psychological and physiological symptoms. Healthcare (Basel). (2021) 9:575. doi: 10.3390/healthcare9050575
29. Hamzelou, J . What covid-19 does to the brain. New Sci. (2022) 253:19. doi: 10.1016/S0262-4079(22)00130-0
30. Ahmad, MS , Shaik, RA , Ahmad, RK , Yusuf, M , Khan, M , Almutairi, AB, et al. "LONG COVID": an insight. Eur Rev Med Pharmacol Sci. (2021) 25:5561–77. doi: 10.26355/eurrev_202109_26669
31. World Health Organization (2022). WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int/
32. Global Burden of Disease Long COVID CollaboratorsWulf Hanson, S , Abbafati, C , Aerts, JG , al-Aly, Z , Ashbaugh, C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. (2022) 328:1604–15. doi: 10.1001/jama.2022.18931
33. Arjun, MC , Singh, AK , Pal, D , Das, K , Alekhya, G , Venkateshan, M, et al. Prevalence, characteristics, and predictors of long COVID among diagnosed cases of COVID-19. medRxiv. (2022). doi: 10.1101/2022.01.04.21268536
34. Taquet, M , Dercon, Q , Luciano, S , Geddes, JR , Husain, M , and Harrison, PJ . Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. (2021) 18:e1003773. doi: 10.1371/journal.pmed.1003773
35. Greenhalgh, T , Knight, M , A’Court, C , Buxton, M , and Husain, L . Management of post-acute covid-19 in primary care. BMJ. (2020) 370:m3026. doi: 10.1136/bmj.m3026
36. Wang, Z , and Yang, L . The therapeutic potential of natural dietary flavonoids against SARS-CoV-2 infection. Nutrients. (2023) 15:3443. doi: 10.3390/nu15153443
37. Aiyegbusi, OL , Hughes, SE , Turner, G , Rivera, SC , McMullan, C , Chandan, JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. (2021) 114:428–42. doi: 10.1177/01410768211032850
38. McNaughton, CD , Austin, PC , Sivaswamy, A , Fang, J , Abdel-Qadir, H , Daneman, N, et al. Post-acute health care burden after SARS-CoV-2 infection: a retrospective cohort study. CMAJ. (2022) 194:E1368–76. doi: 10.1503/cmaj.220728
39. Rajan, S , Khunti, K , Alwan, N , Steves, C , MacDermott, N , Morsella, A, et al. In the wake of the pandemic: Preparing for long COVID. Ottawa: Canadian Medical Association (2021).
41. Yang, L , and Wang, Z . Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur J Med Chem. (2023) 257:115503. doi: 10.1016/j.ejmech.2023.115503
42. Davis, HE , McCorkell, L , Vogel, JM , and Topol, EJ . Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
43. Mann, D . Memory lock; is chemo brain making you forgetful. Int J Radiat Oncol Biol Phys. (1999) 33:179–82.
44. Mitchell, T . The social and emotional toll of chemotherapy - patients' perspectives. Eur J Cancer Care (Engl). (2007) 16:39–47. doi: 10.1111/j.1365-2354.2006.00701.x
45. Mitchell, T , and Turton, P . 'Chemobrain': concentration and memory effects in people receiving chemotherapy - a descriptive phenomenological study. Eur J Cancer Care (Engl). (2011) 20:539–48. doi: 10.1111/j.1365-2354.2011.01244.x
46. Raffa, RB . A proposed mechanism for chemotherapy-related cognitive impairment ('chemo-fog'). J Clin Pharm Ther. (2011) 36:257–9. doi: 10.1111/j.1365-2710.2010.01188.x
47. Theoharides, TC , Cholevas, C , Polyzoidis, K , and Politis, A . Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors. (2021) 47:232–41. doi: 10.1002/biof.1726
48. Chams, N , Chams, S , Badran, R , Shams, A , Araji, A , Raad, M, et al. COVID-19: a multidisciplinary review. Front Public Health. (2020) 8:383. doi: 10.3389/fpubh.2020.00383
49. Umakanthan, S , Sahu, P , Ranade, AV , Bukelo, MM , Rao, JS , Abrahao-Machado, LF, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. (2020) 96:753–8. doi: 10.1136/postgradmedj-2020-138234
50. Theoharides, TC , and Conti, P . COVID-19 and multisystem inflammatory syndrome, or is it mast cell activation syndrome? J Biol Regul Homeost Agents. (2020) 34:1633–6. doi: 10.23812/20-EDIT3
51. Polinder, S , Cnossen, MC , Real, RGL , Covic, A , Gorbunova, A , Voormolen, DC, et al. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front Neurol. (2018) 9:1113. doi: 10.3389/fneur.2018.01113
52. Iverson, GL . Network analysis and precision rehabilitation for the post-concussion syndrome. Front Neurol. (2019) 10:489. doi: 10.3389/fneur.2019.00489
53. Palagini, L , Domschke, K , Benedetti, F , Foster, RG , Wulff, K , and Riemann, D . Developmental pathways towards mood disorders in adult life: is there a role for sleep disturbances? J Affect Disord. (2019) 243:121–32. doi: 10.1016/j.jad.2018.09.011
54. Bernstein, LJ , McCreath, GA , Nyhof-Young, J , Dissanayake, D , and Rich, JB . A brief psychoeducational intervention improves memory contentment in breast cancer survivors with cognitive concerns: results of a single-arm prospective study. Support Care Cancer. (2018) 26:2851–9. doi: 10.1007/s00520-018-4135-z
55. Scorza, KA , and Cole, W . Current concepts in concussion: initial evaluation and management. Am Fam Physician. (2019) 99:426–34.
56. Dani, M , Dirksen, A , Taraborrelli, P , Torocastro, M , Panagopoulos, D , Sutton, R, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med (Lond). (2021) 21:e63–7. doi: 10.7861/clinmed.2020-0896
57. Creswell, JD . Mindfulness interventions. Annu Rev Psychol. (2017) 68:491–516. doi: 10.1146/annurev-psych-042716-051139
58. Ludwig, DS , and Kabat-Zinn, J . Mindfulness in medicine. JAMA. (2008) 300:1350–2. doi: 10.1001/jama.300.11.1350
59. Gok Metin, Z , Karadas, C , Izgu, N , Ozdemir, L , and Demirci, U . Effects of progressive muscle relaxation and mindfulness meditation on fatigue, coping styles, and quality of life in early breast cancer patients: an assessor blinded, three-arm, randomized controlled trial. Eur J Oncol Nurs. (2019) 42:116–25. doi: 10.1016/j.ejon.2019.09.003
60. Arring, NM , Barton, DL , Brooks, T , and Zick, SM . Integrative therapies for Cancer-related fatigue. Cancer J. (2019) 25:349–56. doi: 10.1097/PPO.0000000000000396
61. Park, S , Sato, Y , Takita, Y , Tamura, N , Ninomiya, A , Kosugi, T, et al. Mindfulness-based cognitive therapy for psychological distress, fear of Cancer recurrence, fatigue, spiritual well-being, and quality of life in patients with breast Cancer-a randomized controlled trial. J Pain Symptom Manag. (2020) 60:381–9. doi: 10.1016/j.jpainsymman.2020.02.017
62. Khanpour Ardestani, S , Karkhaneh, M , Stein, E , Punja, S , Junqueira, DR , Kuzmyn, T, et al. Systematic review of mind-body interventions to treat Myalgic encephalomyelitis/chronic fatigue syndrome. Medicina (Kaunas). (2021) 57:652. doi: 10.3390/medicina57070652
63. Acabchuk, RL , Brisson, JM , Park, CL , Babbott-Bryan, N , Parmelee, OA , and Johnson, BT . Therapeutic effects of meditation, yoga, and mindfulness-based interventions for chronic symptoms of mild traumatic brain injury: a systematic review and Meta-analysis. Appl Psychol Health Well Being. (2021) 13:34–62. doi: 10.1111/aphw.12244
64. Azulay, J , Smart, CM , Mott, T , and Cicerone, KD . A pilot study examining the effect of mindfulness-based stress reduction on symptoms of chronic mild traumatic brain injury/postconcussive syndrome. J Head Trauma Rehabil. (2013) 28:323–31. doi: 10.1097/HTR.0b013e318250ebda
65. Bay, E , and Chan, RR . Mindfulness-based versus health promotion group therapy after traumatic brain injury. J Psychosoc Nurs Ment Health Serv. (2019) 57:26–33. doi: 10.3928/02793695-20180924-03
66. Sgalla, G , Cerri, S , Ferrari, R , Ricchieri, MP , Poletti, S , Ori, M, et al. Mindfulness-based stress reduction in patients with interstitial lung diseases: a pilot, single-Centre observational study on safety and efficacy. BMJ Open Respir Res. (2015) 2:e000065–5. doi: 10.1136/bmjresp-2014-000065
67. Jarrott, B , Head, R , Pringle, KG , Lumbers, ER , and Martin, JH . "LONG COVID"-a hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol Res Perspect. (2022) 10:e00911. doi: 10.1002/prp2.911
68. Clinic, M. (2021). Chemo brain. Available at: https://www.mayoclinic.org/diseases-conditions/chemo-brain/diagnosis-treatment/drc-20351065
69. Crook, H , Raza, S , Nowell, J , Young, M , and Edison, P . Long covid-mechanisms, risk factors, and management. BMJ. (2021) 374:n1648. doi: 10.1136/bmj.n1648
70. Roy, D , Song, J , Awad, N , and Zamudio, P . Treatment of unexplained coma and hypokinetic-rigid syndrome in a patient with COVID-19. BMJ Case Rep. (2021) 14:e239781. doi: 10.1136/bcr-2020-239781
71. World Health Organization . Support for rehabilitation: self-management after COVID-19-related illness. Geneva: World Health Organization (2021).
72. Living with Persistent Post-COVID-19 Symptoms . Health Info. Available at: http://www.phsa.ca/health-info/post-covid-19-care-recovery#Self-Care--Info
73. Vance, H , Maslach, A , Stoneman, E , Harmes, K , Ransom, A , Seagly, K, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. (2021) 34:1229–42. doi: 10.3122/jabfm.2021.06.210254
74. Subedi, N , Rawstorn, JC , Gao, L , Koorts, H , and Maddison, R . Implementation of Telerehabilitation interventions for the self-Management of Cardiovascular Disease: systematic review. JMIR Mhealth Uhealth. (2020) 8:e17957. doi: 10.2196/17957
75. Hwang, R , Morris, NR , Mandrusiak, A , Bruning, J , Peters, R , Korczyk, D, et al. Cost-utility analysis of home-based Telerehabilitation compared with Centre-based rehabilitation in patients with heart failure. Heart Lung Circ. (2019) 28:1795–803. doi: 10.1016/j.hlc.2018.11.010
76. Reis, M , Boisvert, I , Beedell, E , and Mumford, V . Auditory training for adult Cochlear implant users: a survey and cost analysis study. Ear Hear. (2019) 40:1445–56. doi: 10.1097/AUD.0000000000000724
77. Alfano, CM , and Rowland, JH . Recovery issues in cancer survivorship: a new challenge for supportive care. Cancer J. (2006) 12:432–43. doi: 10.1097/00130404-200609000-00012
78. Boulos, ME , Colella, B , Meusel, LA , Sharma, B , Peter, MK , Worthington, T, et al. Feasibility of group telerehabilitation for individuals with chronic acquired brain injury: integrating clinical care and research. Disabil Rehabil. (2023):28, 1–13. doi: 10.1080/09638288.2023.2177357
79. Meusel, L-A , Colella, B , Ruttan, L , Tartaglia, MC , and Green, R . Preliminary efficacy and predictors of response to a symptom self-management program for individuals with persistent symptoms of concussion. Brain Inj. (2023) 37:1245–52. doi: 10.1080/02699052.2023.2230873
80. Brown, K , Yahyouche, A , Haroon, S , Camaradou, J , and Turner, G . Long COVID and self-management. Lancet. (2022) 399:355. doi: 10.1016/S0140-6736(21)02798-7
81. Sadio, AJ , Gbeasor-Komlanvi, FA , Konu, RY , Bakoubayi, AW , Tchankoni, MK , Bitty-Anderson, AM, et al. Assessment of self-medication practices in the context of the COVID-19 outbreak in Togo. BMC Public Health. (2021) 21:58. doi: 10.1186/s12889-020-10145-1
82. Quispe-Cañari, JF , Fidel-Rosales, E , Manrique, D , Mascaró-Zan, J , Huamán-Castillón, KM , Chamorro–Espinoza, SE, et al. Self-medication practices during the COVID-19 pandemic among the adult population in Peru: a cross-sectional survey. Saudi Pharm J. (2021) 29:1–11. doi: 10.1016/j.jsps.2020.12.001
83. Shrestha, AB , Karki, S , Timilsina, B , Sherpa, TN , Sapkota, S , Dhakal, B, et al. The scenario of self-medication practices during the covid-19 pandemic; a systematic review. Ann Med Surg (Lond). (2022) 82:104482. doi: 10.1016/j.amsu.2022.104607
84. Albrecht, L , Archibald, M , Arseneau, D , and Scott, SD . Development of a checklist to assess the quality of reporting of knowledge translation interventions using the workgroup for intervention development and evaluation research (WIDER) recommendations. Implement Sci. (2013) 8:1–5. doi: 10.1186/1748-5908-8-52
85. Wild, MG , Ostini, R , Harrington, M , Cavanaugh, KL , and Wallston, KA . Validation of the shortened perceived medical condition self-management scale in patients with chronic disease. Psychol Assess. (2018) 30:1300–7. doi: 10.1037/pas0000572
86. Wild, MG , Wallston, KA , Green, JA , Beach, LB , Umeukeje, E , Wright Nunes, JA, et al. The perceived medical condition self-management scale can be applied to patients with chronic kidney disease. Kidney Int. (2017) 92:972–8. doi: 10.1016/j.kint.2017.03.018
87. Henry, JD , and Crawford, JR . The short-form version of the depression anxiety stress scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. (2005) 44:227–39. doi: 10.1348/014466505X29657
88. Endicott, J , Nee, J , Harrison, W , and Blumenthal, R . Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull. (1993) 29:321–6.
89. Devins, GM , Binik, YM , Hutchinson, TA , Hollomby, DJ , Barré, PE , and Guttmann, RD . The emotional impact of end-stage renal disease: importance of patients' perceptions of intrusiveness and control. Int J Psychiatry Med. (1984) 13:327–43. doi: 10.2190/5DCP-25BV-U1G9-9G7C
90. Devins, GM , Mandin, H , Hons, RB , Burgess, ED , Klassen, J , Taub, K, et al. Illness intrusiveness and quality of life in end-stage renal disease: comparison and stability across treatment modalities. Health Psychol. (1990) 9:117–42. doi: 10.1037/0278-6133.9.2.117
91. Carver, CS , and Scheier, MF . Dispositional optimism. Trends Cogn Sci. (2014) 18:293–9. doi: 10.1016/j.tics.2014.02.003
92. Craig, CL , Marshall, AL , Bauman, AE , Booth, ML , Ainsworth, BE , Pratt, M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
93. Carver, CS . You want to measure coping but your protocol’too long: consider the brief cope. Int J Behav Med. (1997) 4:92. doi: 10.1207/s15327558ijbm0401_6
94. Gierk, B , Kohlmann, S , Kroenke, K , Spangenberg, L , Zenger, M , Brähler, E, et al. The somatic symptom scale–8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med. (2014) 174:399–407. doi: 10.1001/jamainternmed.2013.12179
95. Wallston, KA , Strudler Wallston, B , and DeVellis, R . Development of the multidimensional health locus of control (MHLC) scales. Health Educ Monogr. (1978) 6:160–70. doi: 10.1177/109019817800600107
96. Gosho, M , Hirakawa, A , Noma, H , Maruo, K , and Sato, Y . Comparison of bias-corrected covariance estimators for MMRM analysis in longitudinal data with dropouts. Stat Methods Med Res. (2017) 26:2389–406. doi: 10.1177/0962280215597938
97. Office for National Statistics . Analysis of death registrations not involving coronavirus (COVID-19). England and Wales: Office for National Statistics (2020).
98. Coleman, MT , and Newton, KS . Supporting self-management in patients with chronic illness. Am Fam Physician. (2005) 72:1503–10.
99. Dwarswaard, J , Bakker, EJ , van Staa, A , and Boeije, HR . Self-management support from the perspective of patients with a chronic condition: a thematic synthesis of qualitative studies. Health Expect. (2016) 19:194–208. doi: 10.1111/hex.12346
100. Russell, S , Ogunbayo, OJ , Newham, JJ , Heslop-Marshall, K , Netts, P , Hanratty, B, et al. Qualitative systematic review of barriers and facilitators to self-management of chronic obstructive pulmonary disease: views of patients and healthcare professionals. NPJ Prim Care Respir Med. (2018) 28:2. doi: 10.1038/s41533-017-0069-z
Keywords: Long COVID, COVID-19, rehabilitation, intervention, self-management
Citation: Rybkina J, Jacob N, Colella B, Gold D, Stewart DE, Ruttan LA, Meusel L-A, McAndrews MP, Abbey S and Green R (2024) Self-managing symptoms of Long COVID: an education and strategies research protocol. Front. Public Health. 12:1106578. doi: 10.3389/fpubh.2024.1106578
Edited by:
Sayyed Mohsen Fatemi, York University, CanadaReviewed by:
Pasquale Moretta, IRCCS di Telese Terme (BN), ItalyZhonglei Wang, Qufu Normal University, China
Liyan Yang, Qufu Normal University, China
Copyright © 2024 Rybkina, Jacob, Colella, Gold, Stewart, Ruttan, Meusel, McAndrews, Abbey and Green. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Green, cm9iaW4uZ3JlZW5AdWhuLmNh
 Julia Rybkina
Julia Rybkina Nithin Jacob1
Nithin Jacob1 Mary P. McAndrews
Mary P. McAndrews Robin Green
Robin Green