
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 16 February 2023
Sec. Environmental Health and Exposome
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.998511
 Nurfatehar Ramly1,2
Nurfatehar Ramly1,2 Husna Maizura Ahmad Mahir3
Husna Maizura Ahmad Mahir3 Wan Nurul Farah Wan Azmi1*
Wan Nurul Farah Wan Azmi1* Zailina Hashim4
Zailina Hashim4 Jamal Hisham Hashim5
Jamal Hisham Hashim5 Rafiza Shaharudin1
Rafiza Shaharudin1Arsenic is a carcinogen element that occurs naturally in our environment. Humans can be exposed to arsenic through ingestion, inhalation, and dermal absorption. However, the most significant exposure pathway is via oral ingestion. Therefore, a comparative cross-sectional study was conducted to determine the local arsenic concentration in drinking water and hair. Then, the prevalence of arsenicosis was evaluated to assess the presence of the disease in the community. The study was conducted in two villages, namely Village AG and Village P, in Perak, Malaysia. Socio-demographic data, water consumption patterns, medical history, and signs and symptoms of arsenic poisoning were obtained using questionnaires. In addition, physical examinations by medical doctors were performed to confirm the signs reported by the respondents. A total of 395 drinking water samples and 639 hair samples were collected from both villages. The samples were analyzed using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) to determine arsenic concentration. The results showed that 41% of water samples from Village AG contained arsenic concentrations of more than 0.01 mg/L. In contrast, none of the water samples from Village P exceeded this level. Whilst, for hair samples, 85 (13.5%) of total respondents had arsenic levels above 1 μg/g. A total of 18 respondents in Village AG had at least one sign of arsenicosis and hair arsenic levels of more than 1 μg/g. Factors significantly associated with increased arsenic levels in hair were female, increasing age, living in Village AG and smoking. The prevalence of arsenicosis in the exposed village indicates chronic arsenic exposure, and immediate mitigation action needs to be taken to ensure the wellbeing of the residents in the exposed village.
Arsenic (As) is a bright silver-gray metalloid which is naturally found in the Earth's crust (1, 2). Humans may be exposed to arsenic when the Earth's crust is naturally disturbed, such as through volcanic eruptions and earthquakes, or through industrial activities such as mining, burning of coal at plants and metal smelting activities (3–5). Exposure to high concentrations of arsenic can be toxic and is a public health concern. Most cases of human arsenic-induced toxicity are due to inorganic arsenic exposure. The principal route of exposure to arsenic for the general population is usually through the oral route, primarily in foods and drinking water. Studies have shown that long-term exposure to arsenic in drinking water produces a broad array of health effects (6). Prolonged ingestion (not <6 months) of arsenic above a safe dose will lead to chronic health problems identified as arsenicosis. Arsenicosis is usually exhibited by skin lesions characteristics such as hyperkeratosis, hypomelanosis, and hyperpigmentation (2). Chronic exposure to arsenic is also causally related to a severe peripheral vascular disease known as Blackfoot (7). Arsenic exposure also can affect systemic manifestations such as respiratory, hepatobiliary, cardiovascular, central nervous, gastrointestinal, hematopoietic and endocrine systems (8, 9). According to the International Agency for Research on Cancer (IARC) and US Environmental Protection Agency (USEPA), inorganic arsenic is classified as a carcinogen to human beings (10, 11). Therefore, exposed individuals have increased risks of cancers of the skin, lungs, bladder and kidney (12–14).
In Malaysia, the water supply for water usage primarily originates from rivers, and the population mainly consumes drinking water from tap water, bottled drinking water and bottled mineral water (15, 16). The primary water source in the Perak state of Malaysia is the Perak River. The Perak River is the longest in Perak and the second longest river in Peninsular Malaysia after the Pahang River. It originates from the mountains at the Perak-Kelantan-Thailand border. However, it needs to be treated in water treatment plants (WTP) before it is made potable for the residents. There are several WTP along this river, including the AG WTP. The AG WTP is located in the upper part of the Perak River and can produce 0.22 million liters per day (MLD) drinking water (17). The WTP is one of the oldest WTP and was built in 1976 at the same time as Village AG. The residents here were resettled from Village T, which was inundated when the hydroelectric dam was built in the 1970s. This AG WTP supplies treated water only to residents of AG Village. The Drinking Water Quality Program under the Engineering Division Ministry of Health is responsible for monitoring raw and treated water quality at the WTPs to ensure the consumers receive treated water free from contaminants. However, based on the monitoring data from the previous few years, there has been an occasional violation of the arsenic level in the water from the AG WTP. The presence of arsenic in treated water from the WTP has raised concerns about the potential health impact on the exposed community. Thus, the Ministry of Health and other related departments, such as the Chemistry Department, Department of Environment, Perak Forestry Department, and Perak Water Board, formed a Special Sanitary Survey. The survey aimed to identify the possible sources of arsenic contamination in raw water. Based on the survey, they found several potential activities that could contribute to arsenic in the water, affecting its quality, including mining, timber processing and agriculture activities along the river. Therefore, further evaluation and investigation must be conducted to formulate a solution to this problem. Given that there is no information and monitoring on the level of arsenic at the consumer end, hence, this study was conducted with the aims of (1) to determine the arsenic concentrations in drinking water in Village AG's households and Village P's households, (2) to determine the arsenic concentration in the hair of residents in Village AG and Village P, and lastly, (3) to determine the prevalence of arsenicosis in Village AG and Village P.
In this study, a comparative cross-sectional study was conducted to compare arsenic levels and arsenicosis prevalence between Village AG and Village P. Village AG received water supply from the WTP with high arsenic levels, whereas Village P received from the WTP with low levels of arsenic. The study involved sample collection of drinking water and hair, health surveys and physical examinations. A total of 639 participants (324 from Village AG and 315 from Village P) from 395 households (198 from Village AG and 197 from Village P) were recruited in this study. Then, the arsenic concentrations in drinking water and hair were determined and compared for both villages. The factors that increase the risk of arsenic concentration in hair have also been identified. After that, the prevalence signs and symptoms of arsenicosis were evaluated for both villages. The outcomes of this study offer baseline information for authorities to enhance water quality and ensure the populace's wellbeing.
This study was conducted in Gerik (5.4168°N, 101.1164°E), one of the districts in the state of Perak, Malaysia (Figure 1). The data collection was carried out from September 2018 to October 2019. A comparative cross-sectional study design was utilized in this study to compare arsenic levels between the exposed and non-exposed groups. The exposed group were from Village AG, which received treated water from a WTP with reported violations of arsenic level. The non-exposed group were from Village P, located about 2 km away from Village AG and received treated water from a different WTP with no violations of arsenic level.
The Power and Sample Size Program software (PS version 3.0.1) was used to calculate the sufficient sample size for the cross-sectional study by comparing two proportions. For calculation, we used a power of the study of 80%, with an alpha level of 0.05 at 95% confidence level and also taking into account non-response, we inflated the sample size by 20%. Based on the calculation, 311 respondents are needed from each village to achieve a sufficient sample size. The census conducted by the Hulu Perak District Health Office showed that Village AG has 1,152 residents living in 288 houses, while Village P has about 595 residents in 132 houses. Based on the total number of residents, we estimated the average number of adults per house in Village AG and Village P to be 2.3 and 2.8, respectively. Therefore, to achieve 311 respondents from each village, a total of 135 houses were selected from Village AG and 120 houses from Village P. The study participants were selected from a universal sample of adults aged 18 and above residing in the study village for at least 1 year. Participation was voluntary, and only those given written consent were selected as study subjects. For the sampling of households, we used a random systematic sampling technique. For each village, houses were assigned a number, and SPSS software was used to randomly select the required number of houses in each village for this study.
Before collecting water samples, the taps were turned on for 5 min to flush the water from the piping. Then, 1 L of water was collected directly into the pre-washed polyethylene bottles [pre-washed with 1% nitric acid (HNO3) to eliminate contaminants]. 3 ml of concentrated HNO3 (65%) was added to each water sample to ensure the pH was kept below 2. All the collected samples were transported and stored at 4°C. The analysis of arsenic concentration in tap water was performed according to USEPA Method 6020A using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), ELAN 9000, Perkin Elmer. About 10 ml of water was aliquoted and filtered using a 0.45 mm PTFE membrane to remove the sediments. Then, 0.3 ml HNO3 65% was added to ensure the concentration of aliquots met the standard calibration curve recommended by ICP-MS analytical technique. Dilution was done with 2% HNO3 by preparing 18.2 Ω milliQ Ultra-Pure Water (UPW) with 65% HNO3. The calibration curve for tap water was prepared using standard concentrations of 2, 5, 10, 20, and 50 ppb, prepared from stock solution with 2% nitric HNO3 as diluent.
About 1 g of hair sample was collected from each respondent. Samples were taken at the posterior vertex and nape of the head as close as possible to the scalp using stainless steel scissors (18). Samples were sealed in labeled zipped plastic bags before being transported to the laboratory. Before analysis using ICP-MS, the hair sample was cut into small pieces at least 3 mm in length. Then, samples were washed once with 25 ml acetone, three times with 25 ml deionized water, and lastly with 25 ml acetone (19). Each washing step requires 10 min of shaking using a sonicator. After washing, the samples were dried overnight at 60°C.
Hair samples were digested using Milestone Microwave Laboratory System, ETHOS, with a rotor for 15 Teflon vessels (SK-15 high-pressure rotor). Approximately 0.1 g of dry hair samples were weighed in a clean digestion vessel. Then, 10 ml of HNO3 65% Suprapur (MERCK, Germany) was added. The vessels were closed, tightened and put inside the microwave digestion system. After digestion, the vessels were taken out from the machine and opened carefully inside a fume hood after the system's temperature reached <60°C. The solutions were stored in centrifuge tubes and were diluted up to 50 ml for further analysis.
The calibration curve for hair samples analysis was prepared using standard concentrations of 5, 10, 20, 50, and 100 ppb. Sample blanks were prepared for quality control of every digestion batch to correct the background sample reading from contamination in reagents, filters or ultrapure water. The calibration check standard was analyzed at the beginning and end of the analysis and for every 10 samples for quality control purposes. The analytical method was validated using European Reference Material (ERM) DB001-human hair (Trace Element).
Participation in this study was voluntary, and only those who gave written consent were selected as study subjects. Inclusion criteria were respondents aged 18 years and older who have lived in the area for at least 1 year. For each selected village, a medical team consisting of a medical doctor and a nurse or medical assistant were recruited to interview respondents. The interview was conducted using a standardized questionnaire, and a physical examination was also performed to look for signs of arsenicosis (hyperkeratosis, hypomelanosis, hyperpigmentation, and Mees' line). All team members received training related to the study from a family medicine specialist and a public health specialist. An album of images was provided to each team to help them identify the signs of arsenicosis on the skin and nails. They were also trained to collect hair samples properly.
Statistical analysis was performed using SPSS version 26.0 for Windows (SPSS Inc., Chicago, IL, USA). First, descriptive statistics were calculated to explore and describe the data. Associations between categorical data for signs and symptoms with hair arsenic concentration were then assessed using the chi-square test. Lastly, univariate and multivariate logistic regression analyses were conducted to determine the effect of each risk factor (age, gender, duration of stay, smoking status, study villages and arsenic level in water) on the risk of having high hair arsenic level (more than 1 μg/g) among the respondents. All statistical tests were conducted at a 95% confidence interval, using p = 0.05.
A total of 639 adult respondents from Village AG and Village P consented to participate in this study. The participants included 324 respondents from Village AG and 315 respondents from Village P. Majority of respondents were females in both villages. More than 40% of respondents in both villages were housewives, students, and pensioners. The respondents were between 18 and 93 years old, with a mean age of 45.9 (SD ±14.8) years for Village AG and 46.1 (SD ±16.6) years for Village P. About 254 (78.4%) respondents in Village AG and 186 (59%) of Village P respondents lived there for more than 10 years. The data also showed that in both villages, the respondents were mostly non-smokers (Table 1).
The results of arsenic concentrations in drinking water and hair were summarized in Table 2. The total number of drinking water samples collected from households in Village AG and Village P was 198 and 197, respectively. The results showed that the sample collected from Village AG had the highest arsenic concentration in drinking water (0.0223 mg/L) compared to Village P (0.0017 mg/L). The hair arsenic concentrations among respondents in Village AG ranged from 0.05 to 4.15 μg/g, with a median of 0.65 μg/g. Meanwhile, hair arsenic concentration among respondents from Village P ranged between 0.04 and 1.99 μg/g, with a median of 0.17 μg/g, which was lower than respondents from Village AG.
According to the National Drinking Water Quality Standards (NDWQS) (22), water samples from 81 (41.3%) households in Village AG exceeded the maximum acceptable value of 0.01 mg/L. In contrast, none of the water samples from Village P exceeded this level (Figure 2). In contexts of hair arsenic concentration, the results showed that 82 (25%) of 324 respondents from Village AG and 3 (1%) out of 315 respondents from Village P had levels above 1 μg/g, indicating unsafe arsenic exposure (Figure 3) (20, 21).
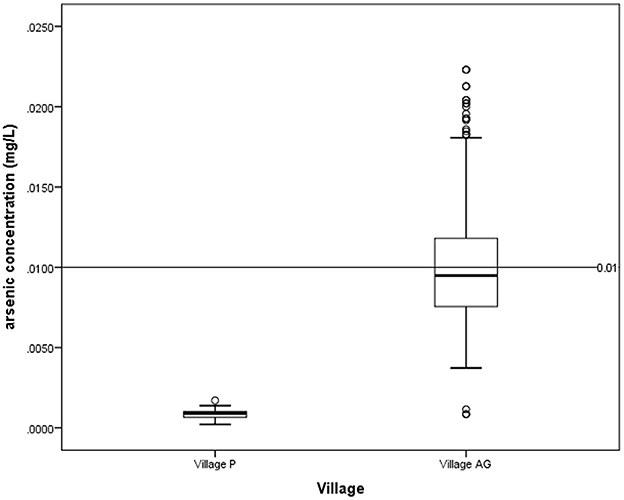
Figure 2. Arsenic concentration in drinking water for village P and village AG based on NDWQS guideline of 0.01 mg/L.
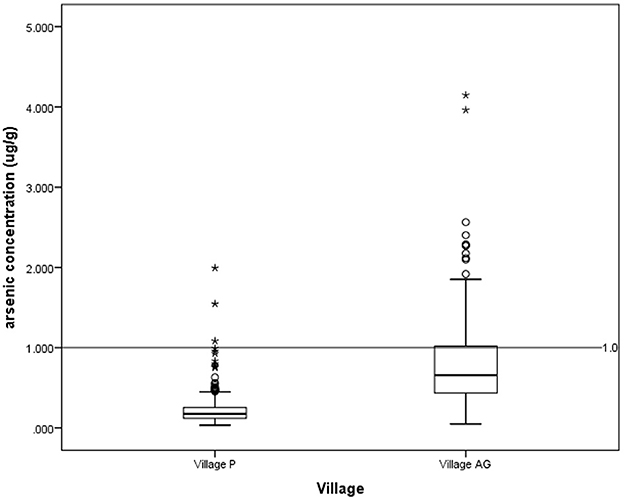
Figure 3. Arsenic concentration in hair for village P and village AG based on a guideline of 1.0 μg/g. *outlier.
Out of the total number of respondents, 554 respondents had safe levels of hair arsenic (<1 μg/g), while 85 respondents had unsafe levels of hair arsenic (≥1 μg/g). Three arsenicosis signs were identified during the survey: hyperkeratosis, hyperpigmentation, and hypomelanosis (Figures 4–6). The results showed that the respondents with unsafe levels of arsenic demonstrated a higher prevalence of hyperkeratosis (10.6%), hyperpigmentation (9.4%), and hypomelanosis (4.7%) than those with low levels of hair arsenic. Hyperkeratosis prevalence showed a significant difference between respondents with high hair arsenic levels and those with low levels. A similar trend was also observed for study villages where the exposed village (Village AG) showed a significantly higher incidence of arsenicosis hyperkeratosis (8.3%), hyperpigmentation (9.7%), and hypomelanosis (4.3%) than those in Village P (Table 3). A confirmed diagnosis was defined when at least one sign of arsenicosis presence and hair arsenic equal to or >1 μg/g (21). Using these criteria, 18 respondents met the requirements, yielding a prevalence rate of 21.2%.
The three most common symptoms reported by respondents were numbness of fingertips, palms, and soles, frequent headaches, and tingling sensation. All these symptoms, with the addition of forgetfulness and frequent nausea, were significantly higher for respondents with elevated hair arsenic levels (≥1 μg/g) than those with lower arsenic levels. When the symptoms were compared between the two villages, the prevalence of having symptoms of arsenicosis was greater in Village AG compared to the control, Village P. The symptoms of frequent stomachache, headache, forgetfulness, tingling sensation, and numbness of fingertips, palms, and soles were significantly higher in Village AG compared to Village P (Table 4).
The result demonstrated that the risk factors of age, female gender, living in Village AG and smoking cigarettes were significantly associated with high hair arsenic levels in this study. The highest risk was living in Village AG, with an adjusted odd ratio (AOR) of 33.03. An increment of 1 year in age increased the risk of elevated hair arsenic levels by 2%. Females in this study had a 6.5-fold risk of having elevated hair arsenic compared to males and smoking also increased the risk of elevated hair arsenic by 4-fold compared to non-smokers (Table 5).
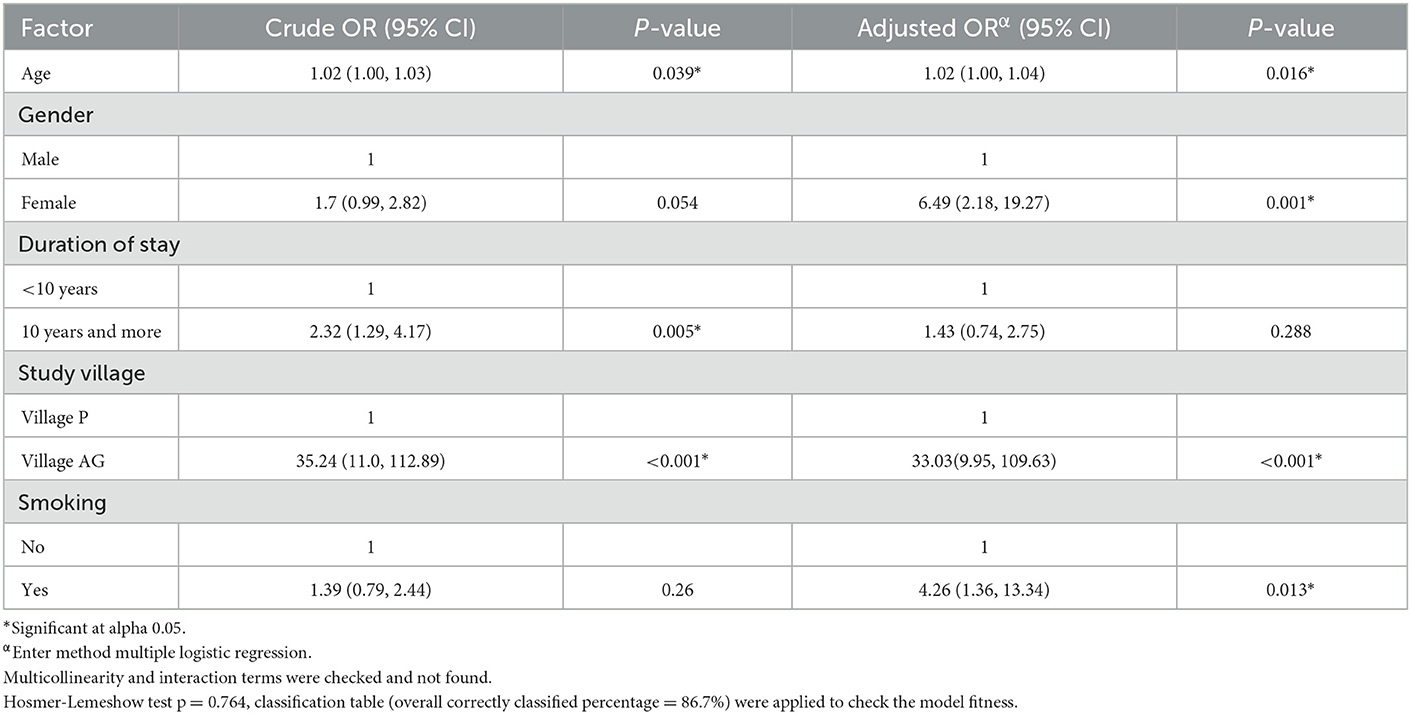
Table 5. Logistic regression analysis to determine factors associated with hair arsenic levels of more than 1 ug/g.
In this study, we found that arsenic concentration in Village AG drinking water was higher than the guidelines for drinking water by WHO and NDWQS (6, 22). However, the arsenic concentration in Village AG was not as high as in other countries such as Cambodia and Bangladesh. For example, Sthiannopkao et al. (23) and Gault et al. (24) reported that the arsenic concentration in Kandal, Cambodia's drinking water ranges from 5–543 to 0.21–943 μg/L, respectively. Meanwhile, in Bangladesh, the British Geological Survey in 1998 shown out of 2022 water samples collected, 35% had arsenic concentrations of more than 50 μg/L, and 8.4% were above 300 μg/L (25). The differences between our study and Bangladesh are probably due to different drinking water sources. In Village AG, the source of drinking water is mainly from the river; meanwhile, in those countries, their primary water sources are groundwater, that believed to have rich-arsenic content. Furthermore, arsenic contamination of the drinking water in Village AG is also believed to be a possibility due to seasonal variations. The Village AG drinking water collections were conducted in September and October during the rainy seasons. This finding is comparable with studies by Egbinola and Amanambu (26) and Savarimuthu et al. (27) showed that high arsenic concentrations were found during the early monsoon season, proving that seasonal disparities exist in contaminant concentration.
Hair is used as the biomarker in this present study to determine human exposure to inorganic arsenic. Compared to the other biomarkers such as urine and blood serum, hair had more advantages as it records chronic exposure to arsenic which occurs a few months before collection (24). Moreover, it is more convenient for collecting, storing, and transporting samples. Based on the findings of elevated arsenic in drinking water and hair of respondents in Village AG, it is highly suggestive that there has been chronic arsenic exposure among the residents as a level of more than 1.0 μg/g in hair usually indicates chronic intoxication (9, 28). Furthermore, many other studies reported that those exposed to prolonged environmental arsenic had a high arsenic concentration in their hair. For example, in the studies in Kandal Province, Cambodia, by Phan et al. (19), Sthiannopkao et al. (23) and Sampson et al. (29), the arsenic in hair ranged from 0.271–30.09, 0.06–30, and 0.05–13.94 μg/g respectively. However, using hair as a biomarker had limitations as the arsenic in hair could be influenced by pathways other than drinking water, such as the consumption of rice and vegetables that are high in arsenic and exposure to exogenous arsenic during bathing.
This study demonstrated that residents with high arsenic levels in the water had a higher incidence of arsenicosis. The result was equivalent to the study conducted in China (38), where villages with poor water supplies and high arsenic in water yielded 13.48–19.67% prevalence rate of arsenicosis. This finding proves that the arsenic level in water is one of the main factors contributing to the incidence of arsenicosis in the community. In this study, the most frequently found sign was hyperkeratosis among the respondents with hair arsenic levels of more than 1 ug/g. This result corresponds to studies in Bangladesh and Iran, where hyperkeratosis was reported to be more prevalent than other signs (30, 31). However, the study by Hashim et al. (21) reported that hypomelanosis was more prominent in Kandal, Cambodia. The differences between our findings and the previous study are probably due to the length of exposure and genetic and nutritional differences in the study area (31). Eighteen respondents from Village AG had their hair arsenic level equal to or more than 1 μg/g. They showed at least one sign of arsenicosis, which fulfilled the criteria of confirmed cases of arsenicosis. These respondents will further be evaluated by a dermatologist and screened for cancer, as chronic arsenicosis could lead to skin, bladder, and kidney cancer.
Arsenicosis symptoms vary, and most of them are the leading cause of morbidity and mortality associated with this disease. There are no specific symptoms of arsenicosis. Therefore, it could be confused with another disease which had similar symptoms. In this study, the prevalence of symptoms such as frequent nausea, forgetfulness, frequent headache, tingling sensation, and numbness of fingertips, palms, and soles was significantly higher for respondents having hair arsenic levels of 1 ug/g and more. Previous studies supported this by stating that symptoms such as nausea could manifest due to chronic arsenic exposure in the gastrointestinal system (9, 32). A systematic review by Brinkel et al. (33) revealed that various studies confirmed that exposure to arsenic will modify the physiology of the brain and might lead to developmental disabilities and intellectual disability; this is because arsenic has the ability to cross the blood-brain barrier and affect the central nervous system. (33). A Taiwan study shows that adolescents' neurobehavioral function may be affected by childhood exposure to arsenic in drinking water (34). In Cambodia, children with high hair arsenic levels experienced a 1.57–4.67 times greater risk of having lower neurobehavioral test scores than those with low hair arsenic levels after adjusting for hair lead, manganese and cadmium (35).
In our study, females demonstrated higher arsenic concentration in hair than males. Most females in this study are housewives; thus, there could be a probability that the exposure to the contaminated drinking water is more remarkable as they used the contaminated water for cooking, drinking, bathing and other. Compared to males who work outside the village, they probably get their drinking water from other sources that are not contaminated with arsenic. This finding differs from the previous study, which reported that males are prone to have high hair arsenic compared to females as they work as a labor and drink more water containing arsenic (3, 36). We also found that smoking can increase the risk of having a high arsenic concentration in hair. A study in Austria which looked into the influence of tobacco on heavy metals in the hair shows that in smokers, the geometric mean of hair arsenic was 0.081 ug/g higher than non-smokers, 0.065 ug/g, thus supporting the finding in this study (37). According to WHO, the tobacco plant can take up natural inorganic arsenic, which is naturally present in soils, thus indirectly can expose smokers to the carcinogenic element. Therefore, smokers with high hair arsenic could probably be exposed to either inhaling tobacco or ingesting water contaminated with arsenic. However, both synergic effects are harmful as they increase lung cancer risk (37).
This study proved that arsenic was present in residents daily drinking water distributed from AG WTP. The study also found that people who live in an area exposed to high levels of As yielding high arsenicosis incidence. Although the arsenic contamination in Village AG is not severe as reported by other countries, the confirmed case of arsenicosis shows an alarming indication of chronic exposure, which could jeopardize public health in future. However, the finding in this study is only the tip of the iceberg of the arsenic pollution problem. Therefore, the policy-making bodies should find the primary source of arsenic pollution in the area to help eradicate the contamination.
Evidence from this study has been reported to the responsible authorities for further mitigation action. Subsequently, the authorities took immediate measures by shutting down the affected water treatment plant. Furthermore, they installed a new pipeline from another uncontaminated water treatment plant to provide arsenic-safe drinking water for residents in Village AG. However, long-term monitoring should be conducted to guarantee the safety of drinking water and track the progressive effectiveness of alternative arsenic-safe water supplies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Medical Research and Ethics Committee, Ministry of Health Malaysia. The patients/participants provided their written informed consent to participate in this study.
NR contributed to the data collection, analysis, and manuscript writing. HA contributed to the funding acquisition, study conception, and data collection. WW contributed to study design and manuscript writing. ZH and JH contributed to data interpretation. RS contributed to the funding acquisition, study conception, and design. All authors reviewed the manuscript.
This research was funded by the Ministry of Health Malaysia under research grant NMRR:18-391-40698.
The authors would like to thank the Director General of Health, Malaysia, for the permission to publish this study. We would like to thank all the healthcare workers (medical doctors, environmental health officers in the water quality unit, nurses, medical assistants, and drivers) from the Hulu Perak Health Department for their assistance in water and hair sample collection. We also like to specially thanks Dr. Asiah binti Ayob, Dr. Marina binti Kamarudin, Dr. Rohaidah binti Ahmad, Mr. Fakrusyi Syakirin bin Zainol Alam and Mrs. Nur Adilla Fatihah binti Mohd Amran for helping to ensure this study went well and smooth. Our deepest gratitude to Mrs. Nadrah bin Naim, Mrs. Cathrinena Anak Robun and Mr. Mohamad Hairulhisham bin Hairi from the Environmental Health Research Center, Institute for Medical Research, for assisting in the sample analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Habashi F. Arsenic, physical and chemical properties BT—encyclopedia of metalloproteins. In: Kretsinger RH, Uversky VN, Permyakov EA, editors. Encyclopedia of Metalloproteins. New York, NY: Springer (2013), pp. 162–163. doi: 10.1007/978-1-4614-1533-6_406
2. James KA, Meliker JR, Nriagu JO. Arsenic. In: Quah E, editor. International Encyclopedia of Public Health, 2nd ed. Oxford: Academic Press (2017). p. 170–175. doi: 10.1016/B978-0-12-803678-5.00025-4
3. Masindi V. Environmental contamination by heavy metals. In: Aglan KL, editor. Heavy Metal. Rijeka: IntechOpen. (2018). doi: 10.5772/intechopen.76082
4. Hasan SE. Medical geology. Encycl Geol. (2021) 9:684–702. doi: 10.1016/B978-0-12-409548-9.12523-0
5. Callender E. Heavy metals in the environment-historical trends. Treat Geochem. (2003) 9:67–105. doi: 10.1016/B0-08-043751-6/09161-1
6. World Health Organization (WHO). Guidelines for Drinking-Water Quality. 4th ed. Geneva: WHO (2017).
7. Gomez-Caminero A, Howe P, Hughes M, Kenyon E, Lewis DR, Moore M, et al. Arsenic and Arsenic Compounds. Environmental Health Criteria 224, World Health Organization (2001). Available online at: http://whqlibdoc.who.int/ehc/WHO_EHC_224.pdf (accessed July 15, 2020).
8. Mazumder DN, Haque R, Ghosh N, De BK, Santra A, Chakraborti D, et al. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int J Epidemiol. (2000) 29:1047–52. doi: 10.1093/ije/29.6.1047
9. Das A, Paul R, Das N. Chronic arsenicosis: clinical features and diagnosis. Arsen Toxic. (2015) 113:375–400. doi: 10.1201/b18734-17
10. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Evaluat Carcinogen Risks Hum. (2012) 100:407–43.
11. USEPA. Chemical Assessment Summary: Arsenic (Inorganic). Iris: USEPA (1988). Available online at: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?&substance_nmbr=278 (accessed June 15, 2020).
12. Chen CJ, Kuo TL, Wu MM. Arsenic and cancers. Lancet. (1988) 331:414–5. doi: 10.1016/S0140-6736(88)91207-X
13. Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. J Toxicol. (2011) 2011:1287. doi: 10.1155/2011/431287
14. Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Heal A Glob Access Sci Sour. (2014) 13:44. doi: 10.1186/1476-069X-13-44
15. Ab Razak NH, Praveena SM, Aris AZ, Hashim Z. Drinking water studies: a review on heavy metal, application of biomarker and health risk assessment (a special focus in Malaysia). J Epidemiol Glob Health. (2015) 5:297–310. doi: 10.1016/j.jegh.2015.04.003
16. Annua ZF, Azmi WN, Ahmad NI, Sham NM, Mahiyuddin WR, Veloo Y, et al. Drinking water quality in Malaysia: a review on its current status. Int J Environ Sci Nat Resour. (2020) 24:6132. doi: 10.19080/IJESNR.2020.24.556132
17. Lembaga Air Perak (2020). Available online at: http://www.lap.com.my/bm/index.php (accessed June 15, 2020).
18. Yáñez J, Fierro V, Mansilla H, Figueroa L, Cornejo L, Barnes RM. Arsenic speciation in human hair: a new perspective for epidemiological assessment in chronic arsenicism. J Environ Monit. (2005) 7:1335–41. doi: 10.1039/b506313b
19. Phan K, Sthiannopkao S, Kim KW, Wong MH, Sao V, Hashim JH, et al. Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res. (2010) 44:5777–88. doi: 10.1016/j.watres.2010.06.021
20. WHO. A Field Guide for Detection, Management and Surveillance of Arsenicosis in South-East Asia Region. Geneva: WHO (2005).
21. Hashim JH, Radzi RS, Aljunid SM, Nur AM, Ismail A, Baguma D, et al. Hair arsenic levels and prevalence of arsenicosis in three Cambodian provinces. Sci Total Environ. (2013) 463–464:1210–1216. doi: 10.1016/j.scitotenv.2013.04.084
22. WHO. Standard Kualiti Air Minum Kebangsaan. Geneva: WHO (2020). Available online at: http://engineering.moh.gov.my/v4/download.php?i=43 (accessed June 15, 2020).
23. Sthiannopkao S, Kim KW, Cho KH, Wantala K, Sotham S, Sokuntheara C, et al. Arsenic levels in human hair, Kandal Province, Cambodia: the influences of groundwater arsenic, consumption period, age and gender. Appl Geochem. (2010) 25:81–90. doi: 10.1016/j.apgeochem.2009.10.003
24. Gault AG, Rowland HA, Charnock JM, Wogelius RA, Gomez-Morilla I, Vong S, et al. Arsenic in hair and nails of individuals exposed to arsenic-rich groundwaters in Kandal province, Cambodia. Sci Total Environ. (2008) 393:168–76. doi: 10.1016/j.scitotenv.2007.12.028
25. Smith AH, Lingas EO, Rahman M. Contamination of drinking water by arsenic in Bangladesh: a public health emergency. BullWorld Health Organ. (2000) 78:1093.
26. Egbinola CN, Amanambu AC. Groundwater contamination in Ibadan, South-West Nigeria. Springerplus. (2014) 3:448. doi: 10.1186/2193-1801-3-448
27. Savarimuthu X, Hira-Smith MM, Yuan Y, von Ehrenstein OS, Das S, Ghosh N, et al. Seasonal variation of arsenic concentrations in Tubewells in West Bengal, India. J Heal Popul Nutr. (2006) 24:277–81.
28. Arnold WJ, Odom RB. Diseases of the Skin, 8th ed. Philadelphia: WB Saunders Co. (1990). p. 879–882.
29. Sampson ML, Bostick B, Chiew H, Hagan JM, Shantz A. Arsenicosis in Cambodia: case studies and policy response. Appl Geochem. (2008) 23:2977–86. doi: 10.1016/j.apgeochem.2008.06.022
30. Kadono T, Inaoka T, Murayama N, Ushijima K, Nagano M, Nakamura S, et al. Skin manifestations of arsenicosis in two villages in Bangladesh. Int J Dermatol. (2002) 41:841–6. doi: 10.1046/j.1365-4362.2002.01668.x
31. Mosaferi M, Yunesian M, Dastgiri S, Mesdaghinia A, Esmailnasab N. Prevalence of skin lesions and exposure to arsenic in drinking water in Iran. Sci Total Environ. (2008) 390:69–76. doi: 10.1016/j.scitotenv.2007.09.035
32. Guha Mazumder D, Dasgupta UB. Chronic arsenic toxicity: studies in West Bengal, India. Kaohsiung J Med Sci. (2011) 27:360–70. doi: 10.1016/j.kjms.2011.05.003
33. Brinkel J, Khan MH, Kraemer A, A. systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Environ Res Public Health. (2009) 6:1609–19. doi: 10.3390/ijerph6051609
34. Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology. (2003) 24:747–53. doi: 10.1016/S0161-813X(03)00029-9
35. Vibol S, Hashim JH, Sarmani S. Neurobehavioral effects of arsenic exposure among secondary school children in the Kandal Province, Cambodia. Environ Res. (2015) 137:329–37. doi: 10.1016/j.envres.2014.12.001
36. Hadi A, Parveen R. Arsenicosis in Bangladesh: prevalence and socio-economic correlates. Public Health. (2004) 118:559–64. doi: 10.1016/j.puhe.2003.11.002
37. Chen CL, Hsu LI, Chiou HY, Hsueh YM, Chen SY, Wu MM, et al. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. J Am Med Assoc. (2004) 292:2984–90. doi: 10.1001/jama.292.24.2984
Keywords: arsenic poisoning, hair, water, prevalence, carcinogens, mass spectrometry
Citation: Ramly N, Ahmad Mahir HM, Wan Azmi WNF, Hashim Z, Hashim JH and Shaharudin R (2023) Arsenic in drinking water, hair, and prevalence of arsenicosis in Perak, Malaysia. Front. Public Health 11:998511. doi: 10.3389/fpubh.2023.998511
Received: 20 July 2022; Accepted: 26 January 2023;
Published: 16 February 2023.
Edited by:
Mohammad Javad Mohammadi, Ahvaz Jundishapur University of Medical Sciences, IranReviewed by:
Anirban Biswas, Nabadwip Vidyasagar College, IndiaCopyright © 2023 Ramly, Ahmad Mahir, Wan Azmi, Hashim, Hashim and Shaharudin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan Nurul Farah Wan Azmi,  bnVydWwuZmFyYWhAbW9oLmdvdi5teQ==
bnVydWwuZmFyYWhAbW9oLmdvdi5teQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.