- Epidemiology Unit, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
The objective of this study was to compare the effect of diabetes and pathologies potentially related to diabetes on the risk of infection and death from COVID-19 among people from Highly-Developed-Country (HDC), including Italians, and immigrants from the High-Migratory-Pressure-Countries (HMPC). Among the population with diabetes, whose prevalence is known to be higher among immigrants, we compared the effect of body mass index among HDC and HMPC populations. A population-based cohort study was conducted, using population registries and routinely collected surveillance data. The population was stratified into HDC and HMPC, according to the place of birth; moreover, a focus was set on the South Asiatic population. Analyses restricted to the population with type-2 diabetes were performed. We reported incidence (IRR) and mortality rate ratios (MRR) and hazard ratios (HR) with 95% confidence interval (CI) to estimate the effect of diabetes on SARS-CoV-2 infection and COVID-19 mortality. Overall, IRR of infection and MRR from COVID-19 comparing HMPC with HDC group were 0.84 (95% CI 0.82–0.87) and 0.67 (95% CI 0.46–0.99), respectively. The effect of diabetes on the risk of infection and death from COVID-19 was slightly higher in the HMPC population than in the HDC population (HRs for infection: 1.37 95% CI 1.22–1.53 vs. 1.20 95% CI 1.14–1.25; HRs for mortality: 3.96 95% CI 1.82–8.60 vs. 1.71 95% CI 1.50–1.95, respectively). No substantial difference in the strength of the association was observed between obesity or other comorbidities and SARS-CoV-2 infection. Similarly for COVID-19 mortality, HRs for obesity (HRs: 18.92 95% CI 4.48–79.87 vs. 3.91 95% CI 2.69–5.69) were larger in HMPC than in the HDC population, but differences could be due to chance. Among the population with diabetes, the HMPC group showed similar incidence (IRR: 0.99 95% CI: 0.88–1.12) and mortality (MRR: 0.89 95% CI: 0.49–1.61) to that of HDC individuals. The effect of obesity on incidence was similar in both HDC and HMPC populations (HRs: 1.73 95% CI 1.41–2.11 among HDC vs. 1.41 95% CI 0.63–3.17 among HMPC), although the estimates were very imprecise. Despite a higher prevalence of diabetes and a stronger effect of diabetes on COVID-19 mortality in HMPC than in the HDC population, our cohort did not show an overall excess risk of COVID-19 mortality in immigrants.
Introduction
Risk factors increasing COVID-19 mortality can act in two main ways: by increasing the probability of infection or by increasing the severity and lethality of the disease following infection. In addition to biological characteristics, a multitude of social and economic factors can influence both the probability of infection and the severity of the disease. Several studies have shown that mortality from COVID-19, as reported by routine statistics, provides an accurate snapshot of deaths in which the main cause was actually SARS-CoV-2 infection and its short-term consequences (1–3).
COVID-19 and ethnicity
The main clinical risk factors for COVID-19 mortality are age, male sex, obesity, and some chronic diseases, including in particular renal failure, diabetes, and dementia (4). The effect of ethnicity and migrant status changes according to context (5, 6). Most of the published studies were based on national data or on large cohorts built in the United Kingdom (UK) and the USA. These studies observed an excess of incidence and to a lesser extent of mortality from COVID-19 in the most deprived groups and in some ethnic minorities (7), particularly populations of Asian origin (8). In the UK, the differential was greater for COVID-19 mortality than for other causes of death (5). In Italy, during the first two waves, there was no overall excess of either infection or mortality in the immigrant population (9–12). Both incidence and mortality were lower in immigrants in the first wave of the pandemic. During the second wave, the risk of infection and mortality in immigrants was similar in females and, in males, slightly higher than in Italians. Only for hospitalizations, an excess risk in immigrants was constantly observed (13, 14).
A study conducted in the USA found some differences in the strength of risk factors for infection—but not for severity of disease—in African Americans compared to whites: a higher incidence in children, a greater effect of prior cancer, a lesser effect of crowded housing, and a greater effect of obesity (15).
Diabetes, obesity, and ethnicity and the effect on COVID-19 mortality
Excess weight and diabetes are negative prognostic factors in COVID-19. A review (16) published at the beginning of the pandemic reported that chronic inflammation, increased coagulation activity, immune response impairment, and potential direct pancreatic damage by SARS-CoV-2 might be among the underlying mechanisms of the association between diabetes and COVID-19 severity. A more recent review (17) focused also on the relationship between body mass index (BMI) and COVID-19-related death and between blood glucose controls on poor COVID-19 outcomes. These factors appear to be useful as a prognostic tool. However, the strength of the association between BMI and COVID-19 severity and mortality varies according to ethnic group (15, 18–20). Diabetes and, in some contexts, obesity show higher prevalence among some ethnic minorities and immigrants from eastern countries, particularly people from South Asia (21). The most deprived populations also often show the poorest glycemic control, possibly because of barriers to effective care and difficulties in reconciling diet regimens and physical activity with economic and time constraints. Nevertheless, epidemiological and clinical evidence suggest that, in South Asians, gaining good glycemic control is more difficult, independently from diet, physical activity, and treatment compliance (22, 23).
Studies carried out in the UK showed that the association between COVID-19 mortality and obesity was stronger in populations originating from South Asia, intermediate in populations of African origin and minor in populations of European origin (19, 24). Differences were reduced by adjusting for pre-clinical conditions (24). This greater effect was also found for people with diabetes. In addition, a study conducted in England in a large cohort of people with diabetes showed that the differences were reduced by adjusting for pre-COVID-19 clinical conditions, suggesting that part of the effect was due to worsened control of diabetes and its complications among the most deprived populations (18). This study also observed that the effect of BMI on COVID-19 mortality was greater in populations of Asian and African origin (18).
Analysis of the effect of BMI and glycemic control in the sub-population of people with diabetes could help to disentangle the associations between ethnicity, diabetes, and COVID-19 mortality. BMI and glycemic control are routinely collected in the Reggio Emilia diabetes registry.
Objective of the study
The objective of this study was to compare the effect of diabetes and of pathologies potentially related to diabetes on the risk of infection and death from COVID-19 among people from Highly-Developed-Countries (HDCs) including Italians, and immigrants from High-Migratory-Pressure-Countries (HMPCs). Among the population with diabetes, we also aimed to compare the effect of glycemic control and BMI in Italians and immigrants.
Materials and methods
Study setting and design
The province of Reggio Emilia has 532,000 inhabitants. A population-based cohort study was conducted. Information was gathered from routine clinical registries and surveillance databases. The study was approved by the Area Vasta Emilia Nord Ethics Committee (Approval No 2020/0045199), which also allowed the population to be included without requiring informed consent, given the retrospective nature of the study.
Study population
All residents aged over 18 years in the province of Reggio Emilia as of December 31, 2019 were included. The outcomes included all infections reported by COVID-19 surveillance from the beginning of the epidemic (first case diagnosed in Reggio Emilia on February 27, 2020) until August 10, 2021. These cases were then followed up for COVID-19 mortality until September 20, 2021.
Data sources
Different information sources were used:
• For the resident population, the local health unit resident population registry, updated in real time by the municipal registries from all municipalities within the province; from this source we took information on residence, deaths and country of birth;
• For the outcomes, the local health unit COVID-19 surveillance system, which provided daily data on SARS-CoV-2 infections, hospitalizations and deaths from COVID-19 to the regional coordination structure and from there to the National Institute of Health. Locally, the system was integrated with the laboratory application, the hospitalization management system, the contact tracing management system and mortality registries;
• For the assessment of pre-existing comorbidities, the hospital information system;
• The variables relating to previous cancers were acquired from the cancer registry (25);
• The presence of diabetes, glycated hemoglobin values (HbA1c) and BMI data were acquired from the diabetes registry (26);
• Data about administered vaccines were retrieved from the local health authority vaccine information system.
A detailed description of record linkage operations is described in the report by Mangone and colleagues (25).
Role of the main variables
Outcomes
• SARS-CoV-2 infection: positive molecular test (or third-generation antigenic test since December 1, 2020). Only first infections were considered and not re-infections.
• Death from COVID-19: death occurring within 30 days from the diagnosis of positivity to SARS-CoV-2.
We focused on mortality and not on fatality rates to measure the impact of diabetes on COVID-19 severity because, based on the assumption of more limited testing in immigrants and consequent undiagnosed disease, the incidence of infection in immigrants may be underestimated, thus overestimating fatality. Actually, in Emilia-Romagna Region, a reduced probability of testing has been demonstrated, especially for women (9) from HMPC populations (27), and increased screening activity related to international traveling has also been observed in people from HPMCs (28).
Stratification and exposures
Migrant status was our stratification variable. This was defined, on the basis of country of birth, as high developed countries (HDC) and high migration pressure countries (HMPC). The HDC population included Italians and immigrants from Europe (Andorra, Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Iceland, Liechtenstein, Luxemburg, Netherlands, Norway, Principality of Monaco, Portugal, Republic of Saint Marin, Spain, Sweden, Switzerland, United Kingdom and Vatican City), North America (United States and Canada), Australia (Australia and New Zeland) and Asia (Israel, Japan and South Korea). Immigrants from all the other countries were considered among the HMPC population (29). Among HMPC a focus was presented about South Asians, because of the known high prevalence of diabetes in this population (21).
A sensitivity analysis restricted to people <65 years of age was performed. The rationale underlying the stratified analysis by age was met in the different age structure of the two populations: people from HMPC had a very low proportion of people over 65. Moreover, a strong interaction was observed between the effect of comorbidity and age on COVID-19 prognosis, thus adjusting may not be sufficient to take into account the difference in age structure.
Type-2 diabetes was our main exposure analysis. In the descriptive analyses, we also considered other diabetes types (Type-1, other, and undefined), but people with other forms of diabetes were excluded from association analyses.
In the sub-population with diabetes, the main determinants of outcomes were the most recent glycemic control and BMI measurements in the period between January 1, 2018, and December 31, 2019. BMI was divided according to the quartiles (<25.90, 25.90–29.07, 29.07–32.90, >32.90).
We also included information about recent comorbidities—such as ischemic heart disease, chronic renal failure, hypertension, obesity, heart failure, arrhythmia, vascular disease and stroke—collected from hospital discharge databases (2015–2019). The Charlson Comorbidity Index (CCI) was calculated based on hospital admissions in the previous 5 years and it was used to evaluate the impact of comorbidity on selected outomes (30). Our CCI incorporated 17 different comorbidities, each of which was weighted according to its potential impact on mortality. This index has been previously applied to COVID-19 patients (31).
Furthermore, the pandemic had different characteristics in different periods: different control measures, different variants, and finally different susceptibility of the populations after the vaccination campaign. For this reason, we added a stratified analysis by calendar period, which may suggest differences if control measures and variants were effect modifiers of the observed associations.
Adjustment variables
Age, sex and COVID-19 vaccination until August 10, 2021 were used as adjustment variables in the models.
Statistical analysis
All the analyses were stratified by place of birth, HDC (including Italy), and HMPC. A focus on South Asians known to have the highest risk and prevalence of diabetes was added. Crude COVID-19 incidence and mortality rates (IRs and MRs) were reported. We reported IRs and MRs per 1,000 persons, incidence and mortality in people from HDC and from HMPC were compared through incidence (IRR) and mortality rate ratios (MRR), with associated 95% confidence intervals (95%CI). Hazard ratios (HRs) with their associated 95% CI adjusted for sex and age were calculated using the Cox proportional hazards model. For the outcome of COVID-19 infections only, a further adjustment for COVID-19 vaccination was considered. COVID-19 vaccination was the only variable included as a time-varying covariate in the model. For COVID-19 mortality, this adjustment was not possible because of the limited number of deaths among vaccinated individuals (Table 1), and thus we presented analyses restricted to non-vaccinated individuals instead of adjusting for vaccine status. Analyses restricted to the diabetic population, stratified by country of birth, were also reported.
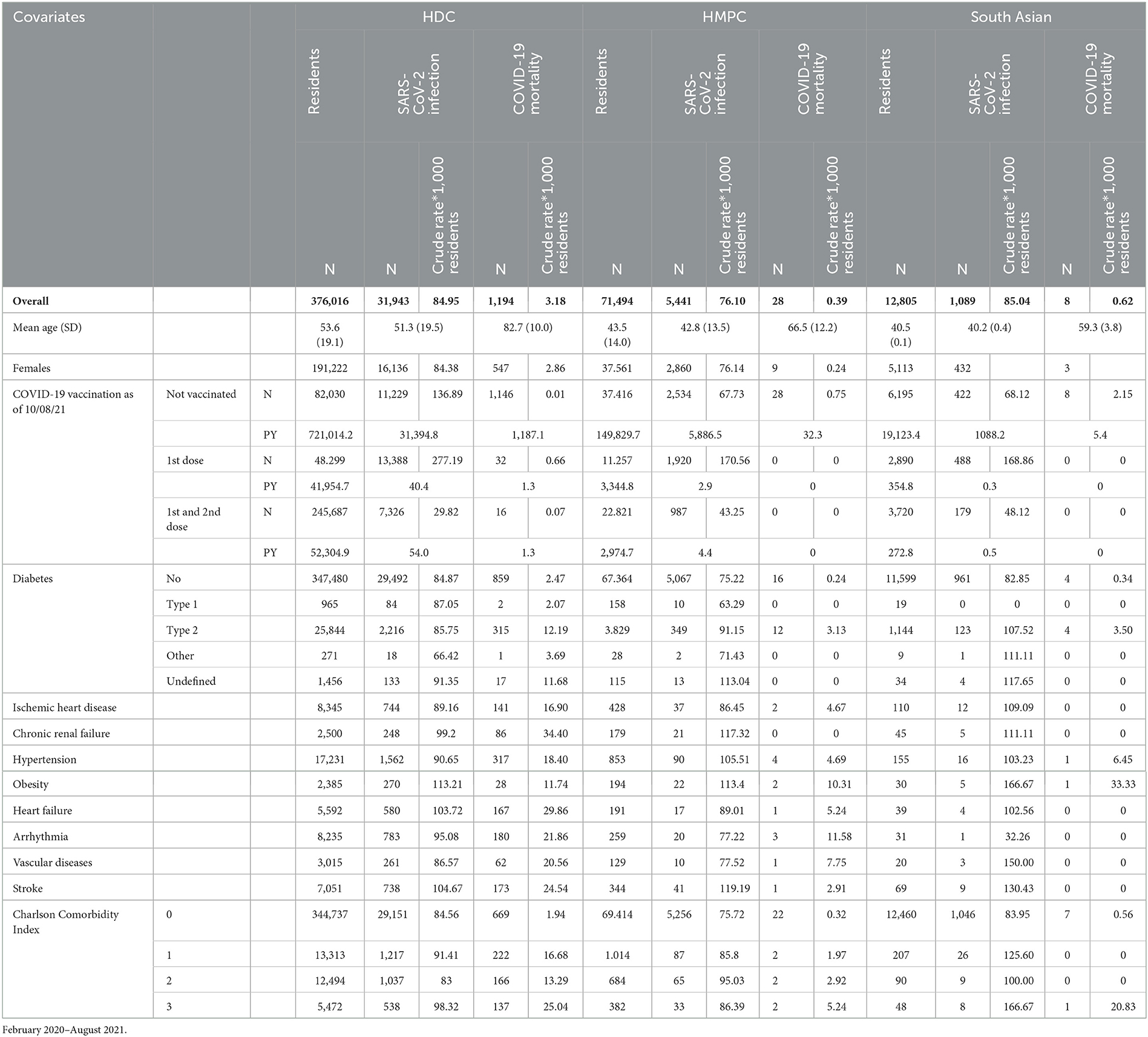
Table 1. Resident population in the province of Reggio Emilia, number of first SARS-CoV-2 infections and deaths from COVID-19, by demographic and clinical characteristics.
We decided not to use the term “significant” or “not-significant” since we did not set a significance threshold and we did not performe formal tests of hypothesis. Confidence intervals should be interpreted as continuous variables and should not be used to reject or accept the null hypothesis according to a pre-fixed threshold.
Sensitivity analyses were performed and were restricted to the population <65 years of age.
We added a stratified analysis according to the following four periods of time (1: February 22, 2020–June 1, 2020; 2: June 2, 2020–December 31, 2020; 3: January 1, 2021–June 30, 2021; 4: July 1, 2021–August 10, 2021), depending on the waves and variants of the infection, and these results are shown in the Supplementary material.
Two direct acyclic graphs (DAG) were built: the first described the causal network from contact with the virus to infection and the second described the causal network leading from infection to COVID-19-related death (Figures 1, 2).
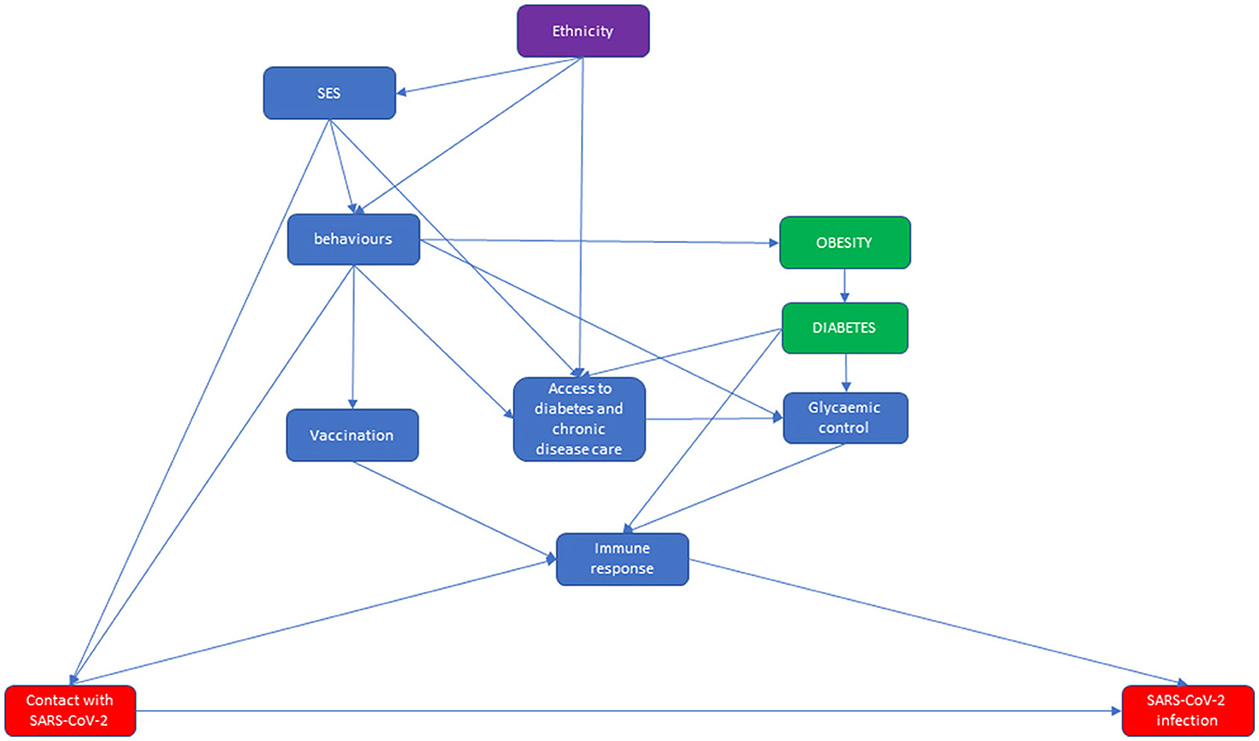
Figure 1. Direct acyclic graph (DAG) of the relationship between ethnicity and diabetes from contact with SARS-CoV-2 to SARS-CoV-2 infection, in which the effect modifier is ethnicity, the exposures are obesity and diabetes, and the outcome is SARS-CoV-2 infection.
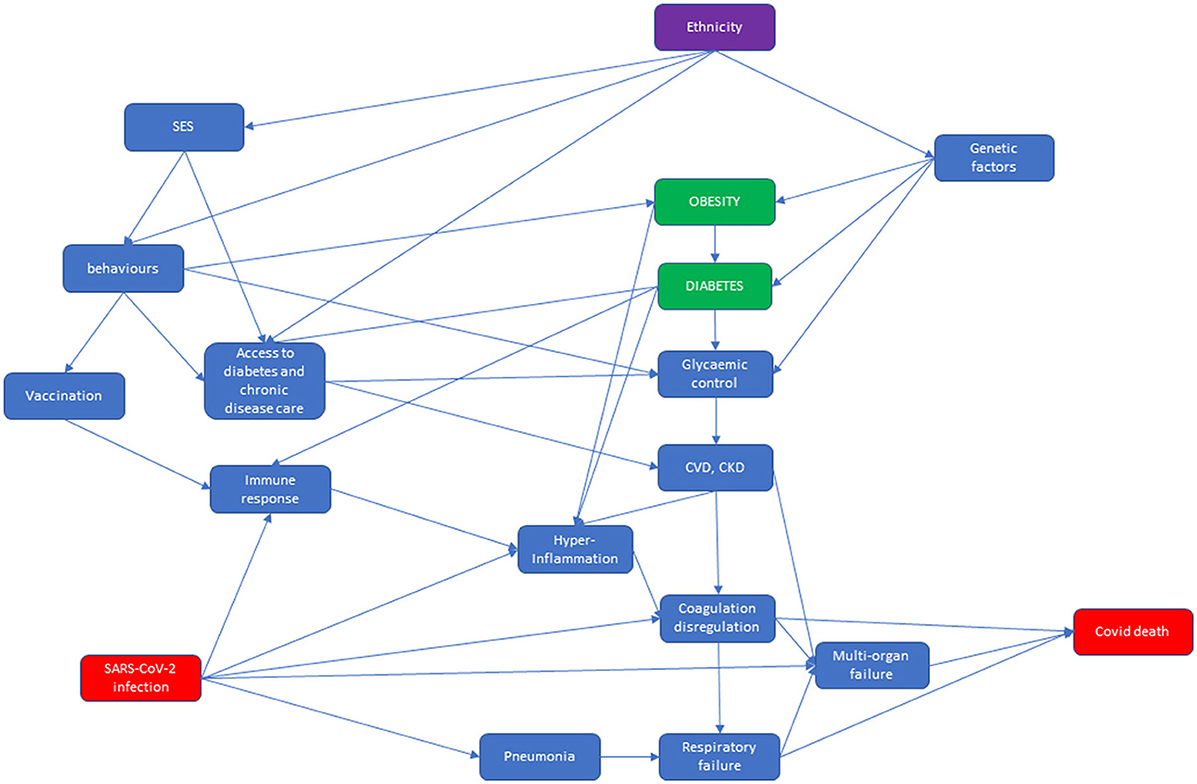
Figure 2. COVID-19 vaccination coverage for HDC and HMPC populations for the adult population residing in Reggio Emilia, as of August 10, 2021.
The first DAG starts from the association between ethnicity and socio-economic status (SES), characterizing immigrant status in Italy for the HMPC population (32). Both ethnicity and SES influence behaviors that can impact obesity [through diet and physical activity (33, 34)], vaccination (35, 36) and—among individuals with diabetes—glycemic control (21) and access to health care (37). Furthermore, linguistic and logistical barriers limit the accessibility of health care for immigrants (37, 38). Behaviors and living and working conditions influence the probability that an individual will be in contact with SARS-CoV-2 (5, 6, 39). Vaccination status, and possibly diabetes (40) and glycemic control (41), influences the ability of the immune system to block the development of a detectable infection once contact has occurred. In this latter step, we had not considered those infections that had not been detected, mostly because they were asymptomatic and did not lead to testing. More generally, the DAG does not account for factors influencing the probability of testing for screening, tracing or mild symptoms. As reported in the rationale for choosing the study outcomes, it is reasonable to think that migrant status could influence the probability of testing (9, 27, 42).
At the top of the second DAG, we have represented pre-existing conditions that might modify the probability of death from COVID-19–reported at the bottom right of the DAG. In Italy, ethnicity is associated with SES, because immigrants have lower SES, which in turn affects behaviors such as physical activity and diet, and they are thus prone to obesity and poor glycemic control. People's behaviors determine vaccination status, which modifies immune response. Moreover, SES and people's behaviors affect access to care for diabetes and other chronic diseases. This is then associated with poor glycemic control and with pre-existing cardiovascular disease (CVD) (37, 43). In addition, genetic factors are linked to diabetes and glycemic control, in particular among the South Asian population (22, 23). Diabetes not only affects the heart and kidneys but hyperglycemia in diabetes is also thought to cause an immune response dysfunction (44). Both these factors are associated with hyperinflammation, coagulation dysregulation, and multi-organ failure (44). SARS-CoV-2 infection is directly associated with pneumonia and respiratory failure, but also with multi-organ failure, and it is indirectly associated with immune response dysfunction, which can evolve into hyperinflammation, and then into coagulation dysregulation. In conclusion, respiratory failure, multi-organ failure, and coagulation dysregulation lead to COVID-19 death (45).
Results
Overall population
On December 31, 2019, 447,510 adults (18+) were residents in the province of Reggio Emilia. Of those individuals, 376,016 (84%) were from HDC populations and 71,494 (16%) were from HMPC populations. Generally, the HMPC group was younger than the HDC group, both in terms of the general population and for those with COVID-19. We counted 31,943 COVID-19 infections among HDC and 5,441 among HMPC population. Of these, 1,194 HDC and 28 HMPC died of COVID-19. Table 1 shows crude COVID-19 IRs and MRs per 1,000 individuals in the HDC and HMPC groups according to demographic and clinical characteristics. A lower incidence (IRR: 0.84 95% CI: 0.82–0.87) was observed in the HMPC group than in the HDC group (Supplementary Table 1). The MMR was also lower for HMPC, but it was based on 28 deaths in HMPC and the estimate was rather imprecise (MRR: 0.67 95% CI: 0.46–0.99).
The risk factor that showed a substantial difference in the strength of the association was obesity, which had a greater effect on mortality among HMPC individuals than among the HDC population (HR for HMPC: 18.92 95% CI: 4.48–78.87; HR for HDC: 3.91 95% CI: 2.69–5.69). A slightly higher effect of type-2 diabetes on SARS-CoV-2 infection was observed in the HMPC group (HR: 1.37 95% CI: 1.22–1.53), and particularly in South Asian individuals (HR: 1.43 95% CI: 1.17–1.75), than in the HDC population (HR: 1.20 95% CI: 1.14–1.25), while a difference in the effect on mortality was observed for type-2 diabetes (HR for HMPC: 3.96 95% CI: 1.82–8.60; HR for HDC: 1.71 95% CI: 1.50–1.95) and arrhythmia (HR for HMPC: 5.59 95% CI: 1.61–19.45; HR for HDC: 1.73 95% CI: 1.47–2.04) among the HMPC population compared to the HDC population. Hypertension and vascular diseases also showed a greater impact on risk of death among HMPC individuals than among the HDC population, but the differences were compatible with random fluctuations (Table 2). COVID-19 vaccination was shown to be a protective factor against SARS-CoV-2 infection among both HDC and HMPC populations, with an increasingly protective effect as the number of doses increased (Table 2).
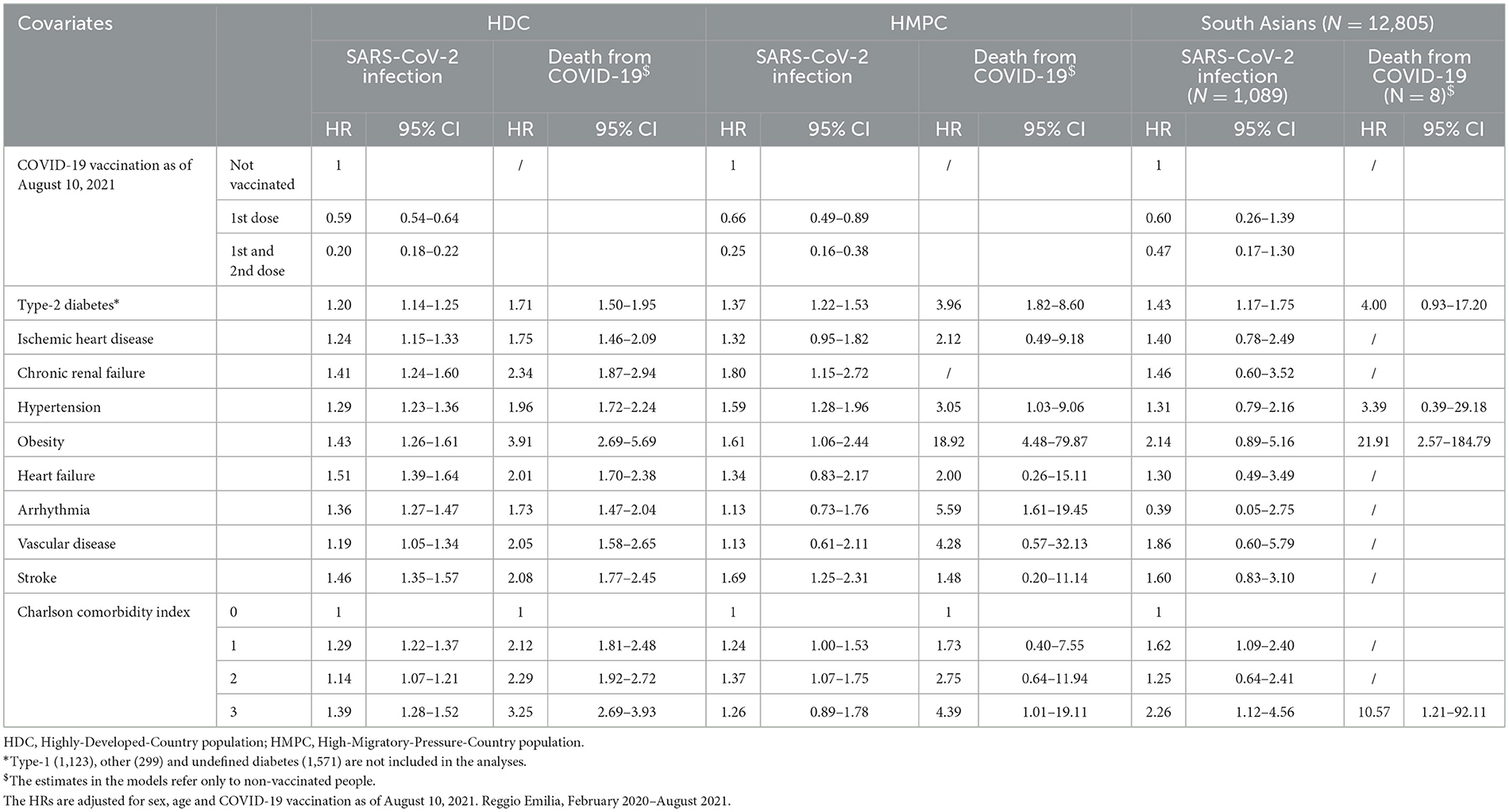
Table 2. Hazard ratios (HRs) of first infections from SARS-CoV-2 and deaths from COVID-19, by demographic and clinical characteristics, stratified by HDC, HMPC and South Asian populations.
If we analyzed the initial months of vaccination against COVID-19 in our cohort, cumulative coverage was higher among HDC individuals than HMPC individuals, for both the first and second doses of the vaccine (Figure 3). As of August 10, 2021, one dose vaccination coverage was 78 and 47% for HDC and HMPC, respectively; while the two-dose vaccination coverage was 65 and 31%, respectively. The risk reduction in relation to SARS-CoV-2 infection was around 80% among HDC individuals and 75% among HMPC individuals with two doses of vaccination.
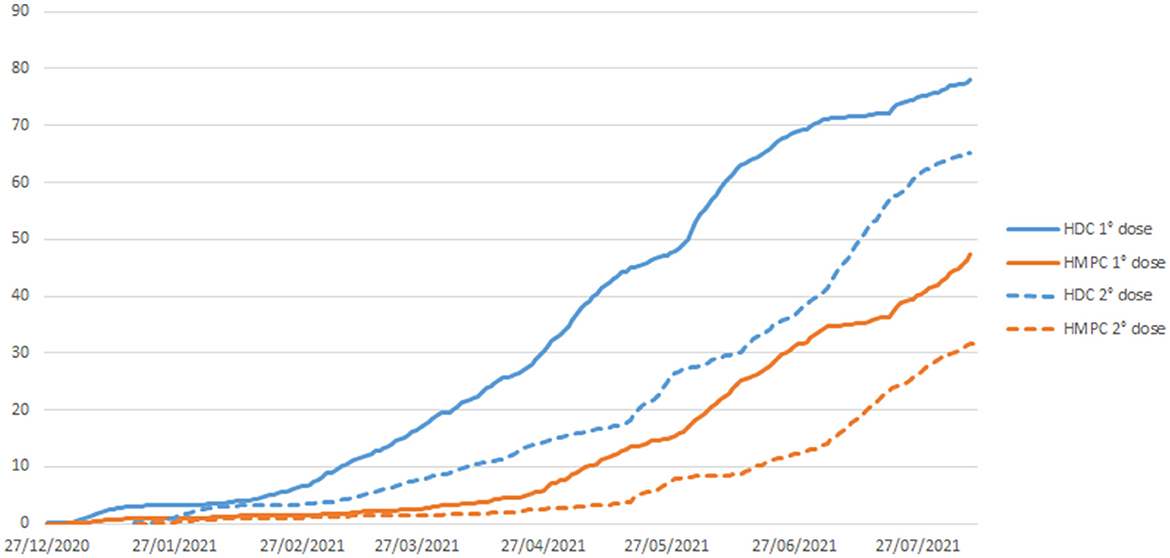
Figure 3. Direct acyclic graph (DAG) of the relationship between ethnicity and diabetes and COVID-19-related death, in which the effect modifier is ethnicity, the exposures are obesity and diabetes, and the outcome is COVID-19-related death.
Diabetic population
Among the residents of the province of Reggio Emilia, 25,844 HDC (87.1%) and 3,829 HMPC (12.9%) individuals had type-2 diabetes (Table 3). Of these, there were 2,216 COVID-19 infections among HDC and 349 among HMPC. COVID-19 deaths were 315 among HDC and 12 among HMPC. Furthermore, the HMPC group showed generally similar incidence (IRR: 0.99 95% CI: 0.88–1.12) and mortality (MRR: 0.89 95% CI: 0.49–1.61) to that of HDC individuals (Supplementary Table 1).
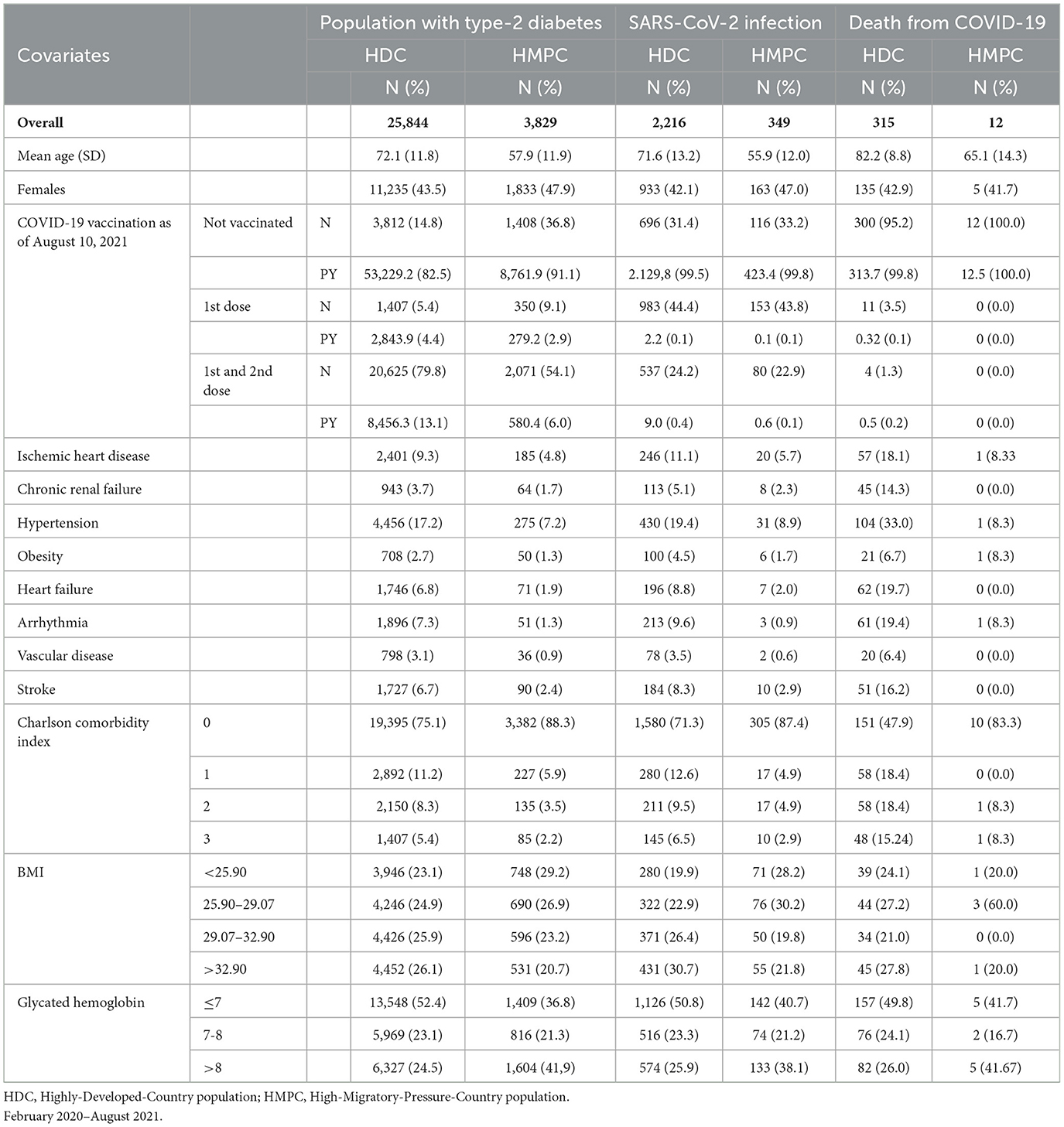
Table 3. Population with Type-2 diabetes residing in the province of Reggio Emilia, numbers and percentages of first infections of SARS-CoV-2 and deaths from COVID-19, by demographic and clinical characteristics.
SARS-CoV-2 infection from COVID-19 showed an association with obesity in both HDC and HMPC individuals with diabetes (HRs: 1.73 95% CI 1.41–2.11 and 1.41 95% CI 0.63–3.17, respectively), even if estimates were very imprecise (Table 4). Finally, in individuals with diabetes, the association between the outcomes and BMI showed a consistent trend only in HDC for incidence, with HR for the upper quartiles of BMI (29.07–32.90) 1.13 (95% CI 0.97–1.32) and for BMI>32.90 HR: 1.29 (95% CI 1.10–1.50). For HMPC, no trend was appreciable. The effect of BMI on mortality was only appreciable for BMI >32.90 with HR: 2.04 (95% CI 1.31–3.19) in HDC, while in HMPC only one death was observed in those with BMI >32.90.
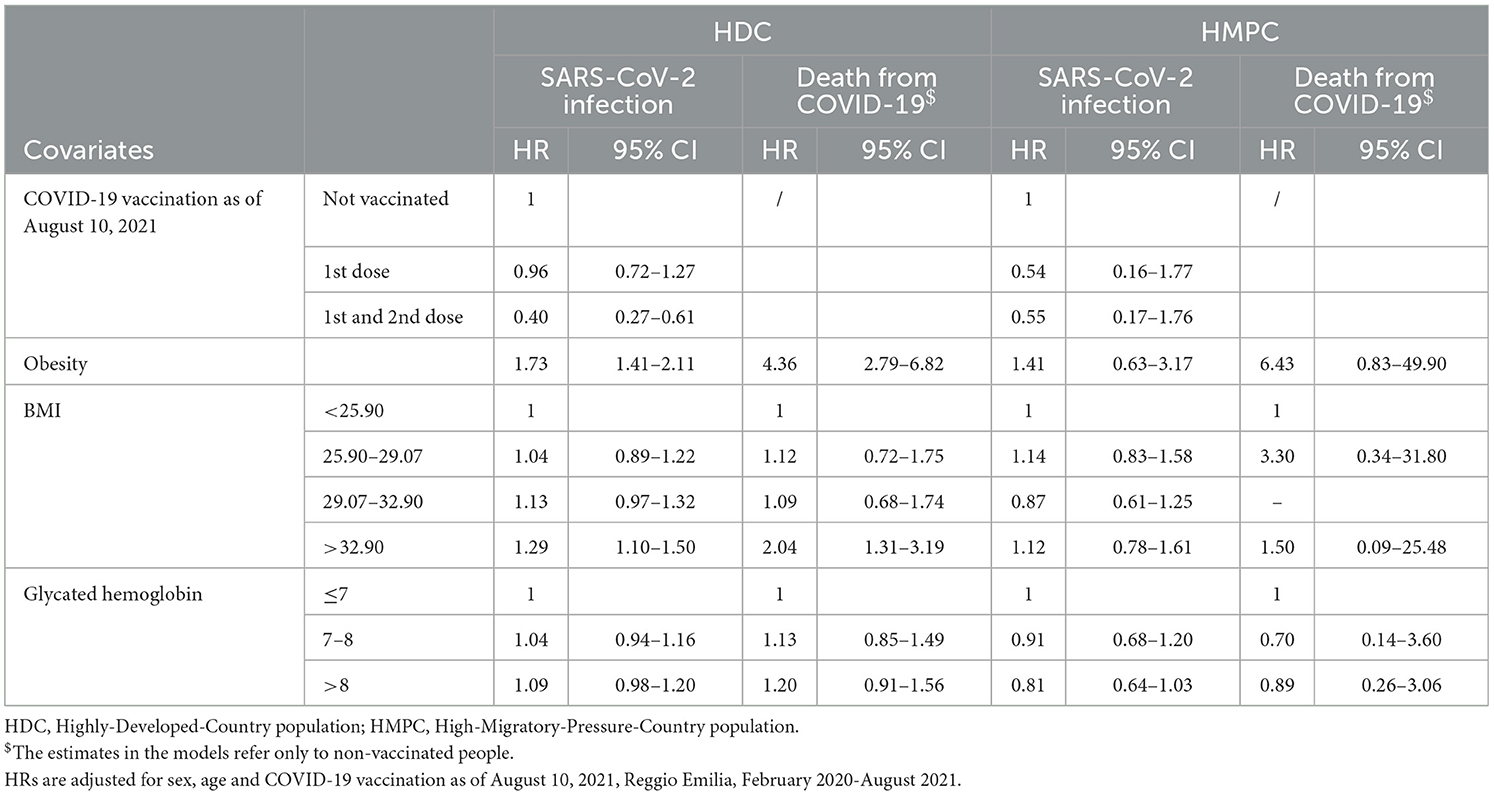
Table 4. Hazard ratios (HRs) of first infections of SARS-CoV-2 and deaths from COVID-19, in the population with Type-2 diabetes, by clinical characteristics, stratified by HDC and HMPC populations.
Glycated hemoglobin showed no association with the two outcomes, except for a slight excess risk of infection for HbA1c levels above 8% appreciable only in HDC and possibly due to random fluctuation (Table 4).
When the analysis was restricted to people <65 years of age, for deaths from COVID-19 among the HDC population, the HR for type-2 diabetes was 3.47 (95% CI: 1.81–6.67), and this became almost double among HMPC, with a HR of 6.12 (95% CI: 1.94–19.32) (Supplementary Table 2).
As for the general population, vaccination against COVID-19 infection also played a protective role among diabetics, resulting in a reduction of 60 and 45%, respectively among HDC and HMPC populations following two doses of vaccination.
Analyses stratified according to four time periods were presented in the Supplementary Tables 3–6.
Discussion
Main results
In our cohort, we did not observe any increase in incidence and mortality in individuals from HMPC, compared to Italians and people from HDC. Our data are in line with previous literature that observed a larger association between diabetes and obesity and COVID-19 mortality among immigrants, particularly from South Asia than in Europeans (4, 8, 18, 19). The effect of diabetes on the risk of infection was slightly higher in the HMPC and South Asian populations than in the HDC population. No substantial difference in the strength of the association was observed between obesity or other comorbidities and SARS-CoV-2 infection. Among individuals with diabetes, the Reggio Emilia cohort did not show any difference in the strength of risk and prognostic factors between the HMPC population and the HDC population, although the very small number of deaths in the HMPC population did not allow to draw any certain.
Limitations of the study and comparison with the international literature
It is worth noting that our results did not confirm the excess of SARS-CoV-2 infections and COVID-19 mortality reported by studies conducted in the UK and the USA for populations of Asian and African origin. Our results are consistent with other Italian studies (46, 47), which also reported a lower incidence of COVID-19 among immigrants (11), while COVID-19 mortality depended on the period considered, alternating phases of slightly lower mortality among immigrants and vice versa (12, 28). This occurred despite the fact that vaccine coverage was lower in the HMPC population than in the HDC population. Stratified analyses by calendar period did not suggest differences in the outcomes for diabetes and pathologies potentially related to diabetes between HDC and HMPC, suggesting control measures at the society level and virus variants were not effect modifiers of the observed associations.
The main limitation of this study is that our estimates of associations concerning the mortality outcome in people from HMPC are extremely imprecise, due to the relatively small number of deaths in this population. A second limitation of the study is that it was not possible to consider the socio-economic and housing conditions of the population, as data on these variables were not available for our cohort. Both types of determinants (socio-economic and clinical) could explain some of the differences in the risk of infection between the HDC and HMPC populations when these differences were present. Studies conducted in the UK showed that by adjusting for socio-economic level, as well as for pre-existing clinical conditions, the excesses of incidence and mortality observed in populations of non-European origin decreased or disappeared, suggesting that some of the worst outcomes were due to the greater state of deprivation of these populations and the worse pre-existing clinical conditions (18, 24). Previous studies found that the effect of comorbidities on COVID-19 mortality was much stronger in younger patients (31). The immigrant population is actually much younger than the Italian population, and part of the phenomenon of the higher impact of specific comorbidities on COVID-19 mortality in immigrants could thus be due to their younger age. With reference to the HDC group, if we restricted the analyses to people aged <65 years, the strength of the associations between diabetes and COVID-19 mortality (and between diabetes, BMI, and COVID-19 mortality, for those individuals with diabetes) was greater than at all ages combined.
Regarding the differences observed in the associations for diabetes between HMPC and HDC populations in SARS-CoV-2 infection, our results were unable to distinguish the portion of the effect caused by SES, behavior and access to care from the portion of the effect caused by the different biological factors and genetic background (Figure 1). However, a previous study on this population (48) had shown that despite the fact that South Asians attended diabetes clinics more than Italians and had a similar level to Italians for compliance with guidelines, they had poorer glycemic control, thus suggesting some biological determinants for the differences observed. Nevertheless we did not observed any overall COVID-19 mortality excess in the HMPC population despite a higher prevalence of diabetes and a stronger effect of diabetes on COVID-19 mortality in this population. This suggests that if any fragility factor due to genetic background is present, its effect on COVID-19 severity is small. The few studies that could adjust, at least in part, for socioeconomic conditions, including crowding and housing conditions found that the excess risk was extremely reduced in immigrants. Furthermore, in studies from UK, part of the excess mortality in non-white people with diabetes was due to worsening pre-existing chronic conditions. It should be considered that immigration in Italy is a relatively recent period, and the healthy migrant effect is still appreciable (48). Thus, it is possible that, even among the immigrants with diabetes, the burden of chronic conditions is still low due to the selection of healthier people among those affording the move to another country (49).
A lower incidence of infection in immigrants may be due to more limited testing and consequent undiagnosed disease. A possible bias in testing has been observed in the Emilia-Romagna Region, but it did not always go in the direction of a lower probability of testing in immigrants. During the first wave, a reduced probability of testing has been demonstrated, especially for women (9) from HMPC populations (27). On the contrary, increased screening activity related to international traveling has been observed in immigrants during the summer of 2020 (28). This difference in the probability of COVID-19 diagnoses led us to not consider the fatality rate of COVID-19 as a reliable outcome to compare the interaction between metabolic risk factors and ethnicity or immigrant status. This is why, despite our conceptual framework presenting the causal chains in two steps, i.e., from contact with the virus to infection and from infection to death, we only presented the associations of factors assessed before infection with infection and with death.
When we focused on individuals with diabetes, the excess in COVID-19 mortality linked to obesity and BMI was similar among the HPMC and HDC populations, as was the effect of glycemic control. This suggests that the stronger effect of obesity on COVID-19 mortality observed in the HMPC population compared with the general population was linked to the higher prevalence of diabetes in the HMPC population. This finding is not consistent with those reported by a large population-based study conducted in a cohort of people with diabetes in England, where the effect of BMI >40 on COVID-19 mortality was stronger among all non-white ethnicities than in those classified as white (11). Nevertheless, our estimates were rather imprecise and confidence intervals included the estimates from the English cohort.
Conclusions
Diabetes and pathologies potentially related to diabetes are a worse risk factor for death from COVID-19 for HMPC individuals and particularly for South Asians than for the HDC population. Although there is evidence that some biological mechanisms contribute to worsening the outcome of COVID-19 in some ethnic groups, the fact that we did not observe an overall excess risk of COVID-19 mortality in immigrants in our cohort (50) suggests that this intrinsic disadvantage is small and does not justify the higher mortality observed in other studies (5, 7, 51), thus redirecting attention to socio-economic and environmental causes.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Researchers who would like to access individual data should present their request, together with a study protocol, to the Area Vasta Emilia Nord Ethics Committee for approval (Y2VyZWdnaW9lbWlsaWFAYXVzbC5yZS5pdA==). Requests to access these datasets should be directed to bGF1cmEuYm9udmljaW5pQGF1c2wucmUuaXQ=.
Reggio Emilia COVID-19 working group
Massimo Costantini, Roberto Grilli, Massimiliano Marino, Giulio Formoso, Debora Formisano, Ivano Venturi, Cinzia Campari, Francesco Gioia, Serena Broccoli, Marta Ottone, Pierpaolo Pattacini, Giulia Besutti, Valentina Iotti, Lucia Spaggiari, Chiara Seidenari, Licia Veronesi, Paola Affanni, Maria Eugenia Colucci, Andrea Nitrosi, Marco Foracchia, Rossana Colla, Marco Massari, Anna Maria Ferrari, Mirco Pinotti, Nicola Facciolongo, Ivana Lattuada, Laura Trabucco, Stefano De Pietri, Giorgio Francesco Danelli, Laura Albertazzi, Enrica Bellesia, Simone Canovi, Mattia Corradini, Tommaso Fasano, Elena Magnani, Annalisa Pilia, Alessandra Polese, Silvia Storchi Incerti, Piera Zaldini, Efrem Bonelli, Bonanno Orsola, Matteo Revelli, Carlo Salvarani, Carmine Pinto, Pamela Mancuso, Francesco Venturelli, Massimo Vicentini, Cinzia Perilli, Elisabetta Larosa, Eufemia Bisaccia, Emanuela Bedeschi, Alessandro Zerbini, and Paolo Giorgi Rossi.
Author contributions
PG designed the study, planned the data analysis, drafted the outline of the manuscript, and critically reviewed and revised the manuscript. MO and LBa contributed to designing the study, conducted the analyses, and drafted the methods and results of the manuscript. LBo contributed to designing the study, to analyzing the data, and to writing the manuscript. All authors approved the final manuscript as submitted.
Acknowledgments
The present paper has been supported by the EU founds within PNRR program: PNRR-MAD-2022-12376546 (LBo). This project was carried out with the technical and financial support of the Italian Ministry of Health - CCM 2020.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.969143/full#supplementary-material
References
1. Riccardo F, Ajelli M, Andrianou XD, Bella A, Manso MD, Fabiani M, et al. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Euro Surveill. (2020) 25:1–11. doi: 10.2807/1560-7917.ES.2020.25.49.2000790
2. Giorgi Rossi P, Broccoli S, Angelini P. Case fatality rate in patients with COVID-19 infection and its relationship with length of follow up. J Clin Virol. (2020) 128:104415. doi: 10.1016/j.jcv.2020.104415
3. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
4. Bhaskaran K, Bacon S, Evans SJ, Bates CJ, Rentsch CT, MacKenna B, et al. Factors associated with deaths due to COVID-19 vs. other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Regional Health. (2021) 6:100109. doi: 10.1016/j.lanepe.2021.100109
5. Nafilyan V, Islam N, Mathur R, Ayoubkhani D, Banerjee A, Glickman M, et al. Ethnic differences in COVID-19 mortality during the first two waves of the Coronavirus Pandemic: a nationwide cohort study of 29 million adults in England. Eur J Epidemiol. (2021) 36:605. doi: 10.1007/s10654-021-00765-1
6. Ayoubkhani D, Nafilyan V, White C, Goldblatt P, Gaughan C, Blackwell L, et al. Ethnic-minority groups in England and Wales—factors associated with the size and timing of elevated COVID-19 mortality: a retrospective cohort study linking census and death records. Int J Epidemiol. (2020) 49:1951–62. doi: 10.1093/ije/dyaa208
7. Reyes MV. The disproportional impact of coVID-19 on African Americans. Health Hum Rights. (2020) 22:299.
8. Sze S, Pan D, Nevill CR, Gray LJ, Martin CA, Nazareth J, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. (2020) 29:100630. doi: 10.1016/j.eclinm.2020.100630
9. Group REC-1W. Prevalence of SARS-CoV-2 (Covid-19) in Italians and in immigrants in an area of Northern Italy (Reggio Emilia). Epidemiol Prev. (2020) 44:304–07. doi: 10.19191/EP20.4.P304.061
10. Amodio E, Battisti M, Maida CM, Zarcone M, Casuccio A, Vitale F. Socio-demographic factors involved in a low-incidence phase of SARS-CoV-2 spread in sicily, Italy. Healthcare. (2021) 9:7. doi: 10.3390/healthcare9070867
11. Maifredi G, Magoni M, Ercolanoni M, Lazzeretti M, Gennaro N, Ferroni E, et al. SARS-CoV-2 epidemic among Italians e resident immigrant population: differential incidence from an interregional multicentre study. Epidemiol Prev. (2022) 46:41–8. doi: 10.19191/EP22.4S1.055
12. Di Girolamo C, Bartolini L, Allotta AV, Cacciani L, Cernigliaro A, Di Napoli A, et al. Mortalità per COVID-19 nella popolazione immigrata in sette Regioni italiane da inizio pandemia a metà luglio 2021. Epidemiol Prev. (2022) 46:59–69. doi: 10.19191/EP22.4S1.057
13. Cacciani L, Calandrini E, Cascini S, Spadea T, Rusciani R, Ercolanoni M, et al. Hospital assistance for COVID-19: a comparison between non-Italian and Italian resident population in five Italian Regions since the beginning of the pandemic until June 2021. Epidemiol Prev. (2022) 46:49–58. doi: 10.19191/EP22.4S1.056
14. Ferroni E, Gennaro N, Amidei CB, Avossa F, Maifredi G, Spadea T, et al. Impact of COVID-19 on the immigrant population in the Veneto Region (Northern Italy), by geographical area of origin. Epidemiol Prev. (2022) 46:81–8. doi: 10.19191/EP22.4S1.059
15. Gu T, Mack JA, Salvatore M, Sankar SP, Valley TS, Singh K, et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open. (2020) 3:e2025197–e2025197. doi: 10.1001/jamanetworkopen.2020.25197
16. Hussain A, Bhowmik B. do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. (2020) 162:108142. doi: 10.1016/j.diabres.2020.108142
17. Pranata R, Henrina J, Raffaello WM, Lawrensia S, Huang I. Diabetes and COVID-19: the past, the present, and the future. Metabolism. (2021) 121:154814. doi: 10.1016/j.metabol.2021.154814
18. Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. (2020) 8:823. doi: 10.1016/S2213-8587(20)30271-0
19. Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O'Rahilly S, Aveyard P, et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. (2021) 9:350–9. doi: 10.1016/S2213-8587(21)00089-9
20. Sattar N, Ho FK, Gill JM, Ghouri N, Gray SR, Celis-Morales CA, et al. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: preliminary findings from UK biobank. Diabetes Metab Syndr. (2020) 14:1149–51. doi: 10.1016/j.dsx.2020.06.060
21. Ballotari P, Caroli S, Ferrari F, Romani G, Marina G, Chiarenza A, et al. Differences in diabetes prevalence and inequalities in disease management and glycaemic control by immigrant status: a population-based study (Italy). BMC Public Health. (2015) 15:87. doi: 10.1186/s12889-015-1403-4
22. Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs. white Caucasians. Int J Obes. (2011) 35:167–87. doi: 10.1038/ijo.2010.135
23. Gujral UP, Pradeepa R, Weber MB, Narayan KMV, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. (2013) 1281:51–63. doi: 10.1111/j.1749-6632.2012.06838.x
24. Yates T, Zaccardi F, Islam N, Razieh C, Gillies CL, Lawson CA, et al. Obesity, ethnicity, and risk of critical care, mechanical ventilation, and mortality in patients admitted to hospital with COVID-19: analysis of the ISARIC CCP-UK cohort. Obesity. (2021) 29:1223–30. doi: 10.1002/oby.23178
25. Mangone L, Gioia F, Mancuso P, Bisceglia I, Ottone M, Vicentini M, et al. Cumulative COVID-19 incidence, mortality and prognosis in cancer survivors: A population-based study in Reggio Emilia, Northern Italy. Int J Cancer. (2021) 149:820–6. doi: 10.1002/ijc.33601
26. Ballotari P, Ranieri SC, Vicentini M, Caroli S, Gardini A, Rodolfi R, et al. Building a population-based diabetes register: an Italian experience. Diabetes Res Clin Pract. (2014) 103:79–87. doi: 10.1016/j.diabres.2013.11.020
27. Profili F, Stasi C, Silvestri C, Ferroni E, Zorzi M, Ventura M, et al. The impact of the COVID-19 pandemic on the Italian and foreign population in the various phases: the results of an interregional multicentre project. Epidemiol Prev. (2022) 46:71–9.
28. Caranci N, Di Girolamo C, Bartolini L, Fortuna D, Berti E, Sforza S, et al. General and COVID-19-related mortality by pre-existing chronic conditions and care setting during 2020 in Emilia-Romagna region, Italy. Int J Environ Res Public Health. (2021) 18:13224. doi: 10.3390/ijerph182413224
29. CCM - Regione Marche e coordinato dalla Regione Marche,. La Salute Della Popolazione Immigrata: Metodologia Di Analisi. (2007). Available online at: https://www.ccm-network.it/documenti_Ccm/prg_area5/Prg_5_Immigrati_metodologia.pdf.pdf
30. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
31. Ferroni E, Rossi PG, Alegiani SS, Trifirò G, Pitter G, Leoni O, et al. Survival of hospitalized COVID-19 patients in Northern Italy: a population-based cohort study by the ITA-COVID-19 Network. Clin Epidemiol. (2020) 12:1337–46. doi: 10.2147/CLEP.S271763
32. Petrelli A, Di Napoli A, Agabiti N, Barbieri G, Bardin A, Bargagli AM, et al. Immigrants' health and socioeconomic inequalities of overall population residing in Italy evaluated through the Italian network of Longitudinal Metropolitan Studies. Epidemiol Prev. (2019) 43:5. doi: 10.19191/EP19.5-6.S1.112
33. Campostrini S, Carrozzi G, Severoni S, Masocco M, Salmaso S, Europe WMHPOotRDWROf, et al. Migrant health in Italy: a better health status difficult to maintain-country of origin and assimilation effects studied from the Italian risk factor surveillance data. Popul Health Metr. (2019) 17:1–11. doi: 10.1186/s12963-019-0194-8
34. Oude Groeniger J, Kamphuis CBM, Mackenbach JP, Beenackers MA, van Lenthe FJ. Are socio-economic inequalities in diet and physical activity a matter of social distinction? A cross-sectional study. Int J Public Health. (2019) 64:1037–47. doi: 10.1007/s00038-019-01268-3
35. Bertoncello C, Ferro A, Fonzo M, Zanovello S, Napoletano G, Russo F, et al. Socioeconomic determinants in vaccine hesitancy and vaccine refusal in Italy. Vaccines. (2020) 8:1–9. doi: 10.3390/vaccines8020276
36. Abba-Aji M, Stuckler D, Galea S, McKee M. Ethnic/racial minorities' and migrants' access to COVID-19 vaccines: a systematic review of barriers and facilitators. J Migr Health. (2022) 5:100086. doi: 10.1016/j.jmh.2022.100086
37. Buja A, Gini R, Visca M, Damiani G, Federico B, Francesconi P, et al. Prevalence of chronic diseases by immigrant status and disparities in chronic disease management in immigrants: a population-based cohort study, Valore Project. BMC Public Health. (2013) 13:1–7. doi: 10.1186/1471-2458-13-504
38. Di Napoli A, Ventura M, Spadea T, Rossi PG, Bartolini L, Battisti L, et al. Barriers to accessing primary care and appropriateness of healthcare among immigrants in Italy. Front Public Health. (2022) 10:47 doi: 10.3389/fpubh.2022.817696
39. Nafilyan V, Islam N, Ayoubkhani D, Gilles C, Katikireddi SV, Mathur R, et al. Ethnicity, household composition and COVID-19 mortality: a national linked data study. J R Soc Med. (2021) 114:182–211. doi: 10.1177/0141076821999973
40. Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. (2016) 4:148–58. doi: 10.1016/S2213-8587(15)00379-4
41. Hayek S, Ben-Shlomo Y, Balicer R, Byrne K, Katz M, Kepten E, et al. Preinfection glycaemic control and disease severity among patients with type 2 diabetes and COVID-19: a retrospective, cohort study. Diabetes Obes Metab. (2021) 23:1995–2000. doi: 10.1111/dom.14393
42. González JLD, Rusciani R, Spadea T, Leoni O, Bortolan F, Cacciani L, et al. Access to SARS-CoV-2 diagnostic tests: are there barriers for the immigrants in Italy? Epidemiol Prev. (2022) 46:33–40. doi: 10.19191/EP22.4S1.054
43. Marchesini G, Bernardi D, Miccoli R, et al. Under-treatment of migrants with diabetes in a universalistic health care system: the ARNO observatory. Nutr Metab Cardiovasc Dis. (2014) 24:393–9. doi: 10.1016/j.numecd.2013.09.012
44. Landstra CP, de Koning EJP. COVID-19 and diabetes: understanding the interrelationship and risks for a severe course. Front Endocrinol. (2021) 12:649525. doi: 10.3389/fendo.2021.649525
45. Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. (2022) 20:270–84. doi: 10.1038/s41579-022-00713-0
46. Petrelli A, Di Napoli A. The impact of COVID-19 on the immigrant population in Italy. Context, methodology and synthesis of the main evidence from the project of the National Institute for Health, Migration and Poverty (INMP) and Italian Regions. Epidemiol Prev. (2022) 46:7–13. doi: 10.19191/EP22.4S1.051
47. Fabiani M, Vescio MF, Bressi M, Mateo-Urdiales A, Petrone D, Spuri M, et al. Differences in the incidence and clinical outcomes of SARS-CoV-2 infection between Italian and non-Italian nationals using routine data. Public Health. (2022) 211:136–43. doi: 10.1016/j.puhe.2022.07.022
48. Pacelli B, Zengarini N, Broccoli S, Caranci N, Spadea T, Di Girolamo C, et al. Differences in mortality by immigrant status in Italy. Results of the Italian network of longitudinal metropolitan studies. Eur J Epidemiol. (2016) 31:691–701. doi: 10.1007/s10654-016-0177-z
49. Razum O, Zeeb H. The'healthy migrant effect'—not merely a fallacy of inaccurate denominator figurese. Int J Epidemiol. (2000) 29:191–2. doi: 10.1093/ije/29.1.191
50. Abolfazl Adli M. Reza Khodaie, Niloofar Hashemzaei SMH. Role of genetic variants and host polymorphisms on COVID-19: From viral entrance mechanisms to immunological reactions. J Med Virol. (2022) 94:1846–65. doi: 10.1002/jmv.27615
51. Ayoubkhani D, Nafilyan V, White C, Goldblatt P, Gaughan C, Blackwell L, et al. Ethnic-minority groups in England and Wales-factors associated with the size and timing of elevated COVID-19 mortality: a retrospective cohort study linking census and death records. Int J Epidemiol. (2021) 49:1951–62.
Keywords: immigrants, SARS-CoV-2 infection, COVID-19 mortality, type-2 diabetes, diabetes register
Citation: Ottone M, Bartolini L, Bonvicini L, Giorgi Rossi P and Reggio Emilia COVID-19 working group (2023) The effect of diabetes on COVID-19 incidence and mortality: Differences between highly-developed-country and high-migratory-pressure-country populations. Front. Public Health 11:969143. doi: 10.3389/fpubh.2023.969143
Received: 14 June 2022; Accepted: 13 February 2023;
Published: 08 March 2023.
Edited by:
Cristina Canova, University Padova, ItalyReviewed by:
Nera Agabiti, Regional Health Service of Lazio, ItalyTeresa Dalla Zuanna, University of Padua, Italy
Copyright © 2023 Ottone, Bartolini, Bonvicini, Giorgi Rossi and Reggio Emilia COVID-19 working group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Bonvicini, bGF1cmEuYm9udmljaW5pQGF1c2wucmUuaXQ=
†These authors have contributed equally to this work and share first authorship
 Marta Ottone†
Marta Ottone† Laura Bonvicini
Laura Bonvicini Paolo Giorgi Rossi
Paolo Giorgi Rossi